Abstract
Context
Despite the expanding clinical utility of antipsychotics beyond psychotic disorders to include depressive, bipolar, and anxiety disorders, reproductive safety data regarding the neurodevelopmental sequelae of fetal antipsychotic exposure are scarce.
Objective
To examine whether intrauterine antipsychotic exposure is associated with deficits in neuromotor performance and habituation in 6-month-old infants.
Design, Setting, and Participants
A prospective controlled study was conducted from December 1999 through June 2008 at the Infant Development Laboratory of the Emory Psychological Center examining maternal-infant dyads (N=309) at 6 months postpartum with pregnancy exposure to antipsychotics (n=22), antidepressants (n = 202), or no psychotropic agents (n = 85). Examiners masked to maternal-infant exposure status administered a standardized neuromotor examination (Infant Neurological International Battery [INFANIB]) that tests posture, tone, reflexes, and motor skills and a visual habituation paradigm using a neutral female face.
Main Outcome Measures
The INFANIB composite score; number of trials required to achieve a 50% decrease in infant fixation during a visual habituation task; and mean time looking at the stimulus across 10 trials.
Results
Infants prenatally exposed to antipsychotics (mean=64.71) showed significantly lower INFANIB scores than those with antidepressant (mean=68.57) or no psychotropic (mean=71.19) exposure, after controlling for significant covariates (F2,281=4.51; P =.01; partial η2=0.033). The INFANIB scores were also significantly associated with maternal psychiatric history, including depression, psychosis, and overall severity/chronicity (P’s<.05) and maternal depression during pregnancy was associated with less efficient habituation (r245=0.16; P <.02). There were no significant differences regarding habituation between medication exposure groups.
Conclusions
Among 6-month-old infants, a history of intrauterine antipsychotic exposure, compared with antidepressant or no psychotropic exposure, was associated with significantly lower scores on a standard test of neuromotor performance, highlighting the need for further scrutiny of the reproductive safety and neurodevelopmental sequelae of fetal antipsychotic exposure. Disentangling medication effects from maternal illness effects, which also contributed, remains a critical challenge.
Approximately two-thirds of women with histories of mental illness give birth.1 Although some research has suggested that pregnancy protects women from psychiatric illness,2 other studies report unchanged or heightened prenatal risk for recurrence of mental disorders including bipolar disorder,3–5 schizophrenia,6 and depression.7 Despite the significant morbidity associated with maternal mental illness during gestation, treatment guidelines are largely speculative with little systematic data assessing the safety and efficacy of prenatal psychotropic therapy.
With their use rising dramatically in recent years,8 antipsychotics are now regarded as one of the most commonly prescribed drug classes in the United States. The array of therapeutic applications for antipsychotics has expanded beyond psychotic disorders to include bipolar, major depressive, and anxiety disorders.9 Thus, a 170% increase over the past decade in antipsychotic use during pregnancy is not surprising.10,11
Although antipsychotics have been shown to cross the placenta,12 there is a paucity of reproductive safety data regarding these medications. Clinical outcome studies following prenatal antipsychotic administration are limited to analyses using small sample sizes of placental passage rates,12 neonatal outcomes,13,14 and the risk for delivery complications and congenital malformations.11,15–19 A previous study reported hypertonicity, tremulousness, and poor motor maturity in neonates within days of delivery following prenatal exposure to first-generation antipsychotics (FGAs).20 Because it is unclear whether these effects persist, the long-term neurodevelopmental impact of fetal central nervous system antipsychotic exposure remains unknown.21
Preclinical studies have demonstrated lasting anatomical and functional alterations in the central nervous system of adult rats prenatally exposed to antipsychotics.22 For example, adult rodents prenatally exposed to antipsychotic medications display deficits in learning and memory acquisition and retention.23 Some studies report neurocognitive effects with specific antipsychotic medications but not others23,24 and pharmacological mechanisms underlying differential effects are poorly understood. Recent clinical reviews conclude that human data are significantly lacking.21
To address this critical gap in the literature, the current study examined the impact of prenatal exposure to antipsychotics, antidepressants, and maternal psychiatric illness on 6-month-old infants. Given evidence of placental passage and based on limited nonhuman animal findings, we hypothesized that infants prenatally exposed to antipsychotics would demonstrate poorer neurodevelopmental outcomes compared with infants with no psychotropic exposure, after accounting for maternal psychiatric status. Infants prenatally exposed to antidepressants but not antipsychotics were included as a second comparison group. Infant neurodevelopmental status was measured using a standardized neuromotor screening instrument and visual habituation paradigm. These specific areas were investigated based on limited human data reporting poor motor maturity in newborns and animal data suggesting potential learning and memory deficits.
METHODS
PARTICIPANTS
Women participating in a naturalistic study of the perinatal course of mental illness at the Emory Women’s Mental Health Program (WMHP) were invited to participate in the current protocol. Women with varied psychiatric histories were referred to the WMHP by community providers including obstetricians, therapists, and other psychiatrists during pregnancy or prior to conception. The WMHP patients were offered an opportunity to participate in an observational study documenting the course of maternal psychiatric symptoms and maternal use of psychotropic medication during gestation. Study participants were not required to receive psychiatric medication or any other form of treatment to participate. At enrollment, all participants received peer-reviewed information providing an overview of the respective risks associated with maternal mental illness and maternal use of psychiatric medication during pregnancy. Women in the WMHP cohort were followed up longitudinally across pregnancy and the postpartum period; psychometric data and maternal report of medication exposure were gathered during all visits. The nonmedicated subsample of the WMHP cohort was supplemented with an additional community control group that was recruited after pregnancy via a mass mailing. Prenatal psychotropic exposure data were collected retrospectively from the community cohort. We have previously reported that postpartum maternal recall of prenatal psychotropic exposure at 6 months postpartum is reliable when compared with the prospective record.25 Although the community control group was recruited on the basis of nonexposure, 1 participant in that group reported antidepressant use during pregnancy and was assigned to the appropriate exposure group. To be included in the study, maternal medication history during pregnancy had to meet 1 of the following criteria: (1) at least 1 antipsychotic medication, (2) at least 1 antidepressant medication, or (3) no psychotropic exposure. There was no minimum level of exposure required for inclusion and dosage and timing varied according to patient need. Exposure data were gathered with regard to number of drugs, duration of treatment (in weeks), and number of weeks exposed during each trimester on a subsample of women (n=212). Dosage information was not incorporated into the analysis because it varies from agent to agent. Mothers prescribed antiepileptic drugs during pregnancy (n=27) were removed from the sample to isolate the potential effects of antipsychotic medications, and mothers only prescribed anxiolytics or hypnotics (n=7) were excluded because of the small group size. Additional exclusion criteria included active maternal DSM-IV substance use disorder (abuse or dependence except for nicotine dependence) within 6 months of conception as determined by Structured Clinical Interview for DSM-IV-TR (SCID) and the presence of infant congenital abnormalities. Multiple gestation was not an exclusion criterion, and the sample included 2 sets of twins.
PROCEDURES
The study was approved by the Emory University institutional review board and conducted from December 1999 through June 2008 at the Infant Development Laboratory of the Emory Psychological Center. All participants gave written informed consent after receiving a complete description of the study and were compensated for their time. The protocol, beginning between 1 and 1:30 PM, proceeded as follows: (1) each mother completed questionnaires while the infant was held by an examiner nearby; (2) a visual habituation paradigm (details later) was administered; (3) an infant stressor paradigm was performed (described previously26); (4) the infant was reunited with the mother and given time to return to a calm state if upset; (5) a trained examiner masked to maternal medication exposure administered a standardized motor assessment (details later); and (6) each mother completed a standardized diagnostic interview (SCID).
MEASURES
Infant Neurological International Battery
The Infant Neurological International Battery (INFANIB)27 assesses neuromotor functioning for infants aged 4 to 18 months. Twenty items (scored 1–5) that assess infant posture, muscle tone, reflexes, and motor abilities are summed to create 1 composite score that reflects overall neuromotor integrity. The INFANIB demonstrates excellent reliability (interrater=0.97 and test-retest=0.95)28 and evidences acceptable sensitivity (90%), specificity (83%), positive predictive value (79%), and negative predictive value (93%).29 Standardized scores are not provided but clinically informative cutoff scores are provided for 3 age groups, including 4 to 8 months old: abnormal, 54 or less; transiently abnormal, 55 to 71; and normal, 72 or more.
Habituation Paradigm
Habituation refers to a decreased response intensity after repeated administration of a stimulus, generally reflecting an infant’s “attempt to process information contained in a stimulus and learn from it.”30(p237) Meta-analysis results indicated modest associations between infant habituation and later IQ.31 However, test-retest reliability is generally less adequate than more standardized infant measures (eg, mean r=0.46 for 4- to 7-month-old infants32), complicating clinical interpretation of such data.
In the current study, the infant sat in an infant seat in a quiet, dimly lit room while the mother and examiner waited behind an occlusion screen. For each trial, a colorful geometric pattern attracted the infant’s gaze. Once the infant fixated on the image, a neutral adult female face was presented until the infant looked away. The intertrial interval varied based on infant gaze to ensure fixation. The examiner, masked to maternal medication status, used a keyboard press to record the start and stop time, indicating the gaze duration for each trial. Interrater reliability calculated for at least 25% of the sample was adequate (κ>0.90). Habituation criteria were met when the infant fixation time decreased by 50% compared with the previous trial. A maximum of 10 trials were presented. Previous data using a similar paradigm indicated that most 5-month-old infants habituated in 5 to 8 trials.33 Infant habituation was operationalized as the number of trials to achieve habituation and the mean time looking at the stimulus. Only infants who completed the task and met criteria for habituation were included in habituation analyses (n=245).
Structured Clinical Interview for DSM-IV-TR
The SCID is a well-validated structured interview used to determine current and lifetime psychiatric diagnoses.34 Interrater reliability based on 10% of the sample was adequate for all major diagnoses (weighted κ’s ranged from 0.75–1.0).
Beck Depression Inventory
The Beck Depression Inventory is a reliable self-report rating scale of current depressive symptoms,35 which our group has recently validated for perinatal use.36 The internal consistency for the Beck Depression Inventory in this study was good to excellent (Cronbach α=.90).
DATA ANALYTIC PLAN
Data analysis was initiated with descriptive analyses examining sample characteristics, including demographics, obstetrical information, psychiatric diagnoses, and medication exposure. Pearson correlations, independent t tests, 1-way analyses of variance, and χ2 analyses tested associations between the dependent variables (INFANIB composite and habituation measures) and potential covariates. Analyses of covariance were performed to test study hypotheses. Multiple regression/partial correlation analyses were conducted to examine treatment duration effects within each exposure group, and post hoc χ2 analyses and logistic regression were conducted to further clarify group differences with respect to published clinical cutoffs. All results reflect 2-tailed tests.
RESULTS
SAMPLE
The sample of 309 mother-infant pairs included 163 male and 146 female infants. Mothers in the community cohort (n=39) were younger (t1,308=2.98; P <.01; mean difference=2.4 years) compared with the WMHP cohort (n=270); otherwise, the 2 cohorts showed similar demographic profiles. Mean (SD) age of the infants after adjusting for gestational age was 181 (15.7) days. Maternal mean (SD) age was 34 (4.4) years, the median education level was college graduate, and 92% of the mothers were married or partnered.
Psychotropic exposures were categorized as follows: antipsychotics, antidepressants, anxiolytics, and hypnotics. Mother-infant dyads were assigned to 1 of 3 prenatal medication exposure groups. Dyads exposed to an antipsychotic during gestation (n=22) were assigned to the antipsychotic exposure group. Antipsychotic exposure included (1) FGA: haloperidol (n = 9); (2) second-generation antipsychotic (SGA) (n=12; n=3 exposed to >1): aripiprazole (n=1), olanzapine (n=5), quetiapine fumarate (n=5), risperidone (n=3), and ziprasidone hydrochloride (n=1); (3) both FGA and SGA: haloperidol and ziprasidone (n=1). This group included 12 exposed to antipsychotic medication during the first trimester, 14 during the second, and 16 during the third. The number of gestational weeks exposed ranged from 2 to 40 (median=32 weeks). This reflects the clinical variability in treatment duration and timing evident in this observational study. Because antipsychotic monotherapy is uncommon when treating patients with mood and anxiety disorders, concurrent treatment with other psychotropic classes was permitted for women assigned to this group: antidepressants, n=17; anxiolytics, n=9; and hypnotics, n=6. Two women received antipsychotic monotherapy during pregnancy. Dyads exposed during gestation to an antidepressant and perhaps other psychotropic agents but not an antipsychotic (n=202) were assigned to the antidepressant exposure group. This group included women taking hypnotics (n=40) and anxiolytics (n=18). The number of gestational weeks exposed in the antidepressant group ranged from 3 to 40 (median=40 weeks). Dyads with no prenatal psychotropic exposure (n=85) were assigned to the no psychotropic exposure group. This group comprised both women in the community control cohort (n=38) and in the WMHP cohort (n=47) and included women with and without positive psychiatric histories. The nonexposed WMHP cohort included women with no history of mental illness (n=8) and those with a history of illness who did not require psychotropic medication during pregnancy (n=39). Most, but not all, women in the community control sample had no history of psychiatric illness (n=24). Sample characteristics by group are displayed in Table 1.
Table 1.
Descriptive Profile of Participants by Prenatal Medication Exposure Group
| No. (%)
|
|||
|---|---|---|---|
| No Psychotropic Exposure (n=85) | Anti-depressant Exposure (n=202) | Antipsychotic Exposure (n=22) | |
| Cohort source | |||
| WMHP (n=270) | 47 (55.3) | 201 (99.5) | 22 (100.0) |
| Community (n=39) | 38 (44.7) | 1 (0.5) | 0 |
| Maternal demographics | |||
| Age, y, mean (SD)a | 32.35 (4.47) 34.13 (4.27) | 34.14 (5.10) | |
| Married | 75 (88.2) | 192 (95.0) | 17 (77.3) |
| Median educationa | College graduate | College graduate | College attended |
| Smoked during pregnancy | 4 (4.71) | 16 (7.92) | 3 (13.64) |
| Infant demographics | |||
| Infant age, d, mean (SD) | 181.6 (16.0) 180.7 (15.6) | 184.3 (16.5) | |
| Male | 44 (51.8) | 110 (54.5) | 9 (40.9) |
| No. of siblings, mean (SD)a | 0.44 (0.66) | 0.87 (0.89) | 1.23 (1.19) |
| Obstetrical course | |||
| No. of delivery complications, mean (SD) | 0.73 (0.94) | 1.07 (1.18) | 0.91 (0.87) |
| Gestational age at delivery, wk, mean (SD) | 39.4 (1.54) | 39.1 (1.54) | 38.9 (1.64) |
| Infant birth weight, kg, mean (SD) | 3.42 (0.52) | 3.34 (0.51) | 3.24 (0.47) |
| Prenatal psychotropic exposure | |||
| Antipsychotic exposurea | 0 | 0 | 22 (100.0) |
| SGA | 0 | 0 | 12 (54.6) |
| FGA | 0 | 0 | 9 (40.9) |
| Both SGA and FGA | 0 | 0 | 1 (4.5) |
| Antidepressant exposurea | 0 | 202 (100.0) | 17 (77.2) |
| Anxiolytic exposurea | 0 | 40 (19.8) | 9 (40.9) |
| Hypnotic exposurea | 0 | 18 (8.9) | 1 (4.5) |
| Postnatal psychotropic exposure | |||
| Breastfed at age 6 moa | 55 (65.5) | 113 (55.9) | 6 (27.3) |
| Antipsychotic exposurea | 1 (1.2) | 5 (2.5) | 4 (18.2) |
| Antidepressant exposurea | 18 (21.2) | 111 (55.0) | 5 (22.7) |
| Psychiatric history | |||
| Lifetime diagnosis | |||
| Depressive disorder | 45 (52.9) | 173 (85.6) | 11 (50.0) |
| Bipolar disorder | 4 (4.7) | 12 (5.9) | 10 (45.5) |
| Anxiety disorder | 30 (35.3) | 120 (59.4) | 16 (72.7) |
| Psychotic disorder | 0 | 5 (2.5) | 2 (9.1) |
| Pregnancy diagnosis | |||
| Depressive disorder | 12 (14.1) | 88 (43.6) | 8 (36.4) |
| Bipolar disorder | 3 (3.5) | 5 (2.5) | 7 (31.8) |
| Anxiety disorder | 13 (15.3) | 56 (27.7) | 11 (50.0) |
| Psychotic disorder | 0 | 5 (2.5) | 2 (9.1) |
Abbreviations: FGA, first-generation antipsychotic; SGA, second-generation antipsychotic; WMHP, Women’s Mental Health Program.
P<.05 (between-group differences based on 1-way analysis of variance or χ2 tests). Number of delivery complications reflects a summed count variable of the following: abnormal fetal/infant heart rate, respiratory distress, presence of meconium, anoxia or hypoxia, abnormal fetal position, postpartum hemorrhage, forceps, vacuum extraction, induced labor, placental previa or placental abruption; marital status reflects a nominal variable of whether the mother was married or partnered; infant age is presented as age in days corrected for gestational age; gestational age reflects number of weeks at delivery; postnatal psychotropic exposure reflects women prescribed medication after birth who were breastfeeding at the time of the 6-month visit; and psychiatric history variables reflect the comorbidity evident in this sample; thus, women may fall into more than 1 cell.
EXAMINING POTENTIAL COVARIATES
Covariates showing a significant association with a dependent variable were included in all subsequent analyses. The following delivery and demographic variables were significantly related to INFANIB scores: infant age (r281=0.14; P =.02), maternal age (r282=−0.16; P <.01), and marital status (t1,280=2.94; P <.01). Maternal age was positively associated with habituation look time (r245=0.17; P<.01) and breastfed infants habituated in fewer trials (t1,243=−2.73; P <.01) and showed shorter habituation look times (t1,243=−2.57; P <.02) compared with nonbreastfed infants. Gestational age at delivery, delivery complications, birth weight, sex, maternal education, and number of children in the home were not related to any dependent variables.
The following psychiatric variables characterizing maternal mental illness showed significant associations with INFANIB scores: a lifetime history of at least 1 major depressive episode or dysthymia (t1,280=2.26; P <.03), a lifetime diagnosis of a psychotic disorder (t1,280=−2.07; P =.04), and a severity/chronicity index (F3,281=4.07; P <.01). For the severity/chronicity composite variable, 1 point was given for each of the following: (1) psychiatric diagnosis lasting longer than 10 years, (2) previous hospitalization, and (3) past or present psychotropic treatment. Psychiatric diagnoses during pregnancy were not significantly associated with INFANIB scores (all P’s>.10), but the number of months depressed during pregnancy was associated with longer habituation look times (r245=0.16; P<.02). The following maternal psychiatric variables were not related to any dependent variables (P’s>.10): bipolar or anxiety diagnosis (lifetime or during pregnancy), number of mood episodes, number of psychotic symptoms, duration of disorder, number of hospitalizations, leave of absence due to mental illness, current treatment status, and previous psychotropic therapy.
Concomitant prenatal exposures to anxiolytics and hypnotics were not associated with INFANIB scores or visual habituation measures (P’s<.05) across the entire sample. Infant psychotropic exposures proximate to participation in the laboratory protocol via lactation were also examined (see Table 1 for descriptions by group). In most cases (92%), psychotropic exposure via breastfeeding was a continuation of prenatal exposure. However, 1 infant in the prenatal no psychotropic exposure group and 5 infants in the prenatal antidepressant exposure groups were postnatally exposed to antipsychotics via lactation. Infants postnatally exposed to an antipsychotic (n=10 across groups) exhibited significantly lower INFANIB scores (t1,280=2.13; P =.03; mean difference=5.45). Regression, controlling for relevant covariates, confirmed a negative association between antipsychotic exposure via lactation and INFANIB scores (β=−0.12; t=−2.04; P=.04). Antidepressant exposure via lactation (n=134 across groups) failed to predict INFANIB scores (data not shown), and associations with the habituation variable were nonsignificant after controlling for the overall effect of breastfeeding (F1,245=2.51; P <.11).
Cohort differences within the control group were examined with respect to obstetrical course and psychiatric history and no differences were found in birth weight, gestational age, pregnancy complications, or delivery complications (P’s >.15). Community controls were less likely to have a lifetime history of depression ( ; P <.01) or anxiety ( ; P <.01), but no differences in the likelihood of depression, anxiety, or mania during pregnancy were noted between cohorts (P’s>.10).
HYPOTHESIS TESTING: PRENATAL PSYCHOTROPIC EXPOSURE AND INFANIB SCORES
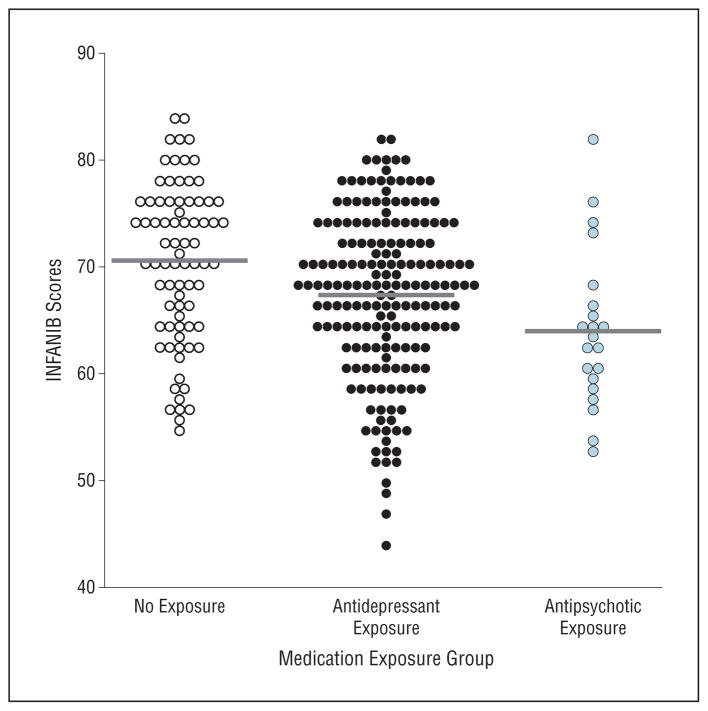
Twenty-seven infants (8.7%) failed to complete the INFANIB because of fatigue or irritability. Attrition was comparable between exposure groups ( ; P =.72). Analyses of covariance revealed a significant medication group effect in INFANIB scores (F2,281=4.51; P =.01; partial η2=0.033) after controlling for infant age and maternal age, marital status, lifetime history of depression/dysthymia, lifetime history of a psychotic disorder, and the psychiatric severity/chronicity index. Post hoc contrasts revealed that infants in the antipsychotic exposure group had significantly lower INFANIB scores (adjusted mean [SE] = 63.86 [1.78]) than those in the antidepressant exposure (P<.01) and no psychotropic exposure groups (P <.01). With regard to INFANIB scores, infants in the no psychotropic exposure group (adjusted mean [SE]=70.12 [1.03]) did not differ significantly from those in the antidepressant exposure group (adjusted mean [SE]=68.58 [0.60]) (P =.35). Scatter-plots of unadjusted INFANIB scores by group are presented in the Figure.
Figure.
Scatterplot of Infant Neurological International Battery (INFANIB) scores for prenatal medication exposure groups.
Because the no psychotropic exposure group included women from 2 recruitment sources, a secondary analysis of covariance was conducted selecting only those from the prospective WMHP cohort to confirm the original findings: antipsychotic-exposed infants had lower INFANIB scores compared with antidepressant-exposed and nonexposed infants after controlling for relevant covariates (F2,244 = 3.67; P < .03; η2= 0.03). Repeated analyses excluding 2 sets of twins showed similar results (data not shown). Repeated analyses excluding 5 women who received medication for less than 5 weeks of their pregnancy also did not change results (data not shown).
Exploratory analyses within the antipsychotic-exposed group were undertaken to better describe medication effects; however, the small sample size significantly limited statistical power. Multiple regression results, controlling for relevant covariates, suggested a trend toward a treatment duration effect using INFANIB scores and number of gestational weeks exposed to antipsychotics (β= −0.34; t = −1.78; P <.11; partial correlation=−0.51). Removing nonsignificant covariates did not improve the model. Analogous exploratory analyses conducted within the antidepressant-exposed group showed a negative association between number of weeks exposed and INFANIB scores (r118=−0.19; P <.03); however, this association was no longer significant accounting for identified covariates (P=.28). Trimester effects were also tested for both exposure groups but no significant associations were noted (data not shown).
Given the observational nature of the study and the small sample sizes, examining specific medication effects was implausible; however, follow-up analyses attempted to disentangle the influence of SGA vs FGA exposure on infant outcomes. After controlling for maternal psychiatric history using the severity/chronicity index, results revealed a modest but nonsignificant difference (F2,21=2.96; P =.10), with adjusted means suggesting lower scores for SGA-exposed infants (mean [SE]=62.9 [1.60]) compared with FGA-exposed infants (mean [SE]=67.1 [1.84]).
A frequency distribution of INFANIB clinical outcomes by exposure group is reported in Table 2. To maximize power given small cell sizes and simplify interpretation of results, the clinical categories were condensed into normal vs not normal. Frequency testing, using a 2×3 χ2 analysis, revealed a significant between-group effect ( ; P <.01): 50% of the nonexposed infants fell in the normal range, compared with 32% of the antidepressant-exposed and 19% of the antipsychotic-exposed infants, respectively. Adjusted odds ratios reflecting the likelihood of a normal score, accounting for significant covariates, were 5.41 for the nonexposed (95% CI, 1.22–24.09; P=.03) and 4.11 (95% CI, 1.05–15.99; P = .04) for the antidepressant-exposed infants, compared with the antipsychotic-exposed infants.
Table 2.
Frequency Distribution of INFANIB Clinical Outcomes for Prenatal Medication Exposure Groupsa
| Prenatal Medication Exposure Group | INFANIB Clinical Outcome, No. (%)
|
||
|---|---|---|---|
| Normal | Transiently Abnormal | Abnormal | |
| No psychotropic exposure | 39 (50.0) | 39 (50.0) | 0 |
| Antidepressant exposure | 59 (32.4) | 113 (62.1) | 11 (6.0) |
| Antipsychotic exposure | 4 (19.0) | 15 (71.5) | 2 (9.5) |
Abbreviation: INFANIB, Infant Neurological International Battery.
Results of 2 ×3 χ2 analysis of INFANIB scores (normal vs abnormal and transiently abnormal) and prenatal medication exposure group: ; P <.01.
HYPOTHESIS TESTING: PRENATAL ANTIPSYCHOTIC EXPOSURE AND HABITUATION
Analyses of the habituation task revealed no significant effect of prenatal medication exposure group on number of trials to habituate (F2,245=1.69; P =.19) or average looking time during the habituation task (F2,245=0.42; P=.66). No dose-response associations were evident in either exposure group for the visual habituation measures (P’s>.15).
COMMENT
Considerable literature supports an adverse impact of mental illness on several domains of obstetrical and infant outcome. Clinicians and patients are confronted with the arduous task of balancing the risks and benefits of psychiatric treatment during pregnancy, underscoring the critical need to improve our understanding of the neurodevelopmental consequences of prenatal psychotropic exposure and maternal mental illness. The results from the current study show that 6-month-old infants exposed prenatally to an antipsychotic demonstrated significantly lower scores on a standardized neuromotor screening measure compared with both antidepressant-exposed infants and infants with no psychotropic exposure. Only 19% of infants prenatally exposed to an antipsychotic demonstrated normal neuromotor performance. Infant outcomes were also negatively associated with indices of maternal psychiatric illness, and taken together, results from the current study raise concerns about the potential impact of both prenatal exposure and maternal psychiatric illness. These results suggest that maternal mental illness and fetal antipsychotic exposure may exert additive adverse effects on subsequent infant neurodevelopment. Disentangling the relative contribution of a neuropsychiatric illness and its treatment on the risk for an adverse developmental outcome remains a daunting challenge. Diagnostic variability within exposure groups limited our ability to further tease apart additive effects of illness and medication exposure.
Examining effects of specific agents was implausible given our sample, but further support for a medication exposure effect is provided by the fact that postpartum antipsychotic exposure at 6 months was independently associated with neuromotor performance and that partial correlations revealed a moderate though nonsignificant association between INFANIB scores and treatment duration within the antipsychotic exposure group. Although antipsychotics differ greatly in their in vivo pharmacodynamic profiles, they uniformly antagonize (to varying degrees) postsynaptic dopamine D2 receptors. Preclinical data indicate that dopaminergic neurons can be identified in the fetal rat brainstem by gestational day 12 or 13,37 suggesting that the ontogenesis of dopaminergic systems may occur early in human fetal development. Prenatal antipsychotic exposure in rodents alters the development of dopamine receptor function38 and reduces dopaminergic binding in regions of the mesocortical pathway.39 Recognizing that recent neuroimaging data indicate that dopamine is phasically released during motor learning, presumably serving to focus the learner and reinforce newly learned motor patterns,40 the potential impact of fetal exposure to agents affecting dopaminergic systems on later neuromotor performance warrants careful scrutiny.
While dopamine antagonism is evident to varying degrees among all antipsychotics, FGAs and SGAs differ with respect to their impact on serotonergic pathways. Although pharmacodynamic profiles also vary among SGAs, as a class, these medications produce extensive blockade of serotonin 5HT2A receptors while stimulating 5-HT1A receptors. Moderately lower neuromotor scores for SGA-exposed as compared with FGA-exposed infants, while not statistically significant, raise questions about the influence of serotonin agonism/antagonism via prenatal exposure on fetal development. Serotonin is present in the fertilized egg and serotonergic neurons are functional early in the fetal brain.41 Both excess and depletion of serotonin during the fetal period can influence brain development and produce maladaptive behavioral profiles in rodents.42,43 Animal data also demonstrate that serotonin depletion results in reduced excitability in motor neurons, which in turn alters posture and intralimb coordination.44 The present study failed to show differences in antidepressant-exposed and nonexposed infants, suggesting that the role of serotonin-dopamine interactions in early brain development deserves further attention. A high proportion of SGA-exposed infants were also exposed to antidepressants but small sample sizes precluded the ability to test statistical interaction. Synergistic effects of prenatal antipsychotic and antidepressant exposure should be the focus of future animal and human research.
Neither prenatal antipsychotic nor prenatal antidepressant exposure was associated with alterations in infant habituation, indicating that these agents may not significantly impact early attention modulation and learning. While habituation measures did correlate with a limited number of demographic and maternal psychiatric variables in the expected direction, reduced statistical power related to unequal sample sizes and methodological aspects of the paradigm may have hindered our ability to detect group differences. For example, the habituation paradigm used a fixed number of trials. Approximately 21% of the infants did not habituate after 10 trials. Thus, the habituation scores were not normally distributed and those who did not habituate were dropped from the analyses. Although no group differences were apparent in infants who failed to habituate, we were still unable to adequately examine “slow habituators,” a theoretically important group. Methods for measuring habituation continue to vary, and while some argue for varying the number of trials to better capture individual differences, others point out that too many trials may increase type I error.45 Future studies would likely benefit from a multimethod approach that incorporates infant behavior (eg, gaze) in combination with heart rate changes to better assess information processing capabilities.46
Strengths of the current study include (1) prospective acquisition of subjects in the groups with prenatal psychotropic exposure; (2) administration of a standardized infant neuromotor assessment by an examiner masked to infant exposure; (3) use of a standardized diagnostic interview to gather extensive data regarding maternal psychiatric illness, enabling analysis to control for maternal mental health factors that may be associated with infant neuromotor performance; (4) inclusion of comparator groups including women with and without histories of mental illness and with and without prenatal exposure to other classes of psychotropic agents; and (5) extensive collection of psychiatric and demographic data supporting careful examination of potential confounds.
Despite the data and rigor of the laboratory protocol and data analyses, the study has several limitations. (1) Demographic homogeneity of the cohort limited generalizability and ability to incorporate more systematic analyses of psychosocial variability (although this strengthened our ability to isolate medication effects). (2) The limited sample size for prenatal antipsychotic exposure, with the antipsychotic exposure group represented by only 22 subjects, precluded isolated analyses of the impact of antipsychotic monotherapy or specific agents. (3) Accession of a significant proportion of the no psychotropic exposure group after delivery (44.7%) precluded prospective prenatal data collection. While we have previously reported that 6-month postnatal recall is reliable with respect to prenatal psychotropic therapy, postnatal recall produces a systematic underreporting of depression during pregnancy.25 However, analyses excluding those with only retrospective prenatal data were consistent with those from the entire sample. (4) Prenatal measurement of maternal symptoms did not address manic, psychotic, or anxiety symptoms but was limited to depressive symptoms. This decision was made a priori based on the high prevalence of depressive symptoms and initial focus on antenatal and postpartum depression. Additional symptom measures have since been added, but small sample sizes preclude the analysis of those data in the current study. (5) The INFANIB, while an appropriate measure of neuromotor performance in 6-month-old infants, only assesses a limited domain of behavior, and normative data are not available. Thus, the clinically predictive value of the differences between groups is unclear. The INFANIB was chosen over other standardized measures such as the Bayley Scales of Infant Development because it focuses on qualitative aspects of motor development, such as posture and tone, rather than discrete developmental tasks, such as sitting independently. Because of the narrow age range of the sample and given potential effects were hypothesized to be mild, a measure that maximized variability was important, and a normed clinical measure was less important. Follow-up studies should focus on the clinical significance and predictive value of these findings. Although useful, the clinically informative cutoff scores used in follow-up analyses are based on (1) limited data for the current age group (95 six-month-olds); (2) a wide age range (eg, 4–8 months), considering the rapidity of developmental change during infancy; and (3) a sample of infants followed up from the neonatal intensive care unit. Therefore, the generalizability of these clinical categories to the current sample is unknown, and despite strong psychometric properties as a continuous measure, analyses using clinical categories should be interpreted with caution.
In summary, these data suggest that prenatal antipsychotic exposure may influence infant neuromotor performance at 6 months of age. These novel data provide an important contribution given the lack of published human data on this topic and the frequency of psychiatric illness requiring pharmacotherapy during pregnancy. It is unknown whether the observed deficits are transient or reflect early evidence of a persistent disruption in neuromotor function. Future investigations are warranted to disentangle the relative contribution of antipsychotic medications, maternal mental illness, concomitant medications, and the broader psychosocial context in the developmental trajectory of high-risk infants. Pending such studies, these data support an additional level of clinical scrutiny in medication selection, treatment planning, and risk/benefit discussions for women with illnesses who may warrant antipsychotic pharmacotherapy during gestation.
Acknowledgments
Funding/Support: This study was supported by a NARSAD Young Investigator Grant Award (Dr Brennan), Emory University Silvio O. Conte Center for the Neurobiology of Mental Disease grant MH58922, Specialized Center of Research on Sex and Gender Effects grant MH68036, and National Institute of Mental Health grant MH88609.
Footnotes
Author Contributions: Dr Brennan had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Role of the Sponsors: The study sponsors had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
Additional Contributions: We thank the research staff for all of their efforts with data collection and coding.
Financial Disclosure: Dr Stowe has received research support from the National Institutes of Health, GlaxoSmithKline, Pfizer, and Wyeth; served on speakers or advisory boards for Pfizer, Eli Lilly, Wyeth, Bristol-Myers Squibb, and GlaxoSmithKline; and received honoraria from Eli Lilly, GlaxoSmithKline, Pfizer, and Wyeth. Dr Newport has received research support from Eli Lilly, GlaxoSmithKline, Janssen, the National Institutes of Health, NARSAD, and Wyeth; served on speakers or advisory boards for AstraZeneca, Eli Lilly, GlaxoSmithKline, Pfizer, and Wyeth; and received honoraria from Astra-Zeneca, Eli Lilly, GlaxoSmithKline, Pfizer, and Wyeth.
References
- 1.Nicholson J, Biebel K, Katz-Leavy J, Williams V. The Prevalence of Parenthood in Adults With Mental Illness: Implications for State and Federal Policymakers, Programs and Providers. Boston: University of Massachusetts Medical School; 2002. [Google Scholar]
- 2.Grof P, Robbins W, Alda M, Berghoefer A, Vojtechovsky M, Nilsson A, Robertson C. Protective effect of pregnancy in women with lithium-responsive bipolar disorder. J Affect Disord. 2000;61(1–2):31–39. doi: 10.1016/s0165-0327(99)00197-4. [DOI] [PubMed] [Google Scholar]
- 3.Freeman MP, Smith KW, Freeman SA, McElroy SL, Kmetz GE, Wright R, Keck PE., Jr The impact of reproductive events on the course of bipolar disorder in women. J Clin Psychiatry. 2002;63(4):284–287. doi: 10.4088/jcp.v63n0403. [DOI] [PubMed] [Google Scholar]
- 4.Viguera AC, Whitfield T, Baldessarini RJ, Newport DJ, Stowe Z, Reminick A, Zurick A, Cohen LS. Risk of recurrence in women with bipolar disorder during pregnancy: prospective study of mood stabilizer discontinuation. Am J Psychiatry. 2007;164(12):1817–1824. doi: 10.1176/appi.ajp.2007.06101639. [DOI] [PubMed] [Google Scholar]
- 5.Newport DJ, Stowe ZN, Viguera AC, Calamaras MR, Juric S, Knight B, Pennell PB, Baldessarini RJ. Lamotrigine in bipolar disorder: efficacy during pregnancy. Bipolar Disord. 2008;10(3):432–436. doi: 10.1111/j.1399-5618.2007.00565.x. [DOI] [PubMed] [Google Scholar]
- 6.Casiano ME, Hawkins DR. Major mental illness and childbearing: a role for the consultation-liaison psychiatrist in obstetrics. Psychiatr Clin North Am. 1987;10(1):35–51. [PubMed] [Google Scholar]
- 7.Cohen LS, Altshuler LL, Harlow BL, Nonacs R, Newport DJ, Viguera AC, Suri R, Burt VK, Hendrick V, Reminick AM, Loughead A, Vitonis AF, Stowe ZN. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA. 2006;295(5):499–507. doi: 10.1001/jama.295.5.499. [DOI] [PubMed] [Google Scholar]
- 8.Kuehn BM. Questionable antipsychotic prescribing remains common, despite serious risks. JAMA. 2010;303(16):1582–1584. doi: 10.1001/jama.2010.453. [DOI] [PubMed] [Google Scholar]
- 9.Alexander GC, Gallagher SA, Mascola A, Moloney RM, Stafford RS. Increasing off-label use of antipsychotic medications in the United States, 1995–2008. Pharmacoepidemiol Drug Saf. 2011;20(2):177–184. doi: 10.1002/pds.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKenna K, Einarson A, Levinson A, Gideon K. Significant changes in antipsychotic drug use during pregnancy. Vet Hum Toxicol. 2004;46(1):44–46. [PubMed] [Google Scholar]
- 11.McKenna K, Koren G, Tetelbaum M, Wilton L, Shakir S, Diav-Citrin O, Levinson A, Zipursky RB, Einarson A. Pregnancy outcome of women using atypical antipsychotic drugs: a prospective comparative study. J Clin Psychiatry. 2005;66(4):444–449. doi: 10.4088/jcp.v66n0406. [DOI] [PubMed] [Google Scholar]
- 12.Newport DJ, Calamaras MR, DeVane CL, Donovan J, Beach AJ, Winn S, Knight BT, Gibson BB, Viguera AC, Owens MJ, Nemeroff CB, Stowe ZN. Atypical antipsychotic administration during late pregnancy: placental passage and obstetrical outcomes. Am J Psychiatry. 2007;164(8):1214–1220. doi: 10.1176/appi.ajp.2007.06111886. [DOI] [PubMed] [Google Scholar]
- 13.Newham JJ, Thomas SH, MacRitchie K, McElhatton PR, McAllister-Williams RH. Birth weight of infants after maternal exposure to typical and atypical antipsychotics: prospective comparison study. Br J Psychiatry. 2008;192(5):333–337. doi: 10.1192/bjp.bp.107.041541. [DOI] [PubMed] [Google Scholar]
- 14.Babu GN, Desai G, Tippeswamy H, Chandra PS. Birth weight and use of olanzapine in pregnancy: a prospective comparative study. J Clin Psychopharmacol. 2010;30(3):331–332. doi: 10.1097/JCP.0b013e3181db8734. [DOI] [PubMed] [Google Scholar]
- 15.Koren G, Cohn T, Chitayat D, Kapur B, Remington G, Reid DM, Zipursky RB. Use of atypical antipsychotics during pregnancy and the risk of neural tube defects in infants. Am J Psychiatry. 2002;159(1):136–137. doi: 10.1176/appi.ajp.159.1.136. [DOI] [PubMed] [Google Scholar]
- 16.Reis M, Källén B. Maternal use of antipsychotics in early pregnancy and delivery outcome. J Clin Psychopharmacol. 2008;28(3):279–288. doi: 10.1097/JCP.0b013e318172b8d5. [DOI] [PubMed] [Google Scholar]
- 17.Bazire S. Psychotropic Drug Directory. Salisbury, England: Five-pin Limited; 2005. [Google Scholar]
- 18.Biswasl PN, Wilton LV, Pearcel GL, Freemantle S, Shakir SAW. The pharmacovigilance of olanzapine: results of a post-marketing surveillance study on 8858 patients in England. J Psychopharmacol. 2001;15(4):265–271. doi: 10.1177/026988110101500405. [DOI] [PubMed] [Google Scholar]
- 19.Goldstein DJ, Corbin LA, Fung MC. Olanzapine-exposed pregnancies and lactation: early experience. J Clin Psychopharmacol. 2000;20(4):399–403. doi: 10.1097/00004714-200008000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Auerbach JG, Hans SL, Marcus J, Maeir S. Maternal psychotropic medication and neonatal behavior. Neurotoxicol Teratol. 1992;14(6):399–406. doi: 10.1016/0892-0362(92)90050-k. [DOI] [PubMed] [Google Scholar]
- 21.Gentile S. Antipsychotic therapy during early and late pregnancy: a systematic review. Schizophr Bull. 2010;36(3):518–544. doi: 10.1093/schbul/sbn107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Costa LG, Steardo L, Cuomo V. Structural effects and neurofunctional sequelae of developmental exposure to psychotherapeutic drugs: experimental and clinical aspects. Pharmacol Rev. 2004;56(1):103–147. doi: 10.1124/pr.56.1.5. [DOI] [PubMed] [Google Scholar]
- 23.Rosengarten H, Quartermain D. Effect of prenatal administration of haloperidol, risperidone, quetiapine and olanzapine on spatial learning and retention in adult rats. Pharmacol Biochem Behav. 2002;72(3):575–579. doi: 10.1016/s0091-3057(02)00727-x. [DOI] [PubMed] [Google Scholar]
- 24.Zuo J, Liu Z, Ouyang X, Liu H, Hao Y, Xu L, Lu X-H. Distinct neurobehavioral consequences of prenatal exposure to sulpiride (SUL) and risperidone (RIS) in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(2):387–397. doi: 10.1016/j.pnpbp.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 25.Newport DJ, Brennan PA, Green P, Ilardi D, Whitfield TH, Morris N, Knight BT, Stowe ZN. Maternal depression and medication exposure during pregnancy: comparison of maternal retrospective recall to prospective documentation. BJOG. 2008;115(6):681–688. doi: 10.1111/j.1471-0528.2008.01701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brennan PA, Pargas R, Walker EF, Green P, Newport DJ, Stowe Z. Maternal depression and infant cortisol: influences of timing, comorbidity and treatment. J Child Psychol Psychiatry. 2008;49(10):1099–1107. doi: 10.1111/j.1469-7610.2008.01914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ellison PH, Horn JL, Browning CA. Construction of an Infant Neurological International Battery (INFANIB) for the assessment of neurological integrity in infancy. Phys Ther. 1985;65(9):1326–1331. doi: 10.1093/ptj/65.9.1326. [DOI] [PubMed] [Google Scholar]
- 28.Einarsson-Backes LM, Stewart KB. Infant neuromotor assessments: a review and preview of selected instruments. Am J Occup Ther. 1992;46(3):224–232. doi: 10.5014/ajot.46.3.224. [DOI] [PubMed] [Google Scholar]
- 29.Soleimani F, Dadkhah A. Validity and reliability of Infant Neurological International Battery for detection of gross motor developmental delay in Iran. Child Care Health Dev. 2007;33(3):262–265. doi: 10.1111/j.1365-2214.2006.00704.x. [DOI] [PubMed] [Google Scholar]
- 30.Fagen JW, Ohr PS. Learning and Memory in Infancy: Habituation, Instrumental Conditioning and Expectancy Formation. Biobehavioral Assessment of the Infant. New York, NY: The Guilford Press; 2001. pp. 233–273. [Google Scholar]
- 31.McCall RB, Carriger MS. A meta-analysis of infant habituation and recognition memory performance as predictors of later IQ. Child Dev. 1993;64(1):57–79. [PubMed] [Google Scholar]
- 32.Columbo J. Infant Cognition: Predicting Later Intellectual Functioning. Thousand Oaks, CA: Sage Publications, Inc; 1993. [Google Scholar]
- 33.Bornstein MH. Habituation of attention as a measure of visual information processing in human infants: summary, systematization, and synthesis. In: Gottlieb G, Krasnegor NA, editors. Measurement of Audition and Vision in the First Year of Postnatal Life: A Methodological Overview. Westport, CT: Ablex Publishing; 1985. pp. 253–300. [Google Scholar]
- 34.Lobbestael J, Leurgans M, Arntz A. Inter-rater reliability of the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID I) and Axis II Disorders (SCID II) Clin Psychol Psychother. 2011;18(1):75–79. doi: 10.1002/cpp.693. [DOI] [PubMed] [Google Scholar]
- 35.Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clin Psychol Rev. 1988;8(1):77–100. doi: 10.1016/0272-7358(88)90050-5. [DOI] [Google Scholar]
- 36.Ji S, Long Q, Newport DJ, Na H, Knight B, Zach EB, Morris NJ, Kutner M, Stowe ZN. Validity of depression rating scales during pregnancy and the postpartum period: impact of trimester and parity. J Psychiatr Res. 2011;45(2):213–219. doi: 10.1016/j.jpsychires.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Voorn P, Kalsbeek A, Jorritsma-Byham B, Groenewegen HJ. The pre- and post-natal development of the dopaminergic cell groups in the ventral mesencephalon and the dopaminergic innervation of the striatum of the rat. Neuroscience. 1988;25(3):857–887. doi: 10.1016/0306-4522(88)90041-3. [DOI] [PubMed] [Google Scholar]
- 38.Scalzo FM, Spear LP. Chronic haloperidol during development attenuates dopamine autoreceptor function in striatal and mesolimbic brain regions of young and older adult rats. Psychopharmacology (Berl) 1985;85(3):271–276. doi: 10.1007/BF00428186. [DOI] [PubMed] [Google Scholar]
- 39.Scalzo FM, Holson RR, Gough BJ, Ali SF. Neurochemical effects of prenatal haloperidol exposure. Pharmacol Biochem Behav. 1989;34(4):721–725. doi: 10.1016/0091-3057(89)90265-7. [DOI] [PubMed] [Google Scholar]
- 40.Brooks DJ. Functional imaging studies on dopamine and motor control. J Neural Transm. 2001;108(11):1283–1298. doi: 10.1007/s007020100005. [DOI] [PubMed] [Google Scholar]
- 41.Herlenius E, Lagercrantz H. Neurotransmitters and neuromodulators during early human development. Early Hum Dev. 2001;65(1):21–37. doi: 10.1016/s0378-3782(01)00189-x. [DOI] [PubMed] [Google Scholar]
- 42.Cases O, Lebrand C, Giros B, Vitalis T, De Maeyer E, Caron MG, Price DJ, Gaspar P, Seif I. Plasma membrane transporters of serotonin, dopamine, and norepinephrine mediate serotonin accumulation in atypical locations in the developing brain of monoamine oxidase A knock-outs. J Neurosci. 1998;18(17):6914–6927. doi: 10.1523/JNEUROSCI.18-17-06914.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sodhi MSK, Sanders-Bush E. Serotonin and brain development. Int Rev Neurobiol. 2004;59:111–174. doi: 10.1016/S0074-7742(04)59006-2. [DOI] [PubMed] [Google Scholar]
- 44.Vinay L, Ben-Mabrouk F, Brocard F, Clarac F, Jean-Xavier C, Pearlstein E, Pflieger J-F. Perinatal development of the motor systems involved in postural control. Neural Plast. 2005;12(2–3):131–139. doi: 10.1155/NP.2005.131. discussion 263–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dannemiller JL. Infant habituation criteria: a Monte Carlo study of the 50% decrement criterion. Infant Behav Dev. 1984;7(2):147–166. doi: 10.1016/S0163-6383(84)80055-7. [DOI] [Google Scholar]
- 46.Columbo J. What habituates in infant visual habituation? a psychophysiological analysis. Infancy. 2010;15(2):107–124. doi: 10.1111/j.1532-7078.2009.00012.x. [DOI] [PubMed] [Google Scholar]