Abstract
Infection with Helicobacter pylori (H. pylori) leads to inflammatory events that can promote gastric cancer development. Immune cells transition from the circulation into the infected mucosa through the interaction of their receptors and ligands in the endothelial compartment. CD44 expression is increased in advanced gastric lesions. However, the association of this molecule with the progression of these lesions over time has not been investigated. In addition, there is a lack of understanding of the CD44-dependent cellular processes that lead to gastritis, and possibly to gastric cancer. Here we studied H. pylori-positive subjects with gastric lesions that ranged from multifocal atrophic gastritis to dysplasia to determine gene expression changes associated with disease progression over a period of six years. We report that CD44 expression is significantly increased in individuals whose gastric lesions progressed along the gastric precancerous cascade. We also show that CD44−/− mice develop less severe and less extensive H. pylori-induced metaplasia, and show fewer infiltrating Gr1+ cells compared to wild type mice. We present data suggesting that CD44 is associated with disease progression. Mechanisms associated with these effects include induction of interferon gamma responses.
Keywords: Inflammation, gastritis, gastric cancer, CD44
1. Introduction
Gastric cancer (GC) is one of the most common and lethal cancers worldwide. In the United States, more than 20,000 new cases of GC and more than 10,000 deaths attributable to the disease are expected in 2015 [1]. Several factors have been associated with the disease, including race/ethnicity, genetic and environmental factors, gender, and age [1–6]. However, infection with Helicobacter pylori (H. pylori) has been described as the strongest factor associated with risk of intestinal type gastric adenocarcinoma, such that the International Agency for Research in Cancer (IARC) has classified H. pylori as a Type I carcinogen [7]. Infection with H. pylori usually occurs early in life [8] and persists, in most cases, without causing any major complications to the human host [9]. However, the infection may lead to a cascade of inflammatory events that trigger the transformation of the normal gastric mucosa into non-atrophic gastritis (NAG), followed by multifocal atrophic gastritis without intestinal metaplasia (MAG), intestinal metaplasia (IM), dysplasia, and finally cancer [10;11]. Despite the high prevalence of H. pylori infection, it is estimated that 1% of infected people will develop non-cardia gastric cancer [12].
Studies of human gastric samples and mice infected with mouse-adapted H. pylori indicate a major role of the immune response in this process [13–15]. We have shown that single nucleotide polymorphisms (SNPs) and SNP haplotypes may be associated with differential risk of more advanced precancerous gastric lesions in certain populations [16–18]. The inflammatory process, in general, involves cellular activation, migration, and infiltration into the inflamed site through the interaction between cellular receptors and ligands on the endothelium (reviewed in[19]). Selectins are major endothelial ligands induced in response to inflammation [20], and of these, E-selectins seem to be especially induced by H. pylori infection [21]. CD44, a cell-adhesion molecule expressed on a great variety of cell types including leukocytes and epithelial cells, has been shown to participate in the migration of inflammatory cells [22–26]. CD44 is a glycoprotein that binds mainly hyaluronan in the extracellular matrix [27], but also other matrix components including collagen, fibronectin, osteopontin and growth factors [28–30]. The most common form of CD44 is of 80–100kD but several other variants generated by splicing and posttranslational modifications are also expressed in various cell types [31;32]. In the gastric epithelium, CD44 has been associated with cell progenitors within the isthmus, which actively proliferate in response to H. pylori- or tamoxifen-induced atrophy [33]. In gastric tissues CD44 expression is weaker in intestinal metaplasia and grows stronger in dysplasia, intramucosal carcinoma and invasive carcinomas [34]. Additionally, 92% of intestinal-type gastric cancers expressed one splice variant of the CD44 molecule (CD44v6) [35]. Interestingly, CD44v4 has been associated with increased migration in tumor cells through endothelial monolayers by interacting with E-selectin [36].
The association of epithelial CD44 in gastric disease progression in a cohort of individuals has not been previously investigated, nor have the mechanisms leading to development of precancerous lesions in response to H. pylori in CD44 + or CD44−/− cells. Here we show that CD44 expression in the gastric mucosa increased over time in individuals who progressed to more advanced precancerous gastric lesions over time. We also show that gastritis progression over time is associated with increased expression of CD44v4 at baseline. In vitro experiments show that H. pylori induces the expression of this marker. In vivo models of H. pylori infection indicate that CD44 is involved in the development of mucous metaplasia, a process that, according to our data, is driven by interferon-gamma (IFN-γ) responses and differential infiltration of Gr1+ cells into the infected gastric mucosa.
2. Materials and methods
2.1. Patient description and microarray analysis
All patients were from an area of high incidence of gastric cancer in the southwest region of Colombia [37;38]. Inclusion criteria have been previously reported and included the presence of MAG or IM, but otherwise in good health, with no major diseases, i.e. cancer [38]. All patients signed a consent form for their participation in the study and the unrestricted use of their biological samples. The study was approved by the Institutional Review Board of Louisiana State University Health Sciences Center and the Committees on Ethics of Universidad del Valle and Hospital Departamental de Nariño in Colombia [38]. For the present study, gastric mucosa biopsy samples at baseline and at 6-year follow-up were compared. Four endoscopic biopsies (two from antrum, one from incisura angularis and one from corpus) were obtained at each time point, and the more advanced histological lesion observed in each set of biopsies was considered the diagnosis. Diagnoses were considered from less to more advanced in the following order: MAG, IM, and dysplasia. Subjects were assigned to three different groups based on the evolution of the gastric pathology at 6 years, as follows: the “No change”, similar diagnosis at baseline and at follow-up; “Regression”, milder gastritis at follow-up; and “Progression”, more advanced lesions over time.
A random sample of 39 subjects was selected for this study among subjects that were H. pylori-positive at baseline, including 11 subjects with no change in diagnosis, 12 with regression and 16 with progression. Baseline biopsy samples and their corresponding 6-year follow-up paired samples containing the most advanced lesion in each set of biopsies were used for microarray analysis. Total RNA was extracted from formalin-fixed paraffin-embedded (FFPE) tissues using the RecoverAll Total Nucleic Acid Isolation from Ambion (Austin, TX), in five 10–20μm-thick tissue sections, as recommended by the vendor. The RNA was resuspended in DEPC-water and quantified by NanoDrop (ThermoFisher Scientific, Waltham, MA). The suitability of the RNA samples was tested by amplifying the gene RPL13A using SYBR green (Life Technologies, Foster City, CA), as recommended by the vendor of the microarray kits (Illumina Inc, San Diego, CA). Briefly, 1 μl cDNA was mixed with 5 μl SYBR green Master Mix, and 250 nM each of forward and reverse primers (Forward 5′-GTACGCTGTGAAGGCATCAA-3′; reverse 5′-GTTGGTGTTCATCCGCTTG-3′) in a total volume of 10 μl and subjected to amplification for 40 cycles at 95°C for 15 sec and 60°C for 2 min. This verification step is required to determine if replicates of the samples are necessary, based on their quality, as recommended by Illumina, as follows: the best reproducibility is found in those samples with a threshold cycle (Ct) of 28 or less, and larger variability in those with Ct values greater than 28 for which technical replicates are mandatory.
For the analysis of the gene profiles, we used the focused DASL (cDNA-mediated Annealing, Selection, extension and Ligation) Cancer Panel Array (Illumina), which allows the profiling of 502 gene transcripts in RNA extracted from FFPE tissues. Briefly, 200 ng of RNA were used to make biotin-labeled cDNA which was later hybridized to bead chips. After hybridization, the chips were washed and scanned in the Illumina BeadArray Scanner to record the intensity of the fluorescence emitted. Before the analysis, the nonspecific binding was removed using the “Detection P value” algorithm that removes background signals based on the emission of negative probes (lacking targets in the human genome but thermodynamically similar to the regular probes). The signal was normalized using the “cubic spline algorithm”, assuming that the distribution of transcripts is similar among samples of the same group. Differential gene expression analysis was done separately per outcome group (No change, Regression or Progression) using the baseline values as reference and the “Illumina Custom Algorithm” that assumes that the intensity of the signals is normally distributed among all replicates under the same condition, as well as multiple testing corrections using the Benjamini and Hochberg false discovery rate (FDR). We then identified the genes that showed statistically significant differences between the baseline and the follow-up signals. The log10 of the ratio between each one of the samples and one internal calibrator (the average signals in all the samples) was obtained to build heat maps, as recommended previously [39;40]. Gene microarray data reported in this paper has been uploaded to the Gene Expression Omnibus (GEO) under reference number GSE69146.
2.2. Bacterial strain and co-culture conditions
H. pylori strain 26695 was used for co-culture experiments. Bacteria were grown for 3 days in CDC anaerobic agar plates supplemented with 5% sheep blood (BD Diagnostics, Sparks, MD) under microaerobic conditions using a Campy Pouch system (BD Diagnostics). Bacteria cultures were harvested and resuspended in PBS. AGS gastric epithelial cells (ATCC CRL-1739, Rockville, MD) were cultured in F-12 medium with 10% FBS at 37°C in an atmosphere of 5% CO2. For H. pylori co-culture experiments, 1 x 106 AGS cells were seeded into 6-well plates containing 2 ml fresh F-12 supplemented with 3% heat-inactivated FBS and cultured for 8 h. The medium was replaced with 2 ml fresh F-12 containing 3% heat-inactivated FBS before inoculation of H. pylori at a multiplicity of infection (MOI) of 20:1. The infected cells were cultured for additional 16 h after which RNA was extracted for real-time PCR analysis.
2.3. Harvest of peritoneal macrophages with thioglycollate
Peritoneal macrophages (PM) from six-to-eight-week old specific pathogen-free female CD44−/− mice (Jackson Laboratories, Bar Harbor ME, stock 005085) and PM from the corresponding wild type (WT) controls (Jackson Laboratories, stock 00664) were collected by peritoneal lavage with PBS three days after the peritoneal injection of thioglycollate (Becton Dickinson, Sparks, MD). Macrophages were seeded onto 6-well plates at 1 x 106 per well in complete RPMI (containing 10% FBS, antibiotics, L-glutamine and 25mM HEPES). After an overnight incubation, the macrophages were infected with the mouse-adapted Sydney strain H. pylori (SS1)[41] at MOI of 20:1 (bacteria:macrophage) for 16 h. Brucella broth was added to control PM. The RNA was extracted with Trizol reagent (Life Technologies) as recommended by the manufacturer; this RNA was later used to generate double-stranded cDNA that served as template to make biotin-labeled RNA which was then used for microarray analysis following the manufacturer’s instructions (Illumina). The processing of the data was done as described above for human samples. The analysis was done separately for WT and CD44−/− PM. We used the “differential expression” algorithm and identified the genes that were significantly different in H. pylori-infected vs non-infected controls (in WT and CD44−/− separately). Metacore software (Thomson Reuters, Philadelphia, PA) was used to enrich for unique and common pathways in infected cells form WT and CD44−/− mice, with the non-infected cells as controls, as previously described [39].
2.4. Real-time PCR analysis
Results from both human and mice microarray analyses were confirmed by real-time PCR as follows: the RNA was converted into cDNA using SuperScript III as recommended by the vendor (Life Technologies) and subjected to real-time PCR using Assays-on-Demand Taqman probes (Life Technologies; human primer-probe set # Hs00153304_m1; mouse primer-probe set # Mm01277163_m1). We compared the signal in the combined “No change” and “Regression” groups versus the “Progression” group. The fold induction of the genes was determined by the 2ΔΔΔCt method using GAPDH as the housekeeping gene and an internal control for normalization.
2.5. Mice infection and processing of stomach tissues
Six- to eight-week-old specific pathogen-free female CD44−/− and WT mice were inoculated by gavage for three consecutive days, with 200 μl of Brucella broth containing 1 x 108 colony forming units (c.f.u) of H. pylori SS1, or with broth only. Mice were fed the Teklad Global rodent diet (2019S) (Harlan Laboratories, Indianapolis, IN) and water ad libitum in accordance with the guidelines of LSUHSC Institutional Animal Care and Use Committee. Seven months after inoculation, mice were euthanized by CO2 inhalation and their stomachs removed, opened along the greater curvature, cut longitudinally into strips, fixed with 10% neutral-buffered formalin, and embedded in paraffin as recommended [42;43].
2.6. Histologic evaluation of mouse gastric mucosa
Hematoxylin- and eosin-stained sections were assessed blindly for pathological changes. Acute and chronic inflammatory infiltration was graded from 0 to 3 in the antrum and corpus independently, using a scoring system based on the updated Sydney System [44]. Mucous metaplasia was scored from 0 to 3 based on the extension of the foamy mucus-containing cells observed in the corpus: the presence of moderate foci of foamy cells replacing less than 1/3 of the parietal cells was scored as 1, large foci affecting between 1/3 and 2/3 of the parietal cells was scored 2, and when the change affected more than 2/3 of the parietal cells the assigned score was 3.
2.7. Immunohistochemistry
The variant 4 of the human CD44 (CD44v4) was detected in biopsies using a 1:200 dilution of a mouse IgG1-k antibody against human CD44v4 from eBioscience (San Diego, CA) and a mach 2 mouse polymer labeled with horseradish peroxidase (HRP) from Biocare Medical Co. (Concord, CA) as secondary antibody solution (overnight at 4°C and 30 mins at room temperature, respectively). The antigen retrieval was done in citrate buffer in a pressure cooker for 20 minutes and the staining done with DAB for 5 mins at room temperature. Gr1 and pStat-1 detection in mouse tissues was performed using the Avidin-Biotin-Peroxidase complex system, according to the manufacturer’s instructions (Vectastain Elite ABC Peroxidase Kit; Vector Laboratories). Antigen retrieval was performed by heating slides in 0.01 M sodium citrate buffer (pH 6.0) to 95°C under vacuum for 40 minutes and allowing them to cool for 30 min at room temperature, then incubating in MeOH/3% H2O2 for 20 min to quench endogenous peroxidase. Slides were incubated with the primary antibodies anti-Gr1, (eBioscience, 1:100) or anti-pStat-1, (SantaCruz, 1:100) overnight, followed by incubation with biotinylated secondary antibodies at room temperature for 1 h, and by avidin-biotin peroxidase complexes for 1 h. Finally, slides were developed using a diaminobenzidine substrate, and counterstained with hematoxylin. Images were collected at 200x and 600x magnification using an Olympus BX61 (UIS2 optical system) microscope equipped with a high resolution Olympus DP72 camera and Standard Cell Sense image capture software.
2.8. Statistical analysis
All statistical analysis was done in GraphPad Prism Software 4.0 using Mann-Whitney or Wilcoxon’s tests, except for the comparison of the infiltration of Gr1 cells into the gastric mucosa for which the R Studio software, version 0.98.1062 was used.
3. Results
3.1. CD44 is associated with progression of the gastric lesions in humans
Baseline and 6-year follow-up gastric samples from 39 individuals were compared by microarray analysis to determine gene patterns associated with evolution of the gastric lesions over time. After normalizing the data, we looked for genes with at least a 50% change between the two time points and p<0.05. Only one gene (ARHA1) in the “No change” group, 17 genes in the “Regression” group and 26 genes in the “Progression” met these criteria. However, after applying FDR correction, only the ARHA1 gene remained significant in the “Regression’ group while four genes, ARHA1, NUMA1, LCN2, and the canonical form of CD44, remained significant in the “Progression” group. Interestingly, these genes have all been associated with cancer risk. For example, mutations in ARHA1 (RHOA) have been repeatedly associated with risk of diffuse gastric cancer [45;46]; NUMA over expression has been significantly associated with disease stage and local metastases to lymph nodes in ovarian cancer [47]; and LCN2, after the activation of the PI3/AKT/NFkB [48], has been shown to form complexes with MMP9 and these, in turn, being associated with reduced survival in gastric cancer [49;50]. Since progression to more advanced gastric lesions may be associated with the development of gastric cancer, and because previous studies had suggested that CD44 may be a marker of advanced gastric lesions [34;35], we wanted to investigate the role of this gene in development of gastric lesions. The transformed Log10 ratios between the follow-up and baseline signals were used to compare the groups. We found a non-significant reduction in the expression of CD44 in the “Regression” group when compared to the “No change”, but a significant difference between the level of CD44 expression between the “Regression” and ”Progression” groups (Figure 1A). To confirm the results of the microarray, we performed real-time PCR in baseline gastric mucosa samples and found that individuals with progression had increased expression of CD44, compared to the group that combined individuals with regression and with no change (Figure 1B). Because all of the subjects studied were infected with H. pylori, we infected the gastric cell line AGS with H. pylori at a MOI of 20:1for 16 hours and found that the infection induced a significant increase in the expression of CD44 (Figure 1C), a finding that is in agreement with previously published data [51]. Interestingly, we found that baseline expression of CD44v4 (both the percentage and intensity) (Figure 1D and 1E, respectively) in the progression group was significantly higher than those in the no change or regression groups. Taken together, our results suggest that infection with H. pylori induces the expression of CD44 and this induction, in turn, is associated with progression to advanced gastric lesions over time. To identify the mechanism of the CD44-mediated progression of gastritis, we infected mice as described in the following sections.
Figure 1. CD44 expression is associated with the progression of gastric premalignant lesions over time and with infection with H. pylori.
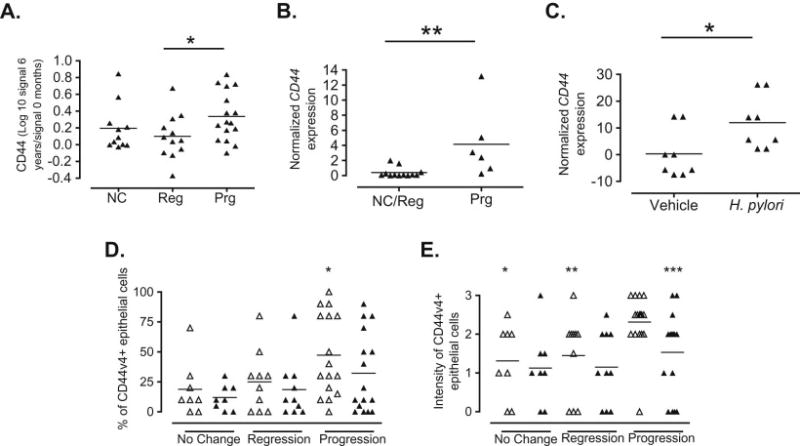
A. CD44 levels were determined by microarray analysis in baseline and 6-year follow-up biopsies from 39 subjects that were grouped based in the evolution of their gastric lesions. CD44 levels were expressed as a log transformation of the ratio between the follow-up and baseline signals in the three comparison groups (NC, no change; Reg, regression; Prg, progression). *p=0.039 when comparing Prg with Reg. B. CD44 expression in baseline biopsies was determined by real-time PCR and normalized to GAPDH expression.*p=0.0057 when comparing Prg to NC/reg. C. AGS cells were infected for 16 hours with H. pylori (26995) at a MOI of 20 and CD44 expression was determined by real-time PCR. *p=0.0379 when comparing H. pylori-infected AGS with non-infected cells. D. Percentage of CD44v4 expression in gastric epithelial cells of baseline and follow-up gastric biopsies of 34 individuals with different outcomes of gastritis over time. *p=0.0295 when compared to no change at baseline. E. Intensity of the CD44v4 expression in gastric tissue of 34 individuals with different gastritis outcome over time. *p=0.0116, **p=0.0159, ***p=0.0188 when compared to baseline progression.
3.2. CD44 is necessary to induce the expression of IFN-γ-inducible genes in response to H. pylori infection
To explore the effect of CD44 after H. pylori infection, we infected WT and CD44−/− PM with H. pylori SS1 and performed microarray analysis as described in Materials and Methods. Infection of PM with H. pylori induces the expression of a set of genes that is different from that of non-infected PM as evidenced by the separation of the two groups in the dendrogram (Figure 2A). Interestingly, H. pylori-infected PM from wild type and CD44 −/− mice further separated, suggesting that the lack of CD44 leads to the induction of different gene pathways in response to the infection. That separation was not observed in the non-infected PM, indicating that this difference was an effect of the infection. Analysis using MetaCore revealed that at least 80% of the gene networks associated with these responses were related to inflammation (Figure 2B; blue bar, genes unique to WT PM; orange bar, genes unique to CD44−/− PM; hatched bar, common genes). As can be observed, genes associated exclusively with CD44−/− mice had non-significant participation in the networks listed, suggesting the importance of CD44 in mounting specific responses to the H. pylori infection (Figure 2B).
Figure 2. Differential gene profiles in wild type and CD44−/− peritoneal macrophages infected with H. pylori.
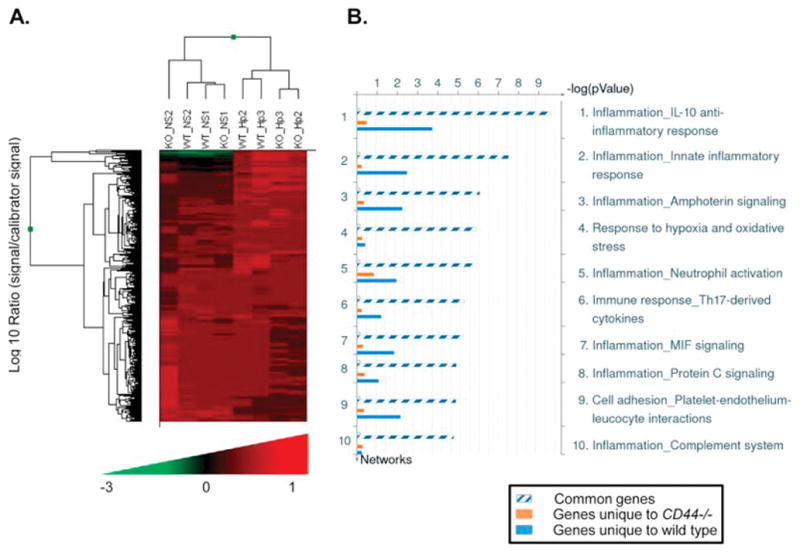
Thioglycollate-induced peritoneal macrophages (PM) obtained from wild type or CD44−/− mice were infected with H. pylori at a MOI of 20 for 16h and gene expression profiles determined by microarray analysis. Two mice were analyzed in each group. A. Dendrogram of gene profiles of wild type (WT) or CD44−/− (KO) PMs infected with H. pylori (Hp) or brucella broth alone (NS). B. Distribution of gene networks in which genes unique to WT PM (blue bar), those unique to CD44−/− PM (orange bar) and common genes participate (hatched bar).
It has been shown that CD44 is essential to mount IFN-γ– induced responses during infections [52], and that overexpression of IFN-γ in the gastric mucosa may lead to the development of advanced pre-cancerous lesions in the stomachs of mice [53]. We investigated the expression of IFN-γ-associated genes in the gastric mucosae of infected and non-infected wild type and CD44−/− mice. These genes included IFN-γ, the interferon-induced protein with tetratricopeptide repeats 2 and 3 (IFIT2 and 3), the immunity-related GTPase family member 2 (IIGP2), the interferon regulatory factor 7 (IRF7) and the signal transducer and activator of transcription 1 (STAT1). We found that none of these genes were up-regulated in the CD44−/− mice in response to H. pylori infection to the same levels as they were in the wild type mice (Figure 3). Thus, only wild type mice showed an infection-induced response of IFN-γ-dependent genes, suggesting that CD44 is needed for this response. It is interesting that there was also loss of induction of Nos2, a pro-inflammatory gene that can be activated by IFN-γ [54;55] suggesting that CD44 may be regulating this interaction. Additional confirmation of this affected pathway in CD44−/− mice was observed when the level of phosphorylation of STAT-1 was studied by immunohistochemistry (IHC), revealing that CD44−/− mice had lower numbers of pSTAT-1 positive cells in their gastric mucosae in response to H. pylori infection when compared to WT mice (Figure 4A). A representative IHC staining for pSTAT-1 is shown in Figure 4B.
Figure 3. CD44- deficient mice failed to up-regulate the expression of IFN-γ and IFN-γ-induced genes following H. pylori infection.
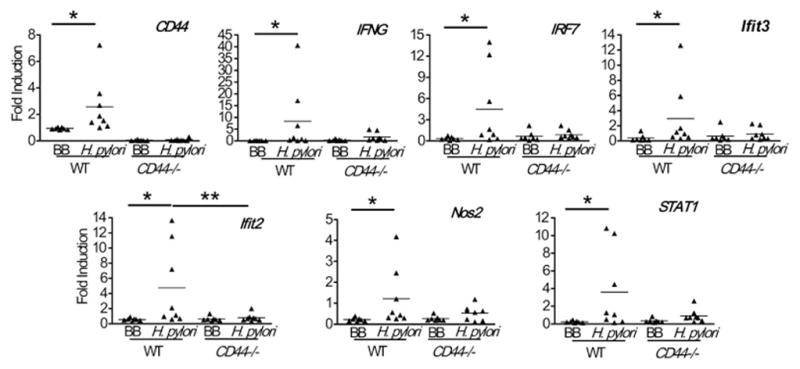
mRNA levels of IFN-γ and the indicated IFN-γ related genes were assessed by real-time PCR in the gastric mucosae of CD44−/− and wild type (WT) mice following infection with H. pylori for 7 months. *, ** p<0.05; BB, Brucella broth.
Figure 4. Reduced expression of pSTAT-1 in CD44−/− mice following H. pylori infection.
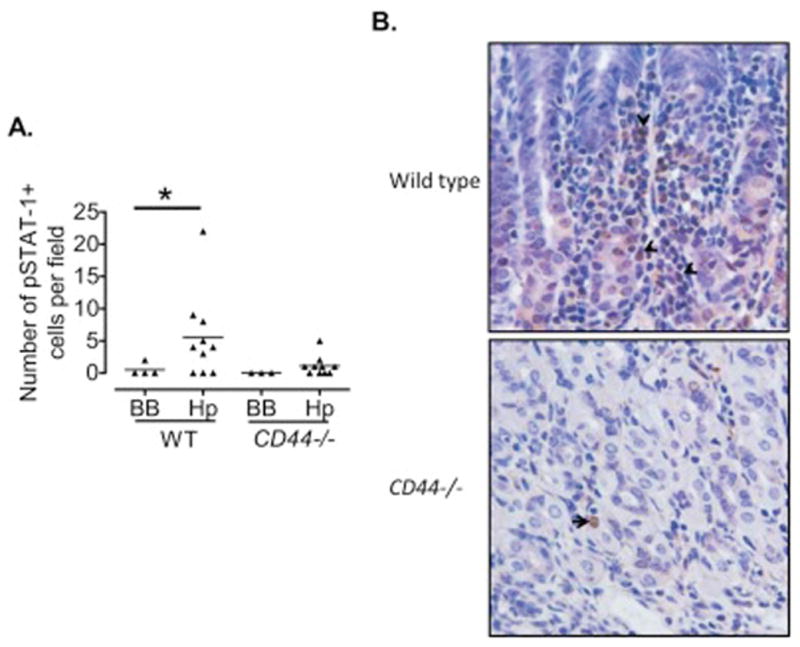
A. The number of pSTAT-1+ cells in 10 high power fields (200X) was assessed using immunohistochemistry in the gastric mucosae of CD44−/− and wild type mice infected with H. pylori for 7 months. B. Representative immunohistochemistry showing pSTAT-1 positive cells in the gastric mucosa (200X magnification). *p=0.07; BB, Brucella broth.
3.3. CD44 is involved in the appearance of mucous metaplasia in mice
Our in vitro and in vivo results suggested that CD44 is crucial to mount an appropriate immune response after infection with H. pylori. Since CD44 has been implicated in the movement of immune cells, namely macrophages and neutrophils, into the inflamed tissues [22–25], we chose to investigate the role of CD44 in the modulation of the gastric inflammatory responses to H. pylori infection in vivo. We infected wild type and CD44−/− mice with H. pylori and determined the levels of mucous metaplasia and inflammatory response. As can be observed in Figure 5A gastric tissue of CD44−/− mice had a significantly lower degree of mucous metaplasia compared to that of wild type mice. Representative staining for mucous metaplasia is shown in Figure 5B, including an inset showing a 40X magnification of a metaplastic gland. This difference between WT and CD44−/− mice is an important finding, because this type of metaplasia has been implicated as a precancerous lesion in mice [56–59]. To verify the presence of this gastric lesion, we stained the gastric biopsies with Alcian Blue-Periodic acid-Schiff (AB-PAS), as recommended [53;60–62] (Figure 5B). AB-PAS staining confirmed the replacement of fundic parietal and chief cells by foamy cells containing abundant mucins. Even though significant differences between the inflammation scores (infiltration of mononuclear and polymorphonuclear cells) in antrum and corpus were observed, no significant differences were noted between WT and CD44−/− mice (Figures 5C and D, respectively).
Figure 5. CD44−/− mice infected with H. pylori developed less mucous metaplasia.
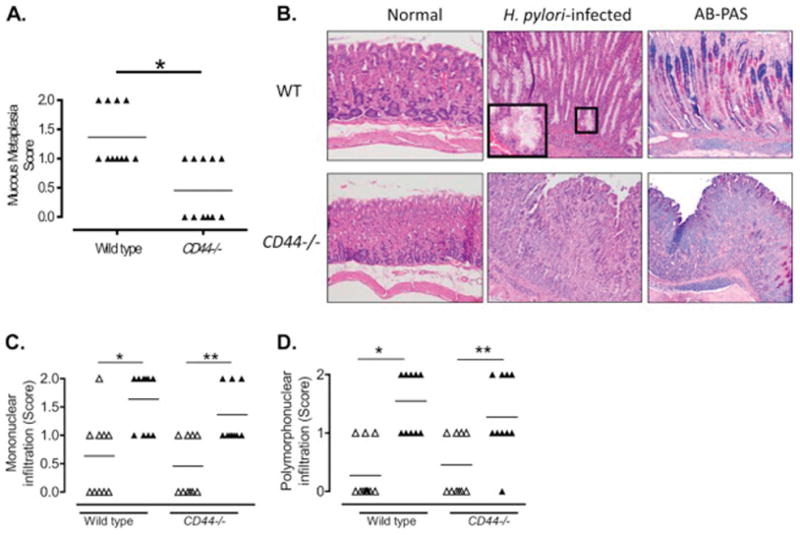
A. Metaplastic changes in the mucosae of the corpus of CD44−/− and WT mice 7 months after inoculation with H. pylori were scored from 0 to 3 as described in Material and Methods. *p=0.016. B. Representative images of H&E and AB-PAS stains showing mucous metaplasia in H. pylori-infected gastric corpus of wild type (WT) mice, but not in H. pylori-infected CD44−/− mice (200X magnification). Inset shows higher magnification of a metaplastic gland. Semiquantitative score of the mononuclear (C, *p=0.004; **p=0.008) and polymorphonuclear (D, *p=0.002; **p=0.027) infiltrate in the antrum and corpus of CD44−/− and WT following infection with H. pylori for 7 months; open triangles, antrum; closed triangles, corpus.
Depletion of Gr1 cells dysregulates the immune response against many pathogens including Helicobacter [63;64]. In mice, the myeloid differentiation antigen Gr1 is highly expressed on neutrophils and thus it has been considered a neutrophil marker. However, in addition to neutrophils other cells including dendritic cells, and myeloid suppressor monocytes also express Gr1[63;65;66]. Recent studies have shown that myeloid–derived suppressor cells (MDSCs) contribute to H. pylori-related inflammation and carcinogenesis [67–69], as well as modulation of IFN-γ responses [70]. To determine whether the levels of Gr1 expression in the gastric mucosa of wild type and CD44−/− infected mice differed, we conducted IHC for Gr1 and found that WT mice infected with H. pylori had a high number of Gr1+ cells infiltrating their gastric mucosa (>30 per high power field) both in the corpus and in the antrum (Figure 6A and B, respectively). Representative IHC staining showing Gr1+ positive cells in the gastric mucosae of H. pylori-infected wild type and CD44−/− mice is shown in Figure 6C.
Figure 6. Decreased influx of Gr1+ myeloid cells in CD44−/− mice upon H. pylori infection.
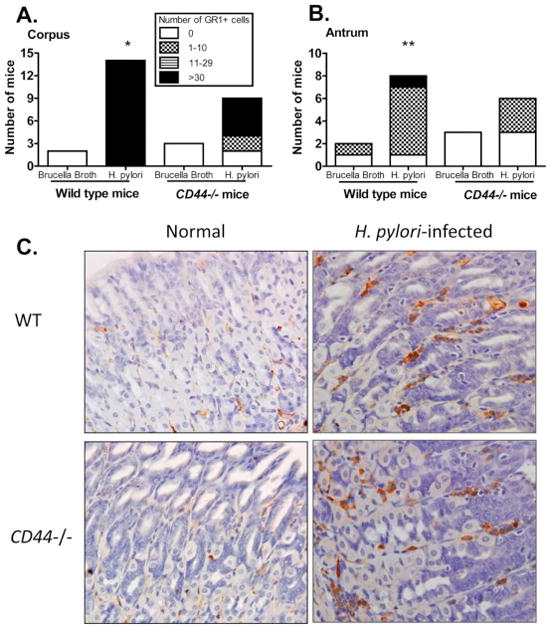
Gr1+ cells were quantified in IHC stains of the mucosae of the corpus (A) and the antrum (B) of CD44−/− and wild type (WT) mice by counting positive cells in 10 fields in each slide (200X magnification). *p=0.037 and **p=0.019 when comparing the number of Gr1+ cells in WT and CD44 −/− in corpus and antrum, respectively; BB, Brucella broth. (C). Representative IHC staining showing Gr1+ positive cells in sections of the gastric mucosae of WT and CD44−/− mice infected with H. pylori and non-infected controls (200X magnification).
4. Discussion
Infiltration of immune cells into the sites of inflammation is a crucial step to mount specific immune responses [19]. In vitro and in vivo data show that H. pylori induces the migration of immune cells [71–78]. The adhesion cell molecule CD44 is one of the molecules that is involved in this immune cell recruitment [23–25;36]. CD44 has been previously shown to be up-regulated in gastric cancer compared to IM and to normal tissue, but the changes in CD44 expression following H. pylori infection and during the evolution of precancerous lesions have not been completely elucidated [34;35;79;80]. In this study, we compared the gene expression signatures of baseline and 6 year follow-up biopsies by microarray and found that individuals with progression of the gastric precancerous lesions over time had significant changes in their expression of CD44. We also found that CD44−/− mice have reduced levels of mucous metaplasia in response to H. pylori infection. This response is accompanied by reduced levels of IFN-γ and IFN-γ-related genes as well as reduced infiltration of Gr1+ cells into the gastric mucosa.
Recent interesting publications have associated the epithelial expression of CD44 with regeneration of atrophy of parietal cells in mice after treatment with tamoxifen [33]. Other studies have shown that CD44 suppresses the production of reactive oxygen species (ROS) while cells expressing variants of this molecule (CD44v8–10) or lacking the marker (CD44−/−) are associated with increased production of ROS [81]. Reduced levels of ROS in CD44+ cells protect CagA, a known H. pylori virulence factor, from degradation by autophagy [82]. These CD44+/CagA+ gastric cells have been proposed as the origin of spasmolytic polypeptide-expressing metaplasia (SPEM) in mice [83]. Our model of mouse infection uses the H. pylori SS1 which, as previously reported, does not translocate cagA into epithelial cells [84] suggesting that other mechanisms in addition to the interaction of CagA and CD44 are involved in gastric tissue damage in response to the infection. Although the expression of gastric epithelial CD44 is associated with the appearance of metaplasia, the role of the immune system is also important. Several publications have demonstrated that the infiltration of IFN-γ–producing Th1 cells into the gastric mucosa during infection is partially responsible for the H. pylori-induced pathology [85;86]. It has been shown that CD44 regulates inflammation by providing stimulatory signals that activate T lymphocytes [87], inducing the secretion of pro-inflammatory molecules by macrophages [87–89], and regulating the production of IFN-γ by CD4 T cells [53]. Our findings expand current knowledge by showing that CD44 may be associated with differential infiltration of Gr1+ cells into infected gastric mucosae.
Recent in vitro evidence shows that different subpopulations of MDSCs, Gr1+/CD11b+ myeloid cells, may induce different CD8+ T-cell responses, including increased IFN-γ [90]. Our results show increased levels of Gr1+ inflammatory cells and increased expression of mucosal IFN-γ and IFN-γ-related genes in response to H. pylori in WT mice but not in CD44−/− mice. This difference suggests that the presence of CD44 is essential to induce an IFNγ-mediated inflammatory response against H. pylori. The full identity of the cells expressing Gr1 and their role in modulating the production of IFN-γ in the H. pylori-infected gastric mucosa has not been totally established.
Taken together, our data suggest that after infection with H. pylori, CD44 is a crucial player in the evolution of the gastric lesions by recruiting cells that can either ameliorate or potentiate immune responses associated with tissue damage. Our results also suggest that modulating CD44 responses may be of benefit to reduce the inflammation induced by H. pylori, making it a potential target for treatment.
Highlights.
CD44 expression is significantly increased in individuals whose gastric lesions progressed along the gastric precancerous cascade.
CD44 is associated with increased Th1 responses, increased tissue damage and higher counts of Gr1+ cells in the gastric tissues of H. pylori infected mice.
We show, for the first time, the association of CD44 expression, Th1 responses, cellular infiltration and tissue damage in H. pylori-induced gastritis.
Acknowledgments
The genomic analysis was done in the LCRC Translational Genomics Core, the IHC staining was done at VUMC and the LCRC Molecular Histopathology and Analytic Microscopy Core. This work was supported in part by a NIH grant P01-CA028842 (to PC), a grant from the National Institute for General Medical Sciences (NIGMS) P20GM10350 subproject 2 (to JZ), and by the Louisiana Cancer Research Consortium (JZ).
Footnotes
Conflict of interest
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Surveillance Epidemiology and End Results. Cancer Facts Statistics, SEER. National Cancer Institute; 2013. 4-10-2008. [Google Scholar]
- 2.Fiedorek SC, Malaty HM, Evans DL, Pumphrey CL, Casteel HB, Evans DJ, Jr, Graham DY. Factors influencing the epidemiology of Helicobacter pylori infection in children. Pediatrics. 1991;88:578–582. [PubMed] [Google Scholar]
- 3.Plummer M, Franceschi S, Munoz N. Epidemiology of gastric cancer. IARC Sci Publ. 2004:311–326. [PubMed] [Google Scholar]
- 4.Campos F, Carrasquilla G, Koriyama C, Serra M, Carrascal E, Itoh T, Nomoto M, Akiba S. Risk factors of gastric cancer specific for tumor location and histology in Cali, Colombia. World J Gastroenterol. 2006;12:5772–5779. doi: 10.3748/wjg.v12.i36.5772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Stefani E, Correa P, Boffetta P, Deneo-Pellegrini H, Ronco AL, Mendilaharsu M. Dietary patterns and risk of gastric cancer: a case-control study in Uruguay. Gastric Cancer. 2004;7:211–220. doi: 10.1007/s10120-004-0295-2. [DOI] [PubMed] [Google Scholar]
- 6.Terry P, Nyren O, Yuen J. Protective effect of fruits and vegetables on stomach cancer in a cohort of Swedish twins. Int J Cancer. 1998;76:35–37. doi: 10.1002/(sici)1097-0215(19980330)76:1<35::aid-ijc7>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 7.IARC. IARC monograph on the evaluation of carcinogenic risks to humans:Schistosomes, liver flukes and Helicobacter pylori. IARC. 1994;61:177–240. [PMC free article] [PubMed] [Google Scholar]
- 8.Banatvala N, Mayo K, Megraud F, Jennings R, Deeks JJ, Feldman RA. The cohort effect and Helicobacter pylori. J Infect Dis. 1993;168:219–221. doi: 10.1093/infdis/168.1.219. [DOI] [PubMed] [Google Scholar]
- 9.Brown LM. Helicobacter pylori: epidemiology and routes of transmission. Epidemiol Rev. 2000;22:283–297. doi: 10.1093/oxfordjournals.epirev.a018040. [DOI] [PubMed] [Google Scholar]
- 10.Correa P, Houghton J. Carcinogenesis of Helicobacter pylori. Gastroenterology. 2007;133:659–672. doi: 10.1053/j.gastro.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 11.Correa P, Piazuelo MB. The gastric precancerous cascade. J Dig Dis. 2012;13:2–9. doi: 10.1111/j.1751-2980.2011.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wroblewski LE, Peek RM, Jr, Wilson KT. Helicobacter pylori and gastric cancer: factors that modulate disease risk. Clin Microbiol Rev. 2010;23:713–739. doi: 10.1128/CMR.00011-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bamford KB, Fan X, Crowe SE, Leary JF, Gourley WK, Luthra GK, Brooks EG, Graham DY, Reyes VE, Ernst PB. Lymphocytes in the human gastric mucosa during Helicobacter pylori have a T helper cell 1 phenotype. Gastroenterology. 1998;114:482–492. doi: 10.1016/s0016-5085(98)70531-1. [DOI] [PubMed] [Google Scholar]
- 14.Mohammadi M, Nedrud J, Redline R, Lycke N, Czinn SJ. Murine CD4 T-cell response to Helicobacter infection: TH1 cells enhance gastritis and TH2 cells reduce bacterial load. Gastroenterology. 1997;113:1848–1857. doi: 10.1016/s0016-5085(97)70004-0. [DOI] [PubMed] [Google Scholar]
- 15.Nedrud JG, Mohammadi M, Blanchard T, Redline R, Czinn SJ. TH1/TH2 lymphocyte responses in Helicobacter infections. In: Hunt R, Tycgat S, editors. Helicobacter pylori. Mechanisms to clinical cure. Kluwer Academics Publishers; Boston: 1998. pp. 101–109. 1998. [Google Scholar]
- 16.Zabaleta J, Camargo MC, Piazuelo MB, Fontham E, Schneider BG, Sicinschi LA, Ferrante W, Balart L, Correa P, Ochoa AC. Association of interleukin-1beta gene polymorphisms with precancerous gastric lesions in African Americans and Caucasians. Am J Gastroenterol. 2006;101:163–171. doi: 10.1111/j.1572-0241.2006.00387.x. [DOI] [PubMed] [Google Scholar]
- 17.Zabaleta J, Schneider BG, Ryckman K, Hooper PF, Camargo MC, Piazuelo MB, Sierra RA, Fontham ET, Correa P, Williams SM, Ochoa AC. Ethnic differences in cytokine gene polymorphisms: potential implications for cancer development. Cancer Immunol Immunother. 2007;57:107–114. doi: 10.1007/s00262-007-0358-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zabaleta J, Camargo MC, Ritchie MD, Piazuelo MB, Sierra RA, Turner SD, Delgado A, Fontham ET, Schneider BG, Correa P, Ochoa AC. Association of haplotypes of inflammation-related genes with gastric preneoplastic lesions in African Americans and Caucasians. Int J Cancer. 2011;128:668–675. doi: 10.1002/ijc.25385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bevilacqua MP, Nelson RM. Selectins. J Clin Invest. 1993;91:379–387. doi: 10.1172/JCI116210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Svensson H, Hansson M, Kilhamn J, Backert S, Quiding-Jarbrink M. Selective upregulation of endothelial E-selectin in response to Helicobacter pylori-induced gastritis. Infect Immun. 2009;77:3109–3116. doi: 10.1128/IAI.01460-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alstergren P, Zhu B, Glogauer M, Mak TW, Ellen RP, Sodek J. Polarization and directed migration of murine neutrophils is dependent on cell surface expression of CD44. Cell Immunol. 2004;231:146–157. doi: 10.1016/j.cellimm.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 23.Aziz KA. CD44 mediates polymorphonuclear leukocyte motility on hyaluronan. Saudi Med J. 2003;24:827–831. [PubMed] [Google Scholar]
- 24.Hollingsworth JW, Li Z, Brass DM, Garantziotis S, Timberlake SH, Kim A, Hossain I, Savani RC, Schwartz DA. CD44 regulates macrophage recruitment to the lung in lipopolysaccharide-induced airway disease. Am J Respir Cell Mol Biol. 2007;37:248–253. doi: 10.1165/rcmb.2006-0363OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khan AI, Kerfoot SM, Heit B, Liu L, Andonegui G, Ruffell B, Johnson P, Kubes P. Role of CD44 and hyaluronan in neutrophil recruitment. J Immunol. 2004;173:7594–7601. doi: 10.4049/jimmunol.173.12.7594. [DOI] [PubMed] [Google Scholar]
- 26.Ilangumaran S, Borisch B, Hoessli DC. Signal transduction via CD44: role of plasma membrane microdomains. Leuk Lymphoma. 1999;35:455–469. doi: 10.1080/10428199909169610. [DOI] [PubMed] [Google Scholar]
- 27.Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990;61:1303–1313. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- 28.Jalkanen S, Jalkanen M. Lymphocyte CD44 binds the COOH-terminal heparin-binding domain of fibronectin. J Cell Biol. 1992;116:817–825. doi: 10.1083/jcb.116.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weber GF, Ashkar S, Glimcher MJ, Cantor H. Receptor-ligand interaction between CD44 and osteopontin (Eta-1) Science. 1996;271:509–512. doi: 10.1126/science.271.5248.509. [DOI] [PubMed] [Google Scholar]
- 30.Jones M, Tussey L, Athanasou N, Jackson DG. Heparan sulfate proteoglycan isoforms of the CD44 hyaluronan receptor induced in human inflammatory macrophages can function as paracrine regulators of fibroblast growth factor action. J Biol Chem. 2000;275:7964–7974. doi: 10.1074/jbc.275.11.7964. [DOI] [PubMed] [Google Scholar]
- 31.Mackay CR, Terpe HJ, Stauder R, Marston WL, Stark H, Gunthert U. Expression and modulation of CD44 variant isoforms in humans. J Cell Biol. 1994;124:71–82. doi: 10.1083/jcb.124.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Screaton GR, Bell MV, Jackson DG, Cornelis FB, Gerth U, Bell JI. Genomic structure of DNA encoding the lymphocyte homing receptor CD44 reveals at least 12 alternatively spliced exons. Proc Natl Acad Sci USA. 1992;89:12160–12164. doi: 10.1073/pnas.89.24.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khurana SS, Riehl TE, Moore BD, Fassan M, Rugge M, Romero-Gallo J, Noto J, Peek RM, Jr, Stenson WF, Mills JC. The hyaluronic acid receptor CD44 coordinates normal and metaplastic gastric epithelial progenitor cell proliferation. J Biol Chem. 2013;288:16085–16097. doi: 10.1074/jbc.M112.445551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Washington K, Gottfried MR, Telen MJ. Expression of the cell adhesion molecule CD44 in gastric adenocarcinomas. Hum Pathol. 1994;25:1043–1049. doi: 10.1016/0046-8177(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 35.Dammrich J, Vollmers HP, Heider KH, Muller-Hermelink HK. Importance of different CD44v6 expression in human gastric intestinal and diffuse type cancers for metastatic lymphogenic spreading. J Mol Med. 1995;73:395–401. doi: 10.1007/BF00240138. [DOI] [PubMed] [Google Scholar]
- 36.Zen K, Liu DQ, Guo YL, Wang C, Shan J, Fang M, Zhang CY, Liu Y. CD44v4 is a major E-selectin ligand that mediates breast cancer cell transendothelial migration. PLoS One. 2008;3:e1826. doi: 10.1371/journal.pone.0001826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Correa P, van Doorn LJ, Bravo JC, Ruiz B, Bravo LE, Realpe JL. Unsuccessful treatment results in survival of less virulent genotypes of Helicobacter pylori in Colombian patients. Am J Gastroenterol. 2000;95:564–566. doi: 10.1111/j.1572-0241.2000.t01-1-01813.x. [DOI] [PubMed] [Google Scholar]
- 38.Correa P, Fontham ET, Bravo JC, Bravo LE, Ruiz B, Zarama G, Realpe JL, Malcom GT, Li D, Johnson WD, Mera R. Chemoprevention of gastric dysplasia: randomized trial of antioxidant supplements and anti-helicobacter pylori therapy. J Natl Cancer Inst. 2000;92:1881–1888. doi: 10.1093/jnci/92.23.1881. [DOI] [PubMed] [Google Scholar]
- 39.Kim SH, Sierra RA, McGee DJ, Zabaleta J. Transcriptional profiling of gastric epithelial cells infected with wild type or arginase-deficient Helicobacter pylori. BMC Microbiol. 2012;12:175. doi: 10.1186/1471-2180-12-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van’t Veer JL, He YD, van’t Veer LJ, Dai H, Hart AA, Voskuil DW, Schreiber GJ, Peterse JL, Roberts C, Marton MJ, Parrish M, Atsma D, Witteveen A, Glas A, Delahaye L, van d, Bartelink VH, Rodenhuis S, Rutgers ET, Friend SH, Bernards R. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347:1999–2009. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- 41.Lee A, O’Rourke J, De Ungria MC, Robertson B, Daskalopoulos G, Dixon MF. A standardized mouse model of Helicobacter pylori infection: introducing the Sydney strain. Gastroenterology. 1997;112:1386–1397. doi: 10.1016/s0016-5085(97)70155-0. [DOI] [PubMed] [Google Scholar]
- 42.Bedoya A, Garay J, Sanzon F, Bravo LE, Bravo JC, Correa H, Craver R, Fontham E, Du JX, Correa P. Histopathology of gastritis in Helicobacter pylori-infected children from populations at high and low gastric cancer risk. Hum Pathol. 2003;34:206–213. doi: 10.1053/hupa.2003.43. [DOI] [PubMed] [Google Scholar]
- 43.Ruiz B, Garay J, Correa P, Fontham ET, Bravo JC, Bravo LE, Realpe JL, Mera R. Morphometric evaluation of gastric antral atrophy: improvement after cure of Helicobacter pylori infection. Am J Gastroenterol. 2001;96:3281–3287. doi: 10.1111/j.1572-0241.2001.05326.x. [DOI] [PubMed] [Google Scholar]
- 44.Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 45.Wang K, Yuen ST, Xu J, Lee SP, Yan HH, Shi ST, Siu HC, Deng S, Chu KM, Law S, Chan KH, Chan AS, Tsui WY, Ho SL, Chan AK, Man JL, Foglizzo V, Ng MK, Chan AS, Ching YP, Cheng GH, Xie T, Fernandez J, Li VS, Clevers H, Rejto PA, Mao M, Leung SY. Whole-genome sequencing and comprehensive molecular profiling identify new driver mutations in gastric cancer. Nat Genet. 2014;46:573–582. doi: 10.1038/ng.2983. [DOI] [PubMed] [Google Scholar]
- 46.Zhou J, Hayakawa Y, Wang TC, Bass AJ. RhoA mutations identified in diffuse gastric cancer. Cancer Cell. 2014;26:9–11. doi: 10.1016/j.ccr.2014.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bruning-Richardson A, Bond J, Alsiary R, Richardson J, Cairns DA, McCormac L, Hutson R, Burns PA, Wilkinson N, Hall GD, Morrison EE, Bell SM. NuMA overexpression in epithelial ovarian cancer. PLoS One. 2012;7:e38945. doi: 10.1371/journal.pone.0038945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koh SA, Lee KH. HGF mediated upregulation of lipocalin 2 regulates MMP9 through nuclear factor-kappaB activation. Oncol Rep. 2015;34:2179–2187. doi: 10.3892/or.2015.4189. [DOI] [PubMed] [Google Scholar]
- 49.Sier CF, Kubben FJ, Ganesh S, Heerding MM, Griffioen G, Hanemaaijer R, van Krieken JH, Lamers CB, Verspaget HW. Tissue levels of matrix metalloproteinases MMP-2 and MMP-9 are related to the overall survival of patients with gastric carcinoma. Br J Cancer. 1996;74:413–417. doi: 10.1038/bjc.1996.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kubben FJ, Sier CF, Hawinkels LJ, Tschesche H, van DW, Zuidwijk K, van der Reijden JJ, Hanemaaijer R, Griffioen G, Lamers CB, Verspaget HW. Clinical evidence for a protective role of lipocalin-2 against MMP-9 autodegradation and the impact for gastric cancer. Eur J Cancer. 2007;43:1869–1876. doi: 10.1016/j.ejca.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 51.Fan XG, Fan XJ, Xia HX, Keeling PW, Kelleher D. Up-regulation of CD44 and ICAM-1 expression on gastric epithelial cells by H. pylori. APMIS. 1995;103:744–748. doi: 10.1111/j.1699-0463.1995.tb01432.x. [DOI] [PubMed] [Google Scholar]
- 52.Blass SL, Pure E, Hunter CA. A role for CD44 in the production of IFN-gamma and immunopathology during infection with Toxoplasma gondii. J Immunol. 2001;166:5726–5732. doi: 10.4049/jimmunol.166.9.5726. [DOI] [PubMed] [Google Scholar]
- 53.Syu LJ, El-Zaatari M, Eaton KA, Liu Z, Tetarbe M, Keeley TM, Pero J, Ferris J, Wilbert D, Kaatz A, Zheng X, Qiao X, Grachtchouk M, Gumucio DL, Merchant JL, Samuelson LC, Dlugosz AA. Transgenic expression of interferon-gamma in mouse stomach leads to inflammation, metaplasia, and dysplasia. Am J Pathol. 2012;181:2114–2125. doi: 10.1016/j.ajpath.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Obonyo M, Guiney DG, Harwood J, Fierer J, Cole SP. Role of gamma interferon in Helicobacter pylori induction of inflammatory mediators during murine infection. Infect Immun. 2002;70:3295–3299. doi: 10.1128/IAI.70.6.3295-3299.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Obonyo M, Guiney DG, Fierer J, Cole SP. Interactions between inducible nitric oxide and other inflammatory mediators during Helicobacter pylori infection. Helicobacter. 2003;8:495–502. doi: 10.1046/j.1523-5378.2003.00171.x. [DOI] [PubMed] [Google Scholar]
- 56.Filipe MI, Munoz N, Matko I, Kato I, Pompe-Kirn V, Jutersek A, Teuchmann S, Benz M, Prijon T. Intestinal metaplasia types and the risk of gastric cancer: a cohort study in Slovenia. Int J Cancer. 1994;57:324–329. doi: 10.1002/ijc.2910570306. [DOI] [PubMed] [Google Scholar]
- 57.Halldorsdottir AM, Sigurdardottrir M, Jonasson JG, Oddsdottir M, Magnusson J, Lee JR, Goldenring JR. Spasmolytic polypeptide-expressing metaplasia (SPEM) associated with gastric cancer in Iceland. Dig Dis Sci. 2003;48:431–441. doi: 10.1023/a:1022564027468. [DOI] [PubMed] [Google Scholar]
- 58.Weis VG, Goldenring JR. Current understanding of SPEM and its standing in the preneoplastic process. Gastric Cancer. 2009;12:189–197. doi: 10.1007/s10120-009-0527-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamaguchi H, Goldenring JR, Kaminishi M, Lee JR. Identification of spasmolytic polypeptide expressing metaplasia (SPEM) in remnant gastric cancer and surveillance postgastrectomy biopsies. Dig Dis Sci. 2002;47:573–578. doi: 10.1023/a:1017920220149. [DOI] [PubMed] [Google Scholar]
- 60.Kim BW, Kim KM, Lee BI, Maeng LS, Choi H, Cho SH, Chae HS, Kim JK, Choi KY, Chung IS. Expression of trefoil peptides in the subtypes of intestinal metaplasia. Peptides. 2004;25:779–783. doi: 10.1016/j.peptides.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 61.Byrne JP, Bhatnagar S, Hamid B, Armstrong GR, Attwood SE. Comparative study of intestinal metaplasia and mucin staining at the cardia and esophagogastric junction in 225 symptomatic patients presenting for diagnostic open-access gastroscopy. Am J Gastroenterol. 1999;94:98–103. doi: 10.1111/j.1572-0241.1999.00778.x. [DOI] [PubMed] [Google Scholar]
- 62.Winter JA, Letley DP, Cook KW, Rhead JL, Zaitoun AA, Ingram RJ, Amilon KR, Croxall NJ, Kaye PV, Robinson K, Atherton JC. A Role for the Vacuolating Cytotoxin, VacA, in Colonization and Helicobacter pylori-Induced Metaplasia in the Stomach. J Infect Dis. 2014 doi: 10.1093/infdis/jiu154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Egan CE, Sukhumavasi W, Bierly AL, Denkers EY. Understanding the multiple functions of Gr-1(+) cell subpopulations during microbial infection. Immunol Res. 2008;40:35–48. doi: 10.1007/s12026-007-0061-8. [DOI] [PubMed] [Google Scholar]
- 64.Ismail HF, Fick P, Zhang J, Lynch RG, Berg DJ. Depletion of neutrophils in IL-10(−/−) mice delays clearance of gastric Helicobacter infection and decreases the Th1 immune response to Helicobacter. J Immunol. 2003;170:3782–3789. doi: 10.4049/jimmunol.170.7.3782. [DOI] [PubMed] [Google Scholar]
- 65.Kusmartsev S, Nefedova Y, Yoder D, Gabrilovich DI. Antigen-specific inhibition of CD8+ T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. J Immunol. 2004;172:989–999. doi: 10.4049/jimmunol.172.2.989. [DOI] [PubMed] [Google Scholar]
- 66.Tacke F, Randolph GJ. Migratory fate and differentiation of blood monocyte subsets. Immunobiology. 2006;211:609–618. doi: 10.1016/j.imbio.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 67.Tu S, Bhagat G, Cui G, Takaishi S, Kurt-Jones EA, Rickman B, Betz KS, Penz-Oesterreicher M, Bjorkdahl O, Fox JG, Wang TC. Overexpression of interleukin-1beta induces gastric inflammation and cancer and mobilizes myeloid-derived suppressor cells in mice. Cancer Cell. 2008;14:408–419. doi: 10.1016/j.ccr.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Asfaha S, Dubeykovskiy AN, Tomita H, Yang X, Stokes S, Shibata W, Friedman RA, Ariyama H, Dubeykovskaya ZA, Muthupalani S, Ericksen R, Frucht H, Fox JG, Wang TC. Mice that express human interleukin-8 have increased mobilization of immature myeloid cells, which exacerbates inflammation and accelerates colon carcinogenesis. Gastroenterology. 2013;144:155–166. doi: 10.1053/j.gastro.2012.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ericksen RE, Rose S, Westphalen CB, Shibata W, Muthupalani S, Tailor Y, Friedman RA, Han W, Fox JG, Ferrante AW, Jr, Wang TC. Obesity accelerates Helicobacter felis-induced gastric carcinogenesis by enhancing immature myeloid cell trafficking and TH17 response. Gut. 2014;63:385–394. doi: 10.1136/gutjnl-2013-305092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mundy-Bosse BL, Lesinski GB, Jaime-Ramirez AC, Benninger K, Khan M, Kuppusamy P, Guenterberg K, Kondadasula SV, Chaudhury AR, La Perle KM, Kreiner M, Young G, Guttridge DC, Carson WE., III Myeloid-derived suppressor cell inhibition of the IFN response in tumor-bearing mice. Cancer Res. 2011;71:5101–5110. doi: 10.1158/0008-5472.CAN-10-2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brisslert M, Enarsson K, Lundin S, Karlsson A, Kusters JG, Svennerholm AM, Backert S, Quiding-Jarbrink M. Helicobacter pylori induce neutrophil transendothelial migration: role of the bacterial HP-NAP. FEMS Microbiol Lett. 2005;249:95–103. doi: 10.1016/j.femsle.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 72.Fujiwara Y, Arakawa T, Fukuda T, Sasaki E, Nakagawa K, Fujiwara K, Higuchi K, Kobayashi K, Tarnawski A. Interleukin-8 stimulates leukocyte migration across a monolayer of cultured rabbit gastric epithelial cells. Effect associated with the impairment of gastric epithelial barrier function. Dig Dis Sci. 1997;42:1210–1215. doi: 10.1023/a:1018850006714. [DOI] [PubMed] [Google Scholar]
- 73.Hansen PS, Madsen PH, Petersen SB, Nielsen H. Inflammatory activation of neutrophils by Helicobacter pylori; a mechanism insensitive to pertussis toxin. Clin Exp Immunol. 2001;123:73–80. doi: 10.1046/j.1365-2249.2001.01368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shimoyama T, Crabtree JE. Mucosal chemokines in Helicobacter pylori infection. J Physiol Pharmacol. 1997;48:315–323. [PubMed] [Google Scholar]
- 75.Conlin VS, Curtis SB, Zhao Y, Moore ED, Smith VC, Meloche RM, Finlay BB, Buchan AM. Helicobacter pylori infection targets adherens junction regulatory proteins and results in increased rates of migration in human gastric epithelial cells. Infect Immun. 2004;72:5181–5192. doi: 10.1128/IAI.72.9.5181-5192.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.de Paulis A, Prevete N, Rossi FW, Rivellese F, Salerno F, Delfino G, Liccardo B, Avilla E, Montuori N, Mascolo M, Staibano S, Melillo RM, D’Argenio G, Ricci V, Romano M, Marone G. Helicobacter pylori Hp(2–20) promotes migration and proliferation of gastric epithelial cells by interacting with formyl peptide receptors in vitro and accelerates gastric mucosal healing in vivo. J Immunol. 2009;183:3761–3769. doi: 10.4049/jimmunol.0900863. [DOI] [PubMed] [Google Scholar]
- 77.Enarsson K, Brisslert M, Backert S, Quiding-Jarbrink M. Helicobacter pylori induces transendothelial migration of activated memory T cells. Infect Immun. 2005;73:761–769. doi: 10.1128/IAI.73.2.761-769.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nagy TA, Frey MR, Yan F, Israel DA, Polk DB, Peek RM., Jr Helicobacter pylori regulates cellular migration and apoptosis by activation of phosphatidylinositol 3-kinase signaling. J Infect Dis. 2009;199:641–651. doi: 10.1086/596660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fan X, Long A, Goggins M, Fan X, Keeling PW, Kelleher D. Expression of CD44 and its variants on gastric epithelial cells of patients with Helicobacter pylori colonisation. Gut. 1996;38:507–512. doi: 10.1136/gut.38.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jang BI, Li Y, Graham DY, Cen P. The Role of CD44 in the Pathogenesis, Diagnosis, and Therapy of Gastric Cancer. Gut Liver. 2011;5:397–405. doi: 10.5009/gnl.2011.5.4.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ishimoto T, Nagano O, Yae T, Tamada M, Motohara T, Oshima H, Oshima M, Ikeda T, Asaba R, Yagi H, Masuko T, Shimizu T, Ishikawa T, Kai K, Takahashi E, Imamura Y, Baba Y, Ohmura M, Suematsu M, Baba H, Saya H. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(-) and thereby promotes tumor growth. Cancer Cell. 2011;19:387–400. doi: 10.1016/j.ccr.2011.01.038. [DOI] [PubMed] [Google Scholar]
- 82.Tsugawa H, Suzuki H, Saya H, Hatakeyama M, Hirayama T, Hirata K, Nagano O, Matsuzaki J, Hibi T. Reactive oxygen species-induced autophagic degradation of Helicobacter pylori CagA is specifically suppressed in cancer stem-like cells. Cell Host Microbe. 2012;12:764–777. doi: 10.1016/j.chom.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 83.Wada T, Ishimoto T, Seishima R, Tsuchihashi K, Yoshikawa M, Oshima H, Oshima M, Masuko T, Wright NA, Furuhashi S, Hirashima K, Baba H, Kitagawa Y, Saya H, Nagano O. Functional role of CD44v-xCT system in the development of spasmolytic polypeptide-expressing metaplasia. Cancer Sci. 2013;104:1323–1329. doi: 10.1111/cas.12236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Crabtree JE, Ferrero RL, Kusters JG. The mouse colonizing Helicobacter pylori strain SS1 may lack a functional cag pathogenicity island. Helicobacter. 2002;7:139–140. doi: 10.1046/j.1083-4389.2002.00071.x. [DOI] [PubMed] [Google Scholar]
- 85.Sawai N, Kita M, Kodama T, Tanahashi T, Yamaoka Y, Tagawa Y, Iwakura Y, Imanishi J. Role of gamma interferon in Helicobacter pylori-induced gastric inflammatory responses in a mouse model. Infect Immun. 1999;67:279–285. doi: 10.1128/iai.67.1.279-285.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Smythies LE, Waites KB, Lindsey JR, Harris PR, Ghiara P, Smith PD. Helicobacter pylori-induced mucosal inflammation is Th1 mediated and exacerbated in IL-4, but not IFN-gamma, gene-deficient mice. J Immunol. 2000;165:1022–1029. doi: 10.4049/jimmunol.165.2.1022. [DOI] [PubMed] [Google Scholar]
- 87.Huet S, Groux H, Caillou B, Valentin H, Prieur AM, Bernard A. CD44 contributes to T cell activation. J Immunol. 1989;143:798–801. [PubMed] [Google Scholar]
- 88.Hodge-Dufour J, Noble PW, Horton MR, Bao C, Wysoka M, Burdick MD, Strieter RM, Trinchieri G, Pure E. Induction of IL-12 and chemokines by hyaluronan requires adhesion-dependent priming of resident but not elicited macrophages. J Immunol. 1997;159:2492–2500. [PubMed] [Google Scholar]
- 89.Tan PH, Santos EB, Rossbach HC, Sandmaier BM. Enhancement of natural killer activity by an antibody to CD44. J Immunol. 1993;150:812–820. [PubMed] [Google Scholar]
- 90.Schouppe E, Mommer C, Movahedi K, Laoui D, Morias Y, Gysemans C, Luyckx A, De BP, Van Ginderachter JA. Tumor-induced myeloid-derived suppressor cell subsets exert either inhibitory or stimulatory effects on distinct CD8+ T-cell activation events. Eur J Immunol. 2013;43:2930–2942. doi: 10.1002/eji.201343349. [DOI] [PubMed] [Google Scholar]


