Abstract
Individuals with schizophrenia and their first-degree relatives have higher rates of type 2 diabetes (T2D) than the general population (18–30 vs. 1.2–6.3%), independent of body mass index and antipsychotic medication, suggesting shared genetic components may contribute to both diseases. The cause of this association remains unknown. Mutations in disrupted in schizophrenia 1 (DISC1) increase the risk of developing psychiatric disorders [logarithm (base 10) of odds = 7.1]. Here, we identified DISC1 as a major player controlling pancreatic β-cell proliferation and insulin secretion via regulation of glycogen synthase kinase-3β (GSK3β). DISC1 expression was enriched in developing mouse and human pancreas and adult β- and ductal cells. Loss of DISC1 function, through siRNA-mediated depletion or expression of a dominant-negative truncation that models the chromosomal translocation of human DISC1 in schizophrenia, resulted in decreased β-cell proliferation (3 vs. 1%; P < 0.01), increased apoptosis (0.1 vs. 0.6%; P < 0.01), and glucose intolerance in transgenic mice. Insulin secretion was reduced (0.5 vs. 0.1 ng/ml; P < 0.05), and critical β-cell transcription factors Pdx1 and Nkx6.1 were significantly decreased. Impaired DISC1 allowed inappropriate activation of GSK3β in β cells, and antagonizing GSK3β (SB216763; IC50 = 34.3 nM) rescued the β-cell defects. These results uncover an unexpected role for DISC1 in normal β-cell physiology and suggest that DISC1 dysregulation contributes to T2D independently of its importance for cognition.—Jurczyk, A., Nowosielska, A., Przewozniak, N., Aryee, K.-E., DiIorio, P., Blodgett, D., Yang, C., Campbell-Thompson, M., Atkinson, M., Shultz, L., Rittenhouse, A., Harlan, D., Greiner, D., Bortell, R. Beyond the brain: disrupted in schizophrenia 1 regulates pancreatic β-cell function via glycogen synthase kinase-3β.
Keywords: insulin, secretion, diabetes, T2D
The continued increase of type 2 diabetes (T2D) is a major public health concern worldwide. In addition to genetic predisposition, obesity and a sedentary lifestyle contribute to its prevalence, and one-third of individuals with hyperinsulinemia, insulin resistance, and impaired glucose tolerance will eventually develop T2D (1, 2). Increased insulin secretion can compensate for insulin resistance; however, reduced action of β cells and a reduction in β-cell mass eventually result in compromised glucose tolerance and T2D develops (3). This reduction of β-cell mass/function may result from increased cell death and/or inadequate proliferation (4).
Intriguingly, individuals with severe mental illnesses such as schizophrenia and bipolar disorder also have higher rates of metabolic syndrome and diabetes than the general population (5). The higher rates of T2D in schizophrenics and their family members (18–30% vs. 1.2–6.3% in the general population) suggest a pathogenetic association between diabetes and these psychiatric disorders (6, 7). Antipsychotic therapies often lead to weight gain and further increase incidence of T2D (8); however, even nonobese schizophrenic patients (with normal weight, body mass index, waist circumference, and waist hip ratio) also show dysregulated insulin secretion (9). In addition, impaired glucose tolerance has been observed in first-episode, drug-naïve patients with schizophrenia (2, 6, 10, 11).
Disrupted in schizophrenia 1 (DISC1) is a well-characterized schizophrenia susceptibility gene that was originally identified in a large Scottish family in which a balanced chromosomal translocation segregated with schizophrenia and related psychiatric disorders (12). Additionally, a mutation in DISC1 was also identified in an American family (13), and multiple DISC1 single-nucleotide polymorphisms associated with mental disorders have been identified, as reviewed elsewhere (14). Genetic linkage studies have confirmed DISC1 involvement with several psychiatric illnesses including schizophrenia, bipolar disorder, and major depression with logarithm (base 10) of odds score 7.1 (15–17). In addition, the blood RNA expression levels of DISC1 and its interacting proteins are decreased in schizophrenic patients as compared with healthy individuals (18). Biochemical studies established DISC1 as a multifunctional protein hub that is required for both neurodevelopment, because of its ability to regulate the proliferation and migration of neuronal progenitors, as well as for normal adult cognitive functioning (19–21).
Because DISC1 was shown to be expressed in pancreas (12), we hypothesized that DISC1 may also play a role in β cells and that defects in DISC1 may predispose individuals to T2D in addition to the known disruption of cognition. Therefore, to investigate a possible genetic link between severe psychiatric disorders such as schizophrenia and impaired glucose homeostasis, we chose to examine the function of DISC1 in vitro in β cells and in vivo in a transgenic mouse model with inducible β-cell-specific expression of a dominant-negative truncation of human DISC1 [truncated human DISC1 (thDISC1)]. Previously, schizophrenia was thought to disrupt central control of blood glucose homeostasis, leading to T2D (22). Here we present an alternative perspective showing that DISC1 disruption impairs mouse β-cell function independently of CNS regulation, thus revealing an important role for DISC1 in normal pancreatic β-cell function and glucose homeostasis in vivo.
MATERIALS AND METHODS
Animals
Mice were housed in the University of Massachusetts facilities on a 12 h light-dark cycle with unlimited access to food and water. ThDISC1 transgenic mice were obtained from The Jackson Laboratory (Bar Harbor, ME, USA). The Tet-On double transgenic system was modified from elsewhere (23) to generate a mouse model of inducible thDISC1 expression in β cells [NOD.Cg-Prkdc < scid > Il2rg < tm1Wjl > Tg(Ins2-rtTA)2Efr/Sz; Tg(tetO-DISC1*)1001Plet/J]. Mice harboring both of these transgenes have doxycycline (DOX)-inducible expression of thDISC1 in pancreatic β cells following dietary administration of 200 mg/kg DOX (Bio-Serv; Flemington, NJ, USA). These mice have a mixed genetic background. To use mice with similar mixed genetic backgrounds for experiments, we used double transgenic homozygote mice without DOX for controls and double transgenic homozygote mice with DOX for experimental animals.
Body weight and fed blood glucose levels were measured weekly using a OneTouch glucometer (LifeScan; Wayne, PA, USA) on blood from a tail snip. Samples for intraperitoneal glucose tolerance tests were collected from mice fasted overnight and at indicated time points after intraperitoneal injection of glucose (2 g/kg body weight). Plasma insulin was measured with a mouse insulin ELISA kit (Alpco; Salem, NH, USA). Glycogen synthase kinase-3β (GSK3β) inhibitor rescue injections were performed after indicated time of DOX or control diet with 4 intraperitoneal injections, every day of SB 216763 (IC50 = 34.3 nM; Sigma-Aldrich, St. Louis, MO, USA) at 2 mg/kg body weight.
Cell culture and gene silencing
The INS1 832/13 insulinoma cell line [gift from Dr. Urano, (Washington University, St. Louis, MO, USA)] was cultured in RPMI 1640 medium containing 10% fetal bovine serum (FBS), penicillin and streptomycin, sodium pyruvate, and β-mercaptoethanol. The MIN6 insulinoma cell line (gift from Dr. Urano) was cultured in DMEM containing 10% FBS, penicillin and streptomycin. Primary humans islets obtained from the IIDP (Integrated Islet Distribution Program, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases Bethesda, MD, USA) were plated onto Matrigel (BD Biosciences; Billerica, MA, USA) coated chamber slides (Thermo Fisher Scientific; Waltham, MA, USA) and cultured in CMRL medium supplemented with 10% FBS, nonessential amino acids, sodium pyruvate, penicillin, and streptomycin. For small interference RNA (siRNA) transfection Lipofectamine 2000 (Invitrogen; Grand Island, NY, USA) was used according to the manufacturer’s protocol. Sequences for siRNA (siDISC1: 5′-AACGGCTGAGCCAAGAGTTGG-3′, 5′-AAGGCAAACACTGTGAAGTGC-3′; SCR: 5′-AATTCTCCGAACGTGTCACGT-3′).
Primary islets, islet perifusion, and insulin content
Pancreatic islets from control or thDISC1 mice were harvested by collagenase digestion as described elsewhere (24). For perifusion experiments, islets were isolated and cultured overnight on nonadhesive petri dishes after which 25 islets were hand selected and loaded for perifusion using a perifusion machine from Biorep Technologies (Miami, FL, USA). The islets were perifused in Kreb’s buffer with 0.17% bovine serum albumin at a flow rate of 100 μl/min throughout the run and flow-through was maintained at 4°C. Perifusion solutions were gassed with 95% O2/5% CO2 and the islet chambers were maintained at 37°C. At the end of the run, the islets were collected from the chambers and placed in acidified ethanol overnight to determine total insulin levels.
Pancreatic insulin content was determined as described elsewhere (25). The whole pancreas was dissected out, weighted, and placed into 1.5% HCl, 70% EtOH solution overnight at −20°C. The tissue was spun the following day and insulin content of the supernatant was determined by ELISA kit (Alpco; Salem, NH, USA).
Quantitative RT-PCR
Mouse and human islets were isolated and RNA extracted with RNeasy Mini kit (Qiagen, Valenica, CA, USA) and reverse transcribed using SuperScript III (Invitrogen). Gene expression was evaluated with qPCR using TaqMan system and TaqMan probes (human ACTB: Hs01060665_g1, DISC1: Hs00962132_m1, GCG: Hs01031536_m1, INS: Hs02741908_m1; mouse ActB: Mm00607939_s1, Disc1: Mm00533313_m1, INS1: Mm01950294_s1, Nkx6.1: Mm00454961_m1, Pdx1: Mm00435565_m1. Probes used for genotyping were as follows: control: apolipoprotein B oIMR 1544:5′-CACGTGGGCTCCAGCATT-3, apolipoprotein B oIMR 3580: 5′-TCACCAGTCATTTCTGCCTTTG-3′, apolipoprotein B probe 13745: 5′-CCAATGGTCGGGCACTGCTCAA-3′; DISC1 forward: 5′-TCTCTGCCATCAGCAGAGTTGAGT-3′, DISC1 reverse: 5′-AGAGCCAAGCGAGAGCCGAATAAA-3′, DISC1 (MGB-FAM): 5′-TGGCTCTCACAGTGCCTTTACCTCAA-3′; rtTA 9342: 5′-GGACGAGCTCCACTTAGACG-3′, rtTA 9343: 5′-CAACATGTCCAGATCGAAATC-3′, rtTA (MGB-FAM): 5′-CTAGCGCGTCGGCATGCG-3′.
Human pancreas and in situ hybridization
Human frozen pancreas sections (4 μm) were obtained from the Network for Pancreatic Organ Donors with Diabetes program (26). Fetal pancreata were obtained from Stem Express (Placerville, CA, USA). The sections were postfixed in 4% paraformaldehyde, blocked in Tris-buffered saline with Tween 20 (50 ml of Tris-buffered saline, 1 g bovine serum albumin, 50 μl Tween, 2% goat serum), and incubated with primary antibodies followed by secondary antibodies. Primary antibodies were anti: actin (1:1000), active/dephosphorylated β-catenin (1:500), total β-catenin [1:1000 immunofluorescence (IF), 1:2000 Westerns, Millipore, Billerica, MA, USA], BrdU (1:200; Accurate Chemicals, Westbury, NY, USA), caspase 3 active (1:500), CK-19 (1:100, Abcam, Cambridge, MA, USA), cyclin D1 (1:250), p9GSK3β (1:500), total GSK3β (1:500, Cell Signaling, Danvers, MA, USA), Disc1 (1:150 IF, 1:250, Westerns), Sox9 (1:200, Novus Biologicals, Littleton, CO, USA), glucagon (1:200, Sigma-Aldrich), insulin (1:250 IF and Westerns, Dako, Carpinteria, CA, USA), Pdx1 (1:200, R&D Systems, Minneapolis, MN, USA). Secondary antibodies were Alexa 488, Alexa 594 (1:1000) and Alexa 647 (1:500) from Invitrogen.
For in situ hybridization (ISH), human paraffin sections from Network for Pancreatic Organ Donors with Diabetes pediatric organ donors were used and ISH was performed according to manufacturer’s recommendations (RNAScope, Advanced Cell Diagnostics, San Francisco, CA, USA). Amplification was applied as recommended followed by counterstaining with Gill Hemoxylin and coverslipping with Cytoseal (Thermo Fisher Scientific). Colocalization with insulin was determined in one donor using the ISH procedure as described without coverslipping before immunofluorescence detection of insulin.
Image acquisition
All images were acquired with a Nikon (Tokyo, Japan) Eclipse Ti series microscope. Images were further analyzed with Nikon Elements image analysis software.
Statistics
Statistical analyses were performed with the Student’s t test. All values were expressed as means ± sem. Differences were considered to be statistically significant when P < 0.05.
Study approval
The Institutional Animal Care and Use Committee of the University of Massachusetts Medical School approved all experimental procedures involving mice.
RESULTS
Endogenous DISC1 expression is enriched in adult human and mouse pancreatic β cells and ducts
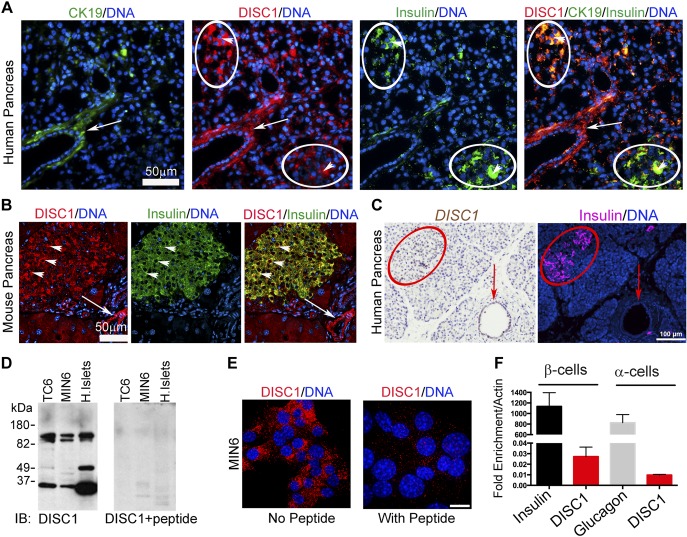
Although DISC1 mRNA expression was reported in adult human pancreas (12), the precise cell types remained unknown. Closer examination of human (Fig. 1A) and mouse (Fig. 1B) pancreas showed DISC1 immunofluorescent staining enriched in insulin-positive β cells and in cytokeratin 19 (CK19)-staining ductal cells; exocrine pancreas showed much less staining. ISH confirmed DISC1 mRNA in human islets and ductal cells, with lower expression in exocrine pancreas (Fig. 1C). The full length DISC1 protein is ∼100 kDa; however, multiple differentially spliced transcripts of DISC1 have been identified (27, 28). By Western blot (Fig. 1D), the previously described ∼100-kDa doublet as well as smaller DISC1 isoforms were observed in murine β-cell lines (TC6 and MIN6) and human islets (29). The functional relevance of DISC1 isoforms is not understood. Specificity of the DISC1 antibody was demonstrated by DISC1-specific peptide blockade for Western blots (Fig. 1D) and immunofluorescence of β cells (Fig. 1E).
Figure 1.
DISC1 localizes to human and mouse pancreatic islets and ducts. A) Immunofluorescence of human pancreas showing DISC1 in β cells (arrowheads) and duct cells (arrow). The islet areas based on insulin staining are circled in white. Ductal cells are stained with CK19. Scale bar, 50 μm. B) Immunofluorescence of mouse pancreas showing DISC1 in β cells (arrowheads) and duct cells (arrow). Scale bar, 50 μm. C) ISH of human pancreas showing high expression of DISC1 in islet (circled in red) and ductal cells (arrow) and low expression in exocrine pancreas. Scale bar, 100 μm. D) Western analysis of DISC1 expression in lysates from mouse β-cell lines (TC6 and MIN6) and human islets blotted with DISC1 antibody ± specific inhibitory peptide. E) Immunofluorescence of MIN6 β cells showing DISC1 staining in the cytosol is competed away with DISC1-specific peptide. Scale bar, 10 μm. F) Quantitative RT-PCR for mRNA levels (relative to actin) of DISC1, insulin, and glucagon expression in FACS sorted β- and α cells from 5 human islet donors.
To verify more precisely which islets cells express DISC1, we fluorescence-activated cell sorting (FACS)-sorted adult human islets into insulin-positive β cells and glucagon-positive α cells and performed quantitative RT-PCR on the sorted cell populations. We show that DISC1 mRNA is expressed in insulin-positive β cells and, to a lesser extent, in glucagon-positive α cells (Fig. 1F); expression levels of insulin and glucagon indicate relative abundance compared to actin.
DISC1 is coexpressed with human pancreatic progenitor markers during development
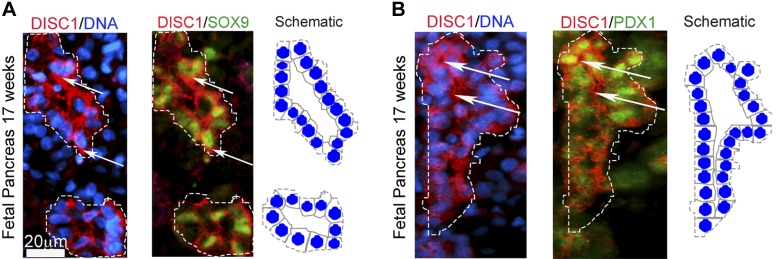
Because DISC1 is important for maintaining the neuronal progenitor pool (19) and we detected DISC1 in ductal cells, the source of pancreatic progenitors during embryonic neogenesis, we tested whether DISC1 also coexpresses with pancreatic progenitors in human fetal pancreas. At 17 wk, DISC1 coexpressed with pancreatic progenitor markers SOX9 (Fig. 2A) and PDX1 (Fig. 2B) in pancreatic epithelium. Most pancreatic ductal cells exhibiting high SOX9 and PDX1 expression also showed high DISC1 expression. Recently published RNA-seq analysis of FACS-sorted fetal pancreas extend our immunofluorescence data showing high DISC1 expression in fetal β cells compared with adult β cells (5.98 ± 1.53 vs. 0.69 ± 0.203 transcripts per million; P < 0.01) (30). Similarly in neurons, DISC1 is enriched in fetal as compared to adult brain (29). Sox9 levels, similar to DISC1, were also enriched in developing pancreas, and PDX1 levels were higher in adult β cells (30). Together, these data suggest an important role for DISC1 in development of human pancreatic progenitors in addition to mature pancreas.
Figure 2.
DISC1 colocalizes with pancreatic progenitors to human fetal pancreatic epithelium. A) Immunofluorescence of human fetal pancreas at 17 wk showing high DISC1 expression with pancreatic progenitors SOX9 and B) PDX1 in growing ductal epithelium (arrows). Scale bar, 20 μm. Schematic representation of the pancreatic epithelial ducts on the right of each image.
thDISC1 transgenic mice have normal body weight but transient hyperglycemia postweaning
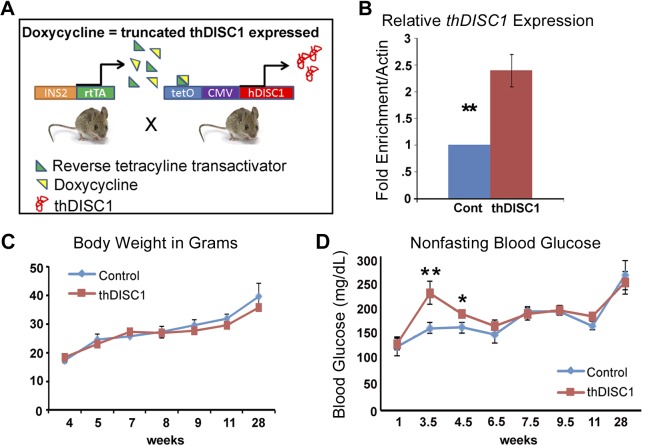
To determine the function of DISC1 specifically in β cells, we used an established thDISC1 transgenic mouse model (engineered to model the effects of the disease-associated chromosomal translocation of DISC1 in schizophrenia) (23). These mice were crossed with mice expressing the tetracycline transactivator under control of the insulin 2 (Ins2) promoter to inducibly express thDISC1 in mature β cells (Fig. 3A). DISC1 truncation acts as a dominant negative in animal models and cell lines by binding to native DISC1 and preventing its interactions with other binding partners (31–34). Experimental animals were derived from homozygous mice continuously provided DOX containing food from the time a plug was observed in the pregnant females, throughout pregnancy and until takedown, although control animals were the same genotype but without DOX in their diet. DOX induction led to β-cell expression of thDISC1 (Fig. 3B). We and others have not observed any toxic effects of DOX, including glucose regulation (data not shown) (35, 36). Transgenic thDISC1 mice were born at predicted mendelian proportion with normal body weights (Fig. 3C). However, between weeks 3.5 to 4.5, thDISC1 mice had significantly elevated blood glucose (Fig. 3D). Although blood glucose levels subsequently normalized after 6 wk of age, transient early hyperglycemia has been reported to result in alterations in gene expression, as well as impaired glucose-stimulated insulin secretion in β cells (37, 38).
Figure 3.
Transgenic thDISC1 mice have elevated blood glucose with normal body weights. A) A schematic representation of transgenic thDISC1 mice in which the expression of thDISC1 is regulated by the presence or absence of DOX. Experimental animals were derived from homozygous mice continuously provided DOX-containing food from the time we observed a plug in pregnant females, through weaning, and until takedown of the resultant pups. Control mice contain homozygote thDISC1 transgene but do not express it without DOX treatment. B) Quantitative RT-PCR for mRNA levels (relative to ActinB) of islets from control and thDISC1 mice. C) Body weights of control and thDISC1 mice, n = 4–11. D) Nonfasting blood glucose levels at selected time points, n = 5–7. Means ± sem. *P < 0.05, Student’s t test; **P < 0.01 vs. corresponding controls.
thDISC1 transgenic mice have reduced plasma insulin levels, impaired glucose tolerance, and decreased pancreatic insulin content
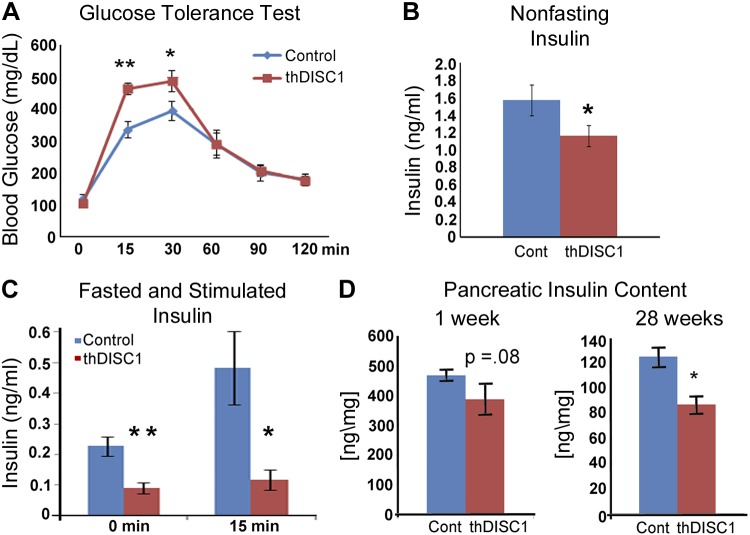
To determine whether our transgenic animals had impaired glucose-stimulated insulin secretion, we first performed a glucose tolerance test. We found that at 6 wk of age thDISC1 mice had significantly impaired glucose tolerance (Fig. 4A), and their nonfasting plasma insulin (Fig. 4B), and fasting and glucose-stimulated levels of plasma insulin (Fig. 4C) were significantly lower. Indeed, β-cell mass, as determined by pancreatic insulin content, was marginally decreased (P = 0.087) in thDISC1 mice early in life, at 1 wk of age, suggesting that the animals may have been born with a deficiency in endocrine pancreas (Fig. 4D) and eventually by 28 wk, β-cell insulin content of thDISC1 was significantly lower than controls (Fig. 4D).
Figure 4.
Transgenic thDISC1 mice are glucose intolerant and have lower levels of insulin. A) Glucose tolerance test at 9 wk, n = 6. B) Nonfasting plasma insulin levels at 6 wk, n = 9–21. C) Fasted and glucose-stimulated plasma insulin levels at 6 wk, n = 4. D) Pancreatic insulin content normalized to weight of pancreatic tissue in 1-wk-old newborns and 28-wk-old adult animals, n = 4–13. Means ± sem. *P < 0.05, Student’s t test; **P < 0.01 vs. corresponding controls.
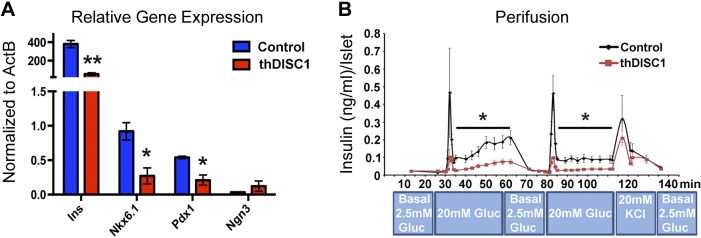
Isolated pancreatic islets from thDISC1 transgenic mice have decreased expression of β-cell-specific transcription factors and insulin and reduced insulin secretion
To investigate the mechanism underlying the decreased insulin levels in thDISC1 mice, we first determined the levels of β-cell-specific transcription factors because DISC1 has recently been identified as a transcriptional regulator of schizophrenia-associated genes in human neural progenitor cells (39). By quantitative RT-PCR analyses, we found that β-cell-specific transcription factors Pdx1 and Nkx6.1, as well as insulin, were significantly down-regulated in islets isolated from thDISC1 mice as compared with control (Fig. 5A). In contrast, the expression of Neurogenin 3, a critical transcription factor for endocrine differentiation and survival early in development (40), was not changed. Decreased expression of β-cell-specific transcription factors could underlie the decreased insulin secretion and subsequent glucose intolerance in thDISC1 mice. In support of this, targeted disruption of the Pdx1 gene leads to diabetes in mice, whereas partial down-regulation leads to decreased insulin expression and secretion and predisposes β cells to apoptosis (41–43).
Figure 5.
Islets isolated from transgenic thDISC1 mice have decreased levels of β-cell-specific transcription factors and secrete less insulin when stimulated with glucose. A) Quantitative RT-PCR for mRNA levels (relative to ActinB) of mouse insulin, Nkx6.1, Pdx1, and Neurogenin 3 in DOX-treated thDISC1 mice (red) as compared with control (blue). B) Islet perifusion at 10 wk, n = 3. Means ± sem. *P < 0.05, Student’s t test; **P < 0.01 vs. corresponding controls.
However, in addition to pancreatic β cells, the INS2 promoter that drives thDISC1 expression shows some detectable expression in brain (44). We did not observe any detectable expression of thDISC1 in the brain (data not shown); however, to dissociate any potential effects of DISC1 disruption in the brain, we performed perifusion analysis on islets isolated from thDISC1 mice. We found that glucose-stimulated insulin secretion was decreased when comparing equivalent numbers of size-matched islets from thDISC1 and control mice (Fig. 5B), indicating an effect of DISC1 disruption on insulin secretion independent of the brain.
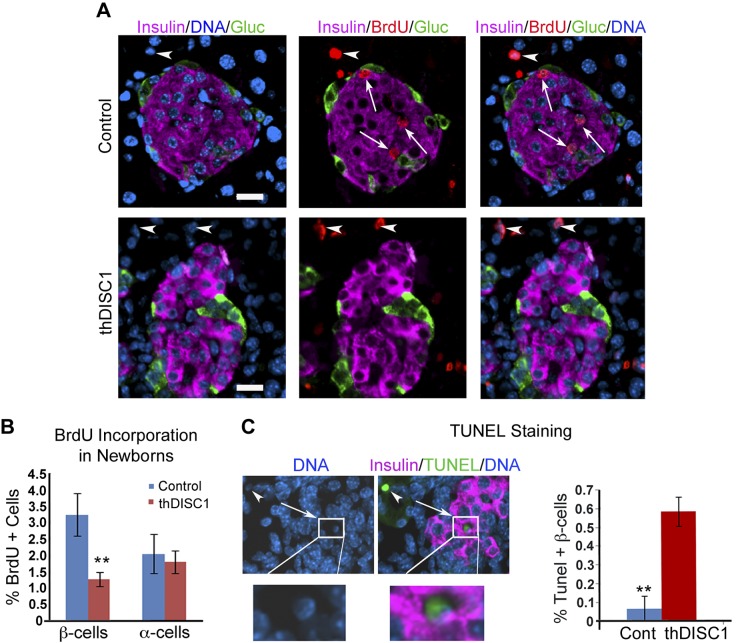
thDISC1 transgenic mice exhibit decreased proliferation and increased apoptosis of β cells
Another factor that could contribute to low insulin levels would be an effect of DISC1 disruption on β-cell proliferation and/or apoptosis. To first test the effect of truncated DISC1 expression on β-cell proliferation, we used newborn animals because proliferation remains high during the postnatal period, thus improving our ability to measure changes in proliferation rate. Indeed, overnight BrdU incorporation was significantly decreased in β cells expressing thDISC1, but not in α cells that lacked transgene expression (Fig. 6A, B). We next assayed for β-cell apoptosis, and found that TUNEL staining in β cells from newborn thDISC1 mice was also increased (Fig. 6C). Decreased proliferation and increased apoptosis could also contribute to the deceased insulin secretion and glucose intolerance observed in thDISC1 mice. However, decreased β-cell numbers cannot account for the decreased insulin secretion observed in isolated islets, where equivalent numbers of size-matched islets from thDISC1 and control mice were used (Fig. 5B). Therefore, together our data indicate that DISC1 is important for both proliferation and survival of β cells, as well as glucose stimulated insulin secretion.
Figure 6.
DISC1 is important for proliferation and survival of pancreatic β cells. A) Postnatal day 2 control and hDISC1 mice pancreas 17 h after BrdU injection stained with insulin (pink), glucagon (green), BrdU (red), and DAPI (blue). BrdU-positive insulin cells (arrows), exocrine pancreas BrdU-positive cells (arrowheads). Scale bar, 20 μm. B) Quantitation of BrdU-positive β cells (total β cells counted: n = 3700 controls and n = 8391 thDISC1) and α cells (total α cells counted: n = 1670 controls and n = 4092 thDISC1) from 4 control and 8 thDISC1 mice. C) TUNEL staining in thDISC1 mouse islets showing TUNEL-positive staining in β cell (arrow); enlargement on the bottom, and TUNEL-positive cell in the exocrine pancreas (arrowhead); Quantitation of percent TUNEL-positive β cells; β cells counted: n = 1130 for control (n = 4 mice) and n = 2487 for thDISC1 (n = 7 mice). Means ± sem. **P < 0.01 vs. corresponding controls.
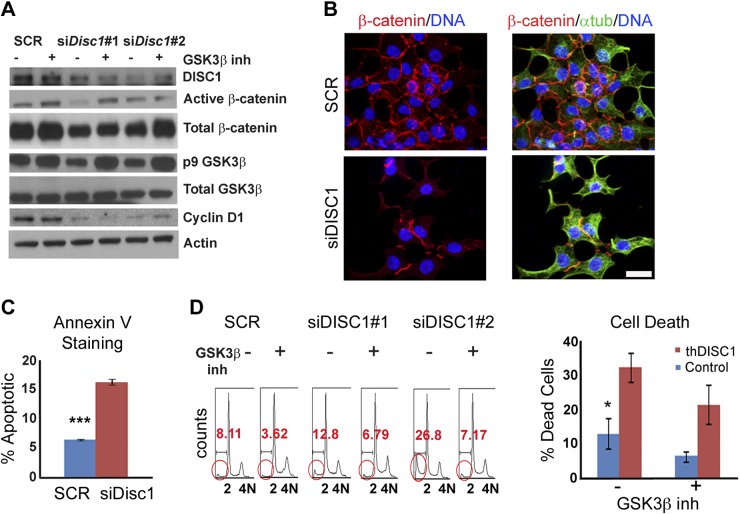
DISC1 regulates proliferation and apoptosis of β cells through GSK3β activity
To determine the mechanism by which DISC1 regulates proliferation of β cells we used specific siRNAs to deplete DISC1 in INS 832/13 insulinoma cells (Fig. 7A). Because DISC1 regulates proliferation of progenitor neurons by direct binding of GSK3β and inhibition of its activity (19), we tested whether DISC1 plays a similar role in β cells. A well-known readout of GSK3β kinase activity is the phosphorylation-dependent proteasomal degradation of β-catenin (45). We show that both active/dephosphorylated and total levels of β-catenin were reduced in DISC1-depleted β cells by Western analysis (Fig. 7A) and immunofluorescence (Fig. 7B), and that β-catenin levels were partially restored with the GSK3β inhibitor, SB-216763 (Fig. 7A). These results are consistent with an increase in active GSK3β with DISC1 depletion, which coincides with the observed decrease (Fig. 7A) in the inactive phospho-Ser 9 (p9) form of GSK3β (46, 47). Cyclin D1, a well-known β-catenin target that promotes cell cycle progression, was also decreased (Fig. 7A). Although treatment with a specific GSK3β inhibitor restored the levels of p9-GSK3β and β-catenin in DISC1-depleted cells, the levels of cyclin D1 were not significantly changed (Fig. 7A), indicating a different mechanism for the down-regulation of cyclin D1.
Figure 7.
Regulation of β-cell proliferation and apoptosis by DISC1 through modulation of GSK3β activity. A) Western blot with indicated antibodies on lysates from INS1 β-cell line after 72 h of scrambled control (SCR) or Disc1 siRNAs plus or minus GSK3β specific inhibitor. B) Immunofluorescence from SCR or Disc1 siRNA-treated INS1 β cells showing lower levels of β-catenin (red) in siDisc1 cells; α-tubulin (green) and DAPI (blue). Scale bar, 10 μm. C) Annexin V staining of INS1 β cells after 48 h of scrambled control and Disc1 siRNA treatment. D) FACS profile showing the dead cells (circled in red) with and without GSK3β-specific inhibitor after 48–72 h of SCR and Disc1 siRNA; SCR (n = 4 different replicates), siDisc1 (n = 8 different replicates), quantitation of dead cells on the right. Means ± sem. *P < 0.05, ***P < 0.001 vs. corresponding controls.
To investigate the mechanism of DISC1’s role in β-cell survival, we again used siRNA technology to deplete DISC1 in vitro. We observed an increase in apoptosis as determined by FACS-based quantitation of Annexin-positive cells (Fig. 7C) and sub-G0/G1 (<2 N) dead cells (Fig. 7D). The cell survival was partially rescued by treatment with a specific GSK3β inhibitor (Fig. 7D). Thus, the increase in β-cell death we observed with DISC1 depletion may be a consequence of GSK3β activation, as previously reported in primary neuronal cultures (48, 49).
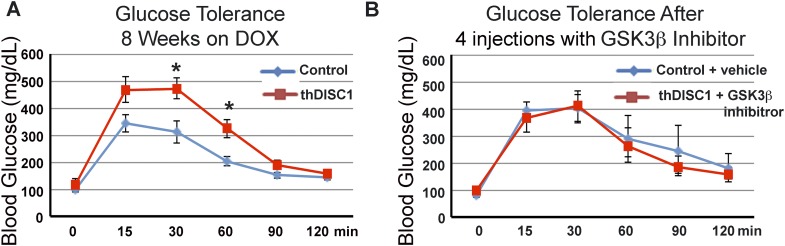
Induction of thDISC1 in adult animals results in glucose intolerance that can be rescued by GSK3β inhibition
Because expression of thDISC1 throughout the pre- and postnatal period disrupted glucose homeostasis (Figs. 3 and 4), we asked if a similar phenotype occurs when thDISC1 is expressed postdevelopment in adult animals. Adult transgenic animals were induced with DOX; after 2 mo, thDISC1 animals showed glucose intolerance (Fig. 8A). Because in vitro GSK3β inhibition was able to rescue the effects of DISC1 depletion on the regulation of GSK3β (Fig. 7A) as well as cell death (Fig. 7D), we asked if in vivo GSK3β inhibition could rescue the glucose intolerance of thDISC1 mice. In the following 1 wk, DOX-induced thDISC1 mice were additionally treated every day with 4 injections of GSK3β inhibitor (2 mg/kg, i.p.); glucose tolerance of these thDISC1 animals was restored and comparable to that of vehicle-treated thDISC1 mice on control diet (Fig. 8B). Our data, in vitro and in vivo showed that activation of GSK3β by disruption of DISC1 and its deleterious effects on pancreatic β-cell survival and function can be rescued by treatment with a specific GSK3β inhibitor.
Figure 8.
Induction of glucose intolerance in adult mice expressing thDISC1 in vivo and rescue with GSK3β inhibitor. A) Glucose tolerance tests of thDISC1 mice after 8 wk of DOX treatment. B) Rescue of glucose intolerance after 4 intraperitoneal injections of the specific GSK3β inhibitor, SB216763 (2 mg/kg) every day beginning within a week of the glucose tolerance test in A), n = 4 mice. Means ± sem, *P < 0.05 vs. corresponding controls.
DISCUSSION
Given that individuals with severe mental illness such as schizophrenia and bipolar disorder also have higher rates of metabolic syndrome and diabetes than the general population (5) and that DISC1, a well-known associated gene for psychiatric disorders localizes to pancreas (12), we set out to determine whether DISC1 plays a role in pancreatic β cells. We have identified DISC1 as a novel regulator of β-cell function. Our in vivo studies with a mouse model expressing dominant-negative thDISC1 demonstrate that DISC1 disruption decreases proliferation and increases apoptosis in β cells of newborn mice, and adult mice have reduced levels of plasma insulin and are glucose intolerant. However, to further ensure that our studies of DISC1 function in β cells were not influenced by the contribution of the nervous system, we performed additional in vitro analyses using 2 different approaches. First, using insulinoma cell lines with siRNA-mediated knockdown of DISC1, we demonstrated that DISC1 depletion resulted in increased apoptosis of β cells, consistent with our studies in newborn mice. Furthermore, DISC1 depletion caused a decrease in active β-catenin, an indicator of decreased cell proliferation, which was rescued by GSK3β inhibition. Second, we isolated pancreatic islets from our thDISC1 mice, and showed by in vitro perifusion that glucose-stimulated insulin secretion was reduced compared with equal numbers of islets from control cells. Importantly, not only do these studies exclude any potential CNS effects, they also demonstrate that β-cell function is impaired using 2 completely different methodologies to disrupt DISC1, namely, siRNA-mediated depletion and transgenic expression of a dominant-negative truncation. Together with our finding that DISC1 expression is enriched in fetal and human pancreatic β cells and ducts, these data indicate that DISC1 could play an important function in human β-cell function and survival. Indeed, these findings illustrate an emerging principle where incongruous coprecipitation of disorders such as schizophrenia and diabetes can now be explained by parallel but independent dysfunction of a single protein in different tissues—brain and islets. A global DISC1 knockout rat has recently become available (SAGE Labs, Boyertown, PA, USA), and it will be interesting to see how β-cell function in these animals compares with our β-cell-specific dominant-negative DISC1 mouse model.
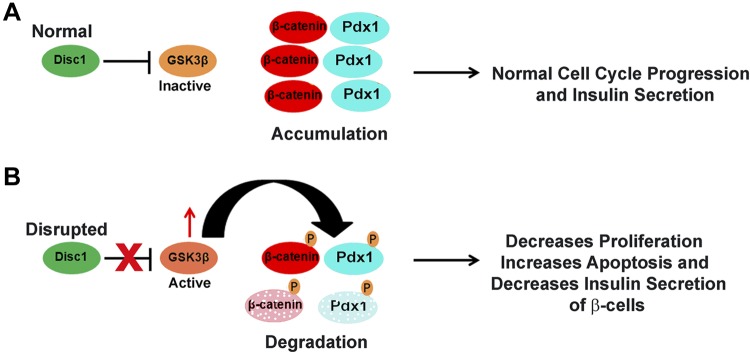
At the cellular level, our data suggest normal DISC1-mediated inhibition of GSK3β activity promotes β-cell proliferation via preservation of β-catenin, as well as β-cell survival and insulin secretion via appropriate maintenance of Pdx1 levels (see model in Fig. 9A). GSK3β has many cellular targets, and we propose that during early neonatal stages when proliferation of β cells is robust, DISC1 inhibition of GSK3β and the subsequent stabilization of β-catenin and Pdx1 could play important roles in the proliferation and survival, respectively, of β cells. Later in life, when proliferation may not be the primary mechanism of compensating for increased insulin demand, the action of Pdx1 on insulin expression and secretion could play a more important role. In support of this, reduction of Pdx1 was shown to reduce β-cell compensatory insulin secretion response to insulin resistance in adult mice (50).
Figure 9.
Model of DISC1 role in β cells. A) Normally endogenous DISC1 inhibits GSK3β leading to accumulation of β-catenin and Pdx1 and activation of the cell cycle and normal regulation of insulin secretion. B) Depletion of DISC1 allows activation of GSK3β that, in turn, phosphorylates β-catenin and Pdx1 for degradation; this leads to decreased proliferation and insulin secretion and increased apoptosis of pancreatic β cells.
Because inhibition of GSK3β plays an important role in pancreatic β-cell function and survival (51, 52), relieving inhibition of GSK3β by disrupting DISC1 could account for our observed deleterious β-cell phenotypes (see model in Fig. 9B). In support of this, glucose intolerance and apoptosis of DISC1-disrupted β cells was rescued by treating thDISC1 mice or DISC1-depleted cells with a specific GSK3β inhibitor. This direct role of DISC1 in glucose homeostasis could explain the increased rates of T2D among people with DISC1-related psychiatric illnesses (53, 54). Diabetes is a multivariable disease and several interacting variables must coalesce for a patient to become diabetic (55, 56). Individuals with mental illnesses may be predisposed to developing T2D due to lifestyle and to genetics, including disruption of DISC1; antipsychotic drug treatments that are known to cause a further increase in T2D could tip the balance toward disease. To our knowledge, this is the first identification of a pleiotropic role, in neurons and β cells, for DISC1 gene whose disruption was previously associated only with mental illnesses. In this study, we now show that DISC1 also plays a novel role in pancreatic β-cell survival and function and provide a molecular link for a prevalence of diabetes in individuals with psychiatric disorders.
Acknowledgments
The authors thank Linda Leehy, Linda Paquin, and Rabia Lidstrom for technical support, and Aldo Rossini and Laura Alonso for their advice and guidance. This work was supported by U.S. National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases Grant U01 DK089572, and the Leona M. and Harry B. Helmsley Charitable Trust Grant 2012PG-T1D018.
Glossary
- CK19
cytokeratin 19
- DISC1
disrupted in schizophrenia 1
- DOX
doxycycline
- FACS
fluorescence-activated cell sorting
- FBS
fetal bovine serum
- GSK3β
glycogen synthase kinase-3β
- IF
immunofluorescence
- ISH
in situ hybridization
- siRNA
small interference RNA
- T2D
type 2 diabetes
- thDISC1
truncated human DISC1
Footnotes
Deceased.
REFERENCES
- 1.Alberti K. G. (1996) The clinical implications of impaired glucose tolerance. Diabet. Med. 13, 927–937 [DOI] [PubMed] [Google Scholar]
- 2.Ryan M. C., Collins P., Thakore J. H. (2003) Impaired fasting glucose tolerance in first-episode, drug-naive patients with schizophrenia. Am. J. Psychiatry 160, 284–289 [DOI] [PubMed] [Google Scholar]
- 3.Henquin J. C., Cerasi E., Efendic S., Steiner D. F., Boitard C. (2008) Pancreatic beta-cell mass or beta-cell function? That is the question! Diabetes Obes. Metab. 10(Suppl 4), 1–4 [DOI] [PubMed] [Google Scholar]
- 4.Weir G. C., Bonner-Weir S. (2013) Islet β cell mass in diabetes and how it relates to function, birth, and death. Ann. N. Y. Acad. Sci. 1281, 92–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chadda R. K., Ramshankar P., Deb K. S., Sood M. (2013) Metabolic syndrome in schizophrenia: Differences between antipsychotic-naïve and treated patients. J. Pharmacol. Pharmacother. 4, 176–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mukherjee S., Schnur D. B., Reddy R. (1989) Family history of type 2 diabetes in schizophrenic patients. Lancet 1, 495 [DOI] [PubMed] [Google Scholar]
- 7.Adams, P. F., and Marano, M. A. (1995) Current estimates from the National Health Interview Survey, 1994. National Center for Health Statistics. Vital Health Stat. 10, 82–90 [PubMed]
- 8.De Hert M., van Winkel R., Van Eyck D., Hanssens L., Wampers M., Scheen A., Peuskens J. (2006) Prevalence of diabetes, metabolic syndrome and metabolic abnormalities in schizophrenia over the course of the illness: a cross-sectional study. Clin. Pract. Epidemol Ment. Health 2, e14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang J., Liu E. Y., Freudenreich O., Goff D., Henderson D. C., Fan X. (2010) Phenotypic characteristics in metabolically obese but normal weight non-diabetic patients with schizophrenia. Schizophr. Res. 124, 49–53 [DOI] [PubMed] [Google Scholar]
- 10.Spelman L. M., Walsh P. I., Sharifi N., Collins P., Thakore J. H. (2007) Impaired glucose tolerance in first-episode drug-naive patients with schizophrenia. Diabet. Med. 24, 481–485 [DOI] [PubMed] [Google Scholar]
- 11.Venkatasubramanian G., Chittiprol S., Neelakantachar N., Naveen M. N., Thirthall J., Gangadhar B. N., Shetty K. T. (2007) Insulin and insulin-like growth factor-1 abnormalities in antipsychotic-naive schizophrenia. Am. J. Psychiatry 164, 1557–1560 [DOI] [PubMed] [Google Scholar]
- 12.Millar J. K., Wilson-Annan J. C., Anderson S., Christie S., Taylor M. S., Semple C. A., Devon R. S., St Clair D. M., Muir W. J., Blackwood D. H., Porteous D. J. (2000) Disruption of two novel genes by a translocation co-segregating with schizophrenia. Hum. Mol. Genet. 9, 1415–1423 [DOI] [PubMed] [Google Scholar]
- 13.Sachs N. A., Sawa A., Holmes S. E., Ross C. A., DeLisi L. E., Margolis R. L. (2005) A frameshift mutation in Disrupted in Schizophrenia 1 in an American family with schizophrenia and schizoaffective disorder. Mol. Psychiatry 10, 758–764 [DOI] [PubMed] [Google Scholar]
- 14.Chubb J. E., Bradshaw N. J., Soares D. C., Porteous D. J., Millar J. K. (2008) The DISC locus in psychiatric illness. Mol. Psychiatry 13, 36–64 [DOI] [PubMed] [Google Scholar]
- 15.Palo O. M., Antila M., Silander K., Hennah W., Kilpinen H., Soronen P., Tuulio-Henriksson A., Kieseppä T., Partonen T., Lönnqvist J., Peltonen L., Paunio T. (2007) Association of distinct allelic haplotypes of DISC1 with psychotic and bipolar spectrum disorders and with underlying cognitive impairments. Hum. Mol. Genet. 16, 2517–2528 [DOI] [PubMed] [Google Scholar]
- 16.Porteous D. (2008) Genetic causality in schizophrenia and bipolar disorder: out with the old and in with the new. Curr. Opin. Genet. Dev. 18, 229–234 [DOI] [PubMed] [Google Scholar]
- 17.Hennah W., Thomson P., McQuillin A., Bass N., Loukola A., Anjorin A., Blackwood D., Curtis D., Deary I. J., Harris S. E., Isometsä E. T., Lawrence J., Lönnqvist J., Muir W., Palotie A., Partonen T., Paunio T., Pylkkö E., Robinson M., Soronen P., Suominen K., Suvisaari J., Thirumalai S., St Clair D., Gurling H., Peltonen L., Porteous D. (2009) DISC1 association, heterogeneity and interplay in schizophrenia and bipolar disorder. Mol. Psychiatry 14, 865–873 [DOI] [PubMed] [Google Scholar]
- 18.Rampino A., Walker R. M., Torrance H. S., Anderson S. M., Fazio L., Di Giorgio A., Taurisano P., Gelao B., Romano R., Masellis R., Ursini G., Caforio G., Blasi G., Millar J. K., Porteous D. J., Thomson P. A., Bertolino A., Evans K. L. (2014) Expression of DISC1-interactome members correlates with cognitive phenotypes related to schizophrenia. PLoS One 9, e99892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mao Y., Ge X., Frank C. L., Madison J. M., Koehler A. N., Doud M. K., Tassa C., Berry E. M., Soda T., Singh K. K., Biechele T., Petryshen T. L., Moon R. T., Haggarty S. J., Tsai L. H. (2009) Disrupted in schizophrenia 1 regulates neuronal progenitor proliferation via modulation of GSK3beta/beta-catenin signaling. Cell 136, 1017–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh K. K., Ge X., Mao Y., Drane L., Meletis K., Samuels B. A., Tsai L. H. (2010) Dixdc1 is a critical regulator of DISC1 and embryonic cortical development. Neuron 67, 33–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishizuka K., Kamiya A., Oh E. C., Kanki H., Seshadri S., Robinson J. F., Murdoch H., Dunlop A. J., Kubo K., Furukori K., Huang B., Zeledon M., Hayashi-Takagi A., Okano H., Nakajima K., Houslay M. D., Katsanis N., Sawa A. (2011) DISC1-dependent switch from progenitor proliferation to migration in the developing cortex. Nature 473, 92–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris L. W., Guest P. C., Wayland M. T., Umrania Y., Krishnamurthy D., Rahmoune H., Bahn S. (2013) Schizophrenia: metabolic aspects of aetiology, diagnosis and future treatment strategies. Psychoneuroendocrinology 38, 752–766 [DOI] [PubMed] [Google Scholar]
- 23.Pletnikov, M. V., Ayhan, Y., Nikolskaia, O., Xu, Y., Ovanesov, M. V., Huang, H., Mori, S., Moran, T. H., Ross, C. A. (2008) Inducible expression of mutant human DISC1 in mice is associated with brain and behavioral abnormalities reminiscent of schizophrenia. [E-pub ahead of print] Mol. Psychiatry 13, 173–186 [DOI] [PubMed]
- 24.Parker D. C., Greiner D. L., Phillips N. E., Appel M. C., Steele A. W., Durie F. H., Noelle R. J., Mordes J. P., Rossini A. A. (1995) Survival of mouse pancreatic islet allografts in recipients treated with allogeneic small lymphocytes and antibody to CD40 ligand. Proc. Natl. Acad. Sci. USA 92, 9560–9564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang C., Suckow A. T., Chessler S. D. (2013) Altered pancreatic islet function and morphology in mice lacking the Beta-cell surface protein neuroligin-2. PLoS One 8, e65711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campbell-Thompson M., Wasserfall C., Kaddis J., Albanese-O’Neill A., Staeva T., Nierras C., Moraski J., Rowe P., Gianani R., Eisenbarth G., Crawford J., Schatz D., Pugliese A., Atkinson M. (2012) Network for Pancreatic Organ Donors with Diabetes (nPOD): developing a tissue biobank for type 1 diabetes. Diabetes Metab. Res. Rev. 28, 608–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Millar J. K., Christie S., Anderson S., Lawson D., Hsiao-Wei Loh D., Devon R. S., Arveiler B., Muir W. J., Blackwood D. H., Porteous D. J. (2001) Genomic structure and localisation within a linkage hotspot of Disrupted In Schizophrenia 1, a gene disrupted by a translocation segregating with schizophrenia. Mol. Psychiatry 6, 173–178 [DOI] [PubMed] [Google Scholar]
- 28.Soares D. C., Carlyle B. C., Bradshaw N. J., Porteous D. J. (2011) DISC1: structure, function, and therapeutic potential for major mental illness. ACS Chem. Neurosci. 2, 609–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schurov I. L., Handford E. J., Brandon N. J., Whiting P. J. (2004) Expression of disrupted in schizophrenia 1 (DISC1) protein in the adult and developing mouse brain indicates its role in neurodevelopment. Mol. Psychiatry 9, 1100–1110 [DOI] [PubMed] [Google Scholar]
- 30.Blodgett D. M., Nowosielska A., Afik S., Pechhold S., Cura A. J., Kennedy N. J., Kim S., Kucukural A., Davis R. J., Kent S. C., Greiner D. L., Garber M. G., Harlan D. M., diIorio P. (2015) Novel observations from next-generation rna sequencing of highly purified human adult and fetal islet cell subsets. Diabetes 64, 3172–3181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cash-Padgett T., Jaaro-Peled H. (2013) DISC1 mouse models as a tool to decipher gene-environment interactions in psychiatric disorders. Front. Behav. Neurosci. 7, e113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kamiya A., Kubo K., Tomoda T., Takaki M., Youn R., Ozeki Y., Sawamura N., Park U., Kudo C., Okawa M., Ross C. A., Hatten M. E., Nakajima K., Sawa A. (2005) A schizophrenia-associated mutation of DISC1 perturbs cerebral cortex development. Nat. Cell Biol. 7, 1167–1178 [DOI] [PubMed] [Google Scholar]
- 33.Newburn E. N., Hyde T. M., Ye T., Morita Y., Weinberger D. R., Kleinman J. E., Lipska B. K. (2011) Interactions of human truncated DISC1 proteins: implications for schizophrenia. Transl. Psychiatry 1, e30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ozeki Y., Tomoda T., Kleiderlein J., Kamiya A., Bord L., Fujii K., Okawa M., Yamada N., Hatten M. E., Snyder S. H., Ross C. A., Sawa A. (2003) Disrupted-in-Schizophrenia-1 (DISC-1): mutant truncation prevents binding to NudE-like (NUDEL) and inhibits neurite outgrowth. Proc. Natl. Acad. Sci. USA 100, 289–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kistner A., Gossen M., Zimmermann F., Jerecic J., Ullmer C., Lübbert H., Bujard H. (1996) Doxycycline-mediated quantitative and tissue-specific control of gene expression in transgenic mice. Proc. Natl. Acad. Sci. USA 93, 10933–10938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pletnikov M. V. (2009) Inducible and conditional transgenic mouse models of schizophrenia. Prog. Brain Res. 179, 35–47 [DOI] [PubMed] [Google Scholar]
- 37.Leahy J. L., Bonner-Weir S., Weir G. C. (1988) Minimal chronic hyperglycemia is a critical determinant of impaired insulin secretion after an incomplete pancreatectomy. J. Clin. Invest. 81, 1407–1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Laybutt D. R., Glandt M., Xu G., Ahn Y. B., Trivedi N., Bonner-Weir S., Weir G. C. (2003) Critical reduction in beta-cell mass results in two distinct outcomes over time. Adaptation with impaired glucose tolerance or decompensated diabetes. J. Biol. Chem. 278, 2997–3005 [DOI] [PubMed] [Google Scholar]
- 39.Wen Z., Nguyen H. N., Guo Z., Lalli M. A., Wang X., Su Y., Kim N. S., Yoon K. J., Shin J., Zhang C., Makri G., Nauen D., Yu H., Guzman E., Chiang C. H., Yoritomo N., Kaibuchi K., Zou J., Christian K. M., Cheng L., Ross C. A., Margolis R. L., Chen G., Kosik K. S., Song H., Ming G. L. (2014) Synaptic dysregulation in a human iPS cell model of mental disorders. Nature 515, 414–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gradwohl G., Dierich A., LeMeur M., Guillemot F. (2000) neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc. Natl. Acad. Sci. USA 97, 1607–1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahlgren U., Jonsson J., Jonsson L., Simu K., Edlund H. (1998) beta-cell-specific inactivation of the mouse Ipf1/Pdx1 gene results in loss of the beta-cell phenotype and maturity onset diabetes. Genes Dev. 12, 1763–1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brissova M., Shiota M., Nicholson W. E., Gannon M., Knobel S. M., Piston D. W., Wright C. V., Powers A. C. (2002) Reduction in pancreatic transcription factor PDX-1 impairs glucose-stimulated insulin secretion. J. Biol. Chem. 277, 11225–11232 [DOI] [PubMed] [Google Scholar]
- 43.Johnson J. D., Ahmed N. T., Luciani D. S., Han Z., Tran H., Fujita J., Misler S., Edlund H., Polonsky K. S. (2003) Increased islet apoptosis in Pdx1+/- mice. J. Clin. Invest. 111, 1147–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wicksteed B., Brissova M., Yan W., Opland D. M., Plank J. L., Reinert R. B., Dickson L. M., Tamarina N. A., Philipson L. H., Shostak A., Bernal-Mizrachi E., Elghazi L., Roe M. W., Labosky P. A., Myers M. G. Jr., Gannon M., Powers A. C., Dempsey P. J. (2010) Conditional gene targeting in mouse pancreatic ß-Cells: analysis of ectopic Cre transgene expression in the brain. Diabetes 59, 3090–3098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aberle H., Bauer A., Stappert J., Kispert A., Kemler R. (1997) Beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 16, 3797–3804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cross D. A., Alessi D. R., Cohen P., Andjelkovich M., Hemmings B. A. (1995) Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378, 785–789 [DOI] [PubMed] [Google Scholar]
- 47.Dajani R., Fraser E., Roe S. M., Young N., Good V., Dale T. C., Pearl L. H. (2001) Crystal structure of glycogen synthase kinase 3 beta: structural basis for phosphate-primed substrate specificity and autoinhibition. Cell 105, 721–732 [DOI] [PubMed] [Google Scholar]
- 48.Takashima A., Noguchi K., Sato K., Hoshino T., Imahori K. (1993) Tau protein kinase I is essential for amyloid beta-protein-induced neurotoxicity. Proc. Natl. Acad. Sci. USA 90, 7789–7793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Busciglio J., Lorenzo A., Yeh J., Yankner B. A. (1995) beta-amyloid fibrils induce tau phosphorylation and loss of microtubule binding. Neuron 14, 879–888 [DOI] [PubMed] [Google Scholar]
- 50.Brissova M., Blaha M., Spear C., Nicholson W., Radhika A., Shiota M., Charron M. J., Wright C. V., Powers A. C. (2005) Reduced PDX-1 expression impairs islet response to insulin resistance and worsens glucose homeostasis. Am. J. Physiol. Endocrinol. Metab. 288, E707–E714 [DOI] [PubMed] [Google Scholar]
- 51.Mussmann R., Geese M., Harder F., Kegel S., Andag U., Lomow A., Burk U., Onichtchouk D., Dohrmann C., Austen M. (2007) Inhibition of GSK3 promotes replication and survival of pancreatic beta cells. J. Biol. Chem. 282, 12030–12037 [DOI] [PubMed] [Google Scholar]
- 52.Stein J., Milewski W. M., Dey A. (2013) The negative cell cycle regulators, p27(Kip1), p18(Ink4c), and GSK-3, play critical role in maintaining quiescence of adult human pancreatic β-cells and restrict their ability to proliferate. Islets 5, 156–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Millar J. K., Christie S., Semple C. A., Porteous D. J. (2000) Chromosomal location and genomic structure of the human translin-associated factor X gene (TRAX; TSNAX) revealed by intergenic splicing to DISC1, a gene disrupted by a translocation segregating with schizophrenia. Genomics 67, 69–77 [DOI] [PubMed] [Google Scholar]
- 54.Su P., Li S., Chen S., Lipina T. V., Wang M., Lai T. K., Lee F. H., Zhang H., Zhai D., Ferguson S. S., Nobrega J. N., Wong A. H., Roder J. C., Fletcher P. J., Liu F. (2014) A dopamine D2 receptor-DISC1 protein complex may contribute to antipsychotic-like effects. Neuron 84, 1302–1316 [DOI] [PubMed] [Google Scholar]
- 55.Stumvoll M., Goldstein B. J., van Haeften T. W. (2005) Type 2 diabetes: principles of pathogenesis and therapy. Lancet 365, 1333–1346 [DOI] [PubMed] [Google Scholar]
- 56.Hu F. B. (2011) Globalization of diabetes: the role of diet, lifestyle, and genes. Diabetes Care 34, 1249–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]