PIK3CA is an oncogene that encodes the p110α component of phosphatidylinositol 3‐kinase (PI3K); it is the second most frequently mutated gene following the TP53 gene. In the clinical setting, PIK3CA mutations may have favorable prognostic value for hormone receptor‐positive breast cancer patients and, during the past few years, PIK3CA mutations of cell‐free DNA (cfDNA) have attracted attention as a potential noninvasive biomarker of cancer. However, there are few reports on the clinical implications of PIK3CA mutations for TNBC patients. We investigated the PIK3CA major mutation status of cfDNA as a noninvasive biomarker of cancer using droplet digital polymerase chain reaction (ddPCR), which has high level sensitivity and specificity for cancer mutation, in early‐stage 49 triple negative breast cancer (TNBC) patients. A total of 12 (24.4%) of 49 patients had PIK3CA mutations of cfDNA. In a median follow up of 54.4 months, the presence of PIK3CA mutations of cfDNA had significant impacts on relapse‐free survival (RFS; P = 0.0072) and breast cancer‐specific survival (BCSS; P = 0.016), according to the log‐lank test. In a Cox proportional hazards model, the presence of PIK3CA mutations of cfDNA had significant prognostic value in the univariate and multivariate analysis. Additionally, the presence of PIK3CA mutations of cfDNA was significantly correlated with positive androgen receptor phosphorylated form depending on PI3K signaling pathway (pAR) which is independent favorable prognostic factors of TNBC. We demonstrated that the presence of PIK3CA major mutations of cfDNA could be a discriminatory predictor of RFS and BCSS in early‐stage TNBC patients and it was associated with PI3K pathway‐dependent AR phosphorylation.
Keywords: Androgen receptor, androgen receptor phosphorylation, droplet digital PCR, PIK3CA mutations of circulating cell‐free DNA, triple negative breast cancer
Short abstract
We demonstrated the presence of PIK3CA major mutations of cfDNA could be discriminatory predictor of RFS and BCSS in early‐stage TNBC patients which may be associated with PI3K pathway dependent AR phosphorylation.
Triple‐negative breast cancer (TNBC) is a breast cancer subtype that lacks detectable expression of estrogen receptor (ER) and progesterone receptor (PgR) and amplification of human epidermal growth factor receptor 2 (HER2). TNBC tumors are generally more aggressive than their ER‐positive counterparts, with higher rates of relapse in the early stages and decreased overall survival rate with it.1, 2 This is because there have not been the targeted therapeutic agents for the multiple independent subtypes of TNBC, in contrast to the cases for endocrine therapy for ER‐positive breast cancer and anti‐HER2 therapy for HER2‐amplified breast cancer. The current standard of care for TNBC is conventional systemic chemotherapy,3 with new therapeutic options being explored for patients suffering from TNBC. Molecular pathology and gene expression profiling are useful for identifying the subtypes of TNBC, developing therapeutic options, and determining the spectrum of somatic mutations.4, 5 PIK3CA, an oncogene that encodes the p110α component of phosphatidylinositol 3‐kinase (PI3K), is the second most frequently mutated gene following the TP53 gene. PIK3CA mutation is present in 20–40% of all breast cancers.6, 7 A meta‐analysis suggested that the clinical implications of PIK3CA mutations may vary according to the status of well‐known molecular markers in breast cancer; namely, ERα, PgR and HER2.8 The androgen receptor (AR), a member of the steroid receptor subfamily, has also been connected with PI3K pathway aberrations, including PIK3CA mutations.9, 10 In the clinical setting, PIK3CA mutations alone may have favorable prognostic value for hormone receptor‐positive breast cancer patients;11, 12, 13, 14 however, there have only been a few reports about the clinical implications of PIK3CA mutations for TNBC patients. For TNBC patients, AR has been known as a positive prognostic marker15, 16, 17, 18, 19, 20 and AR phosphorylation at serine‐213/210 dependent on PI3K/Akt signaling pathway (pAR) is also an independent favorable prognostic marker.21 These findings have aroused interest in investigating PIK3CA mutations’ potential to serve as specific prognostic markers and in their influence on AR signaling pathway in TNBC.
Over the past 10 years, “liquid biopsy” using cell‐free DNA (cfDNA) has been attracting attention as a potential method to noninvasively identify specific mutations within a patient's cancer.22 Several groups have reported that, using various technologies, mutant PIK3CA DNA can be reliably detected in plasma from patients with metastatic breast cancer.23, 24, 25, 26 In particular, the newer technique of droplet digital PCR (ddPCR) can detect and quantify the small mutant gene fragments that are present in 0.02–0.1% of the total amount of DNA assayed. Therefore ddPCR has the potential to detect small amounts of mutations in peripheral blood.23, 27 This is because these technologies partition thousands of individual DNA molecules and then each molecule is amplified and queried for a given mutation.27, 28 We hypothesized that ddPCR could identify PIK3CA mutations in plasma and could verify PIK3CA cfDNA mutations’ potential as a biomarker. There are three frequently recurring “hotspot” PIK3CA mutations in exons 9 and 20, corresponding to the helical (E542K and E545K) and kinase (H1047R) domains, respectively. These hotspot PIK3CA mutations account for 80–90% of all PIK3CA mutations in human malignancies.29
Here, we investigated cfDNA and tissue tumor genomic DNA (gDNA) using next‐generation digital PCR platforms, with high level sensitivity and specificity for cancer mutation detection, and verified the PIK3CA major mutation status of cfDNA as a noninvasive prognostic biomarker in a series of 49 patients with unilateral invasive primary TNBC. In addition, we evaluated the influence of PIK3CA mutations on AR and pAR, which are independent favorable prognostic factors of TNBC.
Materials and Methods
Patients and breast cancer tissues
A total of 49 patients with primary ER‐negative, PgR‐negative and HER2‐negative breast carcinoma, treated at Kumamoto University Hospital between 2003 and 2011, were enrolled in this protocol. Cases were selected if plasma was available. If possible, corresponding fresh frozen (FF) tissue from either a core‐cut biopsy or the surgical excision was analyzed. Informed consent was obtained from all patients before biopsy or surgery. The Ethics Committee of Kumamoto University Graduate School of Medicine (Kumamoto, Japan) approved the study protocol. Neoadjuvant and/or adjuvant treatment was administered in accordance with the recommendations of the St. Gallen international expert consensus on the primary therapy of early breast cancer.30, 31, 32 Neoadjuvant and/or adjuvant treatment was administered to a total of 29 (59.2%) of 49 patients. Neoadjuvant chemotherapy was performed in 11 patients. No therapy was administered to 20 patients. When tumors recurred, patients were treated with chemotherapy (e.g. anthracycline containing regimens, taxanes, capecitabine and vinorelbine). Patients were periodically examined at the Kumamoto University Hospital or affiliated hospitals. The patients were observed every 3 months for 5 years and every 1 year thereafter. Recurrence was defined when positive spots were found by physical examination and/or by imaging diagnosis during the follow‐up period. The median follow‐up period was 54.4 months (range, 4.0–126.4 months).
Sample preparation for fresh frozen samples and plasma
Blood samples were collected at the same time as tissue collection. Blood samples and plasma DNA preparation were performed using a PureLink Viral RNA/DNA Mini Kit (ThermoFisher Scientific, Waltham, MA USA) as per the manufacturer's instructions. Primary tumor samples from a core‐cut biopsy or the surgical excision were immediately frozen by liquid nitrogen and stored as FF samples. Tissue tumor gDNA was extracted from homogenized FF samples, and purified using the All‐prep DNA/RNA/miRNA Universal Kit (Qiagen, Valencia, CA, USA) as per the manufacturer's instructions.
All extracted DNA was quantified using a NanoDrop Spectrophotometer (Thermo Scientific, Tokyo, Japan). Of the 49 patients, all had cfDNA available in plasma, but cancer tissues were not available for 7 (14.2%) of the 49 primary tumor samples (Table 1, Fig. S1).
Table 1.
Patients clinicopathological characteristics with cfDNA PIK3CA mutations or without
| Total | Number of cases (%) | P‐value | ||
|---|---|---|---|---|
| PIK3CA genomic state | ||||
| Mutations | Wild‐type | |||
| Variables | (N = 49) | (N = 12) | (N = 37) | |
| Age at biopsy | ||||
| ≤50 | 9 | 1 (8.3) | 8 (21.6) | 0.3 |
| >50 | 40 | 11 (91.7) | 29 (78.4) | |
| Menopausal state | ||||
| Premenopausal | 8 | 1 (8.3) | 7 (18.9) | 0.38 |
| Postmenopausal | 41 | 11 (91.7) | 30 (81.1) | |
| Tumor size (cm) | ||||
| ≤2 | 20 | 5 (41.7) | 15 (40.5) | 0.94 |
| >2 | 29 | 7 (58.3) | 22 (59.5) | |
| Nodal status | ||||
| Negative | 33 | 7 (58.3) | 26 (70.3) | 0.44 |
| Positive | 16 | 5 (41.7) | 11 (29.7) | |
| Lymphovascular invasion | ||||
| No | 30 | 7 (58.3) | 23 (62.2) | 0.81 |
| Yes | 19 | 5 (41.7) | 14 (37.8) | |
| Histological type | ||||
| Invasive ductal | 36 | 11 (91.7) | 25 (67.6) | 0.23 |
| Invasive lobular | 2 | 0 | 2 (5.4) | |
| Special type | 11 | 1 (8.3)† | 10 (27) ‡ | |
| Histological grade | ||||
| 1, 2 | 18 | 5 (41.7) | 13 (35.1) | 0.68 |
| 3 | 31 | 7 (58.3) | 24 (64.9) | |
| Ki67 LI | ||||
| ≤14 | 7 | 2 (16.7) | 5 (13.5) | 0.81 |
| >14 | 41 | 10 (83.3) | 31 (83.8) | |
| Unknown | 1 | 1 (2.7) | ||
| PIK3CA mutations in tumor tissue gDNA (%) | ||||
| Median (25%, 75%) | 42 | 0.35 (0.15, 11.5) | 0.53 (0.12, 11.6) | 0.86 |
| Chemotherapy received | ||||
| No | 20 | 4 (33.3) | 16 (43.2) | 0.54 |
| Yes | 29 | 8 (66.7) | 21 (56.8) | |
†This is medullary carcinoma. ‡These include 3 metaplastic carcinomas, 3 apocrine carcinomas, 1 medullary carcinomas, 1 spindle cell carcinoma and 2 squamous cell carcinomas. cfDNA, cell‐free DNA; gDNA, genomic DNA; LI, labeling index.
Droplet digital polymerase chain reaction analysis of PIK3CA mutations
Cell‐free DNA and tissue tumor gDNA were analyzed with the QX200 Droplet Digital PCR System (Bio‐Rad Laboratories, Hercules, CA, USA) using TaqMan probes for detecting and quantifying wild‐type PIK3CA, as well as PIK3CA E542K, E545K in exon 9, and PIK3CA H1047R mutations in exon 20. They create thousands of nanoliter‐sized droplets. Each droplet contained one or no copy of the sequence of PIK3CA gene reacted with a pair of primers and two TaqMan probes. One probe was specific to the wild‐type sequence and the other was specific to each PIK3CA mutation. Each primer annealed to the antisense strand of the PIK3CA exon 9 and exon 20. When the region was mutated, the mutant probe was able to anneal to the template, hence giving rise to an FAM signal; this would not occur with the wild‐type‐specific probe. Conversely, when the region was not mutated, the mutant probe was unable to anneal to the template, but the wild‐type‐specific probe was able to, hence giving rise to a VIC‐signal. For this assay, the wild‐type and mutant signals represent the presence of wild‐type and mutant molecules, respectively. The PCR mix for one well was set up by mixing 1 μL DNA sample (5 ng in cfDNA and 20 ng in tissue gDNA), 10 μL 2× ddPCR Supermix (Bio‐Rad laboratories, Hercules, CA, USA) and 0.5 μL 4× TaqMan SNP Genotyping Assays (written in “probes and primers”) in a reaction volume of 20 μL, adjusted with sterile water. After loading a 20‐μL PCR reaction and 70 μL of droplet generation oil to the exclusive chamber, the droplet generator produces approximately 20 000 droplets per sample. After the sample was loaded on a 96‐well plate and heat sealed, it was placed in a thermal cycler for PCR and detection of reporter signals. The cycling profile of the PIK3CA mutant detection assay was as follows: incubation at 95°C for 10 min followed by 40 cycles of 94°C for 30 s and 51°C for 1 min. Analysis of the PCR data were performed using QuantaSoft software (Bio‐Rad laboratories, Hercules, CA, USA). The results of the cfDNA analysis were expressed as the ratio of each PIK3CA mutation to wild‐type because ddPCR could identify a heterogeneous mutation as one event.33 The results of tissue tumor gDNA analysis were expressed as a percentage or fractional abundance of mutant to total (mutant plus wild type) PIK3CA molecules for each sample.
Probes and primers
The ddPCR assay for the detection of the variant types of amino acids 542 and 545 in PIK3CA exon 9 and 1047 in PIK3CA exon 20 consisted of a pair of primers and two TaqMan minor groove binding (MGB) probes, respectively, as described previously.34
Immunohistochemistry
Immunohistochemical staining was carried out on 4‐μm thick tumor sections. Serial sections were prepared from selected blocks and float‐mounted on adhesive‐coated glass slides for ERα, PgR, HER2 and AR staining. The primary antibodies and visualization methods were as previously described.21 This study followed the Reporting Recommendations for Tumor Marker Prognostic Studies criteria.35 ERα, PgR, AR and pAR expression were scored by Histoscore (HS), multiplying the percentage (0–100) of cells stained at a given intensity by the staining intensity score (IS) (0, none; 1, weak; 2, moderate; and 3, intense) and were evaluated as previously (Fig. S2).21 HER2 immunostaining was evaluated using the HercepTest (Dako), with the score (range, 0 to 3+) based on membranous staining and its distribution. Tumors with scores of 3+ or with a ≥2.2‐fold increase in HER2 gene amplification, as determined by FISH, were considered to be positive for HER2 overexpression. Ki‐67 was scored as the percentage of nuclear staining cells out of all cancer cells in the invasive front of the tumor in a 40‐power field (Ki‐67 labeling index); a 14% cut‐off level was adopted.36
Statistical analysis
The χ2‐test or Fisher's exact test was used to assess baseline differences between binary variables. The Spearman rank correlation coefficient was used to assess the correlation among PIK3CA E542K, E545K and H1047R in gDNA and cfDNA. The Kaplan–Meier method was used to estimate the relapse‐free survival (RFS) and breast cancer‐specific survival (BCSS) rates, and differences between survival curves were evaluated using the log‐rank test. Cox's proportional hazards model was used for the univariate and multivariate analysis of prognostic status. P‐values <0.05 were considered significant. All reported P‐values are two‐sided, and confidence intervals (CI) are at the 95% level. All statistics analyses were two‐sided and performed using JMP software version 10.0.1 for Windows (SAS institute Japan, Tokyo, Japan).
Results
Presence of PIK3CA mutations in cell‐free DNA
First, we verified the utility of the ddPCR system using the samples of each PIK3CA mutation‐positive and mutation‐negative case, which were previously verified by the BioMark system (Fluidigm Corporation) with the BioMark qdPCR 37K (Fluidigm Corporation).34 ddPCR could detect each PIK3CA mutation in each positive case, but could also recognize a small quantity in a few negative cases (data not shown). Next, ddPCR was performed in 49 cfDNA samples and 42 corresponding tumor tissue gDNA of early invasive TNBC cases. There was no correlation in the rate of each PIK3CA mutation between cfDNA and corresponding tumor gDNA (Table S3). We set the 75th percentile of the total of the ratio of PIK3CA mutation to wild‐type as a dichotomized cutoff point. A total of 12 (24.4%) of 49 patients had PIK3CA mutations in cfDNA. In the three mutation sites (PIK3CA E542K, E545K and H1047R) that were assessed, seven PIK3CA E542K and seven H1047R were identified, and two cases of those were identified as double mutations, but PIK3CA E545K was not detected (Table S1). The presence of double PIK3CA mutations was reported elsewhere.8 This may reflect a high level of tumoral heterogeneity in TNBC.
Correlation of patient and breast tumor characteristics with the presence of PIK3CA mutations
The median age of the patients was 64 years (29–87 years old; Table S1). The patient characteristics are summarized in Table 1. The presence of PIK3CA mutations of cfDNA had no association with major prognostic factors of breast cancer. The relationship between the presence of PIK3CA mutations and AR and pAR is summarized in Fig. S1 and Table 2. The AR positive rate was 73.3% for the 1% cut‐off level, 69.2% for the 5% cut‐off level, 69.2% for the 10% cut‐off level and 71.4% for the 20% cut‐off level of PIK3CA mutant percentage. Higher AR expression was significantly related to any cut‐off level of PIK3CA mutations of tumor tissue gDNA (P = 0.0005 in 1%, P = 0.005 in 5%, P = 0.005 in 10% and P = 0.046 in 20%, respectively, Fig. S1a), but there was no statistically significant difference between the presence of PIK3CA mutations of cfDNA and AR expression (Table 2). We found that the presence of PIK3CA mutations of cfDNA, in particular, helical domain mutations, was significantly correlated with pAR expression (P = 0.018 and P = 0.03, respectively). However, there was no statistically significant correlation between any cut‐off level of PIK3CA mutations and pAR expression (Fig. S1b).
Table 2.
Correlation of cfDNA PIK3CA mutations with AR and pAR
| Variables | Number of cases (%) | ||||||
|---|---|---|---|---|---|---|---|
| Total | AR | pAR | |||||
| PIK3CA mutation of cfDNA | (N = 49) | Positive | Negative | P‐value | Positive | Negative | P‐value |
| (N = 19) | (N = 30) | (N = 12) | (N = 37) | ||||
| Total | 12 | 5 (26.3) | 7 (23.3) | 0.81 | 6 (50) | 6 (16.2) | 0.018† |
| Wild type | 37 | 14 (73.7) | 23 (76.7) | 6 (50) | 31 (83.8) | ||
| Kinase domain (H1047R) | 7 | 3 (15.8) | 4 (13.3) | 0.81 | 3 (25) | 4 (10.8) | 0.22 |
| Others | 42 | 16 (84.2) | 26 (86.7) | 9 (75) | 33 (89.2) | ||
| Helical domain (E542K, E545K) | 7 | 3 (15.8) | 4 (13.3) | 0.81 | 4 (33.3) | 3 (8.1) | 0.03† |
| Others | 42 | 16 (84.2) | 26 (86.7) | 8 (66.7) | 34 (91.9) | ||
†Factor showing statistical significance. AR, androgen receptor; cfDNA, cell‐free DNA; gDNA; genomic DNA; pAR, AR phosphorylation at serine‐213/210.
Patient outcomes
In the RFS analysis, local recurrences and distant metastases were considered as an event. Fifteen (30.6%) patients experienced breast cancer relapse. A total of 12 patients died of breast cancer, which was regarded as events when analyzing BCSS. The presence of PIK3CA mutations of cfDNA were significant prognostic factors of both RFS (P = 0.0072) and BCSS (P = 0.016; Fig. 1). The presence of PIK3CA kinase domain mutations and helical domain mutations were marginal prognostic factors of both RFS (P = 0.067 and P = 0.052) and BCSS (P = 0.10 and 0.077), respectively (Fig. 2). In this setting, higher AR expression was a marginal prognostic factor of RFS (P = 0.062) and a significant prognostic factor of BCSS (P = 0.022). Positive pAR was a significant prognostic factor in RFS (P = 0.048), but not in BCSS (P = 0.12; data not shown). These were tested using the Kaplan–Meier method and verified by the log‐rank test.
Figure 1.
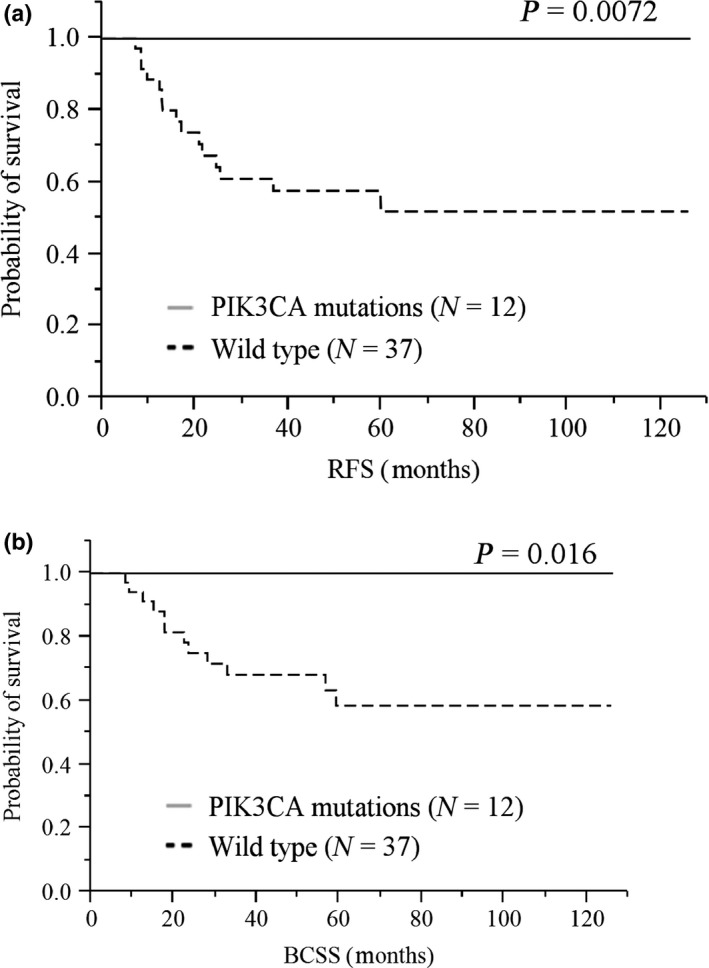
Kaplan–Meier plots of the association of the presence of total PIK3CA mutations of cfDNA with RFS: (a) BCSS and (b) the entire cohort. When the presence of total PIK3CA mutations of cfDNA was defined as either positive or negative, positive cases seemed to have a longer survival than negative ones in log‐lank test: (a) RFS, P = 0.0072; (b) BCSS, P = 0.016. BCSS, breast cancer‐specific survival; cfDNA, cell‐free DNA; RFS, relapse‐free survival.
Figure 2.

Kaplan–Meier plots of the association of the presence of PIK3CA kinase domain mutation (H1047R) of cfDNA with RFS: (a) BCSS; (c) the presence of PIK3CA helical domain mutation (E542K or E544K) of cfDNA with RFS; (b) BCSS; and (d) in the entire cohort. When the presence of PIK3CA kinase and helical domain mutation of cfDNA was defined as either positive or negative, positive cases seemed to have a trend of longer survival than negative ones in the log‐lank test (PIK3CA kinase domain mutation: (a) RFS, P = 0.067, (c) BCSS, P = 0.10 and PIK3CA helical domain mutation; (b) RFS, P = 0.052, (d) BCSS, P = 0.077. BCSS, breast cancer‐specific survival; cfDNA, cell‐free DNA; RFS, relapse‐free survival.
The RFS and BCSS Cox hazard analyses are summarized in Tables 3 and 4. The presence of PIK3CA mutations was a significant predictor of RFS (hazards ratio [HR], 3.88e‐10; 95% CI, 0.28–0.28; P = 0.006) and BCSS (HR, 3.93e‐10; 95% CI, 0.36–0.36; P = 0.022) in the univariate analysis. The presence of PIK3CA kinase domain and helical domain mutations was associated with significantly better RFS (P = 0.014 and P = 0.030) and BCSS (P = 0.0099 and P = 0.018), respectively.
Table 3.
Univariate and multivariate analysis for relapse‐free survival (Cox proportional hazards model)
| Variables | Value | Univariate analysis | Multivariate analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95%CI | P‐value | n | HR | 95%CI | P‐value | n | |||
| Age at biopsy | (ref = ≤50) | >50 | 0.87 | 0.27–3.86 | 0.84 | 49 | 48 | |||
| Menopausal state | (ref = Pre) | Post | 0.71 | 0.22–3.15 | 0.62 | 49 | ||||
| Tumor size (cm) | (ref = ≤2) | >2 | 2.32 | 0.79–8.39 | 0.12 | 49 | ||||
| Nodal status | (ref = 0) | >1 | 1.49 | 0.50–4.15 | 0.45 | 49 | ||||
| Lymphovascular invasion | (ref = No) | Yes | 1.86 | 0.67–5.33 | 0.22 | 49 | ||||
| Histopathology | (ref = Others)† | Invasive ductal | 1.27 | 0.35–3.75 | 0.68 | 49 | ||||
| Histological Grade | (ref = 1,2) | 3 | 3.05 | 0.96–13.44 | 0.057 | 49 | 2.69 | 0.71–17.5 | 0.15 | |
| Ki67 (MIB1) LI | (ref = ≤14%) | >14% | 1.86E+09 | 1.71–1.71 | 0.0121‡ | 48 | 2.25E+09 | 1.15 | 0.040‡ | |
| AR (IHC) | (ref = HS10) | ≥HS10 | 0.31 | 0.07–1.006 | 0.051 | 49 | 0.53 | 0.11–1.84 | 0.34 | |
| pAR | (ref = IS 1 | ≥IS 2 | 0.16 | 0.009–0.82 | 0.024‡ | 49 | 2.6 | 0.11–28.7 | 0.47 | |
| PIK3CA mutation of cfDNA | ||||||||||
| Total | (ref = Wild type) | 3.88E‐10 | 0.28–0.28 | 0.0006‡ | 49 | 2.01E‐10 | 8.41e‐60–0.19 | 0.0003‡ | ||
| Kinase domain | (ref = Others) | 1.48E‐09 | 0.612–0.612 | 0.014‡ | 49 | |||||
| Helical domain | (ref = Others) | 5.10E‐10 | 0.54–0.54 | 0.0099‡ | 49 | |||||
| Chemotherapy | (ref = No) | Yes | 3.12 | 0.99–13.7 | 0.0519 | 49 | ||||
†Others include 2 invasive lobular carcinomas, 3 metaplastic carcinomas, 3 apocrine carcinomas, 2 medullary carcinomas, 1 spindle cell carcinoma and 2 squamous cell carcinomas. ‡Factor showing statistical significance. AR, androgen receptor; cfDNA, cell‐free DNA; HR, hazard ratio; HS, Histoscore; IHC, immunohistochemistry; IS, intensity score; LI, labeling index; pAR, AR phosphorylation at serine‐213/210; 95%CI, 95% confidence interval.
Table 4.
Univariate and multivariate analysis for breast cancer specific survival (Cox proportional hazards model)
| Variables | Value | Univariate analysis | Multivariate analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95%CI | P‐value | n | HR | 95%CI | PV | n | |||
| Age at biopsy | (ref = ≤50) | >50 | 1.26 | 0.33–8.23 | 0.75 | 49 | 48 | |||
| Menopausal state | (ref = Pre) | Post | 1.04 | 0.27–6.80 | 0.95 | 49 | ||||
| Tumor size (cm) | (ref = ≤2) | >2 | 1.58 | 0.49–5.96 | 0.44 | 49 | ||||
| Nodal status | (ref = 0) | >1 | 2.45 | 0.76–7.85 | 0.12 | 49 | ||||
| Lymphovascular invasion | (ref = No) | Yes | 2.39 | 0.76–8.09 | 0.13 | 49 | ||||
| Histopathology | (ref = Others)† | Invasive ductal | 0.79 | 0.26–3.99 | 0.79 | 49 | ||||
| Histological Grade | (ref = 1,2) | 3 | 3.73 | 0.98–24.3 | 0.053 | 49 | 2.1 | 0.55–13.7 | 0.3 | |
| Ki67 (MIB1) LI | (ref = ≤14%) | >14% | 6.58E+08 | 1.16–1.16 | 0.035‡ | 48 | 6.70E+08 | 0.33 ‐ | 0.2 | |
| AR (IHC) | (ref = HS10) | ≥HS10 | 0.13 | 0.007–0.68 | 0.011‡ | 49 | 0.21 | 0.011–1.15 | 0.078 | |
| pAR | (ref = IS 1 | ≥IS 2 | 0.22 | 0.012–1.17 | 0.082 | 49 | ||||
| PIK3CA mutation of cfDNA | ||||||||||
| Total | (ref = Wild type) | 3.93E‐10 | 0.36–0.36 | 0.0022‡ | 49 | 3.62E‐10 | 0.011–0.31 | 0.0012‡ | ||
| Kinase domain | (ref = Others) | 1.50E‐09 | 0.80–0.80 | 0.030‡ | 49 | |||||
| Helical domain | (ref = Others) | 1.38E‐09 | 0.66–0.66 | 0.018‡ | 49 | |||||
| Chemotherapy | (ref = No) | Yes | 3.5 | 0.92–22.7 | 0.067 | 49 | ||||
†Others include 2 invasive lobular carcinomas, 3 metaplastic carcinomas, 3 apocrine carcinomas, 2 medullary carcinomas, 1 spindle cell carcinoma and 2 squamous cell carcinomas. ‡Factor showing statistical significance. AR, androgen receptor; cfDNA, cell‐free DNA; HR, hazard ratio; HS, Histoscore; IHC, immunohistochemistry; IS, intensity score; LI, labeling index; pAR, AR phosphorylation at serine‐213/210; 95%CI, 95% confidence interval.
In the multivariate Cox hazard analysis, after adjustment for histological grade, Ki67 status, AR status, pAR status and PIK3CA mutation status, which were significant prognostic predictors in the univariate analysis, the presence of PIK3CA mutations of cfDNA corresponded to significantly improved RFS times (P = 0.0003; Table 3). After adjustment for histological grade, Ki67 status, AR status and PIK3CA mutation status, the presence of PIK3CA mutations of cfDNA corresponded to significantly improved BCSS times (P = 0.0012; Table 4).
Discussion
This retrospective study demonstrated accurate PIK3CA mutation detection in plasma using ddPCR in patients with early‐stage TNBC. These results would allow for future trials testing the clinical utility of cfDNA as a cancer biomarker to guide individual decisions about adjuvant systemic therapies. The number of circulating mutant DNA is smaller compared to the number of normal circulating cfDNA fragments. This feature makes it difficult to detect and quantify cfDNA and to apply it for meaningful clinical use.37 Therefore, we adopted the ddPCR system, which has a high level of sensitivity and specificity for somatic mutation detection.27 There was no correlation in the rate of each PIK3CA mutation between cfDNA and the corresponding tumor gDNA (Table S3), but this result does not spoil the reliability of the ddPCR method for two reasons. First, we verified the utility of the ddPCR system using the samples of each PIK3CA mutant‐positive and mutant‐negative case, which were previously verified using another digital PCR system.34 Second, this result may support the increasingly recognized problem of tumor heterogeneity. Chu et al. reported that the presence of ESR1 mutations in tumor tissue gDNA did not match them in plasma tumor DNA.38 They suggested that their results were a result of the problem of tumor heterogeneity and were in agreement with a prior report demonstrating differences in ESR1 mutation status between two metastatic sites within the same patient.39 We demonstrated that a total of 12 (24.4%) of 49 TNBC patients had cfDNA PIK3CA mutations. Ohtani et al. analyzed 313 breast cancer patients by similar methodology and reported 22.7% had PIK3CA mutations in cfDNA40 The amount of total detected cfDNA was related to higher tumor burden (P = 0.087), upper stage (P = 0.064) and higher histological grade (P = 0.043; Table S2), as previously described.37 However, the presence of PIK3CA mutations of cfDNA was an independent factor that was not associated with major prognostic factors of breast cancer (Table 1). Furthermore, we found that AR expression was significantly associated with any cut‐off level of PIK3CA mutations of tumor tissue gDNA (Fig. S1). These results indicated that AR‐positive TN breast cancer possessed PIK3CA kinase mutations of tumor gDNA.10 We also looked at the association between pAR expression, which is dependent on the PI3K/Akt signaling pathway, and the presence of PIK3CA mutations of cfDNA (P = 0.018), with the helical domain PIK3CA mutation (P = 0.03) being overrepresented in comparison with the kinase domain mutation (P = 0.022; Table 2), but pAR expression was not related to any cut‐off level of PIK3CA mutations of tumor tissue gDNA. These findings are consistent with the observation that the presence of PIK3CA mutations of cfDNA may reflect PI3K pathway aberrations in AR positive TNBC more precisely because circulating cfDNA reacts, sometimes dramatically, depending on the surrounding environment.37
In the present study with a median follow up of 54.4 months (range, 4.0–126.4 months), the presence of PIK3CA mutations of cfDNA was a positive prognostic factor on both RFS and BCSS analyzed by the log‐lank test (Fig. 1). In a Cox proportional hazards model, TNBC patients with PIK3CA mutations of cfDNA had significant prognostic values for both RFS and BCSS in the univariate and multivariate analyses (Tables 3 and 4). Furthermore, PIK3CA kinase and helical domain mutations were each significant prognostic factors in the univariate Cox analysis: RFS (P = 0.014) and BCSS (P = 0.030) in kinase domain mutation and RFS (P = 0.0099) and BCSS (P = 0.018) in helical domain mutation (see Tables 3 and 4). These data suggested that PIK3CA mutations were significantly associated with a more favorable prognosis,8, 41 and the presence of PIK3CA mutations of cfDNA could be discriminatory predictors of RFS and BCSS in TNBC.
Different from other oncogenes and unique to PIK3CA, it seems that activation of this oncogene might somehow exert a “protective” function for breast cancer patients. The biggest reason is that PIK3CA mutations show significant concordance, possibly pointing to an important interaction with AR signaling pathway which is likely to be associated with a good prognosis in TNBC patients: RFS (P = 0.062) and BCSS (P = 0.022) in AR and RFS (P = 0.048) and BCSS (P = 0.12) in pAR.
PIK3CA mutations of cfDNA are also emerging tumor markers that, in the future, might be used in the process of choosing a treatment. In HER2‐amplified breast cancer, we showed that activation of the PI3K pathway depending on the presence of PIK3CA mutations was associated with a lower frequency of pathological complete response in neoadjuvant treatment.34 Anti‐androgen therapy has been investigated as a treatment for ER/PgR‐negative AR‐positive breast cancer patients42, 43 and is now being explored in this patient population (see clinicaltrials.gov: NCT00468715, NCT00516542, NCT00755885 and NCT00972023). In the preclinical setting, genetic or pharmacological targeting of AR in LAR cells increases the growth inhibitory activity of PI3K inhibitors.43 The same group explored the combination of AR antagonism and PI3K inhibition and found an additive or synergistic effect on AR‐positive TNBC cell growth. Furthermore, resistance to bicalutamide, which is a non‐steroidal anti‐androgen drug, could be reversed by inhibitors of PI3K or the mammalian target of rapamycin (mTOR).44 These findings show that TN breast cancer patients with mutant PIK3CA may benefit from androgen blockade alone or from androgen blockade added to PI3K‐targeted therapies.45 Given that the presence of PIK3CA mutations of cfDNA is a reliable surrogate of pAR expression within TNBC, cfDNA could be adopted as a biomarker without the need for accessing tumor tissues for individual decisions about adjuvant systemic therapies, in particular, anti‐androgen agents.
Our study provides a proof of concept and attests to the feasibility of cfDNA analysis for patients with early stage TNBC; however, our study has some limitations, including that it is a retrospective, single‐institute study with a relatively small patient cohort, and 59.2% (29/49) of our patients had received adjuvant systemic therapies. In addition, we assayed only for hotspot mutations in the PIK3CA gene. We are currently expanding this study to recurrent/metastatic patients and performing broader mutational analyses to obtain additional “markers” to grasp the condition of each patient.
In conclusion, we showed that the presence of PIK3CA major mutations of cfDNA could be a discriminatory predictor of RFS and BCSS. In addition, we found that the presence of PIK3CA major mutations of cfDNA was associated with PI3K pathway‐dependent AR phosphorylation. In the near future, biomarker analysis may clarify the criteria to increase the benefit from systemic therapies in TN patients.
Disclosure Statement
The authors have no conflict of interest to declare.
Supporting information
Fig. S1. Correlation of proportion of PIK3CA mutations of tumor tissue gDNA with androgen receptor (AR) (a) and pAR (b) positive rate. Cut‐off level of PIK3CA mutant percentage to total is set as 1, 5, 10 and 20%.
Fig. S2. Representative staining patterns of androgen receptor (AR) and PI3K/Akt signaling pathway (pAR) immunohistochemistry (magnification ×40).
Table S1. Patients characteristics.
Table S2. The correlation of patients’ characteristics with the quantity of total cell‐free DNA (cfDNA).
Table S3. The concordance rate of PIK3CA mutations between cell‐free DNA (cfDNA) and corresponding tumor tissue DNA.
Acknowledgments
This work was supported in part by a Grant‐in‐Aid (project no. 2459191000) for Scientific Research from the Ministry of Education, Science, and Culture of Japan.
Cancer Sci 106 (2015) 1582–1589
Funding Information
This work was supported in part by a Grant‐in‐Aid project no. 2459191000 for Scientific Research from the Ministry of Education, Science, and Culture of Japan
References
- 1. Lin NU, Vanderplas A, Hughes ME et al Clinicopathologic features, patterns of recurrence, and survival among women with triple‐negative breast cancer in the National Comprehensive Cancer Network. Cancer 2012; 118: 5463–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dent R, Trudeau M, Pritchard KI et al Triple‐negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res 2007; 13: 4429–34. [DOI] [PubMed] [Google Scholar]
- 3. Iwase H, Kurebayashi J, Tsuda H et al Clinicopathological analyses of triple negative breast cancer using surveillance data from the Registration Committee of the Japanese Breast Cancer Society. Breast Cancer 2010; 17: 118–24. [DOI] [PubMed] [Google Scholar]
- 4. Cancer Genome Atlas N . Comprehensive molecular portraits of human breast tumours. Nature 2012; 490: 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shah SP, Roth A, Goya R et al The clonal and mutational evolution spectrum of primary triple‐negative breast cancers. Nature 2012; 486: 395–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Saal LH, Holm K, Maurer M et al PIK3CA mutations correlate with hormone receptors, node metastasis, and ERBB2, and are mutually exclusive with PTEN loss in human breast carcinoma. Cancer Res 2005; 65: 2554–9. [DOI] [PubMed] [Google Scholar]
- 7. Stemke‐Hale K, Gonzalez‐Angulo AM, Lluch A et al An integrative genomic and proteomic analysis of PIK3CA, PTEN, and AKT mutations in breast cancer. Cancer Res 2008; 68: 6084–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cizkova M, Susini A, Vacher S et al PIK3CA mutation impact on survival in breast cancer patients and in ERalpha, PR and ERBB2‐based subgroups. Breast Cancer Res 2012; 14: R28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Doane AS, Danso M, Lal P et al An estrogen receptor‐negative breast cancer subset characterized by a hormonally regulated transcriptional program and response to androgen. Oncogene 2006; 25: 3994–4008. [DOI] [PubMed] [Google Scholar]
- 10. Gonzalez‐Angulo AM, Stemke‐Hale K, Palla SL et al Androgen receptor levels and association with PIK3CA mutations and prognosis in breast cancer. Clin Cancer Res 2009; 15: 2472–8. [DOI] [PubMed] [Google Scholar]
- 11. Pang B, Cheng S, Sun SP et al Prognostic role of PIK3CA mutations and their association with hormone receptor expression in breast cancer: a meta‐analysis. Sci Rep 2014; 4: 6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kalinsky K, Jacks LM, Heguy A et al PIK3CA mutation associates with improved outcome in breast cancer. Clin Cancer Res 2009; 15: 5049–59. [DOI] [PubMed] [Google Scholar]
- 13. Zardavas D, Phillips WA, Loi S. PIK3CA mutations in breast cancer: reconciling findings from preclinical and clinical data. Breast Cancer Res 2014; 16: 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cuorvo LV, Verderio P, Ciniselli CM et al PI3KCA mutation status is of limited prognostic relevance in ER‐positive breast cancer patients treated with hormone therapy. Virchows Arch 2014; 464: 85–93. [DOI] [PubMed] [Google Scholar]
- 15. Peters AA, Buchanan G, Ricciardelli C et al Androgen receptor inhibits estrogen receptor‐alpha activity and is prognostic in breast cancer. Cancer Res 2009; 69: 6131–40. [DOI] [PubMed] [Google Scholar]
- 16. Agoff SN, Swanson PE, Linden H, Hawes SE, Lawton TJ. Androgen receptor expression in estrogen receptor‐negative breast cancer. Immunohistochemical, clinical, and prognostic associations. Am J Clin Pathol 2003; 120: 725–31. [DOI] [PubMed] [Google Scholar]
- 17. He J, Peng R, Yuan Z et al Prognostic value of androgen receptor expression in operable triple‐negative breast cancer: a retrospective analysis based on a tissue microarray. Med Oncol 2012; 29: 406–10. [DOI] [PubMed] [Google Scholar]
- 18. Micello D, Marando A, Sahnane N, Riva C, Capella C, Sessa F. Androgen receptor is frequently expressed in HER2‐positive. ER/PR‐negative breast cancers. Virchows Arch 2010; 457: 467–76. [DOI] [PubMed] [Google Scholar]
- 19. Tang D, Xu S, Zhang Q, Zhao W. The expression and clinical significance of the androgen receptor and E‐cadherin in triple‐negative breast cancer. Med Oncol 2012; 29: 526–33. [DOI] [PubMed] [Google Scholar]
- 20. Hu R, Dawood S, Holmes MD et al Androgen receptor expression and breast cancer survival in postmenopausal women. Clin Cancer Res 2011; 17: 1867–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Takeshita T, Omoto Y, Yamamoto‐Ibusuki M, Yamamoto Y, Iwase H. Clinical significance of androgen receptor and its phosphorylated form in breast cancer. Endocr Relat Cancer 2013; 20: L15–21. [DOI] [PubMed] [Google Scholar]
- 22. Diehl F, Li M, Dressman D et al Detection and quantification of mutations in the plasma of patients with colorectal tumors. Proc Natl Acad Sci USA 2005; 102: 16368–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dawson SJ, Tsui DW, Murtaza M et al Analysis of circulating tumor DNA to monitor metastatic breast cancer. N Engl J Med 2013; 368: 1199–209. [DOI] [PubMed] [Google Scholar]
- 24. Wang C, Ozaki T, Takita R, Uchiyama M. Aryl ether as a Negishi coupling partner: an approach for constructing C‐C bonds under mild conditions. Chemistry 2012; 18: 3482–5. [DOI] [PubMed] [Google Scholar]
- 25. Board RE, Wardley AM, Dixon JM et al Detection of PIK3CA mutations in circulating free DNA in patients with breast cancer. Breast Cancer Res Treat 2010; 120: 461–7. [DOI] [PubMed] [Google Scholar]
- 26. Beaver JA, Jelovac D, Balukrishna S et al Detection of cancer DNA in plasma of patients with early‐stage breast cancer. Clin Cancer Res 2014; 20: 2643–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hindson CM, Chevillet JR, Briggs HA et al Absolute quantification by droplet digital PCR versus analog real‐time PCR. Nat Methods 2013; 10: 1003–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hindson BJ, Ness KD, Masquelier DA et al High‐throughput droplet digital PCR system for absolute quantitation of DNA copy number. Anal Chem 2011; 83: 8604–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Karakas B, Bachman KE, Park BH. Mutation of the PIK3CA oncogene in human cancers. Br J Cancer 2006; 94: 455–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Goldhirsch A, Wood WC, Gelber RD, Coates AS, Thurlimann B, Senn HJ. Meeting highlights: updated international expert consensus on the primary therapy of early breast cancer. J Clin Oncol 2003; 21: 3357–65. [DOI] [PubMed] [Google Scholar]
- 31. Goldhirsch A, Glick JH, Gelber RD et al Meeting highlights: international expert consensus on the primary therapy of early breast cancer 2005. Ann Oncol 2005; 16: 1569–83. [DOI] [PubMed] [Google Scholar]
- 32. Goldhirsch A, Wood WC, Gelber RD et al Progress and promise: highlights of the international expert consensus on the primary therapy of early breast cancer 2007. Ann Oncol 2007; 18: 1133–44. [DOI] [PubMed] [Google Scholar]
- 33. Pekin D, Skhiri Y, Baret JC et al Quantitative and sensitive detection of rare mutations using droplet‐based microfluidics. Lab Chip 2011; 11: 2156–66. [DOI] [PubMed] [Google Scholar]
- 34. Sueta A, Yamamoto Y, Yamamoto‐Ibusuki M et al An integrative analysis of PIK3CA mutation, PTEN, and INPP4B expression in terms of trastuzumab efficacy in HER2‐positive breast cancer. PLoS ONE 2014; 9: e116054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McShane LM, Altman DG, Sauerbrei W et al Reporting recommendations for tumor marker prognostic studies. J Clin Oncol 2005; 23: 9067–72. [DOI] [PubMed] [Google Scholar]
- 36. Goldhirsch A, Wood WC, Coates AS et al Strategies for subtypes–Dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol 2011; 22: 1736–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Diaz LA Jr, Bardelli A. Liquid biopsies: genotyping circulating tumor DNA. J Clin Oncol 2014; 32: 579–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chu D, Paoletti C, Gersch C et al ESR1 mutations in circulating plasma tumor DNA from metastatic breast cancer patients. Clin Cancer Res 2015; doi: 10.1158/1078‐0432.CCR‐15‐0943 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Merenbakh‐Lamin K, Ben‐Baruch N, Yeheskel A et al D538G mutation in estrogen receptor‐alpha: a novel mechanism for acquired endocrine resistance in breast cancer. Cancer Res 2013; 73: 6856–64. [DOI] [PubMed] [Google Scholar]
- 40. Oshiro C, Kagara N, Naoi Y et al PIK3CA mutations in serum DNA are predictive of recurrence in primary breast cancer patients. Breast Cancer Res Treat 2015; 150: 299–307. [DOI] [PubMed] [Google Scholar]
- 41. Perez‐Tenorio G, Alkhori L, Olsson B et al PIK3CA mutations and PTEN loss correlate with similar prognostic factors and are not mutually exclusive in breast cancer. Clin Cancer Res 2007; 13: 3577–84. [DOI] [PubMed] [Google Scholar]
- 42. Hardin C, Pommier R, Calhoun K, Muller P, Jackson T, Pommier S. A new hormonal therapy for estrogen receptor‐negative breast cancer. World J Surg 2007; 31: 1041–6. [DOI] [PubMed] [Google Scholar]
- 43. Lehmann BD, Bauer JA, Schafer JM et al PIK3CA mutations in androgen receptor‐positive triple negative breast cancer confer sensitivity to the combination of PI3K and androgen receptor inhibitors. Breast Cancer Res 2014; 16: 406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Eichhorn PJ, Gili M, Scaltriti M et al Phosphatidylinositol 3‐kinase hyperactivation results in lapatinib resistance that is reversed by the mTOR/phosphatidylinositol 3‐kinase inhibitor NVP‐BEZ235. Cancer Res 2008; 68: 9221–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Garcia‐Echeverria C, Sellers WR. Drug discovery approaches targeting the PI3K/Akt pathway in cancer. Oncogene 2008; 27: 5511–26. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Correlation of proportion of PIK3CA mutations of tumor tissue gDNA with androgen receptor (AR) (a) and pAR (b) positive rate. Cut‐off level of PIK3CA mutant percentage to total is set as 1, 5, 10 and 20%.
Fig. S2. Representative staining patterns of androgen receptor (AR) and PI3K/Akt signaling pathway (pAR) immunohistochemistry (magnification ×40).
Table S1. Patients characteristics.
Table S2. The correlation of patients’ characteristics with the quantity of total cell‐free DNA (cfDNA).
Table S3. The concordance rate of PIK3CA mutations between cell‐free DNA (cfDNA) and corresponding tumor tissue DNA.


