Abstract
Protein disulfide isomerase A6 (PDIA6) interacts with protein kinase RNA-like endoplasmic reticulum kinase (PERK) and inositol requiring enzyme (IRE)-1 and inhibits their unfolded protein response signaling. In this study, shRNA silencing of PDIA6 expression in insulin-producing mouse cells reduced insulin production (5-fold) and, consequently, glucose-stimulated insulin secretion (3–4-fold). This inhibition of insulin release was independent of the PDIA6-PERK interaction or PERK activity. Acute inhibition of PERK did not change the short-term response of β cells to glucose. Rather, PDIA6 affected insulin secretion by modulating one of the activities of IRE1. At 11 mM glucose and lower, the regulated IRE1-dependent decay (RIDD) of the mRNA activity of IRE1 was activated, but not its X-box binding protein (XBP)-1 splicing activity. In the absence of PDIA6, RIDD activity toward insulin transcripts was enhanced up to 4-fold, as shown by molecular assays in cultured cells and the use of a fluorescent reporter in intact islets. Such physiologic activation of IRE1 by glucose contrasted with IRE1 activation by chemical stress, when both IRE1 activities were induced. Thus, whereas the stimulus determines the quality of IRE1 signaling, PDIA6 attenuates multiple enzymatic activities of IRE1, maintaining its signaling within a physiologically tolerable range.—Eletto, D., Eletto, D., Boyle, S., Argon, Y. PDIA6 regulates insulin secretion by selectively inhibiting the RIDD activity of IRE1.
Keywords: β-cell metabolism, unfolded protein response, GSIS
The unfolded protein response (UPR) is not merely a stress response that functions to restore protein homeostasis to cells in toxic environmental conditions. The UPR is also a physiologic signaling and transcriptional system that is activated during normal development and differentiation of multiple cell types and is needed for their biologic functions (1). One of the most striking examples of physiologic UPR is pancreatic β cells. These cells use the UPR sensors Ser/Thr protein kinase inositol-requiring enzyme (IRE)-1, protein kinase RNA-like endoplasmic reticulum kinase (PERK), and activating transcription factor (ATF)-6 (2, 3), to respond to changes in blood glucose and secrete insulin. Their secretion of insulin relies on and is modulated by the UPR machinery daily in a cyclical manner (4). The UPR in β cells is qualitatively different in conditions of hypo- and hyperglycemia. At low glucose concentration, PERK is maximally activated (5), whereas XBP1 splicing by IRE1 is minimal (6, 7), but at high glucose, and in particular, in sustained hyperglycemia, the IRE1 pathway is dominant, and PERK activation subsides (6, 8–10).
Because they are dedicated to efficient secretion of hormones and operate with a high flux of protein synthesis, it is not surprising that β cells are very sensitive to endoplasmic reticulum (ER) stress and maintain their homeostasis via the UPR machinery (11). Sustained hyperglycemia, hyperlipidemia, depletion of ER calcium stores, or expression of mutated insulin (4, 10–12) all increase apoptosis of β cells as a result of excessive ER stress. Whether the β cells cope with these metabolic stresses or die is determined by the integration of signaling pathways emanating from IRE1 and PERK.
PERK is essential for β-cell development and function, as demonstrated by Wolcott-Rallison disease, which is a form of congenital diabetes caused by mutations in PERK (13). The human disease is mimicked by the PERK knockout mouse, in which pancreata progressively deteriorate (14). Ablation of PERK by expression of a dominant–negative mutant dysregulates the ER chaperone system and then reduces insulin gene expression, secretion, and cell proliferation (15). IRE1 is also essential for the development of the secretory machinery of pancreatic cells (16) and, in the mature pancreas, it is necessary for insulin biosynthesis in response to transiently high glucose levels (6). In addition, the IRE1 pathway, together with the AKT pathway, serves as an important defense mechanism against lipotoxic β-cell death (17).
The activation of both of these transmembrane sensors involves similar mechanisms. In their inactive state, both are monomeric and bound on the luminal side of the ER membrane by the chaperone binding immunoglobulin protein (BiP)/GRP78. Upon ER stress, BiP dissociates, IRE1 or PERK dimerize and oligomerize, and their cytoplasmic kinase domains cross-phosphorylate each other and thus enable their proximal enzymatic activities: cleavage of RNAs in the case of IRE1 or phosphorylation of the translation elongation eukaryotic initiation factor (eIF)-2α in the case of PERK (18, 19). Thus, IRE1 primarily regulates target gene expression, whereas PERK exerts a primary translational control. However, when activated, both sensors are engaged in additional activities. IRE1 can initiate JNK pathway signaling by recruitment of the TRAF2 protein to the cytosolic domain (20) and associate with RACK1 (7) and BH3-family proteins (21), and PERK can phosphorylate another substrate, Nrf2 (22), a transcription factor that regulates many redox genes. Therefore, the activation of each sensor per se is insufficient to determine the outcome of the UPR response and requires regulation by interacting proteins (21, 23).
As befitting a physiologic response, the inherent signaling activities of IRE1 and PERK are known to be transient (24). Furthermore, keeping these activities within certain boundaries is an essential feature of the UPR that determines whether a cell will return to homeostasis or enter apoptotic pathways (25–27). The inherent signaling activities tend to become detrimental when the duration of IRE1 and PERK activities are extended, for example, under a chronic hyperglycemic stress (12). The intensity and duration of UPR signaling can both be modulated by interacting proteins, some of which have been characterized [see review (28)]. Almost all of the sensor interacting proteins studied to date are cytosolic and modulate the activities of the kinase and ribonuclease domains of the UPR sensors. Only 2 interactors operate in the lumen of the ER, where the metabolic stress arises and is sensed by the luminal domains of PERK and IRE1. One luminal interactor is the above-mentioned BiP/GRP78. The other is the enzyme protein disulfide isomerase A6 (PDIA6), whose interaction with IRE1 and PERK has been characterized recently by us and others (29, 30). The data show that PDIA6 interacts with IRE1 and PERK, but not with ATF6 (29). Activation of the latter sensor requires another PDI family member, PDIA5 (31). PDIA6 acts as a negative modulator of both IRE1 and PERK in cell culture and in vivo, and when it is silenced, UPR signaling (specifically IRE1 splicing activity) becomes excessive and favors apoptosis (29).
Because β-cell UPR changes depending on the nature of the UPR inducers and because PDIA6 can interact with either IRE1 or PERK (29), the response of β cells to changes in glucose is an interesting scenario in which to explore how PDIA6 regulates UPR signaling. In this study, we used both genetic and pharmacological tools to silence the expression of UPR components to determine which pathway is important for glucose-stimulated insulin secretion (GSIS).
MATERIALS AND METHODS
Cell and tissue culture
293T human embryonic kidney cells from American Type Culture Collection (Manassas, VA, USA) and insulin (INS)-1 832/13 cells (32) from Dr. H.-E. Hohmeier (Duke University School of Medicine, Durham, NC, USA) were maintained, respectively, in DMEM (Mediatech, Manassas, VA, USA), with 10% heat-inactivated fetal bovine serum (FBS; Atlanta Biologicals, Atlanta, GA, USA), 100 U penicillin, 100 U streptomycin, and 292 µg/mL glutamine (Thermo Fisher–Invitrogen, Grand Island, NY, USA), or in RPMI 1640 (Thermo Fisher–Gibco) with 11 mM glucose, 10% heat-inactivated FBS (Atlanta Biologicals, Oakwood, GA, USA), 100 U penicillin, 100 U streptomycin, 292 µg/ml glutamine (Thermo Fisher-Invitrogen), 1 mM sodium pyruvate (Thermo Fisher–Invitrogen), and 55 µM 2-ME. For transient transfections of plasmids, we used Lipofectamine 2000 (Thermo Fisher–Invitrogen), according to the manufacturer’s protocol.
Islets were isolated by the islet cell biology core of the Penn Diabetes Research Center, In brief, mice were anesthetized with sodium pentobarbital (50 mg/kg body weight, i.p.), and pancreatic islets were isolated by collagenase digestion followed by Ficoll density gradient centrifugation. Islets were cultured in RPMI medium similar to that used for INS-1 cells. Cell viability was assessed with a fixable live/dead blue fluorescent stain (Thermo Fisher–Invitrogen).
Chemicals, plasmids, molecular cloning, and mutagenesis
Thapsigargin (TG) was from Calbiochem (San Diego, CA, USA), the pcDNA3-PDIA6-V5 plasmid was a kind gift from Dr. N. Bulleid (University of Glasgow, Scotland) and the pcDNA-PERK WT and K618A were obtained from Addgene (Cambridge, MA, USA). The QuickChange kit (Agilent, Stamford, CT, USA) was used for site-directed mutagenesis.
Insulin content and insulin secretion
INS-1 832/13 cell extracts were assayed for matured insulin with the human-specific insulin RIA kit (HI-14K; EMS-Millipore, Billerica, MA, USA). The values were normalized to protein content, measured by a bicinchoninic acid (BCA) assay. INS-1 832/13 cells were also immunostained for total insulin with mouse monoclonal anti-insulin antibody I-2018 (Sigma-Aldrich, St. Louis, MO, USA). Insulin secretion was assayed as has been reported (6). In brief, INS-1 cells were pretreated with 5 mM glucose in RPMI for 18 h, then for 1 h with 2.5 mM glucose in Krebs Ringer bicarbonate (KRB) buffer [135 mM NaCl, 3.6 mM KCl, 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES; pH 7.4), 5 mM NaHCO3, 0.5 mM NaH3PO4, 0.5 mM MgCl2, and 1.5 mM CaCl2]. The cells were then exposed to KRB buffer containing different glucose concentrations for 3 h. Supernatants were collected, clarified, and assayed for human insulin by radioimmunoassay (RIA). The RIA values were normalized to the lysates’ protein contents.
RT-quantitative PCR
For analysis of the abundance of transcripts by SYBR green, total RNA was extracted with Trizol reagent (Thermo Fisher–Invitrogen) according to the manufacturer’s protocol. One microgram of RNA was retrotranscribed into cDNA with random hexamers (SuperScript II Reverse Transcriptase; Thermo Fisher–Invitrogen). Quantitative PCR (qPCR) was performed with the Power SYBR Green master mix on a StepOne Real-Time PCR system (Thermo Fisher–Applied Biosystems, Foster City, CA, USA). The 2−ΔΔCt method (33) was applied for data analysis. Primer sequences were as follows: β-Actin, CTA CAA TGA GCT GCG TGT GGC (forward), CAG GTC CAG ACG CAG GAT GGC (reverse); Ins1, GTC CTC TGG GAG CCC AAG (forward), ACA GAG CCT CCA CCA GG (reverse); Ins2, ATC CTC TGG GAG CCC CGC (forward), AGA GAG CTT CCA CCA AGT G (reverse); and XBP1s, CTG AGT CCG CAG CAG GTG CAG (forward), ATC CAT GGG AAG ATG TTC TGG (reverse).
For TaqMan qPCR assays, 750 ng of each RNA sample was used per reaction. The following TaqMan gene expression assays were used with TaqMan One-Step RT-PCR Master Mix Reagents (Thermo Fisher): GAPDH (glyceraldehyde-3-phosphate dehydrogenase), Rn99999916_s1; BLOC1S1 (biogenesis of lysosomal organelles complex-1, subunit 1), Rn01527159_m1; and Pmp22 (peripheral myelin protein 22) Rn00566835_m1.
Measurements of XBP1 mRNA splicing and regulated IRE1-dependent decay activity
XBP1 and β-actin mRNAs were reverse-transcribed, PCR amplified, and separated by gel electrophoresis on a 2% agarose (Genemate-Bioexpress, Kaysville, UT, USA) gel (34). Ethidium bromide (Sigma-Aldrich, St. Louis, MO, USA)–stained amplicons were quantified by densitometry with ImageJ software (U. S. National Institutes of Health, Bethesda, MD, USA). In an alternative assay for splicing, we used a tdTomato fluorescent protein reporter (Takara-Clontech, Palo Alto, CA, USA), whose expression is under the control of the XBP1 intron. This reporter was modified from a green fluorescent protein (GFP) reporter that has been described (35).
Mouse islets were allowed to recover for 24 h after isolation and then microporated with the tdTomato XBP-1 splicing reporter by the Neon Transfection System (Thermo Fisher, Waltham, MA, USA). In brief, islets were washed with PBS, resuspended in the proprietary R buffer, and incubated with the reporter plasmid at room temperature for 20 min. Islets were then microporated using 10 µl neon tips 9 (50 V, 30 ms, 2-pulse setting) and replated in growth medium without antibiotic overnight. Expression of the reporter was scored and analyzed with an X-81 inverted microscope (Olympus, Waltham, MA, USA) controlled by Volocity software (Perkin Elmer, Waltham, MA, USA).
Gene silencing
Cells were transduced as reported (36) with Mission short hairpin RNA (shRNA) lentiviruses (Sigma-Aldrich) carrying the following sequences: 1) shPDIA6-clone; TRCN0000111770; 5′-CCGGCTATGAATTGTAGCAGTGAATCTCGAGATTCACTGCTACAATTCATAGTTTTTG-3′; and 2) shERp72; homemade clone 5′-CCGGCCTGAGAGAAGATTACAAGTTCTCGAGAACTTGTAATCTTCTCTCAGGTTTTTG-3′.
Immunoblot analysis, immunoprecipitation, and antibodies
Expression and association of proteins were determined by coimmunoprecipitation and immunoblot analysis as in (36). For protein extractions, cells were washed in ice-cold PBS buffer and lysed in 50 mM Tris (pH 8), 150 mM NaCl, 20 mM iodoacetamide, 5 mM KCl, 5 mM MgCl2, and 1% NP-40 and supplemented with protease (Complete EDTA-Free; Roche, Nutley, NJ, USA) and phosphatase inhibitor cocktails (PhosStop; Roche). Protein concentrations were determined with a BCA assay (Thermo Fisher). Immunoblot analysis was performed with the an Owl Scientific gel transfer module (Thermo Fisher) and 10% 37.5:1 acrylamide/bis-acrylamide polyacrylamide gels. SDS-PAGE was performed with Tris-glycine-SDS buffer and transferred onto 0.45 μm nitrocellulose membrane (Bio-Rad, Hercules, CA, USA) in Tris-glycine buffer plus 20% methanol, with a Genie Blot Module (Thermo Fisher). Nitrocellulose membranes were blocked in 5% nonfat milk in Tris-buffered saline (TBS). For blots to be probed with antibody against phosphoproteins a 1:1 mixture of Odyssey blocking buffer (Li-Cor, Lincoln, NE, USA) and TBS solution were used. The blots were then incubated overnight at 4°C, in 1% nonfat milk/TBS-0.1% Tween (TBS-T) plus the primary antibody. Afterward, the membranes were washed in TBS-T and incubated with the appropriate infrared (IR)-labeled secondary antibody (Li-Cor) in 5% milk/TBS-T. The membranes were washed and scanned using the Odyssey Infrared Imaging System (Li-Cor). Protein bands were visualized, and densitometry determined with Li-Cor Odyssey software. Mouse anti-KDEL (clone 10C4) and rabbit anti-14-3-3 ζ (C-19) antibodies were purchased from Santa Cruz Biotechnology (Dallas, TX, USA). Rabbit anti-PDIA6 and mouse anti-V5 antibodies were purchased from Abcam (Cambridge, United Kingdom) and Thermo Scientific–Invitrogen, respectively. Rabbit anti-PERK (C33E10), rabbit anti-endoplasmic reticulum protein (ERp)72, rabbit phospho-eIF2α (Ser51), and mouse total eIF2α (L57A5) antibodies were from Cell Signaling Technology (Danvers, MA, USA). IR secondary antibodies (used at 1:10,000 dilutions) were from Li-Cor.
Metabolic labeling
INS-1 832/13 cells were starved for 20 min in Met/Cys-free medium, pulsed for 30 or 60 min with 88 μCi [35S]Met/Cys per milliliter. The supernatants were collected at each time point and resolved on 10% acrylamide gels, dried, exposed to a storage phosphor screen, and imaged with a Typhoon Phosphorimager (GE Healthcare, Pittsburgh, PA, USA). Band intensities were quantified with ImageQuant software (GE Healthcare).
RESULTS
We showed in another study that PDIA6 attenuates the signaling activity of both the IRE1 and PERK UPR sensors and that this attenuation is caused by direct interactions with cysteines in IRE1 and in PERK (29). We sought to test the biologic significance of the PDIA6-IRE1 and PDIA6-PERK interactions in cells that use both of these UPR sensors during physiologic responses. We chose to analyze insulin-secreting cells, where IRE1 and PERK are activated, either singly or together, by various metabolic alterations (37, 38). To determine whether the PDIA6-IRE1 and PDIA6-PERK interactions are separable according to the state of the cell, we used viral-mediated silencing of PDIA6 expression in 2 β-cell lines, INS-1 832/13 (32) and Min6 (39), that respond to variations in glucose level by regulated exocytosis of insulin. Infection of either cell line with shRNA-encoding lentivirus led to 60–95% silencing of PDIA6 expression (Figs. 1A, 2A–C, 3B, 4E, 5C). This silencing lasted several weeks, enabling selection of pools of PDIA6-depleted cells. The silencing was specific for PDIA6; expression of PDIA1, PDIA3, and often ERp72 (PDIA4) was not reduced (Fig. 1A; Supplemental Fig. 1A) (29). In some experiments, expression of other genes was enhanced when PDIA6 was silenced, reflecting selective feedback mechanisms that respond to limitation of some, but not all ER components (Fig. 1A; Supplemental Fig. 1; and Discussion in ref. 36). The depletion of PDIA6 did not affect the viability of INS-1 cells (Supplemental Fig. 1B), their protein synthesis capacity (Supplemental Fig. 1C), or their global proteome (Supplemental. Fig, 1C). As we and others have shown, the absence of PDIA6 does not have any discernible impact on glycoprotein biosynthesis, oxidative folding, or secretion (29, 40, 41). Finally, PDIA6-depleted cells were equally sensitive to the chemical ER stress inflicted by TG, tunicamycin, or DTT (Supplemental Fig. 3).
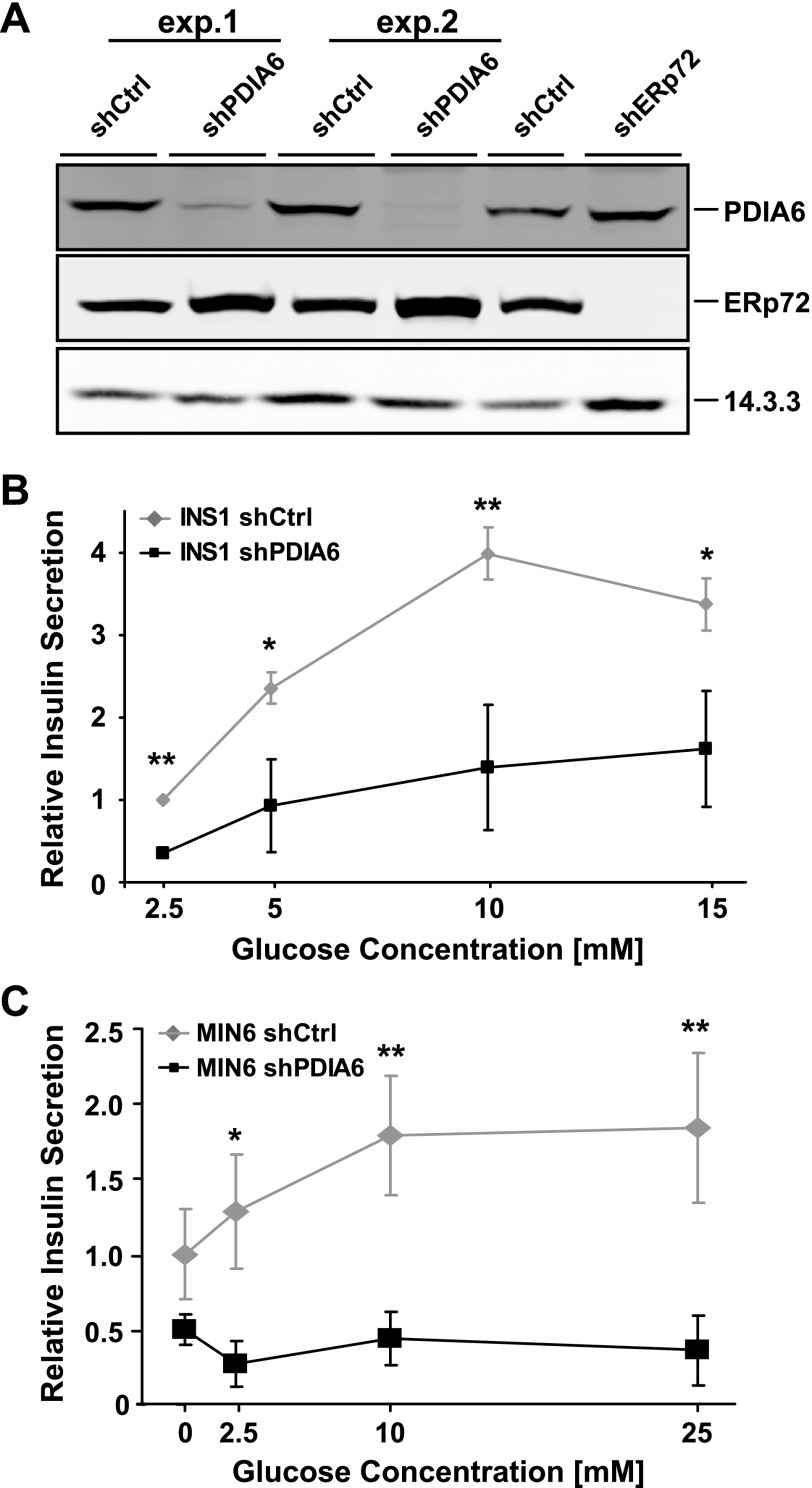
Figure 1.
PDIA6 depletion affects significantly glucose-stimulated insulin secretion. A) INS1 832/13 cells expressing a nontargeting (shCtrl, short hairpin control) or a PDIA6-targeting shRNA were obtained from 2 different infection events. Lysates were immunoblotted to detect the expression level of PDIA6. ERp72 served as a specificity control and 14.3.3 as a loading control. There was progressive recovery of PDIA6 expression over time. B) INS1 cells from (A) were exposed to the indicated glucose concentrations for 3 h. Supernatants were analyzed by RIA to detect human insulin. Each measurement was normalized to the protein content. Data are means ± SD of 3 independent experiments. *P ≤ 0.05; **P ≤ 0.01; Student’s t test; shPDIA6 vs. corresponding shCtrl condition.
Figure 2.
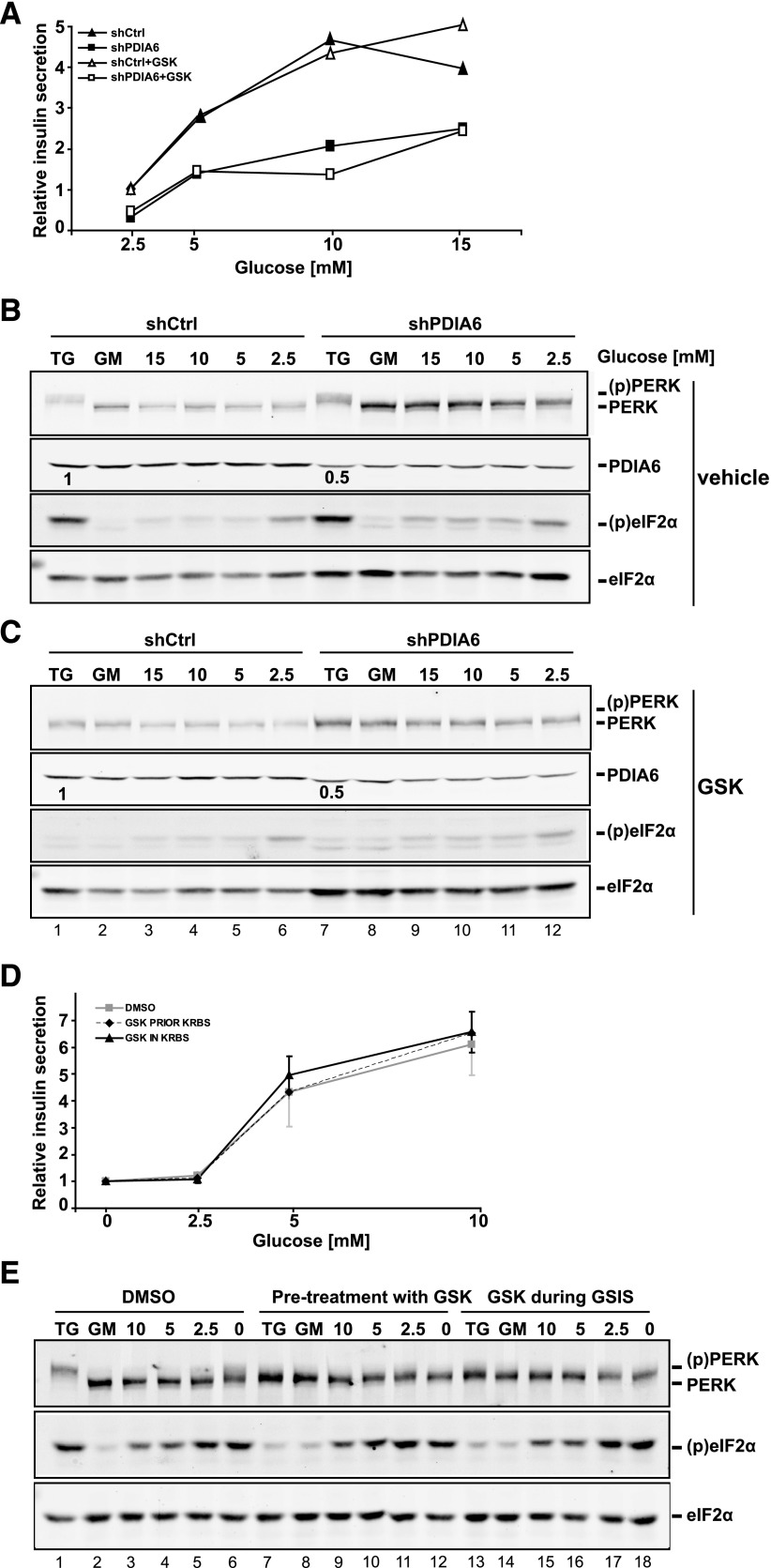
Loss of PDIA6 augments PERK signaling. A) Short hairpin control (shCtrl) and shPDIA6 INS1 832/13 cells were exposed to 500 nM TG for the indicated times. Cell lysates were then immunoblotted to detect PERK, KDEL, total or Ser51-phosphorylated eIF2α, Phospho-signal/total eIF2α ratios (normalized to eIF2α) are showed in the gel (n = 2). B) shCtrl or shPDIA6 MIN6 cells were exposed to 1 μM TG for the indicated times. Cell lysates were then immunoblotted to detect total or Ser51-phosphorylated eIF2α. In both experiments, untreated shCtrl samples served as internal reference. Means ± sd of the phosphosignal/total eIF2α ratios are plotted (n = 3). *P ≤ 0.05. C) Immunoblotting of cells from (B) to show the level of PDIA6 depletion. D) INS1 832/13 cells were exposed to 500 nM TG for the indicated times and in parallel, either pretreated or treated, with the GSK2606414 at the indicated concentrations. PERK, total, or Ser-51-phosphorylated eIF2α levels were assessed by immunoblotting.
Figure 3.
Glucose-stimulated insulin secretion is not mediated by PERK. A) Short hairpin control (shCtrl) or shPDIA6 INS1 cells were exposed to the indicated glucose levels and the human insulin measured in the supernatants after 3 h of exposure by RIA. Each measurement was normalized to the protein content. B and C) Samples in (A) were subjected to immunoblot analysis, to detect PERK, PDIA6, total, or Ser51-phosphorylated eIF2α levels. The second blot in (C) shows cells treated with the GSK 2606414. Data are means ± sd of 3 independent experiments. D) INS1 832/13 cells were either pretreated or treated with the GSK inhibitor during the glucose stimulation. Human insulin was measured as in (A). E) Cell lysates from (D) were analyzed by immunoblot, as in (B–C).
Figure 4.
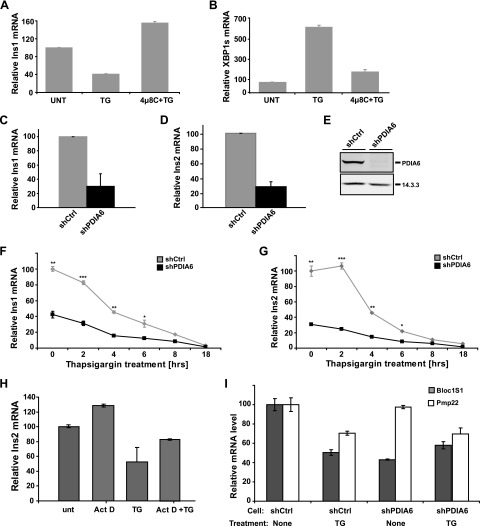
Insulin transcripts decrease in the absence of PDIA6. A, B) INS1 832/13 cells were exposed to 500 nM TG for 8 h in the presence or absence of 30 μM 4μ8C. Ins1 and XBP1 splicing mRNAs were measured by qPCR, with actin as the housekeeping gene. C, D) Ins1 and Ins2 transcripts were measured as above in PDIA6-deficient (shPDIA6) and -sufficient (shCtrl, short hairpin control) INS1 cells. Data are means ± sd of 3 independent experiments. E) Samples in (C, D) were immunoblotted to estimate PDIA6 depletion. F, G) Cells in (C, D) were treated with 500 nM TG for the indicated times, and Ins1 and Ins2 transcripts measured by qPCR. Data are means ± sd of 3 independent experiments. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; Student’s t test; shPDIA6 vs. the corresponding shCtrl condition. H) INS1 832/13 cells were pretreated with 1 μg/ml actinomycin D (Act D) for 30 min and then stressed with 1 μM TG for 5 h, in the continued presence of actinomycin D. Ins2 mRNA was measured by qPCR, with actin as the housekeeping gene. I) Bloc1S1 and Pmp22 transcripts were measured as above in PDIA6-deficient and -sufficient INS1 cells, before and after TG treatment.
Figure 5.
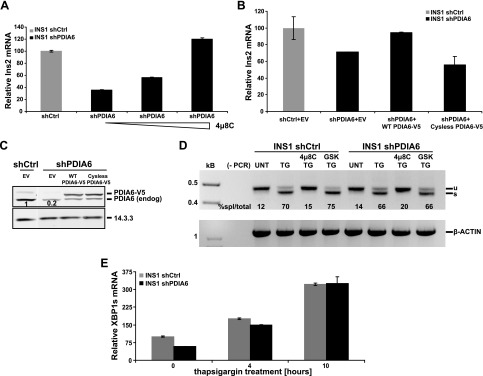
Degradation of insulin transcripts in the absence of PDIA6 is mediated by IRE1. A) PDIA6-depleted INS1 832/13 cells were treated with different concentrations (10, 30, and 90 μM) of 4μ8C for 8 h. Untreated shCtrl samples served as an internal reference. B) PDIA6-depleted INS1 cells were transiently transfected with empty vector (EV) or wild-type (WT) or cysteine-free V5-tagged PDIA6. Ins2 transcript was measured by qPCR at 48 h after transfection. C) Samples in (B) were immunoblotted to detect the level of exogenous PDIA6 over the endogenous. D) PDIA6-sufficient and -depleted INS1 cells were treated with 500 nM TG for 6 h. In addition to TG, one sample was pretreated with 4μ8C and another with the GSK inhibitor as the negative control. Unspliced (u) and spliced (s) XBP1 mRNAs were amplified by RT-PCR. β-Actin served as control for RNA recovery. The percentage of spliced XBP1 out of the total is reported under each lane. E) PDIA6-sufficient and -depleted INS1 cells were treated with 500 nM TG for the indicated times, and XBP1 splicing was analyzed by qPCR.
PDIA6-depleted INS1 cells displayed dramatically reduced secretion of insulin upon glucose stimulation. The basal level of insulin release was consistently ∼2-fold lower in PDIA6-depleted cells, and the dependence of insulin release on glucose concentration was weaker than that of PDIA6-sufficient cells (Fig. 1B). Similar observations were made with PDIA6-depleted Min6 cells (Fig. 1C). The inhibition of insulin release was not attributable to defective processing of proinsulin to insulin, which proceeds even in the absence of PDIA6 (29). The inhibition of GSIS also was not attributable to increased ER stress, because depletion of PDIA6 does not generally induce the expression of UPR target genes in mammalian cells (29, 36, 41) and only occasionally is a target gene up-regulated (e.g., ERp72; Fig. 1A). The inhibition of insulin release was specific, as the secretome of PDIA6-depleted cells does not differ (at least superficially) from the secretome of PDIA6-sufficient cells (Supplemental Fig. 1C).
Because PERK plays a role in INS1 cells’ stress responses, we reconfirmed that, as in other cell types, loss of PDIA6 in INS1 cells augmented eIF2α phosphorylation in response to TG treatment (Fig. 2A), which induces ER stress by inhibiting the sarcoendoplasmic reticulum SR calcium transport ATPase (SERCA) calcium pump (42). In the absence of PDIA6, higher amplitude and longer duration of eIF2α phosphorylation were observed (Fig. 2A). Similarly, in PDIA6-deficient MIN6 cells exposed to TG, the duration of eIF2α phosphorylation increased considerably between 0.5 and 4 h (Fig. 2B, C). Thus, PDIA6 negatively regulates PERK in insulin-secreting cells during external chemical ER stress.
To establish whether the reduction in insulin secretion in the absence of PDIA6 (Fig. 1) and the increased phosphorylation of eIF2α (Fig. 2) were caused by hyperactivation of PERK, we used a small-molecule inhibitor of PERK, GSK2606414 (43). Treatment of INS1 cells with GSK2606414 fully abrogated both the activation of PERK, seen as slower gel mobility compared with inactive PERK, and the phosphorylation of eIF2α in response to TG treatment, and it was equally efficient when added before or together with TG (Fig. 2D). We conclude, therefore, that eIF2α phosphorylation in insulin-producing cell lines subjected to chemical ER stress is largely caused by the activation of PERK, confirming reported results (44).
Because PERK is known to be activated when insulin-secreting cells are exposed to low glucose (5, 44), we next asked how suppression of PDIA6 expression affects PERK activity, not only under chemical ER stress but also during physiologic UPR. PDIA6-sufficient or -deficient INS1 cells were treated with the PERK inhibitor during short-term (3 h) stimulation with various levels of glucose, and the secreted insulin was analyzed by RIA. Although GSIS was reduced in PDIA6-deficient INS1 cells, we were surprised to find that PERK inhibition did not alter insulin secretion in either cell subline (Fig. 3A). To ascertain that the inhibition was efficient, PERK signaling was also reanalyzed by immunoblot. Unlike when PERK was activated by TG treatment, where PERK’s gel mobility shift was abolished in the presence of GSK2606414 (Fig. 3B, compare lanes 1–2 with 7–8), PERK activation was not detectable in INS1 growth medium (GM; 11 mM glucose) or even in low-glucose medium (5 or 2.5 mM glucose; Fig. 3B). This finding was true for both PDIA6-deficient and -sufficient cells (Fig. 3B). To ask whether PERK is important for GSIS, we repeated the experiment in the presence of the PERK inhibitor at 2 different stages, either during the glucose stimulation phase or during the prior adaptation to low glucose (see Materials and Methods). In all these conditions, inhibition of PERK did not affect the secretion of insulin (Fig. 3C–D). When PERK signaling was analyzed by immunoblot analysis, it was activated only marginally. Only in glucose-free medium was the gel mobility shift noticeable, and at that, it was smaller than the shift induced by chemical UPR (Fig. 3E, lanes 1 and 6). In contrast, PERK’s downstream effector eIF2α was activated equally by the metabolic and chemical (TG) stimuli (discussed below). The marginal activation of PERK and the phosphorylation of eIF2α caused by low glucose exposure were observed even in the presence of the PERK inhibitor, in contrast to the effect of the inhibitor under TG stress of the same cells (Fig. 3E). We conclude that the impact of PDIA6 on acute glucose stimulated insulin secretion is not mediated through PERK, one of the known interactors of PDIA6, because GSIS in this model system appears to be independent of PERK.
It is apparent that despite the effective inhibition of PERK, eIF2α is still phosphorylated in a glucose-dependent fashion, and its phosphorylation is refractive to GSK2606414 (Fig. 3C, E). Thus, it is likely that, at low glucose, eIF2α is phosphorylated by a different kinase. An indication that this kinase is general control nonderepressible (GCN)-2 (45) is that eIF2α phosphorylation was much diminished when we performed the GSIS assay, not in the usual Krebs buffer, but rather in GM, which contains amino acids (Supplemental Fig. 2A). This observation was confirmed again by GSK2606414 treatment, which affected only PERK signaling under TG exposure (Supplemental Fig. 2B), reflecting no suppression of insulin secretion (Supplemental Fig. 2C).
Because we concluded that PDIA6 does not inhibit GSIS through its interaction with PERK, we asked whether the effect of silenced PDIA6 on GSIS of INS1 cells is mediated by IRE1. This UPR sensor has been shown to affect insulin transcripts (6, 8, 46, 47). Once IRE1 is activated by exposure to either persistently high glucose (6, 8) or a chemical ER stressor like TG, it degrades insulin mRNA via its regulated IRE1-dependent decay (RIDD) activity (48–50), leading consequently to reduced production of proinsulin (46, 47). Given this knowledge, we wanted to assess whether IRE1 is hyperactivated in the PDIA6-depleted insulinoma cells and to what extent such activation could account for the impaired insulin GSIS. We used a qPCR assay similar to that in Lerner et al. (51), in which insulin mRNAs are the substrates for the RNase activity of IRE1. We confirmed that treatment of INS1 cells with TG causes reduction in the level of the Ins1 transcript (Fig. 4A), in proportion to the time of TG treatment (Fig. 4F). The reduced level was observed, not only with Ins1, but also with Ins2 transcripts (Fig. 4G). The reduction was abolished by a concomitant treatment with TG and the IRE1 inhibitor 4μ8C (Fig. 4A) (52), but not with the transcriptional inhibitor actinomycin D (Fig. 4H). The same PDIA6-deficient INS1 cells with steady-state levels of Ins1 and Ins2 transcripts were reduced 2.5- to 4-fold [compared with PDIA6-sufficient short hairpin control (shCtrl) INS1 cells] and exhibited a 6-fold increase in XBP1 splicing, and this increased activity was abolished when IRE1 was inhibited (Fig. 4B). We conclude that the qPCR assay measures the increase in RIDD activity of IRE1 when PDIA6 is depleted. In support of this conclusion, transcripts of Bloc1S1, which are canonical good substrates for RIDD activity found in most studies (53), were also depleted in PDIA6-deficient INS1 cells, even without TG stress (Fig. 4I). On the other hand, Pmp22 transcripts, which are also RIDD substrates (52), were refractive to PDIA6 depletion in INS1 cells and only became better RIDD substrates with TG stress (Fig. 4I). Thus, the RIDD activity in INS1 cells exhibits substrate selectivity.
As expected for the RIDD activity of IRE1, treating PDIA6-deficient INS1 cells with increasing concentrations of 4μ8C progressively reversed the decline in insulin mRNA levels caused by depletion of PDIA6 (Fig. 5A). The increased RIDD activity in PDIA6-depleted cells was directly caused by the loss of PDIA6, because it was complemented by ectopic expression of a V5-tagged version of active PDIA6, engineered to be resistant to shRNA (Fig. 5B, C). Moreover, the effect on RIDD activity was caused by the enzymatic activity of PDIA6, rather than the expression of the protein, because an inactive version of V5-tagged PDIA6, where all 4 cysteines in the 2 active sites are mutated (40), when expressed at levels similar to those of the active PDIA6, failed to counteract the increased RIDD activity (Fig. 5B, C). The other major activity of IRE1, XBP1 splicing, was unaffected. Both PDIA6-sufficient and -deficient cell lines had the same extent of XBP1 splicing, whether measured by RT-PCR assay or qPCR, either at steady state or under TG treatment (Fig. 5D, E). This result is consistent with our previous observation that PDIA6 activity affects primarily the decay phase of the splicing response (29). We conclude that PDIA6 depletion induces chronic activation of the RIDD activity of IRE1 in INS1 cells, resulting in suppressed expression of insulin mRNAs (and other transcripts). The implication is that the RIDD activity of IRE1, like the XBP-1 splicing activity, has a basal level, and PDIA6 prevents it from becoming too robust.
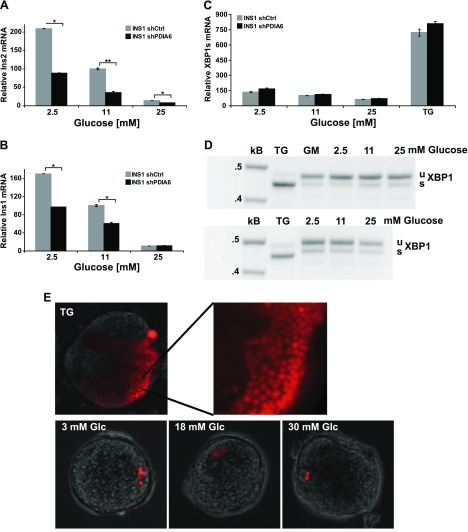
To examine whether the effect of PDIA6 on RIDD activity is limited to chemical ER stress conditions or also operates physiologically, we investigated whether INS1 cells without PDIA6 are more sensitive to changes in glucose level. According to Urano et al. (6, 8), IRE1 is activated in pancreatic β cells by short-term elevation of glucose, and is hyperactivated during prolonged exposure to high glucose, and this IRE1 activation leads to degradation of insulin mRNA. We therefore compared levels of insulin transcripts in PDIA6-sufficient or -deficient cells, under chronic low or high glucose. As illustrated in Fig. 6A, B, INS1 cells exposed to 2.5, 11, or 25 mM glucose for 2 days had progressively lower levels of insulin transcripts, because of the increasing levels of IRE1 activity, in agreement with published work (6, 8, 9). The absence of PDIA6 was additive to the glucose effect, causing a further reduction of insulin transcripts without a corresponding increase in spliced XBP1 (Fig. 6A–C). In the same conditions, the XBP1-splicing activity was minimal and insensitive to glucose level, as shown by the qPCR assay and confirmed by the RT PCR assay (Fig. 6C, D, respectively). The insensitivity of XBP1 splicing to glucose was not just a property of β-cell lines and was also observed in intact islets, as revealed by a fluorescent XBP1 splicing reporter; only a few cells per islet displayed the activated reported when cultured in either low or high glucose for 24 or 48 h (Fig. 6E). In contrast, the splicing activity of these cells was very robust and was displayed by many cells per islet in response to a TG stimulus (Fig. 6C, D). Therefore, the quality of IRE1 activation differs in response to distinct stimuli and when the RIDD activity of IRE1 predominates, PDIA6 attenuates this activity, not only the splicing activity.
Figure 6.
PDIA6 depletion exacerbates chronic glucose exposure–induced RIDD activity. A, B) PDIA6-sufficient and -depleted INS1 832/13 cells were exposed to the indicated concentrations of glucose for 2 days. Ins2 (A) and Ins1 (B) transcripts were measured by qPCR. Each value was normalized to shCtrl cells in 11 mM glucose. Data are means ± sd of 3 independent experiments. *P ≤ 0.05; **P ≤ 0.01; Student’s t test; shPDIA6 vs. the corresponding shCtrl condition. C) XBP1 splicing was measured from samples as in (A) by qPCR. Cells were also treated with 500 nM TG for 6 h as a positive control for induction of XBP1 splicing. D) INS1 832/13 cells were exposed to the indicated concentrations of glucose for 3 days in 2 independent experiments. Unspliced (u) and spliced (s) XBP1 mRNAs were amplified by RT-PCR. β-Actin served as the control for RNA recovery. E) Mouse islets were transfected with the tdTomato XBP1 splicing reporter and then exposed to different glucose concentrations. TG treatment served as the positive control to identify transfected cells, given that only a portion of islet cells are responsive to ER stress.
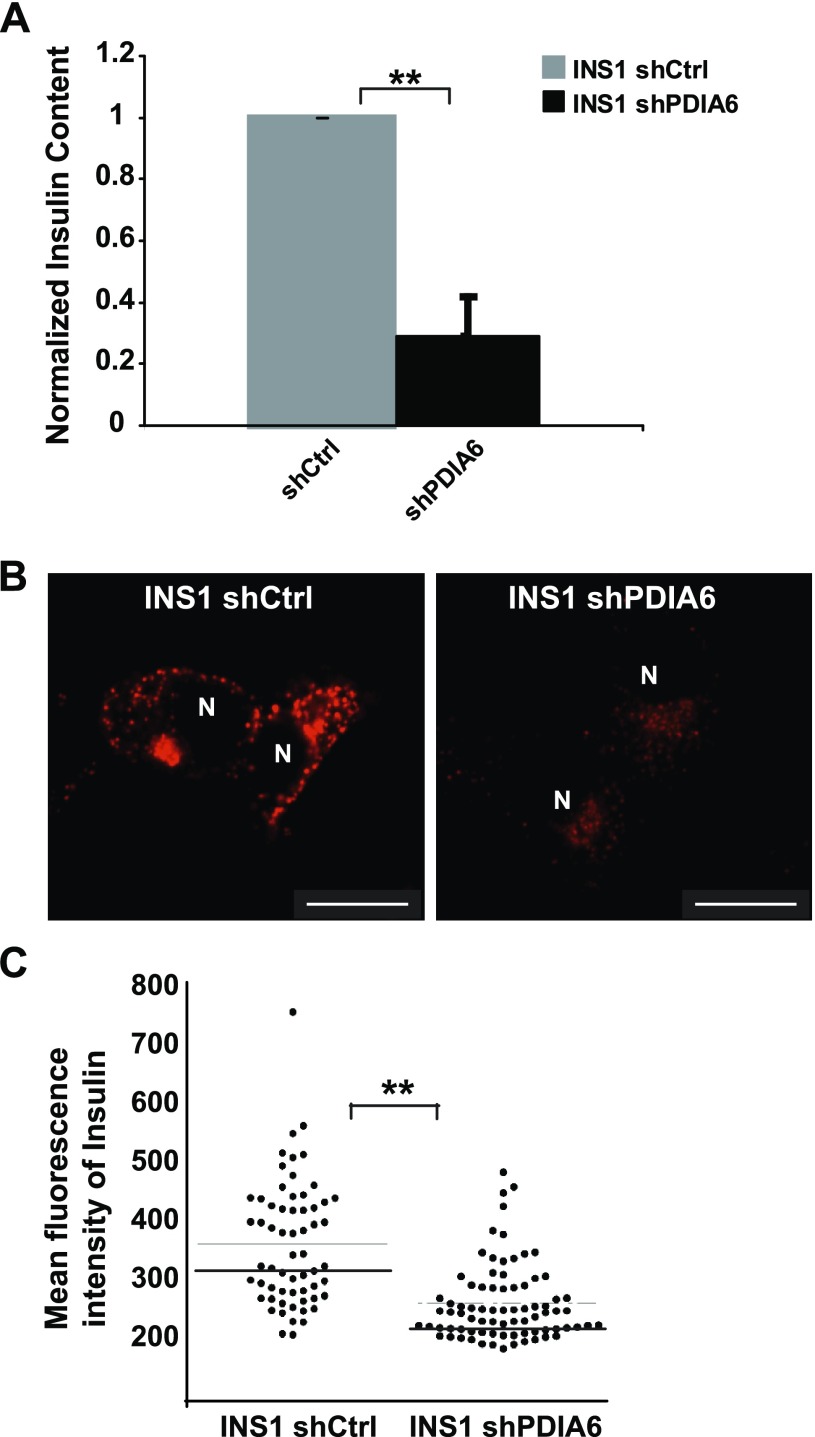
Reduction of insulin mRNA is inherently a longer term coping mechanism that should, over time, lead to lower intracellular insulin protein levels and a lower releasable pool of insulin. We used several approaches to test this prediction. First, the same RIA that was used to measure the released insulin was used to quantify intracellular insulin. Depletion of PDIA6 led to 4–5-fold less total insulin at steady state (Fig. 7A). We also assessed by immunofluorescence the insulin content in both β-cell lines. The observed decrease in overall staining intensity, as well as the change in the distribution of insulin-positive organelles, shows that in the absence of PDIA6, there was a significant depletion of the releasable insulin pool (Fig. 7B, C). This decrease was not caused by a defect in insulin folding, as we have shown (29), and explains the observed reduction in insulin secretion (Fig. 1).
Figure 7.
Intracellular insulin content is compromised in the absence of PDIA6. A) PDIA6-sufficient and -deficient INS1 cells were lysed and analyzed by RIA for the human intracellular insulin. Each measurement was normalized to the protein content. Data are means ± sd of 3 independent experiments. *P ≤ 0.05; **P ≤ 0.01; Student’s t test; shPDIA6 vs. the corresponding shCtrl condition. B) Immunostaining of total insulin in either PDIA6-sufficient or -deficient INS1 cells. C) Quantitation of total fluorescence intensity from cells in (B).
DISCUSSION
We have shown that PDIA6 attenuates the IRE1 and PERK sensors of the UPR and that, at least with respect to IRE1, PDIA6 acts by forming mixed disulfides with Cys residues in the activated sensor (29, 54). We sought to determine whether PDIA6 attenuates the UPR, not only under ectopically imposed chemical stress, but also during physiologic situations that activate the UPR, where PERK and IRE1 are used either alternately or concomitantly, and where more than one activity of each sensor is operational. We examined how PDIA6 regulates the UPR in β cells, because previous work had implied alternate use of UPR sensors in glucose-stimulated insulin secretion from pancreatic β cells. PERK has been shown to be activated by low glucose (5), and IRE1 in response to hyperglycemia (6, 8, 51). In contrast, when pancreatic β cells are exposed to chemically induced UPR, both PERK and IRE1 are activated (9, 55).
Our results show that PDIA6 is essential for GSIS, and its ablation inhibits insulin release profoundly. PDIA6 does not affect the cells’ response to glucose by modulating PERK signaling. We found that PERK was not necessary for acute control of GSIS—as distinct from the ability to mount an insulin response to long-term changes in glucose (37)—because acute pharmacological inhibition of PERK does not affect GSIS. Another conclusion from our data is that short-term glucose stimulation of UPR is distinct mechanistically from chemical stimulation of UPR. TG treatment stimulates PERK, and its stimulation is attenuated by PDIA6, but PERK does not signal in response to changes in glucose. We find that the difference between the 2 responses lies in the differential use of the UPR sensors and their enzymatic activities.
PDIA6 controls insulin release by modulating IRE1, but the activity of IRE1 needed for GSIS is not XBP1 splicing, but rather the RNase activity that degrades selected transcripts including insulin mRNA. At least one vital function of PDIA6 is to limit IRE1’s RIDD activity to a level that allows for a steady-state pool of insulin mRNA. This conclusion is significant for several reasons: 1) It extends the observation of Upton et al. (56) that IRE1 RIDD activity is induced during chemical stress to physiologic UPR induction in β cells; 2) it uncouples the RIDD and XBP1 splicing activities of IRE1 during physiologic UPR; 3) it extends our understanding of PDIA6’s modulation of UPR in showing that it affects not only the XBP1 splicing activity of IRE1 but also its RIDD activity.
Superficially, our observations about the different roles of PERK and eIF2α in GSIS appear to contradict the results in several studies. However, a closer examination of the data reveals common rather than disparate observations. As discussed by Wei et al. (55), most of the conclusions about the roles of PERK in β-cell biology are derived from long-term depletion or ablation experiments. In fact, they discovered that PERK is specifically needed for the development of β cells during the fetal and early neonatal period, but is not necessary at the adult stage to maintain β-cell functions and glucose homeostasis (57). Ablation of PERK via RNA interference (RNAi) in INS 832/13 cells mimicked the major defects in PERK knockout mice and revealed the abnormal expression and redox state of key ER chaperone proteins such as ERp72 and GRP78/BiP (15). However, the time frame necessary for the knockdown was much longer than the time frame examined in our work, which was dictated by the necessity to allow depletion or accumulation of proteins. In contrast, the time frame of our GSIS experiments was too short to reveal up-regulation of UPR targets. One discrepant line of evidence is that overexpression of dominant–negative PERK inhibits eIF2α phosphorylation in glucose-deprived cells (58). Given that the PERK inhibitor does not affect the other 2 UPR signaling pathways, perhaps there is cross-talk among UPR pathways when DN PERK is expressed.
An alternative explanation for the role of PDIA6 in GSIS is that it is needed for protein folding rather than for UPR signaling. However, 3 different experimental approaches failed to detect a role for PDIA6 in protein folding in general (40, 41) and in insulin biosynthesis in particular, which was shown to be normal by pulse–chase analysis (29). PDIA6 was shown to be a cofactor for the chaperone BiP/GRP78 (40), but there is no evidence that BiP and its cofactors are necessary for folding of wild-type insulin. PDIA6 may play a more indirect role in establishing the conditions for protein folding in the ER, for example the oxidizing environment. Indeed, PDIA6 has been shown to associate with peroxiredoxin (PRDX)-4, an enzyme that is necessary for maintaining the redox potential of the ER lumen (59). We have verified by mass spectrometry that PRDX4 is one of PDIA6’s most prominent interacting partners (unpublished data), but this interaction should be analyzed further in the context of β cells.
Another possible role for PDIA6 that is not excluded by our data is that it affects GSIS via PERK, but not in the acute time frame. For example, long-term processes of granule biogenesis, protein traffic, and quality control could be regulated by PERK and modulated by PDIA6 on a time frame not easily testable by our assays. Cavener et al. (57, 60) had proposed that PERK is important for the proper differentiation of β cells and their quality control system. PDIA6 may well be needed to modulate these activities of PERK, which were not directly measured in the current study. It will also be interesting to determine the role of the PDIA6-PERK system in the context of other types of pancreatic metabolic stress, including lipotoxicity and accumulation of misfolded insulins, as is observed in some neonatal forms of diabetes (61, 62).
Yet another possibility, not mutually exclusive with the explanation given herein, is that PDIA6 affects the half-life of short-lived regulatory molecules such as micro-ß-RNAs through its effect on IRE1. Although we focused on insulin transcripts as the target of RIDD activity of IRE1, it is important to note that this activity has already been shown to target the regulatory micro-RNAs (miRs) present in pancreatic β cells (56). Of particular relevance to PDIA6 is miR-322, which was implicated as a RIDD substrate and was also shown to form a Ca++-sensitive regulatory loop with IRE1 and PDIA6 (30). Degradation of miR in addition to mRNAs may have a much more immediate impact on the mechanism of insulin release, and future studies will focus on this pathway in the regulation of GSIS.
The findings in this study show that PDIA6 is a general regulator of both IRE1 and PERK, whose roles come into play when either UPR sensor is activated. When PERK and IRE1 are activated by chemical ER stressors, PDIA6 attenuates respectively the kinase activity of PERK and the XBP1 splicing activity of IRE1, whereas during physiologic changes in glucose, where IRE1 performs only RIDD (and PERK is not required), PDIA6 inhibits this RNase activity in much the same way as it regulates XBP1-splicing activity (29).
Supplementary Material
Acknowledgments
The authors thank Dr. D. C. Fan (The Children's Hospital of Philadelphia, Philadelphia, PA, USA) for help with some of the experiments; Drs. D. Ron (University of Cambridge, Cambridge, United Kingdom), A. Volchuk (University of Toronto, Toronto, ON, Canada), N. Bulleid (University of Glasgow, Glasgow, United Kingdom), E. Snapp (Albert Einstein College of Medicine of Yeshiva University, New York, NY, USA), T.P. Herbert (University of Leicester, Leicester, United Kingdom), and T. Iwawaki (Gumna University, Maebashi, Japan) for generous gifts of cell lines, plasmids, and antibodies; Dr. H.-E. Hohmeier (Duke University School of Medicine, Durham, NC, USA) for the glucose-responsive INS1 832/13 cells and help in their culture; and Drs. T. Gidalevitz, J. Burkhardt, and D. Dersh for comments and suggestions during the work and on the manuscript. This work was supported by U.S. National Institutes of Health, National Institute of Allergy and Infectious Diseases Grant AI-18001 and pilot grants from the University of Pennsylvania’s Diabetes Research Center and the University Research Foundation (to Y.A.), and by a postdoctoral fellowship from the American-Italian Cancer Foundation (to Dan.E.). The authors declare no conflicts of interest.
Glossary
- ATF
activating transcription factor
- BCA
bicinchoninic acid
- BiP
binding immunoglobulin protein
- eIF2α
eukaryotic initiation factor 2, α subunit
- ER
endoplasmic reticulum
- ERp
ER protein
- FBS
fetal bovine serum
- GM
growth medium
- GSIS
glucose-stimulated insulin secretion
- GSK
GlaxoSmithKline
- IR
infrared
- IRE
inositol-requiring enzyme
- miR
micro-RNA
- PDIA6
protein disulfide isomerase-6
- PERK
protein kinase RNA-like endoplasmic reticulum kinase
- qPCR
quantitative PCR
- PRDX
peroxiredoxin
- RIDD
regulated IRE1-dependent decay of mRNA
- shRNA
short hairpin RNA
- shCtrl
short hairpin control
- TBS
Tris-buffered saline
- TBS-T
TBS-Tween
- TG
thapsigargin
- UPR
unfolded protein response
- XBP
X-box-binding protein
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Wu J., Kaufman R. J. (2006) From acute ER stress to physiological roles of the unfolded protein response. Cell Death Differ. 13, 374–384 [DOI] [PubMed] [Google Scholar]
- 2.Engin F., Yermalovich A., Nguyen T., Hummasti S., Fu W., Eizirik D. L., Mathis D., Hotamisligil G. S. (2013) Restoration of the unfolded protein response in pancreatic β cells protects mice against type 1 diabetes. Sci. Transl. Med. 5, 211ra156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teodoro T., Odisho T., Sidorova E., Volchuk A. (2012) Pancreatic β-cells depend on basal expression of active ATF6α-p50 for cell survival even under nonstress conditions. Am. J. Physiol. Cell Physiol. 302, C992–C1003 [DOI] [PubMed] [Google Scholar]
- 4.Herbert T. P. (2007) PERK in the life and death of the pancreatic beta-cell. Biochem. Soc. Trans. 35, 1205–1207 [DOI] [PubMed] [Google Scholar]
- 5.Moore C. E., Omikorede O., Gomez E., Willars G. B., Herbert T. P. (2011) PERK activation at low glucose concentration is mediated by SERCA pump inhibition and confers preemptive cytoprotection to pancreatic β-cells. Mol. Endocrinol. 25, 315–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lipson K. L., Fonseca S. G., Ishigaki S., Nguyen L. X., Foss E., Bortell R., Rossini A. A., Urano F. (2006) Regulation of insulin biosynthesis in pancreatic beta cells by an endoplasmic reticulum-resident protein kinase IRE1. Cell Metab. 4, 245–254 [DOI] [PubMed] [Google Scholar]
- 7.Qiu Y., Mao T., Zhang Y., Shao M., You J., Ding Q., Chen Y., Wu D., Xie D., Lin X., Gao X., Kaufman R. J., Li W., Liu Y. (2010) A crucial role for RACK1 in the regulation of glucose-stimulated IRE1alpha activation in pancreatic beta cells. Sci. Signal. 3, ra7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lipson K. L., Ghosh R., Urano F. (2008) The role of IRE1alpha in the degradation of insulin mRNA in pancreatic beta-cells. PloS One 3, e1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han D., Lerner A. G., Vande Walle L., Upton J. P., Xu W., Hagen A., Backes B. J., Oakes S. A., Papa F. R. (2009) IRE1alpha kinase activation modes control alternate endoribonuclease outputs to determine divergent cell fates. Cell 138, 562–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elouil H., Bensellam M., Guiot Y., Vander Mierde D., Pascal S. M., Schuit F. C., Jonas J. C. (2007) Acute nutrient regulation of the unfolded protein response and integrated stress response in cultured rat pancreatic islets. Diabetologia 50, 1442–1452 [DOI] [PubMed] [Google Scholar]
- 11.Scheuner D., Kaufman R. J. (2008) The unfolded protein response: a pathway that links insulin demand with beta-cell failure and diabetes. Endocr. Rev. 29, 317–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fonseca S. G., Gromada J., Urano F. (2011) Endoplasmic reticulum stress and pancreatic β-cell death. Trends Endocrinol. Metab. 22, 266–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Durocher F., Faure R., Labrie Y., Pelletier L., Bouchard I., Laframboise R. (2006) A novel mutation in the EIF2AK3 gene with variable expressivity in two patients with Wolcott-Rallison syndrome. Clin. Genet. 70, 34–38 [DOI] [PubMed] [Google Scholar]
- 14.Zhang P., McGrath B., Li S., Frank A., Zambito F., Reinert J., Gannon M., Ma K., McNaughton K., Cavener D. R. (2002) The PERK eukaryotic initiation factor 2 alpha kinase is required for the development of the skeletal system, postnatal growth, and the function and viability of the pancreas. Mol. Cell. Biol. 22, 3864–3874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng D., Wei J., Gupta S., McGrath B. C., Cavener D. R. (2009) Acute ablation of PERK results in ER dysfunctions followed by reduced insulin secretion and cell proliferation. BMC Cell Biol. 10, 61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee A. H., Chu G. C., Iwakoshi N. N., Glimcher L. H. (2005) XBP-1 is required for biogenesis of cellular secretory machinery of exocrine glands. EMBO J. 24, 4368–4380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cunha D. A., Gurzov E. N., Naamane N., Ortis F., Cardozo A. K., Bugliani M., Marchetti P., Eizirik D. L., Cnop M. (2014) JunB protects β-cells from lipotoxicity via the XBP1-AKT pathway. Cell Death Differ. 21, 1313–1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bertolotti A., Zhang Y., Hendershot L. M., Harding H. P., Ron D. (2000) Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat. Cell Biol. 2, 326–332 [DOI] [PubMed] [Google Scholar]
- 19.Pincus D., Chevalier M. W., Aragón T., van Anken E., Vidal S. E., El-Samad H., Walter P. (2010) BiP binding to the ER-stress sensor Ire1 tunes the homeostatic behavior of the unfolded protein response. PLoS Biol. 8, e1000415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Urano F., Wang X., Bertolotti A., Zhang Y., Chung P., Harding H. P., Ron D. (2000) Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 287, 664–666 [DOI] [PubMed] [Google Scholar]
- 21.Hetz C., Glimcher L. H. (2009) Fine-tuning of the unfolded protein response: assembling the IRE1alpha interactome. Mol. Cell 35, 551–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cullinan S. B., Zhang D., Hannink M., Arvisais E., Kaufman R. J., Diehl J. A. (2003) Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol. Cell. Biol. 23, 7198–7209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Volchuk A., Ron D. (2010) The endoplasmic reticulum stress response in the pancreatic β-cell. Diabetes Obes. Metab. 12(Suppl 2), 48–57 [DOI] [PubMed] [Google Scholar]
- 24.Lu M., Lawrence D. A., Marsters S., Acosta-Alvear D., Kimmig P., Mendez A. S., Paton A. W., Paton J. C., Walter P., Ashkenazi A. (2014) Cell death. Opposing unfolded-protein-response signals converge on death receptor 5 to control apoptosis. Science 345, 98–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin J. H., Li H., Yasumura D., Cohen H. R., Zhang C., Panning B., Shokat K. M., Lavail M. M., Walter P. (2007) IRE1 signaling affects cell fate during the unfolded protein response. Science 318, 944–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin J. H., Li H., Zhang Y., Ron D., Walter P. (2009) Divergent effects of PERK and IRE1 signaling on cell viability. PLoS One 4, e4170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Korennykh A. V., Egea P. F., Korostelev A. A., Finer-Moore J., Zhang C., Shokat K. M., Stroud R. M., Walter P. (2009) The unfolded protein response signals through high-order assembly of Ire1. Nature 457, 687–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hetz C., Martinon F., Rodriguez D., Glimcher L. H. (2011) The unfolded protein response: integrating stress signals through the stress sensor IRE1α. Physiol. Rev. 91, 1219–1243 [DOI] [PubMed] [Google Scholar]
- 29.Eletto D., Eletto D., Dersh D., Gidalevitz T., Argon Y. (2014) Protein disulfide isomerase A6 controls the decay of IRE1α signaling via disulfide-dependent association. Mol. Cell 53, 562–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Groenendyk J., Peng Z., Dudek E., Fan X., Mizianty M. J., Dufey E., Urra H., Sepulveda D., Rojas-Rivera D., Lim Y., Kim H., Baretta K., Srikanth S., Gwack Y., Ahnn J., Kaufman R. J., Lee S. K., Hetz C., Kurgan L., Michalak M. (2014) Interplay between the oxidoreductase PDIA6 and microRNA-322 controls the response to disrupted endoplasmic reticulum calcium homeostasis. Sci. Signal. 7, ra54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Higa A., Taouji S., Lhomond S., Jensen D., Fernandez-Zapico M. E., Simpson J. C., Pasquet J. M., Schekman R., Chevet E. (2014) Endoplasmic reticulum stress-activated transcription factor ATF6α requires the disulfide isomerase PDIA5 to modulate chemoresistance. Mol. Cell. Biol. 34, 1839–1849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hohmeier H. E., Mulder H., Chen G., Henkel-Rieger R., Prentki M., Newgard C. B. (2000) Isolation of INS-1-derived cell lines with robust ATP-sensitive K+ channel-dependent and -independent glucose-stimulated insulin secretion. Diabetes 49, 424–430 [DOI] [PubMed] [Google Scholar]
- 33.Livak K. J., Schmittgen T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 34.Calfon M., Zeng H., Urano F., Till J. H., Hubbard S. R., Harding H. P., Clark S. G., Ron D. (2002) IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 415, 92–96 [DOI] [PubMed] [Google Scholar]
- 35.Rutkowski D. T., Arnold S. M., Miller C. N., Wu J., Li J., Gunnison K. M., Mori K., Sadighi Akha A. A., Raden D., Kaufman R. J. (2006) Adaptation to ER stress is mediated by differential stabilities of pro-survival and pro-apoptotic mRNAs and proteins. PLoS Biol. 4, e374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eletto D., Maganty A., Eletto D., Dersh D., Makarewich C., Biswas C., Paton J. C., Paton A. W., Doroudgar S., Glembotski C. C., Argon Y. (2012) Limitation of individual folding resources in the ER leads to outcomes distinct from the unfolded protein response. J. Cell Sci. 125, 4865–4875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cavener D. R., Gupta S., McGrath B. C. (2010) PERK in beta cell biology and insulin biogenesis. Trends Endocrinol. Metab. 21, 714–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Papa F. R. (2012) Endoplasmic reticulum stress, pancreatic β-cell degeneration, and diabetes. Cold Spring Harb. Perspect. Med. 2, a007666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ishihara H., Asano T., Tsukuda K., Katagiri H., Inukai K., Anai M., Kikuchi M., Yazaki Y., Miyazaki J. I., Oka Y. (1993) Pancreatic beta cell line MIN6 exhibits characteristics of glucose metabolism and glucose-stimulated insulin secretion similar to those of normal islets. Diabetologia 36, 1139–1145 [DOI] [PubMed] [Google Scholar]
- 40.Jessop C. E., Watkins R. H., Simmons J. J., Tasab M., Bulleid N. J. (2009) Protein disulphide isomerase family members show distinct substrate specificity: P5 is targeted to BiP client proteins. J. Cell Sci. 122, 4287–4295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rutkevich L. A., Cohen-Doyle M. F., Brockmeier U., Williams D. B. (2010) Functional relationship between protein disulfide isomerase family members during the oxidative folding of human secretory proteins. Mol. Biol. Cell 21, 3093–3105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thastrup O., Cullen P. J., Drøbak B. K., Hanley M. R., Dawson A. P. (1990) Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc. Natl. Acad. Sci. USA 87, 2466–2470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Axten J. M., Medina J. R., Feng Y., Shu A., Romeril S. P., Grant S. W., Li W. H., Heerding D. A., Minthorn E., Mencken T., Atkins C., Liu Q., Rabindran S., Kumar R., Hong X., Goetz A., Stanley T., Taylor J. D., Sigethy S. D., Tomberlin G. H., Hassell A. M., Kahler K. M., Shewchuk L. M., Gampe R. T. (2012) Discovery of 7-methyl-5-(1-[3-(trifluoromethyl)phenyl]acetyl-2,3-dihydro-1H-indol-5-yl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine (GSK2606414), a potent and selective first-in-class inhibitor of protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK). J. Med. Chem. 55, 7193–7207 [DOI] [PubMed] [Google Scholar]
- 44.Harding H. P., Zyryanova A. F., Ron D. (2012) Uncoupling proteostasis and development in vitro with a small molecule inhibitor of the pancreatic endoplasmic reticulum kinase, PERK. J. Biol. Chem. 287, 44338–44344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bunpo P., Dudley A., Cundiff J. K., Cavener D. R., Wek R. C., Anthony T. G. (2009) GCN2 protein kinase is required to activate amino acid deprivation responses in mice treated with the anti-cancer agent L-asparaginase. J. Biol. Chem. 284, 32742–32749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Han D., Upton J. P., Hagen A., Callahan J., Oakes S. A., Papa F. R. (2008) A kinase inhibitor activates the IRE1alpha RNase to confer cytoprotection against ER stress. Biochem. Biophys. Res. Commun. 365, 777–783 [DOI] [PubMed] [Google Scholar]
- 47.Lee A. H., Heidtman K., Hotamisligil G. S., Glimcher L. H. (2011) Dual and opposing roles of the unfolded protein response regulated by IRE1alpha and XBP1 in proinsulin processing and insulin secretion. Proc. Natl. Acad. Sci. USA 108, 8885–8890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tirasophon W., Lee K., Callaghan B., Welihinda A., Kaufman R. J. (2000) The endoribonuclease activity of mammalian IRE1 autoregulates its mRNA and is required for the unfolded protein response. Genes Dev. 14, 2725–2736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hollien J., Weissman J. S. (2006) Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science 313, 104–107 [DOI] [PubMed] [Google Scholar]
- 50.Hollien J., Lin J. H., Li H., Stevens N., Walter P., Weissman J. S. (2009) Regulated Ire1-dependent decay of messenger RNAs in mammalian cells. J. Cell Biol. 186, 323–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lerner A. G., Upton J. P., Praveen P. V., Ghosh R., Nakagawa Y., Igbaria A., Shen S., Nguyen V., Backes B. J., Heiman M., Heintz N., Greengard P., Hui S., Tang Q., Trusina A., Oakes S. A., Papa F. R. (2012) IRE1α induces thioredoxin-interacting protein to activate the NLRP3 inflammasome and promote programmed cell death under irremediable ER stress. Cell Metab. 16, 250–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cross B. C., Bond P. J., Sadowski P. G., Jha B. K., Zak J., Goodman J. M., Silverman R. H., Neubert T. A., Baxendale I. R., Ron D., Harding H. P. (2012) The molecular basis for selective inhibition of unconventional mRNA splicing by an IRE1-binding small molecule. Proc. Natl. Acad. Sci. USA 109, E869–E878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bright M. D., Itzhak D. N., Wardell C. P., Morgan G. J., Davies F. E. (2015) Cleavage of BLOC1S1 mRNA by IRE1 is sequence specific, temporally separate from XBP1 splicing, and dispensable for cell viability under acute endoplasmic reticulum stress. Mol. Cell. Biol. 35, 2186–2202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eletto D., Chevet E., Argon Y., Appenzeller-Herzog C. (2014) Redox controls UPR to control redox. J. Cell Sci. 127, 3649–3658 [DOI] [PubMed] [Google Scholar]
- 55.Wei J., Sheng X., Feng D., McGrath B., Cavener D. R. (2008) PERK is essential for neonatal skeletal development to regulate osteoblast proliferation and differentiation. J. Cell. Physiol. 217, 693–707 [DOI] [PubMed] [Google Scholar]
- 56.Upton J. P., Wang L., Han D., Wang E. S., Huskey N. E., Lim L., Truitt M., McManus M. T., Ruggero D., Goga A., Papa F. R., Oakes S. A. (2012) IRE1α cleaves select microRNAs during ER stress to derepress translation of proapoptotic Caspase-2. Science 338, 818–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang W., Feng D., Li Y., Iida K., McGrath B., Cavener D. R. (2006) PERK EIF2AK3 control of pancreatic beta cell differentiation and proliferation is required for postnatal glucose homeostasis. Cell Metab. 4, 491–497 [DOI] [PubMed] [Google Scholar]
- 58.Gomez E., Powell M. L., Bevington A., Herbert T. P. (2008) A decrease in cellular energy status stimulates PERK-dependent eIF2alpha phosphorylation and regulates protein synthesis in pancreatic beta-cells. Biochem. J. 410, 485–493 [DOI] [PubMed] [Google Scholar]
- 59.Tavender T. J., Bulleid N. J. (2010) Molecular mechanisms regulating oxidative activity of the Ero1 family in the endoplasmic reticulum. Antioxid. Redox Signal. 13, 1177–1187 [DOI] [PubMed] [Google Scholar]
- 60.Gupta S., McGrath B., Cavener D. R. (2010) PERK (EIF2AK3) regulates proinsulin trafficking and quality control in the secretory pathway. Diabetes 59, 1937–1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Støy J., Edghill E. L., Flanagan S. E., Ye H., Paz V. P., Pluzhnikov A., Below J. E., Hayes M. G., Cox N. J., Lipkind G. M., Lipton R. B., Greeley S. A., Patch A. M., Ellard S., Steiner D. F., Hattersley A. T., Philipson L. H., Bell G. I.; Neonatal Diabetes International Collaborative Group (2007) Insulin gene mutations as a cause of permanent neonatal diabetes. Proc. Natl. Acad. Sci. USA 104, 15040–15044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Colombo C., Porzio O., Liu M., Massa O., Vasta M., Salardi S., Beccaria L., Monciotti C., Toni S., Pedersen O., Hansen T., Federici L., Pesavento R., Cadario F., Federici G., Ghirri P., Arvan P., Iafusco D., Barbetti F.; Early Onset Diabetes Study Group of the Italian Society of Pediatric Endocrinology and Diabetes (SIEDP) (2008) Seven mutations in the human insulin gene linked to permanent neonatal/infancy-onset diabetes mellitus. J. Clin. Invest. 118, 2148–2156 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.