Abstract
Background
Antibody alone cannot provide optimal protection against many infectious diseases impacting global heath. In these cases, our challenge is to develop innovative vaccines that generate protective populations of memory T cells. However, our studies suggest that current paradigms explaining how memory CD4 T cells provide protection are inadequate. This is likely due to both the paucity of and heterogeneity of memory CD4 T cells observed in vivo, which make analysis extremely difficult.
Summary
Here, we discuss new findings that indicate there is extensive functional heterogeneity within effector and memory CD4 T cell populations both in vivo and in vitro. Using influenza as an example, we also discuss the merits of employing reductionist approaches to explore how unique subsets of CD4 T cells are generated, what mechanisms of protection they use, and where they stand on the axes of differentiation that define T cell subsets.
Keywords: T cell memory, influenza, CD4 T cell
Introduction
The design of effective vaccines against a number of pathogens that threaten human health, including tuberculosis and HIV/AIDS, has proved challenging. An important factor that makes control of these pathogens ineffective is the inability of serum antibody alone to mediate adequate protection. The generation of strong T cell immunity is thus of paramount importance in achieving broader protection. In the case of influenza, where rapid evolution of the epitopes recognized by neutralizing antibodies occurs, this leaves us vulnerable to the possible emergence of a pandemic. T cell immunity against influenza core proteins is not strain specific and there is little drift in T cell epitopes [1], so a degree of protection may be achieved by generating strong T cell immunity. However, while important aspects of memory T cell response have been defined, our understanding of the mechanisms by which they provide optimal protection is limited. This is especially true of CD4 memory T cells for two reasons. First, antigen-specific CD4 T cells are often found at very low frequencies in vivo as compared to CD8 T cells [2], making their analysis difficult, and second, CD4 T cells can be subdivided into an extensive spectrum of subsets, many of which make different products and use different mechanisms to contribute to immunity [3]. Here, we discuss important aspects of this heterogeneity with an emphasis on defining the diverse impacts of CD4 T cell effector and memory responses during influenza challenge. We also discuss the merits of employing homogeneous as well as more heterogeneous CD4 T cell memory populations to determine mechanisms of protection and to gain a fuller appreciation of the complex immunobiology of CD4 T cells.
Characterizing T Cell Diversity
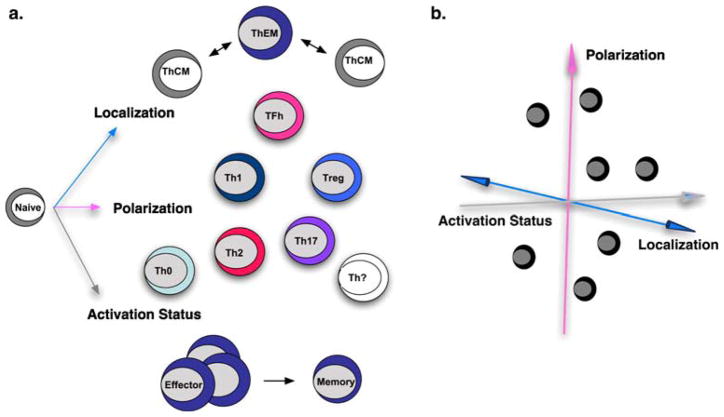
It has long been appreciated that naïve CD4 T cells with identical T cell receptor specificities can develop into distinct subsets depending on variables present during antigen encounter. Several criteria can be employed to classify the heterogeneity of responding T cells. Most often, T cells have been categorized into subsets based on either their polarization with regards to cytokine production and function, to their anatomical location, and to their activation or differentiation status (Fig. 1a). It is a testament to the remarkable flexibility and complexity of CD4 T cell responses that researchers are still defining new criteria by which CD4 T cells can be separated, and new subsets, several of which have yet to be well characterized functionally.
Fig. 1.
a After stimulation with antigen, naïve cells develop into effectors that can be subdivided based on cytokine production and function into numerous subsets (polarization). Activated effectors also differ from naïve and from resting memory cells as judged by several phenotypic markers and functional criteria (activation status). Finally, CD4 T cells with distinct phenotype and function can be categorized by various localization criteria in vivo (localization). b During immune responses against complex pathogens such as influenza, it is difficult to utilize any one axis of differentiation to describe CD4 T cell responses. Instead, it is likely that CD4 T cell responses can be characterized on several axes of differentiation
Distinct cytokine production patterns have long been used to subset stimulated CD4 T cells and they indicate an extensive functional diversity. The best characterized subsets of CD4 T cells are Th1, typified by strong IFNγ production, and Th2, a hallmark of which is IL-4 production [4]. Both clinical and experimental observations underscore the pivotal role that Th1 and Th2 CD4 T cells play in regulating responses against intracellular or extra-cellular pathogens, respectively. However, recently, it has become clear that additional subsets exist and much attention has been given to the characterization of Th17 [5, 6] and T follicular helper (TFH) [7] cells, which produce the hallmark cytokines IL-17 and IL-21, respectively. Th17-polarized CD4 T cells have been found in several situations to enhance inflammatory responses and have been implicated in several autoimmune diseases. However, other evidence suggests that Th17 responses may play beneficial roles against pathogens such as Mycobacterium tuberculosis [8]. ThFH-polarized CD4 T cells express CXCR5, are located primarily within germinal centers, and act as specialized helpers of antibody production. Finally, diverse populations of regulatory CD4 T cells (Treg) have been described, often typified by strong IL-10 and/or TGF-β production [9]. Treg populations have been shown to play critical roles in maintaining peripheral tolerance and in dampening immune responses in general. Importantly though, while highly polarized cytokine-producing CD4 T cells can be generated in vitro, the rigid definitions within this scheme are sometimes blurred during in vivo responses [10].
Several pieces of experimental and clinical evidence have correlated the location of responding CD4 T cells with specialized function. Perhaps the most striking example of subsetting by anatomical location is the division of human central and effector memory introduced by Sallusto and colleagues via differential expression of a variety of surface markers including CCR7, CD27, and CD28 [11]. These subsets also display differential access to tissues with central memory cells (ThCM) having restricted access to non-lymphoid tissue, whereas effector memory (ThEM) can be found within both lymphoid and non-lymphoid tissues. Beyond surface phenotype and localization, central and effector memory T cells also display unique functional qualities upon stimulation. Several observations support a similar division in function of memory T cells in murine models. Recent studies also support the concept that residual antigen depots may drive the development of ‘local’ memory CD4 T cells in lymphoid tissues with distinct properties [12].
A final axis along which CD4 T cells can be defined is based on activation or differentiation status. As already discussed, upon activation, naïve CD4 T cells develop into effectors, capable of secreting a diverse range of cytokines and capable of diverse ‘effector functions’. While the majority of responding effector CD4 T cells die via apoptosis both in vitro and in vivo after antigen/pathogen clearance and the resolution of inflammation, a small number persist as memory cells [13]. The transition from activated effector to resting memory endows memory T cells with unique functional qualities, recirculation patterns, and homeostatic requirements [14–17]. It is also understood, but has not been often studied, that resting memory CD4 T cells, like naïve CD4 T cells, give rise to effectors when they re-encounter antigen [18].
Given the scope of possible T cell fates and the complexity of in vivo responses against pathogens, we believe it is necessary to integrate the different schemes of subsetting CD4 T cells on separate axes of differentiation (Fig. 1b). Thus, it is likely that, during a response, cells that develop and respond are located at multiple positions along each of these three axes, leading to extensive heterogeneity and making it challenging to identify the mechanisms by which each distinct subset of CD4 T cells contributes to protective immune responses. This drives us to consider reductionist approaches to define roles of individual components.
Heterogeneity of CD4 T Cell Responses during Influenza Infection
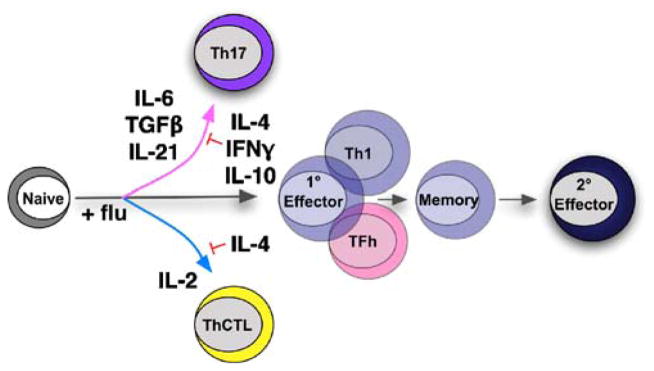
We have documented a wide degree of heterogeneity between responding antigen-specific CD4 T cells during influenza infection [19]. By using adoptive transfer approaches, naïve donor CD4 T cells responding in the spleen, draining lymph node, and lung differ substantially in terms of CFSE dilution, surface phenotype, and functional criteria [20]. Additionally, a striking degree of heterogeneity is seen between donor cells responding within the same organ, even when very low numbers of donor cells are transferred (unpublished observations). The CD4 T cell compartment developed during primary influenza challenge almost certainly represents multiple effectors arrayed along each of the axes of differentiation discussed above. Interestingly, a great deal of the heterogeneity observed during the primary response is retained in the influenza-specific memory CD4 T cell population [19]. Here, we highlight some of the more unexpected aspects of this diversity as it relates to protective CD4 T cell responses against influenza infection (Fig. 2).
Fig. 2.
Our studies have revealed unexpected contributions to protection against influenza employed by CD4 T cell subsets that can be characterized along different axes of differentiation (polarization, activation status, localization). Though the majority of responding virus-specific cells can be characterized as Th1, Th17-polarized cells develop during influenza responses, and in vitro-generated Th17 effectors can protect naïve mice against lethal challenge. In addition, we have identified a ThCTL subset present primarily in the lung that can provide protection via a perforin-dependent cytotoxic mechanism. Finally, we find that memory-derived secondary CD4 T cell effectors employ novel mechanisms of protection independent of helper function and cytotoxic activity during high-dose influenza challenge
We found several years back that CD4 T effectors can develop cytotoxic function in vitro and in vivo in an influenza challenge model [21]. Cytolytic CD4 T cells are found in the lung, but not the spleen and lymph nodes, and kill via a perforin-dependent mechanism that can contribute to protection (Brown and Swain, unpublished observations). More recently, we analyzed requirements for the generation of CD4 effectors with cytolytic ability in vitro. We find that neither Th1 polarizing (IL-12, IFNγ), Th2 polarizing (IL-4), nor Th17 or Treg polarizing (IL-6, TGFβ) cytokines were necessary and that IL-4 suppressed induction of effectors with lytic activity (Brown and Swain, unpublished observations). Based on the unique requirements of this activity, and its in vivo relevance, we suggest that this constitutes a novel CD4 subset, which we propose should be called ThCTL. Interestingly, when in vitro-generated virus-specific Th1-polarized effectors are transferred to naïve hosts, which are then infected with influenza, both ThCTL in the lung and CD4 T cells with the phenotype of TFH in the periphery develop [22].
We also find that Th17 cells may contribute to protection against high doses of influenza (Strutt, McKinstry, and Swain, unpublished observations). IL-10-deficient mice display dramatically increased survival compared to wild-type mice following challenge with lethal doses of virus. Administration of IL-10 receptor blocking antibodies to wild-type mice results in a similar enhancement of survival. Increased survival against influenza in the absence of IL-10 does not correlate with an increased number of responding T cells as compared to wild-type mice, but does correlate with increased expression of a number of Th17-associated cytokines during the peak of infection. These observations suggested that Th17-polarized effectors might be able to provide some immunity to influenza. To test this hypothesis, we generated virus-specific Th17 effectors in vitro using published methods [23], validated their lack of Th1, Th2, and Treg properties, and compared their protective capacity to that of Th1 effectors. Indeed, we found that Th17 were able to reverse weight loss and prevent death. Based on these results, we suggest that ThCTL, Th17, ThFH, and Th1 effectors each play unique and important roles in combating flu.
Finally, we have investigated an overlooked aspect of memory CD4 T cell responses against influenza. We asked whether effectors derived from memory cells (secondary effectors) are equivalent or different functionally from primary effectors derived from naïve CD4 T cells. Our results suggest that secondary effectors are superior to primary effectors functionally, retaining some unique qualities of memory cells such as enhanced cytokine production potential, especially for IL-2 [24]. We find that, during influenza infection, adoptively transferred secondary effectors are capable of protecting otherwise naïve mice via mechanisms not used by primary effectors (McKinstry, Strutt, and Swain, unpublished observations). These results suggest that the generation of secondary effectors represents an important aspect of protective secondary responses, independent of the helper activities usually ascribed to memory CD4 T cell populations.
It is likely that the heterogeneity observed in flu-specific CD4 T cell effectors will reveal additional subsets, and perhaps additional unexpected functions. To this end, we have begun to investigate differences in gene expression between donor cells responding in different organs as well as between primary and secondary effectors.
The Origins and ‘Fixing’ of Heterogeneity
Reductionist in vitro systems have revealed optimal conditions and critical cytokine signals required for the generation of well-polarized Th1, Th2, Th17, and Treg populations from naïve CD4 T cells. Other variables and signals exchanged during the T cell:APC interaction during priming, such as antigen dose and co-stimulatory interactions, are also likely to influence the course of the T cell response. While discrete manipulation in vitro can lead to the definition of how different kinds of signals influence generation of subsets, understanding heterogeneity in vivo is a more difficult challenge. Furthermore, the presence of viral antigen detectable weeks after clearance of influenza infection [25, 26] suggests that virus-specific CD4 T cells can be primed under dramatically different inflammatory conditions. As we and others have discussed recently, whether a naïve cell is recruited early or late into an immune response in vivo can lead to generation of different types of effector and memory CD4 T cells [27, 28].
Once a pathogen is cleared, effectors are widely dispersed throughout the host [29] in a number of very different environments that vary with the type of pathogen encountered, anatomical site, and cytokine milieu. It is unlikely that these disparate situations would present T cells with a common set of signals to instruct their transition to memory. Two key models have been proposed to explain how many effectors contract to a few memory cells. One suggests that a unique subset of responding ‘pre-memory’ cells act as the progenitors of memory [30, 31]. We have favored the concept that a stochastic process occurs whereby some effectors become resting, and thus resistant to programmed death, soon enough to escape contraction [19, 32–34].
We recently investigated how long it takes for an effector CD4 T cell to become a resting memory cell. We compared the phenotype, several important functional criteria, and gene expression profiles of prototypical effector and memory populations to a population of ‘rested effectors’, generated by removing effector cells from antigen and the cytokine milieu of effector cultures for only 3 days. Strikingly, 3-day-rested effectors closely resembled long-term memory CD4 T cells [35]. It is thus likely that many if not all of the important aspects associated with the effector to memory transition are programmed during CD4 T cell activation, similar as to what has been suggested for T cell proliferation and contraction.
The seeming ‘flash freeze’ of heterogeneous donor cell-derived effectors into corresponding heterogeneous memory cells after influenza infection, discussed earlier, is most readily explained within the framework of a rapid transition from effector to memory. These observations lead us to propose a model whereby progressive differentiation of effector CD4 T cells can lead to a level of heterogeneity in the responding population, which is retained in the memory phase via a rapid transition of heterogeneous effectors into similarly heterogeneous memory CD4 T cells [24]. Importantly though, extensive differentiation beyond an as yet undefined threshold has been shown to result in terminally differentiated effector populations [36], unable to enter the memory pool. If effector CD4 T cells in different relative states of differentiation acquire and/or lose different functional abilities, it is likely that an equally diverse memory pool could provide optimal multi-functional recall responses.
Utilizing the Rapid Transition to make Memory Cells in vitro
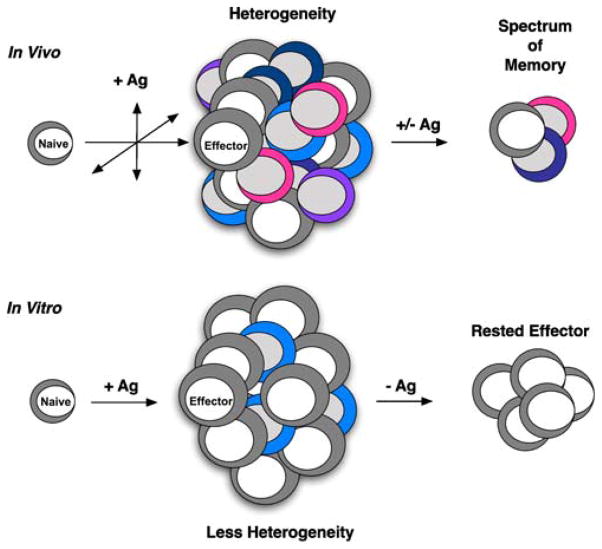
The rapid transition of effector CD4 T cells to memory can also be leveraged in order to generate more homogeneous populations of memory CD4 T cells to use in defining mechanisms of memory cell-mediated immunity (Fig. 3). By generating highly polarized subsets of well-defined effectors in vitro and resting them either in vivo or in vitro for about 3 days, which achieves the effector to memory transition, relatively large and homogeneous populations of memory CD4 T cells can be obtained. These populations can readily be used in reductionist approaches to address mechanisms used by memory CD4 T cells to provide protection. Furthermore, these populations can be used to ask basic questions at a resolution not currently possible utilizing the low numbers of endogenous antigen-specific memory CD4 T cell found following immunization or infection.
Fig. 3.
In vivo , diverse factors impact CD4 T cell priming and lead to a heterogeneous response comprising multiple functional subsets. The transition from effector to memory retains a degree of this heterogeneity in the virus-specific memory CD4 T cell pool. In vitro, defined priming conditions lead to the generation of a more homogeneous effector population, resulting in a similarly homogeneous population of antigen (Ag)-experienced memory CD4 T cells
We have begun to employ subsets of T cell receptor transgenic memory CD4 T cells generated in this manner to ask basic questions about how memory CD4 T cells respond during influenza infection, and how they contribute to protection. In particular, we have used in vitro-generated memory cells as precursors to define the functional potential of secondary effectors during influenza challenge. As mentioned earlier, our results show that secondary effectors contribute in important ways to protection via unique mechanisms independent of traditional helper functions. It is likely that focusing on other aspects of memory CD4 T cell responses will reveal further important roles for distinct memory CD4 T cell subsets during influenza infection.
Summary
Heterogeneity is implicit in immune responses. Understanding the origins of functional memory T cell heterogeneity is important in the design of optimal vaccine strategies. Equally important is an understanding of the full range of functions and unique properties of secondary effectors derived from distinct memory cells. By combining analysis of endogenous primary and secondary T cell responses against complex pathogens such as influenza, which elicit a full spectrum of T cell subsets, and by analyzing in depth the functions of more homogeneous populations of defined subsets of CD4 T cells, such as are obtained by in vitro generation of memory, we believe that we can begin to better understand the complex dynamics of CD4 T cell memory.
Acknowledgments
This work was supported by P01AI04630, P01AI45666, and R56AI967294 and the Trudeau Institute.
References
- 1.Dutton RW, Swain SL, Woodland DL. Vaccines against pandemic influenza. Viral Immunol. 2007;20:326–7. doi: 10.1089/vim.2007.0011. [DOI] [PubMed] [Google Scholar]
- 2.Seder RA, Ahmed R. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nat Immunol. 2003;4:835–42. doi: 10.1038/ni969. [DOI] [PubMed] [Google Scholar]
- 3.Stockinger B, Bourgeois C, Kassiotis G. CD4+ memory T cells: functional differentiation and homeostasis. Immunol Rev. 2006;211:39–48. doi: 10.1111/j.0105-2896.2006.00381.x. [DOI] [PubMed] [Google Scholar]
- 4.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–73. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 5.Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat Rev Immunol. 2008;8:337–48. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- 6.Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T(H)17 cells. Nature. 2008;453:1051–7. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.King C, Tangye SG, Mackay CR. T follicular helper (TFH) cells in normal and dysregulated immune responses. Annu Rev Immunol. 2008;26:741–66. doi: 10.1146/annurev.immunol.26.021607.090344. [DOI] [PubMed] [Google Scholar]
- 8.Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, Shen F, Eaton SM, Gaffen SL, Swain SL, Locksley RM, Haynes L, Randall TD, Cooper AM. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007;8:369–77. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 9.Shevach EM. From vanilla to 28 flavors: multiple varieties of T regulatory cells. Immunity. 2006;25:195–201. doi: 10.1016/j.immuni.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Kelso A, Groves P, Ramm L, Doyle AG. Single-cell analysis by RT-PCR reveals differential expression of multiple type 1 and 2 cytokine genes among cells within polarized CD4+ T cell populations. Int Immunol. 1999;11:617–21. doi: 10.1093/intimm/11.4.617. [DOI] [PubMed] [Google Scholar]
- 11.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–63. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 12.Fazilleau N, Eisenbraun MD, Malherbe L, Ebright JN, Pogue-Caley RR, McHeyzer-Williams LJ, McHeyzer-Williams MG. Lymphoid reservoirs of antigen-specific memory T helper cells. Nat Immunol. 2007;8:753–61. doi: 10.1038/ni1472. [DOI] [PubMed] [Google Scholar]
- 13.Swain SL, Croft M, Dubey C, Haynes L, Rogers P, Zhang X, Bradley LM. From naive to memory T cells. Immunol Rev. 1996;150:143–67. doi: 10.1111/j.1600-065X.1996.tb00700.x. [DOI] [PubMed] [Google Scholar]
- 14.Dutton RW, Bradley LM, Swain SL. T cell memory. Annu Rev Immunol. 1998;16:201–23. doi: 10.1146/annurev.immunol.16.1.201. [DOI] [PubMed] [Google Scholar]
- 15.Agrewala JN, Brown DM, Lepak NM, Duso D, Huston G, Swain SL. Unique ability of activated CD4+ T cells but not rested effectors to migrate to non-lymphoid sites in the absence of inflammation. J Biol Chem. 2007;282:6106–15. doi: 10.1074/jbc.M608266200. [DOI] [PubMed] [Google Scholar]
- 16.Bradley LM, Haynes L, Swain SL. IL-7: maintaining T-cell memory and achieving homeostasis. Trends Immunol. 2005;26:172–6. doi: 10.1016/j.it.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 17.Surh CD, Boyman O, Purton JF, Sprent J. Homeostasis of memory T cells. Immunol Rev. 2006;211:154–63. doi: 10.1111/j.0105-2896.2006.00401.x. [DOI] [PubMed] [Google Scholar]
- 18.Bradley LM, Duncan DD, Yoshimoto K, Swain SL. Memory effectors: a potent, IL-4-secreting helper T cell population that develops in vivo after restimulation with antigen. J Immunol. 1993;150:3119–30. [PubMed] [Google Scholar]
- 19.Swain SL, Agrewala JN, Brown DM, Jelley-Gibbs DM, Golech S, Huston G, Jones SC, Kamperschroer C, Lee WH, McKinstry KK, Roman E, Strutt T, Weng NP. CD4+ T-cell memory: generation and multi-faceted roles for CD4+ T cells in protective immunity to influenza. Immunol Rev. 2006;211:8–22. doi: 10.1111/j.0105-2896.2006.00388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roman E, Miller E, Harmsen A, Wiley J, Von Andrian UH, Huston G, Swain SL. CD4 effector T cell subsets in the response to influenza: heterogeneity, migration, and function. J Exp Med. 2002;196:957–68. doi: 10.1084/jem.20021052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown DM, Dilzer AM, Meents DL, Swain SL. CD4 T cell-mediated protection from lethal influenza: perforin and antibody-mediated mechanisms give a one-two punch. J Immunol. 2006;177:2888–98. doi: 10.4049/jimmunol.177.5.2888. [DOI] [PubMed] [Google Scholar]
- 22.Kamperschroer C, Roberts DM, Zhang Y, Weng NP, Swain SL. SAP enables T cells to help B cells by a mechanism distinct from Th cell programming or CD40 ligand regulation. J Immunol. 2008;181:3994–4003. doi: 10.4049/jimmunol.181.6.3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, Ziegler SF, Littman DR. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236–40. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McKinstry KK, Strutt TM, Swain SL. The effector to memory transition of CD4 T cells. Immunol Res. 2008;40:114–27. doi: 10.1007/s12026-007-8004-y. [DOI] [PubMed] [Google Scholar]
- 25.Jelley-Gibbs DM, Brown DM, Dibble JP, Haynes L, Eaton SM, Swain SL. Unexpected prolonged presentation of influenza antigens promotes CD4 T cell memory generation. J Exp Med. 2005;202:697–706. doi: 10.1084/jem.20050227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jelley-Gibbs DM, Dibble JP, Brown DM, Strutt TM, McKinstry KK, Swain SL. Persistent depots of influenza antigen fail to induce a cytotoxic CD8 T cell response. J Immunol. 2007;178:7563–70. doi: 10.4049/jimmunol.178.12.7563. [DOI] [PubMed] [Google Scholar]
- 27.Jelley-Gibbs DM, Strutt TM, McKinstry KK, Swain SL. Influencing the fates of CD4 T cells on the path to memory: lessons from influenza. Immunol Cell Biol. 2008;86:343–52. doi: 10.1038/icb.2008.13. [DOI] [PubMed] [Google Scholar]
- 28.Catron DM, Rusch LK, Hataye J, Itano AA, Jenkins MK. CD4+ T cells that enter the draining lymph nodes after antigen injection participate in the primary response and become central-memory cells. J Exp Med. 2006;203:1045–54. doi: 10.1084/jem.20051954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reinhardt RL, Khoruts A, Merica R, Zell T, Jenkins MK. Visualizing the generation of memory CD4 T cells in the whole body. Nature. 2001;410:101–5. doi: 10.1038/35065111. [DOI] [PubMed] [Google Scholar]
- 30.Chang JT, Palanivel VR, Kinjyo I, Schambach F, Intlekofer AM, Banerjee A, Longworth SA, Vinup KE, Mrass P, Oliaro J, Killeen N, Orange JS, Russell SM, Weninger W, Reiner SL. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science. 2007;315:1687–91. doi: 10.1126/science.1139393. [DOI] [PubMed] [Google Scholar]
- 31.Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2:251–62. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- 32.Hu H, Huston G, Duso D, Lepak N, Roman E, Swain SL. CD4(+) T cell effectors can become memory cells with high efficiency and without further division. Nat Immunol. 2001;2:705–10. doi: 10.1038/90643. [DOI] [PubMed] [Google Scholar]
- 33.Harrington LE, Janowski KM, Oliver JR, Zajac AJ, Weaver CT. Memory CD4 T cells emerge from effector T-cell progenitors. Nature. 2008;452:356–60. doi: 10.1038/nature06672. [DOI] [PubMed] [Google Scholar]
- 34.Lohning M, Hegazy AN, Pinschewer DD, Busse D, Lang KS, Hofer T, Radbruch A, Zinkernagel RM, Hengartner H. Long-lived virus-reactive memory T cells generated from purified cytokine-secreting T helper type 1 and type 2 effectors. J Exp Med. 2008;205:53–61. doi: 10.1084/jem.20071855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McKinstry KK, Golech S, Lee WH, Huston G, Weng NP, Swain SL. Rapid default transition of CD4 T cell effectors to functional memory cells. J Exp Med. 2007;204:2199–211. doi: 10.1084/jem.20070041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu CY, Kirman JR, Rotte MJ, Davey DF, Perfetto SP, Rhee EG, Freidag BL, Hill BJ, Douek DC, Seder RA. Distinct lineages of T(H)1 cells have differential capacities for memory cell generation in vivo. Nat Immunol. 2002;3:852–8. doi: 10.1038/ni832. [DOI] [PubMed] [Google Scholar]