Abstract
Pheochromocytoma is a very special kind of tumor full of duplicity. On the one hand it represents its own microworld with unique clinical, biochemical and pathological features, while on the other it constitutes a tremendously significant part of whole body system, playing a vital role for practically every organ system. It has a very special character – sometimes like a child it can be sweet and predictable, while at times it can behave like a deadly wild beast, crashing and tearing everything on its path in a fierce rage. It also consists of the amazingly intelligent neuroendocrine cells that possess a magical ability to make miraculous substances of many kinds. But most of all, it is a system that is able to drive our curiosity and the itch of “Cogito, ergo sum” to limitless depths and year by year it still amazes us with new and unexpected discoveries that move our understanding of multiple pathways and metabolic events closer to the ultimate truth. Recent discoveries of succinate dehydrogenase (SHD) and prolyl hydroxylase (PHD) mutations, for example, propelled our understanding of neuroendocrine tumorigenesis as a whole, as well as physiology of mitochondrial respiratory chain and phenomenon of pseudohypoxia in particular. Good old discoveries make their way from dusty repositories to shine with new meaning, appropriate for the current level of knowledge. This acquired wisdom makes us better physicians – knowing the specific expression makeup of catecholamine transporters, GLUTs and SRIFs allows for better tailored imaging and therapeutic manipulations. There are still long ways to go, keeping in mind that pheochromocytoma is but so very special, and we are optimistic and expect many great things to come.
Keywords: pheochromocytoma, paraganglioma, catecholamines, metanephrines, positron emission tomography
Past
There are probably few names that have not been used to describe pheochromocytoma – from somewhat complimenting “great masquerader” to unflattering “treacherous murderer”. These apparently “mixed feelings” relate to the rarity of disease in a population of usual suspects – patients with poorly controlled and labile hypertension of one side and horrific devastation of acute pheochromocytoma crisis that strikes unexpectedly and unsuspectedly, leaving traumatic memories of acute medical disaster that champions any intensivecarestory (Brouwers et al., 2003; Manger and Gifford, 2002; Pacak et al., 2001e). For the sake of simplicity, we will use the term “pheochromocytoma” as an inclusive reference for both the adrenal tumor (true pheochromocytoma) and its extra-adrenal counterpart (paraganglioma), but diverge into more detailed terminology when necessary. Autopsy studies have shown that significant numbers of pheochromocytomas remain undiagnosed until death, and that up to 50% of these unrecognized tumors may have contributed to patient mortality (Sutton et al., 1981). On the other hand, pheochromocytomas represent an amazing pathophysiological phenomenon, where a single tumor comprises part of the diffuse neuroendocrine system (DNES). The history of pheochromocytoma research is rich while its present is dynamic with continuous change in our view on the disease and its management based on rapidly changing knowledge and understanding of the underlying genetics and other mechanisms leading to it.
The initial discovery of pheochromocytoma represents the pinnacle of descriptive medical science and closely associates with the development of neuroendocrinology as a whole. First, adrenal pheochromocytoma was described by Frankel (1886), extra-adrenal pheochromocytoma by Alezais and Peyron (1908), while the name was coined by Pick (1912) for dusky (phaios) colour (chroma) features on staining with chromium salts. It is also important to recognize the visionary work of Alfred Kohn, who between 1898 and 1902 introduced the terms “chromaffin” and “paraganglion” and shaped a paradigm of systemic nature of chromaffin tissue and existence of diffuse paraganglionic system. The association between paroxysmal hypertension and pheochromocytoma was described by L’Abbe et al. (1922). Epinephrine was isolated from a pheochromocytoma by Kelly et al. (1936) and Holton (1949) demonstrated the presence of norepinephrine in a pheochromocytoma. Von Euler and Holtz independently reported the occurrence of norepinephrine in the human body (Holtz et al., 1947; von Euler, 1946a, 1946b). The first successful surgical removal of pheochromocytoma was performed by Roux in 1926 (Manger and Gifford, 1996).
Present
Epidemiology
According to different reviews and statistics, pheochromocytomas account for ~0.05–0.1% of patients with any degree of sustained hypertension (Bravo and Tagle, 2003; Manger and Gifford, 1996, 2002). This probably accounts for only half of persons harbouring the tumor, since about half the patients with pheochromocytoma have only paroxysmal hypertension or are normotensive. With the current prevalence of sustained hypertension in the adult population of Western countries about up to 30% (Epstein and Eckhoff, 1967; McBride et al., 2003; Page, 1976), the prevalence of pheochromocytoma can be estimated at between 1:4500 and 1:1700, with an annual incidence of 3–8 cases per 1 million per year in the general population (Pacak et al., 2001a). Pheochromocytoma occurs at any age, but most often in the fourth and fifth decade, and it occurs equally in men and women. At least 24% are familial (Neumann et al., 2002) and those tumors are often multi-focal and bilateral (Bravo and Tagle, 2003; Manger and Gifford, 2002). Furthermore, malignant pheochromocytoma or paraganglioma may account from 1% to about 90% of cases, depending on the type of the tumor, its localization (adrenal tumors are very rarely metastatic) and genetic background (mainly SDHB-related tumors) (Brouwers et al., 2006; Timmers et al., 2007c). About 10% of patients with pheochromocytoma present with metastatic disease at the time of their initial workup (Scholz et al., 2007).
In children, pheochromocytomas are more frequently familial (40%), extra-adrenal (8–43%), bilateral adrenal (7–53%), or multi-focal or bilateral if located in the adrenal gland (Barontini et al., 2006; Caty et al., 1990; De Krijger et al., 2006; Ludwig et al., 2007; Ross, 2000). These tumors peak at 10–13 years, with a 2:1 male predominance before puberty (Caty et al., 1990; Hume, 1960; Ross, 2000). Previous studies suggested that less than 10% of paediatric pheochromocytomas are malignant (Caty et al., 1990; Kaufman et al., 1983; Reddy et al., 2000; Ross, 2000) with reported mean survival rates of 73% at 3 years and 40–50% at 5 years after diagnosis (Coutant et al., 1999; Hume, 1960; Loh et al., 1997). There are currently new data suggesting that pheochromocytomas in children show much higher genetic incidence (Neumann et al., 2002), are commonly associated with succinate dehydrogenase (SDH) subunit B (mainly in those patients with no family history of pheochromocytoma or paraganglioma) and often lead to metastatic disease even in very young children (Pacak et al., unpublished observations).
Anatomy and physiology
Chromaffin cell ganglia (also called paraganglia) – with adrenal medulla representing the largest of them – are neuroendocrine organs, diffusely scattered throughout the human body and associated with autonomic nervous system explaining why pheochromocytomas may be found practically in any location. It seems that paraganglia may play a role of prenatal regional chemo- and/or oxygen sensor, relying on maternal pressor backup, while after the birth the body needs both functions equally, which is better provided by the adrenal. Abnormalities in postnatal oxygen sensing may play a significant role in the generation of hypoxia or “pseudohypoxia” phenomenon and play a pivotal role in further tumorigenesis (Gottlieb and Tomlinson, 2005).
Pheochromocytomas are derived from the adrenal gland; paragangliomas arise from parasympathetic-associated tissues (most commonly along the cranial nerves and vagus (e.g. glomus tumors, chemodectoma and carotid body tumor) and from extra-adrenal sympathetic-associated chromaffin tissue (often designated extra-adrenal pheochromocytomas). While paragangliomas arise mainly from chromaffin tissue adjacent to sympathetic ganglia in the abdomen and less commonly in the chest or pelvis, both adrenal and extra-adrenal tumors display similar histopathological characteristics.
It is difficult, if not impossible, to distinguish malignant from benign pheochromocytomas based on histopathological features. Only the tumor invasion of tissues and the presence of metastatic lesions (most commonly in the liver, lungs, lymphatic nodes and bones) are consistent with the diagnosis of malignant pheochromocytoma (Yu and Pacak, 2002). Chromaffin cells have the ability to synthesize and secrete various amines and peptides (i.e. adrenocorticotropic hormone (ACTH), chromogranins, neuropeptide Y, calcitonin, angiotensin-converting enzyme, renin, vasoactive intestinal polypeptide, adrenomedullin and atrial natriuretic factor), as well as antigens (Trojanowski and Lee, 1985), protein gene product 9.5 (Thompson et al., 1983), galanin (Bauer et al., 1986), renin (Mizuno et al., 1985), angiotensin-converting enzyme (Gonzalez-Garcia and Keiser, 1990) and synaptophysin (Miettinen, 1987) or parathyroid hormone-related protein (Kimura et al., 1990).
Catecholamines
Biochemical reactions resulting in the production of catecholamines take place in numerous cells throughout the body, including central and peripheral nervous systems, DNES, gastrointestinal tract and kidneys (Eisenhofer and Goldstein, 2004). Pathophysiological relevance of this production depends mostly on the ability of the cell to possess appropriate enzymatic machinery, as well as on the ability of significant volume production of catecholamines. It is also important to appreciate that intracellular catecholamine synthesis parallels simultaneous cellular metabolism of produced catecholamines independently of their release (Eisenhofer et al., 2003a). This metabolic phenomenon serves as a cornerstone of current biochemical diagnostic strategy of pheochromocytomas.
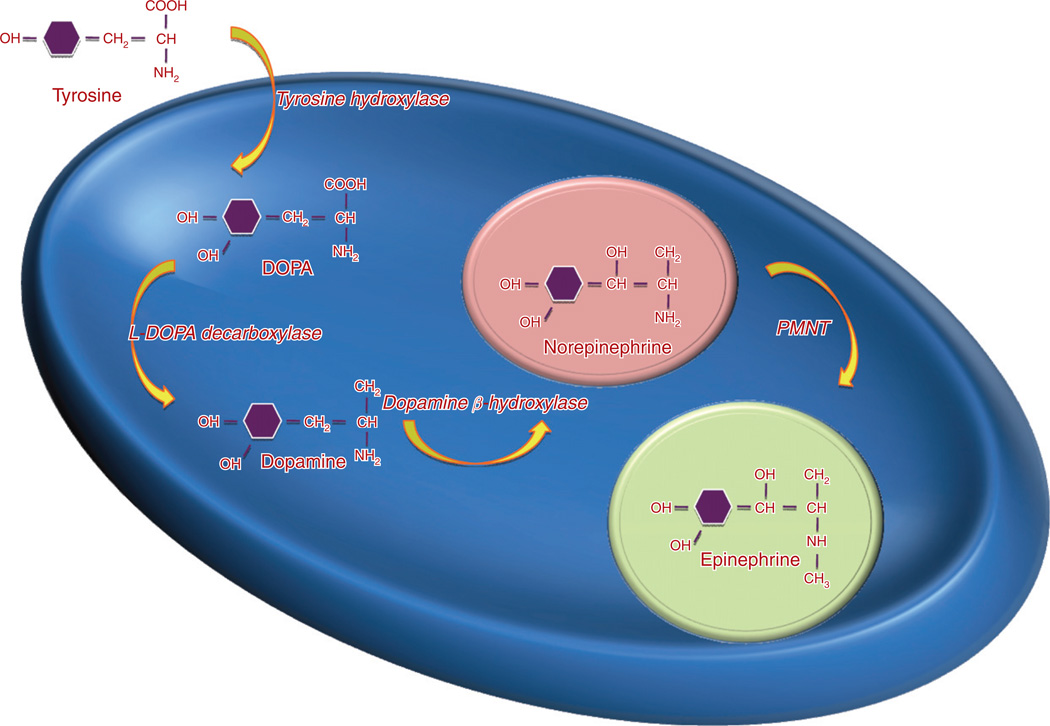
The l-stereoisomer of amino acid tyrosine from a dietary source or following hydroxylation of phenylalanine in the liver serves as a substrate for the initiation of catecholamine synthesis. It is converted to dihydroxyphenylalanine (dopa) by tyrosine hydroxylase (TH) (Fig. 1) and represents the rate-limiting step in catecholamine biosynthesis (Udenfriend, 1966). Tissue expression of this enzyme is largely confined to dopaminergic and noradrenergic neurons of the central nervous system, sympathetic nerves of peripheral nervous system, chromaffin cells of the adrenal medulla and extramedullary paraganglia. It is also expressed in enterochromaffin cells of enteric carcinoids (Jakobsen et al., 2001). The following step represents the production of dopamine by decarboxylation of dopa aromatic l-amino acid decarboxylase. The dopamine formed in neurons and chromaffin cells is translocated from the cytoplasm into vesicular storage granules. Large amounts of dopamine are also produced as an end product of catecholamine synthesis in peripheral non-neuronal cells of the gastrointestinal tract and kidneys (Eisenhofer and Goldstein, 2004). The dopamine formed in noradrenergic neurons and chromaffin cells is converted to norepinephrine by dopamine β-hydroxylase (DBH), an enzyme that is found only in the vesicles of cells that synthesize norepinephrine and epinephrine. In adrenal medullary chromaffin cells, norepinephrine is metabolized by the cytosolic enzyme phenylethanolamine N-methyltransferase (PNMT) to form epinephrine (Axelrod, 1966). Epinephrine is then translocated into chromaffin granules, where the amine is stored awaiting release (Johnson, 1988). Expression of PNMT in extra-adrenal pheochromocytomas is negligent, which explains the preferential production of norepinephrine by these tumors, compared to both norepinephrine and epinephrine production by adrenal pheochromocytomas (Brown et al., 1972).
Fig. 1.
Catecholamine biosynthesis.
Translocation of catecholamines into vesicular granules for storage is facilitated by two vesicular monoamine transporters: VMAT1 and VMAT2 (Fig. 2)(Henry et al., 1998). Catecholamines show higher affinity to VMAT2 and differential expression of these transporters throughout the sympatho-adrenal system may correlate with differences in uptake of metaiodobenzylguanidine (MIBG; see below) (Jakobsen et al., 2001). Storage vesicles represent a complex functional unit that continuously maintains a highly dynamic equilibrium between passive outward leakage of catecholamines into the cytoplasm counterbalanced by VMATs-driven inward active transport (Eisenhofer et al., 2003b). Catecholamines share the acid environment of the storage granule matrix with adenosine triphosphate (ATP), peptides and proteins, the most well known of which are the chromogranins (O’Connor et al., 1994). The chromogranins are ubiquitous components of secretory vesicles, and their widespread presence among endocrine tissues has led to their measurement in plasma as sensitive, albeit relatively non-specific markers of neuroendocrine tumors, including pheochromocytomas or paragangliomas. Two populations of chromaffin cells have been described with morphologically distinct vesicles that preferentially store either norepinephrine or epinephrine, and which release the two catecholamines differentially in response to different stimuli. Most adrenal pheochromocytomas secrete both norepinephrine and epinephrine; about a third exclusively produce norepinephrine, and a much smaller proportion exclusively produce and secrete epinephrine.
Fig. 2.
Catecholamine metabolism.
For a better understanding of the pathophysiology and clinical symptomatology of pheochromocytoma and paraganglioma, one needs to be aware about the following physiological concepts. All the three main catecholamines (dopamine, epinephrine and norepinephrine) have tightly regulated metabolism and organ-specific secretion. While dopamine is a major central neurotransmitter, its peripheral levels can be elevated in rare dopamine-secreting pheochromocytomas. Adrenal gland, on the other hand, normally secretes both epinephrine (predominantly) and norepinephrine directly into the circulation, of which about 90% is rapidly removed by extra-neuronal hepatic monoamine transport system. Paraganglia exclusively secretes norepinephrine, which is a major neurotransmitter within sympathetic nervous system. For norepinephrine released by sympathetic nerves, about 90% is removed back into nerves by neuronal uptake, 5% is removed by extraneuronal uptake and 5% escapes these processes to enter the bloodstream. Overall, these processes result in circulatory half-life for catecholamines of less than 2 minutes (Eisenhofer, 2001).
Catechol-O-methyltransferase (COMT) is responsible for a major pathway of catecholamine metabolism, catalyzing O-methylation of dopamine to methoxytyramine, norepinephrine to normetanephrine and epinephrine to metanephrine, as well as methylation of dihydroxyphenylglycol (DHPG) to 3-methoxy-4-hydroxyphenylglycol (MHPG). COMT is not present in catecholamine-producing neurons. Normetanephrine and metanephrine are produced in small amounts and only at extraneuronal locations, with the single largest source representing adrenal chromaffin cells, which account for over 90% of circulating metanephrine and 24–40% of circulating normetanephrine (Eisenhofer et al., 1995). About 90% of the vesicular monoamine (VMA) formed in the body is produced in the liver, mainly from hepatic uptake and metabolism of circulating DHPG and MHPG (Eisenhofer et al., 1996). In humans, VMA and the sulphate and glucuronide conjugates of MHPG represent the main end products of norepinephrine and epinephrine metabolism and are eliminated mainly by urinary excretion.
Clinical presentation
Most clinical signs and symptoms associated with hypercatecholaminaemia are non-specific, which may in part explain underdiagnosis of phaeochronocytoma (Tables 1 and 2) resulting in extensive differential diagnosis (Manger and Gifford, 1996, 1977; Young and Landsberg, 1998). Although paroxysmal or resistant hypertension is still a major cause for diagnostic workup of pheochromocytoma, there is a steadily increasing proportion of cases found as part of investigation for adrenal incidentalomas or accidentally found extra-adrenal paragangliomas showing few, if any, symptoms. Incidentalomas seem to be associated with lower levels of catecholamines (Kopetschke et al., 2009). At least about 20–30% of patients with pheochromocytoma or paraganglioma are asymptomatic or have only minor signs and symptoms; therefore, the diagnosis is easily missed, often with tragic consequences. There also seems to be a difference in clinical presentation between adrenal and extra-adrenal tumors, driven predominantly by different secretory profile – norepinephrine in extra-adrenal tumors, and epinephrine and norepinephrine in adrenal tumors.
Table 1.
Symptoms and signs of pheochromocytoma
| Clinical setting | Symptoms |
|---|---|
| Multi-system crisis | Hypertension and/or hypotension, multiple organ failure, temperature = 40°C, encephalopathy |
| Cardiovascular | Collapse |
| Hypertensive crisis | |
| Shock or profound hypotension | |
| Acute heart failure | |
| Myocardial infarction | |
| Arrhythmia | |
| Cardiomyopathy | |
| Myocarditis | |
| Dissecting aortic aneurysm | |
| Limb ischaemia, digital necrosis or gangrene | |
| Pulmonary | Acute pulmonary oedema |
| Adult respiratory distress syndrome | |
| Abdominal | Abdominal bleeding |
| Paralytic ileus | |
| Acute intestinal obstruction | |
| Severe enterocolitis and peritonitis | |
| Bowel perforation with generalized peritonitis | |
| Bowel ischaemia | |
| Mesenteric vascular occlusion | |
| Acute pancreatitis | |
| Cholecystitis | |
| Megacolon | |
| Neurological | Hemiplegia |
| Limb weakness | |
| Renal | Acute renal failure |
| Acute pyelonephritis | |
| Severe haematuria | |
| Metabolic | Diabetic ketoacidosis |
| Lactic acidosis |
Table 2.
Differential diagnosis for pheochromocytoma
|
Sustained or paroxysmal hypertension (equally present) is the most common clinical sign (85–90%). Up to 13% of the patients typically present with persistently normal blood pressure (Bravo and Tagle, 2003), but this proportion can be much higher in patients with adrenal incidentalomas or in those who undergo periodic screening for familial pheochromocytoma (Neumann et al., 2002). Pheochromocytoma may also present with hypotension, commonly seen in patients harbouring tumors that are secreting epinephrine or compounds causing vasodilatation, or after higher doses of anti-hypertensive therapy. Hypotension, postural or alternating, may occur secondary to hypovolaemia, abnormal autonomic reflexes, downregulation or differential stimulation of α- and β-adrenergic receptors, or the type of co-secreted neuropeptide (Bravo and Gifford, 1993; Bravo and Tagle, 2003; Bravo et al., 1981). Headache occurs in up to 90% of patients with pheochromocytoma (Manger and Gifford, 1996), being mild or severe, short or long in duration, and may last for up to several days. Excessive generalized sweating occurs in ~60–70% of patients presenting with pheochromocytoma (Manger and Gifford, 1996). Other complaints are palpitations and dyspnea, weight loss despite normal appetite (caused by catecholamine-induced glycogenolysis and lipolysis) or weight gain (constipation), and generalized weakness (Gifford et al., 1985). Some patients present with new and commonly more severe episodes of anxiety or panic attacks (Bravo and Gifford, 1993). Palpitations, anxiety and nervousness are more common in patients with pheochromocytomas that produce epinephrine (Bravo and Gifford, 1993). This set of clinical symptoms has to be differentiated from hyperadrenergic hypertension, anxiety or panic attacks, which is characterized by tachycardia, sweating, anxiety and an increased cardiac output (Esler et al., 1977). These patients often have increased levels of catecholamines in blood and urine and may be excluded by the use of the clonidine suppression test (Bravo et al., 1981).
Pheochromocytoma is known to be associated with a wide range of cardiac complications, including ischemic or hypertrophic cardiomyopathy, supra- and ventricular tachyarrhythmias and acute ischemic events. It is important to suspect pheochromocytoma in case of unexplained acute cardiac event. On the other hand, we have observed only few cardiac complications in patients with long-standing pheochromocytomas or paragangliomas and extensive hypercatecholaminaemia, probably related to significant down-regulation of adrenergic receptors (Pacak et al., unpublished observations).
Pheochromocytoma-induced metabolic or haemodynamic attacks may last from a few seconds to several hours, with intervals between attacks varying widely. A typical paroxysm is characterized by a sudden major increase in blood pressure; a severe, often pounding headache; profuse sweating over most of the body, especially the trunk; palpitations; prominent anxiety or a sense of doom; skin pallor; nausea, with or without emesis; and pain in the abdomen, the chest or other locations where a tumor is usually located (Manger and Gifford, 1996; Plouin et al., 1981). After an episode, patients usually feel drained and exhausted, and some may urinate more frequently. Rarely, paroxysmal blood pressure elevations during diagnostic procedures such as endoscopy, anaesthesia (caused by a sudden fall in blood pressure or any activation of the sympathetic nervous system that occurs during the induction phase of general anaesthesia) or ingestion of food or beverages containing tyramine (e.g. certain cheeses, beers, wines, bananas and chocolate) should arouse immediate suspicion of pheochromocytoma. The use of certain drugs such as histamine, metoclopramide, glucagon, etc. may also precipitate a hypertensive episode (Barancik, 1989; Bittar, 1979; Cook and Katritsis, 1990; Jan et al., 1990; Manger and Gifford, 1996; Page et al., 1969; Schorr and Rogers, 1987; Steiner et al., 1968). Moreover, micturition or bladder distension in the case of a pheochromocytoma of the urinary bladder can cause clinical symptoms and signs of catecholamine excess. These episodes should be differentiated from pseudopheochromocytoma, which refers to the large majority of individuals (often women) with severe paroxysmal hypertension, whether normotensive or hypertensive between episodes, (Kuchel, 1985), related to a short-term activation of the sympathetic nervous system. In contrast to pheochromocytoma, patients with pseudopheochromocytoma more often present with panic attacks or anxiety, flushing, nausea and polyuria (Kuchel, 1985, 2004; White and Baker, 1986).
Less common clinical manifestations include fever of unknown origin (hypermetabolic state) and constipation secondary to catecholamine-induced decrease in intestinal motility (Bouloux and Fakeeh, 1995). Observed flushing is rare, but can follow the vasoconstrictive episode of pallor or Raynaud’s phenomenon, associated with the attack (Manger and Gifford, 1996). Patients may also present with tremor, seizures, hyperglycaemia, hypermetabolism, weight loss (usually only in patients with malignant pheochromocytoma), fever and even mental changes (Manger and Gifford, 1996). The hyperglycaemia is usually mild, occurs with the hypertensive episodes, is accompanied by a subnormal level of plasma insulin (because of α-adrenergic inhibition of insulin release) (Colwell, 1969) and usually does not need treatment; however, it can be sustained and severe enough to require insulin and even to present as diabetic ketoacidosis (Edelman et al., 1992). Hypercalcaemia has been reported in some patients with pheochromocytoma perhaps due to parathyroid hormone-related peptide (Heath and Edis, 1979; Steiner et al., 1968; Stewart et al., 1985). In addition, pheochromocytoma has presented as Cushing’s syndrome with the tumor as the ectopic source of ACTH (Spark et al., 1979). Rarely, pheochromocytoma has produced vasoactive intestinal peptide with resultant watery diarrhoea, hypokalaemia and achlorhydria (Verner–Morrison syndrome) (Viale et al., 1985). Lactic acidosis without shock or sepsis has been reported (Bornemann et al., 1986). We have recently discovered a cohort of patients stating to have experienced exercise-induced nausea and vomiting on the first presentation of disease. We propose that the act of exercising increases the amount of circulating catecholamines in pheochromocytoma/paraganglioma patients and may potentially lead to the activation of the α1- and α2-adrenergic receptors in the area postrema inducing nausea and vomiting (Pacak et al., unpublished observations).
In contrast to adult patients, in whom sustained hypertension is found in only 50% of cases, more than 70–90% of children present with sustained hypertension (Caty et al., 1990; Reddy et al., 2000). Pheochromocytoma is the underlying cause in 1–2% of cases of paediatric hypertension and should be considered after exclusion of the more common causes such as renal diseases and renal artery stenosis (Ross, 2000). Sweating, visual problems, weight loss, and nausea and vomiting are more common in children than in adults (Fonseca and Bouloux, 1993), as are polyuria and polydypsia. In addition, children commonly present with palpitations, anxiety, hyperglycaemia, pallor and/or flushing, while others occasionally present with a reddish blue mottling of the skin and a puffy red and cyanotic appearance of the hands (Manger and Gifford, 1996). Because neuroblastomas (the most common solid tumors in childhood), ganglioneuroblastomas and ganglioneuromas can synthesize and excrete catecholamines and their metabolites, the differential diagnosis of a pheochromocytoma is somewhat difficult or, at times, impossible.
Genetics of pheochromocytoma
Up to 24–28% of pheochromocytomas are inherited (Neumann et al., 2002). Hereditary pheochromocytoma is associated with multiple endocrine neoplasia type 2 (MEN2A or MEN2B), von Recklinghausen’s neurofibromatosis type 1 (NF-1), von Hippel–Lindau (VHL) syndrome and familial paraganglioma caused by germ-line mutations of genes encoding SDH subunits B, C and D (Table 3), as well as recently discovered SDH5 (see below). In general, the traits are inherited in an autosomal-dominant pattern. Tumorigenesis theories and related applications are discussed in the section below.
Table 3.
Familial pheochromocytoma and paraganglioma syndromes
| Syndrome | Gene/protein/pathway | Phenotype | Pheo phenotype |
|---|---|---|---|
| Multiple endocrine neoplasia type 2 | Chr 10 (10q11.2) – AD RET proto-oncogene Receptor tyrosine kinase Growth and differentiation |
MEN2A – MTC Hyperparathyroidism MEN2B – MTC Mucosal neuromas Megacolon Marfanoid habitus Intestinal ganglioneuroma |
Prevalence of 50% Adrenergic phenotype MEN2A – mostly benign Bilateral in 30% MEN2B – higher malignancy rate |
| Neurofibromatosis type 1 (von Recklinghausen’s disease) | Chr 17 (17q11.2) – AD NF1 tumor suppressor gene Neurofibromin Downregulation of p21-ras |
Peripheral neurofibromas Café au lait spots and freckling Iris hamartomas Hyperparathyroidism MTC Hypothalamic tumors |
Prevalence of 0.1–5.7% Adrenergic phenotype |
| Cerebelloretinal haemangioblastomatosis (von Hippel–Lindau syndrome, VHL) | Chr 3 (3p25-26) – AD VHL tumor suppressor gene HIFα Hypoxia-induced pathway |
Retinal angiomas NS haemangioblastomas Renal cell cancer Cystadenomas |
Prevalence of 10–20% Noradrenergic phenotype |
| SDH syndromes | SDHB: Chr 1 (1p36) SDHC: Chr 1 (1q21) SDHD: Chr 11 (11q23) Subunits of MC II Pseudohypoxia-induced pathway Abnormal apoptosis Increased angiogenesis Complex inheritance |
Parasympathetic paraganglioma Sympathetic paraganglioma Renal cell cancer |
Mostly extra-adrenal SDHB – malignant PPT SDHC/D – HNPGL SDHD – multi-focal Mostly noradrenergic phenotype Sometimes dopaminergic phenotype |
Abbreviations: MTC – medullary thyroid cancer; NS – nervous system (in this case – cerebellar and spinal haemangioblastomas); SDH – succinate dehydrogenase; AD – autosomal dominant mode of inheritance; MC II – mitochondrial complex II – component of electron transport chain involved in the Krebs cycle; HNPGL – head and neck paraganglioma.
Multiple endocrine neoplasia type 2 syndrome
MEN2 is an autosomal, dominantly inherited syndrome (Sipple’s syndrome) that consists of pheochromocytoma, medullary carcinoma of the thyroid and hyperparathyroidism (Pacak et al., 2005; Sarosi and Doe, 1968; Sipple, 1961). It affects about 1 in 40,000 individuals and is characterized by medullary thyroid carcinoma, pheochromocytoma and parathyroid hyperplasia/adenoma (Brandi et al., 2001). The syndrome is caused by mutation of RET proto-oncogene located on chromosome 10 (10q11.2), which encodes a receptor tyrosine kinase from glial cell line-derived neurotrophic factor (GDNF) family and is specifically expressed in neural crest-derived cells such as the calcitonin-producing C cells in the thyroid gland and the catecholamine-producing chromaffin cells in the adrenal gland (Mulligan et al., 1993). RET plays a role in normal gastrointestinal neuronal and kidney development (Lore et al., 2001). RET consists of 21 exons with 6 “hot spot exons” (exons 10, 11, 13, 14, 15 and 16) in which mutations are identified in 97% of patients with MEN2. Pheochromocytoma (at least 70% of which are bilateral) develops on a background of adrenomedullary hyperplasia and becomes manifest (e.g. biochemically or on imaging) in about 50% of patients. MEN2-associated pheochromocytomas are almost exclusively benign (with <5% reported to be malignant) and localized to the adrenals (Casanova et al., 1993; Neumann et al., 1993). The peak age is around 40 years, but children as young as 10 years can be affected (Jadoul et al., 1989). Patients with MEN2-related pheochromocytoma often lack sustained hypertension or other symptoms (they occur only in about 50%). Because MEN-related pheochromocytomas secrete epinephrine, stimulation of β-adrenergic receptors causes palpitations and tachycardia. Therefore, their detection is mainly based on elevated plasma metanephrine and epinephrine levels. In patients with pheochromocytomas that produce exclusively normetanephrine, MEN2 is excluded. In addition, as with most epinephrine-secreting pheochromocytomas, the hypertension is more likely to be paroxysmal than sustained. For these reasons the diagnosis is easy to miss.
MEN2B patients have pheochromocytoma; medullary carcinoma of the thyroid; ganglioneuromatosis; multiple mucosal neuromas of eyelids, lips and tongue; and some connective tissue disorders that include marfanoid habitus, scoliosis, kyphosis, pectus excavatum, slipped femoral epiphysis and pes cavus (Khairi et al., 1975). This syndrome also appears to be caused by germ-line mutations in the RET proto-oncogene on chromosome 10, but these mutations affect the tyrosine kinase catalytic site of the protein (Eng et al., 1994). In children with MEN2B-associated pheochromocytomas, a higher risk of malignancy compared with MEN2A or sporadic disease is found (Ross, 2000).
von Hippel–Lindau syndrome
Another neuroectodermal syndrome commonly associated with pheochromocytoma is VHL syndrome, which is caused by mutations in chromosome 3 (3p25-26), which encodes the VHL tumor suppressor gene (Latif et al., 1993). Pheochromocytomas in VHL disease typically develop according to Knudson’s two-hit model, an inherited germ-line mutation of VHL and loss of function of the wild-type allele of the VHL gene. The disease has been divided into two types based on the significant genotype–phenotype correlations observed. Type 1 has mainly large deletions or mutations and expresses the full phenotype of vascular lesions of the retina, cysts or solid tumors in the brain or spinal cord, pancreatic cysts, renal cell carcinoma, epididymal cystadenoma and endolymphatic sac tumors, but no pheochromocytoma. Type 2 has missense mutations, pheochromocytoma and the full phenotype (Chen et al., 1995). Patients are often asymptomatic when they present with other aspects of this disease. This syndrome is quite variable in terms of the different organ systems involved and the extent of involvement from patient to patient and from family to family. Overall, less than 30% of patients with a VHL germ-line mutation develop a pheochromocytoma. Pheochromocytomas as part of the VHL syndrome have an exclusively noradrenergic phenotype reflecting the production of norepinephrine only (Eisenhofer et al., 2001b). These tumors are mainly located intra-adrenally and are bilateral in about 50% of patients, with a less than 7% incidence of metastases. They arise on the background on adrenomedullary hyperplasia. These tumors are commonly found based on periodic annual screening, or during searches for other tumors that are part of this syndrome. Therefore, when detected, these tumors are commonly small and often fail to be detected by nuclear imaging methods. Furthermore, about 80% of pheochromocytomas found in VHL patients during screening are asymptomatic and not associated with hypertension.
Neurofibromatosis type 1
von Recklinghausen’s NF is now divided into two types: NF-1 has neurofibromas of peripheral nerves, whereas NF-2 has central neurofibromas. NF-1 is inherited in an autosomal-dominant pattern. The association with pheochromocytoma is one between a relatively common disease and a rare disease. Thus, although less than 1–2% of patients with NF have pheochromocytoma, about 5% of patients with pheochromocytoma have NF (Kalff et al., 1982). Pheochromocytoma associated with NF-1 is caused by germ-line mutations in chromosome 17 (17q11), where the NF-1 gene encodes for the protein neurofibromin. These mutations lead to inactivation of this tumor suppressor gene and its protein (Colman and Wallace, 1994). Similar mutations introduced into the NF-1 gene in mice lead to pheochromocytoma, which is otherwise rare in these animals (Jacks et al., 1994). Pheochromocytoma in patients with NF-1 is rarely seen in children, because it usually occurs at a later age (around 50 years). Only 12% of NF-1 patients are diagnosed with bilateral and multi-focal pheochromocytomas, and less than 6% of patients have metastatic pheochromocytoma (Walther et al., 1999). The incidence of pheochromocytomas in NF-1 is relatively low (about 1%) compared with other hereditary syndromes, and routine screening of such patients is not generally recommended. However, if a patient with NF-1 has hypertension, then a pheochromocytoma should be considered and excluded (Kalff et al., 1982).
Succinate dehydrogenase gene family
Recently, paraganglioma and pheochromocytoma susceptibility has been associated with germ-line mutations of the SDH gene family (Baysal et al., 2000). The SDH genes (SDHA, SDHB, SDHC and SDHD) encode the four subunits of complex II of the mitochondrial electron transport chain and are also part of the Krebs cycle (Astrom et al., 2003; Gottlieb and Tomlinson, 2005), both essential for the generation of ATP. SDHB and SDHD mutations can lead to complete loss of SDH enzymatic activity, a phenomenon that has been linked to tumorigenesis through upregulation of hypoxic–angiogenetic responsive genes (Gimenez-Roqueplo et al., 2002; van Nederveen et al., 2009). Except for the SDHA gene, mutations of SDHB, SDHC and SDHD genes are associated with the presence of familial paraganglioma or pheochromocytoma. Frameshift, missense and nonsense mutations were identified for the SDH gene family. In recent studies it has been found that about 4–12% of “sporadic” pheochromocytomas and up to 50% of familial pheochromocytomas express either SDHD or SDHB mutation (Astuti et al., 2001). The SDHB, SDHC and SDHD traits are inherited in an autosomal dominant fashion and give rise to familial paraganglioma (PGL) syndromes 4, 3 and 1, respectively (Table 3). The penetrance of these traits is incomplete, however. Furthermore, SDHD-related disease is characterized by maternal genomic imprinting. Due to silencing of the maternal allele by methylation, individuals who inherit a mutation from the mother remain free of paraganglioma, but may still pass on the mutation to their offspring. Although no mutations in the SDHA have been associated with pheochromocytomas yet (most likely due to the fact that two genes encodes for SDHA), mutation of SDH5 was detected in patients with head and neck paragangliomas (Hao et al., 2009).
SDHB mutations predispose to mainly extra-adrenal pheochromocytomas with a high malignant potential, and less frequently to benign parasympathetic head and neck paragangliomas (Amar et al., 2005a; Benn et al., 2006; Brouwers et al., 2006; Havekes et al., 2007; Neumann et al., 2004; Timmers et al., 2007c). SDHD mutations are typically associated with multifocal parasympathetic head and neck paragangliomas and usually benign extra-adrenal and adrenal pheochromocytomas (Benn et al., 2006; Neumann et al., 2004). Metastastic pheochromocytoma is rare in SDHD mutation carriers, but can occur (Havekes et al., 2007; Timmers et al., 2007d). SDHC mutations are rare, and are almost exclusively associated with parasympathetic head and neck paraganglioma (Schiavi et al., 2005), although rare cases of SDHC-associated extra-adrenal pheochromocytoma have been reported (Mannelli et al., 2007; Peczkowska et al., 2008). In a recent large prospective study, Burnichon et al. (2009) supported previous findings of high frequency of SDHD and SDHC mutations in head and neck paragangliomas, as well as higher frequency of abdominal and pelvic disease and overall malignancy in SDHB mutations. We have observed the same high frequency of malignancy in our paediatric population (Pacak et al., unpublished observation). The majority of SDHB-related paragangliomas secrete either norepinephrine or both norepinephrine and dopamine (Timmers et al., 2007c), a profile that is consistent with mainly extra-adrenal tumors. Some SDHB-related PGLs exclusively overproduce dopamine, but not other catecholamines (Eisenhofer et al., 2005; Timmers et al., 2007c). Therefore, measurement of plasma levels of dopamine or its O-methylated metabolite methoxytyramine should be considered in SDHB-related pheochromocytoma. About 10% of SDHB-related sympathetic paragangliomas are “biochemically silent” (Timmers et al., 2007c; Timmers et al., 2009). Both mediastinal paragangliomas and paragangliomas of the large para-aortic paraganglion, described by Emil Zuckerkandl, usually associate with SDHx mutations (Ghayee et al., 2009; Van Nederveen et al., 2006).
Other pheochromocytomas
MEN1 (Wermer’s syndrome) consists of hyperparathyroidism, pituitary adenomas and pancreatic islet cell tumors. Pheochromocytoma is not usually part of this complex; however, the occurrence of pheochromocytoma and pancreatic islet cell tumors has been reported in some families (Carney et al., 1980). Various crossover syndromes have been reported in which pheochromocytoma has been associated with characteristics of MEN1, MEN2A, MEN2B, von Recklinghausen’s neurofibromatosis (NF), VHL and the Zollinger–Ellison syndrome (Cameron et al., 1978).
Pheochromocytoma may also occur as part of Carney’s triad (i.e. gastric leiomyosarcoma, pulmonary chondroma and extra-adrenal pheochromocytoma) (Carney, 1983). The syndrome is very rare; less than 30 cases have been reported, and only 25% of patients manifest all three parts of the triad. It occurs sporadically and is clearly non-familial (Margulies and Sheps, 1988). Recently, a new syndrome called the Stratakis–Carney syndrome, or the “dyad of gastrointestinal stromal tumor and paraganglioma” associated with SDHB, SDHC and SDHD mutations were identified (Pasini et al., 2008).
Diagnosis of pheochromocytoma
The diagnostic algorithm changes continuously in case of pheochromocytoma. For right or wrong, current diagnostic approach is not driven by high clinical suspicion, but rather by the defensive “rule out” nature of modern health-care. Massive number of patients with resistant hypertension, anxiety disorders and adrenal incidentalomas will undergo questionably indicated workup for pheochromocytoma and very few will be found to have one, decreasing the likelihood of pheochromocytoma even in a patient with a positive result. This fact, on the other hand, elevates the need for certainty of right diagnosis to considerable importance, which results in repeated biochemical tests and use of at least two imaging modalities (Ilias and Pacak, 2004; Pacak et al., 2007).
There had also been tremendous developments in diagnostic procedures. Biochemical testing is based not on the episodically secreted catecholamines, but rather on continuously produced metabolites, metanephrines, which significantly increased our ability to “catch” pheochromocytoma. Imaging studies include high-resolution anatomical techniques that enable discovery of smaller adrenal, extra-adrenal and metastatic lesions, while functional studies based on physiology of chromaffin cells enable “metabolic” localization of tumors. Single-photon emission computed tomography (SPECT) techniques together with image fusion opened a new era of diagnosis and treatment for pheochromocytoma.
Diagnostic algorithm had also become multidirectional: in some patients it starts from clinical symptomatology through biochemistry and imaging, while in others it begins by incidental discovery of adrenal mass and continues into “all-possible-adrenal-pathology-rule out” workup. Yet in another group it will be initiated by surgical discovery of adrenal or paraganglial tumor during an unrelated surgery. In such case, pathological examination will become the main diagnostic procedure, followed by metastatic workup.
Biochemical diagnosis of pheochromocytoma
Historically, biochemical testing of pheochromocytoma faced high quality expectations. It had to have high sensitivity with reasonable false-testing parameters, as well as be reliable in detecting relatively small increase in catecholamines. These expectations were driven by potential dreadfulness of misdiagnosis, as well as the fact that catecholamine secretion is mostly episodic and may not correlate with existence of hypertension. Metanephrines, on the other hand, are produced continuously within pheochromocytoma tumor cells, and independently of catecholamine release, thus obviating any need for collection of blood or urine samples during hypertensive episodes (de Jong et al., 2007; Eisenhofer et al., 1999a; Lenders et al., 1995; Lenders et al., 2002; Raber et al., 2000; Sawka et al., 2003; Unger et al., 2006; Vaclavik et al., 2007). Expert recommendations from the International Symposium on Pheochromocytoma for initial biochemical testing include measurements of fractionated metanephrines in urine or plasma, or both, as available (Grossman et al., 2006; Pacak et al., 2007). There was no consensus on whether plasma or urine measurements should be the preferred test. Both tests offer similarly high diagnostic sensitivity (provided appropriate reference ranges are used), so that a negative result for either test appears equally effective for excluding pheochromocytoma. However, because of differences in specificity, tests of plasma free metanephrines exclude pheochromocytoma in more patients without the tumor than do tests of urinary fractionated metanephrines.
The conditions under which blood or urine samples are collected can be crucial to the reliability and interpretation of test results. Blood for measurements of plasma free metanephrines or catecholamines should ideally be collected from patients supine for at least 20 minutes before sampling (Eisenhofer, 2003). The extent of the increase of a positive test result is also crucially important in judging the likelihood of a pheochromocytoma. Most patients with the tumor have increases in plasma or urinary metanephrines well in excess of those more commonly encountered as false-positive results in patients without the tumor. Increases in plasma concentrations of normetanephrine or of metanephrine four times above the upper reference limit are almost non-existing in patients without pheochromocytoma, but occur in about 70–80% of patients with the tumor (Lenders et al., 2002). Similarly, increases in urinary outputs of normetanephrine above 1500 µg/day (8.2 µmol/day) or of metanephrine above 600 µg/day (3.0 mol/day) are rare in patients without pheochromocytoma, but occur in about 70% of patients with the tumor. Dietary constituents or medications can either cause direct analytical interference in measurements of catecholamines and metabolite levels (tricyclic anti-depressants) or may influence the physiological processes that determine these levels (labetalol, buspirone, acetaminophen), as can do renal failure, etc. (Bouloux and Perrett, 1985; Eisenhofer et al., 2003c; Lenders et al., 1993;Roden et al., 2001). In rare dopamine-secreting pheochromocytomas, measurement of plasma methoxytyramine was shown to provide additional diagnostic value (Eisenhofer et al., 2005).
The provacative glucagon test is inherently dangerous and rarely used, while clonidine suppression test may be useful and bear lower risk (Bravo et al., 1979; Grossman et al., 1991; Lawrence, 1967; Lenders et al., 2010; Sheps and Maher, 1966). Clonidine (Catapres) is now the drug used most often in suppression tests to identify a pheochromocytoma (Bravo and Gifford, 1984). It is a centrally acting α2-adrenergic agonist that suppresses central sympathetic nervous outflow, and this normally results in lower levels of plasma catecholamines. Blood is drawn for plasma catecholamines and metanephrines before and 3 hours after the oral administration of clonidine 0.3 mg/70 kg body weight (to maximum of 0.5 mg). Normal plasma norepinephrine or normetanephrine levels, or their respective decrease by 50 or 40% exclude pheochromocytoma (Eisenhofer et al., 2003c).
Pheochromocytomas differ considerably in the rates of catecholamine synthesis, turnover and release, and in the types of catecholamines and metabolites produced. Adrenal pheochromocytomas may produce near exclusively norepinephrine, or both norepinephrine and epinephrine, which will show up in urine as metanephrine and normetanephrine. In contrast, extra-adrenal pheochromocytomas almost invariably produce norepinephrine and urinary normetanephrine only (Brown et al., 1972; Eisenhofer et al., 1999b; Eisenhofer et al., 2008). Because of the considerable variation in catecholamine release among patients with pheochromocytoma, plasma concentrations or urinary excretion of catecholamines are poorly correlated with tumor size (Eisenhofer et al., 1995). In contrast, because of the metabolism of catecholamines within tumors and the independence of this process on catecholamine release, urinary excretion or plasma concentrations of metanephrines show strong positive correlations with tumor size and can be useful in judging the extent and progression of disease (Eisenhofer et al., 1999a; Stenstrom and Waldenstrom, 1985). Malignant pheochromocytoma usually shows norepinephric profile, as well as high tissue, plasma and urinary levels of dopa and dopamine, the immediate precursors of norepinephrine (Goldstein et al., 1986; John et al., 1999; van der Harst et al., 2002).
Anatomical imaging
In most institutions, computed tomography (CT) of the abdomen, either with or without contrast, provides the initial method of localizing both adrenal and extra-adrenal pheochromocytoma because this imaging technique is easy, widely available and relatively inexpensive. CT can be used to localize adrenal tumors 1 cm or larger and extra-adrenal tumors 2 cm or larger (sensitivity is about 95%, but specificity is only about 70%) (Maurea et al., 1996). Pheochromocytomas will usually exhibit a density of more than 10 HU and an inhomogeneous appearance. Radiographic contrast seems to be safe in patients with pheochromocytoma and does not precipitate exaggerated release of catecholamines into circulation (Baid et al., 2009).
Magnetic resonance imaging (MRI) with or without gadolinium enhancement is a very reliable method and may identify more than 95% of tumors; it is superior to CT in detecting extra-adrenal tumors (Schmedtje et al., 1987). On MRI T1 sequences, pheochromocytoma has a signal like that of the liver, kidney and muscle, and can be differentiated with ease from adipose tissue. Chemical shift MRI characterizes adrenal masses based on the presence of fat in benign adenomas and the absence of fat in pheochromocytoma, metastases, haemorrhagic pseudocysts or malignant tumors. The hypervascularity of pheochromocytoma makes them appear characteristically bright, with a high signal on T2 sequence and no signal loss on opposed-phase images. More particularly, almost all pheochromocytomas have a more intense signal than that of the liver or muscle and often more intense than fat on T2-weighted images. However, such intense signals can be elicited by haemorrhage or haematomas, adenomas and carcinomas, so an overlap with pheochromocytoma must be considered and specific additional imaging is needed to confirm that the tumor is pheochromocytoma (Prager et al., 2002). Among the advantages of MRI imaging of pheochromocytoma are its high sensitivity in detecting adrenal disease (93–100%) and the lack of exposure to ionizing radiation (Honigschnabl et al., 2002). However, its overall sensitivity for detection of extra-adrenal, metastatic or recurrent pheochromocytoma is lower compared with that of adrenal disease (90%). Overall, the specificity of MRI is about 70% (Maurea et al., 1996). Anatomical imaging should initially focus on the abdomen and pelvis (Pacak et al., 2007).
Functional imaging
Functional imaging of pheochromocytomas made a significant leap in recent years (Havekes, 2009). These tumors “are made” to be functionally imaged through utilization of specific transporters, which enable cellular accumulation of isotope and thus, imaging of primary or metastatic pheochromocytoma cells. One can further subdivide these into specific, which are based on catecholamine transporters and vesicular monoamine transporters (VMATs), and non-specific, showing increased tissue metabolic activity or expressing somatostatin receptors. The first include 123I- or 131I-MIBG scintigraphy, 18F-fluorodopamine, 18F-dihydroxyphenylalanine (18F-dopa), 11C-hydroxyephedrine and 11C-epinephrine positron emission tomography (PET), enabled by the presence of norepinephrine transporter system on in the cellular membrane and VMATs (Hoegerle et al., 2002; Pacak et al., 2001b; Pacak et al., 2001d; Pacak et al., 2002; Shapiro et al., 2001; Shulkin et al., 1999). Others include 18F-fluorodeoxyglucose (FDG)-PET scanning (based on membrane expression of glucose transporters) or somatostatin receptor scintigraphy (Epelbaum et al., 1995). Although CT and MRI have excellent sensitivity, these anatomic imaging approaches lack the specificity required to unequivocally identify a mass as a pheochromocytoma. The higher specificity of functional imaging – the test of choice is currently 123I-MIBG scintigraphy – offers an approach to overcome the limitations of anatomic imaging (Pacak et al., 2007).
Metaiodobenzylguanidine scintigraphy
Whole-body scanning using MIBG labelled with radioiodine (123I or 131I) is a historic cornerstone of functional imaging for pheochromocytoma. Other tumors arising from neuroendocrine cells – chemodectomas, non-secreting paragangliomas, carcinoids and medullary carcinomas of the thyroid – may also take up 123I- or 131I-MIBG (von Moll et al., 1987). MIBG labelled with 131I provides negative results in over 50% of patients with proven pheochromocytomas (Havekes, 2009). Much better sensitivity is, however, available with MIBG labelled with 123I (Nakatani et al., 2002; Shapiro et al., 2001). Another advantage of 123I over 131I-labelled MIBG is its additional utility for imaging by SPECT. The agent also has a shorter half-life compared with 131I-MIBG (13 hours vs. 8.2 days), so that higher doses can be used (Shapiro et al., 2001). The sensitivity of 123I-MIBG scintigraphy is 92–98% for non-metastatic pheochromocytoma (van der Horst-Schrivers et al., 2006), but only 57–79% for metastases (Timmers et al., 2007b; van der Harst et al., 2001; van der Horst-Schrivers et al., 2006). The accumulation of MIBG can be decreased by several types of drugs: (1) agents that deplete catecholamine stores, such as sympathomimetics, reserpine and labetalol; (2) agents that inhibit cell catecholamine transporters, including cocaine and tricyclic anti-depressants and (3) other drugs such as calcium channel blockers and certain α- and β-adrenergic receptor blockers (Solanki et al., 1992). It is suggested that most of these drugs be withheld for about 2 weeks before undergoing MIBG scintigraphy. Both 123I-MIBG and 131I-MIBG require saturated solution of potassium iodine (SSKI, 100 mg twice a day for 4 or 7 days, respectively) to be used to block thyroid gland accumulation of free 123/131I. The study is relatively expensive, and the patient must usually be scanned at 24 hours and again at either 48 or 72 hours after injection of the radioisotope to determine whether images that appear on the early scan are physiological and will fade, or are tumors and will persist or increase in intensity on the later scan.
Positron emission tomography
PET imaging is done within minutes or hours after injection of short-lived positron-emitting agents. Low radiation exposure and superior spatial resolution are among the advantages of PET, whereas cost and limited availability of the radiopharmaceuticals and PET equipment (including cyclotron) still prohibit more widespread use.
Most PET radiopharmaceuticals used for the detection of pheochromocytoma enter the pheochromocytoma cell using the cell membrane norepinephrine transporter. Dopamine is a better substrate for the norepinephrine transporter than most other amines, including norepinephrine. 18F-fluorodopamine is a positron-emitting analogue of dopamine and was found to be a good substrate for both the plasma membrane and intracellular vesicular transporters in catecholamine-synthesizing cells (Goldstein et al., 1993). It is superior to 123/131I-MIBG in patients with metastatic pheochromocytoma. 11C-hydroxyephedrine and 11C-epinephrine are other PET imaging agents that have been shown to have a limited diagnostic yield because of their less than perfect sensitivity and/or specificity (Shulkin et al., 1992), while 18F-fluorodopa has good sensitivity in detecting metastatic disease and perhaps the highest yield to detect head and neck paragangliomas (Hoegerle et al., 2002; Timmers et al., 2007a).
Increased glucose metabolism characterizes various malignant tumors, and thus the uptake of glucose labelled with 18F-fluoride is useful in the imaging of these tumors. Malignant pheochromocytomas accumulate 18F-FDG more avidly compared to benign pheochromocytomas; nevertheless, FDG cannot distinguish malignant from benign disease. This radiopharmaceutical is non-specific for this tumor; however, it can be useful in those patients in whom other imaging modalities are negative, and in rapidly growing metastatic pheochromocytoma that is becoming undifferentiated, losing the property to accumulate more specific agents (Ilias and Pacak, 2004). Moreover, 18F-FDG PET is the preferred technique for the localization of SDHB-associated metastatic pheochromocytoma – the so-called “flip-flop phenomenon” (Timmers et al., 2007b). Impairment of mitochondrial function due to loss of SDHB function may cause tumor cells to shift from oxidative phosphorylation to “aerobic” glycolysis, a phenomenon known as the “Warburg effect” (Warburg, 1956). Higher glucose requirement because of a switch to less efficient pathways for cellular energy production may explain the increased 18F-FDG uptake by malignant SDHB-related pheochromocytoma.
Octreoscan
Somatostatin receptor scintigraphy using octreotide has also been used in patients with pheochromocytoma (Kaltsas et al., 2001); however, the sensitivity of this imaging modality is low, especially in the detection of solitary tumors, and this modality is inferior to MIBG scintigraphy (Kaltsas et al., 2001). However, in patients with metastatic pheochromocytoma, Octreoscan can be useful, especially in those tumors that express somatostatin receptors and are negative on MIBG scintigraphy and 18F-fluorodopamine-PET (Ilias and Pacak, 2004). Octreoscan can also prove to be a sensitive imaging modality in head and neck paragangliomas but larger studies are needed.
Treatment of pheochromocytoma
The best therapeutic modus operandi for a pheochromocytoma, as with most of other neuroendocrine tumors, is finding an experienced operator (surgeon) (Manger and Gifford, 1977, 1996). In the perfect world, a team consisting of an internist, an anaesthesiologist and a surgeon will medically prepare the patient for a safe surgery, maintain blood pressure within normal values during the surgery and safely withdraw from therapy after the surgery. In the same perfect world, the job of the endocrinologist will be to diagnose pheochromocytoma, localize it, design and execute efficient preoperative therapy for at least 2 weeks prior to surgery and follow up the patient after successful operation. In reality, as usually is the case, the situation is complicated by the fact that many patients have insidious clinical course and will have end organ damage or metastatic disease by the time of diagnosis. In those patients, medical therapy will be chronic, which requires appreciation of possible adverse effects and compliance. On the other hand, some of the tumors will be found during unrelated surgeries and precipitated by manipulation around or at the unsuspected tumor. These tumors usually present with severe intra-operative pheochromocytoma crisis and are extremely hard to manage because of tremendous amount of catecholamines released to the circulation (Mannelli, 2006; Pacak, 2007).
Medical therapy and preparation for surgery
α-Adrenoceptor blockers
Phenoxybenzamine (Dibenzyline; irreversible non-competitive α-adrenoceptor blocker) is most commonly used for preoperative management. The initial dose of long-acting phenoxybenzamine is usually 10 mg twice a day (usual total daily dose is 1 mg/kg); this is increased until the clinical manifestations are controlled, patient becomes normotensive or side effects appear (postural hypertension). Some patients may require much larger doses, and the dosage may be increased in increments of 10–20 mg every 2–3 days. Other α-blocking agents also of use are prazosin (Minipress), terazosin (Hytrin) and doxazosin (Cardura) (Nicholson et al., 1983). All three are specific, competitive and therefore short-acting α1-adrenergic antagonists, and all three have the potential for severe postural hypotension immediately after the first dose. Labetalol (Normodyne or Trandate), a drug with both α- and β-antagonistic activities, may also be used in a dosage of 200–600 mg twice daily (Van Stratum et al., 1983). The advantages of labetalol are that an α-blocker and a β-blocker are given simultaneously, and both oral and intravenous (IV) formulations of the drug are readily available. However, with labetalol, one is forced to use a fixed ratio of α- to β-antagonistic activity (i.e. 1:4 or 1:6), while the desired ratio is about 4:1 or more, which means that an efficient anti-hypertensive dose will almost certainly associate with bradycardia. In some patients it may also cause hypertension (perhaps by its greater effect on β-adrenoceptors than α-adrenoceptors) (Briggs et al., 1978). Pre- or postoperative postural hypotension can be treated with normal or high-salt diet to restore volume depletion.
Others
In patients with clinical manifestations caused by β-adrenoceptor stimulation (e.g. tachycardia or arrhythmias, angina, or nervousness), β-adrenergic receptor blockers such as propranolol, atenolol or metoprolol are indicated. A β-blocking agent should never be used in the absence of an efficient α-blockade because of loss of β-adrenoceptor-mediated vasodilatation, exacerbation of epinephrine-induced vasoconstriction and a resultant serious and life-threatening elevation of blood pressure – an unopposed β-blockade (Eisenhofer et al., 2007). We had previously also suggested that in rare cases a phenomenon of unopposed α-blockade can occur (Kantorovich and Pacak, 2005).
Metyrosine (α-methyl-l-tyrosine) competitively inhibits TH, the rate-limiting step in catecholamine biosynthesis and significantly but not completely depletes catecholamine stores, making pre- and in-surgical blood pressure control easier (Sjoerdsma et al., 1965). Treatment is started at a dosage of 250 mg orally every 6–8 hours, and thereafter the dose is increased by 250–500 mg every 2–3 days or as necessary up to a total dose of 1.5–4.0 g/day. The drug readily crosses the blood–brain barrier and often causes sedation, depression, anxiety, galactorrhea and rarely causes extrapyramidal signs (e.g. parkinsonism) in older patients. Various calcium channel blockers have also been used to control blood pressure both before and during surgery (Colson and Ribstein, 1991). While both α- and β-adrenoceptor blockers may elevate plasma-free normetanephrine levels (Eisenhofer et al., 2003c), calcium channel blockers do not affect plasma metanephrine levels.
Hypertensive crisis
Hypertensive crises can manifest as severe headache, visual disturbances, acute myocardial infarction, congestive heart failure or cerebrovascular accident. It is a true medical emergency and should be treated as such in preferable setting of the intensive care unit that allows continuous monitoring of the patient’s haemodynamic parameters. Phentolamine (Regitine) is one of the choice medications, used as repeated (every 2 minutes) intravenous 5 mg boluses or as a continuous infusion (100 mg of phentolamine in 500 ml of 5% dextrose in water). Alternatively, nifedipine (10 mg orally or sublingually) can also be used to control hypertension, while labetalol should be used with caution (see above). Some drugs (e.g. tricyclic anti-depressants, metoclopramide and naloxone) can cause hypertensive crisis in patients with pheochromocytoma.
Surgery
For most abdominal pheochromocytomas smaller than 6 cm, laparoscopy has replaced laparotomy as the procedure of choice because of significant postoperative benefits (Vargas et al., 1997; Winfield et al., 1998). The operative mortality at the Mayo Clinic from 1980 to 1986 was 1.3%, or 1 in 77 patients (Sheps et al., 1990). The long-term survival of patients after successful removal of a benign pheochromocytoma is essentially the same as that of age-adjusted normals and with improvement in current technique and multi-disciplinary approach, the procedure seems to be relatively safe (Stenstrom et al., 1988).
Postoperative management
Acute withdrawal of hypercatecholaminaemia results in postoperative hypotension, which can be significant especially if both metyrosine and phenoxybenzamine were used preoperatively, because of catecholamine synthesis inhibition by first and long-term blockade by second. Volume replacement is the treatment of choice and the volume of fluid required is often large (0.5–1.5 times the patient’s total blood volume) during the first 24–48 hours after removal of the tumor and lower volumes thereafter (i.e. 125 ml/hour). Postoperative hypertension may be related to pain, volume overload, autonomic instability, essential hypertension or residual tumor. Repeat measurements should be made several weeks after surgery and later follow up yearly, for a total of 5 years, if the patient remains asymptomatic or at any time if symptoms reappear. At least 25% of patients remain hypertensive, but this is usually easily controlled with medication (Amar et al., 2005b; Young and Landsberg, 1998).
Malignant pheochromocytoma
Malignant pheochromocytoma is established by the presence of metastases at the sites where chromaffin cells are normally absent (Linnoila et al., 1990). Pheochromocytoma metastasizes via haematogenous or lymphatic pathways, and the most common metastatic sites are lymph nodes, bone, lung and liver (Bravo, 1994; Glodny et al., 2001; Goldstein et al., 1999; Kopf et al., 2001; Schlumberger et al., 1992). Among all pheochromocytomas, the frequency of malignant pheochromocytomas ranges from 1 to 90% (Amar et al., 2005a; Benn et al., 2006; Brouwers et al., 2006; Glodny et al., 2001; Timmers et al., 2007c; Timmers et al., 2009). It is recognized that one half of malignant tumors present are found at the initial presentation, whereas the second half develop at a median interval of 5.6 years (Mornex et al., 1992). The prevalence of underlying SDHB mutations among patients with malignant pheochromocytoma is 30%, and even higher (48%) if the primary tumor originates from an extra-adrenal abdominal location (Brouwers et al., 2006). Two groups of patients can be distinguished based on the location of metastatic lesions. The first group represents short-term survivors with the presence of metastatic lesions, especially in liver and lungs. Their survival is usually less than 2 years. The second group represents long-term survivors with the presence of bone metastatic lesions. Patients in this group can survive more than 20 years after the initial diagnosis. The overall 5-year survival rate varies between 34 and 60% (John et al., 1999; Mundschenk and Lehnert, 1998). The survival of patients with metastatic disease due to an underlying SDHB mutation is lower in non-SDHB patients (Amar et al., 2007). We have recently found an unexpected high frequency of SDHB mutation in paediatric malignant pheochromocytomas and paragangliomas (Pacak et al., unpublished observation). Recent advances in biochemical testing and nuclear imaging techniques, as discussed previously, have greatly improved our ability to diagnose and localize malignant pheochromocytoma at much earlier stages.
Clinical manifestations of malignant pheochromocytoma are similar to that of its benign counterpart, and patients may have minimal symptoms or symptoms caused by local invasion despite marked hypercatecholaminaemia (Bravo and Gifford, 1984; Glodny et al., 2001; Goldstein et al., 1999; Kopf et al., 2001; Mornex et al., 1992; Schlumberger et al., 1992; Timmers et al., 2007c). Similar to benign pheochromocytomas, malignant pheochromocytomas predominantly secrete norepinephrine (Eisenhofer et al., 2001a; Schlumberger et al., 1992; Stumvoll et al., 1997). However, larger tumor burden associates with higher levels of plasma and urinary metanephrines (Goldstein et al., 1999; Mundschenk and Lehnert, 1998). Immaturity and dedifferentiation of malignant tissue associate with increased dopamine excretion because of an intraneuronal loss of dopamine-β-hydroxylase (Eisenhofer et al., 2001a; John et al., 1999; Mornex et al., 1992; Schlumberger et al., 1992), while normal epinephrine concentrations, together with that of excessive norepinephrine levels result from the tumor’s inability to N-methylate (Stumvoll et al., 1997). No clinical or pathological features reliably predict malignancy, although young age, extra-adrenal tumor location, large tumor size, adrenal pheochromocytomas that fail to take up MIBG and persistent postoperative arterial hypertension have all been associated with an increased likelihood of malignancy (Elder et al., 2003; Glodny et al., 2001; Goldstein et al., 1999; John et al., 1999; Mornex et al., 1992; Rao et al., 2000; Schlumberger et al., 1992; Stumvoll et al., 1997; Yon et al., 2003).
Successful management of malignant pheochromocytoma requires a multi-disciplinary approach (Scholz et al., 2007). The treatment regimen should be individualized to meet the goal of controlling endocrine activity, decreasing tumor burden, and alleviating local symptoms. Pharmacological treatment of malignant pheochromocytoma does not differ from benign disease. However, for patients with multiple metastatic lesions, radical surgical resection is often impossible and at present it is not clear whether it would prolong patients’ survival. However, debulking often results in smaller tumor burden that may respond much better to radio- and chemotherapy, and a significant decrease in catecholamine levels, reflecting improvement of many symptoms and signs.
The first-line systemic treatment for malignant pheochromocytoma is targeted radiotherapy using 131I-MIBG. 131I-MIBG therapy is used in MIBG-positive tumors, especially those that are unresectable as single or multiple doses. The procedure is well tolerated, with minimal toxicity that includes nausea; mild bone marrow suppression, especially thrombocytopenia; mildly elevated liver enzymes; and some renal toxicity. Overall, about one third of patients show partial response (less than 50% reduction of tumor mass) and improvement in symptoms and signs (Loh et al., 1997). Radiotherapy using radiolabelled somatostatin analogues such as [111In]-pentetreotide appears to be largely ineffective in metastatic pheochromocytoma (Lamarre-Cliche et al., 2002), while the use of [90Y-DOTA]-D-Phe1-Tyr3-octreotide needs further investigation (Forster et al., 2001).
In rapidly progressive metastatic pheochromocytoma, chemotherapy rather than MIBG therapy is recommended. A combination of cyclophosphamide, vincristine and dacarbazine showed a 57% complete or partial tumor response, while 79% had either a complete or partial biochemical response (Averbuch et al., 1988). Patients with large tumor burdens may present with massive release of catecholamines – “catecholamine storm” – within the first few hours after administration of the first course of chemotherapy (Quezado et al., 1992).
External beam radiation is used for palliation of chronic pain and symptoms of local compression arising from these tumors (Siddiqui et al., 1988). However, no systemic effects on tumor burden or hormone levels were observed. Successful infarction of pheochromocytoma by embolization has been demonstrated in individual case reports (Takahashi et al., 1999). Recently, radiofrequency ablation, cryotherapy, percutaneous microwave coagulation, embolization, as well as use of anti-angiogenesis medications have been used for the treatment of malignant pheochromocytoma (Pacak et al., 2001c).
Thoughts about future
In general
The future of pheochromocytoma-related science lies in part in its past, while understanding of its pathology changes with better understanding of the physiology of the sympathoadrenal system. It is usually seen as the “emergency response” – fight or flight system, but on the other side paraganglia is an organic part of peripheral nervous system, while norepinephrine is a major central and peripheral neurotransmitter. From this point of view, it seems quite artificial to see pheochromocytoma as just an isolated endocrine pathology. Just by itself, adrenal medulla together with paraganglia represents a truly diffuse neuroendocrine organ. One can suggest that direct secretion of catecholamines into circulation by medulla as opposed to local uptake-based neuronal transmission of paraganglia, together with rapid metabolism, to assure that there will be no significant spillover, subdivides them into functionally different and somewhat functionally unrelated organs. Difference in enzymatic milieu with noradrenergic secretory pattern of paraganglia versus adrenergic/noradrenergic profile of medulla could further support this notion. Development of paragangliomas in chronic hypoxic conditions supports the thought that in addition to peripheral neurotransmission paraganglia still has a “side job” of oxygen sensing. This is especially true for parasympathetic paraganglia, the carotid body, in particular. These paraganglia were thought to predominantly secrete acetylcholine and not catecholamines, but we know now that they do contain catecholamine secretory granules and are capable of secretion, as well as paraganglioma formation.
On genetic testing
The Shakespearian question on whenever “to do or not to do” the genetic testing for patients with pheochromocytomas and paragangliomas hangs as Damocles’ sword above the provider’s head, changing shape and size with every paper on frequency and malignant potential of these tumors. The condition is not as clear as with MEN2, where there is a well-documented correlation between genotype and phenotype, as well as age-related morbidity. In VHL, 98% of pheochromocytomas associate with missense rather than other type of gene mutation, while mutations at codons 634 (2A) and 918 (2B) are mostly “pheochromocytomagenic” in MEN2. Numerous views and opinions have been published lately, but there is still no clear-cut agreement on this issue (Cascon et al., 2009; Gimenez-Roqueplo et al., 2006; Gimenez-Roqueplo et al., 2008; Pigny et al., 2009). Clinical observations and history as well as biochemical phenotype can help guide the necessity of genetic test to some extent. VHL tumors have noradrenergic phenotype, while pheochromocytomas of MEN2 usually have an adrenergic biochemical profile. Thus, we strongly suggest that appropriate genetic testing be done based on clinical presentation, localization of pheochromocytoma, as well as biochemical phenotype. In patients with metastatic pheochromocytoma with primary extra-adrenal tumor in abdomen physicians should think about SDHB gene testing first, while head and neck paraganglioma will be more suggestive of SDHD or SDHC mutations (Amar et al., 2007; Benn et al., 2006; Brouwers et al., 2006; Neumann et al., 2004). Paediatric cases are far more concerning. Recent description of malignant paragangliomas presenting at relatively early age suggest the need for more aggressive approach (Havekes et al., 2008; Prodanov et al., 2009). Our recent unpublished observations of high rate of malignancy in paediatric paraganglioma cases further support this notion. Unless larger studies come up with well-structured guidelines, all paediatric patients without family history of these tumors should be tested for SDHB/D gene mutations.
On being nervous
Let’s go back to the anxious personality. Although acute stress will not result in sustained elevation of catecholamine levels and end-organ damage but severe or prolonged stress might do so. We had recently seen a patient with adrenal incidentaloma and prominent noradrenergic hypercatecholaminaemia (Kantorovich et al., presented at the Endocrine Society Meeting, 2009). This patient was later found to have malignant thymoma-associated paraneoplastic Morvan’s syndrome, which associates, among other features, with overactivity of sympathetic nervous system. Another phenomenon worth attention is catecholamine-induced cardiomyopathy. Traditionally, described as hypertrophic or dilated, it lately expanded to mysterious “broken heart” and Japanese octopus trap-type (tako-tsubo) cardiomyopathies. With no additional pathogenic causative mechanisms other than previously suggested coronary vasospasm or abnormalities in myocardial microvasculature as a result of high catecholamine levels, these clinical entities are widely reported in association with and without pheochromocytomas as classic apical or inverted forms (Bielecka-Dabrowa et al., 2010; de Souza et al., 2008; Kim et al., 2007, Madhavan et al., 2009; Sanchez-Recalde et al., 2006). Of great interest in this context is a recent paper showing that pheochromocytoma-conditioned growth media causes significantly more cardiomyocyte damage then norepinephrine only, suggesting existence of additional factors, secreted by the tumor (Mobine et al., 2009). Our feeling is that in the condition of any severe hypercatecholaminaemia – stress- or pheochromocytoma-related, there are several factors that will predict the extension of cardiac damage – the rapidity and level of catecholamine rise, the known ability of cardiomyocyte adrenergic receptor desensitization and the individual distribution and amount of epicardial sympathetic innervation and endo-/myocardial adrenergic receptors density. This feeling is strongly supported by our recent observation of lack of cardiac abnormalities seen on high-resolution cardiac MRI in series of patients with long-standing pheochromocytomas (Pacak et al., unpublished observations).
On tumorigenesis
Familial pheochromocytoma–paraganglioma syndromes widened our understanding of tumorigenesis significantly, although not without some additional confusion. Currently, two major and seemingly independent, but probably somewhat cross-talking pathways result in pheochromocytoma formation. The first takes place in the neural crest-derived neuroendocrine cells (medullary thyroid cells, parathyroid chief cells, adrenal chromaffin cells, etc.) and results in multiple tumors, related to this embryological system. It is represented by RET and NF1 gene mutations and displays abnormalities in cell cycle and differentiation, still containing numerous spots of “terra incognita”. The second, functional rather than embryonic, shows an avalanche of newly discovered data and relates to hypoxia-driven pathway of tumorigenesis. It started with VHL syndrome, but expanded over recent years into a solid system that significantly extended our understanding of cellular metabolism. Pseudohypoxic conditions of VHL-related mutation in HIF (hypoxia-inducible factor) support both the oxygen sensing function of sympatho-adrenal system and tumorigenic capabilities of “hypoxia”. But it not just a scavenging VHL protein, which when mutated, precludes ubiquitination and further degradation of HIF-1α, which dimerizes with HIF-1β to become a potent hypoxia-driven transcription factor, resulting in pheochromocytoma or paraganglioma. It is even not just HIF-1α anymore. It can be any of oxygen-sensing α units – HIF-1α or HIF-2α – and the whole pathway seems to contain a growing number of spots, susceptible to mutations and associated with similar functional and pathogenic outcome, as well as syndromatic presentation (Ladroue et al., 2008). Addition of SDH system to this pathway is both logical and confusing. Mutation of mitochondrial protein involved in electron transfer would clearly associate with abnormal oxygen sensing and would be expected to induce hypoxic drive, but SDH mutations are also associated with accumulation of succinate through the system’s involvement in Krebs cycle, which generates independent metabolic stress, capable of HIF induction. Another SDH-related confusion is caused by heterogenicity of clinical pictures between different subunits mutations. Of these, SDHB stands alone as the most malignant of all pheochromocytoma–paraganglioma-related conditions. Malignant disease is found in a relatively young age. This brings us back to embryological phenomenon of paraganglia being the leading part of the system at antepartum with relative involution in postpartum period. It is not clear if this malignant phenotype is part of reversal to embryological phenotype or just a dedifferentiation. The last would be more reasonable if one adds the fact that SDHB tumors are more glucose-avid on PET images compared to other pheochromocytomas/paragangliomas. One would also suspect that “Warburg effect” in these tumors is a clear advantageous phenomenon of aggressive malignancy rather than a compensatory manoeuvre of the diseased mitochondria. While one would expect SDH-complex abnormalities to generate potentially more intracellular hypoxia and probably induce higher Warburg effect, recent studies showed that VHL but not SDH-related mutations drive Warburg effect in inherited pheochromocytomas (Favier et al., 2009; Kaelin, 2009). Interesting are findings of association of pheochromocytoma/paraganglioma with abnormalities in p53, which has been previously reported to play a significant role in the generation of the “Warburg effect” (Ma et al., 2007).
On being a part of DNES
There is a definite lack of clear characterization of the last being a part of the DNES. With the unfortunate departure of the amine precursor uptake and dercarboxylation (APUD) system (Andrew et al., 1998), there seems to be no effort to generate a replacement that would be accepted by the field. Neuroendocrine cell can take up any amine precursor and with appropriate the enzymatic machinery it can produce any neuroendocrine product – amine, peptide, etc. It is well known that carcinoids can produce catecholamines. While this fact does not make carcinoid a pheochromocytoma, the functional usefulness of the APUD classification is hard not to miss. They both possess similar uptake, synthetic (amines, peptides, chromogranin) abilities, secretory granules, somatostatin receptors and catecholamine uptake transporters. They definitely differ embryologically with pheochromocytomas and paragangliomas being derivatives of the neural crest (ectoderm), while carcinoids coming from endoderm. Using the term of DNES with or without stressing their functional similarities rather than embryonic differences may ease the pain of artificial division of the field, while it can definitely gain if intellectual forces would join together in the search for better imaging and treatment, which does have some similarities in both cases.
In summary
There are more things to expect from the field in the future. Our understanding of the basic mechanisms of cell function with regard to differential expression of catecholamine transporters on the cell surface, as well as different VMATs in the membrane of the vesicle will allow development of new imaging and treatment modalities in the future, and also the wide use of the latest molecular techniques – as in the case of proteomics approach in the discovery of SDH5 mutation. Better understanding of molecular pathology of SDH mutations will widen our understanding of pathogenesis and better screening and treatment of the disease.
Acknowledgments
The authors would like to express their deep gratitude to Ms. Kathryn S. King for her tireless assistance in the preparation of this chapter.
References
- Alezais H, Peyron A. Un groupe nouveau de tumeurs epitheliales: les paraganglions. Comptes Rendus des Séances et Mémoires de la Société de Biologie (Paris) 1908;65:745–747. [Google Scholar]
- Amar L, Baudin E, Burnichon N, Peyrard S, Silvera S, Bertherat J, et al. Succinate dehydrogenase B gene mutations predict survival in patients with malignant pheochromocytomas or paragangliomas. The Journal of Clinical Endocrinology & Metabolism. 2007;92:3822–3828. doi: 10.1210/jc.2007-0709. [DOI] [PubMed] [Google Scholar]
- Amar L, Bertherat J, Baudin E, Ajzenberg C, Bressac-De Paillerets B, Chabre O, et al. Genetic testing in pheochromocytoma or functional paraganglioma. Journal of Clinical Oncology. 2005a;23:8812–8818. doi: 10.1200/JCO.2005.03.1484. [DOI] [PubMed] [Google Scholar]
- Amar L, Servais A, Gimenez-Roqueplo AP, Zinzindohoue F, Chatellier G, Plouin PF. Year of diagnosis, features at presentation, and risk of recurrence in patients with pheochromocytoma or secreting paraganglioma. The Journal of Clinical Endocrinology & Metabolism. 2005b;90:2110–2116. doi: 10.1210/jc.2004-1398. [DOI] [PubMed] [Google Scholar]
- Andrew A, Kramer B, Rawdon BB. The origin of gut and pancreatic neuroendocrine (APUD) cells – the last word? Journal of Pathology. 1998;186:117–118. doi: 10.1002/(SICI)1096-9896(1998100)186:2<117::AID-PATH152>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Astrom K, Cohen JE, Willett-Brozick JE, Aston CE, Baysal BE. Altitude is a phenotypic modifier in hereditary paraganglioma type 1: Evidence for an oxygen-sensing defect. Human Genetics. 2003;113:228–237. doi: 10.1007/s00439-003-0969-6. [DOI] [PubMed] [Google Scholar]
- Astuti D, Latif F, Dallol A, Dahia PL, Douglas F, George E, et al. Gene mutations in the succinate dehydrogenase subunit SDHB cause susceptibility to familial pheochromocytoma and to familial paraganglioma. The American Journal of Human Genetics. 2001;69:49–54. doi: 10.1086/321282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averbuch SD, Steakley CS, Young RC, Gelmann EP, Goldstein DS, Stull R, et al. Malignant pheochromocytoma: Effective treatment with a combination of cyclophosphamide, vincristine, and dacarbazine. Annals of Internal Medicine. 1988;109:267–273. doi: 10.7326/0003-4819-109-4-267. [DOI] [PubMed] [Google Scholar]
- Axelrod J. Methylation reactions in the formation and metabolism of catecholamines and other biogenic amines. Pharmacological Reviews. 1966;18:95–113. [PubMed] [Google Scholar]
- Baid SK, Lai EW, Wesley RA, Ling A, Timmers HJ, Adams KT, et al. Brief communication: Radiographic contrast infusion and catecholamine release in patients with pheochromocytoma. Annals of Internal Medicine. 2009;150:27–32. doi: 10.7326/0003-4819-150-1-200901060-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barancik M. Inadvertent diagnosis of pheochromocytoma after endoscopic premedication. Digestive Diseases and Sciences. 1989;34:136–138. doi: 10.1007/BF01536169. [DOI] [PubMed] [Google Scholar]
- Barontini M, Levin G, Sanso G. Characteristics of pheochromocytoma in a 4- to 20-year-old population. Annals of the New York Academy of Sciences. 2006;1073:30–37. doi: 10.1196/annals.1353.003. [DOI] [PubMed] [Google Scholar]
- Bauer FE, Hacker GW, Terenghi G, Adrian TE, Polak JM, Bloom SR. Localization and molecular forms of galanin in human adrenals: Elevated levels in pheochromocytomas. The Journal of Clinical Endocrinology & Metabolism. 1986;63:1372–1378. doi: 10.1210/jcem-63-6-1372. [DOI] [PubMed] [Google Scholar]
- Baysal BE, Ferrell RE, Willett-Brozick JE, Lawrence EC, Myssiorek D, Bosch A, et al. Mutations in SDHD, a mitochondrial complex II gene, in hereditary paraganglioma. Science. 2000;287:848–851. doi: 10.1126/science.287.5454.848. [DOI] [PubMed] [Google Scholar]
- Benn DE, Gimenez-Roqueplo AP, Reilly JR, Bertherat J, Burgess J, Byth K, et al. Clinical presentation and penetrance of pheochromocytoma/paraganglioma syndromes. The Journal of Clinical Endocrinology & Metabolism. 2006;91:827–836. doi: 10.1210/jc.2005-1862. [DOI] [PubMed] [Google Scholar]
- Bielecka-Dabrowa A, Mikhailidis DP, Hannam S, Rysz J, Michalska M, Akashi YJ, et al. Takotsubo cardiomyopathy – the current state of knowledge. International Journal of Cardiology. 2010 doi: 10.1016/j.ijcard.2009.11.040. (in press) [DOI] [PubMed] [Google Scholar]
- Bittar DA. Innovar-induced hypertensive crises in patients with pheochromocytoma. Anesthesiology. 1979;50:366–369. doi: 10.1097/00000542-197904000-00019. [DOI] [PubMed] [Google Scholar]
- Bornemann M, Hill SC, Kidd GS., II Lactic acidosis in pheochromocytoma. Annals of Internal Medicine. 1986;105:880–882. doi: 10.7326/0003-4819-105-6-880. [DOI] [PubMed] [Google Scholar]
- Bouloux PG, Fakeeh M. Investigation of phaeochromocytoma. Clinical Endocrinology (Oxford) 1995;43:657–664. doi: 10.1111/j.1365-2265.1995.tb00531.x. [DOI] [PubMed] [Google Scholar]
- Bouloux PM, Perrett D. Interference of labetalol metabolites in the determination of plasma catecholamines by HPLC with electrochemical detection. Clinica Chimica Acta. 1985;150:111–117. doi: 10.1016/0009-8981(85)90261-x. [DOI] [PubMed] [Google Scholar]
- Brandi ML, Gagel RF, Angeli A, Bilezikian JP, Beck-Peccoz P, Bordi C, et al. Guidelines for diagnosis and therapy of MEN type 1 and type 2. The Journal of Clinical Endocrinology & Metabolism. 2001;86:5658–5671. doi: 10.1210/jcem.86.12.8070. [DOI] [PubMed] [Google Scholar]
- Bravo E. Evolving concepts in the pathophysiology, diagnosis, and treatment of pheochromocytoma. Endocrine Reviews. 1994;15:356–368. doi: 10.1210/edrv-15-3-356. [DOI] [PubMed] [Google Scholar]
- Bravo EL, Gifford RW., Jr Current concepts. Pheochromocytoma: Diagnosis, localization and management. The New England Journal of Medicine. 1984;311:1298–1303. doi: 10.1056/NEJM198411153112007. [DOI] [PubMed] [Google Scholar]
- Bravo EL, Gifford RW., Jr Pheochromocytoma. Endocrinology Metabolism Clinics of North America. 1993;22:329–341. [PubMed] [Google Scholar]
- Bravo EL, Tagle R. Pheochromocytoma: State-of-the-art and future prospects. Endocrine Reviews. 2003;24:539–553. doi: 10.1210/er.2002-0013. [DOI] [PubMed] [Google Scholar]
- Bravo EL, Tarazi RC, Fouad FM, Vidt DG, Gifford RW., Jr Clonidine-suppression test: A useful aid in the diagnosis of pheochromocytoma. The New England Journal of Medicine. 1981;305:623–626. doi: 10.1056/NEJM198109103051107. [DOI] [PubMed] [Google Scholar]
- Bravo EL, Tarazi RC, Gifford RW, Stewart BH. Circulating and urinary catecholamines in pheochromocytoma. Diagnostic and pathophysiologic implications. The New England Journal of Medicine. 1979;301:682–686. doi: 10.1056/NEJM197909273011302. [DOI] [PubMed] [Google Scholar]
- Briggs RS, Birtwell AJ, Pohl JE. Hypertensive response to labetalol in phaeochromocytoma. Lancet. 1978;1:1045–1046. doi: 10.1016/s0140-6736(78)90772-9. [DOI] [PubMed] [Google Scholar]
- Brouwers FM, Eisenhofer G, Tao JJ, Kant JA, Adams KT, Linehan WM, et al. High frequency of SDHB germline mutations in patients with malignant catecholamine-producing paragangliomas: Implications for genetic testing. The Journal of Clinical Endocrinology & Metabolism. 2006;91:4505–4509. doi: 10.1210/jc.2006-0423. [DOI] [PubMed] [Google Scholar]
- Brouwers FM, Lenders JW, Eisenhofer G, Pacak K. Pheochromocytoma as an endocrine emergency. Reviews in Endocrine & Metabolic Disorders. 2003;4:121–128. doi: 10.1023/a:1022981801344. [DOI] [PubMed] [Google Scholar]
- Brown WJ, Barajas L, Waisman J, De Quattro V. Ultrastructural and biochemical correlates of adrenal and extra-adrenal pheochromocytoma. Cancer. 1972;29:744–759. doi: 10.1002/1097-0142(197203)29:3<744::aid-cncr2820290331>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Burnichon N, Rohmer V, Amar L, Herman P, Leboulleux S, Darrouzet V, et al. The succinate dehydrogenase genetic testing in a large prospective series of patients with paragangliomas. The Journal of Clinical Endocrinology & Metabolism. 2009;94:2817–2827. doi: 10.1210/jc.2008-2504. [DOI] [PubMed] [Google Scholar]
- Cameron D, Spiro HM, Landsberg L. Zollingerellison syndrome with multiple endocrine adenomatosis type II. The New England Journal of Medicine. 1978;299:152–153. doi: 10.1056/NEJM197807202990315. [DOI] [PubMed] [Google Scholar]
- Carney JA. The triad of gastric epithelioid leiomyosarcoma, pulmonary chondroma, and functioning extra-adrenal paraganglioma: A five-year review. Medicine (Baltimore) 1983;62:159–169. doi: 10.1097/00005792-198305000-00003. [DOI] [PubMed] [Google Scholar]
- Carney JA, Go VL, Gordon H, Northcutt RC, Pearse AG, Sheps SG. Familial pheochromocytoma and islet cell tumor of the pancreas. American Journal of Medicine. 1980;68:515–521. doi: 10.1016/0002-9343(80)90295-8. [DOI] [PubMed] [Google Scholar]
- Casanova S, Rosenberg-Bourgin M, Farkas D, Calmettes C, Feingold N, Heshmati H, et al. Phaeochromocytoma in multiple endocrine neoplasia type 2a: Survey of 100 cases. Clinical Endocrinology. 1993;38:531–537. doi: 10.1111/j.1365-2265.1993.tb00350.x. [DOI] [PubMed] [Google Scholar]
- Cascon A, Lopez-Jimenez E, Landa I, Leskela S, Leandro-Garcia LJ, Maliszewska A, et al. Rationalization of genetic testing in patients with apparently sporadic pheochromocytoma/paraganglioma. Hormone and Metabolic Research. 2009;41:672–675. doi: 10.1055/s-0029-1202814. [DOI] [PubMed] [Google Scholar]
- Caty MG, Coran AG, Geagen M, Thompson NW. Current diagnosis and treatment of pheochromocytoma in children: Experience with 22 consecutive tumors in 14 patients. Archives of Surgery. 1990;125:978–981. doi: 10.1001/archsurg.1990.01410200036004. [DOI] [PubMed] [Google Scholar]
- Chen F, Kishida T, Yao M, Hustad T, Glavac D, Dean M, et al. Germline mutations in the von Hippel–Lindau disease tumor suppressor gene: Correlation with phenotype. Human Mutation. 1995;5:66–75. doi: 10.1002/humu.1380050109. [DOI] [PubMed] [Google Scholar]
- Colman SD, Wallace MR. Neurofibromatosis type 1. European Journal of Cancer. 1994;30A:1974–1981. doi: 10.1016/0959-8049(94)00389-m. [DOI] [PubMed] [Google Scholar]
- Colson P, Ribstein J. Simplified strategy for anesthesia of pheochromocytoma. Annales Françaises d’Anesthésie et de Réanimation. 1991;10:456–462. doi: 10.1016/s0750-7658(05)80849-4. [DOI] [PubMed] [Google Scholar]
- Colwell JA. Inhibition of insulin secretion by catecholamines in pheochromocytoma. Annals of Internal Medicine. 1969;71:251–256. doi: 10.7326/0003-4819-71-2-251. [DOI] [PubMed] [Google Scholar]
- Cook RF, Katritsis D. Hypertensive crisis precipitated by a monoamine oxidase inhibitor in a patient with phaeochromocytoma. British Medical Journal. 1990;300:614. doi: 10.1136/bmj.300.6724.614-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutant R, Pein F, Adamsbaum C, Oberlin O, Dubousset J, Guinebretiere JM, et al. Prognosis of children with malignant pheochromocytoma: Report of 2 cases and review of the literature. Hormone Research. 1999;52:145–149. doi: 10.1159/000023451. [DOI] [PubMed] [Google Scholar]
- De Jong WH, Graham KS, Van Der Molen JC, Links TP, Morris MR, Ross HA, et al. Plasma free metanephrine measurement using automated online solid-phase extraction HPLC tandem mass spectrometry. Clinical Chemistry. 2007;53:1684–1693. doi: 10.1373/clinchem.2007.087114. [DOI] [PubMed] [Google Scholar]
- De Krijger RR, Van Nederveen FH, Korpershoek E, De Herder WW, De Muinck Keizer-Schrama SM, Dinjens WN. Frequent genetic changes in childhood pheochromocytomas. Annals of the New York Academy of Sciences. 2006;1073:166–176. doi: 10.1196/annals.1353.017. [DOI] [PubMed] [Google Scholar]
- De Souza F, Altenburg Odebrecht Curi Gismondi R, Henriques Cunha Neto S, De Mattos MA. Tako-tsubo-like cardiomyopathy and extra-adrenal pheochromocytoma: Case report and literature review. Clinical Research in Cardiology. 2008;97:397–401. doi: 10.1007/s00392-007-0638-1. [DOI] [PubMed] [Google Scholar]
- Edelman ER, Stuenkel CA, Rutherford JD, Williams GH. Diabetic ketoacidosis associated with pheochromocytoma. Cleveland Clinic Journal of Medicine. 1992;59:423–427. doi: 10.3949/ccjm.59.4.423. [DOI] [PubMed] [Google Scholar]
- Eisenhofer G. The role of neuronal and extraneuronal plasma membrane transporters in the inactivation of peripheral catecholamines. Pharmacology & Therapeutics. 2001;91:35–62. doi: 10.1016/s0163-7258(01)00144-9. [DOI] [PubMed] [Google Scholar]
- Eisenhofer G. Editorial: Biochemical diagnosis of pheochromocytoma – is it time to switch to plasma-free metanephrines? The Journal of Clinical Endocrinology & Metabolism. 2003;88:550–552. doi: 10.1210/jc.2002-021913. [DOI] [PubMed] [Google Scholar]
- Eisenhofer G, Aneman A, Hooper D, Rundqvist B, Friberg P. Mesenteric organ production, hepatic metabolism, and renal elimination of norepinephrine and its metabolites in humans. Journal of Neurochemistry. 1996;66:1565–1573. doi: 10.1046/j.1471-4159.1996.66041565.x. [DOI] [PubMed] [Google Scholar]
- Eisenhofer G, Goldstein DS. Peripheral dopamine systems. In: Robertson D, Low P, Burnstock G, Biaggioni I, editors. Primer on the autonomic nervous system. San Diego: Elsevier; 2004. [Google Scholar]
- Eisenhofer G, Goldstein DS, Kopin IJ, Crout JR. Pheochromocytoma: Rediscovery as a catecholamine-metabolizing tumor. Endocrine Pathology. 2003a;14:193–212. doi: 10.1007/s12022-003-0012-4. [DOI] [PubMed] [Google Scholar]
- Eisenhofer G, Goldstein DS, Kopin IJ, Crout JR. Pheochromocytoma: Rediscovery as a catecholamine-metabolizing tumor. Endocrine Pathology. 2003b;14:193–212. doi: 10.1007/s12022-003-0012-4. [DOI] [PubMed] [Google Scholar]
- Eisenhofer G, Goldstein DS, Sullivan P, Csako G, Brouwers FM, Lai EW, et al. Biochemical and clinical manifestations of dopamine-producing paragangliomas: Utility of plasma methoxytyramine. The Journal of Clinical Endocrinology & Metabolism. 2005;90:2086–2075. doi: 10.1210/jc.2004-2025. [DOI] [PubMed] [Google Scholar]
- Eisenhofer G, Goldstein DS, Walther MM, Friberg P, Lenders JW, Keiser HR, et al. Biochemical diagnosis of pheochromocytoma: How to distinguish true-from false-positive test results. The Journal of Clinical Endocrinology & Metabolism. 2003c;88:2656–2666. doi: 10.1210/jc.2002-030005. [DOI] [PubMed] [Google Scholar]
- Eisenhofer G, Huynh TT, Hiroi M, Pacak K. Understanding catecholamine metabolism as a guide to the biochemical diagnosis of pheochromocytoma. Reviews in Endocrine & Metabolic Disorders. 2001a;2:297–311. doi: 10.1023/a:1011572617314. [DOI] [PubMed] [Google Scholar]
- Eisenhofer G, Lenders J, Linehan W, Walther M, Goldstein D, Keiser H. Plasma normetanephrine and metanephrine for detecting pheochromocytoma in von Hippel–Lindau disease and multiple endocrine neoplasia type 2. The New England Journal of Medicine. 1999a;340:1872–1879. doi: 10.1056/NEJM199906173402404. [DOI] [PubMed] [Google Scholar]
- Eisenhofer G, Lenders JW, Linehan WM, Walther MM, Goldstein DS, Keiser HR. Plasma normetanephrine and metanephrine for detecting pheochromocytoma in von Hippel–Lindau disease and multiple endocrine neoplasia type 2. The New England Journal of Medicine. 1999b;340:1872–1879. doi: 10.1056/NEJM199906173402404. [DOI] [PubMed] [Google Scholar]
- Eisenhofer G, Rivers G, Rosas AL, Quezado Z, Manger WM, Pacak K. Adverse drug reactions in patients with phaeochromocytoma: Incidence, prevention and management. Drug Safety. 2007;30:1031–1062. doi: 10.2165/00002018-200730110-00004. [DOI] [PubMed] [Google Scholar]
- Eisenhofer G, Rundqvist B, Aneman A, et al. Regional release and removal of catecholamines and extra-neuronal metabolism to metanephrines. The Journal of Clinical Endocrinology & Metabolism. 1995;80:3009–3017. doi: 10.1210/jcem.80.10.7559889. [DOI] [PubMed] [Google Scholar]
- Eisenhofer G, Siegert G, Kotzerke J, Bornstein SR, Pacak K. Current progress and future challenges in the biochemical diagnosis and treatment of pheochromocytomas and paragangliomas. Hormone and Metabolic Research. 2008;40:329–337. doi: 10.1055/s-2008-1073156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhofer G, Walther MM, Huynh TT, Li ST, Born-stein SR, Vortmeyer A, et al. Pheochromocytomas in von Hippel–Lindau syndrome and multiple endocrine neoplasia type 2 display distinct biochemical and clinical phenotypes. The Journal of Clinical Endocrinology & Metabolism. 2001b;86:1999–2008. doi: 10.1210/jcem.86.5.7496. [DOI] [PubMed] [Google Scholar]
- Elder EE, Xu D, Hoog A, Enberg U, Hou M, Pisa P, et al. KI-67 AND hTERT expression can aid in the distinction between malignant and benign pheochromocytoma and paraganglioma. Modern Pathology. 2003;16:246–255. doi: 10.1097/01.MP.0000056982.07160.E3. [DOI] [PubMed] [Google Scholar]
- Eng C, Smith DP, Mulligan LM, Nagai MA, Healey CS, Ponder MA, et al. Point mutation within the tyrosine kinase domain of the RET proto-oncogene in multiple endocrine neoplasia type 2b and related sporadic tumours. Human Molecular Genetics. 1994;3:237–241. doi: 10.1093/hmg/3.2.237. [DOI] [PubMed] [Google Scholar]
- Epelbaum J, Bertherat J, Prevost G, Kordon C, Meyerhof W, Wulfsen I, et al. Molecular and pharmacological characterization of somatostatin receptor subtypes in adrenal, extraadrenal, and malignant pheochromocytomas. The Journal of Clinical Endocrinology & Metabolism. 1995;80:1837–1844. doi: 10.1210/jcem.80.6.7775631. [DOI] [PubMed] [Google Scholar]
- Epstein FH, Eckhoff RD. The epidemiology of high blood pressure – geographic distributions and etiologic factors. In: Stamler J, Stamler R, Pullman TN, editors. The epidemiology of hypertension. New York: Grune and Stratton; 1967. [Google Scholar]
- Esler M, Julius S, Zweifler A, Randall O, Harburg E, Gardiner H, et al. Mild high-renin essential hypertension: Neurogenic human hypertension? The New England Journal of Medicine. 1977;296:405–411. doi: 10.1056/NEJM197702242960801. [DOI] [PubMed] [Google Scholar]
- Favier J, Briere JJ, Burnichon N, Riviere J, Vescovo L, Benit P, et al. The Warburg effect is genetically determined in inherited pheochromocytomas. Public Library of Science (PLoS) One. 2009;4:e7094. doi: 10.1371/journal.pone.0007094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca V, Bouloux PM. Phaeochromocytoma and paraganglioma. Baillière’s Clinical Endocrinology and Metabolism. 1993;7:509–544. doi: 10.1016/s0950-351x(05)80186-7. [DOI] [PubMed] [Google Scholar]
- Forster GJ, Engelbach MJ, Brockmann JJ, Reber HJ, Buchholz HG, Macke HR, et al. Preliminary data on biodistribution and dosimetry for therapy planning of somatostatin receptor positive tumours: Comparison of (86)Y-DOTATOC and (111)in-DTPA-octreotide. European Journal of Nuclear Medicine. 2001;28:1743–1750. doi: 10.1007/s002590100628. [DOI] [PubMed] [Google Scholar]
- Frankel F. Ein fall von doppelseitigem, vollig latent verlaufenden nebennieren tumor und gleichzeitiger nephritis mit veranderungen am circulationsapparat und retinitis. Virchows Archives. 1886;103:244–263. [Google Scholar]
- Ghayee HK, Havekes B, Corssmit EP, Eisenhofer G, Hammes SR, Ahmad Z, et al. Mediastinal paragangliomas: Association with mutations in the succinate dehydrogenase genes and aggressive behavior. Endocrine-Related Cancer. 2009;16:291–299. doi: 10.1677/ERC-08-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford RW, Jr, Bravo EL, Manger WM. Diagnosis and management of pheochromocytoma. Cardiology. 1985;72:126–130. doi: 10.1159/000173958. [DOI] [PubMed] [Google Scholar]
- Gimenez-Roqueplo AP, Burnichon N, Amar L, Favier J, Jeunemaitre X, Plouin PF. Recent advances in the genetics of phaeochromocytoma and functional paraganglioma. Clinical and Experimental Pharmacology and Physiology. 2008;35:376–379. doi: 10.1111/j.1440-1681.2008.04881.x. [DOI] [PubMed] [Google Scholar]
- Gimenez-Roqueplo AP, Favier J, Rustin P, Rieubland C, Kerlan V, Plouin PF, et al. Functional consequences of a SDHB gene mutation in an apparently sporadic pheochromocytoma. The Journal of Clinical Endocrinology & Metabolism. 2002;87:4771–4774. doi: 10.1210/jc.2002-020525. [DOI] [PubMed] [Google Scholar]
- Gimenez-Roqueplo AP, Lehnert H, Mannelli M, Neumann H, Opocher G, Maher ER, et al. Phaeochromocytoma, new genes and screening strategies. Clinical Endocrinology (Oxford) 2006;65:699–705. doi: 10.1111/j.1365-2265.2006.02714.x. [DOI] [PubMed] [Google Scholar]
- Glodny B, Winde G, Herwig R, Meier A, Kuhle C, Cromme S, et al. Clinical differences between benign and malignant pheochromocytomas. Endocrine Journal. 2001;48:151–159. doi: 10.1507/endocrj.48.151. [DOI] [PubMed] [Google Scholar]
- Goldstein DS, Eisenhofer G, Dunn BB, Armando I, Lenders J, Grossman E, et al. Positron emission tomographic imaging of cardiac sympathetic innervation using 6-[18f]fluorodopamine: Initial findings in humans. Journal of the American College of Cardiology. 1993;22:1961–1971. doi: 10.1016/0735-1097(93)90786-z. [DOI] [PubMed] [Google Scholar]
- Goldstein DS, Stull R, Eisenhofer G, Sisson JC, Weder A, Averbuch SD, et al. Plasma 3,4-dihydroxyphenylalanine (dopa) and catecholamines in neuroblastoma or pheochromocytoma. Annals of Internal Medicine. 1986;105:887–888. doi: 10.7326/0003-4819-105-6-887. [DOI] [PubMed] [Google Scholar]
- Goldstein RE, O’Neill JA, Jr, Holcomb GW, III, Morgan WM, III, Neblett WW, III, Oates JA, et al. Clinical experience over 48 years with pheochromocytoma. Annals of Surgery. 1999;229:755–764. doi: 10.1097/00000658-199906000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Garcia C, Keiser HR. Angiotensin II and angiotensin converting enzyme binding in human adrenal gland and pheochromocytomas. Journal of Hypertension. 1990;8:433–441. doi: 10.1097/00004872-199005000-00007. [DOI] [PubMed] [Google Scholar]
- Gottlieb E, Tomlinson IP. Mitochondrial tumour suppressors: A genetic and biochemical update. Nature Reviews Cancer. 2005;5:857–866. doi: 10.1038/nrc1737. [DOI] [PubMed] [Google Scholar]
- Grossman A, Pacak K, Sawka A, Lenders JW, Harlander D, Peaston RT, et al. Biochemical diagnosis and localization of pheochromocytoma: Can we reach a consensus? Annals of the New York Academy of Sciences. 2006;1073:332–347. doi: 10.1196/annals.1353.038. [DOI] [PubMed] [Google Scholar]
- Grossman E, Goldstein D, Hoffman A, Keiser H. Glucagon and clonidine testing in the diagnosis of pheochromocytoma. Hypertension. 1991;17:733–741. doi: 10.1161/01.hyp.17.6.733. [DOI] [PubMed] [Google Scholar]
- Hao HX, Khalimonchuk O, Schraders M, Dephoure N, Bayley JP, Kunst H, et al. SDH5, a gene required for flavination of succinate dehydrogenase, is mutated in paraganglioma. Science. 2009;325:1139–1142. doi: 10.1126/science.1175689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havekes B, et al. New imaging approaches to phaeochromocytomas and paragangliomas. Clinical Endocinology (Oxford) 2009;72(2):137–145. doi: 10.1111/j.1365-2265.2009.03648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havekes B, Corssmit EP, Jansen JC, Van Der Mey AG, Vriends AH, Romijn JA. Malignant paragangliomas associated with mutations in the succinate dehydrogenase D gene. The Journal of Clinical Endocrinology & Metabolism. 2007;92:1245–1248. doi: 10.1210/jc.2006-1993. [DOI] [PubMed] [Google Scholar]
- Havekes B, Romijn JA, Eisenhofer G, Adams K, Pacak K. Update on pediatric pheochromocytoma. Pediatric Nephrology. 2008;24:943–950. doi: 10.1007/s00467-008-0888-9. [DOI] [PubMed] [Google Scholar]
- Heath H, III, Edis AJ. Pheochromocytoma associated with hypercalcemia and ectopic secretion of calcitonin. Annals of Internal Medicine. 1979;91:208–210. doi: 10.7326/0003-4819-91-2-208. [DOI] [PubMed] [Google Scholar]
- Henry JP, Sagne C, Bedet C, Gasnier B. The vesicular monoamine transporter: From chromaffin granule to brain. Neurochemistry International. 1998;32:227–246. doi: 10.1016/s0197-0186(97)00092-2. [DOI] [PubMed] [Google Scholar]
- Hoegerle S, Nitzsche E, Altehoefer C, Ghanem N, Manz T, Brink I, et al. Pheochromocytomas: Detection with 18f DOPA whole body PET – initial results. Radiology. 2002;222:507–512. doi: 10.1148/radiol.2222010622. [DOI] [PubMed] [Google Scholar]
- Holton P. Noradrenaline in tumors of the adrenal medulla. Journal of Physiology (Paris) 1949;108:525–529. doi: 10.1113/jphysiol.1949.sp004355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtz P, Credner K, Kroneberg G. Uber das sympathicomimetische pressorische prinizip des harns (“urosympathin”) Naunyn-Schmiedebergs Archiv für experimentelle Pathologie und Pharmakologie. 1947;204:228–243. [PubMed] [Google Scholar]
- Honigschnabl S, Gallo S, Niederle B, Prager G, Kaserer K, Lechner G, et al. How accurate is MR imaging in characterisation of adrenal masses: Update of a long-term study. European Journal of Radiology. 2002;41:113–122. doi: 10.1016/s0720-048x(01)00443-0. [DOI] [PubMed] [Google Scholar]
- Hume DM. Pheochromocytoma in the adult and in the child. American Journal of Surgery. 1960;99:458–496. doi: 10.1016/0002-9610(60)90141-0. [DOI] [PubMed] [Google Scholar]
- Ilias I, Pacak K. Current approaches and recommended algorithm for the diagnostic localization of pheochromocytoma. The Journal of Clinical Endocrinology & Metabolism. 2004;89:479–491. doi: 10.1210/jc.2003-031091. [DOI] [PubMed] [Google Scholar]
- Jacks T, Shih TS, Schmitt EM, Bronson RT, Bernards A, Weinberg RA. Tumour predisposition in mice heterozygous for a targeted mutation in Nf1. Nature Genetics. 1994;7:353–361. doi: 10.1038/ng0794-353. [DOI] [PubMed] [Google Scholar]
- Jadoul M, Leo JR, Berends MJ, Ooms EC, Buurke EJ, Vasen HF, et al. Pheochromocytoma-induced hypertensive encephalopathy revealing MEN-IIa syndrome in a 13-year old boy. Implications for screening procedures and surgery. Hormone and Metabolic Research Supplement Series. 1989;21:46–49. [PubMed] [Google Scholar]
- Jakobsen AM, Andersson P, Saglik G, Andersson E, Kolby L, Erickson JD, et al. Differential expression of vesicular monoamine transporter (VMAT) 1 and 2 in gastrointestinal endocrine tumours. Journal of Pathology. 2001;195:463–472. doi: 10.1002/path.973. [DOI] [PubMed] [Google Scholar]
- Jan T, Metzger BE, Baumann G. Epinephrine-producing pheochromocytoma with hypertensive crisis after corticotropin injection. American Journal of Medicine. 1990;89:824–825. doi: 10.1016/0002-9343(90)90233-4. [DOI] [PubMed] [Google Scholar]
- John H, Ziegler WH, Hauri D, Jaeger P. Pheochromocytomas: Can malignant potential be predicted? Urology. 1999;53:679–683. doi: 10.1016/s0090-4295(98)00612-8. [DOI] [PubMed] [Google Scholar]
- Johnson RG., Jr Accumulation of biological amines into chromaffin granules: A model for hormone and neurotransmitter transport. Physiological Reviews. 1988;68:232–307. doi: 10.1152/physrev.1988.68.1.232. [DOI] [PubMed] [Google Scholar]
- Kaelin WG., Jr SDH5 mutations and familial paraganglioma: Somewhere Warburg is smiling. Cancer Cell. 2009;16:180–182. doi: 10.1016/j.ccr.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Kalff V, Shapiro B, Lloyd R, Sisson JC, Holland K, Nakajo M, et al. The spectrum of pheochromocytoma in hypertensive patients with neurofibromatosis. Archives of Internal Medicine. 1982;142:2092–2098. [PubMed] [Google Scholar]
- Kaltsas G, Korbonits M, Heintz E, Mukherjee JJ, Jenkins PJ, Chew SL, et al. Comparison of somatostatin analog and meta-iodobenzylguanidine radionuclides in the diagnosis and localization of advanced neuroendocrine tumors. The Journal of Clinical Endocrinology & Metabolism. 2001;86:895–902. doi: 10.1210/jcem.86.2.7194. [DOI] [PubMed] [Google Scholar]
- Kantorovich V, Pacak K. A new concept of unopposed beta-adrenergic overstimulation in a patient with pheochromocytoma. Annals of Internal Medicine. 2005;142:1026–1028. doi: 10.7326/0003-4819-142-12_part_1-200506210-00023. [DOI] [PubMed] [Google Scholar]
- Kaufman BH, Telander RL, Van Heerden JA, Zimmerman D, Sheps SG, Dawson B. Pheochromocytoma in the pediatric age group: Current status. Journal of Pediatric Surgery. 1983;18:879–884. doi: 10.1016/s0022-3468(83)80040-2. [DOI] [PubMed] [Google Scholar]
- Kelly HM, Piper MC, Wilder RE. Case of paroxysmal hypertension with paraganglioma of the right suprarenal gland. Mayo Clinic Proceedings. 1936;11:65–70. [Google Scholar]
- Khairi MR, Dexter RN, Burzynski NJ, Johnston CC., Jr Mucosal neuroma, pheochromocytoma and medullary thyroid carcinoma: Multiple endocrine neoplasia type 3. Medicine (Baltimore) 1975;54:89–112. [PubMed] [Google Scholar]
- Kim HS, Chang WI, Kim YC, Yi SY, Kil JS, Hahn JY, et al. Catecholamine cardiomyopathy associated with paraganglioma rescued by percutaneous cardiopulmonary support: Inverted takotsubo contractile pattern. Circulation Journal. 2007;71:1993–1995. doi: 10.1253/circj.71.1993. [DOI] [PubMed] [Google Scholar]
- Kimura S, Nishimura Y, Yamaguchi K, Nagasaki K, Shimada K, Uchida H. A case of pheochromocytoma producing parathyroid hormone-related protein and presenting with hypercalcemia. The Journal of Clinical Endocrinology & Metabolism. 1990;70:1559–1563. doi: 10.1210/jcem-70-6-1559. [DOI] [PubMed] [Google Scholar]
- Kopetschke R, Slisko M, Kilisli A, Tuschy U, Wallaschofski H, Fassnacht M, et al. Frequent incidental discovery of phaeochromocytoma: Data from a German cohort of 201 phaeochromocytoma. European Journal of Endocrinology. 2009;161:355–361. doi: 10.1530/EJE-09-0384. [DOI] [PubMed] [Google Scholar]
- Kopf D, Goretzki PE, Lehnert H. Clinical management of malignant adrenal tumors. Journal of Cancer Research and Clinical Oncology. 2001;127:143–155. doi: 10.1007/s004320000170. [DOI] [PubMed] [Google Scholar]
- Kuchel O. Pseudopheochromocytoma. Hypertension. 1985;7:151–158. doi: 10.1161/01.hyp.7.1.151. [DOI] [PubMed] [Google Scholar]
- Kuchel O. New insights into pseudopheochromocytoma and emotionally provoked hypertension. In: Mansoor GA, editor. Secondary hypertension. Totowa: Humana Press; 2004. [Google Scholar]
- L’Abbe M, Tinel J, Doumer A. Crises solaires et hypertension paroxystique en rapport avec une tumeur surrenale. Bulletins et mémoires de la Société médicale des hôpitaux de Paris. 1922;46:982–990. [Google Scholar]
- Ladroue C, Carcenac R, Leporrier M, Gad S, Le Hello C, Galateau-Salle F, et al. PHD2 mutation and congenital erythrocytosis with paraganglioma. The New England Journal of Medicine. 2008;359:2685–2692. doi: 10.1056/NEJMoa0806277. [DOI] [PubMed] [Google Scholar]
- Lamarre-Cliche M, Gimenez-Roqueplo AP, Billaud E, Baudin E, Luton JP, Plouin PF. Effects of slow-release octreotide on urinary metanephrine excretion and plasma chromogranin A and catecholamine levels in patients with malignant or recurrent phaeochromocytoma. Clinical Endocrinology (Oxford) 2002;57:629–634. doi: 10.1046/j.1365-2265.2002.01658.x. [DOI] [PubMed] [Google Scholar]
- Latif F, Tory K, Gnarra J, Yao M, Duh FM, Orcutt ML, et al. Identification of the von Hippel–Lindau disease tumor suppressor gene. Science. 1993;260:1317–1320. doi: 10.1126/science.8493574. [DOI] [PubMed] [Google Scholar]
- Lawrence AM. Glucagon provocative test for pheochromocytoma. Annals of Internal Medicine. 1967;66:1091–1096. doi: 10.7326/0003-4819-66-6-1091. [DOI] [PubMed] [Google Scholar]
- Lenders J, Keiser H, Goldstein D, Willemsen JJ, Friberg P, Jacobs MC, et al. Plasma metanephrines in the diagnosis of pheochromocytoma. Annals of Internal Medicine. 1995;123:101–109. doi: 10.7326/0003-4819-123-2-199507150-00004. [DOI] [PubMed] [Google Scholar]
- Lenders JW, Eisenhofer G, Armando I, Keiser HR, Goldstein DS, Kopin IJ. Determination of metanephrines in plasma by liquid chromatography with electrochemical detection. Clinical Chemistry. 1993;39:97–103. [PubMed] [Google Scholar]
- Lenders JW, Pacak K, Huynh TT, Sharabi Y, Mannelli M, Bratslavsky G, et al. Low sensitivity of glucagon provocative testing for diagnosis of pheochromocytoma. The Journal of Clinical Endocrinology & Metabolism. 2010;95:238–245. doi: 10.1210/jc.2009-1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenders JW, Pacak K, Walther MM, Linehan WM, Mannelli M, Friberg P, et al. Biochemical diagnosis of pheochromocytoma: Which test is best? Journal of American Medical Association. 2002;287:1427–1434. doi: 10.1001/jama.287.11.1427. [DOI] [PubMed] [Google Scholar]
- Linnoila RI, Keiser HR, Steinberg SM, Lack EE. Histopathology of benign versus malignant sympathoadrenal paragangliomas: Clinicopathologic study of 120 cases including unusual histologic features. Human Pathology. 1990;21:1168–1180. doi: 10.1016/0046-8177(90)90155-x. [see comments] [DOI] [PubMed] [Google Scholar]
- Loh KC, Fitzgerald PA, Matthay KK, Yeo PP, Price DC. The treatment of malignant pheochromocytoma with iodine-131 metaiodobenzylguanidine (131i-MIBG): A comprehensive review of 116 reported patients. Journal of Endocrinological Investigation. 1997;20:648–658. doi: 10.1007/BF03348026. [DOI] [PubMed] [Google Scholar]
- Lore F, Talidis F, Di Cairano G, Renieri A. Multiple endocrine neoplasia type 2 syndromes may be associated with renal malformations. Journal of Internal Medicine. 2001;250:37–42. doi: 10.1046/j.1365-2796.2001.00846.x. [DOI] [PubMed] [Google Scholar]
- Ludwig AD, Feig DI, Brandt ML, Hicks MJ, Fitch ME, Cass DL. Recent advances in the diagnosis and treatment of pheochromocytoma in children. American Journal of Surgery. 2007;194:792–796. doi: 10.1016/j.amjsurg.2007.08.028. [DOI] [PubMed] [Google Scholar]
- Ma W, Sung HJ, Park JY, Matoba S, Hwang PM. A pivotal role for p53: Balancing aerobic respiration and glycolysis. Journal of Bioenergetics and Biomembranes. 2007;39:243–246. doi: 10.1007/s10863-007-9083-0. [DOI] [PubMed] [Google Scholar]
- Madhavan M, Borlaug BA, Lerman A, Rihal CS, Prasad A. Stress hormone and circulating biomarker profile of apical ballooning syndrome (takotsubo cardiomyopathy): Insights into the clinical significance of B-type natriuretic peptide and troponin levels. Heart. 2009;95:1436–1441. doi: 10.1136/hrt.2009.170399. [DOI] [PubMed] [Google Scholar]
- Manger WM, Gifford RW., Jr . Pheochromocytoma. New York: Springer-Verlag; 1977. [Google Scholar]
- Manger WM, Gifford RW. Clinical and experimental pheochromocytoma. Cambridge, MA: Blackwell Science; 1996. [Google Scholar]
- Manger WM, Gifford RW. Pheochromocytoma. Journal of Clinical Hypertension. 2002;4:62–72. doi: 10.1111/j.1524-6175.2002.01452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannelli M. Management and treatment of pheochromocytomas and paragangliomas. Annals of the New York Academy of Sciences. 2006;1073:405–416. doi: 10.1196/annals.1353.044. [DOI] [PubMed] [Google Scholar]
- Mannelli M, Ercolino T, Giache V, Simi L, Cirami C, Parenti G. Genetic screening for pheochromocytoma: Should SDHC gene analysis be included? Journal of Medical Genetics. 2007;44:586–587. doi: 10.1136/jmg.2007.051045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies KB, Sheps SG. Carney’s triad: Guidelines for management. Mayo Clinic Proceedings. 1988;63:496–502. doi: 10.1016/s0025-6196(12)65648-1. [DOI] [PubMed] [Google Scholar]
- Maurea S, Cuocolo A, Reynolds JC, Neumann RD, Salvatore M. Diagnostic imaging in patients with paragangliomas: Computed tomography, magnetic resonance and MIBG scintigraphy comparison. Quarterly Journal of Nuclear Medicine. 1996;40:365–371. [PubMed] [Google Scholar]
- Mcbride W, Ferrario C, Lyle PA. Hypertension and medical informatics. Journal of the National Medical Association (JNMA) 2003;95:1048–1056. [PMC free article] [PubMed] [Google Scholar]
- Miettinen M. Synaptophysin and neurofilament proteins as markers for neuroendocrine tumors. Archives of Pathology & Laboratory Medicine. 1987;111:813–818. [PubMed] [Google Scholar]
- Mizuno K, Ojima M, Hashimoto S, Watari H, Tani M, Niimura S, et al. Biochemical identification of renin in human pheochromocytoma. Research Communications in Chemical Pathology and Pharmacology. 1985;50:419–433. [PubMed] [Google Scholar]
- Mobine HR, Baker AB, Wang L, Wakimoto H, Jacobsen KC, Seidman CE, et al. Pheochromocytoma-induced cardiomyopathy is modulated by the synergistic effects of cell-secreted factors. Circulation: Heart Failure. 2009;2:121–128. doi: 10.1161/CIRCHEARTFAILURE.108.813261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mornex R, Badet C, Peyrin L. Malignant pheochromocytoma: A series of 14 cases observed between 1966 and 1990. Journal of Endocrinological Investigation. 1992;15:643–649. doi: 10.1007/BF03345808. [DOI] [PubMed] [Google Scholar]
- Mulligan LM, Kwok JB, Healey CS, Elsdon MJ, Eng C, Gardner E, et al. Germ-line mutations of the RET proto-oncogene in multiple endocrine neoplasia type 2a. Nature. 1993;363:458–460. doi: 10.1038/363458a0. [DOI] [PubMed] [Google Scholar]
- Mundschenk J, Lehnert H. Malignant pheochromocytoma. Experimental and Clinical Endocrinology & Diabetes. 1998;106:373–376. doi: 10.1055/s-0029-1212001. [DOI] [PubMed] [Google Scholar]
- Nakatani T, Hayama T, Uchida J, Nakamura K, Takemoto Y, Sugimura K. Diagnostic localization of extra-adrenal pheochromocytoma: Comparison of (123)I-MIBG imaging and (131)I-MIBG imaging. Oncology Reports. 2002;9:1225–1227. [PubMed] [Google Scholar]
- Neumann HP, Bausch B, Mcwhinney SR, Bender BU, Gimm O, Franke G, et al. Germ-line mutations in nonsyndromic pheochromocytoma. The New England Journal of Medicine. 2002;346:1459–1466. doi: 10.1056/NEJMoa020152. [DOI] [PubMed] [Google Scholar]
- Neumann HP, Berger DP, Sigmund G, Blum U, Schmidt D, Parmer RJ, et al. Pheochromocytomas, multiple endocrine neoplasia type 2, and von Hippel–Lindau disease. The New England Journal of Medicine. 1993;329:1531–1538. doi: 10.1056/NEJM199311183292103. [DOI] [PubMed] [Google Scholar]
- Neumann HP, Pawlu C, Peczkowska M, Bausch B, Mcwhinney SR, Muresan M, et al. Distinct clinical features of paraganglioma syndromes associated with SDHB and SDHD gene mutations. Journal of American Medical Association. 2004;292:943–951. doi: 10.1001/jama.292.8.943. [DOI] [PubMed] [Google Scholar]
- Nicholson JP, Jr, Vaughn ED, Jr, Pickering TG, Resnick LM, Artusio J, Kleinert HD, et al. Pheochromocytoma and prazosin. Annals of Internal Medicine. 1983;99:477–479. doi: 10.7326/0003-4819-99-4-477. [DOI] [PubMed] [Google Scholar]
- O’Connor DT, Wu H, Gill BM, Rozansky DJ, Tang K, Mahata SK, et al. Hormone storage vesicle proteins. Transcriptional basis of the widespread neuroendocrine expression of chromogranin A, and evidence of its diverse biological actions, intracellular and extracellular. Annals of the New York Academy of Sciences. 1994;733:36–45. doi: 10.1111/j.1749-6632.1994.tb17254.x. [DOI] [PubMed] [Google Scholar]
- Pacak K. Preoperative management of the pheochromocytoma patient. The Journal of Clinical Endocrinology & Metabolism. 2007;92:4069–4079. doi: 10.1210/jc.2007-1720. [DOI] [PubMed] [Google Scholar]
- Pacak K, Chrousos GP, Koch CA, Lenders JW, Eisenhofer G. Pheochromocytoma: Progress in diagnosis, therapy, and genetics. In: Margioris A, Chrousos GP, editors. Adrenal disorders. 1st. Totowa: Humana Press; 2001a. [Google Scholar]
- Pacak K, Eisenhofer G, Ahlman H, Bornstein SR, Gimenez-Roqueplo AP, Grossman AB, et al. Pheochromocytoma: Recommendations for clinical practice from the first international symposium. Nature Clinical Practice Endocrinology & Metabolism. 2007;3:92–102. doi: 10.1038/ncpendmet0396. [DOI] [PubMed] [Google Scholar]
- Pacak K, Eisenhofer G, Carrasquillo JA, Chen CC, Li ST, Goldstein DS. 6-[18f]Fluorodopamine positron emission tomographic (PET) scanning for diagnostic localization of pheochromocytoma. Hypertension. 2001b;38:6–8. doi: 10.1161/01.hyp.38.1.6. [DOI] [PubMed] [Google Scholar]
- Pacak K, Eisenhofer G, Carrasquillo JA, Chen CC, Whatley M, Goldstein DS. Diagnostic localization of pheochromocytoma: The coming of age of positron emission tomography. Annals of the New York Academy of Sciences. 2002;970:170–176. doi: 10.1111/j.1749-6632.2002.tb04423.x. [DOI] [PubMed] [Google Scholar]
- Pacak K, Fojo T, Goldstein DS, Eisenhofer G, Walther MM, Linehan WM, et al. Radiofrequency ablation: A novel approach for treatment of metastatic pheochromocytoma. Journal of the National Cancer Institute. 2001c;93:648–649. doi: 10.1093/jnci/93.8.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacak K, Goldstein DS, Doppman JL, Shulkin BL, Udelsman R, Eisenhofer G. A “pheo” lurks: Novel approaches for locating occult pheochromocytoma. The Journal of Clinical Endocrinology & Metabolism. 2001d;86:3641–3646. doi: 10.1210/jcem.86.8.7714. [DOI] [PubMed] [Google Scholar]
- Pacak K, Ilias I, Adams KT, Eisenhofer G. Biochemical diagnosis, localization and management of pheochromocytoma: Focus on multiple endocrine neoplasia type 2 in relation to other hereditary syndromes and sporadic forms of the tumour. Journal of Internal Medicine. 2005;257:60–68. doi: 10.1111/j.1365-2796.2004.01425.x. [DOI] [PubMed] [Google Scholar]
- Pacak K, Linehan WM, Eisenhofer G, Walther MM, Goldstein DS. Recent advances in genetics, diagnosis, localization, and treatment of pheochromocytoma. Annals of Internal Medicine. 2001e;134:315–329. doi: 10.7326/0003-4819-134-4-200102200-00016. [DOI] [PubMed] [Google Scholar]
- Page LB. Epidemiologic evidence on the etiology of human hypertension and its possible prevention. American Heart Journal. 1976;91:527–534. doi: 10.1016/s0002-8703(76)80337-7. [DOI] [PubMed] [Google Scholar]
- Page LB, Raker JW, Berberich FR. Pheochromocytoma with predominant epinephrine secretion. American Journal of Medicine. 1969;47:648–652. doi: 10.1016/0002-9343(69)90195-8. [DOI] [PubMed] [Google Scholar]
- Pasini B, Mcwhinney SR, Bei T, Matyakhina L, Stergiopoulos S, Muchow M, et al. Clinical and molecular genetics of patients with the carney-stratakis syndrome and germline mutations of the genes coding for the succinate dehydrogenase subunits SDHB, SDHC, and SDHD. European Journal of Human Genetics. 2008;16:79–88. doi: 10.1038/sj.ejhg.5201904. [DOI] [PubMed] [Google Scholar]
- Peczkowska M, Cascon A, Prejbisz A, Kubaszek A, Cwikla BJ, Furmanek M, et al. Extra-adrenal and adrenal pheochromocytomas associated with a germline SDHC mutation. Nature Clinical Practice Endocrinology & Metabolism. 2008;4:111–115. doi: 10.1038/ncpendmet0726. [DOI] [PubMed] [Google Scholar]
- Pick L. Das ganglioma embryonale sympathicum (sympathoma embryonale), eine typisch bosartige geschwulstform des sympathischen nervensystems. Klin Wochenschr. 1912;49:16–22. [Google Scholar]
- Pigny P, Cardot-Bauters C, Do Cao C, Vantyghem MC, Carnaille B, Pattou F, et al. Should genetic testing be performed in each patient with sporadic pheochromocytoma at presentation? European Journal of Endocrinology. 2009;160:227–231. doi: 10.1530/EJE-08-0574. [DOI] [PubMed] [Google Scholar]
- Plouin PF, Degoulet P, Tugaye A, Ducrocq MB, Menard J. Screening for phaeochromocytoma: In which hypertensive patients? A semiological study of 2585 patients, including 11 with phaeochromocytoma (author’s transl.) La Nouvelle presse médicale. 1981;10:869–872. [PubMed] [Google Scholar]
- Prager G, Heinz-Peer G, Passler C, Kaczirek K, Schindl M, Scheuba C, et al. Can dynamic gadolinium-enhanced magnetic resonance imaging with chemical shift studies predict the status of adrenal masses? World Journal of Surgery. 2002;26:958–964. doi: 10.1007/s00268-002-6625-9. [DOI] [PubMed] [Google Scholar]
- Prodanov T, Havekes B, Nathanson KL, Adams KT, Pacak K. Malignant paraganglioma associated with succinate dehydrogenase subunit B in an 8-year-old child: The age of first screening? Pediatric Nephrology. 2009;24:1239–1242. doi: 10.1007/s00467-008-1111-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quezado ZN, Keiser HR, Parker MM. Reversible myocardial depression after massive catecholamine release from a pheochromocytoma. Critical Care Medicine. 1992;20:549–551. doi: 10.1097/00003246-199204000-00022. [DOI] [PubMed] [Google Scholar]
- Raber W, Raffesberg W, Bischof M, Scheuba C, Niederle B, Gasic S, et al. Diagnostic efficacy of unconjugated plasma metanephrines for the detection of pheochromocytoma. Archives of Internal Medicine. 2000;160:2957–2963. doi: 10.1001/archinte.160.19.2957. [DOI] [PubMed] [Google Scholar]
- Rao F, Keiser HR, O’Connor DT. Malignant pheochromocytoma: Chromaffin granule transmitters and response to treatment. Hypertension. 2000;36:1045–1052. doi: 10.1161/01.hyp.36.6.1045. [DOI] [PubMed] [Google Scholar]
- Reddy VS, O’Neill JA, Jr, Holcomb GW, III, Neblett WW, III, Pietsch JB, Morgan WM, III, et al. Twenty-five-year surgical experience with pheochromocytoma in children. The American Surgeon. 2000;66:1085–1091. discussion 1092. [PubMed] [Google Scholar]
- Roden M, Raffesberg W, Raber W, Bernroider E, Niederle B, Waldhausl W, et al. Quantification of unconjugated metanephrines in human plasma without interference by acetaminophen. Clinical Chemistry. 2001;47:1061–1067. [PubMed] [Google Scholar]
- Ross JH. Pheochromocytoma. Special considerations in children. Urologic Clinics of North America. 2000;27:393–402. doi: 10.1016/s0094-0143(05)70088-4. [DOI] [PubMed] [Google Scholar]
- Sanchez-Recalde A, Costero O, Oliver JM, Iborra C, Ruiz E, Sobrino JA. Images in cardiovascular medicine. Pheochromocytoma-related cardiomyopathy: Inverted takotsubo contractile pattern. Circulation. 2006;113:111. doi: 10.1161/CIRCULATIONAHA.105.581108. [DOI] [PubMed] [Google Scholar]
- Sarosi G, Doe RP. Familial occurrence of parathyroid adenomas, pheochromocytoma, and medullary carcinoma of the thyroid with amyloid stroma (Sipple’s syndrome) Annals of Internal Medicine. 1968;68:1305–1309. doi: 10.7326/0003-4819-68-6-1305. [DOI] [PubMed] [Google Scholar]
- Sawka AM, Jaeschke R, Singh RJ, Young WF., Jr A comparison of biochemical tests for pheochromocytoma: Measurement of fractionated plasma metanephrines compared with the combination of 24-hour urinary metanephrines and catecholamines. The Journal of Clinical Endocrinology & Metabolism. 2003;88:553–558. doi: 10.1210/jc.2002-021251. [DOI] [PubMed] [Google Scholar]
- Schiavi F, Boedeker CC, Bausch B, Peczkowska M, Gomez CF, Strassburg T, et al. Predictors and prevalence of paraganglioma syndrome associated with mutations of the SDHC gene. Journal of American Medical Association. 2005;294:2057–2063. doi: 10.1001/jama.294.16.2057. [DOI] [PubMed] [Google Scholar]
- Schlumberger M, Gicquel C, Lumbroso J, Tenenbaum F, Comoy E, Bosq J, et al. Malignant pheochromocytoma: Clinical, biological, histologic and therapeutic data in a series of 20 patients with distant metastases. Journal of Endocrinological Investigation. 1992;15:631–642. doi: 10.1007/BF03345807. [DOI] [PubMed] [Google Scholar]
- Schmedtje JFJ, Sax S, Pool JL, Goldfarb RA, Nelson EB. Localization of ectopic pheochromocytomas by magnetic resonance imaging. American Journal of Medicine. 1987;83:770–772. doi: 10.1016/0002-9343(87)90912-0. [DOI] [PubMed] [Google Scholar]
- Scholz T, Eisenhofer G, Pacak K, Dralle H, Lehnert H. Clinical review: Current treatment of malignant pheochromocytoma. The Journal of Clinical Endocrinology & Metabolism. 2007;92:1217–1225. doi: 10.1210/jc.2006-1544. [DOI] [PubMed] [Google Scholar]
- Schorr RT, Rogers SN. Intraoperative cardiovascular crisis caused by glucagon. Archives of Surgery. 1987;122:833–834. doi: 10.1001/archsurg.1987.01400190099022. [DOI] [PubMed] [Google Scholar]
- Shapiro B, Gross MD, Shulkin B. Radioisotope diagnosis and therapy of malignant pheochromocytoma. Trends in Endocrinology and Metabolism. 2001;12:469–475. doi: 10.1016/s1043-2760(01)00492-1. [DOI] [PubMed] [Google Scholar]
- Sheps SG, Jiang NS, Klee GG, Van Heerden JA. Recent developments in the diagnosis and treatment of pheochromocytoma. Mayo Clinic Proceedings. 1990;65:88–95. doi: 10.1016/s0025-6196(12)62113-2. [DOI] [PubMed] [Google Scholar]
- Sheps SG, Maher FT. Comparison of the histamine and tyramine hydrochloride tests in the diagnosis of pheochromocytoma. Journal of American Medical Association. 1966;195:265–267. [PubMed] [Google Scholar]
- Shulkin BL, Thompson NW, Shapiro B, Francis IR, Sisson JC. Pheochromocytomas: Imaging with 2-[fluorine-18]fluoro-2-deoxy-d-glucose PET. Nuclear Medicine. 1999;212:35–41. doi: 10.1148/radiology.212.1.r99jl3035. [DOI] [PubMed] [Google Scholar]
- Shulkin BL, Wieland DM, Schwaiger M, Thompson NW, Francis IR, Haka MS, et al. PET scanning with hydroxyephedrine: An approach to the localization of pheochromocytoma. Journal of Nuclear Medicine. 1992;33:1125–1131. [PubMed] [Google Scholar]
- Siddiqui MZ, Von Eyben FE, Spanos G. High-voltage irradiation and combination chemotherapy for malignant pheochromocytoma. Cancer. 1988;62:686–690. doi: 10.1002/1097-0142(19880815)62:4<686::aid-cncr2820620407>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Sipple JH. The association of pheochromocytoma with carcinoma of the thyroid gland. American Journal of Medicine. 1961;31:163–166. [Google Scholar]
- Sjoerdsma A, Engelman K, Spector S, Udenfriend S. Inhibition of catecholamine synthesis in man with alpha-methyl-tyrosine, an inhibitor of tyrosine hydroxylase. Lancet. 1965;2:1092–1094. doi: 10.1016/s0140-6736(65)90062-0. [DOI] [PubMed] [Google Scholar]
- Solanki KK, Bomanji J, Moyes J, Mather SJ, Trainer PJ, Britton KE. A pharmacological guide to medicines which interfere with the biodistribution of radiolabelled meta-iodobenzylguanidine (MIBG) Nuclear Medicine Communications. 1992;13:513–521. doi: 10.1097/00006231-199207000-00006. [DOI] [PubMed] [Google Scholar]
- Spark RF, Connolly PB, Gluckin DS, White R, Sacks B, Landsberg L. ACTH secretion from a functioning pheochromocytoma. The New England Journal of Medicine. 1979;301:416–418. doi: 10.1056/NEJM197908233010807. [DOI] [PubMed] [Google Scholar]
- Steiner AL, Goodman AD, Powers SR. Study of a kindred with pheochromocytoma, medullary thyroid carcinoma, hyperparathyroidism and Cushing’s disease: Multiple endocrine neoplasia, type 2. Medicine (Baltimore) 1968;47:371–409. doi: 10.1097/00005792-196809000-00001. [DOI] [PubMed] [Google Scholar]
- Stenstrom G, Ernest I, Tisell LE. Long-term results in 64 patients operated upon for pheochromocytoma. Acta Medica Scandinavica. 1988;223:345–352. doi: 10.1111/j.0954-6820.1988.tb15883.x. [DOI] [PubMed] [Google Scholar]
- Stenstrom G, Waldenstrom J. Positive correlation between urinary excretion of catecholamine metabolites and tumour mass in pheochromocytoma: Results in patients with sustained and paroxysmal hypertension and multiple endocrine neoplasia. Acta Medica Scandinavica. 1985;217:73–77. doi: 10.1111/j.0954-6820.1985.tb01637.x. [DOI] [PubMed] [Google Scholar]
- Stewart AF, Hoecker JL, Mallette LE, Segre GV, Amatruda TT, Jr, Vignery A. Hypercalcemia in pheochromocytoma: Evidence for a novel mechanism. Annals of Internal Medicine. 1985;102:776–779. doi: 10.7326/0003-4819-102-6-776. [DOI] [PubMed] [Google Scholar]
- Stumvoll M, Fritsche A, Pickert A, Overkamp D. Features of malignancy in a benign pheochromocytoma. Hormone Research. 1997;48:135–136. doi: 10.1159/000185503. [DOI] [PubMed] [Google Scholar]
- Sutton MG, Sheps SG, Lie JT. Prevalence of clinically unsuspected pheochromocytoma: Review of a 50-year autopsy series. Mayo Clinic Proceedings. 1981;56:354–360. [PubMed] [Google Scholar]
- Takahashi K, Ashizawa N, Minami T, Suzuki S, Sakamoto I, Hayashi K, et al. Malignant pheochromocytoma with multiple hepatic metastases treated by chemotherapy and transcatheter arterial embolization. Internal Medicine. 1999;38:349–354. doi: 10.2169/internalmedicine.38.349. [DOI] [PubMed] [Google Scholar]
- Thompson RJ, Doran JF, Jackson P, Dhillon AP, Rode J. PGP 9.5 – A new marker for vertebrate neurons and neuroendocrine cells. Brain Research. 1983;278:224–228. doi: 10.1016/0006-8993(83)90241-x. [DOI] [PubMed] [Google Scholar]
- Timmers H, Gimenez-Roqueplo AP, Mannelli M, Pacak K. Clinical aspects of SDHx-related pheochromocytoma and paraganglioma. Endocrine-Related Cancer. 2009;16:391–400. doi: 10.1677/ERC-08-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmers HJ, Hadi M, Carrasquillo JA, Chen CC, Martiniova L, Whatley M, et al. The effects of carbidopa on uptake of 6–18f-fluoro-l-DOPA in PET of pheochromocytoma and extraadrenal abdominal paraganglioma. Journal of Nuclear Medicine. 2007a;48:1599–1606. doi: 10.2967/jnumed.107.042721. [DOI] [PubMed] [Google Scholar]
- Timmers HJ, Kozupa A, Chen CC, Carrasquillo JA, Ling A, Eisenhofer G, et al. Superiority of fluorodeoxyglucose positron emission tomography to other functional imaging techniques in the evaluation of metastatic SDHB-associated pheochromocytoma and paraganglioma. Journal of Clinical Oncology. 2007b;25:2262–2269. doi: 10.1200/JCO.2006.09.6297. [DOI] [PubMed] [Google Scholar]
- Timmers HJ, Kozupa A, Eisenhofer G, Raygada M, Adams KT, Solis D, et al. Clinical presentations, biochemical phenotypes, and genotype-phenotype correlations in patients with succinate dehydrogenase subunit B-associated pheochromocytomas and paragangliomas. The Journal of Clinical Endocrinology & Metabolism. 2007c;92:779–786. doi: 10.1210/jc.2006-2315. [DOI] [PubMed] [Google Scholar]
- Timmers HJ, Pacak K, Bertherat J, Lenders JW, Duet M, Eisenhofer G, et al. Mutations associated with succinate dehydrogenase d-related malignant paragangliomas. Clinical Endocrinology (Oxford) 2007d;68:561–566. doi: 10.1111/j.1365-2265.2007.03086.x. [DOI] [PubMed] [Google Scholar]
- Trojanowski JQ, Lee VM. Expression of neurofilament antigens by normal and neoplastic human adrenal chromaffin cells. The New England Journal of Medicine. 1985;313:101–104. doi: 10.1056/NEJM198507113130207. [DOI] [PubMed] [Google Scholar]
- Udenfriend S. Tyrosine hydroxylase. Pharmacological Reviews. 1966;18:43–51. [PubMed] [Google Scholar]
- Unger N, Pitt C, Schmidt IL, Walz MK, Schmid KW, Philipp T, et al. Diagnostic value of various biochemical parameters for the diagnosis of pheochromocytoma in patients with adrenal mass. European Journal of Endocrinology. 2006;154:409–417. doi: 10.1530/eje.1.02097. [DOI] [PubMed] [Google Scholar]
- Vaclavik J, Stejskal D, Lacnak B, Lazarova M, Jedelsky L, Kadalova L, et al. Free plasma metanephrines as a screening test for pheochromocytoma in low-risk patients. Journal of Hypertension. 2007;25:1427–1431. doi: 10.1097/HJH.0b013e32813aeb5a. [DOI] [PubMed] [Google Scholar]
- Van Der Harst E, De Herder WW, Bruining HA, Bonjer HJ, De Krijger RR, Lamberts SW, et al. [(123)I]metaiodobenzylguanidine and [(111)in] octreotide uptake in benign and malignant pheochromocytomas. The Journal of Clinical Endocrinology & Metabolism. 2001;86:685–693. doi: 10.1210/jcem.86.2.7238. [DOI] [PubMed] [Google Scholar]
- Van Der Harst E, De Herder WW, De Krijger RR, Bruining HA, Bonjer HJ, Lamberts SW, et al. The value of plasma markers for the clinical behavior of phaeochromocytomas. European Journal of Endocrinology. 2002;147:85–94. doi: 10.1530/eje.0.1470085. [DOI] [PubMed] [Google Scholar]
- Van Der Horst-Schrivers AN, Jager PL, Boezen HM, Schouten JP, Kema IP, Links TP. Iodine-123 metaiodobenzylguanidine scintigraphy in localising phaeochromocytomas – experience and meta-analysis. Anticancer Research. 2006;26:1599–1604. [PubMed] [Google Scholar]
- Van Nederveen FH, Dinjens WN, Korpershoek E, De Krijger RR. The occurrence of SDHB gene mutations in pheochromocytoma. Annals of the New York Academy of Sciences. 2006;1073:177–182. doi: 10.1196/annals.1353.018. [DOI] [PubMed] [Google Scholar]
- Van Nederveen FH, Gaal J, Favier J, Korpershoek E, Oldenburg RA, De Bruyn EM, et al. An immunohistochemical procedure to detect patients with paraganglioma and phaeochromocytoma with germline SDHB, SDHC, or SDHD gene mutations: A retrospective and prospective analysis. Lancet Oncology. 2009;10:764–771. doi: 10.1016/S1470-2045(09)70164-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Stratum M, Levarlet M, Lambilliotte JP, Lignian H, De Rood M. Use of labetalol during anesthesia for pheochromocytoma removal. Acta Anaesthesiologica Belgica. 1983;34:233–240. [PubMed] [Google Scholar]
- Vargas H, Kavoussi L, Bartlett D, Wagner J, Venzon D, Fraker D, et al. Laparoscopic adrenalectomy: A new standard of care. Urology. 1997;49:673–678. doi: 10.1016/s0090-4295(97)00083-6. [DOI] [PubMed] [Google Scholar]
- Viale G, Dell’Orto P, Moro E, Cozzaglio L, Coggi G. Vasoactive intestinal polypeptide-, somatostatin-, and calcitonin-producing adrenal pheochromocytoma associated with the watery diarrhea (WDHH) syndrome: First case report with immunohistochemical findings. Cancer. 1985;55:1099–1106. doi: 10.1002/1097-0142(19850301)55:5<1099::aid-cncr2820550527>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Von Euler US. Presence of a substance with sympathin E properties in spleen extracts. Acta Physiologica Scandinavica. 1946a;11:168–186. doi: 10.1038/157369b0. [DOI] [PubMed] [Google Scholar]
- Von Euler US. The presence of a sympathomimetic substance in extracts of mammalian heart. Journal of Physiology (Paris) 1946b;105:38–44. [PubMed] [Google Scholar]
- Von Moll L, Mcewan AJ, Shapiro B, Sisson JC, Gross MD, Lloyd R, et al. Iodine-131 MIBG scintigraphy of neuroendocrine tumors other than pheochromocytoma and neuroblastoma. Journal of Nuclear Medicine. 1987;28:979–988. [PubMed] [Google Scholar]
- Walther MM, Herring J, Enquist E, Keiser HR, Linehan WM. von Recklinghausen’s disease and pheochromocytomas. The Journal of Urology. 1999;162:1582–1586. [PubMed] [Google Scholar]
- Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- White WB, Baker LH. Episodic hypertension secondary to panic disorder. Archives of Internal Medicine. 1986;146:1129–1130. [PubMed] [Google Scholar]
- Winfield HN, Hamilton BD, Bravo EL, Novick AC. Laparoscopic adrenalectomy: The preferred choice? A comparison to open adrenalectomy. The Journal of Urology. 1998;160:325–329. doi: 10.1016/s0022-5347(01)62884-2. [DOI] [PubMed] [Google Scholar]
- Yon L, Guillemot J, Montero-Hadjadje M, Grumolato L, Leprince J, Lefebvre H, et al. Identification of the secretogranin II-derived peptide EM66 in pheochromocytomas as a potential marker for discriminating benign versus malignant tumors. The Journal of Clinical Endocrinology & Metabolism. 2003;88:2579–2585. doi: 10.1210/jc.2002-021748. [DOI] [PubMed] [Google Scholar]
- Young JB, Landsberg L. Catecholamines and the adrenal medulla. In: Wilson JD, Foster DW, editors. William’s tesxtbook of endocrinology. 9th. Philadelphia: W.B. Saunders Company; 1998. pp. 665–728. [Google Scholar]
- Yu J, Pacak K. Management of malignant pheochromocytoma. Endocrinologist. 2002;12:291–299. [Google Scholar]