Abstract
How an animal matches feeding to food availability is a key question for energy homeostasis. We addressed this in the nematode Caenorhabditis elegans, which couples feeding to the presence of its food (bacteria) by regulating pharyngeal activity (pumping). We scored pumping in the presence of food and over an extended time course of food deprivation in wild-type and mutant worms to determine the neural substrates of adaptive behavior. Removal of food initially suppressed pumping but after 2 h this was accompanied by intermittent periods of high activity. We show pumping is fine-tuned by context-specific neural mechanisms and highlight a key role for inhibitory glutamatergic and excitatory cholinergic/peptidergic drives in the absence of food. Additionally, the synaptic protein UNC-31 [calcium-activated protein for secretion (CAPS)] acts through an inhibitory pathway not explained by previously identified contributions of UNC-31/CAPS to neuropeptide or glutamate transmission. Pumping was unaffected by laser ablation of connectivity between the pharyngeal and central nervous system indicating signals are either humoral or intrinsic to the enteric system. This framework in which control is mediated through finely tuned excitatory and inhibitory drives resonates with mammalian hypothalamic control of feeding and suggests that fundamental regulation of this basic animal behavior may be conserved through evolution from nematode to human.—Dallière, N., Bhatla, N., Luedtke, Z., Ma, D. K., Woolman, J., Walker, R. J., Holden-Dye, L., O’Connor, V. Multiple excitatory and inhibitory neural signals converge to fine-tune Caenorhabditis elegans feeding to food availability.
Keywords: behavioral plasticity, fasting, glutamate, neuropeptide, UNC-31
All animals must balance energy intake against energy expenditure to thrive. To achieve this balance, animals respond appropriately to changes in food availability and their nutritional status (1). In mammals, distinct hypothalamic circuits respond to multiple converging signals to either increase or decrease food intake (2). In humans, where feeding is influenced by complex motivational states and higher cognitive processing, there is strong evidence for a dominant role of subconscious fundamental biological processes that control eating (3), and understanding these has relevance to understanding eating disorders and obesity.
A model to understand these phenomena can be provided by the free-living nematode Caenorhabditis elegans. This animal provides a highly tractable experimental platform for a temporal analysis of the biological processes that control the response to food and food deprivation (4). C. elegans feeds on bacteria, and its chemosensory systems allow detection of bacterial-associated cues that modulate locomotion and drive worms toward a food source (5–9). In the presence of bacteria, worms alternate between “dwelling” and “roaming” in a probabilistic fashion (10, 11). This behavior is indicative of a changing tone or modulation of the core circuits (6, 12) that mediate food-dependent locomotion. The food dependence of C. elegans behavioral plasticity is further evidenced by paradigms that have tracked changes in behavior following removal from food (13–15). Importantly, these studies provide evidence for chemosensory systems that selectively respond to the removal of food cues (5) and for peptide neuromodulators that underlie temporal aspects of the behavioral response (16). Overall, such studies highlight that C. elegans locomotory behavior is regulated in response to the presence or absence of food through distinct signaling pathways operating across a wide temporal window.
Once a C. elegans worm has located a patch of food, the animals engage their pharyngeal system to actively feed (17–20). The rate of feeding is influenced by the developmental state and the quality of the food (21) but typically the syncytial radial pharyngeal muscle undergoes a rhythmic contraction–relaxation cycle, or pump, at a frequency of 4 to 5 Hz (18). The pumping rate is regulated by a simple microcircuit of 20 neurons enveloped within the basal lamina of the pharyngeal muscle. This in turn connects to the worm’s central nerve ring and upstream chemosensory circuits via the RIP pair of interneurons (22). Previously it has been reported that laser ablation of RIP has surprisingly little effect on pharyngeal function (23). Disconnecting the pharyngeal and extrapharyngeal nervous system in this manner negates the inhibitory effect of light touch on pharyngeal pumping (24), but otherwise the pharynx appears to function without detriment. These observations suggest that neurohormonal and/or intrinsic enteric signals have an important role in the physiological regulation of feeding behavior. Further, laser ablation experiments have assigned functional roles to specific neurons within the pharyngeal microcircuit, in particular MC, which acts as a pacemaker motor neuron (25) to drive the high rate of pumping in the presence of food. The duration of the pharyngeal pump is regulated by inhibitory glutamatergic transmission from the M3 neurons (26). In turn, MC, M3, M4, and the pharyngeal muscle itself are subject to regulation by 5-hydroxytryptamine (5-HT) released from ADF and/or NSM neurons to regulate the microcircuit to permit a sustained high frequency of pumping in the presence of bacteria (25–31). Finally, there is evidence to indicate that pharyngeal myogenic activity may also contribute to pumping, although this pumping could be modulated by humoral release coming from outside the pharyngeal nervous system (32).
These mechanisms detailed above underpin the ability of the worm to actively feed in laboratory conditions; however, it is noteworthy that the pharyngeal neurons also express a range of classic small molecule neurotransmitters, neuropeptides, and receptors (20), which may provide discrete context-dependent regulation of feeding behavior that have not yet been modeled in laboratory behavioral paradigms. In particular, neuropeptides are widely expressed in the pharyngeal nervous system (33–35), and subsets of these exert potent inhibitory or excitatory effects on the isolated pharyngeal system, suggesting they play a role in its physiologic regulation (27, 36–38).
The pharyngeal system is subject to regulation depending on the food context (39). Early studies reported that C. elegans pumping is reduced by at least half in the absence of food, although after more than 4 h of starvation, the worms started to pump again (40) even though there was no food in the vicinity. It has been suggested that this may be a result of the increasing need for food as starvation progresses (40). Furthermore, it was observed that worms carrying mutations in the gene unc-31, which encodes calcium-activated protein for secretion (CAPS), a calcium-binding protein required for exocytosis (41) pumped constitutively in the absence of food (42). Additional work has investigated C. elegans pharyngeal function in different behavioral contexts and during starvation (4, 43–46). These suggest a complex shifting behavioral response of the pharyngeal system, which is dependent on the “on” and “off” food context. However, the temporal aspects of this behavior and the role of discrete neural signals have not yet been defined.
Here we report the feeding behavior of C. elegans in the presence of food and following removal of food over 8 h. Through a systematic analysis of mutants defective in specific neurotransmitter signals, we have assigned roles of neurotransmitters to specific phases of the adaptive behavioral response. These results reveal an interplay between several neurotransmitters and highlight excitatory and inhibitory pathways that converge to regulate context-dependent feeding.
MATERIALS AND METHODS
Caenorhabditis elegans strains and maintenance
The Bristol isolate N2 has been used as the wild-type strain. The following mutants strains were used in this study; VC671 egl-3(ok979), MT150 egl-3(n150), KP2018 egl-21(n476), MT13113 tdc-1(n3419), CB113 unc-17(e113), CB933 unc-17(e245), CB156 unc-25(e156), CB1112 cat-2(e1112), RB1161 tbh-1(ok1196), MT6318 eat-4(n2474), MT6308 eat-4(ky5), DA509 unc-31(e928), and GR1321 tph-1(mg280) were provided by the C. elegans Genetics Center, which is funded by U.S. National Institutes of Health Office of Research Infrastructure Programs (P40 OD010440). RWK102 egl-3(n150);eat-4(n2474) was provided by the R. Komuniecki laboratory (University of Toledo, Toledo, OH, USA). The transgenic strain N2;nEx1997 (Pgpa-16::GFP) was generated by microinjection. Double mutants for eat-4(ky5);unc-31(e169) and unc-31(e928);egl-3(ok979) were generated by mating male eat-4(ky5) and male egl-3(ok979) with hermaphrodites unc-31(e169) and unc-31(e928), respectively. Genotype was confirmed by PCR.
Strains were maintained as previously described (47).
Measuring pharyngeal pumping in the presence and absence of food
Pumping may be scored by direct visual observation of movements of the grinder in the terminal bulb of the pharynx in intact freely moving worms. One anterior and posterior movement of the grinder is scored as a single pharyngeal pump. Experiments were performed on age-synchronized young adult worms (L4 + 1 d old), and mutant experimental groups were paired with a wild-type N2 control group on each day of assay. The pumping of each worm was counted for 1 min on the food-containing plates used to cultivate them from an L4 stage overnight. The worm was then transferred onto a nonfood plate and left for 1 min to clean itself of attached bacteria before being transferred onto a plate lacking food (Fig. 1A). The plates in which pharyngeal pumping off food was observed were 9 cm diameter plates containing 30 ml of nematode growth medium (not more than 2 wk old). After transfer to the second nonfood plate, pumping rate was recorded at periodic intervals as indicated up to a maximum of 8 h. Worms were then placed back onto a fresh food plate and a final score made. During this experiment, there was an unavoidable attrition rate in the worms as some worms of each experimental group were lost because they dried out after migration off the edge of the agar plate.
Figure 1.
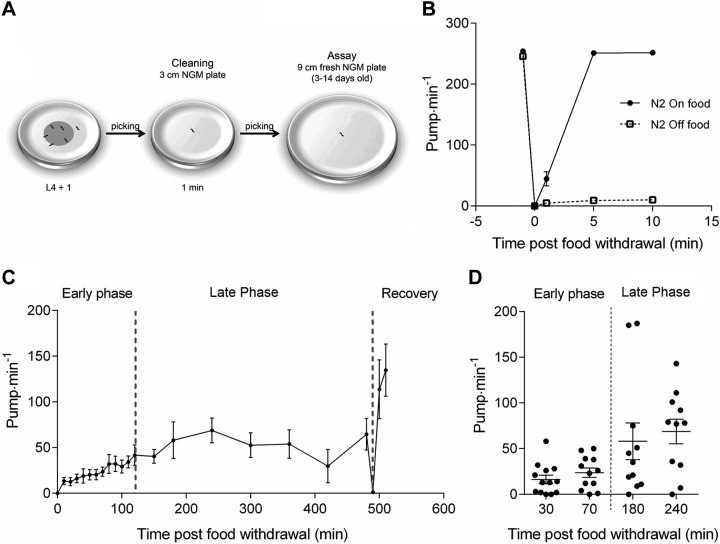
Modulation of pharyngeal pumping on and off an E. coli OP50 food source. A) Schematic representation of the pumping off food assay. B) Pumping rate of well-fed worms before and after pick-mediated transfer to agar plates with or without an OP50 bacterial lawn. The on-food pump rate is halted by the transfer and returns to prepick on food levels when returned to bacteria. There is a reduced but clearly measurable off food pumping rate after the initial pick mediated inhibition. C) Extended time course in which worms handled as in B are monitored off food at indicated time points before being picked back onto a bacterial lawn. The adaptive response in pharyngeal pumping is segregated into 3 phases. These demarcations, defined by visual inspection, are indicated by broken lines highlighting an early phase (0–120 min), a late phase (120–480 min), and a recovery phase (480–500 min). These experiments represent mean of observations made on 13 individual worms. D) Scatter plot of 2 time points of both the early (30 and 70 min) and late phase (180 and 240 min) to illustrate the increased variability during the late phase. Each datum point indicates a measurement made from a single worm.
Laser beam ablation of RIP
Laser beam ablation was performed using a MicroPoint laser (VSL-337; output 337 nm, 142 µJ; Laser Science Inc., Cambridge, MA, USA) mounted to an epifluorescent microscope with Nomarski differential interference contrast optics according to the previously described method (48). N2;nEx1997 (Pgpa-16::GFP) L1/L2 larvae, expressing green fluorescent protein (GFP) in RIP, were mounted on an nematode growth medium agar pad and immobilized with 10 mM sodium azide. RIP neurons were identified and ablated. Ablation was confirmed by visual inspection for GFP fluorescence the next day. Sham-ablated controls were performed at the same time.
Statistical analysis
One-way ANOVA with Bonferroni post hoc test was used to test for significant difference between strains pumping rate on food. Two-way ANOVA with Bonferroni post hoc test was used to test for significant difference between strains, wild-type compared with mutant, over time following removal from food. The periods of the behavioral adaptation to food deprivation were divided into an early phase (from 0 to 120 min post-food withdrawal) and a late phase (from 150 min until transfer back on food) and these periods were analyzed for statistical significance between wild-type and mutant separately. Statistical significance was set at P < 0.05.
RESULTS
Food deprivation inhibits pharyngeal pumping in 2 distinct phases
When C. elegans were placed on a plate replete with OP50 Escherichia coli bacteria, they dwelled and exhibited a pharyngeal pumping rate of more than 200 pumps per minute (Fig. 1B) (25, 49). The food deprivation assay involved the transfer of well-fed worms to an arena without bacterial food. As a control, we measured the pumping rate of well-fed worms transferred to a new bacterial lawn. This control showed that transferring worms induced mechanosensory-mediated inhibition of pumping that was followed by recovery to the normal pumping rate on food, as previously reported (43) (Fig. 1B). When worms were transferred to plates without food, we observed a change in the pumping rate over an 8 h period (Fig. 1C). Initially, pumping was inhibited as seen when worms were picked between food plates. Subsequently, pumping rate remained low but increased during the first 120 min of food deprivation, to about one-quarter of the rate on food (i.e., 50 pumps per minute) (Fig. 1C). We called this the “early phase” of the response to food removal. After the early phase, pumping rate became dramatically more erratic, with the rate of individual worms fluctuating between relatively low (0–20 pumps per minute) and relatively high pump rates (100–200 pumps per minute; Fig. 1D). We called this the “late phase” of the response to food removal. Finally, in each experiment worms were placed back onto food and showed a rapid recovery of pumping (the “recovery phase”).
Acetylcholine increases and glutamate and 5-HT inhibit pumping during food deprivation
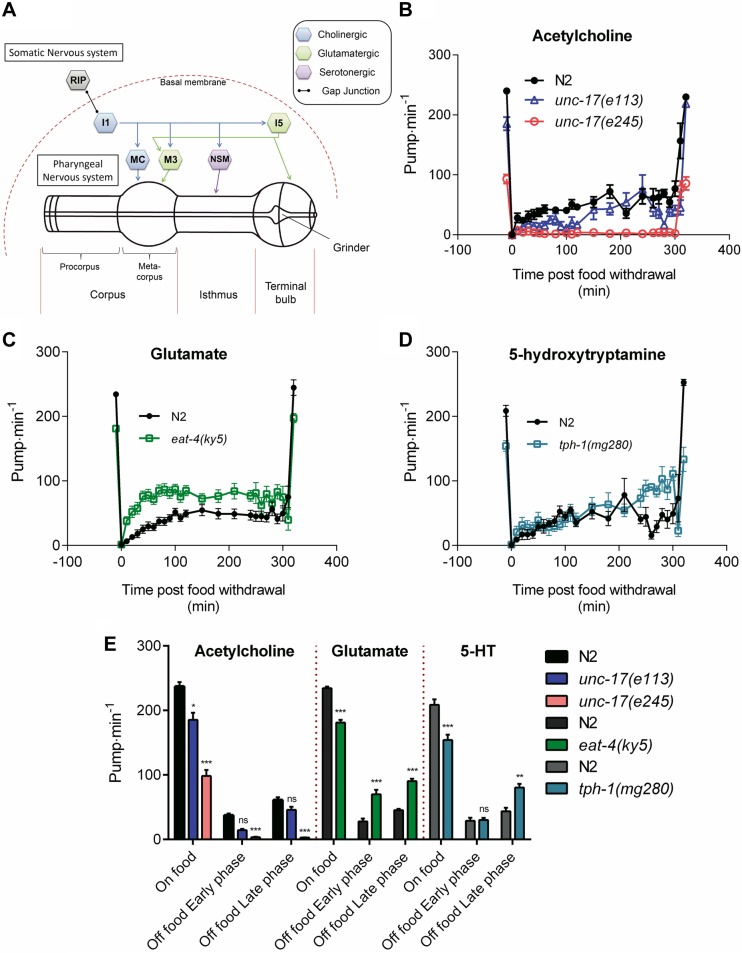
Signaling through acetylcholine, glutamate and 5-HT (or serotonin) neural pathways within the pharyngeal microcircuit (Fig. 2A) have all been reported to be required to maintain a high pumping rate on food (18, 25, 26, 50, 51). Here we used strains deficient in these transmitters, harboring mutations in the vesicular transporters for acetylcholine, unc-17(e113 and e245) (52) or glutamate, eat-4(ky5) (50), or the rate-limiting 5-HT synthesis enzyme, tryptophan hydroxylase [tph-1(mg280)] (44). The acetylcholine analysis uses hypomorphic strains as mutants completely lacking acetylcholine are nonviable (52). In agreement with earlier reports, the deficiencies in these core regulators of pharyngeal function all showed reduced pumping on food relative to wild-type worms (Fig. 2B–E) (44, 53, 54). However, none of these deficiencies completely ablated pumping rate on their own, and with the exception of unc-17(e245), the reduction on food was less than 50%. The situation in the absence of food was strikingly different. Although the unc-17 mutants provided support for an important cholinergic excitatory drive both on and off food, the roles for glutamate and 5-HT were reversed. Thus, in contrast to their stimulatory role in the presence of food, both glutamate and 5-HT have an inhibitory role during food deprivation as indicated by the higher pumping rate in the mutants compared with wild-type control (Fig. 2C–E). Furthermore, there is a temporal aspect to the 5-HT regulation, as this inhibition was only apparently required following food deprivation of longer than 4 h (Fig. 2D, E).
Figure 2.
Comparison of the role played by major pharyngeal transmitters acetylcholine, glutamate, and 5-HT to the pharyngeal pumping rate on and off food. A) The main neural components of the pharyngeal nervous system. The pharynx (composed of a corpus, isthmus, and terminal bulb) and its embedded nervous system are isolated from the rest of the body by a basal membrane, and only physically linked to the somatic nervous system by the RIP-I1 gap junction. MC (cholinergic), M3 (glutamatergic), and NSM (serotonergic) are the main pharyngeal neurons known to control pharyngeal pumping rate in the presence of food. B) Acetylcholine: unc-17(e113) (−26 ± 11 pump min−1; P = 0.029; n = 11 N2, n = 10 e113) and unc-17(e245) (−158 ± 8 pump min−1; P < 0.001; n = 20 N2, n = 20 e245) mutants displays a lower pumping rate than N2 (wild-type) in the presence of food. unc-17(e113) showed no effect on the early phase (P = 0.214; n = 9) or late phase (P = 0.902; n = 7) relative to N2 (n = 6 and n = 3 for early and late phase, respectively). However, unc-17(e245) displayed a reduced pumping rate off food during both early (−48 ± 8 pump min−1; P < 0.001; n = 8 N2 and e245) and late phase (−64 ± 12 pump min−1; P < 0.001; n = 8 N2 and e245) compared with N2. C) Glutamate: eat-4(ky5) displays a lower pumping rate than N2 (−53 ± 5 pump min−1; n = 19 N2 and n = 18 eat-4) in the presence of food (P < 0.001). eat-4(ky5) mutant pumps at a higher rate during the early (P < 0.001; n = 16) and late phase (P < 0.001; n = 7) of food deprivation. Both of these phases are elevated by 45 pump min−1 indicating that eat-4 mutants have a constitutive pumping phenotype. D) 5-HT: tph-1(mg180) displays a lower pump rate (−54 ± 12 pump min−1) than N2 in the presence of food (P < 0.001; n = 8 N2, n = 10 tph-1). No significant effect was observed in absence of food during the early phase (P = 0.8521; n = 8 N2, n = 10 tph-1) but a higher pumping rate (36 pump min−1) was observed during the late phase (P = 0.0362; n = 5 N2, n = 5 tph-1) for these mutants. E) Histogram representing the average pumping rate on food and off food during the early and late phases of B–D. *P < 0.05, **P < 0.01, ***P < 0.001 compared with paired N2.
GABA and dopamine promote pumping during food deprivation
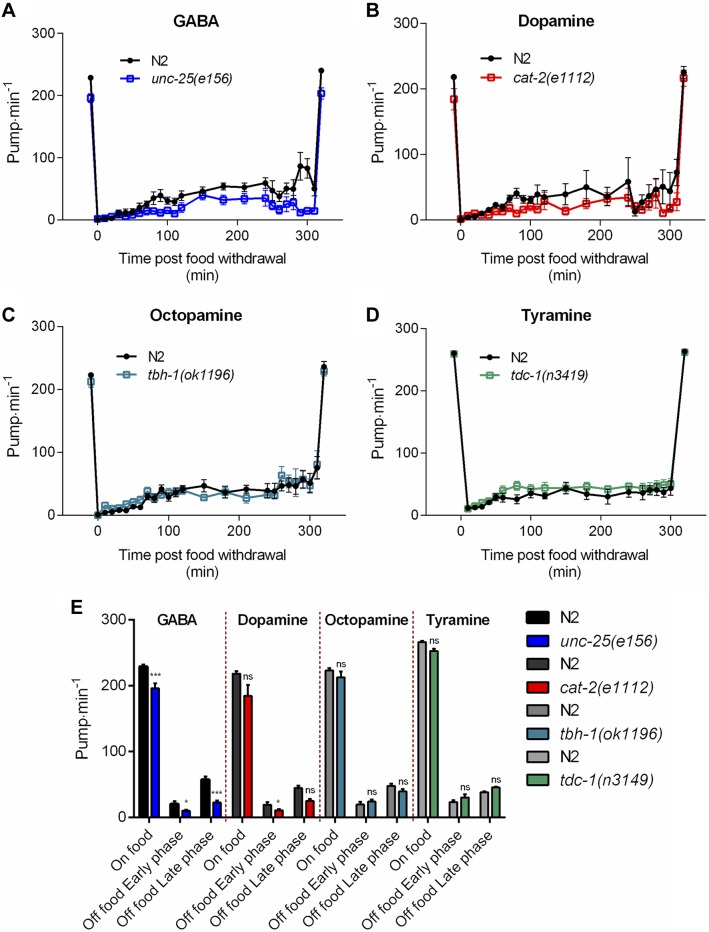
There is no evidence for pharyngeal synthesis of these neurotransmitters (20), but there is potential for them to regulate the pumping via input through the RIP interneurons circuits or humoral signals received by receptors expressed in the pharyngeal nervous system (15, 55, 56). These experiments used strains with mutations in the biosynthetic enzymes glutamic acid decarboxylase (unc-25), tyrosine hydroxylase (cat-2), dopamine β-hydroxylase (tbh-1), and tyrosine decarboxylase (tdc-1) deficient in GABA, dopamine, octopamine, and tyramine, respectively (57–59). We observed a significant reduction in the pumping rate on and off food in mutants lacking the neurotransmitter GABA suggesting it contributes to the excitatory drive in the presence of food (Fig. 3A, E). For the dopamine mutant cat-2, there was a small but significant reduction in pumping during the early phase of food deprivation, again consistent with a net excitatory effect on pharyngeal function during this phase of the behavior (Fig. 3B, E). The behavior of the mutants defective in tyramine and octopamine signaling was indistinguishable from wild-type controls, showing no role for these neurotransmitters in this feeding paradigm (Fig. 3C–E). This is despite the observation that exogenous octopamine has been shown to inhibit pharyngeal pumping (27, 56) and to be involved in food-dependent responses (60–62). This suggests that differences in assay may underlie the difference in function found for octopamine.
Figure 3.
Modulation of pharyngeal pumping by major neurotransmitters that are synthesized extrinsically to the pharynx. A) GABA: unc-25(e156) pump less than wild-type N2 (−32 ± 7 pump min−1; n = 12 N2, n = 10 unc-25) in the presence of food (P < 0.001). A significant reduction of the pumping rate relative to N2 was observed during both early (−10 pump min−1; P = 0.0157; n = 11 N2, n = 9 unc-25) and late phases of food deprivation (−44 pump min−1; P < 0.001; n = 7 N2, n = 7 unc-25). B) Dopamine: cat-2(e1112) mutants display a similar pumping rate to N2 in the presence of food (P = 0.0665; n = 12 N2, n = 13 cat-2). The pump rate is slightly lower (−9 pump min−1; n = 6 N2, n = 7 cat-2) than N2 during the early phase (P = 0.0452), but not the late phase (P = 0.2632; n = 3 N2, n = 6 cat-2) of the food deprivation. C) Octopamine: tbh-1(ok1196) pharyngeal pumping is similar to N2 in the presence (P = 0.2805; n = 13 N2, n = 12 tbh-1) and absence of food (P = 0.1777; n = 12 N2, n = 11 tbh-1 and P = 0.4456; n = 5 N2, n = 6 tbh-1 for the early and late phase, respectively). D) Tyramine: tdc-1(n3419) pumps at a similar rate compared with N2 both in the presence of food (P = 0.3435; n = 17 N2, n = 17 tdc-1) and in the absence of food (P = 0.2577; n = 8 N2, n = 8 tdc-1 and P = 0.483; n = 8 N2, n = 8 tdc-1 for the early and late phase, respectively). E) Histogram representing the average pumping rate on food and off food during the early and late phases of A–D. *P < 0.05, ***P < 0.001 compared with paired N2.
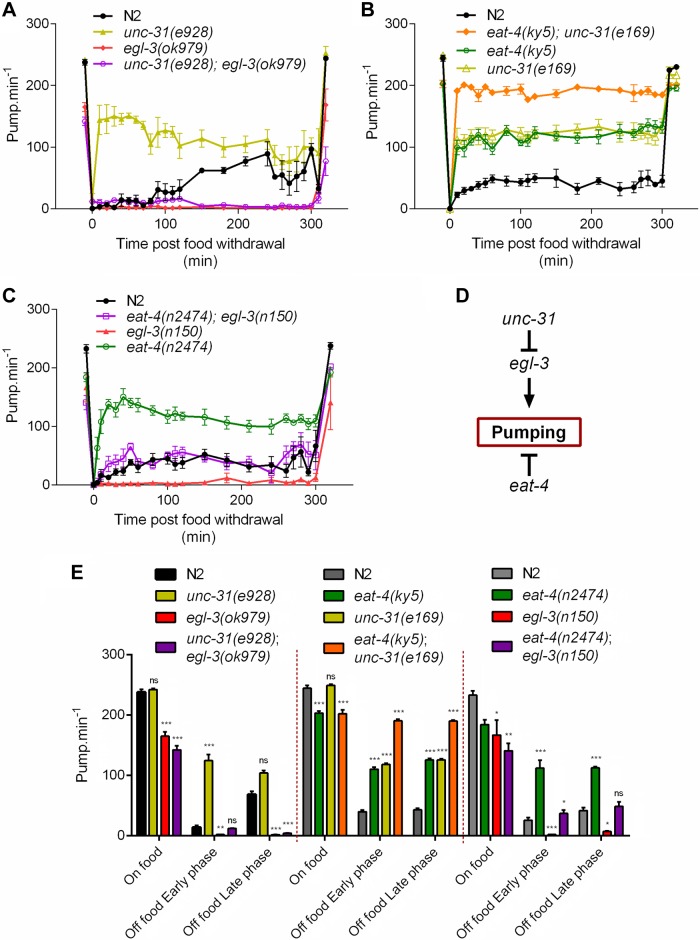
The synaptic protein mutant unc-31 pumps constitutively throughout food deprivation
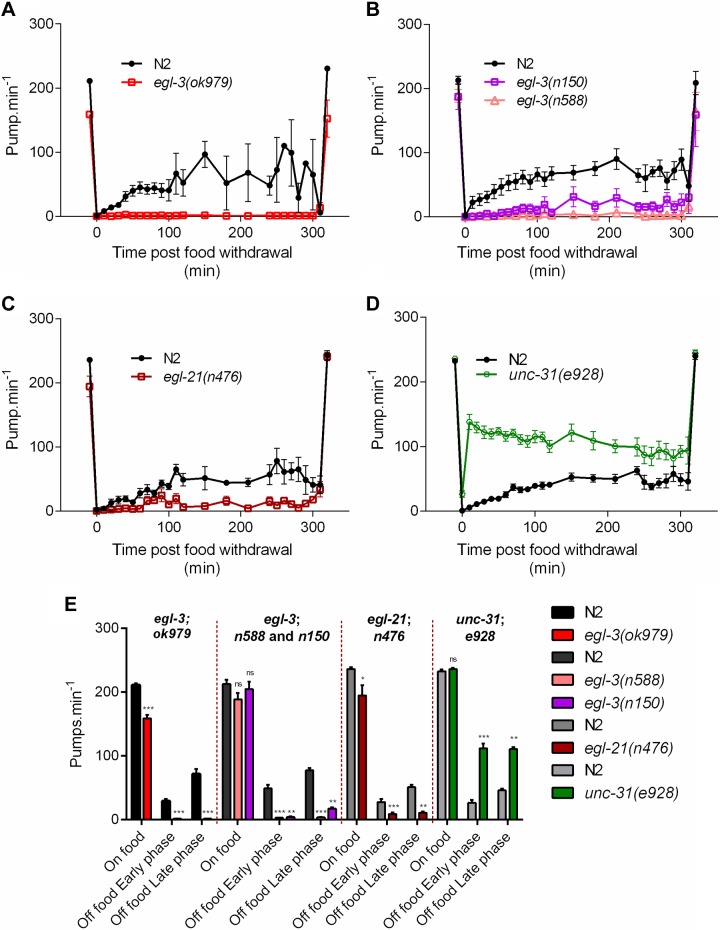
UNC-31 is required for dense core vesicle-mediated exocytosis (63, 64). Our data showing unc-31(e169 and e928) pump at a similar rate to wild-type on food but at a significantly higher rate in the absence of food is consistent with the previous observation that unc-31 mutants exhibit constitutive pharyngeal activity during starvation (42) (Figs. 4D and 5A, B).
Figure 4.
Comparison of pharyngeal pumping on and off food in wild-type N2 and mutants deficient in neuropeptide synthesis and secretion. A) egl-3(ok979) has a reduced pumping rate on food [−52 ± 6 pump min−1; n = 10 N2, n = 10 egl-3(ok979)] relative to wild-type N2 (P < 0.001). In the absence of food there was a significant reduction in the rate in both phases of food deprivation [early and late phase P < 0.001 with −22; n = 5 N2, n = 8 egl-3(ok979) and –70 pump min−1; n = 3 N2, n = 8 egl-3(ok979), respectively]. B) egl-3(n588) [P = 0.0551; n = 12 N2, n = 12 egl-3(n588)] and egl-3(n150) [P = 0.5275; n = 12 N2, n = 10 egl-3(n150)] have no reduction in pumping on food relative to N2. egl-3(n588) (early phase: −46 pump min−1 n = 11 N2, n = 10 egl-3(n588) and late phase: −74 pump min−1; n = 6 N2, n = 8 egl-3(n588) P < 0.001) and egl-3(n150) [early phase: −43 pump min−1 P = 0.0029; n = 12 N2, n = 7 egl-3(n150), late phase: −60 pump min−1 P = 0.0012; n = 6 N2, n = 6 egl-3(n150)] exhibited a significant reduction in pumping in both phases of food deprivation. C) egl-21(n476) displays a reduced pumping in presence of food [−41 ± 17 pump min−1; n = 8 N2, n = 9 egl-21(n476)] relative to N2 (P = 0.0309). In the absence of food there is a significant reduction in both phases of the pharyngeal pumping [early phase: −19 pump min−1 P < 0.001; n = 8 N2, n = 8 egl-21(n476) and late phase: −40 pump min−1 P = 0.0015; n = 5 N2, n = 9 egl-21(n476)]. D) unc-31(e928) pumping rate on food was similar [P = 0.3897; n = 11 N2, n = 9 unc-31(e928)] to the paired N2 control. unc-31(e928) shows a marked increase in its pumping rate above 100 pump min−1 during food deprivation compared with N2, which is significant during the early phase [86 pump min−1 P < 0.001; n = 15 N2, n = 15 unc-31(e928)] and the late phase [65 pump min−1; P = 0.0072; n = 6 N2, n = 10 unc-31(e928)]. E) Histogram representing the average pumping rate on food and off food during the early and late phases of A–D. *P < 0.05, **P < 0.01, ***P < 0.001 compared with paired N2 control.
Figure 5.
Genetic interaction between the mutants that define the extremes of the pumping off food phenotype. A) In the presence of food, the double mutant unc-31(e928);egl-3(ok979) displays a pumping rate similar to egl-3(ok979) single mutant [23 ± 8 pump min−1; P = 0.0669; n = 8 double mutant, n = 8 egl-3(ok979)] but lower than unc-31(e928) [−100 ± 5 pump min−1; P < 0.001; n = 6 unc-31(e928)]. In response to food withdrawal the double mutant unc-31(e928);egl-3(ok979) displays a pumping rate similar to wild-type N2 during the early phase (P = 0.5162; n = 5 N2, n = 8 double mutant), but significantly lower during the late phase (−64 pump min−1; P < 0.001; n = 3 N2, n = 8 double mutant). B) Double mutant eat-4(ky5);unc-31(e169) displays a reduced pumping rate in presence of food compared with wild-type N2 (−42 ± 6 pump min−1; P < 0.001; n = 8). In the absence of food, both the single mutant eat-4(ky5) and unc-31(e928) pump constitutively higher than N2 during both early [71 ± 12 pump min−1; P < 0.001; n = 8 N2, n = 8 eat-4(ky5) and 79 ± 13 pump min−1; P < 0.001; n = 8 N2, n = 8 unc-31(e928), respectively] and late phase (83 ± 14 pump min−1; P < 0.001; n = 7 N2, n = 8 eat-4(ky5) and 83 ± 17 pump min−1; P < 0.001 n = 7 N2, n = 8 unc-31(e928), respectively). The double mutant eat-4(ky5);unc-31(e169) pumps at an even higher rate than the single mutants displaying, compared with N2, an increase of 151 pump min−1 (P < 0.001; n = 8 N2, n = 8 double mutant) for the early phase and 147 pump min−1 (P < 0.001; n = 7 N2, n = 8 double mutant) for the late phase, with a pumping rate reaching around 200 pump min−1. C) No significant difference in the pumping rates on food of the double mutant eat-4(n2474);egl-3(n150) was observed compared with both eat-4(n2474) [P = 0.2691; n = 7 eat-4(n2474), n = 6 double mutant] and egl-3(n150) [P > 0.99; n = 6 egl-3(n150), n = 6 double mutant] single mutants. Double mutants eat-4(n2474);egl-3(n150) have an early phase significantly different from eat-4(n2474) [−75 pump min−1; P < 0.001; n = 5 eat-4(n2474), n = 6 double mutant] and egl-3(n150) [35 pump min−1; P < 0.001; n = 6 egl-3(n150), n = 6 double mutant]. The late phase of eat-4(n2474);egl-3(n150) is also significantly different from eat-4(n2474) [−64 pump min−1; P < 0.001; n = 4 eat-4(n2474), n = 4 double mutant] and egl-3(n150) [41 pump min−1; P < 0.001; n = 5 egl-3(n150), n = 4 double mutant]. D) Schematic model representing the genetic interactions between eat-4, egl-3, and unc-31 in the control of the pharyngeal pumping rate in the absence of food. E) Histogram representing the average pumping rate on food and off food during the early and late phases of A–C. *P < 0.05, **P < 0.01, P < 0.001 compared with N2 control.
Neuropeptide signaling has a net excitatory effect on pumping on and off food
As UNC-31 is a regulator of neuropeptide transmission in C. elegans (65), we expected that mutants deficient in neuropeptide signaling would similarly promote pumping. The worm expresses several important classes of neuropeptides including FMRFamide-like peptides (FLPs) (66, 67) neuropeptide-like peptides (NLPs) (35) and insulin-like peptides (68), many of which have roles in regulating feeding behavior (69). These molecules are synthesized as single-chain polypeptides then proteolytically processed and packaged into secretory granules. Mutants defective in the neuropeptide precursor processing enzymes EGL-3 (70) and EGL-21 (71) are depleted in neuropeptides (72, 73) and provide a useful experimental tool for investigating the net effect of peptidergic signaling on behavior (74).
We tested 3 strains carrying mutations in egl-3. The ok979 allele is a predicted loss of function mutation (72), and worms carrying this mutation showed significantly reduced pumping on food. Strikingly, during food deprivation, egl-3(ok979) mutants pumped rarely if at all (Fig. 4A, E). When the egl-3(ok979) mutants were returned to food following prolonged starvation, their pumping rate returned to the level observed prior to food deprivation. Two additional mutants of egl-3, n150 and n588, showed a similar response to both the presence and absence of food as ok979 (Fig. 4B). The difference in the behavioral phenotype between ok979 and the other alleles, n150 and n588, may be explained by the latter 2 mutations resulting in a differential loss of the neuropeptide complement, as previously suggested from mass spectrometry analysis of these mutants (73).
egl-21(n476) mutants had a reduction in pumping both on and off food; however, the phenotype observed during food deprivation was not as severe as that observed for egl-3(ok979) (Fig. 4C, E). The 2 mutants egl-3 and egl-21 have been investigated using mass spectrometry and they differ in the neuropeptides that are properly processed (72, 73). Taken together, these data indicate that neuropeptide signaling is essential for a low level of pumping in the absence of food (Fig. 4A–E).
Two parallel pathways fine-tune feeding behavior in the absence of food
To investigate the relationship between the genotypes that gave phenotypes during food deprivation (unc-31, egl-3, and eat-4), we generated double mutants. To determine the epistatic relationship between unc-31 and egl-3 mutants, we constructed and tested the unc-31;egl-3 double mutant. We found that the pumping rate unc-31;egl-3 mutants was reduced relative to wild-type pumping both on and off food, similar to the egl-3 single mutant (Fig. 5A).
In contrast, the eat-4;unc-31 double mutant exhibited an extreme pumping off food phenotype in that the constitutive pumping during food deprivation was additive, and the double mutants showed a pumping rate that was similar to that observed in the presence of food (Fig. 5B). This result suggests that the glutamatergic- and unc-31–dependent excitatory drive pumping in the absence of food function in parallel pathways. Also of note is the observation that in the presence of food, the eat-4(ky5);unc-31(e169) double mutant had a pumping rate significantly less than wild-type, similar to eat-4, again consistent with unc-31 and eat-4 lying in parallel pathways.
Finally, we also investigated the eat-4;egl-3 double mutant. In the absence of food, where glutamate has an inhibitory role and EGL-3-processed neuropeptides have a net excitatory effect, the pumping rate in the double mutant is intermediate to either single mutant and phenocopies wild-type (Fig. 5C, E). This result suggests that the glutamatergic and egl-3–dependent pathways likely function in parallel (Fig. 5A).
A simple model consistent with these data is shown in Fig. 5D. unc-31– and eat-4–mediated inhibition of pumping during food deprivation is via 2 distinct routes by which food removal inhibits pharyngeal pumping. The pathway(s) are predicted to be organized such that unc-31 lies upstream of egl-3. This is surprising in the context of the requirement for unc-31 in neuropeptide signaling (64, 65, 75), and it suggests that the functional roles of unc-31 and egl-3 in peptide transmission do not completely overlap.
Laser ablation that disconnects the pharyngeal nervous system from the extrapharyngeal nervous system does not affect pharyngeal pumping
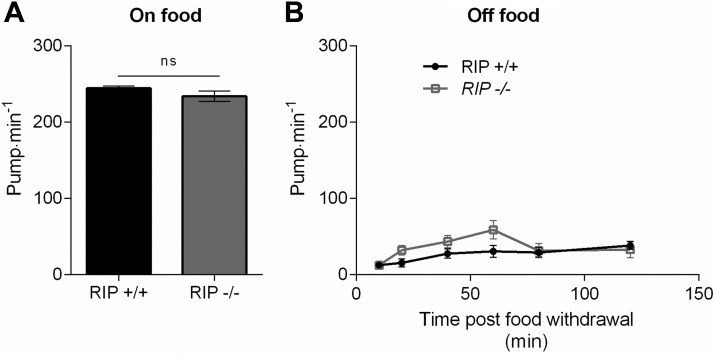
A pair of interneurons designated RIP provides the sole anatomic connection between the pharyngeal nervous system and the main nervous system (22) (Fig. 2A). This neuron could provide a route for chemosensory and mechanosensory cues detected by extrapharyngeal sensory neurons to regulate feeding behavior. The situation is complex as studies investigating the regulation of foraging behavior during food deprivation have shown that food selectively stimulates sensory microcircuits that are distinct from those activated by the removal of food (5, 76). It has previously been suggested that ablation of RIP has little effect on pharyngeal function, suggesting that extrapharyngeal chemosensory circuits either have no role in feeding regulation or function entirely through neurohormonal signals (23). To test whether or not extrapharyngeal inputs might direct the pharyngeal response to food cues in the extended food deprivation paradigm, we investigated pumping in animals in which RIP neurons were ablated with a laser. Ablation of the RIP neurons had no effect on pumping rate either on or off food (Fig. 6A, B). We assessed pumping for up to 120 min post-food withdrawal, and RIP ablation did not have a significant effect. Overall, it appears that extrapharyngeal inputs are not connected to the pharynx by this anatomically direct pathway to mediate feeding behavior.
Figure 6.
Pharyngeal pumping in the presence and absence of food in RIP-ablated worms. Worms expressing GFP under the gpa-16 promoter were subjected to laser ablation to remove the cell bodies of the bilaterally localized RIP neurons that provide the only identified anatomic link between the extra pharyngeal and pharyngeal nervous system. Ablation was performed at L1 or L2 and confirmed 24 h later. L4 ablated worms were picked the day before their pharyngeal pumping rate was measured in the presence (A) and at increasing times after the removal of food (B). There was no difference in the pumping on intact or laser-ablated controls on food (P = 0.189; RIP+/+ n = 14; RIP−/− n = 15). There was a small difference in the pump rates in the absence of food this was not significant (P = 0.496; RIP+/+ n = 12; RIP−/− n = 20).
DISCUSSION
In this study, we carried out a systematic analysis of C. elegans pharyngeal activity in the presence and absence of food. We observed 2 distinct phases of the C. elegans pharyngeal pumping response to food deprivation (1): an early phase (0–120 min) in which pumping is inhibited, and (2) a late phase (120–500 min) in which pumping remains inhibited on average but varies over a wide range (Fig. 7). Food deprivation was initiated by manually removing the worm from food and appears similar to a scenario in which the worm explores its environment after its food source has been depleted. What is the physiological significance of this residual pumping that occurs in the absence of food, given that this pumping does not lead to ingestion of bacteria? One interpretation is that the worm is enacting “fictive feeding,” which facilitates gustatory detection of food in the immediate vicinity (40). Alternatively, pumping in the absence of food might be required to permit fluid intake in the form of “drinking.” The biological significance of this switch in behavior in this late stage of food deprivation is intriguing: it might relate to a change in the worm’s foraging strategy to conserve energy as its nutritional status starts to become compromised (77).
Figure 7.
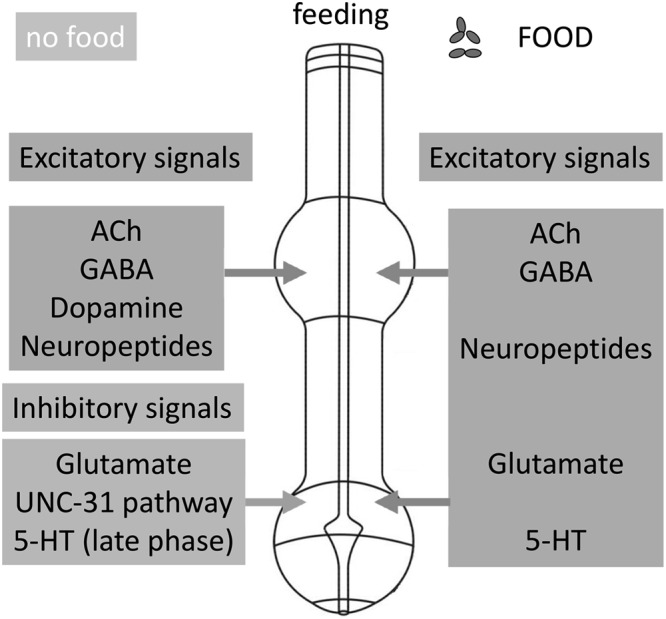
A multimodal framework for the regulation of feeding behavior in C. elegans. A summary of the multiple excitatory and inhibitory neural signals that regulate feeding behavior in the presence of food and during food deprivation. Presentation of food (right) facilitates pharyngeal pumping through several neurotransmitters that operate at the level of the pharynx (ACh, glutamate, 5-HT, neuropeptides) or via neurohormonally mediated signaling (GABA, neuropeptides) directed to the pharynx. The removal of food results in activation of both excitatory and inhibitory pathways that titrate the level of pharyngeal activity to a low level that is about one-fifth of the on-food rate. Key to this regulation is a context-dependent role for glutamate signaling, which in the presence of food is excitatory but in the absence of food provides an inhibition. Independent of the glutamatergic inhibition is an UNC-31–dependent circuit that imposes an inhibitory tone by inhibition of a net excitatory neuropeptidergic drive. The worm’s food, bacteria, provide a complex environmental cue that is able to trigger chemosensory (olfaction, gustation) and mechanosensory modalities. Thus, physical detection of food presentation and removal may be mediated by previously described sensory pathways that utilize established extrapharyngeal sensors. Alternatively, more ill-defined intrapharyngeal detectors may mediate responses to gustatory and/or mechanosensation of bacteria.
Imaging sensory neuron activity has established that presentation and removal of food cues can selectively activate and drive divergent downstream circuits modifying foraging behavior (5). The temporal regulation of feeding behavior following removal from food has interesting parallels to the worm’s regulation of locomotory behavior in which the worm initially exhibits a high frequency of reversals and turns called “local area search,” which transitions to periods of long forward runs (i.e., “dispersal”) (6, 13). The switch of the locomotory behavior from local area search to dispersal occurs earlier during food deprivation than the switch in the pharyngeal behavior from sustained to erratic pumping, suggesting differential regulation of these 2 food deprivation–dependent processes.
Here we have investigated the mechanism, or mechanisms, that lead to a marked reduction in pharyngeal pumping rate when the worm is moved to an environment without food. At its simplest, this suppression could be provided by the absence or reduction of an excitatory signal that normally drives feeding in the presence of food. Alternatively, there may be a mechanism to actively suppress pharyngeal activity in the absence of food, or, in a more complex model, pharyngeal pumping rate could be simultaneously modulated by excitatory and inhibitory signals. Interestingly, in the absence of food, we found mutants that pumped at a higher rate and also mutants that pumped at lower rate compared with wild-type. Therefore, our data are consistent with the latter dual-process model in which food deprivation engages both inhibitory and excitatory regulation to titrate pumping to a lower, off-food, rate.
Evidence for major inhibitory signals required to suppress pharyngeal pumping in the absence of food comes from the mutant unc-31, which pumps at a rate 5 times greater than wild-type off food. This can be explained by unc-31–dependent pathways being activated by food removal and imposing an inhibitory tone and strongly suggests that removal of the food cue actively suppresses pharyngeal function. unc-31 has previously been reported to pump constitutively when starved (42) and as it plays a selective role in coordinating neuropeptide release (64, 65), it might be predicted that mutants with defective peptidergic signaling would show a similar high level of pharyngeal activity in the absence of food. However, contrary to this expectation, we found that during food deprivation the neuropeptide processing mutants egl-3 and egl-21 pump at a very low frequency if at all.
We have considered the possibility that the antonymous behavior of unc-31 and egl-3 in the absence of food is due to each mutant harboring deficits in distinct inhibitory and excitatory neuropeptides, respectively. egl-3 mutants are deficient in FMRFamide-like and neuropeptide-like peptides, FLPs and NLPs (72), but will likely still express neuropeptides belonging to other families (e.g., the insulin-like peptides). unc-31 on the other hand is involved more broadly in peptidergic signaling with evidence for a role in both FLP and insulin-like peptide secretion (64, 65, 75, 78). This different repertoire of residual neuropeptides in unc-31 and egl-3 may provide an explanation for the differential impact of unc-31 and egl-3 mutations on pharyngeal pumping rate. To test this, we made the double mutant unc-31;egl-3 with the prediction that this would exhibit an intermediary phenotype and pump in a similar manner to wild-type in the absence of food if unc-31 and egl-3 each individually resulted in deficits in inhibitory and excitatory neuropeptides. However, the unc-31;egl-3 double mutant pumped at a very low rate off food, similar to egl-3. This epistatic relationship suggests a genetic model in which a distinct inhibitory unc-31-dependent signal functions upstream of an excitatory egl-3-dependent activation of pharyngeal pumping.
We found that the constitutive pumping of unc-31 was phenocopied by the glutamate-deficient mutant eat-4, suggesting that the constitutive pumping in unc-31 may be due to loss of glutamate signaling. This suggestion is based on previous studies showing that the mammalian ortholog of unc-31, CAPS, has a critical role in the vesicle-mediated release of glutamate (79). To test this, we made the double unc-31;eat-4 mutant with the prediction that this would result in no further increase in pumping rate off food. This double mutant pumped at a slightly lower rate than wild-type, similar to eat-4 in the presence of food. Remarkably, in the absence of food, there was no further reduction in pumping rate; it pumps at such a high rate as though the worm has not recognized the absence of food in the environment. Thus the high pumping rate phenotype of unc-31 and eat-4 mutants in the absence of food is additive. Therefore, it is clear that the constitutive pumping of unc-31 cannot be explained by a deficit in glutamate signaling. Furthermore, the phenotype of the unc-31 and eat-4 double mutants, in which they appear to be unreceptive to food removal in terms of their pharyngeal pumping rate, indicate that together these signaling pathways are required to suppress pharyngeal pumping in the absence of food. To further test the relationship between unc-31, egl-3, and eat-4 in regulating pumping off food we tested the double mutant eat-4;egl-3. The double mutant pumped in a similar manner to wild-type off food, and this leads us to suggest that 2 distinct pathways, which have unc-31/egl-3 and eat-4 dependence, respectively, drive inhibition of pumping when worms are off food.
The observation that the neuropeptide-deficient mutants egl-3 and egl-21 pump considerably less than wild-type, particularly during food deprivation, is important as it demonstrates a net excitatory neuropeptidergic drive for pharyngeal pumping both in the presence and absence of food. egl-3 mutants are deficient in over 65 neuropeptides (72), and further work will be required to determine which peptides promote feeding. In electrophysiological recordings from isolated pharynx, a number of neuropeptides were shown to modulate pharyngeal pumping (37, 38). The most potent excitatory neuropeptides are FLP-17A, FLP-17B, and FLP-8. The encoding genes, flp-17 and flp-8, have a limited expression pattern; flp-17 is expressed in the sensory BAG neuron and in the pharyngeal neuron M5, and flp-8 is expressed in sensory neurons ASEL/R, URXL/R, and PVM (33). Our ablations of the RIP interneurons suggest that anatomically mediated point-to-point communication from outside the pharyngeal system is not needed for changes in state-dependent pharyngeal pumping. Taken together, these results suggest an important control from within the pharynx or from external neural pathways acting through volume transmission. Thus, FLP-17A and FLP-17B appear to be good candidates for the neuropeptides endogenous to the pharynx that could promote feeding and fictive feeding as only these peptides are present within the pharyngeal circuit. In addition, both FLP-17 and FLP-8 could act in a neurohormonal fashion to increase pumping rate in the presence and absence of food. Of note in this regard is a recent report of an RNA interference screen for neuropeptides involved in regulating feeding, which has provided evidence for an excitatory opioid-like peptide NLP-24 (80). There are also a number of inhibitory neuropeptides (38), and the scenario could be more complex with the net effect on pharyngeal pumping being determined by the summation of the effects of both inhibitory and excitatory peptides.
In addition to providing insight into the complex regulation of pharyngeal activity in the absence of food, our analysis highlights distinct differences in the neural regulation of feeding behavior on and off food. Thus, although acetylcholine has a core excitatory role both on and off food, the role of glutamate and 5-HT are context dependent, being excitatory in the presence of food but inhibitory in the absence of food.
In summary, our observations show that food removal is actively transduced to inhibit feeding (Fig. 7). Our analysis suggests the worm perceives the removal of food with 2 important consequences. The first is the activation of inhibitory pathways to suppress pumping. Importantly, our genetic analyses argue for 2 pathways, unc-31 and eat-4, acting independently of each other. In addition, our observations with respect to unc-31 cannot readily be explained by its previously reported contributions to either neuropeptide or glutamatergic signaling. Remarkably, in the absence of both unc-31– and eat-4–dependent suppression of pharyngeal pumping, worms pump at the same rate on and off food. In addition to this suppression of pumping, there is an excitatory cholinergic and peptidergic drive. In the absence of food, the peptidergic component is more important in sustaining pumping than on food. This cue is independent of glutamate signaling but appears to lie downstream of unc-31.
In C. elegans, therefore, as in mammalian systems, there is a basic framework for the regulation of feeding behavior in which local and hormonal excitatory and inhibitory systems converge to fine-tune food intake in a context-dependent manner (2). This highlights evolutionary conservation of regulation of this basic animal drive and supports C. elegans as a valuable model in defining the complex way that higher organisms respond to food.
Acknowledgments
The authors are grateful to Richard Komuniecki for providing the C. elegans eat-4;egl-3 double mutant. N.D. and Z.L. were supported by Ph.D. studentships funded by the Gerald Kerkut Charitable Trust. C. elegans strains were provided by the C. elegans Genetics Center, which is funded by U.S. National Institutes of Health Office of Research Infrastructure Programs (P40 OD010440).
Glossary
- 5-HT
hydroxytryptamine
- CAPS
calcium-activated protein for secretion
- GFP
green fluorescent protein
- FLP
FMRFamide-like peptide
- NLP
neuropeptide-like peptide
REFERENCES
- 1.Friedman J. M. (2010) A tale of two hormones. Nat. Med. 16, 1100–1106 [DOI] [PubMed] [Google Scholar]
- 2.Aponte Y., Atasoy D., Sternson S. M. (2011) AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat. Neurosci. 14, 351–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedman J. M. (2009) Obesity: causes and control of excess body fat. Nature 459, 340–342 [DOI] [PubMed] [Google Scholar]
- 4.Greer E. R., Pérez C. L., Van Gilst M. R., Lee B. H., Ashrafi K. (2008) Neural and molecular dissection of a C. elegans sensory circuit that regulates fat and feeding. Cell Metab. 8, 118–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chalasani S. H., Chronis N., Tsunozaki M., Gray J. M., Ramot D., Goodman M. B., Bargmann C. I. (2007) Dissecting a circuit for olfactory behaviour in Caenorhabditis elegans. Nature 450, 63–70 [DOI] [PubMed] [Google Scholar]
- 6.Gray J. M., Hill J. J., Bargmann C. I. (2005) A circuit for navigation in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 102, 3184–3191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pierce-Shimomura J. T., Morse T. M., Lockery S. R. (1999) The fundamental role of pirouettes in Caenorhabditis elegans chemotaxis. J. Neurosci. 19, 9557–9569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ward S. (1973) Chemotaxis by the nematode Caenorhabditis elegans: identification of attractants and analysis of the response by use of mutants. Proc. Natl. Acad. Sci. USA 70, 817–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Werner K. M., Perez L. J., Ghosh R., Semmelhack M. F., Bassler B. L. (2014) Caenorhabditis elegans recognizes a bacterial quorum-sensing signal molecule through the AWCON neuron. J. Biol. Chem. 289, 26566–26573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sawin E. R., Ranganathan R., Horvitz H. R. (2000) C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron 26, 619–631 [DOI] [PubMed] [Google Scholar]
- 11.Flavell S. W., Pokala N., Macosko E. Z., Albrecht D. R., Larsch J., Bargmann C. I. (2013) Serotonin and the neuropeptide PDF initiate and extend opposing behavioral states in C. elegans. Cell 154, 1023–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Bono M., Maricq A. V. (2005) Neuronal substrates of complex behaviors in C. elegans. Annu. Rev. Neurosci. 28, 451–501 [DOI] [PubMed] [Google Scholar]
- 13.Hills T., Brockie P. J., Maricq A. V. (2004) Dopamine and glutamate control area-restricted search behavior in Caenorhabditis elegans. J. Neurosci. 24, 1217–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shingai R. (2000) Durations and frequencies of free locomotion in wild type and GABAergic mutants of Caenorhabditis elegans. Neurosci. Res. 38, 71–84 [DOI] [PubMed] [Google Scholar]
- 15.Tsalik E. L., Hobert O. (2003) Functional mapping of neurons that control locomotory behavior in Caenorhabditis elegans. J. Neurobiol. 56, 178–197 [DOI] [PubMed] [Google Scholar]
- 16.Chalasani S. H., Kato S., Albrecht D. R., Nakagawa T., Abbott L. F., Bargmann C. I. (2010) Neuropeptide feedback modifies odor-evoked dynamics in Caenorhabditis elegans olfactory neurons. Nat. Neurosci. 13, 615–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Avery L., Shtonda B. B. (2003) Food transport in the C. elegans pharynx. J. Exp. Biol. 206, 2441–2457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song B. M., Avery L. (2012) Serotonin activates overall feeding by activating two separate neural pathways in Caenorhabditis elegans. J. Neurosci. 32, 1920–1931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song B. M., Faumont S., Lockery S., Avery L. (2013) Recognition of familiar food activates feeding via an endocrine serotonin signal in Caenorhabditis elegans. eLife 2, e00329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franks C. J., Holden-Dye L., Bull K., Luedtke S., Walker R. J. (2006) Anatomy, physiology and pharmacology of Caenorhabditis elegans pharynx: a model to define gene function in a simple neural system. Invert. Neurosci. 6, 105–122 [DOI] [PubMed] [Google Scholar]
- 21.Shtonda B. B., Avery L. (2006) Dietary choice behavior in Caenorhabditis elegans. J. Exp. Biol. 209, 89–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Albertson D. G., Thomson J. N. (1976) The pharynx of Caenorhabditis elegans. Philos. Trans. R. Soc. Lond. B Biol. Sci. 275, 299–325 [DOI] [PubMed] [Google Scholar]
- 23.Avery L., Thomas J. H. (1997) Feeding and defecation. In C. elegans II (Riddle D. L., Blumenthal T., Meyer B. J., and Priess J. R., eds.), pp. 679–716, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, USA: [PubMed] [Google Scholar]
- 24.Chalfie M., Sulston J. E., White J. G., Southgate E., Thomson J. N., Brenner S. (1985) The neural circuit for touch sensitivity in Caenorhabditis elegans. J. Neurosci. 5, 956–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raizen D. M., Lee R. Y., Avery L. (1995) Interacting genes required for pharyngeal excitation by motor neuron MC in Caenorhabditis elegans. Genetics 141, 1365–1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niacaris T., Avery L. (2003) Serotonin regulates repolarization of the C. elegans pharyngeal muscle. J. Exp. Biol. 206, 223–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rogers C. M., Franks C. J., Walker R. J., Burke J. F., Holden-Dye L. (2001) Regulation of the pharynx of Caenorhabditis elegans by 5-HT, octopamine, and FMRFamide-like neuropeptides. J. Neurobiol. 49, 235–244 [DOI] [PubMed] [Google Scholar]
- 28.Raizen D. M., Avery L. (1994) Electrical activity and behavior in the pharynx of Caenorhabditis elegans. Neuron 12, 483–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hobson R. J., Hapiak V. M., Xiao H., Buehrer K. L., Komuniecki P. R., Komuniecki R. W. (2006) SER-7, a Caenorhabditis elegans 5-HT7-like receptor, is essential for the 5-HT stimulation of pharyngeal pumping and egg laying. Genetics 172, 159–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Z., Li Y., Yi Y., Huang W., Yang S., Niu W., Zhang L., Xu Z., Qu A., Wu Z., Xu T. (2012) Dissecting a central flip-flop circuit that integrates contradictory sensory cues in C. elegans feeding regulation. Nat. Commun. 3, 776 [DOI] [PubMed] [Google Scholar]
- 31.Cunningham K. A., Hua Z., Srinivasan S., Liu J., Lee B. H., Edwards R. H., Ashrafi K. (2012) AMP-activated kinase links serotonergic signaling to glutamate release for regulation of feeding behavior in C. elegans. Cell Metab. 16, 113–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Avery L., Horvitz H. R. (1989) Pharyngeal pumping continues after laser killing of the pharyngeal nervous system of C. elegans. Neuron 3, 473–485 [DOI] [PubMed] [Google Scholar]
- 33.Li C., Kim K., Nelson L. S. (1999) FMRFamide-related neuropeptide gene family in Caenorhabditis elegans. Brain Res. 848, 26–34 [DOI] [PubMed] [Google Scholar]
- 34.Li C., Nelson L. S., Kim K., Nathoo A., Hart A. C. (1999) Neuropeptide gene families in the nematode Caenorhabditis elegans. Ann. N. Y. Acad. Sci. 897, 239–252 [DOI] [PubMed] [Google Scholar]
- 35.Nathoo A. N., Moeller R. A., Westlund B. A., Hart A. C. (2001) Identification of neuropeptide-like protein gene families in Caenorhabditiselegans and other species. Proc. Natl. Acad. Sci. USA 98, 14000–14005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Papaioannou S., Holden-Dye L., Walker R. J. (2008) The actions of Caenorhabditis elegans neuropeptide-like peptides (NLPs) on body wall muscle of Ascaris suum and pharyngeal muscle of C. elegans. Acta Biol. Hung. 59(Suppl), 189–197 [DOI] [PubMed] [Google Scholar]
- 37.Papaioannou S., Holden-Dye L., Walker R. J. (2008) Evidence for a role for cyclic AMP in modulating the action of 5-HT and an excitatory neuropeptide, FLP17A, in the pharyngeal muscle of Caenorhabditis elegans. Invert. Neurosci. 8, 91–100 [DOI] [PubMed] [Google Scholar]
- 38.Papaioannou S., Marsden D., Franks C. J., Walker R. J., Holden-Dye L. (2005) Role of a FMRFamide-like family of neuropeptides in the pharyngeal nervous system of Caenorhabditis elegans. J. Neurobiol. 65, 304–319 [DOI] [PubMed] [Google Scholar]
- 39.Horvitz H. R., Chalfie M., Trent C., Sulston J. E., Evans P. D. (1982) Serotonin and octopamine in the nematode Caenorhabditis elegans. Science 216, 1012–1014 [DOI] [PubMed] [Google Scholar]
- 40.Avery L., Horvitz H. R. (1990) Effects of starvation and neuroactive drugs on feeding in Caenorhabditis elegans. J. Exp. Zool. 253, 263–270 [DOI] [PubMed] [Google Scholar]
- 41.Ann K., Kowalchyk J. A., Loyet K. M., Martin T. F. J. (1997) Novel Ca2+-binding protein (CAPS) related to UNC-31 required for Ca2+-activated exocytosis. J. Biol. Chem. 272, 19637–19640 [DOI] [PubMed] [Google Scholar]
- 42.Avery L., Bargmann C. I., Horvitz H. R. (1993) The Caenorhabditis elegans unc-31 gene affects multiple nervous system-controlled functions. Genetics 134, 455–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Keane J., Avery L. (2003) Mechanosensory inputs influence Caenorhabditis elegans pharyngeal activity via ivermectin sensitivity genes. Genetics 164, 153–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sze J. Y., Victor M., Loer C., Shi Y., Ruvkun G. (2000) Food and metabolic signalling defects in a Caenorhabditis elegans serotonin-synthesis mutant. Nature 403, 560–564 [DOI] [PubMed] [Google Scholar]
- 45.Dwyer D. S., Aamodt E. J. (2013) Insulin/IGF-1 signaling, including class II/III PI3Ks, β-arrestin and SGK-1, is required in C. elegans to maintain pharyngeal muscle performance during starvation. PLoS One 8, e63851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.You Y. J., Kim J., Raizen D. M., Avery L. (2008) Insulin, cGMP, and TGF-β signals regulate food intake and quiescence in C. elegans: a model for satiety. Cell Metab. 7, 249–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brenner S. (1974) The genetics of Caenorhabditis elegans. Genetics 77, 71–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fang-Yen, C., Gabel, C. V., Samuel, A. D. T., Bargmann, C. I., and Avery, L. (2012) Laser microsurgery in Caenorhabditis elegans Methods Cell Biol 107, 177–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walker D. S., Gower N. J. D., Ly S., Bradley G. L., Baylis H. A. (2002) Regulated disruption of inositol 1,4,5-trisphosphate signaling in Caenorhabditis elegans reveals new functions in feeding and embryogenesis. Mol. Biol. Cell 13, 1329–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee R. Y. N., Sawin E. R., Chalfie M., Horvitz H. R., Avery L. (1999) EAT-4, a homolog of a mammalian sodium-dependent inorganic phosphate cotransporter, is necessary for glutamatergic neurotransmission in caenorhabditis elegans. J. Neurosci. 19, 159–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Avery L. (1993) Motor neuron M3 controls pharyngeal muscle relaxation timing in Caenorhabditis elegans. J. Exp. Biol. 175, 283–297 [DOI] [PubMed] [Google Scholar]
- 52.Alfonso A., Grundahl K., Duerr J. S., Han H. P., Rand J. B. (1993) The Caenorhabditis elegans unc-17 gene: a putative vesicular acetylcholine transporter. Science 261, 617–619 [DOI] [PubMed] [Google Scholar]
- 53.Dent J. A., Davis M. W., Avery L. (1997) avr-15 encodes a chloride channel subunit that mediates inhibitory glutamatergic neurotransmission and ivermectin sensitivity in Caenorhabditis elegans. EMBO J. 16, 5867–5879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McKay J. P., Raizen D. M., Gottschalk A., Schafer W. R., Avery L. (2004) eat-2 and eat-18 are required for nicotinic neurotransmission in the Caenorhabditis elegans pharynx. Genetics 166, 161–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sugiura M., Fuke S., Suo S., Sasagawa N., Van Tol H. H. M., Ishiura S. (2005) Characterization of a novel D2-like dopamine receptor with a truncated splice variant and a D1-like dopamine receptor unique to invertebrates from Caenorhabditis elegans. J. Neurochem. 94, 1146–1157 [DOI] [PubMed] [Google Scholar]
- 56.Packham R., Walker R. J., Holden-Dye L. (2010) The effect of a selective octopamine antagonist, epinastine, on pharyngeal pumping in Caenorhabditis elegans. Invert. Neurosci. 10, 47–52 [DOI] [PubMed] [Google Scholar]
- 57.Alkema M. J., Hunter-Ensor M., Ringstad N., Horvitz H. R. (2005) Tyramine functions independently of octopamine in the Caenorhabditis elegans nervous system. Neuron 46, 247–260 [DOI] [PubMed] [Google Scholar]
- 58.Calvo A. C., Pey A. L., Miranda-Vizuete A., Døskeland A. P., Martinez A. (2011) Divergence in enzyme regulation between Caenorhabditis elegans and human tyrosine hydroxylase, the key enzyme in the synthesis of dopamine. Biochem. J. 434, 133–141 [DOI] [PubMed] [Google Scholar]
- 59.McIntire S. L., Jorgensen E., Kaplan J., Horvitz H. R. (1993) The GABAergic nervous system of Caenorhabditis elegans. Nature 364, 337–341 [DOI] [PubMed] [Google Scholar]
- 60.Noble T., Stieglitz J., Srinivasan S. (2013) An integrated serotonin and octopamine neuronal circuit directs the release of an endocrine signal to control C. elegans body fat. Cell Metab. 18, 672–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Suo S., Culotti J. G., Van Tol H. H. M. (2009) Dopamine counteracts octopamine signalling in a neural circuit mediating food response in C. elegans. EMBO J. 28, 2437–2448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yoshida M., Oami E., Wang M., Ishiura S., Suo S. (2014) Nonredundant function of two highly homologous octopamine receptors in food-deprivation-mediated signaling in Caenorhabditis elegans. J. Neurosci. Res. 92, 671–678 [DOI] [PubMed] [Google Scholar]
- 63.Berwin B., Floor E., Martin T. F. J. (1998) CAPS (mammalian UNC-31) protein localizes to membranes involved in dense-core vesicle exocytosis. Neuron 21, 137–145 [DOI] [PubMed] [Google Scholar]
- 64.Speese S., Petrie M., Schuske K., Ailion M., Ann K., Iwasaki K., Jorgensen E. M., Martin T. F. J. (2007) UNC-31 (CAPS) is required for dense-core vesicle but not synaptic vesicle exocytosis in Caenorhabditis elegans. J. Neurosci. 27, 6150–6162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sieburth D., Madison J. M., Kaplan J. M. (2007) PKC-1 regulates secretion of neuropeptides. Nat. Neurosci. 10, 49–57 [DOI] [PubMed] [Google Scholar]
- 66.Peymen K., Watteyne J., Frooninckx L., Schoofs L., Beets I. (2014) The FMRFamide-like peptide family in nematodes. Front. Endocrinol. (Lausanne) 5, 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li C., Kim K. (2014) Family of flp peptides in Caenorhabditis elegans and related nematodes. Front. Endocrinol. (Lausanne) 5, 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lau H. E., Chalasani S. H. (2014) Divergent and convergent roles for insulin-like peptides in the worm, fly and mammalian nervous systems. Invert. Neurosci. 14, 71–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Holden-Dye L., Walker R. J. (2013) The roles of neuropeptides in Caenorhabditis elegans including their importance in the regulation of feeding and metabolism. Protein Pept. Lett. 20, 636–646 [DOI] [PubMed] [Google Scholar]
- 70.Kass J., Jacob T. C., Kim P., Kaplan J. M. (2001) The EGL-3 proprotein convertase regulates mechanosensory responses of Caenorhabditis elegans. J. Neurosci. 21, 9265–9272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jacob T. C., Kaplan J. M. (2003) The EGL-21 carboxypeptidase E facilitates acetylcholine release at Caenorhabditis elegans neuromuscular junctions. J. Neurosci. 23, 2122–2130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Husson S. J., Clynen E., Baggerman G., Janssen T., Schoofs L. (2006) Defective processing of neuropeptide precursors in Caenorhabditis elegans lacking proprotein convertase 2 (KPC-2/EGL-3): mutant analysis by mass spectrometry. J. Neurochem. 98, 1999–2012 [DOI] [PubMed] [Google Scholar]
- 73.Husson S. J., Janssen T., Baggerman G., Bogert B., Kahn-Kirby A. H., Ashrafi K., Schoofs L. (2007) Impaired processing of FLP and NLP peptides in carboxypeptidase E (EGL-21)-deficient Caenorhabditis elegans as analyzed by mass spectrometry. J. Neurochem. 102, 246–260 [DOI] [PubMed] [Google Scholar]
- 74.Mitchell P., Mould R., Dillon J., Glautier S., Andrianakis I., James C., Pugh A., Holden-Dye L., O’Connor V. (2010) A differential role for neuropeptides in acute and chronic adaptive responses to alcohol: behavioural and genetic analysis in Caenorhabditis elegans. PLoS One 5, e10422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Charlie N. K., Schade M. A., Thomure A. M., Miller K. G. (2006) Presynaptic UNC-31 (CAPS) is required to activate the G α(s) pathway of the Caenorhabditis elegans synaptic signaling network. Genetics 172, 943–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zaslaver A., Liani I., Shtangel O., Ginzburg S., Yee L., Sternberg P. W. (2015) Hierarchical sparse coding in the sensory system of Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 112, 1185–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee J. H., Kong J., Jang J. Y., Han J. S., Ji Y., Lee J., Kim J. B. (2014) Lipid droplet protein LID-1 mediates ATGL-1-dependent lipolysis during fasting in Caenorhabditis elegans. Mol. Cell. Biol. 34, 4165–4176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Leinwand S. G., Chalasani S. H. (2013) Neuropeptide signaling remodels chemosensory circuit composition in Caenorhabditis elegans. Nat. Neurosci. 16, 1461–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jockusch W. J., Speidel D., Sigler A., Sørensen J. B., Varoqueaux F., Rhee J.-S., Brose N. (2007) CAPS-1 and CAPS-2 are essential synaptic vesicle priming proteins. Cell 131, 796–808 [DOI] [PubMed] [Google Scholar]
- 80.Cheong M. C., Artyukhin A. B., You Y.-J., Avery L. (2015) An opioid-like system regulating feeding behavior in C. elegans. eLife 4, e06683 [DOI] [PMC free article] [PubMed] [Google Scholar]