Abstract
Reprimo (RPRM), initially identified as a downstream effector of p53-induced cell cycle arrest at G2/M, is a putative tumor suppressor silenced in some types of cancer. In microarrays, the RPRM transcript was repressed 26-fold in gonadotrope (null cell) human pituitary tumors compared with normal pituitary but in the absence of changes in p53. Inhibition of RPRM mRNA was confirmed by RT-PCR in all gonadotrope tumors, most GH samples, and variably in other tumor types. Human pituitary tumors showed no evidence of abnormal promoter hypermethylation as a mechanism of RPRM repression. RPRM stable expression in gonadotrope (LβT2) and GH (GH3) pituitary cells resulted in decreased rates of cell proliferation by 55 and 30%, respectively; however, RPRM reexpression did not alter G2/M transition. In addition, RPRM increased rates of apoptosis in response to growth factor deprivation as assessed by caspase-3 cleavage and nuclear condensation. Clonagenic assays showed a 5.3- and 3.7-fold suppression of colony growth in RPRM-overexpressing LβT2 and GH3 cells, respectively, supporting its role as a tumor suppressor. In cells stably expressing RPRM mRNA, protein levels were actively suppressed due to rapid degradation through ubiquitination and proteasomal targeting. Growth factor withdrawal, as a model of cellular stress, stabilized RPRM protein levels. Together these data suggest that RPRM is transiently up-regulated at a posttranscriptional level in times of cellular stress to restrict cell survival, proliferation, and tumor formation. When RPRM is silenced as in human pituitary tumors, unrestrained growth and tumor progression may occur.
Pituitary tumors are a common intracranial neoplasm, detected in one in 10,000 persons and evident at autopsy in up to 10–20% (1, 2). Clinically, pituitary tumors lead to manifestations of hormone overproduction including acromegaly, Cushing's disease, or amenorrhea due to elevated GH, adrenocorticotropic hormone, and prolactin, respectively (3). In contrast, gonadotrope or null cell tumors were initially thought to be clinically silent; however, these tumors are common in men presenting with erectile dysfunction and hypogonadism (low testosterone levels) with headaches and visual disturbances progressing to blindness (4). Because of their larger size, these pituitary tumors often compromise normal pituitary hormone production and patients have symptoms of panhypopituitarism (1, 2). Local invasion occurs in approximately 50% of gonadotrope tumors, leading to increased risk of residual tumor regrowth and recurrence after primary transsphenoidal surgical resection (5). Although monoclonal in nature, the underlying pathogenesis of these tumors is poorly understood. There are few prognostic biomarkers and no medical therapies exist (2, 4, 6).
Microarray based expression profiling of human pituitary tumors and normal pituitary has been used to identify novel candidates involved in pituitary tumorigenesis or progression. We have previously characterized several oncogenic candidates including bone morphogenic and retinoic acid inducible neuronal protein-3 (Brinp3; FAM5C) (7), epidermal growth factor receptor-associated protein-8 (Eps8), and recently growth arrest and DNA-damage-inducible gene-β (GADD45B) (8). FAM5C (Brinp3) is overexpressed selectively in gonadotrope tumors in which it directs increased proliferation, migration, and survival (7). Eps8 is up-regulated in multiple pituitary tumor subtypes in which it mediates survival, proliferation, and tumorigenicity (3). Few tumor suppressors have been identified in human pituitary tumors, including MEG3A (9, 10), GADD45γ (9), and GADD45β but not GADD45α (8). A DNA microarray screen of individual gonadotrope tumors and normal human pituitaries identified Reprimo (RPRM) as a novel tumor suppressor candidate, and this was chosen for further analysis.
Reprimo (Latin for stop/repress) is a glycosylated cytoplasmic protein that was identified using differential display PCR of wild-type and p53/interferon regulatory factor(IRF)-1-deficient mouse embryonal fibroblasts after X-irradiation and thus classified as a p53-inducible gene (11, 12). Overexpression of RPRM induced G2 arrest of the cell cycle-dependent on inhibition of Cdc2 and nuclear translocation of cyclin B1 (11), suggesting it was a mediator of cell cycle transition downstream of p53 in some systems. The down-regulation of the RPRM transcript is associated with RPRM promoter methylation in some tumors and tumor cell lines including colorectal, gastric, gallbladder, and leukemia (13, 14). Thus, we asked whether RPRM levels were altered in human pituitary tumors and whether the promoter was hypermethylated as a mechanism of its down-regulation as well as the functional significance of modulating the RPRM expression in gonadotrope (LβT2) and GH (GH3) pituitary cells. In contrast to a previous report in fibroblasts (11), RPRM up-regulation had no effect on the G2/M transition of the cell cycle but modulated pituitary cell proliferation, survival, and tumorigenicity. In addition, we report the novel observation that RPRM protein levels are dynamically controlled and that proteasomal degradation is a major pathway for RPRM regulation that is disrupted when its expression is silenced in human pituitary tumors.
Materials and Methods
Reagents
A RPRM overexpression plasmid was generated by cloning the PCR-amplified mouse Rprm open reading frame from αT3 gonadotrope cDNA into pcDNA3 vector (Invitrogen, Grand Island, NY) via KpnI and NotI sites. Oligonucleotide primers used were the following: 5′KpnI mouse Rprm (ACT GAC TGG TAC CAC CAT GAA TTC AGT GCT GGG CAA C) and 3′NotI flag mouse RPRM (TTT TCC TTT TGC GGC CGC TCA TTA CTT GTC ATC GTC GTC CTT GTA GTC GTA GGG TCC CAC GAC CAC TGC). The sequence verified as wild type by comparison with GenBank. Hoechst stain solution was purchased from Sigma (St. Louis, MO). Antibodies used included caspase-3 (Cell Signaling Technology, Danvers, MA; no. 9662), β-tubulin (Abcam, Cambridge, MA; no. ab6046), and Flag (Sigma; F1804).
Characterization of human pituitary tumors and normal pituitary and cell lines
Pituitary tumors samples were obtained from patients at the University of Colorado Hospital at the time of transsphenoidal surgery after informed consent. Portions of the specimens not used for histology and immunohistochemistry were placed in RNAlater (QIAGEN, Valencia, CA) and stored at −80 C. Gonadotrope tumors were characterized by immunostaining. Antisera used in detection include ACTH and GH polyclonal prediluted (Cell Marque, Rocklin, CA), α-subunit monoclonal, 1:400 (Biogenex, San Ramon, CA), FSH (1:50), LH (1:50) monoclonal (Dako, Carpinteria, CA), prolactin polyclonal, prediluted (Ventana, Tucson, AZ). Normal pituitary glands used as controls were obtained at autopsy within 2–18 h of death from the University of Colorado Denver Pathology Department.
Microarray preparation and data generation
Total RNA was isolated from 14 pituitary tumors or nine control pituitaries using TRIzol (Invitrogen), followed by clean-up with a QIAGEN RNeasy minikit. RNA was quantified by spectrophotometry and RNA integrity confirmed with the Agilent Bioanalyzer (Agilent Technologies, Santa Clara, CA). Microarray targets were prepared and labeled from 300 ng of total RNA using the MessageAmp premier RNA amplification kit (Applied Biosystems/Ambion, Austin, TX) following the manufacturer's instructions. Affymetrix HG-U133 plus 2.0 arrays (Santa Clara, CA) were hybridized with 10 μg of cRNA and processed and scanned using standard Affymetrix protocols.
Hybridization intensities were quantified and normalized across all arrays in Partek Genomics Suite software (St. Louis, MO) using the RMA algorithm with GC adjustment (15). Data were filtered using Affymetrix GeneChip Operating Software to retain all genes present in greater than 95% of all the samples (22 of 23 total samples) and remove all genes considered absent. The remaining 38,932 transcripts (of 54,675) were used for all subsequent statistical analysis. Within Partek Genomics Suite, a mixed-model ANOVA was used to estimate the array batch effect, and data were adjusted using the Partek Batch Remover tool. The filtered, batch effect-adjusted data have also been deposited, along with the original Affymetrix CEL files used to generate the raw data, in the GEO database (www.ncbi.nlm.nih.gov/geo/). The CEL files for this experiment have been uploaded into GEO (7).
Reverse transcriptase-polymerase chain reaction
Total RNA was extracted from tissues or cells using TRIzol reagent according to the manufacturer's protocol (Invitrogen). For RT-PCR, RNA (0.5 μg) was reverse transcribed using a Thermo Verso cDNA kit (Fisher Scientific, Pittsburgh, PA). RT-PCR was performed under the following conditions: 94 C for 3 min, 94 C for 30 sec, 60 C for 30 sec, and 72 C for 30 sec for 35 cycles and 72 C for 7 min. The primer sequences used to amplify human RPRM were human RPRM forward 233 Suzuki, 5′-GCA ATCTGCTCATCAAGTCCGAG-3′ and human RPRM reverse 616 Suzuki, 5′-CCCCGCATTCCAAGTAAGTAG3′. The primer sequences used to amplify mouse or rat Rprm were: 5′-GCCTGTTCCTGGTCAACAGT-3′ and 5′-AGTTGATCATGCCTTCGGAC-3′. PCR were performed in the GeneAmp PCR System 9700 (Applied Biosystems, Foster City, CA). The quantity of RPRM mRNA was compared with that of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in the PCR reactions using human GADPH primers 5′-CGGAGTCAACGGATTTGGTCGTAT-3′ and 3′-CAGAAGTGGTGGTACCTCTTCCGA-5′ or mouse GAPDH primers 5′-CGACCCCTTCATTGACCTCA-3′ and 3′-GCCACGACTCATACAGCACC-5′.
RPRM promoter methylation analysis
DNA from tumor or normal pituitary samples were extracted with the DNeasy blood and tissue kit (QIAGEN) followed by bisulfite conversion and desulfonation using the EpiTect kit (QIAGEN). The RPRM promoter was amplified from converted DNA with the primers (5′-GTTTTAGAAGAGTTTAGTTGTTG-3′) and RPRM_MSPFlnkRev1 (5′-CTACTATTAACCAAAAACAAAC-3′) using the Failsafe PCR system (Epicenter, Madison, WI), and amplimers were purified with GeneJET PCR purification kit (Fermentas, Glen Burnie, MD). The amplified promoters were sequenced directly with the primer RPRM_MSPFlnkFwd1 and RPRM_MSPFlnkRev1, allowing the detection of 30 CpG within a 262-bp promoter region. Positive control DNA, CpGenome Universal methylated DNA (Millipore, Billerica, MA), was also amplified and sequenced.
In addition to this approach of DNA sequencing, methylation-specific PCR (MS-PCR) was also performed on bisulfite-modified pituitary DNA using primers specific for methylated (5′-GAAGAGTTTAGTTGTTGCGC-3′ and 5′-ATACCGAACTAAACGCTCACT-3′) and unmethylated (5′-TTAGAAGAGTTTAGTTGTTGTGT-3′and 5′-AAATACCAAACTAAACACTCACTC-3′) DNA. Each PCR amplification was started with 27 ng of template DNA using GoTaq Green master mix (Promega, Madison, WI). The cycle was as follows: 94 C 3 min; 94 C 45 sec, 50 C 45 sec, and 72 C 45 sec for 35 cycles and 72 C for 7 min for a final extension.
Cell culture and transfections
LβT2 mouse pituitary gonadotrope cell lines were obtained from P. Mellon (University of California, San Diego, San Diego, CA). GH3 cells were purchased from the American Type Culture Collection (Manassas, VA). Both of cells were cultured in DMEM (Invitrogen) supplemented with 10% fetal bovine serum (FBS; HyClone, Logan, UT), 100 U/ml penicillin, and 100 μg/ml streptomycin at 37 C in humidified 5% CO2. To generate LβT2 stable transfectants, cells were transfected with the pCDNA3-Rprm-Flag or with pCDNA3, the empty vector control, using Lipofectamine 2000 (Invitrogen) following the manufacturer's protocol. To generate GH3-stable transfectants, GH3 cells were electroporated with vector control or Rprm-Flag constructs. Selection of stably overexpressing Rprm-flag and pCDNA3 control cells was achieved under geneticin selection (Invitrogen) at 600 μg/ml.
Cell cycle analysis
LβT2 and GH3 cells stably transfected with the pcDNA3 vector or pCDNA3-Rprm-flag were grown in complete media (DMEM + 10% FBS) followed by a 16-h starvation in serum-free media (DMEM). The cells were allowed to recover in complete media for 24 h followed by trypsinization, resuspension, and staining with saponin/propidium iodide (PI) (0.3% saponin, 25 μg/ml PI, 10 μg/ml ribonuclease A, and 0.1 mm EDTA). Cell cycle profiles were gathered using a Beckman Coulter FC500 (Fullerton, CA) at the University of Colorado Denver Cancer Center Flow Cytometry Core.
Proliferation assays
To assess proliferation, cell growth was determined using the CellTiter 96 AQueous One Solution cell proliferation assay [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (MTS) (Promega)]. LβT2 and GH3 cells stably transfected with the pcDNA3 vector or pCDNA3-Rprm-flag were trypsinized and counted. Five thousand cells were plated in a 96-well plate (nine replicates for each condition) in complete medium (DMEM + 10% FBS), alongside control wells containing cell-free medium, and incubated for 1, 3, 5, or 7 d after plating. Twenty microliters of CellTiter MTS solution were added to each well, and plates were incubated for 1 h at 37 C and absorbance at 490 nm was measured. The experimental groups were compared via a nonmatched, two-way, repeated-measures ANOVA.
Immunoblot analysis
Cells were harvested in radioimmunoprecipitation assay buffer [150 mm NaCl, 1% Nonidet P-40, 0.5% deoxycholate, 0.1% sodium dodecyl sulfate, 50 mm Tris (pH 8.0)]. Protein lysates were quantified by bicinchoninic assay (Pierce, Rockford, IL) and electrophoresed through sodium dodecyl sulfate polyacrylamide gels. Proteins on the gels were electrotransferred to polyvinyl difluoride membranes using the minitransblotter system (Bio-Rad Laboratories, Hercules, CA). The polyvinyl difluoride membranes were blocked in 3% BSA in Tris-buffered saline and 0.2% Tween 20 (TBST) for 1 h. Primary antibodies were diluted 1:1000 in TBST/0.5% BSA/0.1% NaN3, and the membranes were incubated in primary antibodies at 4 C overnight. Membranes were washed three times in TBST for 10 min each. Horseradish peroxidase-conjugated secondary antibody (Amersham Biosciences, Piscataway, NJ) was diluted 1:3000 in TBST and incubated with the membranes for 1 h at room temperature. The membranes were washed as above and visualized using enhanced chemiluminescence according to the manufacturer's protocol (Pierce).
Apoptosis assays
Cleaved caspase-3 detection
LβT2 and GH3 cells stably overexpressing pcDNA3 vector or pcDNA3-Rprm-flag were exposed to growth factor replete (10% FBS) or deprived (0% FBS) conditions for 48 h, lysates harvested, and immunoblots performed for total and cleaved caspase-3 detection to assess for apoptotic activity.
Detection of nuclear condensation by Hoechst staining: LβT2 and GH3 cells (20 × 104) stably overexpressing pcDNA3 vector or pcDNA3-Rprm-flag were plated in 24-well plates and after overnight growth, cells were exposed to growth factor replete (10% FBS) or deprived (0% FBS) conditions for 48 h. Cells were then fixed in 1% paraformaldehyde for 5 min at room temperature followed by 70% ethanol in glycine buffer [100 mm (pH 3.0)] for 20 min at −20 C. After fixation, cells were washed with PBS three times and then incubated in Hoechst 33258 stain (8 μg/ml in PBS) for 15 min at room temperature. Cells were washed with PBS three times and then coverslips mounted on slides. Stained cells were viewed under a fluorescent microscope (Zeiss Axiovert 200; New York, NY) at ×40. Apoptotic cells were counted as the number of cells with condensed or fragmented chromatin. These cells typically appeared small and rounded and bright blue. One thousand cells were counted from randomly chosen fields. The index of cells with nuclear condensation was expressed as a percentage of total counted cells as an index of apoptosis.
Colony formation assay
LβT2 and GH3 were transiently transfected with pcDNA3 vector or pcDNA3-Rprm-flag. Forty-eight hours after transfection, cells were cultured in serum-replete conditions containing G418 (600 μg/ml). Media were changed every other day. Colonies were stained using Diff-Quik DADE Behring, Newark, DE stain set after 14–21 d and counted at ×20 image under a Zeiss Axiovert 200 microscope. Images of colonies were captured at ×2 using an Olympus (Center Valley, PA) BX51 mounted Microfire digital camera (Tokyo, Japan).
Statistical analysis
Data were analyzed by an unpaired Student's t test and ANOVA with Bonferroni's multiple comparison tests using GraphPad Prism software (San Diego, CA). Values are expressed as means + se. Values of P < 0.05 were considered statistically significant.
Results
RPRM transcripts are decreased in human pituitary tumors compared with normal pituitary
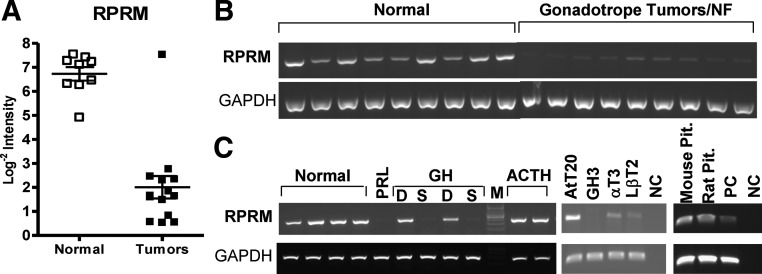
RNA was isolated from 14 gonadotrope tumors (defined as 5% or greater cells with positive immunostaining for FSH, LH, and/or α-subunit) and from nine normal pituitaries at the time of autopsy as previously described (7, 8, 16). RNA integrity was confirmed and cDNA was transcribed, labeled, and hybridized from individual samples on the Affymetrix Human Genome U133 Plus 2.0 arrays. To detect genes with significantly altered expression profiles between the two experimental groups [control pituitaries (n = 9) and gonadotrope tumors (n = 14)], a one-way ANOVA model was used to generate P values for all present genes (38,932 present/54,675 transcripts probed for on the Affymetrix U133 2.0 array). The average RPRM transcript intensity for normal pituitary was 106.18 (log2 transformed = 6.73) compared with 4.02 (log2 transformed = 2.01) in gonadotrope tumors, representing a 26-fold decrease in RPRM expression (Fig. 1A, P = 2.25e-07).
Fig. 1.
DNA Microarray of RPRM transcript and RT-PCR of RPRM mRNA in normal pituitary and pituitary tumors and cell lines. A, RPRM transcript expression levels assessed using Affymetrix U133plus2.0 microarrays in nine normal pituitaries and 14 gonadotrope/nonfunctioning (NF) tumors. Lines indicate average intensity ± se. B and C, RNA was extracted from tumor tissue and cell lines harvested in RNAlater (see Materials and Methods for details). RNA was reverse transcribed and cDNA amplified with primers specific for RPRM and GADPH as an internal control. Tumor types are listed including gonadotrope, prolactin (PRL), GH [sparse (S), or densely granulated (D)] and ACTH. Cell lines include AtT20 (a corticotroph cell line), GH3(a GH cell line), αT3, and LβT2 early and differentiated gonadotrope cell lines. PC, Positive control; NC, negative control; M, marker.
RPRM mRNA is variably repressed in human pituitary tumors dependent on cell type
To confirm the results of the DNA microarray, RT-PCR was performed on samples from human pituitaries and pituitary tumors using probes specific for RPRM and GAPDH as an internal control. RPRM mRNA was consistently down-regulated or absent in human gonadotrope pituitary tumors compared with normal pituitary (Fig. 1B). RPRM transcript was also low in the available prolactinoma sample and the sparsely granulated growth hormone tumor samples tested. However, RPRM mRNA was not suppressed in densely granulated GH or ACTH tumor samples, suggesting that there may be specific regulation of RPRM expression based on pituitary tumor subtype (Fig. 1C). Additionally, RPRM mRNA was variable in different cell lines representative of pituitary tumor subtypes (Fig. 1C).
RPRM down-regulation is not a result of abnormal promoter hypermethylation in human pituitary tumors
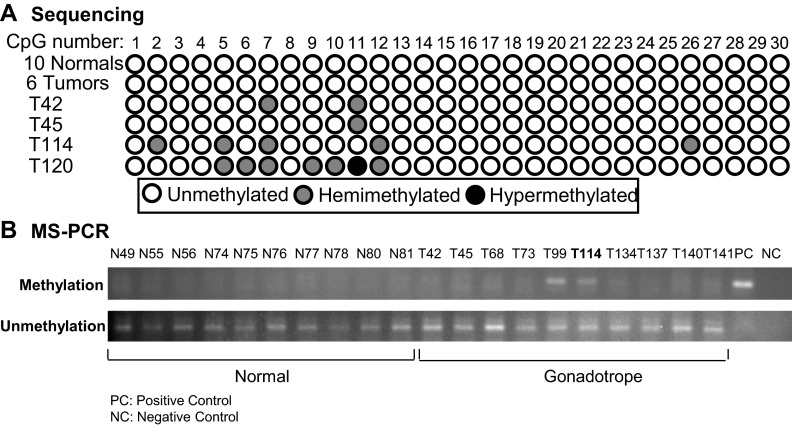
In other tumors, including prostate, hematological, and breast, RPRM expression is repressed by abnormal hypermethylation of its promoter (14, 17, 18). Thus, we characterized 10 additional gonadotrope tumors and normal human pituitary samples for RPRM promoter hypermethylation. Genomic DNA was treated with sodium bisulfite to convert unmethylated cytosine residues to uracil, leaving methylated cytosines unaltered. A 262-bp region of the proximal RPRM promoter containing 30 CpG was amplified by PCR and sequenced in both directions. All normal human pituitary and six tumor samples showed no CpG methylation, and universally methylated control DNA showed complete methylation at all 30 CpG (Fig. 2A). Four tumors had evidence of several hemimethylated CpG, but only one tumor displayed a single homozygously methylated CpG on both strands.
Fig. 2.
Methylation detection assays demonstrate that loss of RPRM in pituitary tumors is not due to hypermethylation of the promoter. Pituitary tumor and normal pituitary genomic DNA was treated with sodium bisulfite to convert unmethylated cytosine residues to uracil, leaving methylated cytosines unaltered. A, A 262-bp region of the proximal RPRM promoter containing 30 CpG was PCR amplified and sequenced in both directions. All normals are not methylated. Some tumors are hemimethylated. Only rare tumors are methylated on both strands. B, Additional tumors and normal sample DNA was amplified with primers specific to methylated or unmethylated DNA. Similarly, normal pituitary samples were not methylated; tumor 99 and 114 are hemimethylated. No tumor was completely methylated.
To confirm these results, MS-PCR was also performed on bisulfite-modified pituitary DNA using primers specific for methylated and unmethylated RPRM DNA on an additional 10 normal pituitary and gonadotrope tumor samples (Fig. 2B). One sample, tumor 114, was available for retesting. In these studies, no methylation was detected in the normal pituitary samples. Two tumors (T99 and T114) showed hemimethylation (bands detectable using both the methylation and unmethylation specific primers, Fig 2B). Tumor 114 also displayed evidence of hemimethylation using the alternative approach above (Fig. 1A). In this sample set, no tumor displayed complete methylation of the CpG sites on both strands. Taken together, these data suggest that in contrast to prior reports of malignant tumor types, inhibition of RPRM expression in pituitary tumors is not a result of promoter hypermethylation.
Creation of model systems to define the functional role of RPRM in pituitary tumors
In the absence of human gonadotrope pituitary cell lines, functional studies were conducted in mouse LβT2 gonadotrope cells and GH3 rat pituitary cells. RPRM levels were low to undetectable in both pituitary tumor cell lines per RT-PCR (Fig. 1C). MS-PCR of genomic DNA from GH3 cells was performed and demonstrated that loss of RPRM in the pituitary cell line was associated with hypermethylation, in contrast to human pituitary tumors (see Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). Vector control (pcDNA3) or Flag-tagged Rprm constructs were stably overexpressed and overexpression confirmed by RT-PCR (Fig. 3, A and B) and immunoblot using a Flag antibody in the absence of available RPRM antibody (see Fig. 5, A and B).
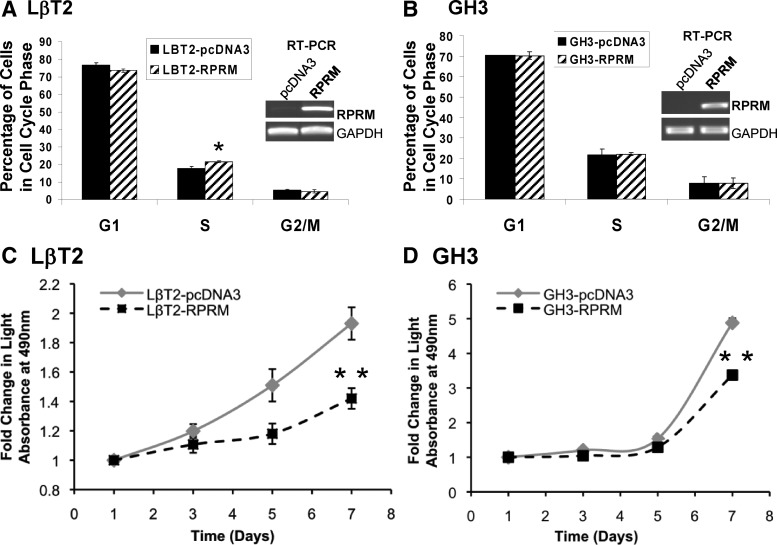
Fig. 3.
Role of Rprm on cell cycle and rates of proliferation in pituitary cells. A and B, Modulating Rprm levels had no effect on G2/M. Cells stably expressing vector or Rprm-Flag were grown in complete media (DMEM + 10% FBS) for 24 h followed by a 16-h starvation in serum-free media (DMEM). Cells were then trypsinized, resuspened, and stained with saponin/PI. Cell cycle profiles were gathered using a Beckman Coulter FC500 (*, P < 0.05). C and D, Overexpression of Rprm in LβT2 and GH3 cells restricted cell proliferation. Cells stably expressing vector or Rprm-Flag were plated in a 96-well plate (nine replicates for each condition) in complete medium (DMEM + 10% FBS) and incubated for 1, 3, 5, or 7 d after plating. Twenty microliters of CellTiter MTS solution was added to each well and plates were incubated for 1 h at 37 C and absorbance at 490 nm was measured. **, P < 0.0001.
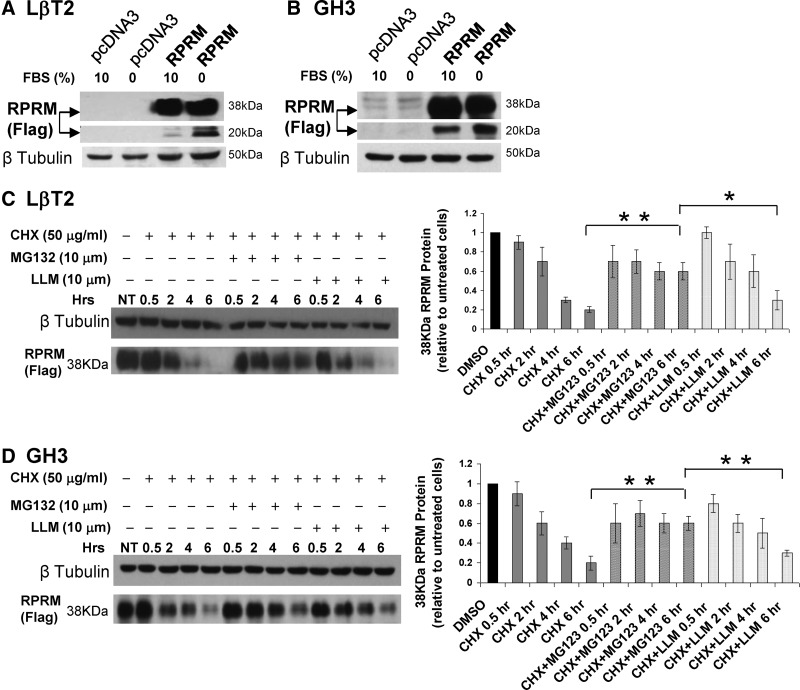
Fig. 5.
Rprm protein is a target of ubiquitination and the rapid in vitro degradation of Rprm protein is blocked by proteasomal inhibition. Figure 5 shows representative immunoblots and compiled data of n = 3 experiments in each pituitary cell type. A and B, Rprm-Flag protein levels in LβT2 and GH3 stable cells were assessed by immunoblot in serum starved vs. complete media. β-Tubulin was as a loading control. C and D, Stable overexpressed Rprm LβT2 or GH3 serum-replete cells were treated with CHX alone or in combination with specific proteasome inhibitor, MG132, or a nonspecific protease (calpain) inhibitor, LLM. Rprm levels were assessed by immunoblot for Flag and β-tubulin was used as a loading control. NT, No treatment. Abundance of 38 kDa Rprm protein upon CHX, MG132, and LLM treatment, expressed as the ratio of Rprm to β-tubulin relative to untreated cells. *, P < 0.05; **, P < 0.01.
RPRM fails to produce a G2/M phase block in pituitary cells
We asked whether RPRM could act as a G2/M phase cell cycle brake in gonadotrope and GH pituitary cells as previously reported in mouse embryonic fibroblasts (10). LβT2 gonadotrope and GH3 cells stably overexpressing the Rprm cDNA were synchronized by serum deprivation for 16 h, recovered in complete media, and analyzed 24 h later for cell cycle profiles. Surprisingly, there was no change in the percentage of cells in G2/M phase between vector and Rprm LβT2 or GH3 cells (Fig. 3, A and B). A small 20% increase in the S phase was observed in gonadotrope cells but not in GH3 cells. Thus, RPRM does not have a significant effect to alter G2/M in pituitary cells. These data suggest that RPRM may perform alternative cellular functions in nonfibroblast derived cells.
RPRM restricts proliferation of gonadotrope pituitary cells
To determine the functional consequences of Rprm overexpression in regard to cell growth, cell proliferation assays were performed in LβT2 and GH3 pituitary cells. Cells stably expressing either a control vector (pcDNA3) or Rprm were grown in 10% serum, and cell growth assays were performed. MTS assays were conducted on d 1, 3, 5, and 7. By 7 d, Rprm overexpressing cells showed a 70 and 51% decrease in proliferation rate in LβT2 and GH3 cells, respectively, compared with controls (P < 0.0001, Fig. 3, C and D). Together these data are consistent with a normal role for RPRM as a brake to dysregulated pituitary cell growth, which is lost with tumorigenesis.
RPRM overexpression amplifies growth factor withdrawal-induced programmed cell death
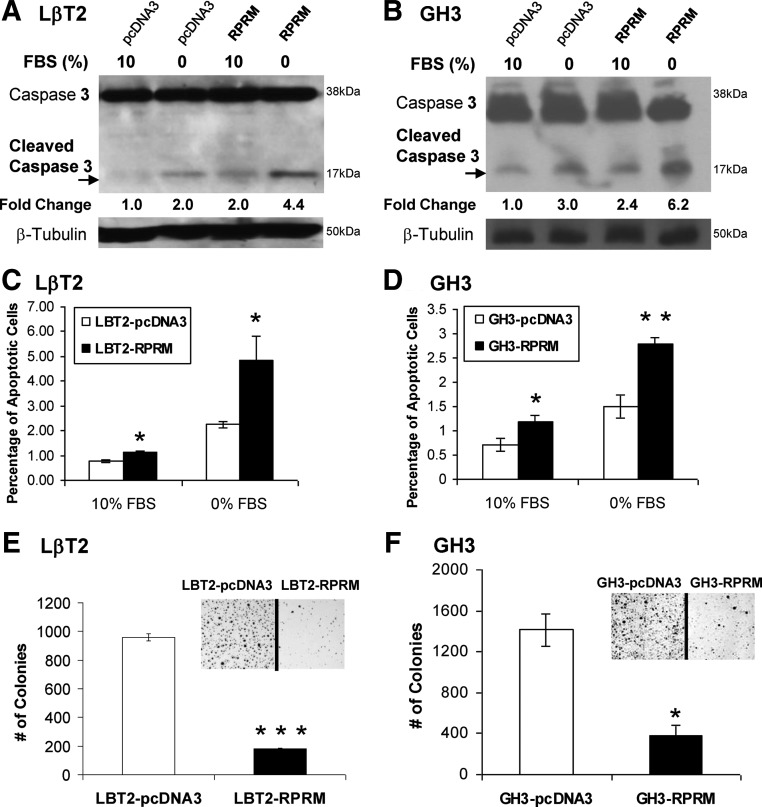
Maintenance and progression of tumorigenesis characteristically requires strategies to survive under growth-restricted conditions. To test whether the loss of RPRM plays such a role in pituitary tumors, LβT2 gonadotrope and GH3 pituitary cells stably expressing vector control or Rprm-Flag were compared in their response to serum withdrawal for 48 h. Cell lysates were harvested and immunoblots performed with an antibody that recognizes both full-length and cleaved caspase-3 as a hallmark of activation of the intrinsic apoptosis pathway triggered by growth factor withdrawal. Vector-containing cells showed increased levels of cleaved caspase-3 (2.0- to 2.5-fold in LβT2 and 3.0- to 5.7-fold in GH3 cells) after 48 h of serum withdrawal. Rprm-overexpressing cells showed increased caspase-3 cleavage in both serum replete (1.9- to 2.0-fold in LβT2 and 1.2- to 2.4-fold in GH3 cells) as well as deprived conditions (4.4- to 6.6-fold and 3.2- to 6.2-fold, respectively), compared with control cells (Fig. 4, A and B, show representative immunoblot for each pituitary cell type of n = 3 experiments).
Fig. 4.
Effects of Rprm on cell survival and tumorigenicity. A and B, Overexpressed of Rprm amplifies serum withdrawal-induced apoptosis in LβT2 and GH3 as assessed by cleaved caspase-3. Cells stably expressing vector or Rprm-Flag were cultured in growth-replete (10% serum) or -deficient (0% serum) for 48 h and lysates harvested. Immunoblots were probed for total and cleaved caspase-3 as an index of apoptotic activity. β-Tubulin was used as a loading control. Panels A and B show representative immunoblots of n = 3 experiments for each cell type showing similar results. C and D, Overexpressed of Rprm amplifies serum withdrawal-induced apoptosis in LβT2 and GH3 as assessed by Hoechst staining of nuclear condensation of cells. Cells stably expressing vector or Rprm-Flag were cultured in growth-replete (10% serum) or -deficient (0% serum) for 48 h and stained with Hoechst. Experiments were repeated three times. *, P < 0.05; **, P < 0.01). E and F, Modulating Rprm levels represses gonadotrope colony formation in LβT2 and GH3 pituitary cells. Cells were transiently transfected with vector and Rprm and then cultured in serum-replete conditions containing G418. Colonies were stained using Diff-Quik stain set and counted after 14–21d at ×20 image under Zeiss Axiovert 200 microscope. Photomicrographs of colonies were taken at ×2 using Olympus BX51 mounted Microfire digital camera. *, P < 0.05; ***, P < 0.00001.
To confirm these findings, nuclear condensation as an index of cell death was assessed by Hoechst staining of apoptotic nuclei before and after growth factor withdrawal. Stably expressing vector control or Rprm-Flag LβT2 or GH3 cells were stained with Hoechst before and after 48 h starvation and nuclear condensation assessed. Rprm-overexpressing cells showed an increase in percentage of apoptotic cells in both serum replete and growth factor withdrawal conditions (LβT2 cells: vector 10 to 0% FBS 0.77 ± 0.07 to 2.24 ± 0.15; RPRM in 10 to 0% FBS 1.11 ± 0.08 to 4.84 ± 0.96. In GH3cells: vector in 10 to 0% FBS: 0.70 ± 0.13 to 1.50 ± 0.23; Rprm 10 to 0% FBS 1.18 ± 0.13 to 2.80 ± 0.11, n = 3, *, P < 0.05, **, P < 0.01) (Fig. 4, C and D). Together these data support the hypothesis that repression of RPRM levels in pituitary tumors may alter the rates of programmed cell death in response to restricted growth environments.
RPRM expression inhibits colony formation in pituitary cells
To characterize RPRM's role in tumorigenicity, clonagenic assays were performed in the context of Rprm overexpression. LβT2 or GH3 cells were transiently transfected with vector (pcDNA3) or Rprm cDNA and selected by G418. Rprm-LβT2 cells demonstrated 5.3-fold fewer colonies than cells containing vector control (959 ± 26 vs. 181 ± 4.9, P < 0.00001, Fig. 4E). Rprm-GH3 cells were similarly affected, forming 3.7-fold fewer colonies than vector control (1414 ± 162 and 384 ± 99, P < 0.05, Fig 4F). Together these data confirm that RPRM has anticlonagenic effects in pituitary cells and suggests that loss of RPRM may allow for continued tumor growth in human pituitary tumors.
RPRM protein levels are regulated posttranslationally by proteasomal targeting
During our studies we observed robust overexpression of Rprm mRNA after transient or stable expression but low levels of the lower-molecular-mass species (Rprm is 12 kDa but is heavily glycosylated, resulting in 20 and 38 kDa species) in growth-replete conditions. Conversely, serum withdrawal induced Rprm protein levels (Fig. 5, A and B). Analysis of previously established protein interactions with RPRM suggested that it can form a physical interaction with the ubiquitin ligase, Cullin 1 (19); thus, we postulated that the ubiquitin-proteasome targeting pathway might play a role in RPRM protein stability in the normal cells to carefully modulate its activity at the posttranslational level. In both LβT2 and GH3 cells with constitutive Rprm-Flag mRNA expression driven by the CMV promoter, transcript levels were robustly overexpressed (Fig. 3, A and B). In contrast, at the protein level the 38-kDa isoform was detected but with diminished expression of the lower-molecular-mass species. Serum deprivation for 48 h, however, induced the 20-kDa Rprm protein isoforms (Fig. 5, A and B).
To determine the stability of the Rprm protein under typical growth-replete conditions and determine the pathway controlling steady-state Rprm levels, LβT2-Rprm and GH3-RPRM cells were incubated with the universal translation inhibitor, cycloheximide, and Rprm protein levels were assessed by immunoblot using the Flag antibody (in the absence of available effective RPRM antibodies) (Fig. 5, C and D, representative immunoblots of n = 3 experiments in each cell line). Rprm-Flag protein levels decreased rapidly upon cycloheximide treatment at between 2 and 4 h, and within 6 h protein levels were nearly undetectable. Addition of the proteasome-specific inhibitor, MG132, leads to stabilization of Rprm protein (Fig. 5, C and D). To address the possible role of non-proteasome-mediated proteolysis in the degradation of Rprm, the impact of a nonspecific protease (calpain) inhibitor, LLM, on Rprm levels was assessed. In replicate experiments (n = 3), retention of Rprm at 6 h in the presence of MG132 was at least 3 times higher than in cycloheximide (CHX)- and CHX/LLM-treated LBT2 and GH3 cells. Coincubation with LLM and CHX (Fig. 5, C and D) resulted in levels of Rprm protein similar to treatment with cycloheximide alone, consistent with the hypothesis that modulation of Rprm protein levels in the absence or presence of growth factors was dependent on an ubiquitin-specific pathway. Figure 6 outlines the potential modulation of Rprm gene expression and posttranslational modification in normal cells that is lost with pituitary tumorigenesis when the expression is silenced.
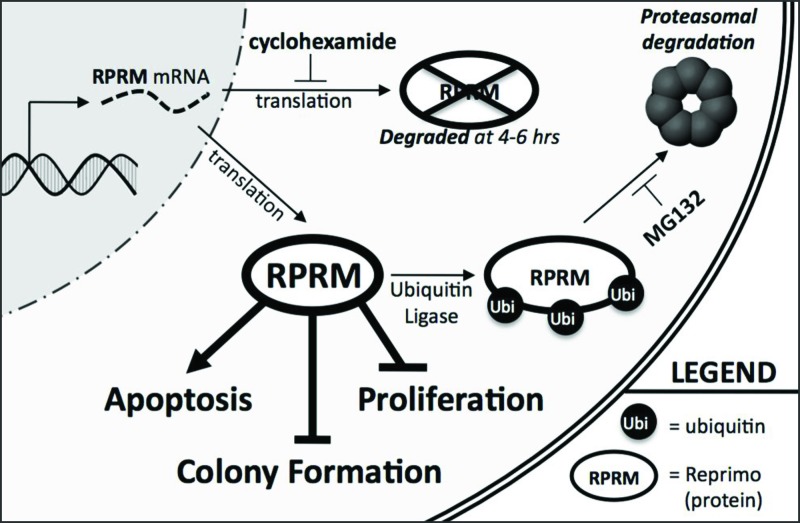
Fig. 6.
Proposed roles of RPRM in pituitary cells. RPRM is regulated posttranslationally through proteasomal-mediated degradation in response to cellular stress and functions normally to restrict pituitary cell survival and proliferation. Permanent silencing of RPRM would augment survival, proliferation, and tumor formation, maintenance, and/or progression.
Discussion
Using microarray analysis of human gonadotrope tumors and normal pituitaries, we identified RPRM as a novel tumor suppressor. RPRM mRNA were low in gonadotrope, sparse GH and prolactin, but not ACTH pituitary tumors compared with normal pituitary. Unlike cell lines and tumors in which promoter methylation is a key epigenetic mechanism in RPRM repression, bisulfite sequencing failed to demonstrate Rprm promoter hypermethylation in any of the gonadotrope tumors analyzed. In functional studies, however, reexpression of Rprm in pituitary cells repressed cell proliferation, increased rates of programmed cell death, and inhibited colony formation, supporting its role as a tumor suppressor. Further analysis demonstrated the novel observation that the Rprm protein levels are normally regulated at the posttranscriptional level with rapid degradation under growth replete conditions and increased protein stability under cellular stress as with growth factor withdrawal. These data suggest that RPRM levels are restricted during pituitary tumorigenesis to prevent its carefully modulated roles, which are transiently activated during stress to mediate cell-specific events.
RPRM was identified as a downstream effector of p53 signaling that could modulate G2/M phase transition in some systems (11). Suzuki et al. (20) found no correlation between p53 status and the methylation of RPRM promoter despite its suppression in tumors, suggesting it may be regulated through alternative pathways. Consistent with this observation, in our pituitary tumor array, RPRM was suppressed 26-fold, in contrast to p53, which was not differentially expressed in gonadotrope tumors at the protein or mRNA level (8). The RPRM gene maps to chromosome 2q23, a locus that often has allelic imbalance in lung, breast, and colon cancers (21–23). Examination of the methylation status of a broad range of cancer cell lines showed that 62% exhibited RPRM loss or repression. Within the repressed lines, 92% demonstrated RPRM hypermethylation (14). In addition, a review of more than 600 tumors showed methylation of the RPRM promoter in 79% of gastric cancers, 57% of lymphomas, 56% of gastric cancers, and 37% of breast cancers, in contrast to significantly lower rates of hypermethylation in brain, ovarian, cervical, and bladder cancer (14), suggesting tissue-specific modulation of RPRM gene expression during tumorigenesis. Methylation was further demonstrated as the primary method of RPRM repression in nine various tumor cell lines, which all demonstrated full restoration of RPRM expression after treatment with 5-Aza-Deoxycytidine (14). More recently, researchers found hypermethylation of RPRM in 54% of prostate cancer samples, but no correlation to Gleason score or prognosis (18). However, epigenetic suppression of RPRM via promoter methylation correlated with progression of Barrett's esophagus to neoplasm (17) and poor prognosis in pancreatic ductal carcinoma (17). In contrast to these studies, in pituitary tumors, RPRM silencing is independent of promoter hypermethylation. RPRM gene dysregulation and silencing could also stem from mutations within tumors. However, in ongoing investigations using copy number microarrays to identify gene amplifications and deletions in 10 gonadotrope tumors, we have not found mutations within or near the RPRM locus at 2q23 (Xu M. and Knox A.J., unpublished observations).
Only one report of the functional effects of RPRM was available before our studies, suggesting in fibroblasts RPRM altered cell cycle at G2/M (11). Investigations in both gonadotrope and GH pituitary cells showed no such effect. However, we demonstrated significant effects of altering the levels of Rprm expression on pituitary cell proliferation, survival, and colony formation, supporting its normal role as a tumor suppressor.
Interestingly, RPRM was recently shown to be repressed by ligand activated estrogen receptor-α via a mechanism that involves histone deacetylase-7 and Forkhead A1 in breast cancer cells (24), suggesting that sex hormone milieu may modulate down-regulation of RPRM in hormone-sensitive tissues such as the pituitary, prostate, and colon. However, in our hands, 17β-estradiol treatment of gonadotrope cells failed to modify endogenous RPRM mRNA levels (data not shown), arguing against the role estrogen receptor-α-mediated RPRM regulation in gonadotrope pituitary cells.
Our data reveal that normally RPRM protein levels are actively modulated at the posttranslational level in pituitary cells. In cells stably overexpressing Rprm cDNA, the Rprm protein levels were increased in cells under stress with removal of growth factors by serum deprivation and then suppressed with growth factor repletion. This modulation of RPRM protein levels is consistent with data concerning other proteins, which are controlled at numerous levels including posttranslational modification and rates of degradation and turnover (25–27). For example, mono- and polyubiquitination of p53 by murine double minute-2 triggers p53 nuclear export and trafficking to proteasomes (25). Also, the proapoptotic protein phorbol-12-myristate-13-acetate-induced protein 1 is stabilized by proteasomal inhibition in tumor but not normal cells, promoting the potential use of proteasomal inhibitors as a targeted therapy (28).
In summary, our data demonstrate that the novel tumor suppressor, RPRM, is repressed in subtypes of human pituitary tumors (Fig. 6). The loss of Rprm, however, is not due to promoter hypermethylation, suggesting cell specific regulation. When reintroduced by overexpression in pituitary cells, Rprm restricts cellular transformation. Changes in Rprm levels modulate rates of cell proliferation and rates of programmed cell death without direct effects on the G2/M transition in the cell cycle. Our findings contribute significantly to the knowledge of RPRM's biology and cellular functions, identifying novel properties that support its normal role in which its expression is actively modulated by cellular stress and as a tumor suppressor, which is silenced in pituitary tumors. In human pituitary tumors, RPRM mRNA loss would lead to decreased RPRM protein preventing its normal role as a brake to proliferation, resulting in protracted cell proliferation and survival contributing to initiation and/or progression of tumorigenesis. Future studies will ask whether its reactivation or reintroduction will alter tumor initiation or progression in vivo.
Acknowledgments
We thank Sierra McNamara for her efforts on this project, Janice Kerr for her manuscript review, and Francisco La Rosa for his assistance with photography. The immortalized mouse gonadotrope cell lines were provided by P. Mellon (University of California, San Diego, San Diego, CA) and the MG132 and LLM by H. Ford (University of University of Colorado Denver Cancer Center Colorado Denver, Aurora, CO). We acknowledge the Tissue Culture, DNA Sequencing, Gene Expression, and Bioinformatics cores for assistance on this project.
This work was supported by Grant VA Merit Review 001 (to M.E.W.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CHX
- cycloheximide
- FBS
- fetal bovine serum
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- MS-PCR
- methylation-specific PCR
- MTS
- 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt
- PI
- propidium iodide
- RPRM
- Reprimo
- TBST
- Tris-buffered saline and 0.2% Tween 20.
References
- 1. Ezzat S, Asa SL, Couldwell WT, Barr CE, Dodge WE, Vance ML, McCutcheon IE. 2004. The prevalence of pituitary adenomas: a systematic review. Cancer 101:613–619 [DOI] [PubMed] [Google Scholar]
- 2. Melmed S. 2003. Mechanisms for pituitary tumorigenesis: the plastic pituitary. J Clin Invest 112:1603–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Knox AJ, Xu M., Wierman ME. Role of Reprimo in pituitary tumorigenesis. 11th International Pituitary Congress, Washington, DC, 2009 [Google Scholar]
- 4. Alexander JM, Biller BM, Bikkal H, Zervas NT, Arnold A, Klibanski A. 1990. Clinically nonfunctioning pituitary tumors are monoclonal in origin. J Clin Invest 86:336–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Meij BP, Lopes MB, Ellegala DB, Alden TD, Laws ER., Jr 2002. The long-term significance of microscopic dural invasion in 354 patients with pituitary adenomas treated with transsphenoidal surgery. J Neurosurg 96:195–208 [DOI] [PubMed] [Google Scholar]
- 6. Ezzat S. 2003. Pituitary tumor pathogenesis—the hunt for novel candidate genes continues. J Clin Endocrinol Metab 88:5116–5118 [DOI] [PubMed] [Google Scholar]
- 7. Shorts-Cary L, Xu M, Ertel J, Kleinschmidt-Demasters BK, Lillehei K, Matsuoka I, Nielsen-Preiss S, Wierman ME. 2007. Bone morphogenetic protein and retinoic acid-inducible neural specific protein-3 is expressed in gonadotrope cell pituitary adenomas and induces proliferation, migration, and invasion. Endocrinology 148:967–975 [DOI] [PubMed] [Google Scholar]
- 8. Michaelis KA, Knox AJ, Xu M, Kiseljak-Vassiliades K, Edwards MG, Geraci M, Kleinschmidt-DeMasters BK, Lillehei KO, Wierman ME. 2011. Identification of growth arrest and DNA-damage-inducible gene β (GADD45β) as a novel tumor suppressor in pituitary gonadotrope tumors. Endocrinology 152:3603–3613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang X, Sun H, Danila DC, Johnson SR, Zhou Y, Swearingen B, Klibanski A. 2002. Loss of expression of GADD45γ, a growth inhibitory gene, in human pituitary adenomas: implications for tumorigenesis. J Clin Endocrinol Metab 87:1262–1267 [DOI] [PubMed] [Google Scholar]
- 10. Zhang X, Zhou Y, Mehta KR, Danila DC, Scolavino S, Johnson SR, Klibanski A. 2003. A pituitary-derived MEG3 isoform functions as a growth suppressor in tumor cells. J Clin Endocrinol Metab 88:5119–5126 [DOI] [PubMed] [Google Scholar]
- 11. Ohki R, Nemoto J, Murasawa H, Oda E, Inazawa J, Tanaka N, Taniguchi T. 2000. Reprimo, a new candidate mediator of the p53-mediated cell cycle arrest at the G2 phase. J Biol Chem 275:22627–22630 [DOI] [PubMed] [Google Scholar]
- 12. Taylor WR, Stark GR. 2001. Regulation of the G2/M transition by p53. Oncogene 20:1803–1815 [DOI] [PubMed] [Google Scholar]
- 13. Wong TS, Kwong DL, Sham JS, Wei WI, Yuen AP. 2005. Methylation status of Reprimo in head and neck carcinomas. Int J Cancer 117:697. [DOI] [PubMed] [Google Scholar]
- 14. Takahashi T, Suzuki M, Shigematsu H, Shivapurkar N, Echebiri C, Nomura M, Stastny V, Augustus M, Wu CW, Wistuba II, Meltzer SJ, Gazdar AF. 2005. Aberrant methylation of Reprimo in human malignancies. Int J Cancer 115:503–510 [DOI] [PubMed] [Google Scholar]
- 15. Wu ZJ, Irizarry RA, Gentleman R, Martinez-Murillo F, Spencer F. 2004. A model-based background adjustment for oligonucleotide expresion arrays. J Am Stat Assoc 99:909–917 [Google Scholar]
- 16. Xu M, Shorts-Cary L, Knox AJ, Kleinsmidt-DeMasters B, Lillehei K, Wierman ME. 2009. Epidermal growth factor receptor pathway substrate 8 is overexpressed in human pituitary tumors: role in proliferation and survival. Endocrinology 150:2064–2071 [DOI] [PubMed] [Google Scholar]
- 17. Hamilton JP, Sato F, Jin Z, Greenwald BD, Ito T, Mori Y, Paun BC, Kan T, Cheng Y, Wang S, Yang J, Abraham JM, Meltzer SJ. 2006. Reprimo methylation is a potential biomarker of Barrett's-Associated esophageal neoplastic progression. Clin Cancer Res 12:6637–6642 [DOI] [PubMed] [Google Scholar]
- 18. Suzuki M, Shigematsu H, Shivapurkar N, Reddy J, Miyajima K, Takahashi T, Gazdar AF, Frenkel EP. 2006. Methylation of apoptosis related genes in the pathogenesis and prognosis of prostate cancer. Cancer Lett 242:222–230 [DOI] [PubMed] [Google Scholar]
- 19. Ewing RM, Chu P, Elisma F, Li H, Taylor P, Climie S, McBroom-Cerajewski L, Robinson MD, O'Connor L, Li M, Taylor R, Dharsee M, Ho Y, Heilbut A, Moore L, Zhang S, Ornatsky O, Bukhman YV, Ethier M, Sheng Y, Vasilescu J, Abu-Farha M, Lambert JP, Duewel HS, Stewart II, Kuehl B, Hogue K, Colwill K, Gladwish K, Muskat B, Kinach R, Adams SL, Moran MF, Morin GB, Topaloglou T, Figeys D. 2007. Large-scale mapping of human protein-protein interactions by mass spectrometry. Mol Syst Biol 3:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Suzuki M, Shigematsu H, Takahashi T, Shivapurkar N, Sathyanarayana UG, Iizasa T, Fujisawa T, Gazdar AF. 2005. Aberrant methylation of Reprimo in lung cancer. Lung Cancer 47:309–314 [DOI] [PubMed] [Google Scholar]
- 21. Otsuka T, Kohno T, Mori M, Noguchi M, Hirohashi S, Yokota J. 1996. Deletion mapping of chromosome 2 in human lung carcinoma. Genes Chromosomes Cancer 16:113–119 [DOI] [PubMed] [Google Scholar]
- 22. Piao Z, Lee KS, Kim H, Perucho M, Malkhosyan S. 2001. Identification of novel deletion regions on chromosome arms 2q and 6p in breast carcinomas by amplotype analysis. Genes Chromosomes Cancer 30:113–122 [PubMed] [Google Scholar]
- 23. Olaru A, Mori Y, Yin J, Wang S, Kimos MC, Perry K, Xu Y, Sato F, Selaru FM, Deacu E, Sterian A, Shibata D, Abraham JM, Meltzer SJ. 2003. Loss of heterozygosity and mutational analyses of the ACTRII gene locus in human colorectal tumors. Lab Invest 83:1867–1871 [DOI] [PubMed] [Google Scholar]
- 24. Malik S, Jiang S, Garee JP, Verdin E, Lee AV, O'Malley BW, Zhang M, Belaguli NS, Oesterreich S. 2010. Histone deacetylase 7 and FoxA1 in estrogen-mediated repression of RPRM. Mol Cell Biol 30:399–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brooks CL, Gu W. 2006. p53 ubiquitination: Mdm2 and beyond. Mol Cell 21:307–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Haupt Y, Maya R, Kazaz A, Oren M. 1997. Mdm2 promotes the rapid degradation of p53. Nature 387:296–299 [DOI] [PubMed] [Google Scholar]
- 27. Kubbutat MH, Jones SN, Vousden KH. 1997. Regulation of p53 stability by Mdm2. Nature 387:299–303 [DOI] [PubMed] [Google Scholar]
- 28. Nikiforov MA, Riblett M, Tang WH, Gratchouck V, Zhuang D, Fernandez Y, Verhaegen M, Varambally S, Chinnaiyan AM, Jakubowiak AJ, Soengas MS. 2007. Tumor cell-selective regulation of NOXA by c-MYC in response to proteasome inhibition. Proc Natl Acad Sci USA 104:19488–19493 [DOI] [PMC free article] [PubMed] [Google Scholar]