Abstract
In solid tumors, decreased absolute lymphocyte count (ALC) at diagnosis was found to be associated with poorer outcome, but there is only limited data on the clinical impact of ALC at the time of diagnosis in acute myeloid leukemia (AML). In this study we evaluated the prognostic value of ALC at time of diagnosis on outcome in 259 patients with AML who responded to induction therapy. Multivariable analysis indicated that higher than normal lymphocyte count at diagnosis was associated with shorter remission (HR 4.06); 95% CI, 2.29 – 7.21; P<0.001), and decreased relapse free and overall survival (HR 3.47; P<0.001 and HR 3.85; P<0.001 respectively). Flow cytometry showed low frequency of natural killer (NK) cells and high frequency of CD4+ T cells (which includes the subset of T regulatory cells; CD4+CD25+Foxp3+ cells) in the high ALC group. Low frequency of NK cells and potentially high frequency of inhibitory T regulatory cells may result in weaker immune responses against residual leukemia remaining after initial response to induction therapy, and thus, may explain the poorer outcome of the high ALC group.
Keywords: Acute myeloid leukemia (AML), absolute lymphocyte count (ALC)
Introduction
Despite advances in diagnosis and therapy, the outcome of acute myeloid leukemia (AML) patients remains poor, with on average less than 30% 5-year overall survival (OS) (1, 2). Although covariates such as age, cytogenetics, and status of the NPM1 and FLT3 genes can give prognostic information beyond this average, their predictive ability is suboptimal (3). Therefore additional factors, which may affect treatment outcomes should be evaluated. Additionally better understanding and modulating the immune system may improve treatment and survival in patients with AML.
In solid tumors, decreased absolute lymphocyte count (ALC) at diagnosis was found to be associated with poorer outcome (4–7), however there is only limited data on the clinical impact of ALC at the time of diagnosis in AML (8). Thus, the primary objective of the current study was to evaluate the prognostic value of initial ALC with respect to AML outcome. After initially noting that ALC was not associated with complete response (CR) rate in 380 newly diagnosed AML patients, we retrospectively evaluated the prognostic value of ALC at time of diagnosis on post induction relapse and survival in patients who achieved CR, CRp, or CRi each +/− minimal residual disease (MRD) after induction therapy.
Patients and Methods
Study Population
In this retrospective study we evaluated all newly diagnosed adult patients treated at the FHCRC between January 2008 and June 2013 and achieved complete remission (CR). Diagnosis of AML and determination of disease status after treatment were based on morphology evaluation of bone marrow aspirate and biopsy, flow cytometry, cytogenetics, and molecular studies in some cases. High intensity induction chemotherapy was defined as any regimen that contained cytarabine at a daily dose of ≥ 500 mg/m2, Cytarabine + Idarubicin (7+3) was considered as intermediate intensity, and lower doses chemotherapy or hypomethylation agents were considered as low intensity therapy. CR was defined as <5% blasts in marrow by morphology with neutrophil and platelet counts > 1000 and >100,000/μl respectively. CRp was defined as CR except platelet count was < 100,000; CRi required a marrow that was at least 10% cellular with < 5% blasts but with neutrophil count <1000/μl. Minimal residual disease (MRD) was defined as less than 5% blasts by morphology but with evidence of any disease by flow cytometry or cytogenetics. All patients provided informed consent for treatment based on standard treatment plans or clinical trials, and Institutional review board approval was obtained to retrospectively collect data from patient records and databases.
Identification of lymphocyte subsets by flow cytometry
Flow cytometry analysis was performed on a modified 4-laser, 10-color Becton Dickinson LSRII flow cytometer (BD Biosciences, San Jose, CA) as previously described at UWMC Hematopathology Laboratory (9). The following laser-fluorochrome combinations were used: (i) 405-nm violet laser (1 color) and Pacific blue (PB); (ii) 488-nm blue laser (5 colors), fluorescein isothiocyanate (FITC), phycoerytherin (PE), PE-Texas red (ECD/PE-TR), PE-cyanine (Cy)-5, and PE-Cy7; (iii) 594-nm yellow laser (1 color) and Alexa Fluor 594 (A594); and (iv) 633-nm red laser (3 colors), allophycocyanin (APC), APC-Alexa Fluor 700 (A700; BD Biosciences), and APC-Cy7. The specific fluorescently labeled antibodies used in this study were obtained primarily from Beckman Coulter (BC, Fullerton, CA) and Becton Dickinson (BD, San Jose, CA). Antibodies used in three tubes were as follows: (i): HLA-DR PB, CD15 FITC, CD33 PE, CD19 PE-TR, CD117 PE-Cy5, CD13 PE-Cy7, CD38 A594, CD34 APC, CD71 APC-A700, and CD45 APC-H7; (ii) HLA-DR PB, CD64 FITC, CD123 PE, CD4 PE-TR, CD14 PE Cy5.5, CD13 PE-Cy7, CD38 A594, CD34 APC, CD16 APC-A700, and CD45 APC-H7; (iii) CD56 Alexa488, CD7 PE, CD5 PE-Cy5, CD33 PE-Cy7, CD38 A594, CD34 APC, and CD45 APC-H7, or CD2 FITC (BC), CD5 PE (BD), CD34 PE-TR (BC), CD56 PE-Cy5 (BC), CD3 PE-Cy7 (BC), CD8 BV421 (Bio Legend), CD4 A594 (BD), CD7 APC (Invitrogen), CD30 APC-A700 (BC), and CD45 APC-H7 (BD). For the analysis, 100-ul aliquots of peripheral blood or bone marrow were processed with a standard whole blood lysing procedure using buffered ammonium chloride, followed by washing with phosphate-buffered saline-bovine serum albumin (PBS-BSA)-azide to remove plasma protein, as previously described (10). 100-ul cell suspension were incubated with titered antibodies, washed with PBS-BSA-azide, and resuspended in 1% paraformaldehyde. Data were analyzed using an in-house software developed by one of the coauthors (B.L.W.). T cell subsets were gated based on T cell antigen expression.
Statistical Analysis
Fisher’s exact test was used to compare categorical covariates and the Wilcoxon rank sum test was used to compare numerical covariates. OS was measured from date of response (CR, CRp, CRi) to the date of death due to any cause with patients last known to be alive censored at the date of last contact. Relapse-free survival (RFS) was measured from date of response to date of relapse (marrow with > 5% morphologic blasts unrelated to blood count recovery) or death due to any cause with patients last known to be alive in CR censored at the date of last contact. The Kaplan-Meier method was used to examine OS and RFS and remission duration with death treated as a competing risk for the latter. Logistic regression was used to model the outcome CR, and Cox regression was used to models the outcomes OS and RFS. Proportional subdistribution hazards regression models were used to model length of CR with death from any cause treated as a competing risk event (11).
Results
Patient characteristics
The study cohort included 259 patients with median age of 56 years (range, 18–84 years). There were 143 patients with de novo AML, and 116 with secondary AML (antecedent hematologic disorder or therapy-related). Using SWOG criteria 11% of the patients had “favorable” karyotype, 51% intermediate, and 29% unfavorable with cytogenetics unknown in the remaining 9%. Intensity of induction therapy was considered high in 41%, intermediate in 39% and low in 20%. In remission patients typically continued these therapies, with 49% receiving an allogeneic hematopoietic cell transplantion (HCT). Median ALC at diagnosis was 1.65 with 12%, 61%, and 27% of patients found to have high, normal, and low ALCs respectively. Patients with high ALC tended to more often have favorable cytogenetics and less often unfavorable cytogenetics than patients with low ALC, they less often had secondary AML and less often received low intensity induction therapy. They also were more likely to achieve CR without MRD. In the entire cohort 69% of the patients achieved CR with neutrophil and platelet count recovery and with no MRD and 20% of the patients had CR with MRD. Medians were 1.95 years for RFS, and 3.55 years for OS. Median follow-up time for censored patients was 1.4 years. Patient Characteristics according to ALC at diagnosis are shown in Table 1.
Table 1. Patient Characteristics.
Median (range) or N (%)
| All patients | Low Initial ALC (≤1 × 103/uL) n = 71 |
Normal Initial ALC (>1 – 4.8 × 103/uL) n = 157 |
High Initial ALC (> 4.8 × 103/uL) n = 31 |
P value | |
|---|---|---|---|---|---|
|
| |||||
| Age, years | 56 (18–84) | 56 (23, 84) | 58 (18, 81) | 51 (19, 70) | 0.002 |
|
| |||||
| Male/Female | 151/108 | 44/27 | 94/63 | 13/18 | 0.14 |
|
| |||||
| WBC at diagnosis, ×103/μL | 6 (0.26, 96.48) | 1.52 (0.26, 119.89) | 7.79 (1.3, 226.94) | 40.5 (22.39, 296.48) | <0.001 |
|
| |||||
| ANC at diagnosis, ×103/μL | 1 (0, 101) | 1 (0, 22) | 1 (0, 101) | 1 (0, 73) | <0.001 |
|
| |||||
| Platelet at diagnosis, ×103/μL | 54 (5, 1698) | 51 (5, 249) | 57 (6, 1698) | 48 (12, 104) | 0.45 |
|
| |||||
| Cytogenetic risk group | <0.001 | ||||
| - Favorable | 29 (11) | 1 (1) | 18 (11) | 10 (32) | |
| - Intermediate | 131 (51) | 33 (46) | 83 (53) | 15 (48) | |
| - Unfavorable | 75 (29) | 28 (39) | 43 (27) | 4 (13) | |
| - Missing | 24 (9) | 9 (13) | 13 (8) | 2 (6) | |
|
| |||||
| Secondary AML | 116 (45) | 47 (66) | 64 (41) | 5 (16) | <0.001 |
|
| |||||
| Intensity of induction therapy | 0.003 | ||||
| - High | 107 (41) | 32 (45) | 60 (38) | 15 (48) | |
| - Intermediate | 100 (39) | 19 (27) | 65 (41) | 16 (52) | |
| - Low | 52 (20) | 20 (28) | 32 (20) | 0 (0) | |
|
| |||||
| CR status | 0.057 | ||||
| - CR | 180 (69) | 42 (59) | 113 (72) | 25 (81) | |
| - CRi | 28 (11) | 13 (18) | 12 (8) | 3 (10) | |
| - CR-MRD | 38 (15) | 9 (13) | 26 (17) | 3 (10) | |
| - CRi-MRD | 13 (5) | 7 (10) | 6 (4) | 0 (0) | |
|
| |||||
| # of patient who underwent HCT | 127 (49) | 45 (63) | 67 (43) | 15 (48) | 0.012 |
Effect of absolute lymphocyte count at time of diagnosis on treatment outcome
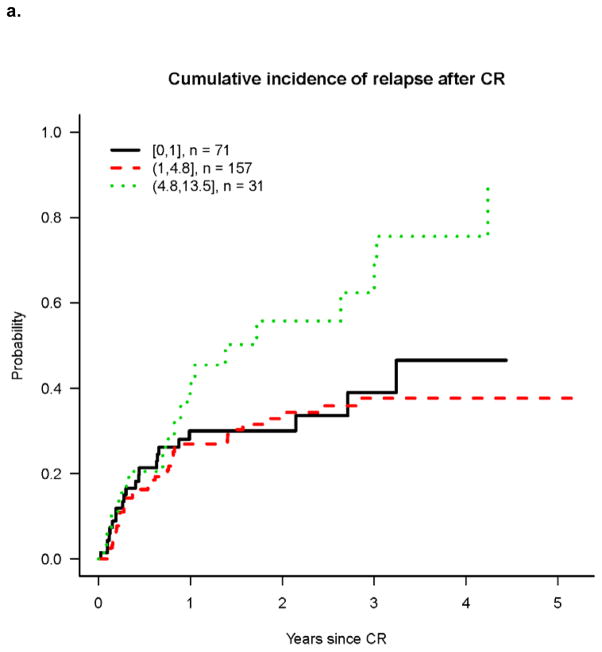
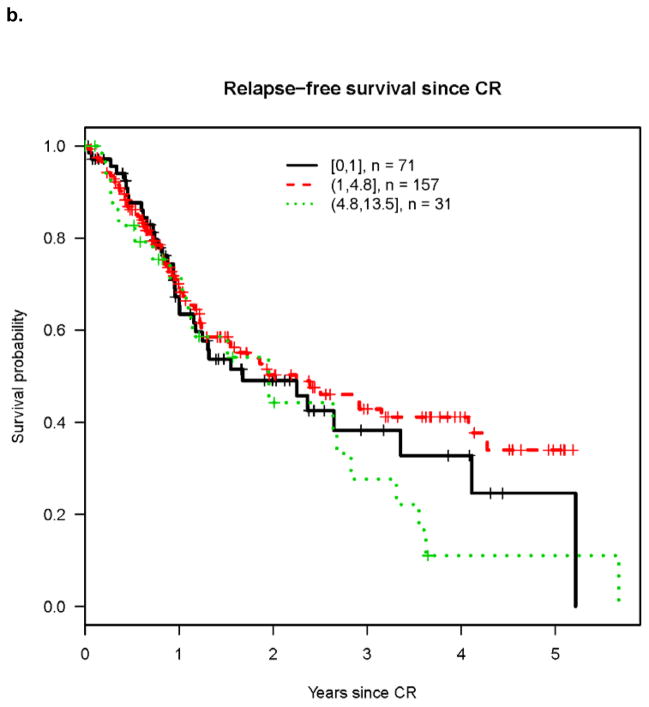
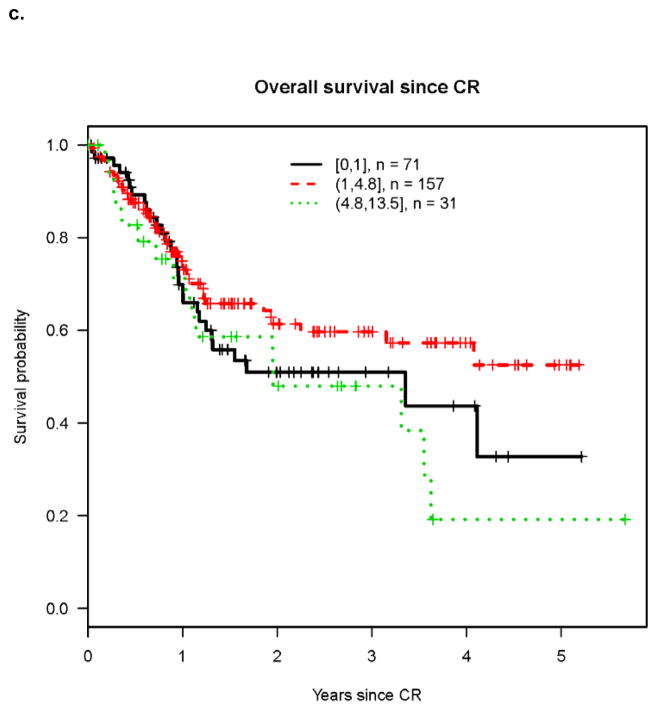
Univariate analysis revealed that cumulative incidence of relapse (CIR) was highest in patients with high presenting ALC (Figure 1a) while differences in RFS and OS were less marked among patients with high, normal and low ALC (Figures 1b and 1c). However on multivariable analysis, high pre-treatment ALC was independently associated with higher CIR, and shorter RFS and OS (Tables 2–4). Hazard rates relative to normal pre-treatment ALC were 4.06 (95% CI, 2.29 – 7.21; P<0.001) for CIR (Table 2), 3.47 (95% CI, 1.78 – 6.77; P<0.001) for RFS (Table 3), and 3.85 (95% CI, 1.85 – 8.01; P<0.001) for OS (Table 4).
Figure 1. Outcomes after achieving CR according to ALC at time of diagnosis.
a. Cumulative incidence of relapse after CR
b. Relapse free survival
c. Overall survival after CR
Table 2.
Multivariable analysis of risk factors for duration of first CR
| Covariate | HR | 95% CI | P-value |
|---|---|---|---|
| Low pre-treatment lymphocytes (ref=normal) | 1.15 | (0.61, 2.19) | 0.67 |
| High pre-treatment lymphocytes (ref=normal) | 4.06 | (2.29, 7.21) | <0.001 |
| Low remission lymphocytes (ref=normal) | 1.56 | (0.91, 2.69) | 0.11 |
| Age at diagnosis (years) | 1.02 | (1, 1.04) | 0.088 |
| Secondary AML (ref = de novo AML) | 1.51 | (0.87, 2.64) | 0.14 |
| Intermediate intensity treatment (ref=high) | 0.79 | (0.43, 1.44) | 0.44 |
| Low intensity treatment (ref = high) | 0.37 | (0.16, 0.89) | 0.027 |
| Favorable cytogenetics (ref = intermediate) | 0.59 | (0.28, 1.27) | 0.18 |
| Unfavorable cytogenetics (ref = intermediate) | 1.28 | (0.7, 2.33) | 0.43 |
| Unknown cytogenetics (ref = intermediate) | 3.32 | (0.87, 12.61) | 0.078 |
| CR-MRD (ref = CR) | 4.69 | (2.34, 9.42) | <0.001 |
| CRi (ref = CR) | 2.28 | (0.91, 5.68) | 0.077 |
| CRi-MRD (ref = CR) | 4.63 | (1.39, 15.41) | 0.013 |
| HCT (ref = no HCT) | 0.51 | (0.3, 0.85) | 0.01 |
| Other NPM1/FLT3 (ref = NPM1+/FLT3-) | 1.26 | (0.65, 2.45) | 0.49 |
| Missing NPM1/FLT3 (ref = NPM1+/FLT3- | 0.83 | (0.36, 1.9) | 0.66 |
| Pre-treatment platelets | 1 | (0.99, 1) | 0.26 |
| Pre-treatment ANC | 1.01 | (0.99, 1.03) | 0.26 |
| Pre-treatment monocytes | 1.01 | (1, 1.03) | 0.11 |
| Response platelets | 1 | (1, 1) | 0.32 |
| Response ANC | 0.97 | (0.94, 1.01) | 0.14 |
| Response monocytes | 1.13 | (0.96, 1.32) | 0.14 |
Table 4.
Multivariable analysis of risk factors for Overall Survival
| Covariate | HR | 95% CI | P-value |
|---|---|---|---|
| Low pre-treatment lymphocytes (ref=normal) | 0.92 | (0.54, 1.57) | 0.75 |
| High pre-treatment lymphocytes (ref=normal) | 3.85 | (1.85, 8.01) | <0.001 |
| Low remission lymphocytes (ref=normal) | 0.56 | (0.33, 0.96) | 0.036 |
| Age at diagnosis (years) | 1.01 | (0.99, 1.03) | 0.17 |
| Secondary AML (ref = de novo AML) | 1.78 | (1.03, 3.07) | 0.038 |
| Intermediate intensity treatment (ref=high) | 0.96 | (0.56, 1.62) | 0.87 |
| Low intensity treatment (ref = high) | 0.62 | (0.31, 1.25) | 0.18 |
| Favorable cytogenetics (ref = intermediate) | 0.42 | (0.16, 1.07) | 0.068 |
| Unfavorable cytogenetics (ref = intermediate) | 1.31 | (0.79, 2.18) | 0.29 |
| Unknown cytogenetics (ref = intermediate) | 2.55 | (0.71, 9.11) | 0.15 |
| CR-MRD (ref = CR) | 3.62 | (1.93, 6.79) | <0.001 |
| CRi (ref = CR) | 2.41 | (1.13, 5.17) | 0.023 |
| CRi-MRD (ref = CR) | 5.03 | (2.11, 11.98) | <0.001 |
| HCT (ref = no HCT) | 0.31 | (0.19, 0.52) | <0.001 |
| Other NPM1/FLT3 (ref = NPM1+/FLT3-) | 3.39 | (1.15, 9.94) | 0.026 |
| Missing NPM1/FLT3 (ref = NPM1+/FLT3- | 3.92 | (1.28, 12) | 0.017 |
| Pre-treatment platelets | 1 | (0.99, 1) | 0.17 |
| Pre-treatment ANC | 1.01 | (0.98, 1.03) | 0.54 |
| Pre-treatment monocytes | 0.99 | (0.97, 1.02) | 0.66 |
| Response platelets | 1 | (1, 1) | 0.78 |
| Response ANC | 0.93 | (0.87, 1.01) | 0.075 |
| Response monocytes | 1.15 | (0.88, 1.51) | 0.29 |
Table 3.
Multivariable analysis of risk factors for Relapse Free Survival
| Covariate | HR | 95% CI | P-value |
|---|---|---|---|
| Low pre-treatment lymphocytes (ref=normal) | 0.95 | (0.6, 1.52) | 0.84 |
| High pre-treatment lymphocytes (ref=normal) | 3.47 | (1.78, 6.77) | <0.001 |
| Low remission lymphocytes (ref=normal) | 0.83 | (0.53, 1.28) | 0.4 |
| Age at diagnosis (years) | 1.02 | (1, 1.04) | 0.033 |
| Secondary AML (ref = de novo AML) | 1.56 | (0.98, 2.5) | 0.063 |
| Intermediate intensity treatment (ref=high) | 0.85 | (0.53, 1.37) | 0.51 |
| Low intensity treatment (ref = high) | 0.64 | (0.35, 1.17) | 0.15 |
| Favorable cytogenetics (ref = intermediate) | 0.52 | (0.24, 1.12) | 0.093 |
| Unfavorable cytogenetics (ref = intermediate) | 1.14 | (0.72, 1.8) | 0.57 |
| Unknown cytogenetics (ref = intermediate) | 2.53 | (0.72, 8.93) | 0.15 |
| CR-MRD (ref = CR) | 4.29 | (2.43, 7.58) | <0.001 |
| CRi (ref = CR) | 2.67 | (1.36, 5.27) | 0.0045 |
| CRi-MRD (ref = CR) | 5.72 | (2.55, 12.79) | <0.001 |
| HCT (ref = no HCT) | 0.32 | (0.21, 0.49) | <0.001 |
| Other NPM1/FLT3 (ref = NPM1+/FLT3-) | 1.75 | (0.84, 3.66) | 0.13 |
| Missing NPM1/FLT3 (ref = NPM1+/FLT3- | 1.99 | (0.91, 4.33) | 0.084 |
| Pre-treatment platelets | 1 | (0.99, 1) | 0.12 |
| Pre-treatment ANC | 1.01 | (0.98, 1.03) | 0.6 |
| Pre-treatment monocytes | 0.99 | (0.97, 1.02) | 0.63 |
| Response platelets | 1 | (1,1) | 0.79 |
| Response ANC | 0.97 | (0.92, 1.02) | 0.24 |
| Response monocytes | 1.17 | (0.96, 1.42) | 0.13 |
Additional covariates that were statistically significantly associated with AML outcome in our cohort were disease status after induction therapy (CR versus CR with MRD or CRi), and HCT (Tables 2,3,4).
Lymphocyte subpopulations
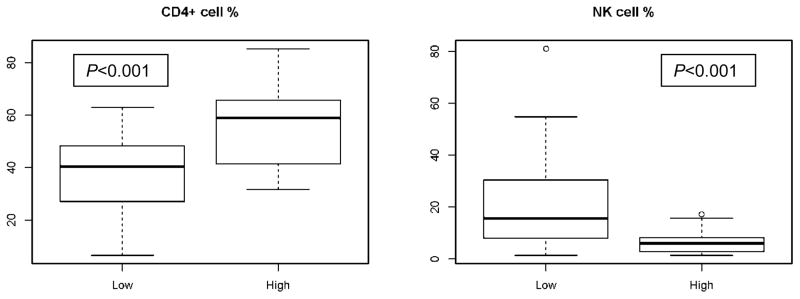
In order to evaluate whether the effect of pretreatment ALC on post induction outcomes reflected the effect of specific lymphocyte subpopulations we reviewed flow cytometry on bone marrow (before and after treatment) and peripheral blood (before treatment) of patients with high ALC and patients with low ALC at time of diagnosis. Pre-treatment flow cytometry evaluation revealed a statistically significant lower frequency of natural killer (NK) cells in the high ALC group in the marrow (median 5% vs 13%; P<0.001) and blood (median 6% vs 14%; P<0.001) (Tables 5a,b). The same was true in marrow samples after recovery from induction therapy (median day 27 post-treatment; range 17–81) (median 6% in the high vs 16% in the low ALC groups; P<0.001). However the pots-recovery marrow of the high ALC group had a higher proportion of total T cells (median 92% vs. 76%, P < 0.001) and CD4+ T cells (medians 59% vs 40%, p < 0.001) than the low ALC group (Table 6). Frequencies of NK cells and CD4+ T cells in post-treatment marrow samples from patients in the high and low ALC groups are shown in figure 2.
Table 5.
Lymphocyte subpopulation pre-treatment in patient with low and high ALC count at diagnosis
| a. Bone marrow | |||
|---|---|---|---|
| Low ALC (n = 30) | High ALC (n=23) | P-value | |
| CD4% | 37 (17, 56) | 39 (21, 70) | 0.27 |
| CD8% | 35 (11, 60) | 37 (31, 44) | 0.47 |
| T-cell% | 74 (44, 98) | 76 (61, 97) | 0.19 |
| B-cell% | 10 (0, 34) | 10 (0, 26) | 0.76 |
| NK cell% | 13 (1, 34) | 5 (2, 13) | <0.001 |
| b. Peripheral blood | |||
|---|---|---|---|
| Low ALC (n =10) | High ALC (n=18) | P-value | |
| CD4% | 42 (26, 50) | 43 (24, 60) | 0.4 |
| CD8% | 22 (14, 41) | 20 (12, 51) | 0.75 |
| T-cell% | 70 (50, 77) | 71 (55, 86) | 0.43 |
| B-cell% | 14 (0, 27) | 17 (8, 30) | 0.2 |
| NK cell% | 14 (5, 42) | 6 (1, 13) | <0.001 |
Table 6.
Lymphocyte subpopulation in bone marrow at post-treatment recovery in patient with low and high ALC count at diagnosis
| Low ALC (n=31) | High ALC (n=19) | P-value | |
|---|---|---|---|
| CD4% | 40 (6, 63) | 59 (32, 85) | <0.001 |
| CD8% | 37 (2, 72) | 29 (13, 61) | 0.99 |
| T-cell% | 76 (9, 94) | 92 (79, 98) | <0.001 |
| B-cell% | 1 (0, 31) | 0 (0, 4) | 0.079 |
| NK cell% | 16 (1, 81) | 6 (1, 16) | <0.001 |
Figure 2.
Distribution of CD4+ T cells and NK cells in marrow after recovery from induction therapy in patients with high and low ALC at time of diagnosis.
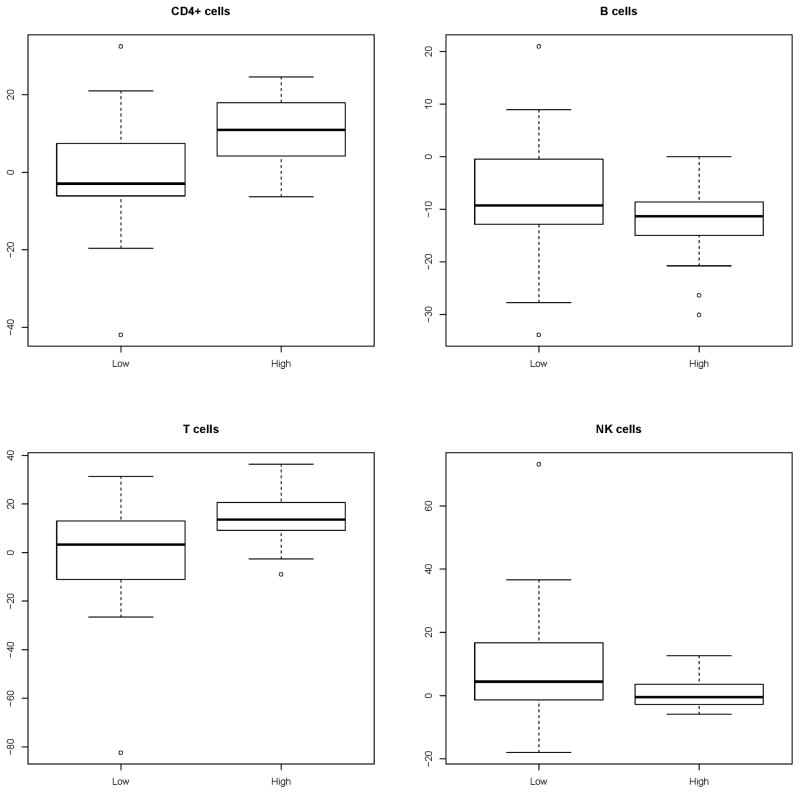
Noting that the frequencies of total T cells and CD4+ T cells were similar pre-treatment in the low and high ALC groups the frequency of CD4+ T cells in the marrow at recovery from induction therapy increased by 11% compared to pre-treatment in the high ALC group but decreased by 3% in the low ALC group (P=0.0043). There were no statistically significantly relevant differences between the high and low ACL groups in the changes in frequencies of total T cells, B cells or NK cells between pretreatment and marrow recovery after induction therapy (Figure 3). NK cell frequencies were statistically significantly lower in the high ALC group compared to the low ALC group both before and after treatment.
Figure 3.
Changes in lymphocyte subsets frequencies in bone marrow at recovery from induction therapy compared to pre-treatment frequencies in the low and high ALC groups.
Discussion
Our principal finding was that high pre-treatment ALC was independently associated with a higher CIR and shorter RFS and OFS and together with response and MRD status at response was the factor most predictive of these outcomes. The correlation between elevated lymphocyte count at time of diagnosis and poorer outcome is surprising and is not consistent with data from colorectal, breast, renal and ovarian cancers where elevated lymphocyte count at diagnosis has been associated with better outcome (4–7). However, similar to our findings, Le Jeune and colleagues demonstrated association between high ALC at time of diagnosis and poorer AML outcome (8).
It is likely that subpopulation(s) of lymphocytes may be responsible for the effect of pre-treatment lymphocyte count on outcome. Evaluation of marrow flow cytometry showed lower frequency of NK cells before and after treatment, and higher frequency of total T cells and CD4+ post treatment in the high ALC group compared to the low ALC group. Lower frequency of NK cells and higher frequency of CD4+ T cells (which includes the subpopulation of inhibitory T regulatory cells (Tregs; CD4+CD25+Foxp3+ cells)) in the high ALC group may lead to weaker immune responses against residual leukemia cells after induction therapy. A potential anti-leukemic activity of NK cells has been demonstrated in several studies (12–14). Additionally, it has been shown that AML microenvironment contains a variety of immune suppressive elements, including Tregs, which inhibit the cytotoxic capacity of anti-AML reactive cytotoxic T cells (15–18). Several studies have demonstrated increased Tregs frequency in bone marrow and peripheral blood of AML patients compared to normal controls (19–22), and an association between high Treg frequency and poor AML prognosis has also been shown. (21, 22). Additionally, Tregs appear to be the T cell subset least affected by induction chemotherapy for AML (19, 23). Although we did not specifically analyze Tregs by flow cytometry our finding of increased frequency of CT4+ T cells in the high ALC group after treatment may reflect a high frequency of Tregs in this patient population. Thus, the combination of lower NK cell frequency and the potential higher frequency of Tregs after treatment in the high ALC group may result in a weaker anti-AML immune response against residual disease remaining in patients in remission and may lead to the shorter CRs and decreased survival of the high ALC group compared to the low ALC group.
Other cofactors that were found to be associated with AML outcome in our multivariate model were response to treatment (CR versus CR with MRD or CRi) and HCT. As previously noted (3) when response to treatment and MRD status at response are taken into account in our analysis, cytogenetic characteristics and age were not statistically significantly associated with outcome, likely due to the stronger effect of the response to treatment. The unfavorable effect of high intensity treatment (tables 2–4) is more difficult to understand, but it is plausible that patients put into CR with higher intensity therapy are patients with more aggressive disease, who would not have been put into CR by lower intensity therapy and hence are more likely to relapse once in CR.
A principal limitation of our study is the relatively short follow-up time (median 1.4 years). Additionally, as in many AML studies, the data were collected retrospectively, the patient population and treatment were heterogeneous and methods and timing of follow-up were not standardized. Because of the study’s retrospective nature, flow cytometry data are limited and do not include the specific frequency of Tregs, but only CD4+T cells, which includes the subpopulation of Tregs. Despite those limitations, we think that this study further supports a role of the immune response in outcome of AML in remission even outside the setting of HCT.
In summary, our results demonstrate that high lymphocyte count at time of AML diagnosis is associated with shorter CR after induction therapy and decreased survival. This may be explained by low frequency of NK cells and potentially high frequency of Treg cells, which may result in weaker immune response against residual disease remaining after response to initial treatment.
Acknowledgments
We thank the patients who participated. We thank members of the research and clinical staff at the FHCRC and referring physicians for their contribution to the care of our AML patients.
Footnotes
Financial Disclosure: The authors declare no competing financial interests
References
- 1.Kayser S, Zucknick M, Dohner K, Krauter J, Kohne CH, Horst HA, et al. Monosomal karyotype in adult acute myeloid leukemia: prognostic impact and outcome after different treatment strategies. Blood. 2012;119(2):551–8. doi: 10.1182/blood-2011-07-367508. Epub 2011/11/19. [DOI] [PubMed] [Google Scholar]
- 2.Liersch R, Muller-Tidow C, Berdel WE, Krug U. Prognostic factors for acute myeloid leukaemia in adults--biological significance and clinical use. British journal of haematology. 2014;165(1):17–38. doi: 10.1111/bjh.12750. Epub 2014/02/04. [DOI] [PubMed] [Google Scholar]
- 3.Walter RB, Othus M, Burnett AK, Lowenberg B, Kantarjian HM, Ossenkoppele GJ, et al. Resistance prediction in AML: analysis of 4601 patients from MRC/NCRI. HOVON/SAKK, SWOG and MD Anderson Cancer Center; Leukemia: 2014. Epub 2014/08/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ceze N, Thibault G, Goujon G, Viguier J, Watier H, Dorval E, et al. Pre-treatment lymphopenia as a prognostic biomarker in colorectal cancer patients receiving chemotherapy. Cancer chemotherapy and pharmacology. 2011;68(5):1305–13. doi: 10.1007/s00280-011-1610-3. Epub 2011/03/31. [DOI] [PubMed] [Google Scholar]
- 5.Vicente Conesa MA, Garcia-Martinez E, Gonzalez Billalabeitia E, Chaves Benito A, Garcia Garcia T, Vicente Garcia V, et al. Predictive value of peripheral blood lymphocyte count in breast cancer patients treated with primary chemotherapy. Breast. 2012;21(4):468–74. doi: 10.1016/j.breast.2011.11.002. Epub 2011/11/29. [DOI] [PubMed] [Google Scholar]
- 6.Fumagalli LA, Vinke J, Hoff W, Ypma E, Brivio F, Nespoli A. Lymphocyte counts independently predict overall survival in advanced cancer patients: a biomarker for IL-2 immunotherapy. J Immunother. 2003;26(5):394–402. doi: 10.1097/00002371-200309000-00002. Epub 2003/09/16. [DOI] [PubMed] [Google Scholar]
- 7.Williams KA, Labidi-Galy SI, Terry KL, Vitonis AF, Welch WR, Goodman A, et al. Prognostic significance and predictors of the neutrophil-to-lymphocyte ratio in ovarian cancer. Gynecologic oncology. 2014;132(3):542–50. doi: 10.1016/j.ygyno.2014.01.026. Epub 2014/01/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le Jeune C, Bertoli S, Elhamri M, Vergez F, Borel C, Huguet F, et al. Initial absolute lymphocyte count as a prognostic factor for outcome in acute myeloid leukemia. Leukemia & lymphoma. 2014;55(4):855–62. doi: 10.3109/10428194.2013.813504. Epub 2013/06/22. [DOI] [PubMed] [Google Scholar]
- 9.Wood B. 9-color and 10-color flow cytometry in the clinical laboratory. Archives of pathology & laboratory medicine. 2006;130(5):680–90. doi: 10.5858/2006-130-680-CACFCI. Epub 2006/05/11. [DOI] [PubMed] [Google Scholar]
- 10.Kussick SJ, Fromm JR, Rossini A, Li Y, Chang A, Norwood TH, et al. Four-color flow cytometry shows strong concordance with bone marrow morphology and cytogenetics in the evaluation for myelodysplasia. American journal of clinical pathology. 2005;124(2):170–81. doi: 10.1309/6PBP-78G4-FBA1-FDG6. Epub 2005/07/26. [DOI] [PubMed] [Google Scholar]
- 11.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association. 1999;94(446):496–509. [Google Scholar]
- 12.Siegler U, Kalberer CP, Nowbakht P, Sendelov S, Meyer-Monard S, Wodnar-Filipowicz A. Activated natural killer cells from patients with acute myeloid leukemia are cytotoxic against autologous leukemic blasts in NOD/SCID mice. Leukemia. 2005;19(12):2215–22. doi: 10.1038/sj.leu.2403985. Epub 2005/10/15. [DOI] [PubMed] [Google Scholar]
- 13.Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105(8):3051–7. doi: 10.1182/blood-2004-07-2974. Epub 2005/01/06. [DOI] [PubMed] [Google Scholar]
- 14.Bachanova V, Cooley S, Defor TE, Verneris MR, Zhang B, McKenna DH, et al. Clearance of acute myeloid leukemia by haploidentical natural killer cells is improved using IL-2 diphtheria toxin fusion protein. Blood. 2014;123(25):3855–63. doi: 10.1182/blood-2013-10-532531. Epub 2014/04/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buggins AG, Milojkovic D, Arno MJ, Lea NC, Mufti GJ, Thomas NS, et al. Microenvironment produced by acute myeloid leukemia cells prevents T cell activation and proliferation by inhibition of NF-kappaB, c-Myc, and pRb pathways. J Immunol. 2001;167(10):6021–30. doi: 10.4049/jimmunol.167.10.6021. Epub 2001/11/08. [DOI] [PubMed] [Google Scholar]
- 16.Orleans-Lindsay JK, Barber LD, Prentice HG, Lowdell MW. Acute myeloid leukaemia cells secrete a soluble factor that inhibits T and NK cell proliferation but not cytolytic function--implications for the adoptive immunotherapy of leukaemia. Clinical and experimental immunology. 2001;126(3):403–11. doi: 10.1046/j.1365-2249.2001.01692.x. Epub 2001/12/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou Q, Bucher C, Munger ME, Highfill SL, Tolar J, Munn DH, et al. Depletion of endogenous tumor-associated regulatory T cells improves the efficacy of adoptive cytotoxic T-cell immunotherapy in murine acute myeloid leukemia. Blood. 2009;114(18):3793–802. doi: 10.1182/blood-2009-03-208181. Epub 2009/09/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gasteiger G, Kastenmuller W. Foxp3+ Regulatory T-cells and IL-2: The Moirai of T-cell Fates? Frontiers in immunology. 2012;3:179. doi: 10.3389/fimmu.2012.00179. Epub 2012/07/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ersvaer E, Liseth K, Skavland J, Gjertsen BT, Bruserud O. Intensive chemotherapy for acute myeloid leukemia differentially affects circulating TC1, TH1, TH17 and TREG cells. BMC immunology. 2010;11:38. doi: 10.1186/1471-2172-11-38. Epub 2010/07/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X, Zheng J, Liu J, Yao J, He Y, Li X, et al. Increased population of CD4(+)CD25(high), regulatory T cells with their higher apoptotic and proliferating status in peripheral blood of acute myeloid leukemia patients. European journal of haematology. 2005;75(6):468–76. doi: 10.1111/j.1600-0609.2005.00537.x. Epub 2005/11/30. [DOI] [PubMed] [Google Scholar]
- 21.Szczepanski MJ, Szajnik M, Czystowska M, Mandapathil M, Strauss L, Welsh A, et al. Increased frequency and suppression by regulatory T cells in patients with acute myelogenous leukemia. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15(10):3325–32. doi: 10.1158/1078-0432.CCR-08-3010. Epub 2009/05/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shenghui Z, Yixiang H, Jianbo W, Kang Y, Laixi B, Yan Z, et al. Elevated frequencies of CD4(+) CD25(+) CD127lo regulatory T cells is associated to poor prognosis in patients with acute myeloid leukemia. International journal of cancer Journal international du cancer. 2011;129(6):1373–81. doi: 10.1002/ijc.25791. Epub 2010/11/26. [DOI] [PubMed] [Google Scholar]
- 23.Kanakry CG, Hess AD, Gocke CD, Thoburn C, Kos F, Meyer C, et al. Early lymphocyte recovery after intensive timed sequential chemotherapy for acute myelogenous leukemia: peripheral oligoclonal expansion of regulatory T cells. Blood. 2011;117(2):608–17. doi: 10.1182/blood-2010-04-277939. Epub 2010/10/12. [DOI] [PMC free article] [PubMed] [Google Scholar]