Abstract
The present study attempted to identify T helper epitope long peptides capable of inducing cytotoxic T lymphocytes (CTL) from Lck antigen (p56Lck), the src family tyrosine kinase, which is known to be aberrantly expressed in metastatic cancers cells, in order to develop a long peptide‐based cancer vaccine for HLA‐A2+ cancer patients. Based on the biding motif to the HLA‐DR and HLA‐A2 alleles, 94 peptides were prepared from the Lck antigen. These peptides were screened for their reactivity to immunoglobulin G (IgG) from plasma of cancer patients, followed by testing of their ability to induce both CD4+ and CD8+ T lymphocytes showing not only peptide‐specific IFN‐γ production but cytotoxicity against HLA‐A2+ cancer cells from peripheral blood mononuclear cells (PBMC) of HLA‐A2+ cancer patients. Among 94 peptides tested, the three T helper epitope long peptides and their inner CTL epitope short peptides with HLA‐A2 binding motifs were frequently recognized by IgG of cancer patients, and efficiently induced both CD4+ IFN‐γ+ and CD8+ IFN‐γ+ T lymphocytes. Patients' PBMC stimulated with these long peptides showed cytotoxicity against HLA‐A2+ Lck+ cancer cells in HLA‐class I and HLA‐class II dependent manners. These three peptides might be useful for long peptide‐based vaccines for HLA‐A2+cancer patients with Lck+ tumor cells.
Keywords: Antitumor effector cells and their induction, T lymphocyte epitopes, tumor antigens, tyrosin kinese p56(lck), vaccination therapy
Recent advances in cancer immunotherapy revealed that immune checkpoint inhibitors induced durable clinical responses for at least one‐fifth of various types of advanced cancer patients.1, 2, 3 However, the clinical activity of immune checkpoint inhibitors was dependent on the presence of T lymphocytes at tumor sites. Therefore, clinical benefits could not be expected in cancer patients whose tumors had no or fewer tumor infiltrating lymphocytes. We previously reported that personalized peptide vaccination (PPV) rapidly induced CD45RO+ activated lymphocytes into tumor sites, and had clinical benefits in advanced prostate cancer patients and bladder cancer patients.4, 5, 6 These results suggest that sequential cancer therapy involving PPV followed by immune checkpoint inhibitors could be more efficacious than the monotherapy.
Although a large number of clinical trials have identified CTL epitope short peptides capable of inducing CD8+ cytotoxic T lymphocytes (CTL) in the past two decades, these vaccines have not yet been approved as cancer vaccines.7, 8 One of the reasons for this failure could be that these CTL‐directed short peptides rarely induce tumor‐specific T helper (Th) immunity,9 which is pivotal for the efficient eradication of solid tumors.9, 10, 11, 12, 13, 14 CD4+ Th lymphocytes facilitate the activation of TAA‐specific CTL, and are essential for the generation and maintenance of long‐lasting CD8+ T lymphocyte responses.10, 11, 12 Furthermore, CD4+ Th lymphocytes by themselves can eliminate both HLA‐Class II expressing tumor cells and non‐expressing tumor cells.13, 14 These results suggest that the cancer vaccine with the peptides capable of both tumor‐specific Th and CTL activity could be clinically effective as therapeutic cancer vaccines. However, the Th epitope peptides capable of inducing CTL activity have rarely been identified.15, 16
One of the appropriate TAA for this purpose could be the Lck antigen (p56Lck), the src family tyrosine kinase essential for T‐cell development and function, because it is aberrantly expressed in metastatic and invasive cancer cells, and contributes not only to the process of neoplastic transformation, but to the anchorage‐independent growth of TGF‐β‐initiated tumor cells through transcription of p56Lck with a type I promoter.17, 18, 19 Consequently, we tried herein to identify Lck‐derived Th epitope long peptides applicable for HLA‐A2+ cancer patients as a cancer vaccine.
Materials and Methods
Samples, cell lines and peptides
Peripheral blood mononuclear cells (PBMC; n = 21) and plasma samples (n = 32) were collected from patients with advanced cancer of various histological types using the method reported previously.6, 20, 21 The present study was approved by the Kurume University Ethical Committees: informed consent was obtained from all subjects prior to sample collection. The HLA‐A02:01/24:02 SW620 colorectal carcinoma, HLA‐A01:01/02:01 LNCaP and HLA‐A11:01/24:02 SQ‐1 lung squamous cell carcinoma cell lines were used in the present study.
To predict HLA class II (DR) binding of human Lck‐derived peptides, the amino acid sequence of the human Lck protein was analyzed by computer algorithm (MULTIPRED), as reported previously.22 For selection of CTL epitope short peptides included in these Lck‐derived HLA‐class II (DR) binding peptides, we also utilized the information of computer algorithm of Lck‐derived CTL epitope sequences presented by JLA‐A02:01. Information on the sequences presented by HLA‐A02:06 and HLA‐A02:07, which are found with relatively high frequency in the Japanese population, was also included. As a result, 94 peptides, consisting of the 44 Th epitope long peptides with HLA class II‐binding motifs and their 50 CTL epitope short peptides with HLA‐A02:01 binding motifs, were prepared (Table S1). These short peptides also possessed A02:06 and A02:07 binding motifs. These peptides had more than 90% purity and were purchased from Eurofins Operon (Tokyo, Japan).
IFN‐γ production, IFN‐γ ELISPOT, cytotoxicity and immunoglobulin assays
Lck‐derived peptides were directly used to stimulate PBMC in vitro for IFN‐γ production and IFN‐γ producing T‐cells induction assays. Purified antigen‐presenting cells were not added in this assay because we have previously reported this IFN‐γ producing T‐cell induction method, which was patented as a new technology.23 Monocytes and B lymphocytes in PBMC were considered to work as antigen‐presenting cells in this assay.
If it is possible to postulated that IFN‐γ production by CD4+ or CD8+ T lymphocytes was considered to be Lck peptide‐specific T lymphocytes capable of producing IFN‐γ after stimulation with the corresponding Lck peptide, the percentage of IFN‐γ producing CD4+ lymphocytes or IFN‐γ producing CD8+ T lymphocytes within the CD4+ or CD8+ T lymphocyte population was at least twice as high as that without peptide stimulation by means of flow cytometory. For the present study, FITC‐CD4 antibody, PE‐CD8 antibody and PE‐Cy7‐anti‐human IFN‐γ antibody were purchased from BD Pharmingen (San Diego, CA, USA).
Detection of the activity of long peptides to induce the relevant short peptide‐specific CTL was carried out according to a previously reported method with several modifications.23 PBMC (1 × 105 cells per well) were incubated with 10 μg/mL of each long peptide in quadruplicate in a U‐bottomed‐type 96‐well microculture plate (Nunc, Roskilde, Denmark) in 200 μL of culture medium. The culture medium consisted of 45% RPMI 1640, 45% AIM‐V medium (Life Technologies, Gaithersburg, MD, USA), 10% FCS, 20 units/mL of interleukin‐2 and 0.1 mmol/L MEM nonessential amino acid solution (Life Technologies). Every 3 or 4 days, half the culture medium was removed and replaced with new medium containing the corresponding peptide (20 μg/mL) and 20 units/mL interleukin‐2. On the 15th day of culture, the cultured cells were separated into four wells. Two wells were used for the culture with the corresponding peptide‐pulsed T2 cells, and the other two were used for the culture with HIV peptide‐pulsed T2 cells. After an 18‐h incubation, the supernatant was collected and the level of IFN‐γ was determined by ELISA.
An IFN‐ γ ELISPOT assay optimized to measure Lck‐specific CTL responses was performed according to the manufacturer's instructions as reported previously.14, 15, 16 Briefly, 0.5 million PBMC were stimulated with/without the indicated peptide (10 μg/mL) and cultured in OpTmizer T Cell Expansion SFM (Invitrogen, Carlsbad, CA, USA) containing 10% FBS (MP Biologicals, Solon, OH, USA) and IL‐2 (20 IU/mL; AbD Serotec, Kidlington, UK) for 4 days. After 4 days incubation at 37°C, PBMC were harvested, washed and seeded in four replicate wells at a density of 100 000 cells per well in a Multiscreen 96‐well plate (Millipore, Frankfurter Straβe, Germany) coated with an IFN‐γ capture antibody (Mabtech, Nacka Strand, Sweden, mAb‐1‐D1K). Further antibody incubations and development of the ELISPOT was done according to the manufacturer's instructions (Mabtech, mAb‐7‐B6‐1‐Biotin). Spots were counted by an ELISPOT reader (CTL‐ImmunoSpot S5 Series; Cellular Technology, Shaker Heights, OH, USA). Anti‐Human HLA‐ABC (eBiosciences, San Diego, CA, USA), Anti‐Human HLA‐DR, DP and DQ (BD Biosciences), and mouse IgG2a isotype control (eBiosciences) were used to determine the HLA molecules involved in antigen presentation.22, 24
Peptide‐stimulated PBMC were tested for their cytotoxicity against SW620, LNCaP or SQ‐1 tumor cells using a standard 6‐h 51Cr release assay as reported previously.6, 20, 21 Ten μg/mL of either anti‐HLA‐class I (W6/32: mouse IgG2a), anti‐HLA‐DR (L243: mouse IgG2a) or anti‐CD14 (H14: mouse IgG2a) monoclonal antibody (mAb), as an irrelevant control, was added into the wells at the initiation of the culture to determine the molecules involved in the cytotoxicity.
The levels of peptide‐specific IgG levels in plasma were measured by multiplex bead suspension array using the Luminex system (Luminex, Austin, TX, USA), as reported previously.25 In brief, 100 times diluted plasma was incubated with 100 μL of peptide‐coupled color‐coded beads for 1.5 h at 30°C. To detect IgG, after washing, the beads were incubated with 100 μL of biotinylated goat anti‐human IgG (gamma chain‐specific; Vector Laboratories, Burlingame, CA, USA) Ab for 1 h at 30°C. After washing, the beads were incubated with 100 μL of streptavidin‐PE (Life Technologies, Carlsbad, CA, USA) for 30 min at 30°C, followed by washing and detection of fluorescence intensity unit (FIU) on the beads using the Luminex system.
Statistical analysis
The statistical significance of the data was determined using a two‐tailed Student's t‐test. P‐values of less than 0.05 were considered to be statistically significant.
Results
Identification of long peptides capable of inducing both CD4+ IFN‐ γ+ and CD8+ IFN‐ γ+ lymphocytes
Ninety‐four Lck‐derived peptides selected on their binding motifs to the HLA‐DR and HLA‐A2 molecules were examined for their reactivity to IgG of plasma samples from advanced cancer patients (n = 32). IgG reactive to the Lck‐derived peptide was judged positive when the FIU in 1:100‐diluted plasma exceeded 50 FIU. The 17 peptides were more frequently reactive to the patients' IgG than the other 77 Lck‐derived peptides. Results for the 17 peptides are shown in Table 1.
Table 1.
IgG responses and induction of CD4+ IFN‐γ+ and CD8+IFN‐γ+ lymphocytes
| Peptide | HLA | IgG† | CD4+INF‐γ+ ‡ | CD8+INF‐γ+ ‡ |
|---|---|---|---|---|
| Positive (%) | ||||
| Lck33–47 | DR | 34 | 50 | 75 |
| Lck37–47 | A2 | 6 | 75 | 25 |
| Lck175–189 | DR | 44 | 25 | 75 |
| Lck177–186 | A2 | 38 | 50 | 75 |
| Lck190–204 | DR | 50 | 25 | 75 |
| Lck194–204 | A2 | 59 | 25 | 75 |
| Lck242–256 | DR | 44 | 50 | 25 |
| Lck246–254 | A2 | 50 | 25 | 75 |
| Lck289–303 | DR | 47 | 50 | 50 |
| Lck290–298 | A2 | 22 | 25 | 75 |
| Lck374–388 | DR | 34 | 25 | 50 |
| Lck375–383 | A2 | 34 | 50 | 75 |
| Lck377–384 | A2 | 16 | 75 | 50 |
| Lck414–428 | DR | 63 | 50 | 50 |
| Lck415–423 | A2 | 16 | 50 | 75 |
| Lck487–501 | DR | 47 | 75 | 50 |
| Lck489–497 | A2 | 63 | 50 | 75 |
†Positive percentages among the 32 patients are shown. ‡Positive percentages among four patients are shown.
The PBMC of the HLA‐A2+ cancer patients were then stimulated in vitro with each of these 17 peptides followed by examination of their IFN‐γ secretion. The IFN‐γ production was judged as positive when the percentage of CD4+ IFN‐γ+ or CD8+ IFN‐γ+ lymphocytes stimulated with the peptide exceeded that for the non‐peptide control sample. The Lck33–47 long peptide induced CD4+ IFN‐γ+ and CD8+ IFN‐γ+ lymphocytes from 2 and 3 of 4 patients tested, respectively (Table 1). Representative profiles of CD4+ IFN‐γ+ and CD8+ IFN‐γ+ lymphocytes are shown in the Figure 1. The inner Lck37–47 short peptide with HLA‐A2 binding motifs also induced CD4+ IFN‐γ+ and CD8+ IFN‐γ+ lymphocytes from 3 and 1 of 4 patients, respectively. Similarly, the Lck374–388 long peptide induced CD4+ IFN‐γ+ and CD8+ IFN‐γ+ lymphocytes from 1 and 2 of 4 patients, respectively. The two inner Lck375–383 and Lck377–384 short peptides induced CD4+ IFN‐γ+ and CD8+ IFN‐γ+ lymphocytes with higher frequencies (2 or 3 of 4 patients), respectively. Furthermore, the Lck487–501 long peptide induced CD4+ IFN‐γ+ and CD8+ IFN‐γ+ lymphocytes from 3 and 2 of 4 patients, respectively. The inner Lck489–497 short peptide also induced CD4+ IFN‐γ+ and CD8+ IFN‐γ+ lymphocytes from 2 and 3 of 4 patients, respectively. The other four long peptides could also induce CD4+ IFN‐γ+ and CD8+ IFN‐γ+ lymphocytes, but their positive percentages did not exceed any of the three Th epitope peptides shown above from the viewpoint of their own activity and the activity of the inner peptides with HLA‐A2 binding motifs.
Figure 1.
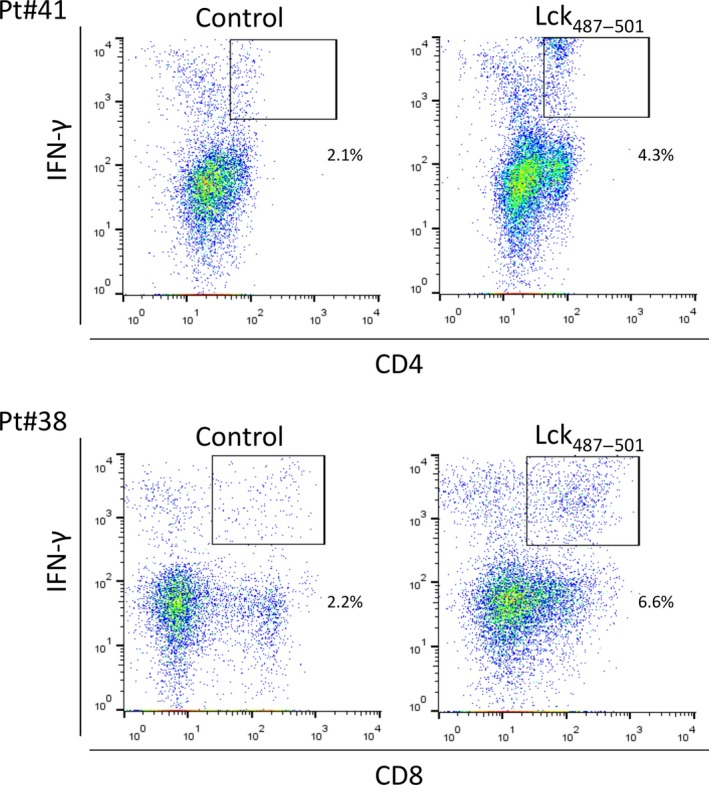
Induction of IFN‐γ producing T lymphocytes. For induction of IFN‐γ producing T cells, peripheral blood mononuclear cells (PBMC) of HLA‐A2+ cancer patients (n = 4) were stimulated in vitro with each of Lck‐derived peptides followed by staining with anti‐ CD4, CD8 and IFN‐γ antibodies. Representative profiles of CD4+ IFN‐γ+ and CD8+ IFN‐γ+ T lymphocytes are shown in this figure. Detailed results are given in the Results section.
The mean and SD of CD4+ IFN‐γ+ cells induced with Lck33–47 and Lck487–501 peptide in PBMC from four cancer patients were 3.2 ± 3.5 and 2.2 ± 2.1, respectively. There was only one positive response compared to the control in the case of Lck374–388, peptide stimulation. The mean and SD of CD8+ IFN‐γ+ cells induced with Lck33–47, Lck374–388 and Lck487–501 peptide were 10.2 ± 2.7, 6.5 ± 1.6 and 11.4 ± 7.0, respectively. Relatively large deviations were due to individual variations among patients.
Induction of peptide‐specific cytotoxic T lymphocytes to HLA‐A2+ cancer cells
The three long peptides were then tested for their ability to induce peptide‐specific CTL from the PBMC of HLA‐A2+ advanced cancer patients (n = 18). Peptide‐specific CTL were judged to have been successfully induced when P < 0.05 or there was a twofold difference in IFN‐γ spot counts as compared with the control. As a result, Lck33–47, Lck374–388 and Lck487–501 were found to have induced peptide‐reactive IFN‐γ producing T‐cells from the PBMC of 3 of 9 (33%), 7 of 14 (50%), and 7 of 9 (78%) of the HLA‐A2+ cancer patients tested, respectively (Table 2). In contrast, the peptide‐specific IFN‐γ producing T‐cells were not induced from the PBMC of either of the two HLA‐A2+ healthy donors tested (data not shown).
Table 2.
Induction of peptide‐specific IFN‐γ producing T‐cellsa
| ID | HLA | Genotype | Lck33–47 | Lck374–388 | Lck487–501 |
|---|---|---|---|---|---|
| Pt#12 | A2/A11 | 02:10/11:01 | N/A | 0 | 589 (41) |
| Pt#13 | A2/A33 | 02:06/33:03 | N/A | 15 (4) | 198 (4) |
| Pt#15 | A2/A24 | 02:07/24:02 | N/A | 378 (14) | 574 (14) |
| Pt#17 | A2/A24 | 02:01/24:02 | N/A | 0 | 0 |
| Pt#18 | A2/A24 | 02:06/24:02 | 429 (298) | 342 (298) | N/A |
| Pt#19 | A2/A24 | 02:06/24:02 | 20 (7) | 52 (7) | N/A |
| Pt#20 | A2/A2 | 02:01/02:06 | N/A | 291 (2) | 105 (2) |
| Pt#22 | A2/A26 | 02:01/26:01 | 0 | N/A | N/A |
| Pt#23 | A2/A31 | 02:06/31:01 | 0 | N/A | N/A |
| Pt#24 | A2/A26 | 02:01/26:01 | 73 (12) | 0 | N/A |
| Pt#26 | A2/A2 | 02:01/02:06 | 0 | 0 | N/A |
| Pt#27 | A2/A2 | 02:01/02:06 | 0 | N/A | N/A |
| Pt#28 | A2/A2 | 02:01/02:03 | 0 | 102 (40) | N/A |
| Pt#29 | A2/A24 | 02:01/24:02 | 0 | N/A | N/A |
| Pt#32 | A2/A24 | 02:06/24:02 | N/A | 0 | 0 |
| Pt#33 | A2/A24 | 02:06/24:02 | N/A | 0 | 523 (9) |
| Pt#34 | A2/A2 | 02:01/02:07 | N/A | 171 (3) | 25 (2) |
| Pt#35 | A2/A24 | 02:01/24:02 | N/A | 0 | 489 (1) |
| CTL induction (%) | 3/9 (33%) | 7/14 (50%) | 7/9 (78%) | ||
Cell number showing IFN‐γ spots per 105 peripheral blood mononuclear cells (PBMC) are given in the table. Non‐specific cell number was put in parentheses. N/A, not available.
The A2 genotypes of these patients were 02:01 (n = 10), 02:03 (n = 1), 02:06 (n = 9), 02:07 (n = 2) and 02:10 (n = 1), respectively. All three of the long peptides exhibited the activity to induce IFN‐γ producing T‐cells in both A02:01 and A02:06 cancer patients. In addition, Lck487–501 had the activity in both A02:07 and A02:10 cancer patients, while Lck374–388 had the activity in an A02:07 patient.
We then tested the ability of the long peptide to induce the relevant short peptide‐specific CTL from the PBMC of HLA‐A2+ advanced cancer patients using the peptide‐pulsed T2 cells as an indicator cell (n = 4). The successful induction of peptide‐specific CTL was judged to be positive when a significant value of P < 0.05 was reached by a two‐tailed Student's t‐test or when the ratio of IFN‐γ spots with the relevant peptide to those with the HIV peptide was more than 1.5. As a result, Lck33–47, Lck374–388 and Lck487–501 induced the relevant short peptide‐reactive IFN‐γ producing T‐cells from the PBMC of 3 of 4, 3 of 4 on Lck375–383 and 1 of 4 on Lck377–384, and 2 of 4 of the HLA‐A2+ cancer patients tested, respectively (Table 3).
Table 3.
Induction of peptide‐specific IFN‐γ producing T‐cellsa
| Stimulated peptide | Lck33–47 | Lck374–388 | Lck487–501 | ||
|---|---|---|---|---|---|
| T2 loaded peptide | Lck37–47 | Lck375–383 | Lck377–384 | Lck489–497 | |
| ID | HLA | IFN‐γ (pg/mL) | |||
| Pt#3 | A2 | 224 (152) | 0 | 0 | 0 |
| Pt#2 | A2/A33 | 23 (51) | 52 (32) | 0 | 14 (6) |
| Pt#5 | A2 | 0 | 82 (63) | 0 | 43 (27) |
| Pt#6 | A2/A24 | 95 (59) | 69 (45) | 84 (54) | 0 |
PBMC from HLA‐A2+ cancer patients were stimulated in vitro with the indicated peptide as described in the Materials and Methods. On day 15, the cultured peripheral blood mononuclear cells (PBMC) were tested for their reactivity to T2 cells which were pre‐pulsed with the relevant short peptides. The HIV peptide was used as a control.
The expression of Lck antigen in these three cell lines was confirmed by RT‐PCR (data not shown). We then determined whether or not HLA‐A2+ PBMC stimulated with each of these long peptides could show cytotoxicity against HLA‐A2+ Lck+ cancer cells (SW620). HLA‐A2− Lck+ SQ‐1 cells were used as a negative control. The PBMC from the three patients exhibited significantly higher levels of cytotoxicity against SW620 than against SQ‐1 after the stimulation with each of the corresponding peptides, respectively (Fig. 2a).
Figure 2.
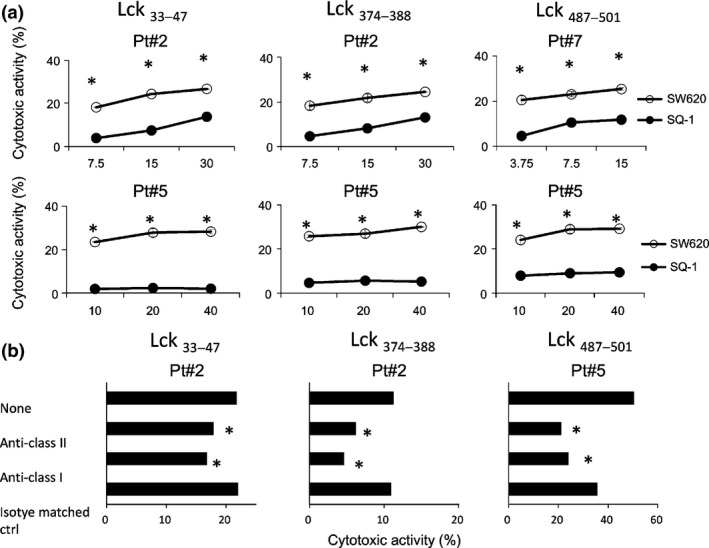
Cytotoxicity of peptide‐stimulated peripheral blood mononuclear cells (PBMC). (a) PBMC from the three HLA‐A2+ cancer patients were stimulated in vitro with each of the Lck33–47, Lck374–388 and Lck487–501 peptides followed by testing of their cytotoxicity against HLA‐A2+ Lck+ SW620 and HLA‐A2− Lck+ SQ‐1 cells by a 6‐h 51Cr release assay at three different effector to target cell ratios. Representative results are shown in the figure. *P < 0.05 (statistically significant). (b) To determine the HLA class I‐restricted or class II‐restricted manner, 10 μg/mL of either anti‐HLA‐class I (W6/32: mouse IgG2a), anti‐HLA‐DR (L243: mouse IgG2a) or anti‐CD14 (H14: mouse IgG2a) mAb, as an irrelevant control, were added into wells at the initiation of the culture. Representative results are shown in the figure. *P < 0.05 (statistically significant). The genotypes of Pt.2, Pt.5 and Pt.7 were HLA‐DR04:05/13:02 and HLA‐A02:06/33:03, HLA‐DR04:03/09:01 and HLA‐A02:01/02:06, and HLA‐DR09:01/14:05 and HLA‐A02:01/31:01, respectively.
We further investigated whether or not the recognition of SW620 cells by these Lck peptide‐specific T lymphocytes proceeded in an HLA‐class I or HLA‐II dependent manner. The representative results are shown in Figure 2(b). The cytotoxicity was significantly inhibited by the addition of anti‐HLA‐class I mAb and anti‐HLA‐class II mAb, but not by the addition of an isotype matched irrelevant mAb, respectively.
We lastly confirmed the Lck specific cytotoxicity of long peptide‐induced CTL with HLA‐A2+ Lck− cancer cells (LNCaP), HLA‐A2+ Lck+ cancer cells (SW620) and HLA‐A2− Lck+ cells (SQ‐1) as target cells. As a result, HLA‐A2+ PBMC from patient 2 stimulated with Lck374–388 or Lck487–501 showed significantly high levels of cytotoxicity against HLA‐A2+ Lck+ cancer cells (SW620) as compared to those against the others (Fig. 3). Those of patient 5 stimulated with Lck33–47 showed higher levels of cytotoxicity to SW620 cells as compared to that to LNCaP cells, and showed no cytotoxicity to SQ‐1 cells.
Figure 3.
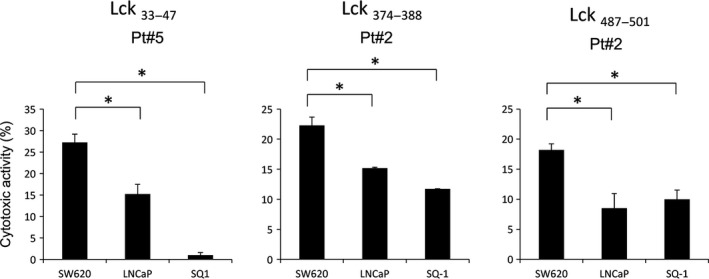
Lck‐specific cytotoxicity of peptide‐stimulated peripheral blood mononuclear cells (PBMC). PBMC from the three HLA‐A2+ cancer patients were stimulated vitro with each of the Lck33–47, Lck374–388 and Lck487–501 peptides followed by testing of their cytotoxicity against HLA‐A2+ Lck+ SW620, HLA‐A2+ Lck− LNCaP, and HLA‐A2− Lck+ SQ‐1 cells by a 6‐h 51Cr release assay at the 15:1, 10:1 and 5:1 of effector to target cell ratios, respectively. Representative results at the 10:1 ratio are shown in the figure. *P < 0.05 (statistically significant).
Discussion
We newly identified the three Lck‐derived Th epitope peptides capable of inducing both CD4+ and CD8+ T lymphocytes, which showed not only peptide‐specific IFN‐γ production but cytotoxicity against HLA‐A2+ cancer cells from PBMC of HLA‐A2+ cancer patients. In addition, their inner short peptides with HLA‐A2 binding motifs also had the same ability. Their cytotoxicity against cancer cells was inhibited by the addition of anti‐HLA‐class I mAb and anti‐HLA‐class II mAb, suggesting the involvement of both molecules. Therefore, these long peptides might be more potent for induction of anti‐tumor immunity as compared to the conventional short peptides.
To better understand the involvement of HLA‐class II molecules in the cytotoxicity, we have examined both HLA‐class I and HLA‐class II expression on SW620 cells used for the study after incubation with or without 10 μg of IFN‐γ because the HLA class II expression was reported as negative.25, 26 It was undetectable in culture without IFN‐γ, but became detectable in SW620 cells when cultured in the presence of IFN‐γ, although the expression level was minimal (data not shown). Therefore, the blocking effects of anti‐HLA‐DR antibody on cytotoxicity mediated by the induced T cells might be explained in part by the assumption that the stimulated T cells (both CD8+ and CD4+ T cells) responded to naturally‐processed CTL epitope short peptides that shared the homology with the inner peptides of Lck‐derived long peptides in a HLA‐A2‐restricted manner, and then produced IFN‐γ in culture, which in turn induced the HLA‐class II expression on SW620 cells. The other more likely explanation could be that anti‐DR antibody bound to DR molecules on the stimulated CD4+ Th1 lymphocytes, which recognized naturally processed CTL epitope short peptides sharing the homology with the inner peptides of Lck‐derived long peptides in a HLA‐A2‐restricted manner, which in turn inhibited both the CD4+ Th1‐mediated cytotoxicity against SW620 tumor cells and their IFN‐γ production. Although this issue shall be clarified by further investigation in the near future, these results are consistent with the recent reports of CD4+ Th lymphocytes‐mediated direct cytotoxicity against both HLA‐Class II expressing tumor cells and non‐expressing tumor cells.13, 14
It is also important to investigate the percentages of peptide‐induced CD4+ T and CD8+ T lymphocytes used in the cytotoxic assay. However, we could not run a flow cytometry primarily because of limited numbers of available effector cells. To better understand the relative contribution level of CD8+ T lymphocytes in this cytotoxicity, we tested the cytotoxicity against the peptide loaded T2 cells that expressed HLA‐A2, but not HLA‐class II antigen. As a result, the percent cytotoxicity at an effector to target ratio of 10 to 1 was around 10% in PBMC stimulated with Lck374–388 and also 10% in PBMC stimulated with Lck487–501 peptide (data not shown), which are lower percentages than those shown in the Figure 2(a). These results suggest that the contribution level of CD8+ T lymphocytes in the cytotoxicity was almost equal to that of CD4+ T lymphocytes, which is mostly consistent with the results for the inhibition levels by anti‐class I or anti‐class II antibody shown in Figure 2(b).
Referring to HLA‐class II prediction (EpiTOP SYFPEIT and NIH), Lck33–47, Lck374–388 and Lck487–501 peptide epitopes cover DRB1*01, DRB1*03, DRB1*04, DRB1*07, DRB1*08, DRB1*09, DRB1*11, DRB1*12, DRB1*13, DRB1*14, DRB1*15 and DRB1*16. The frequencies of these HLA‐DR in the Japanese population are 5.84, 0.15, 23.9, 0.34, 12.58, 14.28, 2.55, 5.58, 6.55, 8.47, 18.44 and 0.9% based on the results of the Allele Frequencies in Worldwide Populations website (http://www.allelefrequencies.net/default.asp). Therefore, each of these long peptides could be applicable as HLA‐class II‐directed peptide vaccines for the vast majority (95%) of the Japanese population. These peptides could also be applicable for the majority of Asians and White people based on information from the same prediction studies.
All the three long peptides exhibited the activity to induce CTL in both A02:01 and A02:06 cancer patients. In addition, Lck387–501 had the activity in both A02:07 and A02:10 cancer patients, while Lck374–388 had the activity in an A02:07 patient. These results suggest that each of these long peptides had the activity to induce CTL activity in HLA‐A2 cancer patients irrespective of their genotypes, although detailed studies with more samples are needed. The reactivity of these long peptides for different HLA‐A2 genotypes could be in part due to the inner short peptides with binding motifs to different HLA‐A2 genotypes (data not shown).
We found three Lck‐derived long peptides with both Th and CTL activities. These peptides might be useful for long peptide‐based vaccines for HLA‐A2+ metastatic cancer patients.
Disclosure Statement
Kyogo Itoh received research funding from Taiho Pharmaceutical Co., Ltd., for which he also received bureau honorarium and is a consultant/advisory board member. Akira Yamada is a Board member of the Green Peptide Co., Ltd. No potential conflicts of interests were declared by the other authors.
Supporting information
Table S1. Lck‐peptides binding to HLA‐DR and‐ A*02:01 alleles.
Acknowledgments
This study was supported in part by a grant from the Developing Innovation Systems Program for Fostering Regional Innovation (Global Type), a research program of the Project for Development of Innova‐tive Research on Cancer Therapeutics (P‐Direct), and by Sendai Kousei Hospital (to KI), and a JSPS KAKENHI Grant number 24791452 and 26861105 from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to SM).
Cancer Sci 106 (2015) 1493–1498
Funding Information Developing Innovation Systems Program for Fostering Regional Innovation (Global Type); Project for Development of Innovative Research on Cancer Therapeutics (P‐Direct), Sendai Kousei Hospital; JSPS KAKENHI (24791452, 26861105). Ministry of Education, Culture, Sports, Science and Technology of Japan; Taiho Pharmaceutical Co., Ltd.
References
- 1. Hodi FS, O'Day SJ, McDermott DF et al Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363: 711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Topalian SK, Hodi FS, Brahmer JR et al Safety, activity, and immune correlates of anti‐PD‐1 antibody in cancer. N Engl J Med 2012; 366: 2443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Powles T, Eder JP, Fine GD et al MPDL3280A (anti‐PD‐L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 2014; 515: 558–62. [DOI] [PubMed] [Google Scholar]
- 4. Noguchi M, Yao A, Harada M et al Immunological evaluation of neoadjuvant peptide vaccination before radical prostatectomy for patients with localized prostate cancer. Prostate 2007; 67: 933–42. [DOI] [PubMed] [Google Scholar]
- 5. Matsumoto K, Noguchi M, Satoh T et al A phase I study of personalized peptide vaccination for advanced urothelial carcinoma patients who failed treatment with methotrexate, vinblastine, adriamycin and cisplatin. BJU Int 2011; 108: 831–8. [DOI] [PubMed] [Google Scholar]
- 6. Noguchi M, Kakuma T, Uemura H et al A randomized phase II trial of personalized peptide vaccine plus low dose estramustine phosphate (EMP) versus standard dose EMP in patients with castration resistant prostate cancer. Cancer Immunol Immunother 2010; 59: 1001–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cheever MA, Allison JP, Ferris AS et al The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res 2009; 15: 5323–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Noguchi M, Sasada T, Itoh K. Personalized peptide vaccination: a new approach for advanced cancer as therapeutic cancer vaccine. Cancer Immunol Immunother 2013; 62: 919–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Harada M, Gohara R, Matsueda S et al In vivo evidence that peptide vaccination can induce HLA‐DR‐restricted CD4+ T cells reactive to a class I tumor peptide. J Immunol 2004; 172: 2659–67. [DOI] [PubMed] [Google Scholar]
- 10. Melief CJ, van der Burg SH. Immunotherapy of established (pre)malignant disease by synthetic long peptide vaccines. Nat Rev Cancer 2008; 8: 351–60. [DOI] [PubMed] [Google Scholar]
- 11. Bos R, Sherman LA. CD4+ T‐cell help in the tumor milieu is required for recruitment and cytolytic function of CD8+ T lymphocytes. Cancer Res 2010; 70: 8368–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Quezada SA, Simpson TR, Peggs KS et al Tumor‐reactive CD4+ T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J Exp Med 2010; 207: 637–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Braum€uller H, Wieder T, Breuner E et al T helper cell cytokines drive cancer into senescence. Nature 2013; 494: 361–5. [DOI] [PubMed] [Google Scholar]
- 14. Haaheth OAW, Tveita AA, Fauskanger M et al How do CD4+ T cells detect and eliminate tumor cells that either lack or express MHC class II molecules. Front Immunol 2014; 174: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tomita Y, Yuno A, Tsukamoto H et al Identification of promiscuous KIF20A long peptides bearing both CD4+ and CD8+ T‐cell epitopes: KIF20A‐specific CD4+ T‐cell immunity in patients with malignant tumor. Clin Cancer Res 2013; 15: 4508–20. [DOI] [PubMed] [Google Scholar]
- 16. Tomita Y, Yuno A, Tsukamoto H et al Identification of CDCA1‐derived long peptides bearing both CD41 and CD81 T‐cell epitopes: CDCA1‐specific CD41 T‐cell immunity in cancer patients. Int J Cancer 2013; 12: 123–36. [DOI] [PubMed] [Google Scholar]
- 17. Harashima N, Tanaka K, Sasatomi T et al Recognition of the Lck tyrosine kinase as a tumor antigen by cytotoxic T lymphocytes of cancer patients with distant metastases. Eur J Immunol 2001; 31: 323–32. [DOI] [PubMed] [Google Scholar]
- 18. Amundadottir LT, Leder P. Signal transduction pathways activated and required for mammary carcinogenesis in response to specific oncogenes. Oncogene 1998; 12: 737–46. [DOI] [PubMed] [Google Scholar]
- 19. Leung S, Miyamoto NG. Differential expression of two classes of lck transcripts upon phorbol ester treatment of human leukemic T cells. J Cell Physiol 1991; 148: 344–52. [DOI] [PubMed] [Google Scholar]
- 20. Terasaki M, Shibui S, Narita Y et al A phase I trial of personalized peptide vaccine for HLA‐A24 patients with recurrent or progressive glioblastoma multiforme. J Clin Oncol 2011; 20: 337–44. [DOI] [PubMed] [Google Scholar]
- 21. Sasada T, Yamada A, Noguchi M, Itoh K. Personalized peptide vaccine for treatment of advanced cancer. Curr Med Chem 2014; 21: 2332–45. [DOI] [PubMed] [Google Scholar]
- 22. Fukui A, Matsueda S, Kawano K et al Identification of B cell epitopes reactive to human papillomavirus type‐16L1‐derived peptides. Virol J 2012; 9: 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hida N, Maeda Y, Katagiri K et al A simple culture protocol to detect peptide‐specific cytotoxic T lymphocyte precursors in the circulation. Cancer Immunol Immunother 2002; 51: 219–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Matsueda S, Takedatsu H, Yao A et al Identification of peptide vaccine candidates for prostate cancer patients with HLA‐A3 supertype alleles. Clin Cancer Res 2005; 11: 6933–43. [DOI] [PubMed] [Google Scholar]
- 25. Matsueda S, Komatsu N, Kusumoto K et al Humoral immune responses to CTL epitope peptides from tumor‐associated antigens are widely detectable in humans: a new biomarker for overall survival of patients with malignant diseases. Dev Comp Immunol 2013; 41: 68–76. [DOI] [PubMed] [Google Scholar]
- 26. Dohlsten M, Hedlund G, Segren S et al Human major histocompatibility complex class II‐negative colon carcinoma cells present staphylococcal superantigens to cytotoxic T lymphocytes: evidence for a novel enterotoxin receptor. Eur J Immunol 1991; 21: 1229–33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Lck‐peptides binding to HLA‐DR and‐ A*02:01 alleles.


