Abstract
After establishing a biochemical diagnosis, pheochromocytomas and extra-adrenal paragangliomas (PPGLs) can be localized using different anatomical and functional imaging modalities. These include computed tomography, magnetic resonance imaging, single-photon emission computed tomography (SPECT) using 123I-metaiodobenzylguanidine or 111In-DTPA-pentetreotide, and positron emission tomography (PET) using 6-[18F]-fluorodopamine (18F-FDA), 6-[18F]-fluoro-L-3,4-dihydroxyphenylalanine (18F-DOPA), and 2-[18F]-fluoro-2-deoxy-D-glucose. We review the currently available data on the performance of anatomical imaging, SPECT, and PET for the detection of (metastatic) PPGL as well as parasympathetic head and neck paragangliomas. We show that there appears to be no ‘gold-standard’ imaging technique for all patients with (suspected) PPGL. A tailor-made approach is warranted, guided by clinical, biochemical, and genetic characteristics. In the current era of a growing number of PET tracers, PPGL imaging has moved beyond tumor localization towards functional characterization of tumors.
Keywords: FDG PET, MIBG scintigraphy, FDOPA PET, FDA PET
Introduction
Pheochromocytomas and paragangliomas (PPGLs) are rare neuroendocrine tumors that derive from sympathetic chromaffin tissue in the adrenal medulla and from the extra-adrenal paraganglial system of the thorax and abdomen [1]. PPGLs arising from the adrenal medulla are commonly referred to as pheochromocytomas. Typical locations for extra-adrenal abdominal and thoracic PPGLs are: 1. the Zuckerkandl body, a sympathetic ganglion located at the root of the inferior mesenteric artery, 2. the sympathetic plexus of the urinary bladder, the kidneys and the heart, and 3. the sympathetic ganglia or the aortopulmonary body in the mediastinum. Head and neck paragangliomas (HNPGLs), also called glomus tumors, arise from parasympathetic paraganglia, mainly from the glomus caroticum, glomus jugulare, glomus tympanicum, and glomus vagale.
The majority of PPGLs synthesize, metabolize, store, and secrete catecholamines, whereas HNPGLs usually do not. Most common symptoms and signs of catecholamine excess due to PPGLs include headache, palpitations, diaphoresis, and sustained or paroxysmal hypertension. Symptoms and sign of HNPGLs rather relate to the tumor’s space-occupying effects, including cranial nerve damage.
PPGLs occur sporadically or in association with familial poly-tumor syndromes: multiple endocrine neoplasia type 2 (MEN2); von Hippel-Lindau (VHL) syndrome; neurofibromatosis type 1 (NF1); and paraganglioma syndromes associated with mutations of genes encoding subunits of the succinate dehydrogenase (SDH) complex, in particular subunits B (SDHB) and D (SDHD) [2, 3]. Reported frequencies of germline mutations of the above genes among patients with PPGLs range from 27 % to 32 % [4], and are likely to increase as further tumor susceptibility genes are identified. Most recently, mutations of genes encoding the SDH complex assembly factor 2 (SDHAF2), transmembrane protein 127 (TMEM127), SDHA, and MYC associated factor X (MAX) have been identified as further hereditary causes of PPGLs, ranking PPGLs as tumors most commonly associated with known gene mutations [5 – 7]. It is important to note that PPGLs with an underlying SDHB mutation are strongly associated with an aggressive behavior and the development of metastatic disease [8].
Before any localization of these tumors is initiated, it is now well accepted that the presence of these tumors needs to be proven biochemically first. It is recommended that plasma and/or 24-hour urine concentrations of metanephrines are measured since their sensitivity is more than 97 % for detecting PPGLs [9].
In patients with a biochemically established diagnosis of PPGL, anatomical and functional imaging are critical for a) primary tumor localization; b) the detection of multiple primary tumors that often come with above mentioned genetic disorders; and c) the detection of metastases commonly seen in SDHB-related PPGLs, guiding the optimal choice between curative surgery and palliative treatment options. First line anatomical imaging modalities for PPGL imaging include computed tomography (CT) or magnetic resonance imaging (MRI). MRI is a preferred method in children, young adults, pregnant women, and in those with cardiac PPGLs. CT and MRI provide a high sensitivity and allow precise tumor delineation, which is critical for pre-surgical evaluation. The specificity of these techniques is limited, however, and often must be coupled with the use of PPGL specific functional imaging. The exception to such a rule would be epinephrine-secreting tumors, since these are almost always located in adrenal glands and a positive CT or MRI points towards these tumors without the need of any functional imaging. However, it should be noted that in tumors with size over 5–6 cm, the likelihood of metastases at initial presentation is high and the whole body PPGL-specific functional imaging is recommended.
PPGLs possess unique characteristics regarding the uptake of highly specific radiotracers. Functional imaging is complimentary to anatomical imaging and provides specific information about the tumor’s functional characteristics. Nuclear medicine scanning techniques include planar scintigraphy, single-photon emission computed tomography (SPECT) and positron emission tomography (PET). These imaging modalities involve the use of radiotracers, which are taken up by the tumor cells through the targeting of specific transporter systems or peptide receptors on the cell membrane. The most widely used radiotracers for PPGL and HNPGL scintigraphy are 123I-labeled metaiodobenzylguanidine (123I-MIBG) and 111In-DTPA-pentetreotide scintigraphy. They have long been considered as the ‘gold standard’ modalities. However, novel PET tracers have become available in the recent years, some of which have been proven to be very useful for the functional and highly specific imaging of PPGL. These include 6-[18F]-fluorodopamine (18F-FDA), 6-[18F]-fluoro-L-3,4-dihydroxyphenylalanine (18F-DOPA), and 2-[18F]-fluoro-2-deoxy-D-glucose (18F-FDG).
We provide a review of the techniques that are currently available for the anatomical and functional imaging of PPGs and compare the diagnostic performance of these techniques for the detection of nonmetastatic and metastatic PPGLs as well as HNPGLs. We also explore the links between genotype-specific tumor biology and functional imaging results and address the future role of nuclear medicine in the localization and characterization of PPGs and HNPGs. Based on the available data, we provide recommendations for the optimal approach to PPGL imaging in clinical practice.
Anatomical Imaging
There are no large comparative studies on the performance of CT vs. MRI for the localization of PPGL. From our own experience, extra-adrenal PPGLs of the chest and abdomen may be better detected by CT than by MRI. Potential disadvantages of CT, however, include radiation exposure and the risk of contrast nephropathy. For the localization of HNPGL, it is well established that MRI is the preferred anatomical imaging technique.
Functional Imaging
In conjunction with planar imaging, SPECT provides cross sectional functional imaging data. The SPECT data can be fused with CT images for anatomic correlation, which are now routinely obtained on hybrid SPECT/CT instruments. In recent years, SPECT/CT has become more widely available and has the advantage of simultaneous acquisition of both morphological and functional data, increasing diagnostic confidence in image interpretation and enhancing sensitivity in some cases. However, these examinations are associated with practical constraints such as long imaging times, protection of the thyroid with non-radioactive iodine and need for withdrawals of interfering medications and gastrointestinal tract artifacts requiring bowel cleansing in some cases. The main disadvantage is probably the still low-resolution of the SPECT image, which is prone to artifacts and attenuation, limiting the ability to detect tiny lesions. SPECT also does not provide a quantifiable estimate of the tumor metabolism (tracer uptake). Thus, PET imaging has been growing rapidly in the imaging of PGLs, paralleled by great efforts towards the development of new tracers. The sensitivity and resolution of PET is superior to that of SPECT scintigraphy (the current theoretical resolution on phantoms is 4–5 mm, and in true clinical setting close to 7–10 mm). PET is also a quantitative imaging technique. Most commonly, the Standardized Uptake Value (SUV) is used to estimate the degree of tracer concentration in a defined region. In cases of small lesions, the partial volume phenomenon affects images both qualitatively and quantitatively with underestimation of the SUV, and sometimes missing such lesions. However, detectability of sub-centimetric lesions remains possible in case of high tracer avidity with favorable signal-to-noise ratio. Similar to SPECT-CT, PET is combined with CT for attenuation correction and co-localization.
Functional Imaging Targets in PPGL
Lesions detected by anatomical imaging can be specifically identified as PPGL by functional imaging agents that target the catecholamine synthesis, storage, and secretion pathways of chromaffin tumor cells [10]. These techniques include 123/131I-MIBG SPECT and 18F-FDA PET. 123/131I-MIBG and 18F-FDA target the norepinephrine transporter of the PPGL cell membrane and the vesicular monoamine transporters in the membrane of intracellular vesicles. These transporters facilitate the re-uptake and storage of catecholamines, respectively. The PET tracers 11C-epinephrine and 11C-hydroxyephedrine are alternatives that accumulate in tumor cells through the same mechanisms, but are of limited use for clinical imaging because of their (very) short half-life [11, 12].
18F-DOPA PET can be used for the imaging of the striatal system and neuroendocrine tumors such as carcinoids, but also for PPGL and HNPGL. The target of 18F-DOPA is the large amino acid transporter involved in the uptake of amine precursors.
Other, less-specific targets for PPGL imaging are the somatostatin receptors and glucose transporters. For somatostatin receptor-based imaging, 111In-DTPA-pentetreotide is available for SPECT and 68Ga-DOTA-TOC/TATE/NOC for PET/(CT). 18F-FDG PET provides an index of intracellular glucose metabolism. 18F-FDG is taken up by the tumor cell through the glucose transporters (6–8).
Sensitivity and Specificity
The use of 123I-MIBG is preferred over 131I-MIBG because of its higher sensitivity, lower radiation exposure, and improved imaging quality with SPECT [13]. The energy of 123I photons is better suited to detection with gamma cameras and, the absence of emission of beta particles, as well as the shorter isotope half-life (13 h vs. 8 days), lead to reduced dosimetry and authorize the administration of higher activity. Reported sensitivities of 123I-MIBG scintigraphy for localizing nonmetastatic PPGL vary between 77 and 98 % [14 – 16]. MIBG uptake by normal adrenal gland tissue might obscure small lesions. Overall, the sensitivity of MIBG imaging appears to be lower in extra-adrenal PGLs. The specificity of 123I-MIBG SPECT approaches 100 % [17]. Normal adrenal medulla is visualized with 131I-MIBG in about 10 % of cases and with 123I-MIBG in 50–80 % of cases. For the detection of metastatic lesions of PPGL, the sensitivity of 123I-MIBG is much lower, that is, only 50–79 % [14, 18]. Despite the low sensitivity for detecting PPGL metastases, a very important advantage of using 123I-MIBG scintigraphy in the setting of metastatic disease is the fact that it can identify patients who possibly benefit from palliative treatment with therapeutic doses of 131I-MIBG [19].
18F-DOPA PET has a high sensitivity for the localization of nonmetastatic PPGL [14, 20 – 22] and HNPGL [23 – 25]. Reported sensitivities vary between 81–100 %. No specificity data are available. The diagnostic accuracy of 18F-DOPA PET is improved when using carbidopa, an inhibitor of DOPA decarboxylase. Carbidopa enhances the sensitivity of 18F-DOPA PET for PPGL by increasing the tumor-to-background ratio of tracer uptake [26]. The performance of 18F-DOPA PET appears to be disappointing, however, in case of metastatic PPGL, especially SDHB-related cases, with sensitivities of only 45 % and 20 %, respectively (lesion-based analysis) [14].
For 18F-FDG PET, sensitivities up to 88 % for primary nonmetastatic PPGL were reported. When using a radiotracer that targets any tissue with an increase glucose metabolism, specificity might be of concern. Specificity in the context of PPGL imaging, however is as high as ~ 90 % and is similar to the specificity of 123I-MIBG SPECT and 18F-DOPA PET (unpublished results). 18F-FDG PET is highly sensitive for the detection of PPGL metastases, especially SDHB-related cases (region-based sensitivity 97 % in reference to CT/MRI) [18] (Fig. 1).
Fig. 1.
18F-FDA PET-CT (left panels) and 18F-FDG PET-CT (right panels) images in a 35-year-old male with retroperitoneal metastases of an SDHB-related PPGL. CT images (upper panels), axial PET images (middle panels), axial PET-CT fused images (lower panels).
18F-FDA was initially developed at the National Institutes of Health for functional imaging of the sympathetic nervous system and later evaluated as a new imaging tool for PPGL. 18F-FDA PET was shown to have a high sensitivity for both primary tumors (77–100 %) and metastases (77–90 %) [14, 17, 27]. The specificity of 18F-FDA PET exceeds 90 % [17]. The distinction between adrenal PPGL and normal adrenal glands is facilitated by the quantification of 18F-FDA uptake using SUVs [28]. So far, 18F-FDA is only available as an experimental imaging agent at the National Institutes of Health.
The sensitivity of somatostatin receptor scintigraphy with 111In-DTPA-pentetreotide is lower than that of 123I/131I-MIBG in PPGLs [29 – 31]. However, considering HNPGLs, several studies have demonstrated the superiority of 111In-DTPA-pentetreotide scintigraphy as compared to 123I/131I-MIBG, with sensitivities of 89–100 % and 18–50 %, respectively [32 – 37]. SPECT imaging with 111In-DTPA-pentetreotide is currently the most widely available scintigraphy modality for HNPGLs. However, its sensitivity needs to be revised downwards in patients with hereditary syndromes because some additional lesions can be at the millimeter stage and may not be detectable by conventional scintigraphy. For PET imaging of HNPGL, 18F-DOPA has been shown to be superior to other tracers [25].
The results of our previously reported study with a head-to-head comparison of the sensitivities between different functional imaging modalities [14] are presented in Table 1.
Table 1.
Sensitivity of functional imaging.
| Nonmetastatic PPGL (20 patients) | |||||
|---|---|---|---|---|---|
| CT and/or MRI | 18F-DOPA | 18F-FDA | 123I-MIBG | 18F-FDG | |
| With ref. to histologically confirmed lesions | 100 % (26/26) | 81 % (21/26) | 77 % (20/26) | 77 % (20/26) | 88 % (23/26) |
|
| |||||
| Sensitivities are not significantly different between functional imaging modalities | |||||
|
| |||||
| Metastatic PPGL (28 patients) | |||||
| CT and/or MRI | 18F-DOPA A | 18F-FDA B | 123I-MIBG C | 18F-FDG D | |
|
| |||||
| With ref. to lesions on CT and/or MRI | – | 45 % (96/211) | 76 % (161/211) | 57 % (106/187) | 74 % (157/211) |
Adapted with permission from [12], Copyright 2009, The Endocrine Society
A vs. B, A vs. C, A vs. D, B vs. C, C vs. D: p < 0.01. B vs. D: p = 0.760
Imaging across Hereditary Syndromes
Imaging results appear to be largely determined by the underlying genotypes and related tumor cell characteristics. There is evidence of differential expression of cellular targets for radiopharmaceuticals. For example, it was shown that there is lower expression of the cell membrane norepinephrine transporter system in VHL-related PPGL cells than in MEN2-related tumor cells [38]. Considering a higher affinity of 18F-FDA than 123I-MIBG for these transporters, it is no surprise that 18F-FDA PET is superior to 123I-MIBG SPECT in the context of VHL syndrome [39].
There also appears to be a link between tumor biology and imaging. SDHB -mutations are associated with PPGLs of a parti cularly high malignant potential. 18F-FDG PET has an excellent sensitivity for SDHB-associated metastatic PPGL [40, 41] (Fig. 2). 18F-FDG accumulation is an index of increased tissue glucose metabolism and, as a marker of tumor viability, the degree of 18F-FDG uptake usually reflects tumor aggressiveness. 18F-FDG uptake by PPGL does not appear to be merely an indicator of a high metabolic rate due to malignancy per se, but may rather be directly linked to SDHB -specific tumor biology [14]. The SDHB gene encodes for subunit B of the mitochondrial SDH complex II, a key enzyme in oxidative phosphorylation. SDHB mutations can lead to complete loss of SDH enzymatic activity in malignant PPGL, with upregulation of hypoxic-angiogenetic responsive genes [42]. Impairment of mitochondrial function due to loss of SDHB function may cause tumor cells to shift from oxidative phosphorylation to aerobic glycolysis, a phenomenon known as the “Warburg effect” [43]. Higher glucose requirement because of a switch to less efficient pathways for cellular energy production may perhaps explain the increased 18F-FDG uptake by malignant SDHB -related PPGL.
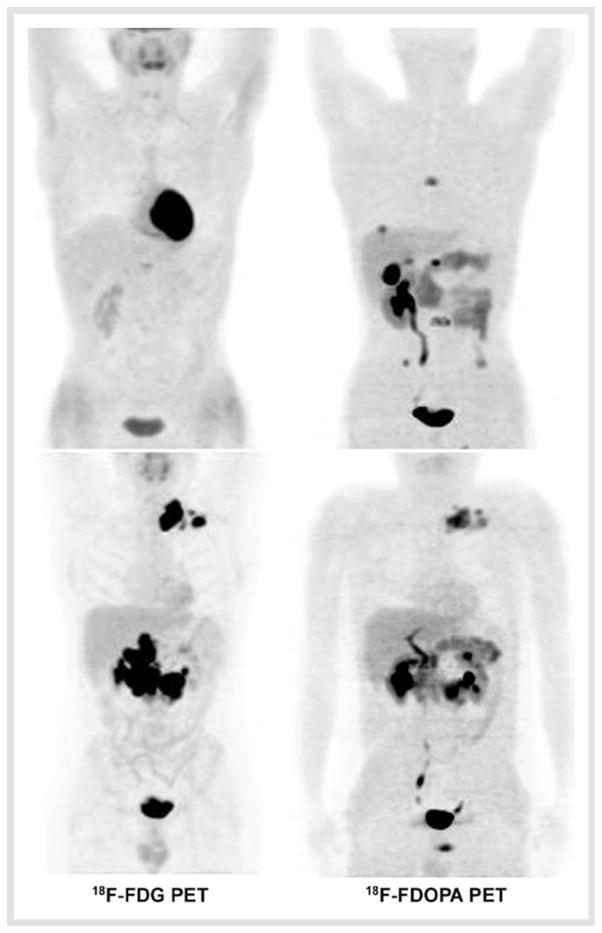
Fig. 2.
Comparison between 18F-FDOPA and 18F-FDG PET in 2 patients with metastatic PPGL (maximal intensity projection images). SDHB-negative patient (upper images). SDHB-positive patient (lower images). 18F-FDOPA was clearly superior to 18F-FDG PET in SDHB-negative PPGL and vice versa in the SDHB-related case.
Recommendations for Clinical practice
The current review shows that there appears to be no ‘gold-standard’ imaging technique for all patients with (suspected) PPGL and/or (suspected) HNPGL. A tailor-made approach to the anatomical and functional imaging of the individual patient is clearly warranted. The choices to be made by the practicing physician depend on many factors, including the aim of the investigation, clinical parameters including age and known hereditary syndrome, renal function (contrast nephropathy), (anticipated) radiation burden, the results of previous imaging (tumor size and location, suspicion of metastases), biochemical findings, preference of the patient, local availability of scanning systems and insurance issues. Based on the currently reviewed data and our own experience, we recommend the following principles for the approach to imaging. In patients with a biochemically established diagnosis of PPGL and a low likelihood of metastases (small tumors, adrenal location, adrenergic phenotype, non-SDHB) we recommend to perform a CT scan of the abdomen alone. In addition, 18F-FDG PET can be useful for functional tumor characterization, providing clues for an underlying hereditary syndrome. 18F-FDG uptake appears to be particularly high in SDHx and VHL-related PPGLs. In patients with a biochemically established diagnosis, when the aim is to rule out metastases, we recommend to perform a CT of the neck, chest, abdomen and pelvis in combination with 18F-FDG PET or 18F-FDA PET. 123I-MIBG SPECT needs to be performed in patients with established metastatic disease in order determine whether the tumor lesions are 123I-MIBG avid and whether the patient qualifies for palliative 131I-MIBG treatment. We do not recommend the use of 111In-DTPA-pentetreotide scintigraphy as a first-line imaging tool for PPGL due to lack of sensitivity. The role of novel somatostatin receptor-based PET scanning such as 68Ga-DOTA-peptides awaits further evaluation.
For patients in whom a HNPGL is suspected or needs to be ruled out (SDHx mutation carriers), an MRI of the head and neck is the scan of choice. For functional imaging of HNPGL, 18F-DOPA PET and somatostatin-receptor based imaging such as 111In-DTPA-pentetreotide appear to be most effective.
Future Perspectives
As outlined above current imaging approaches using either anatomical or functional imaging are directed towards the precise localization of PPGLs. However, with exciting recent introductions of new radiopharmaceuticals a new era is approaching for imaging cancer, including neuroendocrine tumors such as PPGLs. Soon it is expected that we will move from classic tumor localization towards tumor characterization. This is well supported by recent discoveries about how to assess angiogenesis [44], apoptosis [45], hypoxia [46], and other tumor specific cellular characteristics. The information obtained from these studies will serve to facilitate choice of appropriate drug regimens. This includes predicting therapeutic responses and discontinuation of treatment if no “targeted” pathway is affected. This will secure a cost-effective approach, minimize side effects, and “buy” valuable time for any patient in need of transfer to another form of treatment. Lack of response to indicate the latter can be expected to be revealed much earlier than by CT or MRI (usually 3–6 months). Personalized medicine will become a reality supported to the greatest extent by the use of functional imaging directed towards the characterization of any tumors including PPGLs. This at least initially requires a multi-institutional effort tightly coupled to close collaboration with the radiopharmaceutical industry as well as patient support groups. The future of new functional imaging is bright and it is very close to realization.
References
- 1.Lenders JW, Eisenhofer G, Mannelli M, Pacak K. Phaeochromocytoma. Lancet. 2005;366:665–675. doi: 10.1016/S0140-6736(05)67139-5. [DOI] [PubMed] [Google Scholar]
- 2.Timmers HJ, Gimenez-Roqueplo AP, Mannelli M, Pacak K. Clinical aspects of SDHx-related pheochromocytoma and paraganglioma. Endocr Relat Cancer. 2009;16:391–400. doi: 10.1677/ERC-08-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Timmers HJ, Kozupa A, Eisenhofer G, Raygada M, Adams KT, Solis D, Lenders JW, Pacak K. Clinical presentations, biochemical phenotypes, and genotype-phenotype correlations in patients with succinate dehydrogenase subunit B-associated pheochromocytomas and paragangliomas. J Clin Endocrinol Metab. 2007;92:779–786. doi: 10.1210/jc.2006-2315. [DOI] [PubMed] [Google Scholar]
- 4.Mannelli M, Castellano M, Schiavi F, Filetti S, Giacche M, Mori L, Pignataro V, Bernini G, Giache V, Bacca A, Biondi B, Corona G, Di Trapani G, Grossrubatscher E, Reimondo G, Arnaldi G, Giacchetti G, Veglio F, Loli P, Colao A, Ambrosio MR, Terzolo M, Letizia C, Ercolino T, Opocher G. Clinically guided genetic screening in a large cohort of italian patients with pheochromocytomas and/or functional or nonfunctional paragangliomas. J Clin Endocrinol Metab. 2009;94:1541–1547. doi: 10.1210/jc.2008-2419. [DOI] [PubMed] [Google Scholar]
- 5.Bayley JP, Kunst HP, Cascon A, Sampietro ML, Gaal J, Korpershoek E, Hinojar-Gutierrez A, Timmers HJ, Hoefsloot LH, Hermsen MA, Suarez C, Hussain AK, Vriends AH, Hes FJ, Jansen JC, Tops CM, Corssmit EP, de Knijff P, Lenders JW, Cremers CW, Devilee P, Dinjens WN, de Krijger RR, Robledo M. SDHAF2 mutations in familial and sporadic paraganglioma and phaeochromocytoma. Lancet Oncol. 2010;11:366–372. doi: 10.1016/S1470-2045(10)70007-3. [DOI] [PubMed] [Google Scholar]
- 6.Qin Y, Yao L, King EE, Buddavarapu K, Lenci RE, Chocron ES, Lechleiter JD, Sass M, Aronin N, Schiavi F, Boaretto F, Opocher G, Toledo RA, Toledo SP, Stiles C, Aguiar RC, Dahia PL. Germline mutations in TMEM127 confer susceptibility to pheochromocytoma. Nature Genet. 2010;42:229–233. doi: 10.1038/ng.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Comino-Mendez I, Gracia-Aznarez FJ, Schiavi F, Landa I, Leandro-Garcia LJ, Leton R, Honrado E, Ramos-Medina R, Caronia D, Pita G, Gomez-Grana A, de Cubas AA, Inglada-Perez L, Maliszewska A, Taschin E, Bobisse S, Pica G, Loli P, Hernandez-Lavado R, Diaz JA, Gomez-Morales M, Gonzalez-Neira A, Roncador G, Rodriguez-Antona C, Benitez J, Mannelli M, Opocher G, Robledo M, Cascon A. Exome sequencing identifies MAX mutations as a cause of hereditary pheochromocytoma. Nature Genet. 2011;43:663–667. doi: 10.1038/ng.861. [DOI] [PubMed] [Google Scholar]
- 8.Benn DE, Gimenez-Roqueplo AP, Reilly JR, Bertherat J, Burgess J, Byth K, Croxson M, Dahia PL, Elston M, Gimm O, Henley D, Herman P, Murday V, Niccoli-Sire P, Pasieka JL, Rohmer V, Tucker K, Jeunemaitre X, Marsh DJ, Plouin PF, Robinson BG. Clinical presentation and penetrance of pheochromocytoma/paraganglioma syndromes. J Clin Endocrinol Metab. 2006;91:827–836. doi: 10.1210/jc.2005-1862. [DOI] [PubMed] [Google Scholar]
- 9.Lenders JW, Pacak K, Walther MM, Linehan WM, Mannelli M, Friberg P, Keiser HR, Goldstein DS, Eisenhofer G. Biochemical diagnosis of pheochromocytoma: which test is best? JAMA. 2002;287:1427–1434. doi: 10.1001/jama.287.11.1427. [DOI] [PubMed] [Google Scholar]
- 10.Ilias I, Shulkin B, Pacak K. New functional imaging modalities for chromaffin tumors, neuroblastomas and ganglioneuromas. Trends Endocrinol Metab. 2005;16:66–72. doi: 10.1016/j.tem.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 11.Shulkin BL, Wieland DM, Schwaiger M, Thompson NW, Francis IR, Haka MS, Rosenspire KC, Shapiro B, Sisson JC, Kuhl DE. PET scanning with hydroxyephedrine: an approach to the localization of pheochromocytoma. J Nucl Med. 1992;33:1125–1131. [PubMed] [Google Scholar]
- 12.Mann GN, Link JM, Pham P, Pickett CA, Byrd DR, Kinahan PE, Krohn KA, Mankoff DA. [11C]metahydroxyephedrine and [18F]fluorodeoxyglucose positron emission tomography improve clinical decision making in suspected pheochromocytoma. Ann Surg Oncol. 2006;13:187–197. doi: 10.1245/ASO.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 13.Lynn MD, Shapiro B, Sisson JC, Beierwaltes WH, Meyers LJ, Ackerman RM, Mangner TJ. Pheochromocytoma and the normal adrenal medulla: improved visualization with I-123 MIBG scintigraphy. Radiology. 1985;155:789–792. doi: 10.1148/radiology.155.3.4001380. [DOI] [PubMed] [Google Scholar]
- 14.Timmers HJ, Chen CC, Carrasquillo JA, Whatley M, Ling A, Havekes B, Eisenhofer G, Martiniova L, Adams KT, Pacak K. Comparison of 18F-fluoro-L-DOPA. 18F-fluoro-deoxyglucose, and 18F-fluorodopamine PET and 123I-MIBG scintigraphy in the localization of pheochromocytoma and paraganglioma. J Clin Endocrinol Metab. 2009;94:4757–4767. doi: 10.1210/jc.2009-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Der Horst-Schrivers AN, Jager PL, Boezen HM, Schouten JP, Kema IP, Links TP. Iodine-123 metaiodobenzylguanidine scintigraphy in localising phaeochromocytomas–experience and meta-analysis. Anticancer Res. 2006;26:1599–1604. [PubMed] [Google Scholar]
- 16.Taieb D, Sebag F, Hubbard JG, Mundler O, Henry JF, Conte-Devolx B. Does iodine-131 meta-iodobenzylguanidine (MIBG) scintigraphy have an impact on the management of sporadic and familial phaeochromocytoma? Clin Endocrinol (Oxf) 2004;61:102–108. doi: 10.1111/j.1365-2265.2004.02077.x. [DOI] [PubMed] [Google Scholar]
- 17.Timmers HJ, Eisenhofer G, Carrasquillo JA, Chen CC, Whatley M, Ling A, Adams KT, Pacak K. Use of 6-[18F]-fluorodopamine positron emission tomography (PET) as first-line investigation for the diagnosis and localization of non-metastatic and metastatic phaeochromocytoma (PHEO) Clin Endocrinol (Oxf) 2009;71:11–17. doi: 10.1111/j.1365-2265.2008.03496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Timmers HJ, Kozupa A, Chen CC, Carrasquillo JA, Ling A, Eisenhofer G, Adams KT, Solis D, Lenders JW, Pacak K. Superiority of fluorodeoxyglucose positron emission tomography to other functional imaging techniques in the evaluation of metastatic SDHB-associated pheochromocytoma and paraganglioma. J Clin Oncol. 2007;25:2262–2269. doi: 10.1200/JCO.2006.09.6297. [DOI] [PubMed] [Google Scholar]
- 19.Loh KC, Fitzgerald PA, Matthay KK, Yeo PP, Price DC. The treatment of malignant pheochromocytoma with iodine-131 metaiodobenzylguanidine (131I-MIBG): a comprehensive review of 116 reported patients. J Endocrinol Invest. 1997;20:648–658. doi: 10.1007/BF03348026. [DOI] [PubMed] [Google Scholar]
- 20.Fiebrich HB, Brouwers AH, Kerstens MN, Pijl ME, Kema IP, de Jong JR, Jager PL, Elsinga PH, Dierckx RA, van der Wal JE, Sluiter WJ, de Vries EG, Links TP. 6-[F-18]Fluoro-L-dihydroxyphenylalanine positron emission tomography is superior to conventional imaging with (123)I-metaiodobenzylguanidine scintigraphy, computer tomography, and magnetic resonance imaging in localizing tumors causing catecholamine excess. J Clin Endocrinol Metab. 2009;94:3922–3930. doi: 10.1210/jc.2009-1054. [DOI] [PubMed] [Google Scholar]
- 21.Hoegerle S, Nitzsche E, Altehoefer C, Ghanem N, Manz T, Brink I, Reincke M, Moser E, Neumann HP. Pheochromocytomas: detection with 18F DOPA whole body PET–initial results. Radiology. 2002;222:507–512. doi: 10.1148/radiol.2222010622. [DOI] [PubMed] [Google Scholar]
- 22.Taieb D, Tessonnier L, Sebag F, Niccoli-Sire P, Morange I, Colavolpe C, De Micco C, Barlier A, Palazzo FF, Henry JF, Mundler O. The role of 18F-FDOPA and 18F-FDG-PET in the management of malignant and multifocal phaeochromocytomas. Clin Endocrinol (Oxf) 2008;69:580–586. doi: 10.1111/j.1365-2265.2008.03257.x. [DOI] [PubMed] [Google Scholar]
- 23.Hoegerle S, Ghanem N, Altehoefer C, Schipper J, Brink I, Moser E, Neumann HP. 18F-DOPA positron emission tomography for the detection of glomus tumours. Eur J Nucl Med Mol Imaging. 2003;30:689–694. doi: 10.1007/s00259-003-1115-3. [DOI] [PubMed] [Google Scholar]
- 24.Charrier N, Deveze A, Fakhry N, Sebag F, Morange I, Gaborit B, Barlier A, Carmona E, De Micco C, Garcia S, Mancini J, Palazzo FF, Lavieille J, Zanaret M, Henry JF, Mundler O, Taieb D. Comparison of [111In]pentetreotide-SPECT and [18F]FDOPA-PET in the localization of extra-adrenal paragangliomas: The case for a patient-tailored use of nuclear imaging modalities. Clin Endocrinol (Oxf) 2011;74:21–29. doi: 10.1111/j.1365-2265.2010.03893.x. [DOI] [PubMed] [Google Scholar]
- 25.King KS, Chen CC, Alexopoulos DK, Whatley MA, Reynolds JC, Patronas N, Ling A, Adams KT, Xekouki P, Lando H, Stratakis CA, Pacak K. Functional Imaging of SDHx-Related Head and Neck Paragangliomas: Comparison of 18F-Fluorodihydroxyphenylalanine, 18F-Fluorodopamine, 18F-Fluoro-2-Deoxy-D-Glucose PET, 123I-Metaiodobenzylguanidine Scintigraphy, and 111In-Pentetreotide Scintigraphy. J Clin Endocrinol Metab. 2011;96:2779–2785. doi: 10.1210/jc.2011-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Timmers HJ, Hadi M, Carrasquillo JA, Chen CC, Martiniova L, Whatley M, Ling A, Eisenhofer G, Adams KT, Pacak K. The effects of carbidopa on uptake of 6-18F-Fluoro-L-DOPA in PET of pheochromocytoma and extraadrenal abdominal paraganglioma. J Nucl Med. 2007;48:1599–1606. doi: 10.2967/jnumed.107.042721. [DOI] [PubMed] [Google Scholar]
- 27.Pacak K, Palkovits M. Stressor specificity of central neuroendocrine responses: implications for stress-related disorders. Endocr Rev. 2001;22:502–548. doi: 10.1210/edrv.22.4.0436. [DOI] [PubMed] [Google Scholar]
- 28.Timmers HJ, Carrasquillo JA, Whatley M, Eisenhofer G, Chen CC, Ling A, Linehan WM, Pinto PA, Adams KT, Pacak K. Usefulness of standardized uptake values for distinguishing adrenal glands with pheochromocytoma from normal adrenal glands by use of 6-18F-fluorodopamine PET. J Nucl Med. 2007;48:1940–1944. doi: 10.2967/jnumed.107.043281. [DOI] [PubMed] [Google Scholar]
- 29.Ilias I, Chen CC, Carrasquillo JA, Whatley M, Ling A, Lazurova I, Adams KT, Perera S, Pacak K. Comparison of 6-18F-fluorodopamine PET with 123I-metaiodobenzylguanidine and 111in-pentetreotide scintigraphy in localization of nonmetastatic and metastatic pheochromocytoma. J Nucl Med. 2008;49:1613–1619. doi: 10.2967/jnumed.108.052373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaltsas GA, Mukherjee JJ, Grossman AB. The value of radiolabelled MIBG and octreotide in the diagnosis and management of neuroendocrine tumours. Ann Oncol. 2001;12(Suppl 2):S47–S50. doi: 10.1093/annonc/12.suppl_2.s47. [DOI] [PubMed] [Google Scholar]
- 31.van der Harst E, de Herder WW, Bruining HA, Bonjer HJ, de Krijger RR, Lamberts SW, van de Meiracker AH, Boomsma F, Stijnen T, Krenning EP, Bosman FT, Kwekkeboom DJ. [(123)I]metaiodobenzylguanidine and [(111)In]octreotide uptake in begnign and malignant pheochromocytomas. J Clin Endocrinol Metab. 2001;86:685–693. doi: 10.1210/jcem.86.2.7238. [DOI] [PubMed] [Google Scholar]
- 32.Bustillo A, Telischi F, Weed D, Civantos F, Angeli S, Serafini A, Whiteman M. Octreotide scintigraphy in the head and neck. Laryngoscope. 2004;114:434–440. doi: 10.1097/00005537-200403000-00010. [DOI] [PubMed] [Google Scholar]
- 33.Duet M, Sauvaget E, Petelle B, Rizzo N, Guichard JP, Wassef M, Le Cloirec J, Herman P, Tran Ba Huy P. Clinical impact of somatostatin receptor scintigraphy in the management of paragangliomas of the head and neck. J Nucl Med. 2003;44:1767–1774. [PubMed] [Google Scholar]
- 34.Koopmans KP, Jager PL, Kema IP, Kerstens MN, Albers F, Dullaart RP. 111In-octreotide is superior to 123I-metaiodobenzylguanidine for scintigraphic detection of head and neck paragangliomas. J Nucl Med. 2008;49:1232–1237. doi: 10.2967/jnumed.107.047738. [DOI] [PubMed] [Google Scholar]
- 35.Muros MA, Llamas-Elvira JM, Rodriguez A, Ramirez A, Gomez M, Arraez MA, Valencia E, Vilchez R. 111In-pentetreotide scintigraphy is superior to 123I-MIBG scintigraphy in the diagnosis and location of chemodectoma. Nucl Med Commun. 1998;19:735–742. doi: 10.1097/00006231-199808000-00003. [DOI] [PubMed] [Google Scholar]
- 36.Schmidt M, Fischer E, Dietlein M, Michel O, Weber K, Moka D, Stennert E, Schicha H. Clinical value of somatostatin receptor imaging in patients with suspected head and neck paragangliomas. Eur J Nucl Med Mol Imaging. 2002;29:1571–1580. doi: 10.1007/s00259-002-0939-6. [DOI] [PubMed] [Google Scholar]
- 37.Telischi FF, Bustillo A, Whiteman ML, Serafini AN, Reisberg MJ, Gomez-Marin O, Civantos FJ, Balkany TJ. Octreotide scintigraphy for the detection of paragangliomas. Otolaryngol Head Neck Surg. 2000;122:358–362. doi: 10.1016/S0194-5998(00)70048-9. [DOI] [PubMed] [Google Scholar]
- 38.Huynh TT, Pacak K, Brouwers FM, Abu-Asab MS, Worrell RA, Walther MM, Elkahloun AG, Goldstein DS, Cleary S, Eisenhofer G. Different expression of catecholamine transporters in phaeochromocytomas from patients with von Hippel-Lindau syndrome and multiple endocrine neoplasia type 2. Eur J Endocrinol. 2005;153:551–563. doi: 10.1530/eje.1.01987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaji P, Carrasquillo JA, Linehan WM, Chen CC, Eisenhofer G, Pinto PA, Lai EW, Pacak K. The role of 6-[18F]fluorodopamine positron emission tomography in the localization of adrenal pheochromocytoma associated with von Hippel-Lindau syndrome. Eur J Endocrinol. 2007;156:483–487. doi: 10.1530/EJE-06-0712. [DOI] [PubMed] [Google Scholar]
- 40.Taieb D, Sebag F, Barlier A, Tessonnier L, Palazzo FF, Morange I, Niccoli-Sire P, Fakhry N, De Micco C, Cammilleri S, Enjalbert A, Henry JF, Mundler O. 18F-FDG avidity of pheochromocytomas and paragangliomas: a new molecular imaging signature? J Nucl Med. 2009;50:711–717. doi: 10.2967/jnumed.108.060731. [DOI] [PubMed] [Google Scholar]
- 41.Timmers HJ, Kozupa K, Chen CC, Carrasquillo JA, Ling A, Eisenhofer G, Adams KT, Solis D, Lenders JW, Pacak K. Superiority of fluorodeoxyglucose positron emission tomography to other functional imaging techniques in the evaluation of metastatic SDHB-associated pheochromocytoma and paraganglioma. J Clin Oncol. 2007;26:2262–2269. doi: 10.1200/JCO.2006.09.6297. [DOI] [PubMed] [Google Scholar]
- 42.Gimenez-Roqueplo AP, Favier J, Rustin P, Rieubland C, Kerlan V, Plouin PF, Rotig A, Jeunemaitre X. Functional consequences of a SDHB gene mutation in an apparently sporadic pheochromocytoma. J Clin Endocrinol Metab. 2002;87:4771–4774. doi: 10.1210/jc.2002-020525. [DOI] [PubMed] [Google Scholar]
- 43.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 44.Battle MR, Goggi JL, Allen L, Barnett J, Morrison MS. Monitoring tumor response to antiangiogenic sunitinib therapy with 18F-fluciclatide, an 18F-labeled alphaVbeta3-integrin and alphaV beta5-integrin imaging agent. J Nucl Med. 2011;52:424–430. doi: 10.2967/jnumed.110.077479. [DOI] [PubMed] [Google Scholar]
- 45.Blankenberg FG. In vivo detection of apoptosis. J Nucl Med. 2008;49(Suppl 2):81S–95S. doi: 10.2967/jnumed.107.045898. [DOI] [PubMed] [Google Scholar]
- 46.Hendrickson K, Phillips M, Smith W, Peterson L, Krohn K, Rajendran J. Hypoxia imaging with [F-18] FMISO-PET in head and neck cancer: Potential for guiding intensity modulated radiation therapy in overcoming hypoxia-induced treatment resistance. Radiother Oncol. 2011;101:369–375. doi: 10.1016/j.radonc.2011.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]