Abstract
Progress in high throughput “-omic” techniques now allows the simultaneous measurement of expression levels of thousands of genes and promises the improved understanding of the molecular biology of diseases such as cancer. Detection of the dysfunction of molecular pathways in diseases requires healthy control tissue. This is difficult to obtain from pheochromocytomas (PHEOs), rare chromaffin tumors derived from adrenal medulla. The two options for obtaining adrenal tissue are: (1) whole organ removal post-mortem or during radical nephrectomy; (2) removal during PHEO surgery. Access to high quality normal adrenal tissue is limited. Removal of whole adrenals during nephrectomy is rare, because of improved surgical techniques. For adrenals removed post-mortem, the lag time to proper organ perfusion causes uncontrolled tissue degradation. Adjacent normal adrenal tissue can almost never be obtained from resected PHEOs, because they often replace the entire medulla or are well-encapsulated. If a margin of normal adrenal is attached to a resected PHEO, it seldom contains any medulla. The clean separation of medulla and cortex is further complicated, because their border is convoluted, and because adult adrenal consists of ~90% cortex. Thus, the quality of separation has to be evaluated with specific medullary and cortical markers. We describe the successful dissection of highly pure, medullary tissue from adrenals snap-frozen upon resection during radical nephrectomy or after brain death. Separation quality has been verified by quantitative reverse transcription with polymerase chain reaction for the medullary enzymes, tyrosine hydroxylase, and chromogranin A, and for the cortical enzyme, steroidogenic acute regulator.
Keywords: Adrenal medulla, Chromogranin A, Steroidogenic acute regulator, Pheochromocytoma, Chromaffin cell, Human
Introduction
Tumorigenesis and metastasising are complex mechanisms involving multiple interlinked cellular pathways. Knowledge of these pathways can be increased with well-designed microarray, proteomic, and metabolomic studies. The best results will be obtained if the tissue of tumor origin (e.g., adjacent tissue) is procured and examined together with the tumor tissue. Ideally, tumor tissue is compared with adjacent non-tumorous tissue from the same patient. However, obtaining adjacent adrenal medulla from a surgical specimen is almost impossible for pheochromocytomas (PHEO), catecholamine-producing tumors arising from the adrenal medulla (Pacak et al. 2001). PHEOs often replace the entire medulla. If a rim of normal adrenal tissue is present on a removed tumor, it is generally identified as adrenal cortex. In the rare case that adrenal tissue containing medulla is removed during surgery, the clean separation of adrenal medulla from cortical tissue is extremely difficult, because the medulla accounts for just 10% of the volume of the adult adrenal gland. Furthermore, the border between the cortex and medulla is convoluted, increasingly so with age (Quinan and Berger 1933; Kreiner 1982; Schinner and Bornstein 2005). Because of the lack of healthy adrenal medulla control samples, the molecular biology of PHEOs has commonly been studied by comparing different hereditary types of PHEOs or by comparing adrenal to extra-adrenal PHEOs. Thus, in the present study, our aim has been to obtain highly pure human adrenal medulla tissue that can be used as comparative tissue in the study of PHEO. Moreover, well-separated adrenal medulla and cortical tissue will be valuable in establishing new model systems to study cross-talk between these developmentally distinct tissues.
Here, we describe the dissection of highly pure adrenal medulla tissue, as verified by quantitative reverse transcription with polymerase chain reaction (qRT-PCR) for the medullary enzymes, tyrosine hydroxylase (TH) and chromogranin A (CgA), and for the cortical enzyme, steroidogenic acute regulator (StAR). Adrenal expression of StAR has been shown to be restricted to cortical cells (Pollack et al. 1997), whereas the expression of CgA and TH is limited to medullary chromaffin cells. The presence of CgA is routinely used as a marker of neuroendocrine cells.
Materials and methods
Tissue
Human normal adrenal glands (n=7), from six anonymous organ donors without any indication of adrenal dysfunction or tumor, were collected during radical nephrectomy or within 2–5 h after confirmed brain death at the Department of Urology, School of Medicine, Comenius University, Bratislava, Slovakia. Patient age, gender, and cause of adrenalectomy are summarized in Table 1. Whole adrenal glands were snap-frozen upon removal and stored and shipped at −80°C.
Table 1.
Patient information (M male, F female, NK not known)
| Adrenal identification number | Gender | Age | Cause of adrenalectomy |
|---|---|---|---|
| 1 | M | 61 | Renal cell carcinoma |
| 2 | NK | NK | NK |
| 3 | F | 53 | Multiorgan harvest |
| 4 | F | 72 | Kidney transplantation |
| 5 | F | 72 | Kidney transplantation |
Separation of cortex and medulla was performed as previously described (Tischler et al. 1980; Hansen et al. 1988). To prevent tissue from cracking during processing, the adrenals were allowed to warm slightly in a Petri dish on wet ice. The adrenals were cut centrally, perpendicular to the longest axis. The cut surface was visually inspected for the presence of adrenal medulla. The border between the cortex and medulla was easily identified by the red/brown color of the cortical zona reticularis. When sufficient gray/pink medulla for separation was visible, several sections of 3–5 mm in width were taken. Otherwise, the tissue was again cut perpendicular to the longest axis at a distance of 1 cm from the first cut, until an area with a wider section of medulla was found. The tissue cuts were examined under the dissection microscope, and the gray/pink medulla was carefully cut out of the surrounding cortex with size 11 scalpel blades. Medulla, cortex, and inseparable mixtures of medullary and cortical tissue were collected in separate containers for RNA extraction. The tissue was kept frozen on dry ice at all times.
qRT-PCR protocol
For RNA extraction, the tissue was homogenized in Trizol (Invitrogen, Carlsbad, Calif., USA). After the addition of chloroform and separation of the chloroform and water phase by centrifugation at 12,000g, the aqueous phase was brought to a concentration of 37% ethanol, and the RNA was captured by using the RNeasy mini kit (Qiagen, Valencia, Calif., USA) following the manufacturer’s instructions. The amount of RNA of each sample was assessed spectrophotometrically with the NanoDrop ND 1000 (Thermo Scientific, Wilmington, Del., USA).
The SuperScript First Strand kit (Invitrogen) was used for RT of 0.5 μg total RNA into cDNA, following the product manual. Quantitation of StAR, TH, and CgA was performed by using the 7500 RT PCR System and Taqman Gene expression assays (Hs00264912_m1, Hs00165941_m1, Hs00154441_m1; Applied Biosystems, Foster City, Calif., USA). The relative expression of CgA to StAR was calculated based on the delta-delta Ct method.
Estimation of cortical contamination
To estimate the expression levels of CgA and StAR in the human adrenal medulla and cortex, expressed sequence tag (EST)-based transcript per million (TPM) values for CgA and StAR were used from the NCBI-UniGene-EST Profile Viewer. On the assumption that the cDNA for the EST analysis was generated from representative normal adrenal samples, i.e., those consisting of 90% cortex and 10% medulla, the CgA to StAR ratio for a 1:1 mixture of absolutely pure cortical and medullary tissue was calculated as follows: (TPMCgA*90)/(TPMStAR*10)=(450*90)/(11013*10)=0.36775. The percentage of cortical tissue for each sample was calculated by using this estimator (Table 2).
Table 2.
Estimated percentage of cortical tissue in each sample
| Adrenal identification number | Cortex | Medulla | Inseparable cortex plus medulla |
|---|---|---|---|
| 1 | 95.56 | 0.37 | 24.25 |
| 2 | 95.31 | 4.94 | 55.58 |
| 3 | 90.51 | 4.51 | 23.70 |
| 4 | 98.24 | 4.86 | 28.27 |
| 5 | 92.61 | 4.27 | 25.63 |
Results and discussion
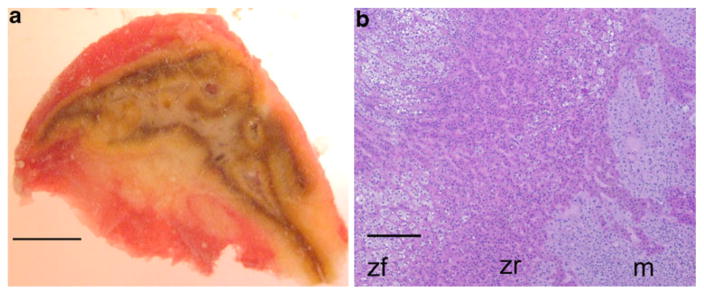
The medulla comprised a continuous area large enough for separation in five of the seven adrenal glands examined. In the two remaining adrenals, convolution of the cortex and medulla was so prominent that separation was not attempted. We successfully separated medullary tissue from these five glands by using a dissection microscope and eye-surgical instruments. Clean separation was difficult because of cortical islands within the medulla, the convoluted border between cortical tissue and medulla, and the central vein, which is mostly surrounded by a layer of cortical cells traversing the medulla (Fig. 1; Kreiner 1982).
Fig. 1.
Convoluted character of adrenal medulla and cortex. a Central section of adrenal number 5. The gray-pink area is medulla, whereas the yellow and brown tissue is of cortical origin. The adrenal is tightly embedded in a capsule of connective tissue and fat. b Hematoxylin and eosin staining of a section of adrenal number 1. Cortical zona fisciculata (zf) and zona reticularis (zr) cells overlap with medullary (m) cells at the border. Bar 50 m (a), 100 μm (b)
The separation quality was validated by qRT-PCR as previously described for mouse tissue (Powers et al. 2007). TH and CgA levels in all samples correlated well with each other. The definitive estimation of separation quality was based on the expression of CgA, because its medullary expression level turned out to be in the same range as that of StAR in the cortex. The separation led to samples of medullary tissue showing ratios of CgA to StAR that were 202 to 5833 times higher than were ratios from cortical tissue (Fig. 2).
Fig. 2.
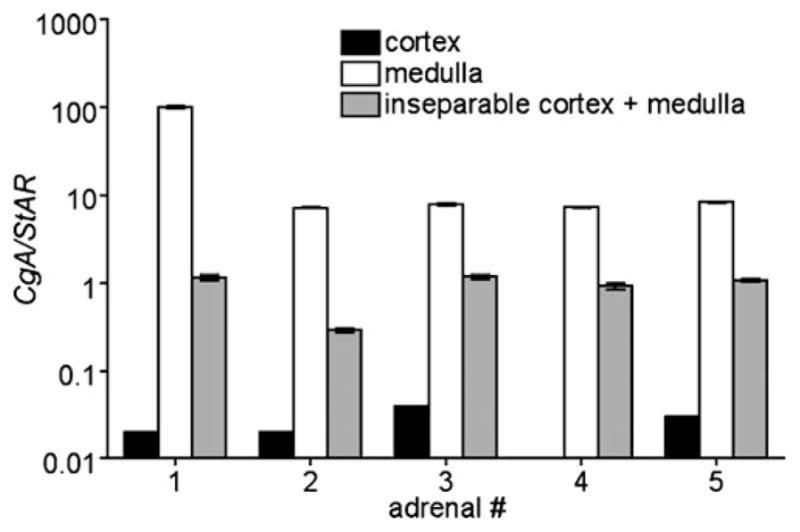
Relative mRNA level of CGA to StAR in cortex (black), medulla (white), and inseparable cortex and medulla tissue (gray) of five different adrenals (numbered 1–5, #)
To estimate the amount of tissue cross-contamination, knowledge of the average CgA expression level of medullary cells and the average StAR expression level in cortical cells is necessary. We estimated the expression ratio of CgA to StAR for a virtual 1:1 mixture of pure human adrenal medulla and cortex based on published EST analysis data. From this approximation, we estimated that all medulla samples that we had collected contained less than 5% cortical contamination (Table 2); thus, the separation of clean adrenal medulla was highly efficient.
A technical consideration in this study is that StAR mRNA may be differentially expressed in different zones of the adrenal cortex. In the rat adrenal cortex, the highest expression of StAR mRNA has been found in the zona reticularis and fasciculata (Peters et al. 1998). Contrarily, StAR protein immunostaining in human adrenals shows the least intense signal in the zona reticularis (Pollack et al. 1997). In any case, differences in StAR expression between the cortical zones could hypothetically influence the calculated amount of cortical contamination, because the contaminating tissue consists mostly of zona reticularis, which is in direct contact with the medulla. However, the impact on the estimation of cortical contamination will be minimal because all cortical cells, independent of zone, express high levels of StAR in comparison to medullary cells, which do not express StAR.
Highly pure, normal adrenal medulla tissue is required as healthy control tissue to study PHEO tumor biology. In addition, highly pure medullary and cortical tissue may be of great value in developing new in vitro models that will help in improving our understanding of the hormonal cross–talk between cortical and medullary cells. However, the obtaining of healthy medullary tissue provides a challenge, because: (1) the improvement of surgical techniques means that the removal of healthy whole adrenal glands during nephrectomy has become exceedingly rare; (2) normal adrenal tissue is seldom present in surgical specimens of adrenal tumors; (3) the adrenal medulla composes only about 10% of the volume of an adult adrenal gland, so that the presence of adrenal medulla in an adrenal tumor specimen almost never occurs; and (4) the boundary between cortex and medullary cells is typically convoluted, making separation difficult or impossible.
After the hurdle of tissue access is overcome, other criteria need to be met in order to obtain high quality adrenal medulla specimens, viz., optimal tissue preservation (1) during sample collection, (2) throughout the separation of cortex and medulla, and (3) during the process of separation quality control.
Preservation of tissue protein and RNA through rapid freezing is mandatory. In the past, adrenal glands have been collected from nephrectomies or post-mortem from kidney donations and autopsies (Jozan et al. 2007; Crickard et al. 1982). However, the rapid retrieval of post-mortem tissue is seldom achieved. Usually, a delay of several hours occurs. To avoid bias caused by uncontrolled degradation processes in the control tissue, fresh-frozen human tumor tissue should only be compared with equivalent fresh-frozen control tissue. To address these critical problems and to ensure the optimal preservation of tissue protein and RNA, we have used tissue from patients that were undergoing kidney surgery or from a patient that had died in the hospital from causes unrelated to adrenal dysfunction. The circulation of the deceased patient was supported after brain death until organ removal. The adrenal glands were immediately snap-frozen after removal.
The quality of RNA and protein extracts depends on rapid preservation through snap-freezing and on the maintenance of the frozen state during processing. Here, the tissue was kept below 0°C at all times, in order to minimize tissue degradation.
Because of the presence of cortical islands within the medulla and the convoluted borders between the cortex and medulla, the verification of the quality of separation is mandatory. Over the past 30 years, adrenal medullary tissue has been used to obtain cultures of primary human chromaffin cells for developmental and functional studies with and without digestion before physical separation from the cortex (Tischler and Slayton 1983; Tischler et al. 1985; Powers et al. 1998, 2009; Cavadas et al. 2001) and for therapeutic transplantation in multiple sclerosis (Lopez-Lozano et al. 1989) and chronic pain patients (Lazorthes et al. 1995; Sagen et al. 1993). In some older publications, the identity of the collected cells was not verified (e.g., Saria et al. 1980; Corder and Lowry 1982; Gaspar et al. 1989). Other authors have verified chromaffin cell origin by testing the response to acetylcholine in vitro (Grazzini et al. 1999), by measuring catecholamine levels (Evans et al. 1983), by testing for TH immunoreactivity (Tischler and Slayton 1983; Tischler et al. 1985; Powers et al. 1998, 2009; Cavadas et al. 2001; Lazorthes et al. 1995; Bés et al. 1998), or by labeling with neutral red and/or the catecholamine fluorescence technique (Hansen et al. 1988; Crickard et al. 1982; Lopez-Lozano et al. 1989). Most of these approaches are well suited for cultured cells but are not practical for tissue extracts. In vivo measurements cannot be performed on frozen tissue. Admittedly, a fragment of separated medulla can be used for TH immunoreactivity, neutral red staining, or the catecholamine fluorescence technique before RNA or protein extraction. However, the outcome will only represent the tested fragment. Alternatively, catecholamine levels can be measured after tissue homogenization. However, because of their membrane solubility, an additional measurement of cortical hormones will not allow an estimate of the degree of cortical contamination. Furthermore, optimal protection from degradation during the simultaneous extraction of catecholamines with RNA or protein will provide a challenge. For our purposes, these validation techniques are impracticable, because the degree of cortical contamination cannot be correctly estimated.
Highly pure, normal adrenal medulla tissue is crucial in “-omics” type studies of PHEO. In addition, the separation of fresh adrenal medullary and cortical cells will be an important first step in generating a human model system to study further the cross-talk between these organs during stress and development (Schinner and Bornstein 2005; Bornstein et al. 2000; Ehrhart-Bornstein and Bornstein 2008; Ehrhart-Bornstein et al. 1998, 2000). Here, we have presented the separation and validation of the purity of adrenal medullary RNA extracts. This RNA will be highly valuable in microarray studies. Comparable evaluations of the protein levels can be performed by enzyme-linked immunosorbent assay or Western blot for the use of tissue samples in proteomics studies.
Acknowledgments
This study was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, Md., USA and, in part, by a grant from the Pheo Para Alliance (to A.S.T).
We thank Frank Leopardi and Xiaojie Zhang (NIDDK) for permission to use their microdissection microscope.
Footnotes
No conflicts of interest arose during this work.
Contributor Information
Stephanie M. J. Fliedner, Program in Reproductive and Adult Endocrinology, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda MD 20892, USA
Jan Breza, Department of Urology, School of Medicine, Comenius University, 84248 Bratislava, Slovakia.
Richard Kvetnansky, Institute of Experimental Endocrinology, Slovak Academy of Sciences, Bratislava 83306, Slovakia.
James F. Powers, Department of Pathology, Tufts Medical Center, Boston, MA 02111, USA
Arthur S. Tischler, Department of Pathology, Tufts Medical Center, Boston, MA 02111, USA
Robert Wesley, Department of Health and Human Services, Clinical Center, National Institutes of Health, Bethesda, MD 20892, USA.
Maria Merino, Laboratory of Surgical Pathology, National Cancer Institute, National Institutes of Health, Bethesda, MD 20892, USA.
Hendrik Lehnert, 1st Department of Medicine, University of Lübeck, 23538 Lübeck, Germany.
Karel Pacak, Email: karel@mail.nih.gov, Program in Reproductive and Adult Endocrinology, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda MD 20892, USA. Section on Medical Neuroendocrinology, Reproductive and Adult Endocrinology Program, NICHD, NIH, Building 10, CRC, 1-East, Room 1-3140, 10 Center Drive, MSC-1109, Bethesda, MD 20892-1109, USA.
References
- Bés JC, Tkaczuk J, Czech KA, Tafani M, Bastide R, Caratero C, Pappas GD, Lazorthes Y. One-year chromaffin cell allograft survival in cancer patients with chronic pain: morphological and functional evidence. Cell Transplant. 1998;7:227–238. doi: 10.1177/096368979800700301. [DOI] [PubMed] [Google Scholar]
- Bornstein SR, Tian H, Haidan A, Böttner A, Hiroi N, Eisenhofer G, McCann SM, Chrousos GP, Roffler-Tarlov S. Deletion of tyrosine hydroxylase gene reveals functional interdependence of adrenocortical and chromaffin cell system in vivo. Proc Natl Acad Sci USA. 2000;97:14742–14747. doi: 10.1073/pnas.97.26.14742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavadas C, Silva AP, Mosimann F, Cotrim MD, Ribeiro CA, Brunner HR, Grouzmann E. NPY regulates catecholamine secretion from human adrenal chromaffin cells. J Clin Endocrinol Metab. 2001;86:5956–5963. doi: 10.1210/jcem.86.12.8091. [DOI] [PubMed] [Google Scholar]
- Corder R, Lowry PJ. Large-molecular-weight somatostatin in human adrenal medullary tissue. Biosci Rep. 1982;2:397–403. doi: 10.1007/BF01119302. [DOI] [PubMed] [Google Scholar]
- Crickard K, Fujii DK, Jaffe RB. Isolation and identification of human fetal adrenal medullary cells in vitro. J Clin Endocrinol Metab. 1982;55:1143–1148. doi: 10.1210/jcem-55-6-1143. [DOI] [PubMed] [Google Scholar]
- Ehrhart-Bornstein M, Bornstein SR. Cross-talk between adrenal medulla and adrenal cortex in stress. Ann N Y Acad Sci. 2008;1148:112–117. doi: 10.1196/annals.1410.053. [DOI] [PubMed] [Google Scholar]
- Ehrhart-Bornstein M, Hinson JP, Bornstein SR, Scherbaum WA, Vinson GP. Intraadrenal interactions in the regulation of adrenocortical steroidogenesis. Endocr Rev. 1998;19:101–143. doi: 10.1210/edrv.19.2.0326. [DOI] [PubMed] [Google Scholar]
- Ehrhart-Bornstein M, Haidan A, Alesci S, Bornstein SR. Neurotransmitters and neuropeptides in the differential regulation of steroidogenesis in adrenocortical-chromaffin co-cultures. Endocr Res. 2000;26:833–842. doi: 10.3109/07435800009048606. [DOI] [PubMed] [Google Scholar]
- Evans CJ, Erdelyi E, Weber E, Barchas JD. Identification of pro-opiomelanocortin-derived peptides in the human adrenal medulla. Science. 1983;221:957–960. doi: 10.1126/science.6308766. [DOI] [PubMed] [Google Scholar]
- Gaspar L, Chan JS, Seidah NG, Chretien M. Peptides related to the N-terminus of pro-opiomelanocortin in the human adrenal medulla. Clin Invest Med. 1989;12:90–98. [PubMed] [Google Scholar]
- Grazzini E, Breton C, Derick S, Andres M, Raufaste D, Rickwaert F, Boccara G, Colson P, Guérineau NC, Serradeil-le Gal C, Guillon G. Vasopressin receptors in human adrenal medulla and pheochromocytoma. J Clin Endocrinol Metab. 1999;84:2195–2203. doi: 10.1210/jcem.84.6.5775. [DOI] [PubMed] [Google Scholar]
- Hansen JT, Notter MF, Okawara SH, Gash DM. Organization, fine structure, and viability of the human adrenal medulla: considerations for neural transplantation. Ann Neurol. 1988;24:599–609. doi: 10.1002/ana.410240503. [DOI] [PubMed] [Google Scholar]
- Jozan S, Aziza J, Châtelin S, Evra C, Courtade-Saïdi M, Parant O, Sol JC, Zhou H, Lazorthes Y. Human fetal chromaffin cells: a potential tool for cell pain therapy. Exp Neurol. 2007;205:525–535. doi: 10.1016/j.expneurol.2007.03.020. [DOI] [PubMed] [Google Scholar]
- Kreiner E. Weight and shape of the human adrenal medulla in various age groups. Virchows Arch. 1982;397:7–15. doi: 10.1007/BF00430889. [DOI] [PubMed] [Google Scholar]
- Lazorthes Y, Bès JC, Sagen J, Tafani M, Tkaczuk J, Sallerin B, et al. Transplantation of human chromaffin cells for control of intractable cancer pain. Acta Neurochir Suppl. 1995;64:97–100. doi: 10.1007/978-3-7091-9419-5_21. [DOI] [PubMed] [Google Scholar]
- Lopez-Lozano JJ, Brera B, Abascal J, Bravo G. Preparation of adrenal medullary tissue for transplantation in Parkinson’s disease: a new procedure. Technical note. J Neurosurg. 1989;71:452–454. doi: 10.3171/jns.1989.71.3.0452. [DOI] [PubMed] [Google Scholar]
- Pacak K, Linehan WM, Eisenhofer G, Walther MM, Goldstein DS. Recent advances in genetics, diagnosis, localization, and treatment of pheochromocytoma. Ann Intern Med. 2001;134:315–329. doi: 10.7326/0003-4819-134-4-200102200-00016. [DOI] [PubMed] [Google Scholar]
- Peters B, Clausmeyer S, Obermüller N, Woyth A, Kränzlin B, Gretz N, Peters J. Specific regulation of StAR expression in the rat adrenal zona glomerulosa: an in situ hybridization study. J Histochem Cytochem. 1998;46:1215–1222. doi: 10.1177/002215549804601101. [DOI] [PubMed] [Google Scholar]
- Pollack SE, Furth EE, Kallen CB, Arakane F, Kiriakidou M, Kozarsky KF, Strauss JF., 3rd Localization of the steroidogenic acute regulatory protein in human tissues. J Clin Endocrinol Metab. 1997;82:4243–4251. doi: 10.1210/jcem.82.12.4445. [DOI] [PubMed] [Google Scholar]
- Powers JF, Tsokas P, Tischler AS. The ret-activating ligand GDNF is differentiative and not mitogenic for normal and neoplastic human chromaffin cells in vitro. Endocr Pathol. 1998;9:325–331. doi: 10.1007/BF02739692. [DOI] [PubMed] [Google Scholar]
- Powers JF, Evinger MJ, Zhi J, Picard KL, Tischler AS. Pheochromocytomas in Nf1 knockout mice express a neural progenitor gene expression profile. Neuroscience. 2007;147:928–937. doi: 10.1016/j.neuroscience.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Powers JF, Picard KL, Tischler AS. RET expression and neuron-like differentiation of pheochromocytoma and normal chromaffin cells. Horm Metab Res. 2009;41:710–714. doi: 10.1055/s-0029-1224136. [DOI] [PubMed] [Google Scholar]
- Quinan C, Berger AA. Observations on human adrenals with especial reference to the relative weight of the normal medulla. Ann Intern Med. 1933;6:1180–1192. [Google Scholar]
- Sagen J, Pappas GD, Winnie AP. Alleviation of pain in cancer patients by adrenal medullary transplants in the spinal subarachnoid space. Cell Transplant. 1993;2:259–266. doi: 10.1177/096368979300200310. [DOI] [PubMed] [Google Scholar]
- Saria A, Wilson SP, Molnar A, Viveros OH, Lembeck F. Substance P and opiate-like peptides in human adrenal medulla. Neurosci Lett. 1980;20:195–200. doi: 10.1016/0304-3940(80)90145-7. [DOI] [PubMed] [Google Scholar]
- Schinner S, Bornstein SR. Cortical-chromaffin cell interactions in the adrenal gland. Endocr Pathol. 2005;16:91–98. doi: 10.1385/ep:16:2:091. [DOI] [PubMed] [Google Scholar]
- Tischler AS, Slayton VW. Cholera toxin does not prevent neurite outgrowth from adult human chromaffin cells in culture. Exp Cell Res. 1983;143:454–456. doi: 10.1016/0014-4827(83)90072-1. [DOI] [PubMed] [Google Scholar]
- Tischler AS, DeLellis RA, Biales B, Nunnemacher G, Carabba V, Wolfe HJ. Nerve growth factor-induced neurite outgrowth from normal human chromaffin cells. Lab Invest. 1980;43:399–409. [PubMed] [Google Scholar]
- Tischler AS, Lee YC, Perlman RL, Costopoulos D, Bloom SR. Production of “ectopic” vasoactive intestinal peptide-like immunoreactivity in normal human chromaffin cell cultures. Life Sci. 1985;37:1881–1886. doi: 10.1016/0024-3205(85)90005-0. [DOI] [PubMed] [Google Scholar]