Abstract
Gastric cancer incidence and mortality have been decreasing in Japan. These decreases are likely due to a decrease in prevalence of Helicobacter pylori infection. Our aim was to characterize the trends in prevalence of H. pylori infection focused on birth‐year. We carried out a cross‐sectional study that included 4285 subjects who were born from 1926 to 1989. We defined H. pylori infection by the serum H. pylori antibody titer. Individuals having H. pylori infection and those with negative H. pylori antibody titer and positive pepsinogen test were defined as high‐risk individuals for gastric cancer. We estimated the birth‐year percent change (BPC) of the prevalence by Joinpoint regression analysis. The prevalence of H. pylori infection among the subjects born from 1927 to 1949 decreased from 54.0% to 42.0% with a BPC of −1.2%. It was followed by a rapid decline in those born between 1949 (42.0%) and 1961 (24.0%) with a BPC of −4.5%, which was followed by those born between 1961 (24.0%) and 1988 (14.0%) with a BPC of −2.1%. The proportion of high‐risk individuals for gastric cancer among the subjects born from 1927 to 1942 decreased from 62.0% to 55.0% with a BPC of −0.8%. A subsequent rapid declining trend was observed in those born between 1942 (55.0%) and 1972 (18.0%) with a BPC of −3.6%, and then it became stable. These remarkable declining trends in the prevalence of H. pylori infection by birth‐year would be useful to predict the future trend in gastric cancer incidence in Japan.
Keywords: Gastric cancer, Helicobacter pylori antibody, Helicobacter pylori infection, high‐risk individuals, pepsinogen
Helicobacter pylori (H. pylori) infection is regarded as a major risk factor for gastric cancer.1, 2 It is typically acquired in childhood through unsanitary an environment.3, 4 Once infection is established, it usually lasts for life. Chronic infection with H. pylori has certain carcinogenicity which induces gastric cancer through chronic atrophic gastritis.5, 6
In Japan, the age‐adjusted incidence and mortality rate of gastric cancer have been declining over the past 30 years.7, 8 These decreasing trends have been thought to be due to a decline in the prevalence of H. pylori infection over time. The prevalence examined in the early 1990s in Sapporo city was over 70% among those aged 50 years and over.9 Another study carried out in Kyushu reported that the prevalence of H. pylori infection increased with age, and the prevalence among those aged 50 years and over was 60–70% during the investigation period of 2002–2006.10 This cross‐sectional study was conducted again in 2007–2011, showing a prevalence of 40–60% among those aged 50 years and over.11 The difference in the prevalence of H. pylori infection in the same geographic area and age group over time might be due to the birth cohort effect. Similarly, Ueda et al.12 recently reported changes in the prevalence of H. pylori infection in 10‐year birth cohorts between the 1940s and 1980s, showing a birth cohort effect with declining tendency of the prevalence in the Japanese population. However, the degree of change by a single birth year effect remains to be clarified.
In this study, we undertook a cross‐sectional study to characterize trends by birth‐year in the prevalence of H. pylori infection and the proportion of high‐risk individuals for developing gastric cancer in Japan, which would provide useful information to estimate the future trend of gastric cancer incidence.
Materials and Methods
Study subjects
We recruited participants through the Hospital‐based Epidemiological Research Program III at Aichi Cancer Center (HERPACC III) from November 2005 to March 2013. Briefly, all first‐visit outpatients at Aichi Cancer Center Hospital (Nagoya, Japan) aged 20–79 years were asked to fill out a self‐administered questionnaire regarding their lifestyle before development of the current symptom, and were also asked to provide blood samples.13, 14 Approximately 66.4% of the outpatients provided written informed consent to participate in the HERPACC III study carried out during this period. The process of subject selection from HERPACC III participants is shown in Figure 1. A total of 11 559 participants, born between 1926 and 1989, filled out the self‐administered questionnaire and provided blood samples between November 2005 and March 2013. Of these, we selected 3828 participants who were born between 1931 and 1989, and who were recruited between September 2010 and March 2013 through HERPACC III. As the number of participants born between 1926 and 1936 was small, we additionally selected 870 individuals who were born in this period from those recruited between November 2005 and August 2010. The data were entered into the HERPACC database, which was periodically linked to the hospital‐based cancer registry system until March 2014, to update the data on cancer incidence. Among the 4698 selected participants, we excluded 336 gastric cancer patients and one with mucosa‐associated lymphoid tissue (MALT) lymphoma who were diagnosed within 1 year after recruitment, 68 individuals whose stored serum volume was insufficient for measurement of H. pylori antibody and pepsinogen levels, and eight persons in whom the result of the pepsinogen test could not be determined. The remaining 4285 subjects (male, n = 2431; female, n = 1854) were included in this study (Fig. 1). During the 1‐year follow‐up period, we identified 259 colorectal cancer cases (6.0%), 186 esophagus cancer cases (4.3%), 67 liver cancer cases (1.6%), 101 pancreatic cancer cases (2.4%), 339 lung cancer cases (7.9%), 221 head and neck cancer cases (5.2%), and 826 cases with other cancers (19.3%) among the 4285 study subjects, and the remaining 2286 subjects (53.3%) were designated as cancer‐free subjects. As there was little evidence for the association between H. pylori infection and risk of cancer except gastric cancer and MALT lymphoma, we included patients who developed cancers other than these two cancer types in our study.
Figure 1.
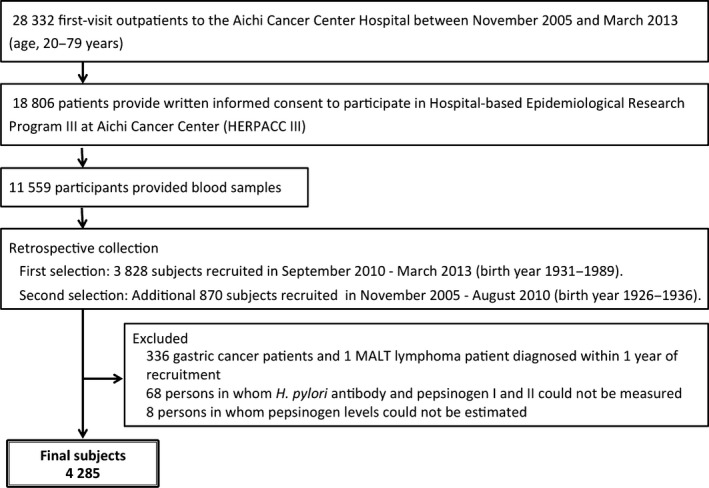
Flowchart showing the selection of eligible subjects for the study of prevalence of Helicobacter pylori infection and proportion of high‐risk individuals for gastric cancer. The study was undertaken within the framework of the Hospital‐based Epidemiology Research Program III at Aichi Cancer Center (Nagoya, Japan).
This study was approved by the Institutional Ethics Committee at Aichi Cancer Center (approval no. 19‐7, 15 August 2005).
Determination of H. pylori antibody titer and pepsinogen levels
The H. pylori antibody titer and pepsinogen levels were measured in the blood sample obtained at the time of the first visit. The serum H. pylori antibody titer was measured using an enzyme immunoassay kit (E‐plate Eiken H. pylori antibody or E‐plate Eiken H. pylori antibody II; Eiken Kagaku, Tokyo, Japan). Positivity for H. pylori antibody was defined as higher than 10 U/mL H. pylori antibody titer. This cut‐off value in the kit showed the sensitivity and specificity to be 90.7% and 91.5%, respectively, when the 13C urea breath test was used for validation.15 Individuals with H. pylori infection were defined as H. pylori antibody‐positive individuals.
Serum pepsinogen levels were measured using the latex agglutination reaction kit (LZ test Eiken pepsinogen I and LZ test Eiken pepsinogen II; Eiken Kagaku). We adopted the level of pepsinogen I ≤ 70 ng/mL and the ratio of pepsinogen I/II ≤ 3.0 as showing a positive result in the pepsinogen test. The cut‐off value was reported to provide a sensitivity and specificity for chronic gastritis of 87.5% and 84.9%, respectively, validated by histological examination.16
In the natural history of H. pylori infection, some H. pylori antibody‐positive individuals develop chronic atrophic gastritis with negative conversion of H. pylori antibody and positive conversion of the result of the pepsinogen test.1, 17 Patients with positive H. pylori antibody and those with chronic atrophic gastritis have a high risk of developing gastric cancer.18 Therefore, we defined both H. pylori antibody‐positive individuals and pepsinogen test‐positive individuals with negative H. pylori antibody as high‐risk individuals for gastric cancer.
Statistical methods
We calculated the prevalence of H. pylori infection and proportion of high‐risk individuals for gastric cancer using the three‐birth‐year moving‐average method. The moving‐average prevalence in the first birth‐year (1927) was calculated as the sum of the number of positive individuals born in the first three birth‐years (1926, 1927, 1928) divided by the total number of subjects born in those three birth‐years. The following moving‐average prevalence was calculated by using the subsequent three adjoining birth‐years (1927, 1928, 1929). We calculated the moving‐average prevalence in the last birth‐year (1988) using the last three birth‐years (1987, 1988, 1989). Their trends were characterized by Joinpoint regression analysis,19 which is widely used to analyze trends over time. The technique identifies the time point(s), also referred to as joinpoint(s), at which there is a statistically significant change in the trend. We assigned the birth‐year as an independent variable and the prevalence of H. pylori infection and the proportion of high‐risk individuals for gastric cancer as dependent variables while setting a maximum of five joinpoints. Then, all possible combinations of the joinpoints were tried to evaluate the best‐fitting number of joinpoints to maximize the log‐likelihood estimate.19 The resulting trend segments, as delimited in time by joinpoints, were described by the birth‐year percent change (BPC), that is, the slope of the line segment. In describing the trends, the terms “increase” or “decrease” were used when the slope (BPC) of the trend was statistically significant (P < 0.05); otherwise, the terms “stable” or “level” were used.
Joinpoint regression analysis was carried out using the Joinpoint Regression Program version 4.1.0 provided by the Surveillance, Epidemiology, and End Results Program (National Cancer Institute; http://surveillance.cancer.gov/joinpoint/). All statistical analyses were performed with 95% confidence intervals (CI); statistical significance was set at P < 0.05.
Results
Table 1 shows the number of study subjects according to birth‐year and age at the time of recruitment in this study. The median birth‐year of the 4285 study subjects was 1948. The age (mean ± SD) of the subjects was 60.5 ± 12.8 years (males, 64.1 ± 11.1 years; females, 55.8 ± 13.4 years). There were 1607 persons who were positive for H. pylori antibody (37.5%; 95% CI, 36.1–39.0%) and 198 persons who had a positive pepsinogen test but were negative for H. pylori antibody (4.6%; 95% CI, 4.0–5.3%). Table S1 shows the prevalence of H. pylori infection and the proportion of high‐risk individuals for gastric cancer in each of the 13 birth cohorts that classified the subjects from 1926–29 to 1985–89.
Table 1.
Number of study subjects by birth‐year and age at the time of recruitment to this study of prevalence of Helicobacter pylori infection in a Japanese population
| Birth‐year | Age, years | ||||||
|---|---|---|---|---|---|---|---|
| 70–79 | 60–69 | 50–59 | 40–49 | 30–39 | 20–29 | Total | |
| 1926–1929 | 76 | 0 | 0 | 0 | 0 | 0 | 76 |
| 1930–1934 | 519 | 0 | 0 | 0 | 0 | 0 | 519 |
| 1935–1939 | 579 | 18 | 0 | 0 | 0 | 0 | 597 |
| 1940–1944 | 205 | 307 | 0 | 0 | 0 | 0 | 512 |
| 1945–1949 | 0 | 654 | 0 | 0 | 0 | 0 | 654 |
| 1950–1954 | 0 | 218 | 270 | 0 | 0 | 0 | 488 |
| 1955–1959 | 0 | 0 | 354 | 0 | 0 | 0 | 354 |
| 1960–1964 | 0 | 0 | 162 | 196 | 0 | 0 | 358 |
| 1965–1969 | 0 | 0 | 0 | 291 | 0 | 0 | 291 |
| 1970–1974 | 0 | 0 | 0 | 104 | 127 | 0 | 231 |
| 1975–1979 | 0 | 0 | 0 | 0 | 120 | 0 | 120 |
| 1980–1984 | 0 | 0 | 0 | 0 | 29 | 24 | 53 |
| 1985–1989 | 0 | 0 | 0 | 0 | 0 | 32 | 32 |
| Total | 1379 | 1197 | 786 | 591 | 276 | 56 | 4285 |
Figure 2 and Table 2 show the trends in the prevalence of H. pylori infection and the proportion of high‐risk individuals for gastric cancer using Joinpoint regression analysis. There were two significant joinpoints in the prevalence of H. pylori infection in 1949 and 1961 (Fig. 2a). The prevalence of H. pylori infection in subjects born between 1927 and 1949 decreased from 54.0% to 42.0%, with a BPC of −1.2% (95% CI, −1.6% to −0.8%). It was followed by a rapid decline in those born between 1949 (42.0%) and 1961 (24.0%) with a BPC of −4.5% (95% CI, −6.0% to −3.0%). The third decreasing trend was observed between 1961 (24.0%) and 1988 (14.0%) with a BPC of −2.1% (95% CI, −3.3% to −0.8%) (Table 2).
Figure 2.
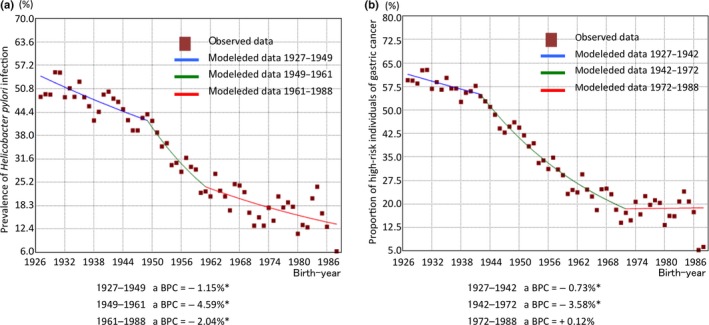
Trends in the prevalence of Helicobacter pylori infection (a) and proportion of high‐risk individuals for gastric cancer (b) by three‐birth‐year moving‐average method in Joinpoint regression analysis. *Statistical significance was set at P < 0.05. BPC, birth‐year percent change.
Table 2.
Results of Joinpoint regression analysis of the trends of prevalence of Helicobacter pylori infection and proportion of high‐risk individuals for gastric cancer in a Japanese population
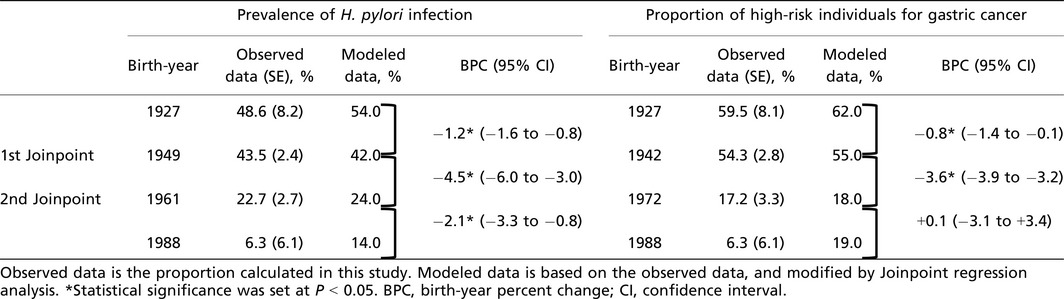
As for the proportion of high‐risk individuals for gastric cancer, there were two significant joinpoints in 1942 and 1972 (Fig. 2b). The proportion of high‐risk individuals for gastric cancer in the subjects born between 1927 and 1942 decreased from 62.0% to 55.0%, and the BPC of the period was −0.8% (95% CI, −1.4% to −0.1%). A subsequent rapid declining trend was observed in those born between 1942 (55.0%) and 1972 (18.0%) with a BPC of −3.6% (95% CI, −3.9% to −3.2%). It was followed by a stable trend until 1988 (BPC, +0.1% [95% CI, −3.1% to +3.4%]) (Table 2).
Discussion
Our results showed that the prevalence of H. pylori infection in the Japanese population decreased as the birth year increased, with a drastic decline in those born between 1949 and 1961. A similar decreasing trend was observed in the proportion of high‐risk individuals for gastric cancer, with a rapid decline among those born between 1942 and 1972. The different first joinpoints between the two proportions (1949 vs 1942) might be due to the latency of chronic inflammation. The negative conversion of H. pylori antibody and positive conversion of pepsinogen test accompany the development of chronic atrophic gastritis with increasing age.20, 21
The drastic decline in the prevalence of H. pylori infection by birth‐year can be explained by the change in sanitary conditions during childhood, when H. pylori infection is predominantly acquired. The main routes of transmission of H. pylori infection are known to be person‐to‐person transmission22, 23, 24, 25 and the waterborne route by drinking well water.4, 26, 27 In particular, close intrafamilial contact, including mother/parent‐to‐child, sibling‐to‐sibling, and spouse‐to‐spouse has been consistently demonstrated as a risk factor for transmission of H. pylori infection.1 In a population‐based study, the risk of H. pylori infection in children increased according to the number of positive parents when the number of the children was the same,25 and large sibship size was associated with increased risk of H. pylori infection in childhood.28 Similarly in Japan, family size and the number of older siblings were reported to show significant positive correlations with the risk of H. pylori infection during childhood.23 The number of live births and the number of persons per household decreased from the 1950s in Japan,29 which might be correlated with the decrease in the prevalence of H. pylori infection in our data. Drinking well water in childhood is reported to be another risk factor for H. pylori infection.4, 26 A previous study in Japan reported that the prevalence of H. pylori infection increased with longer period of drinking well water in childhood.30 There was rapid development of distribution of municipal water supply in Japan from 26.2% of households in 1950 to 80.8% of households in 1970.31 In those days, Japan experienced a drastic economic expansion that brought improvements in social infrastructure, including water supply. The rapid decline in the proportion of those with positive H. pylori antibody among those born between 1949 and 1961 in our study was possibly attributed to these drastic improvements in sanitary conditions.
H. pylori infection is a major cause of gastric cancer. The population attributable fraction (PAF) of H. pylori infection for gastric cancer incidence (the fraction of gastric cancer incident cases that is attributable to H. pylori infection) was estimated to be 84.1% from data in a Japanese cohort study.32 As a result of the high PAF, the trend in prevalence of birth‐year‐specific H. pylori infection would be closely linked to the trends in gastric cancer incidence and mortality rate in Japan. A previous age‐period‐cohort analysis of Japanese gastric cancer mortality showed that there was a cohort effect for the accelerated declining trend among those born after 1940.7, 33 This result is in accordance with our finding that the prevalence of H. pylori infection rapidly declined in those born after 1949.
There are some limitations to this study. First, our study subjects were all first‐visit outpatients at our hospital. To avoid selection bias, we excluded 336 gastric cancer patients and one MALT lymphoma patient as having high probability of a past history of H. pylori infection. As a result, our study subjects included 46.7% of patients who developed other cancers during the 1‐year follow‐up period and 53.3% of cancer‐free subjects who had mostly been referred for detailed examination of the possibility of cancer. Although there was little evidence of any association between H. pylori infection and cancer except gastric cancer and MALT lymphoma, the representativeness of our study subjects might have some vulnerability. Therefore, we re‐analyzed the prevalence of H. pylori infection in only cancer‐free subjects. As a result, the first joinpoint was in 1950, and the first and second BPC was −1.1% and −3.9%, respectively. This trend was similar to the result that was obtained when cancer subjects except for gastric cancer and MALT lymphoma subjects were included. Therefore, we considered that inclusion of cancer patients in our analysis would not lead to selection bias.
Second, as the recruiting period of the subjects was limited, there would be potential for contamination of the age‐effect in our study. However, as H. pylori infection is predominantly acquired in childhood, we thought that the impact of the age‐effect would be limited.
Third, the relatively low sensitivity and specificity of the serum H. pylori antibody titer kit and the pepsinogen test we used in this study might attenuate the accuracy of the prevalence. However, the limited accuracy would not influence the trend in prevalence of birth‐year‐specific H. pylori infection and the trend in birth‐year‐specific proportion of high‐risk individuals for gastric cancer as the accuracy is thought to be consistent regardless of the subjects’ age distribution.
Finally, three‐quarters of our study subjects consisted of those who lived in Aichi Prefecture (75.2%). A previous study revealed that there was geographic variation in the prevalence of H. pylori infection in Japan, and that it was relatively low in Aichi Prefecture.12 As this area has been one of the largest metropolitan areas in Japan, the spread of sanitary infrastructure, including water supply, is considered to have occurred earlier than in other areas in Japan. This condition might have shifted the first joinpoint, making a rapid declining trend towards older birth year in this study.
In summary, the prevalence of H. pylori infection in our study population decreased with increasing birth year. A dramatic decline in the prevalence of H. pylori infection was observed in those born between 1949 and 1961. As the PAF of H. pylori infection for gastric cancer is considerably large, this declining trend in prevalence of H. pylori infection would contribute to projection of the future trend in gastric cancer incidence in Japan. Fortunately, the Japanese government expanded national health insurance coverage for eradication therapy of H. pylori from patients with gastric/duodenal ulcer to those with chronic atrophic gastritis in February 2013. As this situation might have introduced a period effect to reduce the H. pylori‐infected populations, we need to continue monitoring the prevalence of H. pylori infection in the Japanese population.
Disclosure Statement
The authors have no conflict of interest.
Supporting information
Table S1. Prevalence of Helicobacter pylori infection and the proportion of high‐risk individuals for gastric cancer in each of the 13 birth cohorts.
Acknowledgments
The authors are grateful to the staff members of the Division of Epidemiology and Prevention at Aichi Cancer Center Research Institute for their assistance in this study. This study was supported in part by a Grant‐in‐Aid for Scientific Research on Priority Areas of Cancer (No. 17015018) and on Innovative Areas (No. 221S0001) from the Ministry of Education, Science, Sports, Culture and Technology of Japan, Health and Labor Science Research Grants on Research for Promotion of Cancer Control (Political Research for Cancer Control, H26‐political‐general‐019), FALCO Biosystems Ltd. Tokai‐chuo Laboratory, and Takeda Science Foundation.
Cancer Sci 106 (2015) 1738–1743
Funding Information
Ministry of Education, Science, Sports, Culture and Technology of Japan; Health, Labour and Welfare Ministry of Japan; FALCO Biosystems Ltd, Tokai‐chuo Laboratory; Takeda Science Foundation.
References
- 1. International Agency for Research on Cancer . IARC monographs on the evaluation of carcinogenic risks to humans. IARC Sci Publ 2012; 100B: 385–435. [PMC free article] [PubMed] [Google Scholar]
- 2. Uemura N, Okamoto S, Yamamoto S et al Helicobacter pylori infection and the development of gastric cancer. N Engl J Med 2001; 345: 784–9. [DOI] [PubMed] [Google Scholar]
- 3. Queiroz DM, Carneiro JG, Braga‐Neto MB et al Natural history of Helicobacter pylori infection in childhood: eight‐year follow‐up cohort study in an urban community in northeast of Brazil. Helicobacter 2012; 17: 23–9. [DOI] [PubMed] [Google Scholar]
- 4. Klein PD, Graham DY, Gaillour A, Opekun AR, Smith EO. Water source as risk factor for Helicobacter pylori infection in Peruvian children. Gastrointestinal Physiology Working Group. Lancet 1991; 337: 1503–6. [DOI] [PubMed] [Google Scholar]
- 5. Asaka M, Takeda H, Sugiyama T, Kato M. What role does Helicobacter pylori play in gastric cancer? Gastroenterology 1997; 113 (6 Suppl): S56–60. [DOI] [PubMed] [Google Scholar]
- 6. Yoshida T, Kato J, Inoue I et al Cancer development based on chronic active gastritis and resulting gastric atrophy as assessed by serum levels of pepsinogen and Helicobacter pylori antibody titer. Int J Cancer 2014; 134: 1445–57. [DOI] [PubMed] [Google Scholar]
- 7. Tanaka M, Ma E, Tanaka H, Ioka A, Nakahara T, Takahashi H. Trends of stomach cancer mortality in Eastern Asia in 1950–2004: comparative study of Japan, Hong Kong and Singapore using age, period and cohort analysis. Int J Cancer 2012; 130: 930–6. [DOI] [PubMed] [Google Scholar]
- 8. Katanoda K, Hori M, Matsuda T et al An updated report on the trends in cancer incidence and mortality in Japan, 1958–2013. Jpn J Clin Oncol 2015; 45: 390–401. [DOI] [PubMed] [Google Scholar]
- 9. Asaka M, Kimura T, Kudo M et al Relationship of Helicobacter pylori to serum pepsinogens in an asymptomatic Japanese population. Gastroenterology 1992; 102: 760–6. [DOI] [PubMed] [Google Scholar]
- 10. Shiota S, Murakami K, Fujioka T, Yamaoka Y. Population‐based strategies for Helicobacter pylori‐associated disease management: a Japanese perspective. Expert Rev Gastroenterol Hepatol 2010; 4: 149–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shiota S, Murakawi K, Suzuki R, Fujioka T, Yamaoka Y. Helicobacter pylori infection in Japan. Expert Rev Gastroenterol Hepatol 2013; 7 (1): 35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ueda J, Gosho M, Inui Y et al Prevalence of Helicobacter pylori infection by birth year and geographic area in Japan. Helicobacter 2014; 19: 105–10. [DOI] [PubMed] [Google Scholar]
- 13. Tajima K, Hirose K, Inoue M, Takezaki T, Hamajima N, Kuroishi T. A model of practical cancer prevention for out‐patients visiting a hospital: the Hospital‐based Epidemiologic Research Program at Aichi Cancer Center (HERPACC). Asian Pac J Cancer Prev 2000; 1: 35–47. [PubMed] [Google Scholar]
- 14. Hamajima N, Matsuo K, Saito T et al Gene‐environment interactions and polymorphism studies of cancer risk in the Hospital‐based Epidemiologic Research Program at Aichi Cancer Center II (HERPACC‐II). Asian Pac J Cancer Prev 2001; 2: 99–107. [PubMed] [Google Scholar]
- 15. Kikuchi S, Miwa H. Efficancy of direct ELISA kit “E Plate ‘Eiken’ H. pylori Antibody” on diagnosis of Helicobacter pylori infection, (in Japan). Jpn J Med Pharm Sci 2000; 43: 581–6. [Google Scholar]
- 16. Samloff IM, Varis K, Ihamaki T, Siurala M, Rotter JI. Relationships among serum pepsinogen I, serum pepsinogen II, and gastric mucosal histology. A study in relatives of patients with pernicious anemia. Gastroenterology 1982; 83 (1 pt 2): 204–9. [PubMed] [Google Scholar]
- 17. Ohata H, Kitauchi S, Yoshimura N et al Progression of chronic atrophic gastritis associated with Helicobacter pylori infection increases risk of gastric cancer. Int J Cancer 2004; 109: 138–43. [DOI] [PubMed] [Google Scholar]
- 18. Miki K, Ichinose M, Shimizu A et al Serum pepsinogens as a screening test of extensive chronic gastritis. Gastroenterol Jpn 1987; 22: 133–41. [DOI] [PubMed] [Google Scholar]
- 19. Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med 2000; 19: 335–51. [DOI] [PubMed] [Google Scholar]
- 20. Kimura K. Chronological transition of the fundic‐pyloric border determined by stepwise biopsy of the lesser and greater curvatures of the stomach. Gastroenterology 1972; 63: 584–92. [PubMed] [Google Scholar]
- 21. Asaka M, Sugiyama T, Nobuta A, Kato M, Takeda H, Graham DY. Atrophic gastritis and intestinal metaplasia in Japan: results of a large multicenter study. Helicobacter 2001; 6: 294–9. [DOI] [PubMed] [Google Scholar]
- 22. Watanabe Y, Kurata JH, Mizuno S et al Helicobacter pylori infection and gastric cancer. A nested case‐control study in a rural area of Japan. Dig Dis Sci 1997; 42: 1383–7. [DOI] [PubMed] [Google Scholar]
- 23. Ueda M, Kikuchi S, Kasugai T, Shunichi T, Miyake C. Helicobacter pylori risk associated with childhood home environment. Cancer Sci 2003; 94: 914–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Goodman KJ, Correa P. Transmission of Helicobacter pylori among siblings. Lancet 2000; 355: 358–62. [DOI] [PubMed] [Google Scholar]
- 25. Dominici P, Bellentani S, Di Biase AR et al Familial clustering of Helicobacter pylori infection: population based study. BMJ 1999; 319: 537–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hegarty JP, Dowd MT, Baker KH. Occurrence of Helicobacter pylori in surface water in the United States. J Appl Microbiol 1999; 87: 697–701. [DOI] [PubMed] [Google Scholar]
- 27. Sasaki K, Tajiri Y, Sata M et al Helicobacter pylori in the natural environment. Scand J Infect Dis 1999; 31: 275–9. [DOI] [PubMed] [Google Scholar]
- 28. Gao L, Weck MN, Raum E, Stegmaier C, Rothenbacher D, Brenner H. Sibship size, Helicobacter pylori infection and chronic atrophic gastritis: a population‐based study among 9444 older adults from Germany. Int J Epidemiol 2010; 39: 129–34. [DOI] [PubMed] [Google Scholar]
- 29. Ministry of Health LaW . Annual Reports on Health and Welfare (1998 edition). 1998; 1(1). ( http://www.mhlw.go.jp/toukei_hakusho/hakusho/kousei/1998/dl/03.pdf)
- 30. Karita M, Teramukai S, Matsumoto S. Risk of Helicobacter pylori transmission from drinking well water is higher than that from infected intrafamilial members in Japan. Dig Dis Sci 2003; 48: 1062–7. [DOI] [PubMed] [Google Scholar]
- 31. Water Supply Division, Health Service Bureau, Ministry of Health Laboura and Welfare. [Cited Coverage of the water supply system] Available from URL: http://www.mhlw.go.jp/file/06-Seisakujouhou-10900000-Kenkoukyoku/0000077465.pdf.
- 32. Sasazuki S, Inoue M, Iwasaki M et al Effect of Helicobacter pylori infection combined with CagA and pepsinogen status on gastric cancer development among Japanese men and women: a nested case‐control study. Cancer Epidemiol Biomarkers Prev 2006; 15: 1341–7. [DOI] [PubMed] [Google Scholar]
- 33. Ito Y, Ioka A, Nakayama T, Tsukuma H, Nakamura T. Comparison of trends in cancer incidence and mortality in Osaka, Japan, using an age‐period‐cohort model. Asian Pac J Cancer Prev 2011; 12: 879–88. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Prevalence of Helicobacter pylori infection and the proportion of high‐risk individuals for gastric cancer in each of the 13 birth cohorts.


