Abstract
Regional lymph node metastasis in head and neck squamous cell carcinoma (HNSCC) is a crucial event for its progression, associated with a high rate of mortality. Sialidase, a key enzyme for the regulation of cellular sialic acids through catalyzing the initial step of degradation of glycoproteins and glycolipids, has been implicated in cancer progression. To facilitate the development of novel treatments for HNSCC, we have investigated whether sialidase is involved in the progression of this cancer. We found plasma membrane‐associated sialidase (NEU3) to be significantly upregulated in tumor compared to non‐tumor tissues; particularly, an increase in its mRNA levels was significantly associated with lymph node metastasis. To understand the mechanisms, we analyzed the NEU3‐mediated effects on the malignant phenotype using squamous carcinoma HSC‐2 and SAS cells. NEU3 promoted cell motility and invasion, accompanied by the increased expression of MMP‐9, whereas NEU3 silencing or the activity‐null mutant did not. NEU3 enhanced phosphorylation of epidermal growth factor receptor (EGFR), and an EGFR inhibitor, AG1478, abrogated the NEU3‐induced MMP9 augmentation. These findings identify NEU3 as a participant in HNSCC progression through the regulation of EGFR signaling and thus as a potential target for inhibiting EGFR‐mediated tumor progression.
Keywords: Epidermal growth factor receptor, head and neck squamous cell carcinoma, lymph node metastasis, MMP9, sialidase
Head and neck squamous cell carcinoma (HNSCC) includes epithelial malignancies of the oral cavity, pharynx, and larynx, and is the sixth most common cancer worldwide.1, 2 Despite extensive advances in research and treatment, the 5‐year overall survival rates has not improved significantly in the last 50 years. Head and neck squamous cell carcinoma is characterized by a highly invasive and metastatic malignancy, and the high mortality rate is attributed to metastatic diseases at regional and distant sites. A more detailed analysis of the molecular mechanisms underlying its invasiveness is required for the development of novel treatment strategies. To improve HNSCC treatment, we have attempted to identify the molecules involved in its progression.
Alteration in glycosylation is a characteristic feature of tumorigenesis, and aberrant sialylation, in particular, has been implicated in the malignant phenotype with reference to metastatic potential and invasiveness.3, 4 Sialidases are key enzymes for the control of cellular sialic acid contents, by catalyzing the removal of sialic acid residues from their carbohydrate portions. Among the four types of human sialidases so far identified (NEU1–NEU4), which differ in major subcellular localization and enzymatic properties, as well as in positioning on the chromosomes,5 the plasma membrane‐associated sialidase NEU3 appears to play particular roles in controlling transmembrane signaling by the modulation of gangliosides,6 and its aberrant expression is closely related to the pathogenesis of cancer.7 We previously shown that NEU3 is upregulated in tumor compared to adjacent non‐tumor tissues in colon, renal, prostate, and ovarian cancers.8, 9, 10, 11 NEU3 enhances cancer cell survival,8, 12 cell migration and attachment,13 and actually enhances the epidermal growth factor (EGF)‐stimulated tyrosine phosphorylation of EGF receptor (EGFR).12
In the present study, we investigated whether any form of human sialidase is involved in the progression of HNSCC by determining the sialidase expression in surgical specimens. We found that plasma membrane‐associated sialidase NEU3 was markedly upregulated in cancer tissues, especially in association with lymph node metastasis, so it is probably a molecule that promotes metastasis through EGFR signaling activation. Targeting the sialidase may thus be a therapeutic approach for this cancer.
Materials and Methods
Patient samples
Surgical specimens were obtained from 30 consecutive HNSCC patients from April 1999 to October 2000 who underwent radical therapy at Miyagi Cancer Center Hospital. Informed consent was obtained from each patient to allow the use of portions of tissue for research purposes, and the study was approved by the Committee on Human Rights in Research at Miyagi Cancer Center (Natori, Japan). The clinical characteristics of these patients are listed in Table 1. The stage grouping and the TNM system of the patients were defined according to the International Union Against Cancer (UICC; 1997).
Table 1.
Clinical characterization of head and neck squamous cell carcinoma patients (n = 30)
| Age, years/gender | Tumor site | Stage | TNM classification | |
|---|---|---|---|---|
| 1 | 74/M | Hypopharynx | IV | T4N2cMO |
| 2 | 60/F | Tongue | III | T2N1M0 |
| 3 | 61/M | Tongue | IV | T3N2bM0 |
| 4 | 61/M | Hypopharynx | IV | T3N2bM0 |
| 5 | 50/M | Gingiva | IV | T4N2bM0 |
| 6 | 27/M | Mesopharynx | IV | T3N2bM0 |
| 7 | 68/M | Tongue | IV | rT4N0M0 |
| 8 | 67/F | Tongue | II | T2N0M0 |
| 9 | 80/M | Larynx | IV | T4N0M0 |
| 10 | 46/M | Tongue | IV | rN3M0 |
| 11 | 61/M | Mesopharynx | II | T2N0M0 |
| 12 | 48/M | Mesopharynx | IV | T3N2bM0 |
| 13 | 67/M | Hypopharynx | IV | T4N2M0 |
| 14 | 55/M | Mesopharynx | IV | T2N2bM0 |
| 15 | 70/F | Hypopharynx | IV | T2N2bM0 |
| 16 | 56/M | Maxilla | III | T3N0M0 |
| 17 | 85/M | Lip | IV | T3N2cM0 |
| 18 | 48/M | Larynx | I | rT1bNOM0 |
| 19 | 65/M | Tongue | III | T2N1M0 |
| 20 | 58/M | Gingiva | IV | T4N2bM0 |
| 21 | 63/M | Tongue | II | T2N0M0 |
| 22 | 77/M | Tongue | III | T3N1M0 |
| 23 | 76/M | Larynx | IV | T4N2cM0 |
| 24 | 52/F | Tongue | I | T1N0M0 |
| 25 | 48/M | Maxilla | IV | T4N2cM0 |
| 26 | 56/F | Tongue | I | T1N0M0 |
| 27 | 64/M | Mesopharynx | IV | T3N2bM0 |
| 28 | 62/M | Larynx | IV | T4N0M0 |
| 29 | 64/M | Mesopharynx | IV | rT2N0M0 |
| 30 | 72/M | Mesopharynx | IV | T2N2bM0 |
Cell culture
Oral squamous cell carcinoma HSC‐2 and SAS cells were obtained from the Cell Resource Center for Biomedical Research, Tohoku University (Sendai, Japan). Cells were cultured in DMEM supplemented with 10% FBS (Invitrogen, Grand Island, NY, USA) at 37°C in a 5% CO2 atmosphere.
Antibodies
Antibodies for phospho‐EGFR (Y‐845), phospho‐ERK, and ERK, from Cell Signaling Technology (Danvers, MA, USA), EGFR from Santa Cruz Biotechnology (Santa Cruz, CA, USA), and a monoclonal anti‐NEU3, prepared as described previously,14 were used in immunoblotting analysis.
Quantitative RT‐PCR analysis
Real‐time PCR was carried out according to the methods described previously.12 The sequence primers are listed in Table S1. The expression of glyceraldehyde‐3‐phosphate dehydrogenase was determined as an internal control.
Plasmids, siRNA, and transfection
Sialidase expression vectors were constructed by subcloning NEU3 cDNA into an expression vector pCAGGS vector. Transient cDNA transfection was accomplished using FuGENE (Promega, Madison, WI, USA) for HSC‐2 and SAS cells. For the NEU3 silencing, specific siRNA synthesized by Dharmacon (Lafayette, CO, USA) as described12 was transfected using RNAiMAX (Invitrogen), and its efficiency was evaluated by RT‐PCR.
Sialidase activity assay
Cell homogenates and the particulate fractions of tissue homogenates were prepared and assayed for sialidases NEU1 and NEU3 as described previously.8 Briefly, for the assays, NEU1 sialidase activity was evaluated with synthetic substrate 4‐methylumbelliferyl‐neuraminic acid (4MU‐NeuAc) at pH 4.6 at 37°C for 30–60 min, and the 4‐methylumbelliferone released was determined fluorometrically. NEU3 activity was assayed with GM3 gangliosides as a substrate in the presence of 0.1% Triton X‐100. The assays with the tissue particulates as the enzyme source were determined by the thiobarbituric acid method after passing through an AG1X‐2 minicolumn. One unit was defined as the amount of enzyme that cleaved 1 nmol sialic acid/h. Protein concentrations were determined by dye‐binding assay (Bio‐Rad Laboratories, Hercules, CA, USA).
Immunoblotting
Cells were treated with or without EGF (100 ng/mL), washed with PBS and lysed in cold lysis buffer (50 mM HEPES [pH 7.5], 150 mM NaCl, 1% Nonidet P40, 2 mM EDTA, 7.5 μg/mL aprotinin, 10 μg/mL leupeptin, 10 mM NaF, 2 mM orthovanadate, and 2 mM PMSF). After centrifugation (12 000 g for 15 min), cellular lysates were subjected to SDS‐PAGE and immunoblotting. For EGFR inhibition, the cells were treated with 10 μM of specific inhibitor AG1478 (Calbiochem, La Jolla, CA, USA).
Immunohistochemistry
Removed tissues were fixed in 10% neutral buffered formaldehyde for 3 days, routinely processed for embedding in paraffin, and sectioned at a thickness of 2.5 mm. The sections were incubated with anti‐monoclonal NEU3 antibody.
Gelatin zymographic assay
The levels of gelatinases, MMP2 and MMP9, were measured by zymographic assay. Cells were cultured with serum‐free medium for 16 h, and the conditioned medium collected was mixed with SDS buffer without reducing reagent. After SDS‐PAGE on gels containing 0.1% gelatin (Sigma‐Aldrich, St. Louis, MO, USA), the gels were washed with 2.5% Triton X‐100 in Tris‐HCl (pH 8.0), incubated with Tris‐HCl (pH 8.0) containing 0.5 mM CaCl2 and 1 mM ZnCl2 at 37°C for 16 h, and then stained with 0.1% Coomassie R‐250 (Bio‐Rad Laboratories). Proteins with gelatinolytic activity were visualized as clear zones in an otherwise blue gel.
Cell motility and invasion assays
The assays for cell motility and invasion were carried out as previously described.15 Cell motility assays were carried out with cell culture inserts (Corning, Tewksbury, MA, USA). At 24 h after transfection, cells were seeded at 2.5 × 105/well onto their upper surface membranes and the lower chambers were filled with medium containing 10% FBS. After 24 h the cells were fixed and stained with Wright–Giemsa solution and all those present on the lower surfaces of the membranes were counted under a microscope. For the assay of invasive potential, 1 × 106 cells were incubated for 24 h with Biocoat Matrigel Invasion Chambers (Corning).
Thin‐layer chromatography
Glycolipids were extracted from cells as described elsewhere,9 fractionated by thin‐layer chromatography on high‐performance thin‐layer chromatography plates (Merck, Darmstadt, Germany) and visualized with orcinol–H2SO4.
Statistical analysis
Results are expressed as mean ± SD. All values were compared using Student's t‐test.
Results
Increased expression of sialidase NEU3 is associated with lymph node involvement
Among the four human sialidases, NEU1, NEU3, and NEU4 generally show evident expression,5 whereas the level of NEU2 is extremely low and hardly detectable.16 NEU4 expression is especially strong in liver, colon, and brain, but weak in other tissues,17, 18 as assessed by quantitative real‐time RT‐PCR. On the basis of these previous studies, we determined the expression levels of major sialidases, NEU3 and NEU1, by RT‐PCR. Figure 1(a,b) shows the results of NEU3 and NEU1 expression in matched tumor (T) and adjacent non‐tumor (N) mucosa as the T/N ratio. Increases in NEU3 and NEU1 mRNA levels in tumor compared with non‐tumor tissue were detected in 22 (73%) and 18 (60%) in 30 cases, with T/N ratios of 6.67 ± 6.79 and 3.11 ± 3.94, respectively. According to the clinical features of the HNSCC patients, T/N ratios for NEU3 and NEU1 were analyzed, as shown in Table 2. Interestingly, a statistically significant difference in NEU3 mRNA level was observed between cases of lymph node metastasis N0 and N1–N3 (P = 0.019), indicating a close association between NEU3 expression and lymph node metastasis. To confirm whether the sialidase activity level changes in association with the metastasis, the activity assays were carried out using ganglioside GM3 for NEU3, and 4MU‐NeuAc for NEU1 as the substrate, although the values do not exactly represent the activity of each sialidase type because of substrate redundancy among the sialidases. As shown in Figure 1(c,d), the activity levels were compared between tumor and adjacent non‐tumor mucosa in 23 of the above 30 cases. The activities of putative NEU3 and NEU1 were significantly higher in the tumor tissues than in mucosa (T/N ratios, 4.01 ± 3.92 and 2.26 ± 1.86, respectively), although there was no statistically significant correlation with the clinical features, including lymph node involvement, for the activity levels of NEU3 and NEU1. This may be attributable to the involvement of some NEU4‐dependent activity that could have been included, despite such activity being expected to be low, because NEU4 can act on both GM3 and 4MU‐NeuAc.
Figure 1.
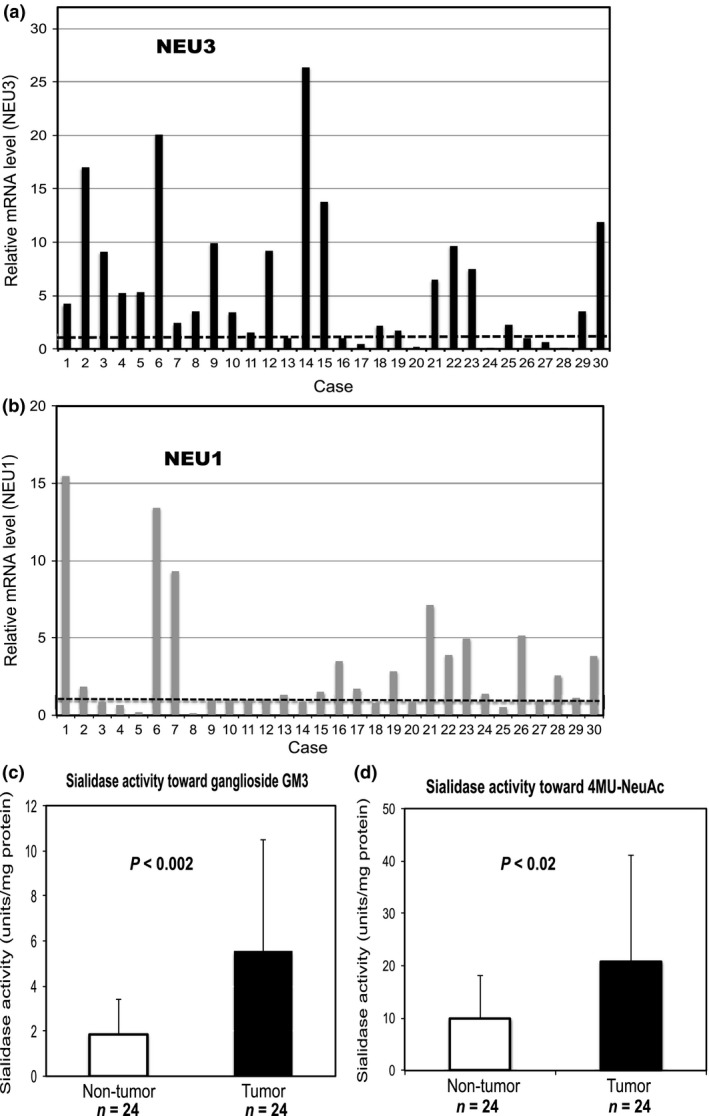
Sialidase expression in matched tumor and adjacent non‐tumor mucosa samples in head and neck squamous cell carcinoma. (a,b) Increased sialidase NEU3 (a) and NEU1 (b) mRNA levels in tumor compared with non‐tumor mucosa. The endogenous levels were determined by quantitative RT‐PCR. Dotted lines indicate a tumor/non‐tumor ratio of one in each graph. (c) Increased sialidase activity toward ganglioside GM3 as the substrate in tumor compared with non‐tumor mucosa (5.36 ± 5.12 and 1.84 ± 1.57 units/mg protein, respectively; P = 0.0028). (d) Increased sialidase activity toward 4MU‐NeuAc as the substrate in the tumor compared to those in non‐tumor mucosa (21.08 ± 20.79 and 10.15 ± 8.33 units/mg protein, respectively; P = 0.024). For evaluation of the apparent NEU3 and NEU1 activities, sialidase assays were carried out using ganglioside GM3 and 4MU‐NeuAc, respectively.
Table 2.
Clinical features of head and neck squamous cell carcinoma patients and association to sialidase NEU3 mRNA level in lymph node metastasis
| TNM category | No. of patients | T/N ratio | P‐value | |
|---|---|---|---|---|
| NEU3 | ||||
| Total | 30 | 6.67 ± 6.79 | ||
| Stage | I–II | 6 | 2.96 ± 4.04 | 0.055 |
| III–IV | 24 | 6.88 ± 6.82 | ||
| Tumor size | T1–T2 | 12 | 7.36 ± 7.88 | 0.580 |
| T3–T4 | 17 | 6.06 ± 6.53 | ||
| Lymph node metastasis | N0 | 11 | 2.81 ± 2.89 | 0.019a |
| N1–N3 | 19 | 7.79 ± 7.17 | ||
| NEU1 | ||||
| Total | 30 | 3.11 ± 3.94 | ||
| Stage | I–II | 6 | 2.61 ± 2.59 | 0.470 |
| III–IV | 24 | 3.23 ± 4.20 | ||
| Tumor size | T1–T2 | 12 | 2.17 ± 2.03 | 0.190 |
| T3–T4 | 17 | 3.16 ± 4.05 | ||
| Lymph node metastasis | N0 | 11 | 3.01 ± 2.84 | 0.490 |
| N1–N3 | 19 | 3.16 ± 4.45 | ||
Significant difference was observed between lymph node metastasis N0 and N1–N3 in sialidase NEU3 mRNA level.
To verify the marked expression of NEU3, immunohistochemical staining was then carried out with anti‐NEU3 mAb. The tumor tissues of 10 cases having strong NEU3 mRNA expression were chosen for the analysis. As shown in Figure 2, the representative results of immunohistochemical analyses indicated marked expression of NEU3, with strong staining in tumor tissues, but almost no expression in non‐tumor tissues. All of the 10 cases examined gave similar patterns. These results together show that sialidase NEU3 was significantly increased in terms of its mRNA level as well as its activity and protein levels in tumor tissues compared with those in the mucosa; in particular, it had strong association with lymph node involvement. We then focused on NEU3 in terms of its relationship to lymph node metastasis.
Figure 2.
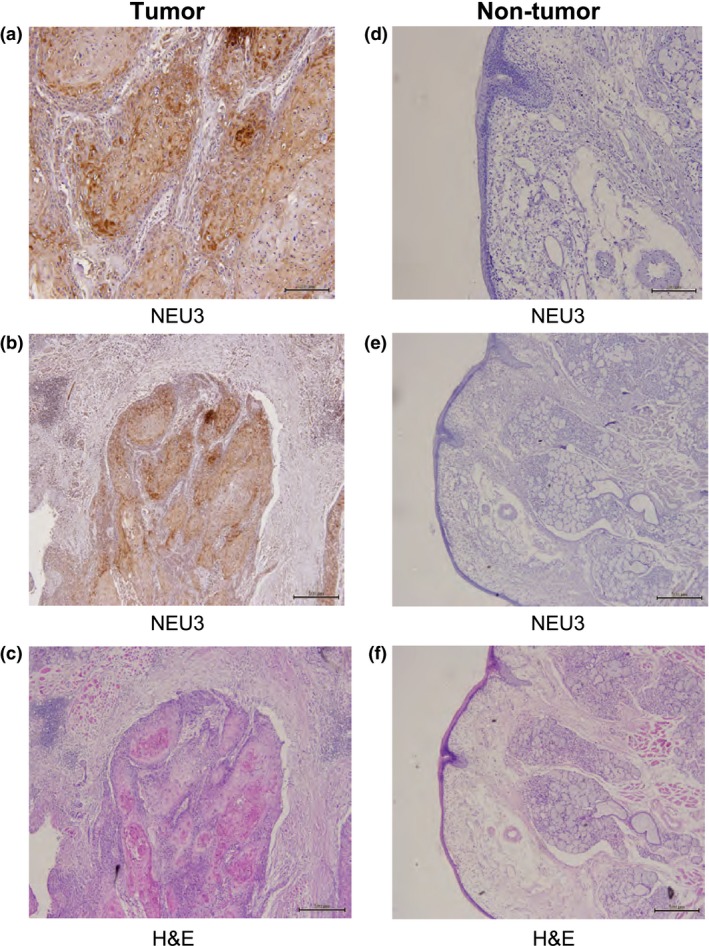
Immunohistochemistry of tumor and non‐tumor tissues from head and neck squamous cell carcinoma patients with anti‐NEU3 mAb. Sections from tumor and non‐tumor tissues were stained with anti‐NEU3 mAb (a,b,d,e) and H&E (c,f). Bar = 200 μm (a,d), and 500 μm (b,c,e,f).
Upregulation of NEU3 enhances cell migration and invasion in squamous cell carcinoma
To elucidate the role of NEU3 upregulation in HNSCC, oral squamous cell carcinoma HSC‐2 and SAS cells were used to overexpress or silence the NEU3 gene. Endogenous expression of the four sialidases in the cells was first evaluated by quantitative RT‐PCR (Fig. 3a). To compare the levels among these forms, a standard curve for each cDNA was generated by serial dilution of the pBluescript vector containing the gene encoding the entire ORF, as described previously.18 As expected, NEU2 was not detectable and the level of NEU4 was extremely low, being at most only one‐thousandth of the levels of NEU1 and NEU3 in these cells, suggesting little involvement of NEU2 and NEU4 in the increased sialidase expression in the carcinomas. It should be noted here that the carcinoma cells showed an NEU3 level of approximately one‐quarter that of NEU1, probably due to its upregulation in carcinogenesis, because the NEU3 level is generally one‐tenth to one‐twentieth that of NEU1 in normal tissues.
Figure 3.
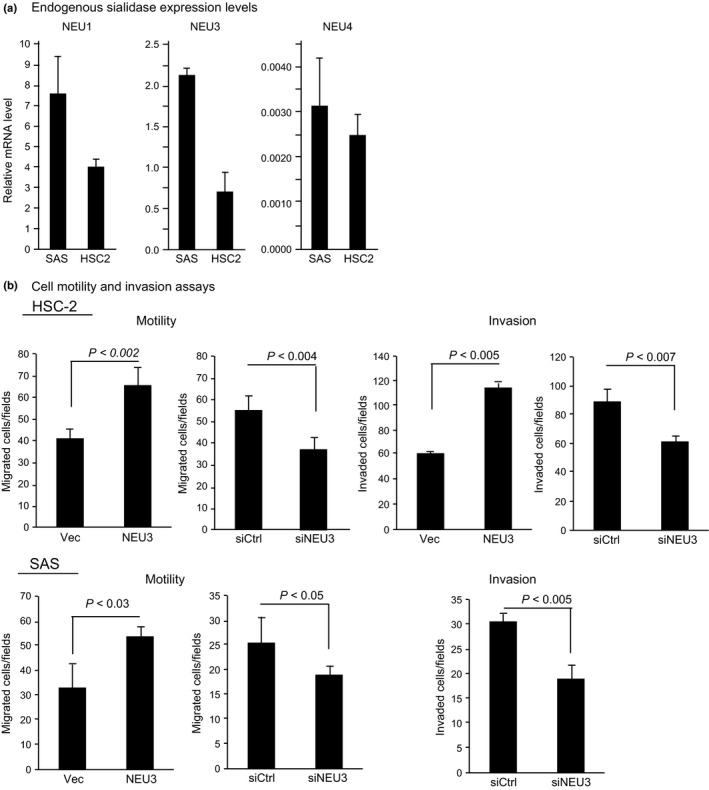
Regulation of cell motility and invasion by sialidase NEU3 in squamous cell carcinoma HSC‐2 and SAS cells. (a) Evaluation of endogenous sialidase mRNA levels in the two carcinoma cell lines by quantitative RT‐PCR. (b) Enhanced cell motility and invasion in NEU3 transiently transfected cells (NEU3) and its suppression in NEU3 silenced cells (siNEU3). The sialidase activities with GM3 substrate were 158.7 ± 49.6 and 2.5 ± 1.1 units/mg protein for HSC‐2 cells and 138 ± 37 and 5.5 ± 2.1 units/mg protein for SAS cells in NEU3‐transfected cells (30–40% transfection efficiency assessed by simultaneous transfection with GFP‐tagged cDNA) and vector control cells (Vec), respectively. The efficiency levels of NEU3 silencing were 78% and 85% in terms of mRNA in HSC‐2 and SAS cells, respectively. siCtrl, control siRNA.
To determine the relationship between NEU3 upregulation and the invasive phenotype of the two squamous cell carcinoma cells, cell migration and invasiveness were determined (Fig. 3b). NEU3 transient transfection yielded an increase of GM3 hydrolyzing activity by approximately 60‐ and 25‐fold in HSC‐2 and SAS cells, respectively, and apparently enhanced the cell motility compared with the vector control in both these cells. In contrast, its silencing resulted in a significant decrease of cell motility in HSC‐2 and SAS cells (78% and 85% reduction in mRNA levels, respectively). For the invasion assay, both HSC‐2 and SAS cells showed decreased invasiveness after NEU3 silencing. Cell proliferation ability was hardly changed by NEU3 alteration in these cells (data not shown).
NEU3 alters MMP expression through EGFR signaling
To understand the mechanism by which NEU3 affects cell motility and invasion, we then studied the expression of several genes associated with these cellular phenotypes, including epithelial–mesenchymal transition and MMP genes in HSC‐2 and SAS cells, by RT‐PCR. Despite lacking statistical significance, E‐cadherin expression levels showed a tendency to decrease with NEU3 overexpression and, in contrast, a tendency to increase with NEU3 silencing in HSC‐2 cells. N‐cadherin expression was clearly reduced in NEU3‐silenced cells (Fig. 4a, upper). In SAS cells, NEU3 significantly increased, and its silencing decreased N‐cadherin levels (Fig. 4a lower). However, the alteration of NEU3 expression did not show any particular changes in cell morphology, nor constant influence on the gene expression of Snail, Twist, or Vimentin in these cells. As a number of reports have described that MMP is essential to the invasiveness of HNSCC,19, 20 the expression of membrane‐type 1 (MT1)‐MMP, MMP2, and MMP9 was also examined in these cells (Fig. 4b). Although NEU3 overexpression enhanced only MMP9 expression, silencing of NEU3 caused significant decreases in both MMP2 and MMP9, and no change in MT1‐MMP, in both HSC‐2 and SAS cells, indicating that the alteration of NEU3 expression profoundly influences MMP expression. To define the mechanism behind the NEU3‐mediated effect on MMP in more detail, we examined the impact of EGF on MMP expression, because EGFR activation has been found to occur with NEU3 upregulation12 and also to be the most common genetic event in oral squamous cell carcinoma1, 21, 22 causing MMP stimulation.23, 24 Figure 5(a) shows that NEU3 silencing downregulated the expression of MMP9 and, in particular, caused a marked decline in its EGF‐induced upregulation in both carcinoma cell lines, whereas MMP2 expression was hardly affected by EGF addition (data not shown). On the basis of these results, MMP9 expression was evaluated in terms of whether it is influenced by EGFR activation. Consistent with these findings on mRNA levels, gelatin zymography analyses confirmed that MMP9 secretion was increased in a manner dependent on EGF and was decreased by NEU3 silencing (Fig. 5b). Next, EGFR signaling was investigated in these cells. Figure 5(c) shows that NEU3 enhanced the phosphorylation of EGFR and subsequent activation of ERK and Akt in HSC‐2 cells, whereas NEU3 silencing in SAS cells resulted in the opposite effect. Furthermore, NEU3‐mediated elevation of MMP9 was abrogated by EGFR inhibitor AG1478 in HSC‐2 and SAS cells, indicating the involvement of NEU3 in the regulation of MMP expression, probably through EGFR signaling (Fig. 5d). It should be noted here that, even without EGF, NEU3 slightly but significantly elevated MMP9 expression in both cells, which is likely to be attributed to NEU3‐mediated activation of the EGFR pathway under the same conditions, as shown in Figure 5(c).
Figure 4.
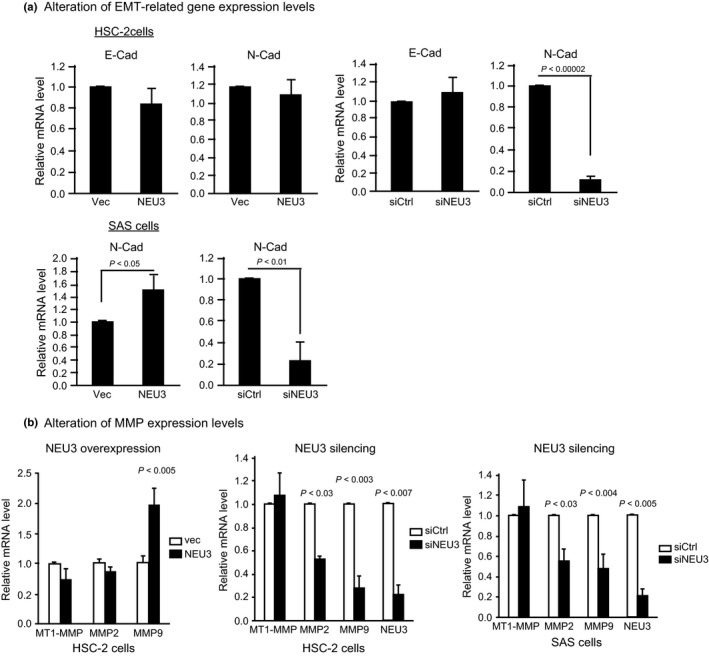
Regulation of epithelial–mesenchymal transition (EMT)‐related and MMP gene expression by NEU3 in squamous cell carcinoma HSC‐2 and SAS cells. (a) Alterations of E‐cadherin (E‐Cad) and N‐cadherin (N‐Cad) mRNA levels in NEU3‐overexpressing or NEU3‐silenced HSC‐2 cells. (b) Alterations of membrane‐type 1 (MT1)‐MMP, MMP2, and MMP9 mRNA levels in NEU3‐overexpressing HSC‐2 cells and in NEU3‐silenced HSC‐2 and SAS cells. siCtrl, control; Vec, vector.
Figure 5.
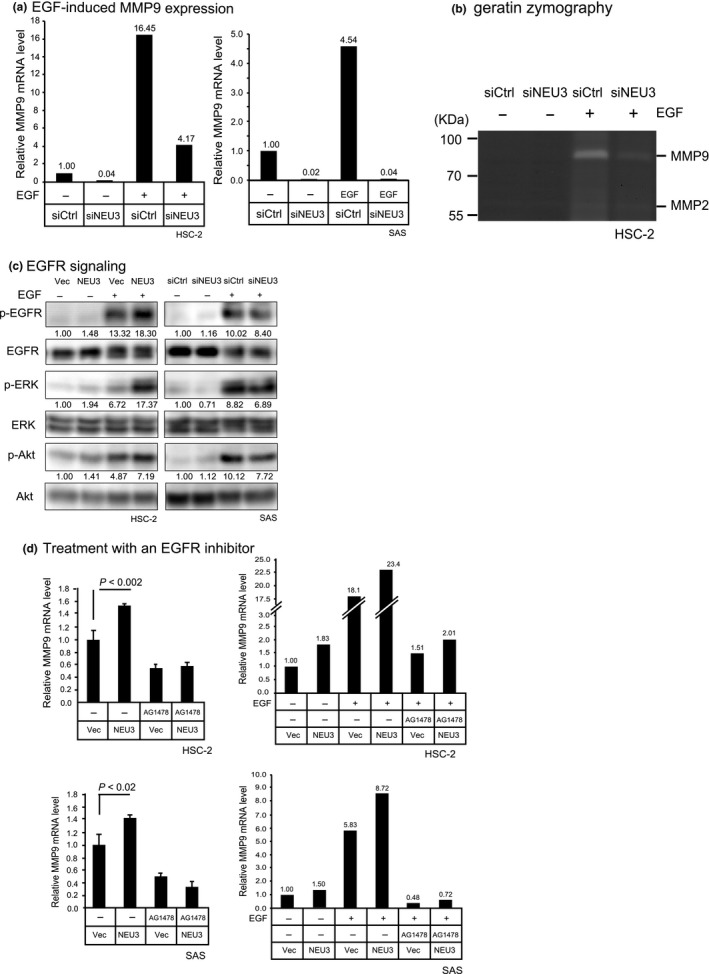
Involvement of NEU3 in epidermal growth factor (EGF)‐induced elevation of MMP9 expression in head and neck squamous cell carcinoma cells. (a) Attenuation of EGF‐induced MMP9 elevation in NEU3‐silenced HSC‐2 and SAS cells in terms of mRNA levels. (b) Attenuation of EGF‐induced MMP2 and MMP9 secretion and activity by NEU3 silencing in HSC‐2 cells, as assessed by gelatin zymography. (c) Enhanced phosphorylation of EGF receptor (EGFR), ERK, and Akt by NEU3 overexpression and its reduction by NEU3 silencing, as assessed by Western blotting. Each value shown under the blot represents a value relative to that in the vector control (Vec) without EGF. (d) Abrogation of NEU3‐mediated as well as EGF‐induced augmentation of MMP9 expression by EGFR inhibitor AG1478.
NEU3‐mediated enhancement of cell migration requires its catalytic activity
Human NEU3 is a ganglioside hydrolyzing sialidase5 and has recently been proved to activate EGFR signaling through its catalytic activity.25 Alterations of the cellular ganglioside pattern in NEU3‐overexpressing HSC‐2 cells were analyzed by thin‐layer chromatography. As shown in Figure 6(a), NEU3 overexpression resulted in a decrease in the glycolipid with similar mobility to GM3, a good substrate of NEU3, together with a slight increase in that with similar mobility to lactosylceramide, a product of NEU3, in HSC‐2 cells, as confirmed statistically by three independent experiments (Fig. 6a, lower). To verify whether the cell motility is altered by glycolipid changes, GM3 was then added exogenously to the chamber. Figure 6(b) shows the GM3 significantly reduced the cell motility, as expected. Although these glycolipid changes are generally observed in most cells by NEU3 overexpression,25 the activity‐null mutants did not show any change in glycolipids. In this context, we used one of these NEU3 mutants (Y370C) to assess the requirement for this molecule's activity for NEU3‐mediated increased cell migration. Compared with the cells transfected with wild‐type NEU3, the mutant did not enhance cell migration, similar to the control cells. These results suggest that NEU3‐dependent glycolipid changes induced by its catalytic action may be necessary for its enhancement of cell migration (Fig. 6c), although other mechanisms including the molecular interaction of NEU3 with EGFR25 or phosphatidic acid26 cannot be excluded in the cellular event.
Figure 6.
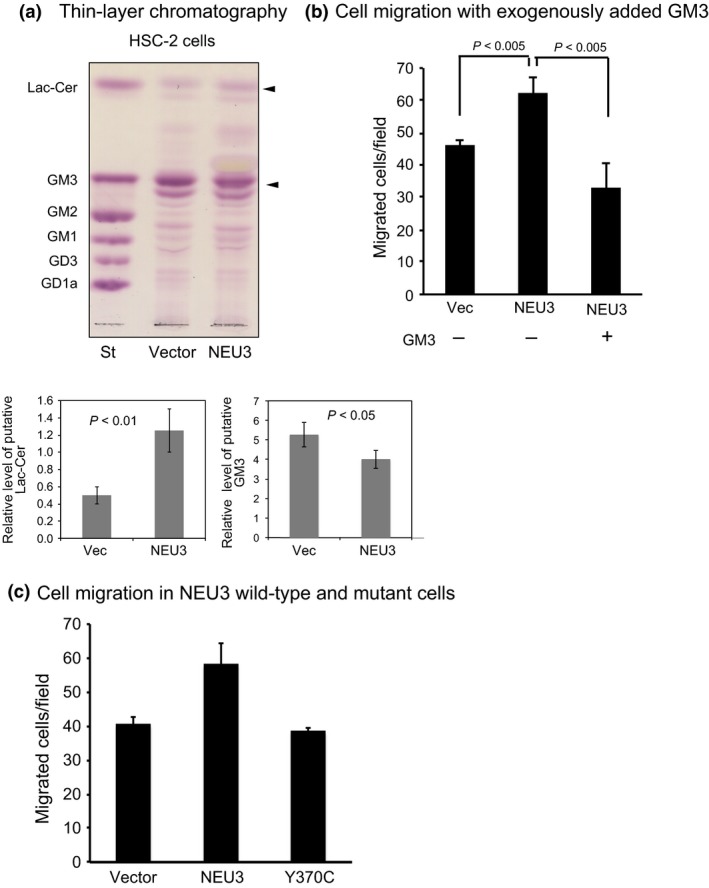
Requirement of NEU3 catalytic activity for the enhanced motility of HSC‐2 cells. (a) Analysis of altered glycolipids as a result of NEU3 catalytic reaction by thin‐layer chromatography. The relative changes in putative lactosylceramide (Lac‐Cer) and GM3 were quantified in independent experiments carried out in triplicate, as shown in the graphs below. St, standard gangliosides. (b) The effects of exogenously added GM3 on cell motility. The chambers were filled with medium containing GM3 at final concentrations of 20 μM, and the medium in lower chambers additionally contained 10% FBS. (c) Abrogation of NEU3‐mediated increase in cell motility in NEU3 mutant (Y370)‐transfected cells (sialidase activity, 1.9 ± 0.5 units/mg protein).
Discussion
Lymph node metastasis is a critical event in HNSCC progression. Identification of the molecules associated with metastasis would provide information to facilitate diagnosis and treatment of this cancer. Molecules involved in regulation of tumor metastasis have been focused on the ECM, such as fibronectin27 and laminin,28 and on their transmembrane matrix receptors, integrins.29 Cell surface glycoproteins including cadherin30 and neural cell adhesion molecule31 have also been thought to mediate the tissue‐specific recognition process that is impaired in tumor progression and metastasis. In addition to these glycoproteins, gangliosides, components of cell‐surface membranes, exert a wide variety of biological functions, including alterations associated with cell growth, differentiation, and tumorigenesis.4 Sialidases are key enzymes to regulate degradation of glycoproteins and gangliosides by the removal of sialic acid residues. In the present study, we investigated sialidase expression and found ganglioside‐specific sialidase NEU3 to be significantly upregulated in specimens of HNSCC patients with lymph node metastasis. NEU1 might also influence the malignant phenotype through degradation of glycoproteins, although there was no statistical significance in our findings. Our previous observations showed that NEU3 was upregulated in various human cancers and its involvement in the expression of malignant properties differed depending on the cancer types. However, the present study revealed a novel function of NEU3 in HNSCC, namely, participation in the regulation of MMP2 and MMP9 expression through activation of EGFR signaling, leading to enhanced cell invasion and migration.
Previous observations by others have identified the clinically correlated biomarkers, including EGFR and MMP9, as predictors of prognosis in HNSCC.1, 19, 20, 21, 22, 23, 24 Our results here show that NEU3 is essentially involved in regulation of the expression and activation of these genes upstream of EGFR. Aberrant upregulation of NEU3 in this carcinoma probably elevates the phosphorylation of EGFR and subsequently leads to ERK activation, followed by MMP activation and secretion, ending with enhanced cell invasion. It is of particular interest that NEU3 32 as well as EGFR, MMP2, and MMP9 33 are all target genes of transcription factors Sp1 and Sp3, which were initially considered as constitutive activators of housekeeping genes and other TATA‐less genes, but are now known to play crucial roles in regulating the transcription of genes involved in cell growth control and tumorigenesis.33 Therefore, the activation of EGFR signaling and consequent increased expression of MMP caused by NEU3 upregulation seem to influence each other in a positive feedback manner, because Sp1 and Sp3 can also be activated through Ras/ERK signals.
Consistent with our recent data,25 we showed that NEU3‐mediated activation of EGF signaling occurs in a manner dependent on the sialidase catalytic reaction, with the putative product lactosylceramide possibly affecting EGFR phosphorylation. Figure 6 reveals that abrogation of NEU3‐mediated enhancement of cell motility occurred in an NEU3 activity‐null mutant. In addition to the deletion of NEU3 activity, the present data provided evidence that NEU3 silencing reversed the malignant phenotype, probably due to accumulation of GM3, as evidenced by results of the experiment with exogenously added GM3. Chemoprevention trials with erlotinib or cetuximab aimed at EGFR inhibition have been encouraging but remain unclear.2 The present findings identify NEU3 as a pivotal molecule regulating the EGFR signaling pathway and MMP expression upstream of EGFR through ganglioside modulation, which is a signaling event closely associated with lymph node metastasis of HNSCC. Thus, NEU3 is a potential target for the development of cancer treatment.
Disclosure Statement
The authors have no conflict of interest.
Supporting information
Table S1. Primer sequences used in this study.
Acknowledgments
We are grateful to Mr. T. Wada (Miyagi Cancer Center) for his valuable technical assistance. This study was supported in part by Grants‐in‐Aid for Scientific Research on Innovative Areas (No. 23110002) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Cancer Sci 106 (2015) 1544–1553
Funding InformationThis study was supported in part by Grants‐in‐Aid for Scientific Research on Innovative Areas (No. 23110002) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- 1. Leemans CR, Braakhuis BJ, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev Cancer 2011; 11: 9–22. [DOI] [PubMed] [Google Scholar]
- 2. Dionne KR, Warnakulasuriya S, Zain RB, Cheong SC. Potentially malignant disorders of the oral cavity: current practice and future directions in the clinic and laboratory. Int J Cancer 2015; 136: 503–15. [DOI] [PubMed] [Google Scholar]
- 3. Lau KS, Dennis JW. N‐Glycans in cancer progression. Glycobiology 2008; 18: 750–60. [DOI] [PubMed] [Google Scholar]
- 4. Hakomori SI, Handa K. GM3 and cancer. Glycoconj J 2015; 32: 1–8. [DOI] [PubMed] [Google Scholar]
- 5. Miyagi T, Yamaguchi K. Mammalian sialidases: physiological and pathological roles in cellular functions. Glycobiology 2012; 22: 880–96. [DOI] [PubMed] [Google Scholar]
- 6. Miyagi T, Wada T, Yamaguchi K, Hata K, Shiozaki K. Plasma membrane‐associated sialidase as a crucial regulator of transmembrane signaling. J Biochem 2008; 144: 279–85. [DOI] [PubMed] [Google Scholar]
- 7. Miyagi T, Takahashi K, Hata K, Shiozaki K, Yamaguchi K. Sialidase significance for cancer progression. Glycoconj J 2012; 29: 567–77. [DOI] [PubMed] [Google Scholar]
- 8. Kakugawa Y, Wada T, Yamaguchi K et al Up‐regulation of plasma membrane‐associated ganglioside sialidase (Neu3) in human colon cancer and its involvement in apoptosis suppression. Proc Natl Acad Sci USA 2002; 99: 10718–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ueno S, Saito S, Wada T et al Plasma membrane‐associated sialidase is up‐regulated in renal cell carcinoma and promotes interleukin‐6‐induced apoptosis suppression and cell motility. J Biol Chem 2006; 281: 7756–64. [DOI] [PubMed] [Google Scholar]
- 10. Kawamura S, Sato I, W ada T et al Plasma membrane‐associated sialidase (NEU3) regulates progression of prostate cancer to androgen‐independent growth through modulation of androgen receptor signaling. Cell Death Differ 2012; 19: 170–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nomura H, Tamada Y, Miyagi T et al Expression of NEU3 (plasma membrane‐associated sialidase) in clear cell adenocarcinoma of the ovary: its relationship with T factor of pTNM classification. Oncol Res 2006; 16: 289–97. [DOI] [PubMed] [Google Scholar]
- 12. Wada T, Hata K, Yamaguchi K et al A crucial role of plasma membrane‐associated sialidase in the survival of human cancer cells. Oncogene 2008; 26: 2483–90. [DOI] [PubMed] [Google Scholar]
- 13. Kato K, Shiga K, Yamaguchi K et al Plasma membrane‐associated sialidase (NEU3) differentially regulates integrin‐mediated cell proliferation through laminin‐ and fibronectin‐derived signalling. Biochem J 2006; 394: 647–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang Y, Yamaguchi K, Wada T et al A close association of the ganglioside‐specific sialidase Neu3 with caveolin in membrane microdomains. J Biol Chem 2002; 277: 26252–9. [DOI] [PubMed] [Google Scholar]
- 15. Tokuyama S, Moriya S, Taniguchi SI, Yasui A, Miyazaki JI, Miyagi T. Suppression of pulmonary metastasis in mouse B16 melanoma cells. Int J Cancer 1997; 73: 410–5. [DOI] [PubMed] [Google Scholar]
- 16. Koseki K, Wada T, Hosono M et al Human cytosolic sialidase NEU2 – low general tissue expression but involvement in PC‐3 prostate cancer cell survival. Biochem Biophys Res Commun 2012; 428: 142–9. [DOI] [PubMed] [Google Scholar]
- 17. Monti E, Bassi MT, Bresciani R et al Molecular cloning and characterization of NEU4, the fourth member of the human sialidase gene family. Genomics 2004; 83: 445–53. [DOI] [PubMed] [Google Scholar]
- 18. Yamaguchi K, Hata K, Koseki K et al Evidence for mitochondrial localization of a novel human sialidase (NEU4). Biochem J 2005; 390: 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Katayama K, Bandoh N, Kishibe K et al Expression of matrix metalloproteinases in early‐stage oral squamous cell carcinoma as predictive indicators for tumor metastases and prognosis. Clin Cancer Res 2004; 10: 634–40. [DOI] [PubMed] [Google Scholar]
- 20. Liu Z, Li L, Yang Z et al Increased expression of MMP9 is correlated with poor prognosis of nasopharyngeal carcinoma. BMC Cancer 2010; 10: 270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhou Z, Temam S, Oh M et al Global expression‐based classification of lymph node metastasis and extracapsular spread of oral tongue squamous cell carcinoma. Neoplasia 2006; 8: 925–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sheu JJ, Hua CH, Wan L et al Functional genomic analysis identified epidermal growth factor receptor activation as the most common genetic event in oral squamous cell carcinoma. Cancer Res 2009; 69: 2568–76. [DOI] [PubMed] [Google Scholar]
- 23. Ohnishi Y, Lieger O, Attygalla M, Iizuka T, Kakudo K. Effects of epidermal growth factor on the invasion activity of the oral cancer cell lines HSC3 and SAS. Oral Oncol 2008; 44: 1155–9. [DOI] [PubMed] [Google Scholar]
- 24. Zuo JG, Zhu W, Li MY et al Activation of EGFR promotes squamous carcinoma SCC10A cell migration and invasion via inducing EMT‐like phenotype change and MMP‐9‐mediated degradation of E‐cadherin. J Cell Biochem 2011; 112: 2508–17. [DOI] [PubMed] [Google Scholar]
- 25. Yamamoto K, Takahashi K, Shiozaki K et al Potentiation of epidermal growth factor‐mediated oncogenic transformation by sialidase NEU3 leading to Src activation. PLoS ONE 2015; 10: e0120578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shiozaki K, Takahashi K, Hosono M et al Phosphatidic acid‐mediated activation and translocation to the cell surface of sialidase NEU3, promoting signaling for cell migration. FASEB J 2015; 29: 2099–111. [DOI] [PubMed] [Google Scholar]
- 27. Hynes RO, Yamada KM. Fibronectins: multifunctional modular proteins. J Cell Biol 1982; 95: 369–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Martin GR, Timpl R. Laminin and other basement membrane components. Annu Rev Cell Biol 1987; 3: 57–85. [DOI] [PubMed] [Google Scholar]
- 29. Hynes RO. Integrins: a family of cell surface receptors. Cell 1987; 48: 549–54. [DOI] [PubMed] [Google Scholar]
- 30. Takeichi M. The cadherins: cell–cell adhesion molecules controlling animal morphogenesis. Development 1988; 102: 639–55. [DOI] [PubMed] [Google Scholar]
- 31. Cunningham BA, Hemperly JJ, Murray BA, Prediger EA, Brackenbury R, Edelman GM. Neural cell adhesion molecules: structure, immunoglobulin‐like domain, cell surface modulation, and alternative RNA splicing. Science 1987; 236: 799–806. [DOI] [PubMed] [Google Scholar]
- 32. Yamaguchi K, Koseki K, Shiozaki M, Shimada Y, Wada T, Miyagi T. Regulation of plasma membrane‐associated sialidase NEU3 gene by Sp1/Sp3 transcription factors. Biochem J 2010; 430: 107–17. [DOI] [PubMed] [Google Scholar]
- 33. Wierstra I. Sp1: emerging roles – beyond constitutive activation of TATA‐less housekeeping genes. Biochem Biophys Res Commun 2008; 372: 1–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Primer sequences used in this study.


