Abstract
Background
Chronic exposure to estradiol-17β (E2) in adult female rats increases mean arterial pressure by stimulating superoxide production in the rostral ventrolateral medulla (RVLM). However the mechanisms behind this phenomenon are unknown. We hypothesized that E2 exposure induces the gene expression of cytokines, chemokines and NADPH oxidase (Nox) in the RVLM that promotes superoxide production and aging would exacerbate this effect.
Methods
Young adult (3-4 month old) and middle-aged (6-8 month old) female Sprague Dawley rats were sham-implanted (YS and MS respectively) or implanted s.c. with slow-release E2 pellets (20ng of E2/day for 90 days; YE and ME respectively). Blood pressure (BP) was measured during the last 3 weeks of exposure in a separate set of rats. At the end of treatment, the animals were sacrificed and RVLM was isolated from the brainstem. PCR array and Quantitative RT-PCR were performed with the tissue to quantify genes associated with hypertension and superoxide production. Superoxide dismutase (SOD) activity was also measured in the RVLM from a different set of animals.
Results
E2 exposure increased mean arterial pressure in both YE and ME animals. Inflammatory genes such as interleukin-1β, interleukin-6 and monocyte chemoattractant protein-1 were significantly up-regulated in the RVLM of ME treated female rats compared to YS rats, but not in YE rats. Endothelin-1 (ET-1) gene was up-regulated in the RVLM of both YE and ME rats that were exposed to E2. Furthermore, chronic E2 treatment increased the mRNA levels of Nox1 and Nox2 genes in the RVLM of YE but not ME animals. SOD activity was reduced in MA animals, compared to young animals. E2 treatment had no significant effect on SOD activity.
Conclusion
Chronic E2 exposure stimulates the expression of inflammatory genes in older animals and increases the expression of Nox subunits in the RVLM of younger animals. SOD activity was reduced in older animals. This suggests increased superoxide production in younger animals, but reduced superoxide elimination in older animals. On the other hand, E2 exposure stimulates ET-1 expression in both young and aging animals. These findings suggest that hypertension caused by chronic E2 exposure may involve different molecular mediators in young and aging animals, however ET-1 and superoxide could be common mediators for both age groups.
Keywords: estrogen, RVLM, cytokines, endothelin, NAPDH oxidase, blood pressure
1. Introduction
Women are chronically exposed to exogenous estrogens through the environment and/or hormonal preparations. Estrogen was believed to be cardioprotective and was being prescribed for post-menopausal women to delay the onset of cardiovascular diseases, neurodegenerative diseases, and osteoporosis (Posthuma et al., 1994). However, studies from the Women's Health Initiative on the effects of hormone replacement therapy (HRT) in postmenopausal women have found that HRT increased the risk for several cardiovascular diseases including stroke, coronary heart disease etc(Moolman, 2006). Moreover, young women on oral contraceptive pills containing estrogenic preparations are reported to have increases in blood pressure (Woods, 1988). Taken together, these studies suggest that long-term exposure to estrogenic preparations may affect cardiovascular health in women.
Recently, we reported that chronic exposure to low levels (20ng/day) of estradiol-17β (E2) elevated mean arterial pressure (MAP) in young female Sprague-Dawley rats. This effect was associated with an increase in superoxide production in the rostral ventrolateral medulla (RVLM), the vasomotor center in the brainstem that is responsible for tonic and reflex control of arterial pressure. In that study, we also treated rats with an anti-oxidant, resveratrol, which completely reversed E2-induced increase in superoxide production and MAP (Subramanian et al., 2011). However, the central mechanisms by which chronic E2 treatment increases superoxide production and MAP are still unclear.
The association between oxidative stress and hypertension has been well documented in various animal models of hypertension (Fujita et al., 2007; Kang et al., 2009; Nagae et al., 2009) and human hypertension (Tsuda). Recently, brain cytokines have been implicated in the pathogenesis of hypertension. Pro-inflammatory cytokines (PICs) such as Interleukin-1β (IL-1β), Interleukin-6 (IL-6) and Tumor necrosis factor-α (TNF-α) are known to increase the levels of nicotinamide—adenine dinucleotide phosphate (NADPH) oxidase to promote superoxide production in brain regions that regulate blood pressure (Kang et al., 2009). Conversely, increasing the activity of superoxide dismutase, an enzyme that breaks down superoxides, has been shown to reduce the levels of PICs and blood pressure in a hypertensive animal model (Oliveira-Sales et al., 2010). These studies suggest that the interaction between cytokines and reactive oxygen species (ROS) play an important role in the development of hypertension. We hypothesized that a similar mechanism could play a role in the hypertension that results from chronic E2 exposure.
In addition to PICs, endothelin-1 (ET-1), a potent vasoconstrictor, could potentially contribute to the development of hypertension (Gao et al., 2005; Just et al., 2007) (Li et al., 2003). ET-1 is believed to cause hypertension by increasing superoxide production through NADPH oxidase-dependent mechanisms (Amiri et al., 2004; Duerrschmidt et al., 2000; Fei et al., 2000). Besides ET-1, Angiotensin II (AngII) could be another likely mediator of E2-induced hypertension. Central administration of AngII increases NADPH oxidase expression and the production of superoxide in the RVLM (Gao et al., 2005) and this could play a critical role in neurogenic hypertension (Oliveira-Sales et al., 2010). Taken together, these studies indicate that a variety of molecular mediators are likely to be involved in the generation of superoxide in the RVLM, which in turn can lead to the development of hypertension. Moreover, there could be alterations in the rate at which superoxide is generated and eliminated (Maier and Chan, 2002).
Therefore, we hypothesized that the gene expression of one or more of these mediators would increase in the RVLM after chronic E2 exposure. Since, aging is also associated with oxidative stress and inflammation in the brain (Gemma et al., 2007), we hypothesized that aging would have an additive effect on E2-induced alterations in gene expression, making older animals more prone to hypertension.
2. Results
2.1. Effect of E2 exposure on blood pressure in young and aging animals
Diastolic blood pressure (DBP; Mean±S.E.; mm Hg) in YS animals was 97.13±3 and was 103.37±2.55 with E2 exposure. In MA animals, DBP was 102.16±2.45 and 109.64±4.1 in sham implanted and E2-treated rats respectively. There was no significant difference in DBP between the different treatment groups (Fig 1a). In contrast, systolic blood pressure (SBP, Mean±S.E.; mm Hg) in the YS group was 115.33±2.96 and increased significantly to 127.65±3.55 in the YE group (p<0.05). MA animals also had higher SBP compared to the YS group (137.37±4.8 and 133.45±4.99 in MS and ME groups respectively; p<0.01). However, there was no difference with E2 treatment in the MA group (Fig 1b). Mean arterial pressure (MAP; Mean±S.E.; mm Hg) was 102.71±3.1 in the YS group and increased to 120.61±4.9 in the YE group. E2 exposure produced an increase in MAP in the ME group as well (118.65±4.6), but MAP in the MS group (113.45±3.9) was not different from that in the YS group (Fig 1c).
Fig 1.
Blood pressure changes in young and middle aged animals sham implanted (YS and MS respectively), or implanted with E2 pellets (YE and ME respectively) are shown here. Diastolic pressure is presented in Panel A, Systolic pressure in Panel B and Mean arterial pressure in Panel C. * indicates significant difference (p<0.05) from the YS (young-sham implanted) group.
2.2. Effect of E2 exposure on the expression of estrogen receptor genes in the RVLM
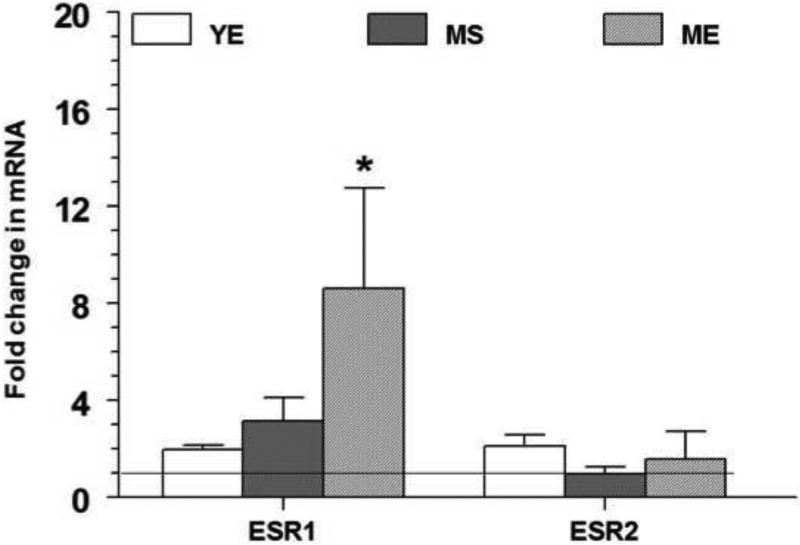
Changes in the expression of estrogen receptor (ESR) genes in the different treatment groups are depicted in fig 2. Fold changes (Mean±S.E.) in all the groups were normalized to the YS group that is represented by the horizontal line at 1. Chronic E2 treatment resulted in 8-fold up-regulation of ESR1 gene expression in the ME group (8.6±4.1, p<0.005) compared to both YE and MS groups (2.0±0.2 and 3.1±1.0 respectively, p< 0.05). No changes were seen in ESR2 gene expression. Analysis using two-way ANOVA revealed a significant E2 treatment (p=0.02) and aging effect (p=0.005) on ESR1 expression.
Fig 2.
The mRNA expression levels of estrogen receptor alpha gene (ESR1) and estrogen receptor beta gene (ESR2) in the RVLM after 90 days of E2 exposure are shown above. The fold change for each mRNA was calculated by the comparative CT method using 2−ΔΔCt. The Ct values of all the genes were normalized to β-actin and the Ct values from different groups were expressed relative to that in young intact sham rats. * denotes significant difference (p<0.05) from control (YS) group.
2.3. Effect of E2 exposure on the expression of inflammation related genes in the RVLM
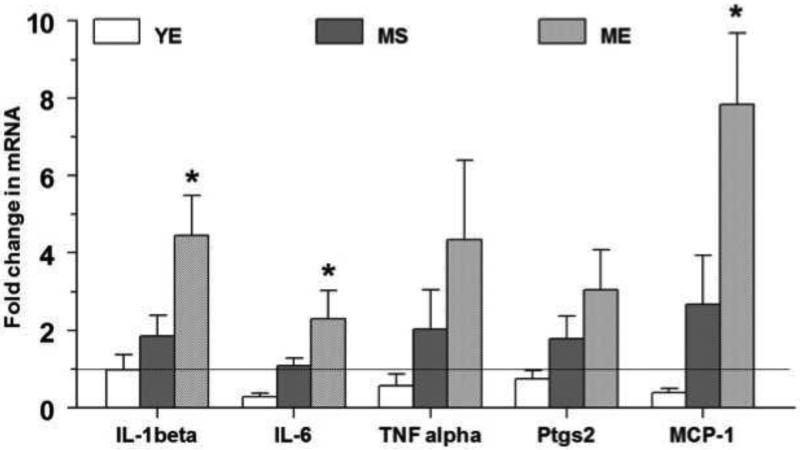
Fold change in the expression of inflammation-related genes after chronic E2 exposure in young and middle aged animals in the RVLM is shown in figure 3. Among the proinflammatory cytokines, IL-1β and IL-6 were up-regulated in ME animals. The mRNA expression (Fold change; Mean±S.E.) of IL-1β was up-regulated 4 fold in the RVLM of the ME group, (4.5±1.0; p<0.005) compared to the YS group, the YE group (1.0±0.4) and the MS group (1.8±0.5; p<0.05). Similarly in ME animals, IL-6 was up-regulated by 2 fold in the RVLM (2.3±0.7; p<0.05) and was significantly different from the YE (0.3±0.1; p<0.05) and the MS groups (1.0±0.2; p<0.05). Although the gene expression of other inflammatory mediators such as TNF-α and cyclooxygenese-2 (PTGS2) showed a trend to increase in ME animals, they did not attain statistical significance (p=0.09 and p=0.05 respectively). The gene expression of the chemokine, MCP-1 was significantly up-regulated in ME animals (7.8±1.9; p<0.005) compared to the YE (0.4±0.1) and MS (2.7±1.3) groups. Overall, it appears that E2 exposure did not affect PIC gene expression in young animals, but older animals exposed chronically to E2 have elevated levels of IL-1β, IL-6 and MCP-1 expression in the RVLM. Aging per se had modest effects on the expression of these genes in the RVLM.
Fig 3.
The fold change in the gene expression of interleukin-1beta (IL-1β), interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), cyclooxygenese-2 (PTGS2) and monocyte chemoattractant factor (MCP-1) in the RVLM after 90 days of E2 exposure are shown above. The fold change for each mRNA was calculated by the comparative CT method using 2−ΔΔCt. The Ct values of all the genes were normalized to β-actin and the Ct values from different groups were expressed relative to that in young intact sham rats. * denotes significant difference (p<0.05) from control (YS) group.
Two-way ANOVA was performed to determine the effects of aging and E2 treatment individually. There were significant effects of E2 treatment, aging and E2 treatment × aging interaction on IL-1β expression (p=0.04, p=0.002, and p=0.04 respectively) and MCP-1 gene expression (p=0.04, p=0.0008, and p=0.01 respectively). For IL-6 gene expression, there was a significant effect of aging and E2 treatment × aging interaction (p=0.006 and p=0.01, respectively).
2.4. Effect of E2 exposure on genes regulating blood pressure in the RVLM
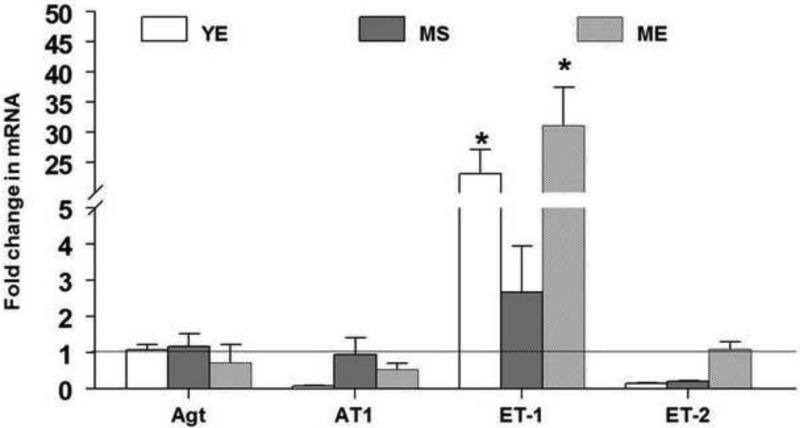
Changes in the mRNA levels of genes regulating blood pressure after chronic E2 exposure are shown in figure 4. Among the genes involved in the regulation of blood pressure, ET-1 was up-regulated in the YE and ME groups. While the mRNA expression (Fold change; Mean±S.E.) of ET-1 was up-regulated by 23 fold in the YE group (23.1±4.1; p<0.0005) it increased by 31 fold in the ME group (31.1±6.3; p<0.0001) compared to the YS group. Within the same age groups, ME animals were also significantly different from the MS (2.7±1.3; p<0.005) animals. However, there were no significant differences between the YS and MS groups. In contrast to ET-1, ET-2 expression was down regulated in the YE group (0.02±0.01; p<0.0005) and MS animals (0.2±0.02; p<0.0005) compared to YS animals. However there were no changes in ET-2 gene expression in the ME group. No changes were seen in the mRNA expression of angiotensinogen and angiotensin II receptor, type 1b genes. It appears that E2 exposure increases the expression of ET-1 in the RVLM regardless of age.
Fig 4.
The fold change in the gene expression of angiotensinogen (Agt), angiotensin II receptor, type 1b (AT1), endothelin-1 (ET-1), endothelin-2 (ET-2) in the RVLM after 90 days of E2 exposure are shown above. The fold change for each mRNA was calculated by the comparative CT method using 2−ΔΔCt. The Ct values of all the genes were normalized to β-actin and the Ct values from different groups were expressed relative to that in young intact sham rats. * denotes significant difference (p<0.05) from control (YS) group.
Two-way ANOVA revealed a significant effect of E2 treatment on ET-1 mRNA levels (p<0.0001). ET-2 was affected by E2 treatment × aging interaction (p<0.0001) than by E2 treatment or aging alone.
2.5. Effect of E2 exposure on oxidative stress-related genes in the RVLM
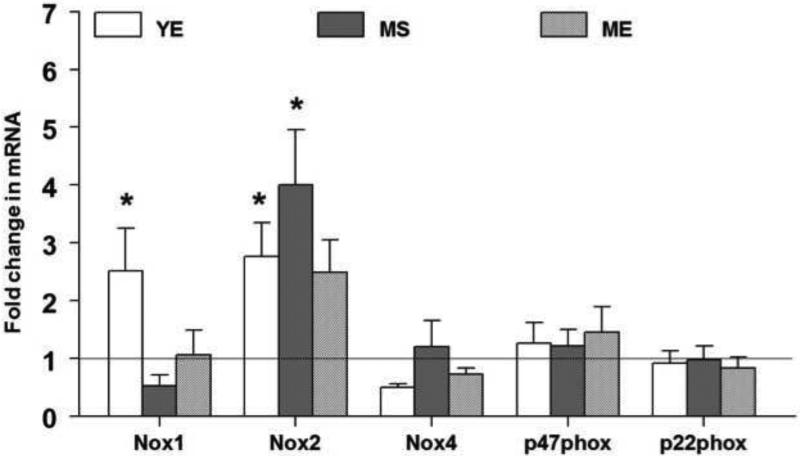
The mRNA levels of different NAPDH oxidase subunits are expressed as a ratio of β-actin mRNA in control and E2 treated animals in Fig 5. Among the different NADPH oxidase subunits, Nox1 and Nox2 were up-regulated predominantly in the YE group. Expression of Nox1 mRNA (Fold change; Mean±S.E.) was up-regulated by 2 fold in the YE group (2.5±0.7, p<0.01) when compared to the MS and ME groups (0.5±0.2 and 1.1±0.4 respectively, p<0.05). Nox2 gene expression was up-regulated significantly by 2 fold in the YE group (2.8±0.6, p<0.05) and by 4 fold in MS group (4.0±0.9, p<0.002) but not in the ME group (2.5±0.6) compared to YS animals. No changes were seen in Nox4, p47phox or p22phox genes in any of the treatment groups. It appears that E2 exposure produces modest increases in the expression of Nox 1 and Nox 2. While Nox2 expression increases in both young and aging animals, Nox 1 expression is limited to young animals exposed to E2.
Fig 5.
The mRNA expression levels of NADPH oxidase subunits in the RVLM in response to 90 days of E2 exposure are shown above. The fold change for each mRNA was calculated by the comparative CT method using 2−ΔΔCt. The Ct values of all the genes were normalized to β-actin and the Ct values from different groups were expressed relative to that in young intact sham rats. * denotes significant difference (p<0.05) from control (YS) group.
Two-way ANOVA showed a significant E2 treatment (p=0.02) and aging effect (p=0.03) for Nox1 gene expression. A significant effect of aging (p=0.04) and aging × E2 interaction (p=0.02) was observed for Nox2 gene expression.
2.6. Effect of E2 exposure on Superoxide Dismutase activity
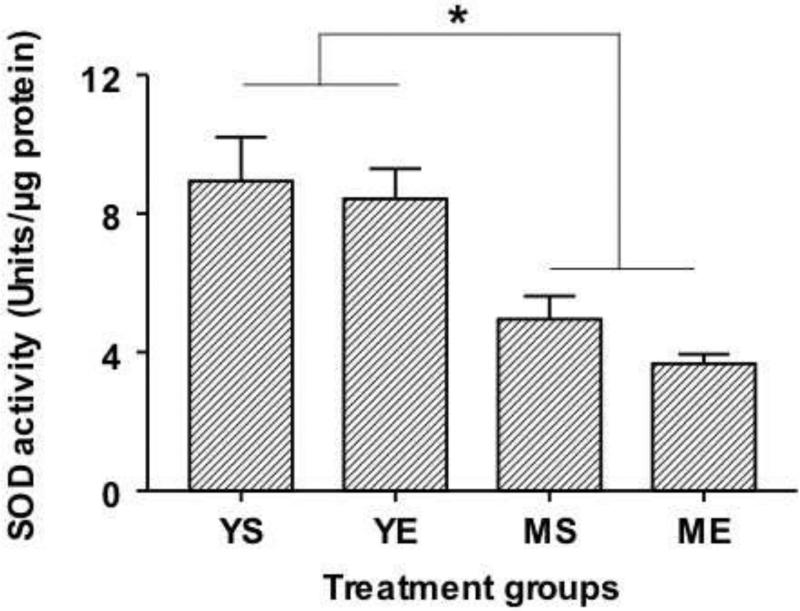
Overall, young animals had higher SOD activity in the RVLM compared to aging animals. SOD activity (Units/μg protein: Mean±SE) in YE animals (8.43±0.86) and YS rats (8.93±1.27) were significantly higher than that measured in MS (4.95±0.6) and ME animals (3.67±0.27) (p<0.01). E2 treatment did not decrease SOD activity in young and aging animals. Two-way ANOVA revealed a significant effect of age on SOD activity (F=24.13; p<0.001)
3. Discussion
Our results suggest that chronic E2 exposure increases the gene expression of PICs like IL-1β, IL-6 and the chemokine MCP-1 in the RVLM of older rats. Chronic E2 exposure also stimulated ET-1 mRNA expression in both age groups. Finally, we found that Nox1 and Nox2 subunits of NADPH oxidase were up-regulated in the RVLM with chronic E2 treatment in young animals correlating well with increased superoxide production reported in our previous study (Subramanian et al., 2011) and with the increase in blood pressure observed in the current study. These results suggest that the effects of chronic E2 exposure on the RVLM are highly specific relative to age and the molecular mediators that are affected and these genes are likely to play a role in the development of hypertension that is observed in these animals.
The RVLM is an important cardiovascular center in the brain stem that regulates the basal sympathetic tone and in turn, blood pressure (Ross et al., 1984). Inflammatory changes in the RVLM have been associated with the development of hypertension (Shi et al., 2010 a,b). Previously, we reported that chronic exposures to E2 in young animals can cause hypertension, increase superoxide anion levels in the RVLM and that this effect could be reversed by treatment with an antioxidant, resveratrol (Subramanian et al., 2011). The mechanism by which superoxide levels increase in this model is not clear. We hypothesized that an increase in PICs could play an important role in this phenomenon since another study in spontaneous hypertensive rats also reported an increase in PIC expression in the RVLM (Agarwal et al., 2011). However, we found that chronic E2 treatment significantly increased IL-1β, IL-6 and MCP-1 gene expression in middle aged, but not young animals. This could suggest that older animals are more susceptible to the pro-inflammatory actions of E2.
The source of PICs in the RVLM is unknown. There is evidence to suggest that astrocytes and glial cells could be potential contributors to cytokines and chemokines in the brain (Dinapoli et al., 2010; Huang et al., 2008). Recently, we reported that E2 exposure can increase astrocyte activity and IL-1β levels in the hypothalamus (Mohankumar et al., 2011). A similar phenomenon may be in operation in the RVLM. It is likely that E2 produces its effect by binding to E2 receptors in the RVLM. To test this, we measured the expression of E2 receptors, ESR1 and 2, and found that E2 exposure preferentially increased ESR1 expression in middle aged but not in young animals. The fact that E2 exposure increased certain cytokines and ESR1 expression only in middle aged animals suggests that cytokines could not have contributed to the hypertension that we observed in young rats after E2 exposure in our previous study. Therefore it becomes important to study the role of other mediators such as ET-1 and Ang-II in E2-induced hypertension.
Central ET-1 plays a role in modulating sympathetic tone and blood pressure (Rossi and Maliszewska-Scislo, 2008). In the brain, ET-1 is produced by glial cells and vascular endothelial cells (Durieu-Trautmann et al., 1993; MacCumber et al., 1990). Microinjection of ET-1 in the RVLM produced an increase in blood pressure (Mosqueda-Garcia et al., 1995) providing a direct link between ET-1 in the RVLM and hypertension. Therefore it is likely that E2-induced increases in ET-1 expression in the RVLM could contribute to hypertension. In the present study, ET-1 gene expression increased in both young and middle aged animals suggesting that ET-1 could mediate the effects of chronic E2 exposure independent of age. In addition to ET-1, central renin-angiotensin system has also been implicated in hypertension (Cuadra et al., 2010). However, we did not observe any changes in the expression of angiotensinogen or the AngII receptor in the RVLM suggesting that the renin-angiotensin system is an unlikely mediator of chronic E2-induced hypertension.
One of the central mechanisms by which PICs and ET-1 could contribute to hypertension is through oxidative stress characterized by NADPH oxidase-mediated generation of superoxide (Amiri et al., 2004; Duerrschmidt et al., 2000; Fei et al., 2000). Previously, we have demonstrated increases in superoxide levels in the RVLM in young rats exposed to E2 (Subramanian et al., 2011). In the present study, we observed an increase in the expression of Nox1 and Nox2 subunits of NADPH oxidase in young animals after chronic E2 exposure suggesting that NADPH oxidase could be a possible source of superoxide in the RVLM. In contrast to young animals, aging appeared to produce a different pattern of Nox gene expression. In this group, only Nox2 was affected with increases apparent even in the sham group (MS). Although Nox 2 expression appeared to increase in the ME group, it was not statistically significant suggesting that aging-induced increase in Nox 2 expression perhaps masked the effect of E2 exposure.
The generation of superoxide by NADPH oxidase is quite complex. In order to produce superoxide a catalytic core needs to be formed from one of the Nox isoforms and p22phox (Sumimoto et al., 2005). However this core complex, gets activated only after the addition of p47phox or GTPase Rac (Babior et al., 2002). In the present study we observed a significant increase in the gene expression of Nox1 and 2 in the RVLM after E2 treatment in young animals, but there was no associated change in the other subunits, so it is unclear how the increased expression of Nox1 and 2 translates into increased superoxide generation in this model. Besides gene expression, protein levels of these subunits and their activity could also provide important clues to the generation of superoxides. It is also possible that an entirely different mechanism involving xanthine oxidase and uncoupled NOS (Li et al., 2003) could participate in E2-induced superoxide generation. We examined the role of SOD which unlike the other enzymes mentioned above, is involved in scavenging superoxides (Maier and Chan, 2002). It is likely that an imbalance between superoxide generation and scavenging mechanisms could result in a net increase in superoxide levels (Salminen and Paul, 2014). Our results suggest that E2 treatment may potentiate superoxide generation in young animals by stimulating Nox1 and 2. In older animals, however, SOD activity remains significantly low indicating that superoxide scavenging mechanisms may be severely affected with aging.
In conclusion, the present study suggests that chronic E2 exposure produces age-dependent changes in the expression of inflammatory mediators and oxidative stress-related genes in the RVLM of female rats. Results from the present study also imply that older women who are on estrogen containing hormone replacement therapy could be more prone to neuroinflammation. These findings correlate well with the systolic and mean arterial pressures that were measured in these animals. Further mechanistic studies are needed to establish a causal relationship between ET-1, PICs and NADPH oxidase subunits in the pathogenesis of E2-induced hypertension.
4. Materials and Methods
4.1. Animals
Young (Y; 3-4 month old) and middle-aged (MA; 6-8 month old) female Sprague-Dawley rats were obtained from Harlan Sprague-Dawley Inc. (Indianapolis, IN). Animals were housed in temperature (23 ± 2° C) and light controlled (lights on from 0600 to 1800h) rooms with ad libitum food and water. Estrous cycles were monitored by vaginal cytology and animals that were cycling regularly were used in the experiment. Animals were used in accordance with the NIH's Guide for the Care and Use of Laboratory Animals and the protocols were approved by the Animal Care and Use Committee at Michigan State University.
4.2. Treatment
Animals were either sham-implanted or implanted s.c. with 90-day custom-made slow-release E2 pellets (n=6 per group) (1.8μg; 20ng/day; Innovative Research America, Sarasota, FL) as described earlier (Kasturi et al., 2009),(Mohankumar et al., 2011). This yielded 4 treatment groups: Young animals that were sham-implanted (YS) or implanted with E2 pellets (YE) and middle aged animals that were sham-implanted (MS) or implanted with E2 pellets (ME). By 90 days of exposure, all E2-implanted rats were in a state of constant estrous. A second set of animals were sham-implanted or implanted with E2 pellets as described above. During the last 2 weeks of treatment, animals were subjected to blood pressure measurement using a SC1000 Hatteras tail-cuff blood pressure monitoring system as described below. At the end of treatment, control and E2-treated animals were sacrificed at noon on the day of estrous. The brainstem was removed, frozen on dry ice and stored at −70°C until sectioning.
4.3. Blood pressure measurement
Blood pressure was measured using a Hatteras SC1000 Blood pressure analysis system (Hatteras, Cary, NC http://www.hatterasinstruments.com/sc1000.shtml) as described elsewhere (7737724). Briefly, the system is comprised of a master control unit that is connected to a tail cuff and a photodiode sensor assembly attached to a heated platform maintained at 37.7 °C. The control unit is also connected to a computer that has the software for data analysis (SC1000 communications program, V2.5). The master control unit inflates and deflates the cuff, and integrates analog data obtained from the photodiode sensor. At the time of blood pressure measurement, the animal was placed in a bucket with a heat lamp for 10 minutes to increase body temperature. It was then placed in a plexiglass restraint tube on the heated platform and its tail was passed through the inflatable cuff and secured to the platform with tape. The photodiode sensor assembly was secured over the tail. The system measures blood pressure in rats by determining what pressure in an inflatable tail cuff will cut off arterial blood flow to the tail. The sensor assembly detects the pulse based on the blood flow and the software converts the analog information into digital waveforms. Detection of thirty consecutive waveform peaks prompts the system to begin measurements. The machine performs 5 preliminary cycles to help the rat adjust to the inflation of the tail cuff after which 10 measurement cycles are initiated and the systolic, diastolic, mean arterial pressure and pulse rate are obtained. Animals were subjected to frequent blood pressure measurements over 2 weeks before obtaining a final reading a week before sacrifice.
4.4. Microdissection of RVLM
Serial coronal sections (300 μm thick) of the brainstem were obtained on RNAse-free slides using a cryostat (Slee Mainz, London, UK). The RVLM was microdissected by the Palkovits's micropunch technique using a 500-μm-diameter punch. A rat brain stereotaxic atlas was used as a reference (Paxinos and Watson, 1987). Tissue samples included all subdivisions of the RVLM.
4.5. RNA extraction and cDNA synthesis
RNA was extracted from tissue punches using the MELT Total Nucleic Acid Isolation System (Ambion Inc, Austin, TX) according to the manufacturer's instructions. RNA was eluted in a volume of 500 μl, after on-bead Turbo DNAse digestion. The quality of the RNA was assessed using a Nanodrop spectrophotometer prior to cDNA synthesis. First strand cDNA was synthesized by reverse transcribing 400 ng of total RNA using a RT2 First Strand Kit (SABiosciences, Frederick, MD) following the manufacturer's protocol.
4.6. qPCR array
The cDNA from the previous step was used to perform a quantitative PCR array. We used Applied Biosystems 7500 system to run the RT2 profiler custom PCR array, Cat # CAPR09289A (SA Biosciences, Frederick, MD) according to the manufacturer's specifications. This array included primers for the following genes: Interleukin-1 beta (IL-1 beta), interleukin-6 (IL-6), tumor necrosis factor alpha (TNF alpha), prostaglandin-endoperoxidase synthase 2 (Ptgs2), monocyte chemoattractant protein 1 (MCP1), estrogen receptor alpha (ESR1), estrogen receptor beta (ESR2), angiotensinogen, Ang II receptor, type 1b, ET-1, ET-2 and beta actin (β-actin). The procedure for qPCR array has been described before (22036618). Briefly, the cDNA was mixed with the SYBR Green/ROX Master Mix (SABiosciences, Frederick, MD) and the appropriate amount of RNA nuclease-free water in PCR plates and were spun at 1500 rpm for 1 minute. PCR was performed using the following 2-step cycling program: 1 cycle at 95°C for 10 minutes, and 40 cycles at 92°C for 15 seconds and 60°C for 1 minute. The mRNA expression of each gene was normalized to the expression of the housekeeping gene β-actin, and the values in different treatment groups were expressed relative to the data obtained from the YS group according to the 2−ΔΔCT method.
4.7. qRT-PCR
A second set of cDNA samples were used to perform quantitative real-time PCR (qRT-PCR). Briefly, the cDNA was mixed with RT2 Real-Time PCR SYBR Green/ROX Master Mix (SABiosciences, Frederick, MD), and the appropriate amount of RNA nuclease-free water. The volume/well contained 12.5 μL of PCR master mix, 2 μL of cDNA, 1 μL each of forward and reverse primer and 8.5 μL of water to obtain a total reaction volume of 25 μL. The forward and reverse primers for NADPH oxidase subunits (Nox1. Nox2, Nox4, p47phox, p22phox) were purchased from Integrated DNA Technologies (Coralville, IA). A control RT reaction with no cDNA was included for each primer. The reactions were performed in the Applied Biosystems 7500 Real-Time PCR System (Applied Biosystems, Carlsbad, CA) using the 2-step cycling program as described above. At the end of amplification, a melting curve analysis was done by heating the PCR products to 65°-95°C and held for 15 sec at increments of 0.2°C. Fluorescence was measured to confirm the presence of a single amplification product. After obtaining the CT values, the values were normalized to those of the housekeeping gene, β- actin. Fold change in the gene expression in different treatment groups were expressed in terms of CT values in the Y+S group according to the 2−ΔΔCT method. Primer sequences that were used to verify PCR products for Nox1, Nox2, Nox 4, p47phox and p22phox genes are provided in table 1. The other genes that were analyzed were part of the qPCR array from SABiosciences and the primer sequences are proprietary.
Table 1.
Primer sequences used for the verification of PCR products with accession numbers.
| Gene | Sequence | NCBI Accession no. |
|---|---|---|
| NOX 1 | For: 5′ TGAACAACAGCACTCACCAATGCC 3′ Rev: 5′ AGTTGTTGAACCAGGCAAAGGCAC 3′ |
AF152963 |
| NOX 2 | For: 5′ GTGGAGTGGTGTGTGAATGC 3′ Rev: 5′ TCCACGTACAATTCGCTCAG 3′ |
AF298656 |
| NOX4 | For: 5′ TCATGGATCTTTGCCTGGAGGGTT 3′ Rev: 5′ AGGTCTGTGGGAAATGAGCTTGGA 3′ |
AY027527 |
| p47phox | For: 5′ AGGTTGGGTCCCTGCATCCTATTT 3′ Rev: 5′ TGGTTACATACGGTTCACCTGCGT 3′ |
AF260779 |
| p22phox | For: 5′ TGTTGCAGGAGTGCTCATCTGTCT 3′ Rev: 5′ AGGACAGCCCGGACGTAGTAATTT 3′ |
U18729 |
| ß-actin | For: 5′ GGCTACAGCTTCACCACCAC 3′ Rev: 5′ TACTCCTGCTTGCTGATCCAC 3′ |
V01217 |
4.8. Measurement of Superoxide dismutase activity in the RVLM
Tissue samples that were obtained by Palkovits’ microdissection were analyzed for SOD activity using the SOD activity colorimetric assay kit supplied by Arbor Assays (Ann Arbor, MI). Samples were homogenized in assay buffer and used in duplicates in the assay as per the manufacturer's instructions. The sensitivity of the assay was 0.044U/ml. Protein concentrations in the sample were measured using a micro BCA assay (Thermo Scientific Pierce, Waltham, MA). SOD activity in tissues were expressed as Units/μg protein.
4.9. Statistical analysis
All statistical analysis was performed using Statview software (Version 5.0). One way ANOVA was used to analyze differences in blood pressure readings followed by post hoc Fisher's LSD test. All gene expression values were expressed as fold change relative to Y+S animals before they were compared by ANOVA. The individual effects of aging and E2 treatment were analyzed using two-way ANOVA followed by post hoc Fisher's LSD test. A p-value of <0.05 was considered statistically significant.
Fig 6.
SOD activity in the RVLM after 90 day exposure to E2 in young and aging animals are shown above. * denotes significant difference (p<0.05) from the young animals (YS and YE groups).
Highlights for review.
This manuscript describes the effects of chronic exposure to estradiol in young and middle aged female rats.
Estradiol produced marked increases in blood pressure in young and aging animals.
There was a corresponding increase in the expression of endothelin-1 mRNA and changes in the expression of other genes in the brainstem.
Acknowledgement
This work was supported by NIH AG027697 and partially by MSU AgBioresearch (to P.S. MohanKumar). A major part of the work was completed at Michigan State University in partial fulfillment of Dr. Subramanian's PhD degree.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: Authors have no conflict of interest to declare
References
- Agarwal D, Welsch MA, Keller JN, Francis J. Chronic exercise modulates RAS components and improves balance between pro- and anti-inflammatory cytokines in the brain of SHR. Basic Res Cardiol. 2011;106:1069–85. doi: 10.1007/s00395-011-0231-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiri F, Virdis A, Neves MF, Iglarz M, Seidah NG, Touyz RM, Reudelhuber TL, Schiffrin EL. Endothelium-restricted overexpression of human endothelin-1 causes vascular remodeling and endothelial dysfunction. Circulation. 2004;110:2233–40. doi: 10.1161/01.CIR.0000144462.08345.B9. [DOI] [PubMed] [Google Scholar]
- Babior BM, Lambeth JD, Nauseef W. The neutrophil NADPH oxidase. Arch Biochem Biophys. 2002;397:342–4. doi: 10.1006/abbi.2001.2642. [DOI] [PubMed] [Google Scholar]
- Cuadra AE, Shan Z, Sumners C, Raizada MK. A current view of brain reninangiotensin system: Is the (pro)renin receptor the missing link? Pharmacol Ther. 2010;125:27–38. doi: 10.1016/j.pharmthera.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinapoli VA, Benkovic SA, Li X, Kelly KA, Miller DB, Rosen CL, Huber JD, O'Callaghan JP. Age exaggerates proinflammatory cytokine signaling and truncates signal transducers and activators of transcription 3 signaling following ischemic stroke in the rat. Neuroscience. 2010;170:633–44. doi: 10.1016/j.neuroscience.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerrschmidt N, Wippich N, Goettsch W, Broemme HJ, Morawietz H. Endothelin-1 induces NAD(P)H oxidase in human endothelial cells. Biochem Biophys Res Commun. 2000;269:713–7. doi: 10.1006/bbrc.2000.2354. [DOI] [PubMed] [Google Scholar]
- Durieu-Trautmann O, Federici C, Creminon C, Foignant-Chaverot N, Roux F, Claire M, Strosberg AD, Couraud PO. Nitric oxide and endothelin secretion by brain microvessel endothelial cells: regulation by cyclic nucleotides. J Cell Physiol. 1993;155:104–11. doi: 10.1002/jcp.1041550114. [DOI] [PubMed] [Google Scholar]
- Fei J, Viedt C, Soto U, Elsing C, Jahn L, Kreuzer J. Endothelin-1 and smooth muscle cells: induction of jun amino-terminal kinase through an oxygen radical-sensitive mechanism. Arterioscler Thromb Vasc Biol. 2000;20:1244–9. doi: 10.1161/01.atv.20.5.1244. [DOI] [PubMed] [Google Scholar]
- Fujita M, Ando K, Nagae A, Fujita T. Sympathoexcitation by oxidative stress in the brain mediates arterial pressure elevation in salt-sensitive hypertension. Hypertension. 2007;50:360–7. doi: 10.1161/HYPERTENSIONAHA.107.091009. [DOI] [PubMed] [Google Scholar]
- Gao L, Wang W, Li YL, Schultz HD, Liu D, Cornish KG, Zucker IH. Sympathoexcitation by central ANG II: roles for AT1 receptor upregulation and NAD(P)H oxidase in RVLM. Am J Physiol Heart Circ Physiol. 2005;288:H2271–9. doi: 10.1152/ajpheart.00949.2004. [DOI] [PubMed] [Google Scholar]
- Gemma C, Vila J, Bachstetter A, Bickford PC. Oxidative Stress and the Aging Brain: From Theory to Prevention. 2007 [PubMed] [Google Scholar]
- Huang Y, Henry CJ, Dantzer R, Johnson RW, Godbout JP. Exaggerated sickness behavior and brain proinflammatory cytokine expression in aged mice in response to intracerebroventricular lipopolysaccharide. Neurobiol Aging. 2008;29:1744–53. doi: 10.1016/j.neurobiolaging.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just A, Olson AJ, Whitten CL, Arendshorst WJ. Superoxide mediates acute renal vasoconstriction produced by angiotensin II and catecholamines by a mechanism independent of nitric oxide. Am J Physiol Heart Circ Physiol. 2007;292:H83–92. doi: 10.1152/ajpheart.00715.2006. [DOI] [PubMed] [Google Scholar]
- Kang YM, Ma Y, Zheng JP, Elks C, Sriramula S, Yang ZM, Francis J. Brain nuclear factor-kappa B activation contributes to neurohumoral excitation in angiotensin II-induced hypertension. Cardiovasc Res. 2009;82:503–12. doi: 10.1093/cvr/cvp073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasturi BS, MohanKumar SM, Sirivelu MP, MohanKumar PS. Chronic exposure to low levels of oestradiol-17beta affects oestrous cyclicity, hypothalamic norepinephrine and serum luteinising hormone in young intact rats. J Neuroendocrinol. 2009;21:568–77. doi: 10.1111/j.1365-2826.2009.01873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Fink GD, Watts SW, Northcott CA, Galligan JJ, Pagano PJ, Chen AF. Endothelin-1 increases vascular superoxide via endothelin(A)-NADPH oxidase pathway in low-renin hypertension. Circulation. 2003;107:1053–8. doi: 10.1161/01.cir.0000051459.74466.46. [DOI] [PubMed] [Google Scholar]
- MacCumber MW, Ross CA, Snyder SH. Endothelin in brain: receptors, mitogenesis, and biosynthesis in glial cells. Proc Natl Acad Sci U S A. 1990;87:2359–63. doi: 10.1073/pnas.87.6.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier CM, Chan PH. Role of superoxide dismutases in oxidative stress and neurodegenerative disorders. Neuroscientist. 2002;8:323–334. doi: 10.1177/107385840200800408. [DOI] [PubMed] [Google Scholar]
- Mohankumar SM, Kasturi BS, Shin AC, Balasubramanian P, Gilbreath ET, Subramanian M, Mohankumar PS. Chronic estradiol exposure induces oxidative stress in the hypothalamus to decrease hypothalamic dopamine and cause hyperprolactinemia. Am J Physiol Regul Integr Comp Physiol. 2011 doi: 10.1152/ajpregu.00481.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moolman JA. Unravelling the cardioprotective mechanism of action of estrogens. Cardiovasc Res. 2006;69:777–80. doi: 10.1016/j.cardiores.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Mosqueda-Garcia R, Yates K, O'Leary J, Inagami T. Cardiovascular and respiratory effects of endothelin in the ventrolateral medulla of the normotensive rat. Hypertension. 1995;26:263–71. doi: 10.1161/01.hyp.26.2.263. [DOI] [PubMed] [Google Scholar]
- Nagae A, Fujita M, Kawarazaki H, Matsui H, Ando K, Fujita T. Sympathoexcitation by oxidative stress in the brain mediates arterial pressure elevation in obesity-induced hypertension. Circulation. 2009;119:978–86. doi: 10.1161/CIRCULATIONAHA.108.824730. [DOI] [PubMed] [Google Scholar]
- Oliveira-Sales EB, Colombari DS, Davisson RL, Kasparov S, Hirata AE, Campos RR, Paton JF. Kidney-induced hypertension depends on superoxide signaling in the rostral ventrolateral medulla. Hypertension. 2010;56:290–6. doi: 10.1161/HYPERTENSIONAHA.110.150425. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Co-ordinates. Academic Press; NewYork: 1987. [Google Scholar]
- Posthuma WF, Westendorp RG, Vandenbroucke JP. Cardioprotective effect of hormone replacement therapy in postmenopausal women: is the evidence biased? BMJ. 1994;308:1268–9. doi: 10.1136/bmj.308.6939.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross CA, Ruggiero DA, Park DH, Joh TH, Sved AF, Fernandez-Pardal J, Saavedra JM, Reis DJ. Tonic vasomotor control by the rostral ventrolateral medulla: effect of electrical or chemical stimulation of the area containing C1 adrenaline neurons on arterial pressure, heart rate, and plasma catecholamines and vasopressin. J Neurosci. 1984;4:474–94. doi: 10.1523/JNEUROSCI.04-02-00474.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi NF, Maliszewska-Scislo M. Role of paraventricular nucleus vasopressin V1A receptors in the response to endothelin 1 activation of the subfornical organ in the rat. J Physiol Pharmacol. 2008;59(Suppl 8):47–59. [PMC free article] [PubMed] [Google Scholar]
- Salminen LE, Paul LH. Oxidative stress and genetic markers of suboptimal antioxidant defense in the aging brain: a theoretical review. Rev Neurosci. 2014;25:805–819. doi: 10.1515/revneuro-2014-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi P, Diez-Freire C, Jun JY, Qi Y, Katovich MJ, Li Q, Sriramula S, Francis J, Sumners C, Raizada MK. Brain microglial cytokines in neurogenic hypertension. Hypertension. 2010;56:297–303. doi: 10.1161/HYPERTENSIONAHA.110.150409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi P, Raizada MK, Sumners C. Brain cytokines as neuromodulators in cardiovascular control. Clin Exp Pharmacol Physiol. 2010;37:e52–7. doi: 10.1111/j.1440-1681.2009.05234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian M, Balasubramanian P, Garver H, Northcott C, Zhao H, Haywood JR, Fink GD, MohanKumar SM, MohanKumar PS. Chronic estradiol-17beta exposure increases superoxide production in the rostral ventrolateral medulla and causes hypertension: reversal by resveratrol. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1560–8. doi: 10.1152/ajpregu.00020.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumimoto H, Miyano K, Takeya R. Molecular composition and regulation of the Nox family NAD(P)H oxidases. Biochem Biophys Res Commun. 2005;338:677–86. doi: 10.1016/j.bbrc.2005.08.210. [DOI] [PubMed] [Google Scholar]
- Tsuda K. Oxidative stress and membrane fluidity of red blood cells in hypertensive and normotensive men: an electron spin resonance investigation. Int Heart J. 51:121–4. doi: 10.1536/ihj.51.121. [DOI] [PubMed] [Google Scholar]
- Woods JW. Oral contraceptives and hypertension. Hypertension. 1988;11:II11–5. doi: 10.1161/01.hyp.11.3_pt_2.ii11. [DOI] [PubMed] [Google Scholar]