Abstract
To evaluate mortality reduction from gastric cancer by endoscopic screening, we undertook a population‐based cohort study in which both radiographic and endoscopic screenings for gastric cancer have been carried out. The subjects were selected from the participants of gastric cancer screening in two cities in Japan, Tottori and Yonago, from 2007 to 2008. The subjects were defined as participants aged 40–79 years who had no gastric cancer screening in the previous year. Follow‐up of mortality was continued from the date of the first screening to the date of death or up to December 31, 2013. A Cox proportional hazards model was used to estimate the relative risk (RR) of gastric cancer incidence, gastric cancer death, all cancer deaths except gastric cancer death, and all‐causes death except gastric cancer death. The number of subjects selected for endoscopic screening was 9950 and that for radiographic screening was 4324. The subjects screened by endoscopy showed a 67% reduction of gastric cancer compared with the subjects screened by radiography (adjusted RR by sex, age group, and resident city = 0.327; 95% confidence interval [CI], 0.118–0.908). The adjusted RR of endoscopic screening was 0.968 (95%CI, 0.675–1.387) for all cancer deaths except gastric cancer death, and 0.929 (95%CI, 0.740–1.168) for all‐causes death except gastric cancer death. This study indicates that endoscopic screening can reduce gastric cancer mortality by 67% compared with radiographic screening. This is consistent with previous studies showing that endoscopic screening reduces gastric cancer mortality.
Keywords: Cohort study, gastric cancer screening, mortality reduction, upper gastrointestinal endoscopy, upper gastrointestinal X‐ray
In 2012, approximately 1 million new cases of gastric cancer were recorded worldwide, and half of these cases occurred in Eastern Asian countries.1 The mortality rates from gastric cancers in Eastern Asian countries were also higher than those in other countries, with rates of 24 per 100 000 men and 9.8 per 100 000 women. Clearly, the burden of gastric cancer cannot be ignored in Eastern Asian countries; this also holds true in Eastern European countries and South America, which also have high incidences of gastric cancer.
Recently, upper gastrointestinal endoscopy has been increasingly used in clinical practice and as a standardized examination procedure for gastrointestinal diseases. In some Asian countries, opportunistic cancer screening for gastric cancer using upper gastrointestinal endoscopy (i.e., endoscopic screening) has gradually increased.2 In fact, high detection rates of gastric cancer have been reported with endoscopic screening in local areas of Eastern Asian countries.3, 4 Although endoscopic screening for gastric cancer has already been introduced in Korean national programs,5 evidence for mortality reduction from gastric cancer screening using endoscopy was unclear when endoscopic screening was introduced in the early 2000s.6 In Japan, gastric cancer screening using upper gastrointestinal X‐ray with barium meal (i.e., radiographic screening) has been carried out as a national program since 1983.7 Several case–control and cohort studies have reported consistent results showing mortality reduction from gastric cancer by radiographic screening in Japan.6 Recently, several municipalities have introduced endoscopic screening as an option for gastric cancer screening. In fact, the possibility of reducing mortality from gastric cancer by endoscopic screening was shown by several studies.8, 9, 10, 11, 12 However, discussions regarding the effectiveness of endoscopic screening continue. To effectively introduce endoscopic screening for gastric cancer in communities, evidence regarding its effectiveness must be accumulated.13
We undertook a population‐based cohort study in Tottori and Yonago cities in Japan, where radiographic and endoscopic screenings for gastric cancer have been carried out for 15 years, to evaluate mortality reduction from gastric cancer by endoscopic screening.
Methods
Screening programs
Endoscopic screening for gastric cancer has been carried out in Tottori and Yonago since 2000. Local governments have performed radiographic screening and endoscopic screening for gastric cancer in both cities. All individuals aged 40 years and above can participate in the gastric cancer screening programs. There is no upper age limit for the target population for gastric cancer screening. Individuals can choose either endoscopy or radiography for gastric cancer screening based on their preference. Although the introduction of endoscopic screening has increased, the participation rate in gastric cancer screening involving both methods has remained at approximately 25%.14
Physicians who carried out the endoscopic screening were approved by the local committee for gastric cancer screening based on certain requirements.14 Although endoscopic screening has been performed in clinical settings, the results have been evaluated based on monitor screen review by the local committee, including experienced endoscopists in each city.
Target group
The study subjects were selected from the participants of gastric cancer screening in Tottori and Yonago between 2007 and 2008. There were 28 782 participants in Tottori and 23 753 participants in Yonago. The subjects were defined as participants aged 40–79 years who had no gastric cancer screening in the previous year. The following cases were excluded: (i) subjects who had registry duplication; and (ii) subjects who had a history of gastric cancer. The selected subjects were divided into two groups, the endoscopic screening group and radiographic screening group, according to the first screening method used from 2007 to 2008.
Outcomes
The primary outcome of the study was gastric cancer mortality. All cancer deaths except gastric cancer death and all‐causes deaths except gastric cancer death were assessed to ensure comparability between the two groups. Mortality data were obtained by linkage to the residential registrations of each city and the Tottori Cancer Registry (Tottori, Japan). The incidence of gastric cancer was identified from the Tottori Cancer Registry. Follow‐up of gastric cancer incidence and mortality was continued from the date of the first screening to the date of gastric cancer diagnosis or up to December 31, 2013.
Statistical analysis
Differences in the proportion of both screening groups were compared using the χ2‐test and Student's t‐test. A Cox proportional hazards model was used to estimate the relative risk (RR) of incident gastric cancer, gastric cancer death, all cancer deaths except gastric cancer death, and all‐causes deaths except gastric cancer death. Unadjusted and adjusted RRs by sex, age group, and resident city were calculated. The cumulative hazard values of gastric cancer incidence and mortality were estimated by the Nelson–Aalen method and plotted on graphs. All test statistics were two‐tailed, and P‐values of <0.05 were considered to indicate a statically significant difference. Analyses were carried out using stata 13.0 (STATA, College Station, Texas, USA).
This study was approved by the Institutional Review Board of the National Cancer Center of Japan (Tokyo, Japan).
Results
The procedure used for the selection of the target population is shown in Figure 1. A total of 52 535 subjects participated in gastric cancer screening in Tottori and Yonago from 2007 to 2008. Of these subjects, 5720 were not within the target age group for the analysis. Those subjects excluded from the target group were more than 80 years old at the first screening, which was not the actual target for cancer screening. A total of 14 394 subjects were selected as they had no gastric cancer screening history in the previous year. Three patients who had duplication on the participant list for gastric cancer screening were excluded from the target group for the analysis. There were 117 subjects who were identified as having a history of gastric cancer by linkage to a local cancer registry, and they were also excluded from the target group. The remaining 14 274 subjects were finally divided into two groups according to the first screening procedure as follows: endoscopic screening group (n = 9950), and radiographic screening group (n = 4324).
Figure 1.
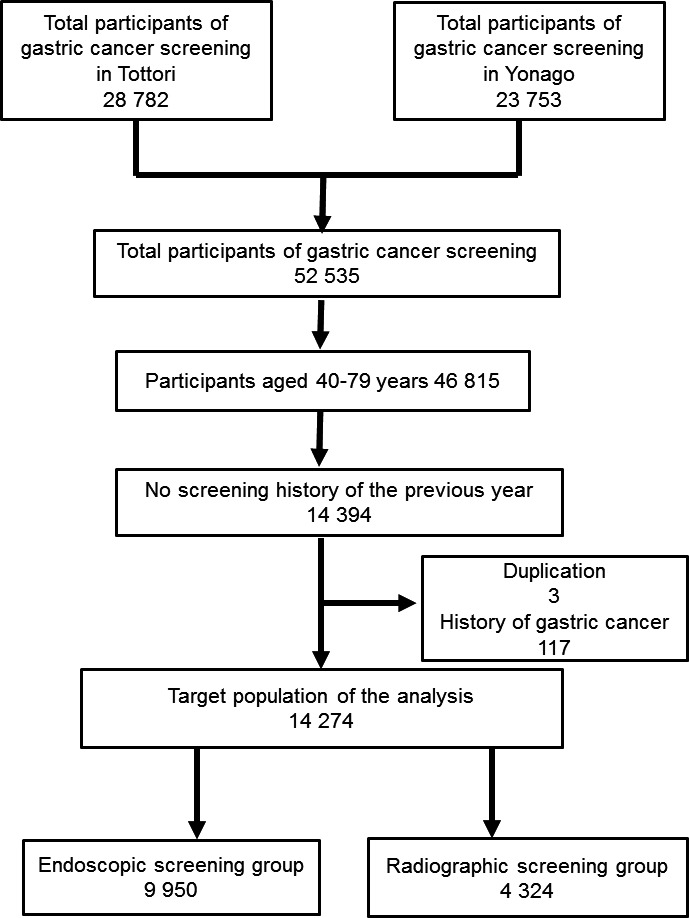
Flowchart of the selection process for the study target group to compare endoscopic and radiographic screening for gastric cancer. A total of 52 535 subjects participated in gastric cancer screening in Tottori and Yonago (Japan) from 2007 to 2008, of which 5720 participants were not within the target age for the analysis (aged more than 80 years at the time of first screening). A total of 14 394 subjects were selected as they had no gastric screening history of the previous year. Three patients who had duplication on the participant list of gastric cancer screening were excluded from 2007 to 2008 the target group of the analysis. There were 117 subjects who were identified as having a history of gastric cancer by linkage to a local cancer registry and they were also excluded from the target group. The remaining 14 274 subjects were divided into two groups according to the first screening procedure: endoscopic screening group (n = 9950), and radiographic screening group (n = 4324).
The results of the comparison of the basic characteristics of the endoscopic screening group and radiographic screening group are shown in Table 1. The sex and age distributions were significantly different between the two groups. The proportion of female subjects was significantly higher than that of male subjects in both groups. The proportion of the ≥70 years age group was significantly lower in the radiographic screening group than in the endoscopic screening group (P < 0.001). During the 6‐year follow‐up period, the screening frequency was 2.3 for the endoscopic screening group and 2.2 for the radiographic screening group (P = 0.988). During the follow‐up period, very few subjects of the endoscopic screening group had also been screened by radiography. In contrast, subjects in the radiographic screening group had two screenings on average, one radiographic and one endoscopic screening.
Table 1.
Comparison of participants between endoscopic screening and radiographic screening for gastric cancer
| Endoscopic screening | Radiographic screening | P‐value | |||
|---|---|---|---|---|---|
| n | (%) | n | (%) | ||
| Total | 9950 | 4324 | |||
| Sex | |||||
| Male | 3589 | (36.1) | 1454 | (33.6) | 0.005 |
| Female | 6361 | (63.9) | 2870 | (66.4) | |
| Age, years | |||||
| 40–49 | 1174 | (11.8) | 593 | (13.7) | |
| 50–59 | 1959 | (19.7) | 1086 | (25.1) | <0.001 |
| 60–69 | 3793 | (38.1) | 1551 | (35.9) | |
| 70–79 | 3024 | (30.4) | 1094 | (25.3) | |
| City | |||||
| Tottori | 5564 | (55.9) | 2945 | (68.1) | <0.001 |
| Yonago | 4386 | (44.1) | 1379 | (31.9) | |
| Screening frequency during follow‐up period, average | |||||
| Total | 2.3 | 2.2 | 0.988 | ||
| Endoscopy | 2.2 | 0.9 | <0.001 | ||
| Radiography | 0.1 | 1.3 | <0.001 | ||
During the 6‐year follow‐up period, 127 gastric cancers were diagnosed in the endoscopic screening group and 41 gastric cancers in the radiographic screening group (Table 2). Approximately half of the subjects were aged 70–79 years and the proportions of the age group were nearly equal in both screening groups (P = 0.365). Although the proportion of localized cancers was higher in the endoscopic screening group than in the radiographic screening group, the stage distribution was similar in both groups (P = 0.276).
Table 2.
Comparison of detected gastric cancers between endoscopic screening and radiographic screening
| Endoscopic group | Radiographic group | P‐value | |||
|---|---|---|---|---|---|
| n | (%) | n | (%) | ||
| Total number of detected cancer | 127 | 41 | |||
| Sex | |||||
| Male | 87 | (68.5) | 25 | (61.0) | 0.374 |
| Female | 40 | (31.5) | 16 | (39.0) | |
| Age group, years | |||||
| 40–49 | 3 | (2.4) | 1 | (2.4) | |
| 50–59 | 9 | (7.1) | 4 | (9.8) | 0.365 |
| 60–69 | 57 | (44.9) | 12 | (29.3) | |
| 70–79 | 58 | (45.7) | 24 | (58.5) | |
| City | |||||
| Tottori | 55 | (43.3) | 19 | (46.3) | 0.734 |
| Yonago | 72 | (56.7) | 22 | (53.7) | |
| Stage | |||||
| Localized | 98 | (77.2) | 30 | (73.2) | 0.276 |
| Regional | 8 | (6.3) | 6 | (14.6) | |
| Distant | 4 | (3.1) | 2 | (4.9) | |
| Unknown | 17 | (13.4) | 3 | (7.3) | |
| Pathology | |||||
| Intestine | 99 | (78.0) | 27 | (65.9) | 0.158 |
| Diffuse | 20 | (15.7) | 12 | (29.3) | |
| Others and unknown | 8 | (6.3) | 2 | (4.9) | |
The mean follow‐up period was 66.6 ± 0.9 months (95% confidence interval [CI], 66.4–66.7). The gastric cancer incidence was 233.7 per 100 000 person‐years in the endoscopic screening group and 172.1 per 100 000 person‐years in the radiographic screening group (Table 3). Although the gastric cancer incidence of the endoscopic screening group was higher than that of the radiographic screening group, it was not significantly different (unadjusted RR = 1.168, 95%CI, 0.804–1.695; adjusted RR = 0.988, 95%CI, 0.679–1.438). During the follow‐up years, cumulative hazard values of gastric cancer incidence were nearly equal between the radiographic screening group and the endoscopic screening group (Fig. 2a).
Table 3.
Relative risks (RR) and 95% confidence intervals (CI) of endoscopic screenings
| Outcome | Screening method | No. of cases | Person‐years | Rate (per 100 000 person‐years) | Unadjusted RR | (95%CI) | Adjusted RR† | (95%CI) |
|---|---|---|---|---|---|---|---|---|
| Gastric cancer incidence | ||||||||
| Radiographic screening | 41 | 23 824 | 172.1 | 1.000 | 1.000 | |||
| Endoscopic screening | 127 | 54 353 | 233.7 | 1.168 | (0.804–1.695) | 0.988 | (0.679–1.438) | |
| Gastric cancer death | ||||||||
| Radiographic screening | 8 | 24 183 | 33.1 | 1.000 | 1.000 | |||
| Endoscopic screening | 7 | 55 002 | 12.7 | 0.384 | (0.139–1.060) | 0.327 | (0.118–0.908) | |
| All cancer deaths‡ | ||||||||
| Radiographic screening | 41 | 24 183 | 169.5 | 1.000 | 1.000 | |||
| Endoscopic screening | 111 | 55 002 | 201.8 | 1.197 | (0.837–1.713) | 0.968 | (0.675–1.387) | |
| All‐causes deaths§ | ||||||||
| Radiographic screening | 104 | 24 183 | 430.1 | 1.000 | 1.000 | |||
| Endoscopic screening | 264 | 55 002 | 480.0 | 1.121 | (0.893–1.407) | 0.929 | (0.740–1.168) |
†Adjusted by sex, age group (40–59 years, 60–69 years, and 70–79 years), and resident city. ‡All cancer deaths excluding gastric cancer death. §All‐causes deaths excluding gastric cancer death.
Figure 2.
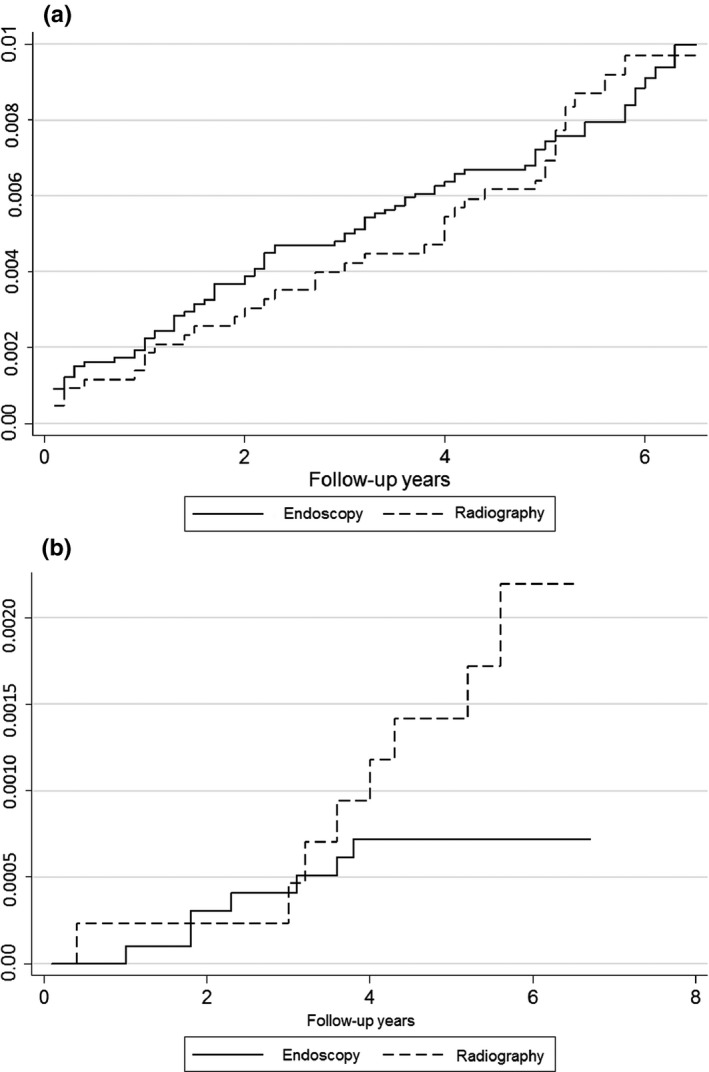
Cumulative hazard values of gastric cancer incidence (a) and mortality (b) in follow‐up years, estimated by the Nelson–Aalen method. Cumulative hazard values were compared between the endoscopic and radiographic screening groups.
After the 6‐year follow‐up period, seven subjects from the endoscopic screening group and eight from the radiographic screening group died of gastric cancer. The gastric cancer death rate was 33.1 per 100 000 person‐years in the endoscopic screening group and 12.7 per 100 000 person‐years in the radiographic screening group (Table 3). Although the unadjusted RR was not statistically significant (unadjusted RR = 0.384, 95%CI, 0.139–1.060), the subjects screened by endoscopy showed a 67% mortality reduction from gastric cancer compared with the subjects screened by radiography when the RR was adjusted by sex, age group, and resident city (adjusted RR = 0.327, 95%CI, 0.118–0.908). The cumulative hazard of gastric cancer mortality became nearly similar in both screening groups until 3 years of follow‐up, but the difference subsequently widened (Fig. 2b).
After the 6‐year follow‐up period, 111 subjects of the endoscopic screening group and 41 subjects of the radiographic screening group died from all cancer deaths excluding gastric cancer death. The all cancer deaths excluding gastric cancer death were 201.8 per 100 000 person‐years in the endoscopic screening group and 169.5 per 100 000 person‐years in the radiographic screening group (Table 3). A total of 264 subjects of the endoscopic screening group and 104 subjects of the radiographic screening group died from all‐causes deaths excluding gastric cancer death. The all‐causes deaths excluding gastric cancer death was 480.0 per 100 000 person‐years in the endoscopic screening group and 430.1 per 100 000 person‐years in the radiographic screening group (Table 3). The adjusted RR of the endoscopic screening group was 0.968 (95%CI, 0.675–1.387) for all cancer deaths except gastric cancer death and 0.929 (95%CI, 0.740–1.168) for all‐causes deaths except gastric cancer death.
Discussion
The present results suggest that endoscopic screening can reduce mortality from gastric cancer by 67% compared with radiographic screening. Although upper gastrointestinal endoscopy has been commonly used for diagnostic examinations in clinical settings, evidence for cancer screening has remained controversial. This has limited its use to opportunistic screening in clinical settings even if high detection rates of gastric cancer can be expected.2 We have recently published the results of our community‐based case–control study evaluating the effectiveness of endoscopic screening for gastric cancer. The findings of our previous study suggest a 30% reduction in gastric cancer mortality by endoscopic screening within 36 months before the date of gastric cancer diagnosis.10 A nested case–control study from Korea reported a 57% mortality reduction by endoscopic screening based on the national database.11 Hosokawa et al.15 reported a 78% mortality reduction from gastric cancer by endoscopic screening compared with radiographic screening based on a 5‐year follow‐up period. The age distribution of the target population was younger in the endoscopic screening group than in the radiographic screening group. Although the present study has a different study design or background from these previous studies, the results consistently demonstrate mortality reduction from gastric cancer by endoscopic screening.
The possibility of reducing mortality from gastric cancer by radiographic screening has been mainly reported in Japan.6 Although radiographic equipment for the upper gastrointestinal series has been improved, the sensitivity range of radiographic screening has remained from 80% to 90%.16, 17, 18, 19 To evaluate mortality reduction from gastric cancer by radiographic screening, case–control studies were mostly carried out until 1995, and then cohort studies were started for follow‐up from the early 1990s. The subjects compared in these studies were individuals who had no screening history and had been treated by the usual care as needed. In 1996, the total number of upper gastrointestinal endoscopy procedures carried out was 73 879 in hospitals and 149 848 in outpatient clinics per month.20 However, the total number of upper gastrointestinal endoscopic examinations carried out in 2011 increased to 521 936 in hospitals and 392 773 in outpatient clinics per month,20 with endoscopic examination becoming a more common technique in medical services in Japan. The Japanese health insurance system covers most of the medical services except screening programs. However, the opportunity to be examined by endoscopy has rapidly increased according to the increase in the total number of upper gastrointestinal endoscopy procedures conducted. A recent case–control study particularly showed that mortality reduction could not be obtained by radiographic screening.10 The impact of radiographic screening may be decreased depending on the periods when the evaluation studies were carried out. Therefore, to evaluate the effectiveness of endoscopic screening for gastric cancer, radiographic screening can be used for comparison.
Although the gastric cancer mortality in the endoscopic screening group was found to be lower than that in the radiographic screening group, the gastric cancer incidence and the stage distribution of diagnosed cancer were similar in both screening groups. As the proportion of the unknown stage of the endoscopic screening group was higher than that of the radiographic screening group, there might be more patients with early stage cancer included in the endoscopic screening group than in the radiographic screening group. In Japanese studies, the proportion of early stage cancer, which constitutes tumor showing invasion within the gastric submucosa, based on the definition of the Japanese Gastric Cancer Association,21 was usually approximately 70% in the radiographic screening group22 and more than 80% in the endoscopic screening group.23 Hosokawa et al.15 previously reported that the detection rate of early cancer was higher in the endoscopic screening group than in the radiographic screening group, and the stage distribution was different in both groups. Endoscopy can diagnose more early stage cancers that can be treated by endoscopic surgical dissection. In fact, endoscopic surgical dissection has been carried out for approximately half of early stage cancers detected by endoscopic screening.23 The difference in the cumulative hazard of gastric cancer mortality widened after 3 years from the first screening. This indicates that the detection of early stage cancer was initially achieved and then the gap of cumulative hazard of gastric cancer mortality widened between endoscopic screening and radiographic screening. Early stage gastric cancer takes approximately 44 months to become advanced stage gastric cancer.24 This fact has to be taken into consideration when aiming for mortality reduction from gastric cancer by endoscopic screening. Although detecting more early stage gastric cancer is advantageous for endoscopic screening, cases of overdiagnosis might also be included. Currently, there are no reports of overdiagnosis by gastric cancer screening using radiography and endoscopy. However, the numbers of cancers detected by endoscopic screening have reportedly been twice the expected numbers.25 These excess cases include overdiagnosis cases and early stage cancers that progress to advanced stage cancers. To further validate evidence of the effectiveness of endoscopic screening for gastric cancer, additional studies to evaluate mortality reduction from gastric cancer by endoscopic screening are warranted.
The relative risks of all cancer mortality excluding gastric cancer death and all‐cause mortality excluding gastric cancer death were nearly equal between the endoscopic screening group and the radiographic screening group. However, to compare mortality reduction from gastric cancer between endoscopic screening and radiographic screening, the background difference should be considered between the endoscopic screening group and the radiographic screening group. Endoscopic screening has been carried out in clinical practice in Tottori prefecture. The age of the participants in the endoscopic screening group was more advanced than that of the participants in the radiographic screening group.10 Individuals aged more than 70 years could be screened by physicians using endoscopy in their own private practice. As the number of younger people with family physicians was fewer than older people with family physicians, there was little opportunity for the younger people to be tested in clinical practice. Helicobacter pylori infection is a major cause of gastric cancer,26 and the difference of the age group also affects the H. pylori infection rate. Although the H. pylori infection rate has decreased in Japan, the rate has remained higher in individuals aged 70 years and over than in individuals aged 40–69 years.27 As the proportion of individuals aged ≥70 years was higher in the endoscopic screening group than in the radiographic screening group, the risk for gastric cancer might be higher in endoscopic screening than in radiographic screening. However, we could not obtain the H. pylori infection rates at the first screening in both screening groups. Lifestyle behaviors could also be a risk factor for gastric cancer; in particular, high salt intake and smoking are associated with gastric cancer.28, 29, 30 The smoking rate is reportedly higher in Tottori prefecture than the national average and the rates decrease according to age in men and women.31 Salt intake in Tottori prefecture is reportedly similar to the national average and the differences of the age group are small.31 Although Fukao et al.32 reported differences in family history and smoking between participants and non‐participants in gastric cancer screening, the difference in the backgrounds between the endoscopic and radiographic screening groups is unclear. We could not use the results of the questionnaire survey at the screening participation because there were no questions regarding salt intake or smoking.
This study has additional limitations. First, the quality of the Tottori Cancer Registry was not optimal as the percentage of death‐certification‐only cases was 15.1% in 2007, which was lower than the national average.33 In Japan, cancer registries have not yet been prepared at the national level, and the registry method, as of 2014, has not yet been standardized.34, 35 As the registration of gastric cancers remains insufficient, differences in the detected cancers by each screening group might not have been fully clarified. Second, there was no information as to whether or not the patients participated in opportunistic screenings. Third, the subjects of the radiographic screening group had been screened by endoscopy once during the follow‐up period. In the study areas, people could choose either endoscopy or radiography as the screening method at the individual level. It was difficult to divide the screening method completely during the follow‐up period. Therefore, the results might suggest a comparison between higher intensive and lower intensive endoscopic screening. Finally, subgroup analysis could not be adequately carried out because of the small sample size.
The incidence of gastric cancer has been decreasing and a predicted additional decrease is anticipated because of a decrease in the H. pylori infection rate,27, 36, 37 However, as the participation rate in gastric cancer screening has decreased, its impact on mortality reduction has become limited. Although the participation rate in radiographic screening for gastric cancer has sunk below 10%,38 there is a possibility of improving the participation rate by the introduction of endoscopic screening as an option for gastric cancer screening. Notably, the participation rate is reportedly approximately 25% in municipalities that have already introduced endoscopic screening.22, 39 However, according to the change in the incidence of gastric cancer, the possibility of a new screening system should be investigated considering the risk factors for gastric cancer.
In conclusion, the present study suggests that endoscopic screening can reduce mortality from gastric cancer by 67% compared with radiographic screening. The results consistently support mortality reduction from gastric cancer by endoscopic screening described by previous studies. Although this indicates the effectiveness of endoscopic screening for gastric cancer, several limitations, including self‐selection bias, remain, and prudent interpretation of the finding is needed. Thus far, endoscopic screening for gastric cancer has shown promising results. Endoscopic screening therefore deserves further comprehensive evaluation to reliably confirm its effectiveness and how its optimal use can be strategically promoted.
Disclosure Statement
The authors have no conflict of interest.
Acknowledgments
This study was supported solely by a Grant‐in‐Aid for Research for Promotion of Cancer Control Programs from the Japanese Ministry of Health, Labour and Welfare (H27‐Toku‐Shitei‐005). We thank the Tottori Cancer Registry office, and the local governments of Tottori and Yonago for their cooperation with the study. We are grateful to Dr. Edward F. Barroga, Associate Professor and Senior Medical Editor of Tokyo Medical University, for editing the English manuscript. We also thank Ms. Kanoko Matsushima, Ms. Junko Asai, and Ms. Mayumi Kobayashi for research assistance.
Cancer Sci 106 (2015) 1744–1749
Funding Information
Ministry of Health, Labor and Welfare of Japan.
References
- 1. International Agency for Research on Cancer . GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012, 2012. [Cited 10 Jul 2015.] Available from URL: http://globocan.iarc.fr/.
- 2. Leung WK, Wu MS, Kakugawa Y et al Screening for gastric cancer in Asia: current evidence and practice. Lancet Oncol 2008; 9: 279–87. [DOI] [PubMed] [Google Scholar]
- 3. Tashiro A, Sano M, Kinameri K et al Comparing mass screening techniques for gastric cancer in Japan. World J Gastroenterol 2006; 12: 4873–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lu YF, Liu ZC, Li ZH et al Esophageal/gastric cancer screening in high‐risk populations in Henan Province, China. Asian Pac J Cancer Prev 2014; 5: 1419–22. [DOI] [PubMed] [Google Scholar]
- 5. Kim Y, Jun JK, Choi KS et al Overview of the national cancer screening programme and the cancer screening status in Korea. Asian Pac J Cancer Prev 2011; 12: 725–30. [PubMed] [Google Scholar]
- 6. Hamashima C, Shibuya D, Yamazaki H et al The Japanese guidelines for gastric cancer screening. Jpn J Clin Oncol 2008; 38: 259–67. [DOI] [PubMed] [Google Scholar]
- 7. Oshima A. A critical review of cancer screening programs in Japan. Int J Technol Assess Health Care 1994; 10: 346–58. [DOI] [PubMed] [Google Scholar]
- 8. Matsumoto S, Yamasaki K, Tsuji K et al Results of mass endoscopic examination for gastric cancer in Kamigoto Hospital, Nagasaki Prefecture. World J Gastroenterol 2007; 13: 4316–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hosokawa O, Miyanaga T, Kaizaki Y et al Decreased death from gastric cancer by endoscopic screening: association with a population‐based cancer registry. Scand J Gastroenterol 2008; 43: 1112–5. [DOI] [PubMed] [Google Scholar]
- 10. Hamashima C, Ogoshi K, Okamoto M et al A community‐based, case–control study evaluating mortality reduction from gastric cancer by endoscopic screening in Japan. PLoS ONE 2013; 8: e79088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cho BY. Evaluation of the validity of current national health screening programs and plans to improve the system. Seoul: Seoul University, 2014; 741–58 (in Korean). [Google Scholar]
- 12. Hamashima C, Ogoshi K, Narisawa R et al Impact of endoscopic screening on mortality reduction from gastric cancer. World J Gantroenterol 2015; 21: 2460–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hamashima C. Have we comprehensively evaluated the effectiveness of endoscopic screening for gastric cancer? Asian Pac J Cancer Prev 2015; 16: 3591–2. [DOI] [PubMed] [Google Scholar]
- 14. Shabana M, Hamashima C, Nishida M et al Current status and evaluation of endoscopic screening for gastric cancer. Jpn J Cancer Det Diag 2010; 17: 229–35. (In Japanese). [Google Scholar]
- 15. Hosokawa O, Shinbo T, Matsuda K et al Impact of opportunistic endoscopic screening on the decrease of mortality from gastric cancer. J Gatsroenterol Cancer Screen 2011; 49: 401–9. (In Japanese). [Google Scholar]
- 16. Hamashima C, Okamoto M, Shabana M et al Sensitivity of endoscopic screening for gastric cancer by the incidence method. Int J Cancer 2013; 133: 653–9. [DOI] [PubMed] [Google Scholar]
- 17. Murakami R, Tsukuma H, Ubukata T et al Estimation of validity of mass screening program for gastric cancer in Osaka, Japan. Cancer 1990; 65: 1255–60. [DOI] [PubMed] [Google Scholar]
- 18. Abe S, Shibuya D, Noguchi T et al An estimate of the false‐negative rate of mass‐screening for gastric carcinoma. J Gatsroenterol Cancer Screen 2000; 38: 475–82. (In Japanese). [Google Scholar]
- 19. Yamamoto K, Yamazaki H, Kuroda C et al Diagnostic validity of high‐density barium sulfate in gastric cancer screening: follow‐up of screenees by record linkage with the Osaka Cancer Registry. J Epidemiol 2010; 20: 287–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ministry of Health, Labour and Welfare . Medical Institute Survey, 2011, 2012. [Cited 10 Jul 2015.] Available from URL: http://www.e-stat.go.jp/SG1/estat/GL08020101.do?_toGL08020101_&tstatCode=000001030908&requestSender=dsearch.
- 21. Japanese Gastric Cancer Association . Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011; 14: 101–12. [DOI] [PubMed] [Google Scholar]
- 22. Kitagawa S, Miyagawa K, Iriguchi Y et al The report of gastroenterological screening in 2012. J Gatsroenterol Cancer Screen 2015; 53: 60–86. (In Japanese). [Google Scholar]
- 23. Ogoshi K, Narisawa R, Kato T et al Evaluation of endoscopic screening for gastric cancer in Niigata City: the reduction of the mortality rate. J Gatsroenterol Cancer Screen 2009; 47: 531–41. (In Japanese). [Google Scholar]
- 24. Tsukuma H, Oshima A, Narahara H et al Natural history of early gastric cancer: a non‐concurrent, long term, follow up study. Gut 2000; 47: 618–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hamashima C, Sobue T, Muramatsu Y et al Comparison of observed and expected numbers of detected cancers in the research center for cancer prevention and screening program. Jpn J Clin Oncol 2006; 36: 301–8. [DOI] [PubMed] [Google Scholar]
- 26. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans Helicobacter pylori In: International Agency for Research on Cancer , eds. IARC Monographs on the Evaluation of Carcinogenic Risk to Humans. Biological Agents. Volume 100b. A Review of Human Cartinogeogens. Lyon: IARC press, 2012; 385–436. [Google Scholar]
- 27. Hirayama Y, Kawai T, Otaki J, Kawakami K, Harada Y. Prevalence of Helicobacter pylori infection with healthy subjects in Japan. J Gastroenterol Hepatol 2014; 29(Suppl 4): 16–9. [DOI] [PubMed] [Google Scholar]
- 28. Tsugane S, Sasazuki S. Diet and the risk of gastric cancer: review of epidemiological evidence. Gastric Cancer 2007; 10: 75–83. [DOI] [PubMed] [Google Scholar]
- 29. Nishino Y, Inoue M, Tsuji I et al Tobacco smoking and gastric cancer risk: an evaluation based on a systematic review of epidemiologic evidence among the Japanese population. Jpn J Clin Oncol 2006; 36: 800–7. [DOI] [PubMed] [Google Scholar]
- 30. Charvat H, Sasazuki S, Inoue M et al Prediction of the 10‐year probability of gastric cancer occurrence in the Japanese population: the JPHC study cohort II. Int J Cancer 2015. (in press). doi:10.1002/ijc.29705. [DOI] [PubMed] [Google Scholar]
- 31. Tottori prefecture government. Nutrition and health survey 2010. Tottori. 2011. 25‐130.
- 32. Fukao A, Hisamichi S, Komatsu S et al Comparison of characteristics between frequent participants and non‐participants in screening programs for stomach cancer. Tohoku J Exp Med 1995; 166: 459–69. [DOI] [PubMed] [Google Scholar]
- 33. Sobue T, Matsuda T, Shibata A et al Monitoring of Cancer Incidence in Japan 2007. Tokyo: National Cancer Center, Center for Cancer Control and Information Services, 2012. [Google Scholar]
- 34. Matsuda T, Sobue T. Recent trends in population‐based cancer registries in Japan: the Act on Promotion of Cancer Registries and drastic changes in the historical registry. Int J Clin Oncol 2015; 20: 11–20. [DOI] [PubMed] [Google Scholar]
- 35. Okamoto N. A history of the cancer registration system in Japan. Int J Clin Oncol 2008; 13: 90–6. [DOI] [PubMed] [Google Scholar]
- 36. National Cancer Center . Center for Cancer Control and Information Services. [Cited 1 Sep 2015.] Available from URL: http://ganjoho.ncc.go.jp/professional/statistics/index.html
- 37. Sobue T, ed. White Paper Cancer Statistics 2012. Tokyo: Shinohara Press, 2012. [Google Scholar]
- 38. Ministry of Health, Labour and Welfare . The Report of Health Promotion and Community Health 2013. [Cited 1 Sep 2015.] Available from URL: http://www.e-stat.go.jp/SG1/estat/GL08020101.do?_toGL08020101_&tstatCode=000001030884&requestSender=dsearch.
- 39. Shabana M, Hamashima C, Nishida M, Miura K, Kishimoto T. Current status and evaluation of endoscopic screening for gastric cancer. Jpn J Cancer Det Diagn 2010; 17: 229–35. (In Japanese). [Google Scholar]


