Abstract
Bone minerals are acquired during growth and are key determinants of adult skeletal health. During puberty, the serum levels of growth hormone (GH) and its downstream effector IGF-1 increase and play critical roles in bone acquisition. The goal of the current study was to determine how bone cells integrate signals from the GH/IGF-1 to enhance skeletal mineralization and strength during pubertal growth. Osteocytes, the most abundant bone cells, were shown to orchestrate bone modeling during growth. We used dentin matrix protein (Dmp)-1-mediated Ghr knockout (DMP-GHRKO) mice to address the role of the GH/IGF axis in osteocytes. We found that DMP-GHRKO did not affect linear growth but compromised overall bone accrual. DMP-GHRKO mice exhibited reduced serum inorganic phosphate and parathyroid hormone (PTH) levels and decreased bone formation indices and were associated with an impaired response to intermittent PTH treatment. Using an osteocyte-like cell line along with in vivo studies, we found that PTH sensitized the response of bone to GH by increasing Janus kinase-2 and IGF-1R protein levels. We concluded that endogenously secreted PTH and GHR signaling in bone are necessary to establish radial bone growth and optimize mineral acquisition during growth.—Liu, Z., Kennedy, O. D., Cardoso, L., Basta-Pljakic, J., Partridge, N. C., Schaffler, M. B., Rosen, C. J., Yakar, S. DMP-1-mediated Ghr gene recombination compromises skeletal development and impairs skeletal response to intermittent PTH.
Keywords: growth hormone receptor, parathyroid hormone, fibroblast growth factor-23, osteocyte, microcomputed tomography
Osteoporosis is a major public health concern. In the United States, 1 in 2 females and 1 in 4 males aged over 50 yr will have an osteoporosis-related bone fracture in their lifetime. The disease prevalence and related healthcare costs, $14–18 billion annually at present, are growing exponentially (1). Currently, the clinical management of osteoporosis and age-related bone disease is limited to symptomatic treatments that are directed mainly at slowing the rate of bone loss with agents that inhibit bone resorption (2, 3). One promising approach to combating osteoporosis is the optimization of peak bone acquisition during growth (4). Increasing evidence suggests that the risk of osteoporosis is programmed during puberty (5, 6). An increase in bone mass occurs via endochondral bone formation in puberty that is largely regulated by the growth hormone (GH)/IGF-1 axis. Epidemiologic data suggest that the skeletal sensitivity to GH, which is programmed early in life, determines peak bone acquisition and is predictive of bone loss during later life (7).
Both GH and IGF-1 exert their effects on bone by binding to their receptors on chondrocytes, osteoblasts, and osteoclasts. IGF-1 increases osteogenic cell proliferation, differentiation, and matrix deposition. GH mainly affects bone length (in IGF-1-dependent and -independent manners) via its receptor on chondrocytes [reviewed in Tahimic et al. (8)]. However, studies by us and others demonstrated that GH also affects the development of long-bone diaphyses and the trabecular bone compartment. In the past few years, the roles of the GH/IGF-1 axis in osteocytes have been identified. Osteocytes, the most abundant cells in bone, orchestrate skeletal growth and remodeling via the secretion of fibroblast growth factor (FGF)23 and sclerostin (SOST) and engages in cross-talk with osteoblasts and osteoclasts on the bone surfaces and with stromal cells in the bone marrow. Osteocytes function as mechanosensors and regulators of mineral homeostasis (9). Despite early work showing that Igf1 is expressed in osteocytes and is produced in response to mechanical loading, the involvement of osteocyte-specific IGF-1 receptor (IGF-1R) or GH receptor (GHR) in bone deposition has not been reported.
Mineralization of the bone matrix depends on calcium intake. Parathyroid hormone (PTH) regulates calcium homeostasis primarily by stimulating the conversion of 25(OH)-vitamin D to the active 1,25 cholecalciferol [1,25(OH)2–vitamin D3]. PTH-receptor (PTHR)-1-null mice (10) die in midgestation and PTH-null mice (11) show dysmorphic bones during fetal development, clearly suggesting that PTH plays a role in skeletal morphogenesis. Adult PTH-null mice show mild reductions in bone length but marked decreases in trabecular bone volume (BV) (12). Dentin matrix protein (DMP)-1-mediated PTHR gene recombination in mice [osteocyte-PPR knockout (Ocy-PPRKO)] results in increased bone mineral density (BMD) and trabecular and cortical bone traits at 12 wk of age, suggesting that PTHR on mature osteoblasts and osteocytes is necessary for normal bone modeling (13). On the other hand, mice with postnatal conditional ablation of PTHR in osteocytes (10 kb DMP1-Cre-Ert2) demonstrated osteopenia by 4–6 wk of age, as evidenced by reductions in trabecular BV and elevations in Sost gene expression in bone and SOST in serum (14). The discrepancy between these 2 models may suggest different roles of PTHR during skeletal morphogenesis and adulthood, but it also emphasizes the gap in our understanding of PTH action on the skeleton.
PTH has both anabolic and catabolic effects on bone. Its nocturnal secretion is thought to be anabolic, whereas the sustained secretion of high levels of PTH is thought to be catabolic. Numerous studies have shown that IGF-1 is a critical mediator of the anabolic actions of PTH. In particular, PTH induces Igf1 gene expression in bone (15, 16) and promotes osteoblast differentiation by increasing IGF-1 production (17–19). Despite increasing evidence that the actions of PTH on bone involve the IGF-1 system, the full mechanism is not yet resolved. In addition, little is known about the interaction between PTH and the GH/IGF-1 axis during early pubertal growth (when GH peaks). Unfortunately, clinical studies on the physiology of endogenous PTH secretion and its effects on the skeleton during pubertal growth in normal subjects are scarce.
The current study was conducted to determine how osteocytes integrate signals from the GH/IGF-1 pathway with anabolic PTH stimuli during bone acquisition. We hypothesized that the actions of GHR, independent of its effects on the growth plates and in addition to its direct effects on bone cells, integrate with PTH actions to increase bone mass during pubertal growth.
MATERIALS AND METHODS
Animals
Several mouse models, in which Ghr, Igf1r, or Igf1 were floxed by 2 loxP sites and Cre recombinase was driven by the DMP-1(10kb) promoter, were used. All mice were in the C57BL/6J (B6) genetic background. Weaned mice were allocated randomly into cages according to their sex. Mice were housed 2–5 animals per cage in a facility with 12 h light–dark cycles and free access to food and water. The different analyses were performed in prepubertal mice (4 wk of age), in mice at peak puberty (8 wk of age), and in young adults (16 wk of age), when peak bone mass is achieved.
For histomorphometric analyses, the animals were injected intraperitoneally with calcein (10 mg/kg) 2 and 8 or 10 d before they were euthanized. For intermittent (i)PTH treatment, mice were injected subcutaneously with 80 μg/kg/d PTH1–34 (Bachem Americas, Inc., Torrance, CA, USA) from 4 to 8 wk of age. For the acute response to GH, mice were injected intraperitoneally with 0.25 mg/kg recombinant mouse (m)GH (cat. no. cyt-540; ProSpec, East Brunswick, NJ, USA) and euthanized 15 min after injection. All procedures involving mice were reviewed and approved by the Institutional Animal Care and Use Committee of the New York University School of Medicine; the Public Health Service animal welfare assurance identification number is A3435-01. The program is licensed under the U.S. Department of Agriculture as research facility No. 465.
Hormone measurements
Serum and plasma were collected via orbital bleeding immediately after the animals were euthanized between 8 and 10 am. Serum IGF-1 levels were measured with commercial radioimmunoassays (20, 21). Plasma osteocalcin (OC) levels were measured with a commercial radioimmunoassay (American Laboratory Products Company, Inc., Salem, NH, USA). Serum GH levels were measured with commercial ELISA kits (EMD Millipore, Billerica, MA, USA). Serum mouse PTH (cat. no. 60-3900; Immunotopics, San Clemente, CA, USA) and SOST levels were measured with ELISA kits (cat. no. E92864Mu ; USCN Life Science Inc.). Serum FGF23 (cat. no. 60-6300; Immunotopics) and 1,25-vitamin D (cat. no. MBS731103; MyBiosource, San Diego, CA, USA) levels were measured with ELISA kits. Urine inorganic phosphate (Pi) and creatinine levels were determined with a colorimetric assay (cat. no. 0830-125 and 0430-120, respectively; Stanbio, Boerne, TX, USA).
Gene expression
Total RNA was extracted from tissues with Trizol (Thermo Fisher-Invitrogen, Carlsbad, CA, USA) or with the RNeasy Plus kit (cat. no. 74134; Qiagen, Valencia, CA, USA;). RNA samples (1 μg) were reverse transcribed with a cDNA synthesis system (cat. no. 18080-051; Thermo Fisher-Invitrogen), and quantitative real-time PCR was performed with SYBR master mix (cat. no.4385612; Thermo Fisher-Applied Biosystems, Foster City, CA, USA) on a CFX384 real-time machine (Bio-Rad, Hercules, CA, USA) with a C1000 Touch Thermal Cycle detection system, according to the manufacturer’s instructions. Transcript levels were assayed 3 times in each sample, and the ratio of the fold change between experimental and control samples was calculated relative to GAPDH or 18S, as indicated. The primer sequences used for real-time PCR are presented in Supplemental Table 4.
IDG-SW3 cell line
Cells were maintained and processed according to a published protocol (22). In brief, for cell-line maintenance, cells were seeded on collagen-coated flasks in α-minimum essential medium (MEM) containing l-glutamine and nucleosides (cat. no. 12571-063; Thermo Fisher–Gibco, Grand Island, NY, USA) supplemented with heat inactivated 10% fetal bovine serum (FBS), 100 U/ml penicillin, 100 μg/ml streptomycin, and 2500 U IFN-γ in 100 ml medium and incubated at 33°C with 5% CO2. For cell-line differentiation, the cells were seeded on collagen-coated plates in α-MEM containing l-glutamine and nucleosides supplemented with heat-inactivated 10% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, 50 µg/ml ascorbic acid, and 4 mM β-glycerophosphate (without IFN-γ) and incubated at 37°C with 5% CO2. All experiments were performed with differentiated cells that had been maintained for ∼20 d in differentiation medium; ∼85% of the cells were green fluorescent protein (GFP)+. The cAMP inducers forskolin (cat. no. sc-3562; Santa Cruz Biotechnology, Santa Cruz, CA, USA), isobutylmethylxanthine (IBMX, cat. no. sc-201188; Santa Cruz Biotechnology), and isoproterenol (cat. no. 1747; Tocris Bioscience, Ellisville, MO, USA) and the PKA (H89, cat. no. 9844; Cell Signaling Technology, Danvers, MA, USA) and PKC (GF109203X, cat. no. 203290; EMD Millipore) inhibitors were used at the indicated concentrations in the figure legends.
Cellular proteins were extracted in modified 3-[(3-cholamidopropyl)dimethyl-ammonio]-1-propanesulfonate (CHAPS) buffer [50 mM Tris (pH 7.4), 150 mM NaCl, 1 mM EDTA, 1.25% CHAPS, 2 mM NaF, 10 mM Na-pyrophosphate, 8 mM β-glycerophosphate, 1 mM Na-orthovanadate, and complete protease inhibitor cocktail (cat. no. 04693132001; Roche, Indianapolis, IN, USA)]. Cell lysates (20–30 μg) were separated on 4–20% gradient SDS-polyacrylamide gels (cat. no. NP0335; Thermo Fisher–Life Technologies) and transferred to nitrocellulose membranes (cat. no. 170-4158; Bio-Rad). Proteins were detected using primary antibodies against GHR (cat. no. G8919; Sigma-Aldrich, St. Louis, MO, USA); and STAT5 (cat. no. 9363), p-STAT5 (cat. no. 9359), p-Janus kinase (Jak)-2 (cat. no. 3771), JAK2 (cat. no. 3773) IGF-1R (cat. no. 3027), tubulin (cat. no. 2148), and actin (cat. no. 4970); followed by secondary antibodies (cat. no. 7074; all from Cell Signaling Technology).
Proteins were extracted from cortical bone shells and other tissues using modified CHAPS buffer. Proteins were separated on 4–20% gradient SDS-polyacrylamide gels (cat. no. NP0335; Thermo Fisher–Life Technologies) and transferred to nitrocellulose membranes (cat. no. 170-4158; Bio-Rad). They were then detected with the above antibodies.
Microcomputed tomography
Microcomputed tomography (micro-CT) was performed according to published guidelines (23). The left femora were scanned with the high-resolution 1172 SkyScan micro-CT system (SkyScan, Kontich, Belgium). Images were acquired using a 10 MP digital detector, 10 W energy (100 kV and 100 μA), and a 0.5 mm aluminum filter with a 6.7 μm image voxel size. A fixed global threshold method was used based on the manufacturer’s recommendations and preliminary studies, which showed that mineral variation between groups was not high enough to warrant adaptive thresholds. The cortical region of interest was selected as the 2.0 mm middiaphyseal region directly below the third trochanter, which includes the middiaphysis and more proximal cortical regions. The following cortical bone measurements were made: total cross-sectional area (Tt.Ar), cortical bone area (Ct.Ar), marrow area (Ma.Ar), cortical bone thickness (Ct.Th), polar moment of inertia (J0), and tissue mineral density (TMD). The trabecular measurements included the bone volume relative to the total volume (BV/TV), BMD, trabecular number (Tb.N), trabecular spacing (Tb.Sp), and trabecular thickness (Tb.Th). Additional whole-bone measurements were made, including femoral length (Le), robustness (Tt.Ar/Le), and relative cortical area (RCA; Ct.Ar/Tt.Ar).
Histomorphometry
Histomorphometry was performed according to published guidelines (24). Eight-week-old animals were injected twice with calcein (10 mg/kg i.p.) 2 and 8 or 10 d before they were euthanized. The femurs were fixed in 10% neutral-buffered formalin, embedded in polymethylmethacrylate (PMMA), and sectioned (80 µm thickness) at the middiaphysis with a low-speed diamond-coated wafering saw (Leica, Bannockburn, IL, USA). The sections were affixed to either glass or acrylic slides with nonfluorescing mounting medium. The final section thicknesses after polishing were 30–40 μm. All measurements were performed with an OsteoMeasure system (Osteometrics, Atlanta, GA, USA) in accordance with standard protocols. Sections were imaged with a digital camera attached to a visible light/fluorescence microscope (Axioplan2; Zeiss AxioVision, Thornwood, NY, USA). Thin plastic sections were obtained from the distal femur for trabecular bone analyses. The sections were stained with toluidine blue for osteoblast cells count.
Histology
The right tibiae were fixed in 10% zinc formalin and decalcified with 10% EDTA. The decalcified bones were then processed for paraffin-embedded sectioning (5 μm sections) and stained with hematoxylin and eosin, or incubated with anti-cathepsin K H-50 antibodies (1:100 dilution, cat. no. sc-30056; Santa Cruz Biotechnology;). Positive staining was detected using the horseradish peroxidase-3,3′-diaminobenzidine system (cat. no. cts005; R&D Systems, Minneapolis, MN, USA).
Statistical analysis
Data are presented as means ± sem. Significant differences (P < 0.05) between groups were confirmed with Student’s t tests, unless otherwise indicated.
Blinding
Scientists were blinded to sample identifications in all experimental procedures.
Justification of sample size
Sample size analysis demonstrated that 10 mice per group was sufficient for precise pair-wise comparisons (Student’s t test; α = 0.05; β = 0.20; n = 4–16) and 1-way ANOVA (α = 0.05; β = 0.20; 3 comparisons; n = 6–11) (R Stats Package; Eidgenössische Technische Hochschule, Zürich, Switzerland; http://stat.ethz.ch/R-manual/R-patched/library/stats/html/00Index.html) in which the effect size (ES) = (minimum significant difference in the means)/(mean standard deviation of the groups), a measure of intermutant variability, ranges from 2.0 to 1.0. Observations made using literature searches and our own studies in different IGF mutant mice revealed that the ES statistic is always >2.0, and on average >5.0, owing to small standard deviations. Therefore, a sample size of n = 10 (based on the confidence interval) is sufficient for the accurate comparison of the sample groups in these studies. Nevertheless, the sample number was increased when unusually large standard deviations were observed.
RESULTS
Dmp-1(10kb)-mediated Ghr gene deletion does not affect overall body growth
We used the cre/loxP system to determine how GHR regulates skeletal acquisition, architecture, and strength. Transgenic mice expressing Cre recombinase driven by the DMP-1 (10 kb) promoter (25–27) were crossed with GHR-floxed mice (28, 29). Control mice were homozygous for the floxed Ghr alleles, whereas Dmp-1-mediated Ghr knockout (GHRKO) mice were homozygous for floxed Ghr allele and carried the DMP1-Cre transgene. Using a non–quantitative PCR approach, we found that the Ghr recombinant allele (null allele) was detected only in the bone and muscle of DMP-GHRKO mice, but not in the liver, spleen, kidney, intestine, gonadal fat, lung, or heart (Supplemental Fig. 1A). In past reports, investigators used the same DMP-derived Cre to demonstrate that gene recombination occurs not only in osteocytes but also in mature osteoblasts. Therefore, we refer to our mice as DMP-GHRKO, while bearing in mind that recombination occurs in both mature osteoblasts and osteocytes. To further validate the DMP-GHRKO model, we assessed Ghr gene expression in crude RNA extracted from cortical shells (likely including periosteal surface and endosteal surface cellularity, such as osteoblasts in osteoid) of control and DMP-GHRKO mice. We found a 50% reduction in Ghr gene expression in bone (Supplemental Fig. 1B). Consequently, we injected mice with mouse recombinant GH and examined phosphorylation of STAT5b, a downstream mediator of GHR, in bone 15 min after injection (Supplemental Fig. 1C, top). The control mice exhibited GH-induced STAT5b phosphorylation, whereas it was absent in bones extracted from DMP-GHRKO or total GHRKO mice (Supplemental Fig. 1C; bottom).
DMP-1-mediated Ghr gene inactivation did not affect osteocyte gross morphology, as evaluated in plastic thin sections (10 μm) of cortical and trabecular bone stained with toluidine blue (Supplemental Fig. 1D). Osteocyte density in trabecular bone was calculated at the femoral distal metaphysis and in cortical bone at the femoral middiaphysis. We found that the total number of lacunae and the number of occupied (full, with apparent nucleolus) lacunae significantly decreased in the DMP-GHRKO mice in both compartments, suggesting that the GHR plays a role in osteocyte differentiation or viability. As will be shown below, gene expression studies have shown that the expression of FGF23, SOST, DMP-1, and phosphate-regulating endopeptidase homolog X-linked (PHEX), markers of osteocyte differentiation, were elevated in DMP-GHRKO mice. Thus, our next step was to assess whether DMP-GHRKO osteocytes exhibit increased apoptosis by immunohistochemistry of cleaved caspase 3 (a marker of apoptosis). However, because bone sections of the DMP-GHRKO mice showed significant reductions in the total number of lacunae and in the number of cell-occupied lacunae (Supplemental Fig. 1D), we did not reveal differences in the number of caspase 3+ cells between controls and DMP-GHRKO (data not shown). It is conceivable that GHR in osteocytes is involved in cell viability. However, further investigations into that matter are warranted.
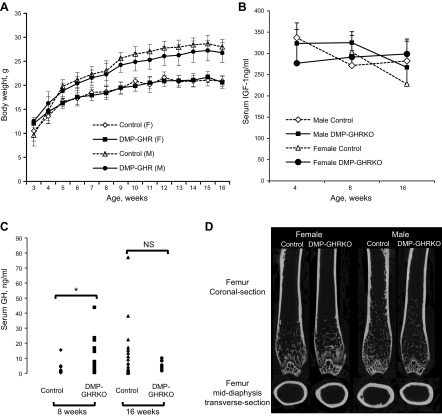
Mice were observed for up to 16 wk of age. Bodyweight, which was recorded once a week starting from 3 wk of age, did not differ significantly between DMP-GHRKO and control mice of either sex (Fig. 1A). Organ weights taken at the time of euthanasia also showed no significant differences (Supplemental Table 1). The serum levels of IGF-1 were measured at 4, 8, and 16 wk of age (Fig. 1B) and did not differ between control and DMP-GHRKO mice of both sexes. Serum GH levels (obtained from a single blood sample) were increased at 8 wk in DMP-GHRKO mice, but were not different at 16 wk of age (Fig. 1C).
Figure 1.
DMP-GHRKO mice had normal body weight. A) Control and DMP-GHRKO mice of both sexes were weighed weekly from 3 to 16 wk of age (n > 10 mice per age, sex, and genotype). B) Serum IGF-1 levels were measured at 4, 8, and 16 wk of age in male and female control and DMP-GHRKO mice (n ≥ 6 mice per age, sex, and genotype, tested by 2-way ANOVA). C) Serum GH levels were measured at 8 and 16 wk of age in control and DMP-GHRKO female mice (male serum GH levels were not different between control and DMP-GHRKO mice; data not shown) (n ≥ 7 mice per age and genotype). NS, not significant. D) Representative micro-CT images of femurs from 16-wk-old male and female mice. Data are presented as means ± sd. *P < 0.05; Student’s t test.
Dmp-1(10kb)-mediated Ghr gene deletion impairs bone accrual
Cortical bone architecture was evaluated in the femoral middiaphysis of 16-wk-old male and female mice with micro-CT (Table 1). Dmp-1-mediated Ghr gene deletion did not affect linear growth. However, there was a significant decrease (∼12%) in total cross-sectional bone area (Tt.Ar) that contributed to a slender (less robust) bone phenotype in both male and female DMP-GHRKO mice (Fig. 1D and Table 1). In addition, both male and female bones had a reduced J0 (Table 1). The differences in morphology between the DMP-GHRKO and control mice remained significant after the traits were corrected for body weight by linear regression analysis. Trabecular bone morphology was assessed at the distal femur (Table 1), which revealed that Dmp-1-mediated Ghr gene deletion reduced the amount of trabecular bone in both sexes. This finding was evidenced by the decreased BV relative to the TV (%BV/TV), reduced BMD, decreased Tb.N, and increased Tb.Sp.
TABLE 1.
Micro-CT analyses of femora dissected from 16-wk-old control and DMP-GHRKO male and female mice
| Parameter | Female |
Male |
||||||
|---|---|---|---|---|---|---|---|---|
| Control | DMP-GHRKO | P | Change (%) | Control | DMP-GHRKO | P | Change (%) | |
| Sample size (n) | 9 | 13 | 17 | 16 | ||||
| Body weight (g) | 20.70 ± 0.99 | 20.57 ± 1.22 | 0.71 | 0.62 | 27.95 ± 1.56 | 26.67 ± 2.03 | 0.06 | 4.57 |
| Bone length (mm) | 14.87 ± 0.13 | 14.91 ± 0.37 | 0.86 | 0.26 | 15.36 ± 0.60 | 15.09 ± 0.37 | 0.12 | 0.59 |
| Cortical bone, femoral middiaphysis | ||||||||
| Tt.Ar (mm2) | 1.50 ± 0.08 | 1.32 ± 0.07 | <0.01 | −12.14 | 1.98 ± 0.25 | 1.76 ± 0.15 | <0.01 | −15.5 |
| Ct.Ar (mm2) | 0.76 ± 0.03 | 0.72 ± 0.04 | 0.07 | 4.6 | 0.92 ± 0.07 | 0.80 ± 0.08 | <0.01 | −15.59 |
| RCA (%) | 50.90 ± 2.85 | 55.26 ± 3.66 | <0.01 | +8.57 | 47.00 ± 4.32 | 46.20 ± 5.60 | 0.63 | 0.44 |
| Ct.Th (mm) | 0.16 ± 0.01 | 0.16 ± 0.00 | 0.86 | 0.37 | 0.18 ± 0.01 | 0.17 ± 0.00 | <0.01 | −7.64 |
| Ma.Ar (mm2) | 0.73 ± 0.07 | 0.59 ± 0.07 | <0.01 | −19.93 | 1.05 ± 0.20 | 0.95 ± 0.16 | 0.11 | 15.44 |
| Robustness (mm−1) | 0.09 ± 0.00 | 0.08 ± 0.00 | 0.01 | −9.49 | 0.12 ± 0.01 | 0.11 ± 0.01 | 0.01 | −14.97 |
| J0 (mm−4) | 0.34 ± 0.02 | 0.30 ± 0.03 | 0.01 | −10.46 | 0.47 ± 0.07 | 0.39 ± 0.07 | <0.01 | −26.14 |
| TMD (g/cc) | 1.25 ± 0.03 | 1.22 ± 0.03 | 0.27 | 1.72 | 1.57 ± 0.25 | 1.66 ± 0.24 | 0.41 | 1.24 |
| Trabecular bone, distal femur | ||||||||
| BV/TV, (%) | 4.38 ± 1.07 | 3.24 ± 0.80 | <0.01 | −26.01 | 11.59 ± 3.62 | 8.68 ± 3.23 | 0.02 | −25.07 |
| Tb.Th (mm) | 0.039 ± 0.008 | 0.047 ± 0.002 | <0.01 | +20.61 | 0.051 ± 0.005 | 0.049 ± 0.004 | 0.19 | 4.59 |
| Tb.N (mm−1) | 1.16 ± 0.39 | 0.68 ± 0.17 | <0.01 | −41.47 | 2.24 ± 0.62 | 1.75 ± 0.58 | 0.02 | −21.68 |
| Tb.Sp (mm) | 0.25 ± 0.04 | 0.28 ± 0.03 | 0.06 | 11.94 | 0.19 ± 0.01 | 0.21 ± 0.03 | 0.03 | +11.08 |
| BMD (g/cc) | 0.10 ± 0.01 | 0.10 ± 0.01 | 0.40 | 7.15 | 0.25 ± 0.09 | 0.22 ± 0.07 | 0.30 | 13.23 |
Data are means ± sd. Significance was set at P < 0.05; Student’s t test.
To rule out any contribution of partial Ghr gene recombination in muscle to the changes observed in the bones of DMP-GHRKO mice, we analyzed femurs from muscle creatine kinase muscle-specific GHRKO (MCK-GHRKO) mice, which we characterized in past studies (28, 30, 31). The complete Ghr gene deletion specifically in muscle (95% gene recombination) did not affect bone morphology in both male and female mice (Table 2). Therefore, it is unlikely that the skeletal phenotype of the DMP-GHRKO mice was affected by partial gene recombination in muscle. Furthermore, the subcutaneous injection of mouse recombinant GH stimulated the phosphorylation of STAT5b, a downstream mediator of GHR, in the muscles of both control and DMP-GHRKO mice 15 min after injection, suggesting that the GHR pathway is intact in the muscles of the DMP-GHRKO mice (Supplemental Fig. 1C).
TABLE 2.
Micro-CT analyses of femora dissected from 16-wk-old control and MCK-GHRKO male and female mice
| Parameter | Female |
Male |
||||
|---|---|---|---|---|---|---|
| Control | MCK-GHRKO | P | Control | MCK-GHRKO | P | |
| Sample size (n) | 6 | 6 | 7 | 8 | ||
| Cortical bone, femoral middiaphysis | ||||||
| Tt.Ar (mm2) | 1.64 ± 0.15 | 1.80 ± 0.09 | 0.93 | 1.88 ± 0.17 | 1.94 ± 0.16 | 0.51 |
| Ct.Ar (mm2) | 0.79 ± 0.06 | 0.83 ± 0.05 | 0.76 | 0.89 ± 0.08 | 0.89 ± 0.07 | 0.99 |
| RCA (%) | 48.32 ± 2.38 | 46.47 ± 1.56 | 0.28 | 47.45 ± 2.52 | 45.97 ± 1.42 | 0.17 |
| Ct.Th (mm) | 0.186 ± 0.01 | 0.185 ± 0.00 | 0.43 | 0.18 ± 0.00 | 0.18 ± 0.00 | 0.73 |
| Ma.Ar (mm2) | 0.85 ± 0.10 | 0.96 ± 0.06 | 0.91 | 0.99 ± 0.11 | 1.04 ± 0.09 | 0.28 |
| J0 (mm−4) | 0.325 ± 0.05 | 0.387 ± 0.04 | 0.60 | 0.439 ± 0.07 | 0.453 ± 0.07 | 0.72 |
| TMD (g/cc) | 1.74 ± 0.04 | 1.75 ± 0.03 | 0.49 | 1.79 ± 0.07 | 1.77 ± 0.03 | 0.46 |
| Trabecular bone, distal femur | ||||||
| BV/TV (%) | 6.77 ± 0.67 | 7.68 ± 1.63 | 0.23 | 15.56 ± 3.87 | 16.11 ± 3.42 | 0.76 |
| Tb.Th (mm) | 0.045 ± 0.002 | 0.045 ± 0.003 | 0.78 | 0.052 ± 0.00 | 0.050 ± 0.00 | 0.31 |
| Tb.N (mm−1) | 1.507 ± 0.161 | 1.67 ± 0.255 | 0.19 | 2.93 ± 0.54 | 3.18 ± 0.59 | 0.37 |
| Tb.Sp (mm) | 0.257 ± 0.008 | 0.251 ± 0.009 | 0.27 | 0.202 ± 0.023 | 0.18 ± 0.014 | 0.08 |
| BMD (g/cc) | 0.255 ± 0.010 | 0.272 ± 0.025 | 0.20 | 0.393 ± 0.050 | 0.396 ± 0.043 | 0.89 |
Data are means ± sd. Significance was set at P < 0.05; Student’s t test.
To better evaluate the roles of the GH/IGF-1 axis in osteocytes, we undertook a similar approach and generated mice with a Dmp-1-mediated Igf1r gene deletion. Dmp-1-mediated Igf1r knockout (DMP-IGF-1RKO) mice were followed up to 16 wk of age, and their body weight (Supplemental Fig. 2) and organ weights (data not shown) did not differ from those of control mice of either sex. Likewise, serum IGF-1 levels in DMP-IGF-1RKO mice were similar to those in controls (Supplemental. Fig. 2). Micro-CT analyses of femurs of 16-wk-old male and female mice (Table 3) revealed a significant reduction in the amount of cortical bone. This finding was evidenced by marked reductions in the Ct.Ar and a reduced Ct.Th in both sexes. Notably, however, Tt.Ar was increased, and consequently the RCA was decreased by ∼20% in both male and female mice. The decrease in RCA was accompanied by 40–50% increases in the Ma.Ar compared with controls. The trabecular bone compartment exhibited a mild phenotype in females, where the %BV/TV was reduced. The bone phenotype of the DMP-IGF-1RKO mice differs from that of the DMP-GHRKO mice. The bones of the DMP-GHRKO mice are more slender than those of the controls. In DMP-GHRKO females, cortical thickness was similar to that of the controls, whereas marrow area decreased, suggesting inhibition of periosteal bone formation but normal endosteal bone formation. In males, the slender bones of the DMP-GHRKO mice associated with decreased cortical bone thickness and increased marrow area, suggesting inhibition of periosteal bone formation with increased endosteal bone resorption. In contrast, in both sexes of the DMP-IGF-1RKO mice, we found increases in total cross-sectional areas, but significant reductions in bone area and marked reductions in cortical bone thickness with enlarged marrow area. This phenotype likely indicates increased endosteal resorption. Thus, in both models, we found overall decreased bone accrual, but these may be related to different mechanisms, although it is conceivable that IGF-1R and GHR have overlapping and distinct effects on osteocytes.
TABLE 3.
Micro-CT analyses of femurs dissected from 16-wk-old control and DMP-IGF-1R male and female mice
| Parameter | Female |
Male |
||||||
|---|---|---|---|---|---|---|---|---|
| Control | DMP-IGF-1RKO | P | Change (%) | Control | DMP-IGF-1RKO | P | Change (%) | |
| Sample size (n) | 7 | 10 | 15 | 11 | ||||
| Body weight (g) | 21.97 ± 0.86 | 21.07 ± 1.30 | 0.13 | 4.06 | 27.59 ± 2.68 | 25.44 ± 3.13 | 0.07 | 7.79 |
| Bone length (mm) | 15.46 ± 0.25 | 15.17 ± 0.39 | 0.12 | 1.87 | 15.09 ± 0.58 | 15.12 ± 0.20 | 0.89 | 0.18 |
| Cortical bone, femoral middiaphysis | ||||||||
| Tt.Ar (mm2) | 1.39 ± 0.07 | 1.58 ± 0.12 | <0.01 | +14.08 | 1.45 ± 0.13 | 1.72 ± 0.16 | <0.01 | +19.20 |
| Ct.Ar (mm2) | 0.77 ± 0.03 | 0.71 ± 0.05 | 0.02 | −7.93 | 0.83 ± 0.09 | 0.76 ± 0.07 | 0.04 | −9.07 |
| RCA (%) | 55.40 ± 2.29 | 44.70 ± 2.09 | <0.01 | −19.30 | 57.60 ± 2.56 | 44.10 ± 3.69 | <0.01 | −23.40 |
| Ct.Th (mm) | 0.17 ± 0.00 | 0.16 ± 0.00 | <0.01 | −8.63 | 0.17 ± 0.00 | 0.15 ± 0.00 | <0.01 | −11.25 |
| Ma.Ar (mm2) | 0.62 ± 0.05 | 0.87 ± 0.08 | <0.01 | +41.39 | 0.61 ± 0.05 | 0.96 ± 0.12 | <0.01 | +57.77 |
| Robustness (mm−1) | 0.09 ± 0.00 | 0.10 ± 0.00 | <0.01 | +17.01 | 0.09 ± 0.00 | 0.11 ± 0.00 | <0.01 | +14.61 |
| J0 (mm−4) | 0.31 ± 0.02 | 0.29 ± 0.04 | 0.23 | 7.27 | 0.41 ± 0.10 | 0.35 ± 0.06 | 0.12 | 14.13 |
| Trabecular bone, distal femur | ||||||||
| BV/TV (%) | 4.72 ± 1.01 | 2.91 ± 0.81 | <0.01 | −38.23 | 11.05 ± 3.90 | 9.95 ± 2.22 | 0.42 | 9.99 |
| Tb.Th (mm) | 0.044 ± 0.004 | 0.041 ± 0.001 | 0.10 | 6.65 | 0.049 ± 0.004 | 0.045 ± 0.002 | 0.01 | −8.62 |
| Tb.N (mm–1) | 1.08 ± 0.25 | 0.70 ± 0.19 | <0.01 | −34.50 | 2.20 ± 0.62 | 2.20 ± 0.41 | 0.98 | 0.15 |
| Tb.Sp (mm) | 0.25 ± 0.02 | 0.25 ± 0.02 | 0.91 | 0.52 | 0.20 ± 0.02 | 0.17 ± 0.01 | <0.01 | −13.15 |
| BMD (g/cc) | 0.11 ± 0.02 | 0.11 ± 0.01 | 0.97 | 0.22 | 0.16 ± 0.04 | 0.20 ± 0.02 | 0.02 | +21.28 |
Data are means ± sd. Significance was set at P < 0.05; Student’s t tests.
A similar approach was used to knock down the gene for the IGF-IR ligand—namely, IGF-1—in bone. However, gene recombination was detected in almost all tissues (Supplemental Fig. 3). Here again, serum IGF-1 levels in DMP-IGF-1KO mice were similar to those in controls (Supplemental Fig. 3). The skeletal phenotype of both male and female mice at 16 wk of age revealed significant decreases in total cross-sectional area in the males, whereas in the females, we observed minor increases in total cross-sectional area in DMP-IGF-1KO mice when compared to that in the controls (Supplemental Table 2). It is possible that, despite detection of gene recombination in various tissues, serum IGF-1 and bone matrix–trapped IGF-1 compensated for the lack of Igf1 expression in osteocytes. Note that these results are in contrast to those already published (32, 33). The differences between both studies may be the result of variations in animal facility conditions and handling, but also of different breeding strategies—namely, controlling for the heterozygosity of the DMP-driven Cre transgene.
Dmp-1(10kb)-mediated Ghr gene deletion results in decreased bone mineral apposition and bone formation rates
The following bone metabolic studies were performed only on female mice, even though both sexes of DMP-GHRKO mice showed impaired bone morphology by micro-CT. To understand the cellular mechanisms involved in the reduced bone accrual in the DMP-GHRKO mice, we performed histomorphometry of femurs dissected from 8-wk-old female DMP-GHRKO mice and calculated the dynamic indices of bone formation rate (BFR) and mineral apposition rate (MAR) of cortical bone (Table 4). Our analyses revealed significant reductions in MAR (65%) and BFR (61%) at the endosteal surface of DMP-GHRKO mice compared with controls. The periosteal surface was less active, and the dynamic parameters varied such that no significant differences were apparent between DMP-GHRKO and control mice.
TABLE 4.
Dynamic indices of bone formation in 8-wk-old control and DMP-GHRKO female mice
| Parameter | Control | DMP-GHRKO | P | Change (%) |
|---|---|---|---|---|
| Sample size (n) | 10 | 10 | ||
| Endosteal surface | ||||
| Mineralized surface (%) | 57.1 ± 8.8 | 47.3 ± 14.9 | 0.092 | 17.16 |
| MAR (μm/d) | 3.33 ± 2.02 | 1.15 ± 1.04 | <0.01 | −65.46 |
| BFR/BS (μm/d) | 0.49 ± 0.24 | 0.19 ± 0.18 | <0.01 | −61.22 |
| Periosteal surface | ||||
| Mineralized surface (%) | 15.8 ± 11.7 | 24.9 ± 24.6 | 0.308 | 57.59 |
| MAR (μm/d) | 1.16 ± 1.29 | 1.19 ± 1.61 | 0.695 | 2.58 |
| BFR/BS (μm/d) | 0.05 ± 0.05 | 0.09 ± 0.10 | 0.262 | 80.00 |
Values are presented as means ± sd. Significance was accepted at P < 0.05 (Student’s t tests). BS, bone surface.
DMP-GHRKO associates with reduced serum Pi levels and increased Fgf23 gene expression in bone
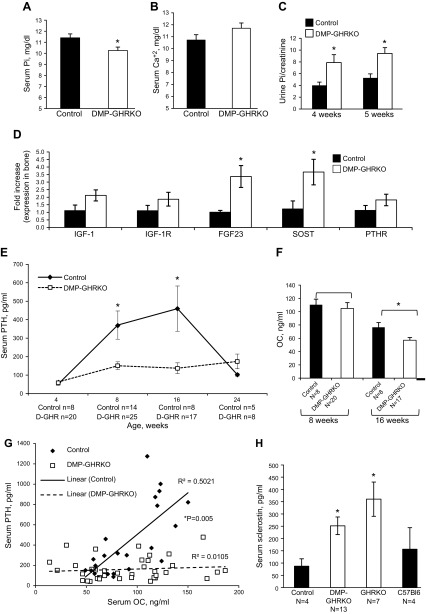
To understand the systemic effects of DMP-GHRKO, we first measured Ca2+ and Pi levels in serum harvested from 8-wk-old female mice. The DMP-GHRKO mice exhibited mild but significant hypophosphatemia compared with controls (Fig. 2A), whereas serum Ca2+ levels did not differ between groups (Fig. 2B). Urine Pi levels determined in 4- and 5-wk-old female mice revealed that, at both ages, Pi excretion significantly increased in the DMP-GHRKO mice as compared to controls and may contribute to the decreased serum Pi levels (Fig. 2C). The reduced serum Pi levels in DMP-GHRKO mice suggest that the regulation of Pi homeostasis was impaired. FGF23 is principally expressed in osteocytes and is responsible for Pi metabolism. Indeed, there was a 2-fold increase in FGF23 gene expression in the bones of 8-wk-old DMP-GHRKO female mice compared with that in the controls (Fig. 2D).
Figure 2.
DMP-GHRKO mice exhibited impaired mineral metabolism. A) Serum Pi levels were measured in female control and DMP-GHRKO mice at 8 wk of age (n ≥ 10 mice per genotype). B) Serum Ca+2 levels were measured in 8-wk-old female control and DMP-GHRKO mice (n ≥ 10 mice per genotype). C) Pi excretion in urine samples was measured in female mice between 4–5 wk of age and corrected to creatinine (n ≥ 10 mice per genotype). D) Gene expression was measured in the cortical bones of 8-wk-old control and DMP-GHRKO mice (n = 5 mice per genotype). E) Serum PTH levels were measured at 4, 8, 16, and 24 wk of age in control and DMP-GHRKO female mice. F) Serum OC levels were measured at 8 and 16 wk of age in control and DMP-GHRKO mice. G) Variation in PTH and OC levels in control and DMP-GHRKO mice at 8 and 16 wk of age. There was a linear relationship between PTH and OC in control mice, but not in DMP-GHRKO mice. H) Serum SOST levels in 8-wk-old control (floxed Ghr), DMP-GHRKO, GHR-null (GHRKO), and wild-type (C57Bl6) female mice (tested by 2-way ANOVA with Tukey’s post hoc test). Data are means ± sem. *P < 0.05; (A–F) Student’s t-test.
DMP-GHRKO mice show low levels of serum PTH after puberty
The relationship between FGF23 and PTH remains unclear, and there are contradictory reports showing that PTH exerts stimulatory (34) or inhibitory (35) effects on FGF23 secretion and vice versa (36). We measured PTH levels in female DMP-GHRKO mice at 4, 8, 16, and 24 wk of age. Although PTH levels increased in female control mice after puberty, female DMP-GHRKO mice exhibited unexpectedly low levels of serum PTH (Fig. 2E). The data suggest that the ablation of GHR action in bone blunts PTH secretion in female mice during pubertal growth (4–8 wk in mice), likely via secondary mechanisms. Because the differences in serum PTH in the DMP-GHRKO mice appeared at 8 wk of age, we also assessed bone morphology by micro-CT at 8 and 4 wk of age. At 4 wk of age we did not detect differences between controls and DMP-GHRKO female mice (data not shown). However, at 8 wk of age, female DMP-GHRKO mice showed a slender bone phenotype, similar to that observed at 16 wk (Supplemental Table 3). Together, these data suggest that the bone phenotype appeared likely during pubertal growth and may indicate interactions between sex steroids and GHR in osteocytes.
To understand whether the increases in PTH during the growth of control mice correlate with bone turnover indices, we measured serum OC levels at 8 and 16 wk of age. There were no significant differences in OC between control and DMP-GHRKO female mice at 8 wk. However, at 16 wk, OC levels in DMP-GHRKO mice were reduced by ∼20% (Fig. 2F). Furthermore, there was a linear relationship between PTH and OC during puberty and young adulthood (8–16 wk) in control mice, but not in the serum of the DMP-GHRKO mice (Fig. 2G). These data suggest that increases in PTH after puberty likely are essential to meet the calcium needs for bone modeling and that the blunted increase in PTH in the DMP-GHRKO mice contributes partially to their compromised bone metabolism.
DMP-GHRKO mice exhibit an impaired response to anabolic PTH treatment during puberty
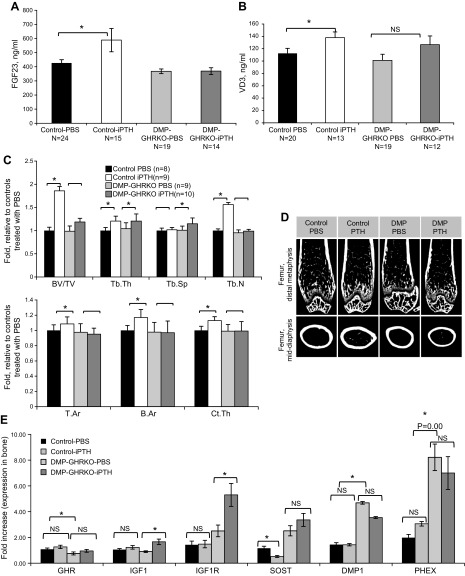
To determine the impact of iPTH treatment on bone remodeling, we treated female DMP-GHRKO and control mice with iPTH during puberty (80 μg/kg/d from 4 to 8 wk of age). We first evaluated basal and iPTH-induced changes in serum FGF23 and 1,25-vitamin D. Despite finding a 2-fold increase in mRNA levels of FGF23 in bones from DMP-GHRKO mice, we did not detect increased serum levels of FGF23 in the basal state (Fig. 3A). We note that the sensitivity of real-time PCR differs from that of the ELISA assay, which measures full-length FGF23 and C-terminal fragments. Hence, there is not always a clear correlation between RNA and protein levels. However, in response to iPTH treatment we found a significant increase in FGF23 levels in control mice that was not apparent in the DMP-GHRKO mice, suggesting impaired response to PTH. Likewise, we found that serum levels of 1,25-vitamin D increased in control mice in response to iPTH, whereas DMP-GHRKO did not show a significant increase in serum 1,25-vitamin D after iPTH (Fig. 3B), probably because of a large variability in serum 1,25-vitamin D in response to iPTH. We note that in physiologic conditions PTH is secreted in a nocturnal fashion, which may be important for modulating bone cell activity. iPTH, used to promote experimental anabolic response in bone, does not necessarily mimic physiologic conditions.
Figure 3.
DMP-GHRKO mice had an impaired bone response to iPTH. Female mice were injected once daily with 80 μg/kg PTH from 4 to 8 wk of age. A) Serum FGF23 levels in female control and the DMP-GHRKO mice treated with iPTH or vehicle from 4 to 8 wk. B) Serum 1,25-vitamin D levels in female control and DMP-GHRKO mice treated with iPTH or vehicle from 4 to 8 wk. C) Femurs were dissected at 8 wk of age and analyzed with micro-CT (the detailed traits are shown in Table 5). Fold change in bone traits obtained by micro-CT (in response to iPTH). The traits obtained from mice treated with PBS were used as the baseline. D) Micro-CT images of control and DMP-GHRKO bones from mice treated with PBS or iPTH. E) Gene expression in cortical bones from control and DMP-GHRKO female mice treated with PBS or iPTH from 4 to 8 wk of age. NS, not significant. Data are presented as means ± sem. *P < 0.05.
Bone response to iPTH was assessed in femur of female mice by micro-CT. The control mice exhibited increased Ct.Ar and Ct.Th in response to iPTH, whereas the female DMP-GHRKO mice had a blunted response to PTH in the cortical bone compartment (Fig. 3C, D and Table 5). Similarly, control mice exhibited an 85% increased trabecular %BV/TV in response to iPTH, whereas female DMP-GHRKO mice had only a trend toward an increased %BV/TV (by 20%), which did not reach significance. We note that in the PBS-treated cohort we did not detect differences between controls and DMP-GHRKO female mice by micro-CT, which may relate to daily handling and injections over a period of 4 wk.
TABLE 5.
Micro-CT and dynamic histomorphometry analyses of femurs of female mice treated with iPTH (80 μg/kg/d) from 4 to 8 wk of age
| Parameter | Control PBS | Control iPTH | P | Change (%) | DMP-GHRKO PBS | DMP-GHRKO iPTH | P | Change (%) |
|---|---|---|---|---|---|---|---|---|
| Sample size (n) | 8 | 9 | 9 | 10 | ||||
| Body weight (g) | 17.00 ± 1.09 | 17.96 ± 1.59 | 0.170 | 5.38 | 17.41 ± 1.81 | 16.56 ± 1.28 | 0.248 | 5.16 |
| Bone length (mm) | 8.36 ± 0.42 | 8.54 ± 0.28 | 0.309 | 2.12 | 8.42 ± 0.36 | 8.39 ± 0.17 | 0.804 | 0.38 |
| Cortical bone, femoral middiaphysis | ||||||||
| Tt.Ar (mm2) | 1.55 ± 0.11 | 1.68 ± 0.14 | 0.056 | 8.49 | 1.51 ± 0.17 | 1.48 ± 0.11 | 0.629 | 2.20 |
| Ct.Ar (mm2) | 0.61 ± 0.04 | 0.72 ± 0.06 | <0.01 | +16.93 | 0.60 ± 0.08 | 0.60 ± 0.09 | 0.965 | 0.28 |
| RCA (%) | 39.93 ± 2.27 | 42.94 ± 0.68 | <0.01 | +7.52 | 39.92 ± 2.25 | 40.77 ± 3.40 | 0.532 | 2.13 |
| Ct.Th (mm) | 0.14 ± 0.00 | 0.16 ± 0.00 | <0.01 | +12.95 | 0.14 ± 0.01 | 0.14 ± 0.01 | 0.833 | 1.06 |
| Ma.Ar (mm2) | 0.93 ± 0.09 | 0.96 ± 0.07 | 0.523 | 2.90 | 0.91 ± 0.10 | 0.87 ± 0.04 | 0.370 | 3.86 |
| Robustness (mm−1) | 0.11 ± 0.00 | 0.12 ± 0.00 | 0.044 | +8.12 | 0.11 ± 0.00 | 0.11 ± 0.00 | 0.356 | 3.33 |
| J0 (mm−4) | 0.252 ± 0.03 | 0.319 ± 0.06 | <0.01 | +26.40 | 0.244 ± 0.05 | 0.235 ± 0.04 | 0.73 | 3.46 |
| TMD (g/cc) | 1.677 ± 0.06 | 1.668 ± 0.07 | 0.79 | 0.55 | 1.710 ± 0.10 | 1.688 ± 0.06 | 0.59 | 1.25 |
| Trabecular bone,distal femur | ||||||||
| BV/TV (%) | 7.01 ± 1.75 | 13.03 ± 5.43 | <0.01 | +85.85 | 6.92 ± 1.25 | 8.31 ± 4.33 | 0.365 | 20.13 |
| Tb.Th, (mm) | 0.041 ± 0.00 | 0.049 ± 0.00 | <0.01 | +20.55 | 0.043 ± 0.00 | 0.049 ± 0.00 | <0.01 | +15.86 |
| Tb.N (mm−1) | 1.68 ± 0.34 | 2.62 ± 1.11 | 0.036 | +56.10 | 1.60 ± 0.22 | 1.66 ± 0.86 | 0.838 | 3.84 |
| Tb.Sp (mm) | 0.24 ± 0.03 | 0.24 ± 0.04 | 0.822 | 1.80 | 0.24 ± 0.01 | 0.27 ± 0.03 | 0.031 | +13.75 |
| BMD (g/cc) | 0.26 ± 0.02 | 0.34 ± 0.06 | <0.01 | +28.59 | 0.27 ± 0.02 | 0.28 ± 0.04 | 0.485 | 4.83 |
| Histomorphometry, trabecular bone, distal femur | ||||||||
| Sample size (n) | 4 | 4 | 7 | 7 | ||||
| Mineralized surface (%) | 32.38 ± 1.25 | 26.77 ± 6.65 | 0.15 | 17.32 | 28.03 ± 4.57 | 32.66 ± 7.38 | 0.18 | 16.5 |
| MAR (μm/d) | 2.33 ± 0.27 | 3.48 ± 0.60 | <0.01 | +49.21 | 2.58 ± 0.43 | 2.636 ± 0.53 | 0.85 | 1.83 |
| BFR/BV (μm/d/mm) | 44.63 ± 5.63 | 48.90 ± 10.08 | 0.48 | 9.56 | 41.09 ± 10.02 | 45.31 ± 14.17 | 0.53 | 10.26 |
| Osteoclast/BS (n/10 mm) | 7.4 ± 2.9 | 11.7 ± 4.88 | 0.18 | 173.61 | 16.9 ± 5.1 | 26.5 ± 3.05 | 0.01 | +266.37 |
| Osteoblast/BS (n/mm) | 37.87 ± 13.66 | 58.94 ± 7.54 | 0.44 | 155.60 | 21.39 ± 10.74 | 31.10 ± 4.80 | 0.01 | +145.39 |
Values are presented as means ± sd. Significance was set P < 0.05; Student’s t tests. BS, bone surface.
Furthermore, trabecular bone histomorphometry was performed at the femur distal diaphysis (Table 5). Data revealed that MAR increased significantly (∼50%) in control mice, but was unchanged in DMP-GHRKO mice treated with iPTH. BFR/BV increased by ∼10% in both control and DMP-GHRKO female mice treated with iPTH, but it did not reach significance (Table 5). iPTH increases bone remodeling via stimulation of osteoblastic activity (measured by MAR/BFR) and recruitment of osteoclasts. To address whether the number of osteoclasts changes in response to iPTH we stained 10 μm paraffin-embedded sections with anti-cathepsin K antibody and recorded the number of osteoclasts per bone surface. We found that in the basal state (mice treated with PBS) the number of osteoclasts on the bone surface increased by 1.7-fold in DMP-GHRKO mice, compared to the number in the controls (Table 5). Upon iPTH treatment DMP-GHRKO bones showed an ∼2.5-fold increase in osteoclasts, whereas in controls the increase was not significant. Using plastic thin sections (10 μm), we also counted the number of osteoblasts on the trabecular bone surface. We found that, in the basal state, DMP-GHRKO mice had a significantly (P = 0.05) lower number of osteoblasts on the bone surface when compared to the controls (Table 5). This result suggests that, in the basal state, fewer osteoblasts are recruited to bone surface, leading to decreased MAR/BFR. However, in response to iPTH, both controls and the DMP-GHRKO mice showed an ∼50% increase in the number of osteoblasts on the bone surface. Nonetheless, our micro-CT data suggest that the DMP-GHRKO mice have poor response to iPTH. Thus, it is conceivable that the increased number of osteoclasts in the DMP-GHRKO mice contributes to overall impaired trabecular bone modeling.
Previous studies demonstrated that PTH stimulates Igf1 gene expression in osteoblasts and thereby contributes to bone cell proliferation and function (37). Therefore, bones were dissected ∼12 h after the last vehicle or iPTH injection, and the expression of candidate genes that might mediate the effects of iPTH was studied using real-time PCR. Despite the insignificant morphologic changes in response to iPTH, the DMP-GHRKO female mice exhibited significant increases in bone Igf1 and Igf1r gene expression (Fig. 3E), suggesting that GHR-independent mechanisms play important roles. Another gene candidate that may explain the blunted response to iPTH in the DMP-GHRKO mice is Sost. Initial data revealed that the expression of Sost, a negative regulator of bone accrual, increased by 2-fold in the naïve DMP-GHRKO mice (Fig. 2D). This increase correlated with an increase in serum SOST levels (Fig. 2H). Treating control female mice with iPTH inhibited Sost expression (Fig. 3E), whereas expression remained high in DMP-GHRKO mice. Likewise, gene expression of DMP-1 and PHEX did not change in response to iPTH treatment in control or DMP-GHRKO mice. However, in the basal state (untreated mice), DMP-1 and PHEX gene expression increased 4–8-fold in the DMP-GHRKO mice, suggesting an impaired matrix mineralization and impaired phosphate metabolism, respectively. Last, we note that FGF23 gene expression in bone after iPTH varied largely. In general, we observed a tendency toward reductions in FGF23 gene expression in response to iPTH in both controls and DMP-GHRKO mice, but the decrease did not reach significance.
PTH sensitizes osteocyte-like cells to GH action in vitro
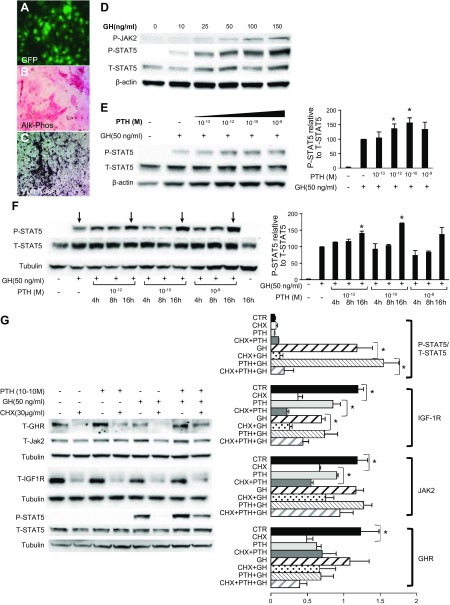
To further investigate the possible interaction between PTH and GH in bone, we used the IDG-SW3 osteocyte cell line (22), which was generated by Dr. L. Bonewald (University of Missouri, Kansas City, MO, USA). These cells expressed GFP under the control of the DMP-1 promoter; they became increasingly fluorescent as differentiation progressed, and ∼85% cells expressed DMP-GFP at day 21 (Fig. 4A). After 3 wk in differentiation medium, IDG-SW3 cells exhibited increased alkaline phosphatase (Alk-Phos) activity (Fig. 4B) and matrix mineralization, as detected with Von Kossa staining (Fig. 4C). Data revealed that GH stimulated JAK2 and STAT5 phosphorylation in a dose-dependent manner in IDG-SW3 cells (Figs. 4D). Exposing IDG-SW3 cells to increasing concentrations of PTH enhanced acute GH-induced STAT5 phosphorylation (Figs. 4E).
Figure 4.
PTH sensitizes IDG-SW3 osteocyte-like cells to GH. IDG-SW3 cells were seeded on collagen-coated plates and grown to 70% confluence (21 d) in differentiation medium. A–C) At 21 d in culture IDG-SW3 cells exhibited increased fluorescence (A) increased Alk-Phos staining (B), and increased Von Kossa staining (C). D) GH treatment induced STAT5b and JAK2 phosphorylation in IDG-SW3 cells in a dose-dependent manner. Cultures were serum starved overnight and stimulated with GH for 10 min. Phospho-STAT5b and phospho-JAK2 were detected by Western blot analysis. E) PTH sensitized IDG-SW3 cells to GH. Cultures were stimulated with the indicated concentrations of PTH, and 18 h later were stimulated with 50 ng/ml GH for 10 min. Phospho-STAT5b was detected by Western blot analysis. F) GH-induced STAT5 phosphorylation increased 16 h after the addition of PTH to IDG-SW3 cells. Cultures were stimulated with the indicated concentrations of PTH followed by 50 ng/ml GH for 10 min at 4, 8, and 16 h. Phospho-STAT5b was detected by Western blot analysis. G) The augmentation of GH-induced STAT5 phosphorylation by PTH was inhibited by CHX. Cultures were treated with 10−10 M PTH and 16 h later were stimulated with 50 ng/ml GH for 10 min in the presence or absence of CHX. Phospho (P)-STAT5b, total (T)-STAT5b, JAK2, and GHR were detected by Western blot analysis. Data are presented as means ± sem of 4 independent experiments. *P < 0.05.
To determine the time course of the effects of PTH on GH action, cells were pretreated with a single dose of PTH, and GH-dependent STAT5 phosphorylation was assessed 4, 8, and 16 h later. STAT5 phosphorylation was increased 16 h after stimulation with PTH (10−12 and 10−10 M), suggesting the involvement of transcriptional or translational mechanisms (Fig. 4F). The addition of 30 μg/ml cycloheximide (CHX; an inhibitor of protein translation) 60 min before treatment with PTH blunted the GH-dependent STAT5 phosphorylation but did not affect total STAT5 levels, indicating that de novo protein synthesis is necessary for the effects of PTH on GH action (Fig. 4G). We further tested whether the levels of GHR, JAK2, and IGF-1R changed in response to PTH and CHX treatment. We found that IGF-1R levels were inhibited in response to CHX and correlated with the decreased STAT5 phosphorylation. Likewise, JAK2 and GHR protein levels decreased in response to CHX but did not correlate with STAT5 phosphorylation. Western blot analysis of GHR suggested that GHR protein levels are regulated by PTH; however, quantification of 4 independent experiments did not support that notion. When IDG-SW3 cells were exposed to PTH over 4 or 8 d, increases in GH-dependent STAT5 phosphorylation (in response to PTH) or increases in IGF-1R protein levels were not evident (Supplemental Fig 4).
To assess whether PTH-induced increased cAMP levels mediates the increased STAT5 phosphorylation in the presence of PTH, IDG-SW3 cells were incubated with the cAMP inducers forskolin (an adenylate cyclase activator), IBMX, a cAMP phosphodiesterase inhibitor), or isoproterenol (a β-receptor agonist that increases cAMP levels) (Supplemental Fig 5A). The induction of cAMP 16 h before GH stimulation with 10−10 or 10−8 M forskolin, 10−8 or 10−6 M IBMX, or 10−10 or 10−8 M isoproterenol sensitized IDG-SW3 cells to GH, as determined by increased STAT5 phosphorylation compared with GH alone. Furthermore, use of H-89 (30 μM) or GF109203X (5 μM) to inhibit the downstream mediators of PTHR1, PKA, and PKC, respectively, showed a decrease in GH-dependent STAT5 phosphorylation (Supplemental Fig. 5B).
Next, we used real-time PCR to evaluate the expression of Ghr, Igf1, and Igf1r in response to PTH. There were no significant changes in the gene expression of Igf1, Ghr, or Igf1r between 4 and 24 h after PTH treatment (Supplemental Fig 5C). However, IGF-1R protein levels increased significantly 8 h after stimulation with 10−8 M PTH (Supplemental Fig 5D), suggesting that PTH increases de novo IGF-1R protein synthesis in vitro.
PTH sensitizes bone cells to GH actions in vivo
To determine whether our in vitro observations also occur in vivo, we treated 4-wk-old control and DMP-GHRKO mice with PTH (80 μg/kg/d) for 3–4 wk. Before the mice were euthanized, they were injected with 0.25 mg/kg recombinant mouse GH, and the bones were dissected after 15 min. STAT5b phosphorylation, JAK2, and IGF-1R protein expression were then measured in bone protein extracts by Western blot analysis.
GH-induced STAT5b phosphorylation in control mice treated with iPTH increased compared with GH-induced STAT5b phosphorylation in PBS-treated control mice; however, the change did not reach statistical significance (Fig. 5A). Control mice exhibited a significant increase in JAK2 and IGF-1R protein levels after iPTH treatment (Fig. 5B). Note that real-time quantitative PCR did not reveal any increase in Igf1r gene expression in the control mice. In contrast, the DMP-GHRKO mice did not show significant changes in JAK2 or IGF-1R protein levels and exhibited large variability between samples. Overall, these data suggest that osteocyte-specific GHR is necessary for bone anabolic response to PTH during growth and likely involves increases in IGF-1R protein levels.
Figure 5.
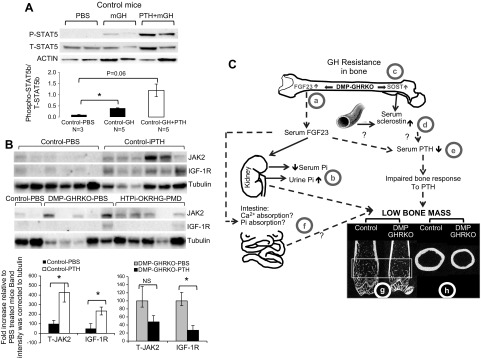
PTH sensitizes bone to GH in vivo. A) Four-week-old control female mice were treated with PTH (80 μg/kg/d) for 3–4 wk, left unfed overnight, injected with 0.25 mg/kg mGH, and euthanized 15 min thereafter. STAT5b phosphorylation (P-STAT5b) and total STAT5b (T-STAT5b), levels were detected by Western blot analysis. Data are presented as means ± SEM; *P < 0.05; ANOVA with Tukey’s post hoc test. B) IGF-1R and JAK2 protein levels were detected in bone protein extracts from female mice treated as described above using Western blot analysis. Four-week-old DMP-GHRKO mice were treated with iPTH (80 μg/kg/d) for 3–4 wk. NS, not significant. Data are presented as means ± sem. *P < 0.05; Student’s t test. C) DMP-GHRKO (GH resistance) in osteoblasts and osteocytes was associated with impaired trabecular and cortical bone accrual during growth. The mechanism may be related to increased skeletal FGF23 from DMP-1-mediated Ghr gene recombination (DMP-GHRKO) (a) or reductions in serum Pi levels and increased urine Pi excretion (b). Likewise, GH resistance in bone was associated with increased Sost gene expression in bone (c) and elevated serum SOST (d). Changes in FGF23 and SOST may contribute directly or indirectly to a blunted serum PTH level during pubertal growth (e). This decrease in turn can result in blunted intestinal absorption of Ca2+ and Pi (f) in the DMP-GHRKO mice because of the inability of 1,25 OHD to respond to PTH, possibly from changes in FGF23.
DISCUSSION
DMP-1-mediated Ghr gene recombination impairs bone acquisition
Herein, we present comprehensive analysis of the bone phenotype in a mouse model of DMP-1-mediated GHRKO (DMP-GHRKO), which has a targeted Ghr gene deletion in mature osteoblasts and osteocytes. DMP-1-mediated Ghr gene recombination (DMP-GHRKO) did not affect overall body weight or body composition and did not affect serum GH or IGF-1 levels during growth. Micro-CT analyses revealed that DMP-GHRKO results in a more slender bone phenotype, evident by the decreased total cross-sectional area at the femur middiaphysis and by significantly decreased J0.
To understand more fully the relative roles of GH and IGF-1 in osteocytes, we used a similar approach to inactivate the IGF-1R or the IGF-1 with DMP-1-derived Cre. A DMP-IGF-1RKO mouse bears a compromised bone phenotype with a significantly reduced bone area and cortical bone thickness. DMP-IGF-1RKO mice exhibited significant increases in marrow area, suggesting increased endosteal bone resorption. These data are consistent with the bone phenotype reported in osteoblast-specific IGF-1RKO mice (OC promoter-driven Cre), which exhibited reduced mineralization and compromised bone accrual (38), and with ex vivo studies of calvarial osteoblasts from these mice, which demonstrated that IGF-1R is essential for the anabolic effects of GH in osteoblasts (39). Although both the DMP-GHRKO and the DMP-IGF-1RKO mice show significant reductions in bone tissue at the middiaphyses compared with controls, their bone phenotypes have different origins. We speculate that the slender bones of the DMP-GHRKO result from inhibition of periosteal bone apposition, while the thinner cortices of the DMP-IGF-1RKO result from increased endosteal bone resorption. Ablation of the ligand IGF-1 resulted in Igf1 gene recombination in multiple tissues. DMP-IGF-1KO mice had normal serum levels of IGF-1, and showed significant decreases in total cross-sectional area in males when compared to controls. We postulate that, despite gene recombination in bone, serum IGF-1 compensated for the lack of local IGF-1 production and thereby resulted in a normal phenotype. This hypothesis is supported by our study showing that IGF-1 deposited in the bone matrix contributes to bone formation and maintenance during aging (40), as well as by our study in IGF-1KO- hepatic IGF-1 transgenic (HIT) mice, which have a global Igf1 gene deletion but carry an Igf1 transgene in the liver (41, 42). In IGF-1KO-HIT mouse serum IGF-1 alone compensated for the absence of tissue IGF-1, both morphologically and mechanically. However, our observations contradict those in other studies (32, 33, 43) showing that DMP-1-mediated Igf1 gene recombination results in an overall decrease in bone size and significant reductions in total, trabecular, and cortical BMD.
Studies in mice with osteoblast-specific IGF-1R ablation revealed a reduced BFR and mineralization defects during growth (3–6 wk of age) (38). In contrast, the overexpression of IGF-1 in osteoblasts increased the BFR 2-fold, leading to a 30% increase in trabecular BV (44), and protected the bone microstructure from low-protein diet-induced bone loss (45). The current study revealed significantly decreased (∼60%) MAR and BFR on the endosteal surface at the middiaphysis of 8-wk-old DMP-GHRKO femurs in female mice. We found that reductions in BFR and MAR were associated with a decreased number of osteoblasts on the bone surface, whereas the number of osteoclasts increased, suggesting impaired bone modeling in the DMP-GHRKO mice during pubertal growth. Our data, together with existing evidence, suggest that both GH and IGF-1 have significant effects on matrix deposition and mineralization in mature osteoblasts and osteocytes.
DMP-1-mediated Ghr gene recombination is associated with mild hypophosphatemia and decreased serum PTH levels
The ablation of GHR in bone was associated with increased FGF23 gene expression in bone, decreased serum Pi levels, and reduced serum PTH levels during growth, without changes in serum calcium. The relationship between the GH/IGF-1 and PTH axes and their effects on bone modeling is controversial. Conflicting data have been reported in patients with acromegaly, where serum GH and IGF-1 levels are extremely elevated and bone formation indices are increased, with some studies reporting low serum PTH levels and others reporting no change (46). In contrast, PTH levels are increased in patients with GH deficiency (GHD) and in GH-deficient lit/lit mice, where both serum GH and IGF-1 levels are reduced, and BMD is compromised. Patients with GHD (47) and postmenopausal women with osteoporosis (48) treated with GH exhibited increased PTH sensitivity in target organs and improved bone mineral metabolism, whereas treating lit/lit mice with GH decreased serum PTH levels (49). Furthermore, the roles of endogenous PTH in bone modeling during growth are unclear. It has been postulated that during midpuberty, when bone formation is rapid, increases in PTH are essential to meet the calcium requirements. However, it is unknown how changes in serum PTH levels are related to BMD and bone structural and strength variables. It is well established that African Americans have a greater cortical volumetric BMD, mass, and size than do Caucasians (50). This superior bone phenotype of African Americans is established early in pubertal growth and is associated with higher serum PTH levels (50). Similarly, a longitudinal study of 69 pubertal Caucasian subjects demonstrated that high (but within the normal range) PTH levels correlate with increased periosteal expansion of the tibia, which was evidenced by increased bone area (51). In the current study, serum PTH levels correlated linearly with serum OC during puberty and young adulthood (8–16 wk) in control mice, and the increased PTH was associated with increased OC and bone formation. In contrast, there was no relationship between serum PTH levels and OC in DMP-GHRKO mice, suggesting that physiologic increases in PTH levels during skeletal growth are associated with increases in levels of bone turnover markers, which may be necessary for increased morphologic traits and BMD. Thus, it is conceivable that GHR in mature osteoblasts and osteocytes is necessary for coupling the effects of PTH and OC in bone during pubertal growth, although the exact mechanism has yet to be discovered.
The anabolic response of bone to iPTH is impaired in mice with DMP-1-mediated Ghr gene recombination
Several studies have revealed that the GH/IGF-1 axis plays important roles in the anabolic response to PTH. However, the molecular mechanisms underlying this response are unclear. PTH increases Igf1 gene expression in bone (15, 16), which may be essential for PTH-mediated osteoblast proliferation and maturation (17, 52, 53) and for the antiapoptotic effects of PTH (54). In the current study, DMP-GHRKO mice exhibited a poor response to 4 wk of iPTH treatment during growth. Although the DMP-GHRKO mice showed an increased number of osteoblasts on bone surfaces after iPTH, the overall anabolic response was minimal and may be related to an ∼2-fold increase in the number of osteoclasts. In contrast, control mice exhibited elevated trabecular BV, thickness, and number, as well as increased cortical bone morphology. Nevertheless, the bone expression of Igf1 and Igf1r increased significantly after the administration of iPTH, suggesting GHR-independent effects of iPTH on bone. In another of our studies, the skeletal response of mice with elevated serum IGF-1 levels (HIT mice) to PTH was significantly higher than that of control mice, suggesting synergy between IGF-1 and PTH on bone (41). In sharp contrast, the absence of tissue IGF-1 in a mouse model with global Igf1 gene ablation but carrying a hepatic Igf1 transgene (IGF-1KO-HIT mouse) resulted in a bone response to iPTH that was identical with that of the PTH-treated control mice. We concluded that tissue IGF-1 is essential for enhancing the anabolic actions of PTH on bone, but can be compensated for by increased serum IGF-1 levels. We should note here that both the HIT and IGF-1KO-HIT mice had intact IGF-1R and GHR in all cells.
In a study in hypophysectomized (HX) rats, treatment with the combination of PTH and GH resulted in substantial bone deposition at both the periosteal and endocortical surfaces, leading to increased BMD (55). GH had anabolic effects on the periosteal surface of the cortical shell and enhanced bone formation without suppressing bone resorption, whereas PTH acted mainly on the endosteal surfaces and stimulated trabecular bone formation and cortical bone thickening. Combined intervention with GH and PTH had synergistic effects and increased the overall bone properties. A similar approach was taken in a study of ovariectomy (OVX)-induced bone loss in aged rats (56). Similar to HX rats, treatment with GH increased periosteal bone apposition in the vertebrae and femoral middiaphysis, whereas PTH acted on the endocortical envelope and decreased the marrow cavity in the aged OVX rats. Combination therapy had synergistic effects and enhanced bone strength at all skeletal sites. In the current study, iPTH was associated with increased serum FGF23 and vitamin D and with increased MAR of the trabecular surfaces of the femur distal metaphysis in control mice, but not in DMP-GHRKO mice, suggesting that the anabolic response to iPTH necessitates some degree of an intact GHR in mature osteoblasts and osteocytes.
Studies in mice revealed that the administration of PTH reduces Sost gene expression in bone by 50% 2 h after injection (57–59) and that this reduction is associated with the anabolic effects of PTH on bone (60, 61). In humans, intermittent PTH treatment reduces Sost gene expression by 15% (62). The current study revealed a 2-fold increase in Sost gene expression in the bones of 8-wk-old DMP-GHRKO female mice and an ∼45% increase in serum SOST levels. This increase in SOST may partially explain the impaired bone phenotype of the DMP-GHRKO mice. In addition, iPTH treatment reduced Sost gene expression in the bones of control mice but not DMP-GHRKO mice. The lack of decrease in Sost gene expression after iPTH may partially explain the blunted anabolic effects of PTH in growing DMP-GHRKO mice.
PTH sensitizes osteocytes to GH
Our in vivo studies suggest interplay between the GH and PTH axes and indicate that both are essential for the establishment of peak bone mass. To explore the possible molecular mechanisms involved in the interaction between GHR and PTHR, we studied IDG-SW3 osteocyte-like cells in vitro. The data clearly demonstrated that IDG-SW3 cells responded to GH, as evidenced by increased STAT5b phosphorylation. In addition, treatment with different concentrations of PTH sensitized cells to GH, and STAT5b phosphorylation increased compared with exposure to GH alone. This increase was evident 16 h after the addition of PTH and was inhibited by CHX, suggesting that the effects of PTH on GHR action involve translational events. In addition, the effects of PTH were likely mediated by increased cAMP because increased GHR-mediated STAT5b phosphorylation was also evident after the addition of forskolin, IBMX, or isoproterenol.
In a study of the UMR106-01 osteoblast-like cell line, Ghr gene expression increased by >2-fold 24 h after stimulation with PTH, and Igf1 gene expression increased by 10- and 11-fold after 1 and 12 h of exposure to PTH, respectively. This effect persisted for 24 h, where Igf1 gene expression increased by 4-fold compared with the control (63). Similarly, in studies of muscle cells, IGF-1 gene and protein expression increased in response to activation of the cAMP/PKA pathway (64, 65). Therefore, we assessed whether PTH enhances GHR activation by increasing Ghr, Igf1, or Igf1r gene expression in IDG-SW3 osteocyte-like cells. However, we did not detect significant changes in Igf1, Igf1r, or Ghr gene expression 24 h after PTH stimulation. IGF-1R protein levels increased unexpectedly 8 h after treatment with PTH and may contribute to the enhanced GH-mediated STAT5 phosphorylation. A series of studies by Dr. S. J. Frank’s laboratory (University of Alabama at Birmingham, Birmingham, AL, USA) (66–70) suggested that IGF-IR participates in the proximal steps of GH signaling. These studies demonstrate that GH promotes the formation of a complex, including GHR, JAK2, and IGF-1R (68, 70), and that silencing the IGF-1R results in a marked reduction in GH-induced proximal signaling and downstream gene expression (66–69). In addition, the current in vivo data from DMP-GHRKO mice (Table 3), suggest that some of the effects of the GHR in osteocytes are IGF-1R dependent. Validation of our in vitro data obtained in IDG-SW3 cells in mice indicate that the levels of JAK2 and IGF-1R increased in bone after treatment with iPTH in control mice, whereas this effect was blunted in the DMP-GHRKO mice.
In summary, in our study, DMP-1-mediated Ghr gene ablation in mature osteoblasts, and osteocytes compromised bone accrual during pubertal growth. DMP-GHRKO mice showed a slender bone phenotype with reduced trabecular bone fraction, which was associated with a decrease in osteocyte lacunae in cortical and trabecular bone and a decrease in osteoblasts and an increase in osteoclasts on trabecular bone surfaces. Despite the decrease in osteocyte lacunae, DMP-GHRKO mice showed elevated expression levels of the osteocyte-specific markers SOST and FGF23, suggesting that GHR in osteocytes may be involved in cell viability rather than cell differentiation. GH resistance in bone (DMP-GHRKO) (Fig. 5C) was associated with reductions in serum Pi levels and increased urine Pi excretion. These effects of DMP-1-mediated Ghr gene recombination on regulation of FGF23 gene expression and mineral metabolism are surprising and require further investigation. Another surprising finding was the transient reductions in serum PTH levels between 4–8 wk in the DMP-GHRKO female mice, suggesting that bone accrual during pubertal growth requires both GH and PTH signaling in bone. Nonetheless, we should note that the reductions in PTH do not fully explain the bone phenotype of the DMP-GHRKO mice, because treatment with exogenous iPTH did not normalize bone traits.
Supplementary Material
Acknowledgments
This work was supported by U.S. National Institutes of Health (NIH), National Institute of Diabetes and Digestive and Kidney Diseases Grant DK100246 (to S.Y.); NIH National Institute of Arthritis and Musculoskeletal and Skin Diseases Grants AR041210 and AR057139 (to M.B.S.); and Binational Science Foundation Grant 2013282 (to S.Y.). The respective roles of the authors: Z.L. conducted all biochemical and gene expression studies, including serum hormone measurements and iPTH treatment; O.K. performed histomorphometry and micro-CT; L.C. and J.B.P. performed and analyzed micro-CT data; M.B.S. and C.J.R. consulted on study design, data analyses, and interpretation; and S.Y. was principle investigator, oversaw all experiments and data collection, and wrote the paper. All authors concurred with the contents of the manuscript. The material submitted for publication has not been reported and is not under consideration for publication elsewhere. The authors declare no conflicts of interest.
Glossary
- α-MEM
α-minimum essential medium
- Alk-Phos
alkaline phosphatase
- BFR
bone formation rate
- BMD
bone mineral density
- BV/TV
the bone volume relative to the total volume
- CHAPS
3-[(3-cholamidopropyl)dimethyl-ammonio]-1-propanesulfonate
- CHX
cycloheximide
- Ct.Ar
cortical bone area
- Ct.Th
cortical bone thickness
- DMP
dentin matrix protein
- DMP-GHRKO
Dmp-1-mediated Ghr knockout
- DMP-IGF-1RKO
Dmp-1-mediated Igf1r knockout
- ES
effect size
- FBS
fetal bovine serum
- FGF
fibroblast growth factor
- GFP
green fluorescent protein
- GH
growth hormone
- GHD
GH-deficient
- GHR
growth hormone receptor
- HIT
hepatic IGF-1 transgenic
- HX
hypophysectomized
- IBMX
isobutylmethylxanthine
- IGF-1R
IGF-1 receptor
- iPTH
intermittent PTH
- J0
polar moment of inertia
- JAK
Janus kinase
- Le
femoral length
- Ma.Ar
marrow area
- MAR
mineral apposition rate
- MCK-GHRKO
muscle creatine kinase muscle-specific Ghr knockout
- micro-CT
micro-computed tomography
- OC
osteocalcin
- Ocy-PPRKO
osteocyte-PPR knockout
- OVX
ovariectomy
- PHEX
phosphate-regulating endopeptidase homolog X-linked
- Pi
inorganic phosphate
- PTH
parathyroid hormone
- PTHR
PTH receptor 1
- RCA
Ct.Ar/Tt.Ar, relative cortical area
- SOST
sclerostin
- Tb.N
trabecular number
- Tb.Sp
trabecular spacing
- Tb.Th
trabecular thickness
- TMD
tissue mineral density
- Tt.Ar
total cross-sectional area
- Tt.Ar/Le
robustness
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Harvey N., Dennison E., Cooper C. (2010) Osteoporosis: impact on health and economics. Nat. Rev. Rheumatol. 6, 99–105 [DOI] [PubMed] [Google Scholar]
- 2.Baron R., Hesse E. (2012) Update on bone anabolics in osteoporosis treatment: rationale, current status, and perspectives. J. Clin. Endocrinol. Metab. 97, 311–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khosla S. (2013) Pathogenesis of age-related bone loss in humans. J. Gerontol. A Biol. Sci. Med. Sci. 68, 1226–1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Erlandson M. C., Kontulainen S. A., Chilibeck P. D., Arnold C. M., Faulkner R. A., Baxter-Jones A. D. (2012) Former premenarcheal gymnasts exhibit site-specific skeletal benefits in adulthood after long-term retirement. J. Bone Miner. Res. 27, 2298–2305 [DOI] [PubMed] [Google Scholar]
- 5.Ferrari S., Rizzoli R., Slosman D., Bonjour J. P. (1998) Familial resemblance for bone mineral mass is expressed before puberty. J. Clin. Endocrinol. Metab. 83, 358–361 [DOI] [PubMed] [Google Scholar]
- 6.Cooper C., Cawley M., Bhalla A., Egger P., Ring F., Morton L., Barker D. (1995) Childhood growth, physical activity, and peak bone mass in women. J. Bone Miner. Res. 10, 940–947 [DOI] [PubMed] [Google Scholar]
- 7.Dennison E. M., Syddall H. E., Rodriguez S., Voropanov A., Day I. N., Cooper C.; Southampton Genetic Epidemiology Research Group (2004) Polymorphism in the growth hormone gene, weight in infancy, and adult bone mass. J. Clin. Endocrinol. Metab. 89, 4898–4903 [DOI] [PubMed] [Google Scholar]
- 8.Tahimic C. G., Wang Y., Bikle D. D. (2013) Anabolic effects of IGF-1 signaling on the skeleton. Front. Endocrinol. (Lausanne) 4, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonewald L. F. (2011) The amazing osteocyte. J. Bone Miner. Res. 26, 229–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lanske B., Karaplis A. C., Lee K., Luz A., Vortkamp A., Pirro A., Karperien M., Defize L. H., Ho C., Mulligan R. C., Abou-Samra A. B., Jüppner H., Segre G. V., Kronenberg H. M. (1996) PTH/PTHrP receptor in early development and Indian hedgehog-regulated bone growth. Science 273, 663–666 [DOI] [PubMed] [Google Scholar]
- 11.Miao D., He B., Karaplis A. C., Goltzman D. (2002) Parathyroid hormone is essential for normal fetal bone formation. J. Clin. Invest. 109, 1173–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xue Y., Karaplis A. C., Hendy G. N., Goltzman D., Miao D. (2005) Genetic models show that parathyroid hormone and 1,25-dihydroxyvitamin D3 play distinct and synergistic roles in postnatal mineral ion homeostasis and skeletal development. Hum. Mol. Genet. 14, 1515–1528 [DOI] [PubMed] [Google Scholar]
- 13.Powell W. F. Jr., Barry K. J., Tulum I., Kobayashi T., Harris S. E., Bringhurst F. R., Pajevic P. D. (2011) Targeted ablation of the PTH/PTHrP receptor in osteocytes impairs bone structure and homeostatic calcemic responses. J. Endocrinol. 209, 21–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saini V., Marengi D. A., Barry K. J., Fulzele K. S., Heiden E., Liu X., Dedic C., Maeda A., Lotinun S., Baron R., Pajevic P. D. (2013) Parathyroid hormone (PTH)/PTH-related peptide type 1 receptor (PPR) signaling in osteocytes regulates anabolic and catabolic skeletal responses to PTH. J. Biol. Chem. 288, 20122–20134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pfeilschifter J., Laukhuf F., Müller-Beckmann B., Blum W. F., Pfister T., Ziegler R. (1995) Parathyroid hormone increases the concentration of insulin-like growth factor-I and transforming growth factor beta 1 in rat bone. J. Clin. Invest. 96, 767–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watson P., Lazowski D., Han V., Fraher L., Steer B., Hodsman A. (1995) Parathyroid hormone restores bone mass and enhances osteoblast insulin-like growth factor I gene expression in ovariectomized rats. Bone 16, 357–365 [DOI] [PubMed] [Google Scholar]
- 17.Wang Y., Nishida S., Boudignon B. M., Burghardt A., Elalieh H. Z., Hamilton M. M., Majumdar S., Halloran B. P., Clemens T. L., Bikle D. D. (2007) IGF-I receptor is required for the anabolic actions of parathyroid hormone on bone. J. Bone Miner. Res. 22, 1329–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bikle D. D., Sakata T., Leary C., Elalieh H., Ginzinger D., Rosen C. J., Beamer W., Majumdar S., Halloran B. P. (2002) Insulin-like growth factor I is required for the anabolic actions of parathyroid hormone on mouse bone. J. Bone Miner. Res. 17, 1570–1578 [DOI] [PubMed] [Google Scholar]
- 19.Miyakoshi N., Kasukawa Y., Linkhart T. A., Baylink D. J., Mohan S. (2001) Evidence that anabolic effects of PTH on bone require IGF-I in growing mice. Endocrinology 142, 4349–4356 [DOI] [PubMed] [Google Scholar]
- 20.Yakar S., Rosen C. J., Beamer W. G., Ackert-Bicknell C. L., Wu Y., Liu J. L., Ooi G. T., Setser J., Frystyk J., Boisclair Y. R., LeRoith D. (2002) Circulating levels of IGF-1 directly regulate bone growth and density. J. Clin. Invest. 110, 771–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yakar S., Rosen C. J., Bouxsein M. L., Sun H., Mejia W., Kawashima Y., Wu Y., Emerton K., Williams V., Jepsen K., Schaffler M. B., Majeska R. J., Gavrilova O., Gutierrez M., Hwang D., Pennisi P., Frystyk J., Boisclair Y., Pintar J., Jasper H., Domene H., Cohen P., Clemmons D., LeRoith D. (2009) Serum complexes of insulin-like growth factor-1 modulate skeletal integrity and carbohydrate metabolism. FASEB J. 23, 709–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woo S. M., Rosser J., Dusevich V., Kalajzic I., Bonewald L. F. (2011) Cell line IDG-SW3 replicates osteoblast-to-late-osteocyte differentiation in vitro and accelerates bone formation in vivo. J. Bone Miner. Res. 26, 2634–2646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bouxsein M. L., Boyd S. K., Christiansen B. A., Guldberg R. E., Jepsen K. J., Müller R. (2010) Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res. 25, 1468–1486 [DOI] [PubMed] [Google Scholar]
- 24.Dempster D. W., Compston J. E., Drezner M. K., Glorieux F. H., Kanis J. A., Malluche H., Meunier P. J., Ott S. M., Recker R. R., Parfitt A. M. (2013) Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 28, 2–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakashima T., Hayashi M., Fukunaga T., Kurata K., Oh-Hora M., Feng J. Q., Bonewald L. F., Kodama T., Wutz A., Wagner E. F., Penninger J. M., Takayanagi H. (2011) Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat. Med. 17, 1231–1234 [DOI] [PubMed] [Google Scholar]
- 26.Xiao Z., Dallas M., Qiu N., Nicolella D., Cao L., Johnson M., Bonewald L., Quarles L. D. (2011) Conditional deletion of Pkd1 in osteocytes disrupts skeletal mechanosensing in mice. FASEB J. 25, 2418–2432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kramer I., Halleux C., Keller H., Pegurri M., Gooi J. H., Weber P. B., Feng J. Q., Bonewald L. F., Kneissel M. (2010) Osteocyte Wnt/beta-catenin signaling is required for normal bone homeostasis. Mol. Cell. Biol. 30, 3071–3085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vijayakumar A., Wu Y., Sun H., Li X., Jeddy Z., Liu C., Schwartz G. J., Yakar S., LeRoith D. (2012) Targeted loss of GHR signaling in mouse skeletal muscle protects against high-fat diet-induced metabolic deterioration. Diabetes 61, 94–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu Y., Liu C., Sun H., Vijayakumar A., Giglou P. R., Qiao R., Oppenheimer J., Yakar S., LeRoith D. (2011) Growth hormone receptor regulates β cell hyperplasia and glucose-stimulated insulin secretion in obese mice. J. Clin. Invest. 121, 2422–2426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vijayakumar A., Buffin N. J., Gallagher E. J., Blank J., Wu Y., Yakar S., LeRoith D. (2013) Deletion of growth hormone receptors in postnatal skeletal muscle of male mice does not alter muscle mass and response to pathological injury. Endocrinology 154, 3776–3783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vijayakumar A., Wu Y., Buffin N. J., Li X., Sun H., Gordon R. E., Yakar S., LeRoith D. (2012) Skeletal muscle growth hormone receptor signaling regulates basal, but not fasting-induced, lipid oxidation. PLoS One 7, e44777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sheng M. H., Lau K. H., Baylink D. J. (2014) Role of osteocyte-derived insulin-like growth factor I in developmental growth, modeling, remodeling, and regeneration of the bone. J. Bone Metab. 21, 41–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sheng M. H., Zhou X. D., Bonewald L. F., Baylink D. J., Lau K. H. (2013) Disruption of the insulin-like growth factor-1 gene in osteocytes impairs developmental bone growth in mice. Bone 52, 133–144 [DOI] [PubMed] [Google Scholar]
- 34.Lavi-Moshayoff V., Wasserman G., Meir T., Silver J., Naveh-Many T. (2010) PTH increases FGF23 gene expression and mediates the high-FGF23 levels of experimental kidney failure: a bone parathyroid feedback loop. Am. J. Physiol. Renal Physiol. 299, F882–F889 [DOI] [PubMed] [Google Scholar]
- 35.Kawata T., Imanishi Y., Kobayashi K., Miki T., Arnold A., Inaba M., Nishizawa Y. (2007) Parathyroid hormone regulates fibroblast growth factor-23 in a mouse model of primary hyperparathyroidism. J. Am. Soc. Nephrol. 18, 2683–2688 [DOI] [PubMed] [Google Scholar]
- 36.Ben-Dov I. Z., Galitzer H., Lavi-Moshayoff V., Goetz R., Kuro-o M., Mohammadi M., Sirkis R., Naveh-Many T., Silver J. (2007) The parathyroid is a target organ for FGF23 in rats. J. Clin. Invest. 117, 4003–4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCarthy T. L., Centrella M. (2001) Local IGF-I expression and bone formation. Growth Horm. IGF Res. 11, 213–219 [DOI] [PubMed] [Google Scholar]
- 38.Zhang M., Xuan S., Bouxsein M. L., von Stechow D., Akeno N., Faugere M. C., Malluche H., Zhao G., Rosen C. J., Efstratiadis A., Clemens T. L. (2002) Osteoblast-specific knockout of the insulin-like growth factor (IGF) receptor gene reveals an essential role of IGF signaling in bone matrix mineralization. J. Biol. Chem. 277, 44005–44012 [DOI] [PubMed] [Google Scholar]
- 39.DiGirolamo D. J., Mukherjee A., Fulzele K., Gan Y., Cao X., Frank S. J., Clemens T. L. (2007) Mode of growth hormone action in osteoblasts. J. Biol. Chem. 282, 31666–31674 [DOI] [PubMed] [Google Scholar]
- 40.Xian L., Wu X., Pang L., Lou M., Rosen C. J., Qiu T., Crane J., Frassica F., Zhang L., Rodriguez J. P., Jia X., Yakar S., Xuan S., Efstratiadis A., Wan M., Cao X (2012) Matrix IGF-1 maintains bone mass by activation of mTOR in mesenchymal stem cells. Nat. Med. 18, 1095–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elis S., Courtland H. W., Wu Y., Fritton J. C., Sun H., Rosen C. J., Yakar S. (2010) Elevated serum IGF-1 levels synergize PTH action on the skeleton only when the tissue IGF-1 axis is intact. J. Bone Miner. Res. 25, 2051–2058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elis S., Courtland H. W., Wu Y., Rosen C. J., Sun H., Jepsen K. J., Majeska R. J., Yakar S. (2010) Elevated serum levels of IGF-1 are sufficient to establish normal body size and skeletal properties even in the absence of tissue IGF-1. J. Bone Miner. Res. 25, 1257–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lau K. H., Baylink D. J., Zhou X. D., Rodriguez D., Bonewald L. F., Li Z., Ruffoni D., Müller R., Kesavan C., Sheng M. H. (2013) Osteocyte-derived insulin-like growth factor I is essential for determining bone mechanosensitivity. Am. J. Physiol. Endocrinol. Metab. 305, E271–E281 [DOI] [PubMed] [Google Scholar]
- 44.Zhao G., Monier-Faugere M. C., Langub M. C., Geng Z., Nakayama T., Pike J. W., Chernausek S. D., Rosen C. J., Donahue L. R., Malluche H. H., Fagin J. A., Clemens T. L. (2000) Targeted overexpression of insulin-like growth factor I to osteoblasts of transgenic mice: increased trabecular bone volume without increased osteoblast proliferation. Endocrinology 141, 2674–2682 [DOI] [PubMed] [Google Scholar]
- 45.Brennan-Speranza T. C., Rizzoli R., Kream B. E., Rosen C., Ammann P. (2011) Selective osteoblast overexpression of IGF-I in mice prevents low protein-induced deterioration of bone strength and material level properties. Bone 49, 1073–1079 [DOI] [PubMed] [Google Scholar]
- 46.Shah R., Licata A., Oyesiku N. M., Ioachimescu A. G. (2012) Acromegaly as a cause of 1,25-dihydroxyvitamin D-dependent hypercalcemia: case reports and review of the literature. Pituitary 15(Suppl 1), S17–S22 [DOI] [PubMed] [Google Scholar]
- 47.Ahmad A. M., Thomas J., Clewes A., Hopkins M. T., Guzder R., Ibrahim H., Durham B. H., Vora J. P., Fraser W. D. (2003) Effects of growth hormone replacement on parathyroid hormone sensitivity and bone mineral metabolism. J. Clin. Endocrinol. Metab. 88, 2860–2868 [DOI] [PubMed] [Google Scholar]
- 48.Joseph F., Ahmad A. M., Ul-Haq M., Durham B. H., Whittingham P., Fraser W. D., Vora J. P. (2008) Effects of growth hormone administration on bone mineral metabolism, PTH sensitivity and PTH secretory rhythm in postmenopausal women with established osteoporosis. J. Bone Miner. Res. 23, 721–729 [DOI] [PubMed] [Google Scholar]
- 49.Kasukawa Y., Baylink D. J., Wergedal J. E., Amaar Y., Srivastava A. K., Guo R., Mohan S. (2003) Lack of insulin-like growth factor I exaggerates the effect of calcium deficiency on bone accretion in mice. Endocrinology 144, 4682–4689 [DOI] [PubMed] [Google Scholar]
- 50.Warden S. J., Hill K. M., Ferira A. J., Laing E. M., Martin B. R., Hausman D. B., Weaver C. M., Peacock M., Lewis R. D. (2013) Racial differences in cortical bone and their relationship to biochemical variables in black and white children in the early stages of puberty. Osteoporos. Int. 24, 1869–1879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tylavsky F. A., Ryder K. M., Li R., Park V., Womack C., Norwood J., Carbone L. D., Cheng S. (2007) Preliminary findings: 25(OH)D levels and PTH are indicators of rapid bone accrual in pubertal children. J. Am. Coll. Nutr. 26, 462–470 [DOI] [PubMed] [Google Scholar]
- 52.Kostenuik P. J., Harris J., Halloran B. P., Turner R. T., Morey-Holton E. R., Bikle D. D. (1999) Skeletal unloading causes resistance of osteoprogenitor cells to parathyroid hormone and to insulin-like growth factor-I. J. Bone Miner. Res. 14, 21–31 [DOI] [PubMed] [Google Scholar]
- 53.Nishida S., Yamaguchi A., Tanizawa T., Endo N., Mashiba T., Uchiyama Y., Suda T., Yoshiki S., Takahashi H. E. (1994) Increased bone formation by intermittent parathyroid hormone administration is due to the stimulation of proliferation and differentiation of osteoprogenitor cells in bone marrow. Bone 15, 717–723 [DOI] [PubMed] [Google Scholar]
- 54.Jilka R. L., Weinstein R. S., Bellido T., Roberson P., Parfitt A. M., Manolagas S. C. (1999) Increased bone formation by prevention of osteoblast apoptosis with parathyroid hormone. J. Clin. Invest. 104, 439–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guevarra M. S., Yeh J. K., Castro Magana M., Aloia J. F. (2010) Synergistic effect of parathyroid hormone and growth hormone on trabecular and cortical bone formation in hypophysectomized rats. Horm. Res. Paediatr. 73, 248–257 [DOI] [PubMed] [Google Scholar]
- 56.Mosekilde L., Tornvig L., Thomsen J. S., Orhii P. B., Banu M. J., Kalu D. N. (2000) Parathyroid hormone and growth hormone have additive or synergetic effect when used as intervention treatment in ovariectomized rats with established osteopenia. Bone 26, 643–651 [DOI] [PubMed] [Google Scholar]
- 57.Silvestrini G., Ballanti P., Leopizzi M., Sebastiani M., Berni S., Di Vito M., Bonucci E. (2007) Effects of intermittent parathyroid hormone (PTH) administration on SOST mRNA and protein in rat bone. J. Mol. Histol. 38, 261–269 [DOI] [PubMed] [Google Scholar]
- 58.Bellido T., Ali A. A., Gubrij I., Plotkin L. I., Fu Q., O’Brien C. A., Manolagas S. C., Jilka R. L. (2005) Chronic elevation of parathyroid hormone in mice reduces expression of sclerostin by osteocytes: a novel mechanism for hormonal control of osteoblastogenesis. Endocrinology 146, 4577–4583 [DOI] [PubMed] [Google Scholar]
- 59.Keller H., Kneissel M. (2005) SOST is a target gene for PTH in bone. Bone 37, 148–158 [DOI] [PubMed] [Google Scholar]
- 60.Gooi J. H., Pompolo S., Karsdal M. A., Kulkarni N. H., Kalajzic I., McAhren S. H., Han B., Onyia J. E., Ho P. W., Gillespie M. T., Walsh N. C., Chia L. Y., Quinn J. M., Martin T. J., Sims N. A. (2010) Calcitonin impairs the anabolic effect of PTH in young rats and stimulates expression of sclerostin by osteocytes. Bone 46, 1486–1497 [DOI] [PubMed] [Google Scholar]
- 61.Kramer I., Loots G. G., Studer A., Keller H., Kneissel M. (2010) Parathyroid hormone (PTH)-induced bone gain is blunted in SOST overexpressing and deficient mice. J. Bone Miner. Res. 25, 178–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Drake M. T., Srinivasan B., Mödder U. I., Peterson J. M., McCready L. K., Riggs B. L., Dwyer D., Stolina M., Kostenuik P., Khosla S. (2010) Effects of parathyroid hormone treatment on circulating sclerostin levels in postmenopausal women. J. Clin. Endocrinol. Metab. 95, 5056–5062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Qin L., Qiu P., Wang L., Li X., Swarthout J. T., Soteropoulos P., Tolias P., Partridge N. C. (2003) Gene expression profiles and transcription factors involved in parathyroid hormone signaling in osteoblasts revealed by microarray and bioinformatics. J. Biol. Chem. 278, 19723–19731 [DOI] [PubMed] [Google Scholar]
- 64.Kravchenko I. V., Furalyov V. A., Popov V. O. (2012) Stimulation of mechano-growth factor expression by myofibrillar proteins in murine myoblasts and myotubes. Mol. Cell. Biochem. 363, 347–355 [DOI] [PubMed] [Google Scholar]
- 65.Kravchenko I. V., Furalyov V. A., Lisitsina E. S., Popov V. O. (2011) Stimulation of mechano-growth factor expression by second messengers. Arch. Biochem. Biophys. 507, 323–331 [DOI] [PubMed] [Google Scholar]
- 66.Gan Y., Paterson A. J., Zhang Y., Jiang J., Frank S. J. (2014) Functional collaboration of insulin-like growth factor-1 receptor (IGF-1R), but not insulin receptor (IR), with acute GH signaling in mouse calvarial cells. Endocrinology 155, 1000–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gan Y., Zhang Y., Buckels A., Paterson A. J., Jiang J., Clemens T. L., Zhang Z. Y., Du K., Chang Y., Frank S. J. (2013) IGF-1R modulation of acute GH-induced STAT5 signaling: role of protein tyrosine phosphatase activity. Mol. Endocrinol. 27, 1969–1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ma F., Wei Z., Shi C., Gan Y., Lu J., Frank S. J., Balducci J., Huang Y. (2011) Signaling cross talk between growth hormone (GH) and insulin-like growth factor-I (IGF-I) in pancreatic islet β-cells. Mol. Endocrinol. 25, 2119–2133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gan Y., Zhang Y., Digirolamo D. J., Jiang J., Wang X., Cao X., Zinn K. R., Carbone D. P., Clemens T. L., Frank S. J. (2010) Deletion of IGF-I receptor (IGF-IR) in primary osteoblasts reduces GH-induced STAT5 signaling. Mol. Endocrinol. 24, 644–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huang Y., Kim S. O., Yang N., Jiang J., Frank S. J. (2004) Physical and functional interaction of growth hormone and insulin-like growth factor-I signaling elements. Mol. Endocrinol. 18, 1471–1485 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.