Abstract
Lack of appropriate biomarkers has hampered early detection of urothelial cancer (UC), therefore, development of biomarkers for its diagnosis at earlier stages is of importance. Laminin‐332 (Ln‐332, formerly Ln‐5), a component of basement membranes, consists of Ln‐α3, Ln‐β3, and Ln‐γ2 polypeptides. However, monomeric Ln‐γ2 alone is frequently expressed in malignant neoplasms. If Ln‐γ2 is also expressed in UC and secreted into the urine, its detection could be useful for UC diagnosis. Here, we evaluated Ln‐γ2 levels from 60 patients with urinary diseases (including UC) by Western blotting, and detected it in approximately 53% of UC cases. Using immunohistochemistry, we confirmed Ln‐γ2 expression in UC tissues that were positive for Ln‐γ2, whereas Ln‐α3 expression was absent. We next developed a sandwich enzyme‐linked immunosorbent assay and applied it for screening 39 patients with non‐muscle invasive UC and 61 patients with benign urologic diseases. The Ln‐γ2 levels were higher in UC patients than in those with benign urologic diseases. Ln‐γ2 was detected even in patients with earlier stages of UC, such as Ta, T1, or carcinoma in situ. The sensitivity of Ln‐γ2 testing for UC was 97.4%, and the specificity was 45.9%, using a cut‐off of 0.5 μg/g∙crn. Ln‐γ2 had greater diagnostic value for detecting non‐muscle invasive UC compared to conventional urine cytology and available biomarkers for UC, and may be useful as a urine biomarker for the diagnosis and monitoring of UC.
Keywords: Biomarker, diagnosis, laminin‐γ2, urine, urothelial cancer (UC)
Urothelial cancer (UC) incidence is increasing with the increase in the aged population, with an age‐standardized annual incidence of 7.6/100 000 in Japan.1, 2 Among these patients, 70% are diagnosed at the non‐invasive stage, with the rest at the invasive stage. Even though non‐invasive UC is endoscopically resected, 70% of these cases experience recurrence, and the other 30% progress.2 To reduce the recurrence rate, diagnostic methods that allow earlier diagnosis is considered important. However, lack of appropriate tumor biomarkers for UC presents an obstacle to diagnosis.
Urine cytology and cystoscopy are currently the major methods used for UC diagnosis. Although urine cytology can accurately diagnose invasive or high‐grade UC, its sensitivity is not adequate to diagnose non‐muscle invasive UC (NMIUC; approximately 70% of all UC cases),3 because cancer cells are rarely released into urine at the earlier stages and its sensitivity for low‐grade tumors is approximately 40%.4 Cystoscopy is a powerful method for UC diagnosis, as it can facilitate biopsy for a definite UC diagnosis by histology, but is invasive and painful. As approximately 70% of patients who received transurethral resection of their bladder tumor (TUR‐Bt) experience recurrence within 2 years, 3‐monthly cystoscopic examination is necessary for monitoring recurrence;2, 5 this represents a severe burden for most aged patients.
Urinary tumor biomarkers for UC have been identified,6 among which nuclear matrix protein 22 (NMP22) and bladder tumor antigen (BTA) have been approved for diagnostic purposes by the Japanese Ministry of Health, Labor, and Welfare. NMP22 is a nuclear protein that is selectively expressed in UC, whereas BTA are produced by digestion of extracellular matrices in the UC basement membranes. However, the sensitivities of NMP22 and BTA are 32–92% and 53–89%, respectively, and their specificities are 51–94% and 53–89%, respectively.2, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 Furthermore, BTA and NMP22 yield a high pseudopositive ratio for hematuria, urinary infections, and urolithiasis patients; therefore, these tests are not used routinely for diagnosis of NMIUC.2, 11 Thus, development of new tumor markers for UC that can also replace cystoscopy for routine monitoring examination is desirable.
Ln‐γ2, a component of Ln‐332,17 is an interesting candidate tumor biomarker, as it is expressed in cancerous tissues. Ln‐332, an adhesive molecule of the basement membranes of normal epithelia, is composed of three polypeptides encoded by different genes, namely Ln‐α3, Ln‐β3, and Ln‐γ2. In normal epithelium, Ln‐γ2 is exclusively expressed as a component of the heterotrimeric form (Ln‐332) and plays a crucial role in maintaining static epithelial structures in vivo.17 In malignant cancer cells, however, Ln‐γ2 is expressed as a monomeric form, rather than as a heterotrimer. Monomeric Ln‐γ2 was originally identified in gastric adenocarcinoma cells,18 and its expression has been detected in budding or disseminating tumor cells in gastric carcinoma tissues.18 Similar results have been observed in other carcinomas, such as cancer of the pancreas, stomach, tongue, colorectal, lung, cervix, and esophagus.18, 19, 20, 21, 22 Furthermore, the N‐terminal Ln‐γ2 fragment has been detected in sera from patients with invasive pancreatic carcinoma (by sandwich ELISA, using an anti‐Ln‐γ2 N‐terminal fragment mAb), but the fragment was absent in the sera from non‐invasive pancreatic cancer patients.23, 24 The N‐terminal portion of Ln‐γ2 is released by proteolytic action of MMPs.25, 26 Particularly, the fragment cleaved off by MMP14 can bind to the ErbB1 receptor and so potentially contributes to motility and proliferation of breast carcinoma cells.26, 27 Based on these previous observations, we considered it plausible that UC would also express Ln‐γ2 as a monomer, which would then be secreted into the urine, making Ln‐γ2 a useful new biomarker for UC.
In this study, we detected Ln‐γ2 from UC patients by Western blotting, and developed a quantitative ELISA to measure Ln‐γ2 in patients. Our results indicated that Ln‐γ2 has a better diagnostic potential compared to NMP22, BTA, and urine cytology and, therefore, may be useful as a sensitive biomarker for diagnosis of NMIUC.
Materials and Methods
Clinical specimens
Between July 2008 and March 2012, urine specimens obtained from patients with UC and benign urologic diseases were obtained. All patients provided written informed consent, and the study design was approved by our institutional review boards (24‐139, Kochi Medical School Hospital, Kochi, Japan; 20‐52‐0123, Institute of Medical Science, University of Tokyo, Tokyo, Japan). Urothelial cancer patients were histopathologically diagnosed using specimens from TUR‐Bt or nephron‐ureterectomy; other patients with benign urologic diseases (no evidence of malignancy during repeated examinations over a ≥ 1‐year period) had benign prostatic hyperplasia, urolithiasis, or urinary tract infection.
Western blot analysis
Ln‐γ2 was detected in UC patients’ urine samples by Western blotting, using an anti‐human Ln‐γ2 mAb (D4B5; Chemicon, Temecula, CA, USA) under non‐reducing conditions. This mAb reacts with both heterotrimeric Ln‐332 and monomeric Ln‐γ2 (seen at different molecular weights on Western blotting). Briefly, proteins in approximately 80 μL urine were concentrated by ice‐cold acetone precipitation, and the precipitate was dissolved in 1 × SDS‐PAGE sample buffer (without 2‐mercaptoethanol). The proteins were then subjected to SDS‐PAGE and blotted onto a PVDF membrane. The membrane was then blocked with 5% dry milk/PBS/0.05% Tween‐20 (PBS‐T) for 1 h at room temperature, and treated with D4B5 for 3 h at room temperature. All other experimental procedures have been described in our previous report.28
Immunohistochemistry
Tumor tissues were resected by cystoscopy at the Kochi Medical School Hospital, and the tissue specimens were immediately fixed in 10% formalin and embedded in paraffin. Four micrometer‐thick paraffin sections were mounted on aminoacyl silane‐coated glass slides, deparaffinized, rehydrated, and immersed in 0.3% hydrogen peroxide‐containing methanol for inactivation of intrinsic peroxidase. All sections were then treated with Protease XXIV (Sigma, St. Louis, MO, USA) for 15 min at room temperature, and then incubated with the anti‐Ln‐α3 (Oriental Yeast, Tokyo, Japan) and anti‐Ln‐γ2 (D4B5; Chemicon) mAbs (1 h at 37°C for each mAb). The labeled antigens were subsequently detected using a DAKO Envision kit (Dako Japan, Tokyo, Japan), and visualized via the 3,3′‐diaminobenzidine reaction. All other experimental procedures have been described in our previous report.28
Sandwich ELISA for Ln‐γ2
The sandwich ELISA assay, using D4B5 as a capture mAb and an anti‐Ln‐γ2 detection polyclonal antibody (pAb) (2778), has been previously described.29 The 2778 pAb reacts with the domain‐III fragment of Ln‐γ2, and was a kind gift from Professor Vito Quaranta (Vanderbilt Medical Center, Nashville, TN, USA).26 D4B5 mAb was coated on a 96‐assay plate (1 μg/mL in PBS at 4°C); pre‐immune mouse serum was also used for coating the plate as a negative control. After blocking with 5% BSA in PBS, monomeric Ln‐γ2 at various concentrations was added to the wells, and allowed to react with each antibody. Each well was washed 3 × with PBS‐T; the 2778 pAb (0.5 μg/mL) was then added and the plate incubated for 1 h. Bound antibodies were then detected using HRP‐conjugated anti‐mouse IgG (Vector Laboratories, Burlingame, CA, USA). Finally, the absorbance of the p‐nitrophenyl‐phosphate (Sigma)‐filled wells was measured at 405 nm, using a Bio‐Rad spectrophotometer (Hercules, CA). Ln‐γ2 concentration was determined using a purified Ln‐γ2 standard curve, as previously described.29
Analysis of creatinine, NMP22, and BTA in urine
Urinary creatinine, NMP22, and BTA levels were analyzed by SRL (Tokyo, Japan). Creatinine was measured using a colorimetric assay, using Determiner‐L CRE (Kyowa Medex, Tokyo Japan). The BTA status (positive or negative) was detected using a latex agglutination assay for qualitative detection in urine (V‐BTA test; Polymedco, Cortlandt, NY, USA).9, 30 NMP22 was measured by sandwich ELISA, using the qualitative Alere NMP‐22 test (Alere Medical, Tokyo, Japan),27 which has a cut‐off value of 12.0 U/mL.
Urine cytology, pT stage, and tumor grade
Classification of urine cytology, pT staging, and tumor grading were carried out at the Laboratory of Diagnostic Pathology at Kochi Medical School. Cytology was evaluated for positive indicators of malignancy, and cases of UC were histologically classified into five categories (class I–V).31 Both class IV and V cases were designated as malignant tumors. The pT stage and tumor grade were evaluated following General Rules for UC.31
Statistical analysis
All data were analyzed using Medcalc software (version 10; MedCalc Software, Ostend, Belgium), and a P‐value of <0.05 was considered statistically significant. All data were reported as median ± SE or as number (percentage); median values were compared using the Mann‐Whitney U‐test. Receiver operating characteristic (ROC) curves, Youden index plots, and dot graphs were used to analyze the areas under the curves (AUC).32 We also calculated the sensitivity, specificity, positive‐predictive value, and negative‐predictive value of the various diagnostic methods.
Results
Detection of Ln‐γ2 by Western blotting
Urine samples were collected from 60 outpatients in our hospital; these included cases of UC and benign disease; diagnostic information for each specimen is shown in Table S1. The collected urine samples were concentrated and analyzed for Ln‐γ2 by Western blotting using a specific anti‐Ln‐γ2 mAb. As the analysis was carried out under non‐reducing conditions, heterotrimeric Ln‐332, composed of Ln‐α3, ‐β3, and ‐γ2 linked by disulfide bonds, was detected as a 560‐kDa band, and monomeric Ln‐γ2 and its proteolytic fragment (defined as Ln‐γ2’) were seen as 150‐kDa and 130‐kDa broad bands, respectively (Fig. 1A).29 We also observed several bands <100 kDa, which may be non‐limited digestive fragments of Ln‐γ2. The positive ratios (%) for Ln‐γ2 are summarized in Figure 1(B). Approximately 53% of UC patients showed detectable Ln‐γ2, and the positive ratios were 11.1%, 16.7%, and 18.2% for prostate cancer, renal cell cancer, and urologic diseases, respectively (Fig. 1B).
Figure 1.
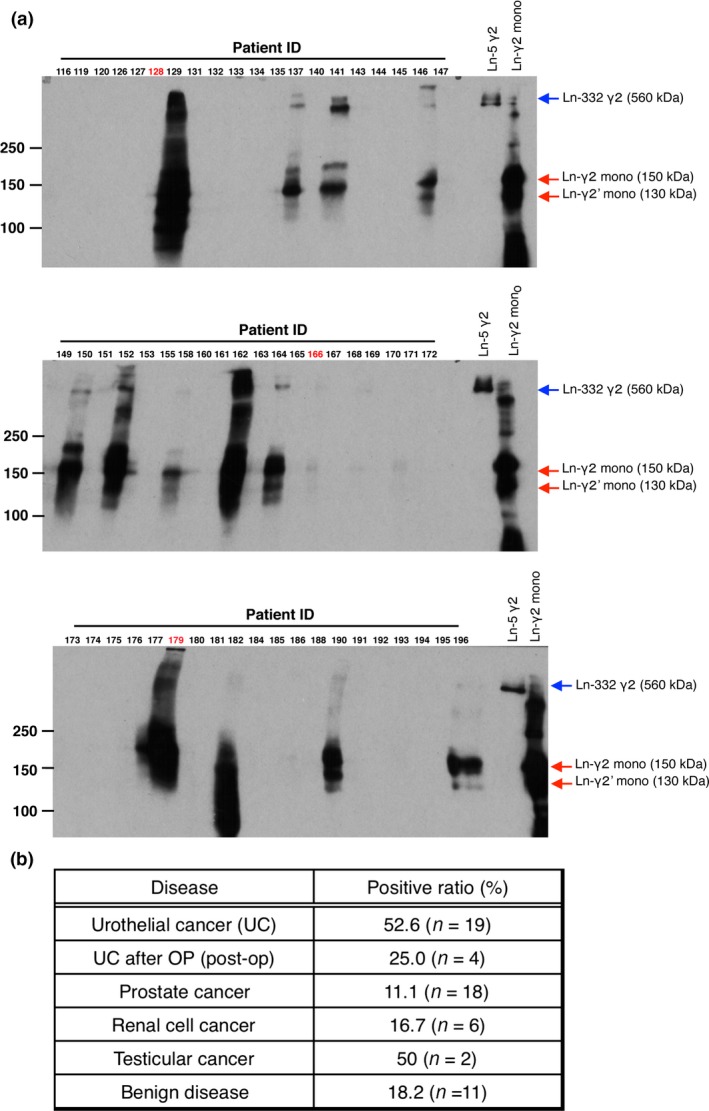
Detection of laminin‐γ2 (Ln‐γ2) in urine from urothelial cancer patients by Western blotting. (A) Sixty urine samples (80 μL) were concentrated using ice‐cold acetone and were subjected to Western blotting using D4B5 mAb under non‐reducing conditions. Ln‐332 and monomeric Ln‐γ2 (Ln‐γ2 mono) were used as positive controls. Red arrows indicate monomeric Ln‐γ2 and its oligomeric forms; blue arrows indicate Ln‐332γ2. Urothelial cancer tissues (red numbers) were subsequently analyzed using immunohistochemistry. (B) Summary of the Ln‐γ2‐positive urine ratio (%) from patients with malignant and benign urologic disease.
Expression of Ln‐γ2 in UC tissue
As Ln‐γ2 is most likely derived from UC tissue, we analyzed surgically resected cancer tissues obtained from three representative patients in whom Ln‐γ2 levels were negative (patient 128), low (patient 166), and high (patient 179) in Western blotting (Fig. 1A). Representative immunohistochemistry data are presented in Figure 2(A). As expected from the presence of Ln‐γ2, expression of the protein in UC tissue was confirmed for patient 166 and patient 179 (Figs. 2A,S1). Compared to these muscle‐invasive UC (MIUC) patients, Ln‐γ2 expression was not apparent in the tissue from patient 128 with NMIUC (Fig. 2B). Notably, Ln‐α3 was not detected in the region where Ln‐γ2 was detected in the tissues of patient 166 and patient 179, whereas Ln‐α3 was co‐localized with Ln‐γ2 at the basement membranes of the skin squamous cell carcinoma (Fig. 2B). Therefore, Ln‐γ2 appears to be expressed as a monomer in UC tissues and, most likely, the Ln‐γ2 secreted from the tissue appears in the urine.
Figure 2.
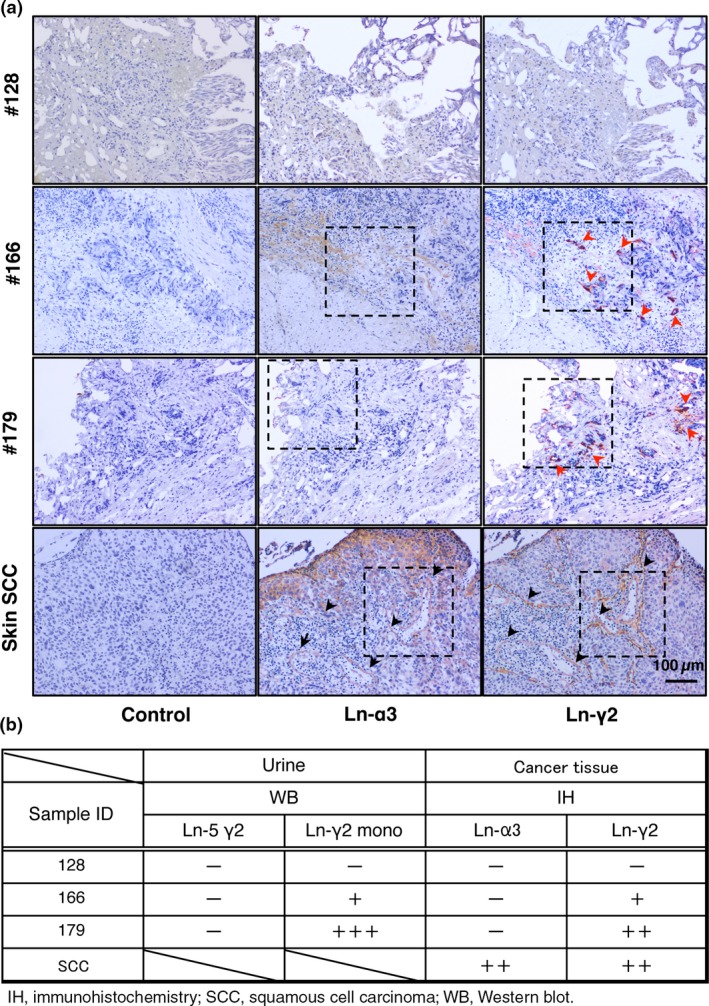
Detection of laminin (Ln‐α3 or ‐γ2) in urothelial cancer (UC) by immunohistochemistry (IH). (A) IH analysis of Ln‐α3 and ‐γ2 (components of Ln‐332). Red arrowheads indicate muscle invasive UC tissue expressing Ln‐γ2 in patients 166 and 179, but not in patient 128 (non‐muscle invasive UC). Black arrowheads indicate basement membranes expressing Ln‐α3 and ‐γ2 in skin squamous cell carcinoma (SCC). Representative views of specimens are indicated with dashed lines. (B) Ln‐α3 and ‐γ2 expression in UC specimens. WB, Western blot.
Quantitative analysis of Ln‐γ2 from UC patients
To analyze Ln‐γ2 quantitatively, we developed a sandwich ELISA using specific antibodies against Ln‐γ2. The standard curve for Ln‐γ2 showed a linear dose‐dependent increase (0–10 ng/mL; Fig. S2). Then, we used this method to analyze 100 urine samples from 39 NMIUC patients and 61 patients with benign urologic diseases (67 men and 33 women) (Table 1). All cases of cancer were histologically diagnosed as UC. The 39 NMIUC cases included 37 bladder cancers, 1 renal pelvic cancer, and 1 ureter cancer, with 21 primary and 18 recurrent cases. Among the patients with recurrence, 13 of 18 cases did not have any treatment after tumor resection. In contrast, 5 cases had received treatment with bladder instillation of bacille Calmette‐Guerin (BCG), and these specimens were collected after 3 months of the BCG treatment regimen. The 61 patients with benign urologic disease included 18 cases of benign prostatic hyperplasia, 12 cases of overactive bladder, 6 cases of neurogenic bladder, and 25 other benign urologic diseases.
Table 1.
Characteristics of evaluated patients with urinary diseases (n = 100)
| No. of patients | |
|---|---|
| Urothelial cancer | 39 |
| Cancer sites | |
| Bladder | 37 |
| Renal pelvis | 1 |
| Ureter | 1 |
| History | |
| Primary | 21 |
| Recurrence | 18 |
| Benign diseases | |
| Benign prostate hyperplasia | 18 |
| Overactive bladder | 12 |
| Neurogenic bladder | 6 |
| Others | 25 |
To standardize the urine specimens, we adjusted the Ln‐γ2 levels to the urine creatinine level (Ln‐γ2/crn; Table 2). The median Ln‐γ2 levels were 0.67 ± 0.58 and 2.07 ± 2.86 μg/g∙crn (P = 0.004) in benign urologic disease and UC, respectively. Median NMP22 levels were 3.97 ± 2.08 and 25.9 ± 71.6 U/mL in benign urologic disease and UC (P = 0.02), respectively.
Table 2.
Comparison of median values of creatinine‐corrected urinary laminin‐γ2 (Ln‐γ2/crn), Ln‐2/crn, and nuclear matrix protein 22 (NMP22) between benign disease and non‐muscle invasive urothelial carcinoma (UC)
| Tumor markers | Benign (n = 61) | UC (n = 39) | P‐value |
|---|---|---|---|
| Median ± SE | Median ± SE | ||
| Ln‐γ2 (ng/mL) | 0.48 ± 0.46 | 1.18 ± 0.16 | 0.010 |
| Ln‐γ2/crn (μg/g∙crn) | 0.67 ± 0.58 | 2.07 ± 2.86 | 0.004 |
| NMP22 (U/mL) | 3.97 ± 2.08 | 25.90 ± 71.60 | 0.020 |
P‐values were calculated using the Mann–Whitney U‐test.
Ln‐γ2 was almost undetectable for NMIUC patient 128, whereas it was detected for MIUC patients 166 and 179 by Western blotting (Fig. 1A). Therefore, it was of particular interest to know whether the Ln‐γ2 levels of patient 128 were detectable by our ELISA. The Ln‐γ2 values of ELISA for the urine specimens of patient 128, 166, and 179 were 7.5, 12.0 and 42.3 ng/mL, respectively, indicating that this method is sufficiently sensitive to diagnose NMIUC patients using their urine specimens.
Receiver operating characteristic analysis
Next, we compared the diagnostic sensitivity of Ln‐γ2 and Ln‐γ2/crn as a biomarker for diagnosis of UC by ROC analysis. The ROC curves created for Ln‐γ2 and Ln‐γ2/crn revealed that the AUC for Ln‐γ2/crn (0.78) was larger than that for Ln‐γ2 (0.73), although not significantly (P > 0.1) (Fig. 3A). We selected the Ln‐γ2/crn curve, and used the Youden index to select a cut‐off value.33 This analysis revealed that the maximum Youden index is 143.98 (Fig. 3B), which corresponded to a cut‐off value of 0.5 μg/g∙crn (Fig. 4).
Figure 3.
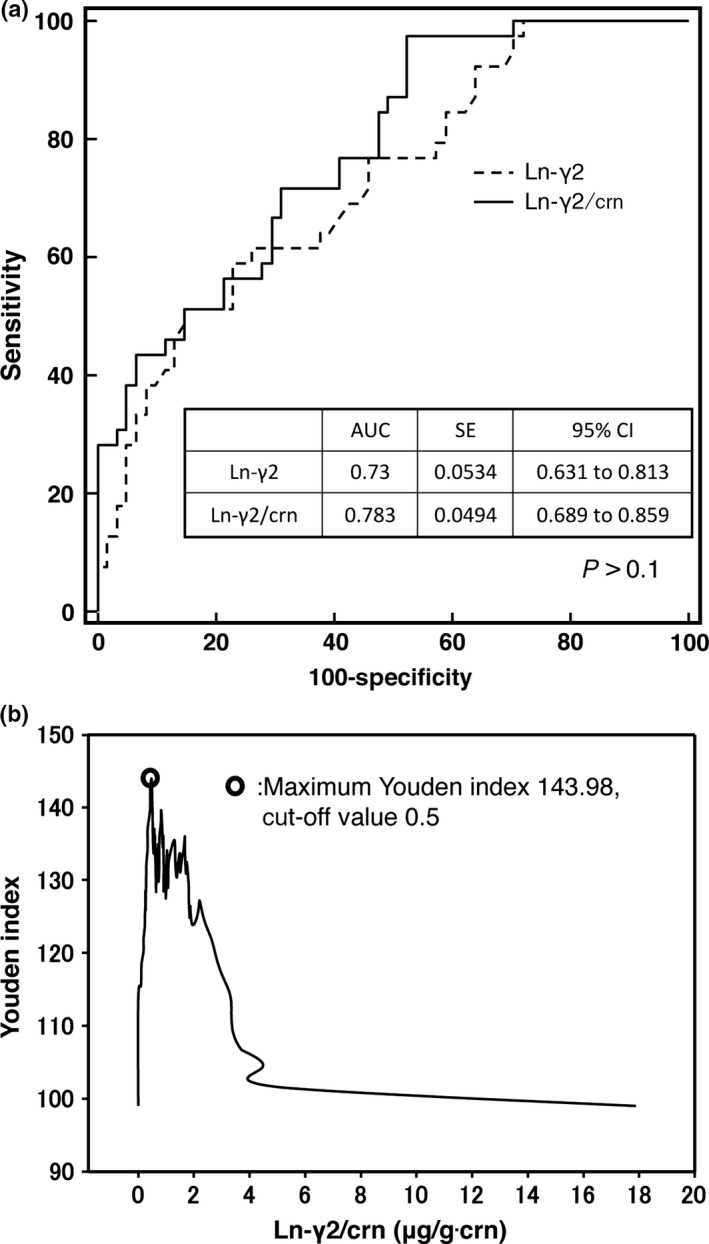
(A) Receiver operating characteristic (ROC) curves for urine laminin‐γ2 (Ln‐γ2) and creatinine‐corrected Ln‐γ2 (Ln‐γ2/crn), for detecting non‐muscle invasive urothelial cancer, among 39 patients with urothelial cancer and 61 patients with urologic diseases. The area under the curve (AUC) for urine Ln‐γ2/crn was higher than that for Ln‐γ2 (0.783 and 0.73, respectively; P > 0.05). (B) A cut‐off value of 0.5 μg/g∙crn was selected using the maximum Youden index. CI, confidence interval.
Figure 4.
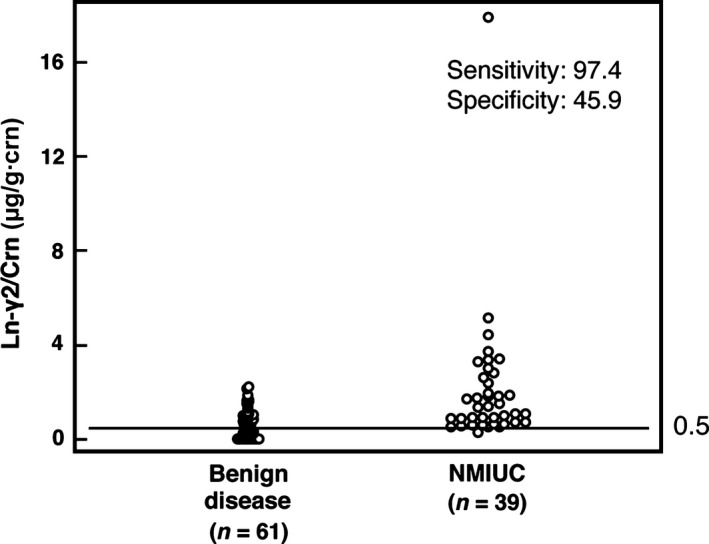
Dot plot of creatinine‐corrected urine laminin‐γ2 (Ln‐γ2/crn) in patients with urologic diseases. Dot plots of Ln‐γ2/crn in 39 non‐muscle invasive urothelial cancer (NMIUC) patients and 61 patients with other urologic diseases. The mean urine concentration of Ln‐γ2/crn in NMIUC patients was significantly higher than in patients with benign urologic disease (2.07 ± 2.86 and 0.67 ± 0.58 μg/g∙crn, respectively; P = 0.004). Using a cut‐off value of 0.5 μg/g∙crn, the sensitivity and specificity for detecting NMIUC were 97.4 and 45.9, respectively.
Comparison of diagnostic accuracy of Ln‐γ2/crn, NMP22, BTA, and cytology
The accuracy of Ln‐γ2/crn, NMP22, BTA, and cytology for UC diagnosis is summarized in Table 3. The sandwich ELISA for Ln‐γ2 was more sensitive than Western blotting (Fig. S2, Table 3), and the cut‐off values for Ln‐γ2/crn and NMP22 were defined as 0.5 μg/g∙crn and 12 U/mL, respectively. The sensitivity values for Ln‐γ2/crn, NMP22, and BTA were 97.4%, 25.6%, and 25.6%, and their specificity values were 45.9%, 98.4%, and 98.4%, respectively. Although Ln‐γ2/crn had the lowest positive predictive value (Ln‐γ2/crn, 53.5%; NMP22, 90.9%; BTA, 90.9%; Table 3), it also had the highest negative predictive value (Ln‐γ2/crn, 96.6%; NMP22, 67.4%; BTA, 67.4%). Ln‐γ2/crn, NMP22, and BTA were analyzed for all 100 specimens, although urine cytology was carried out only for 56 specimens. Therefore, although cytology had a high specificity and positive‐predictive value, those results could not be directly compared to the results for Ln‐γ2/crn, NMP22, and BTA.
Table 3.
Validity of creatinine‐corrected urinary laminin‐γ2 (Ln‐γ2/crn), nuclear matrix protein 22 (NMP22), bladder tumor antigen (BTA), and urinary cytology in predicting urothelial cancer
| No. of patients | Cut‐off | Sensitivity, % | Specificity, % | Positive‐predictive value, % | Negative‐predictive value, % | |
|---|---|---|---|---|---|---|
| Ln‐γ2/crn | 100 | 0.5 | 97.4 | 45.9 | 53.5 | 96.6 |
| NMP22 | 100 | 12.0 | 25.6 | 98.4 | 90.9 | 67.4 |
| BTA | 100 | +/− | 25.6 | 98.4 | 90.9 | 67.4 |
| Urinary cytology | 52 | +/− | 66.7 | 100.0 | 100.0 | 68.8 |
Positive (+) / Negative (−)
Urothelial cancer patients were classified according to tumor grade, T‐stage, concurrent carcinoma in situ (CIS), and primary or recurrent tumor status, and the sensitivities of Ln‐γ2/crn, NMP22, and BTA were evaluated for each category (Table 4). In this analysis, Ln‐γ2/crn had a higher sensitivity than NMP22 and BTA for all categories. For example, the Ln‐γ2/crn sensitivity was 94.4% for low‐grade tumors, compared to 27.8% for both NMP22 and BTA. However, no intragroup differences were observed for sensitivity in cases with low‐ and high‐grade tumors. At the Ta stage, the sensitivity of Ln‐γ2/crn was 95.2%, which was higher than that for NMP22 and BTA (23.8% and 23.8%, respectively). All biomarkers showed higher sensitivities for the advanced tumor stages. In all the stages, NMP22 and BTA showed lower sensitivity than Ln‐γ2/crn, as was also the case for primary and recurrent UC.
Table 4.
Sensitivity and specificity of creatinine‐corrected urinary laminin‐γ2 (Ln‐γ2/crn), nuclear matrix protein 22 (NMP22), bladder tumor antigen (BTA), according to tumor variable
| Variable | No. of patients | Ln‐γ2/crn, % | NMP22, % | BTA, % |
|---|---|---|---|---|
| Tumor grade | ||||
| Low | 18 | 94.4/45.9 | 27.8/98.4 | 27.8/98.4 |
| High | 21 | 100.0/45.9 | 23.8/98.4 | 23.8/98.4 |
| Tumor stage | ||||
| Ta | 21 | 95.2/46.0 | 23.8/98.4 | 23.8/98.4 |
| T1 | 7 | 100.0/45.9 | 37.5/98.4 | 37.5/98.4 |
| Concurrent CIS | 14 | 100.0/45.9 | 21.4/98.4 | 28.6/98.4 |
| Non‐concurrent CIS | 25 | 96.0/45.9 | 28.0/98.4 | 24.0/98.4 |
| Primary/recurrence | ||||
| Primary | 21 | 95.2/45.9 | 33.3/98.4 | 23.8/98.4 |
| Recurrence | 18 | 94.4/45.9 | 27.8/98.4 | 27.8/98.4 |
Values indicate sensitivity (%)/specificity (%). CIS, carcinoma in situ.
Next, we evaluated Ln‐γ2/crn, NMP22, and BTA using logistic regression to identify significant parameters. The overall model‐fit analysis provided a P‐value of <0.05, and no significant difference was observed for the Hosmer‐Lemeshow test. Multiple logistic regression analyses revealed odds ratios of 20.603, 7.796, and 7.796 for Ln‐γ2/crn, NMP22, and BTA, respectively (Table 5). However, we excluded cytology data from the multivariate analyses, due to the lower number of cases with cytology data.
Table 5.
Logistic regression analysis for detecting non‐muscle invasive urothelial cancer
| B | P‐value | OR | 95% CI | |
|---|---|---|---|---|
| Ln‐γ2/crn | 3.025 | 0.004a | 20.603 | 2.61–162.12 |
| NMP22 | 2.054 | 0.064 | 7.796 | 0.884–8.727 |
| BTA | 2.054 | 0.064 | 7.796 | 0.884–8.727 |
P < 0.05. B, regression coefficient; BTA, bladder tumor antigen; CI, confidence interval; Ln‐γ2/crn, urinary Ln‐γ2 levels adjusted to the urine creatinine level; NMP22, nuclear matrix protein 22; OR, odds ratio.
Discussion
The number of patients with UC is increasing in developed countries, due to their increasing aged populations.32 However, no appropriate tumor marker for UC diagnosis has been developed, and the commercially available tumor markers (e.g., NMP22 and BTA) show a high false positive ratio for patients with urinary diseases with hematuria, inflammation, or stones, and are thus unsuitable for patients who have undergone TUR‐Bt.2, 11, 13, 15 As surgical resection remains the only way to cure UC, it highlights the particular importance of an early diagnosis, especially for monitoring recurrent UC after TUR‐Bt. In this study, we show that Ln‐γ2 levels are increased in UC patients, suggesting that Ln‐γ2 is useful as a new biomarker for UC. Based on creatinine‐adjusted Ln‐γ2 levels (as measured using sandwich ELISA), we determined a urinary concentration of 0.5 μg/g∙crn as an optimal cut‐off value for UC screening.
When we compared the sensitivities of Ln‐γ2/crn, NMP22, BTA, and urine cytology, we found that Ln‐γ2/crn had the highest sensitivity for UC. In our study, NMP22 and BTA showed relatively low sensitivities compared to those previously reported,2, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 possibly, at least in part, because our data was obtained only from primary and recurrent NMIUC patients (MIUC patients were excluded), and control data was from patients with benign urologic diseases. Moreover, five recurrence cases with bladder BCG instillation were included in this study. Therefore, BCG treatment may have affected the outcome of NMP22 or BTA. Furthermore, urinary conditions, such as hematuria, pyuria, or proteinuria, which frequently occur in benign urologic diseases, may decrease the sensitivity of NMP22 and BTA. Although further verification of urinary conditions is needed, the present results indicated that Ln‐γ2/crn was superior to the other tests in terms of sensitivity and could be used to diagnose NMIUC under any urinary conditions.
In contrast, urine cytology showed moderate sensitivity compared to the other tumor markers. However, the sensitivity of urine cytology is strongly dependent on the experience of the cytologist or pathologist and is usually in the range of 40–60%, depending on the hospital. Thus, the low sensitivity of current UC screening methods likely precludes an early diagnosis of UC. In contrast, Ln‐γ2/crn showed sensitivities of 85.7%, 100%, 100%, and 100% for patients with Ta, T1, and CIS, which were higher than the sensitivities for the other UC tumor markers. Furthermore, Ln‐γ2/crn was equally sensitive for cases of primary and recurrent UC. Even though Ln‐γ2/crn had a lower specificity compared to the other tumor markers, this depends on the cut‐off values used. Thus, despite the small sample size, we believe that Ln‐γ2/crn is an attractive biomarker for NMIUC. We are currently attempting to improve the specificity of Ln‐γ2/crn (by developing an automated highly sensitive assay method and using fresh urine samples), and are preparing an extended clinical study to establish the diagnostic value of Ln‐γ2/crn for NMIUC in larger patient cohorts.
In addition to its potential usefulness for screening UC patients, Ln‐γ2/crn may also be useful as a prognostic biomarker after TUR‐Bt. Unfortunately, up to 80% of NMIUC patients experience recurrence within 2 years of TUR‐Bt, and these patients must typically undergo invasive cystoscopy every 3 months to monitor recurrence. Unlike NMP22 and BTA, Ln‐γ2/crn had a high sensitivity for patients with UC recurrence after TUR‐Bt, as nearly 100% of patients with recurrent UC could be identified using Ln‐γ2 levels. This was typically represented in a patient with NMIUC (high‐grade pTa CIS), where Ln‐γ2/crn levels decreased to below the cut‐off value after TUR‐Bt and then subsequently increased, and in whom recurrence was eventually found by cystoscopy approximately 1 year later (Case 1, Fig. S3). In contrast, in another case, Ln‐γ2/crn levels decreased to below the cut‐off value after TUR‐Bt, but remained low (Case 2, Fig. S3), and no recurrence has yet been noted. It is of note that Ln‐γ2 levels increased before definite diagnosis of recurrence by pathological examination. To monitor recurrence after TUR‐Bt patients usually require periodic monitoring by cystoscopy. It may be possible to substitute testing for Ln‐γ2/crn for the invasive cystoscopy after TUR‐Bt, greatly relieving the burden of patients. However, further clinical studies are necessary to validate these findings.
In conclusion, we detected monomeric Ln‐γ2 and related forms in urine from UC patients. We developed a quantitative ELISA for Ln‐γ2 and used it to detect Ln‐γ2 from NMIUC, renal pelvic cancer, and ureteric cancer patients. These findings strongly indicate that Ln‐γ2 is an attractive biomarker for detecting early stages of UC and for monitoring recurrence after TUR‐Bt, which may provide relief for patients from the painful cystoscopic approach to monitoring recurrence.
Disclosure Statement
N. Koshikawa and M. Seiki received research funding from Abbott Laboratories (Chicago, IL, USA). The other authors have no conflict of interest.
Supporting information
Fig. S1. Detection of laminin (Ln)‐α3 or ‐γ2 in urothelial cancer tissues by immunohistochemistry.
Fig. S2. Quantitative analysis of laminin‐γ2 (Ln‐γ2) by sandwich ELISA.
Fig. S3. Two cases with creatinine‐corrected urine laminin‐γ2 (Ln‐γ2/crn) levels measured during the postoperative course, highlighting the relationship between urothelial cancer recurrence and Ln‐γ2/crn values.
Table S1. Diagnosis of patients with urologic diseases.
Acknowledgments
We thank Dr. D. Hoshino (Kanagawa Cancer Center) for helpful discussions, and Dr. M. Sakaguchi (Kochi Medical School) for assistance with the statistical analysis. This work was supported by a Grant‐in‐Aid for Scientific Research (C) (N.K.) and an Adaptable and Seamless Technology Transfer Program through Target‐driven R&D (A‐STEP) (N.K. and T.S.), and a Grant‐in‐Aid for Scientific Research (S) (M.S.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Cancer Sci 106 (2015) 1730–1737
Funding Information
Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- 1. Watanabe J, Nishiyama A, Ogawa O. Bladder cancer practice guideline. IGAKUTOSHO 2009; 1: 1. [Google Scholar]
- 2. Babjuk M, Burger M, Zigeuner R et al EAU guidelines on non‐muscle‐invasive urothelial carcinoma of the bladder: update 2013. Eur Urol 2013; 64: 639–53. [DOI] [PubMed] [Google Scholar]
- 3. Garbett EA, Reed MW, Stephenson TJ, Brown NJ. Proteolysis in human breast cancer. Mol Pathol 2000; 53: 99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Badalament RA, Hermansen DK, Kimmel M et al The sensitivity of bladder wash flow cytometry, bladder wash cytology, and voided cytology in the detection of bladder carcinoma. Cancer 1987; 60: 1423–7. [DOI] [PubMed] [Google Scholar]
- 5. Dobrowolska‐Glazar B, Glazar W, Dobrowolski Z, Lipczynski W. Bladder cancer biomarkers. Przegl Lek 2010; 67: 479–83. [PubMed] [Google Scholar]
- 6. Sturgeon CM, Duffy MJ, Hofmann BR et al National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines for use of tumor markers in liver, bladder, cervical, and gastric cancers. Clin Chem 2010; 56(6): e1–48. [DOI] [PubMed] [Google Scholar]
- 7. Soloway MS, Briggman V, Carpinito GA et al Use of a new tumor marker, urinary NMP22, in the detection of occult or rapidly recurring transitional cell carcinoma of the urinary tract following surgical treatment. J Urol 1996; 156(2 Pt 1): 363–7. [DOI] [PubMed] [Google Scholar]
- 8. Miyanaga N, Akaza H, Ishikawa S et al Clinical evaluation of nuclear matrix protein 22 (NMP22) in urine as a novel marker for urothelial cancer. Eur Urol 1997; 31: 163–8. [DOI] [PubMed] [Google Scholar]
- 9. Miyanaga N, Akaza H, Kameyama S et al Significance of the BTA test in bladder cancer: a multicenter trial. BTA Study Group Japan. Int J Urol 1997; 4: 557–60. [DOI] [PubMed] [Google Scholar]
- 10. Stampfer DS, Carpinito GA, Rodriguez‐Villanueva J et al Evaluation of NMP22 in the detection of transitional cell carcinoma of the bladder. J Urol 1998; 159: 394–8. [DOI] [PubMed] [Google Scholar]
- 11. Lahme S, Bichler KH, Feil G, Zumbragel A, Gotz T. Comparison of cytology and nuclear matrix protein 22 (NMP 22) for the detection and follow‐up of bladder‐cancer. Adv Exp Med Biol 2003; 539(Pt A): 111–19. [DOI] [PubMed] [Google Scholar]
- 12. Ponsky LE, Sharma S, Pandrangi L et al Screening and monitoring for bladder cancer: refining the use of NMP22. J Urol 2001; 166(1): 75–8. [PubMed] [Google Scholar]
- 13. Murphy WM, Rivera‐Ramirez I, Medina CA, Wright NJ, Wajsman Z. The bladder tumor antigen (BTA) test compared to voided urine cytology in the detection of bladder neoplasms. J Urol 1997; 158: 2102–6. [DOI] [PubMed] [Google Scholar]
- 14. Nguyen CT, Jones JS. Defining the role of NMP22 in bladder cancer surveillance. World J Urol 2008; 26(1): 51–8. [DOI] [PubMed] [Google Scholar]
- 15. Poulakis V, Witzsch U, De Vries R, Altmannsberger HM, Manyak MJ, Becht E. A comparison of urinary nuclear matrix protein‐22 and bladder tumour antigen tests with voided urinary cytology in detecting and following bladder cancer: the prognostic value of false‐positive results. BJU Int 2001; 88: 692–701. [DOI] [PubMed] [Google Scholar]
- 16. Lammers RJ, Hendriks JC, Rodriguez FO, Witjes WP, Palou J, Witjes JA. Prediction model for recurrence probabilities after intravesical chemotherapy in patients with intermediate‐risk non‐muscle‐invasive bladder cancer, including external validation. World J Urol 2015: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Miyazaki K. Laminin‐5 (laminin‐332): unique biological activity and role in tumor growth and invasion. Cancer Sci 2006; 97(2): 91–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Koshikawa N, Moriyama K, Takamura H et al Overexpression of laminin gamma2 chain monomer in invading gastric carcinoma cells. Cancer Res 1999; 59: 5596–601. [PubMed] [Google Scholar]
- 19. Kagesato Y, Mizushima H, Koshikawa N et al Sole expression of laminin gamma 2 chain in invading tumor cells and its association with stromal fibrosis in lung adenocarcinomas. Japn J Cancer Res 2001; 92: 184–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Olsen J, Kirkeby LT, Brorsson MM et al Converging signals synergistically activate the LAMC2 promoter and lead to accumulation of the laminin gamma 2 chain in human colon carcinoma cells. Biochem J 2003; 371(Pt 1): 211–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pyke C, Romer J, Kallunki P et al The gamma 2 chain of kalinin/laminin 5 is preferentially expressed in invading malignant cells in human cancers. Am J Pathol 1994; 145: 782–91. [PMC free article] [PubMed] [Google Scholar]
- 22. Lim SC, Zhang S, Ishii G et al Predictive markers for late cervical metastasis in stage I and II invasive squamous cell carcinoma of the oral tongue. Clin Cancer Res 2004; 10(1 Pt 1): 166–72. [DOI] [PubMed] [Google Scholar]
- 23. Katayama A, Bandoh N, Kishibe K et al Expressions of matrix metalloproteinases in early‐stage oral squamous cell carcinoma as predictive indicators for tumor metastases and prognosis. Clin Cancer Res 2004; 10: 634–40. [DOI] [PubMed] [Google Scholar]
- 24. Katayama M, Sekiguchi K. Laminin‐5 in epithelial tumour invasion. J Mol Histol 2004; 35: 277–86. [DOI] [PubMed] [Google Scholar]
- 25. Giannelli G, Falk‐Marzillier J, Schiraldi O, Stetler‐Stevenson WG, Quaranta V. Induction of cell migration by matrix metalloprotease‐2 cleavage of laminin‐5. Science 1997; 277: 225–8. [DOI] [PubMed] [Google Scholar]
- 26. Koshikawa N, Giannelli G, Cirulli V, Miyazaki K, Quaranta V. Role of cell surface metalloprotease MT1‐MMP in epithelial cell migration over laminin‐5. J Cell Biol 2000; 148: 615–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schenk S, Hintermann E, Bilban M et al Binding to EGF receptor of a laminin‐5 EGF‐like fragment liberated during MMP‐dependent mammary gland involution. J Cell Biol 2003; 161: 197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Koshikawa N, Minegishi T, Nabeshima K, Seiki M. Development of a new tracking tool for the human monomeric laminin‐gamma 2 chain in vitro and in vivo. Cancer Res 2008; 68: 530–6. [DOI] [PubMed] [Google Scholar]
- 29. Koshikawa N, Minegishi T, Sharabi A, Quaranta V, Seiki M. Membrane‐type matrix metalloproteinase‐1 (MT1‐MMP) is a processing enzyme for human laminin gamma 2 chain. J Biol Chem 2005; 280(1): 88–93. [DOI] [PubMed] [Google Scholar]
- 30. Hwang EC, Choi HS, Jung SI, Kwon DD, Park K, Ryu SB. Use of the NMP22 BladderChek test in the diagnosis and follow‐up of urothelial cancer: a cross‐sectional study. Urology 2011; 77: 154–9. [DOI] [PubMed] [Google Scholar]
- 31. Seiji N, Shojiro M, Yasuyuki Y. General Rule for Clinical and Pathological Studies on Renal Pelvic, Ureteral and Bladder Cancer, 1st edn Tokyo: Kanehara‐publisher, 2011; 17–18. [Google Scholar]
- 32. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013; 63(1): 11–30. [DOI] [PubMed] [Google Scholar]
- 33. Schisterman EF, Perkins NJ, Liu A, Bondell H. Optimal cut‐point and its corresponding Youden Index to discriminate individuals using pooled blood samples. Epidemiology 2005; 16(1): 73–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Detection of laminin (Ln)‐α3 or ‐γ2 in urothelial cancer tissues by immunohistochemistry.
Fig. S2. Quantitative analysis of laminin‐γ2 (Ln‐γ2) by sandwich ELISA.
Fig. S3. Two cases with creatinine‐corrected urine laminin‐γ2 (Ln‐γ2/crn) levels measured during the postoperative course, highlighting the relationship between urothelial cancer recurrence and Ln‐γ2/crn values.
Table S1. Diagnosis of patients with urologic diseases.


