Abstract
Idiopathic pulmonary fibrosis is a devastating lung disease with limited treatment options. The signaling molecule adenosine is produced in response to injury and serves a protective role in early stages of injury and is detrimental during chronic stages of disease such as seen in lung conditions such as pulmonary fibrosis. Understanding the association of extracellular adenosine levels and the progression of pulmonary fibrosis is critical for designing adenosine based approaches to treat pulmonary fibrosis. The goal of this study was to use various models of experimental lung fibrosis to understand when adenosine levels are elevated during pulmonary fibrosis and whether these elevations were associated with disease progression and severity. To accomplish this, extracellular adenosine levels, defined as adenosine levels found in bronchioalveolar lavage fluid, were determined in mouse models of resolvable and progressive pulmonary fibrosis. We found that relative bronchioalveolar lavage fluid adenosine levels are progressively elevated in association with pulmonary fibrosis and that adenosine levels diminish in association with the resolution of lung fibrosis. In addition, treatment of these models with dipyridamole, an inhibitor of nucleoside transporters that potentiates extracellular adenosine levels, demonstrated that the resolution of lung fibrosis is blocked by the failure of adenosine levels to subside. Furthermore, exacerbating adenosine levels led to worse fibrosis in a progressive fibrosis model. Increased adenosine levels were associated with elevation of IL-6 and IL-17, which are important inflammatory cytokines in pulmonary fibrosis. These results demonstrate that extracellular adenosine levels are closely associated with the progression of experimental pulmonary fibrosis and that this signaling pathway may mediate fibrosis by regulating IL-6 and IL-17 production.—Luo, F., Le, N.-B., Mills, T., Chen, N.-Y., Karmouty-Quintana, H., Molina, J. G., Davies, J., Philip, K., Volcik, K. A., Liu, H., Xia, Y., Eltzschig, H. K., Blackburn, M. R. Extracellular adenosine levels are associated with the progression and exacerbation of pulmonary fibrosis.
Keywords: bleomycin, dipyridamole, IPF, IL-17, IL-6
Idiopathic pulmonary fibrosis (IPF) is a progressive, restrictive, and deadly lung disease affecting hundreds of thousands of patients in the United States (1). IPF is characterized by damage to alveolar epithelial cells, abnormal tissue repair, formation of fibroblastic foci, and extracellular matrix deposition (2). Patients with IPF exhibit a median survival of 2 to 3 yr after diagnosis (3), and despite decades of research, available treatments are limited (4). Thus, there is an urgent need to better understand the pathogenesis of IPF to aid the development of new treatments for this deadly disease.
We hypothesized that the signaling molecule adenosine is associated with the progression of pulmonary fibrosis and that understanding the association between adenosine elevations and the pathogenesis of fibrotic lung disease will provide important insight into the use of adenosine based therapeutics for the treatment of IPF (5). Extracellular adenosine levels become elevated after tissues injury where they mediate cellular activity by engaging cell surface adenosine receptors (6, 7). Adenosine signaling events serve to orchestrate different responses in acute and chronic tissue injury settings (8, 9). In acute injury, adenosine inhibits inflammation, protects from hypoxia- and ischemia-induced tissue damage, and diminishes the release of inflammatory cytokines (10–13). However, in chronic injury, adenosine is detrimental by promoting excessive tissue repair and fibrotic responses (14, 15). Work from our laboratory has shown that inhibiting adenosine signaling during active lung fibrosis can attenuate fibrotic injury in the lung (14, 15). However, there is an incomplete understanding of when during the progression of fibrotic lung disease adenosine levels become elevated and whether or not adenosine elevations are associated with the degree of pulmonary fibrosis. Understanding these parameters is particularly important given the diverse protective and detrimental roles of this signaling pathway and the need to determine when activation or blockade of the adenosine signaling pathway would be useful for the treatment of pulmonary fibrosis.
The goal of this study was to use various models of experimental lung fibrosis to understand when adenosine levels are elevated during pulmonary fibrosis and whether these elevations were associated with disease progression and severity. Exposing mice to a single intratracheal injection with bleomycin (BLM) results in progressive lung fibrosis, followed by fibrotic resolution (16). In contrast, repeated intraperitoneal injections of BLM result in progressive pulmonary fibrosis that is not resolvable (17, 18). Using these models, we found that extracellular adenosine levels are progressively elevated in association with pulmonary fibrosis and that adenosine levels diminish in association with the resolution of lung fibrosis. In addition, treatment of these models with dipyridamole (DP), an inhibitor of nucleoside transporters that potentiates extracellular adenosine levels (19), demonstrated that the resolution of lung fibrosis is blocked by the failure of adenosine levels to subside, and exacerbating adenosine levels leads to worse fibrosis. Together, these studies provide the first in vivo evidence that extracellular adenosine levels are associated with the progression and severity of pulmonary fibrosis.
MATERIALS AND METHODS
Animals
Wild-type C57BL/6 female mice (8–10 wk old) and male mice (4–5 wk old) were purchased from Harlan Industries (Indianapolis, IN, USA). Maintenance and care of mice were in accordance with guidelines set by the Animal Welfare Committee at the University of Texas Health Science Center at Houston. All the experiments were reviewed and approved by the University of Texas Health Science Center at Houston Animal Welfare Committee.
BLM and DP administration
To examine resolvable fibrosis, wild-type C57BL/6 female mice were exposed to BLM (2.5 U/kg) (Teva Pharmaceuticals, North Wales, PA, USA) by oropharyngeal aspiration as previously described (20). Briefly, mice were anesthetized by isoflurane gas (3.5 lpm) with 2 lpm oxygen flow. A total of 50 μl of BLM or PBS were pipetted into the pharynx. Mice were humanely killed at the indicated time points. For the examination of progressive fibrosis, wild-type C57BL/6 male mice were administered BLM (0.035 U/g) twice a week for 33 d by intraperitoneal injection as previously described (18). Mice were humanely killed at the indicated time points. For dipuridamole (DP) injection, DP (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in corn oil with 10% ethanol and 2% of DMSO. Starting from the indicated time points, DP and its vehicle control were administered by intraperitoneal injection every 12 h until animals were killed.
Bronchoalveolar lavage fluid, cell differentials, and histology
Bronchoalveolar lavage fluid (BALF) was collected as previously described (21). Briefly, mice were anesthetized and their lungs were lavaged 4 times with 0.3 ml PBS containing a cocktail to prevent the metabolism of extracellular adenosine. The cocktail consisted of 10 µM DP (Sigma-Aldrich), 10 µM ADA-inhibitor deoxycoformycin (R&D Systems, Minneapolis, MN, USA), and 10 µM αβ-methylene ADP (Sigma-Aldrich). A 50 μl aliquot was separated for cell quantification with a hemocytometer. The rest of the BALF was centrifuged (3000 g × 5 min) and the supernatant collected and stored for further analysis. Cell pallets were resuspended and cytospun onto microscope slides. After the lavage, lungs were pressure inflated, fixed in 10% formalin, and embedded in paraffin. Five-micrometer sections were cut for histologic analysis.
Adenosine measurement
Adenosine levels in BALF were measured as previously described (22). One hundred microliters of BALF supernatant was analyzed by HPLC where adenosine peaks were identified and quantified using known external standard curves.
Arterial oxygen saturation
Arterial oxygen saturation was measured as previously described (18). Neck hair was shaved and a collar clip light sensor was set up on conscious mice. Pulse MouseOx software analysis (Starr Life Sciences, Oakmont, PA, USA) was used to measure and record the real-time oxygen saturation percent of functional arterial hemoglobin following the manufacturer’s instructions.
Real-time quantitative PCR
Total RNA was isolated from frozen mouse lung tissues using Trizol reagent (Life Technologies, Grand Island, NY, USA). RNA samples were then treated with DNAse (ArticZymes, Tromsø, Norway) and cDNAs were prepared using an iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA). Real-time quantitative PCR (RT-qPCR) was performed on a LightCycler 96 system (Roche Life Science, Branford, CT, USA) using SYBR green (Roche Life Science). Primers sequences for specific genes are as follows: m-collagen-1, forward: GCCAAGAAGACATCCCTGAAG, reverse: TCATTGCATTGCACGTCATC; m-fibronectin, forward: GCTCAGCAAATCGTGCAGC, reverse: CTAGGTAGGTCCGTTCCCACT; m-IL-6, forward: TAGTCCTTCCTACCCCAATTTCC, reverse: TTGGTCCTTAGCCACTCCTTC; m-TNF-α, forward: CACCACCATCAAGGACTCAA, reverse: TCCAGCCTCATTCTGAGACA; and m-Tbet, forward: GCCAGGGAACCGCTTATATG, reverse: GACGATCATCTGGGTCACATTGT.
ELISA
IL-17 was quantified in protein lysates prepared from mouse lungs using a mouse IL-17 DuoSet ELISA kit (R&D Systems). Protein lysates (10 µl) were diluted in reagent diluent (90 µl) and subjected to ELISA analysis based on the manufacturer’s instructions.
Western blot analysis
Mouse lung tissues were pulverized and protein was extracted using RIPA lysis buffer (50 mM Tris-HCl PH 7.4, 150 mM NaCl, 1% NP-40) containing a protease inhibitor cocktail (Thermo Fisher Scientific, Waltham, MA, USA). Thirty micrograms of protein from each sample was loaded onto 4–12% Tris-glycine SDS polyacrylamide gels (Bio-Rad) for electrophoresis and then transferred onto PVDF membranes (GE Healthcare, Life Sciences, Pittsburgh, PA, USA). Membranes were then blocked with 5% nonfat milk for 1 h at room temperature, washed with Tris-buffered saline–Tween 20, and incubated with primary fibronectin antibodies (1:2000 rabbit; Sigma-Aldrich) or anti–glyceraldehyde phosphate dehydrogenase antibodies (1:20,000 mouse; Life Technologies) overnight at 4°C. Membranes were then washed, incubated with corresponding secondary antibodies conjugated to horseradish peroxidase (Cell Signaling Technology, Danvers, MA, USA) for 1 h at room temperature, and developed with Amersham ECL Prime Western Blotting Detection Reagent (GE Healthcare, Life Sciences).
Histology and fibrosis assessment
Mouse lungs were inflated with 10% buffered formalin and fixed at 4°C overnight. Lungs were dehydrated and embedded in paraffin, and 5 mm tissue sections were collected on microscope slides. Masson trichrome staining (EM Science, Gibbstown, NJ, USA) was performed according to the manufacturer’s instructions. Stained slides were analyzed using a modified Ashcroft scale optimized for mouse lung sections (18).
Hydroxyproline assay
Frozen lung tissue was pulverized, and a hydroxyproline colorimetric assay kit (BioVision, San Francisco, CA, USA) was used to assess collagen quantification. Briefly, pulverized lung tissues were rehydrated in 100 µl of dH2O for every 10 mg of samples and then incubated with the same volume of 12 N HCl at 120°C for 3 h. Next, samples were centrifuged at 10,000 g × 3 min, and the supernatant was collected for further analysis following the manufacturer’s protocol.
Statistical analysis
Statistical analysis was conducted with the Student’s t test and 1-way ANOVA, followed by Sidak’s multiple comparison in GraphPad Prism 6.0 software (GraphPad Software, La Jolla, CA, USA). Statistical significance was defined as P < 0.05.
RESULTS
Characterization of resolvable and progressive lung fibrosis
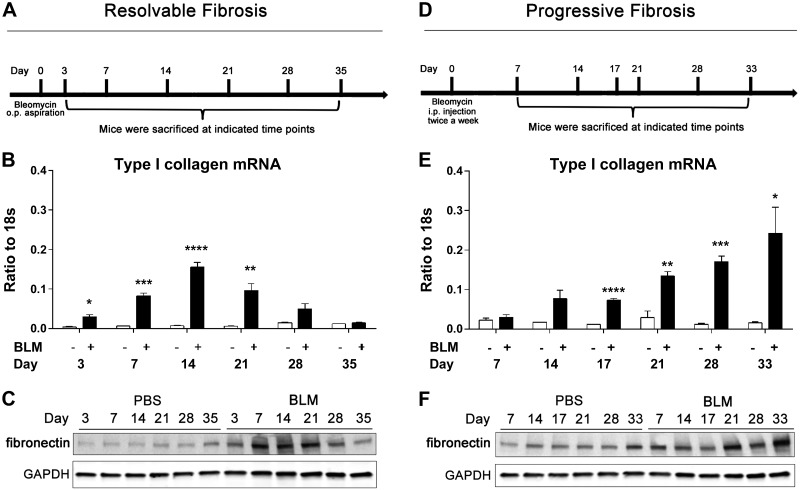
We began by characterizing 2 models of BLM-induced lung fibrosis models designed to exhibit resolvable of progressive pulmonary fibrosis. Mice were exposed to a single oropharyngeal aspiration of BLM and were killed at the indicated time points (Fig. 1A). Assessment of fibrosis revealed that type I α-1 collagen mRNA expression was elevated and reached peak levels at d 14 and decreased to control levels by d 35 (Fig. 1B). In addition, fibronectin protein expression was up-regulated from d 3 to 21 and recovered to control levels from d 28 to 35 (Fig. 1C). BALF cell differentials demonstrated that macrophage, neutrophil, and lymphocyte cell numbers were initially up-regulated and then gradually decreased to control levels (Supplemental Fig. 1A–C). These findings are consistent with previous studies demonstrating that a single-dose of BLM induces lung fibrosis that is resolvable (23, 24).
Figure 1.
Resolvable and progressive fibrosis induced by BLM. A) Wild-type C57BL/6 female mice (n = 5) were exposed to BLM (2.5 U/kg) by oropharyngeal aspiration. Mice were killed at indicated time points after exposure. B) Collagen 1–α1 mRNA expression measured by RT-qPCR. C) Western blot analysis of fibronectin expression in whole lung protein lysates. D) Wild-type C57BL/6 male mice (n = 5) were administered BLM (0.035 U/g) by intraperitoneal injection 2 times per week. Mice were killed at described time points after injection. E) Collagen 1–α1 mRNA expression examined by RT-qPCR. F) Western blot analysis of fibronectin expression in whole lung protein lysates. RT-qPCR data are presented as means ± sem. *P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.0001 for difference between control and BLM groups. Western blot analysis data are representative of n = 5 mice from each group.
To induce progressive and nonresolvable lung fibrosis, mice were injected intraperitoneally with BLM twice a week and were killed at various time points (Fig. 1D). Assessment of fibrosis revealed that type I α-1 collagen mRNA expression and fibronectin protein expression were progressively up-regulated from d 17 to 33 (Fig. 1E, F). Furthermore, BALF cell differentials revealed a persistent increase of macrophages, neutrophils, and lymphocytes (Supplemental Fig. 1E, F). These findings highlight the progressive nature of fibrosis in this model.
BALF adenosine levels were associated with lung fibrosis
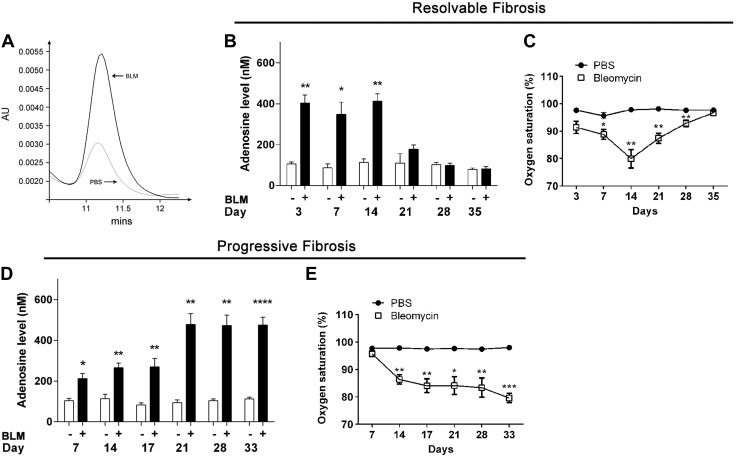
In our previous studies, we identified that whole lung tissue adenosine levels are elevated in models of chronic lung disease (14, 15). However, the level of extracellular adenosine and its association with the progression of fibrosis has not been investigated. To address this knowledge gap, we measured levels of extracellular adenosine, as defined by relative adenosine levels in BALF, gathered from our mouse models of resolvable and progressive pulmonary fibrosis. In the resolvable fibrosis model, BALF adenosine levels were elevated from d 3 to 14 and decreased from d 21 to 35 (Fig. 2A). In the progressive fibrosis model, there was an up-regulation of BALF adenosine from d 7, and the levels remained elevated throughout the experiment until d 33 (Fig. 2B). In contrast, arterial oxygen saturation, which is used as a measurement of lung functionality, demonstrated reduced oxygen saturation from d 3 to 14 and increased levels from d 21 to 35 (Fig. 2C). This was consistent with the continuous decrease in arterial oxygen saturation from d 7 to 33 (Fig. 2D). These findings, together with the examination of fibrotic metrics shown in Fig. 1, indicate that BALF adenosine elevations are associated with the degree of pulmonary fibrosis seen in these models of pulmonary fibrosis.
Figure 2.
BALF adenosine levels and arterial oxygen saturation in resolvable and progressive fibrosis models. A) Wild-type C57BL/6 female mice (n = 5) were exposed to BLM (2.5 U/kg) by oropharyngeal aspiration and then killed at indicated time points. BALF was collected and adenosine levels examined by HPLC. B) Arterial oxygen saturation in mice. C) Wild-type C57BL/6 male mice (n = 5) were exposed to BLM (0.035 U/g) by intraperitoneal injection 2 times per week and then killed at indicated time points. BALF was collected and adenosine levels examined by HPLC. D) Arterial oxygen saturation in mice. All data are presented as means ± sem. *P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.0001 for difference between control and BLM groups.
DP exacerbated pulmonary fibrosis
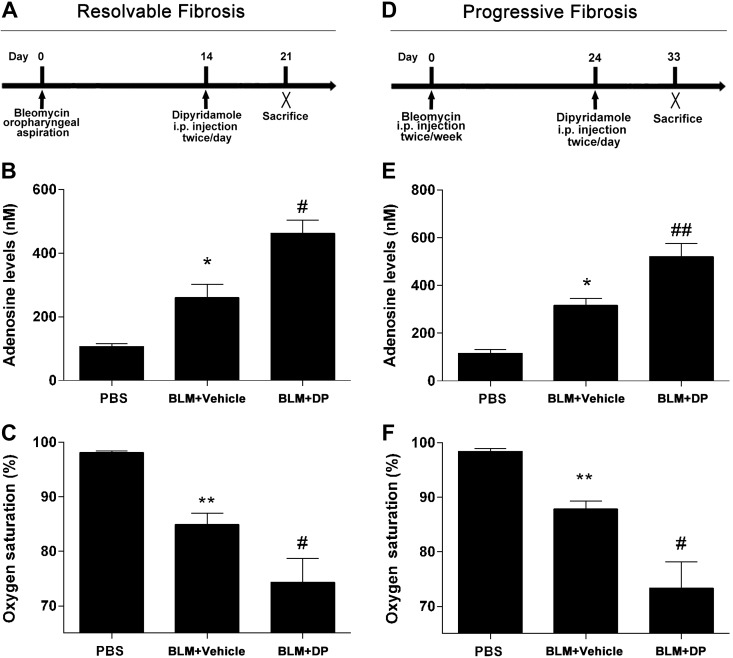
To further investigate the association between extracellular adenosine levels and pulmonary fibrosis, we injected mice exposed to BLM with DP (5 mg/kg), an inhibitor of extracellular adenosine uptake known to increase extracellular adenosine levels (19). In the resolvable model of pulmonary fibrosis, treatment with DP prevented the decrease in BALF adenosine levels normally seen during stages of resolution (Fig. 3A, B). Interestingly, this exacerbation of adenosine levels was associated with further reduced mouse arterial oxygen saturation (Fig. 3C) as opposed to improvements seen with resolution (Fig. 2C). DP injection in the progressive model of pulmonary fibrosis resulted in an up-regulation of BALF adenosine levels and repressed mouse arterial oxygen saturation (Fig. 3D, E). Furthermore, BALF cell differentiation indicated that DP increased the levels of neutrophils and lymphocytes but did not affect the number of macrophages (Supplemental Fig. 2).
Figure 3.
BALF adenosine levels and diminished arterial oxygen saturation after DP treatment. A) Wild-type C57BL/6 female mice (n = 5) were exposed to BLM (2.5 U/kg) by oropharyngeal aspiration. DP (5 mg/kg) and its vehicle control were administered by intraperitoneal injection 2 times per day after 14 d of BLM exposure. Mice were killed at d 21. B) BALF adenosine levels examined by HPLC. C) Arterial oxygen saturation in mice. D) Wild-type C57BL/6 male mice (n = 5) were administered BLM (0.035 U/g) by intraperitoneal injection 2 times per week. DP (5 mg/kg), and its vehicle control was administered by intraperitoneal injection 2 times per day after 24 d of BLM exposure. Mice were killed at d 33. E) Adenosine levels in BALF. F) Arterial oxygen saturation in mice. All data are presented as means ± sem. *P < 0.05, **P < 0.01 for difference between PBS and BLM + vehicle groups; #P < 0.05, ##P < 0.01 for difference between BLM + vehicle and BLM + DP groups.
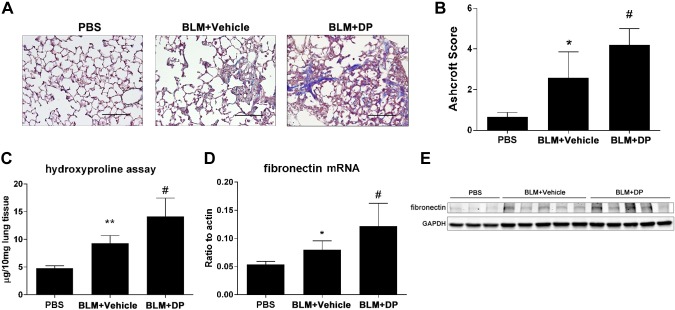
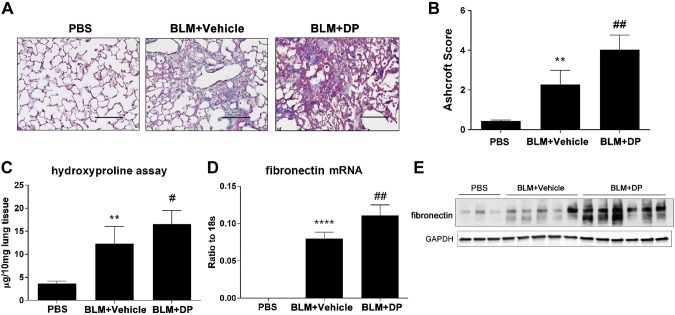
Next, we examined lung fibrosis in these 2 models after DP injection. In the resolution model, DP treatment was associated with heightened pulmonary fibrosis at later stages instead of resolution. This was indicated by Masson trichrome staining of mouse lungs where increased collagen deposition and a more distorted lung structure were evident (Fig. 4A). The overall degree of fibrosis was assessed by Ashcroft scoring (Fig. 4B), and a hydroxyproline assay was used to quantify whole lung collagen levels (Fig. 4C). Furthermore, fibronectin mRNA and protein expression were up-regulated in response to DP treatment (Fig. 4D, E). In the progressive model of pulmonary fibrosis, DP treatment also exacerbated metrics of pulmonary fibrosis, including increased trichrome staining (Fig. 5A), worsened histologic analysis (Fig. 5B), and increased collagen (Fig. 5C) and fibronectin levels (Fig. 5D, E). Collectively, these findings indicated that the degree of pulmonary fibrosis is closely associated with extracellular adenosine levels. Furthermore, preventing the decrease in adenosine levels in the resolution model prevented the resolution of pulmonary fibrosis, indicating a direct role for this signaling model in the promotion of pulmonary fibrosis.
Figure 4.
Exacerbated lung fibrosis in resolvable fibrosis model after DP treatment. Wild-type C57BL/6 female mice (n = 5) were exposed to BLM (2.5 U/kg) by oropharyngeal aspiration. DP (5 mg/kg) and its vehicle control were administered by intraperitoneal injection 2 times per day after 14 d of BLM exposure. Mice were killed at d 21. A) Masson trichrome staining for visualization of collagen deposition (blue). Sections are representative of n = 5 mice from each group. Scale bars, 200 µm. B) Pulmonary fibrosis was evaluated by Ashcroft method. C) Hydroxyproline measurement in lung tissue. D) Fibronectin mRNA expression examined by RT-qPCR. E) Western blot analysis for fibronectin expression in whole lung protein lysate. All data are representative of duplicate repeats of experiments and presented as means ± sem. *P < 0.05, **P < 0.01 for difference between PBS and BLM + vehicle groups; #P < 0.05 for difference between BLM + vehicle and BLM + DP groups.
Figure 5.
Worsened lung fibrosis in progressive fibrosis model after DP treatment. Wild-type C57BL/6 male mice (n = 5) were administered BLM (0.035 U/g) by intraperitoneal injection 2 times per week. DP (5 mg/kg), and its vehicle control was administered by intraperitoneal injection twice a day after 24 d of BLM exposure. Mice were killed at d 33. A) Masson trichrome staining showing collagen deposition (blue). Sections are representative of 5 mice from each group. Scale bars, 200 µm. B) Lung fibrosis was evaluated by Ashcroft method. C) Hydroxyproline measurement in lung tissue. D) Fibronectin mRNA expression examined by RT-qPCR. E) Western blot analysis for fibronectin expression in whole lung protein lysate. All data represent duplicate repeats of experiments and are presented as means ± sem. **P < 0.01, ****P < 0.0001 for difference between PBS and BLM + vehicle groups; #P < 0.05, ##P < 0.01 for difference between BLM + vehicle and BLM + DP groups.
DP treatment increased BLM-induced IL-6 and IL-17 expression
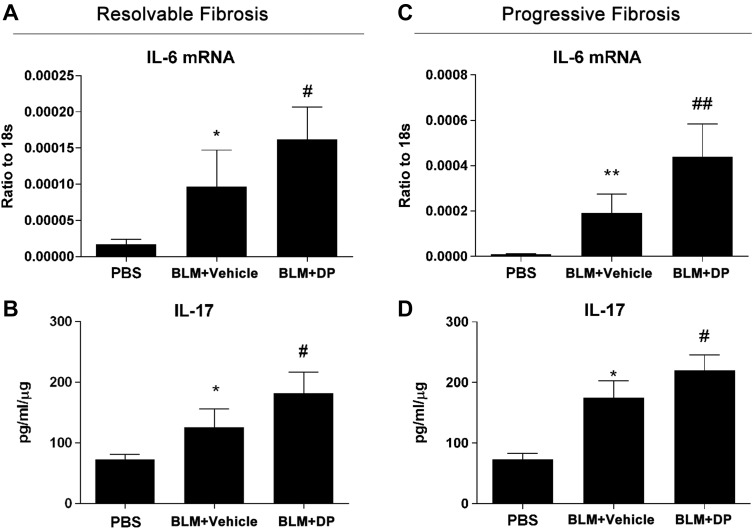
Inflammatory cytokines such as IL-6 and IL-17 play crucial roles in the pathogenesis of pulmonary fibrosis (25, 26). Adenosine has been identified as a crucial mediator for inflammation (13). To further identify the mechanism or mechanisms by which adenosine regulates BLM-induced pulmonary fibrosis, we examined the expression of several key cytokines during both resolvable and progressive fibrotic lung disease. In our resolvable fibrosis model, RT-qPCR showed a robust elevation of IL-6 mRNA expression induced by BLM and a further induction by DP (Fig. 6A). BLM-induced IL-17 expression was also increased by DP (Fig. 6B). Furthermore, TNF-α was associated with the resolution of BLM-induced lung fibrosis (27, 28). DP dampened BLM-induced TNF-α expression, which could explain the persistent fibrosis mediated by DP (Supplemental Fig. 3A). Furthermore, T-bet, a cytokine promoting CD4+ T helper (Th)1 cell proliferation, was found to be induced by BLM yet repressed by DP (Supplemental Fig. 3B). Meanwhile, in the progressive model of pulmonary fibrosis, IL-6 and IL-17 expression was induced by BLM and further elevated by DP (Fig. 6C, D). In summary, these findings suggest that DP treatment and subsequent adenosine elevations could regulate BLM-induced lung fibrosis by affecting the levels of these inflammatory cytokines.
Figure 6.
Enhanced BLM-induced IL-6 and IL-17 expression after DP treatment. Wild-type C57BL/6 male mice (n = 5) were exposed to BLM and DP as described for resolvable and progressive fibrosis models. A, B) In resolvable fibrosis model, IL-6 mRNA and IL-17 expression levels were measured by ELISA. C, D) In progressive fibrosis model, IL-6 mRNA and IL-17 expression levels were measured by ELISA. All data represent duplicate repeats of experiments and are presented as means ± sem. *P < 0.05, **P < 0.01 for difference between PBS and BLM + vehicle groups; #P < 0.05, ##P < 0.01 for difference between BLM + vehicle and BLM + DP groups.
DISCUSSION
Understanding pathways involved in promoting the progression of pulmonary fibrosis could provide insight into the development of novel approaches for the treatment of this deadly condition. The excessive generation of adenosine in chronic injury situations has been suggested to promote excessive tissue remodeling and fibrosis (5). This has been suggested in lung fibrosis (15) as well as other tissues, including liver (29), kidney (30), skin (31, 32), and penis (33, 34). However, the profibrotic effects of adenosine have to be balanced with studies demonstrating that the generation of extracellular adenosine can play a protective role in acute injury situations (35–37). Indeed, in the lung, therapeutic approaches to enhance or block adenosine signaling have shown that activation of adenosine signaling pathways can improve acute lung injury (35, 38, 39), while blockade of adenosine signaling pathways can attenuate aspects of chronic lung disease, including pulmonary fibrosis (14, 40). Thus, there is an important need to understand when adenosine levels become elevated in certain lung disease conditions in order to better guide adenosine-based therapeutic approaches. The focus of this study was to better understand the association between the progression of pulmonary fibrosis and extracellular adenosine levels in the lung. We utilized different models of pulmonary fibrosis as well as DP treatment to exacerbate extracellular adenosine levels. Our results demonstrated that BALF adenosine levels are closely associated with the progression of pulmonary fibrosis. The levels of adenosine presented should be viewed as relative levels as opposed to absolute levels, in that it is difficult to account for the impact of sample dilution when recovering lavage specimens. Furthermore, we found that DP exacerbates BLM-induced pulmonary fibrosis. This is a novel finding that supports previous observations that adenosine is beneficial in acute injury and detrimental in chronic injury, given that DP has previously been shown to only protect against acute injury (41, 42). Taken together, this study provides valuable information on the temporal regulation of extracellular adenosine during the progression of pulmonary fibrosis.
BLM-induced lung injury is a well-established model to simulate lung fibrosis in mice (16). Pulmonary fibrosis induced by a single dose of BLM is resolvable, whereas fibrosis observed in IPF is progressive (23, 24). Therefore, comparing molecular changes between resolution and fibrotic phases provides valuable information to identify important regulators of fibrotic lung disease. We identified that BALF adenosine levels were elevated in mouse lungs as early as 3 d after BLM oropharyngeal aspiration. These adenosine elevations likely result from the release of adenine nucleotides into the alveolar space, where they are converted extracellular adenosine by nucleotidases (43). These adenosine elevations could provide a protective mechanism in response to the massive inflammation induced by BLM, despite adenosine up-regulation being associated with the progression of lung fibrosis. Lung fibrosis was resolved when adenosine levels were diminished at d 21 after BLM oropharyngeal aspiration. In contrast, in a repetitive intraperitoneal injection model where fibrosis is progressive and nonresolvable, BALF adenosine levels remained high and increased as fibrosis progressed.
IPF is characterized by repetitive lung injury and abnormal repair (1). In this context, our findings suggest that a sustained adenosine up-regulation followed by repetitive injury may play a critical role in the pathogenesis of IPF. To date, it has not been possible to directly measure the levels of extracellular adenosine in IPF patients due to difficulty in accurately measuring adenosine, which is highly labile. However, based on the findings in this study, it is likely that approaches to block adenosine signaling in IPF would be most effective in stages where fibrosis is well established. Potential therapeutic approaches could include the use of exogenous adenosine deaminase enzyme therapy to lower extracellular adenosine levels (44) or treatment with selective Adora2B selective antagonists (5). Adenosine deaminase enzyme therapy has been successfully used in mouse models of chronic lung disease to lower adenosine levels in the lung and to provide improvement in the degree of lung inflammation and fibrosis seen (15, 45, 46). The Adora2B is 1 of 4 cell surface adenosine receptors (13), and it has received considerable attention as a target in IPF (5). The levels of the Adora2B are elevated in lung tissue from IPF patients (47), and treatment of mice with selective Adora2B antagonists (9, 14) or genetic removal of this receptor (8) is associated with diminished fibrosis. The current study clearly associates the most severe stages of fibrosis in mouse models with maximum levels of adenosine in the BALF, which indicates that the aforementioned therapeutics will be effective in conditions of severe pulmonary fibrosis, such as that seen in IPF.
To further evaluate the impact of adenosine on lung fibrosis, we used DP, an equilibrative nucleoside transporter (ENT) inhibitor, to up-regulate adenosine levels. ENTs are important regulators of extracellular adenosine levels, and inhibition of ENTs in mice is associated with increased adenosine mediated phenotypes (41). Furthermore, it has been reported that ENT down-regulation is protective in ventilation or lipopolysaccharide induced acute lung injury (48). Thus, pharmacologic or genetic regulation of ENTs is an effective means to regulate extracellular adenosine levels. Our data suggest that ENT inhibition effectively elevates adenosine levels in the fibrotic lung and in so doing exacerbates pulmonary fibrosis, most likely through previously identified Adora2B-mediated mechanisms (14, 40). To our knowledge, this is the first study to evaluate the impact of ENTs on chronic lung injury such as pulmonary fibrosis. In addition to impacts on extracellular adenosine signaling, DP could also lead to alterations in intracellular adenosine levels that could in turn affect intracellular S-adenosylhomocysteine/S-adenosylmethionine ratios and therefore impact inflammatory cytokine expression or other cellular functions by affecting methylation and transcription (49–51). Finally, it must be taken into consideration that DP can also inhibit phosphodiesterase activity and increase cAMP levels with a drug concentration causing 50% inhibition of approximately 50 µM (52), which could also affect cellular function. However, serum DP levels will be much lower than 50 µM, considering the concentration (5 mg/kg) used in this study, and therefore it should not cause systemic phosphodiesterase inhibition (53, 54). Conversely, the dose used in the current study is efficient to inhibit ENT1 and ENT2 activity (drug concentration causing 50% inhibition for ENTs: ENT1 = 5.0 ± 0.9 nM and ENT2 = 356 ± 13 nM). Taken together, we believe the dose of DP used in this study is appropriate for ENT inhibition.
It is intriguing that adenosine has a bilateral effect in different phases of injury. One of the possible explanations is adenosine differentially induces inflammatory cytokines during chronic injury. This is supported by our data that DP further elevates BLM-induced IL-6 and IL-17 expression. It has been reported that the adenosine Adora2B receptor promotes Th17 differentiation (55), and it is likely that adenosine induces IL-17 expression through its Adora2B receptor in chronic lung injury, thus contributing to lung fibrosis. Additional studies are needed to explore the impact of IL-17 in adenosine-mediated fibrogenic phenotypes in the lungs. Furthermore, TNF-α has been shown to play a critical role in the resolution of BLM-induced lung fibrosis (27, 28). Consistent with previous studies, we have observed that BLM-induced TNF-α is inhibited by DP. It has been reported that Th1 cytokines inhibit fibrosis progression (56). T-bet, a molecule promoting Th1 cell proliferation, was found to be reduced by DP treatment. Additional evidence suggest that adenosine signaling plays an important role in promoting the differentiation of alternately activated macrophages, a macrophage subtype that has been shown to contribute to pulmonary fibrosis (18). Recently it was shown that genetic deletion of Adora2B on macrophages was associated with reduced pulmonary fibrosis, further suggesting that increase in adenosine levels in alveolar space where these cells are found, may contribute to fibrosis by activated alternatively activated macrophages. These are examples of mechanisms by which elevations in adenosine levels could drive the progression of fibrotic lung disease.
In conclusion, results from this study demonstrate that extracellular adenosine levels are closely associated with the progression of pulmonary fibrosis. Furthermore, DP exacerbates BLM-induced lung fibrosis, possibly by up-regulating the inflammatory cytokines IL-6 and IL-17. These studies provide additional evidence that adenosine drives the progression of pulmonary fibrosis and suggest that approaches to dampen this pathway during stages of active fibrosis could provide therapeutic benefit.
Supplementary Material
Acknowledgments
This work was supported by U.S. National Institutes of Health, National Heart, Lung, and Blood Institute Grants R01-HL070952 and P01-HL114457 (to M.R.B.).
Glossary
- BALF
bronchoalveolar lavage fluid
- BLM
bleomycin
- DP
dipyridamole
- ENT
equilibrative nucleoside transporter
- IPF
idiopathic pulmonary fibrosis
- RT-qPCR
real-time quantitative PCR
- Th
T helper
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Nalysnyk L., Cid-Ruzafa J., Rotella P., Esser D. (2012) Incidence and prevalence of idiopathic pulmonary fibrosis: review of the literature. Eur. Respir. Rev. 21, 355–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.King T. E. Jr., Pardo A., Selman M. (2011) Idiopathic pulmonary fibrosis. Lancet 378, 1949–1961 [DOI] [PubMed] [Google Scholar]
- 3.Raghu G., Collard H. R., Egan J. J., Martinez F. J., Behr J., Brown K. K., Colby T. V., Cordier J. F., Flaherty K. R., Lasky J. A., Lynch D. A., Ryu J. H., Swigris J. J., Wells A. U., Ancochea J., Bouros D., Carvalho C., Costabel U., Ebina M., Hansell D. M., Johkoh T., Kim D. S., King T. E. Jr., Kondoh Y., Myers J., Müller N. L., Nicholson A. G., Richeldi L., Selman M., Dudden R. F., Griss B. S., Protzko S. L., Schünemann H. J.; ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis (2011) An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am. J. Respir. Crit. Care Med. 183, 788–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.King T. E. Jr., Bradford W. Z., Castro-Bernardini S., Fagan E. A., Glaspole I., Glassberg M. K., Gorina E., Hopkins P. M., Kardatzke D., Lancaster L., Lederer D. J., Nathan S. D., Pereira C. A., Sahn S. A., Sussman R., Swigris J. J., Noble P. W.; ASCEND Study Group (2014) A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N. Engl. J. Med. 370, 2083–2092 [DOI] [PubMed] [Google Scholar]
- 5.Zhou Y., Schneider D. J., Blackburn M. R. (2009) Adenosine signaling and the regulation of chronic lung disease. Pharmacol. Ther. 123, 105–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohta A., Sitkovsky M. (2001) Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature 414, 916–920 [DOI] [PubMed] [Google Scholar]
- 7.Colgan S. P., Eltzschig H. K., Eckle T., Thompson L. F. (2006) Physiological roles for ecto-5′-nucleotidase (CD73). Purinergic Signal. 2, 351–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou Y., Schneider D. J., Morschl E., Song L., Pedroza M., Karmouty-Quintana H., Le T., Sun C. X., Blackburn M. R. (2011) Distinct roles for the A2B adenosine receptor in acute and chronic stages of bleomycin-induced lung injury. J. Immunol. 186, 1097–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karmouty-Quintana H., Xia Y., Blackburn M. R. (2013) Adenosine signaling during acute and chronic disease states. J. Mol. Med. 91, 173–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Synnestvedt K., Furuta G. T., Comerford K. M., Louis N., Karhausen J., Eltzschig H. K., Hansen K. R., Thompson L. F., Colgan S. P. (2002) Ecto-5′-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J. Clin. Invest. 110, 993–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hart M. L., Gorzolla I. C., Schittenhelm J., Robson S. C., Eltzschig H. K. (2010) SP1-dependent induction of CD39 facilitates hepatic ischemic preconditioning. J. Immunol. 184, 4017–4024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bouma M. G., van den Wildenberg F. A., Buurman W. A. (1996) Adenosine inhibits cytokine release and expression of adhesion molecules by activated human endothelial cells. Am. J. Physiol. 270, C522–C529 [DOI] [PubMed] [Google Scholar]
- 13.Blackburn, M. R., Vance, C. O., Morschl, E., and Wilson, C. N. (2009) Adenosine receptors and inflammation. Handb. Exp. Pharmacol. 193, 215–269 [DOI] [PubMed]
- 14.Sun C. X., Zhong H., Mohsenin A., Morschl E., Chunn J. L., Molina J. G., Belardinelli L., Zeng D., Blackburn M. R. (2006) Role of A2B adenosine receptor signaling in adenosine-dependent pulmonary inflammation and injury. J. Clin. Invest. 116, 2173–2182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chunn J. L., Molina J. G., Mi T., Xia Y., Kellems R. E., Blackburn M. R. (2005) Adenosine-dependent pulmonary fibrosis in adenosine deaminase-deficient mice. J. Immunol. 175, 1937–1946 [DOI] [PubMed] [Google Scholar]
- 16.Moore B. B., Hogaboam C. M. (2008) Murine models of pulmonary fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 294, L152–L160 [DOI] [PubMed] [Google Scholar]
- 17.Le T. T., Karmouty-Quintana H., Melicoff E., Le T. T., Weng T., Chen N. Y., Pedroza M., Zhou Y., Davies J., Philip K., Molina J., Luo F., George A. T., Garcia-Morales L. J., Bunge R. R., Bruckner B. A., Loebe M., Seethamraju H., Agarwal S. K., Blackburn M. R. (2014) Blockade of IL-6 Trans signaling attenuates pulmonary fibrosis. J. Immunol. 193, 3755–3768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karmouty-Quintana H., Philip K., Acero L. F., Chen N. Y., Weng T., Molina J. G., Luo F., Davies J., Le N. B., Bunge I., Volcik K. A., Le T. T., Johnston R. A., Xia Y., Eltzschig H. K., Blackburn M. R. (2015) Deletion of ADORA2B from myeloid cells dampens lung fibrosis and pulmonary hypertension. FASEB J. 29, 50– 60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ko K. R., Ngai A. C., Winn H. R. (1990) Role of adenosine in regulation of regional cerebral blood flow in sensory cortex. Am. J. Physiol. 259, H1703–H1708 [DOI] [PubMed] [Google Scholar]
- 20.Luo F., Zhuang Y., Sides M. D., Sanchez C. G., Shan B., White E. S., Lasky J. A. (2014) Arsenic trioxide inhibits transforming growth factor-β1-induced fibroblast to myofibroblast differentiation in vitro and bleomycin induced lung fibrosis in vivo. Respir. Res. 15, 51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davies J., Karmouty-Quintana H., Le T. T., Chen N. Y., Weng T., Luo F., Molina J., Moorthy B., Blackburn M. R. (2014) Adenosine promotes vascular barrier function in hyperoxic lung injury. Physiol. Rep. 2, e12155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wakamiya M., Blackburn M. R., Jurecic R., McArthur M. J., Geske R. S., Cartwright J. Jr., Mitani K., Vaishnav S., Belmont J. W., Kellems R. E., et al. (1995) Disruption of the adenosine deaminase gene causes hepatocellular impairment and perinatal lethality in mice. Proc. Natl. Acad. Sci. USA 92, 3673–3677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phan S. H., Armstrong G., Sulavik M. C., Schrier D., Johnson K. J., Ward P. A. (1983) A comparative study of pulmonary fibrosis induced by bleomycin and an O2 metabolite producing enzyme system. Chest 83(5, Suppl), 44S–45S [DOI] [PubMed] [Google Scholar]
- 24.Izbicki G., Segel M. J., Christensen T. G., Conner M. W., Breuer R. (2002) Time course of bleomycin-induced lung fibrosis. Int. J. Exp. Pathol. 83, 111–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pedroza M., Schneider D. J., Karmouty-Quintana H., Coote J., Shaw S., Corrigan R., Molina J. G., Alcorn J. L., Galas D., Gelinas R., Blackburn M. R. (2011) Interleukin-6 contributes to inflammation and remodeling in a model of adenosine mediated lung injury. PLoS One 6, e22667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mi S., Li Z., Yang H. Z., Liu H., Wang J. P., Ma Y. G., Wang X. X., Liu H. Z., Sun W., Hu Z. W. (2011) Blocking IL-17A promotes the resolution of pulmonary inflammation and fibrosis via TGF-beta1-dependent and -independent mechanisms. J. Immunol. 187, 3003–3014 [DOI] [PubMed] [Google Scholar]
- 27.Redente E. F., Keith R. C., Janssen W., Henson P. M., Ortiz L. A., Downey G. P., Bratton D. L., Riches D. W. (2014) Tumor necrosis factor-α accelerates the resolution of established pulmonary fibrosis in mice by targeting profibrotic lung macrophages. Am. J. Respir. Cell Mol. Biol. 50, 825–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuroki M., Noguchi Y., Shimono M., Tomono K., Tashiro T., Obata Y., Nakayama E., Kohno S. (2003) Repression of bleomycin-induced pneumopathy by TNF. J. Immunol. 170, 567–574 [DOI] [PubMed] [Google Scholar]
- 29.Peng Z., Fernandez P., Wilder T., Yee H., Chiriboga L., Chan E. S., Cronstein B. N. (2008) Ecto-5′-nucleotidase (CD73)-mediated extracellular adenosine production plays a critical role in hepatic fibrosis. Nucleosides Nucleotides Nucleic Acids 27, 821–824 [DOI] [PubMed] [Google Scholar]
- 30.Chunn J. L., Mohsenin A., Young H. W., Lee C. G., Elias J. A., Kellems R. E., Blackburn M. R. (2006) Partially adenosine deaminase–deficient mice develop pulmonary fibrosis in association with adenosine elevations. Am. J. Physiol. Lung Cell. Mol. Physiol. 290, L579–L587 [DOI] [PubMed] [Google Scholar]
- 31.Fernández P., Perez-Aso M., Smith G., Wilder T., Trzaska S., Chiriboga L., Franks A. Jr., Robson S. C., Cronstein B. N., Chan E. S. (2013) Extracellular generation of adenosine by the ectonucleotidases CD39 and CD73 promotes dermal fibrosis. Am. J. Pathol. 183, 1740–1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fernández P., Trzaska S., Wilder T., Chiriboga L., Blackburn M. R., Cronstein B. N., Chan E. S. (2008) Pharmacological blockade of A2A receptors prevents dermal fibrosis in a model of elevated tissue adenosine. Am. J. Pathol. 172, 1675–1682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mi T., Abbasi S., Zhang H., Uray K., Chunn J. L., Xia L. W., Molina J. G., Weisbrodt N. W., Kellems R. E., Blackburn M. R., Xia Y. (2008) Excess adenosine in murine penile erectile tissues contributes to priapism via A2B adenosine receptor signaling. J. Clin. Invest. 118, 1491–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wen J., Jiang X., Dai Y., Zhang Y., Tang Y., Sun H., Mi T., Phatarpekar P. V., Kellems R. E., Blackburn M. R., Xia Y. (2010) Increased adenosine contributes to penile fibrosis, a dangerous feature of priapism, via A2B adenosine receptor signaling. FASEB J. 24, 740–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eckle T., Koeppen M., Eltzschig H. K. (2009) Role of extracellular adenosine in acute lung injury. Physiology (Bethesda) 24, 298–306 [DOI] [PubMed] [Google Scholar]
- 36.Eltzschig H. K., Sitkovsky M. V., Robson S. C. (2013) Purinergic signaling during inflammation. N. Engl. J. Med. 368, 1260 [DOI] [PubMed] [Google Scholar]
- 37.Idzko M., Ferrari D., Eltzschig H. K. (2014) Nucleotide signalling during inflammation. Nature 509, 310–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eckle T., Grenz A., Laucher S., Eltzschig H. K. (2008) A2B adenosine receptor signaling attenuates acute lung injury by enhancing alveolar fluid clearance in mice. J. Clin. Invest. 118, 3301–3315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eckle T., Faigle M., Grenz A., Laucher S., Thompson L. F., Eltzschig H. K. (2008) A2B adenosine receptor dampens hypoxia-induced vascular leak. Blood 111, 2024–2035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karmouty-Quintana H., Zhong H., Acero L., Weng T., Melicoff E., West J. D., Hemnes A., Grenz A., Eltzschig H. K., Blackwell T. S., Xia Y., Johnston R. A., Zeng D., Belardinelli L., Blackburn M. R. (2012) The A2B adenosine receptor modulates pulmonary hypertension associated with interstitial lung disease. FASEB J. 26, 2546–2557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eckle T., Hughes K., Ehrentraut H., Brodsky K. S., Rosenberger P., Choi D. S., Ravid K., Weng T., Xia Y., Blackburn M. R., Eltzschig H. K. (2013) Crosstalk between the equilibrative nucleoside transporter ENT2 and alveolar Adora2b adenosine receptors dampens acute lung injury. FASEB J. 27, 3078–3089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zimmerman M. A., Tak E., Ehrentraut S. F., Kaplan M., Giebler A., Weng T., Choi D. S., Blackburn M. R., Kam I., Eltzschig H. K., Grenz A. (2013) Equilibrative nucleoside transporter (ENT)-1-dependent elevation of extracellular adenosine protects the liver during ischemia and reperfusion. Hepatology 58, 1766–1778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Volmer J. B., Thompson L. F., Blackburn M. R. (2006) Ecto-5′-nucleotidase (CD73)-mediated adenosine production is tissue protective in a model of bleomycin-induced lung injury. J. Immunol. 176, 4449–4458 [DOI] [PubMed] [Google Scholar]
- 44.Blackburn M. R. (2003) Too much of a good thing: adenosine overload in adenosine-deaminase-deficient mice. Trends Pharmacol. Sci. 24, 66–70 [DOI] [PubMed] [Google Scholar]
- 45.Blackburn M. R., Volmer J. B., Thrasher J. L., Zhong H., Crosby J. R., Lee J. J., Kellems R. E. (2000) Metabolic consequences of adenosine deaminase deficiency in mice are associated with defects in alveogenesis, pulmonary inflammation, and airway obstruction. J. Exp. Med. 192, 159–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blackburn M. R., Lee C. G., Young H. W., Zhu Z., Chunn J. L., Kang M. J., Banerjee S. K., Elias J. A. (2003) Adenosine mediates IL-13-induced inflammation and remodeling in the lung and interacts in an IL-13-adenosine amplification pathway. J. Clin. Invest. 112, 332–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou Y., Murthy J. N., Zeng D., Belardinelli L., Blackburn M. R. (2010) Alterations in adenosine metabolism and signaling in patients with chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis. PLoS One 5, e9224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morote-Garcia J. C., Köhler D., Roth J. M., Mirakaj V., Eldh T., Eltzschig H. K., Rosenberger P. (2013) Repression of the equilibrative nucleoside transporters dampens inflammatory lung injury. Am. J. Respir. Cell Mol. Biol. 49, 296–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pfalzer A. C., Choi S. W., Tammen S. A., Park L. K., Bottiglieri T., Parnell L. D., Lamon-Fava S. (2014) S-Adenosylmethionine mediates inhibition of inflammatory response and changes in DNA methylation in human macrophages. Physiol. Genomics 46, 617–623 [DOI] [PubMed] [Google Scholar]
- 50.Song Z., Uriarte S., Sahoo R., Chen T., Barve S., Hill D., McClain C. (2005) S-Adenosylmethionine (SAMe) modulates interleukin-10 and interleukin-6, but not TNF, production via the adenosine (A2) receptor. Biochim. Biophys. Acta 1743, 205–213 [DOI] [PubMed] [Google Scholar]
- 51.Chiang P. K., Gordon R. K., Tal J., Zeng G. C., Doctor B. P., Pardhasaradhi K., McCann P. P. (1996) S-Adenosylmethionine and methylation. FASEB J. 10, 471–480 [PubMed] [Google Scholar]
- 52.Mills D. C., Smith J. B. (1971) The influence on platelet aggregation of drugs that affect the accumulation of adenosine 3′:5′-cyclic monophosphate in platelets. Biochem. J. 121, 185–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim H. H., Sawada N., Soydan G., Lee H. S., Zhou Z., Hwang S. K., Waeber C., Moskowitz M. A., Liao J. K. (2008) Additive effects of statin and dipyridamole on cerebral blood flow and stroke protection. J. Cereb. Blood Flow Metab. 28, 1285–1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chan T. C., Coppoc G. L., Zimm S., Cleary S., Howell S. B. (1988) Pharmacokinetics of intraperitoneally administered dipyridamole in cancer patients. Cancer Res. 48, 215–218 [PubMed] [Google Scholar]
- 55.Wilson J. M., Kurtz C. C., Black S. G., Ross W. G., Alam M. S., Linden J., Ernst P. B. (2011) The A2B adenosine receptor promotes Th17 differentiation via stimulation of dendritic cell IL-6. J. Immunol. 186, 6746–6752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wynn T. A. (2011) Integrating mechanisms of pulmonary fibrosis. J. Exp. Med. 208, 1339–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.