Abstract
In May 2014, the U.S. National Institutes of Health (NIH) announced that it will ensure that investigators account for sex as a biological variable (SABV) in NIH-funded preclinical research as part of the agency’s rigor and transparency initiative. Herein, I describe in more detail the rationale behind the SABV policy component and provide additional detail about policy goals. In short, studying both sexes is a guiding principle in biomedical research that will expand knowledge toward turning discovery into health. NIH expects that considering SABV in preclinical research will help to build a knowledge base that better informs the design of clinical research and trials in humans. Integrating the practice of studying both sexes in preclinical research will, over time, expand our currently incomplete knowledge base that plays a critical role in informing the development of sex- and gender-appropriate medical care for women and men.—Clayton, J. A. Studying both sexes: a guiding principle for biomedicine.
Keywords: gender, NIH, sex-based biology, inclusion, SABV
Many medical professionals can attest to the fact that for decades, the default human model subject was a “70-kg male.” There has also been a preponderant focus on male animals in preclinical research (1, 2). The results of mostly single-sex investigations, in addition to the many studies that have not reported the sex of animals, cells, or tissues used, have contributed to an ambiguous evidence base about sex-based influences on biology and health. Researchers have assumed that fundamental biology includes only those molecular, biochemical, and physiologic characteristics that are shared, or the same, between males and females. However, it is becoming increasingly clear that instead, fundamental biology encompasses those characteristics that are both shared and different.
As has been published elsewhere, one area of current focus at NIH is ensuring both rigor and transparency in taxpayer-funded research (3). In May 2014, the National Institutes of Health (NIH) announced that the agency will ensure that investigators account for sex as a basic biological variable (SABV) in NIH-funded preclinical research (4), and policy changes are underway. It is worth noting that the United States lags behind Europe in many cases in this arena (5–9). Accurate and detailed reporting of experimental conditions, including but not limited to sex, is a key aspect of responsible experimental practice. Established guidelines on the use of research animals in biomedical research stipulate reporting of research animal age, sex, weight, and life stage, as well as characteristics of the research animal’s environment (10, 11).
Considering SABV is not the same as looking for sex differences. In its efforts to enhance reproducibility and transparency by expecting investigators to consider SABV, NIH will not require any specific research design or method for accomplishing this goal. Rather, the existing state of knowledge in a particular scientific area and the specific research question under study will both affect how an investigator considers sex and other basic biological variables. On that note, it is important to point out that NIH policy changes to ensure the consideration of basic biological variables like sex do not imply the necessary doubling of research animals in every experiment, contrary to what some in the research community have assumed. It is true, however, that investigators aiming to differentiate sex effects—that is, to look explicitly for sex differences—may require larger numbers of animals, or equal numbers of animals of both sexes, for adequate power to detect statistically significant effects. Typically, these types of projects grow from preliminary data that provide hints of sex-based influences that generate a testable hypothesis in larger sample sizes. Notably, this concept applies to detecting differences attributed to a range of biological variables including age, genetic strain, environmental conditions, or others. Such studies, ongoing for many years, have contributed to an important body of knowledge about how sex differences affect various physiologic processes and health conditions. Adding to this knowledge base will tell us even more about how sex affects human biology beyond the traditional notion of reproduction.
Not only is studying both sexes in biomedical research good science, it also offers unique opportunities for biomedical discovery. Herein, I present a contextual summary of studying both sexes across the biomedical spectrum and also briefly describe strategies and experimental design approaches to help researchers get started in this area without limiting ongoing inquiry.
STUDYING BOTH SEXES ACROSS THE RESEARCH SPECTRUM
Sex and gender inform various levels of research distinctly. Although sex is a biological attribute relating to genetics, physiology, or anatomy and defines cells and organisms as “male” or “female,” gender references behavioral, social, and cultural domains relevant to humans. Because gender is less amenable to investigation in preclinical work, the rest of this discussion focuses on assessing the variable, sex.
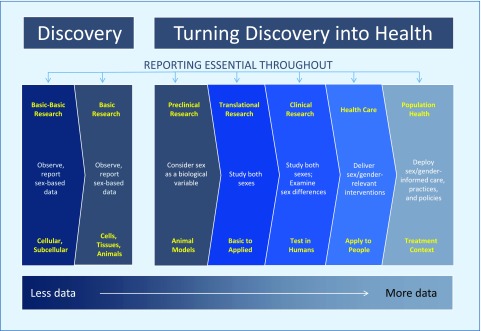
From basic research to clinical care, studying both sexes can be viewed as a guiding principle to aid in experimental design, hypothesis generation and testing, and in quantifying and expanding knowledge toward turning discovery into health for both women and men (Fig. 1). The NIH mission spans basic discovery to health, and it is instructive to consider how sex and gender factors can be addressed distinctly from cells to selves to populations.
Figure 1.
Studying both sexes across the biomedical research continuum.
One overarching feature of considering sex (and gender) in biomedical research is the essentiality of reporting at every stage. Transparency in research calls for accounting fully and accurately for all relevant biological variables—even if they are not elements of the hypothesis. Telling other investigators about the use of females and males enhances the knowledge base for future studies and helps prevent unnecessary and costly duplication of experiments.
As I noted earlier, a comparatively small but very productive cadre of researchers has for many years focused investigations on the detection of sex differences. This body of work has been foundational for our current knowledge base about sex-based biology. Such studies have led to the identification of clinically meaningful sex differences that affect gene expression to organ dysfunction. For example, a recent pain study employing male and female animals showed that males and females use different types of immune cells to convey pain signals (12). Other work has shown that male and female cells employ distinct ischemic cell death pathways in a preclinical stroke model (13, 14).
However, although sex differences may be the most obvious and visible example of the role of sex in health, it is likely that sex (and gender) exert less overt—yet biologically significant—influences on physiology and pathology. Thus, studying the influences of sex more generally is a distinct goal from looking for sex differences, only, between females and males.
Basic research
The goal of basic and “basic-basic” research at the level of molecules to cells is to define and characterize components and principles. At this stage, typically the most research-appropriate SABV activity is to observe and report sex-based data. This stage of research is about discovery, and in this realm, sex is not a determinant, but rather one piece of a larger whole—a data point or an element of an observation. Certain proteins, for example, come in female and male “versions” that can differ by several amino acids (15). Moreover, males and females “read” their genomes differently due to Y chromosome-linked genes that encode dosage-sensitive, broadly expressed regulators of transcription, translation, and protein stability (16). In isolation, such information cannot imply any sex-based effect on larger biological units, such as cells, tissues, or organs. Also, even though every cell has a sex (17), transcriptional, translational, and other processes may be influenced by components of animal serum present in cell culture medium. Phenol red, for example, can exert estrogenic effects. Cultured cells of different sexes may also have different responses to cytotoxic agents such as nitrosative stress and excitotoxicity (18). Previous work in mice has revealed sex-specific differences in the regenerative potential of muscle-derived stem cells (19); whether these differences will pertain to human cell therapy remains unknown but at the very least invites speculation. In basic research, reporting the sex of cells and tissues, when that information is available and reliable, is an important aspect of transparency and reproducibility. Determining the sex of cells reliably has been previously described (20, 21). Detailed guidelines on how to configure biological experiments that address sex as a variable in cell lines and tissue samples are available (Fig. 1) (22).
Preclinical research
In preclinical research with animals where the results aim to inform human health, sex should be accounted for directly in experimental design. At this level of NIH-funded research, investigators are seeking knowledge related to health intentionally: sex is a basic experimental variable in any preclinical study with implications for human males and human females.
Traditionally, one hesitation for the routine use of female animals in preclinical research has been concern about the complexities of the female estrus cycle; however, a recent meta-analysis that examined variability among female and male rodents across a broad array of biological traits counters the validity of this assumption (23). Nonetheless, hormonal cycling is a biological variable to be considered and various approaches exist to do this when appropriate. To answer these questions requires knowledge of how to assess and/or manipulate the hormonal condition of the subjects in the experiment appropriately within the context of the research question at hand (24–26). Although many models and approaches have been described (see Table 1 for a brief list), one is the 4-core genotype mouse model that enables an investigator to vary sex chromosome complement (XX vs. XY) to observe differential effects without confounding hormonal differences (27). The 4 genotypes are XX gonadal males, XX gonadal females, XY gonadal males, and XY gonadal females. Use of this model system can reveal differential effects of ovarian and testicular secretions, as well as identify interactions between chromosomal and hormonal effects. Four-core genotype mice are made by deletion of the testis-determining Sry from the Y chromosome and insertion of an Sry transgene onto an autosome in the same mice. Analysis of results can be accomplished using a 2 × 2 comparison (Sry+ vs. Sry−; XX vs. XY) that fits the 2-way ANOVA test. This model system has demonstrated nonhormonal sex differences in behaviors, gene expression, and disease susceptibility (28). Another strategy to assess hormonal effects genetically is the use of animal models with sex-hormone receptor knockouts. (29, 30).
TABLE 1.
Research designs for considering SABV in preclinical studies
| Research method |
Attributes |
Reference |
| Single-variable design | Single experiment, single effect | Shaw et al. (56) |
| Complete/fractional factorial design | Enables more than one independent variable | Collins et al. (57) |
| Randomized block design | Variability within blocks is less than variability between blocks | Festing (58) |
| Ancillary variable design | Sex captured but not an independent variable | Collins et al. (57) |
| 4-Core genotype model | Differentiates between gonadal and nonhormonal sex effects (mice only) | De Vries et al. (27) |
| Hormone depletion/replacement | Identifies hormonal effects without estrous cycling | Greenspan et al. (59) |
| Sex-hormone receptor knockouts | Whole-animal (mouse) approach to study hormone effects | Kerkhofs et al. (30); Walker et al. (29) |
Translational and clinical research
Late translational and clinical research involving human subjects requires studying both sexes, and in many cases, looking for sex-different outcomes that may affect interventional strategies. At the clinical stage, gender effects may also be relevant. Many examples of sex- and gender-based findings have affected diagnostic, prevention, and treatment strategies for women and men (31–33).
One area ripe for exploration is sex- and gender-informed technology and biomedical engineering. For example, although models of the human body originally employed a 50th-percentile European and North American male to guide the development of various products including crash-test dummies, recently instituted federal regulations (34) now require automakers to use petite female crash dummies in frontal automotive crash tests to more accurately model women drivers and passengers. It has been shown that women on average sit closer to the steering wheel, which increases risk for internal injury in frontal collisions (35). Another example of the relevance of sex and gender in medical technology is imaging modalities such as MRI, which capture female and male tissue differently (36).
In August 2014, the U.S. Food and Drug Administration issued a guidance document on the study and evaluation of sex-specific data in medical device clinical studies (37). This guidance aims to improve the quality and consistency of medical-device performance data in both sexes. This information is useful for patients and providers, as well as researchers. Recently published textbooks on gender medicine are also useful resources for learning more (38–41).
Knowledge gained from clinical studies is applied to individuals and populations in the wide range of settings in which people receive care. Much of this research extends beyond the direct reach of NIH funding and also has significant relevance to health policy. Gender, in particular, is of great importance at these stages, and various national and international bodies have invested significant resources in this arena. Several organizations in Canada and Europe have been paying attention to sex and gender concerns in biomedical research (42). The World Health Organization’s Strategy for Integrating Gender Analysis and Actions calls for mainstreaming a gender perspective at all levels, from research to legislation to policies and programs—in both economic and societal spheres. The notion of mainstreaming highlights the importance of disaggregating data and considering the influence of sex and gender on the health of women and men.
INCLUDING SEX IN EXPERIMENTAL DESIGN AND REPORTING: GETTING STARTED
The vast majority of respondents to a Request for Information issued by NIH in September 2014 agreed that consideration of SABV is an issue affecting the reproducibility, rigor, and/or the generalizability of research findings (43). Despite agreement that SABV is good science, however, scientists and other stakeholders are concerned about practical matters, such as cost, as well as constraints on methodological and experimental design. More than half of respondents suggested that NIH could offer tangible resources to help with SABV policy implementation. The NIH Office of Research on Women’s Health and the NIH Office of Extramural Research have taken into account this input in developing SABV training resources and scientific tools such as courses, workshops, and online resources to help applicants, reviewers, and NIH program staff.
Considering SABV in preclinical research need not be onerous or overly time-consuming, and several recent publications feature practical strategies, checklists, and toolkits (24, 44–48). Possible first steps include the following actions that have been recently summarized by Ritz et al. and other groups (44). 1) Enrich a literature search on the research topic of interest by adding terms such as sex, gender, male, and female. 2) Survey epidemiologic findings; sex-skewed disease prevalence may suggest underlying sex- or gender-based influences on physiologic or pathologic processes. 3) Conduct pilot studies, such as adding a hormone treatment to tissue cultures. Such small interventions can provide preliminary evidence for the potential role of sex in the scientific area of interest, and suggestive findings can be pursued through further studies that are powered to test for a sex difference. 4) Report sex-based data and any identified sex-based influences, so that other investigators may pursue further studies. 5) Include both females and males in test groups; this can be facilitated through the use of certain experimental designs, discussed previously, that enable interrogation of multiple independent variables.
A predominant concern among NIH stakeholders is that considering SABV in preclinical research will cost more money. Addressing the influence of sex in biomedical research with animals does not necessarily imply an increase in costs. Rather, well-designed research either directly tests or controls for variables that might influence outcome, and sex is one such variable among many that must be controlled to obtain valid data. As always, each applicant must assess the characteristics of the test system, analytic approaches, and other factors in determining his or her research design. It is difficult to generalize costs by considering impacts on individual studies and laboratories. Another worthwhile consideration is the cost of including or not including sex as a biological variable across the research continuum. For example, late-stage clinical studies that draw from a body of research that is incomplete due to lack of consideration of a key biological variable such as sex may have human costs and/or may contribute to missteps in drug development.
Even when costs increase, however, these increased expenditures accrue additional benefits derived from new insights, improved therapies, and reduced risk of toxicity surprises incurred later when an incomplete evidence base fails to adequately inform expensive late-stage clinical trials. Some costs may be expected to decrease, however. Single-sex housing costs are often greater for male mice, due to aggressive behavior in group-housed males (49). Using both sexes of mice can actually reduce the cost of maintaining a mouse colony (50). To be sure, selecting the appropriate preclinical model for the question of interest is central to the inquiry process and requires in-depth consideration of research design to economize animal use without surrendering rigor. Individual investigators are in the best position to configure the most rigorous experimental design that considers the role of sex in the context of the specific scientific question of interest.
CONCLUSIONS
Studying both sexes is a guiding principle in biomedicine. Beginning in 2016, NIH applicants will be expected to account for the possible role of sex as a biological variable in vertebrate animal and human studies. The NIH has assembled and posted online materials that address experimental design, randomization, blinding, and sample-size calculation, among others, to help investigators integrate SABV into their research (51). Curriculum projects are also underway to enrich medical school teaching of the necessity of understanding both sexes and to inform the delivery of sex- and gender-appropriate clinical care (52–54). The NIH Office of Research on Women’s Health, in cooperation with the U.S. Food and Drug Administration Office of Women's Health, also offers a free online course, “The Science of Sex and Gender in Human Health” (55).
A continual growth in knowledge of the influence of sex at molecular, cellular, and biochemical levels and the various ways that sex exerts influence will inform the design and conduct of additional biomedical research, which is imperative to the NIH mission of turning discovery into health. Understanding scientific findings in the context of sex—be they similarities, differences, and/or complex nuances—is crucial for correctly applying research-derived knowledge toward achieving our ultimate objectives.
Acknowledgments
The author acknowledges Dr. Alison Davis for intellectual and writing contributions to this paper.
Glossary
- NIH
U.S. National Institutes of Health
- SABV
sex as a biological variable
REFERENCES
- 1.Beery A. K., Zucker I. (2011) Sex bias in neuroscience and biomedical research. Neurosci. Biobehav. Rev. 35, 565–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yoon D. Y., Mansukhani N. A., Stubbs V. C., Helenowski I. B., Woodruff T. K., Kibbe M. R. (2014) Sex bias exists in basic science and translational surgical research. Surgery 156, 508–516 [DOI] [PubMed] [Google Scholar]
- 3.Collins F. S., Tabak L. A. (2014) Policy: NIH plans to enhance reproducibility. Nature 505, 612–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clayton J. A., Collins F. S. (2014) Policy: NIH to balance sex in cell and animal studies. Nature 509, 282–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Irish Research Council. Irish Research Council Gender Strategy & Action Plan (2013–2020). Retrieved October 2, 2015, from http://www.research.ie/sites/default/files/irish_research_council_gender_action_plan_2013_-2020.pdf
- 6.Klinge I. (2007) Bringing gender expertise to biomedical and health-related research. Gend. Med. 4 (Suppl B), S59–S63 [DOI] [PubMed] [Google Scholar]
- 7.Canadian Institutes of Health Research Institute of Gender and Health. Strategic Research Priorities. Retrieved October 26, 2015, from http://www.cihr-irsc.gc.ca/e/35752.html
- 8.genSET, Gender in Science. Retrieved October 26, 2015, from http://www.genderinscience.org/
- 9.U.S. National Institutes of Health. Consideration of Sex as a Biological Variable in NIH-funded Research (NOT-OD-15-102). Retrieved October 2, 2015, from http://grants.nih.gov/grants/guide/notice-files/NOT-OD-15-102.html
- 10.U.S. National Research Council Institute for Laboratory Animal Research (2011) Guidance for the Description of Animal Research in Scientific Publications, National Academies Press, Washington, DC [PubMed] [Google Scholar]
- 11.National Centre for the Replacement, Refinement, and Reduction of Animals in Research. The ARRIVE Guidelines. Available at www.nc3rs.org.uk/ARRIVE [PMC free article] [PubMed]
- 12.Sorge R. E., Mapplebeck J. C., Rosen S., Beggs S., Taves S., Alexander J. K., Martin L. J., Austin J. S., Sotocinal S. G., Chen D., Yang M., Shi X. Q., Huang H., Pillon N. J., Bilan P. J., Tu Y., Klip A., Ji R. R., Zhang J., Salter M. W., Mogil J. S. (2015) Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat. Neurosci. 18, 1081–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCullough L. D., Zeng Z., Blizzard K. K., Debchoudhury I., Hurn P. D. (2005) Ischemic nitric oxide and poly (ADP-ribose) polymerase-1 in cerebral ischemia: male toxicity, female protection. J. Cereb. Blood Flow Metab. 25, 502–512 [DOI] [PubMed] [Google Scholar]
- 14.Yuan M., Siegel C., Zeng Z., Li J., Liu F., McCullough L. D. (2009) Sex differences in the response to activation of the poly (ADP-ribose) polymerase pathway after experimental stroke. Exp. Neurol. 217, 210–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fisher E. M., Beer-Romero P., Brown L. G., Ridley A., McNeil J. A., Lawrence J. B., Willard H. F., Bieber F. R., Page D. C. (1990) Homologous ribosomal protein genes on the human X and Y chromosomes: escape from X inactivation and possible implications for Turner syndrome. Cell 63, 1205–1218 [DOI] [PubMed] [Google Scholar]
- 16.Bellott D. W., Hughes J. F., Skaletsky H., Brown L. G., Pyntikova T., Cho T. J., Koutseva N., Zaghlul S., Graves T., Rock S., Kremitzki C., Fulton R. S., Dugan S., Ding Y., Morton D., Khan Z., Lewis L., Buhay C., Wang Q., Watt J., Holder M., Lee S., Nazareth L., Alföldi J., Rozen S., Muzny D. M., Warren W. C., Gibbs R. A., Wilson R. K., Page D. C. (2014) Mammalian Y chromosomes retain widely expressed dosage-sensitive regulators. Nature 508, 494–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pardue M., and Wizemann T., eds (2001) Exploring the Biological Contributions to Human Health: Does Sex Matter? National Academy Press, Washington, DC: [PubMed] [Google Scholar]
- 18.Du L., Bayir H., Lai Y., Zhang X., Kochanek P. M., Watkins S. C., Graham S. H., Clark R. S. (2004) Innate gender-based proclivity in response to cytotoxicity and programmed cell death pathway. J. Biol. Chem. 279, 38563–38570 [DOI] [PubMed] [Google Scholar]
- 19.Deasy B. M., Lu A., Tebbets J. C., Feduska J. M., Schugar R. C., Pollett J. B., Sun B., Urish K. L., Gharaibeh B. M., Cao B., Rubin R. T., Huard J. (2007) A role for cell sex in stem cell-mediated skeletal muscle regeneration: female cells have higher muscle regeneration efficiency. J. Cell Biol. 177, 73–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang-Chu M. M., Yu M., Haverty P. M., Koeman J., Ziegle J., Lee M., Bourgon R., Neve R. M. (2015) Human biosample authentication using the high-throughput, cost-effective SNPtrace(TM) system. PLoS One 10, e0116218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lorsch J. R., Collins F. S., Lippincott-Schwartz J. (2014) Cell biology. Fixing problems with cell lines. Science 346, 1452–1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stanford University and European Union. Gendered Innovations Tissues and Cells Checklist. Retrieved October 2, 2015, from http://genderedinnovations.stanford.edu/methods/tissues_cells.html
- 23.Prendergast B. J., Onishi K. G., Zucker I. (2014) Female mice liberated for inclusion in neuroscience and biomedical research. Neurosci. Biobehav. Rev. 40, 1–5 [DOI] [PubMed] [Google Scholar]
- 24.Becker J. B., Arnold A. P., Berkley K. J., Blaustein J. D., Eckel L. A., Hampson E., Herman J. P., Marts S., Sadee W., Steiner M., Taylor J., Young E. (2005) Strategies and methods for research on sex differences in brain and behavior. Endocrinology 146, 1650–1673 [DOI] [PubMed] [Google Scholar]
- 25.Becker J. B., Robinson T. E., Lorenz K. A. (1982) Sex differences and estrous cycle variations in amphetamine-elicited rotational behavior. Eur. J. Pharmacol. 80, 65–72 [DOI] [PubMed] [Google Scholar]
- 26.Feder H. H. (1981) Estrous cyclicity in mammals. In Neuroendocrinology of Reproduction: Physiology and Behavior (Adler N. T., ed.), pp. 279–348, Plenum Press, New York [Google Scholar]
- 27.De Vries G. J., Rissman E. F., Simerly R. B., Yang L. Y., Scordalakes E. M., Auger C. J., Swain A., Lovell-Badge R., Burgoyne P. S., Arnold A. P. (2002) A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. J. Neurosci. 22, 9005–9014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arnold A. P., Chen X. (2009) What does the “four core genotypes” mouse model tell us about sex differences in the brain and other tissues? Front. Neuroendocrinol. 30, 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walker V. R., Korach K. S. (2004) Estrogen receptor knockout mice as a model for endocrine research. ILAR J. 45, 455–461 [DOI] [PubMed] [Google Scholar]
- 30.Kerkhofs S., Denayer S., Haelens A., Claessens F. (2009) Androgen receptor knockout and knock-in mouse models. J. Mol. Endocrinol. 42, 11–17 [DOI] [PubMed] [Google Scholar]
- 31.Ridker P. M., Cook N. R., Lee I. M., Gordon D., Gaziano J. M., Manson J. E., Hennekens C. H., Buring J. E. (2005) A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N. Engl. J. Med. 352, 1293–1304 [DOI] [PubMed] [Google Scholar]
- 32.Young E. A., Kornstein S. G., Marcus S. M., Harvey A. T., Warden D., Wisniewski S. R., Balasubramani G. K., Fava M., Trivedi M. H., John Rush A. (2009) Sex differences in response to citalopram: a STAR*D report. J. Psychiatr. Res. 43, 503–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farkas R. H., Unger E. F., Temple R. (2013) Zolpidem and driving impairment--identifying persons at risk. N. Engl. J. Med. 369, 689–691 [DOI] [PubMed] [Google Scholar]
- 34.U.S. Government Publishing Office (2011) U.S. Code of Federal Regulations: 49 CFR U, -2RE Side Impact Crash Test Dummy, 50TH Percentile Adult Male. Retrieved October 2, 2015 from http://www.gpo.gov/fdsys/granule/CFR-2011-title49-vol7/CFR-2011-title49-vol7-part572-subpartU
- 35.Augenstein J., Perdeck E., Bahouth G. T., Digges K. H., Borchers N., Bauer P. (2005) Injury identification: priorities for data transmitted. In Proceedings of the 19th International Technical Conference on the Enhanced Safety of Vehicles (ESV), U.S. Department of Transportation National Highway Traffic Safety Administration, Washington, DC [Google Scholar]
- 36.Biswal B. B., Mennes M., Zuo X. N., Gohel S., Kelly C., Smith S. M., Beckmann C. F., Adelstein J. S., Buckner R. L., Colcombe S., Dogonowski A. M., Ernst M., Fair D., Hampson M., Hoptman M. J., Hyde J. S., Kiviniemi V. J., Kötter R., Li S. J., Lin C. P., Lowe M. J., Mackay C., Madden D. J., Madsen K. H., Margulies D. S., Mayberg H. S., McMahon K., Monk C. S., Mostofsky S. H., Nagel B. J., Pekar J. J., Peltier S. J., Petersen S. E., Riedl V., Rombouts S. A., Rypma B., Schlaggar B. L., Schmidt S., Seidler R. D., Siegle G. J., Sorg C., Teng G. J., Veijola J., Villringer A., Walter M., Wang L., Weng X. C., Whitfield-Gabrieli S., Williamson P., Windischberger C., Zang Y. F., Zhang H. Y., Castellanos F. X., Milham M. P. (2010) Toward discovery science of human brain function. Proc. Natl. Acad. Sci. USA 107, 4734–4739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.U.S. Food and Drug Administration. Evaluation of Sex-Specific Data in Medical Device Clinical Studies - Guidance for Industry and Food and Drug Administration Staff. Retrieved October 2, 2015, from http://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/UCM283707.pdf
- 38.Legato M. (2009) Principles of Gender-Specific Medicine, 2nd Ed., Elsevier/Academic Press, Waltham, MA, USA [Google Scholar]
- 39.Oertelt-Prigione, S., and Regitz-Zagrosek, V. (2012) Sex and Gender Aspects in Clinical Medicine, Springer Science and Business Media, New York
- 40.Schenk-Gustafsson K., DeCola P. R., Pfaff S. W., Plsetsky D. S. (2012) Handbook of Clinical Gender Medicine, Karger; Publishers, Stockholm, Sweden [Google Scholar]
- 41.Regitz-Zagrosek V., ed (2012) Sex and Gender Differences in Pharmacology, Springer Science and Business Media, New York: [Google Scholar]
- 42.Rabesandratana T. (2014) Adding sex-and-gender dimensions to your research. Science (Science Careers). Retrieved October 26, 2015, from http://sciencecareers.sciencemag.org/career_magazine/previous_issues/articles/2014_03_13/caredit.a1400067 [Google Scholar]
- 43.U.S. National Institutes of Health. NIH Request for Information: Consideration of Sex as a Biological Variable in Biomedical Research, Analysis of Public Comments. Retrieved October 2, 2015, from http://orwh.od.nih.gov/about/director/pdf/RFIFinalReport20150520.pdf
- 44.Ritz S. A., Antle D. M., Côté J., Deroy K., Fraleigh N., Messing K., Parent L., St-Pierre J., Vaillancourt C., Mergler D. (2014) First steps for integrating sex and gender considerations into basic experimental biomedical research. FASEB J. 28, 4–13 [DOI] [PubMed] [Google Scholar]
- 45.McCullough L. D., de Vries G. J., Miller V. M., Becker J. B., Sandberg K., McCarthy M. M. (2014) NIH initiative to balance sex of animals in preclinical studies: generative questions to guide policy, implementation, and metrics. Biol. Sex Differ. 5, 15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holdcroft A. (2007) Integrating the dimensions of sex and gender into basic life sciences research: methodologic and ethical issues. Gend. Med. 4(Suppl B), S64–S74 [DOI] [PubMed] [Google Scholar]
- 47.Canadian Institutes of Health Research. The CIHR Institute of Gender and Health - Integrating Gender and Sex in Health Research Webinar Series. Accounting for Sex and Gender in Research with Cells or Animals. Retrieved October 2, 2015, from http://circle.ubc.ca/handle/2429/41731
- 48.Stanford University. Gendered Innovations. Retrieved October 2, 2015, from http://genderedinnovations.stanford.edu/
- 49.Van Loo P. L., Mol J. A., Koolhaas J. M., Van Zutphen B. F., Baumans V. (2001) Modulation of aggression in male mice: influence of group size and cage size. Physiol. Behav. 72, 675–683 [DOI] [PubMed] [Google Scholar]
- 50.The Jackson Laboratory. Breeding Strategies for Maintaining Colonies of Laboratory Mice. Retrieved October 26, 2015, from http://www.research.uci.edu/forms/docs/iacuc/JAX-breeding-strategies.pdf
- 51.U.S. National Institutes of Health. Principles and Guidelines for Reporting Preclinical Research. Retrieved October 26, 2015, from http://www.nih.gov/science/reproducibility/principles-guidelines.htm
- 52.Sex and Gender Women’s Health Collaborative. Curriculum and Training. Retrieved October 26, 2015, from http://sgwhc.org/sex-gender-resources/curriculumtraining/#sthash.VMvXjIN5.dpbs
- 53.Miller V. M., Rice M., Schiebinger L., Jenkins M. R., Werbinski J., Núñez A., Wood S., Viggiano T. R., Shuster L. T. (2013) Embedding concepts of sex and gender health differences into medical curricula. J. Womens Health (Larchmt.) 22, 194–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Laura W. Bush Institute for Women's Health. “Y Does X Make a Difference?” Online Continuing Medical Education and Certificate Program, Retrieved October 2, 2015, from http://www.laurabushinstitute.org/professional-education.aspx
- 55.U.S. National Institutes of Health Office of Research on Women's Health. Online Education on Sex and Gender Differences. Retrieved October 2, 2015, from http://orwh.od.nih.gov/resources/cme.asp
- 56.Shaw R., Festing M. F., Peers I., Furlong L. (2002) Use of factorial designs to optimize animal experiments and reduce animal use. ILAR J. 43, 223–232 [DOI] [PubMed] [Google Scholar]
- 57.Collins L. M., Dziak J. J., Li R. (2009) Design of experiments with multiple independent variables: a resource management perspective on complete and reduced factorial designs. Psychol. Methods 14, 202–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Festing M. F. (2014) Randomized block experimental designs can increase the power and reproducibility of laboratory animal experiments. ILAR J. 55, 472–476 [DOI] [PubMed] [Google Scholar]
- 59.Greenspan J. D., Craft R. M., LeResche L., Arendt-Nielsen L., Berkley K. J., Fillingim R. B., Gold M. S., Holdcroft A., Lautenbacher S., Mayer E. A., Mogil J. S., Murphy A. Z., Traub R. J.; Consensus Working Group of the Sex, Gender, and Pain SIG of the IASP (2007) Studying sex and gender differences in pain and analgesia: a consensus report. Consensus Working Group of the Sex, Gender, and Pain SIG of the IASP. Pain 132(Suppl 1), S26–S45 [DOI] [PMC free article] [PubMed] [Google Scholar]