Abstract
Anecdotal reports in the press and epidemiological studies suggest that deployment to Iraq and Afghanistan may be associated with respiratory diseases and symptoms in U.S. military personnel and veterans. Exposures during military operations were complex, but virtually all service members were exposed to high levels of respirable, geogenic dust. Inhalation of other dusts has been shown to be associated with adverse health effects, but the pulmonary toxicity of ambient dust from Iraq has not been previously studied. The relative toxicity of Camp Victory dust was evaluated by comparing it to particulate matter from northern Kuwait, a standard U.S. urban dust, and crystalline silica using a single intratracheal instillation in rats. Lung histology, protein levels, and cell counts were evaluated in the bronchoalveolar lavage fluid 1–150 d later. The Iraq dust provoked an early significant, acute inflammatory response. However, the level of inflammation in response to the Iraq dust, U.S. urban dust, and Kuwait dust rapidly declined and was nearly at control levels by the end of the study At later times, animals exposed to the Iraq, U.S. urban, or Kuwait dusts showed increased small airway remodeling and emphysema compared to silica-exposed and control animals without evidence of fibrosis or premalignant changes. The severity and persistence of pulmonary toxicity of these three dusts from the Middle East resemble those of a U.S. urban dust and are less than those of silica. Therefore, Iraq dust exposure is not highly toxic, but similar to other poorly soluble low-toxicity dusts.
Recent articles in the popular press implied that there is a direct relationship between exposure to dust and burn pit smoke during deployment to Iraq and Afghanistan and development of serious lung disease by military personnel (Drummond 2013; Kennedy 2009; 2010; Peeples 2013; Risen 2010; Shane 2010). There are also reports in the peer-reviewed literature of military personnel with post-deployment respiratory disease (King et al. 2011), and epidemiological findings of increased respiratory symptoms and asthma in deployed compared with nondeployed service members (Abraham et al. 2014; Smith et al. 2009; Szema et al. 2010; 2011). These observations have raised concerns that some service members who were deployed to southwest Asia (SWA) may suffer from respiratory dysfunction related to deployment, which is difficult to diagnose and of unknown etiology (McAndrew et al. 2012; Quigley et al. 2012).
There are few quantitative exposure data for military personnel during Operation Iraqi Freedom/Operation Enduring Freedom, and exposures in the military operational environment are complex, involving field dust, pit burning, spores, munition combustion products, diesel exhaust, and various other chemicals (Rose 2012; Korzeniewski et al. 2013). Consequently, it has proved challenging to investigate the association of postdeployment respiratory disease with particular exposures or events in SWA. However, a conspicuous exposure that affected virtually all service members deployed to SWA—and that ranks among the top deployment-related health concerns for veterans (Teichman 2012)—is to the ubiquitous ambient particulate matter (PM). Airborne PM concentrations in SWA exceed environmental, occupational, and military exposure guidelines (Weese and Abraham 2009; Engelbrecht et al 2009a). Adverse health effects, including cardiovascular and pulmonary disease, are known consequences of exposure to high levels of PM with aerodynamic diameter of less than 10 μm (PM10) and to a greater degree from PM of less than 2.5 μm (PM2.5) (Brocato et al 2014; Chang et al 2015; Pope and Dockery 2006; Tsai and Yang 2013). The severity of disease depends on the amount and duration of the exposure, physical and chemical properties of PM, and underlying health of exposed individuals (Davidson, et al. 2005; Valavanidis et al. 2008; Ghio et al 2012).
Respiratory symptoms associated with exposure to mineral dusts have been known for decades (Morman and Plumlee 2013). Airborne Saharan dust has been associated with increased morbidity (Alessandrini et al. 2013; Ameida-Silva et al 2013) and mortality in Mediterranean Europe (Karanasiou et al. 2012). Similar findings were noted regarding desert dust originating in the Gobi in Asia (NRC 2010a; Esmaeil et al. 2014). Desert lung syndrome, a nonoccupational pneumoconiosis, was described in populations exposed to dust in the Negev desert (Bar-Ziv and Goldberg 1974) and in Saudi Arabia (Hawass 1987). An acute hyperergic pulmonary condition, referred to as Desert Storm pneumonitis or El Eskan disease, occurred in military personnel who were co-exposed to pigeon droppings and high levels of fine sand dust during deployment to Saudi Arabia (Korenyi-Both et al. 1992; Intitute of Medicine [IOM] 2007). Upper respiratory complaints were reported in military personnel deployed during Operation Desert Shield (Richards et al. 1993). Increases in in-theater medical encounters for asthma (Roop et al. 2007) and respiratory symptoms during Operations Iraqi Freedom and Enduring Freedom (Abraham et al. 2012) were also described. In 2003, there was an unexplained case cluster of severe acute pneumonitis with elevated eosinophils in military personnel deployed in or near Iraq (Shorr et al. 2004).
The levels and composition of ambient aerosols present in the Middle East and SWA have been characterized recently (Engelbrecht et al. 2009a; 2009b) and ambient PM concentrations exceed the U.S. Army Public Health Command (USAPHC) 1-yr air Military Exposure Guideline (MEG) value (15 μg/m3) (U.S. Army Public Health Command 2013) for PM2.5. The concentrations of potentially hazardous chemical constituents in the dusts may exceed levels seen in natural PM from the southwestern United States by as much as 10-fold, although they do not exceed National Institute for Safety and Health (NIOSH) exposure limits (Engelbrecht et al. 2009a). While Hamad et al. (2014) suggested that the bioavailablity of toxic metals in soils from Baghdad is restricted, silica and metals present in the high levels of ambient PM in SWA (Engelbrecht et al. 2009a; 2009b) are cause for concern, as these components might induce inflammation and oxidative stress, resulting in respiratory disease.
Two earlier studies addressed the toxicity of geogenic PM from SWA using surrogates for natural, ambient PM. In the first, rats were exposed to settled dust collected at Camp Buehring in northern Kuwait (Wilfong et al. 2011) by intratracheal (IT) instillation, and in the second, rats received a whole-body exposure for 2 wk using aerosolized milled surface soil collected at Camp Victory, Baghdad, Iraq (Dorman et al. 2012). Neither study found marked pathological effects as a result of exposure.
To test the toxicity of natural, ambient PM from SWA, respirable dust at Camp Victory was collected using high-volume air samplers, and two IT instillation studies were performed in rats using this material. The first was a time-course study using PM collected during 2008 at Camp Victory, Baghdad, Iraq. The second was a comparative analysis of the dusts collected during 2008 and 2009 at Camp Victory, the dust from Camp Buehring, a standard U.S. urban PM (NIST SRM 1648a), and crystalline silica as a positive control. Examination of bronchoalveolar lavage fluid (BALF) and fixed lung tissue was used to assess pulmonary injury. Although the Camp Victory and Kuwait dusts are not highly toxic, they do produce an early, brief, intense inflammatory response and show a weak tendency to initiate emphysematous changes in the lungs of exposed rats, but not to the extent observed with U.S. urban dust.
METHODS
Animals
Eight- to 10-wk-old male Sprague-Dawley rats (Hla:SD CVF) were obtained from Hilltop Labs (Scottsdale, PA). The rats were acclimated for at least 7 d prior to initiation of the study, housed under a standard 12-h light/dark cycle at 20–25°C, and were given food and water ad libitum at NIOSH facilities in Morgantown, WV. At the time of exposure, the rats weighed 280–300 g. Research was conducted in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animals and experiments involving animals and adhered to principles stated in the Guide for the Care and Use of Laboratory Animals (NRC 2010b) in facilities that are fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care, International.
Dust Collection and Analysis
High-volume air samplers (TE-6070 PM-10, Tisch Environmental, Village of Cleves, OH) were used to collect 24 ± 1-h samples of ambient PM10 on 8 × 11-inch PTFE filters with a 3-μm pore size (VWR, Brisbane, CA) at Camp Victory, Baghdad, Iraq, from March 26 to May 1, 2008 (Ir8) and from April 24 to 27, 2009 (Ir9). The elevation of the inlet port, relative to the ground, for the air sampler was approximately 57 inches. The air samplers were ruggedized at the U.S. Army Public Health Command to withstand high temperatures and ambient dust levels prevailing at Camp Victory. The dust was collected using the reference method for the determination of particulate matter as PM10 in the atmosphere (U.S. Congress 1971). Filters were shipped and stored in Mylar bags at ambient temperature. To recover the dust, filters were shaken on an on a IEC Sieve Shaker (Fritsch, Germany) for 15 min in a Mylar bag. The loose dust in the bag was transferred to the shaker’s recovery pan and any dust remaining on the filters was scraped into the recovery pan. All dust obtained was transferred to a labeled scintillation vial and weighed. Kuwait dust (Ku) was collected at PAD#15 Camp Buerhing in Udairi, Kuwait, in June 2004, and size fractionated to ≤10 μm before use (Wilfong et al 2011). A sample of the Ku dust was generously provided by Lieutenant Commander Vishwesh Mokashi, Naval Medical Research Unit–Dayton. Crystalline silica dust was a gift of U.S. Silica (Berkeley Springs, WV) and size fractionated to ≤10 μm before use. U.S. urban particulate matter (SRM 1648a) was purchased from the National Institute of Standards and Technology (NIST, Gaithersburg, MD) and collected in the St. Louis, MO, area during 1976–1977. Particle sizes for SRM 1648a at the 10th, 50th, and 90th percentile (percent by volume of particles smaller than the value) are 1.35 μm, 5.85 μm, and 30.1 μm, respectively (National Institute of Standards and Technology 2012).
Samples of the Ir8, Ir9, and Ku dusts were analyzed for metal and crystalline silica content using NIOSH Methods 7303 and 7500 (National Institute for Occupational Safety and Health 1996) by Bureau Veritas North American, Inc. (Novi, MI). Briefly, crystalline silica (quartz, cristobalite, tridymite) was analyzed by x-ray diffraction with standard solutions for limit of detection/limit of quantification (LOD/LOQ) determination prepared from a NIST traceable reference standard (SRM 1878a, Respirable Alpha Quartz). Metals content was analyzed by inductively coupled plasma–atomic emission spectrometry (ICP-AES).
Study Design
This IT instillation study consisted of two phases. The first phase was designed to evaluate the toxicity of Ir8 dust using crystalline silica as the positive control over time. Rats were exposed to 2.5, 5, or 10 mg/kg body weight (bw) of Ir8 dust or silica. Exposure levels were selected based on prior experience with many dusts at NIOSH in a range that was comparable to that used by Wilfong et al. (2011). Lung specimens were collected at 1, 3, 7, 30, 60, 120, and 150 d after exposure for 5- and 10-mg/kg bw exposures, and at 30, 60, 120, and 150 d for the 2.5-mg/kg bw exposures. The times of collection were based on prior experience with dust exposures at NIOSH and also selected to fill in the gap between the 7-d and 6-mo exposures reported by Wilfong et al. (2011). Experience and pilot studies suggested that, at short times, a 2.5-mg/ kg bw exposure would not provide much information in addition to the 5- and 10-mg exposures. Nevertheless, the effects of this low-level exposure at long times were examined in the event that the exposures produced effects with long latency (see Supplemental File 1). Bronchoalveolar lavage fluid (BALF) was collected from the rats in the 5- and 10-mg/kg bw groups at each time except 150 d. Four or five experimental and control rats were used for most conditions. In our analysis the results of what were originally designed as range-finding experiments for d 1–7 are also reported. These exposures used two to five animal,s depending on the exposure conditions (Supplemental File 1).
The second phase was designed to compare potentially persistent effects of exposure using both the Ir8 and Ir9 dusts as well as the dust from northern Kuwait (Ku) previously studied by Wilfong and coworkers (2011), and the well-described urban dust from the United States purchased from NIST, described earlier. The effects of exposure to 2.5-, 5-, and 10-mg/kg bw instillations of dust were examined at 60, 120, and 150 d after exposure. However, because the amount of material was limited, it was not possible to test the Ir8 and Ku samples under all conditions: Rats were exposed to 10 mg/kg bw Pad 15 Kuwait dust (Ku) and Ir8 for 120 and 150 d only. Lung tissue for histopathology was collected for all exposures and time points; BALF was collected only from rats exposed to Ir9 and NIST. Five separate rats were used for histopathology and BALF collection at each exposure condition and time point.
Intratracheal Instillation
Particles were suspended in sterile, endotoxin- and Ca2+, Mg2+-free phosphate-buffered saline (PBS), pH 7.4. The rats were anesthetized with an intraperitoneal (ip) injection (28 mg/kg bw) of sodium methohexital (Brevital, Eli Lilly and Company, Indianapolis, IN) and were IT instilled using a 20-gauge, 4-inch ball-tipped animal feeding needle.
Bronchoalveolar Lavage (BALF) and Cell Differentials
At the designated time post exposure, rats were anesthetized with a 26% pentobarbital solution (Sleep-Away, Fort Dodge Animal Health, New York, NY) ip (2 ml /kg bw) and exsanguinated by cutting the vena cava and descending aorta. A tracheal cannula was inserted and bronchoalveolar lavage (BAL) of lungs was performed through the cannula using ice-cold PBS (pH 7.4) containing 5.5 mM D-glucose.
The first lavage was 6 ml and subsequent lavages used 8 ml until a total of 50 ml lavage fluid was collected. The first BAL fluid (BALF) was kept separate from the rest of the lavage fluid. BAL cells were isolated by centrifugation (650 × g, 10 min, 4°C). An aliquot of the acellular supernatant from the first BALF was decanted, transferred to tubes, and frozen at −20°C; the remaining BALF was kept at 4°C for analysis of lactate dehydrogenase (LDH) activity and serum albumin levels. The acellular supernatants from the remaining lavage samples were decanted and discarded. BALF cells were resuspended in HEPES-buffered medium (10 mM N-[2-hydroxyethylpiperazine-N’-2-ethane sulfonic acid], 145 mM NaCl, 5 mM KCl, 1 mM CaCl2, 5.5 mM D-glucose; pH 7.4), centrifuged a second time (650 × g, 10 min, 4°C), and supernatant was decanted and discarded. The BALF cell pellet was then resuspended in HEPES-buffered medium and placed on ice. Cell counts of alveolar macrophages (AM) and polymorphonuclear leukocytes (PMN) were obtained using a Coulter Multisizer II (Coulter Electronics, Hialeah, FL).
Cytospin preparations of BALF cells were made using 0.1 × 106 total phagocytes (AM + PMN) in 200 μl HEPES-buffered solution with a Shandon Elliot cytocentrifuge (speed control = 80 for 5 min). The cytospin preparations were stained with modified Wright–Giemsa stain, and cell differentials were determined by light microscopy. Differential cell counts were calculated by multiplying the total cell counts (AM + PMN) obtained from the Coulter Counter by cell differential percentage obtained from the cytospin preparations. Cell count data are presented in Supplemental File 2.
Tissue Damage Assays
Total protein and albumin were determined in the first acellular lavage fluid using a dye-based assay kit (Roche Diagnostics, Indianapolis, IN) performed on a COBAS MIRA Plus autoanalyzer (Roche Diagnostics) according to the manufacturer’s instructions; quantitation employed a protein standard solution (Sigma-Aldrich, St. Louis, MO). Lactate dehydrogenase (LDH) activity was determined in the first acellular lavage fluid using an enzyme-activity-based assay kit performed on the COBAS MIRA Plus autoanalyzer (Roche Diagnostics) according to the manufacturer’s instructions. Protein measurement data are presented in Supplemental File 3.
Histology
Animals were euthanized as described earlier and unlavaged lungs were inflated with formalin, routinely processed for light microscopy, and stained with hematoxylin and eosin (H&E), Masson’s trichrome, and periodic acid Schiff (PAS)/Alcian blue. Pathologic features over a wider range that reflected inflammation, fibrosis, small-airway disease, emphysema, and premalignant changes were graded on a 5-point scale, combining severity and extent of disease (0, no effect, to 5, a severe effect), by two board-certified pathologists with extensive experience of animal toxicology, using standardized criteria developed by NIOSH pathologists (Hubbs et al. 2002; 2008). Interobserver correspondence was assessed using Pearson’s r. Correlation was good (Supplemental File 4) except in the case of the granulomas feature in the comparative study, where the scores all grouped at or below 1. The dust in the lungs was evaluated by incident and polarized light on a scale of 0–3 for birefringent and opaque particles to establish that the animals had been dosed correctly.
Statistics
Data were checked for normality of distribution using the Shapiro–Wilk test. Data that were normally distributed (total cell counts, AM number, levels of total protein and albumin) were analyzed using a one-way analysis of variance (ANOVA) with post hoc Dunnett’s t-test where variances were equal (p < .05). Data that were not normally distributed (PMN leukocyte counts, lymphocyte counts, LDH activity, and all histopathology endpoints) were analyzed using the nonparametric Kruskal–Wallis test with a post hoc Wilcoxon ranks test. No multitest correction was performed. For the Kruskal–Wallis test, animals were analyzed for differences across dusts within exposure levels, using all time points for each exposure level employing the average of the two pathologists’ scores. Each day plus dose exposure condition had its own controls; that is, day 7 + 5 mg/kg and day 7 + 10 mg/kg each had its own unexposed control. For d 1–7 in the time-course experiment, data from control animals for all dust exposure conditions within each day (n = 8) were pooled to increase the number of control animals for comparison. These conditions were originally intended only for range-finding, and some exposures used only one control animal per day. Otherwise, in the time-course project, exposed animals were compared with day + dose matched (dose-matched) controls. In the comparative project, experimental animals were compared with day-matched controls. Data were analyzed using SPSS v. 16 (SPSS, Inc., Chicago IL) or R 3.0.0 (http://www.r-project.org). The criterion for significance was set at p < .05.
RESULTS
Dust Analysis
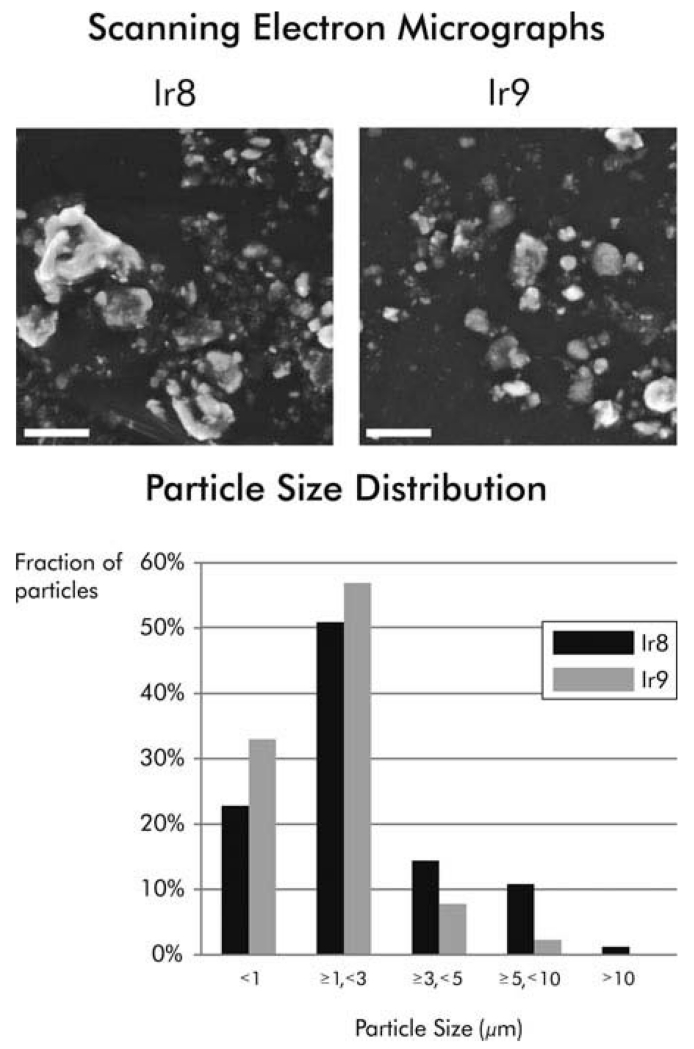
The Ir8 and Ir9 dusts from Camp Victory were first characterized and found to be similar in appearance, size distribution (Figure 1), and composition, but different in chemical constituency from the Ku and NIST dusts (Table 1). The Ir8 and Ir9 dusts contain 3.5 and 8.6 wt% silica, respectively, and 3.3 and 8.6 wt% quartz. No cristobalite or tridymite was detected. Consistent with observations by Engelbrecht and coworkers (2009a), freshly fractured quartz was not observed. The primary elements detected in Ir8 and Ir9 were calcium (Ca) (11.8%), iron (Fe) (2.8%), magnesium (Mg) (2.4%), and aluminum (Al) (2%), in descending order. Similarly, ambient PM2.5 samples collected at Camp Victory in 2006–2007 contained primarily Ca, Al, Fe, and Mg (Engelbrecht et al. 2009a). Ca, Cu, Mg, and Zn were abundant in the Ku material, and NIST PM contained primarily Ca, S, Fe, and Al, with similar amounts of Fe and Al. (Table 1). High levels of lead were also found in the NIST dust (National Institute of Standards and Technology 2012).
FIGURE 1.
Physical characterization of Ir8 and Ir9 dusts. (A) Representative scanning electron micrographs of dusts collected at Camp Victory in 2008 (Ir8) and 2009 (Ir9) are shown. Magnification is 8000×. (B) The particle size distribution of the dusts was determined using scanning electron microscopy. Scale bars are 10 μm.
TABLE 1.
Elemental Composition of Dusts
| Camp Victory 2008 (Ir8) |
Camp Victory 2009 (Ir9) |
Camp Buehring (Ku) |
NIST 1648A |
||||
|---|---|---|---|---|---|---|---|
| Element | mg/kg | LOD/LOQ (mg/kg) |
mg/kg | LOD/LOQ (mg/kg) |
mg/kg | LOD/LOQ (mg/kg) |
Mean±SD (mg/kg) |
| Aluminum | 19500 | 2.0/8.2 | 19000 | 20/69 | 6600 | 8/30 | 34,300±1300 |
| Antimony | ND | 4/14 | (10) | 10/45 | (27) | 20/68 | 45±1 |
| Arsenic | ND | 7/23 | ND | 20/52 | ND | 20/68 | 116±4 |
| Barium | 180 | 0.2/0.66 | 160 | 0.20/0.72 | 190 | 0.3/1.3 | |
| Beryllium | ND | 0.1/0.45 | 0.28 | 0.04/0.13 | ND | 0.5/0.22 | |
| Boron | 161±9 | ||||||
| Bromine | 502±10 | ||||||
| Cadmium | 1.4 | 0.2/0.8 | 1.0 | 0.20/0.63 | (0.84) | 0.8/2.6 | 74±2 |
| Calcium | 118,000 | 4/15 | 110,000 | 90/310 | 150,000 | 20/62 | 58400±1900 |
| Cerium | 55±2 | ||||||
| Cesium | 3.4±0.2 | ||||||
| Chlorine | 4543±47 | ||||||
| Chromium | 95 | 1.0/3.3 | 83 | 0.4/1.4 | 39 | 0.8/2.5 | 102±13 |
| Cobalt | 20 | 0.2/1.0 | 18 | 0.5/1.5 | 6.7 | 0.3/1.2 | 18±0.7 |
| Copper | 99 | 0.4/1.3 | 120 | 0.30/0.85 | 640,000 | 20/59 | 610±70 |
| Iron | 27,700 | 5/16 | 26,000 | 2.0/5.9 | 9900 | 20/84 | 39,200±2100 |
| Lanthanum | ND | 0.2/0.63 | ND | 0.3/0.97 | 7.6 | 0.5/2.2 | 39±3 |
| Lead | 52 | 2.0/6.2 | 74 | 4/15 | 42 | 5/14 | 6600±300 |
| Lithium | (16.9) | 0.7/2.4 | 31 | 0.5/1.7 | 8.7 | 1.0/3.2 | |
| Magnesium | 23,600 | 1.0/3.7 | 24,000 | 2.0/5.8 | 19,000 | 3/12 | 8100±100 |
| Manganese | 703 | 0.3/1.0 | 680 | 0.04/0.12 | 270 | 2.0/5.1 | 790±44 |
| Molybdenum | (7.7) | 1.0/3.6 | ND | 1.0/4.9 | ND | 3.0/9.2 | |
| Nickel | 145 | 0.8/2.6 | 130 | 3.0/9.4 | 54 | 3/11 | 81±7 |
| Phosphorus | 1075 | 10/35 | 710 | 7/23 | 4400 | 10/43 | |
| Potassium | 5290 | 5/15 | 5900 | 90/300 | 1600 | 100/320 | 10600±500 |
| Rubidium | 51±2 | ||||||
| Selenium | ND | 10/40 | ND | 30/97 | ND | 50/180 | 28±1 |
| Silicon | 12,800±400 | ||||||
| Silver | ND | 0.3/1.0 | ND | 0.2/0.5 | (3.6) | 1.0/3.8 | 6±0.3 |
| Sodium | 4240±60 | ||||||
| Strontium | 327 | 0.2/0.51 | 320 | 0.20/0.59 | 2600 | 0.08/3.0 | 215±17 |
| Sulfur | 55100±3600 | ||||||
| Tellurium | (31)* | 5/15 | (15) | 7/25 | (23) | 10/35 | |
| Thallium | (16)* | 3/20 | ND | 20/50 | ND | 20/76 | |
| Tin | ND | 4/13 | ND | 4/13 | ND | 8/27 | |
| Titanium | 478 | 0.2/0.51 | 220 | 0.08/0.27 | 260 | 0.5/1.4 | 4021±86 |
| Tungsten | 4.6±0.3 | ||||||
| Vanadium | 63 | 0.3/1.1 | 58 | 0.4/1.4 | 38 | 0.8/3.0 | 127±11 |
| Yttrium | 12 | 0.1/0.48 | 11 | 0.09/0.30 | 5.8 | 0.08/0.26 | |
| Zinc | 113 | 0.6/2.0 | 120 | 0.4/1.2 | 12000 | 3/10 | 4800±270 |
| Zirconium | 15 | 0.9/2.9 | 11 | 0.2/0.5 | 5.7 | 0.20/0.65 | |
Note. The elemental composition of the SWA dusts was determined as described in Methods. For the SWA dusts, values in parentheses are estimates lying between the limit of detection (LOD) and limit of quantitation (LOQ). ND indicates not detected. The composition of the NIST material was determined by NIST. Empty cells indicate that an assay was not done.
Using light microscopy, it was confirmed that Ir8, Ir9, Ku, and NIST dusts had been successfully instilled into the lungs of the animals and were uniformly distributed to all lobes and deposited in the distal lung (alveolar ducts and alveoli). Occasional particles and granulomas were observed in the pulmonary lymph nodes. The amount of visible Ir8 and Ir9 dust in the lungs declined over the course of the study.
By light microscopy, both opaque and birefringent particles, some bright and elongate consistent with silicates, were noted in lungs of Ir8- and Ir9-exposed animals. With polarized light, dust was seen to consist of a mixture of opaque, possibly carbonaceous, particles that were very fine together with mineral particles showing a range of shapes, birefringence brightness, and color, indicating a mixture of silicates and silica. Spore-like particles and fragments of biologic material were also occasionally seen in the lungs of Ir8 dust-exposed animals. Camp Victory was situated near Baghdad, Iraq, and urban combustion products are likely present in the material, although this was not determined. Engelbrecht and coworkers (2009a) described particles in ambient dust from Camp Victory that seem to be combustion products from oil, gasoline, and/or natural gas. The appearance of the Ir9 sample was similar to the Ir8 sample. No ferruginous particles were seen. The NIST dust-exposed animals showed numerous brown and blackish opaque particles within AM, as well as round to ovoid structures measuring 5–10 μm in diameter that stained with PAS, suggesting these might be spores or other biological material. The Ku dust-exposed animals displayed a diffuse brownish discoloration of the cytoplasm of AM but minimal opaque or birefringent material by light microscopy. Particles were not visible in the lungs of silica-exposed animals, in keeping with the weak birefringence of crystalline silica and the small particle size of this dust. None of the control rats demonstrated evidence of dust deposits in their lungs by either light or polarized microscopy.
BALF Cytology and Biochemistry
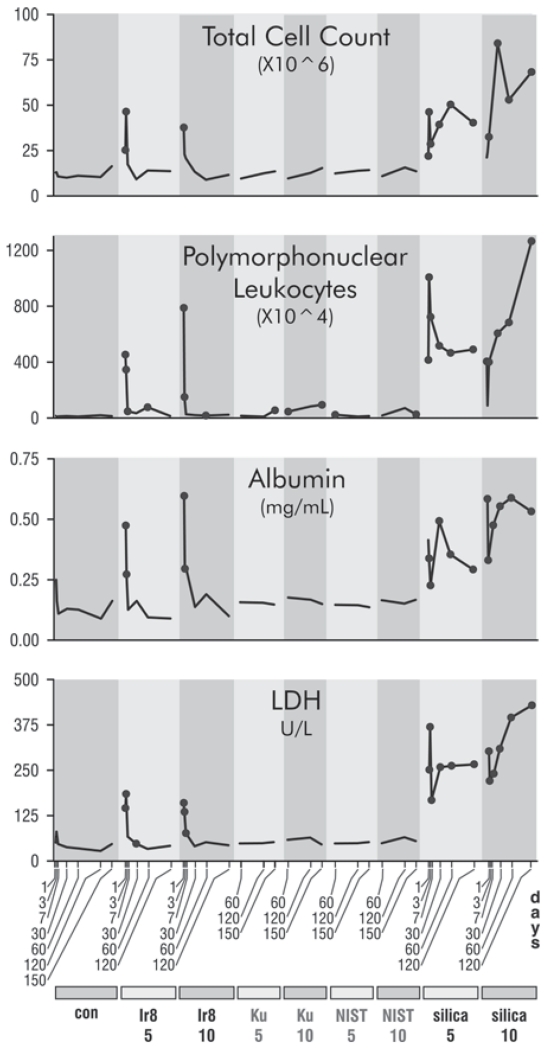
To characterize cell infiltration and inflammation, total and differential cell counts from the combined lavage samples for each animal were determined. The cell counts for the Ir8 dust were initially high, but rapidly declined (Figure 2). In contrast, silica stimulated an increased cell count that remained high throughout the study period. The total cell count was dominated by AM at all times (Supplemental File 2), but the overall pattern of initially elevated but falling cell counts holds generally true for lymphocytes and also PMN leukocytes (Figure 2 and Supplemental File 2). The observed response to silica is consistent with the known mechanisms of silica-mediated toxicity (Castranova et al. 2002).
FIGURE 2.
Time courses of responses in BALF to dust instillation. Dots indicate time points that are statistically different from matched controls for each time point at p ≤ .05 in a Kruskall–Wallis test with a Wilcoxon ranks post hoc analysis. No multitest correction was performed. Dusts are indicated at the bottom of the plot, con (PBS control), Ir8 (Camp Victory 2008 dust collection), Ku (Camp Buehring dust), NIST (U.S. urban dust), and silica (silica). The numbers below the dust identifiers indicate the amount of dust exposure (mg/kg bw). Gray dust identifiers indicate data from the comparative experiment and black from the time course experiment. Gray dust identifiers indicate data from the comparative experiment and black from the time course experiment.
To assess vascular and tissue damage, albumin, total protein, and LDH activity levels were determined in the first acellular lavage samples (LDH and albumin are shown in Figure 2; also see Supplemental File 3). BALF albumin levels were significantly increased in Ir8- and silica-exposed rats on d 1 and 3, but thereafter only silica-exposed rats exhibited elevated albumin levels. Total protein, which closely tracked albumin levels, was significantly elevated in Ir8- and silica-exposed rats in the first week, but only in silica-exposed rats afterward (Supplemental File 3). The continued elevation of total protein and albumin levels in the silica-exposed rats resulted from persistent increased vascular permeability, indicative of a continuing inflammatory response (Cotran and Majno 1964). The decreased levels of total protein and albumin after the first week in the Ir8-exposed rats suggest that the initial inflammatory response produced by dust exposure had resolved. Lavage LDH activity levels were significantly increased in both the Ir8- and silica-exposed rats on d 1 and 3, although more pronounced in silica-exposed rats. After the first week, only silica-exposed rats exhibited elevated LDH activity levels demonstrating continuing tissue injury. There were no significant alterations in albumin or LDH seen in lavage samples from Ir9, Ku, and NIST exposures.
While the effects of Ir9, Ku, and NIST dust at early time points were not investigated, the similarity of the composition of Ir8 and Ir9 suggests that responses would be similar, and Wilfong et al. (2011) noted a similar pattern of response previously for Ku material with early indications of a severe inflammatory response over the first 3 d that declines to control levels by 6 mo.
There are clear indications of an early acute inflammatory response and potential tissue injury following exposure to both Iraq dust and silica. However, the duration and magnitude of this response are markedly greater following silica than Iraq dust exposure. At the later time points, there is evidence of low-level inflammation manifested as a small persistent increase in PMN leukocyte number for all the dusts.
Histology
Inflammation
Inflammation was assessed by evaluating the tissue sections for alveolitis, the presence and extent of granulomas, interstitial inflammation, macrophage infiltration, and alveolar lipoproteinosis. Although an occasional control rat showed a small focus of chronic inflammation, none of the control animals showed acute or chronic inflammation or evidence of dust deposits. Figure 3 depicts a section of a normal rat lung at 150 d for reference.
FIGURE 3.
Normal lung from animals 150 d after saline exposure. A hematoxylin and eosin stained section of whole rat lung (4×) views is shown. A terminal bronchiole (TB) and a few alveolar ducts (AD) are indicated.
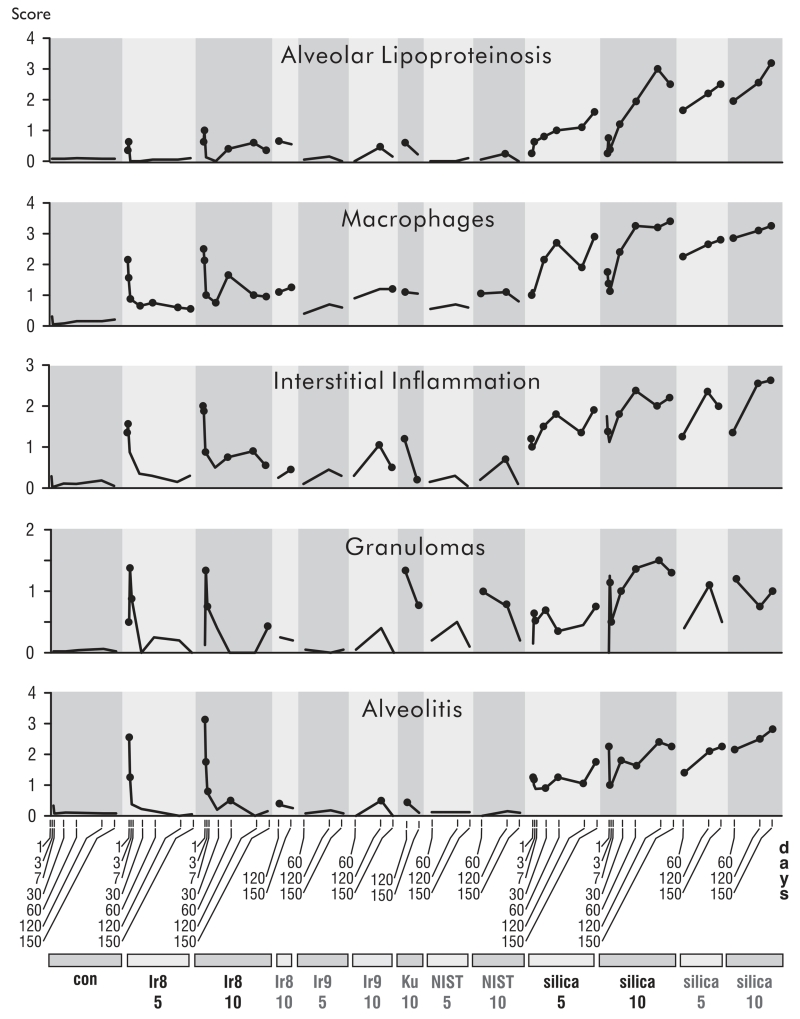
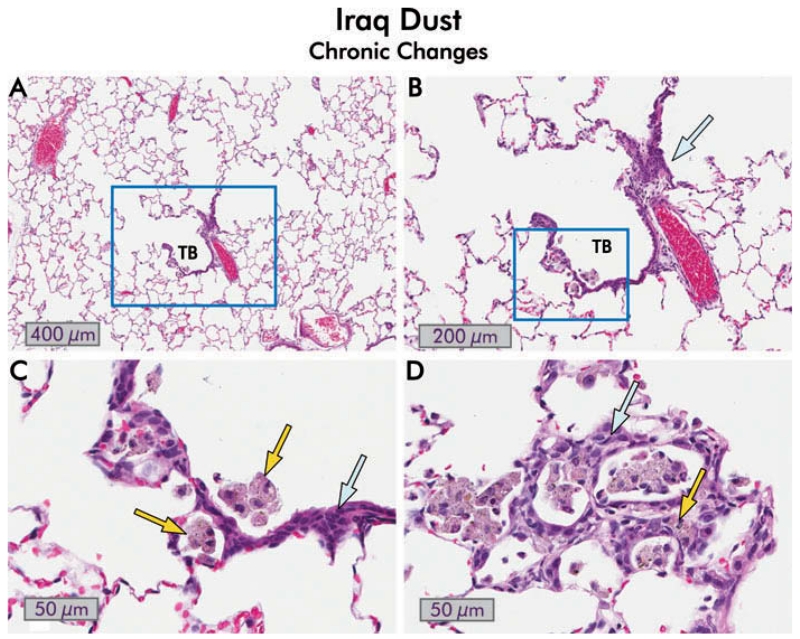
The histological observations over the time course of inflammation paralleled the cytological and biochemical analysis of BALF. Animals exposed to Ir8 dust showed an intense, early inflammatory response that largely subsided by 7 d (Figure 4). This early inflammatory response included alveolar wall damage with edema fluid and extensive eosinophilic and neutrophilic infiltrates (Figure 5). Occasional granulomas were also observed. Macrophages showing degenerative changes including foamy cytoplasm and apoptosis were also elevated in Ir8-exposed rats in the first week but declined to minimal levels thereafter (Figure 6).
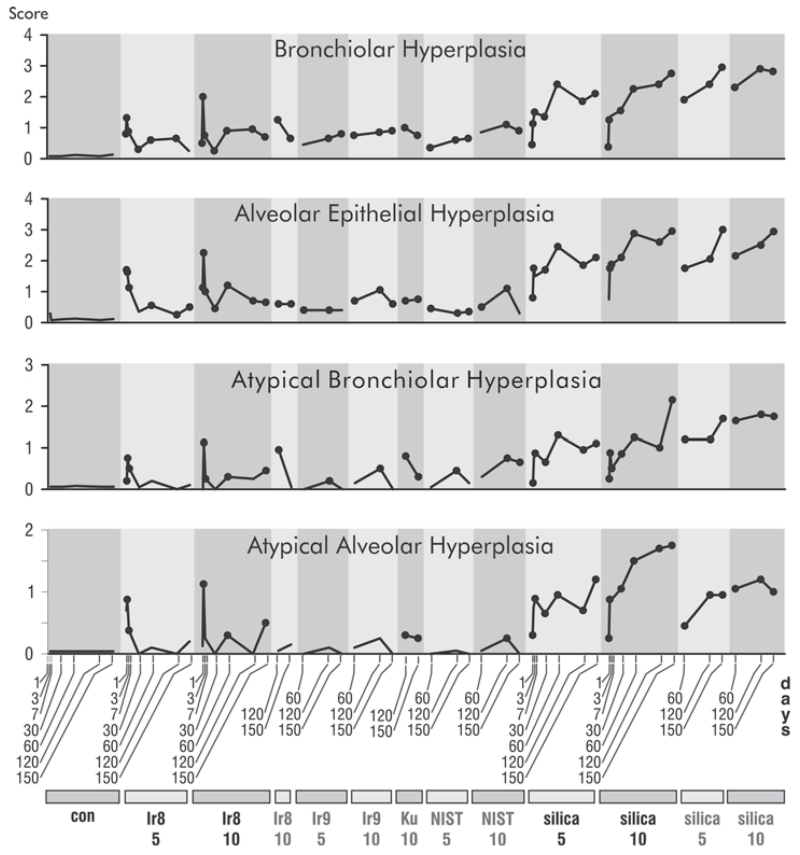
FIGURE 4.
Time courses of responses in inflammatory histopathological features to dust instillation. The vertical axis is the average semiquantitative severity score. Dots indicate time points that are statistically different from matched controls (p ≤ .05) in post hoc testing without multitest correction. The scores for the unexposed controls for all conditions and experiments were combined. Dusts are indicated at the bottom of the plot, con (PBS control), Ir8 (Camp Victory 2008 dust collection), Ir9 (2009 Camp Victory dust collection), Ku (Camp Buehring dust), NIST (U.S. urban dust), and silica (silica dust). The numbers below the dust identifiers indicate amount of dust instilled (mg/kg bw). Dust identifiers in black indicate results from the time course experiment, and gray identifiers indicate results from the comparative study.
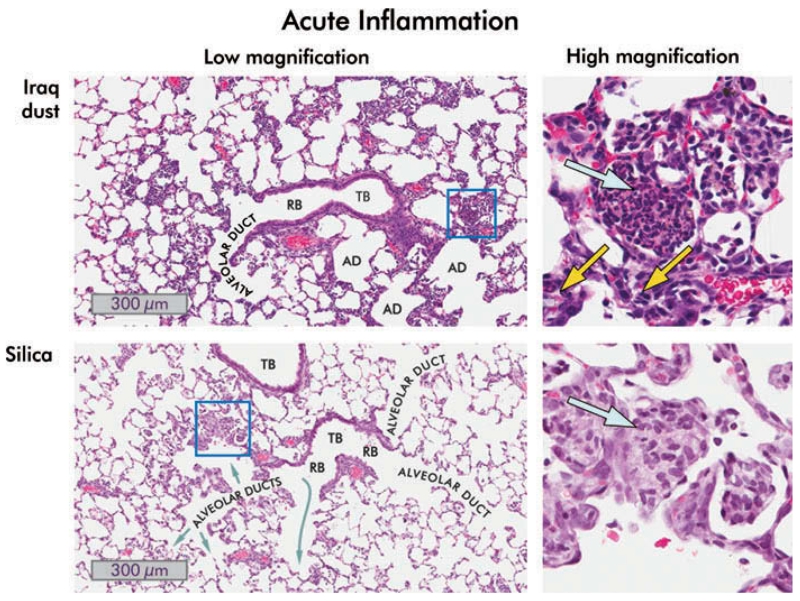
FIGURE 5.
Inflammation in the lungs of rats one day following instillation of 10 mg/kg bw Iraq or silica dust. Low- (8×) and high-magnification (40×) views of hematoxylin and eosin stained lung sections are shown. Iraq dust: A terminal bronchiole (TB), a respiratory bronchiole (RB), and alveolar ducts (AD) are shown. Many alveoli adjacent to the bronchiole are filled with inflammatory cells, predominately neutrophils and eosinophils. In the higher magnification view of the boxed area, multinucleated polymorphonuclear leukocytes and eosinophils are present (blue arrow), and acute inflammatory cells are visible in the interstitium (yellow arrows). Silica: The low-magnification image contains two small terminal bronchioles together with alveolar ducts. The alveoli are filled with neutrophils and, predominately, macrophages. The high-magnification view of the boxed area shows the intra-alveolar exudate in which macrophages predominate (blue arrow). The epithelium lining the alveoli is hyperplastic.
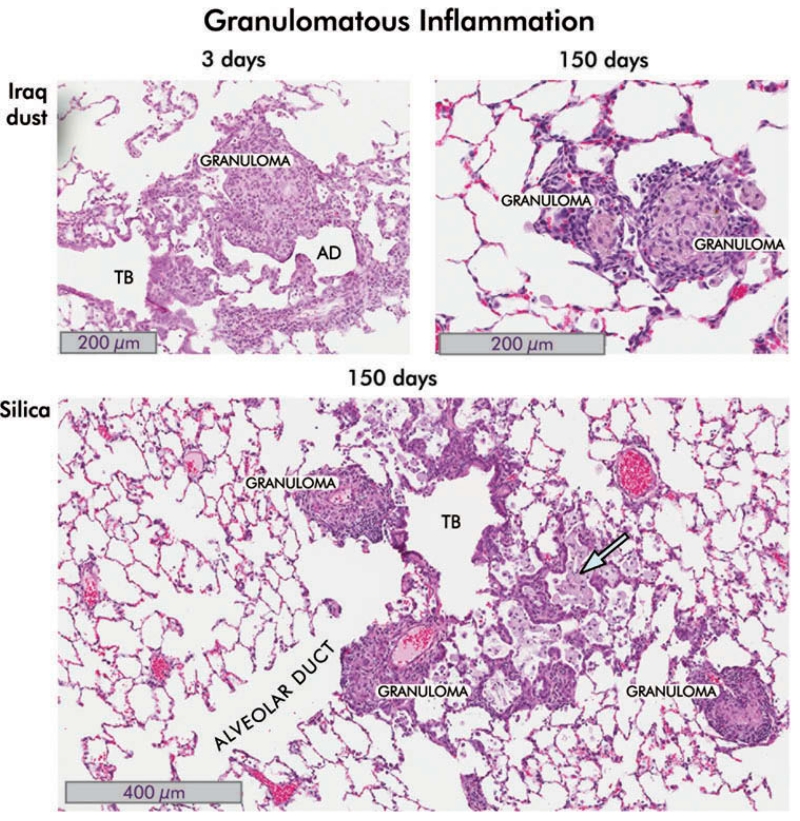
FIGURE 6.
Granulomatous inflammation in the lungs of rats instilled with Iraq and silica dusts. Hematoxylin and eosin-stained sections are shown. Iraq dust. At 3 d following intratracheal injection of 5 mg/kg bw of Ir8 dust, there is a localized inflammatory response around the alveolar ducts (AD) in which a granuloma is seen. The surrounding alveoli appear relatively normal. There is moderate interstitial acute and chronic inflammation around the lesion together with alveolar epithelial hyperplasia. The terminal bronchiole (TB) is indicated (8×). By 150 d, the inflammation is substantially reduced (see Figures 3 and 4 and the following). In the section shown, two small granulomas are seen, persisting in an otherwise normal lung. Incident light microscopy revealed brown and black particles and polarizing light microscopy revealed birefringent particles consistent with silicates (20×). Silica. In this section of lung taken from rat 150 d after instillation of 150 mg/kg bw of silica dust, there are several well-formed granulomas, intra-alveolar foamy macrophages (blue arrow), interstitial and intra-alveolar inflammation, distortion of the small airways and bronchiole, and alveolar epithelial proliferative changes (8×).
In contrast, silica-exposed rats displayed a progressive increase in inflammation over the entire 150-d experiment for all features evaluated (Figures 2 and 4). The acute alveolitis was primarily neutrophilic. It was accompanied by a substantial macrophage response that rose over the first 30 d and persisted at a high level through 150 d. Foamy macrophages showing apoptosis were commonly seen, and individual macrophage cell necrosis with apoptotic bodies associated with particle phagocytosis were also found. Interstitial inflammation was progressive, was primarily lymphocytic, and centered on small blood vessels. Granulomas were seen at all time periods in silica-exposed animals both in lung parenchyma and in pulmonary lymph nodes (Figures 5, 6, and 7, panels A–D).
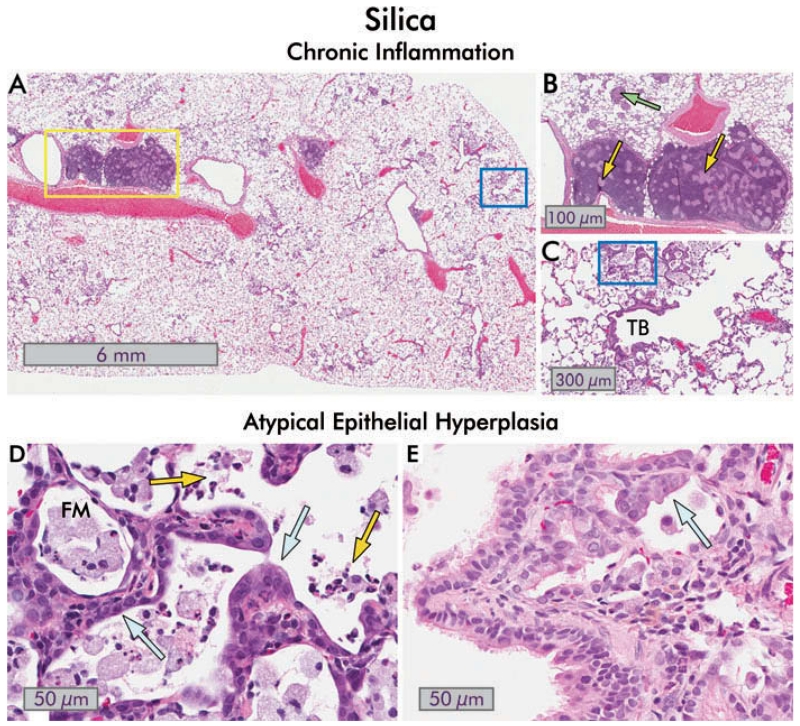
FIGURE 7.
Chronic inflammation, small airways disease and bronchiolar epithelial changes in silica-exposed rats. (A) Section of hematoxylin and eosin stained rat lung 150 d after exposure to 10 mg/kg bw silica dust. Areas of focal inflammation are present, and enlarged; inflamed lymph nodes are obvious (yellow box; 1×). (B) Close-up view of the enlarged peribronchial lymph nodes from (A). Pale granulomas are present within the lymph nodes (yellow arrows), and discrete granulomas are also seen in within the lung parenchyma (green arrow; 8×). (C) Higher magnification view of blue boxed area in (A). There is continuing inflammation with collections of foamy macrophages in the alveoli, foci of lipoproteinosis, and chronic and intra-alveolar inflammation. The terminal bronchiole (TB) is distorted and slightly constricted. Mild interstitial fibrosis is present (8×). (D) Close-up of boxed area in (C) showing atypical alveolar epithelial hyperplasia (blue arrows). The presence of degenerate foamy macrophages (FM) and acute inflammatory cells (neutrophils and eosinophils; yellow arrows) in the alveoli indicates continued toxicity (40×). (E) An example of atypical bronchiolar epithelial hyperplasia in the lung of a different rat 120 d after exposure to 10 mg/kg bw silica is shown. The bronchiolar epithelium extends into the alveoli with abnormal orientation of the epithelium, and cytological atypia are evident (blue arrow; 40×).
Somewhat variable evidence of low-level inflammation at 60-150 d was observed for all dusts other than silica, based on macrophage responses and the presence of granulomas (Figure 4). Macrophages and granulomatous inflammation were more frequent in NIST and Ku dust-exposed animals and were associated with sites of dust deposition. In the NIST and Ku dust-exposed animals, granulomas were often of the foreign body type.
Proliferative and Preneoplastic Changes
Alveolar epithelial and bronchiolar hyperplasia were initially marked in the presence of Ir8 dust (Figure 8; see Figure 6, Iraq dust) but declined to negligible levels over the course of the experiment. In contrast, there was a progressive increase in these features in silica-exposed animals (Figure 8; see Figure 6, silica). All dusts produced some degree of alveolar epithelial and bronchiolar hyperplasia, but the response was strongest in silica-exposed animals. Bronchiolar hyperplasia was largely confined to the proximal acinar zone, the region of maximal dust deposition.
FIGURE 8.
Time courses of proliferative and pre-neoplastic changes in response to dust exposure. Legend is as in Figure 4.
Low levels of atypical alveolar and bronchiolar hyperplasia (Figure 8) were noted in the Ir8-exposed animals shortly after IT instillation, but a tendency to decline over the course of the experiment was found. In the case of silica-exposed animals the magnitude of the response increased throughout the study (Figure 8; see Figure 7D and 7E). These forms of dysplasia are known to be premalignant in the rat (Green et al. 2007), but as the Iraq, NIST, and and Ku dusts cleared relatively rapidly, with almost complete resolution of the proliferative epithelial changes, it is unlikely that these lesions would progress to adenomas and adenocarcinomas.
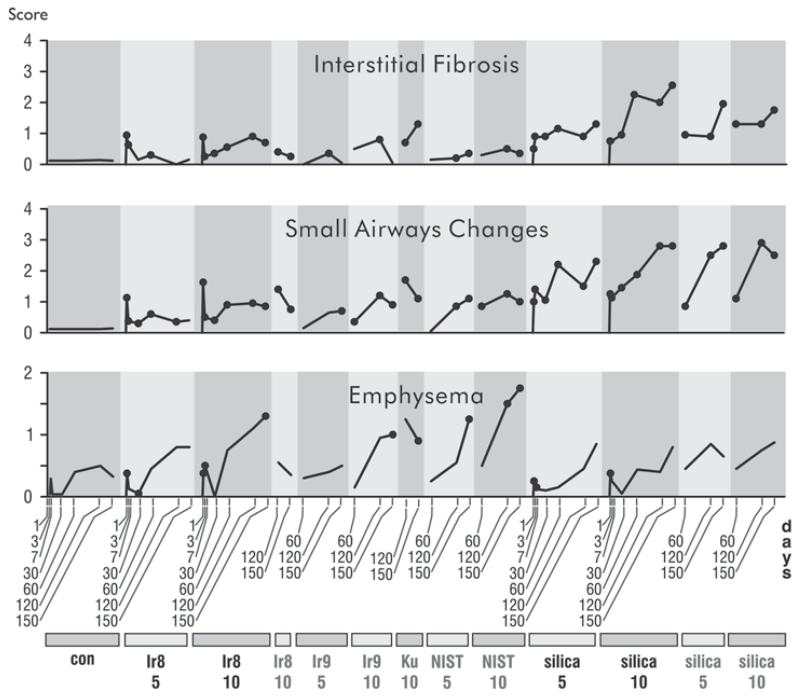
Emphysema, Small-Airway Changes, and Interstitial Fibrosis
Although some older control animals displayed enlarged air spaces, rats exposed to Iraq and Ku dusts showed significantly more emphysema (Figure 9) than controls. The emphysema was centriacinar, primarily involving the alveolar ducts (Figure 10). Emphysema was most severe in animals exposed to the U.S. urban dust from St. Louis (NIST) and least severe in the silica-exposed animals.
FIGURE 9.
Time courses of emphysematous, interstitial fibrotic, and small airways responses to dust exposure. Legend is as in Figure 4.
FIGURE 10.
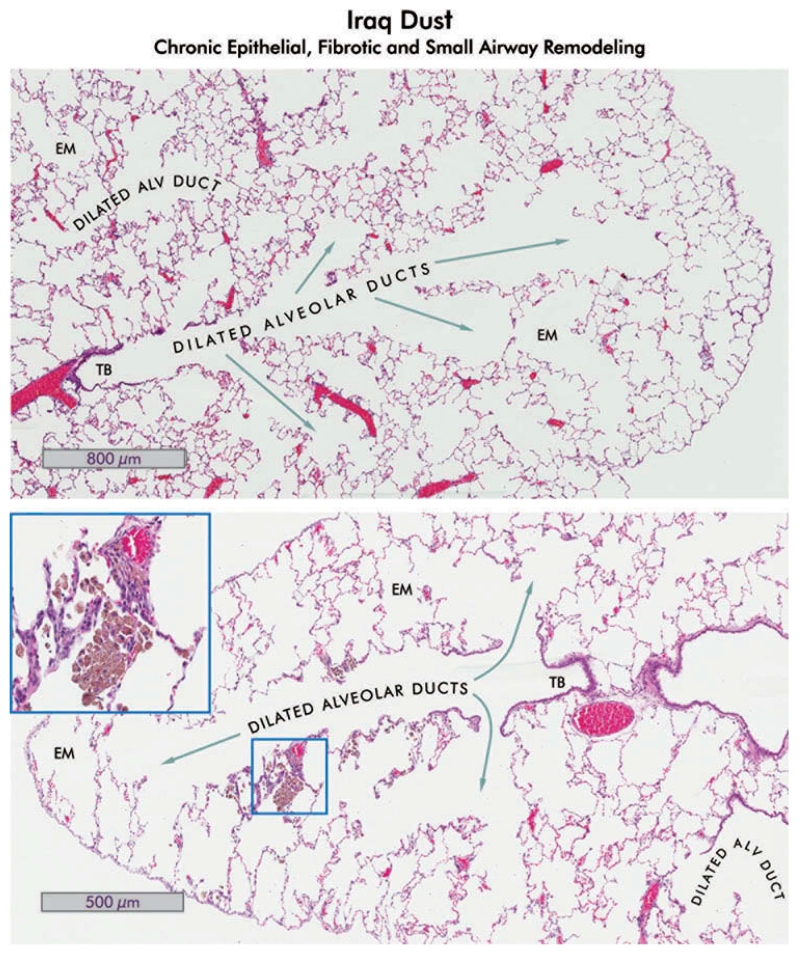
Alveolar duct dilation, emphysema, and Iraq dust in lungs of rats which received 10 mg/kg bw Ir8 dust, 150 d after exposure. Hematoxylin and eosin-stained sections of rat lung are shown. Upper: The lung shows dilation of alveolar ducts and emphysema (EM). No lesions of pneumoconiosis are present, indicating total clearance of the dust in this region. The terminal bronchiole is indicated (TB; 4×). Lower: The central bronchiole with small airways extending nearly to the pleura are shown in the lung of a different rat. The bronchiole and alveolar ducts are dilated, and small areas of emphysema (EM) are evident (4×). There are small clusters of pigmented macrophages containing dust in some of the alveoli (inset, 40×). The epithelium adjacent to the macrophages is slightly hyperplastic and the interstitium is mildly thickened. Compare with the normal lung shown in Figure 3.
Small airway lesions comprising inflammatory, fibrotic, and architectural remodeling were seen in the distal terminal respiratory bronchioles and proximal alveolar ducts of all dust-exposed animals (Figure 9). The lesions were present at 60 d postexposure and were well established at 120 and 150 d. The fibrotic component predominated in the silica-exposed animals, where it was associated with narrowing of the alveolar ducts (Figure 7C). In contrast, the small airway lesions in the Ir8, Ir9, NIST, and Ku dust-exposed animals were associated with dilation of the proximal alveolar ducts (Figure 10). In these animals the small airway lesions developed at sites of maximal dust deposition, even though at 60 d the dust had been largely cleared from those sites (Figure 11). At earlier time points, there was evidence of a marked acute inflammatory reaction to all treatments in this region of the lung.
FIGURE 11.
Chronic changes following exposure to Iraq dust. (A) Hematoxylin and eosin stained section of a rat lung 150 d after exposure to 10 mg/kg bw Iraq dust. The terminal bronchiole (TB) in this section appears normal although the associated alveolar ducts are slightly dilated, and there is mild emphysema. There is minimal inflammation and fibrosis (2×). (B) Close-up of boxed area in (A). The terminal bronchiole appears normal, and there is no extension of the bronchiolar epithelium into the alveoli. There is mild interstitial inflammation (blue arrow) and focal accumulation of macrophages in the boxed area (8×). (C) Higher magnification view of the boxed area in (B). There are a few alveolar macrophages containing dust (yellow arrows), and mild alveolar hyperplasia of the epithelium adjacent to the macrophages (yellow arrows), but no evidence of atypia (40×). (D) Alveolar changes in the lung of a different rat 150 d after exposure to Iraq dust. Only very occasional accumulations of dust in macrophages were evident in this animal. The epithelial changes are hyperplastic with minimal dysplasia (blue arrow). There is mild chronic inflammation (yellow arrow) but no evidence of fibrosis (40×).
Silica was the most fibrogenic of all dusts. In silica-exposed animals fibrosis extended out from the centriacinar region and progressed over time. In contrast, the other dusts produced a mild fibrotic reaction in the centers of acini with minimal progression. Interestingly, there seemed to be an inverse relationship between fibrosis and emphysema, perhaps because the fibrosis stiffens the interstitium and prevents dilation.
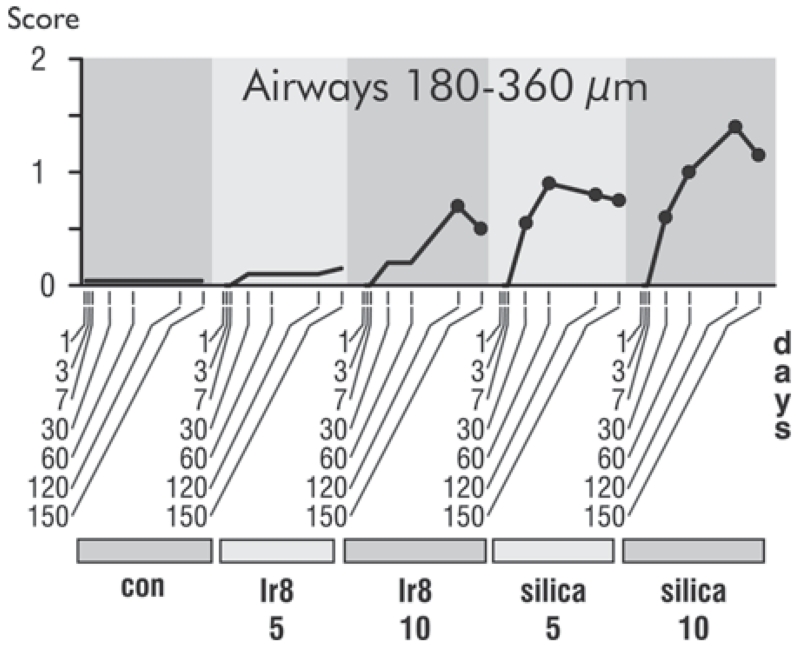
Effects in Midsized Airways and Vasculature
Because King et al. (2011) reported an association between deployment to SWA and constrictive bronchiolitis in a series of cases at Vanderbilt University Medical Center, airways 180–360 μm in diameter were examined for evidence of this feature (Figure 12). Constrictive bronchiolitis was not seen in this study in the sense of circumferential fibrosis with luminal compromise. However, mild peribronchiolar chronic inflammation with mild fibrosis did occur at the highest dose of Ir8 dust and in silica-exposed animals. King and colleagues (2011) also reported changes in pulmonary arterioles. Lungs of the rats were carefully examined in this study in an effort to determine whether similar lesions were present, but the rats tended to display variable pulmonary arteriole structure and artery anatomy; many of the control rats showed some thickening of their pulmonary arteries. Differences in the vasculature appeared to be age related (data not shown).
FIGURE 12.
Time courses of changes in mid-sized airways in response dust exposure. Legend is as in Figure 4.
DISCUSSION
Dust Composition and Toxicity
Ir8, Ir9, NIST, and Ku are mixed-composition dusts containing a relatively small amount of crystalline silica that does not appear to be freshly fractured and may be embedded in clay minerals (Engelbrecht et al. 2009a). The distribution of elements in the Ir dust resembles that of the Ku dusts (Table 1), and while measurements of the content of the Camp Victory soil used by Dorman et al., (2012) were not presented, the composition of Camp Victory soil and ambient dust is similar (Engelbrecht et al. 2009a; 2009b). The dusts also appear to have a large clay mineral component based on mineralogical analysis (Engelbrecht et al. 2009a; 2009b) and elemental composition (National Institute of Science and Technology 2012). Given the similarities in composition of the mixed Ir, Ku, and even the NIST dusts, it is not surprising that the observed responses to exposure to these dusts are similar in comparison with the response to pure crystalline silica. There are several possible reasons for this difference in toxicity. The NIST and SWA dusts are mixed, with clay components that may moderate the effects of silica (Chen et al. 2005; IPCS 2005; Love et al. 1999; Le Bouffant et al. 1988), and silica represents only a small fraction of the total SWA and NIST dust mass. Finally, the presence of silica may be masked from the lung if silica is embedded in clay minerals (Engelbrecht et al. 2009a).
The differences in the toxicities of silica, NIST, and SWA dusts might also be related to differences in clearance of dust from the lungs. Macrophages preferentially remove silica particles with modified or contaminated surfaces more effectively than pure silica (Rainey et al. 1994), and slow clearance of silica may have perpetuated the acute inflammatory, proliferative, and fibrotic responses in the lung. In contrast, the relatively rapid clearance of other dusts, as indicated by light microscopy, produced lesions confined to the center of the acinus (small-airway changes and emphysema) where dust was originally deposited.
Pulmonary Responses
While there was an initial inflammatory response in the environmental dust-exposed rats, there was also a substantial recovery, as shown by return of cell counts and protein levels in BALF to control or near control levels, consistent with progressive clearance of this dust over the course of the experiment. Silica also induced an initial acute inflammatory response, but the inflammation persisted for the 150 d of the study rather than resolving, and it was associated with proliferative, pre-malignant and fibrotic changes.
Similar results were reported with Asian desert dusts (Naota et al. 2013) and by Wilfong and coworkers (2011) using Ku dust at 7 d and 180 d following exposure. In our experiments, inflammation in animals exposed to the environmental dusts did not fully resolve in all rats even at the longest time points, presumably because of incomplete clearance of the dusts over the course of the experiment.
In general the inflammatory profiles in our exposures are similar to those that Wilfong et al. (2011) reported using either Ku dust or pure, fine TiO2 particles, a relatively inert material. Since the compositions of the ambient Ku, Ir, and NIST dusts differ somewhat from one another and from TiO2 particles, it seems likely that much, if not all, of the early inflammatory response observed in this study is related to particle deposition rather than to composition of particles. As a further example, a similar time course of inflammatory response was recently reported for nanosilver particles, another compositionally unrelated material (Seiffert et al. 2015).
Nevertheless, a possible contribution to the toxicity of the dusts from soluble components cannot be excluded since extracts of SWA dusts (including one from Camp Victory) have been shown to be cytotoxic and to provoke inflammatory responses when instilled into lungs of rats (Taylor et al. 2013). Soluble components have been implicated in the toxicity of other materials including inhaled fly ash and North American ambient dusts (Adamson et al. 2004; Chen and Lippmann 2009; Dreher et al. 1997; Prieditis and Adamson 2002; Pritchard et al. 1996). However, the bioavailablity of toxic chemicals in complex matrices needs to be considered in making risk assessments of exposure, since it can vary based on the composition of the material (Hamad et al. 2014).
While finding pulmonary emphysema in animals exposed to the U.S. urban dust is not surprising, it was striking that there was small-airways injury and emphysema in lungs of rodents exposed to the Camp Victory and Ku dusts. Wilfong and colleagues (2011) did not report any evidence of lung injury at their longest time point, 6 mo. In the present study, the emphysema effect was small and greatest at late time points; it is possible that differences between their study design and ours may account for the variation. In our study, it is noteworthy that emphysema was present even though few particles could be discerned in the areas of parenchyma with emphysema, indicating initial acute injury in its pathogenesis rather than ongoing irritation from persistent particles. Evidence for this concept is summarized in a review on the pathogenesis of chronic obstructive pulmonary disease by Tuder and Petrache (2012). The concept of self-amplifying injury loops following oxidative stress and inflammation is considered by some to account for the progression of emphysema despite cessation of exposure.
The body of data for the effects of the mineral component of field dusts on human lung histology is not extensive (Morman and Plumlee 2013), but Schenker et al. (2009) and Pinkerton et al. (2000) collected lungs at autopsy from agricultural workers from California’s Central Valley who had not died of respiratory disease, and evaluated the prevalence of disease and microscopic evidence of mineral dust exposure. Although a histopathological diagnosis of emphysema was statistically significantly associated with the presence of mineral dust in the lung, by an order of magnitude, pneumoconiosis and mineral dust small-airways disease had the highest odds ratios (adjusted for smoking and age) for association with mineral dust exposure. The field workers in these studies resided in the Central Valley for a few to more than 20 years, and repeated chronic exposure might account for differences from our observations in this study. Exposure and health risk data for the local populations in SWA are scant (Rabee 2014), and comparisons either with animal data or the limited human subjects data cannot presently be made.
King and colleagues (2011) described a bronchiolitis in a case series of soldiers seen at Vanderbilt University Medical Center that was characterized by thickening of membranous bronchioles with fibrosis, chronic inflammation and peribronchiolar deposition of grayish-black pigment, and luminal narrowing in 64% of airways in soldiers returning from Iraq and Afghanistan. Such a response in the exposed rats in our study was not detected. There were lesions in the small and mid-size airways, but these differed from those described in the soldiers returning from Iraq and Afghanistan. The differences in the lesions may be accounted for by differences in anatomy between human and rodent lungs or perhaps in routes of exposure. Rats were exposed to a single bolus injection, while soldiers would be exposed by inhalation occurring over several months or years. The soldiers were also exposed to wide array of potential inhalational hazards in the operational environment. King and colleagues (2011) suggested that exposures to various combustion products—burn pit smoke, gases and particles from explosives, incinerated human waste, gases from a large sulfur fire near where many of their patients were stationed—may have contributed to the development of disease. This study also reported changes in human pulmonary arterioles. The rats tended to display variable pulmonary arterioles and artery anatomy, and many of the control rats showed thickening of the arteries, and no differences could be discerned between arteries of rats exposed to Iraq dusts and control animals.
Large quantities of dusts were instilled into the lungs of the rats to provoke readily identifiable responses to the exposure. Wilfong et al. (2011) calculated that a deployed service member might inhale 1.7 mg/d of dust with a peak of 5.4 mg during dust storms. On a per unit body mass scale, rats in this study were exposed to 100- to 500-fold higher than that dose in a single exposure. Under these conditions, Iraq dust elicited an intense but brief acute inflammatory response followed by recovery. The intensity of the early inflammatory response suggests that cumulative exposures might lead to exacerbations of asthma, stimulation of preexisting conditions, or hypersensitivity pneumonitis. In the future it will be important to examine the effects of repeated or chronic exposures to lower level dust exposures in order to better understand the adverse consequences of exposure during deployments. In terms of risk assessments relevant to military service in SWA, the toxicity of dusts from other locations also needs to be evaluated, particularly since troops deployed to different locations in SWA demonstrated different rates of medical encounters for respiratory symptoms (Abraham et al. 2014). Further, possible interactions between exposures to ambient PM and burn pit combustion products (Institute of Medicine 2011) have not yet been adequately explored.
Limitations
Although this study determined the relative pulmonary toxicity of field dust from military sites in Southwest Asia, there are limitations to the experimental design. First, due to the limited amount of Iraq dust available, pulmonary exposure was by IT instillation rather than the preferred method of inhalation exposure. The appropriate use of IT instillation for toxicology testing has been addressed by a panel of experts from the Inhalation Specialty Section of the Society of Toxicology that concluded that IT instillation serves a valid mode in hazard identification (Driscoll et al. 2000). Second, service personnel in Iraq were exposed not only to field dust, but also to pit burning, spores, and other pollutants (Rose 2012; Korzeniewski et al. 2013). In light of the current study, evaluation of coexposure to field dust and particles generated during pit burning is warranted. Lastly, pulmonary exposure to particles was shown to produce dysfunction of the systemic and coronary microvasculature (LeBlanc et al. 2009; Nurkiewicz et al. 2004; Brocato et al 2014). Therefore, evaluation of the cardiovascular effects of pulmonary exposure to Iraq dust is of interest.
CONCLUSIONS
Our results appear to be generally consistent with reports associating deployment to SWA with postdeployment asthma, bronchitis and dyspnea, and with epidemiological findings of increased frequency of respiratory symptoms and asthma in deployed compared with nondeployed service members (Abraham et al. 2012; Smith et al. 2009; Szema et al. 2010; 2011). Although all these reports are in accord with one another, observations that increases in respiratory symptoms may be associated with deployment itself (Abraham et al 2014) and a recent analysis of self-reported data from the National Health Study for a New Generation of US Veterans (Barth et al. 2014), which found a rise in incidence of sinusitis but not bronchitis and asthma in veterans who had been deployed, confound this issue. The bulk of evidence presented here indicates that Iraq dust is not unusually toxic even when delivered in a large bolus, but the development of small airway lesions and emphysema in some animals late in the study raises the possibility that there may be persistent adverse effects following exposure to high levels of Iraq dust. Few U.S. service members are now deployed, but dust and other air pollution exposures continue for SWA nationals. Assessments of health risks from these exposures are beginning to be made, but both exposure and health data are limited (Rabee 2014), and the long-term consequences of exposure for military personnel who deployed to SWA and SWA nationals are uncertain.
Supplementary Material
Acknowledgments
FUNDING
This article is dedicated to the memory of Dr. Val Vallyathan. The research could not have been accomplished without the assistance of the following individuals from the U.S. Army Public Health Command: Joe Sutphin and Paul Hoppe, who ruggedized and tested the air samplers; LTC Ron Ross, who collected the samples in theater; and James Sheehy, who managed the acquisition of materiel and logistics. The authors gratefully acknowledge the technical support of Donna Pack and Christine Baer (Excet, Inc., USACEHR). The research described herein was sponsored by the U.S. Army Medical Research and Materiel Command, Military Operational Medicine Research Program.
Footnotes
DISCLAIMER
The views, opinions, and/or findings contained in this report are those of the authors and should not be construed as official National Institute for Occupational Safety and Health/Centers for Disease Control and Prevention (NIOSH/CDC) or Department of the Army position, policy, or decision, unless so designated by other official documentation. Citations of commercial organizations or trade names in this report do not constitute an official NIOSH/CDC or Department of the Army endorsement or approval of the products or services of these organizations.
Supplemental data for this article can be accessed http://dx.doi.org/10.1080/15287394.2015.1072611
The authors declare they have no competing interests.
Color versions of one or more of the figures in the article can be found online at www.tandfonline.com/uteh
REFERENCES
- Abraham JH, DeBakey SF, Reid L, Zhou J, Baird CP. Does deployment to Iraq and Afghanistan affect respiratory health of US military personnel? J. Occup. Environ. Med. 2012;54:740–745. doi: 10.1097/JOM.0b013e318252969a. [DOI] [PubMed] [Google Scholar]
- Abraham JH, Eick-Cost A, Clark LL, Hu Z, Baird CP, DeFraites R, Tobler SK, Richards EE, Sharkey JM, Lipnick RJ, Ludwig SL. A retrospective cohort study of military deployment and postdeployment medical encounters for respiratory conditions. Mil. Med. 2014;179:540–546. doi: 10.7205/MILMED-D-13-00443. [DOI] [PubMed] [Google Scholar]
- Adamson IY, Prieditis H, Vincent R. Soluble and insoluble air particle fractions induce differential production of tumor necrosis factor alpha in rat lung. Exp. Lung Res. 2004;30:355–368. doi: 10.1080/01902140490438933. [DOI] [PubMed] [Google Scholar]
- Alessandrini ER, Stafoggia M, Faustini A, Gobbi GP, Forastiere F. Saharan dust and the association between particulate matter and daily hospitalisations in Rome, Italy. Occup. Environ. Med. 2013;70:432–434. doi: 10.1136/oemed-2012-101182. [DOI] [PubMed] [Google Scholar]
- Almeida-Silva M, Almeida SM, Freitas MC, Pio CA, Nunes T, Cardoso J. Impact of Sahara dust transport on Cape Verde atmospheric element particles. J. Toxicol. Environ. Health A. 2013;76:240–251. doi: 10.1080/15287394.2013.757200. [DOI] [PubMed] [Google Scholar]
- Bar-Ziv J, Goldberg GM. Simple siliceous pneumoconiosis in Negev Bedouins. Arch. Environ. Health. 1974;29:121–126. doi: 10.1080/00039896.1974.10666548. [DOI] [PubMed] [Google Scholar]
- Barth SK, Dursa EK, Peterson MR, Schneiderman A. Prevalence of respiratory diseases among veterans of operation enduring freedom and operation Iraqi freedom: Results from the national health study for a new generation of U.S. Veterans. Mil. Med. 2014;179:241–245. doi: 10.7205/MILMED-D-13-00338. [DOI] [PubMed] [Google Scholar]
- Brocato J, Sun H, Shamy M, Kluz T, Alghamdi MA, Khoder MI, Chen L-C, Costa M. Pariculate matter from Saudi Arabia induces genes involved in inflammation, metabolic syndrome and atherosclerosis. J Toxicol Environ Health A. 2014;77:751–766. doi: 10.1080/15287394.2014.892446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castranova V, Porter D, Millecchia L, Ma JYC, Hubbs AF, Teass A. Effect of inhaled crystalline silica in a rat model: Time course of pulmonary reactions. Mol. Cell. Biochem. 2002;234/235:177–184. [PubMed] [Google Scholar]
- Chang CC, Chen PS, Yang CY. Short-term effects of fine particulate air pollution on hospital admissions for cardiovascular diseases: A case-crossover study in a tropical city. J. Toxicol. Environ. Health A. 2015;78:267–277. doi: 10.1080/15287394.2014.960044. [DOI] [PubMed] [Google Scholar]
- Chen LC, Lippmann M. Effects of metals within ambient air particulate matter (PM) on human health. Inhal. Toxicol. 2009;21:1–31. doi: 10.1080/08958370802105405. [DOI] [PubMed] [Google Scholar]
- Chen W, Hnizdo E, Chen JQ, Attfield MD, Gao PF, Hearl FF, Lu JF, Wallace WE. Risk of silicosis in cohorts of Chinese tin and tungsten miners, and pottery workers (I): An epidemiological study. Am. J. Ind. Med. 2005;48:1–9. doi: 10.1002/ajim.20174. [DOI] [PubMed] [Google Scholar]
- Cotran RS, Majno G. The delayed and prolonged vascular leakage in inflammation. Pathology. 1964;45:261–281. [PMC free article] [PubMed] [Google Scholar]
- Davidson CI, Phalen RF, Solomon PA. Airborne particulate matter and human health: A review. Aerosol Sci. Technol. 2005;39:737–749. [Google Scholar]
- Dorman DC, Mokashi V, Wagner DJ, Olabisi AO, Wong BA, Moss OR, Centeno JA, Guandalini G, Jackson DA, Dennis WE, Lewis JA, Thomas RS, Chapman GD. Biological responses in rats exposed to cigarette smoke and Middle East sand (dust) Inhal. Toxicol. 2012;24:109–124. doi: 10.3109/08958378.2011.647413. [DOI] [PubMed] [Google Scholar]
- Dreher KL, Jaskot RH, Lehmann JR, Richards JH, McGee JK, Ghio AJ, Costa DL. Soluble transition metals mediate residual oil fly ash induced acute lung injury. J. Toxicol. Environ. Health. 1997;50:285–305. [PubMed] [Google Scholar]
- Driscoll KE, Costa DL, Hatch G, Henderson R, Oberdorster G, Salem H, Schlesinger RB. Intratracheal instillation as an exposure technique for evaluation of respiratory tract toxicity: Uses and limitations. Toxicol. Sci. 2000;55:24–35. doi: 10.1093/toxsci/55.1.24. [DOI] [PubMed] [Google Scholar]
- Drummond K. [April 8, 2015];Ring of fire: Why our military’s toxic burn pits are making soldiers sick. The Verge. 2013 Oct 28; http://www.theverge.com/2013/10/28/4771164/the-next-agent-orange-why-burn-pits-are-making-soldiers-sick.
- Engelbrecht JP, McDonald EV, Gillies JA, Jayanty RK, Casuccio G, Gertler AW. Characterizing mineral dusts and other aerosols from the Middle East—Part 1: Ambient sampling. Inhal. Toxicol. 2009a;21:297–326. doi: 10.1080/08958370802464273. [DOI] [PubMed] [Google Scholar]
- Engelbrecht JP, McDonald EV, Gillies JA, Jayanty RK, Casuccio G, Gertler AW. Characterizing mineral dusts and other aerosols from the Middle East—Part 2: Grab samples and re-suspensions. Inhal. Toxicol. 2009b;21:327–336. doi: 10.1080/08958370802464299. [DOI] [PubMed] [Google Scholar]
- Esmaeil N, Gharagozloo M, Rezaei A, Grunig G. Dust events, pulmonary diseases and immune system. Am. J. Clin. Exp. Immunol. 2014;3:20–29. [PMC free article] [PubMed] [Google Scholar]
- Ghio AJ, Carraway MS, Madden MC. Composition of air pollution particles and oxidative stress in cells, tissues, and living systems. J. Toxicol. Environ. Health B. 2012;15:1–21. doi: 10.1080/10937404.2012.632359. [DOI] [PubMed] [Google Scholar]
- Green FH, Vallyathan V, Hahn FF. Comparative pathology of environmental lung disease: An overview. Toxicol. Pathol. 2007;35:136–147. doi: 10.1080/01926230601132055. [DOI] [PubMed] [Google Scholar]
- Hamad SH, Schauer JJ, Shafer MM, Al-Rheem EA, Skaar PS, Heo J, Tejedor-Tejedor I. Risk assessment of total and bioavailable potentially toxic elements (PTEs) in urban soils of Baghdad. Sci. Total Environ. 2014;495:39–48. doi: 10.1016/j.scitotenv.2014.06.006. [DOI] [PubMed] [Google Scholar]
- Hawass ND. An association between ‘desert lung’ and cataract—A new syndrome. Br. J. Ophthalmol. 1987;71:694–697. doi: 10.1136/bjo.71.9.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbs AF, Battelli LA, Goldsmith WT, Porter DW, Frazer D, Friend S, Schwegler-Berry D, Mercer RR, Reynolds JS, Grote A, Castranova V, Kullman G, Fedan JS, Dowdy J, Jones WG. Necrosis of nasal and airway epithelium in rats inhaling vapors of artificial butter flavoring. Toxicol, Appl. Pharmacol. 2002;185:128–135. doi: 10.1006/taap.2002.9525. [DOI] [PubMed] [Google Scholar]
- Hubbs AF, Goldsmith WT, Kashon ML, Frazer DF, Mercer RR, Battelli LA, Kullman GJ, Schwegler-Berry DF, Friend S, Castranova V. Respiratory toxicologic pathology of inhaled diacetyl in Sprague-Dawley rats. Toxicol Pathol. 2008;36:330–344. doi: 10.1177/0192623307312694. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine . Diseases and agents of special concern to veterans of the Gulf War, Operation Iraqi Freedom, and Operation Enduring Freedom. In: Mitchell AE, Sivitz LB, Black RE, editors. Infectious diseases. National Academies Press; Washington, DC: 2007. chap. 6. [Google Scholar]
- Institute of Medicine . Long term consequences of exposure to burn pits in Iraq and Afghanistan. National Academies Press; Washington, DC: 2011. [Google Scholar]
- International Programme on Chemical Safety . Bentonite, kaolin, and selected clay minerals. World Health Organization; Geneva, Switzerland: 2005. [Google Scholar]
- Karanasiou A, Moreno N, Moreno T, Viana M, de Leeuw LF, Querol X. Health effects from Sahara dust episodes in Europe: Literature review and research gaps. Environ. Int. 2012;47:107–114. doi: 10.1016/j.envint.2012.06.012. [DOI] [PubMed] [Google Scholar]
- Kennedy K. Army Times. Gannett Government Media; Jun 30, 2009. Lung disease of soldiers linked to burn pits. [Google Scholar]
- Kennedy K. Army Times. Gannett Government Media; Jan 20, 2010. Balad burn pit harmed troops living 1 mile away. [Google Scholar]
- King MS, Eisenberg R, Newman JH, Tolle JJ, Harrell FE, Jr., Nian H, Ninan M, Lambright ES, Sheller JR, Johnson JE, Miller RF. Constrictive bronchiolitis in soldiers returning from Iraq and Afghanistan. N. Engl. J. Med. 2011;365:222–230. doi: 10.1056/NEJMoa1101388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korenyi-Both AL, Molnar AC, Fidelus-Gort R. Al Eskan disease: Desert Storm pneumonitis. Mil. Med. 1992;157:452–462. [PubMed] [Google Scholar]
- Korzeniewski K, Nitsch-Osuch A, Chcialowski A, Korsak J. Environmental factors, immune changes and respiratory diseases in troops during military activities. Respir. Physiol. Neurobiol. 2013;187:118–122. doi: 10.1016/j.resp.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bouffant L, Addison J, Bolton RE, Bruch J, Bruyet B, Danuel H, Davis JMG, Degueldre G, Demarez J, Dodgson J, Gormley IP, Hadden GG, Kovacs MP, Martin JC, Reisner MTR, Robertson A, Rosmanith J. Compared in vitro and in vivo toxicity of coal mine dusts. Relationship with mineralogical composition. Ann. Occup. Hyg. 1988;32:611–620. J. [Google Scholar]
- LeBlanc AJ, Cumston JL, Chen BT, Frazer D, Castranova V, Nurkiewicz TR. Nanoparticle inhalation impairs endothelium-dependent vasodilation in subepicardial arterioles. J. Toxicol. Environ. Health A. 2009;72:1576–1584. doi: 10.1080/15287390903232467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love RG, Waclawski EP, Maclaren WM, Wetherill GZ, Groat SK, Porteous RH, Soutar SA. Risks of respiratory disease in the heavy clay industry. Occup. Environ. Med. 1999;56:124–133. doi: 10.1136/oem.56.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAndrew LM, Teichman RF, Osinubi OY, Jasien JV, Quigley KS. Environmental exposure and health of Operation Enduring Freedom/Operation Iraqi. 2012. [DOI] [PubMed] [Google Scholar]
- Morman SA, Plumlee GS. The role of airborne mineral dusts in human disease. Aeolian Res. 2013;9:203–212. [Google Scholar]
- Naota M, Shiotsu S, Shimada A, Kohara Y, Morita T, Inoue K, Takano H. Pathological study of chronic pulmonary toxicity induced by intratracheally instilled Asian sand dust (kosa) Toxicol. Pathol. 2013;41:48–62. doi: 10.1177/0192623312452490. [DOI] [PubMed] [Google Scholar]
- National Institute for Occupational Safety and Health . NIOSH manual of analytical methods. U.S. Government Printing Office; Washington, DC: 1996. [Google Scholar]
- National Institute of Standards and Technology Certificate of Analysis Standard Reference Material 1648a. 2012 https://www-s.nist.gov/srmors/view_detail.cfm?srm=1648A.
- National Research Council . Review of the Department of Defense Enhanced Particulate Matter Surveillance Program report. National Academies Press; Washington, DC: 2010a. Health and surveillance needs; p. 50. The Committee for Review of the DOD’s Enhanced Particulate Matter Surveillance Program Report. [PubMed] [Google Scholar]
- National Research Council . Guide for the care and use of laboratory animals. National Academy Press; Washington, DC: 2010b. [Google Scholar]
- Nurkiewicz TR, Poerter DW, Barger M, Castranova V, Boegehold MA. Particulate matter exposure impairs systemic microvascular endothelium-dependent dilation. Environ. Health Perspect. 2004;112:1299–1306. doi: 10.1289/ehp.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeples L. [April 8, 2015];Gulf War Syndrome, other illnesses among veterans may be due to toxic environments. The Huffington Post. 2013 http://www.huffingtonpost.com/2013/02/07/gulf-war-syndrome-veterans_n_2634838.html.
- Pinkerton KE, Green FH, Saiki C, Vallyathan V, Plopper CG, Gopal V, Hung D, Bahne EB, Lin SS, Menache MG, Schenker MB. Distribution of particulate matter and tissue remodeling in the human lung. Environ. Health Perspect. 2000;108:1063–1069. doi: 10.1289/ehp.001081063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope CI, Dockery D. Health effects of fine particulate air pollution: Lines that connect. J. Air Waste Manage. Assoc. 2006;56:709–742. doi: 10.1080/10473289.2006.10464485. [DOI] [PubMed] [Google Scholar]
- Prieditis H, Adamson IY. Comparative pulmonary toxicity of various soluble metals found in urban particulate dusts. Exp. Lung Res. 2002;28:563–576. doi: 10.1080/01902140290096782. [DOI] [PubMed] [Google Scholar]
- Pritchard RJ, Ghio AJ, Lehmann JR, Winsett DW, Tepper JS, Park P, Gilmour MI, Dreher KL, Costa DL. Oxidant generation and lung Injury after particulate air pollutant exposure increase with the concentrations of associated metals. Inhal. Toxicol. 1996;8:457–477. [Google Scholar]
- Quigley KS, McAndrew LM, Almeida L, D’Andrea EA, Engel CC, Hamtil H, Ackerman AJ. Prevalence of environmental and other military exposure concerns in Operation Enduring Freedom and Operation Iraqi Freedom veterans. J. Occup. Environ. Med. 2012;54:659–664. doi: 10.1097/JOM.0b013e3182570506. [DOI] [PubMed] [Google Scholar]
- Rabee AM. Estimating the health risks associated with air pollution in Baghdad City, Iraq. Environ. Monit. Assess. 2014;187:1–12. doi: 10.1007/s10661-014-4203-x. [DOI] [PubMed] [Google Scholar]
- Rainey LC, Bolsaitis P, Dirsa B, Vander Sande JB. Characterization by scanning transmission electron microscopy of silica articles from alveolar macrophages of coal miners. Environ. Health Perspect. 1994;102:862–868. doi: 10.1289/ehp.94102862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards AL, Hyams C, Watts DM, Rozmajzl PJ, Woody JN, Merrell BR. Respiratory disease among military personnel in Saudi Arabia during Operation Desert Shield. Am. J. Public Health. 1993;83:1326–1329. doi: 10.2105/ajph.83.9.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risen J. Veterans sound alarm over burn pit exposure. New York Times. 2010 Aug 6; http://www.nytimes.com/2010/08/07/us/07burn.html.
- Roop SA, Niven AS, Calvin BE, Bader J, Zacher LL. The prevalence and impact of respiratory symptoms in asthmatics and nonasthmatics during deployment. Mil. Med. 2007;172:1264–1269. doi: 10.7205/milmed.172.12.1264. [DOI] [PubMed] [Google Scholar]
- Rose CS. Military service and lung disease. Clin. Chest. Med. 2012;33:705–714. doi: 10.1016/j.ccm.2012.09.001. [DOI] [PubMed] [Google Scholar]
- Schenker MB, Pinkerton KE, Mitchell D, Vallyathan V, Elvine-Kreis B, Green FH. Pneumoconiosis from agricultural dust exposure among young California farmworkers. Environ. Health Perspect. 2009;117:988–994. doi: 10.1289/ehp.0800144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiffert J, Hussain F, Wiegman C, Li F, Bey L, Baker W, Porter A, Ryan MP, Chang Y, Gow A, Zhang J, Zhu J, Tetley TD, Chung KF. Pulmonary toxicity of instilled silver nanoparticles: Influence of size, coating and rat strain. PLoS ONE. 2015;10:e0119726. doi: 10.1371/journal.pone.0119726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shane L., III Study: Respiratory illness higher near infamous Balad burn pit. Stars and Stripes. 2010 Jul 1; http://www.stripes.com/news/middle-east/crisis-in-iraq/study-respiratory-illnesses-higher-near-infamous-balad-burn-pit-1.109538.
- Shorr AF, Scoville SL, Cersovsky SB, Shanks GD, Ockenhouse CF, Smoak BL, Carr WW, Petruccelli BP. Acute eosinophilic pneumonia among US Military personnel deployed in or near Iraq. J. Am. Med. Assoc. 2004;292:2997–3005. doi: 10.1001/jama.292.24.2997. [DOI] [PubMed] [Google Scholar]
- Smith B, Wong CA, Smith TC, Boyko EJ, Gackstetter GD. Newly reported respiratory symptoms and conditions among military personnel deployed to Iraq and Afghanistan: a prospective population-based study. Am. J. Epidemiol. 2009;170:1433–1442. doi: 10.1093/aje/kwp287. [DOI] [PubMed] [Google Scholar]
- Szema AM, Peters MC, Weissinger KM, Gagliano CA, Chen JJ. New-onset asthma among soldiers serving in Iraq and Afghanistan. Allergy Asthma Proc. 2010;31:67–71. doi: 10.2500/aap.2010.31.3383. [DOI] [PubMed] [Google Scholar]
- Szema AM, Salihi W, Savary K, Chen JJ. Respiratory symptoms necessitating spirometry among soldiers with Iraq/Afghanistan war lung injury. J. Occup. Environ. Med. 2011;53:961–965. doi: 10.1097/JOM.0b013e31822c9f05. [DOI] [PubMed] [Google Scholar]
- Taylor K, Foster ML, Law JM, Centeno JA, Fornero E, Henderson MS, Trager SA, Stockelman MG, Dorman DC. Assessment of geographical variation in the respiratory toxicity of desert dust particles. Inhal. Toxicol. 2013;25:405–416. doi: 10.3109/08958378.2013.797524. [DOI] [PubMed] [Google Scholar]
- Teichman R. Exposures of concern to veterans returning from Afghanistan and Iraq. J. Occup. Environ. Med. 2012;54:677–681. doi: 10.1097/JOM.0b013e318259c1ce. [DOI] [PubMed] [Google Scholar]
- Tuder RM, Petrache I. Pathogenesis of chronic obstructive pulmonary disease. J. Clin. Invest. 2012;122:2749–2755. doi: 10.1172/JCI60324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai SS, Yang CY. Fine particulate air pollution and hospital admissions for pneumonia in a subtropical city: Taipei, Taiwan. J. Toxicol. Environ. Health A. 2014;77:192–201. doi: 10.1080/15287394.2013.853337. [DOI] [PubMed] [Google Scholar]
- U.S. Army Public Health Command . Technical guide 230 Environmental health risk assessment and chemical exposure guidelines for deployed military personnel. U.S. Army Public Health Command; Aberdeen Proving Ground, MD: 2013. [Google Scholar]
- U.S. Congress, 92nd . Clean Air Act, Appendix J to Part 50. 36 FR 22384. U.S. Government Printing Office; Washington, DC: 1971. http://www.ecfr.gov/cgi-bin/retrieveECFR?gp=1&SID=cc839b875cfc420e6b52da404523928a&ty=HTML&h=L&mc=true&n=pt40.2.50&r=PART. [Google Scholar]
- Valavanidis A, Fiotakis K, Vlachogianni T. Airborne particulate matter and human health: toxicological assessment and importance of size and composition of particles for oxidative damage and carcinogenic mechanisms. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2008;26:339–362. doi: 10.1080/10590500802494538. [DOI] [PubMed] [Google Scholar]
- Weese CB, Abraham JH. Potential health implications associated with particulate matter exposure in deployed settings in southwest Asia. Inhal. Toxicol. 2009;21:291–296. doi: 10.1080/08958370802672891. [DOI] [PubMed] [Google Scholar]
- Wilfong ER, Lyles M, Rietcheck RL, Arfsten DP, Boeckman HJ, Johnson EW, Doyle TL, Chapman GD. The acute and long-term effects of Middle East sand particles on the rat airway following a single intratracheal instillation. J. Toxicol. Environ. Health A. 2011;74:1351–1365. doi: 10.1080/15287394.2010.516239. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.