Abstract
Mitochondria, being the principal source of cellular energy, are vital for cell life. Yet, ironically, they are also major mediators of cell death, either by necrosis or apoptosis. One means by which these adverse effects occur is through the mitochondrial permeability transition (mPT) whereby the inner mitochondrial membrane suddenly becomes excessively permeable to ions and other solutes, resulting in a collapse of the inner membrane potential, ultimately leading to energy failure and cell necrosis. The mPT may also bring about the release of various factors known to cause apoptotic cell death. The principal factors leading to the mPT are elevated levels of intracellular Ca2+ and oxidative stress. Characteristically, the mPT is inhibited by cyclosporin A. This article will briefly discuss the concept of the mPT, its molecular composition, its inducers and regulators, agents that influence its activity and describe the consequences of its induction. Lastly, we will review its potential contribution to acute neurological disorders, including ischemia, trauma, and toxic-metabolic conditions, as well as its role in chronic neurodegenerative conditions such as Alzheimer’s disease, Parkinson’s disease, Huntington’s disease and amyotrophic lateral sclerosis.
Keywords: Alzheimer’s disease, ammonia, amyotrophic lateral sclerosis, apoptosis, calcium homeostasis, cell death, cyclosporin A, excitotoxicity, hepatic encephalopathy, Huntington’s disease, ischemia, manganese, mitochondria, mitochondrial permeability transition, oxidative stress, Parkinson’s disease, Reye’s syndrome, trauma
1. Introduction
Mitochondria play a central role in cellular bioenergetics and are often referred to as the “powerhouse" of the cell due to their primary role in the generation of ATP required for cellular functions. Approximately 98% of oxygen in the cell is consumed by mitochondria through oxidative phosphorylation in order to generate ATP (Hatefi, 1985). Such continuous oxidative reactions also produces reactive oxygen species (ROS) that are implicated in various pathological conditions (Salganik et al., 1994). Mitochondria also act as high-capacity Ca2+ sinks by transporting calcium from the cytosol for the regulation of key enzymes (dehydrogenases) of the citric acid cycle, and they also act as temporary stores of this ion (Nicholls and Budd 2000; Nicholls, 2005). The Ca2+ from the cytosol is normally transported into mitochondria by an electrogenic uniporter while its efflux from mitochondria is mediated by the Na+/Ca2+ exchanger (Gunter and Gunter, 2001).
Recent studies have also implicated mitochondria in cell death mechanisms, since mitochondrial dysfunction results in the release of factors that initiate, amplify and execute various signals resulting in apoptotic cell death (Kroemer and Reed, 2000). Additionally, mitochondrial dysfunction and associated bioenergetic failure can lead to abnormal cellular ion homeostasis, as a result of which cells undergo swelling and cellular disruption, eventually leading to necrotic death (Nieminen, 2003).
The outer membrane of mitochondria is permeable to small solutes and ions, while the inner membrane is virtually impermeable and forms a barrier between the cytosol and mitochondrial matrix. Electrons are transferred from reduced nucleotides to various intermediates of the electron transport chain during which protons are pumped across the inner membrane from the matrix into the inter membrane space. This proton transport creates a transmembrane potential (ΔΨm) (Tedeschi, 1980) which provides the motive force required for ATP synthesis, as well as for facilitating the selective entry of ions such as Ca2+ (Siliprandi et al., 1983).
In conditions of increased Ca2+ loading, especially when accompanied by oxidative stress and a fall in adenine nucleotides, mitochondria undergo a phenomenon referred to as the permeability transition (mPT). The mPT is traditionally defined as a phenomenon associated with the opening of a proteinaceous permeability transition pore (referred to as the pore in the rest of the article) located in the inner mitochondrial membrane allowing solutes with molecular masses of up to 1500 Da to enter or exit the mitochondrial matrix. This opening results in osmotic swelling of the mitochondrial matrix, dissipation of the ΔΨm, cessation of the ATP synthesis, and the release of cytochrome c and other apoptogenic factors (Zoratti and Szabo, 1995).
2. The mitochondrial permeability transition
While the concept that mitochondria undergo swelling in the presence of various substances was known for the past 50 years, it was not until the classic work of Haworth and Hunter (1979) who demonstrated that Ca2+ induces swelling in mitochondria, a phenomenon they referred to as a "Ca2+-induced transition", that the mPT acquired scientific interest. Such transition (i.e., transition to swelling) appeared to be directly proportional to the external Ca2+ concentration.
Interest in the mPT increased further when it was shown that the mPT could be specifically blocked by the immunosuppressive agent cyclosporin A (CsA) (Crompton at al., 1988). Such inhibition of the mPT by CsA involves its interaction with the mitochondrial matrix protein cyclophilin D (CyP-D) thereby preventing it from binding to the adenine nucleotide translocator (ANT) (Halestrap et al., 1997a). It should be emphasized that the action of CsA on the mPT is not mediated by its well known calcineurin inhibitory effect (Liu et al., 1991) (its mode of action in immunosuppression), but rather by its direct interaction with components of the pore.
2.1. Composition of the mitochondrial permeability transition pore
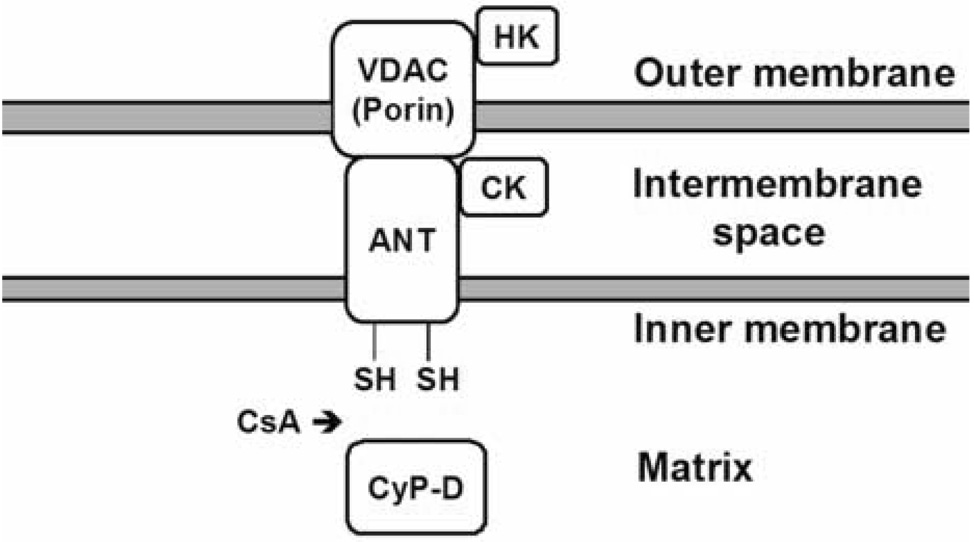
Despite extensive studies, the precise composition of the pore is still unknown. The pore is believed to be a voltage-dependent channel that is formed by a set of mitochondrial proteins located on the inner membrane, mitochondrial matrix, as well as on the outer membrane (Zoratti and Szabo, 1995). While the proteins involved in pore formation remain to be defined, they are generally believed to include the voltage-dependent anion channel (VDAC), an outer mitochondrial membrane protein (Szabo and Zoratti, 1993); the ANT, an inner mitochondrial membrane protein (Halestrap and Davidson, 1990); and CyP-D, residing in the mitochondrial matrix (Griffiths and Halestrap, 1991). Under conditions favorable for the mPT, component proteins assemble to form the pore. It has been suggested that binding of the matrix protein CyP-D with the ANT changes the conformation of the ANT, allowing it to interact with the VDAC located at contact sites of the outer and inner mitochondrial membranes thereby creating the pore (Crompton et al., 2002). A schematic diagram depicting the putative structure of pore is shown in Figure 1.
Figure 1.
Proposed structure of the PT pore. CsA blocks pore opening by inhibiting the binding of cyclophilin D (CyP-D) to the adenine nucleotide translocator (ANT). VDAC, voltage-dependent anion channel; HK, hexokinase; CK, creatine kinase. Modified from Rama Rao et al., Metab. Brain Dis. 18, 113–127, 2003.
VDAC is a 32 kDa protein that exists as dimer on the outer mitochondrial membrane that allows the entry and exit of various metabolites required for mitochondrial metabolism (Shoshan-Barmatz and Gincel, 2003). While VDAC has been shown to interact with the ANT in pore formation (Szabo and Zoratti, 1993), its involvement in the pore has been recently questioned since mitochondria isolated from VDAC knock-out mice undergo a similar degree of mitochondrial swelling induced by Ca2+ (Krauskopf et al., 2006). While this study argues against the participation of VDAC in pore formation, these authors nevertheless suggested the possibility that more than one isoform of VDAC may be involved in pore formation.
The ANT is a 30 kDa protein located on the inner mitochondrial membrane, which is mainly involved in the exchange of ATP with ADP. The ANT appears to be involved in pore formation and such involvement is related to its binding with the matrix protein CyP-D (Woodfield et al., 1998). Several studies employing reconstituted ANT (Brustovetsky and Klingenberg, 1996), inhibitors of the ANT (Ruck et al., 1998), and mPT inducers (Checler, 1999) have shown the involvement of ANT in pore formation. However, Kokozska et al. (2004) demonstrated that mitochondria isolated from liver of ANT null mice still underwent Ca2+-induced swelling. These authors proposed instead that the ANT may be a regulator of the pore. The precise role of ANT in pore formation still needs to be better defined.
Cyclophilin-D (CyP-D) is an 18 kDa mitochondrial matrix protein, which as noted above, has been proposed to represent the major target of CsA’s inhibitory action on mPT. The binding of CyP-D to the ANT is largely regulated by matrix Ca2+ which acts as a signal for the translocation of CyP-D to the inner membrane (Connern and Halestrap, 1994). Consistent with this view, recent studies employing transgenic mice in which the CyP-D gene has been deleted, showed a critical role of CyP-D in pore opening. Nakagawa et al. (2005) and Baines et al. (2005) reported that mitochondria isolated from transgenic mice lacking Cyp-D are resistant to CsA-sensitive induction of the mPT as compared to wild type mitochondria. Basso et al. (2005) have shown that mitochondria derived from CyP-D deficient mice continue to undergo mPT but the Ca2+ requirement for mPT induction was twice as high as that of normal mitochondria.
Other proteins implicated in the pore formation include the peripheral benzodiazepine receptor, an outer mitochondrial membrane protein (McEnery et al., 1992) and hexokinase (Beutner, et al., 1998), but their precise involvement in pore formation is still not clear. Creatine kinase has also been proposed to be a component of the pore by its interaction with VDAC and the ANT (see Figure 1) (Brdiczka et al., 1998). Creatine can stabilize creatine kinase into an octomeric forms and such octomers have been shown to inhibit activation of the mPT (O'Gorman et al., 1996).
He and Lemasters (2002) have recently put forward a novel concept that two modes of the mPT exist - a CsA-sensitive mode, and an unregulated (CsA-insensitive) mPT that appears to result from the aggregation of multiple integral membrane proteins as a result of oxidative damage. How these aggregates interact with the “traditional” members of the pore is not clear.
2.2 Measurement of the mPT
Opening of the pore has been widely demonstrated in isolated mitochondrial fractions. Crompton et al. (1998) were the first to establish that such preparations when exposed to various concentrations of Ca2+ undergo swelling in a CsA-sensitive manner and such swelling can be assayed as a decrease in optical density. Another in vitro assay measures the CsA-sensitive dissipation of the membrane potential by determining extramitochondrial levels of triphenylphosphonium ion (TPP+) with a TPP+-sensitive electrode (Ross et al., 2005).
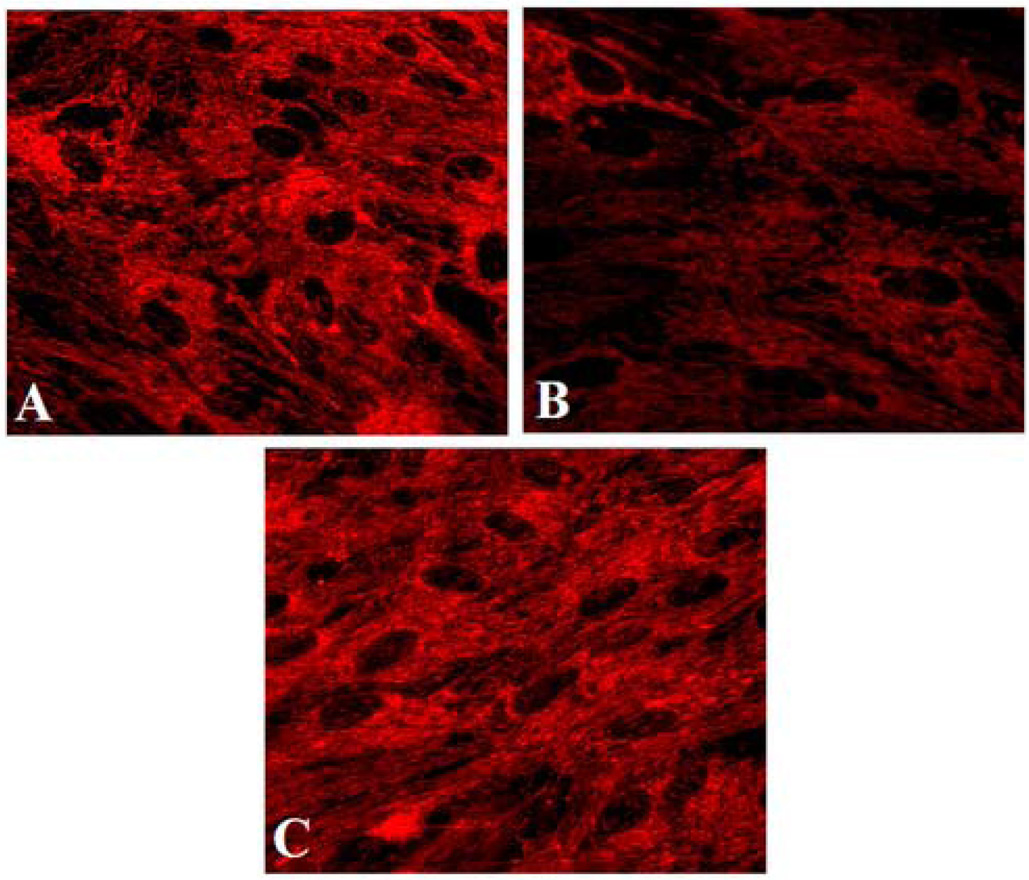
Measurement of the mitochondrial ΔΨm in intact cells was made possible with the advent of potentiometric fluorescent dyes. These include rhodamine 123, terametylrhodamine ethyl ester (TMRE) and its methylated form TMRM, 5,5',6,6'-tretachloro-1,1',2,3'-tetraethylbenzamidazoocarbocyanine (JC1), DiOC6 and DASPMI. An example of this methodology is shown in Figure 2. These cationic lipophilic dyes accumulate in the mitochondria following the Nernst equation, as changes in the ΔΨm alter the distribution of these dyes between cytosol and mitochondria (Duchen, 2004).
Figure 2.
Fluorescence images of cultured astrocytes treated with ammonia for 24 h. Astrocytes were loaded with TMRE (25 nM; 15 min) and images were captured and examined with a fluorescence microscope. A. Control astrocytes show prominent fluorescence. B. Astrocytes treated with ammonia show decreased fluorescence, consistent with depolarization of the mitochondrial membrane potential. C. Astrocytes treated with CsA (1 µM) show fluorescence similar to that of the control.
Petronilli et al. (1999) first employed the calcein fluorescence method to demonstrate the induction of the mPT. This method allows one to directly visualize permeability changes in mitochondria in situ. Kerr et al. (1999) developed the 2-deoxy-glucose-6-phosphate (2-DG-6-P) entrapment method to determine pore opening. This method may be particularly useful when investigating the mPT in vivo.
2.3 Inducers of the mPT
In conditions associated with high cytosolic levels of Ca2+, mitochondria have a great capacity to accumulate Ca2+ via the uniporter, an inner membrane protein channel that transports Ca2+ as a function of the ΔΨm (Siliprandi et al., 1983). Under conditions of heightened cytosolic calcium content, the uniporter can transport large quantities of Ca2+ (as it may not be saturated due to its channel properties), resulting in a Ca2+ overload of mitochondria and opening of the pore (Halestrap, 2006). How increased mitochondrial Ca2+ induces pore opening is not clear. One proposed mechanism involves the facilitation of CyP-D binding to the ANT by Ca2+ (Halestrap and Brennerb, 2003).
Oxidative stress is another major factor in the induction of the mPT (Halestrap et al., 1997b; Kowaltowski et al., 2001). It had been earlier shown that the release of Ca2+ from mitochondria (a phenomenon later confirmed as the mPT) was accelerated by oxidation of pyridine nucleotides (NADPH) (Lehninger et al., 1978), suggesting that mPT induction is strongly dependent on the redox state of the mitochondria. Such oxidation of pyridine nucleotides also diminishes glutathione levels, thereby decreasing the activity of glutathione peroxidase and resulting in free radical production and induction of the mPT (Kowaltowski et al., 2001). Additionally, ROS-mediated oxidation of thiol groups on mitochondrial proteins associated with the pore has been shown to result in pore opening (Halestrap et al., 1997b).
Additional factors important in the opening of the pore include an alkaline matrix pH, diminution of the adenine nucleotide pools, and reduction of inorganic phosphate (Pi) levels (Bernardi et al., 1992).
2.4 Agents that regulate the mPT
In addition to CsA, bongkrekic acid, an inhibitor of the ANT, is a potent inhibitor of the mPT (Brustovetsky et al., 1996). Several other agents are known to inhibit the mPT, however, the specificity of these agents to the mPT is doubtful. For instance, creatine (O'Gorman et al., 1997) and carnitine (Zanelli et al., 2005) are reported to inhibit the mPT, however, both are also known to increase high-energy phosphate stores (Ratnakumari et al., 1993; Brustovetsky et al., 2001; Klivenyi et al., 2004). Similarly, minocycline, a tetracycline derivative, inhibits the mPT and mPT-associated events such as the mitochondrial release of cytochrome c (Wang et al., 2003). However, it is also able to inhibit caspase-inducible form of nitric oxide synthase, as well as p38 MAP kinase (see (Mecocci et al., 1994; Zhu et al., 2002) and references therein), and such effects appear not to be mediated by the mPT. Other agents, including coenzyme Q (Fontaine et al., 1998), tamoxifen (Kimelberg et al., 2003), tricyclics and heterocyclics (desipramine, trifluoperazine) (Stavrovskaya et al., 2004), melatonin (Andrabi et al., 2004), promethazine (Stavrovskaya et al., 2004) all have to variable extent been shown to inhibit the mPT, but many of these agents also have antioxidant properties. The precise mechanisms by which these agents exert inhibitory effects on the mPT (directly or indirectly) still need to be more carefully assessed.
2.5 Consequences of the mPT
The major consequence of the opening of the MPT pore is dissipation of the mitochondrial inner membrane potential resulting in uncoupling of the oxidative phosphorylation and failure to synthesize ATP. Such malfunction of the electron transport chain results in ROS production (Votyakova and Reynolds, 2005). Thus, while oxidative stress is a major cause of the mPT, it can also be a consequence of the mPT.
The involvement of the mPT in the apoptotic cell death pathway has largely been studied by examining the release of cytochrome c (Liu et al., 1996) which activates procaspase 9 to stimulate downstream events related to apoptosis (Jemmerson et al., 2005). Additionally, certain apoptotic factors such as AIF and Smac/Diablo are also released (Brustovetsky et al., 2005). The efflux of cytochrome c involves members of the pro-apoptotic Bcl-2 family such as BAX, BAD and BID (Green and Reed, 1998). However, the mechanism by which BID and BAX association causes cytochrome c efflux is still not clear and whether such an event is mediated by the mPT remains controversial. While disagreement exist regarding the precise role of the mPT in mediating apoptosis, CsA has been shown to block this process (Crompton, 2003;Green and Kroemer, 2004). Recent studies by Schinzel et al. (2005) demonstrated that apoptosis continues to occur in embryonic fibroblasts in CyP-D null mice which have not undergone the mPT after a calcium stimulus, suggesting that the mPT is not important in the mediation of apoptosis.
Induction of the mPT can clearly result in necrotic cell death since the mPT leads to cessation of the ATP synthesis leading to loss of ion homeostasis, cell disintegration and death.
3. Neurological disorders
Many acute and chronic CNS disorders have common pathogenetic factors, including the involvement of excitotoxicity, oxidative stress, disturbances in calcium homeostasis and mitochondrial dysfunction. All these factors create an ideal environment for induction of the mPT. The discovery that CsA was able to inhibit the mPT initiated a flurry of research activity aimed at not only better understanding this process, but more importantly, investigating its possible involvement in disease and its potential as a therapeutic target.
The following sections briefly summarizes contemporary knowledge regarding the role of the mPT in neurological diseases. It must be cautioned that much of the supporting evidence implicating the mPT in disease is largely based on the protective effect of CsA and other mPT inhibiting agents. While such protection is suggestive, it is not definitive as CsA possesses other effects, in particular calcineurin inhibition (Liu et al., 1991). Unfortunately, many in vivo studies have not excluded calcineurin inhibitory effects. Agents inhibiting the mPT other than CsA have been used that are far less specific than CsA as mPT inhibitors, so that invoking the mPT based on beneficial therapeutic action may not be sufficient. Further, many of the studies have employed cell cultures. Although crucial in teasing apart mechanistic considerations, such studies do not prove that mechanistic events identified in culture are operative in vivo. Taking these caveats into account, a body of data has nevertheless emerged indicating that the mPT may be a major factor in disease pathogenesis.
3.1 Excitotoxicity
Excitotoxicity represents a cascade of events triggered by glutamate and other NMDA receptor agonists resulting in the excessive entry of Ca2+ ions into neurons, leading to the activation of destructive hydrolytic enzymes, mitochondrial injury, ROS formation and ultimately cell death (Choi, 1992; Coyle Putterfarcken, 1993). White and Reynolds (1996) and Dubinsky and Levi (1998) showed that such treatment resulted in the accumulation of large quantities of Ca2+ within mitochondria causing a dissipation of the ΔΨm. The mitochondrial depolarization was blocked by CsA, indicating the probable involvement of the mPT. Schinder et al. (1996) further showed that CsA provided cytoprotection against excitotoxic injury in neuronal cultures. However, Ruiz et al., (2000) noted that while CsA protects against excitotoxicity, CsA derivatives that do not bind to calcineurin had a smaller effect on survival than CsA, suggesting that calcineurin inhibition by CsA also plays a part in such neuroprotection. FK506, a calcineurin inhibitor that has no effect on the mPT (Liu et al., 1991), has also been shown to protect against excitotoxicity (Dawson et al., 1993; Ankarcrona et al., 1996) indicating that calcineurin-mediated events also contribute to excitotoxic neuronal death. Thus, while it is clear that mPT represents an important component of the NMDA receptor-mediated excitotoxic cascade, it is by no means the only factor responsible for such injury.
In vivo studies examining the role of the mPT have been hampered by the limited transport of CsA across the BBB. Nevertheless, in vivo support for the role of the mPT in excitotoxic injury was provided by Santos et al. (2003) who systemically injected mice with the excitotoxin kainic acid, a procedure well known to cause cell death of hippocampal neurons. This study showed that CsA almost completely eliminated neuronal cell death, whereas FK506 had no effect. However, Maciel et al. (2003) observed no protection by CsA in an in vivo model of quinolinic acid-mediated excitotoxic lesions in rodents. The reason for the failure of CsA to protect against quinolinic acid is not known.
3.2 Ischemia
The involvement of mitochondria in ischemic cell death is well known, and has been recently extensively reviewed (Fiskum et al., 1999; Kristian, 2004). In the ischemic core mitochondria depolarize and their capacity for oxidative phosphorylation is acutely and irreversibly lost (Siesjö, 1992). Ischemia is consequently associated with a severe energy crisis leading to a profound drop in ATP/ADP levels, a build up of lactic acid, and a fall in intracellular pH (Siesjö, 1985, 1992). Such energy failure also leads to a disruption of Ca2+ homeostasis resulting in an elevation of intracellular Ca2+ levels (Kruman and Mattson, 1999). However, little of this calcium will enter mitochondria as the latter have become depolarized thus removing the driving force for Ca2+ entry into that organelle.
Consequently, during the ischemic phase, conditions may not be optimal for induction of the mPT as calcium is not able to enter mitochondria in sufficient amounts to create the mPT, and because of the acidosis which is known to inhibit the mPT (Friberg and Wieloch, 2002; Kristian, 2004). Rather, it is during the period of reperfusion that Ca2+ can be sequestered in re-energized mitochondria (Hansen and Zeuthen, 1981) and stimulate the formation of ROS. pH will briefly return to normal as lactic acid levels decrease and the activation of pH regulatory pumps occur (Halestrap, 2006). All of these conditions now create an ideal environment for opening of the pore. It should be noted that while acidosis inhibits the mPT, in the setting of ischemia, there in an increased matrix influx of inorganic phosphate. The increase in phosphate, which is known to promote the mPT, appears to overcome the inhibitory effect of acidosis on the mPT (Kristian et al., 2001).
Oxygen-glucose deprivation (OGD) represents a useful in vitro model of ischemia (Goldberg and Choi, 1993). Khaspekov et al. (1999) showed that mouse hippocampal neuronal cultures transiently exposed to OGD for 90 min, followed by 24 h of reoxygenation, exhibited extensive neuronal degeneration. Preincubation of these cultures with CsA diminished neuronal death by 30–50%. Likewise, the CsA analogue, N-methyl-valine-4-cyclosporin A (N-Me-Val-CsA), a potent blocker of the mPT with no significant calcineurin inhibitory activity, decreased cell death by an even greater amount (70–80%). Such findings clearly establish a vital role of the mPT in ischemic cell death in vitro. Using the OGD model of ischemia, MacGregor et al. (2003) showed that CsA afforded similar protection against edema formation whereas FK506 did not. The mPT was detected in cultured astrocytes after OGD exposure, although it took much longer for it to occur as compared to cultured neurons (Reichert et al., 2001).
Consistent with the above findings, CsA has been shown to exert potent neuroprotection in global, as well as in focal ischemia (reviewed in Friberg and Wieloch, 2002). Because of the limited capacity of CsA to cross the BBB, investigators inserted a syringe needle into the brain parenchyma so as to disrupt the BBB. To address the issue of whether the effect of CsA was mediated by closure of the pore or by calcineurin inhibition, the investigators employed N-Me-Val-CsA and found that this agent also diminished infarct size (Matsumoto et al., 1999). The same group subsequently showed that CsA, but not FK506, blocked the mPT after middle cerebral artery occlusion (Yoshimoto et al., 2001).
A major advance in this field occurred with the development of transgenic mice deficient in CyP-D. As CyP-D is a critical component of the pore (see above), the use of these mice obviates many of the difficulties associated with mPT inhibitors. These mice were not susceptible to the mPT induced by the addition of calcium, and displayed a dramatic reduction in brain infarct size after acute middle cerebral artery occlusion and reperfusion, strongly supporting a central role of the mPT in cerebral ischemia (Schinzel et al., 2005).
A number of agents that have mPT inhibitory effects were reported to have beneficial effects in models of stroke, including bongkrekic acid (Muranyi and Li, 2005), melatonin (Andrabi et al., 2004), tricyclics and heterocyclics (e.g., desipramine, trifluoperazine), the antihistaminic promethazine and minocycline (Yrjanheikki et al., 1998; Stavrovskaya et al., 2004). Tamoxifen has mPT inhibitory effects (Moreira et al., 2005) and has recently been shown to have neuroprotective properties in stroke (Kimelberg et al., 2003; Feng et al., 2004). The specificity of these agents to the mPT is doubtful. Nevertheless, these studies support the concept that the mPT is a critical aspect of tissue injury associated with ischemia.
3.3 Hypoglycemia
In contrast to ischemia, cerebral blood flow is maintained in hypoglycemia, and there is also no lactic acid accumulation (Auer and Siesjö, 1988). When severe, membrane ionic gradients cannot be maintained, intracellular Ca2+ levels increase (Harris et al., 1984) the intracellular redox state shifts towards oxidation (Auer and Siesjö, 1988), and oxidative stress develops (Liu et al., 2003). All of these events may eventually cause the mPT. Indeed, Friberg et al. (1998) showed that CsA significantly reduced brain damage when administered prior to insulin-induced hypoglycemic coma. The marked swelling of dendrites and mitochondria in neurons of the dentate gyrus of the hypocampus was abrogated by CsA, while FK506 exhibited no protection.
3.4 Trauma
Mechanisms responsible for tissue injury in CNS trauma remain poorly understood. The initial mechanical injury (primary insult) results in the loss of membrane integrity and membrane depolarization, thereby initiating a cascade of molecular and cellular events over the succeeding hours and days (secondary insult) and resulting in the loss of ion homeostasis (in particular Ca2+), development of brain edema, ischemia, hyperthermia, inflammation, glutamate-induced excitotoxicity, mitochondrial dysfunction, energy failure, and production of free radicals. All of these events will ultimately result in cell/tissue injury and death. For general reviews on pathogenetic mechanisms in traumatic brain injury (TBI), see Rey et al. (2002) and Raghupathi (2004). It is of interest that many of the factors believed to be instrumental in the mechanism TBI, are also known to induce the mPT.
Investigations into the role of the mPT in TBI were initiated by Povlishock and colleagues (Okonkwo et al., 1999; Buki et al., 1999) who found that CsA was able to attenuate trauma-induced axonal lesions. A follow-up study by the same group, however, found that FK506 was as effective as CsA (Singleton et al., 2001). Although not excluding the involvement of the mPT in these lesions, calcineurin inhibition appears to, at least in part, explain the beneficial effects of CsA.
Scheff and Sullivan (1999) observed that CsA significantly reduced the amount of cortical damage following TBI in rats and mice, while FK506 failed to protect against the cortical damage. The same research group subsequently identified the mPT in mitochondria isolated from traumatized brain (Sullivan et al., 1999). Improvement in mitochondrial function was shown by Signoretti et al. (2004). Together, these findings clearly indicate that a significant portion of the cortical damage after TBI is mediated by the mPT.
Interestingly, CsA was shown not to be effective in spinal cord trauma (Rabchevsky, et al., 2001). While explanations for the disparity between brain and spinal cord with regard to the involvement of the mPT following trauma is not known, differences between cord and brain mitochondria were subsequently identified that might explain the differential responsiveness to CsA (Sullivan et al., 2004). These investigators found that superoxide production, lipid peroxidation, and mitochondrial DNA oxidation were higher in spinal cord mitochondria as compared to cortical mitochondria. Complex I enzyme activity and respiration in spinal cord mitochondria was also found to be reduced and the threshold for calcium-induced mPT was also reduced.
3.5. Toxic-metabolic
(a) Hepatic encephalopathy
Hepatic encephalopathy (HE) is a complex neuropsychiatric syndrome resulting from severe liver failure. It may manifest in a chronic form (portal-systemic encephalopathy), which usually occurs in the setting of alcoholic liver cirrhosis. HE may also occur acutely (acute liver failure) following viral hepatitis, and drug-induced hepatotoxicity. Acute HE has an extremely poor prognosis with a mortality rate of about 90%, and the only effective treatment is an emergency liver transplantation. For review of clinical aspects of HE, see Jones and Weissenborn, 1997).
Although mechanisms responsible for HE remain elusive, ammonia is generally considered a key factor in its pathogenesis (Hazell and Butterworth, 1999), with astrocytes being the principal target of ammonia neurotoxicity (Norenberg, 1998). The involvement of astrocytes is likely due to the fact that ammonia in brain is primarily metabolized by glutamine synthetase (Cooper and Plum, 1987), an enzyme predominantly localized in astrocytes (Norenberg and Martinez-Hernandez, 1979). Accordingly, high levels of glutamine in brain is a characteristic feature of HE (Albrecht and Norenberg, 2006).
Many factors that are conducive to the induction of the mPT are also known to be implicated in the mechanism of HE, including oxidative/nitrosative stress (Norenberg et al., 2004), elevation in intracellular Ca2+ (Rose et al., 2005), and alkaline pH (Cooper and Plum, 1987). Recent studies have demonstrated the induction of the mPT by pathophysiological concentrations of ammonia in cultured astrocytes (Bai et al., 2001; Rama Rao et al., 2003b; Rama Rao et al., 2005b) (see Figure 2). Such ammonia-induced free radical production and the mPT were both blocked methionine sulfoximine (Murthy et al., 2001; Bai et al., 2001), an inhibitor of glutamine synthetase, invoking the possibility that glutamine rather than ammonia per se is responsible for these events. Studies employing clinically relevant concentrations of glutamine (6–8 mM), have shown that glutamine causes free radical production (Jayakumar et al. 2004), and induces the mPT in cultured astrocytes (Rama Rao et al., 2003c), but not in cultured neurons (Rama Rao et al., 2005a). These changes were blocked by 6-diazo-5-oxo-L-norleucine (DON), an inhibitor of mitochondrial phosphate-activated glutaminase, suggesting that the hydrolysis of glutamine in mitochondria represents the basis for the free radical production and the mPT in ammonia-treated astrocytes (reviewed in Albrecht and Norenberg, 2006).
Induction of the mPT by ammonia has been shown to exert a major impact on astrocyte properties (Rama Rao et al., 2003b; Norenberg et al., 2005), including the development of cell swelling, a critical component of the brain edema associated with acute HE (Norenberg, 1977; Traber et al., 1987), as well as inhibition of glutamate uptake (Jayakumar et al., 2006). CsA was shown to reduce both ammonia-induced astrocyte swelling (Rama Rao et al., 2003a) as well as correct the glutamate uptake inhibition in cultured astrocytes (Jayakumar et al., 2006). While the direct role of the mPT in in vivo models of HE has not been established, recent unpublished studies (Jayakumar et al.) have shown that CsA protected against brain edema in an acute model of HE, and that such protection was not detected with FK506, supporting the involvement of the mPT in the brain edema associated with acute HE.
(b) Manganese
Manganese is known to cause CNS injury leading to Parkinson’s disease-like neurological abnormalities (parkinsonism) (Wennberg et al., 1992). Manganese also has been implicated in the pathogenesis of chronic hepatic encephalopathy (Hazell and Butterworth, 1999). Manganism is also an occupational health problem in workers employed in welding factories, manganese mines and ferro-alloy plants (Kaiser, 2003a). The widespread use of the manganese derivative, methylcyclopentadienyl manganese tricarbonyl (MMT) as an anti-knock agent in gasoline has evolved into a major environmental issue (Kaiser, 2003b).
Although mechanisms of manganese neurotoxicity are not completely understood, chronic exposure of various cell types to manganese resulted in oxidative stress and mitochondrial energy failure, factors implicated in the induction of the mPT. Neurotoxic concentrations of manganese (Mn3+) induce the mPT in cultured astrocytes, while in cultured neurons such induction is delayed and less severe (Rama Rao et al., 2004). This differential effect could be due to the inherently higher capacity of astrocytes to accumulate manganese as compared to neurons (Aschner et al., 1992). Studies also demonstrated that various antioxidants significantly blocked manganese-induced mPT, supporting a role of oxidative stress in this process (Rama Rao et al., 2004).
(c) Reye’s syndrome
Reye’s syndrome is a lethal childhood disorder that usually occurs following viral infections. Mitochondria in brain and liver had been shown to be structurally and morphologically affected in Reye’s syndrome patients (Partin et al., 1971). Aspirin has been strongly implicated in the pathogenesis of Reye’s syndrome (Trost and Lemasters, 1996), and salicylate, the hydrolyzed product of aspirin, has been shown to induce the mPT in mitochondria (Trost and Lemasters, 1996).
3.6. Degenerative diseases
Neurodegenerative disorders comprise a heterogeneous group of chronic, age-related conditions that are associated with a disease-specific topographic loss of neurons, astrogliosis and microgliosis. These conditions are inexorably progressive, of unknown etiology and unfortunately, without known cures. Common features to neurodegenerative conditions appear to be mitochondrial dysfunction and bioenergetic failure (Fiskum et al., 1999; Cassarino et al., 1999), oxidative stress (Lin and Beal, 2006) (possibly related to bioenergetic failure), and iron deposition (Zecca et al., 2004) which is commonly involved in the generation of ROS (Fenton reaction).
A factor common to the degenerative diseases is aging, which represents the greatest risk factor for these disorders. The aging process is associated with the accumulation of mitochondrial DNA mutations and appears to be the chief source of ROS (Lin and Beal, 2006). In normal aging mitochondrial DNA damage accumulates (Mecocci et al., 1993) and mitochondrial respiratory function decreases (Yen et al., 1989). Aged cells have been shown to have elevated intracellular calcium levels (Squier and Bigelow, 2000) and the activation of the mPT by calcium is enhanced with age (Mather and Rottenberg, 2000).
A number of events associated with neurodegenerative conditions, acting in concert, create conditions conducive to induction of the mPT. These include mitochondrial failure, oxidative stress, disruption of calcium homeostasis and aging, all of which will be highlighted in conditions to be presented in the following sections.
(a) Alzheimer’s disease
Alzheimer's disease (AD) is a progressive neurodegenerative disorder and the leading cause of late-onset dementia in adults. About 10% of cases are familial with mutations in the amyloid precursor protein (APP), and presenilin-1 and -2, which may be part of the γ-secretase complex. The pathological hallmarks of AD are extracellular amyloid "neuritic" plaques (Glenner, 1989), which are composed of aggregates of β-amyloid (Aβ), a 39–42 amino acid peptide derived from the proteolytic breakdown of APP (Selkoe, 2001).
Oxidative stress has been increasingly recognized as a factor in the pathogenesis of AD (see Behl, 1999; Perry et al., 2000). There is abundant evidence that Aβ is toxic and plays a crucial role in AD pathogenesis (Selkoe, 2000), and that such toxicity is enhanced when the Aβ peptide becomes aggregated (Puttfarcken et al., 1996). A major aspect of Aβ toxicity is the promotion of oxidative stress. The Aβ peptide appears to be an important source of free radicals, which in turn, enhances Aβ aggregation (Dyrks et al., 1992), thereby making Aβ more toxic. Additional sources of free radicals in AD may come from activation of NADPH oxidase, a source of superoxide anion and H2O2, possibly derived from glial activation (Abramov et al., 2004), as well as from mitochondrial dysfunction (see below). Noteworthy, there is evidence of oxidative damage to mitochondrial DNA in Alzheimer's disease (Mecocci et al., 1994; Rodrigues et al., 2001), and such oxidative damage to mitochondria may bring about additional free radical formation.
Aβ, including its aggregated form, is also known to bring about a dysregulation of Ca2+ homeostasis (Mattson et al., 1992; Sheehan et al., 1997). Such dyregulation may contribute to the mitochondrial impairment observed in AD (Lin and Beal, 2006) and after exposure of cells to Aβ (Pereira et al., 1998; Parks et al., 2001). Recent studies have identified Aβ in mitochondria (Anandatheerthavarada et al., 2003; Hansson et al., 2004; Manczak et al., 2006) as well as presenilins and the γ-secretase complex (Hansson et al., 2004). These proteins likely exert untoward effects on mitochondria.
As documented above, AD and Aβ have been associated with oxidative stress, calcium dysregulation and mitochondrial dysfunction, thereby making conditions ripe for mPT induction. It is therefore not surprising that several studies have shown that exposure of isolated mitochondria to neurotoxic Aβ peptides lead to a drop in ΔΨm, matrix swelling and impaired respiration in the presence of Ca2+ (Parks et al., 2001; Moreira et al., 2002). These changes were blocked by CsA. Additionally, use of PC12 cell lines expressing the presenilin-1 mutation (L286V) exhibited increased sensitivity to apoptosis when exposed to complex II inhibitors, and such action of mutant presenilin-1 was prevented by CsA (Keller et al., 1998).
(b) Parkinson’s disease
Parkinson’s disease (PD) is characterized by progressive rigidity, poverty of movement (bradykinesia), tremor, and postural instability. The condition is due to the loss of melanin-containing dopaminergic neurons in the substantia nigra and other sites. Other histopathological features include Lewy bodies, which are eosinophilic cytoplasmic inclusions composed largely of α-synuclein (Lang and Lozano, 1998). The mechanism responsible for the neurodegeneration in PD is not known. Environmental factors and genetic susceptibility, however, are strongly suspected to be involved, and oxidative stress and mitochondrial dysfunction have emerged as critical mediators of the neuronal damage in PD (for reviews, see Blum et al., 2001; Beal, 2003). There is also evidence for excitotoxicity in the mechanism of PD (Beal, 1998).
Mitochondrial involvement in PD was initially proposed when it was shown that the parkinsonian toxin, 1-methyl-4-phenyl pyridinium ion (MPP+) derived from 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), inhibits complex I of the mitochondrial electron transport chain (Nicklas et al., 1985). MPTP has proven to be an excellent agent to clarify pathogenetic mechanisms in PD (Kopin, 1992; Singer et al., 1993). Its administration to primates and rodents recapitulates many of the clinical, biochemical and pathological features of PD.
A reduction in complex I activity, impaired cellular energy metabolism and mitochondrial function along with excessive production of ROS were identified in patients with PD (Beal, 2000). MPP+ has been shown to cause mitochondrial depolarization (Cleren et al., 2005), and rotanone, another complex I inhibitor, has also been shown to cause similar clinical and pathological changes to that seen in humans with PD (Greenamyre et al., 2003). A number of genes have been associated with PD including α-synuclein, parkin, UCH-L1, DJ-1 and others, and all appear to have important interactions with mitochondria (reviewed in Lin and Beal, 2006).
Since oxidative stress and mitochondrial dysfunction are features of both human and experimental PD, it was reasonable to expect that the mPT might be involved in PD. MPP+ causes mitochondrial swelling and the release of cytochrome c, which was inhibited by CsA (Cassarino and Bennett, 1999). Packer et al. (1996) showed that exposure of MPP+ to mitochondria to caused a CsA-sensitive calcium efflux and membrane depolarization. Additionally, another parkinsonian neurotoxin, N-methyl(R)salsolinol, aminoindan, was shown to mediate an mPT-dependent apoptosis, while CsA attenuated the degeneration of dopaminergic neurons induced by 6-hydroxydopamine in mice (Akao et al., 2002). Both rotenone and MPP+ have been shown to induce apoptosis in PC12 cells, and such effect was attenuated by CsA and N-Me-4-Val-CsA (Seaton et al., 1998). DOPAL (3,4-dihydroxyphenylacetaldehyde), a monoamine oxidase metabolite of dopamine, also has been shown to be a potent inducer of the mPT in isolated mitochondria derived from PC12 cells (Kristal et al., 2001).
A number of agents with mPT inhibitory effects have been shown to attenuate the toxic effects of parkinsonian toxins. Promethazine, an antihistamine known to delay the onset of the mPT (Stavrovskaya et al., 2004), prevented the MPP+-induced mitochondrial depolarization, inhibited the Ca2+-induced mPT in isolated brain mitochondria and markedly attenuated the loss of nigral neurons (Cleren et al., 2005). Creatine has been shown to protect against MPP+-induced neuronal loss (Matthews et al., 1999; Andres et al., 2005) and against 6-hydroxydopamine neuronal loss (Andres et al., 2005). Similarly, minocycline was found to exert neuroprotective effects against MPTP toxicity both in vivo and in vitro (Du et al., 2001; Wu et al., 2002).
(c) Huntington’s disease
Huntington’s disease (HD) is a hereditary autosomal dominant progressive neurodegenerative fatal disorder with an onset at 35–40 years and an average survival of 15–20 years after the onset of the disease. It is clinically characterized by progressive motor disorder (choreiform movements) and behavioral and cognitive impairments. The disease largely affects the striatum and to a lesser extent the cerebral cortex (Vonsattel and DiFiglia, 1998).
The disease is due to a mutation in the huntingtin (htt) gene located on chromosome 4 resulting in expanded CAG repeats (coding for glutamine) (The Huntington's Disease Collaborative Research Group, 1993). Patients with HD have CAG repeats varying from 36–86 (average 46). The length of the polyglutamine extensions correlates with lower age of onset, severity of the disease and the higher density of ubiquitin-positive neuronal intranuclear inclusions (Walling et al., 1998). The prevailing hypothesis is that expanded glutamine repeats confers a toxic “gain of function”.
Functional disturbances in mitochondrial bioenergetics are a common feature of HD models and humans with PD (for review, see Grunewald and Beal, 1999). The complex II inhibitors 3-nitropropionic acid (3-NPA) and malonate cause striatal lesions and energy impairment similar to HD (Beal et al., 1993; Andreassen et al., 2000). Lymphoblasts from patients with HD treated with complex II or IV inhibitors had greater mitochondrial depolarization than control lymphoblasts. Interestingly, the severity of depolarization correlated with length of glutamine repeats and with the ability of mitochondria to depolarize at lower calcium concentration than control mitochondria (Sawa et al., 1999; Panov et al., 2002). As in other neurodegenerative diseases, there is a substantial role for oxidative stress (Browne et al., 1999) and excitotoxicity in HD (for reviews, see Whetsell and Shapira 1993; Beal, 1994).
Precisely how Htt brings about these mitochondrial changes is not well understood, although recent studies provide important clues. Mutant Htt has been found to be associated with various cellular organelles, including mitochondria (Gutekunst et al., 1998). Bae et al (2005) presented evidence for the involvement of the transcription factor p53 in the mitochondria-associated cellular dysfunction. Mutant Htt with expanded polyglutamine bound to p53 and upregulated levels of nuclear p53 in neuronal cultures. The p53 levels were also found to be increased in the brains of Htt transgenic mice and HD patients. Inhibiting p53 with pifithrin-alpha, RNA interference, or genetic deletion prevented the mitochondrial membrane depolarization and cytotoxicity in HD cells, as well as the decreased respiratory complex IV activity of transgenic mice. This important study provides a crucial link between nuclear and mitochondrial events in HD. Similarly, Choo et al. (2004) showed that the mutant Htt may directly interact with the outer mitochondrial membrane and affect its functions. They further demonstrated that mutant Htt induced the mPT in isolated mouse liver mitochondria as well as promoted cytochrome c release, both of which were blocked by CsA. Mutant Htt also significantly decreased the Ca2+ threshold necessary to trigger mPT pore opening.
Disturbances in calcium homestasis have been identified in HD. In a transgenic model of HD, the calcium buffering capacity of neurons expressing mutant Htt was found to be impaired (Hodgson et al., 1999; Panov et al., 2003). Synthetic peptides containing glutamine repeats capable of crossing the plasma and nuclear membranes of sympathetic neurons brought about elevated cytosolic Ca2+ levels, and decreased ATP levels (Suzuki and Koike, 2005).
There is evidence that HD neurotoxins and cells carrying the mutant Htt gene are prone to develop the mPT. The complex II inhibitor malonate has been shown to induce the mPT in isolated brain mitochondria (Fernandez-Gomez et al., 2005), while CsA protected striatal neurons in vitro and in vivo from 3-NPA toxicity. Synthetic peptides containing glutamine repeats capable of crossing the plasma and nuclear membranes were able to induce the mPT (Suzuki and Koike, 2005). Striatal neurons carrying the HD transgene were more susceptible to cell death after treatment with 3-NPA (Ruan et al., 2004), and such treatment was associated with a greater loss of ΔΨm compared with wild-type cells. CsA diminished 3-NPA-induced cell death and prevented the loss of ΔΨm. Mutant Htt was also shown to induce mPT pore opening which was associated with the release of cytochrome c and was blocked by CsA (Choo et al., 2004). Zeron et al. (2004) found that primary striatal neurons carrying mutant Htt were more sensitive to NMDA receptor-mediated neurotoxicity than were normal neurons, and that such toxicity was attenuated by either CsA or bongkrekic acid.
In keeping with the potential involvement of the mPT in various models of HD, creatine was found to protect against malonate and 3-NPA in vivo models of HD (Matthews et al., 1998), as well as in transgenic mouse models of HD (Ferrante et al., 2000; Andreassen et al., 2001). Minocycline was reported to delay disease progression and to inhibit caspase-1 and caspase-3 mRNA upregulation in the R6/2 mouse model of HD (Chen et al., 2000).
(d) Amyotrophic lateral sclerosis
Amyotrophic lateral sclerosis (ALS) is an age-dependent progressive disorder resulting from degeneration of motor neurons in the ventral horns of the spinal cord, brainstem and motor cortex, along with associated degeneration of the corticospinal tract. This leads to progressive skeletal muscle atrophy, weakness, paralysis, and death frequently due to respiratory failure within 2 to 5 years of onset (Rowland and Schneider, 2001). About 10% of ALS cases are familial and approximately 20% of these have mutations in superoxide dismutase 1 (SOD1) (Rosen et al., 1993). Familial and sporadic forms have indistinguishable clinical and histopathological features (Gurney et al., 1994; Wong et al., 1995). Over 90 different SOD1 mutations have been identified, the majority of which show normal SOD1 activity (Radunovic and Leigh, 1996), suggesting that the mutations lead to a “gain of function”. The cause of motor neuron death in ALS is unknown (for reviews on potential mechanisms involving mitochondria, calcium, oxidative stress and excitotoxicity, see Cleveland and Rothstein, 2001; Menzies et al., 2002).
The involvement of mitochondria in ALS have been recently reviewed by (Menzies et al., 2002; Xu et al., 2004). Such involvement in ALS was initially suggested by the widespread mitochondrial vacuolation identified in the early phase of motor neuron degeneration (Gurney et al., 1994; Wong, et al., 1995; Higgins et al., 2003). Similar structural mitochondrial abnormalities have been observed in humans with sporadic and familial ALS (Sasaki et al., 1990; Siklos et al., 1996).
Cells expressing the G93A SOD1 mutation show a significant loss of mitochondrial membrane potential, an increase in cytosolic Ca2+ concentration (Carri et al., 1997). Mutant SOD1 (a cytosolic protein) has been shown to be imported into mitochondria (Okado-Matsumoto and Fridovich, 2001; Jaarsma et al., 2001; Mattiazzi et al., 2002; Higgins et al., 2002), and it has been suggested that this localization may induce cell death (Takeuchi et al., 2002). Higgins et al. (2003) found that the mitochondrial vacuolar patterns in transgenic mice expressing mutant SOD1G93A originate from the expansion of the mitochondrial intermembrane space and that these vacuoles were bounded by SOD1. As mutated SOD1 has a propensity to undergo aggregation (Julien, 2001), the authors speculated that aggregation of mutant SOD1 may elicit mitochondrial degeneration. Mattiazzi et al. (2002) found mitochondrial abnormalities in oxidative phosphorylation in mice possessing the G93A mutated SOD1.
Using a transgenic mouse model of ALS (SOD1-G93A) in which weakness appears at 3 months of age, and death by 5 months, Keep et al. (2001) and Karlsson et al. (2004) showed that intraventricular administration of CsA prolonged the survival of these mice as compared to vehicle-treated controls. Histologically, there was significant preservation of cervical and lumbar spine motor neurons
The effectiveness of minocycline in mice with ALS expressing the mutant human G93A SOD1 transgene has been examined (Zhu et al., 2002). Minocycline delayed the onset of the disease and extended the survival time. These authors also found that minocycline inhibited the mPT-mediated cytochrome c release as well as blocked mitochondrial swelling upon the addition of Ca2+. Klivenyi et al. (1999) found that G93A transgenic mice treated with creatine showed a dose-dependent improvement in motor performance, an extended survival time and displayed a reduction in oxidative cellular injury.
4. Concluding remarks
The mPT has evolved over the past decade as major factor in the mediation of cell injury and death. Factors associated with its induction include loss of calcium homeostasis leading to elevated intracellular calcium levels, oxidative stress, mitochondrial dysfunction and energy failure. These events are common in many CNS disorders and are often triggered by excitotoxicity. When sufficiently severe, these events may coalesce to create the permeability transition, adding further insult to an already compromised cell.
Much data has accrued to implicate the mPT in a host of acute and chronic neurological conditions. Nevertheless, caution must be exercised when interpreting the results of these studies. There are technical difficulties, and caveats to be considered when determining the presence of the mPT in vivo as well as in cultured cells. Additionally, much of the current data implicating the mPT in neurologic disease has come from cell culture studies exposed to putative neurotoxins which, while suggestive, still leaves unanswered the significance of the mPT in the in vivo condition. Lastly, it is often less than clear whether the mPT was the cause or the consequence of the disorder being investigated.
With these reservations aside, the potential involvement of the mPT in neurological conditions adds an exciting new dimension to mechanisms of neurologic disease and provides attractive and novel approaches for the therapy of these devastating CNS disorders for which no cures are currently available.
Acknowledgments
This work was supported by the Department of Veterans Affairs and NIH Grant No. DK063311. K.V.R. is the recipient of the American Association for the Study of Liver Disease/American Liver Foundation Grants.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abramov AY, Canevari L, Duchen MR. Beta-amyloid peptides induce mitochondrial dysfunction and oxidative stress in astrocytes and death of neurons through activation of NADPH oxidase. J. Neurosci. 2004;24:565–575. doi: 10.1523/JNEUROSCI.4042-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akao Y, Maruyama W, Shimizu S, Yi H, Nakagawa Y, Shamoto-Nagai M, Youdim MB, Tsujimoto Y, Naoi M. Mitochondrial permeability transition mediates apoptosis induced by N-methyl(R)salsolinol, an endogenous neurotoxin, is inhibited by Bcl-2 and rasagiline, N-propargyl-1(R)-aminoindan. J. Neurochem. 2002;82:913–923. doi: 10.1046/j.1471-4159.2002.01047.x. [DOI] [PubMed] [Google Scholar]
- Albrecht J, Norenberg MD. Glutamine: a Trojan horse in ammonia neurotoxicity. Hepatology. 2006;44:788–794. doi: 10.1002/hep.21357. [DOI] [PubMed] [Google Scholar]
- Anandatheerthavarada HK, Biswas G, Robin MA, Avadhani NG. Mitochondrial targeting and a novel transmembrane arrest of Alzheimer's amyloid precursor protein impairs mitochondrial function in neuronal cells. J. Cell Biol. 2003;161:41–54. doi: 10.1083/jcb.200207030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrabi SA, Sayeed I, Siemen D, Wolf G, Horn TF. Direct inhibition of the mitochondrial permeability transition pore: a possible mechanism responsible for anti-apoptotic effects of melatonin. FASEB J. 2004;18:869–871. doi: 10.1096/fj.03-1031fje. [DOI] [PubMed] [Google Scholar]
- Andreassen OA, Dedeoglu A, Ferrante RJ, Jenkins BG, Ferrante KL, Thomas M, Friedlich A, Browne SE, Schilling G, Borchelt DR, Hersch SM, Ross CA, Beal MF. Creatine increase survival and delays motor symptoms in a transgenic animal model of Huntington's disease. Neurobiol. Dis. 2001;8:479–491. doi: 10.1006/nbdi.2001.0406. [DOI] [PubMed] [Google Scholar]
- Andreassen OA, Ferrante RJ, Hughes DB, Klivenyi P, Dedeoglu A, Ona VO, Friedlander RM, Beal MF. Malonate and 3-nitropropionic acid neurotoxicity are reduced in transgenic mice expressing a caspase-1 dominant-negative mutant. J. Neurochem. 2000;75:847–852. doi: 10.1046/j.1471-4159.2000.0750847.x. [DOI] [PubMed] [Google Scholar]
- Andres RH, Huber AW, Schlattner U, Perez-Bouza A, Krebs SH, Seiler RW, Wallimann T, Widmer HR. Effects of creatine treatment on the survival of dopaminergic neurons in cultured fetal ventral mesencephalic tissue. Neuroscience. 2005;133:701–713. doi: 10.1016/j.neuroscience.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Ankarcrona M, Dypbukt JM, Orrenius S, Nicotera P. Calcineurin and mitochondrial function in glutamate-induced neuronal cell death. FEBS Lett. 1996;394:321–324. doi: 10.1016/0014-5793(96)00959-3. [DOI] [PubMed] [Google Scholar]
- Aschner M, Gannon M, Kimelberg HK. Manganese uptake and efflux in cultured rat astrocytes. J. Neurochem. 1992;58:730–735. doi: 10.1111/j.1471-4159.1992.tb09778.x. [DOI] [PubMed] [Google Scholar]
- Auer RN, Siesjö BK. Biological differences between ischemia, hypoglycemia, epilepsy. Ann. Neurol. 1988;24:699–707. doi: 10.1002/ana.410240602. [DOI] [PubMed] [Google Scholar]
- Bae BI, Xu H, Igarashi S, Fujimuro M, Agrawal N, Taya Y, Hayward SD, Moran TH, Montell C, Ross CA, Snyder SH, Sawa A. p53 mediates cellular dysfunction and behavioral abnormalities in Huntington's disease. Neuron. 2005;47:29–41. doi: 10.1016/j.neuron.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Bai G, Rama Rao KV, Murthy ChRK, Panickar KS, Jayakumar AR, Norenberg MD. Ammonia induces the mitochondrial permeability transition in primary cultures of rat astrocytes. J. Neurosci. Res. 2001;66:981–991. doi: 10.1002/jnr.10056. [DOI] [PubMed] [Google Scholar]
- Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- Basso E, Fante L, Fowlkes J, Petronilli V, Forte MA, Bernardi P. Properties of the permeability transition pore in mitochondria devoid of Cyclophilin D. J. Biol. Chem. 2005;280:18558–18561. doi: 10.1074/jbc.C500089200. [DOI] [PubMed] [Google Scholar]
- Beal MF. Huntington's disease, energy, excitotoxicity. Neurobiol. Aging. 1994;15:275–276. doi: 10.1016/0197-4580(94)90132-5. [DOI] [PubMed] [Google Scholar]
- Beal MF. Excitotoxicity and nitric oxide in Parkinson's disease pathogenesis. Ann. Neurol. 1998;44:S110–S114. doi: 10.1002/ana.410440716. [DOI] [PubMed] [Google Scholar]
- Beal MF. Energetics in the pathogenesis of neurodegenerative diseases. Trends Neurosci. 2000;23:298–304. doi: 10.1016/s0166-2236(00)01584-8. [DOI] [PubMed] [Google Scholar]
- Beal MF. Bioenergetic approaches for neuroprotection in Parkinson's disease. Ann. Neurol. 2003;53(Suppl 3):S39–S47. doi: 10.1002/ana.10479. [DOI] [PubMed] [Google Scholar]
- Beal MF, Brouillet E, Jenkins B, Henshaw R, Rosen B, Hyman BT. Age-dependent striatal excitotoxic lesions produced by the endogenous mitochondrial inhibitor malonate. J. Neurochem. 1993;61:1147–1150. doi: 10.1111/j.1471-4159.1993.tb03633.x. [DOI] [PubMed] [Google Scholar]
- Behl C. Alzheimer's disease and oxidative stress: Implications for novel therapeutic approaches. Prog. Neurobiol. 1999;57:301–323. doi: 10.1016/s0301-0082(98)00055-0. [DOI] [PubMed] [Google Scholar]
- Bernardi P, Vassanelli S, Veronese P, Colonna R, Szabo I, Zoratti M. Modulation of the mitochondrial permeability transition pore: effect of protons and divalent cations. J. Biol. Chem. 1992;267:2934–2939. [PubMed] [Google Scholar]
- Beutner G, Ruck A, Riede B, Brdiczka D. Complexes between porin, hexokinase, mitochondrial creatine kinase and adenylate translocator display properties of the permeability transition pore. Implication for regulation of permeability transition by the kinases. Biochim Biophys. Acta. 1998;1368:7–18. doi: 10.1016/s0005-2736(97)00175-2. [DOI] [PubMed] [Google Scholar]
- Blum D, Torch S, Lambeng N, Nissou M, Benabid AL, Sadoul R, Verna JM. Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: contribution to the apoptotic theory in Parkinson's disease. Prog. Neurobiol. 2001;65:135–172. doi: 10.1016/s0301-0082(01)00003-x. [DOI] [PubMed] [Google Scholar]
- Brdiczka D, Beutner G, Ruck A, Dolder M, Wallimann T. The molecular structure of mitochondrial contact sites. Their role in regulation of energy metabolism and permeability transition. BioFactors. 1998;8:235–242. doi: 10.1002/biof.5520080311. [DOI] [PubMed] [Google Scholar]
- Browne SE, Ferrante RJ, Beal MF. Oxidative stress in Huntington's disease. Brain Pathol. 1999;9:147–163. doi: 10.1111/j.1750-3639.1999.tb00216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brustovetsky N, Brustovetsky T, Dubinsky JM. On the mechanisms of neuroprotection by creatine and phosphocreatine. J. Neurochem. 2001;76:425–434. doi: 10.1046/j.1471-4159.2001.00052.x. [DOI] [PubMed] [Google Scholar]
- Brustovetsky N, Klingenberg M. Mitochondrial ADP/ATP carrier can be reversibly converted into a large channel by Ca2+ Biochemistry. 1996;35:8483–8488. doi: 10.1021/bi960833v. [DOI] [PubMed] [Google Scholar]
- Brustovetsky T, Antonsson B, Jemmerson R, Dubinsky JM, Brustovetsky N. Activation of calcium-independent phospholipase A (iPLA) in brain mitochondria and release of apoptogenic factors by BAX and truncated BID. J. Neurochem. 2005;94:980–994. doi: 10.1111/j.1471-4159.2005.03248.x. [DOI] [PubMed] [Google Scholar]
- Buki A, Okonkwo DO, Povlishock JT. Postinjury cyclosporin A administration limits axonal damage and disconnection in traumatic brain injury. J. Neurotrauma. 1999;16:511–521. doi: 10.1089/neu.1999.16.511. [DOI] [PubMed] [Google Scholar]
- Carri MT, Ferri A, Battistoni A, Famhy L, Gabbianelli R, Poccia F, Rotilio G. Expression of a Cu,Zn superoxide dismutase typical of familial amyotrophic lateral sclerosis induces mitochondrial alteration and increase of cytosolic Ca2+ concentration in transfected neuroblastoma SH-SY5Y cells. FEBS Lett. 1997;414:365–368. doi: 10.1016/s0014-5793(97)01051-x. [DOI] [PubMed] [Google Scholar]
- Cassarino DS, Bennett JP., Jr An evaluation of the role of mitochondria in neurodegenerative diseases: mitochondrial mutations and oxidative pathology, protective nuclear responses, cell death in neurodegeneration. Brain Res. Rev. 1999a;29:1–25. doi: 10.1016/s0165-0173(98)00046-0. [DOI] [PubMed] [Google Scholar]
- Cassarino DS, Parks JK, Parker WD, Jr, Bennett JP., Jr The parkinsonian neurotoxin MPP+ opens the mitochondrial permeability transition pore and releases cytochrome c in isolated mitochondria via an oxidative mechanism. Biochim. Biophys. Acta. 1999b;1453:49–62. doi: 10.1016/s0925-4439(98)00083-0. [DOI] [PubMed] [Google Scholar]
- Checler F. Presenilins: structural aspects and posttranslational events. Molec. Neurobiol. 1999;19:255–265. doi: 10.1007/BF02821716. [DOI] [PubMed] [Google Scholar]
- Chen M, Ona VO, Li M, Ferrante RJ, Fink KB, Zhu S, Bian J, Guo L, Farrell LA, Hersch SM, Hobbs W, Vonsattel JP, Cha JH, Friedlander RM. Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington disease. Nature Med. 2000;6:797–801. doi: 10.1038/77528. [see comment] [DOI] [PubMed] [Google Scholar]
- Choi DW. Excitotoxic cell death. J. Neurobiol. 1992;23:1261–1276. doi: 10.1002/neu.480230915. [DOI] [PubMed] [Google Scholar]
- Choo YS, Johnson GV, MacDonald M, Detloff PJ, Lesort M. Mutant huntingtin directly increases susceptibility of mitochondria to the calcium-induced permeability transition and cytochrome c release. Hum. Molec. Genet. 2004;13:1407–1420. doi: 10.1093/hmg/ddh162. [DOI] [PubMed] [Google Scholar]
- Cleren C, Starkov AA, Calingasan NY, Lorenzo BJ, Chen J, Beal MF. Promethazine protects against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine neurotoxicity. Neurobiol. Dis. 2005;20:701–708. doi: 10.1016/j.nbd.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Cleveland DW, Rothstein JD. From Charcot to Lou Gehrig: deciphering selective motor neuron death in ALS. Nat. Rev. Neurosci. 2001;2:806–819. doi: 10.1038/35097565. [DOI] [PubMed] [Google Scholar]
- Connern CP, Halestrap AP. Recruitment of mitochondrial cyclophilin to the mitochondrial inner membrane under conditions of oxidative stress that enhance the opening of a calcium-sensitive non-specific channel. Biochem. J. 1994;301:321–324. doi: 10.1042/bj3020321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper AJL, Plum F. Biochemistry and physiology of brain ammonia. Physiol. Rev. 1987;67:440–519. doi: 10.1152/physrev.1987.67.2.440. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Puttfarcken PS. Oxidative stress, glutamate, neurodegenerative disorders. Science. 1993;262:689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- Crompton M. On the involvement of mitochondrial intermembrane junctional complexes in apoptosis. Curr. Med. Chem. 2003;10:1473–1484. doi: 10.2174/0929867033457197. [DOI] [PubMed] [Google Scholar]
- Crompton M, Barksby E, Johnson N, Capano M. Mitochondrial intermembrane junctional complexes and their involvement in cell death. Biochimie. 2002;84:143–152. doi: 10.1016/s0300-9084(02)01368-8. [DOI] [PubMed] [Google Scholar]
- Crompton M, Ellinger H, Costi A. Inhibition by cyclosporin A of a Ca2+-dependent pore in heart mitochondria activated by inorganic phosphate and oxidative stress. Biochem. J. 1988;255:357–360. [PMC free article] [PubMed] [Google Scholar]
- Crompton M, Virji S, Ward JM. Cyclophilin-D binds strongly to complexes of the voltage- dependent anion channel and the adenine nucleotide translocase to form the permeability transition pore. Eur. J. Biochem. 1998;258:729–735. doi: 10.1046/j.1432-1327.1998.2580729.x. [DOI] [PubMed] [Google Scholar]
- Dawson TM, Steiner JP, Dawson VL, Dinerman JL, Snyder SH. Immunosuppresant FK506 enhances phosphorylation of nitric oxide synthase and protects against glutamate neurotoxicity. Proc. Natl. Acad. Sci. USA. 1993;90:9808–9812. doi: 10.1073/pnas.90.21.9808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Ma Z, Lin S, Dodel RC, Gao F, Bales KR, Triarhou LC, Chernet E, Perry KW, Nelson DL, Luecke S, Phebus LA, Bymaster FP, Paul SM. Minocycline prevents nigrostriatal dopaminergic neurodegeneration in the MPTP model of Parkinson's disease. Proc. Natl. Acad. Sci. 2001;98:14669–14674. doi: 10.1073/pnas.251341998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubinsky JM, Levi Y. Calcium-induced activation of the mitochondrial permeability transition in hippocampal neurons. J. Neurosci. Res. 1998;53:728–741. doi: 10.1002/(SICI)1097-4547(19980915)53:6<728::AID-JNR10>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Duchen MR. Roles of mitochondria in health and disease. Diabetes. 2004;53(Suppl 1):S96–S102. doi: 10.2337/diabetes.53.2007.s96. [DOI] [PubMed] [Google Scholar]
- Dyrks T, Dyrks E, Hartmann T, Masters C, Beyreuther K. Amyloidogenicity of beta A4 and beta A4-bearing amyloid protein precursor fragments by metal-catalyzed oxidation. J. Biol. Chem. 1992;267:18210–18217. [PubMed] [Google Scholar]
- Feng Y, Fratkins JD, LeBlanc MH. Treatment with tamoxifen reduces hypoxic-ischemic brain injury in neonatal rats. Eur. J. Pharmacol. 2004;484:65–74. doi: 10.1016/j.ejphar.2003.10.048. [DOI] [PubMed] [Google Scholar]
- Fernandez-Gomez FJ, Galindo MF, Gomez-Lazaro M, Yuste VJ, Comella JX, Aguirre N, Jordan J. Malonate induces cell death via mitochondrial potential collapse and delayed swelling through an ROS-dependent pathway. Brit. J. Pharmacol. 2005;144:528–537. doi: 10.1038/sj.bjp.0706069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrante RJ, reassen OA, Jenkins BG, Dedeoglu A, Kuemmerle S, Kubilus JK, Kaddurah-Daouk R, Hersch SM, Beal MF. Neuroprotective effects of creatine in a transgenic mouse model of Huntington's disease. J. Neurosci. 2000;20:4389–4397. doi: 10.1523/JNEUROSCI.20-12-04389.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiskum G, Murphy AN, Beal MF. Mitochondria in neurodegeneration: acute ischemia and chronic neurodegenerative diseases. J. Cereb. Blood Flow Metab. 1999;19:351–369. doi: 10.1097/00004647-199904000-00001. [DOI] [PubMed] [Google Scholar]
- Fontaine E, Ichas F, Bernardi P. A ubiquinone-binding site regulates the mitochondrial permeability transition pore. J. Biol. Chem. 1998;273:25734–25740. doi: 10.1074/jbc.273.40.25734. [DOI] [PubMed] [Google Scholar]
- Friberg H, Ferrand-Drake M, Bengtsson F, Halestrap AP, Wieloch T. Cyclosporin A, but not FK506, protects mitochondria and neurons against hypoglycemic damage and implicates the mitochondrial permeability transition in cell death. J. Neurosci. 1998;18:5151–5159. doi: 10.1523/JNEUROSCI.18-14-05151.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friberg H, Wieloch T. Mitochondrial permeability transition in acute neurodegeneration. Biochimie. 2002;84:241–250. doi: 10.1016/s0300-9084(02)01381-0. [DOI] [PubMed] [Google Scholar]
- Glenner GG. Amyloid β protein and the basis for Alzheimer's disease. Prog. Clin. Biol. Res. 1989;317:857–868. [PubMed] [Google Scholar]
- Goldberg MP, Choi DW. Combined oxygen and glucose deprivation in cortical cell culture: calcium-dependent and calcium-indpendent mechanisms of neuronal injury. J. Neurosci. 1993;13:3510–3524. doi: 10.1523/JNEUROSCI.13-08-03510.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305:626–629. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- Greenamyre JT, Betarbet R, Sherer TB. The rotenone model of Parkinson's disease: genes, environment and mitochondria. Parkinsonism Related Dis. 2003;9(Suppl 2):S59–S64. doi: 10.1016/s1353-8020(03)00023-3. [DOI] [PubMed] [Google Scholar]
- Griffiths EJ, Halestrap AP. Further evidence that cyclosporin A protects mitochondria from calcium overload by inhibiting a matrix peptidyl-prolyl cis-trans isomerase. Implications for the immunosuppressive and toxic effects of cyclosporin. Biochem. J. 1991;274:611–614. doi: 10.1042/bj2740611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunewald T, Beal MF. Bioenergetics in Huntington's disease. Ann. NY Acad. Sci. 1999;893:203–213. doi: 10.1111/j.1749-6632.1999.tb07827.x. [DOI] [PubMed] [Google Scholar]
- Gunter TE, Gunter KK. Uptake of calcium by mitochondria: transport and possible function. IUBM Life. 2001;52:197–204. doi: 10.1080/15216540152846000. [DOI] [PubMed] [Google Scholar]
- Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, Caliendo J, Hentati A, Kwon YW, Deng HX, Chen W, Zhai P, Sufit R, Siddique T. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- Gutekunst CA, Li SH, Yi H, Ferrante RJ, Li XJ, Hersch SM, Gutekunst CA, Li SH, Yi H, Ferrante RJ, Li XJ, Hersch SM. The cellular and subcellular localization of huntingtin-associated protein 1 (HAP1): comparison with huntingtin in rat and human. J. Neurosci. 1998;18:7674–7686. doi: 10.1523/JNEUROSCI.18-19-07674.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap AP. Calcium, mitochondria and reperfusion injury: a pore way to die. Biochem. Soc. Trans. 2006;34:232–237. doi: 10.1042/BST20060232. [DOI] [PubMed] [Google Scholar]
- Halestrap AP, Brennerb C. The adenine nucleotide translocase: a central component of the mitochondrial permeability transition pore and key player in cell death. Curr. Med. Chem. 2003;10:1507–1525. doi: 10.2174/0929867033457278. [DOI] [PubMed] [Google Scholar]
- Halestrap AP, Conner CP, Griffiths EJ, Kerr PM. Cyclosporin A binding to mitochondrial cyclophilin inhibits the permeability transition pore and protects hearts from ischemia/reperfusion injury. Mol. Cell. Biochem. 1997a;174:167–172. [PubMed] [Google Scholar]
- Halestrap AP, Davidson AM. Inhibition of Ca2(+)-induced large-amplitude swelling of liver and heart mitochondria by cyclosporin is probably caused by the inhibitor binding to mitochondrial-matrix peptidyl-prolyl cis-trans isomerase and preventing it interacting with the adenine nucleotide translocase. Biochem. J. 1990;268:153–160. doi: 10.1042/bj2680153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap AP, Woodfield KY, Connern CP. Oxidative stress, thiol reagents, membrane potential modulate the mitochondrial permeability transition by affecting nucleotide binding to the adenine nucleotide translocase. J. Biol. Chem. 1997b;272:3346–3354. doi: 10.1074/jbc.272.6.3346. [DOI] [PubMed] [Google Scholar]
- Hansen AJ, Zeuthen T. Extracellular ion concentrations during spreading depression and ischemia in the rat brain cortex. Acta Physiol. Scand. 1981;113:437–445. doi: 10.1111/j.1748-1716.1981.tb06920.x. [DOI] [PubMed] [Google Scholar]
- Hansson CA, Frykman S, Farmery MR, Tjernberg LO, Nilsberth C, Pursglove SE, Ito A, Winblad B, Cowburn RF, Thyberg J, Ankarcrona M. Nicastrin, presenilin, APH-1, PEN-2 form active gamma-secretase complexes in mitochondria. J. Biol. Chem. 2004;279:51654–51660. doi: 10.1074/jbc.M404500200. [DOI] [PubMed] [Google Scholar]
- Harris RJ, Wieloch T, Symon L, Siesjö BK. Cerebral extracellular calcium activity in severe hypoglycemia: relation to extracellular potassium and energy state. J. Cereb. Blood Flow Metab. 1984;4:187–193. doi: 10.1038/jcbfm.1984.27. [DOI] [PubMed] [Google Scholar]
- Hatefi Y. The mitochondrial electron transport and oxidative phosphorylation system. Annu. Rev. Biochem. 1985;54:1015–1069. doi: 10.1146/annurev.bi.54.070185.005055. [DOI] [PubMed] [Google Scholar]
- Haworth RA, Hunter DR. The Ca2+-induced membrane transition in mitochondria. II. Nature of the Ca2+ trigger site. Arch. Biochem. Biophys. 1979;195:460–467. doi: 10.1016/0003-9861(79)90372-2. [DOI] [PubMed] [Google Scholar]
- Hazell AS, Butterworth RF. Hepatic encephalopathy: An update of pathophysiologic mechanisms. Proc. Soc. Exp. Biol. Med. 1999;222:99–112. doi: 10.1046/j.1525-1373.1999.d01-120.x. [DOI] [PubMed] [Google Scholar]
- He L, Lemasters JJ. Regulated and unregulated mitochondrial permeability transition pores: a new paradigm of pore structure and function? FEBS Letters. 2002;512:1–7. doi: 10.1016/s0014-5793(01)03314-2. [DOI] [PubMed] [Google Scholar]
- Higgins CM, Jung C, Ding H, Xu Z. Mutant Cu, Zn superoxide dismutase that causes motoneuron degeneration is present in mitochondria in the CNS. J. Neurosci. 2002;22:RC215. doi: 10.1523/JNEUROSCI.22-06-j0001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins CM, Jung C, Xu Z. ALS-associated mutant SOD1G93A causes mitochondrial vacuolation by expansion of the intermembrane space and by involvement of SOD1 aggregation and peroxisomes. BMC Neurosci. 2003;4:16–30. doi: 10.1186/1471-2202-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson JG, Agopyan N, Gutekunst CA, Leavitt BR, LePiane F, Singaraja R, Smith DJ, Bissada N, McCutcheon K, Nasir J, Jamot L, Li XJ, Stevens ME, Rosemond E, Roder JC, Phillips AG, Rubin EM, Hersch SM, Hayden MR. A YAC mouse model for Huntington's disease with full-length mutant huntingtin, cytoplasmic toxicity, selective striatal neurodegeneration. Neuron. 1999;23:181–192. doi: 10.1016/s0896-6273(00)80764-3. [DOI] [PubMed] [Google Scholar]
- Jaarsma D, Rognoni F, van Duijn W, Verspaget HW, Haasdijk ED, Holstege JC. CuZn superoxide dismutase (SOD1) accumulates in vacuolated mitochondria in transgenic mice expressing amyotrophic lateral sclerosis-linked SOD1 mutations. Acta Neuropathol. 2001;102:293–305. doi: 10.1007/s004010100399. [DOI] [PubMed] [Google Scholar]
- Jayakumar AR, Panickar KS, Murthy ChRK, Norenberg MD. Oxidative stress and MAPK phosphorylation mediate ammonia-induced cell swelling and glutamate uptake inhibition in cultured astrocytes. J. Neurosci. 2006;26:4774–4784. doi: 10.1523/JNEUROSCI.0120-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayakumar AR, Rama Rao KV, Norenberg MD. Glutamine-induced free radical production in cultured astrocytes. Glia. 2004;46:296–301. doi: 10.1002/glia.20003. [DOI] [PubMed] [Google Scholar]
- Jemmerson R, Dubinsky JM, Brustovetsky N. Cytochrome C release from CNS mitochondria and potential for clinical intervention in apoptosis-mediated CNS diseases. Antiox. Redox Signal. 2005;7:1158–1172. doi: 10.1089/ars.2005.7.1158. [DOI] [PubMed] [Google Scholar]
- Jones EA, Weissenborn K. Neurology and the liver. J. Neurol. Neurosurg. Psychiatry. 1997;63:279–293. doi: 10.1136/jnnp.63.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien JP. Amyotrophic lateral sclerosis. Unfolding the toxicity of the misfolded. Cell. 2001;104:581–591. doi: 10.1016/s0092-8674(01)00244-6. [DOI] [PubMed] [Google Scholar]
- Kaiser J. State Court to rule on manganese fume claims. Science. 2003a;300:927. doi: 10.1126/science.300.5621.927. [DOI] [PubMed] [Google Scholar]
- Kaiser J. Manganese: a high-octane dispute. Science. 2003b;300:926–928. doi: 10.1126/science.300.5621.926. [DOI] [PubMed] [Google Scholar]
- Karlsson J, Fong KS, Hansson MJ, Elmer E, Csiszar K, Keep MF. Life span extension and reduced neuronal death after weekly intraventricular cyclosporin injections in the G93A transgenic mouse model of amyotrophic lateral sclerosis. J. Neurosurg. 2004;101:128–137. doi: 10.3171/jns.2004.101.1.0128. [DOI] [PubMed] [Google Scholar]
- Keep M, Elmer E, Fong KS, Csiszar K. Intrathecal cyclosporin prolongs survival of late-stage ALS mice. Brain Res. 2001;894:327–331. doi: 10.1016/s0006-8993(01)02012-1. [DOI] [PubMed] [Google Scholar]
- Keller JN, Guo Q, Holtsberg FW, Bruce-Keller AJ, Mattson MP. Increased sensitivity to mitochondrial toxin-induced apoptosis in neural cells expressing mutant presenilin-1 is linked to perturbed calcium homeostasis and enhanced oxyradical production. J. Neurosci. 1998;18:4439–4450. doi: 10.1523/JNEUROSCI.18-12-04439.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr PM, Suleiman MS, Halestrap AP. Reversal of permeability transition during recovery of hearts from ischemia and its enhancement by pyruvate. Am. J. Physiol. 1999;276:H496–H502. doi: 10.1152/ajpheart.1999.276.2.H496. [DOI] [PubMed] [Google Scholar]
- Khaspekov L, Friberg H, Halestrap A, Viktorov I, Wieloch T. Cyclosporin A and its nonimmunosuppressive analogue N-Me-Val-4-cyclosporin A mitigate glucose/oxygen deprivation-induced damage to rat cultured hippocampal neurons. Eur. J. Neurosci. 1999;11:3194–3198. doi: 10.1046/j.1460-9568.1999.00743.x. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK, Jin Y, Charniga C, Feustel PJ. Neuroprotective activity of tamoxifen in permanent focal ischemia. J. Neurosurg. 2003;99:138–142. doi: 10.3171/jns.2003.99.1.0138. [DOI] [PubMed] [Google Scholar]
- Klivenyi P, Calingasan NY, Starkov A, Stavrovskaya IG, Kristal BS, Yang L, Wieringa B, Beal MF. Neuroprotective mechanisms of creatine occur in the absence of mitochondrial creatine kinase. Neurobiol. Dis. 2004;15:610–617. doi: 10.1016/j.nbd.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Klivenyi P, Ferrante RJ, Bogdanov MB, Klein AM, reassen OA, Mueller G, Werner M, Kaddurah-Daouk R, Beal MF. Neuroprotective effects of creatine in a transgenic animal model of amyotrophic lateral sclerosis. Nat. Med. 1999;5:347–350. doi: 10.1038/6568. [DOI] [PubMed] [Google Scholar]
- Kokoszka JE, Waymire KG, Levy SE, Sligh JE, Cai J, Jones DP, MacGregor GR, Wallace DC. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature. 2004;427:461–465. doi: 10.1038/nature02229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopin IJ. Features of the dopaminergic neurotoxin MPTP. Ann. NY Acad. Sci. 1992;648:96–104. doi: 10.1111/j.1749-6632.1992.tb24527.x. [DOI] [PubMed] [Google Scholar]
- Kowaltowski AJ, Castilho RF, Vercesi AE. Mitochondrial permeability transition and oxidative stress. FEBS Lett. 2001;495:12–15. doi: 10.1016/s0014-5793(01)02316-x. [DOI] [PubMed] [Google Scholar]
- Krauskopf A, Eriksson O, Craigen WJ, Forte MA, Bernardi P. Properties of the permeability transition in VDAC1(−/−) mitochondria. Biochem. Biophys. Acta. 2006;1757:590–595. doi: 10.1016/j.bbabio.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Kristal BS, Conway AD, Brown AM, Jain JC, Ulluci PA, Li SW, Burke WJ. Selective dopaminergic vulnerability: 3,4-dihydroxyphenylacetaldehyde targets mitochondria. Free Rad. Biol. Med. 2001;30:924–931. doi: 10.1016/s0891-5849(01)00484-1. [DOI] [PubMed] [Google Scholar]
- Kristian T. Metabolic stages, mitochondria and calcium in hypoxic/ischemic brain damage. Cell Calcium. 2004;36:221–233. doi: 10.1016/j.ceca.2004.02.016. [DOI] [PubMed] [Google Scholar]
- Kristian TB, Bernardi P, Siesjö BK. Acidosis promotes the permeability transition in energized mitochondria: implications for reperfusion injury. J. Neurotrauma. 2001;18:1059–1074. doi: 10.1089/08977150152693755. [DOI] [PubMed] [Google Scholar]
- Kroemer G, Reed JC. Mitochondrial control of cell death. Nature Med. 2000;6:513–519. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]
- Kruman II, Mattson MP. Pivotal role of mitochondrial calcium uptake in neural cell apoptosis and necrosis. J. Neurochem. 1999;72:529–540. doi: 10.1046/j.1471-4159.1999.0720529.x. [DOI] [PubMed] [Google Scholar]
- Lang AE, Lozano AM. Parkinson's disease. First of two parts. New Engl. J. Med. 1998;339:1044–1053. doi: 10.1056/NEJM199810083391506. [DOI] [PubMed] [Google Scholar]
- Lehninger AL, Vercesi A, Bababunmi EA. Regulation of Ca2+ release from mitochondria by the oxidation-reduction state of pyridine nucleotides. Proc. Natl. Acad. Sci. USA. 1978;75:1690–1694. doi: 10.1073/pnas.75.4.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- Liu J, Farmer JD, Jr, Lane WS, Friedman J, Weissman I, Schreiber SL. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991;66:807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- Liu Y, Song XD, Liu W, Zhang TY, Zuo J. Glucose deprivation induces mitochondrial dysfunction and oxidative stress in PC12 cell line. J. Cell. Molec. Med. 2003;7:49–56. doi: 10.1111/j.1582-4934.2003.tb00202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor DG, Avshalumov MV, Rice ME. Brain edema induced by in vitro ischemia: causal factors and neuroprotection. J. Neurochem. 2003;85:1402–1411. doi: 10.1046/j.1471-4159.2003.01772.x. [DOI] [PubMed] [Google Scholar]
- Maciel EN, Kaminski Schierle GS, Hansson O, Brundin P, Castilho RF. Cyclosporin A and Bcl-2 do not inhibit quinolinic acid-induced striatal excitotoxicity in rodents. Exp. Neurol. 2003;183:430–437. doi: 10.1016/s0014-4886(03)00165-1. [DOI] [PubMed] [Google Scholar]
- Manczak M, Anekonda TS, Henson E, Park BS, Quinn J, Reddy PH. Mitochondria are a direct site of A beta accumulation in Alzheimer's disease neurons: implications for free radical generation and oxidative damage in disease progression. Hum. Molec. Genet. 2006;15:1437–1449. doi: 10.1093/hmg/ddl066. [DOI] [PubMed] [Google Scholar]
- Mather M, Rottenberg H. Aging enhances the activation of the permeability transition pore in mitochondria. Biochem. Biophys. Res. Commun. 2000;273:603–608. doi: 10.1006/bbrc.2000.2994. [DOI] [PubMed] [Google Scholar]
- Matsumoto S, Friberg H, Ferrand-Drake M, Wieloch T. Blockade of the mitochondrial permeability transition pore diminishes infarct size in the rat after transient middle cerebral artery occlusion. J. Cereb. Blood Flow Metab. 1999;19:736–741. doi: 10.1097/00004647-199907000-00002. [DOI] [PubMed] [Google Scholar]
- Matthews RT, Ferrante RJ, Klivenyi P, Yang L, Klein AM, Mueller G, Kaddurah-Daouk R, Beal MF. Creatine and cyclocreatine attenuate MPTP neurotoxicity. Exp. Neurol. 1999;157:142–149. doi: 10.1006/exnr.1999.7049. [DOI] [PubMed] [Google Scholar]
- Matthews RT, Yang L, Jenkins BG, Ferrante RJ, Rosen BR, Kaddurah-Daouk R, Beal MF. Neuroprotective effects of creatine and cyclocreatine in animal models of Huntington's disease. J. Neurosci. 1998;18:156–163. doi: 10.1523/JNEUROSCI.18-01-00156.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattiazzi M, D'Aurelio M, Gajewski CD, Martushova K, Kiaei M, Beal MF, Manfredi G. Mutated human SOD1 causes dysfunction of oxidative phosphorylation in mitochondria of transgenic mice. J. Biol. Chem. 2002;277:29626–29633. doi: 10.1074/jbc.M203065200. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Cheng B, Davis D, Bryant K, Lieberburg I, Rydel RE. beta-Amyloid peptides destabilize calcium homeostasis and render human cortical neurons vulnerable to excitotoxicity. J. Neurosci. 1992;12:376–389. doi: 10.1523/JNEUROSCI.12-02-00376.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEnery MW, Snowman AM, Trifiletti RR, Snyder SH. Isolation of the mitochondrial benzodiazepine receptor: association with the voltage-dependent anion channel and the adenine nucleotide carrier. Proc. Natl. Acad. Sci. USA. 1992;89:3170–3174. doi: 10.1073/pnas.89.8.3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecocci P, MacGarvey U, Beal MF. Oxidative damage to mitochondrial DNA is increased in Alzheimer's disease. Ann. Neurol. 1994;36:747–751. doi: 10.1002/ana.410360510. [DOI] [PubMed] [Google Scholar]
- Mecocci P, MacGarvey U, Kaufman AE, Koontz D, Schoffner JM, Wallace DC, Beal MF. Oxidative damage to mitochondrial DNA shows marked age-dependent increases in human brain. Ann. Neurol. 1993;34:609–616. doi: 10.1002/ana.410340416. [DOI] [PubMed] [Google Scholar]
- Menzies FM, Ince PG, Shaw PJ. Mitochondrial involvement in amyotrophic lateral sclerosis. Neurochem. Int. 2002;40:543–551. doi: 10.1016/s0197-0186(01)00125-5. [DOI] [PubMed] [Google Scholar]
- Moreira PI, Custodio JB, Oliveira CR, Santos MS. Brain mitochondrial injury induced by oxidative stress-related events is prevented by tamoxifen. Neuropharmacology. 2005;48:435–447. doi: 10.1016/j.neuropharm.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Moreira PI, Santos MS, Moreno A, Rego AC, Oliveira C. Effect of amyloid beta-peptide on permeability transition pore: a comparative study. J. Neurosci. Res. 2002;69:257–267. doi: 10.1002/jnr.10282. [DOI] [PubMed] [Google Scholar]
- Muranyi M, Li PA. Bongkrekic acid ameliorates ischemic neuronal death in the cortex by preventing cytochrome c release and inhibiting astrocyte activation. Neurosci. Lett. 2005;384:277–281. doi: 10.1016/j.neulet.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Murthy ChRK, Rama Rao KV, Bai G, Norenberg MD. Ammonia induced production of free radicals in primary cultures of rat astrocytes. J. Neurosci. Res. 2001;66:282–288. doi: 10.1002/jnr.1222. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T, Tsujimoto Y. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434:652–658. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- Nicholls DG. Mitochondria and cell signaling. Cell Calcium. 2005;38:311–317. doi: 10.1016/j.ceca.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Nicholls DG, Budd SL. Mitochondria and neuronal survival. Physiol. Rev. 2000;80:315–360. doi: 10.1152/physrev.2000.80.1.315. [DOI] [PubMed] [Google Scholar]
- Nicklas WJ, Vyas I, Heikkila RE. Inhibition of NADH-linked oxidation in brain mitochondria by 1-Methyl-4-phenylpyridine, a metabolite of the neurotoxin, 1-methyl- 4-phenyl-1,2,3,6-tetrahydropyridine. Life Sci. 1985;36:2503–2508. doi: 10.1016/0024-3205(85)90146-8. [DOI] [PubMed] [Google Scholar]
- Nieminen AL. Apoptosis and necrosis in health and disease: role of mitochondria. Int. Rev. Cytol. 2003;224:29–55. doi: 10.1016/s0074-7696(05)24002-0. [DOI] [PubMed] [Google Scholar]
- Norenberg MD. A light and electron microscopic study of experimental portal-systemic (ammonia) encephalopathy. Progression and reversal of the disorder. Lab. Invest. 1977;36:618–627. [PubMed] [Google Scholar]
- Norenberg MD. Astroglial dysfunction in hepatic encephalopathy. Metab. Brain Dis. 1998;13:319–335. doi: 10.1023/a:1020688925901. [DOI] [PubMed] [Google Scholar]
- Norenberg MD, Jayakumar AR, Rama Rao KV. Oxidative stress in the pathogenesis of hepatic encephalopathy. Metab. Brain Dis. 2004;19:313–329. doi: 10.1023/b:mebr.0000043978.91675.79. [DOI] [PubMed] [Google Scholar]
- Norenberg MD, Martinez-Hernandez A. Fine structural localization of glutamine synthetase in astrocytes of rat brain. Brain Res. 1979;161:303–310. doi: 10.1016/0006-8993(79)90071-4. [DOI] [PubMed] [Google Scholar]
- Norenberg MD, Rama Rao KV, Jayakumar AR. Mechanisms of ammonia-induced astrocyte swelling. Metab. Brain Dis. 2005;20:303–318. doi: 10.1007/s11011-005-7911-7. [DOI] [PubMed] [Google Scholar]
- O'Gorman E, Beutner G, Dolder M, Koretsky AP, Brdiczka D, Wallimann T. The role of creatine kinase in inhibition of mitochondrial permeability transition. FEBS Lett. 1997;414:253–257. doi: 10.1016/s0014-5793(97)01045-4. [DOI] [PubMed] [Google Scholar]
- O'Gorman E, Beutner G, Wallimann T, Brdiczka D. Differential effects of creatine depletion on the regulation of enzyme activities and on creatine-stimulated mitochondrial respiration in skeletal muscle, heart, brain. Biochim. Biophys. Acta. 1996;1276:161–170. doi: 10.1016/0005-2728(96)00074-6. [DOI] [PubMed] [Google Scholar]
- Okado-Matsumoto A, Fridovich I. Subcellular distribution of superoxide dismutases (SOD) in rat liver: Cu,Zn-SOD in mitochondria. J. Biol. Chem. 2001;276:38388–38393. doi: 10.1074/jbc.M105395200. [DOI] [PubMed] [Google Scholar]
- Okonkwo DO, Buki A, Siman R, Povlishock JT. Cyclosporin A limits calcium-induced axonal damage following traumatic brain injury. NeuroReport. 1999;10:353–358. doi: 10.1097/00001756-199902050-00026. [DOI] [PubMed] [Google Scholar]
- Packer MA, Miesel R, Murphy MP. Exposure to the parkinsonian neurotoxin 1-methyl-4-phenylpyridinium (MPP+) and nitric oxide simultaneously causes cyclosporine A-sensitive mitochondrial calcium efflux and depolarization. Biochem. Pharmacol. 1996;51:267–273. doi: 10.1016/0006-2952(95)02165-5. [DOI] [PubMed] [Google Scholar]
- Panov AV, Burke JR, Strittmatter WJ, Greenamyre JT. In vitro effects of polyglutamine tracts on Ca2+-dependent depolarization of rat and human mitochondria: relevance to Huntington's disease. Arch. Biochem. Biophys. 2003;410:1–6. doi: 10.1016/s0003-9861(02)00585-4. [DOI] [PubMed] [Google Scholar]
- Panov AV, Gutekunst CA, Leavitt BR, Hayden MR, Burke JR, Strittmatter WJ, Greenamyre JT. Early mitochondrial calcium defects in Huntington's disease are a direct effect of polyglutamines. Nat. Neurosci. 2002;5:731–736. doi: 10.1038/nn884. [DOI] [PubMed] [Google Scholar]
- Parks JK, Smith TS, Trimmer PA, Bennett JP, Jr, Parker WD., Jr Neurotoxic Abeta peptides increase oxidative stress in vivo through NMDA-receptor and nitric-oxide-synthase mechanisms, inhibit complex IV activity and induce a mitochondrial permeability transition in vitro. J. Neurochem. 2001;76:1050–1056. doi: 10.1046/j.1471-4159.2001.00112.x. [DOI] [PubMed] [Google Scholar]
- Partin JC, Schubert WK, Partin JS. Mitochondrial ultrastucture in Reye's syndrome (encephalopathy and fatty degeneration of the viscera) N. Engl. J. Med. 1971;285:1339–1343. doi: 10.1056/NEJM197112092852402. [DOI] [PubMed] [Google Scholar]
- Pereira C, Santos MS, Oliveira C. Mitochondrial function impairment induced by amyloid beta-peptide on PC12 cells. NeuroReport. 1998;9:1749–1755. doi: 10.1097/00001756-199806010-00015. [DOI] [PubMed] [Google Scholar]
- Perry G, Nunomura A, Hirai K, Takeda A, Aliev G, Smith MA. Oxidative damage in Alzheimer's disease: the metabolic dimension. Int. J. Devl. Neurosci. 2000;18:417–421. doi: 10.1016/s0736-5748(00)00006-x. [DOI] [PubMed] [Google Scholar]
- Petronilli V, Miotto G, Canton M, Brini M, Colonna R, Bernardi P, Di Lisa M. Transient and long-lasting openings of the mitochondrial permeability transition pore can be monitored directly in intact cells by changes in mitochondrial calcein fluorescence. Biophys.J. 1999;765:725–754. doi: 10.1016/S0006-3495(99)77239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puttfarcken PS, Manelli AM, Neilly J, Frail DE. Inhibition of age-induced β-amyloid neurotoxicity in rat hippocampal cells. Exp. Neurol. 1996;138:73–81. doi: 10.1006/exnr.1996.0048. [DOI] [PubMed] [Google Scholar]
- Rabchevsky AG, Fugaccia I, Sullivan PG, Scheff SW. Cyclosporin A treatment following spinal cord injury to the rat: behavioral effects and stereological assessment of tissue sparing. J. Neurotrauma. 2001;18:513–522. doi: 10.1089/089771501300227314. [DOI] [PubMed] [Google Scholar]
- Radunovic A, Leigh PN. Cu/Zn superoxide dismutase gene mutations in amyotrophic lateral sclerosis: correlation between genotype and clinical features. J. Neurol. Neurosurg. Psychiat. 1996;61:565–572. doi: 10.1136/jnnp.61.6.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghupathi R. Cell death mechanisms following traumatic brain injury. Brain Pathol. 2004;14:215–222. doi: 10.1111/j.1750-3639.2004.tb00056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rama Rao KV, Chen M, Simard J, Norenberg MD. Suppression of ammonia-induced astrocyte swelling by cyclosporin A. J. Neurosci. Res. 2003a;74:891–897. doi: 10.1002/jnr.10755. [DOI] [PubMed] [Google Scholar]
- Rama Rao KV, Jayakumar AR, Norenberg MD. Ammonia neurotoxicity: role of the mitochondrial permeability transition. Metab. Brain Dis. 2003b;18:113–127. doi: 10.1023/a:1023858902184. [DOI] [PubMed] [Google Scholar]
- Rama Rao KV, Jayakumar AR, Norenberg MD. Induction of the mitochondrial permeability transition in cultured astrocytes by glutamine. Neurochem. Int. 2003c;43:517–523. doi: 10.1016/s0197-0186(03)00042-1. [DOI] [PubMed] [Google Scholar]
- Rama Rao KV, Jayakumar AR, Norenberg MD. Differential response of glutamine in cultured neurons and astrocytes. J. Neurosci. Res. 2005a;79:193–199. doi: 10.1002/jnr.20295. [DOI] [PubMed] [Google Scholar]
- Rama Rao KV, Jayakumar AR, Norenberg MD. Role of oxidative stress in the ammonia-induced mitochondrial permeability transition in cultured astrocytes. Neurochem. Int. 2005b;47:31–38. doi: 10.1016/j.neuint.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Rama Rao KV, Norenberg MD. Manganese induces the mitochondrial permeability transition in cultured astrocytes. J. Biol. Chem. 2004;279:32333–32338. doi: 10.1074/jbc.M402096200. [DOI] [PubMed] [Google Scholar]
- Ratnakumari L, Qureshi IA, Butterworth RF. Effect of L-carnitine on cerebral and hepatic energy metabolites in congenitally hyperammonemic sparse-fur mice and its role during benzoate therapy. Metabol: Clini & Expe. 1993;8:1039–1046. doi: 10.1016/0026-0495(93)90020-o. [DOI] [PubMed] [Google Scholar]
- Reichert SA, Kim-Han JS, Dugan LL. The mitochondrial permeability transition pore and nitric oxide synthase mediate early mitochondrial depolarization in astrocytes during oxygen-glucose deprivation. J. Neurosci. 2001;21:6608–6616. doi: 10.1523/JNEUROSCI.21-17-06608.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey SK, Dixon CE, Banik NL. Molecular mechanisms in the pathogenesis of traumatic brain injury. Histol. Histopathol. 2002;97:1373–1377. doi: 10.14670/HH-17.1137. [DOI] [PubMed] [Google Scholar]
- Rodrigues CM, Sola S, Brito MA, Brondino CD, Brites D, Moura JJ. Amyloid beta-peptide disrupts mitochondrial membrane lipid and protein structure: protective role of tauroursodeoxycholate. Biochem. Biophys. Res. Commun. 2001;281:468–474. doi: 10.1006/bbrc.2001.4370. [DOI] [PubMed] [Google Scholar]
- Rose C, Kresse W, Kettenmann H. Acute insult of ammonia leads to calcium-dependent glutamate release from cultured astrocytes, an effect of pH. J. Biol. Chem. 2005;280:20937–20944. doi: 10.1074/jbc.M412448200. [DOI] [PubMed] [Google Scholar]
- Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, O'Regan JP, Deng HX, Brown R. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- Ross MF, Kelso GF, Blaikie FH, James AM, Cocheme HM, Filipovska A, Da Ros T, Hurd TR, Smith RA, Murphy MP. Lipophilic triphenylphosphonium cations as tools in mitochondrial bioenergetics and free radical biology. Biochem. Russia. 2005;70:222–230. doi: 10.1007/s10541-005-0104-5. [DOI] [PubMed] [Google Scholar]
- Rowland LP, Shneider NA. Amyotrophic lateral sclerosis. New Engl. J. Med. 2001;344:1688–1700. doi: 10.1056/NEJM200105313442207. [DOI] [PubMed] [Google Scholar]
- Ruan Q, Lesort M, MacDonald ME, Johnson GV. Striatal cells from mutant huntingtin knock-in mice are selectively vulnerable to mitochondrial complex II inhibitor-induced cell death through a non-apoptotic pathway. Hum. Molec. Genet. 2004;13:669–681. doi: 10.1093/hmg/ddh082. [DOI] [PubMed] [Google Scholar]
- Ruck A, Dolder M, Wallimann T, Brdiczka D. Reconstituted adenine nucleotide translocase forms a channel for small molecules comparable to the mitochondrial permeability transition pore. FEBS Lett. 1998;426:97–101. doi: 10.1016/s0014-5793(98)00317-2. [DOI] [PubMed] [Google Scholar]
- Ruiz F, Alvarez G, Ramos M, Hernandez M, Bogonez E, Satrustegui J. Cyclosporin A targets involved in protection against glutamate excitotoxicity. Eur. J. Pharmacol. 2000;404:29–39. doi: 10.1016/s0014-2999(00)00584-7. [DOI] [PubMed] [Google Scholar]
- Salganik RI, Shabalina IG, Solovyova NA, Kolosova NG, Solovyov VN, Kolpakov AR. Impairment of respiratory functions in mitochondria of rats with an inherited hyperproduction of free radicals. Biochem. Biophys. Res. Commun. 1994;205:180–185. doi: 10.1006/bbrc.1994.2647. [DOI] [PubMed] [Google Scholar]
- Santos JB, Schauwecker PE. Protection provided by cyclosporin A against excitotoxic neuronal death is genotype dependent. Epilepsia. 2003;44:995–1002. doi: 10.1046/j.1528-1157.2003.66302.x. [DOI] [PubMed] [Google Scholar]
- Sasaki S, Maruyama S, Yamane K, Sakuma H, Takeishi M. Ultrastructure of swollen proximal axons of anterior horn neurons in motor neuron disease. J. Neurol. Sci. 1990;97:233–240. doi: 10.1016/0022-510x(90)90221-8. [DOI] [PubMed] [Google Scholar]
- Sawa A, Wiegand GW, Cooper J, Margolis RL, Sharp AH, Lawler JF, Jr, Greenamyre JT, Snyder SH, Ross CA. Increased apoptosis of Huntington disease lymphoblasts associated with repeat length-dependent mitochondrial depolarization. Nature Med. 1999;5:1194–1198. doi: 10.1038/13518. [DOI] [PubMed] [Google Scholar]
- Scheff SW, Sullivan PG. Cyclosporin A significantly ameliorates cortical damage following experimental traumatic brain injury in rodents. J. Neurotrauma. 1999;16:783–792. doi: 10.1089/neu.1999.16.783. [DOI] [PubMed] [Google Scholar]
- Schinder AF, Olson EC, Spitzer NC, Montal M. Mitochondrial dysfunction is a primary event in glutamate neurotoxicity. J. Neurosci. 1996;16:6125–6133. doi: 10.1523/JNEUROSCI.16-19-06125.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinzel AC, Takeuchi O, Huang Z, Fisher JK, Zhou Z, Rubens J, Hetz C, Danial NN, Moskowitz MA, Korsmeyer SJ. Cyclophilin D is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia. Proc. Natl. Acad. Sci. USA. 2005;102:12005–12010. doi: 10.1073/pnas.0505294102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaton TA, Cooper JM, Schapira AH. Cyclosporin inhibition of apoptosis induced by mitochondrial complex I toxins. Brain Res. 1998;809:12–17. doi: 10.1016/s0006-8993(98)00790-2. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Toward a comprehensive theory for Alzheimer's disease. Hypothesis: Alzheimer's disease is caused by the cerebral accumulation and cytotoxicity of amyloid beta-protein. Ann. N. Y. Acad. Sci. 2000;924:17–25. doi: 10.1111/j.1749-6632.2000.tb05554.x. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's disease: genes, proteins, therapy. Physiol. Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- Sheehan JP, Swerdlow RH, Miller SW, Davis RE, Parks JK, Parker WD, Tuttle JB. Calcium homeostasis and reactive oxygen species production in cells transformed by mitochondria from individuals with sporadic Alzheimer's disease. J. Neurosci. 1997;17:4612–4622. doi: 10.1523/JNEUROSCI.17-12-04612.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoshan-Barmatz V, Gincel D. The voltage-dependent anion channel: characterization, modulation, role in mitochondrial function in cell life and death. Cell Biochem. Biophys. 2003;39:279–292. doi: 10.1385/CBB:39:3:279. [DOI] [PubMed] [Google Scholar]
- Siesjö BK. Acid-base homeostasis in the brain: physiology, chemistry, neurochemical pathology. Prog. Brain Res. 1985;63:121–154. doi: 10.1016/S0079-6123(08)61980-9. [DOI] [PubMed] [Google Scholar]
- Siesjö BK. Pathophysiology and treatment of focal cerebral ischemia. Part I: Pathophysiology. J. Neurosurg. 1992;77:169–184. doi: 10.3171/jns.1992.77.2.0169. [DOI] [PubMed] [Google Scholar]
- Signoretti S, Marmarou A, Tavazzi B, Dunbar J, Amorini AM, Lazzarino G, Vagnozzi R. The protective effect of cyclosporin A upon N-acetylaspartate and mitochondrial dysfunction following experimental diffuse traumatic brain injury. J. Neurotrauma. 2004;21:1154–1167. doi: 10.1089/neu.2004.21.1154. [DOI] [PubMed] [Google Scholar]
- Siklos L, Engelhardt J, Harati Y, Smith RG, Joo F, Appel SH. Ultrastructural evidence for altered calcium in motor nerve terminals in amyotropic lateral sclerosis. Ann. Neurol. 1996;39:203–216. doi: 10.1002/ana.410390210. [DOI] [PubMed] [Google Scholar]
- Siliprandi D, Siliprandi N, Toninello A. On the relationship between calcium and phosphate transport, transmembrane potential and acetoacetate-induced oxidation of pyridine nucleotides in rat-liver mitochondria. Eur. J. Biochem. 1983;130:173–175. doi: 10.1111/j.1432-1033.1983.tb07133.x. [DOI] [PubMed] [Google Scholar]
- Singer TP, Ramsay RR, Sonsalla PK, Nicklas WJ, Heikkila RE. Biochemical mechanisms underlying MPTP-induced and idiopathic parkinsonism. New vistas. Adv. Neurol. 1993;60:300–305. [PubMed] [Google Scholar]
- Singleton RH, Stone JR, Okonkwo DO, Pellicane AJ, Povlishock JT. The immunophilin ligand FK506 attenuates axonal injury in an impact-acceleration model of traumatic brain injury. J. Neurotrauma. 2001a;18:607–614. doi: 10.1089/089771501750291846. [DOI] [PubMed] [Google Scholar]
- Singleton RH, Stone JR, Okonkwo DO, Pellicane AJ, Povlishock JT. The immunophilin ligand FK506 attenuates axonal injury in an impact-acceleration model of traumatic brain injury. J. Neurotrauma. 2001b;18:607–614. doi: 10.1089/089771501750291846. [DOI] [PubMed] [Google Scholar]
- Squier TC, Bigelow DJ. Protein oxidation and age-dependent alterations in calcium homeostasis. Front. Biosci. 2000;5:D504–D526. doi: 10.2741/squier. [DOI] [PubMed] [Google Scholar]
- Stavrovskaya IG, Narayanan MV, Zhang W, Krasnikov BF, Heemskerk J, Young SS, Blass JP, Brown AM, Beal MF, Friedlander RM, Kristal BS. Clinically approved heterocyclics act on a mitochondrial target and reduce stroke-induced pathology. J. Exp. Med. 2004;200:211–222. doi: 10.1084/jem.20032053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PG, Rabchevsky AG, Keller JN, Lovell M, Sodhi A, Hart PP, Scheff SW. Intrinsic differences in brain and spinal cord mitochondria: Implication for therapeutic interventions. J. Comp. Neurol. 2004;474:524–534. doi: 10.1002/cne.20130. [DOI] [PubMed] [Google Scholar]
- Sullivan PG, Thompson MB, Scheff SW. Cyclosporin A attenuates acute mitochondrial dysfunction following traumatic brain injury. Exp. Neurol. 1999;160:226–234. doi: 10.1006/exnr.1999.7197. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Koike T. Early apoptotic and late necrotic components associated with altered Ca2+ homeostasis in a peptide-delivery model of polyglutamine-induced neuronal death. J. Neurosci. Res. 2005;80:549–561. doi: 10.1002/jnr.20461. [DOI] [PubMed] [Google Scholar]
- Szabo I, Zoratti M. The mitochondrial permeability transition pore may comprise VDAC molecules I. Binary structure and voltage dependence of the pore. FEBS Lett. 1993;330:201–205. doi: 10.1016/0014-5793(93)80273-w. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Kobayashi Y, Ishigaki S, Doyu M. Mitochondrial localization of mutant superoxide dismutase 1 triggers caspase-dependent cell death in a cellular model of familial amyotrophic lateral sclerosis. J. Biol. Chem. 2002;277:50966–50972. doi: 10.1074/jbc.M209356200. [DOI] [PubMed] [Google Scholar]
- Tedeschi H. The mitochondrial membrane potential. Biol. Rev. Cambridge Phil. Soc. 1980;55:171–206. doi: 10.1111/j.1469-185x.1980.tb00692.x. [DOI] [PubMed] [Google Scholar]
- The Huntington's Disease Collaborative Research Group. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. Cell. 1993;72:971–983. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- Traber PG, Dal Canto MC, Ganger D, Blei AT. Electron microscopic evaluation of brain edema in rabbits with galactosamine-induced fulminant hepatic failure: ultrastructure and integrity of the blood-brain barrier. Hepatology. 1987;7:1272–1277. doi: 10.1002/hep.1840070616. [DOI] [PubMed] [Google Scholar]
- Trost LC, Lemasters JJ. The mitochondrial permeability transition: A new pathophysiological mechanism for Reye's syndrome and toxic liver injury. J. Pharmacol. Exp. Ther. 1996;278:1000–1005. [PubMed] [Google Scholar]
- Vonsattel JP, DiFiglia M. Huntington disease. J. Neuropathol. Exp. Neurol. 1998;57:369–384. doi: 10.1097/00005072-199805000-00001. [DOI] [PubMed] [Google Scholar]
- Votyakova TV, Reynolds IJ. Ca2+-induced permeabilization promotes free radical release from rat brain mitochondria with partially inhibited complex I. J. Neurochem. 2005;93:526–537. doi: 10.1111/j.1471-4159.2005.03042.x. [DOI] [PubMed] [Google Scholar]
- Walling HW, Baldassare JJ, Westfall TC. Molecular aspects of Huntington's disease. J. Neurosci. Res. 1998;54:301–308. doi: 10.1002/(SICI)1097-4547(19981101)54:3<301::AID-JNR1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhu S, Drozda M, Zhang W, Stavrovskaya IG, Cattaneo E, Ferrante RJ, Kristal BS, Friedlander RM. Minocycline inhibits caspase-independent and -dependent mitochondrial cell death pathways in models of Huntington's disease. Proc. Natl. Acad. Sci. USA. 2003;100:10483–10487. doi: 10.1073/pnas.1832501100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wennberg A, Hagman M, Johansson L. Preclinical neurophysiological signs of parkinsonism in occupational manganese exposure. Neurotoxicology. 1992;13:271–274. [PubMed] [Google Scholar]
- Whetsell WO, Shapira NA. Biology of disease: neuroexcitation, excitotoxicity and human neurological disease. Lab. Invest. 1993;68:372–387. [PubMed] [Google Scholar]
- White RJ, Reynolds IJ. Mitochondrial depolarization in glutamate-stimulated neurons: an early signal specific to excitotoxic exposure. J. Neurosci. 1996;16:5688–5697. doi: 10.1523/JNEUROSCI.16-18-05688.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong PC, Pardo CA, Borchelt DR, Lee MK, Copeland NG, Jenkins NA, Sisodia SS, Cleveland DW, Price DL. An adverse property of a familial ALS-linked SOD1 mutation causes motor neuron disease characterized by vacuolar degeneration of mitochondria. Neuron. 1995;14:1105–1116. doi: 10.1016/0896-6273(95)90259-7. [DOI] [PubMed] [Google Scholar]
- Woodfield K, Ruck A, Brdiczka D, Halestrap AP. Direct demonstration of a specific interaction between cyclophilin-D and the adenine nucleotide translocase confirms their role in the mitochondrial permeability transition. Biochem. J. 1998;336:287–290. doi: 10.1042/bj3360287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu DC, Jackson-Lewis V, Vila M, Tieu K, Teismann P, Vadseth C, Choi DK, Ischiropoulos H, Przedborski S. Blockade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson disease. J. Neurosci. 2002;22:1763–1771. doi: 10.1523/JNEUROSCI.22-05-01763.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Jung C, Higgins C, Levine J, Kong J. Mitochondrial degeneration in amyotrophic lateral sclerosis. J. Bioenerg. Biomembr. 2004;36:395–399. doi: 10.1023/B:JOBB.0000041774.12654.e1. [DOI] [PubMed] [Google Scholar]
- Yen TC, Chen YS, King KL, Yeh SH, Wei YH. Liver mitochondrial respiratory functions decline with age. Biochem. Biophys. Res. Commun. 1989;165:944–1003. doi: 10.1016/0006-291x(89)92701-0. [DOI] [PubMed] [Google Scholar]
- Yoshimoto T, Uchino H, He QP, Ping AL, Siesjö BK. Cyclosporin A, but not FK506, prevents the downregulation of phosphorylated Akt after transient focal ischemia in the rat. Brain Res. 2001;899:148–158. doi: 10.1016/s0006-8993(01)02220-x. [DOI] [PubMed] [Google Scholar]
- Yrjanheikki J, Keinanen R, Pellikka M, Hokfelt T, Koistinaho J. Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc. Natl. Acad. Sci. USA. 1998;95:15769–15774. doi: 10.1073/pnas.95.26.15769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanelli SA, Solenski NJ, Rosenthal RE, Fiskum G. Mechanisms of ischemic neuroprotection by acetyl-L-carnitine. Ann. NY Acad. Sci. 2005;1053:153–161. doi: 10.1196/annals.1344.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecca L, Youdim MB, Riederer P, Connor JR, Crichton RR. Iron, brain ageing and neurodegenerative disorders. Nature Rev. Neurosci. 2004;5:863–873. doi: 10.1038/nrn1537. [DOI] [PubMed] [Google Scholar]
- Zeron MM, Fernandes HB, Krebs C, Shehadeh J, Wellington CL, Leavitt BR, Baimbridge KG, Hayden MR, Raymond LA. Potentiation of NMDA receptor-mediated excitotoxicity linked with intrinsic apoptotic pathway in YAC transgenic mouse model of Huntington's disease. Molec. Cell. Neurosci. 2004;25:469–479. doi: 10.1016/j.mcn.2003.11.014. [DOI] [PubMed] [Google Scholar]
- Zhu S, Stavrovskaya IG, Drozda M, Kim BY, Ona V, Li M, Sarang S, Liu As, Hartley DM, Wu dC, Gullans S, Ferrante RJ, Przedborski S, Kristal BS, Friedlander RM. Minocycline inhibits cytochrome c release and delays progression of amyotrophic lateral sclerosis in mice. Nature. 2002;417:74–78. doi: 10.1038/417074a. [DOI] [PubMed] [Google Scholar]
- Zoratti M, Szabo I. The mitochondrial permeability transition. Biochim. Biophys. Acta. 1995;1241:139–176. doi: 10.1016/0304-4157(95)00003-a. [DOI] [PubMed] [Google Scholar]