Abstract
Findings from five independent studies – with close to 350 patients with pheochromocytoma and more than 2 500 in whom the tumor was excluded – indicate that measurements of plasma free metanephrines provide an overall diagnostic sensitivity of 98 % and specificity of 92 %. The recommendation that initial testing for the tumor should always include measurements of either plasma or urinary fractionated metanephrines results from recognition of the high diagnostic sensitivity of measurements of plasma metanephrines. The few patients with pheochromocytoma in whom the test may not yield a positive result include those with very small tumors or microscopic disease and others with tumors that do not produce norepinephrine and epinephrine. Such patients are typically normotensive and do not exhibit symptoms of catecholamine excess. Additional measurements of methoxytyramine can be useful for detecting those tumors that produce only dopamine. Suboptimal diagnostic specificity and difficulties in distinguishing true- from false-positive elevations of plasma metanephrines remain challenges for diagnosis. Improvements in analytical technology (e.g., liquid chromatography with tandem mass spectrometry) and new strategies for follow-up testing provide possible solutions to these problems. The single most important remaining clinical care challenge is the development of effective cures for patients with malignant disease. Current treatments, none of which are truly satisfactory, include chemotherapy and radiopharmaceutical therapy with 131I-labelled m-iodobenzylguanidine or radioactive somatostatin analogues. Improvements in treatment may in the future come from several fronts, but proof of efficacy ideally will require well-coordinated multicenter prospective trials in larger numbers of patients than in previous studies.
Keywords: pheochromocytoma, paraganglioma, metanephrines, m-iodobenzylguanidine, malignant pheochromocytoma, metastases
Introduction
Pheochromocytomas and paragangliomas are neuroendocrine tumors derived respectively from adrenal chromaffin cells and extra-adrenal paraganglia. The tumors represent a rare cause of secondary hypertension, a result of their capacity to produce and secrete catecholamines. As a consequence of the unpredictable, often explosive nature of this secretion and the additional actions of co-secreted neuropeptides, the tumors can present with a host of symptoms and variable often confusing clinical manifestations [1]. The ensuing complications make these tumors potentially lethal if not diagnosed and treated appropriately.
Improvements in diagnosis of pheochromocytomas and paragangliomas (which for purposes of simplicity will be referred together hence forth as pheochromocytomas) have been facilitated by advances in our understanding of the physiology of catecholamine systems combined with progress in analytical chemistry, radiology, and nuclear medicine [2]. Because the tumors are most often benign, surgical resection usually provides an effective treatment. There, however, remains no adequate method to distinguish malignant from benign disease. Ineffective available treatments for malignant disease and lack of consensus about how to apply recent scientific and medical advances to improve diagnosis and treatment of patients with malignant disease remain important unresolved problems.
This article reviews recent progress concerning the biochemical diagnosis and treatment of benign and malignant pheochromocytomas and outlines some of the challenges that lie ahead for continuing improvements in clinical evaluation and care of patients with these tumors.
Current Progress in Diagnosis
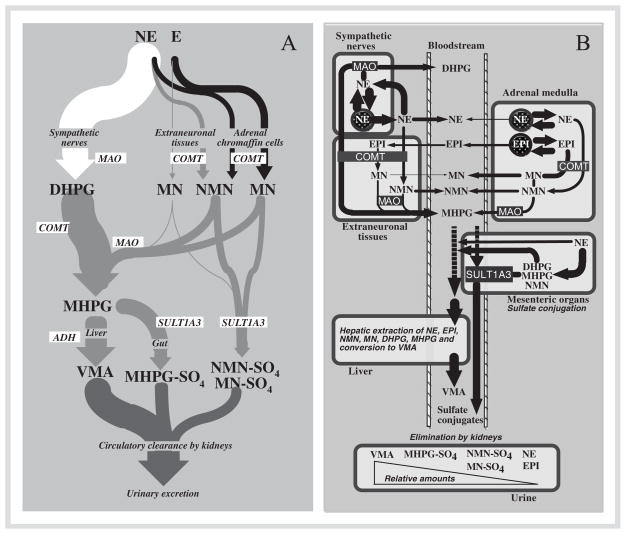
Appropriate appreciation of the utility of the many available biochemical tests used for diagnosis of pheochromocytoma can benefit considerably from a correct understanding of the pathways for synthesis, storage, release, and metabolism of catecholamines (Fig. 1). Most norepinephrine – the predominant catecholamine produced in the body – is synthesized in sympathetic nerves and deaminated there by the enzyme, monoamine oxidase, to 3,4-dihydroxyphenylglycol (DHPG). Under basal conditions of low sympathetic nerve activity (e.g., under stress-free conditions in healthy subjects resting supine) most DHPG is formed after leakage of norepinephrine from storage vesicles into the neuronal cytoplasm, but some is formed after reuptake back into nerves. Only a relatively small proportion of the nor-epinephrine released by sympathetic nerves escapes neuronal reuptake to be removed and metabolized at extraneuronal sites, or alternatively enter the circulation. Thus, the main pathway of norepinephrine metabolism is intraneuronal deamination to form DHPG, followed by extraneuronal O-methylation to form 3-methoxy-4-hydroxyphenylglycol, and finally in the liver further oxidation to form vanillylmandelic acid, the major metabolic end-product excreted in urine [3].
Fig. 1.
Diagrams showing the three main pathways for metabolism of catecholamines derived from sympathoneuronal or adrenalmedullary sources (panel A) and further illustrating the regional nature of catecholamine metabolism in different compartments (panel B). The sympathoneuronal pathway (shown in white in panel A) is the major pathway of norepinephrine metabolism and involves intraneuronal deamination of norepinephrine leaking from storage granules or of norepinephrine recaptured after release by sympathetic nerves (see panel B). The extraneuronal pathway (shown in grey in panel A) is a relatively minor pathway of metabolism of catecholamines released from sympathetic nerves or from the adrenal medulla, but is important for further processing of metabolites produced by neuronal and adrenalmedullary pathways. The adrenalmedullary pathway (shown in black in panel A) involves O-methylation of catecholamines leaking from storage granules into the cytoplasm of adrenalmedullary cells (panel B). Sulfate conjugation of catecholamines and catecholamine metabolites occurs mainly in mesenteric organs, whereas production of vanillylmandelic acid (VMA) occurs mainly in the liver. These represent the main metabolites excreted in urine. Abbreviations: NE: norepinephrine; E: epinephrine; DHPG: 3,4-dihydroxyphenylglycol; MN: metanephrine; NMN: normetanephrine; MHPG: 3-methoxy-4-hydroxyphenylglycol; VMA: vanillylmandelic acid; NMN-SO4: normetanephrine sulfate; MN-SO4: metanephrine sulfate; MHPG-SO4: 3-methoxy-4-hydroxyphenylglycol sulfate; MAO: monoamine oxidase; COMT: catechol-O-methyltransferase; ADH: alcohol dehydrogenase; SULT1A3: sulfotransferase type 1A3.
Extraneuronal pathways are minor routes of metabolism of catecholamines and lead to the formation of O-methylated metabolites through the actions of the enzyme, catechol-O-methyltransferase [3]. Absence of this enzyme in catecholamine-producing neurons means that the O-methylated metabolites are only produced at non-neuronal sites. At these sites metanephrine is formed from epinephrine, normetanephrine from norepinephrine, and methoxytyramine from dopamine. As a consequence of early spectrophotometric methods of measurement, where the former two metabolites were measured together, it is important to clarify that the term “metanephrines” in the plural form refers to both normetanephrine and metanephrine, but does not include methoxytyramine, the O-methylated metabolite of dopamine.
In addition to the commonly considered neuronal and extraneuronal pathways, there is also a third pathway of catecholamine metabolism and second source of O-methylated metabolites from within the adrenal medulla. Unlike sympathetic nerves, which do not contain catechol-O-methyltransferase, the presence of this enzyme within adrenal medullary cells leads to significant local production of metanephrines, again as in sympathetic nerves, from catecholamines leaking from storage vesicles [3]. Over 90 % of circulating metanephrine is formed in the adrenal medulla from epinephrine leaking from storage vesicles and less than 10 % from extraneuronal metabolism of epinephrine after release from the adrenals. For circulating normetanephrine, up to 40 % is formed from norepinephrine metabolized in adrenal medullary cells, the rest from extraneuronal metabolism, mainly of norepinephrine released by sympathetic nerves. The free metanephrines and methoxytyramine are further sulfate conjugated by a specific sulfotransferase isoenzyme located mainly in gastrointestinal tissues. In contrast to the free metanephrines, usually measured in plasma, the sulfate-conjugated metanephrines are the main form measured in urine.
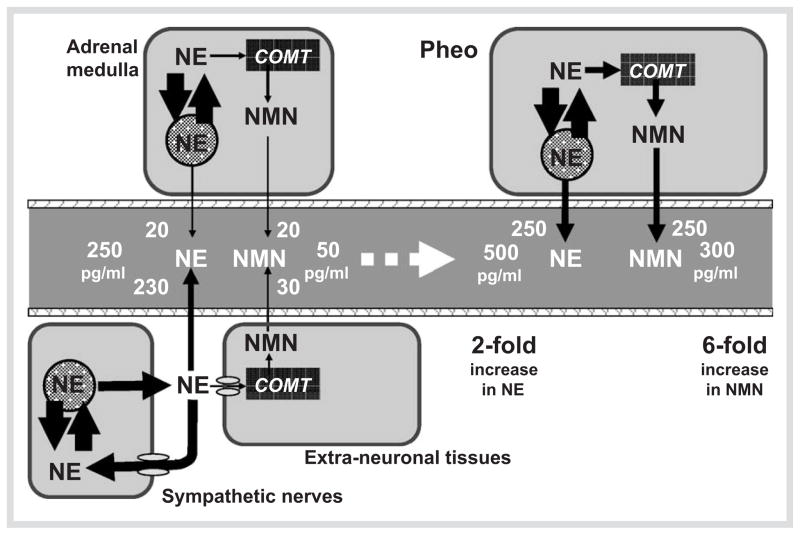
Since pheochromocytomas (like the chromaffin cells from which they are derived) express catechol-O-methyltransferase, these tumors also produce large amounts of metanephrines continuously from the catecholamines leaking from chromaffin granules [4]. This continuous production of O-methylated metabolites provides diagnostic advantages for measurements of metanephrines over the parent catecholamines, the latter often released by tumors intermittently or in negligible amounts. Minimal secretion of catecholamines is particularly evident for tumors that produce epinephrine; these are more often associated with normal plasma and urinary catecholamine levels than tumors that produce only norepinephrine. As shown in Fig. 2, there is also a mathematical reason for why measurements of normetanephrine at least are diagnostically superior to measurements of norepinephrine. Since the normal adrenal medulla contributes up to 40 % of all circulating normetanephrine, but less than 10 % to circulating norepinephrine, a tumor that produces these compounds in the same proportions as normal chromaffin cells can be expected to produce a larger fold increase above normal in levels of normetanephrine than of norepinephrine.
Fig. 2.
Diagrams illustrating the mathematical explanation for the superior sensitivity of plasma free normetanephrine (NMN) than of norepinephrine (NE) for detection of pheochromocytoma. Values on the left show the relative amounts of norepinephrine and normetanephrine in the bloodstream derived from sympathetic nerves and the adrenal medulla and the resulting usual plasma concentrations (250 pg/ml for NE and 50 pg/ ml for NMN). Values on the right illustrate the effects of apheochromocytoma on relative plasma concentrations of norepinephrine and normetanephrine assuming similar proportional production as in adrenal medullary cells. Due to the larger proportion of circulating normetanephrine(40 %) than norepinephrine (8 %) derived from the adrenal medulla, a 2-fold increase in plasma norepinephrine concentrations due to a pheochromocytoma would be associated with a larger 6-fold increase in plasma normetanephrine concentrations.
Correct understanding of the pathways of catecholamine metabolism has provided a rationale for many subsequent studies examining the utility of measurements of plasma free metanephrines for diagnosis of pheochromocytoma [5–11]. At the NIH, the superiority of plasma free metanephrines over other tests was established in a series of 3 published reports [5, 6, 8], culminating in a study involving over 850 patients, including 214 with pheochromocytoma [8]. Plasma free metanephrines showed superior combined diagnostic sensitivity and specificity over all other tests examined, including urinary and plasma catecholamines, urinary vanillylmandelic acid and urinary total and fractionated metanephrines. The high diagnostic sensitivity of measurements of plasma free metanephrines has now been independently confirmed by 4 other groups of investigators [7, 9–11]. All together, the five independent studies – with 336 patients with pheochromocytoma and close to 2 500 patients in whom the tumor was excluded – have indicated an overall diagnostic sensitivity of 98 % and specificity of 92 % for measurements of plasma free metanephrines (Table 1).
Table 1.
Sensitivity and specificity of measurements of plasma fractionated metanephrines for diagnosis of pheochromocytoma
| Center | Method | Diagnosis | URL (pg/ml) | Diag Accuracy | Ref. | |||
|---|---|---|---|---|---|---|---|---|
| P-Yes | P-No | NMN | MN | Sens | Spec | |||
| NIH | HPLC | 214 | 644 | 112 | 61 | 99 % | 89 % | [8] |
| Vienna | HPLC | 17 | 14 | 121 | 61 | 100 % | 100 % | [7] |
| Mayo Clinic | HPLC | 56 | 445 | 165 | 99 | 96 % | 85 % | [9] |
| University of Essen | RIA | 24 | 126 | 126 | 37 | 96 % | 79 % | [10] |
| Sternberk Hospital | HPLC | 25 | 1 235 | 112 | 61 | 100 % | 97 % | [11] |
| All Centers | – | 336 | 2 464 | – | – | 98 % | 92 % | – |
Abbreviations: P-Yes: number of patients with pheochromocytoma; P-No: number of patients in whom pheochromocytoma was excluded; URL: upper reference limits of reference intervals; NMN: normetanephrine; MN: metanephrine; Diag Accuracy: Diagnostic accuracy; Sens: Diagnostic sensitivity; Spec: Diagnostic specificity
Normal plasma concentrations of both plasma free normetanephrine and metanephrine are rarely found in patients with pheochromocytoma. Exceptions include very small or microscopic tumors found during routine screening in patients with a hereditary predisposition or a past history of pheochromocytoma [8]. Paragangliomas that produce only dopamine or no catecholamines at all represent other exceptions [12, 13]. These exceptions all, however, involve patients who do not present with the typical signs and symptoms of catecholamine excess and who also usually do not show elevations of urinary or plasma norepinephrine and epinephrine or any of the metabolites of these two catecholamines.
As a result of the increasingly widely recognized superior diagnostic sensitivity of plasma free metanephrines over other tests of catecholamine excess, it was relatively straightforward to reach a consensus at the 2005 International Symposium on Pheochromocytoma on several recommendations for biochemical testing in patients with suspected tumors [2]:
The first and most important recommendation was that initial testing for the tumor should always include measurements of either plasma free or urinary fractionated metanephrines.
The second recommendation was that reference intervals for initial tests of plasma or urinary fractionated metanephrines should be established primarily to ensure optimum diagnostic sensitivity, with specificity a secondary consideration.
Third, testing algorithms should not simply rely on a binary approach for test interpretation (i.e., whether a test result is negative or positive), but should instead take advantage of the continuous nature of biochemical test results.
Fourth, interpretation of positive test results in the “grey area” requires consideration – and where possible elimination – of causes of false-positive results before further confirmatory testing is initiated.
The fifth recommendation was that imaging studies to search for a pheochromocytoma should usually only be initiated once biochemical or other evidence of the tumor is reasonably compelling.
Further details concerning the above recommendations are available in the Symposium Proceedings [14], and at the website for the Pheochromocytoma Research Support Organization (http://www.pressor.org/information.htm).
Future Challenges for Effective Diagnosis
Although a negative test result for measurements of plasma free metanephrines effectively rules out pheochromocytoma, obviating the need for any further immediate testing, there remain a number of other problems to overcome before efficient diagnosis in every patient tested for the tumor is possible. There also remain several areas of disagreement requiring resolution, ideally with further recommendations derived from a consensus reached from a meeting of experts in the field.
The current recommendation that initial testing should always include either plasma free or urinary fractionated metanephrines, with no consensus reached on the preferred test [2, 14], is in part a response to the wider availability of the urinary than of the plasma test, but also reflects some disagreement and confusion concerning the accuracy and relative advantages of the two tests. The conclusion in the report by Sawka et al. [9] that measurements of plasma free metanephrines provide good diagnostic sensitivity, but suffer from suboptimal specificity, did not take into account the effects of seated posture [15]. In that study tests of urinary metanephrines were also restricted to spectrophotometric measurements of total metanephrines (i.e, the sum of metanephrine and normetanephrine), which showed relatively good specificity, but poor sensitivity. This has subsequently led to a common misconception that urinary fractionated metanephrines should also provide high specificity [16]. The study by Lenders et al. [8], however, showed that although the diagnostic sensitivity of urinary fractionated metanephrines was nearly as good as for plasma free metanephrines (97 vs. 99 %), specificity was much lower (69 vs. 89 %) for the urinary than the plasma test. In contrast, for urinary total metanephrines, sensitivity was only 77 % and specificity 93 %.
Certainly, adjusting the upper reference limits upwards, as advocated by Perry et al. [17], can provide reasonable diagnostic specificity for urinary fractionated metanephrines; however, this is at the cost of reduced diagnostic sensitivity and is not in line with the recommendation outlined above that reference intervals should be established primarily for optimum sensitivity, with specificity a secondary consideration.
Nevertheless, overall differences in the diagnostic accuracy of plasma free versus urinary fractionated metanephrines are much smaller than those of both tests compared to measurements of catecholamines and other metabolites [8]. As reviewed elsewhere [14], measurements of plasma and urinary fractionated metanephrines have different practical advantages and disadvantages, which should also be considered. For example, difficulties in obtaining valid or accurate urine collections in anuric kidney failure patients or in children provide practical advantages for plasma free metanephrines over urinary metanephrines.
Introduction of methods for measurements of urinary free metanephrines may also offer advantages over the more commonly used measurements where an acid hydrolysis deconjugation step is employed and measurements reflect mainly urinary levels of sulfate-conjugated metanephrines [18]. Since it is the free metanephrines that are formed within tumor tissue, measurements of these metabolites, rather than the sulfate conjugates, should offer a more direct and accurate marker for pheochromocytoma than measurements of the more easily measured and much higher concentrations of deconjugated metanephrines.
Differences in instrumental analysis and susceptibility of the various methods to analytical interferences are also likely to have an important influence on ever troublesome false-positive results with attendant impact on test specificity. Currently available methods for measurements of plasma and urinary fractionated metanephrines include enzyme immunoassays (EIA), radio immunoassays (RIA), liquid chromatography with electrochemical detection (LCEC), and more recently, liquid chromatography with tandem mass spectrometry (LC-MS/MS) [19, 20]. These methods have different practical advantages and disadvantages, but with the superior analytical specificity achieved with LC-MS/MS methods it can be expected that such methods should also offer improvements in diagnostic specificity. Indeed, a study from the Mayo Clinic, where LC-MS/MS has now replaced LCEC methods, indicated 1 034 positive results for plasma free metanephrines out of a total of 24 204 plasma samples analyzed, yielding a diagnostic specificity of more than 96 % [21]. This compares with previously reported diagnostic specificities for plasma free metanephrines measured by LCEC at the same center of 85 % [9], and at the NIH of 89 % [8]. In further contrast, for plasma free metanephrines measured by RIA, the diagnostic specificity was 79 % [10].
With the above in mind, any future studies designed to assess the relative diagnostic advantages of measurements of plasma and urinary metanephrines will need to consider the various measurement methods along with the actual nature of the O-methylated metabolites being measured (i.e., free vs. deconjugated metanephrines). It is possible that improved accuracy and analytical specificity of certain methods of analysis over others (e.g., LC-MS/MS vs. LCEC, EIA, and RIA) might be more important in determining differences in diagnostic accuracy than the nature of the O-methylated metabolites actually measured by the various methods. Standardization among laboratories performing testing for metanephrines and availability of appropriate quality control reference materials represent other challenges requiring future consideration.
Irrespective of the methodological considerations outlined above, the need for approaches and algorithms to distinguish false positive from true-positive results continues to be a problem that can benefit from further recommendations beyond those already reached at the First International Symposium on Pheochromocytoma. In patients with positive test results, where increases in plasma or urinary normetanephrine or meta-nephrine are above a certain magnitude, a pheochromocytoma may be so likely so as to render follow-up biochemical testing unnecessary. In these patients the immediate task is to locate the tumor. While it was also agreed that follow-up biochemical testing rarely should be necessary in patients with negative results for plasma free metanephrines, there remain different opinions about the most suitable follow-up tests in patients with results that fall within the “grey area” (i.e., positive test results below the cutoffs that provide 100 % specificity). Further recommendations in this direction may be difficult to reach without carefully designed and implemented prospective multi-center studies that not only take into account the reliability of any subsequent diagnosis, but also the relative costs.
Regardless of the analytical method (e.g., LC-MS/MS and LCEC), drugs such as tricyclic antidepressants and phenoxybenzamine – with pharmacological actions to increase plasma and urinary norepinephrine and normetanephrine – should first be excluded as causes of false-positive test results [22]. In the NIH study where the diagnostic specificity of plasma free metanephrines was 89 %[8], these two drugs were identified as causing up to 45 % of all false-positive results [22]. In a more recent series of over 1200 patients, where tricyclic antidepressants were withdrawn and doses of adrenergic blockers were reduced before testing, the diagnostic specificity of plasma free metanephrines reached 96.7 % with a sensitivity of 100 % [11].
Once medications and other causes of false-positive results have been considered and ruled out, the clonidine-suppression test combined with measurements of plasma free metanephrines has been advocated as an effective method for distinguishing false-positive from true-positive elevations of normetanephrine [22]. This method, however, cannot be used to distinguish false- from true-positive elevations in metanephrine. More recently, a study from the Mayo clinic advocated the use of urine fractionated metanephrines and chromogranin A as follow-up tests [21].
A further challenge to diagnostic testing, that has arisen from advances in molecular genetics and increasing recognition of the importance of mutations of genes for succinate dehydrogenase B, C, and D (SDHB, SDHC, and SDHD), is how best to detect paragangliomas that do not produce (synthesize) any norepinephrine or epinephrine, and as a consequence do not show elevations of either normetanephrine or metanephrine. In those patients where tumors produce only dopamine, additional measurements of methoxytyramine can be useful [12]. However, whether such measurements should be considered in all patients with suspected pheochromocytoma is debatable. Similarly, for those nonfunctional paragangliomas that do not produce or contain any appreciable catecholamines, measurements of chromogranin A may be useful [13], but again whether this measurement should be included for all patients with suspected pheochromocytomas is again debatable.
Current Progress in Treatment
Most pheochromocytomas are readily cured by surgery, but once resected cure is not guaranteed and no patient should be misled into believing they are cured and that no further follow-up is necessary [23]. Recurrence can occur locally, at another single site, or may involve malignant disease (defined as meta-static spread to sites where chromaffin cells are normally absent, e.g., lungs, liver, bone). In one series of 171 patients followed up after surgical resection of a chromaffin cell tumor, 29 patients (17 % ) had recurrent or new tumors, which were malignant in 15 (9 %) cases [24].
Follow-up biochemical testing performed once a year was advocated to ensure prompt diagnosis of local recurrence or metastatic disease. Follow-up testing is especially important for patients with extra-adrenal paragangliomas where the likelihood of recurrences and malignancy is particularly high [25]. Patients with tumors due to mutations of the SDHB gene have a high susceptibility to develop malignant disease [26]. Unfortunately, there is currently no cure for malignant disease and the 5 year survival is less than 50 % [25].
Surgery is rarely curative for malignant disease but can be appropriate for lesions in life threatening or debilitating anatomic locations. Surgical debulking may also reduce the risk from high levels of catecholamines, but the value of this before further treatment of disseminated disease by chemotherapy or radio-pharmaceutical therapy is unproven. Alternatives to surgical resection for treatment of isolated lesions include external beam radiation, cryoablation, radiofrequency ablation, and transcatheter arterial embolization [27, 28].
The principal treatments for disseminated malignant disease, none of which offer an effective cure, include chemotherapy with a combination of cyclophosphamide, vincristine, and dacarbazine (CVD chemotherapy) or other chemotherapeutic agents, and radiopharmaceutical therapy with 131I-labelled m-iodobenzylguanidine (131I-MIBG) or radioactive somatostatin analogues.
The first study on the effectiveness of CVD chemotherapy, carried out by Averbuch et al. [29], yielded 2 out of 14 patients who showed a complete response and 6 a partial response. As reviewed elsewhere [30], since that initial study there have been at least 5 other reports essentially confirming this response rate to similar chemotherapy regimens. The treatment, however, is associated with significant side effects, including leuko- and thrombocytopenia, paresthesias, nausea, and vomiting, and is not well tolerated.
Other chemotherapeutic agents that have been employed in the treatment of malignant pheochromocytoma include the combination of etoposide and cisplatin [31], cisplatin and 5-fluorouracil [32], cytokine arabinoside [33], anthracine plus CVD [34], and most recently, temozolomide and thalidomide [35]. In general, the CVD scheme appears the most effective, though remissions are usually short and often followed by complete relapse. Nevertheless, individualized chemotherapy can be useful for palliative care and may lengthen survival.
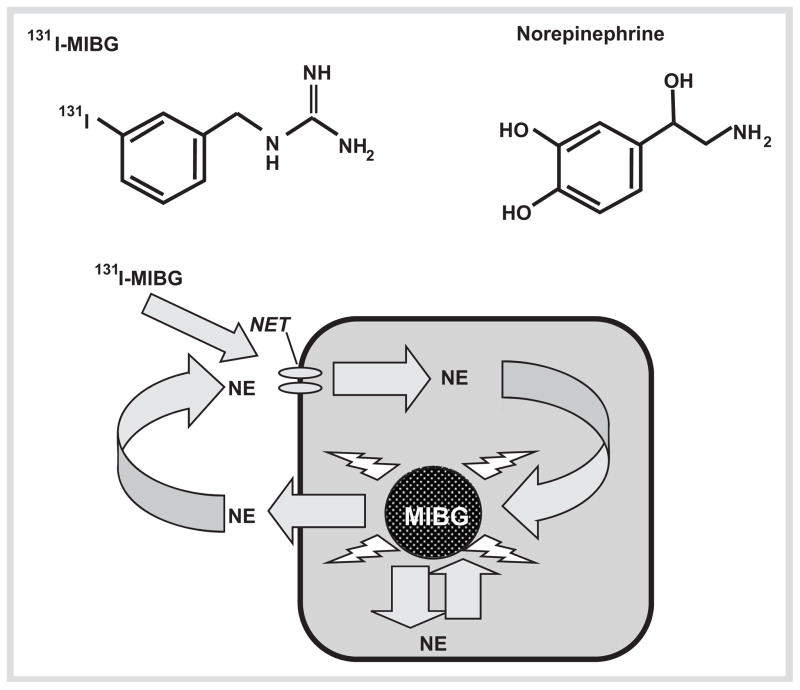
131I-MIBG radiotherapy provides a well tolerated valuable alternative treatment with generally less severe side effects than CVD chemotherapy. Because of a similar structure to norepinephrine, the radiopharmaceutical is taken up into pheochromocytoma cells by the cell membrane norepinephrine transporter, and then sequestered into vesicles where it is retained, exerting radiation-induced cell death (Fig. 3). As extensively reviewed elsewhere [30, 36], there have been numerous studies assessing the utility of the agent since its first use in the early 80 s. However, almost all studies have been small and retrospective in nature, with comparisons made difficult by variability in administered doses and dose scheduling. The overall full recovery rate has only been 4 %, but the agent is nevertheless considered a valuable treatment option. The high dose therapy utilized by Fitzgerald and colleagues [37] appears to yield improved response rates, but significant thrombocytopenia can be a problem.
Fig. 3.
Diagram illustrating the structural similarities between MIBG and norepinephrine (NE) and the mode of uptake and storage of MIBG in chromaffin tumor cells that express the cell membrane nor-epinephrine transporter (NET).
Although both unlabeled and radiolabeled somatostatin analogues provide a valuable treatment option for many neuroendocrine tumors, particularly those of the gastropancreatic system, the results of therapeutic trials for pheochromocytoma have not been encouraging to date [38]. In part, this appears due to low and variable expression of somatostatin type 2 receptors, the receptor type with higher affinity to currently available somatostatin analogues [39]. Data, however, are limited and it is possible that metastases due to extra-adrenal paragangliomas may respond better to this form of treatment than those derived from intra-adrenal tumors.
Future Challenges for Effective Treatment
Improvements in the treatment of malignant pheochromocytoma may in the future come from several fronts, but proof of efficacy ideally will require well-coordinated multicenter prospective trials in larger numbers of patients than in past studies [40]. Treatments with agents that target specific pathways identified as crucial to malignant transformation or survival of metastasizing cells are likely to provide the most appropriate solution for effective treatment. Such advances may be best facilitated from molecular profiling of tumors with follow-up studies in experimental model systems to test efficacy of potential therapeutic agents on the targeted pathway before application in patients. In the mean time, efforts directed towards affected patients may best benefit from refinements and advances in current treatment options.
The development of no-carrier added high specific activity 131I-MIBG offers one strategy for improved treatment efficacy of this form of radiotherapy [41, 42]. Data from preclinical studies has indicated higher uptake by tissues and tumors of the no-carrier added 131I-MIBG than of the lower specific activity form [41]. Data in humans also indicates that no carrier added 123I-MIBG yields higher myocardial uptake than the low specific activity form [43], but whether there is also higher uptake in tumors has been unclear [44].
Placement of angiography-guided catheters to the arterial inflow of affected organ systems provides another approach that can further facilitate targeting of radiopharmaceuticals to tumors [45]. Use of this approach resulted in up to a 4-fold increase in the tumor-to-whole body ratio of delivery of 131I-MIBG in patients with carcinoids [45]. Although unlikely to offer significant overall enhancement of tumor uptake of the radiopharmaceutical in patients with widely disseminated metastatic disease, the approach might prove useful for individualized treatment of patients with metastases in discrete locations (e.g., the liver).
Alternative substrates to MIBG for the norepinephrine transporter and storage system represent another avenue for improvements in therapeutic efficacy for radiotherapies targeting this system [46]. 3-[211At]astatobenzylguanidine ([211At] MABG), 3-[211At]-astato-4-fluorobenzylguanidine, 4-fluoro-3-[131I]iodobenzylguanidine, and 3-[131I]iodo-4-methylbenzylguanidine, are all analogues of MIBG developed as alternative radiopharmaceuticals for targeting tumors that express the norepinephrine transporter and storage system [47–50]. Among these analogues, the alpha-emitting characteristics and prolonged tissue retention of [211 At]MABG, may make this a particularly promising agent for treatment of such neuroendocrine tumors [51, 52].
Approaches aimed at increasing expression of key components of the norepinephrine transporter and storage systems may provide another strategy to improve delivery of 131I-MIBG and other radiolabeled analogues to tumor cells. Work here has mainly been directed at norepinephrine transporter gene transfer strategies using viral constructs [53–55]. Other strategies in this direction that might be effective include targeting the promoter regions of the norepinephrine transporter gene or increasing the cell-surface expression of the transporter via N-glycosylation regulatory mechanisms [56]. Nevertheless, in addition to the cell membrane norepinephrine transporter, it seems that numbers of neurosecretory granules and the presence of vesicular monoamine transporters (VMATs) are also crucially important for uptake and retention of radiolabeled MIBG [57, 58]. Thus, strategies aimed at sensitizing neuroendocrine tumors to drugs targeting the norepinephrine transport and storage system could benefit from approaches that increase expression of all key components of the system.
As described above, lack of efficacy of radiolabeled somatostatin analogues for treatment of malignant pheochromocytoma appears due to the low and variable expression of somatostatin type 2 receptors targeted by those analogues. Malignant pheochromocytomas do, however, show more consistent expression of somatostatin type 3 or 5 than type 2 receptors [39]; thus, it is possible that analogues with broader receptor specificity might be more effective therapeutic agents than currently available radiopharmaceuticals.
Because pheochromocytomas and paragangliomas comprise a highly heterogeneous group of tumors, it is becoming increasingly apparent that no single therapy will work in every patient with malignancy [40]. Thus, patients with metastases that cannot be localized by MIBG scintigraphy are much less likely to respond to 131 I-MIBG radiotherapy than patients with tumors that are localized by this imaging modality. The use of functional imaging as an aid to clinical decision-making and quantification of components of systems targeted by therapies (e.g., vesicular monoamine transporters and somatostatin receptors) provide some of the approaches proposed for tailoring therapies to individual patients [59, 60].
As indicated by several studies there can often be discordant results in the localization of metastases by MIBG and somatostatin receptor imaging, positron emission tomographic (PET) scanning with 18F-fluorodopamine and PET scanning with 18F-fluorodeoxyglucose [61–64]. Patients with malignant tumors secondary to mutations of the SDHB gene in particular tend to show superior results by 18F-fluorodeoxyglucose PET scanning than by other modalities [64]. In these patients, many of whom appear to have rapidly progressive disease and a poorly developed norepinephrine uptake and storage system, use of CVD chemotherapy may provide a better response than 131I-MIBG radiotherapy.
Use of combination therapies, including chemotherapy and 131I-MIBG radiotherapy, or therapies that target both somatostatin receptor and norepinephrine uptake and storage systems represent other avenues proposed to improve therapeutic response [40, 65, 66].
Future progress in alternative therapies to those described above will hopefully evolve from gene and protein expression profiling studies seeking to characterize differences between benign and malignant pheochromocytomas [67–69]. As reviewed elsewhere, there are already several leads on markers of malignancy and targets for treatment from studies comparing malignant with benign pheochromcytoma [70]. Such targets include heat shock protein 90, human telomerase reverse transcriptase, vascular endothelial growth factor and other components of hypoxia-angiogenesis pathways. Increased understanding of the pathways involved in tumorigenesis may also come from approaches looking at the downstream effects of mutated genes that predispose to pheochromocytoma. Effects of mutations of the SDHB gene, involving accumulation of succinate with resulting inhibition of α-ketoglutarate-dependent enzymes, represent one avenue for exploration of possible treatments for malignancies in patients with these mutations [71, 72]. Although prognosis is currently dim, there is hope on the horizon for future patients with malignant pheochromocytoma.
References
- 1.Manger WM. An overview of pheochromocytoma: history, current concepts, vagaries, and diagnostic challenges. Ann N Y Acad Sci. 2006;1073:1–20. doi: 10.1196/annals.1353.001. [DOI] [PubMed] [Google Scholar]
- 2.Pacak K, Eisenhofer G, Ahlman H, Bornstein SR, Gimenez-Roqueplo AP, Grossman AB, Kimura N, Mannelli M, MacNicol AM, Tischler AS. Pheochromocytoma: recommendations for clinical practice from the First International Symposium. Nat Clin Pract Endocrinol Metab. 2007;3:92–102. doi: 10.1038/ncpendmet0396. [DOI] [PubMed] [Google Scholar]
- 3.Eisenhofer G, Kopin IJ, Goldstein DS. Catecholamine metabolism: a contemporary view with implications for physiology and medicine. Pharmacol Rev. 2004;56:331–349. doi: 10.1124/pr.56.3.1. [DOI] [PubMed] [Google Scholar]
- 4.Eisenhofer G, Keiser H, Friberg P, Mezey E, Huynh TT, Hiremagalur B, Ellingson T, Duddempudi S, Eijsbouts A, Lenders JW. Plasma meta-nephrines are markers of pheochromocytoma produced by catechol-O-methyltransferase within tumors. J Clin Endocrinol Metab. 1998;83:2175–2185. doi: 10.1210/jcem.83.6.4870. [DOI] [PubMed] [Google Scholar]
- 5.Lenders JW, Keiser HR, Goldstein DS, Willemsen JJ, Friberg P, Jacobs MC, Kloppenborg PW, Thien T, Eisenhofer G. Plasma metanephrines in the diagnosis of pheochromocytoma. Ann Intern Med. 1995;123:101–109. doi: 10.7326/0003-4819-123-2-199507150-00004. [DOI] [PubMed] [Google Scholar]
- 6.Eisenhofer G, Lenders JW, Linehan WM, Walther MM, Goldstein DS, Keiser HR. Plasma normetanephrine and metanephrine for detecting pheochromocytoma in von Hippel-Lindau disease and multiple endocrine neoplasia type 2. N Engl J Med. 1999;340:1872–1879. doi: 10.1056/NEJM199906173402404. [DOI] [PubMed] [Google Scholar]
- 7.Raber W, Raffesberg W, Bischof M, Scheuba C, Niederle B, Gasic S, Waldhausl W, Roden M. Diagnostic efficacy of unconjugated plasma metanephrines for the detection of pheochromocytoma. Arch Intern Med. 2000;160:2957–2963. doi: 10.1001/archinte.160.19.2957. [DOI] [PubMed] [Google Scholar]
- 8.Lenders JW, Pacak K, Walther MM, Linehan WM, Mannelli M, Friberg P, Keiser HR, Goldstein DS, Eisenhofer G. Biochemical diagnosis of pheochromocytoma: which test is best? JAMA. 2002;287:1427–1434. doi: 10.1001/jama.287.11.1427. [DOI] [PubMed] [Google Scholar]
- 9.Sawka AM, Jaeschke R, Singh RJ, Young WF., Jr A comparison of biochemical tests for pheochromocytoma: Measurement of fractionated plasma metanephrines compared with the combination of 24-hour urinary metanephrines and catecholamines. J Clin Endocrinol Metab. 2003;88:553–558. doi: 10.1210/jc.2002-021251. [DOI] [PubMed] [Google Scholar]
- 10.Unger N, Pitt C, Schmidt IL, Walz MK, Schmid KW, Philipp T, Mann K, Petersenn S. Diagnostic value of various biochemical parameters for the diagnosis of pheochromocytoma in patients with adrenal mass. Eur J Endocrinol. 2006;154:409–417. doi: 10.1530/eje.1.02097. [DOI] [PubMed] [Google Scholar]
- 11.Vaclavik J, Stejskal D, Lacnak B, Lazarova M, Jedelsky L, Kadalova L, Janosova M, Frysak Z, Vlcek P. Free plasma metanephrines as a screening test for pheochromocytoma in low-risk patients. J Hypertens. 2007;25:1427–1431. doi: 10.1097/HJH.0b013e32813aeb5a. [DOI] [PubMed] [Google Scholar]
- 12.Eisenhofer G, Goldstein DS, Sullivan P, Csako G, Brouwers FM, Lai EW, Adams KT, Pacak K. Biochemical and clinical manifestations of dopamine-producing paragangliomas: utility of plasma methoxytyramine. J Clin Endocrinol Metab. 2005;90:2068–2075. doi: 10.1210/jc.2004-2025. [DOI] [PubMed] [Google Scholar]
- 13.Timmers HJ, Kozupa A, Eisenhofer G, Raygada M, Adams KT, Solis D, Lenders JW, Pacak K. Clinical presentations, biochemical phenotypes, and genotype-phenotype correlations in patients with succinate dehydrogenase subunit B-associated pheochromocytomas and paragangliomas. J Clin Endocrinol Metab. 2007;92:779–786. doi: 10.1210/jc.2006-2315. [DOI] [PubMed] [Google Scholar]
- 14.Grossman A, Pacak K, Sawka A, Lenders JW, Harlander D, Peaston RT, Reznek R, Sisson J, Eisenhofer G. Biochemical diagnosis and localization of pheochromocytoma: can we reach a consensus? Ann N Y Acad Sci. 2006;1073:332–347. doi: 10.1196/annals.1353.038. [DOI] [PubMed] [Google Scholar]
- 15.Lenders JW, Willemsen JJ, Eisenhofer G, Ross HA, Pacak K, Timmers HJ, Sweep CG. Is supine rest necessary before blood sampling for plasma metanephrines? Clin Chem. 2007;53:352–354. doi: 10.1373/clinchem.2006.076489. [DOI] [PubMed] [Google Scholar]
- 16.Lee JA, Zarnegar R, Shen WT, Kebebew E, Clark OH, Duh QY. Adrenal incidentaloma, borderline elevations of urine or plasma metanephrine levels, and the “subclinical” pheochromocytoma. Arch Surg. 2007;142:870–873. doi: 10.1001/archsurg.142.9.870. discussion 873–874. [DOI] [PubMed] [Google Scholar]
- 17.Perry CG, Sawka AM, Singh R, Thabane L, Bajnarek J, Young WF., Jr The diagnostic efficacy of urinary fractionated metanephrines measured by tandem mass spectrometry in detection of pheochromocytoma. Clin Endocrinol (Oxf) 2007;66:703–708. doi: 10.1111/j.1365-2265.2007.02805.x. [DOI] [PubMed] [Google Scholar]
- 18.Boyle JG, Davidson DF, Perry CG, Connell JM. Comparison of diagnostic accuracy of urinary free metanephrines VMA, and catecholamines and plasma catecholamines for diagnosis of pheochromocytoma. J Clin Endocrinol Metab. 2007;92:4602–4608. doi: 10.1210/jc.2005-2668. [DOI] [PubMed] [Google Scholar]
- 19.Lagerstedt SA, O’Kane DJ, Singh RJ. Measurement of plasma free metanephrine and normetanephrine by liquid chromatography-tandem mass spectrometry for diagnosis of pheochromocytoma. Clin Chem. 2004;50:603–611. doi: 10.1373/clinchem.2003.024703. [DOI] [PubMed] [Google Scholar]
- 20.Jong WH, de Graham KS, Molen JC, van der Links TP, Morris MR, Ross HA, Vries EG, de Kema IP. Plasma free metanephrine measurement using automated online solid-phase extraction HPLC tandem mass spectrometry. Clin Chem. 2007;53:1684–1693. doi: 10.1373/clinchem.2007.087114. [DOI] [PubMed] [Google Scholar]
- 21.Algeciras-Schimnich A, Preissner CM, Young WF, Jr, Singh RJ, Grebe SK. Plasma chromogranin A or urine fractionated metanephrines follow-up testing improves the diagnostic accuracy of plasma fractionated metanephrines for pheochromocytoma. J Clin Endocrinol Metab. 2008;93:91–95. doi: 10.1210/jc.2007-1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eisenhofer G, Goldstein DS, Walther MM, Friberg P, Lenders JW, Keiser HR, Pacak K. Biochemical diagnosis of pheochromocytoma: How to distinguish true- from false-positive test results. J Clin Endocrinol Metab. 2003;88:2656–2666. doi: 10.1210/jc.2002-030005. [DOI] [PubMed] [Google Scholar]
- 23.Mannelli M. Management and treatment of pheochromocytomas and paragangliomas. Ann N Y Acad Sci. 2006;1073:405–416. doi: 10.1196/annals.1353.044. [DOI] [PubMed] [Google Scholar]
- 24.Amar L, Servais A, Gimenez-Roqueplo AP, Zinzindohoue F, Chatellier G, Plouin PF. Year of diagnosis, features at presentation, and risk of recurrence in patients with pheochromocytoma or secreting paraganglioma. J Clin Endocrinol Metab. 2005;90:2110–2116. doi: 10.1210/jc.2004-1398. [DOI] [PubMed] [Google Scholar]
- 25.John H, Ziegler WH, Hauri D, Jaeger P. Pheochromocytomas: can malignant potential be predicted? Urology. 1999;53:679–683. doi: 10.1016/s0090-4295(98)00612-8. [DOI] [PubMed] [Google Scholar]
- 26.Gimenez-Roqueplo AP, Favier J, Rustin P, Rieubland C, Crespin M, Nau V, Khau Van Kien P, Corvol P, Plouin PF, Jeunemaitre X. Mutations in the SDHB gene are associated with extra-adrenal and/or malignant phaeochromocytomas. Cancer Res. 2003;63:5615–5621. [PubMed] [Google Scholar]
- 27.Takahashi K, Ashizawa N, Minami T, Suzuki S, Sakamoto I, Hayashi K, Tomiyasu S, Sumikawa K, Kitamura K, Eto T, Yano K. Malignant pheochromocytoma with multiple hepatic metastases treated by chemotherapy and transcatheter arterial embolization. Intern Med. 1999;38:349–354. doi: 10.2169/internalmedicine.38.349. [DOI] [PubMed] [Google Scholar]
- 28.Pacak K, Fojo T, Goldstein DS, Eisenhofer G, Walther MM, Linehan WM, Bachenheimer L, Abraham J, Wood BJ. Radiofrequency ablation: a novel approach for treatment of metastatic pheochromocytoma. J Natl Cancer Inst. 2001;93:648–649. doi: 10.1093/jnci/93.8.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Averbuch SD, Steakley CS, Young RC, Gelmann EP, Goldstein DS, Stull R, Keiser HR. Malignant pheochromocytoma: effective treatment with a combination of cyclophosphamide, vincristine, and dacarbazine. Ann Intern Med. 1988;109:267–273. doi: 10.7326/0003-4819-109-4-267. [DOI] [PubMed] [Google Scholar]
- 30.Scholz T, Eisenhofer G, Pacak K, Dralle H, Lehnert H. Clinical review: Current treatment of malignant pheochromocytoma. J Clin Endocrinol Metab. 2007;92:1217–1225. doi: 10.1210/jc.2006-1544. [DOI] [PubMed] [Google Scholar]
- 31.Schlumberger M, Gicquel C, Lumbroso J, Tenenbaum F, Comoy E, Bosq J, Fonseca E, Ghillani PP, Aubert B, Travagli JP, et al. Malignant pheochromocytoma: clinical, biological, histologic and therapeutic data in a series of 20 patients with distant metastases. J Endocrinol Invest. 1992;15:631–642. doi: 10.1007/BF03345807. [DOI] [PubMed] [Google Scholar]
- 32.Srimuninnimit V, Wampler GL. Case report of metastatic familial pheochromocytoma treated with cisplatin and 5-fluorouracil. Cancer Chemother Pharmacol. 1991;28:217–219. doi: 10.1007/BF00685513. [DOI] [PubMed] [Google Scholar]
- 33.Iwabuchi M, Oki Y, Nakamura H. Palliative chemotherapy for malignant pheochromocytoma: symptomatic palliation of two cases. Intern Med. 1999;38:433–435. doi: 10.2169/internalmedicine.38.433. [DOI] [PubMed] [Google Scholar]
- 34.Nakane M, Takahashi S, Sekine I, Fukui I, Koizumi M, Kage K, Ito Y, Aiba K, Horikoshi N, Hatake K, Ishikawa Y, Ogata E. Successful treatment of malignant pheochromocytoma with combination chemotherapy containing anthracycline. Ann Oncol. 2003;14:1449–1451. doi: 10.1093/annonc/mdg358. [DOI] [PubMed] [Google Scholar]
- 35.Kulke MH, Stuart K, Enzinger PC, Ryan DP, Clark JW, Muzikansky A, Vincitore M, Michelini A, Fuchs CS. Phase II study of temozolomide and thalidomide in patients with metastatic neuroendocrine tumors. J Clin Oncol. 2006;24:401–406. doi: 10.1200/JCO.2005.03.6046. [DOI] [PubMed] [Google Scholar]
- 36.Chrisoulidou A, Kaltsas G, Ilias I, Grossman AB. The diagnosis and management of malignant phaeochromocytoma and paraganglioma. Endocr Relat Cancer. 2007;14:569–585. doi: 10.1677/ERC-07-0074. [DOI] [PubMed] [Google Scholar]
- 37.Fitzgerald PA, Goldsby RE, Huberty JP, Price DC, Hawkins RA, Veatch JJ, Dela Cruz F, Jahan TM, Linker CA, Damon L, Matthay KK. Malignant pheochromocytomas and paragangliomas: a phase II study of therapy with high-dose 131I-metaiodobenzylguanidine (131I-MIBG) Ann N Y Acad Sci. 2006;1073:465–490. doi: 10.1196/annals.1353.050. [DOI] [PubMed] [Google Scholar]
- 38.Lamarre-Cliche M, Gimenez-Roqueplo AP, Billaud E, Baudin E, Luton JP, Plouin PF. Effects of slow-release octreotide on urinary metanephrine excretion and plasma chromogranin A and catecholamine levels in patients with malignant or recurrent phaeochromocytoma. Clin Endocrinol (Oxf) 2002;57:629–634. doi: 10.1046/j.1365-2265.2002.01658.x. [DOI] [PubMed] [Google Scholar]
- 39.Mundschenk J, Unger N, Schulz S, Hollt V, Steinke R, Lehnert H. Somatostatin receptor subtypes in human pheochromocytoma: subcellular expression pattern and functional relevance for octreotide scintigraphy. J Clin Endocrinol Metab. 2003;88:5150–5157. doi: 10.1210/jc.2003-030262. [DOI] [PubMed] [Google Scholar]
- 40.Ahlman H. Malignant pheochromocytoma: state of the field with future projections. Ann N Y Acad Sci. 2006;1073:449–464. doi: 10.1196/annals.1353.049. [DOI] [PubMed] [Google Scholar]
- 41.Mairs RJ, Cunningham SH, Russell J, Armour A, Owens J, MacKellar K, Gaze MN. No-carrier-added iodine-131-MIBG: evaluation of a therapeutic preparation. J Nucl Med. 1995;36:1088–1095. [PubMed] [Google Scholar]
- 42.Boyd M, Ross S, Owens J, Hunter D, Babich J, Zalutsky MR, Hamilton TG, Bell S, Mairs RJ. Preclinical evaluation of no-carrier-added [131I]Meta-Iodobenzyl Guanidine, for the treatment of tumours transfected with the noradrenaline transporter gene. Lett Drug Des Discovery. 2004;1:1–5. [Google Scholar]
- 43.Verberne HJ, de Bruin K, Habraken JB, Somsen GA, Eersels JL, Moet F, Booij J, van Eck-Smit BL. No-carrier-added versus carrier-added 123I-metaiodobenzylguanidine for the assessment of cardiac sympathetic nerve activity. Eur J Nucl Med Mol Imaging. 2006;33:483–490. doi: 10.1007/s00259-005-0022-1. [DOI] [PubMed] [Google Scholar]
- 44.Owens J, Bolster AA, Prosser JE, Cunningham S, Mairs RJ, Neilly JB, Reed NS, Hilditch TE. No-carrier-added 123I-MIBG: an initial clinical study in patients with phaeochromocytoma. Nucl Med Commun. 2000;21:437–440. doi: 10.1097/00006231-200005000-00005. [DOI] [PubMed] [Google Scholar]
- 45.Brogsitter C, Pinkert J, Bredow J, Kittner T, Kotzerke J. Enhanced tumor uptake in neuroendocrine tumors after intraarterial application of 131I-MIBG. J Nucl Med. 2005;46:2112–2116. [PubMed] [Google Scholar]
- 46.Raffel DM, Jung YW, Gildersleeve DL, Sherman PS, Moskwa JJ, Tluczek LJ, Chen W. Radiolabeled phenethylguanidines: novel imaging agents for cardiac sympathetic neurons and adrenergic tumors. J Med Chem. 2007;50:2078–2088. doi: 10.1021/jm061398y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vaidyanathan G, Friedman HS, Keir ST, Zalutsky MR. Evaluation of meta-[211At] astatobenzylguanidine in an athymic mouse human neuroblastoma xenograft model. Nucl Med Biol. 1996;23:851–856. doi: 10.1016/0969-8051(96)00115-1. [DOI] [PubMed] [Google Scholar]
- 48.Vaidyanathan G, Zhao XG, Strickland DK, Zalutsky MR. No-carrier-added iodine-131-FIBG: evaluation of an MIBG analog. J Nucl Med. 1997;38:330–334. [PubMed] [Google Scholar]
- 49.Vaidyanathan G, Zhao XG, Larsen RH, Zalutsky MR. 3-[211At]astato-4-fluorobenzylguanidine: a potential therapeutic agent with prolonged retention by neuroblastoma cells. Br J Cancer. 1997;76:226–233. doi: 10.1038/bjc.1997.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vaidyanathan G, Welsh PC, Vitorello KC, Snyder S, Friedman HS, Zalutsky MR. A 4-methyl-substituted meta-iodobenzylguanidine analogue with prolonged retention in human neuroblastoma cells. Eur J Nucl Med Mol Imaging. 2004;31:1362–1370. doi: 10.1007/s00259-004-1596-8. [DOI] [PubMed] [Google Scholar]
- 51.Fullerton NE, Boyd M, Ross SC, Pimlott SL, Babich J, Kirk D, Zalutsky MR, Mairs RJ. Comparison of radiohaloanalogues of meta-iodobenzylguanidine (MIBG) for a combined gene- and targeted radiotherapy approach to bladder carcinoma. Med Chem. 2005;1:611–618. doi: 10.2174/157340605774598090. [DOI] [PubMed] [Google Scholar]
- 52.Vaidyanathan G, Affleck DJ, Alston KL, Zhao XG, Hens M, Hunter DH, Babich J, Zalutsky MR. A kit method for the high level synthesis of [211At]MABG. Bioorg Med Chem. 2007;15:3430–3436. doi: 10.1016/j.bmc.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boyd M, Cunningham SH, Brown MM, Mairs RJ, Wheldon TE. Noradrenaline transporter gene transfer for radiation cell kill by 131I meta-iodobenzylguanidine. Gene Ther. 1999;6:1147–1152. doi: 10.1038/sj.gt.3300905. [DOI] [PubMed] [Google Scholar]
- 54.Altmann A, Kissel M, Zitzmann S, Kubler W, Mahmut M, Peschke P, Haberkorn U. Increased MIBG uptake after transfer of the human nor-epinephrine transporter gene in rat hepatoma. J Nucl Med. 2003;44:973–980. [PubMed] [Google Scholar]
- 55.Mairs RJ, Ross SC, MacCluskey AG, Boyd M. A transfectant mosaic xenograft model for evaluation of targeted radiotherapy in combination with gene therapy in vivo. J Nucl Med. 2007;48:1519–1526. doi: 10.2967/jnumed.107.042226. [DOI] [PubMed] [Google Scholar]
- 56.Ramamoorthy S, Melikian HE, Qian Y, Blakely RD. Biosynthesis, N-glycosylation, and surface trafficking of biogenic amine transporter proteins. Methods Enzymol. 1998;296:347–370. doi: 10.1016/s0076-6879(98)96026-8. [DOI] [PubMed] [Google Scholar]
- 57.Bomanji J, Levison DA, Flatman WD, Horne T, Bouloux PM, Ross G, Britton KE, Besser GM. Uptake of iodine-123 MIBG by pheochromocytomas, paragangliomas, and neuroblastomas: a histopathological comparison. J Nucl Med. 1987;28:973–978. [PubMed] [Google Scholar]
- 58.Kolby L, Bernhardt P, Levin-Jakobsen AM, Johanson V, Wangberg B, Ahlman H, Forssell-Aronsson E, Nilsson O. Uptake of meta-iodobenzyl-guanidine in neuroendocrine tumours is mediated by vesicular monoamine transporters. Br J Cancer. 2003;89:1383–1388. doi: 10.1038/sj.bjc.6601276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bomanji JB, Hyder SW, Gaze MN, Gacinovic S, Costa DC, Coulter C, Ell PJ. Functional imaging as an aid to decision-making in metastatic paraganglioma. Br J Radiol. 2001;74:266–269. doi: 10.1259/bjr.74.879.740266. [DOI] [PubMed] [Google Scholar]
- 60.Kolby L, Bernhardt P, Johanson V, Wangberg B, Muth A, Jansson S, Forssell-Aronsson E, Nilsson O, Ahlman H. Can quantification of VMAT and SSTR expression be helpful for planning radionuclide therapy of malignant pheochromocytomas? Ann N Y Acad Sci. 2006;1073:491–497. doi: 10.1196/annals.1353.051. [DOI] [PubMed] [Google Scholar]
- 61.van der Harst E, de Herder WW, Bruining HA, Bonjer HJ, de Krijger RR, Lamberts SW, van de Meiracker AH, Boomsma F, Stijnen T, Krenning EP, Bosman FT, Kwekkeboom DJ. [(123)I]metaiodobenzylguanidine and [(111)In]octreotide uptake in begnign and malignant pheochromocytomas. J Clin Endocrinol Metab. 2001;86:685–693. doi: 10.1210/jcem.86.2.7238. [DOI] [PubMed] [Google Scholar]
- 62.Ezuddin S, Fragkaki C. MIBG and FDG PET findings in a patient with malignant pheochromocytoma: a significant discrepancy. Clin Nucl Med. 2005;30:579–581. doi: 10.1097/01.rlu.0000170060.52675.14. [DOI] [PubMed] [Google Scholar]
- 63.Mamede M, Carrasquillo JA, Chen CC, Del Corral P, Whatley M, Ilias I, Ayala A, Pacak K. Discordant localization of 2-[18F]-fluoro-2-deoxy-D-glucose in 6-[18F]-fluorodopamine- and [(123)I]-metaiodobenzylguanidine-negative metastatic pheochromocytoma sites. Nucl Med Commun. 2006;27:31–36. doi: 10.1097/01.mnm.0000189780.54658.e8. [DOI] [PubMed] [Google Scholar]
- 64.Timmers HJ, Kozupa A, Chen CC, Carrasquillo JA, Ling A, Eisenhofer G, Adams KT, Solis D, Lenders JW, Pacak K. Superiority of fluorodeoxyglucose positron emission tomography to other functional imaging techniques in the evaluation of metastatic SDHB-associated pheochromocytoma and paraganglioma. J Clin Oncol. 2007;25:2262–2269. doi: 10.1200/JCO.2006.09.6297. [DOI] [PubMed] [Google Scholar]
- 65.Sisson JC, Shapiro B, Shulkin BL, Urba S, Zempel S, Spaulding S. Treatment of malignant pheochromocytomas with 131-I metaiodobenzyl-guanidine and chemotherapy. Am J Clin Oncol. 1999;22:364–370. doi: 10.1097/00000421-199908000-00008. [DOI] [PubMed] [Google Scholar]
- 66.Vaidyanathan G, Affleck DJ, Norman J, O’Dorisio S, Zalutsky MR. A radio-iodinated MIBG-octreotate conjugate exhibiting enhanced uptake and retention in SSTR2-expressing tumor cells. Bioconjug Chem. 2007;18:2122–2130. doi: 10.1021/bc700240r. [DOI] [PubMed] [Google Scholar]
- 67.Brouwers FM, Petricoin EF, 3rd, Ksinantova L, Breza J, Rajapakse V, Ross S, Johann D, Mannelli M, Shulkin BL, Kvetnansky R, Eisenhofer G, Walther MM, Hitt BA, Conrads TP, Veenstra TD, Mannion DP, Wall MR, Wolfe GM, Fusaro VA, Liotta LA, Pacak K. Low molecular weight proteomic information distinguishes metastatic from benign pheochromocytoma. Endocr Relat Cancer. 2005;12:263–272. doi: 10.1677/erc.1.00913. [DOI] [PubMed] [Google Scholar]
- 68.Anouar Y, Yon L, Guillemot J, Thouennon E, Barbier L, Gimenez-Roqueplo AP, Bertherat J, Lefebvre H, Klein M, Muresan M, Grouzmann E, Plouin PF, Vaudry H, Elkahloun AG. Development of novel tools for the diagnosis and prognosis of pheochromocytoma using peptide marker immunoassay and gene expression profiling approaches. Ann N Y Acad Sci. 2006;1073:533–540. doi: 10.1196/annals.1353.057. [DOI] [PubMed] [Google Scholar]
- 69.Brouwers FM, Elkahloun AG, Munson PJ, Eisenhofer G, Barb J, Linehan WM, Lenders JW, De Krijger R, Mannelli M, Udelsman R, Ocal IT, Shulkin BL, Bornstein SR, Breza J, Ksinantova L, Pacak K. Gene expression profiling of benign and malignant pheochromocytoma. Ann N Y Acad Sci. 2006;1073:541–556. doi: 10.1196/annals.1353.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Eisenhofer G, Bornstein SR, Brouwers FM, Cheung NK, Dahia PL, De Krijger RR, Giordano TJ, Greene LA, Goldstein DS, Lehnert H, Manger WM, Maris JM, Neumann HP, Pacak K, Shulkin BL, Smith DI, Tischler AS, Young WF., Jr Malignant pheochromocytoma: current status and initiatives for future progress. Endocr Relat Cancer. 2004;11:423–436. doi: 10.1677/erc.1.00829. [DOI] [PubMed] [Google Scholar]
- 71.Gottlieb E, Tomlinson IP. Mitochondrial tumour suppressors: a genetic and biochemical update. Nat Rev Cancer. 2005;5:857–866. doi: 10.1038/nrc1737. [DOI] [PubMed] [Google Scholar]
- 72.Smith EH, Janknecht R, Maher LJ., 3rd Succinate inhibition of {alpha}-ketoglutarate-dependent enzymes in a yeast model of paraganglioma. Hum Mol Genet. 2007;16:3136–3148. doi: 10.1093/hmg/ddm275. [DOI] [PubMed] [Google Scholar]