Abstract
Background
Measurements of plasma normetanephrine and metanephrine provide a useful diagnostic test for phaeochromocytoma, but this depends on appropriate reference intervals. Upper cut-offs set too high compromise diagnostic sensitivity, whereas set too low, false-positives are a problem. This study aimed to establish optimal reference intervals for plasma normetanephrine and metanephrine.
Methods
Blood samples were collected in the supine position from 1226 subjects, aged 5–84 y, including 116 children, 575 normotensive and hypertensive adults and 535 patients in whom phaeochromocytoma was ruled out. Reference intervals were examined according to age and gender. Various models were examined to optimize upper cut-offs according to estimates of diagnostic sensitivity and specificity in a separate validation group of 3888 patients tested for phaeochromocytoma, including 558 with confirmed disease.
Results
Plasma metanephrine, but not normetanephrine, was higher (P < 0.001) in men than in women, but reference intervals did not differ. Age showed a positive relationship (P < 0.0001) with plasma normetanephrine and a weaker relationship (P = 0.021) with metanephrine. Upper cut-offs of reference intervals for normetanephrine increased from 0.47 nmol/L in children to 1.05 nmol/L in subjects over 60 y. A curvilinear model for age-adjusted compared with fixed upper cut-offs for normetanephrine, together with a higher cut-off for metanephrine (0.45 versus 0.32 nmol/L), resulted in a substantial gain in diagnostic specificity from 88.3% to 96.0% with minimal loss in diagnostic sensitivity from 93.9% to 93.6%.
Conclusions
These data establish age-adjusted cut-offs of reference intervals for plasma normetanephrine and optimized cut-offs for metanephrine useful for minimizing false-positive results.
Introduction
Measurements of plasma free metanephrines, normetanephrine and metanephrine, have become a widely endorsed first-line screening test for phaeochromocytomas and paragangliomas.1–4 Superior diagnostic utility of the test over measurements of catecholamines and other metabolites is explained by the production of metanephrines within chromaffin cells, a process that occurs secondary to leakage of the parent catecholamines from vesicular stores into the cytoplasm where the presence of membrane-bound catechol-O-methyltransferase ensures immediate metabolism.5 Noradrenaline is converted to normetanephrine and adrenaline to metanephrine. The process is continuous and independent of catecholamine secretion, which in some chromaffin cell tumours can be minimal or intermittent.
The diagnostic advantages of measuring the metanephrines compared with catecholamines and other metabolites formed in non-chromaffin tissues is supported by numerous studies establishing a high level of reliability of positive test results for the metabolites in patients with tumours that produce either or both noradrenaline and adrenaline.6–13 Exceptions include normotensive and asymptomatic patients with very small tumours8 and some extra-adrenal paragangliomas that do not produce catecholamines14 or produce only dopamine, in which case diagnosis can be achieved using measurements of plasma methoxytyramine.15
As with almost all laboratory tests, reliability of measurements of plasma metanephrines for diagnosis of phaeochromocytoma is dependent on use of appropriately established reference intervals. Upper cut-offs for reference intervals set too high compromise diagnostic sensitivity, whereas false-positives are a problem when upper cut-offs are set too low. Due to the usually low pretest prevalence of phaeochromocytoma, false-positive results typically far outnumber true-positive results and are a problem for test interpretation.16 Consequently, borderline positive results are not always appropriately followed up,17 underlining a need for testing strategies and reference intervals that minimize false-positive results without compromising diagnostic sensitivity.
Because upright posture increases sympathoadrenal release of catecholamines and thus plasma concentrations of metanephrines,18–20 it is recommended that blood sampling for these measurements should be carried out after at least 20 min of supine rest.1,21,22 This is particularly important for normetanephrine, which is the metabolite most susceptible to influences of sympathoadrenal activation and also principally produced by phaeochromocytomas. Despite recommendations to draw blood in the supine position, reference intervals for plasma metanephrines are often established from samples drawn in the seated position.12,23–25 Consequently, reported upper cut-offs of reference intervals for plasma metanephrines vary widely, for normetanephrine from anywhere between 0.61 and 1.18 nmol/L.7,8,12,13,23–25
In this study, we sought to establish robust reference intervals for plasma free metanephrines using data from a population of 1226 subjects, all sampled in the supine position. To ascertain the appropriateness of different groups for establishing reference intervals, subjects included children, normotensive volunteers, hypertensive subjects and patients in whom catecholamine-producing tumours had been ruled out. We further examined the potential influences of age and gender based on previous observations concerning these variables and plasma free metanephrines.7,18,23,26,27 Finally, from the latter analyses, we explored different models to optimize upper cut-offs of reference intervals according to estimates of diagnostic sensitivity and specificity in a separate group of 3888 patients tested for phaeochromocytoma, including 558 with confirmed disease.
Subjects and methods
Subjects
The 1226 subjects for the reference population (Table 1) included 116 children, 317 normotensive volunteers, 258 hypertensives and 535 patients originally tested for phaeochromocytoma in whom the tumour was ruled out by follow-up or imaging studies as described elsewhere.8 The latter group was further divided into two subgroups according to whether or not testing was primarily based on signs and symptoms of catecholamine excess. These two subgroups included 331 patients tested because of an incidental mass found on imaging, an underlying germline mutation or a previous history of the tumour and 204 patients in whom tumours were originally suspected because of signs and symptoms of catecholamine excess. Subjects taking medications known to raise plasma concentrations of metanephrines (e.g. tricyclic antidepressants) were excluded. Subjects contributing to the reference population were enrolled under clinical protocols with written informed consent.
Table 1.
Characteristics of reference and validation populations
| Reference population (n = 1226) | Child volunteers | Normotensive volunteers | Hypertensive subjects | Patient group 1* | Patient group 2* |
|---|---|---|---|---|---|
| N | 116 | 317 | 258 | 331 | 204 |
| Age (median and range) | 13 (5–17) | 36 (18–73) | 48 (18–84) | 39 (18–77) | 50 (18–81) |
| Gender (F/M) | 59/57 | 168/149 | 138/120 | 193/138 | 121/83 |
| Validation population (n = 3888) | Patients with phaeochromocytoma | Patients without phaeochromocytoma |
|---|---|---|
| N | 558 | 3330 |
| Age (median and range) | 44 (6–89) | 54 (4–96) |
| Gender (F/M) | (277/281) | (1960/1367)† |
Group 1 represent patients tested because of an incidental mass found on imaging, an underlying germline mutation or a previous history of the tumour; patient group 2 represent patients tested because of signs and symptoms suggestive of phaeochromocytoma
For three patients there was no gender assignment
Data from the reference population were used to establish models for reference intervals, which were tested in a separate validation population of 3888 patients (Table 1). These patients included 558 with phaeochromocytoma or paraganglioma confirmed either by pathological examination of resected tumour tissue or for patients with unresectable metastatic disease, by functional imaging evidence of metastases according to previously described criteria.8,28 This group included 365 patients described elsewhere28 and another 193 more recently diagnosed cases. The other 3330 patients tested for phaeochromocytoma were not established to have tumours. Most of these patients (n = 3044) were tested at Dresden or Nijmegen as part of their routine clinical care. Use of these data received Local Ethics Committee approval subject to anonymity of the source of the data, restricted to age, gender and measured plasma concentrations of metanephrines.
Collection of blood samples
Blood samples from subjects of the reference population were collected after at least 20 min of supine rest. Subjects fasted and abstained from caffeinated and decaffeinated beverages overnight and avoided taking acetaminophen for five days before blood sampling. Samples of blood were transferred into tubes containing heparin as anticoagulant and placed on ice until centrifuged (4°C) to separate plasma, which was stored at −80°C until assayed. The same instructions were provided for blood samples collected for the validation population, but these were collected largely under routine conditions rather than under more strictly controlled research conditions.
Laboratory analyses
Measurements of plasma metanephrines were performed at three of the participating centres (National Institutes of Health, University of Dresden and Radboud University Nijmegen Medical Center) using a liquid chromatography with electrochemical detection (LC-ECD) procedure first established at the National Institutes of Health,29 and transferred to laboratories at Nijmegen and Dresden. Accuracy and precision of the LC-ECD procedures, as transferred to the two latter routine laboratories, was established from participation in an interlaboratory quality assurance programme described elsewhere.30 The results of six cycles of this programme from 2009 to 2011 established minimal bias and a high level of precision for measurements as revealed by interassay coefficients of variation averaging 5.1% for Dresden and 5.8% for Nijmegen (Supplementary Table 1; please see http://acb.rsmjournals.com/lookup/suppl/doi:10.1258/acb.2012.012066/-/DC1). Comparison of Nijmegen and Dresden data indicated relationships for both metabolites close to the lines of identity, with no differences for metanephrine, but with 4.5% lower (P < 0.0001) concentrations of normetanephrine recorded at Nijmegen than at Dresden (Supplementary Figure 1; http://acb.rsmjournals.com/lookup/suppl/doi:10.1258/acb.2012.012066/-/DC1).
Statistics
Because plasma concentrations of metanephrines are non-normally distributed, results for these analytes are presented as medians with reference intervals first established using the 2.5 and 97.5 percentiles of distributions.31 Spearman’s test was used to examine significance of correlations and Wilcoxon and Kruskal–Wallis tests were used for respective comparisons between two or multiple groups. Where the latter indicated significance, differences between individual groups were assessed by analysis of variance (ANOVA) in conjunction with Tukey’s honestly significant difference test following a power transformation to normalize data. Influences of multiple variables (i.e. age, subject group and gender) were similarly examined by ANOVA.
From the above analyses and considerations of previous reports of positive relations of plasma normetanephrine to age,7,18,23,27 various models for age-adjusted reference intervals were tested in the validation population of patients with and without phaeochromocytoma. A linear model was first generated by linear quantile regression analysis. Models were also generated by curvilinear fits to the nor-metanephrine data of the reference group, with further optimization of both sensitivity and specificity carried out with additional consideration of normetanephrine and metanephrine in the validation group. Models were tested based on calculations of diagnostic sensitivity and specificity as described elsewhere.8
Results derived from the above models were compared with those for traditional static (age-unadjusted) reference intervals determined from the 97.5 percentiles of distributions for the entire reference population. Another comparison was included using the formula described by Sawka et al.27 for an age-adjusted fractionated metanephrine score derived from a multivariable logistic regression model. For these comparisons, we applied several (n = 14) pairwise proportion tests with Bonferroni-adjusted P values (Padjusted = 0.05/14 = 0.00357). Statistical analyses utilized the JMP statistics software package (SAS Institute Inc., Cary, NC, USA) and R.32
Results
Reference population: influence of gender
Plasma concentrations of normetanephrine showed no significant differences between men and women, whereas median concentrations of metanephrine were 29% higher (P < 0.0001) in men than in women (Table 2). Upper and lower limits of reference intervals, as determined from respective 97.5 and 2.5 percentiles, nevertheless showed negligible differences among genders.
Table 2.
Medians and reference intervals (2.5 and 97.5 percentiles) for plasma normetanephrine and metanephrine according to gender and six age groups
| N | Age (years) Median |
Normetanephrine (nmol/L)
|
Metanephrine (nmol/L)
|
|||||
|---|---|---|---|---|---|---|---|---|
| Median | 97.5 percentile | 2.5 percentile | Median | 97.5 percentile | 2.5 percentile | |||
| All subjects | 1226 | 41.0 | 0.298 | 0.706 | 0.120 | 0.147 | 0.325 | 0.031 |
| Women | 679 | 40.2 | 0.293 | 0.710 | 0.125 | 0.132* | 0.315 | 0.035 |
| Men | 547 | 41.0 | 0.302 | 0.704 | 0.120 | 0.170† | 0.329 | 0.030 |
| 5–17 y | 116 | 13.2 | 0.248* | 0.470 | 0.048 | 0.172† | 0.333 | 0.045 |
| 18–29 y | 229 | 24.7 | 0.251* | 0.588 | 0.118 | 0.137* | 0.264 | 0.034 |
| 30–39 y | 232 | 34.5 | 0.273*† | 0.618 | 0.126 | 0.138* | 0.304 | 0.014 |
| 40–49 y | 283 | 45.0 | 0.300† | 0.687 | 0.115 | 0.147*† | 0.324 | 0.031 |
| 50–59 y | 241 | 53.0 | 0.362§ | 0.747 | 0.136 | 0.157† | 0.375 | 0.046 |
| >60 y | 125 | 65.4 | 0.355§ | 1.047 | 0.137 | 0.163† | 0.358 | 0.051 |
Presence of different symbols (*†§) indicates differences (P < 0.005) in normetanephrine or metanephrine between men and women or among different age groups
Reference population: influence of age
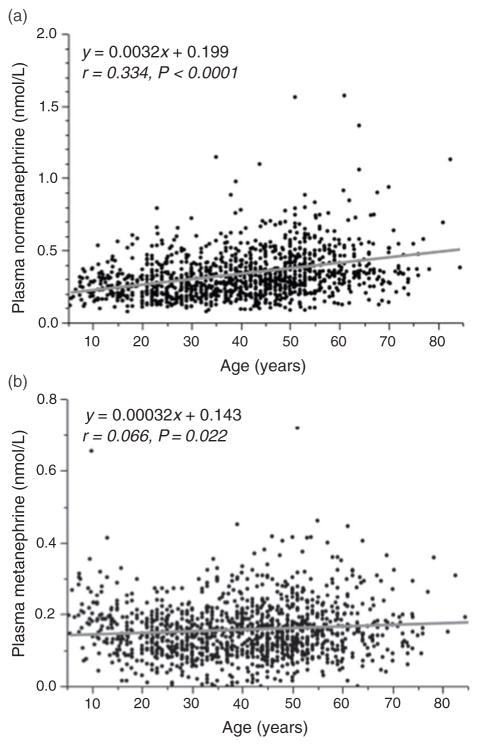
Plasma concentrations of normetanephrine showed a consistent increase (P < 0.0001) with advancing age (Table 2). Upper cut-offs for reference intervals, as determined from the 97.5 percentiles, also showed age-associated increases with more than a doubling from upper limits of 0.470 nmol/L in children to 1.047 nmol/L in subjects over 60 y. The association of advancing age with increased plasma concentration of normetanephrine was manifested by a positive relationship (r = 0.334, P < 0.0001) between age and plasma normetanephrine (Figure 1a).
Figure 1.
Scatter plots showing relationships of age with plasma concentrations of normetanephrine (a) and metanephrine (b) for subjects of the reference population (n = 1226)
A positive relationship was also observed between age and plasma concentrations of metanephrine (Figure 1b), but compared with that for normetanephrine (Figure 1a), this relationship was weak (r = 0.066, P = 0.022) and disturbed by concentrations in children that were higher (P < 0.0002) than in each of the two next highest adult age groups, but not different from subsequent more elderly groups (Table 2). Upper cut-offs for reference intervals for plasma metanephrine showed less than 30% differences among the various age groups.
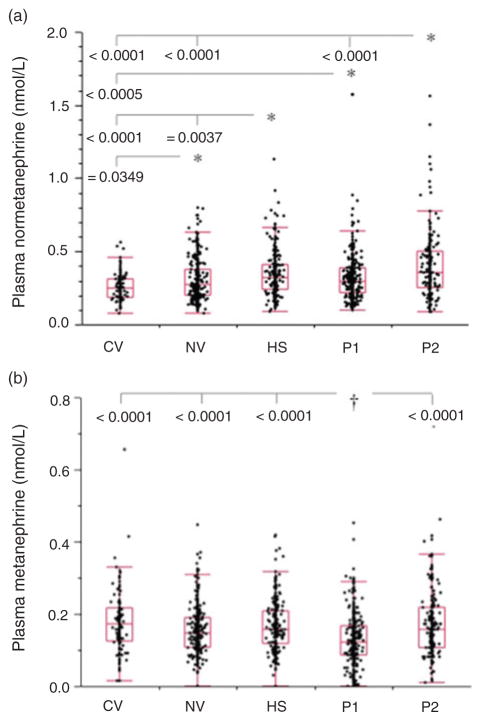
Reference population: influence of subject type
Plasma concentrations of normetanephrine and metanephrine both showed significant (P < 0.0001) differences among the five subject groups that comprised the reference population (Figure 2). Plasma concentrations of normetanephrine were higher (P < 0.05) in each of the four adult groups than in the paediatric group (Figure 2a). Among adult groups, plasma concentrations of normetanephrine were higher (P < 0.0001) in patient group 2, tested for phaeochromocytoma because of signs and symptoms of catecholamine excess, than in normotensive volunteers and those in patient group 1, tested for phaeochromocytoma because of an incidental mass found on imaging, an underlying germ-line mutation or a previous history of the tumour. The hypertensive group also had higher (P = 0.0037) plasma concentrations of normetanephrine than normotensive volunteers.
Figure 2.
Box plots showing distributions of plasma concentrations of normetanephrine (a) and metanephrine (b) among the five subgroups of the reference population. CV, child volunteers; NV, normotensive heathy volunteers; HS, hypertensive subjects; P1, patient group 1 (tested for phaeochromocytoma because of an incidental mass found on imaging, an underlying germline mutation or a previous history of the tumour); P2, patient group 2 (tested for phaeochromocytoma because of signs and symptoms of catecholamine excess). *†Indicates groups with higher* and lower† concentrations than other connected groups at the indicated levels of significance
After correcting for influences of age, almost all differences in plasma concentrations of normetanephrine among the five subject groups were no longer apparent. The exception was patient group 2, for which plasma concentrations of normetanephrine remained higher than in normotensive volunteers (P = 0.0083).
For metanephrine, plasma concentrations were lower (P < 0.0001) in patient group 1 than in each of the four other groups (Figure 2b). These differences remained significant (P < 0.0001) after correcting for influences of gender and age.
Validation population characteristics
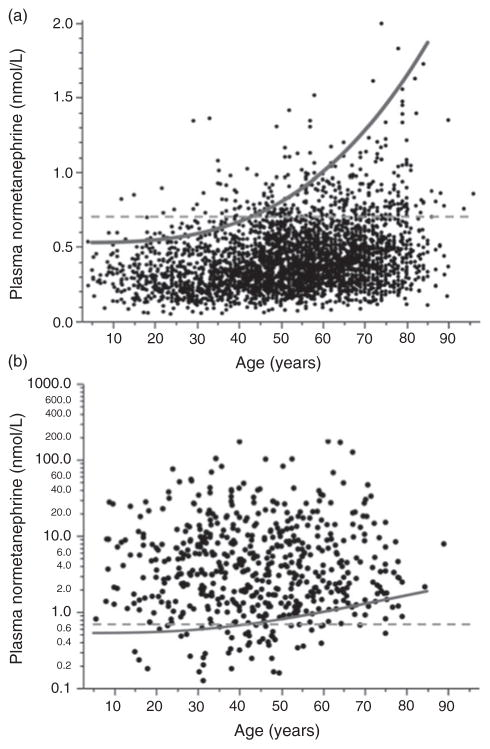
Plasma concentrations of normetanephrine for patients in the validation population without phaeochromocytoma showed a positive relationship (r = 0.277, P < 0.0001) with age (Figure 3a), albeit with respective 9% and 19% higher values for the slope and y-intercept compared with subjects in the reference population (Figure 1a). In contrast, for patients with phaeochromocytoma, plasma concentrations of normetanephrine were widely scattered over a three orders of magnitude concentration range, without any evident relationship with age (Figure 3b).
Figure 3.
Scatter plots showing relationships of age with plasma concentrations of normetanephrine for patients of the validation population without (a) and with (b) phaeochromocytoma. The dashed horizontal lines serve to illustrate the static age-unadjusted upper cut-off for plasma normetanephrine (0.706 nmol/L) determined from the 97.5 percentiles of the combined reference population (Table 2). The curved line serves to illustrate age-adjusted cut-offs for plasma normetanephrine (UCNMN) according to the equation, UCNMN = 2.074 × 10−6age3 + 0.540, established for the curvilinear model. Note that for patients with phaeochromocytoma (b), data for plasma normetanephrine are plotted using a logarithmic scale
As in the reference population, plasma concentrations of metanephrine also showed a shallow positive relationship (r = 0.103, P < 0.0001) with age for patients in the validation population without phaeochromocytoma (data not shown). Plasma concentrations of metanephrine for this group were also 20% higher (P < 0.0001) in men than women, but again as in the reference population, the 97.5 percentiles showed little difference between men and women (0.346 versus 0.335 nmol/L).
Reference intervals for optimized diagnostic test performance
Use of fixed upper cut-offs for both normetanephrine (0.706 nmol/L) and metanephrine (0.325 nmol/L), estimated from the 97.5 percentiles of the reference population, yielded a diagnostic sensitivity of 93.9% and a specificity of 88.3% for patients in the validation population (Table 3). Use of the same upper cut-offs for metanephrine together with a linear model in which upper cut-offs for normetanephrine (UCNMN) were estimated from a regression equation (UCNMN = 7.99 × 10−3age + 0.358) resulted in a small but significant (P = 0.0001) gain in diagnostic specificity (91.2%) with no change in sensitivity. According to this model, upper cut-offs for normetanephrine increase linearly from 0.397 nmol/L for a five-year-old to 0.957 nmol/L for a 75-year-old.
Table 3.
Diagnostic test performance of plasma metanephrines with different upper cut-offs and models adjusting for age
| Model | Upper cut-offs (nmol/L)
|
Test performance
|
||
|---|---|---|---|---|
| NMN | MN | Sensitivity (%) | Specificity (%) | |
| Fixed – 97.5 percentiles | 0.706 | 0.325 | 93.9* | 88.3* |
| Age-dependent linear model | Variable | 0.325 | 93.9* | 91.2† |
| Age-dependent curvilinear model | Variable | 0.325 | 93.7* | 93.6§ |
| Age-dependent curvilinear model | Variable | 0.446 | 93.6* | 96.0¶ |
| Age-adjusted score model | NA | NA | 79.5† | 99.9# |
NMN, normetanephrine; MN, metanephrine; NA, not applicable (based on a score)
Presence of different symbols (*†§¶#) indicates differences (P < 0.0003) in sensitivity or specificity among different models. Presence of the same symbol indicates no difference
Variable upper cut-offs for plasma NMN (UCNMN) for the age-dependent linear model were defined by the equation UCNMN = 7.99 × 10−3age + 0.358
Variable upper cut-offs for plasma NMN for the age-dependent curvilinear model were defined by the equation UCNMN = 2.07 × 10−6 age3 + 0.540 and are shown for two upper cut-offs for MN
The age-adjusted scored model is defined by the equation [−4.188 + −0.07age + 4.516MN + 3.129NMN] as described by Sawka et al.27
A further significant (P = 0.0002) improvement in diagnostic specificity (93.4%), with a minimal non-significant drop in sensitivity (93.7%), was gained using an optimized curvilinear model (UCNMN = 2.07 × 10−6age3 + 0.540), as illustrated in Figure 3 and documented in Table 3. According to this model, upper cut-offs for normetanephrine increase curvilinearly from 0.540 nmol/L for a five-year-old to 1.415 nmol/L for a 75-year-old. A further significant (P < 0.0001) gain in diagnostic specificity (96.0%) with no overall significant loss in sensitivity (93.6%) was obtained by increasing the upper cut-offs for metanephrine from 0.325 to 0.446 nmol/L. Application of the score model described by Sawka et al.27 resulted in a substantial gain (P < 0.0001) in diagnostic specificity (99.9%) over that of all other models, but was associated with a large drop (P < 0.0001) in diagnostic sensitivity (79.4%).
Discussion
The data covered in this report, based on unprecedented numbers of both reference subjects and patients tested for phaeochromocytoma, provide useful information for improving diagnosis of these potentially lethal neuroendocrine tumours. This information is not only relevant for routine laboratories offering tests of plasma free metanephrines for diagnosis of these tumours, but also provides an aid for clinicians to facilitate improved interpretation of test results, particularly those within 50% of either side of the highly divergent upper cut-offs currently in use among different laboratories.
Variation between laboratories in reference intervals for plasma free metanephrines reflects in part differences in the conditions of blood sampling. This is particularly important for plasma normetanephrine, which as the metabolite of noradrenaline is much more responsive to changes in sympathetic nerve activity than metanephrine, the metabolite of adrenaline, which is formed mainly within adrenal medullary cells independently of adrenal adrenaline release.5,18 Upright posture is a well-established potent stimulus for release of noradrenaline by sympathetic nerves to constrict vessels and prevent any posture-associated fall in blood pressure. Upright posture is not only associated with increased plasma concentrations of catecholamines, but also those of their O-methylated metabolites.19,20,33 It is therefore unsurprising that upper cut-offs for metanephrines determined from blood collected in the seated position12,23–25 are up to two-fold higher than those determined from samples collected in the supine position.34
Recognition of the influence of posture on plasma metanephrines has led to recommendations that to ensure optimum diagnostic sensitivity reference intervals should be established from samples taken in the supine position.19,22 To minimize false-positive results, it is also recommended that blood sampling during testing should be carried out in the supine position, or when this is not possible, repeated in the supine position if sampling in the seated position returns a positive result.1
Despite the above recommendations, most centres sample blood and continue to use reference intervals established from samples taken in the seated position. Although this may provide higher diagnostic specificity than reference intervals established in the less convenient supine position, it is at the expense of optimum sensitivity and does not negate a paramount need for establishing reference intervals from samples taken in the supine position. The present study not only addresses this need, but also establishes that with appropriate age-adjustments, diagnostic specificity can be optimized with no significant loss in sensitivity.
As shown in Figure 3a, the gain in diagnostic specificity associated with age-adjusted reference intervals for normetanephrine largely reflects a change in interpretation of test results from a false-positive to a true-negative status for patients over 40 y. As further illustrated in Table 3 and Figure 3b, the gain in diagnostic specificity is associated with a non-significant drop in diagnostic sensitivity associated with a slight increase in the proportion of older patients with phaeochromocytoma in whom the diagnosis would be missed counterbalanced by a small increase in that of younger patients in whom tumours would then be detected instead of missed using conventional upper cut-offs. These relative age-associated gains and losses for detection of tumours should be considered in relation to the potential ramifications of the slight overall drop in diagnostic sensitivity associated with implementation of age-adjusted upper cut-offs for plasma normetanephrine.
In addition to providing needed reference intervals for samples obtained in the supine position, another issue that the present study addresses is the appropriateness of different groups selected for reference intervals.31,35 It is generally considered that reference intervals should be established using healthy, disease-free individuals. However, essential hypertensives are suggested to provide a more relevant population for establishing reference intervals for diagnosis of phaeochromocytoma. A problem with this is that hypertension does not characterize all patients with these tumours,4 particularly those in whom discovery is based on an incidental mass found during imaging, an underlying germline mutation or a previous history of the tumour. As we now show here, although the various groups examined do appear to show differences in plasma concentrations of normetanephrine, these differences largely disappear after consideration of age.
The one group that with age-correction continued to show higher concentrations of normetanephrine was that originally tested because of signs and symptoms of catecholamine excess. This likely reflects underlying clinical conditions in this population that raise sympathetic nerve activity and levels of metabolites of the neurotransmitter, noradrenaline, released by those nerves. Presumably this also partly accounts for why specificity for the validation population, at 88.3%, was lower than expected using the 97.5 percentiles of the reference population if it were assumed that both populations were matched. Other contributing factors include potential differences in preanalytical conditions,31 in particular the more likely well-controlled nature of blood sampling under strict clinical protocols for the reference than the validation population. Although the appropriate instructions for sampling were provided, it is unlikely that these, and particularly the stipulated 20-min supine rest period, were always followed in the routine clinical environment. On the other hand, this does represent the ‘real world’ situation for laboratory testing, underscoring a limitation of diagnostic studies performed under strict clinical protocols.
With the above in mind, our previous descriptions of upper cut-offs of 0.61 nmol/L for normetanephrine and 0.31 nmol/L for metanephrine8,34 must be reconsidered. Although such cut-offs may be appropriate for young patients and those undergoing blood sampling under strictly controlled conditions, such reference intervals in general provide unacceptably low diagnostic specificity in the ‘real world’ situation of laboratory testing. This provides some justification for the higher cut-offs used by other laboratories.11–13,23–25 Nevertheless, as we show here, such higher cut-offs do not appear appropriate for younger patients; an age-adjustment to the cut-offs, as already documented for urinary metanephrines in children,36,37 provides a solution for measurements of normetanephrine in plasma for both children and adults.
In addition to normetanephrine, cut-offs must also be considered for metanephrine. However, for most phaeochromocytomas, diagnosis is readily achieved from elevated results for normetanephrine.7,8 Measurements of metanephrine are usually of secondary importance, but remain crucial for tumours characterized by high rates of conversion of noradrenaline to adrenaline. For such tumours, elevations of metanephrine may represent the principal or only positive result among the pair of measurements and are also important to consider for genotype-phenotype pro-filing of underlying mutations.38 Consequently, our modelling established that use of diagnostic cut-offs for metanephrine of 0.446 nmol/L, 37% higher than those estimated from the traditionally used 97.5 percentiles, further optimized diagnostic specificity.
Application of an age-adjusted score model, described previously by Sawka et al.,27 to our own data resulted in a substantial gain in diagnostic specificity close to 100%, albeit with considerable loss in sensitivity (79.5%). Our own modelling, which combined age-adjusted upper cut-offs for plasma normetanephrine and optimized cut-offs for metanephrine, also resulted in a considerable gain in diagnostic specificity, but without unacceptable loss in diagnostic sensitivity.
The lower diagnostic sensitivity reported in this study than in past studies6–13 in part reflects use of higher upper cut-offs, but more importantly, our improving identification of patients with dopamine-producing paragangliomas and others with non-functional tumours due to mutations of succinate dehydrogenase subunit B and D genes.14,28 Increasing numbers of patients with such tumours are now being identified by routine periodic screening, which includes imaging studies and additional measurements of methoxytyramine for individualized testing consequent to the presence of germline mutations of specific tumour-susceptibility genes.4
In summary, the present study establishes robust reference intervals for plasma metanephrines with age-adjusted cut-offs for normetanephrine that should considerably minimize the need for follow-up of false-positive results. With modern day computerized reporting systems, it should be a simple matter for laboratories to provide individualized upper cut-offs based on date-of-birth data included with test requests. Some consideration must, however, also be given to interlaboratory differences in measured values, but this is now easily ascertained through participation in interlaboratory proficiency programmes available on an international basis and which promise achievement of improved agreement among participating laboratories.30 Any utility of the equation for age-associated cut-offs established here for use by other laboratories should be first validated against those reference intervals already in use and, as required, adjusted according to the performance of the method employed by such laboratories.
Acknowledgments
Funding: This work was supported by the Deutsche Forschungsgesellschaft (EI855/1/1), the Institute of Cardiology, Warsaw, Poland (Grant no: 2.21/VII/12) and the intramural programme of the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Thanks are extended to Carmen Berndt for technical assistance.
Footnotes
Competing interests: None.
Ethical approval: Patients were enrolled into the study under approvals from Institutional Review Boards of the Technical University of Dresden, Dresden, Germany (EK189062010), the Faculty of Medicine at the University of Leipzig, Leipzig, Germany (051/2010/08032010), the Institute of Cardiology, Warsaw, Poland (1233/08112010) and the National Institutes of Health, Bethesda, USA (00-CH-0093, NCT00004847).
Guarantor: GE.
Contributorship: GE, PL, MH, GS and JWML conceived the study and together with RD, AV, AP, AJ and KP were involved in protocol development and/or gaining ethical approval. All authors contributed to patient recruitment and/or data retrieval and analysis. GE wrote the first draft of the manuscript. All authors reviewed and edited the manuscript and approved the final version.
References
- 1.Pacak K, Eisenhofer G, Ahlman H, et al. Pheochromocytoma: recommendations for clinical practice from the First International Symposium. Nat Clin Pract Endocrinol Metab. 2007;3:92–102. doi: 10.1038/ncpendmet0396. [DOI] [PubMed] [Google Scholar]
- 2.Peaston RT, Ball S. Biochemical detection of phaeochromocytoma: why are we continuing to ignore the evidence? Ann Clin Biochem. 2008;45:6–10. doi: 10.1258/acb.2007.007116. [DOI] [PubMed] [Google Scholar]
- 3.Whiting MJ, Doogue MP. Advances in biochemical screening for phaeochromocytoma using biogenic amines. Clin Biochem Rev. 2009;30:3–17. [PMC free article] [PubMed] [Google Scholar]
- 4.Eisenhofer G. Screening for pheochromocytomas and paragangliomas. Curr Hypertens Rep. 2012;14:130–7. doi: 10.1007/s11906-012-0246-y. [DOI] [PubMed] [Google Scholar]
- 5.Eisenhofer G, Keiser H, Friberg P, et al. Plasma metanephrines are markers of pheochromocytoma produced by catechol-O-methyltransferase within tumors. J Clin Endocrinol Metab. 1998;83:2175–85. doi: 10.1210/jcem.83.6.4870. [DOI] [PubMed] [Google Scholar]
- 6.Raber W, Raffesberg W, Bischof M, et al. Diagnostic efficacy of unconjugated plasma metanephrines for the detection of pheochromocytoma. Arch Intern Med. 2000;160:2957–63. doi: 10.1001/archinte.160.19.2957. [DOI] [PubMed] [Google Scholar]
- 7.Sawka AM, Jaeschke R, Singh RJ, Young WF., Jr A comparison of biochemical tests for pheochromocytoma: measurement of fractionated plasma metanephrines compared with the combination of 24-hour urinary metanephrines and catecholamines. J Clin Endocrinol Metab. 2003;88:553–8. doi: 10.1210/jc.2002-021251. [DOI] [PubMed] [Google Scholar]
- 8.Lenders JW, Pacak K, Walther MM, et al. Biochemical diagnosis of pheochromocytoma: which test is best? JAMA. 2002;287:1427–34. doi: 10.1001/jama.287.11.1427. [DOI] [PubMed] [Google Scholar]
- 9.Unger N, Pitt C, Schmidt IL, et al. Diagnostic value of various biochemical parameters for the diagnosis of pheochromocytoma in patients with adrenal mass. Eur J Endocrinol. 2006;154:409–17. doi: 10.1530/eje.1.02097. [DOI] [PubMed] [Google Scholar]
- 10.Vaclavik J, Stejskal D, Lacnak B, et al. Free plasma metanephrines as a screening test for pheochromocytoma in low-risk patients. J Hypertens. 2007;25:1427–31. doi: 10.1097/HJH.0b013e32813aeb5a. [DOI] [PubMed] [Google Scholar]
- 11.Hickman PE, Leong M, Chang J, Wilson SR, McWhinney B. Plasma free metanephrines are superior to urine and plasma catecholamines and urine catecholamine metabolites for the investigation of phaeochromocytoma. Pathology. 2009;41:173–7. doi: 10.1080/00313020802579284. [DOI] [PubMed] [Google Scholar]
- 12.Peaston RT, Graham KS, Chambers E, van der Molen JC, Ball S. Performance of plasma free metanephrines measured by liquid chromatography-tandem mass spectrometry in the diagnosis of pheochromocytoma. Clin Chim Acta. 2010;411:546–52. doi: 10.1016/j.cca.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 13.Procopiou M, Finney H, Akker SA, et al. Evaluation of an enzyme immunoassay for plasma-free metanephrines in the diagnosis of catecholamine-secreting tumors. Eur J Endocrinol. 2009;161:131–40. doi: 10.1530/EJE-09-0172. [DOI] [PubMed] [Google Scholar]
- 14.Timmers HJ, Pacak K, Huynh TT, et al. Biochemically silent abdominal paragangliomas in patients with mutations in the SDHB gene. J Clin Endocrinol Metab. 2008;93:4826–32. doi: 10.1210/jc.2008-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eisenhofer G, Goldstein DS, Sullivan P, et al. Biochemical and clinical manifestations of dopamine-producing paragangliomas: utility of plasma methoxytyramine. J Clin Endocrinol Metab. 2005;90:2068–75. doi: 10.1210/jc.2004-2025. [DOI] [PubMed] [Google Scholar]
- 16.Yu R, Wei M. False positive test results for pheochromocytoma from 2000 to 2008. Exp Clin Endocrinol Diabetes. 2009;118:400–4. doi: 10.1055/s-0029-1237699. [DOI] [PubMed] [Google Scholar]
- 17.Anas SS, Vasikaran SD. An audit of management of patients with borderline increased plasma-free metanephrines. Ann Clin Biochem. 2010;47:554–8. doi: 10.1258/acb.2010.010131. [DOI] [PubMed] [Google Scholar]
- 18.Eisenhofer G, Friberg P, Pacak K, et al. Plasma metadrenalines: do they provide useful information about sympathoadrenal function and catecholamine metabolism? Clin Sci (Lond) 1995;88:533–42. doi: 10.1042/cs0880533. [DOI] [PubMed] [Google Scholar]
- 19.Lenders JW, Willemsen JJ, Eisenhofer G, et al. Is supine rest necessary before blood sampling for plasma metanephrines? Clin Chem. 2007;53:352–4. doi: 10.1373/clinchem.2006.076489. [DOI] [PubMed] [Google Scholar]
- 20.de Jong WH, Eisenhofer G, Post WJ, Muskiet FA, de Vries EG, Kema IP. Dietary influences on plasma and urinary metanephrines: implications for diagnosis of catecholamine-producing tumors. J Clin Endocrinol Metab. 2009;94:2841–9. doi: 10.1210/jc.2009-0303. [DOI] [PubMed] [Google Scholar]
- 21.Grossman A, Pacak K, Sawka A, et al. Biochemical diagnosis and localization of pheochromocytoma: can we reach a consensus? Ann N Y Acad Sci. 2006;1073:332–47. doi: 10.1196/annals.1353.038. [DOI] [PubMed] [Google Scholar]
- 22.Eisenhofer G, Lenders J. Rapid circulatory clearances and half-lives of plasma free metanephrines. Clin Endocrinol. 2012;77:484–5. doi: 10.1111/j.1365-2265.2012.04340.x. [DOI] [PubMed] [Google Scholar]
- 23.Lagerstedt SA, O’Kane DJ, Singh RJ. Measurement of plasma free metanephrine and normetanephrine by liquid chromatography-tandem mass spectrometry for diagnosis of pheochromocytoma. Clin Chem. 2004;50:603–11. doi: 10.1373/clinchem.2003.024703. [DOI] [PubMed] [Google Scholar]
- 24.Heider EC, Davis BG, Frank EL. Nonparametric determination of reference intervals for plasma metanephrine and normetanephrine. Clin Chem. 2004;50:2381–4. doi: 10.1373/clinchem.2004.035089. [DOI] [PubMed] [Google Scholar]
- 25.de Jong WH, Graham KS, van der Molen JC, et al. Plasma free metanephrine measurement using automated online solid-phase extraction HPLC tandem mass spectrometry. Clin Chem. 2007;53:1684–93. doi: 10.1373/clinchem.2007.087114. [DOI] [PubMed] [Google Scholar]
- 26.Roden M, Raffesberg W, Raber W, et al. Quantification of unconjugated metanephrines in human plasma without interference by acetaminophen. Clin Chem. 2001;47:1061–7. [PubMed] [Google Scholar]
- 27.Sawka AM, Thabane L, Gafni A, Levine M, Young WF., Jr Measurement of fractionated plasma metanephrines for exclusion of pheochromocytoma: can specificity be improved by adjustment for age? BMC Endocr Disord. 2005;5:1. doi: 10.1186/1472-6823-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eisenhofer G, Lenders JW, Siegert G, et al. Plasma methoxytyramine: a novel biomarker of metastatic pheochromocytoma and paraganglioma in relation to established risk factors of tumor size, location and SDHB mutation status. Eur J Cancer. 2012;48:1739–49. doi: 10.1016/j.ejca.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lenders JW, Eisenhofer G, Armando I, Keiser HR, Goldstein DS, Kopin IJ. Determination of metanephrines in plasma by liquid chromatography with electrochemical detection. Clin Chem. 1993;39:97–103. [PubMed] [Google Scholar]
- 30.Pillai D, Callen S. Pilot quality assurance programme for plasma metanephrines. Ann Clin Biochem. 2010;47:137–42. doi: 10.1258/acb.2009.009153. [DOI] [PubMed] [Google Scholar]
- 31.Ceriotti F, Hinzmann R, Panteghini M. Reference intervals: the way forward. Ann Clin Biochem. 2008;46:8–17. doi: 10.1258/acb.2008.008170. [DOI] [PubMed] [Google Scholar]
- 32.R Development Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2011. See http://www.R-project.orglast checked 30 July 2012. [Google Scholar]
- 33.Deutschbein T, Unger N, Jaeger A, Broecker-Preuss M, Mann K, Petersenn S. Influence of various confounding variables and storage conditions on metanephrine and normetanephrine levels in plasma. Clin Endocrinol (Oxf) 2010;73:153–60. doi: 10.1111/j.1365-2265.2009.03761.x. [DOI] [PubMed] [Google Scholar]
- 34.Eisenhofer G, Lenders JW, Linehan WM, Walther MM, Goldstein DS, Keiser HR. Plasma normetanephrine and metanephrine for detecting pheochromocytoma in von Hippel-Lindau disease and multiple endocrine neoplasia type 2. N Engl J Med. 1999;340:1872–9. doi: 10.1056/NEJM199906173402404. [DOI] [PubMed] [Google Scholar]
- 35.Ritchie RF, Palomaki G. Selecting clinically relevant populations for reference intervals. Clin Chem Lab Med. 2004;42:702–9. doi: 10.1515/CCLM.2004.120. [DOI] [PubMed] [Google Scholar]
- 36.Griffin A, O’Shea P, FitzGerald R, O’Connor G, Tormey W. Establishment of a paediatric age-related reference interval for the measurement of urinary total fractionated metanephrines. Ann Clin Biochem. 2011;48:41–4. doi: 10.1258/acb.2010.010062. [DOI] [PubMed] [Google Scholar]
- 37.Davidson DF, Hammond PJ, Murphy DL, Carachi R. Age-related medical decision limits for urinary free (unconjugated) metadrenalines, catecholamines and metabolites in random urine specimens from children. Ann Clin Biochem. 2011;48:358–6. doi: 10.1258/acb.2011.011023. [DOI] [PubMed] [Google Scholar]
- 38.Eisenhofer G, Lenders JW, Timmers H, et al. Measurements of plasma methoxytyramine, normetanephrine, and metanephrine as discriminators of different hereditary forms of pheochromocytoma. Clin Chem. 2011;57:411–20. doi: 10.1373/clinchem.2010.153320. [DOI] [PMC free article] [PubMed] [Google Scholar]