Abstract
Meniscal tears are the most common knee injury, and partial meniscectomies are the most common orthopaedic surgical procedure. The injured meniscus has an impaired ability to distribute load and resist tibial translation. Partial or complete loss of the meniscus promotes early development of chondromalacia and osteoarthritis. The primary goal of treatment for meniscus-deficient knees is to provide symptomatic relief, ideally to delay advanced joint space narrowing, and ultimately, joint replacement. Surgical treatments, including meniscal allograft transplantation (MAT), high tibial osteotomy (HTO), and distal femoral osteotomy (DFO), are options that attempt to decrease the loads on the articular cartilage of the meniscus-deficient compartment by replacing meniscal tissue or altering joint alignment. Clinical and biomechanical studies have reported promising outcomes for MAT, HTO, and DFO in the postmeniscectomized knee. These procedures can be performed alone or in conjunction with ligament reconstruction or chondral procedures (reparative, restorative, or reconstructive) to optimize stability and longevity of the knee. Complications can include fracture, nonunion, patella baja, compartment syndrome, infection, and deep venous thrombosis. MAT, HTO, and DFO are effective options for young patients suffering from pain and functional limitations secondary to meniscal deficiency.
Keywords: meniscus deficient, high tibial osteotomy, meniscal allograft transplantation, meniscus
Meniscal tears are the most common knee injury, resulting in approximately 61 meniscectomies per 100,000 patients annually.63 Every year, approximately 700,000 partial meniscectomies are performed in the United States, which results in an annual direct medical cost of approximately US$4 billion.17,101 Meniscal tears occur more often in men than women, with ratios ranging from 2.5:1 to 4:1.35 Approximately one-third of all tears are associated with an anterior cruciate ligament (ACL) injury, more commonly in patients aged 11 to 30 years, as a result of a traumatic athletic injury.31,99 Degenerative meniscal tears are more common in patients aged 40 to 65 years and can occur from gradual loss of inherent meniscal physiology, chronic wear, activities of daily living, athletic activities, and sometimes trivial twisting events.35
Clinical symptoms include pain, effusion, locking, catching, and loss of motion. Degenerative meniscal tears are initially managed conservatively but may require surgical intervention. Meniscal repairs and partial meniscectomies attempt to preserve meniscal function; however, patient-specific factors such as age, concomitant injury, location and size of the tear, degree of injury, recurrent meniscal injury, and tear pattern frequently require subtotal or total meniscectomy. Although infrequent today, total meniscectomy was historically a common procedure, and many of these patients are now presenting with arthritis, pain, and loss of function. Meniscal deficiency in turn can lead to premature, progressive osteoarthritis of the ipsilateral compartment, with resultant radiographic joint space narrowing.4,48,56,90
Historical Perspective
In 1948, Fairbank29 reported radiographic changes, graded 0 through 4, after meniscectomy and stressed the importance of the meniscus in protecting the articular cartilage and joint.119 Load testing by Krause et al53 noted that once the meniscus is removed, stress acting across the joint significantly increased, confirming its role in hoop stresses, load transmission, and energy absorption. Baratz et al13 further confirmed the effect of meniscectomy on load transmission and found a linear relationship between the amount of meniscus removed and the increase in contact stresses. Shelbourne and Dickens96 radiographically evaluated 49 patients 12 years after isolated medial meniscectomy for a bucket-handle tear. They reported that the mean joint space decreased 1.2 ± 0.5 mm, with 4 patients showing decrease over 2 mm compared with 0.2 mm in the unaffected knee. Evidence regarding the negative long-term effects of meniscectomies led to research exploring surgical procedures to prevent or delay these changes, such as meniscal allograft transplantation (MAT), high tibial osteotomies (HTOs), and distal femoral osteotomies (DFOs).20,24 In this article, we examine the current concepts surrounding the options for treating the meniscus-deficient knee, including the anatomy and biomechanics of the meniscus, clinical evaluation, conservative and surgical treatment options, and functional rehabilitation.
Anatomy And Biomechanics
The medial and lateral menisci are fibrocartilaginous structures composed of 70% water and 60% to 70% collagen (90% type I) by dry weight, organized primarily circumferentially and radially.35,92 Only the outer 10% to 30% of the meniscus is vascular, supplied primarily by the perimeniscular capillary plexus from the superior and inferior branches of the medial and lateral geniculate arteries.11,54,82,92 The medial meniscus is C-shaped, with capsular attachments to the femur and tibia via the deep medial collateral ligament (MCL), to the tibia anterior to the ACL, to the tibia via the coronary ligament, and to the lateral meniscus via the intermeniscal ligament.5,35,82 In contrast, the lateral meniscus is more semicircular and covers a larger portion of the tibial articular surface. The anterior horn is attached to the transverse meniscal ligament and to the tibial eminence just posterior to the insertion of the ACL. The posterior horn is attached to the tibia in the intercondylar region and to the medial femoral condyle via the ligaments of Humphrey (anterior to the posterior cruciate ligament [PCL]) and Wrisberg (posterior to the PCL) when present.82
Primary functions of the menisci include load distribution and stability. The medial and lateral menisci transmit 50% and 70%, respectively, of their compartmental loads in extension, increasing to 85% and 90%, respectively, in knee flexion. After medial meniscectomy, contact stresses increase 100%, whereas after a lateral meniscectomy, contact stresses increase 200% to 350%.8,12,35,54,63,113,118 A review of 210 patients 10 to 22 years after meniscectomy found that those with abnormal leg alignment showed significantly more degenerative changes in the knee.4 Covall and Wasilewski22 further demonstrated at 5.4 years after meniscectomy that 50% of patients in varus alignment showed grade 1 Fairbank changes, and 43% showed changes of greater than grade 2.
The menisci also play a key role in joint stability, especially in the ACL-deficient knee. The medial meniscus acts as a secondary stabilizer to anterior tibial translation and bears increased loads in the ACL-deficient knee; therefore, a medial meniscectomy in an ACL-deficient knee can result in increased anterior tibial translation up to 58% at 90° of flexion.35 Trojani et al112 found that in 121 patients, 70% underwent a meniscectomy either before, during, or after primary or revision ACL reconstruction; meniscectomy at any point resulted in significantly reduced subjective knee scores and pivot shift control, further confirming the role of the meniscus as a knee stabilizer. Biomechanically, Spang et al106 also found that meniscal allograft transplantation can restore tibial displacement. Using a transducer placed in the ACL of 10 human cadaveric knees, they recorded strain during anterior-posterior cycles at 30°, 60°, and 90° of knee flexion in the native knee, after meniscectomy, and after meniscal transplantation. They found that tibial displacement was significantly increased after meniscectomy at 60° and 90° of flexion but was restored after meniscal transplantation. Finally, the anterior and posterior horns of the meniscus are innervated with mechanoreceptors that play a role in proprioceptive feedback, especially during the extremes of motion.35
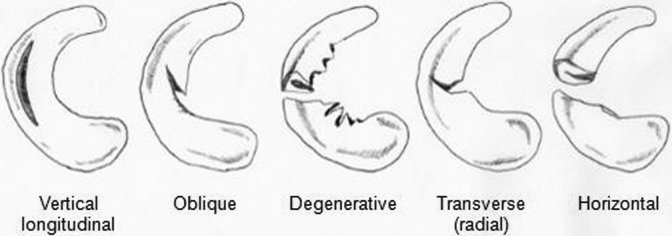
Acute meniscal tears may be radial (transverse), oblique, horizontal, complex, vertical (longitudinal), or bucket handle (complete vertical longitudinal) or may be complex with 1 or more patterns (Figure 1). Tear morphology can have a significant effect on contact pressures. Ode et al69 demonstrated in a cadaveric study that complete radial tears significantly increase mean contact pressure and decrease contact area compared with the intact state. Additionally, in chronically ACL-deficient knees, the medial meniscus bears increased loads leading to degenerative tears.57,72 Meniscal tears are often classified according to location of the tear relative to the blood supply or the location of the tear, as in the Cooper classification (Figure 2).21,109
Figure 1.
Common patterns of meniscal tears.
Figure 2.

Cooper classification of meniscal tears: Radial zones are divided into areas A, B, and C for the medial meniscus (from posterior to anterior) and into areas D, E, and F for the lateral meniscus (from anterior to posterior). The 4 circumferential zones are 0 for the meniscocapsular junction, 1 for the outer third, 2 for the middle third, and 3 for the inner third.
Chondral lesions can result after loss of the meniscus or concomitantly in an acute injury and are graded using the Outerbridge arthroscopic classification.70 These injuries occur most commonly on the femoral surface but can also occur on the tibial surface or patella; adjacent lesions can occur on the tibia and femur, known as “kissing lesions.” Addressing concomitant chondral injury may be necessary in the meniscus-deficient knee and can be done in conjunction with procedures to address the affected compartment. Additionally, in an in vivo model, Szomor et al107 showed that after a meniscectomy, macroscopic damage to the articular cartilage was significantly decreased if a meniscal transplant was performed compared with no treatment. The size of the area of cartilage damage was reduced by approximately 50% after a meniscal transplantation compared with meniscectomy alone.
Postmeniscectomy, radiographic and magnetic resonance imaging (MRI) evaluation have also revealed significant wear and degeneration of cartilage, soft tissue, and subchondral bone.15,19,90,123 In a retrospective analysis of 29 patients who had undergone isolated arthroscopic partial medial or lateral meniscectomy, Williams et al123 showed that at a minimum of 5 years postoperatively, Outerbridge grades 2 to 4 chondral lesions were noted in 64% of medial compartments and in 33% of lateral compartments, despite excellent clinical outcomes. By comparison, Shelbourne and Dickens97 found that only 12% of patients had radiographic evidence of joint space narrowing >2 mm at 12-year follow-up. Imaging analysis can help guide treatment in determining the optimal treatment course and potential need for concurrent procedures.
Although patients may be able to return to high-level sports after meniscectomy, long-term results suggest that meniscectomy significantly reduces the longevity of an athlete’s career. Kim et al51 retrospectively reviewed 56 athletes who were either elite, competitive, or recreational players and found that elite and competitive athletes returned to activity approximately 54 days after meniscectomy, while recreational athletes returned 88 days after surgery. Jorgensen et al48 prospectively followed 147 athletes who had a meniscectomy, looking for joint degeneration and level of sporting activity. They found that 4.5 years after operation, 40% had radiographic evidence of degeneration, increasing to 89% at 14.5 years; 8% of patients showed definitive osteoarthritis, 46% of athletes had given up or reduced their sporting activity, and 6.5% had changed their occupation.
Clinical Evaluation
History and Physical Examination
Diagnosis of meniscal deficiency can frequently be made clinically based on careful history, physical examination, and appropriate diagnostic tests. History taking should focus on patient age, pain, subsequent pain and swelling, complaints of locking or catching, and loss of motion. Prior treatments should be elucidated, which could include injection, therapy, anti-inflammatories, surgeries, and any previous imaging studies. Particular attention should be paid to the time course of symptoms, relation of symptoms to surgical intervention, and difference in symptom character. For example, patients will typically have sharp pain or mechanical symptoms that lead them to undergo meniscectomy. After a pain-free interval, the patient develops “toothache”-type pain that is more dull and nagging. This is referred to as the postmeniscectomy syndrome. Joint effusions may be present but will often be transient and vary based on activity level. While injections may not always provide long-term relief, a transient response may indicate the likelihood of a positive response after surgery.
Physical examination should begin with having the patient change into shorts and analyze his or her gait for decreased stance phase on the affected leg, any dynamic varus thrust, or limping.95 Lower extremity alignment should be evaluated with the patient standing facing the examiner. Subtle thrusts may be accentuated by having the patient walk faster or walk backward. The knee should be inspected for surgical scars and assessed for the presence of a joint effusion, and the quadriceps circumference should be evaluated bilaterally to determine the chronicity of the problem and rehabilitation potential. Passive and active range of motion should be checked bilaterally in the prone position. The joint-line, femoral condyle, pes anserinus, Gerdy tubercle, and patella should be palpated to assess for tenderness. Ligament stability should be assessed, including the ACL (Lachman and pivot-shift examinations55), PCL, medial and lateral collateral ligaments (MCL, LCL), and posterior lateral corner (PLC). If the patient has malalignment, it should be determined whether this is passively correctable as these patients may have success with bracing compared with those with fixed deformities. Compared with an acute meniscal tear, after meniscectomy, specialty examinations such as the McMurray test, Apley grind test, the Thessaly test, and others are less commonly present.
Imaging Studies
Radiographs
Radiographic imaging should include a weightbearing anteroposterior (AP) view in full extension, posteroanterior (PA) views at 30° or 45° of flexion (Rosenberg views), skyline, lateral, and mechanical axis view. Fairbank changes may also be seen on radiographs, which include formation of a ridge on the femoral condyle, joint space narrowing, and flattening of the femoral condyle.29 Rosenberg views are more sensitive and specific than conventional extension radiographs at detecting narrowing of the joint space.85 The Kellgren-Lawrence radiographic scale of joint space narrowing aids in evaluation; however, Bin Abd Razak et al14 found that correlation between the Kellgren-Lawrence scale and arthroscopic evidence of articular cartilage damage was low, at 0.32. Although these radiographs cannot confirm the diagnosis of a meniscal tear, they are useful for determining bony pathology and joint space narrowing. Radiographs should also be evaluated for surgical implants from prior procedures.
Magnetic Resonance Imaging
MRI is the most powerful tool for evaluating the meniscectomized patient, both to evaluate the affected compartment as well as to look for concomitant ligamentous injuries.123 The meniscus normally appears as a uniformly low-signal triangular structure. After a meniscectomy, if greater than 25% to 30% of the meniscus has been resected, the criteria for assessment of a new tear changes compared with a native knee.80 T2-weighted images should also be used to examine the subchondral bone for the presence of subchondral edema. MRI can also be used to assess the presence of subchondral sclerosis, bony edema, condylar squaring, and osteophytes, which may indicate progression to chondromalacia and osteoarthritis.123
Treatment
The goals of treatment of patients with symptomatic meniscal deficiency are primarily to provide symptomatic relief during daily activities, as relief with higher level activities is less predictable. Additionally, treatment aims to improve patient functioning. Ideally, treatment would prevent further progression of osteoarthritis, although current literature has not reliably proven this.
Nonsurgical Approaches
In the setting of postmeniscectomy syndrome, there are essentially no absolute indications for immediate surgical intervention. Therefore, nonoperative management should be thoroughly exhausted in this patient population prior to proceeding with surgery. Nonsurgical management includes activity modification, weight loss, nonsteroidal anti-inflammatory medications (NSAIDs), physical therapy, unloading braces, and injections.28,36,81 Many patients with postmeniscectomy syndrome can be successfully managed with nonoperative measures.
Corticosteroid injections may provide short-term relief and facilitate active participation in physical therapy.40 Additionally, use of viscosupplementation via intra-articular injection of hyaluronic acid (hyaluronan) may provide short-term relief through its anti-inflammatory, local analgesic, and chondroprotective effects, although evidence is mixed.122 However, although injections may provide short-term relief and aid in some meniscal repair, they may also have toxic effects on chondrocytes. Dragoo et al25 have shown in vitro that a single dose of corticosteroids results in significant chondrocyte cell death. In a meta-analysis, Watterson and Esdaile122 reviewed randomized trials comparing use of viscosupplementation to placebo; of the 9 trials comparing hyaluronan to placebo, 6 studies found a significant difference between treatment and placebo. In 2 of 4 studies comparing hyaluronan to conservative modalities, there was again a significant clinical difference. Similarly, the evidence for use of platelet-rich plasma (PRP), bone marrow aspirates (BMAs), and mesenchymal stem cells (MSCs) in isolation as injections or in conjunction with surgical repair techniques is still controversial.10,46,127 MSCs have been shown to enhance meniscal repair.9,27,41,42,127 In a randomized, controlled, double-blind study, Vangsness et al114 found that a single injection of MSCs 7 to 10 days after meniscectomy could result in up to a 24% increase in meniscal volume on MRI as compared with controls.
Unloading braces can be trialed, with the aim to decrease compressive forces transmitted to the femoral-tibial joint surfaces by applying a varus or valgus force.75,78 In a crossover study, Horlick and Loomer43 treated patients with medial compartment osteoarthritis with 3 conditions (no brace, brace in neutral alignment, and valgus-producing brace) for 6 weeks. They found using visual analog scales of pain that only bracing with valgus alignment resulted in decreased pain.43 In a randomized controlled trial, Kirkley et al52 treated patients with either medical treatment (control group), a neoprene sleeve, or a valgus-alignment brace. At 6-month follow-up, significant improvement in the osteoarthritis index was noted with valgus-producing braces. The use of off-loader braces is important in the meniscectomized patient for therapeutic and diagnostic purposes. As osteotomies are significant surgical procedures, this is a good way to have patients understand how their knee might feel after realignment. However, in the patient with neutral alignment and postmeniscectomy syndrome, there is minimal role for bracing.
Meniscal Transplantation
Indications
Meniscal allograft transplantation (MAT) can help to restore native biomechanics and stability to the knee in postmeniscectomy patients.45 Improved function after MAT is due to an increase in intra-articular contact area and a decrease in peak contact pressures across the knee. Indications and contraindications for MAT are noted in Table 1.92 Contraindications for MAT include Outerbridge grade 3 or 4 articular damage, diffuse arthritic changes, squaring or flattening of the femoral condyle or tibial plateau, significant osteophyte formation, untreated tibiofemoral subluxation, inflammatory arthritis, synovial disease, previous or active joint infection, or marked obesity.57
TABLE 1.
Patient Selection for Meniscal Allograft Transplantation
| Ideal Candidate | Contraindications |
|---|---|
| • Age <40 y | • Age >50 y |
| • Absent or nonfunctioning meniscus | • Varus/valgus malalignment |
| • Pain with activity | • Knee instability |
| • Normal mechanical alignment | • Bony architecture changes |
| • Outerbridge grade <2 articular changes | • Inflammatory arthritis |
| • Synovial disease | |
| • Obesity |
Sizing and Allograft Preparation
Success of MAT is highly dependent on many variables, especially accurate size matching of the allograft to the native meniscus when using a bone bridge or plugs. Sizing of meniscal allografts can be done using radiographs, MRI, or computed tomography (CT) scans. Accurate sizing is critical, as oversized allografts lead to greater forces across the articular cartilage and may cause extrusion with inadequate transmission of compressive loads across the knee. Undersized allografts lead to excessive load and poor congruity with the femoral condyle. Postmeniscectomy, the contralateral knee can be used for sizing; however, variability between opposite knees has been reported.47 Radiographs with a standardized marker can be used to estimate meniscal width, which has been shown to be the greatest predictor of matching native contact pressures, although MRI is more accurate.44,57,94 Meniscal length may be measured on lateral radiographs as determined by the AP dimension of the ipsilateral tibial plateau. After correction, these measurements are then multiplied by 0.8 for the medial and 0.7 for the lateral meniscus.44,57,94
There is a small risk of disease transmission with MAT. As the cellular component of menisci are believed to be immunoprivileged, clinical evidence for tissue rejection is rare.82 Secondary processing of graft tissue after harvest may include debridement, ultrasonic pulsatile washing, and use of ethanol to denature proteins, which further decreases the risk for disease transmission. Chemical or radiation treatment of grafts is only performed by a small number of tissue banks given concerns for graft compromise. Four common methods of preservation after graft harvest are cryopreservation, fresh-frozen, fresh, and freeze-drying. Additionally, sterilization techniques can be used, cleansing graft tissue using both mechanical processes through oscillating positive and negative pressure and chemical processes that remove blood and pathogenic microorganisms. Fresh-frozen is the most commonly used preservation technique.59 Fresh allografts are often favored as they contain the highest number of viable cells, which may have a beneficial effect in maintaining the extracellular matrix and mechanical integrity of the allograft.82 In an animal model, McNickle et al64 evaluated the tissue integrity of aseptic grafts compared with sterilized grafts and native tissue. Four months after implantation, aseptic and sterilized grafts had similar cell viabilities (approximately 60%) and both had decreased compressive strength compared with native menisci, confirming that sterilization provided an additional level of safety without compromising tissue integrity. However, due to limited availability, this may not be an option for all patients, in which case fresh-frozen or cryopreserved grafts are preferred. Freeze-drying has been shown in follow-up MRI analysis to be associated with the highest percentage of graft shrinkage, and on second-look arthroscopy, had increased degeneration.82,117
Surgical Technique
Surgical technique in MAT continues to evolve and may be performed arthroscopically, open, or in a combination with a mini-arthrotomy for graft insertion. Along with meniscus transplantation, various bony and soft tissue attachments can be harvested including separate anterior and posterior bone plugs and bone bridges, such as key hole, trough, dovetail, and bridge-in-slot variations.57 A bone bridge between the anterior and posterior horns is almost always used for lateral MAT.67 In medial MAT, anchoring can be accomplished with bone plugs, which allows for minor modifications, or a bone bridge.67 Fixation can also be accomplished with soft tissue fixation alone or with use of suture fixation. Abat et al1 evaluated 88 MAT procedures that obtained fixation with either suture-only technique (n = 33) compared with bone plugs (n = 55). At 40-month follow-up, MRI evaluation showed a greater percentage of extruded meniscal body with suture fixation, with no difference in functional outcome.
To perform MAT, the patient is positioned supine with a tourniquet placed high on the thigh. Knee arthroscopy should be performed first to examine for any concomitant cartilage or ligamentous pathology. The meniscus of the operative compartment should be debrided to a 1- to 2-mm bleeding peripheral rim. A mini-arthrotomy is then made adjacent to the patellar tendon on the affected side in line with the insertion sites of the anterior and posterior meniscal horns. The incision should be made one-third above and two-thirds below the joint line. An MCL release is sometimes performed to reduce pressure and facilitate passage of the bone plug into a tight knee as it does not alter contact mechanics.50 An ipsilateral posteromedial incision (or posterolateral for lateral transplantation) is also made anterior to the semitendinosus and semimembranosus tendons, creating an interval between the posteromedial aspect of the capsule anterior to the gastrocnemius and semitendinosus tendons. The transplantation slot is prepared, with orientation following normal anatomy. A line is made connecting the center of the anterior and posterior horn attachments sites, and a 4-mm bur is used along this line to make a reference slot in the tibial plateau. The slot height and width will equal the dimension of the bur, and the alignment in the sagittal plane should parallel the slope of the tibial plateau. A guide pin is then placed just distal and parallel to the reference slot, taking care not to violate the posterior cortex. The pin is overreamed with a 7- or 8-mm cannulated drill, and a box-cutter osteotome is used to widen the trough to 7 to 8 mm and deepened to 10 mm.
Our preferred technique is to utilize a bone bridge to secure the meniscal graft to the tibial plateau. The allograft is debrided, and the meniscal allograft is then prepared to achieve the desired width and length as determined by the slot preparation (Figure 3). The bone bridge is then cut to a width of 7 mm and a height of 10 mm, allowing for bone extension to the anterior horn to aid in graft insertion. For meniscal insertion, a single-barrel, zone-specific meniscal repair cannula should be placed through the contralateral portal, and a Nitinol suture passing pin is placed through the capsular attachment site of the posterior and middle thirds of the meniscus. Through the anterior arthrotomy site, the proximal end of the Nitinol pin is withdrawn, the allograft traction sutures are passed through the loop of the Nitinol pin, and the bundle of pins and traction sutures are withdrawn through the accessory incision. The meniscal allograft is then pulled into the joint through the anterior arthrotomy, and the bone bridge is advanced into the tibial slot. The meniscus is manually reduced, with varus/valgus and flexion/extension stresses placed to aid in graft reduction. For fixation, a guide wire is placed between the bone bridge and medial eminence side of the slot, a tap is inserted over the guide wire, and a 7 × 25–mm bioabsorbable cortical interference screw is passed and secured. Finally, the graft is attached to the capsule using 8 to 10 standard inside-out vertical mattress sutures (Figure 4). Standard closure is then performed.56,57,67,92
Figure 3.
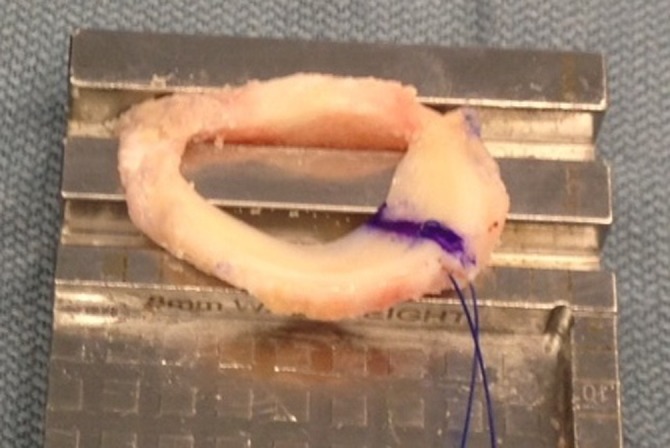
Intraoperative photographs of meniscal allograft transplantation.
Figure 4.
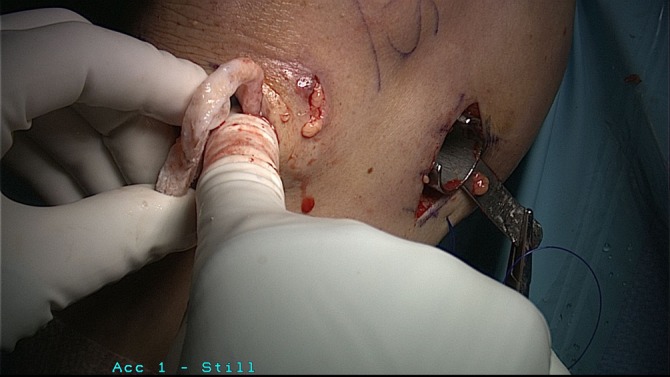
Placement of bone block in meniscal allograft transplantation.
No clear rehabilitation protocol exists; the initiation of weightbearing and range of motion exercises remains controversial. Patellar mobilization, therapeutic modalities, and quadriceps/hamstring strengthening can be initiated immediately as this will not impact the transplanted meniscus. Bracing, limited weightbearing, and protected range of motion are often recommended for the first 6 weeks to allow healing of the meniscus (Table 2).17,82
TABLE 2.
Functional Rehabilitation for Meniscal Allograft Transplantation
| Phase 1. Weeks 0-2: Protected weightbearing, hinged knee brace locked at 0°-90° of flexion |
| • Goal is to full extension |
| Phase 2. Weeks 2-6: Progression to full weightbearing |
| • Allowed full range of motion, strengthening, and closed- chain strengthening |
| Phase 3. Weeks 6-16 |
| • Progression to low-impact activities at 3 months |
| • Return to full activities at 4 months |
Results
Graft revascularization and cellular repopulation are essential to optimum graft functioning and restoration of biomechanics. Human retrieval studies have shown that transplants are partially repopulated by host cells.83,84 Histological evaluation of removed allograft tissue has shown decreased cellularity and growth factor production, which may partially account for the high rate of tears in allografts.79 In a recent systematic review, Verdonk et al117 reported that 75% to 90% of patients achieve fair to excellent clinical results after MAT with or without concomitant procedures. In a survivorship analysis of 100 viable meniscal transplants, Verdonk et al115 reported that mean survival time for medial or lateral transplantation was 11.6 years, as measured by pain or poor functioning.
Complications can be common and include arthrofibrosis with possible need for knee manipulation or arthroscopic debridement, loss of bony fixation, meniscal tears, failure to heal to the periphery, continued pain, and limited range of motion.59 In a retrospective review of 172 patients who underwent MAT, McCormick et al61 reported a 32% reoperation rate, with 59% of these being a simple arthroscopic debridement, and a 95% allograft survival rate at a mean of 5 years. Noyes et al66 found that 29 of 96 transplanted menisci had to be removed less than 2 years after transplantation. Cameron and Saha20 and Rath et al79 also found that 6 of 67 and 8 of 22 implants, respectively, required reoperation for symptomatic tears. Shelton and Dukes100 found that loss of bone plug fixation occurred in 1 of 15 patients 6 days after implantation.
High Tibial Osteotomy
Indications
HTO also presents as an option for patients suffering from unicompartmental arthrosis postmeniscectomy. Indications for HTO are noted in Table 3.7,86,124,125 Unicompartmental knee arthroplasty (UKA) is an alternative to HTO for the patient with unicompartmental tibiofemoral disease, and total knee arthroplasty (TKA) is an option for older patients and for those with global arthritis.
TABLE 3.
Patient Selection for High Tibial Osteotomy
| Ideal Candidate | Contraindications |
|---|---|
| • Age <60 y | • Severe articular damage |
| • Good range of motion | • Tricompartmental arthrosis |
| • No ligamentous instability | • Patellofemoral arthrosis |
| • Isolated medial compartment arthrosis | • Decreased range of motion |
| • Varus alignment |
Surgical Techniques
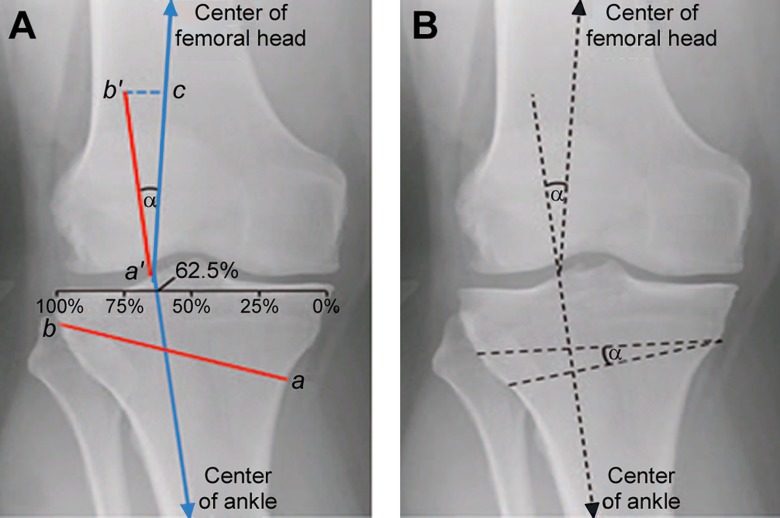
Surgical techniques include closing-wedge osteotomy, opening-wedge osteotomy, dome osteotomy, progressive callus distraction, and chevron osteotomy; the most common are the closing- and opening-wedge osteotomies. In preoperative planning, the angular degree of deformity can be calculated according to the technique described by Dugdale et al.26 The weightbearing method is performed using standing, mechanical axis, weightbearing films. The tibial plateau is divided into percentages (0% to 100%, with 0% as the medial side and 100% as the lateral side), and a line is drawn from the center of the femoral head to the center of the talar dome, which normally passes through a point located at 62.5% of the tibial width and equates to 3° to 5° of mechanical valgus (Figure 5, line c). In planning for an open-wedge HTO, a line is drawn from this point to the center of the femoral head, and another line from this point to the center of the ankle joint (Figure 5).8,32,34,124 The angle between the 2 lines represents the angle of correction (Figure 5). A second measurement that can be used to template for HTO uses a full-length standing mechanical axis (with markers to correct for view magnification) radiograph to determine the wedge height using trigonometric principles to place the mechanical axis at the desired location within the joint.
Figure 5.
Angle of correction for a high tibial osteotomy, α. (A) To determine the angle of correction for an opening wedge osteotomy, a line was first drawn from a point located at 62.5% of the width of the tibial plateau to the center of the femoral head (line a′c). A second line was drawn from this point to the center of the ankle. The angle formed by the intersection of these line is the correction angle (α). An osteotomy line was then defined from 4 cm below the medial joint line to the tip of the fibular head (line ab). This line segment (ab) was then transferred to the rays of the angle α to obtain a′b′ and a′c, with the distance between (line b′c) corresponding to the opening that should be achieved medially at the osteotomy site. (B) Using the same principles in (A), the angle of correction α was measured. In contrast to an opening-wedge osteotomy, the osteotomy site and angle of correction were transferred to the proximal tibia to form a triangle with a lateral base.
The osteotomy line is measured from medial (about 4 cm below the joint line) to lateral (tip of the fibular head). This measurement is transferred to both rays of the angle of correction from the vertex (equal to the osteotomy length), which are then connected by another line, which corresponds to the opening that should be achieved medially at the osteotomy site.86 Planning is similar in closing-wedge osteotomy, as the angle of deformity is calculated in the same manner, but the osteotomy itself consists of 2 cuts. Intra-articular fractures are a risk of all HTO techniques and can impair stability as well as articular surface congruency. Pearls for HTO are listed in Table 4.
TABLE 4.
Pearls for High Tibial Osteotomya
| • To minimize the risk of fracture, carry the apex of the osteotomy cut to within 10 mm of the far cortex. Leave the proximal fragment at least 15 mm thick. |
| • If medial or lateral hinge fractures or intra-articular fractures occur, achieve stable fixation with a locked plate, additional screws, or a plate to reduce the risk of loss of correction and nonunion. |
| • Use a drain at the incision site to minimize the risk of compartment syndrome. |
| • Deep venous thrombosis prophylaxis is recommended after HTO. |
| • Distal fibular osteotomy (15 cm distal to fibular head) decreases the risk of peroneal nerve injury. |
| • During LCW osteotomy, use rigid internal fixation and aggressive postoperative mobilization to help prevent patellar tendon contracture and patella baja. |
aHTO, high tibial osteotomy; LCW, lateral closing-wedge.
Medial Opening-Wedge Osteotomy
In the varus postmeniscectomized knee, HTO aims to produce valgus realignment of the proximal tibial articular surface. The patient is positioned supine on a radiolucent operating table with a tourniquet placed high around the thigh. Knee arthroscopy is performed to assess for any associated chondral and ligamentous pathology. Once arthroscopy is completed, a 5-cm longitudinal incision is made extending from 1 cm below the medial joint line between the medial border of the tibial tubercle and the posteromedial border of the tibia. Sharp dissection is performed down to the sartorius fascia, and the pes anserinus is retracted distally with a blunt retractor, exposing the superficial MCL. The MCL is retracted posteromedially, thereby exposing the tibial cortex. Alternatively, it can be transected and later repaired. The posterior neurovascular structures are now at risk and should be protected by passing a blunt retractor on bone deep to the MCL. Next, the patellar tendon is retracted laterally. A guide wire is then inserted under fluoroscopic guidance. The wire should enter approximately 4 cm distal to the joint line, passing from the anteromedial tibia aiming for 1 cm below the tip of the fibular head on the coronal view. On the sagittal view, the slope of the osteotomy should mimic the proximal tibial joint slope.
The tibial osteotomy is performed using a small oscillating saw, beginning just distal to the guide pin to avoid extension of the osteotomy into the joint and taking care to not cut past the medial and posteromedial cortices. The surgeon must remember the natural triangular shape of the tibia when making the bone cuts with the saw, as it comes to a point anteriorly and has much more bone posteriorly to avoid perforating the anterolateral cortex. Graduated thin flexible osteotomes are then used to advance the osteotomy to within 1 cm of the lateral tibial cortex using fluoroscopic guidance. A gentle valgus force can be applied to the leg to check the mobility of the osteotomy. If there is insufficient opening with valgus stress, 2 or 3 osteotomes can be stacked on one another at the osteotomy site to achieve the desired opening and minimize the risk of intra-articular fractures. A calibrated osteotome is then used to open the osteotomy to the desired opening. A long alignment rod can be used to assess the accuracy of the calculated preoperative wedge size, and when centered over the hip and ankle joints, should lie at approximately 62.5% of the tibial width at the level of the knee.86 Once the desired correction is obtained, plating is performed and the wedges are removed. Plating options include short or long, locked or unlocked, and with or without a spacer. The size of the metal block should be selected to match the opening created with the calibrated wedges. The defect is then grafted using bone graft of choice; iliac crest autograft or allograft are recommended for openings measuring >10 mm and bone substitutes for smaller corrections.34,86,124 Typically, patients with ACL-deficient knees undergo ACL reconstruction months prior to the osteotomy.
The advantages of a medial opening-wedge osteotomy include stable fixation, relative surgical ease in obtaining the desired amount of angular correction, and less extensive surgical dissection adding leg length. Furthermore, there is no need for mobilization of the proximal fibula and less operative risk to the peroneal nerve. Disadvantages of medial opening-wedge HTO include intraoperative fracture, loss of fixation, nonunion, delayed union leading to instability, and delayed weightbearing up to 6 to 8 weeks.34 Additionally, if distraction plate fixation is used with autograft, there will be morbidity from the autograft harvest site. To reduce the chance of morbidity from autologous bone marrow harvest, other options to fill the osseous gap can include synthetic bone substitutes (hydroxyapatite, β-tricalcium phosphate, bone cement) with or without PRP, growth factors, and bone marrow stromal cells.7 If an external fixator is used, potential for pin site infection is introduced, which could jeopardize subsequent TKA.125
Lateral Closing-Wedge Osteotomy
Historically, lateral closing-wedge osteotomy was common in treatment of varus malalignment but has fallen out of favor recently due to the higher risk for complications and imprecision in achieving the desired angle of correction. However, newer calibration methods and cutting guides still make this technique a viable option. In comparison with medial opening-wedge osteotomy, the initial incision for a lateral closing-wedge osteotomy is L-shaped, with the vertical limb along the lateral edge of the tibial tubercle and the horizontal limb parallel and 1 cm distal to the lateral joint line. After blunt dissection, either a fibular osteotomy distal to the fibular head or complete excision of the fibular head is performed, disrupting the proximal tibiofibular joint. Next, a laterally based wedge is removed, with saw cuts to within 1 cm of the medial cortex. After fluoroscopic confirmation, fixation of the osteotomy is achieved with staples driven from lateral to medial just anterior to the fibula, or for more rigid fixation, a laterally applied contoured locking plate or T-plate can be used.6,86
After both opening- and closing-wedge HTO, the knee is usually placed into a range of motion brace set at 0° to 90°, and the patient is restricted to touch weightbearing for 6 weeks. Weightbearing and range of motion are gradually increased from 6 to 12 weeks to allow for healing of the osteotomy (Table 5).86
TABLE 5.
Functional Rehabilitation for Distal Femoral Osteotomy/High Tibial Osteotomya
| Phase 1. Immediate in-hospital and home convalescence care for 0-2 weeks |
| • HKB locked in extension except when using CPM |
| • Goal is 90° of flexion and full extension by second postoperative week |
| Phase 2. Nonweightbearing while the osteotomy site heals for 2-6 weeks |
| • Hip girdle strengthening (straight leg raises) |
| Phase 3. Gradual and progressive weightbearing and strengthening after bone healing for 6-12 weeks |
| Phase 4. Return to full activities at 3-9 months |
| • Begin with low-impact activities (bicycle, elliptical) |
| • Progress to high-impact activities at 6 months |
aCPM, continuous passive motion; HKB, hinged knee brace.
The advantage of lateral closing-wedge osteotomy is that it produces apposition of 2 broad native metaphyseal surfaces, increasing healing potential and stability.125 In early practice, lateral closing-wedge osteotomy was performed with freehand cuts and stabilized with bone staples or cylinder casts, leading to imprecision in the amount of correction and potential patella baja. More recently, integration of calibrated cutting guides and rigid internal fixation devices have improved results and precision.86 Intraoperative fracture is similarly risky in lateral closing-wedge osteotomy as opening-wedge osteotomy. Disadvantages of lateral closing-wedge HTO include proximal tibiofibular joint disruption, peroneal nerve injury, difficulty with subsequent total knee arthroplasty, loss of bone stock, leg-length discrepancy, and patellar baja.
Other Osteotomies
The dome osteotomy is not commonly performed, as it is more technically demanding to create a curved osteotomy and avoid injury to the patellar tendon. It is indicated for a larger correction (>18-20 mm of opening or closing or angle of correction >20°).86 A major advantage of the dome osteotomy is it allows for concomitant anteriorization of the tibial tuberosity, alleviating specific types of patellofemoral disease. In the chevron osteotomy, a wedge is removed medially and added laterally followed by rigid plate fixation. This technique is less often used as it is more invasive and technically challenging. In the callus distraction technique, an opening-wedge osteotomy is created and progressively distracted with an external fixator (Ilizarov technique).86
Results
Clinical success of HTO slowly deteriorates with time; however, the mean range of effectiveness is typically more than 7 to 10 years, which, in select candidates, can provide valuable time before a UKA or TKA.7,39 Imprecise correction of preoperative angular deformity is the biggest contributor to HTO failure; overcorrection is more desirable than undercorrection.18,103 In a systematic review of HTO, Harris et al39 identified 57 studies (4344 knees) of isolated HTO. They found that across the studies, survival of isolated HTO gradually decreased over time up to a 20-year follow-up. Survival of isolated HTO was 92.4%, 84.5%, 77.3%, and 72.3% at 5, 10, 15, and 20 years of follow-up, respectively. Harris et al39 also found 4 studies that directly compared medial opening-wedge osteotomy with lateral closing-wedge osteotomy, with no difference in survivorship or clinical outcomes in long-term follow-up after more than 2 years. In a randomized clinical trial, Luites et al60 compared 42 patients treated with either a medial opening-wedge or lateral closing-wedge osteotomy and found no difference in time to regain knee function and full weightbearing and no related translation of bone based on radiostereometry. Song et al104 similarly retrospectively compared outcomes of both medial opening and lateral closing osteotomy techniques at 3-year follow-up and found no significant difference in anterior knee pain, patellar alignment, or patellofemoral arthritis. Preston et al76 compared outcomes of TKA in 275 patients after medial opening- versus lateral closing-wedge osteotomy and found no significant difference in survivorship, pain, or functional outcomes between techniques. Overall, HTO is an effective procedure for the physiologically young patient allowing for return to impact activities and decreased pain, with no significant differences seen between medial opening-wedge and lateral closing-wedge osteotomies. In a case series of 65 patients with a mean 3-year follow-up after HTO, Salzmann et al91 found that 90% of patients were engaged in sports at the same frequency and duration as preoperatively. However, it should be noted that as patients age, many go on to require total knee arthroplasty, the results of which are affected by previous high tibial osteotomy. Parvizi et al73 found a very high rate of radiographic evidence of loosening in patients with total knee arthroplasties after high tibial osteotomy.
Distal Femoral Osteotomy
Indications
Although less common, isolated lateral compartment osteoarthritis can occur after meniscectomy. In these cases, HTO and MAT are less commonly described. However, distal femoral varus osteotomy (DFO) is an option for select patients, with the goal of correcting the mechanical axis of the lower limb to 0° to 3°.108 The indications for DFO are seen in Table 6.3,23,120,121 Preoperative radiographic assessment is similar to that described by Dugdale et al26 for HTO. Using AP radiographs, the weightbearing line is placed at a selected position 48% to 50% across the width of the tibial plateau from medial to lateral. The desired correction angle is formed by the angle between a line from the center of the femoral head to 50% of the width of the tibia and a line from the center of the talus to the 50% coordinate.77
TABLE 6.
Patient Selection for Distal Femoral Osteotomy
| Ideal Candidate | Contraindications |
|---|---|
| • Physiologically young | • Severe articular damage |
| • Valgus deformity of >12°-15° | • Tricompartmental arthrosis |
| • Joint-line obliquity >10° | • Inflammatory arthritis |
| • Flexion of at least 90° | • Decreased range of motion |
| • <15° flexion contracture |
Surgical Technique
DFO can be approached using either a medial closing osteotomy or, more commonly, a lateral opening-wedge osteotomy.2,77,120 The patient is positioned supine on the table with the knee flexed to 30°. After diagnostic arthroscopy, a 15-cm longitudinal lateral incision is made starting 2 cm distal to the lateral epicondyle, approximately at the Gerdy tubercle, and extending proximally. The vastus lateralis is reflected laterally, and a guide wire is inserted on the lateral aspect of the femur 3 to 4 cm proximal to the epicondyle, directed to a point just proximal to the medial epicondyle. An oscillating saw is used for the osteotomy and extended using an osteotome to ensure a hinge of at least 1 cm of medial cortex. The osteotomy is opened to obtain the planned correction, and a plate is then used with 2 distal and 4 proximal screws to secure the osteotomy.62,77,89,110 The gap created can be similarly filled with autografts or allografts as described for medial opening-wedge osteotomy. Rehabilitation protocol is similar to high tibial osteotomy, with limited range of motion for the first 6 weeks. Complications of DFO are similar to HTO and include delayed union or nonunion, fracture, rotational deformity, infection, vascular injury, and deep venous thrombosis.87,88 DFO has been established for treatment of isolated lateral compartment arthritis in select patients, with a mean survivorship of 80% at 10-year follow-up30,33,120 In addition, the Ilizarov method can be used in which a distracting external fixator is applied after osteotomy formation, with adjustments made to distract callus formation.37
Staged Versus Concomitant Procedures
MAT and HTO
Postmeniscectomized patients with malalignment may not achieve optimum results with cartilage salvage procedures alone.38 An HTO performed concomitantly with meniscal transplantation may both unload the affected compartment and improve outcomes of the transplant.71,113 Concomitant MAT and HTO can be considered if there are no architectural changes in the femoral condyle or areas of full-thickness cartilage loss >10 mm on the weightbearing zone.71 The MAT is performed first, as the varus and valgus stresses needed during implantation could jeopardize the osteotomy. If an opening-wedge osteotomy is performed, it should be done as distally as possible, at least 1.5 cm below the bottom of the tibial slot.57 Amendola5 and Bonasia and Amendola16 have further delineated the technique for concomitant meniscal transplant and high tibial osteotomy. Verdonk et al115,116 reported on the 10-year survivorship of patients who underwent isolated medial meniscal transplantation (MMT) compared with MMT and HTO. They found that the survivorship was 83.3% for MMT + HTO compared with 74.2% for MMT alone; however, it is unclear which factor led to the increased survivorship. In a systematic review, Harris et al39 found 3 studies that examined the outcomes of combined HTO and MAT. They found 91% survival at 5-year follow-up in 113 knees.
ACL Reconstruction and Meniscal Treatment
In the young, active patient, concomitant injury to the meniscus and ACL is common. There are an estimated 200,000 ACL injuries yearly, and approximately 40% to 60% of these cases involve concomitant meniscal tears.68 Meniscal transplantation can be combined with ACL reconstruction in symptomatically painful and unstable knees. The ACL tibial tunnel is first drilled as vertically and obliquely as possible, followed by the femoral tunnel. The meniscal slot is prepared, and femoral fixation of the ACL graft is completed. Then, the meniscal transplantation is performed followed finally by the tibial fixation of the ACL graft.57 Success of ACL reconstruction is affected by the status of the meniscus. In a retrospective study of 235 patients at 8-year follow-up, the International Knee Documentation Committee (IKDC) rating was normal for 87% of patients with both menisci present, 70% in patients with partial or total lateral meniscectomies, 63% for those with partial or total medial meniscectomies, and 50% for patients with both menisci removed.98 Addressing meniscal injury and ACL deficiency concurrently can lead to optimal outcomes in select patients. A retrospective review of 28 patients who underwent concomitant meniscal transplantation and ACL reconstruction showed the combined procedure was beneficial for patients with chronic ACL deficiency or failed reconstruction, with 85% having normal subjective assessments and 90% having normal Lachman and pivot-shift tests at a mean of 2.8 years of follow-up.93
In cases of subacute or chronic ACL deficiency with varus malalignment, HTO can be combined with ACL reconstruction in a single-stage operation.65,74 When performing a combined HTO and ACL reconstruction, the osteotomy should be performed first and the tibial slope should be reduced, as an increase in posterior tibial slope has been associated with an increase in ACL reconstruction failure.58,105 Posterior plate positioning during the osteotomy is preferred to reduce tibial slope and avoid interference between the proximal screws and tibial tunnel. In a retrospective review of 29 patients with chronic knee instability and medial compartmental arthritis, Trojani et al111 found that at 6-year follow-up, 70% of patients had relief of pain and 80% had restored knee stability, enabling return to sports. Similarly, Zaffagnini et al126 retrospectively reported on 32 patients who underwent concurrent ACL reconstruction and closing-wedge lateral HTO. At a mean of 6.5 years of follow-up, 2 patients were considered failures, osteoarthritis progression was recorded as severe in 22% of patients, and no patients underwent revision osteotomy, ACL revision, UKA, or TKA.
Cartilage Restoration and Meniscal Treatment
Cartilage reparative procedures should also be considered if Outerbridge grade 3 or 4 lesions are present. Cartilage lesions in the meniscal weightbearing zones can predispose to meniscal allograft failure, and cartilage restoration should therefore be performed concurrently. Restorative procedures such as autologous chondrocyte implantation (ACI), reparative procedures such as microfracture, or reconstructive procedures such as osteochondral auto- or allograft can augment meniscal transplantation to optimize results. All steps of the meniscal transplantation should be performed first; however, care should be taken to avoid damage to the anterior horn of the transplanted meniscus during osteochondral grafting. In a systematic review, Harris et al38 found 6 studies that examined concurrent MAT and cartilage restoration with either ACI (n = 73), osteochondral allograft (n = 20), osteochondral autograft transfer system (OATS; n = 17), or microfracture (n = 3). In 4 of 6 studies, overall outcomes from combined surgeries were the same as either procedure performed alone; in 2 studies, outcomes were inferior to either procedure performed alone. Also, 12% of patients required revision surgery, most commonly due to failure of the MAT. Similarly, a systematic review of 9 studies analyzed concurrent HTO and articular cartilage surgery. There was a 98% survival at 5-year follow-up, with no significant difference between opening- and closing-wedge HTO when combined with articular cartilage surgery.39
Meniscal Replacement
Although not approved for use in the United States, other options for the meniscectomized knee exist in Europe, including collagen meniscal implants, polymers, hydrogels, and stem cells. Collagen meniscal implants are enriched with hyaluronic acid, chondroitin sulfate, and glycosaminoglycans to mimic the properties of the native meniscus.17,49 Early clinical studies have shown these implants to be safe and improve patient activity. Synthetic polymers, another form of meniscal scaffolds, use polyurethane to give biomechanical strength as well as polycaprolactone to allow for tissue integration.102 Hydrogels have also been suggested as a solution to meniscal replacement; however, in animal studies, their wear properties have questioned safety and reliability in a clinical trial.17
Conclusion
The meniscus plays an important role in maintaining knee stability and slowing the development of osteoarthritis. Unfortunately, a large portion of meniscal tears are irreparable, leading to partial or total meniscectomy. A meniscus-deficient knee leads to pain and decreased activity, which can be debilitating for young, active patients. Correction of limb alignment and preserving cartilage by restoring joint mechanics can help to slow the progression to osteoarthritis. High tibial osteotomy, meniscal allograft transplantation, and distal femoral osteotomy are viable options for select patients after meniscectomy. With appropriate patient selection and meticulous surgical technique, excellent outcomes with infrequent complications have been described. Future work and clinical trials should explore the use of concomitant high tibial osteotomy and meniscal allograft transplant in the postmeniscectomized knee and further elucidate the long-term outcomes of these procedures.
Footnotes
One or more of the authors has declared the following potential conflicts of interest or source of funding: A.B.Y. receives research support from Arthex Inc and NuTech. B.R.B. receives research support from Arthrex, Conmed Linvatec, DJ Orthopaedics, Ossur, Smith & Nephew, and Tornier and receives publishing royalties from SLACK Inc. B.J.C. receives research support from Aesculap/B.Braun, Arthrex, Cytori, Medipost, Ossur, Smith & Nephew, Tornier, and Zimmer; is a paid consultant for Arthrex, Regentis, and Zimmer; receives royalties from Arthrex, DJ Orthopaedics, Elsevier, Saunders/Mosby-Elsevier, and SLACK Inc; and has stock/stock options in Carticept and Regentis.
References
- 1. Abat F, Gelber PE, Erquicia JI, Pelfort X, Gonzalez-Lucena G, Monllau JC. Suture-only fixation technique leads to a higher degree of extrusion than bony fixation in meniscal allograft transplantation. Am J Sports Med. 2012;40:1591–1596. [DOI] [PubMed] [Google Scholar]
- 2. Aglietti P, Menchetti PP. Distal femoral varus osteotomy in the valgus osteoarthritic knee. Am J Knee Surg. 2000;13:89–95. [PubMed] [Google Scholar]
- 3. Aglietti P, Stringa G, Buzzi R, Pisaneschi A, Windsor RE. Correction of valgus knee deformity with a supracondylar V osteotomy. Clin Orthop Relat Res. 1987;217:214–220. [PubMed] [Google Scholar]
- 4. Allen PR, Denham RA, Swan AV. Late degenerative changes after meniscectomy. Factors affecting the knee after operation. J Bone Joint Surg Br. 1984;66:666–671. [DOI] [PubMed] [Google Scholar]
- 5. Amendola A. Knee osteotomy and meniscal transplantation: indications, technical considerations, and results. Sports Med Arthrosc. 2007;15:32–38. [DOI] [PubMed] [Google Scholar]
- 6. Amendola A. Unicompartmental osteoarthritis in the active patient: the role of high tibial osteotomy. Arthroscopy. 2003;19(suppl 1):109–116. [DOI] [PubMed] [Google Scholar]
- 7. Amendola A, Bonasia DE. Results of high tibial osteotomy: review of the literature. Int Orthop. 2010;34:155–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Amis AA. Biomechanics of high tibial osteotomy. Knee Surg Sports Traumatol Arthrosc. 2013;21:197–205. [DOI] [PubMed] [Google Scholar]
- 9. Angele P, Johnstone B, Kujat R, et al. Stem cell based tissue engineering for meniscus repair. J Biomed Mater Res A. 2008;85:445–455. [DOI] [PubMed] [Google Scholar]
- 10. Anz AW, Hackel JG, Nilssen EC, Andrews JR. Application of biologics in the treatment of the rotator cuff, meniscus, cartilage, and osteoarthritis. J Am Acad Orthop Surg. 2014;22:68–79. [DOI] [PubMed] [Google Scholar]
- 11. Arnoczky SP, Warren RF. Microvasculature of the human meniscus. Am J Sports Med. 1982;10:90–95. [DOI] [PubMed] [Google Scholar]
- 12. Bai B, Shun H, Yin ZX, Liao ZW, Chen N. Changes of contact pressure and area in patellofemoral joint after different meniscectomies. Int Orthop. 2012;36:987–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Baratz ME, Fu FH, Mengato R. Meniscal tears: the effect of meniscectomy and of repair on intraarticular contact areas and stress in the human knee. A preliminary report. Am J Sports Med. 1986;14:270–275. [DOI] [PubMed] [Google Scholar]
- 14. Bin Abd Razak HR, Heng HY, Cheng KY, Mitra AK. Correlation between radiographic and arthroscopic findings in Asian osteoarthritic knees. J Orthop Surg (Hong Kong). 2014;22:155–157. [DOI] [PubMed] [Google Scholar]
- 15. Bolano LE, Grana WA. Isolated arthroscopic partial meniscectomy. Functional radiographic evaluation at five years. Am J Sports Med. 1993;21:432–437. [DOI] [PubMed] [Google Scholar]
- 16. Bonasia DE, Amendola A. Combined medial meniscal transplantation and high tibial osteotomy. Knee Surg Sports Traumatol Arthrosc. 2010;18:870–873. [DOI] [PubMed] [Google Scholar]
- 17. Brophy RH, Matava MJ. Surgical options for meniscal replacement. J Am Acad Orthop Surg. 2012;20:265–272. [DOI] [PubMed] [Google Scholar]
- 18. Brouwer RW, Bierma-Zeinstra SM, van Raaij TM, Verhaar JA. Osteotomy for medial compartment arthritis of the knee using a closing wedge or an opening wedge controlled by a Puddu plate. A one-year randomised, controlled study. J Bone Joint Surg Br. 2006;88:1454–1459. [DOI] [PubMed] [Google Scholar]
- 19. Burks RT, Metcalf MH, Metcalf RW. Fifteen-year follow-up of arthroscopic partial meniscectomy. Arthroscopy. 1997;13:673–679. [DOI] [PubMed] [Google Scholar]
- 20. Cameron JC, Saha S. Meniscal allograft transplantation for unicompartmental arthritis of the knee. Clin Orthop Relat Res. 1997;337:164–171. [DOI] [PubMed] [Google Scholar]
- 21. Cooper DE, Arnoczky SP, Warren RF. Meniscal repair. Clin Sports Med. 1991;10:529–548. [PubMed] [Google Scholar]
- 22. Covall DJ, Wasilewski SA. Roentgenographic changes after arthroscopic meniscectomy: five-year follow-up in patients more than 45 years old. Arthroscopy. 1992;8:242–246. [DOI] [PubMed] [Google Scholar]
- 23. Coventry MB. Osteotomy about the knee for degenerative and rheumatoid arthritis. J Bone Joint Surg Am. 1973;55:23–48. [PubMed] [Google Scholar]
- 24. Cummins JF, Mansour JN, Howe Z, Allan DG. Meniscal transplantation and degenerative articular change: an experimental study in the rabbit. Arthroscopy. 1997;13:485–491. [DOI] [PubMed] [Google Scholar]
- 25. Dragoo JL, Danial CM, Braun HJ, Pouliot MA, Kim HJ. The chondrotoxicity of single-dose corticosteroids. Knee Surg Sports Traumatol Arthrosc. 2012;20:1809–1814. [DOI] [PubMed] [Google Scholar]
- 26. Dugdale TW, Noyes FR, Styer D. Preoperative planning for high tibial osteotomy. The effect of lateral tibiofemoral separation and tibiofemoral length. Clin Orthop Relat Res. 1992;274:248–264. [PubMed] [Google Scholar]
- 27. Duygulu F, Demirel M, Atalan G, et al. Effects of intra-articular administration of autologous bone marrow aspirate on healing of full-thickness meniscal tear: an experimental study on sheep. Acta Orthop Traumatol Turc. 2012;46:61–67. [DOI] [PubMed] [Google Scholar]
- 28. Fabricant PD, Jokl P. Surgical outcomes after arthroscopic partial meniscectomy. J Am Acad Orthop Surg. 2007;15:647–653. [DOI] [PubMed] [Google Scholar]
- 29. Fairbank TJ. Knee joint changes after meniscectomy. J Bone Joint Surg Br. 1948;30B:664–670. [PubMed] [Google Scholar]
- 30. Finkelstein JA, Gross AE, Davis A. Varus osteotomy of the distal part of the femur. A survivorship analysis. J Bone Joint Surg Am. 1996;78:1348–1352. [DOI] [PubMed] [Google Scholar]
- 31. Fitzgibbons RE, Shelbourne KD. “Aggressive” nontreatment of lateral meniscal tears seen during anterior cruciate ligament reconstruction. Am J Sports Med. 1995;23:156–159. [DOI] [PubMed] [Google Scholar]
- 32. Fujisawa Y, Masuhara K, Shiomi S. The effect of high tibial osteotomy on osteoarthritis of the knee. An arthroscopic study of 54 knee joints. Orthop Clin North Am. 1979;10:585–608. [PubMed] [Google Scholar]
- 33. Gardiner A, Richmond JC. Periarticular osteotomies for degenerative joint disease of the knee. Sports Med Arthrosc. 2013;21:38–46. [DOI] [PubMed] [Google Scholar]
- 34. Gomoll AH. High tibial osteotomy for the treatment of unicompartmental knee osteoarthritis: a review of the literature, indications, and technique. Phys Sportsmed. 2011;39(3):45–54. [DOI] [PubMed] [Google Scholar]
- 35. Greis PE, Bardana DD, Holmstrom MC, Burks RT. Meniscal injury: I. Basic science and evaluation. J Am Acad Orthop Surg. 2002;10:168–176. [DOI] [PubMed] [Google Scholar]
- 36. Greis PE, Homstrom MC, Bardana DD, Burks RT. Meniscal injury: II. Management. J Am Acad Orthop Surg. 2002;10:177–187. [DOI] [PubMed] [Google Scholar]
- 37. Gugenheim JJ, Jr, Brinker MR. Bone realignment with use of temporary external fixation for distal femoral valgus and varus deformities. J Bone Joint Surg Am. 2003;85-A:1229–1237. [DOI] [PubMed] [Google Scholar]
- 38. Harris JD, Cavo M, Brophy R, Siston R, Flanigan D. Biological knee reconstruction: a systematic review of combined meniscal allograft transplantation and cartilage repair or restoration. Arthroscopy. 2011;27:409–418. [DOI] [PubMed] [Google Scholar]
- 39. Harris JD, McNeilan R, Siston RA, Flanigan DC. Survival and clinical outcome of isolated high tibial osteotomy and combined biological knee reconstruction. Knee. 2013;20:154–161. [DOI] [PubMed] [Google Scholar]
- 40. Hepper CT, Halvorson JJ, Duncan ST, Gregory AJ, Dunn WR, Spindler KP. The efficacy and duration of intra-articular corticosteroid injection for knee osteoarthritis: a systematic review of level I studies. J Am Acad Orthop Surg. 2009;17:638–646. [DOI] [PubMed] [Google Scholar]
- 41. Horie M, Driscoll MD, Sampson HW, et al. Implantation of allogenic synovial stem cells promotes meniscal regeneration in a rabbit meniscal defect model. J Bone Joint Surg Am. 2012;94:701–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Horie M, Sekiya I, Muneta T, et al. Intra-articular injected synovial stem cells differentiate into meniscal cells directly and promote meniscal regeneration without mobilization to distant organs in rat massive meniscal defect. Stem Cells. 2009;27:878–887. [DOI] [PubMed] [Google Scholar]
- 43. Horlick SG, Loomer RL. Valgus knee bracing for medial gonarthrosis. Clin J Sport Med. 1993;3:251–255. [Google Scholar]
- 44. Huang A, Hull ML, Howell SM, Haut Donahue T. Identification of cross-sectional parameters of lateral meniscal allografts that predict tibial contact pressure in human cadaveric knees. J Biomech Eng. 2002;124:481–489. [DOI] [PubMed] [Google Scholar]
- 45. Hutchinson ID, Moran CJ, Potter HG, Warren RF, Rodeo SA. Restoration of the meniscus: form and function. Am J Sports Med. 2014;42:987–998. [DOI] [PubMed] [Google Scholar]
- 46. Ishida K, Kuroda R, Miwa M, et al. The regenerative effects of platelet-rich plasma on meniscal cells in vitro and its in vivo application with biodegradable gelatin hydrogel. Tissue Eng. 2007;13:1103–1112. [DOI] [PubMed] [Google Scholar]
- 47. Johnson DL, Swenson TM, Livesay GA, Aizawa H, Fu FH, Harner CD. Insertion-site anatomy of the human menisci: gross, arthroscopic, and topographical anatomy as a basis for meniscal transplantation. Arthroscopy. 1995;11:386–394. [DOI] [PubMed] [Google Scholar]
- 48. Jorgensen U, Sonne-Holm S, Lauridsen F, Rosenklint A. Long-term follow-up of meniscectomy in athletes. A prospective longitudinal study. J Bone Joint Surg Br. 1987;69:80–83. [DOI] [PubMed] [Google Scholar]
- 49. Kaleka CC, Debieux P, da Costa Astur D, Arliani GG, Cohen M. Updates in biological therapies for knee injuries: menisci. Curr Rev Musculoskelet Med. 2014;7:247–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kim JG, Lee YS, Bae TS, et al. Tibiofemoral contact mechanics following posterior root of medial meniscus tear, repair, meniscectomy, and allograft transplantation. Knee Surg Sports Traumatol Arthrosc. 2013;21:2121–2125. [DOI] [PubMed] [Google Scholar]
- 51. Kim SG, Nagao M, Kamata K, Maeda K, Nozawa M. Return to sport after arthroscopic meniscectomy on stable knees. BMC Sports Sci Med Rehabil. 2013;5:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kirkley A, Webster-Bogaert S, Litchfield R, et al. The effect of bracing on varus gonarthrosis. J Bone Joint Surg Am. 1999;81:539–548. [DOI] [PubMed] [Google Scholar]
- 53. Krause WR, Pope MH, Johnson RJ, Wilder DG. Mechanical changes in the knee after meniscectomy. J Bone Joint Surg Am. 1976;58:599–604. [PubMed] [Google Scholar]
- 54. Laible C, Stein DA, Kiridly DN. Meniscal repair. J Am Acad Orthop Surg. 2013;21:204–213. [DOI] [PubMed] [Google Scholar]
- 55. Lane CG, Warren R, Pearle AD. The pivot shift. J Am Acad Orthop Surg. 2008;16:679–688. [DOI] [PubMed] [Google Scholar]
- 56. LaPrade RF, Wills NJ, Spiridonov SI, Perkinson S. A prospective outcomes study of meniscal allograft transplantation. Am J Sports Med. 2010;38:1804–1812. [DOI] [PubMed] [Google Scholar]
- 57. Lee AS, Kang RW, Kroin E, Verma NN, Cole BJ. Allograft meniscus transplantation. Sports Med Arthrosc. 2012;20:106–114. [DOI] [PubMed] [Google Scholar]
- 58. Li Y, Hong L, Feng H, Wang Q, Zhang H, Song G. Are failures of anterior cruciate ligament reconstruction associated with steep posterior tibial slopes? A case control study. Chin Med J (Engl). 2014;127:2649–2653. [PubMed] [Google Scholar]
- 59. Lubowitz JH, Verdonk PC, Reid JB, 3rd, Verdonk R. Meniscus allograft transplantation: a current concepts review. Knee Surg Sports Traumatol Arthrosc. 2007;15:476–492. [DOI] [PubMed] [Google Scholar]
- 60. Luites JW, Brinkman JM, Wymenga AB, van Heerwaarden RJ. Fixation stability of opening- versus closing-wedge high tibial osteotomy: a randomised clinical trial using radiostereometry. J Bone Joint Surg Br. 2009;91:1459–1465. [DOI] [PubMed] [Google Scholar]
- 61. McCormick F, Harris JD, Abrams GD, et al. Survival and reoperation rates after meniscal allograft transplantation: analysis of failures for 172 consecutive transplants at a minimum 2-year follow-up. Am J Sports Med. 2014;42:892–897. [DOI] [PubMed] [Google Scholar]
- 62. McDermott AG, Finklestein JA, Farine I, Boynton EL, MacIntosh DL, Gross A. Distal femoral varus osteotomy for valgus deformity of the knee. J Bone Joint Surg Am. 1988;70:110–116. [PubMed] [Google Scholar]
- 63. McDermott ID, Amis AA. The consequences of meniscectomy. J Bone Joint Surg Br. 2006;88:1549–1556. [DOI] [PubMed] [Google Scholar]
- 64. McNickle AG, Wang VM, Shewman EF, Cole BJ, Williams JM. Performance of a sterile meniscal allograft in an ovine model. Clin Orthop Relat Res. 2009;467:1868–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Naudie DD, Amendola A, Fowler PJ. Opening wedge high tibial osteotomy for symptomatic hyperextension-varus thrust. Am J Sports Med. 2004;32:60–70. [DOI] [PubMed] [Google Scholar]
- 66. Noyes FR, Barber-Westin SD, Butler DL, Wilkins RM. The role of allografts in repair and reconstruction of knee joint ligaments and menisci. Instr Course Lect. 1998;47:379–396. [PubMed] [Google Scholar]
- 67. Noyes FR, Barber-Westin SD, Rankin M. Meniscal transplantation in symptomatic patients less than fifty years old. J Bone Joint Surg Am. 2005;87(suppl 1):149–165. [DOI] [PubMed] [Google Scholar]
- 68. Noyes FR, Heckmann TP, Barber-Westin SD. Meniscus repair and transplantation: a comprehensive update. J Orthop Sports Phys Ther. 2012;42:274–290. [DOI] [PubMed] [Google Scholar]
- 69. Ode GE, Van Thiel GS, McArthur SA, et al. Effects of serial sectioning and repair of radial tears in the lateral meniscus. Am J Sports Med. 2012;40:1863–1870. [DOI] [PubMed] [Google Scholar]
- 70. Outerbridge RE. The etiology of chondromalacia patellae. J Bone Joint Surg Br. 1961;43-B:752–757. [DOI] [PubMed] [Google Scholar]
- 71. Packer JD, Rodeo SA. Meniscal allograft transplantation. Clin Sports Med. 2009;28:259–283. [DOI] [PubMed] [Google Scholar]
- 72. Papageorgiou CD, Gil JE, Kanamori A, Fenwick JA, Woo SL, Fu FH. The biomechanical interdependence between the anterior cruciate ligament replacement graft and the medial meniscus. Am J Sports Med. 2001;29:226–231. [DOI] [PubMed] [Google Scholar]
- 73. Parvizi J, Hanssen AD, Spangehl MJ. Total knee arthroplasty following proximal tibial osteotomy: risk factors for failure. J Bone Joint Surg Am. 2004;86-A:474–479. [DOI] [PubMed] [Google Scholar]
- 74. Phisitkul P, Wolf BR, Amendola A. Role of high tibial and distal femoral osteotomies in the treatment of lateral-posterolateral and medial instabilities of the knee. Sports Med Arthrosc. 2006;14:96–104. [DOI] [PubMed] [Google Scholar]
- 75. Pollo FE, Jackson RW. Knee bracing for unicompartmental osteoarthritis. J Am Acad Orthop Surg. 2006;14:5–11. [DOI] [PubMed] [Google Scholar]
- 76. Preston S, Howard J, Naudie D, Somerville L, McAuley J. Total knee arthroplasty after high tibial osteotomy: no differences between medial and lateral osteotomy approaches. Clin Orthop Relat Res. 2014;472:105–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Puddu G, Cipolla M, Cerullo G, Franco V, Gianni E. Osteotomies: the surgical treatment of the valgus knee. Sports Med Arthrosc. 2007;15:15–22. [DOI] [PubMed] [Google Scholar]
- 78. Rannou F, Poiraudeau S, Beaudreuil J. Role of bracing in the management of knee osteoarthritis. Curr Opin Rheumatol. 2010;22:218–222. [DOI] [PubMed] [Google Scholar]
- 79. Rath E, Richmond JC, Yassir W, Albright JD, Gundogan F. Meniscal allograft transplantation. Two- to eight-year results. Am J Sports Med. 2001;29:410–414. [DOI] [PubMed] [Google Scholar]
- 80. Recht MP, Kramer J. MR imaging of the postoperative knee: a pictorial essay. Radiographics. 2002;22:765–774. [DOI] [PubMed] [Google Scholar]
- 81. Richmond J, Hunter D, Irrgang J, et al. Treatment of osteoarthritis of the knee (nonarthroplasty). J Am Acad Orthop Surg. 2009;17:591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Rijk PC. Meniscal allograft transplantation—part I: background, results, graft selection and preservation, and surgical considerations. Arthroscopy. 2004;20:728–743. [DOI] [PubMed] [Google Scholar]
- 83. Rijk PC. Meniscal allograft transplantation—part II: alternative treatments, effects on articular cartilage, and future directions. Arthroscopy. 2004;20:851–859. [DOI] [PubMed] [Google Scholar]
- 84. Rodeo SA, Seneviratne A, Suzuki K, Felker K, Wickiewicz TL, Warren RF. Histological analysis of human meniscal allografts. A preliminary report. J Bone Joint Surg Am. 2000;82-A:1071–1082. [DOI] [PubMed] [Google Scholar]
- 85. Rosenberg TD, Paulos LE, Parker RD, Coward DB, Scott SM. The forty-five-degree posteroanterior flexion weight-bearing radiograph of the knee. J Bone Joint Surg Am. 1988;70:1479–1483. [PubMed] [Google Scholar]
- 86. Rossi R, Bonasia DE, Amendola A. The role of high tibial osteotomy in the varus knee. J Am Acad Orthop Surg. 2011;19:590–599. [DOI] [PubMed] [Google Scholar]
- 87. Rudan JF, Simurda MA. High tibial osteotomy. A prospective clinical and roentgenographic review. Clin Orthop Relat Res. 1990;255:251–256. [PubMed] [Google Scholar]
- 88. Rudan JF, Simurda MA. Valgus high tibial osteotomy. A long-term follow-up study. Clin Orthop Relat Res. 1991;268:157–160. [PubMed] [Google Scholar]
- 89. Saithna A, Kundra R, Getgood A, Spalding T. Opening wedge distal femoral varus osteotomy for lateral compartment osteoarthritis in the valgus knee. Knee. 2014;21:172–175. [DOI] [PubMed] [Google Scholar]
- 90. Salata MJ, Gibbs AE, Sekiya JK. A systematic review of clinical outcomes in patients undergoing meniscectomy. Am J Sports Med. 2010;38:1907–1916. [DOI] [PubMed] [Google Scholar]
- 91. Salzmann GM, Ahrens P, Naal FD, et al. Sporting activity after high tibial osteotomy for the treatment of medial compartment knee osteoarthritis. Am J Sports Med. 2009;37:312–318. [DOI] [PubMed] [Google Scholar]
- 92. Sekiya JK, Ellingson CI. Meniscal allograft transplantation. J Am Acad Orthop Surg. 2006;14:164–174. [DOI] [PubMed] [Google Scholar]
- 93. Sekiya JK, Giffin JR, Irrgang JJ, Fu FH, Harner CD. Clinical outcomes after combined meniscal allograft transplantation and anterior cruciate ligament reconstruction. Am J Sports Med. 2003;31:896–906. [DOI] [PubMed] [Google Scholar]
- 94. Shaffer B, Kennedy S, Klimkiewicz J, Yao L. Preoperative sizing of meniscal allografts in meniscus transplantation. Am J Sports Med. 2000;28:524–533. [DOI] [PubMed] [Google Scholar]
- 95. Shelbourne KD. The art of the knee examination: where has it gone? J Bone Joint Surg Am. 2010;92:e9. [DOI] [PubMed] [Google Scholar]
- 96. Shelbourne KD, Dickens JF. Digital radiographic evaluation of medial joint space narrowing after partial meniscectomy of bucket-handle medial meniscus tears in anterior cruciate ligament-intact knees. Am J Sports Med. 2006;34:1648–1655. [DOI] [PubMed] [Google Scholar]
- 97. Shelbourne KD, Dickens JF. Joint space narrowing after partial medial meniscectomy in the anterior cruciate ligament-intact knee. J Am Acad Orthop Surg. 2007;15:519–524. [DOI] [PubMed] [Google Scholar]
- 98. Shelbourne KD, Gray T. Results of anterior cruciate ligament reconstruction based on meniscus and articular cartilage status at the time of surgery. Five- to fifteen-year evaluations. Am J Sports Med. 2000;28:446–452. [DOI] [PubMed] [Google Scholar]
- 99. Shelbourne KD, Martini DJ, McCarroll JR, VanMeter CD. Correlation of joint line tenderness and meniscal lesions in patients with acute anterior cruciate ligament tears. Am J Sports Med. 1995;23:166–169. [DOI] [PubMed] [Google Scholar]
- 100. Shelton WR, Dukes AD. Meniscus replacement with bone anchors: a surgical technique. Arthroscopy. 1994;10:324–327. [DOI] [PubMed] [Google Scholar]
- 101. Sihvonen R, Paavola M, Malmivaara A, et al. Arthroscopic partial meniscectomy versus sham surgery for a degenerative meniscal tear. N Engl J Med. 2013;369:2515–2524. [DOI] [PubMed] [Google Scholar]
- 102. Smith BD, Grande DA. The current state of scaffolds for musculoskeletal regenerative applications. Nat Rev Rheumatol. 2015;11:213–222. [DOI] [PubMed] [Google Scholar]
- 103. Smith TO, Sexton D, Mitchell P, Hing CB. Opening- or closing-wedged high tibial osteotomy: a meta-analysis of clinical and radiological outcomes. Knee. 2011;18:361–368. [DOI] [PubMed] [Google Scholar]
- 104. Song IH, Song EK, Seo HY, Lee KB, Yim JH, Seon JK. Patellofemoral alignment and anterior knee pain after closing- and opening-wedge valgus high tibial osteotomy. Arthroscopy. 2012;28:1087–1093. [DOI] [PubMed] [Google Scholar]
- 105. Sonnery-Cottet B, Mogos S, Thaunat M, et al. Proximal tibial anterior closing wedge osteotomy in repeat revision of anterior cruciate ligament reconstruction. Am J Sports Med. 2014;42:1873–1880. [DOI] [PubMed] [Google Scholar]
- 106. Spang JT, Dang AB, Mazzocca A, et al. The effect of medial meniscectomy and meniscal allograft transplantation on knee and anterior cruciate ligament biomechanics. Arthroscopy. 2010;26:192–201. [DOI] [PubMed] [Google Scholar]
- 107. Szomor ZL, Martin TE, Bonar F, Murrell GA. The protective effects of meniscal transplantation on cartilage. An experimental study in sheep. J Bone Joint Surg Am. 2000;82:80–88. [DOI] [PubMed] [Google Scholar]
- 108. Terry GC, Cimino PM. Distal femoral osteotomy for valgus deformity of the knee. Orthopedics. 1992;15:1283–1289. [DOI] [PubMed] [Google Scholar]
- 109. Terzidis IP, Christodoulou A, Ploumis A, Givissis P, Natsis K, Koimtzis M. Meniscal tear characteristics in young athletes with a stable knee: arthroscopic evaluation. Am J Sports Med. 2006;34:1170–1175. [DOI] [PubMed] [Google Scholar]
- 110. Thein R, Bronak S, Thein R, Haviv B. Distal femoral osteotomy for valgus arthritic knees. J Orthop Sci. 2012;17:745–749. [DOI] [PubMed] [Google Scholar]
- 111. Trojani C, Elhor H, Carles M, Boileau P. Anterior cruciate ligament reconstruction combined with valgus high tibial osteotomy allows return to sports. Orthop Traumatol Surg Res. 2014;100:209–212. [DOI] [PubMed] [Google Scholar]
- 112. Trojani C, Sbihi A, Djian P, et al. Causes for failure of ACL reconstruction and influence of meniscectomies after revision. Knee Surg Sports Traumatol Arthrosc. 2011;19:196–201. [DOI] [PubMed] [Google Scholar]
- 113. Van Thiel GS, Frank RM, Gupta A, et al. Biomechanical evaluation of a high tibial osteotomy with a meniscal transplant. J Knee Surg. 2011;24:45–53. [DOI] [PubMed] [Google Scholar]
- 114. Vangsness CT, Jr, Farr J, 2nd, Boyd J, Dellaero DT, Mills CR, LeRoux-Williams M. Adult human mesenchymal stem cells delivered via intra-articular injection to the knee following partial medial meniscectomy: a randomized, double-blind, controlled study. J Bone Joint Surg Am. 2014;96:90–98. [DOI] [PubMed] [Google Scholar]
- 115. Verdonk PC, Demurie A, Almqvist KF, Veys EM, Verbruggen G, Verdonk R. Transplantation of viable meniscal allograft. Survivorship analysis and clinical outcome of one hundred cases. J Bone Joint Surg Am. 2005;87:715–724. [DOI] [PubMed] [Google Scholar]
- 116. Verdonk PC, Verstraete KL, Almqvist KF, et al. Meniscal allograft transplantation: long-term clinical results with radiological and magnetic resonance imaging correlations. Knee Surg Sports Traumatol Arthrosc. 2006;14:694–706. [DOI] [PubMed] [Google Scholar]
- 117. Verdonk R, Volpi P, Verdonk P, et al. Indications and limits of meniscal allografts. Injury. 2013;44(suppl 1):S21–S27. [DOI] [PubMed] [Google Scholar]
- 118. Verma NN, Kolb E, Cole BJ, et al. The effects of medial meniscal transplantation techniques on intra-articular contact pressures. J Knee Surg. 2008;21:20–26. [DOI] [PubMed] [Google Scholar]
- 119. Veth RP. Clinical significance of knee joint changes after meniscectomy. Clin Orthop Relat Res. 1985;198:56–60. [PubMed] [Google Scholar]
- 120. Wang JW, Hsu CC. Distal femoral varus osteotomy for osteoarthritis of the knee. J Bone Joint Surg Am. 2005;87:127–133. [DOI] [PubMed] [Google Scholar]
- 121. Wang JW, Hsu CC. Distal femoral varus osteotomy for osteoarthritis of the knee. Surgical technique. J Bone Joint Surg Am. 2006;88(suppl 1, pt 1):100–108. [DOI] [PubMed] [Google Scholar]
- 122. Watterson JR, Esdaile JM. Viscosupplementation: therapeutic mechanisms and clinical potential in osteoarthritis of the knee. J Am Acad Orthop Surg. 2000;8:277–284. [DOI] [PubMed] [Google Scholar]
- 123. Williams RJ, 3rd, Warner KK, Petrigliano FA, Potter HG, Hatch J, Cordasco FA. MRI evaluation of isolated arthroscopic partial meniscectomy patients at a minimum five-year follow-up. HSS J. 2007;3:35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Wolcott M, Traub S, Efird C. High tibial osteotomies in the young active patient. Int Orthop. 2010;34:161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Wright JM, Crockett HC, Slawski DP, Madsen MW, Windsor RE. High tibial osteotomy. J Am Acad Orthop Surg. 2005;13:279–289. [DOI] [PubMed] [Google Scholar]
- 126. Zaffagnini S, Bonanzinga T, Grassi A, et al. Combined ACL reconstruction and closing-wedge HTO for varus angulated ACL-deficient knees. Knee Surg Sports Traumatol Arthrosc. 2013;21:934–941. [DOI] [PubMed] [Google Scholar]
- 127. Zellner J, Mueller M, Berner A, et al. Role of mesenchymal stem cells in tissue engineering of meniscus. J Biomed Mater Res A. 2010;94:1150–1161. [DOI] [PubMed] [Google Scholar]