Abstract
The locus coeruleus (LC), the brainstem noradrenergic nucleus that is the sole source of norepinephrine (NE) in the forebrain, is one of the first structures affected in Alzheimer’s disease. Experimental ablation of the LC exacerbates, while increasing NE abates, AD-like neuropathology and cognitive deficits in animal models of the disease. Some neuroprotective effects of NE appear to be mediated by tropomyosin-related kinase B (TrkB), the canonical receptor for brain-derived neurotrophic factor (BDNF). Here, we report that NE dosedependently protected primary cortical and LC neurons from amyloid-β (Aβ) toxicity. The neuroprotective effects of NE were fully prevented by the Trk receptor antagonist K252a but only partially attenuated by adrenergic receptor antagonists and not mimicked by adrenergic agonists. Activation of TrkB by NE in cortical and LC neurons was confirmed by immunoblot and immunocytochemistry for phospho-TrkB. These results indicate that NE can activate TrkB and protect against Aβ toxicity, at least in part, via adrenergic receptor-independent mechanisms, and have implications for the consequences of LC degeneration in AD and potential therapies for the disease.
Keywords: norepinephrine, brain-derived neurotrophic factor, Alzheimer’s disease, amyloid-β, tropomyosin-related kinase B, neuroprotection, locus coeruleus
Introduction
Alzheimer’s disease (AD) is the most common cause of dementia and is characterized pathologically by β-amyloid plaques, tau neurofibrillary tangles, and neuronal death in the brain. While loss of neurons in the entorhinal cortex, hippocampus, and basal forebrain is considered central to AD pathogenesis, LC degeneration and loss of forebrain norepinephrine (NE) is ubiquitous in AD, occurs early, and correlates better with plaques, tangles, and cognitive impairment than neuronal death in other brain regions [1–3]. In fact, a series of recent papers indicates that aberrant tau in the LC is the first detectable sign of AD-like neuropathology in the human brain [4–8]. Experimental LC lesions in amyloid β-protein precursor (AβPP) transgenic mice also lead to more severe AD-like forebrain neuropathology and memory deficits, while increasing NE transmission partially ameliorates neuroinflammation, Aβ load, and cognitive impairment [9–13].
While most evidence points to a neuroprotective role of NE in AD, the underlying mechanisms are unclear. In some cases, adrenergic receptors, particularly the β-subtype, have been implicated. For example, β-adrenergic agonists have potent anti-inflammatory properties, can protect cultured neurons from Aβ toxicity, and enhance Aβ phagocytosis by microglia, while β-adrenergic antagonists have the opposite effects [12, 14–16]. However, in some cases, the ability of NE to protect cultured neurons from AD-related insults such as oxidative stress and Aβ cannot be fully mimicked by adrenergic agonists or blocked by adrenergic antagonists, suggesting the existence of non-canonical signaling mechanisms [16–19]. Moreover, the neuroprotective effects of NE have been assessed in hippocampal neurons, cortical neurons, and immortalized cell lines, but not LC neurons. Although amyloid burden in the brainstem is relatively low, it is very high in LC projection areas like the hippocampus and cortex. One theory to explain the death of LC neurons in AD is that amyloid damages LC axons and terminals in the forebrain, which over time leads to cell body death in the brainstem; thus, assessing amyloid toxicity in cultured LC neurons, which retain processes, could provide important insight into LC degeneration in AD.
Tropomyosin-related kinase B (TrkB), the cognate receptor for brain-derived neurotrophic factor (BDNF), has emerged as a candidate for mediating the neuroprotective effects of NE in the context of AD. Activation of TrkB by BDNF or other agonists can protect neurons from Aβ toxicity [20–24], while blockade of TrkB attenuates the neuroprotective effects of NE against Aβ toxicity in culture [16]. The purpose of this study was to examine both cortical and LC neurons to further investigate the relative contributions of TrkB and adrenergic receptor activation to the neuroprotective effect of NE against Aβ toxicity.
Material and Methods
Primary neuron culture
All experiments were approved by the Emory Institutional Animal Care and Use Committee. Primary rat cortical neurons and LC neurons were cultured as previously described [25]. Briefly, E17 rat pups were decapitated, and cortex was extirpated, cross-chopped, and suspended by pipetting for gentle separation in 5% fetal calf serum (FCS), 5% horse serum (HS) DMEM. The cell suspension was then centrifuged at 250 xg for 5 min. This operation was repeated again. Cells were seeded into 10 polyethyleneimine-coated dishes and 12-well plates including coated coverslips and incubated at 37°C in 5% CO2/95% air. After 3 h, culture medium was changed to Neurobasal containing B-27 supplement (Invitrogen, Grand Island, NY) and incubated for 4 d. For maintenance, half the medium was changed to fresh Neurobasal/B27 every 4 d. After a week, the dished cultured neurons were used in experiments. Approximately 15–25% of the neurons in the LC cultures were noradrenergic, as assessed by TH immunostaining (data not shown). Primary cortical neuron cultures were also made from TrkB F616A knock-in mice, as described [19].
To assess the effect of NE on Aβ-induced toxicity, NE (50–500 nM) was added to the medium 20 min before Aβ treatment. Neurons were then exposed to 10–40 μM Aβ25–35 or Aβ1–42 and incubated for 18 h in serum-free OptiMem, as described [16, 24]. Aβ25–35 was dissolved in DMSO and applied without pre-aggregation, which results in the rapid formation of oligomeric and protofibril intermediates [26, 27], while Aβ1–42 was dissolved in N2 medium and preaggregated for 4 days at 37°C, as described [16, 24]. Caspase activity was detected by Caspase-Glo 3/7 assay (Promega, Madison, WI). Neuronal apoptosis was detected with the in situ cell death detection kit (Roche Diagnostics, Indianapolis, IN). The apoptotic index was expressed as the percentage of TUNEL-positive neurons out of the total number of MAP2-positive neurons. To assess the effect of TrkB and adrenergic receptor antagonists, neurons were pretreated with the Trk antagonist K252a (100 nM) or a cocktail of the α2-adrenergic antagonist yohimbine (10 μM) + the α1-adrenergic antagonist prazosin (50 nM) + the β-adrenergic antagonist propranolol (10 μM) 30 min prior to NE or isoproterenol (β-adrenergic agonist, 100 μM) exposure. Because some of these experiments were part of a larger panel of studies testing various agents in Aβ toxicity assays, the “control” and “Aβ” bars in Fig. 2B are the same as in Fig. 1C of our previously published paper [24].
Immunofluorescence staining
For immunofluorescence and TUNEL staining in the Aβ experiments, cells were incubated overnight at 4°C with anti-MAP2 or anti-TH antibody. After being washed with TBS, the cells were incubated with Alexa Fluor 488-coupled secondary antibodies. The cells were then incubated with TUNEL reagent for 1 h at room temperature (RT). After a PBS wash, images were acquired through an AxioCam camera on an Axiovert 200M microscope (Zeiss). For the TrkB experiments, primary cortical and LC neurons were seeded on poly-L-lysine-coated coverslips in 12-well dishes. After 7 DIV, the neurons were treated with 100 ng/ml BDNF (Peptron; Daejeon, Republic of Korea) or 100 nM NE for 30 min, and then washed with PBS. Cells were fixed with 3% formaldehyde in PBS at RT for 15 min, then permeabilized and blocked with 0.4% Triton X-100 and 2% FBS in PBS at RT for 15 min, washed with PBS three times, and treated with anti-MAP2 (1: 200) or anti-TH (1:250) and anti-pTrkB 706 antibody (1:100) overnight. After staining with FITC-or rhodamine-conjugated secondary antibody, the coverslips were mounted on slides. Fluorescent images were taken by Olympus IX71 fluorescence microscope.
Immunoprecipitation and Western blot
Rat primary cultured cortical neurons were treated with vehicle, BDNF (100 ng/ml) or NE (500 nM) for 20 min and then harvested in ice-cold homogenization buffer [10 mM sodium-β-glycerophosphate, 50 mM Tris-Cl (pH 7.4), 150 mM NaCl, 1 mM EDTA (pH 8.0), 1% Triton X-100, 1.5 mM Na3VO4, 50 mM NaF, 10 mM sodium-pyrophosphate, pH 7.4] containing protease inhibitors. Total protein from rat neurons was mixed in 800 μl lysis buffer and added to a tube with 25 μl bed volume of Protein A/G beads (Santa Cruz Biotechnology, Dallas, TX) and 1 mg anti-TrkB antibody (1:1000). The mixture was rotate overnight at 4°C, then beads were washed 3–4 times with 1 ml homogenization buffer. Beads were incubated with 30 μl of 1x loading buffer for 5 min at 95°C. Eluted sample proteins were separated by SDS-PAGE (9% acrylamide) and transferred to nitrocellulose membranes (BioRad, Hercules, CA) electrophoretically. Membranes were blocked in TBS/0.1% Tween-20(TBST)/5%milk for 30 min at RT and incubated overnight at 4°C with anti-PY99 antibody (1:1000) in 3% BSA/TBST incubation buffer. Blots were washed 3 times and incubated for 1 h at RT with horseradish peroxidase-conjugated goat anti-mouse secondary IgG (1:2000, Fisher Scientific). The pTrkB band was visualized by enhanced chemiluminescence (Thermo Scientific/Pierce, Rockford, IL) on x-ray film.
Reagents
Adrenergic compounds, K252a, and Aβ25–35 were obtained from Sigma-Aldrich (St. Louis, MO), BDNF was obtained from Peptron (Daejeon, Republic of Korea), and 1NMPP1 was obtained from Toronto Research Chemicals (Toronto, Ontario). Antibodies used were antityrosine hydroxylase (EMD Millipore, Billerica, MA), anti-MAP2 (Sigma-Aldrich, St. Louis, MO) anti-TrkB (BioVision, Milpitas, CA and Cell Signaling, Danvers, MA), anti-pTrkB 706 (Santa Cruz Biotechnology, Inc., Dallas, TX), anti-pTrkB 817 (Epitomics, Burlingame, CA), and anti-PY99 (Santa Cruz Biotechnology).
Statistical Analysis
All data are presented as mean ± SEM. Statistical analysis were performed using either Student’s t-tests were used when comparing 2 groups, and ANOVA followed by Sidak’s or Tukey’s posthoc tests was used when comparing more than 2 groups using Prism 6.0 for Macintosh (Graphpad, La Jolla, CA). Data were normally distributed. The level of significance was set at p<0.05.
Results
Norepinephrine protects cortical neurons from Aβ toxicity
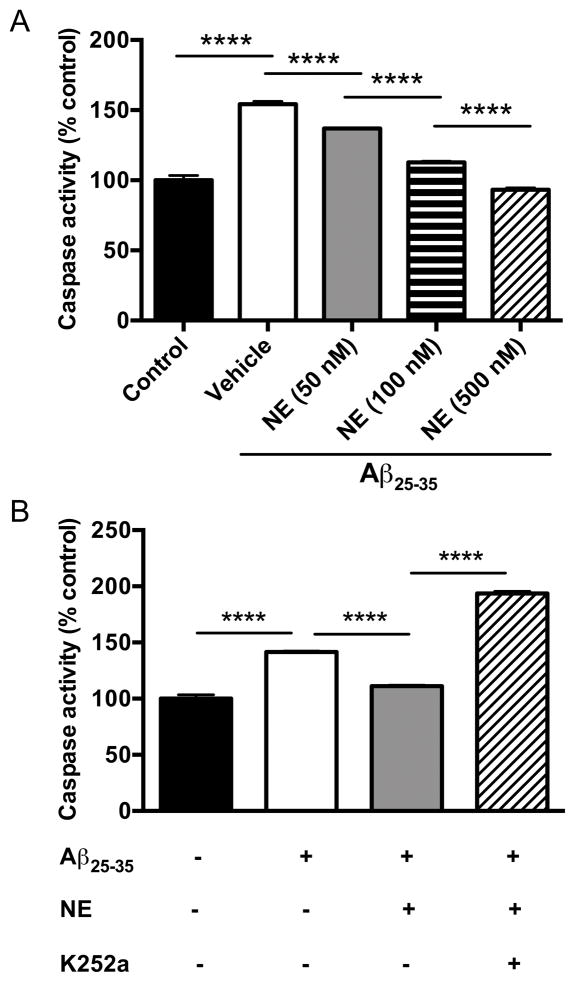
It has been reported that NE can protect cultured hippocampal neurons from Aβ toxicity in a Trk receptor-dependent manner. To confirm and extend these results, we exposed cultured cortical neurons to the Aβ25–35 fragment and assessed toxicity in the presence of NE with or without the Trk receptor antagonist K252a. The increase in caspase-3 activity following Aβ25–35 treatment (20 μM) was dose-dependently suppressed by NE (50–500 nM) in cortical neurons (one-way ANOVA: F4,10 = 520, p<0.0001) (Fig. 1A), and the protective effect of NE (100 nM) was abolished by K252a (100 nM; one-way ANOVA: F3,8 = 1293, p<0.0001) (Fig. 1B).
Fig. 1. Norepinephrine attenuates Aβ25–35-induced neurotoxicity in cultured cortical neurons.
(A) Cultured cortical neurons were exposed to Aβ25–35 (20 μM) for 18 h in the absence or presence of NE (50–500 nM). Shown is the mean ± SEM caspase activity (n=3). (B) Cultured cortical neurons were exposed to Aβ25–35 (20 μM) for 18 h in the presence or absence of NE (100 nM) and the Trk inhibitor K252a (100 nM). Shown is the mean ± SEM caspase activity (n=3). ****p<0.0001.
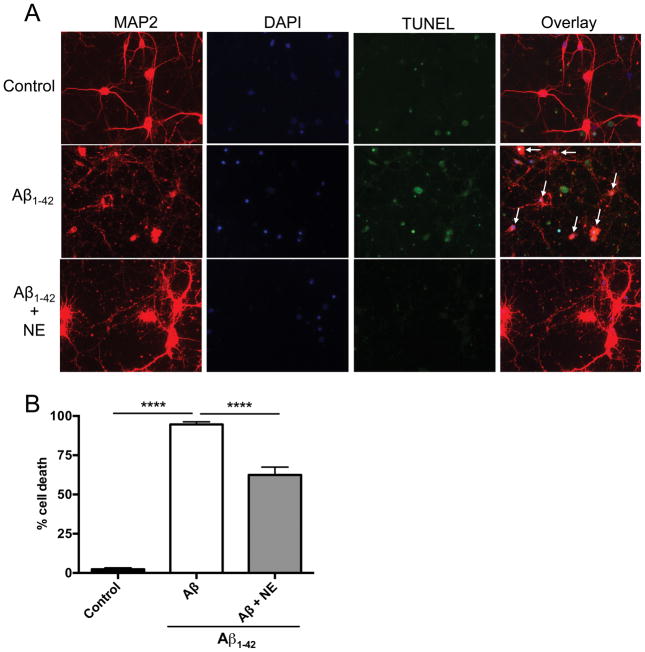
Although Aβ25–35 is commonly used in primary culture studies, it is a synthetic fragment not found endogenously in AD brains. To confirm our results with full length Aβ, we assessed the effects of NE on Aβ1–42 toxicity in cortical neurons. NE significantly reduced cell death elicited by 10 μM Aβ1–42 (one-way ANOVA: F2,9 = 932.80, p<0.0001) (Fig. 2), although the rescue was not complete. Cell death was still higher than control in cultures treated with Aβ1–42 + NE, and some of the surviving neurons looked abnormal (hypertophic cell bodies and thickened projections) (Fig. 2). Similar results were obtained using a higher dose of Aβ1–42 (20 μM) (data not shown).
Fig. 2. Norepinephrine attenuates Aβ1–42-induced neurotoxicity in cultured cortical neurons.
Cultured cortical neurons were exposed to pre-aggregated Aβ1–42 (10 μM) for 18 h in the presence or absence of NE (500 nM). Neurons were immunostained with the neuronal marker MAP2 (red) and the nuclear marker DAPI (blue), and neuronal apoptosis was detected by TUNEL staining (green). Shown are (A) representative immunosfluorescent images, and (B) the mean ± SEM percentage of apoptotic cells (n=4 experiments, 150–200 total cells examined per condition). White arrows indicate apoptotic neurons. ****p<0.0001.
Protection of locus coeruleus neurons from Aβ toxicity by norepinephrine requires Trk receptor activation but not canonical adrenergic receptors
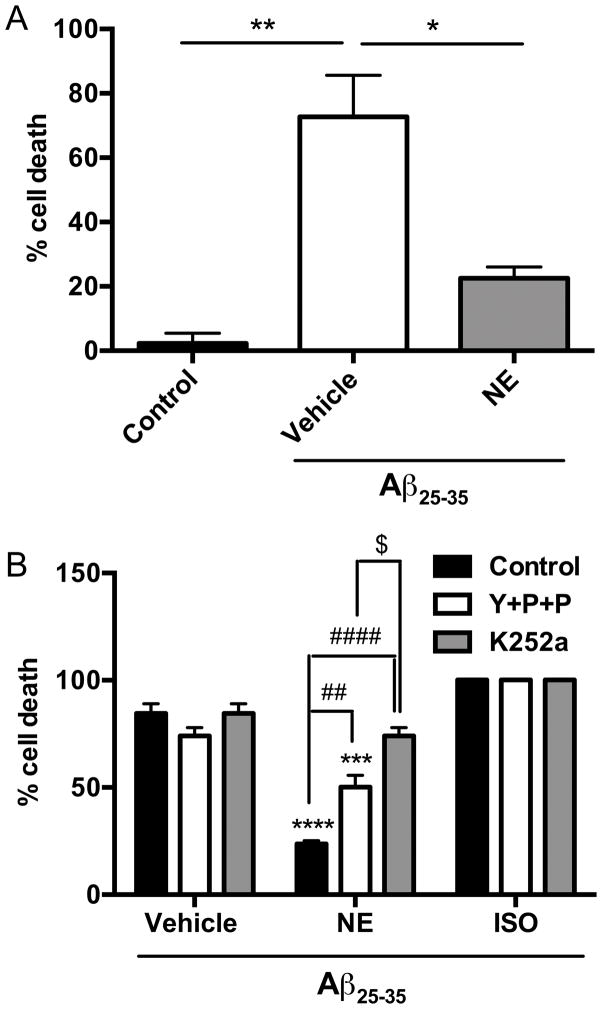
Because LC degeneration is a hallmark of AD, occurs early in the disease, and exacerbates AD-like neuropathology and cognitive deficits in AβPP transgenic mice, identifying ways of protecting these neurons is of high clinical significance. We have shown before that the small molecule TrkB agonist 7,8-dihydroxyflavone (7,8-DHF) can protect LC neurons from Aβ toxicity, but the effects of NE have never been tested. Similar to what we observed in cortical neurons, NE (500 nM) protected against Aβ25–35 (40 μM)-induced cell death in LC neurons in a TrkB-dependent manner (one-way ANOVA: F2,3 = 41.97, p<0.01) (Fig. 3A).
Fig. 3. Attenuation of Aβ25–35-induced neurotoxicity in cultured locus coeruleus neurons requires TrkB but is partially independent of adrenergic receptors.
(A) LC neurons were exposed to Aβ25–35 (40 μM) for 18 h in the presence or absence of NE (500 nM). Shown is the mean ± SEM percentage of apoptotic cells determined by TUNEL staining (n=2). *p<0.05, **p<0.01. (B) LC neurons were exposed to Aβ25–35 (40 μM) for 18 h in the presence or absence of NE (100 nM) or the β-adrenergic receptor agonist isoproterenol (ISO, 100 μM), with or without K252a (100 nM) or a cocktail (Y+P+P) of the α2-adrenergic antagonist yohimbine (10 μM) + the α1-antagonist prazosin (50 nM) + the β-antagonist propranolol (10 μM). Shown is the mean ± SEM percentage of apoptotic cells determined by TUNEL staining (n=2). ***p<0.001, ****p<0.0001 compared to vehicle; ##p<0.01, ####p<0.0001 compared to NE alone; $p<0.05 compared to NE with Y+P+P.
It has been reported that the ability of NE to protect cultured hippocampal neurons from Aβ toxicity may not always involve canonical β, α1, or α2 G protein-coupled adrenergic receptors, and our results support this idea. The neuroprotective properties of NE (100 nM) in LC neurons were only partially attenuated by a cocktail of α1 (prazosin, 50 nM) + α2 (yohimbine, 10 μM) + β (propranolol, 10 μM) adrenergic receptor antagonists and were not recapitulated by the β-agonist isoproterenol (10 μM) (Fig. 3B). By contrast, the Trk receptor antagonist K252a (100 nM) completely abolished the pro-survival effects of NE (Fig. 3B). A two-way ANOVA showed a main effect of antagonist pretreatment (F2,9 = 19.33, p<0.001), agonist treatment (F2,9 = 173.6, p<0.0001), and a pretreatment x treatment interaction (F4,9 = 19.72, p<0.001). Posthoc tests revealed a significant protective effect of NE (q = 18.02, p < 0.0001) and NE + adrenergic receptor antagonist cocktail (q = 10.20, p < 0.001), but not NE + K252a (q = 3.13, p > 0.05), compared to vehicle, and cell death was significantly higher in the NE + K252a group compared to the NE + adrenergic antagonist cocktail group (q = 7.08, p < 0.05). Isoproterenol had no effect on Aβ toxicity under any condition.
Norepinephrine induces TrkB phosphorylation in primary cortical and locus coeruleus neurons
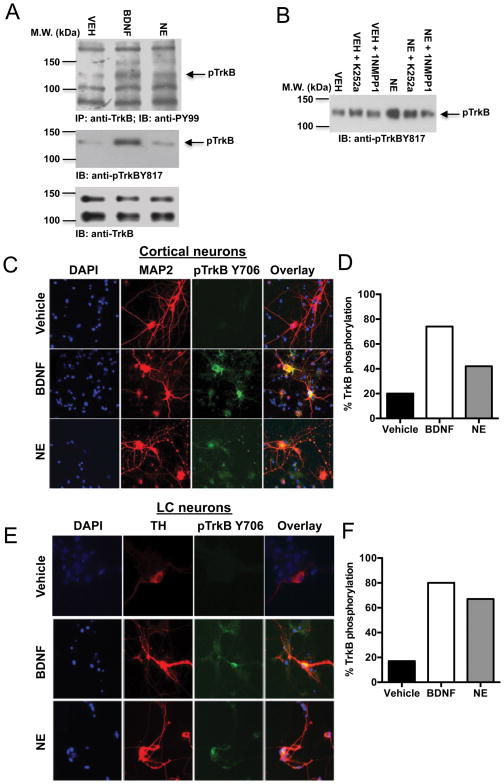
Upon agonist stimulation, TrkB undergoes dimerization and autophosphorylation. To confirm that NE treatment caused bona fide TrkB activation, we conducted immunoprecipitation assays with cortical neuron lysates. BDNF (100 ng/ml) and NE (500 nM) elicited evident TrkB tyrosine phosphorylation (Fig 4A, top panel). Immunoblotting analysis with neuronal lysates using an antibody against p-TrkB 817 also showed that both BDNF and NE escalated TrkB activation compared to control (Fig 4A, middle panel). Total TrkB levels were unaffected by treatment (Fig. 4A, bottom panel). To further explore whether NE can selectively provoke TrkB activation, we employed TrkB F616A knock-in mice. Whereas wild-type TrkB neurons can be inhibited by K252a, TrkB F616A can be selectively blocked by the specific inhibitor 1NMPP1, resulting in a virtual TrkB-null phenotype [28, 29]. We found that NE-provoked TrkB phosphorylation was selectively reduced by 1NMPP1, but not K252a, in TrkB F616A neurons (Fig 4B). Immunofluorescence staining confirmed that both BDNF and NE provoked robust TrkB phosphorylation in primary cortical (Fig. 4C) and LC (Fig. 4D) neurons.
Fig. 4. NE triggers TrkB phosphorylation in cultured cortical and locus coeruleus neurons.
(A) Primary cortical neurons were treated with vehicle, BDNF (100 ng/ml), or NE (500 nM) for 15 min. Total TrkB was immunoprecipitated with anti-TrkB antibody, followed by immunoblotting analysis with anti-PY99 antibody (top panel). Total lysates were also analyzed with anti-pTrkB 817 antibody (middle panel) and anti-TrkB antibody (bottom panel). (B) Primary cortical neurons from TrkB F616A knock-in mice were incubated with vehicle, K252a (100 nM), or 1NMPPI (100 nM) for 2 h and then treated with vehicle or NE (500 nM) for 15 min, followed by immunoblotting analysis with anti-pTrkB 817 antibody. Primary cortical (C, D) and LC (E, F) neurons were pretreated with vehicle, BDNF (100 ng/ml) or NE (500 nM) for 15 min. Neurons were fixed and costained with the nuclear marker DAPI (blue), the neuronal marker MAP2, or the catecholaminergic marker TH (red), and anti-pTrkB 706 (green). Shown are representative immunofluorescent images (C, E) and % of the 150–200 total neurons counted for each condition that were also pTrkB-positive (D, F).
Discussion
Some neuroprotective properties of NE depend on the activation of canonical adrenergic receptors, while others appear to be mediated by independent mechanisms. Here we show that NE triggers TrkB phosphorylation and that TrkB, but not adrenergic receptors, is essential for the ability of NE to protect cultured cortical and LC neurons from Aβ toxicity.
Neuroprotective effects of NE in AD
As discussed in the Introduction, LC degeneration and loss of forebrain NE is ubiquitous in postmortem AD brains. LC pathology is evident as early as mild cognitive impairment, a prodromal stage of AD, and perhaps even decades before symptom onset. Experimental lesions of the LC exacerbate, while increasing NE levels ameliorate, AD-like neuropathology in rodent AβPP models of AD.
Some neuroprotective effects of NE against Aβ toxicity involve adrenergic receptors, most notably the β-adrenergic receptor. For instance, the β-adrenergic receptor agonist isoproterenol ameliorates neuroinflammation produced by Aβ in vivo [14]. In cultured cells, activation of β-adrenergic receptors facilitates the phagocytosis of Aβ and attenuates Aβ-induced cell death [12, 16], while β-adrenergic antagonists abolish the ability of NE to block Aβ-induced mitochondrial membrane depolarization and caspase activation [16]. α-adrenergic drugs were ineffective in these assays. On the other hand, the increase in survival of cholinergic neurons exposed to oxidative stress following NE treatment could not be mimicked by adrenergic receptor agonists nor blocked by adrenergic receptor antagonists [17], and the antagonists also failed to alter the antioxidant effects of NE in Aβ-exposed cells [16]. A recent study showed that the ability of NE to protect against Aβ-mediated oxidative stress and toxicity in SK-N-SH cells could not be blocked by a cocktail of α-and β-antagonists [19]. Thus, at least some neuroprotective properties of NE appear to be mediated by canonical adrenergic receptorindependent mechanisms.
The neuroprotective effects of NE require TrkB activation
A loss of neurotrophic support is also associated with AD. For example, both BDNF and TrkB levels are decreased in postmortem AD brains [30], while we and others have shown that activation of TrkB signaling is neuroprotective in animal and cellular models of AD [22–24, 31]. Importantly, TrkB is required for the neuroprotective effects of NE in cultured hippocampal and hNT neurons following Aβ exposure [16]. To extend these findings, we assessed the contribution of adrenergic receptors and TrkB to NE-mediated protection against Aβ toxicity in cortical and LC neurons. We found that the ability of NE to prevent Aβ-induced cell death was fully prevented by the Trk antagonist K252a but only partially attenuated by adrenergic antagonists and not recapitulated by isoproterenol. These results suggest that canonical G protein-coupled adrenergic receptors and TrkB may act in concert to transduce NE-mediated neuroprotection. While many similarities between our data and those reported by Counts and Mufson [16] exist, the adrenergic receptor-independent effects of NE on Aβ-induced neuronal death were more pronounced in our experiments. Some potential explanations are differences in cell type examined (hippocampal neurons and a neuronal cell line vs. LC neurons) and cell death assay used (Live/Dead cell viability assay or propidium iodide vs. TUNEL staining).
Activation of TrkB by NE
To further investigate the relationship between NE and TrkB, we assessed TrkB activation in cultured cortical and LC neurons and found that NE provoked robust TrkB phosphorylation as measured by western blot and immunocytochemistry, although the exact nature of the interaction remains to be elucidated. There are several ways that NE could activate TrkB. First, NE may function via increases in BDNF, as reported previously for protection against Aβ toxicity [16]. Alternatively, NE may transactivate TrkB via Src family kinase activity, as shown for adenosine, pituitary adenylate cyclase-activating peptide, and zinc [32–35]. Finally, it has not escaped our notice that NE and the small molecule TrkB agonist 7,8-dihydroxyphenylserine [36] share structural similarities (i.e. a catechol ring), and thus a direct physical interaction cannot be ruled out. Interestingly, the neuroprotective effect of NE against oxidative stress in cultured cholinergic neurons and SK-N-SH cells also appears to be mediated by the catechol ring [17, 19].
The nature and pattern of NE-TrkB interactions that we observed is reminiscent of those that occur during noradrenergic neuron development. The specification of NE neurons requires NE and TrkB activation but not canonical adrenergic receptors [37–40]. Thus, NE-mediated activation of TrkB may be important for multiple aspects of neuronal development and function.
Implications for Alzheimer’s disease
There is an overall loss of trophic support in AD; BDNF and TrkB levels are reduced, while cell death is rampant in postmortem AD brains. LC pathology appears very early in AD, and loss of LC neurons and forebrain NE are pathological and neurochemical hallmarks of the disease. Our data suggest that LC degeneration could exacerbate AD neuropathology because NE activation of TrkB is depressed. BDNF is co-expressed in LC neurons [41, 42], and BDNF released from noradrenergic terminals has neurotrophic properties [43, 44]. Thus, LC degeneration might deprive the brain of two TrkB activators. BDNF itself promotes noradrenergic neuron survival in aged animals [45, 46], suggesting that progressive loss of autocrine support could hasten the degeneration of this nucleus during AD progression. We and others have shown that TrkB agonists ameliorate AD-like neuropathology in AβPP transgenic mice [23, 24]. Drugs such as L-3,4-dihydroxyphenylserine that elevate NE levels are also neuroprotective in animal models of AD [10, 13], perhaps in part because they increase TrkB activation, and are potential therapies for disease treatment in humans.
Acknowledgments
This work was funded by the National Institutes of Health (AG025688 to DW and KY, DC010204 to KY).
Footnotes
The authors declare no conflicts of interest.
References
- 1.Chalermpalanupap T, Kinkead B, Hu WT, Kummer MP, Hammerschmidt T, Heneka MT, Weinshenker D, Levey AI. Targeting norepinephrine in mild cognitive impairment and Alzheimer’s disease. Alzheimers Res Ther. 2013;5:21. doi: 10.1186/alzrt175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weinshenker D. Functional consequences of locus coeruleus degeneration in Alzheimer’s disease. Curr Alzheimer Res. 2008;5:342–345. doi: 10.2174/156720508784533286. [DOI] [PubMed] [Google Scholar]
- 3.Wilson RS, Nag S, Boyle PA, Hizel LP, Yu L, Buchman AS, Schneider JA, Bennett DA. Neural reserve, neuronal density in the locus ceruleus, and cognitive decline. Neurology. 2013;80:1202–1208. doi: 10.1212/WNL.0b013e3182897103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braak H, Del Tredici K. Where, when, and in what form does sporadic Alzheimer’s disease begin? Curr Opin Neurol. 2012;25:708–714. doi: 10.1097/WCO.0b013e32835a3432. [DOI] [PubMed] [Google Scholar]
- 5.Braak H, Del Tredici K. The pathological process underlying Alzheimer’s disease in individuals under thirty. Acta Neuropathol. 2011;121:171–181. doi: 10.1007/s00401-010-0789-4. [DOI] [PubMed] [Google Scholar]
- 6.Braak H, Del Tredici K. Alzheimer’s pathogenesis: is there neuron-to-neuron propagation? Acta Neuropathol. 2011;121:589–595. doi: 10.1007/s00401-011-0825-z. [DOI] [PubMed] [Google Scholar]
- 7.Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol. 2011;70:960–969. doi: 10.1097/NEN.0b013e318232a379. [DOI] [PubMed] [Google Scholar]
- 8.Elobeid A, Soininen H, Alafuzoff I. Hyperphosphorylated tau in young and middle-aged subjects. Acta Neuropathol. 2012;123:97–104. doi: 10.1007/s00401-011-0906-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hammerschmidt T, Kummer MP, Terwel D, Martinez A, Gorji A, Pape HC, Rommelfanger KS, Schroeder JP, Stoll M, Schultze J, Weinshenker D, Heneka MT. Selective Loss of Noradrenaline Exacerbates Early Cognitive Dysfunction and Synaptic Deficits in APP/PS1 Mice. Biol Psychiatry. 2013;73:454–463. doi: 10.1016/j.biopsych.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heneka MT, Nadrigny F, Regen T, Martinez-Hernandez A, Dumitrescu-Ozimek L, Terwel D, Jardanhazi-Kurutz D, Walter J, Kirchhoff F, Hanisch UK, Kummer MP. Locus ceruleus controls Alzheimer’s disease pathology by modulating microglial functions through norepinephrine. Proc Natl Acad Sci U S A. 2010;107:6058–6063. doi: 10.1073/pnas.0909586107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heneka MT, Ramanathan M, Jacobs AH, Dumitrescu-Ozimek L, Bilkei-Gorzo A, Debeir T, Sastre M, Galldiks N, Zimmer A, Hoehn M, Heiss WD, Klockgether T, Staufenbiel M. Locus ceruleus degeneration promotes Alzheimer pathogenesis in amyloid precursor protein 23 transgenic mice. J Neurosci. 2006;26:1343–1354. doi: 10.1523/JNEUROSCI.4236-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalinin S, Gavrilyuk V, Polak PE, Vasser R, Zhao J, Heneka MT, Feinstein DL. Noradrenaline deficiency in brain increases beta-amyloid plaque burden in an animal model of Alzheimer’s disease. Neurobiol Aging. 2007;28:1206–1214. doi: 10.1016/j.neurobiolaging.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 13.Kalinin S, Polak PE, Lin SX, Sakharkar AJ, Pandey SC, Feinstein DL. The noradrenaline precursor L-DOPS reduces pathology in a mouse model of Alzheimer’s disease. Neurobiol Aging. 2012;33:1651–1663. doi: 10.1016/j.neurobiolaging.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heneka MT, Galea E, Gavriluyk V, Dumitrescu-Ozimek L, Daeschner J, O’Banion MK, Weinberg G, Klockgether T, Feinstein DL. Noradrenergic depletion potentiates beta-amyloid-induced cortical inflammation: implications for Alzheimer’s disease. J Neurosci. 2002;22:2434–2442. doi: 10.1523/JNEUROSCI.22-07-02434.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klotz L, Sastre M, Kreutz A, Gavrilyuk V, Klockgether T, Feinstein DL, Heneka MT. Noradrenaline induces expression of peroxisome proliferator activated receptor gamma (PPARgamma) in murine primary astrocytes and neurons. J Neurochem. 2003;86:907–916. doi: 10.1046/j.1471-4159.2003.01909.x. [DOI] [PubMed] [Google Scholar]
- 16.Counts SE, Mufson EJ. Noradrenaline activation of neurotrophic pathways protects against neuronal amyloid toxicity. J Neurochem. 2010;113:649–660. doi: 10.1111/j.1471-4159.2010.06622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Traver S, Salthun-Lassalle B, Marien M, Hirsch EC, Colpaert F, Michel PP. The neurotransmitter noradrenaline rescues septal cholinergic neurons in culture from degeneration caused by low-level oxidative stress. Mol Pharmacol. 2005;67:1882–1891. doi: 10.1124/mol.104.007864. [DOI] [PubMed] [Google Scholar]
- 18.Troadec JD, Marien M, Darios F, Hartmann A, Ruberg M, Colpaert F, Michel PP. Noradrenaline provides long-term protection to dopaminergic neurons by reducing oxidative stress. J Neurochem. 2001;79:200–210. doi: 10.1046/j.1471-4159.2001.00556.x. [DOI] [PubMed] [Google Scholar]
- 19.Jhang KA, Lee EO, Kim HS, Chong YH. Norepinephrine provides short-term neuroprotection against Abeta1–42 by reducing oxidative stress independent of Nrf2 activation. Neurobiol Aging. 2014 doi: 10.1016/j.neurobiolaging.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 20.Iwasaki Y, Negishi T, Inoue M, Tashiro T, Tabira T, Kimura N. Sendai virus vector-mediated brain-derived neurotrophic factor expression ameliorates memory deficits and synaptic degeneration in a transgenic mouse model of Alzheimer’s disease. J Neurosci Res. 2012;90:981–989. doi: 10.1002/jnr.22830. [DOI] [PubMed] [Google Scholar]
- 21.Arancibia S, Silhol M, Mouliere F, Meffre J, Hollinger I, Maurice T, Tapia-Arancibia L. Protective effect of BDNF against beta-amyloid induced neurotoxicity in vitro and in vivo in rats. Neurobiol Dis. 2008;31:316–326. doi: 10.1016/j.nbd.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 22.Nagahara AH, Merrill DA, Coppola G, Tsukada S, Schroeder BE, Shaked GM, Wang L, Blesch A, Kim A, Conner JM, Rockenstein E, Chao MV, Koo EH, Geschwind D, Masliah E, Chiba AA, Tuszynski MH. Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer’s disease. Nat Med. 2009;15:331–337. doi: 10.1038/nm.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Devi L, Ohno M. 7,8-dihydroxyflavone, a small-molecule TrkB agonist, reverses memory deficits and BACE1 elevation in a mouse model of Alzheimer’s disease. Neuropsychopharmacology. 2012;37:434–444. doi: 10.1038/npp.2011.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Z, Liu X, Schroeder JP, Chan CB, Song M, Yu SP, Weinshenker D, Ye K. 7,8-dihydroxyflavone prevents synaptic loss and memory deficits in a mouse model of Alzheimer’s disease. Neuropsychopharmacology. 2014;39:638–650. doi: 10.1038/npp.2013.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan CB, Liu X, Pradoldej S, Hao C, An J, Yepes M, Luo HR, Ye K. Phosphoinositide 3-kinase enhancer regulates neuronal dendritogenesis and survival in neocortex. J Neurosci. 2011;31:8083–8092. doi: 10.1523/JNEUROSCI.1129-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giuffrida ML, Grasso G, Ruvo M, Pedone C, Saporito A, Marasco D, Pignataro B, Cascio C, Copani A, Rizzarelli E. Abeta(25–35) and its C-and/or N-blocked derivatives: copper driven structural features and neurotoxicity. J Neurosci Res. 2007;85:623–633. doi: 10.1002/jnr.21135. [DOI] [PubMed] [Google Scholar]
- 27.Millucci L, Raggiaschi R, Franceschini D, Terstappen G, Santucci A. Rapid aggregation and assembly in aqueous solution of A beta (25–35) peptide. J Biosci. 2009;34:293–303. doi: 10.1007/s12038-009-0033-3. [DOI] [PubMed] [Google Scholar]
- 28.Chen X, Ye H, Kuruvilla R, Ramanan N, Scangos KW, Zhang C, Johnson NM, England PM, Shokat KM, Ginty DD. A chemical-genetic approach to studying neurotrophin signaling. Neuron. 2005;46:13–21. doi: 10.1016/j.neuron.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 29.Bishop AC, Ubersax JA, Petsch DT, Matheos DP, Gray NS, Blethrow J, Shimizu E, Tsien JZ, Schultz PG, Rose MD, Wood JL, Morgan DO, Shokat KM. A chemical switch for inhibitor-sensitive alleles of any protein kinase. Nature. 2000;407:395–401. doi: 10.1038/35030148. [DOI] [PubMed] [Google Scholar]
- 30.Zhang F, Kang Z, Li W, Xiao Z, Zhou X. Roles of brain-derived neurotrophic factor/tropomyosin-related kinase B (BDNF/TrkB) signalling in Alzheimer’s disease. J Clin Neurosci. 2012;19:946–949. doi: 10.1016/j.jocn.2011.12.022. [DOI] [PubMed] [Google Scholar]
- 31.Nagahara AH, Mateling M, Kovacs I, Wang L, Eggert S, Rockenstein E, Koo EH, Masliah E, Tuszynski MH. Early BDNF treatment ameliorates cell loss in the entorhinal cortex of APP transgenic mice. J Neurosci. 2013;33:15596–15602. doi: 10.1523/JNEUROSCI.5195-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee FS, Chao MV. Activation of Trk neurotrophin receptors in the absence of neurotrophins. Proc Natl Acad Sci U S A. 2001;98:3555–3560. doi: 10.1073/pnas.061020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee FS, Rajagopal R, Kim AH, Chang PC, Chao MV. Activation of Trk neurotrophin receptor signaling by pituitary adenylate cyclase-activating polypeptides. J Biol Chem. 2002;277:9096–9102. doi: 10.1074/jbc.M107421200. [DOI] [PubMed] [Google Scholar]
- 34.Rajagopal R, Chen ZY, Lee FS, Chao MV. Transactivation of Trk neurotrophin receptors by G-protein-coupled receptor ligands occurs on intracellular membranes. J Neurosci. 2004;24:6650–6658. doi: 10.1523/JNEUROSCI.0010-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang YZ, Pan E, Xiong ZQ, McNamara JO. Zinc-mediated transactivation of TrkB potentiates the hippocampal mossy fiber-CA3 pyramid synapse. Neuron. 2008;57:546–558. doi: 10.1016/j.neuron.2007.11.026. [DOI] [PubMed] [Google Scholar]
- 36.Jang SW, Liu X, Yepes M, Shepherd KR, Miller GW, Liu Y, Wilson WD, Xiao G, Blanchi B, Sun YE, Ye K. A selective TrkB agonist with potent neurotrophic activities by 7,8-dihydroxyflavone. Proc Natl Acad Sci U S A. 2010;107:2687–2692. doi: 10.1073/pnas.0913572107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sieber-Blum M. Inhibition of the adrenergic phenotype in cultured neural crest cells by norepinephrine uptake inhibitors. Dev Biol. 1989;136:372–380. doi: 10.1016/0012-1606(89)90263-7. [DOI] [PubMed] [Google Scholar]
- 38.Sieber-Blum M, Ren Z. Norepinephrine transporter expression and function in noradrenergic cell differentiation. Mol Cell Biochem. 2000;212:61–70. [PubMed] [Google Scholar]
- 39.Zhang JM, Dix J, Langtimm-Sedlak CJ, Trusk T, Schroeder B, Hoffmann R, Strosberg AD, Winslow JW, Sieber-Blum M. Neurotrophin-3-and norepinephrine-mediated adrenergic differentiation and the inhibitory action of desipramine and cocaine. J Neurobiol. 1997;32:262–280. [PubMed] [Google Scholar]
- 40.Holm PC, Rodriguez FJ, Kresse A, Canals JM, Silos-Santiago I, Arenas E. Crucial role of TrkB ligands in the survival and phenotypic differentiation of developing locus coeruleus noradrenergic neurons. Development. 2003;130:3535–3545. doi: 10.1242/dev.00565. [DOI] [PubMed] [Google Scholar]
- 41.Smith MA, Makino S, Altemus M, Michelson D, Hong SK, Kvetnansky R, Post RM. Stress and antidepressants differentially regulate neurotrophin 3 mRNA expression in the locus coeruleus. Proc Natl Acad Sci U S A. 1995;92:8788–8792. doi: 10.1073/pnas.92.19.8788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Numan S, Lane-Ladd SB, Zhang L, Lundgren KH, Russell DS, Seroogy KB, Nestler EJ. Differential regulation of neurotrophin and trk receptor mRNAs in catecholaminergic nuclei during chronic opiate treatment and withdrawal. J Neurosci. 1998;18:10700–10708. doi: 10.1523/JNEUROSCI.18-24-10700.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alonso-Vanegas MA, Fawcett JP, Causing CG, Miller FD, Sadikot AF. Characterization of dopaminergic midbrain neurons in a DBH:BDNF transgenic mouse. J Comp Neurol. 1999;413:449–462. doi: 10.1002/(sici)1096-9861(19991025)413:3<449::aid-cne7>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 44.Fawcett JP, Bamji SX, Causing CG, Aloyz R, Ase AR, Reader TA, McLean JH, Miller FD. Functional evidence that BDNF is an anterograde neuronal trophic factor in the CNS. J Neurosci. 1998;18:2808–2821. doi: 10.1523/JNEUROSCI.18-08-02808.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luellen BA, Bianco LE, Schneider LM, Andrews AM. Reduced brain-derived neurotrophic factor is associated with a loss of serotonergic innervation in the hippocampus of aging mice. Genes Brain Behav. 2007;6:482–490. doi: 10.1111/j.1601-183X.2006.00279.x. [DOI] [PubMed] [Google Scholar]
- 46.Matsunaga W, Shirokawa T, Isobe K. BDNF is necessary for maintenance of noradrenergic innervations in the aged rat brain. Neurobiol Aging. 2004;25:341–348. doi: 10.1016/S0197-4580(03)00093-9. [DOI] [PubMed] [Google Scholar]