Abstract
INTRODUCTION
The survival impact of incomplete lung cancer resection has never been systematically quantified, nor has the value of post-operative adjuvant therapy in this setting.
METHODS
We evaluated lung cancer resections in the National Cancer Data Base from 2004 to 2011 to determine factors associated with margin involvement. We compared the survival of patients with and without positive margins, and evaluated the impact of postoperative adjuvant therapy.
RESULTS
Of 112,998 resections over 8 years, 5335 (4.7%) had positive margins. Patient demographic and clinical factors associated with increased adjusted odds ratio (aOR) of incomplete resection included black race (p=0.006), age-based Medicare insurance (p=0.006), urban residence (p=0.01), squamous histology, high tumor grade, tumor overlapping more than 1 lobe, and advanced pathologic stage (p<0.001 for all clinical factors). Community Cancer Programs (p=0.002), institutions with high proportions of under-insured patients (p=0.01), and institutions with lower cancer resection volumes (p=0.006), also had increased aOR. The crude 5-year survival rate of patients with complete vs. incomplete resection was 58.5% v 33.8% (p<.001). After incomplete resection, adjuvant chemotherapy was associated with improved 5-year survival across all stages (p<0.01); radiotherapy was associated with worse survival in stage I patients (p<.001).
CONCLUSIONS
Margin involvement significantly impaired survival after lung cancer resection, irrespective of stage. Causative institutional and provider practices should be identified, to minimize this adverse outcome. Postoperative adjuvant chemotherapy mitigated the mortality risk independently of stage, whilst postoperative radiotherapy exacerbated the risk in patients with stage I disease. These findings need validation in prospective trials.
Keywords: non-small cell, positive margins, quality of care, outcomes, surgical resection, survival
INTRODUCTION
Lung cancer is the oncologic scourge of the present age, causing 160,000 annual deaths in the US, 28% of all US cancer mortality.1 Only 17% of all patients diagnosed with lung cancer survive up to 5 years.1 The overwhelming majority of long-term survivors have had surgery as a component of their treatment. However, majority of patients who undergo surgery for lung cancer die within 5 years.2 Improving surgical outcomes is therefore a viable strategy for improving overall lung cancer survivorship.
It is believed that attaining the benefit of surgical resection of non-small-cell lung cancer requires complete (R0) resection of all evident disease.3 However, ‘complete resection’ in lung cancer has been ill-defined, with ongoing controversy about the optimal extent of lung parenchymal resection 4 and nodal examination.5–9 Even the prognostic implication of a positive resection margin has been questioned.5,6,10 Current practice guidelines recognize that patients with microscopic (R1) or macroscopic (R2) positive resection margins are at high risk.3 But this risk has never been systematically quantified.11
Practice guidelines recommend re-resection as the preferred response to margin positivity for patients with stage I and II disease, but this is infeasible in many cases, and surgeons are often reluctant to subject patients to re-resection in any case. 3 Alternative options to surgery are post-operative adjuvant radiation therapy, chemotherapy, and the combination of modalities in certain situations.3,11 However, postoperative radiotherapy increases mortality risk in patients with completely resected pathologic N0 or N1 non-small-cell lung cancer.12 The benefit of radiotherapy has never been clearly demonstrated in patients with positive margins.11 The role of chemotherapy in managing patients with positive margins is even less well-defined, as clinical trials of adjuvant chemotherapy typically exclude patients with positive resection margins.13,14 Practice guidelines in this area rely predominantly on expert opinion,3,5 contradictory single-institutional reports,10,15–22 ad-hoc secondary analyses of patient subsets in clinical trials designed to answer other questions,6 or literature reviews.11
We analyzed the US National Cancer Data Base (NCDB) to establish the proportion of non-R0 resections in a contemporary multi-year national cohort. We also sought to examine factors associated with resection margin positivity, to definitively quantify the survival implications of resection with positive margins, and to examine the survival impact of non-surgical adjuvant therapies.
MATERIALS AND METHODS
DATA SOURCES
The NCDB, jointly sponsored by the American College of Surgeons and the American Cancer Society, is a clinical oncology database sourced from hospital registry data that are collected in more than 1,500 Commission on Cancer-accredited facilities. NCDB data are used to analyze and track patients with malignant neoplastic diseases, their treatments, and outcomes. Data represent approximately 70 percent of newly diagnosed cancer cases in the US.23 This study was exempted from the informed consent requirement because it analyzes a pre-existing, de-identified dataset.
STUDY SUBJECTS
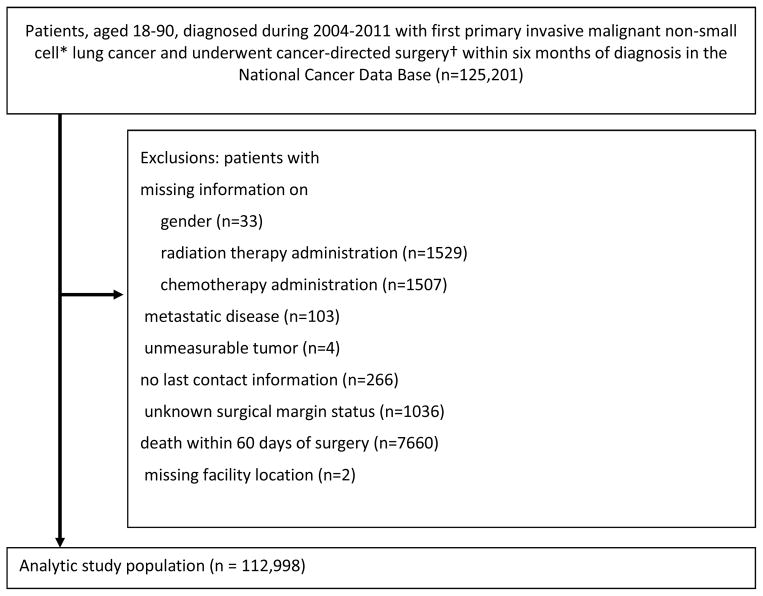
We selected patients aged 18 to 90 years, with a first primary non-small-cell lung cancer diagnosis (International Classification of Disease for Oncology, 9th edition [ICD-9-CM] site codes C34.0 – C34.9), pathologic stage I – IIIA, diagnosed from 2004 to 2011, who underwent cancer-directed surgery in Commission on Cancer-accredited reporting facilities within 6 months of diagnosis (Fig 1). We excluded patients with missing information on gender, radiation therapy administration, chemotherapy administration, those with metastatic disease, unmeasurable tumor, no last contact information, and unknown margin status. Because we were interested in the quality of oncologic resection, we excluded patients who died within 60 days postoperatively.
Figure 1.
Patient selection schema. *Non-small cell histology was identified through International Classification of Diseases for Oncology, 3 rd version (ICD-O-3) histology codes: 8010–8040, 8050 – 8076, 8140, 8143, 8211, 8230–8231, 8246, 8250 – 8260, 8310, 8320, 8323, 8430, 8470 – 8490, 8550 – 8573, 8980, 8981. †Cancer-directed surgery was identified through site-specific surgical codes (21, 22, 30 – 70), including sub-lobectomy, lobectomy, bi-lobectomy, and pneumonectomy.
STUDY OUTCOMES AND COVARIATES
The primary outcome was margin status (positive or negative) identified from the final status of the surgical margins after resection of the primary tumor. Positive margin was defined if residual tumor (R1, R2, or ‘not otherwise specified’) was recorded in the pathology report. Secondary outcomes were overall-survival rates at 1, 3, 5 years in patients categorized by margin status. Patients were censored if they were lost to follow-up or still alive at the end of the study period.
We examined associations between margin status and patient-level clinical and demographic covariates, as well as institutional covariates. Patient-level demographic covariates included age, sex, race, insurance status, US census region of residence, residence in a rural or urban location, median income level in patients’ neighborhood of residence, year of cancer diagnosis and co-morbidities. Patient-level clinical covariates included disease characteristics such as tumor histology, grade, size, T-category, N-category, aggregate American Joint Committee on Cancer (AJCC) pathologic stage, and the treatment characteristics extent of resection, and lymph node examination. Institutional covariates included facility type, facility location by census division, proportion of institutional patients with no insurance or insured by Medicaid (in quartiles), lung cancer volume as a proportion of all institutional cancer surgery volume (in quartiles), and total annual cancer surgery volume (in quartiles).
In secondary analyses, we evaluated the association between preoperative adjuvant therapy use and margin positivity. We also examined survival following postoperative adjuvant therapy in groups of patients stratified by stage. Adjuvant therapy, including chemotherapy, radiotherapy or both, was identified if commenced within six months before, or after, surgery. Chemotherapy was identified if administered as single- or multiple-agents. Radiotherapy was identified if administered at the reporting facility or elsewhere.
STATISTICAL ANALYSIS PLAN
Descriptive analyses were conducted to summarize patient and institutional characteristics. Chi-square tests were used to determine significance of differences according to margin status. The yearly trend in the incidence of surgical resection with positive margins was evaluated using the Cochran-Armitage test. Uni- and multivariate logistic regression analyses were conducted to evaluate associations between patient and institutional characteristics and positive margins. The multivariate model included the aggregate pathologic stage, and because this is based on the pathological T, N and M category, we did not separately adjust for T, N, M stage in order to avoid problems with multicollinearity. The results of multivariate logistic regression adjusting either aggregate pathologic stage or T, N, and M categories were similar, therefore we presented results with aggregate pathologic stage in the model.
In addition, to determine whether use of pre-operative adjuvant therapy might be associated with positive margin, three multivariate logistic regression analyses were performed for patients who received pre-operative chemotherapy, radiotherapy, or chemoradiation versus those without preoperative adjuvant therapy, respectively.
To examine the survival impact of resection with positive margins, 5-year overall survival distributions in patients stratified by margin status were estimated using the Kaplan-Meier method and compared using the log-rank test. Additional stratification by T-category, tumor size and aggregate AJCC stage was conducted to control possible confounding. Furthermore, to assess the survival impact of postoperative adjuvant therapy use in patients with a positive margin, 5-year overall survival rates, stratified by postoperative treatment and aggregate AJCC stage, were estimated using the Kaplan-Meier method. Patients who received preoperative adjuvant therapy were excluded from all analyses of the effects of postoperative adjuvant therapy.
To evaluate the impact of including R2 and indeterminate cases as margin-positive, we conducted sensitivity analyses by excluding patients with R2 and/or indeterminate cases. We also analyzed the impact on our results of excluding patients who died within 30, 60, and 90 days postoperatively. Because the results were similar, we have reported data using the 60-day exclusion window. All tests of significance were 2-sided, and p-values <0.05 were considered statistically significant. All analyses were conducted using SAS Version 9.4 (Cary, NC).
RESULTS
PATIENT DEMOGRAPHIC AND CLINICAL CHARACTERISTICS AND LIKELIHOOD OF MARGIN POSITIVITY
Our study cohort consists of 112,998 individuals, of whom 5,335 (4.7%) had margin-positive resection (Table 1). The annual margin-positivity rate was stable over the 8-year time span (2004–2011), ranging between 4.4%, in 2007, and 5.2% in 2010 (trend-test p= 0.07). Fifty-seven percent of margin-positive cases were R1, 4% were R2, and 39% were not specified.
Table 1.
Factors associated with margin positive resection (univariate and multivariate analysis).
|
|
|
|
|||||
|---|---|---|---|---|---|---|---|
| Categories | Patients, N(col%) | Margin- Positive Patients, N(row %) | p-value | Unadjusted OR (95% CI) | p-value | Adjusted OR (95% CI) | p-value |
|
|
|
|
|||||
| Patient Characteristics | N=112998 | N=5335 (4.7) | |||||
| Age | |||||||
| 18–49 | 6300 (5.6) | 341 (5.4) | <.001 | 1 | 1 | ||
| 50–64 | 36940 (32.7) | 1836 (5.0) | 0.91(0.81–1.03) | 0.14 | 1(0.88–1.13) | 0.99 | |
| 65–74 | 42857 (37.9) | 1915 (4.5) | 0.82(0.73–0.92) | <.001 | 0.84(0.72–0.99) | 0.03 | |
| 75–90 | 26901 (23.8) | 1243 (4.6) | 0.85(0.75–0.96) | 0.008 | 0.89(0.76–1.05) | 0.18 | |
| Gender | |||||||
| Male | 54806 (48.5) | 2837 (5.2) | <.001 | 1 | |||
| Female | 58192 (51.5) | 2498 (4.3) | 0.82(0.78–0.87) | <.001 | 1(0.95–1.06) | 0.96 | |
| Race/Ethnicity | |||||||
| Non-Hispanic, White | 88945 (78.7) | 4111 (4.6) | <.001 | 1 | 1 | ||
| Hispanic | 2464 (2.2) | 119 (4.8) | 1.05(0.87–1.26) | 0.63 | 0.99(0.81–1.2) | 0.88 | |
| Black | 9354 (8.3) | 531 (5.7) | 1.24(1.13–1.36) | <.001 | 1.15(1.04–1.27) | 0.006 | |
| Other | 2931 (2.6) | 131 (4.5) | 0.97(0.81–1.15) | 0.70 | 0.98(0.81–1.18) | 0.80 | |
| Missing | 9304 (8.2) | 443 (4.8) | 1.03(0.93–1.14) | 0.54 | 1.05(0.94–1.16) | 0.41 | |
| Diagnosis Year | |||||||
| 2004 | 12859 (11.4) | 606 (4.7) | 0.06 | 1 | 1 | ||
| 2005 | 13891 (12.3) | 638 (4.6) | 0.97(0.87–1.09) | 0.64 | 1.02(0.9–1.14) | 0.78 | |
| 2006 | 14216 (12.6) | 662 (4.7) | 0.99(0.88–1.11) | 0.83 | 1.02(0.91–1.15) | 0.73 | |
| 2007 | 14117 (12.5) | 618 (4.4) | 0.93(0.83–1.04) | 0.19 | 0.99(0.88–1.11) | 0.81 | |
| 2008 | 14244 (12.6) | 676 (4.8) | 1.01(0.9–1.13) | 0.90 | 1.07(0.95–1.2) | 0.28 | |
| 2009 | 14193 (12.6) | 659 (4.6) | 0.99(0.88–1.1) | 0.79 | 1.04(0.93–1.17) | 0.48 | |
| 2010 | 15114 (13.4) | 791 (5.2) | 1.12(1–1.25) | 0.05 | 0.95(0.85–1.06) | 0.35 | |
| 2011 | 14364 (12.7) | 685 (4.8) | 1.01(0.91–1.13) | 0.83 | 0.89(0.79–0.99) | 0.04 | |
| Insurance | |||||||
| Uninsured | 2185 (1.93) | 133 (6.1) | <.001 | 1.32(1.1–1.59) | 0.003 | 1.07(0.88–1.3) | 0.49 |
| Medicaid | 4822 (4.27) | 274 (5.7) | 1.23(1.08–1.4) | 0.002 | 1.03(0.9–1.18) | 0.70 | |
| Younger Medicare | 6392 (5.66) | 313 (4.9) | 1.05(0.93–1.19) | 0.43 | 0.99(0.87–1.13) | 0.87 | |
| Older Medicare | 58853 (52.08) | 2723 (4.6) | 0.99(0.93–1.05) | 0.73 | 1.16(1.04–1.3) | 0.006 | |
| Government | 220 (0.19) | 10 (4.6) | 0.97(0.51–1.84) | 0.93 | 0.69(0.36–1.33) | 0.27 | |
| Private | 39028 (34.54) | 1824 (4.8) | 1 | 1 | |||
| Missing | 1498 (1.33) | 58 (3.9) | 0.82(0.63–1.07) | 0.15 | 0.86(0.65–1.13) | 0.27 | |
| Median Income–Quartile 2000 | |||||||
| <$30,000 | 14702 (13.01) | 751 (5.1) | <.001 | 1.19(1.09–1.3) | <.001 | 1.09(0.98–1.21) | 0.11 |
| $30,000– $34,999 | 20829 (18.43) | 1079 (5.2) | 1.21(1.12–1.31) | <.001 | 1.14(1.04–1.24) | 0.004 | |
| $35,000– $45,999 | 31072 (27.5) | 1498 (4.8) | 1.12(1.05–1.21) | 0.001 | 1.05(0.98–1.14) | 0.18 | |
| $46,000+ | 40252 (35.62) | 1736 (4.3) | 1 | 1 | |||
| Missing | 6143 (5.44) | 271 (4.4) | 1.02(0.9–1.17) | 0.72 | 1.08(0.89–1.31) | 0.46 | |
| Rural/Urban | |||||||
| Rural | 21159 (18.73) | 1030 (4.9) | 0.20 | 1.03(0.96–1.11) | 0.35 | 0.9(0.83–0.98) | 0.01 |
| Urban | 84715 (74.97) | 3995 (4.7) | 1 | 1 | |||
| Unknown | 7124 (6.3) | 310 (4.4) | 0.92(0.82–1.04) | 0.16 | 0.98(0.82–1.17) | 0.81 | |
| Comorbidity | |||||||
| 0 | 53437 (47.29) | 2454 (4.6) | 0.11 | 1 | 1 | ||
| 1 | 40806 (36.11) | 1993 (4.9) | 1.07(1–1.13) | 0.04 | 1.07(1–1.14) | 0.05 | |
| 2+ | 18755 (16.6) | 888 (4.7) | 1.03(0.95–1.12) | 0.42 | 1.02(0.94–1.11) | 0.63 | |
| Census Region | |||||||
| Northeast | 23892 (21.14) | 946 (4.0) | <.001 | 1 | 1 | ||
| Midwest | 30404 (26.91) | 1626 (5.4) | 1.37(1.26–1.49) | <.001 | 1.55(0.93–2.6) | 0.10 | |
| South | 44324 (39.23) | 2038 (4.6) | 1.17(1.08–1.27) | <.001 | 1.17(0.74–1.86) | 0.51 | |
| West | 14250 (12.61) | 718 (5.0) | 1.29(1.17–1.42) | <.001 | 0.98(0.48–1.97) | 0.94 | |
| Missing | 128 (0.11) | 7 (5.5) | 1.4(0.65–3.01) | 0.39 | 1.45(0.61–3.45) | 0.41 | |
| Histology | |||||||
| NOS | 347 (0.31) | 18 (5.2) | <.001 | 1.37(0.85–2.2) | 0.20 | 1.05(0.65–1.72) | 0.84 |
| Large Cell | 5320 (4.71) | 261 (4.9) | 1.29(1.13–1.47) | <.001 | 0.98(0.86–1.13) | 0.78 | |
| Squamous | 33768 (29.88) | 2094 (6.2) | 1.65(1.56–1.75) | <.001 | 1.38(1.29–1.47) | <.001 | |
| Other | 5851 (5.18) | 357 (6.1) | 1.62(1.45–1.82) | <.001 | 1.34(1.19–1.51) | <.001 | |
| Adeno | 67712 (59.92) | 2605 (3.9) | 1 | 1 | |||
| Tumor Grade | |||||||
| well/moderately differentiated | 64772 (57.32) | 2528 (3.9) | <.001 | 1 | 1 | ||
| poorly/undifferentiated | 42668 (37.76) | 2557 (6.0) | 1.57(1.48–1.66) | <.001 | 1.14(1.07–1.21) | <.001 | |
| Unknown | 5558 (4.92) | 250 (4.5) | 1.16(1.02–1.33) | 0.03 | 0.98(0.85–1.13) | 0.79 | |
| Tumor Size | |||||||
| ≤3cm | 68906 (60.98) | 2136 (3.1) | <.001 | 1 | 1 | ||
| >3cm–≤5cm | 27971 (24.75) | 1706 (6.1) | 2.03(1.9–2.17) | <.001 | 1.65(1.54–1.77) | <.001 | |
| >5cm | 15518 (13.73) | 1422 (9.2) | 3.15(2.94–3.38) | <.001 | 1.94(1.79–2.1) | <.001 | |
| Unknown | 603 (0.53) | 71 (11.8) | 4.17(3.25–5.36) | <.001 | 2.47(1.89–3.22) | <.001 | |
| Lymph Node examined | |||||||
| Yes | 107002 (94.69) | 4858 (4.5) | <.001 | 1 | 1 | ||
| No | 5888 (5.21) | 467 (7.9) | 1.81(1.64–2) | <.001 | 2.14(1.9–2.41) | <.001 | |
| Unknown | 108 (0.1) | 10 (9.3) | 2.15(1.12–4.12) | 0.02 | 1.77(0.9–3.47) | 0.10 | |
| Primary Site | |||||||
| C340– Main bronchus | 725 (0.64) | 88 (12.1) | <.001 | 2.79(2.22–3.49) | <.001 | 1.4(1.1–1.78) | 0.007 |
| C341–upper lobe | 67878 (60.07) | 3207 (4.7) | 1 | 1 | |||
| C342–Middle lobe | 5451 (4.82) | 268 (4.9) | 1.04(0.92–1.19) | 0.52 | 1.19(1.04–1.35) | 0.01 | |
| C343–Lower lobe | 35317 (31.25) | 1464 (4.2) | 0.87(0.82–0.93) | <.001 | 0.83(0.78–0.89) | <.001 | |
| C348–Overlapping lesion | 1883 (1.67) | 182 (9.7) | 2.16(1.84–2.52) | 0.06 | 1.35(1.15–1.59) | <.001 | |
| C349–Lung NOS | 1744 (1.54) | 126 (7.2) | 1.57(1.31–1.89) | <.001 | 1.15(0.95–1.39) | 0.16 | |
| Pathologic Stage Group | |||||||
| Stage I | 79614 (70.46) | 1893 (2.4) | <.001 | 1 | 1 | ||
| Stage II | 21550 (19.07) | 1950 (9.1) | 4.09(3.83–4.36) | <.001 | 3.79(3.53–4.07) | <.001 | |
| Stage III | 11834 (10.47) | 1492 (12.6) | 5.92(5.52–6.36) | <.001 | 5.77(5.34–6.23) | <.001 | |
| TNM Path T #880 | |||||||
| T1 | 55320 (48.96) | 1115 (2.0) | <.001 | 1 | |||
| T2 | 48028 (42.5) | 2509 (5.2) | 2.68(2.49–2.88) | <.001 | |||
| T3 | 8300 (7.35) | 1516 (18.3) | 10.86(10.02–11.79) | <.001 | |||
| T4 | 667 (0.59) | 146 (21.9) | 13.62(11.23–16.52) | <.001 | |||
| Unknown | 683 (0.6) | 49 (7.2) | 3.76(2.79–5.06) | <.001 | |||
| TNM Path N #890 | |||||||
| N0 | 85272 (75.46) | 2818 (3.3) | <.001 | 1 | |||
| N1 | 16187 (14.33) | 1342 (8.3) | 2.65(2.47–2.83) | <.001 | |||
| N2 | 9289 (8.22) | 975 (10.5) | 3.43(3.18–3.7) | <.001 | |||
| NX | 2021 (1.79) | 178 (8.8) | 2.83(2.41–3.31) | <.001 | |||
| Unknown | 229 (0.2) | 22 (9.6) | 3.11(2–4.83) | <.001 | |||
| Extent of resection | |||||||
| Sublobar | 15671 (13.87) | 971 (6.2) | <.001 | 1.53(1.43–1.65) | <.001 | 1.96(1.80–2.15) | <.001 |
| Lobe/bilobectomy | 91017 (80.55) | 3757 (4.1) | 1 | 1 | |||
| Pneumonectomy | 6310 (5.58) | 607 (9.6) | 2.47(2.26–2.71) | <.001 | 0.98(0.89–1.09) | 0.71 | |
| Facility Characteristics Facility type | |||||||
| Community Cancer Program | 8248 (7.3) | 497 (6.0) | <.001 | 1.62(1.42–1.84) | <.001 | 1.34(1.12–1.61) | 0.002 |
| Comprehensive | 53784 (47.6) | 2647 (4.9) | <.001 | ||||
| Community Cancer Program | 1.31(1.18–1.44) | 1.23(1.1–1.38) | <.001 | ||||
| Teaching/Research | 27546 (24.38) | 1187 (4.3) | 1.14(1.02–1.26) | 0.02 | 1.06(0.95–1.19) | 0.28 | |
| NCI Program/Network | 13418 (11.87) | 512 (3.8) | 1 | 1 | |||
| 7–Other | 10002 (8.85) | 492 (4.9) | 1.3(1.15–1.48) | <.001 | 1.23(1.07–1.4) | 0.003 | |
| Proportion of Medicaid/Uninsured patients | |||||||
| Q1 (low) | 26715 (23.64) | 1157 (4.3) | 0.003 | 1 | 1 | ||
| Q2 | 30129 (26.66) | 1433 (4.8) | 1.1(1.02–1.19) | 0.02 | 1.07(0.98–1.16) | 0.13 | |
| Q3 | 31222 (27.63) | 1497 (4.8) | 1.11(1.03–1.2) | 0.008 | 1.08(0.99–1.18) | 0.07 | |
| Q4 (high) | 24932 (22.06) | 1248 (5.0) | 1.16(1.07–1.26) | <.001 | 1.13(1.03–1.24) | 0.01 | |
| Lung cancer resection as proportion of all surgery | |||||||
| Q1 (low) | 2690 (2.38) | 137 (5.1) | <.001 | 1.13(0.95–1.35) | 0.18 | 1.14(0.95–1.38) | 0.15 |
| Q2 | 22445 (19.86) | 1175 (5.2) | 1.17(1.08–1.25) | <.001 | 1.24(1.15–1.34) | <.001 | |
| Q3 | 38529 (34.1) | 1789 (4.6) | 1.03(0.96–1.09) | 0.42 | 1.09(1.02–1.16) | 0.02 | |
| Q4 (high) | 49334 (43.66) | 2234 (4.5) | 1 | 1 | |||
| Total cancer surgery volume | |||||||
| Q1 (low) | 3377 (2.99) | 206 (6.1) | <.001 | 1.45(1.26–1.68) | <.001 | 1.17(0.95–1.43) | 0.15 |
| Q2 | 13357 (11.82) | 739 (5.5) | 1.31(1.21–1.42) | <.001 | 1.17(1.05–1.3) | 0.006 | |
| Q3 | 26773 (23.69) | 1413 (5.3) | 1.25(1.17–1.33) | <.001 | 1.19(1.1–1.28) | <.001 | |
| Q4 (high) | 69491 (61.5) | 2977 (4.3) | 1 | 1 | |||
| Census Division | |||||||
| New England | 6993 (6.19) | 273 (3.9) | <.001 | 1 | 1 | ||
| Middle Atlantic | 17065 (15.1) | 681 (4.0) | 1.02(0.89–1.18) | 0.75 | 1.03(0.89–1.19) | 0.72 | |
| East North Central | 21333 (18.88) | 1182 (5.5) | 1.44(1.26–1.65) | <.001 | 0.87(0.51–1.47) | 0.59 | |
| West North Central | 9521 (8.43) | 443 (4.7) | 1.2(1.03–1.4) | 0.02 | 0.76(0.45–1.3) | 0.31 | |
| South Atlantic | 26279 (23.26) | 1124 (4.3) | 1.1(0.96–1.26) | 0.17 | 0.88(0.54–1.42) | 0.60 | |
| East South Central | 10175 (9) | 531 (5.2) | 1.36(1.17–1.57) | <.001 | 1.03(0.63–1.67) | 0.92 | |
| West South Central | 7583 (6.71) | 385 (5.1) | 1.32(1.12–1.54) | <.001 | 0.98(0.6–1.6) | 0.93 | |
| Mountain | 3628 (3.21) | 210 (5.8) | 1.51(1.26–1.82) | <.001 | 1.41(0.69–2.9) | 0.35 | |
| Pacific | 10421 (9.22) | 506 (4.9) | 1.26(1.08–1.46) | 0.003 | 1.16(0.57–2.38) | 0.68 | |
| Treatment Received radiation | |||||||
| No | 101731 (90.03) | 3000 (3.0) | <.001 | ||||
| Yes | 11267 (9.97) | 2335 (20.7) | |||||
| Received Chemotherapy | |||||||
| No | 83513 (73.91) | 2638 (3.2) | <.001 | ||||
| Yes | 29485 (26.09) | 2697 (9.2) | |||||
Several patient-level demographic characteristics were independently associated with higher adjusted odds of having incomplete resection (Table 1). These include black race, older patients with Medicare insurance coverage, and residence in an urban area. Associated patient-level clinical factors were squamous and ‘other’ histology (in comparison to adenocarcinoma), poor histologic differentiation, larger tumors, lymph node involvement (or unknown lymph node status), tumors overlapping multiple lobes, located in the middle lobe, or in the main-stem bronchus, and advanced stage (in terms of T-category, N-category or aggregate stage). Patients with sub-lobar resections were more likely to have positive margins compared to those who had lobectomy or pneumonectomy.
INSTITUTIONAL CHARACTERISTICS AND LIKELIHOOD OF MARGIN POSITIVITY
Surgery performed at institutions designated as Community Cancer Programs, and Comprehensive Community Cancer Programs was more likely to be associated with positive margins than surgery performed at Teaching/Research institutions or those designated as National Cancer Institute Programs or Networks (Table 1). Institutions with a higher proportion of patients with Medicaid or no insurance were more likely to have incomplete resections than those with the lowest proportion of underinsured patients. In addition, institutions which performed more lung cancer surgery as a proportion of all surgery, and those with higher volumes of cancer surgery in general, had significantly lower odds of performing incomplete resections. Sensitivity analyses, in which we excluded patients with R2 and/or unspecified R-status, revealed results similar to our primary analyses (supplemental digital content Table 1.docx).
PATTERN OF ADJUVANT THERAPY USE
Radiotherapy was administered to 11,267 patients (10%) in the whole cohort, and 2335 (43.8%) of those with positive margins, in whom it was deployed preoperatively in 206 (8.8%), and postoperatively in 2104 (90.1%). Chemotherapy was administered to 29,845 patients (26.1%) in the whole cohort. This included 2697 (50.6%) patients with positive resection margins, in whom it was used pre-operatively in 277 (10.3%), and post-operatively in 2319 (86%).
NEOADJUVANT THERAPY AND INCIDENCE OF INCOMPLETE RESECTION
There was a strong association between the use of neoadjuvant therapy and the occurrence of resection with positive margins (Table 2). After adjusting for other factors associated with margin positivity, patients who received preoperative radiotherapy had a significantly greater likelihood of margin positivity than those who did not receive neoadjuvant therapy (adjusted Odds Ratio [aOR] 1.59, 95% confidence interval [CI], 1.17–2.15, p=0.003.
Table 2.
Pre-operative adjuvant therapy and the likelihood of margin positive resection.
|
|
|||||
|---|---|---|---|---|---|
| Total N | Margin Positive | Likelihood of Margin Positive | |||
| N (%) | p value | aOR* (95% CI) | p value | ||
| Pre-operative radiation | <.001 | ||||
| Yes | 590 | 51(8.6) | 1.59(1.17–2.15) | 0.003 | |
| No | 107660 | 4896(4.6) | 1 | ||
| Pre-operative chemotherapy | <.001 | ||||
| Yes | 1640 | 124(7.6) | 1.19(0.98 –1.45) | 0.08 | |
| No | 107660 | 4896(4.6) | 1 | ||
| Pre-operative chemoradiation | <.001 | ||||
| Yes | 1653 | 151(9.1) | 1.17(0.98–1.41) | 0.09 | |
| No | 107660 | 4896(4.6) | 1 | ||
Odds ratio adjusted for age at diagnosis, gender, race/ethnicity, insurance, median income level, urban/rural, histology, tumor grade, tumor size, primary site, T stage, N stage, Surgical type, facility type, proportion of Medicaid/Uninsured, proportion of Lung Cancer surgery, Volume of cancer surgery
SURVIVAL IMPACT OF INCOMPLETE RESECTION
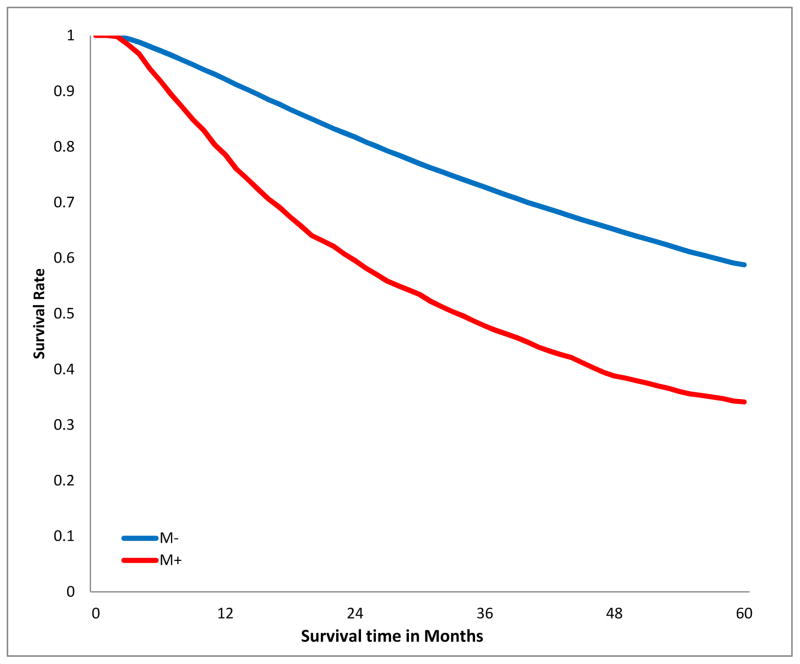
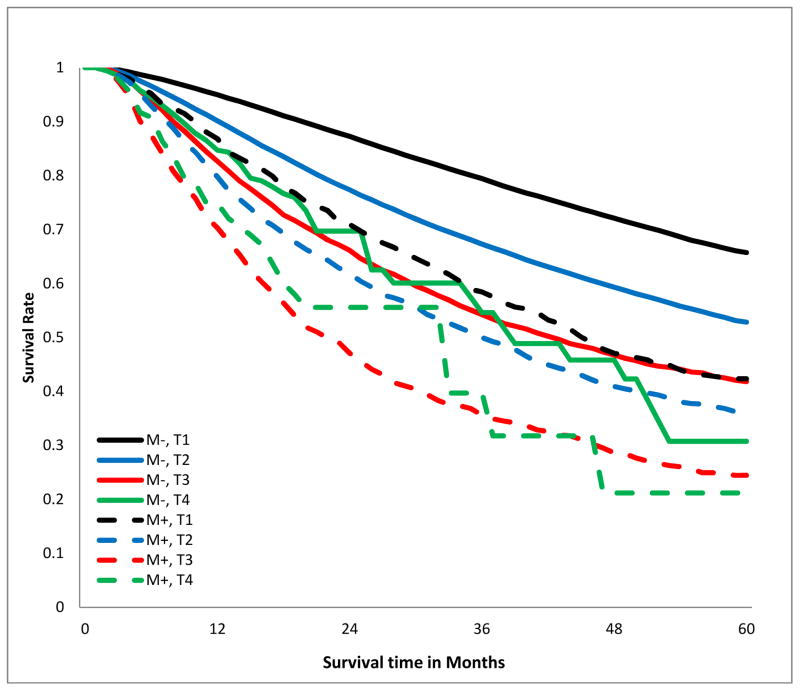
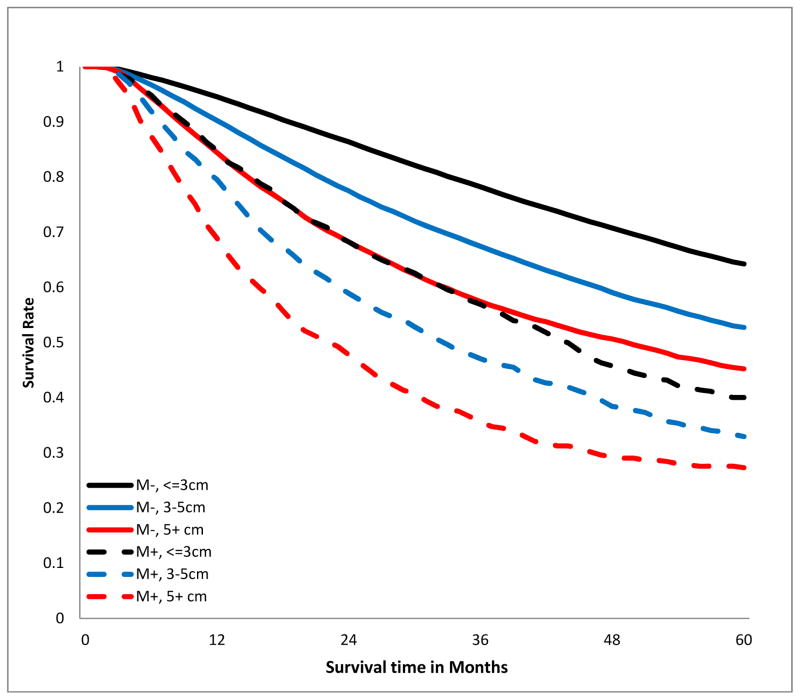
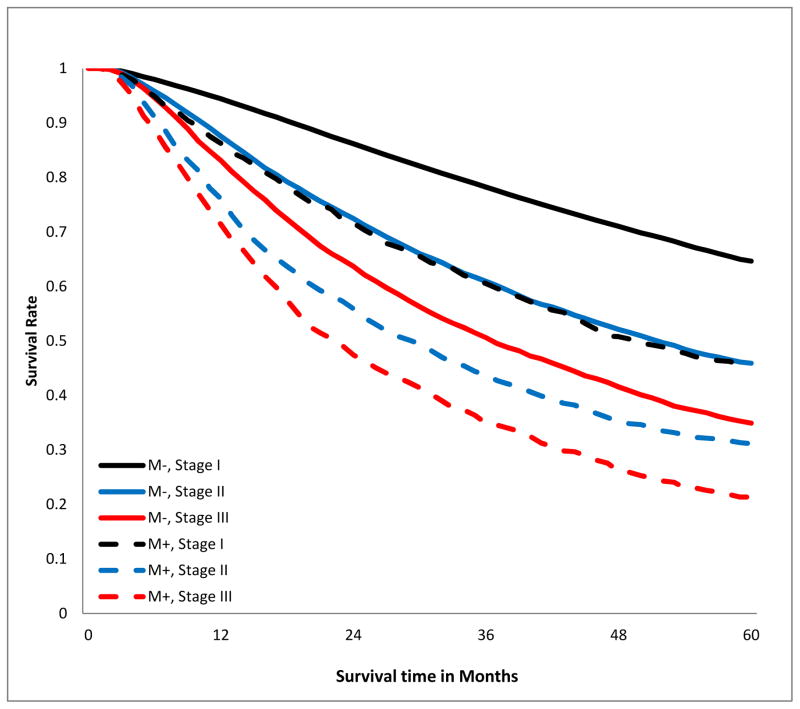
The crude overall 5-year survival rate of patients with R0 resection was 58.5%, compared to 33.8% in those with positive margins, log-rank p<0.001, Fig 2a). This survival difference persisted when patients were stratified by T-category (Fig 2b), tumor size (Fig 2c), and aggregate AJCC stage (Fig 2d). Notably, margin-positive patients with pT1 disease had a survival curve overlapping that of patients with pT3 disease who had an R0 resection (Fig 2b). The survival curve of patients with margin-positive stage I disease overlapped that of patients with stage II who had an R0 resection. Patients with incompletely resected stage II disease had worse survival than those with completely resected stage III disease (Fig 2d). The survival detriment was consistent at 1, 3, and 5 years (supplemental digital content Table 2.docx).
Figure 2.
Kaplan-Meier estimates of 5-year overall survival curves stratified by margin status. A.)Crude; B.) stratified by T-category; C.) stratified by tumor size; D.) stratified by American Joint Committee on Cancer aggregate Tumor Node Metastasis stage. M−, margin-negative patients; M+ margin-positive patients.
Sensitivity analyses, in which margin-positive patients with R1 resection were analyzed separately from those with R2 and unspecified R-status revealed similar results (supplemental digital content Table 3.docx). Results were also similar irrespective of whether eligibility was limited to patients surviving past post-operative day 30, 60, or 90 (supplemental digital content Table 4).
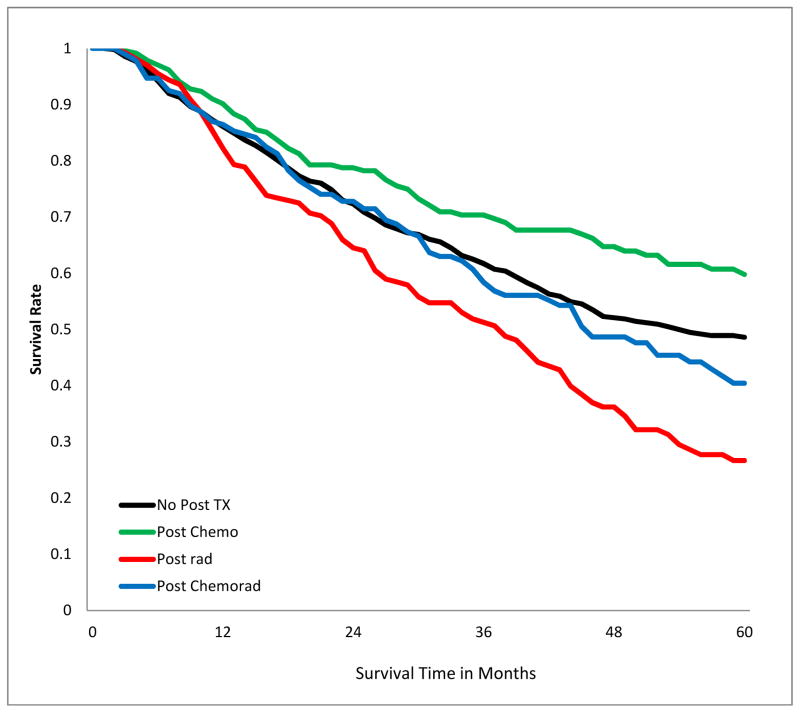
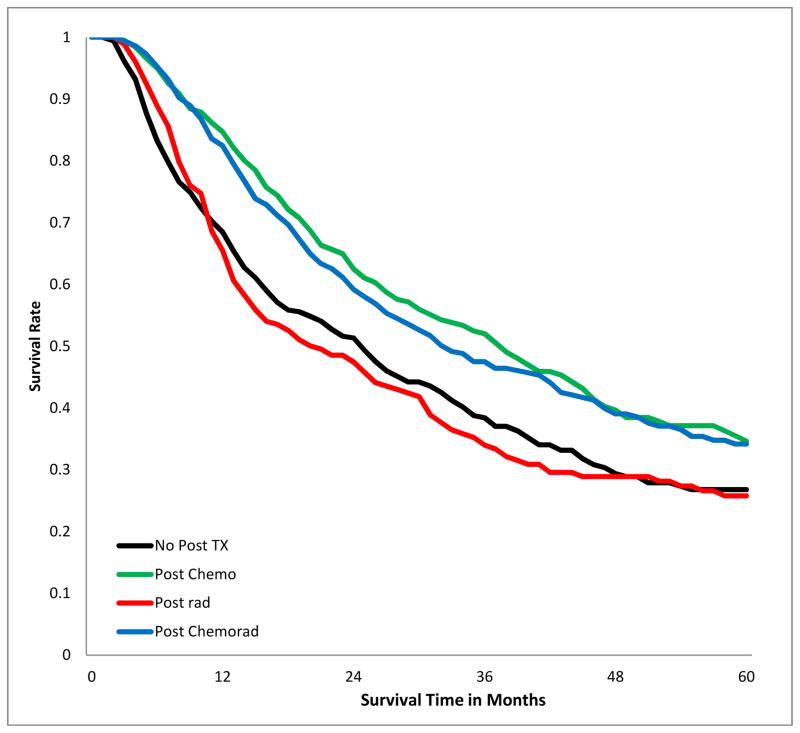
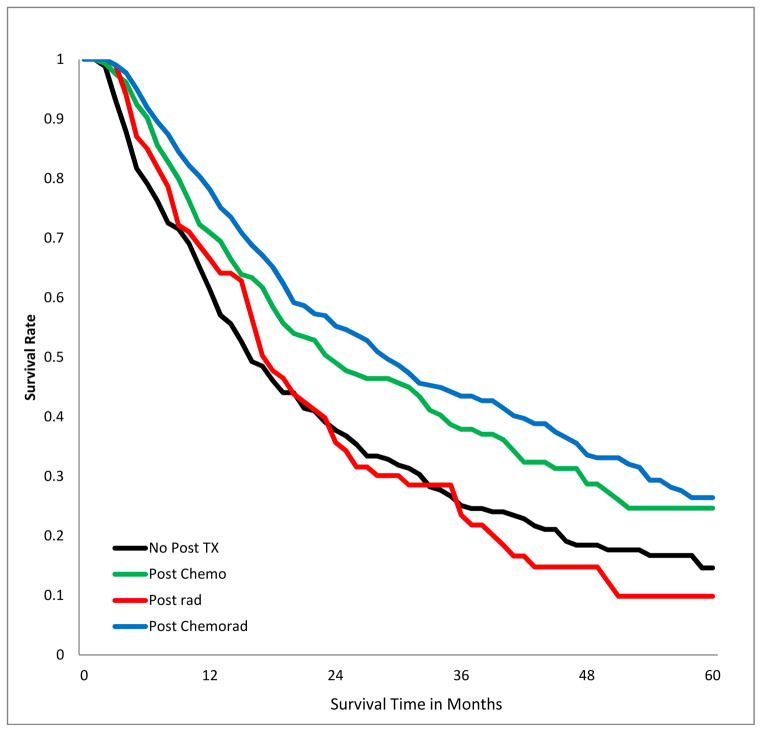
SURVIVAL IMPACT OF POST-OPERATIVE ADJUVANT THERAPY IN PATIENTS WITH POSITIVE MARGINS
The 5-year overall survival of patients with positive margins varied by stage and treatment modality (Fig 3a–c, Table 3). Receipt of chemotherapy was associated with better survival, irrespective of stage. Radiotherapy was associated with significantly worse survival in patients with stage I (p<.001), had no significant impact in patients with stage II and III disease. Chemo-radiation had no significant impact in patients with stage I, but was associated with improved survival in patients with stage II and III disease (p<0.001). The results were similar when margin-positive patients with R1 resection were analyzed separately from those with R2 or unspecified R-status (supplemental digital content Table 5.docx), and also when the analysis population excluded patients who died within 30, 60, and 90 days post201 operatively (supplemental digital content Table 6.docx).
Figure 3.
Kaplan-Meier estimates of 5-Year overall survival of patients with incomplete lung cancer resection, stratified by post-operative adjuvant treatment and Stage. A) stage I patients; B) stage II; C) stage III.
Table 3.
Five-year overall survival rates after incomplete lung cancer resection, stratified by stage and post-operative treatment.
| Five-year Overall Survival Rate | |||||||
|---|---|---|---|---|---|---|---|
| Stage | No adjuvant therapy | Chemotherapy | P* | Radiotherapy | P* | Chemo-radiation | P* |
| I | 0.49 | 0.60 | 0.008 | 0.27 | <0.001 | 0.40 | 0.34 |
| II | 0.27 | 0.35 | <0.001 | 0.26 | 0.66 | 0.34 | <0.001 |
| III | 0.15 | 0.25 | <0.001 | 0.10 | 0.97 | 0.26 | <0.001 |
Log-rank survival comparison to similar stage patients without adjuvant therapy.
DISCUSSION
The goal of all curative-intent surgery in oncology is to achieve a disease-free plane of tissue at the microscopic margin of resection, thereby increasing assurance that no disease has been left behind. The adverse survival impact of resection with positive margins has been confirmed for many different types of cancer. Indeed, the rate of resection with positive margins has been proposed as a marker of the quality of rectal cancer care.24 Since at least 2004, the College of American Pathologists has mandated inclusion of the resection margin status in all lung cancer resection pathology reports. Tacit acknowledgement of the negative impact of incomplete resection of lung cancer has stimulated development of algorithms for the care of such patients.3
However, the lung cancer literature has been ambiguous on the prognostic implications of margin involvement. Some studies suggest a negative impact on survival,15,16,18,20–22,25–33 others suggest little, or no, impact.5,10,17,19,34–36 Furthermore, the role of postoperative adjuvant therapy in this situation is unclear. Most reports indicate no benefit from adjuvant radiotherapy.15–22,30,32 Some even recommended it, despite evidence of detrimental effects.20,31 All previous reports were small, single-institutional studies, ranging in size from 4 to 216 cases. Indeed, the cumulative number of cases in the 28 English-language reports from 1945 to 2010 is approximately 1,227.6,10,11,15–22,25–38 Our 8-year NCDB cohort is >4-fold the sum of cases in all previous English-language reports.
The size of our cohort has enabled us to definitively address several open questions. The reported margin-positive resection rate ranged from 1.2 to 17% in the existing literature.10,38 Our dataset, representing a heterogeneous group of institutions covering >70% of the US population, establishes an aggregate annual margin-positive rate consistently under 6%. In addition, we have identified factors associated with the risk of incomplete resection, quantified its negative survival impact, and provided data on the benefit of postoperative adjuvant therapy in this situation.
Consistent with previous reports, we found that the risk of incomplete resection advances with tumor stage.11,16,21,22,32,38 However, contrary to prior reports, we show that resection with positive margins is not rare in patients with stage I and II non-small-cell lung cancer.16 These patients represented 35.5% and 36.5% of our cohort. Also contrary to previous reports, the negative survival impact of incomplete resection was independent of stage.21,22,30 Indeed, the impact of incomplete resection was equivalent to at least 1 level of AJCC stage advancement. So, stage I non-R0 patients’ survival was similar to stage II R0 patients, and stage II non-R0 patients’ survival was worse than stage IIIA R0 patients (Fig 2D).
We clarify that squamous cell histology is associated with a higher risk for incomplete resection than adenocarcinoma, contrary to certain previous reports.16,38 This is probably related to the generally more proximal location of these tumors. Patients who undergo lobectomy, bilobectomy or pneumonectomy are at relatively similarly lower risk than recipients of sublobar resection. The association between use of preoperative adjuvant therapy and incomplete resection is probably an example of ‘confounding by indication’. Patients deemed by clinical staging parameters to be at high risk for incomplete resection are more likely to be offered preoperative adjuvant therapy.
We found a strong association between non-clinical characteristics and the risk of incomplete resection. Resection margin status appears to be a lung cancer healthcare outcome disparity, associated with patient demographic and institutional characteristics. The specific patient, institutional and provider-level practices driving this association need to be elucidated, to provide a pathway to meaningful quality improvement.39 We can only speculate on the reasons for this association. One possibility is that certain patients seek care from less proficient providers with access to fewer resources. For example, racial minorities seek care from less well-trained providers, who are less able to provide high-quality care, and who have less access to high-quality specialists, diagnostic imaging, and non-emergency care.40 Reducing the negative impact of incomplete resection requires incidence-reduction by eliminating modifiable contributory practices, and post-incident risk-mitigation.
Irrespective of stage, postoperative adjuvant chemotherapy is associated with reduced mortality risk after incomplete resection, suggesting the need to consider it for all such patients. However, a key finding of this study is that, contrary to current recommendations, postoperative adjuvant radiation may be profoundly harmful to patients with incompletely resected stage I non-small-cell lung cancer.3,11 Clinical trials to confirm these findings, and identify the best adjuvant therapy options for the different subsets of patients with incomplete resection, would be ideal. Such trials will be difficult to conduct, partly because providers and institutions with access to large numbers of eligible patients are unlikely to be actively engaged in clinical trials. However, this may be a suitable challenge for the National Cancer Institute’s Community Oncology Research Program.
Our study is limited by the retrospective nature of the analysis, and the unavailability of some important details about institutional practices (such as intraoperative use of frozen section pathology examination). In addition, we lack information on the anatomic site of margin involvement, the proportion of margin-positive cases with carcinoma in-situ at the margin, and disease recurrence patterns. Further, it is unclear in 39% of cases if the resection was R1 or R2, although our sensitivity analysis suggests that most were probably R1 resection cases. In any case, our results are consistent, even when the known R1 cases are analyzed separately from the R2 and unspecified R cases. Finally, we lack information on the criteria for selection of the various adjuvant therapy options. Nevertheless, the size of the dataset has enabled us to resolve the decades-long debate about the survival impact of incomplete lung cancer resection, and provides evidentiary guidance for developers of clinical management algorithms. Future work should identify causal links with provider and institutional practice. The adjuvant therapy options after incomplete resection of non-small-cell lung cancer should also be subjected to prospective clinical trials.
Supplementary Material
Supplemental digital content Table 1: Sensitivity analysis excluding R2 or undetermined R patients in predicting the likelihood of margin positive
Supplemental digital content Table 2. Survival rates of patients with complete and incomplete lung cancer resections at 1, 3, and 5 years.
Supplemental digital content Table 3: sensitivity analysis. Survival rates of patients with complete and incomplete lung cancer resections separated by R1, R2+R1/R2 unknown status at 1, 3, 5 years.
Supplemental digital content Table 4: sensitivity analysis. Impact of limiting analysis to patients surviving past post-operative day 30, 60, and 90 on 1, 3, and 5 year overall survival rates of patients with and without positive resection margins unstratified cohort. The proportion of patients with positive margins in the 30, 60, or 90-day post-operative survival cohorts was 4.76%, 4.72%, and 4.65%, respectively.
Supplemental Table 5: sensitivity analysis. Five-year overall survival rates after incomplete lung cancer resection with R1 cases analyzed separately from cases with R2+R1/R2 unknown status. Data stratified by stage and post-operative treatment.
Supplemental digital content Table 6: sensitivity analysis. Impact of limiting analysis to patients surviving past post-operative day 30, 60, and 90 on the five-year overall survival rates after incomplete lung cancer resection, stratified by stage and post-operative treatment.
Acknowledgments
This study used the National Cancer Data Base (NCDB). The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the American College of Surgeons and the Commission on Cancer in the creation of the National Cancer Data Base. The data used in the study are derived from a de-identified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the authors.
RUO was partially supported by RO1CA172253 and PCORI IH-1304-6147. CCL and AJ were supported by American Cancer Society Intramural Research Department.
This work was (partially) supported through a Patient-Centered Outcomes Research Institute (PCORI) Award (IH-1304-6147).
Footnotes
Dr. Osarogiagbon has served as a consultant for Roche/Genentech, and speaker for Pfizer and Roche/Genentech. All other authors have no conflicts of interest.
DISCLAIMER: All statements in this report, including its findings and conclusions, are solely those of the authors and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute (PCORI), its Board of Governors or Methodology Committee.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Pfannschmidt J, Muley T, Bulzebruck H, et al. Prognostic assessment after surgical resection for non-small cell lung cancer: experiences in 2083 patients. Lung Cancer. 2007;55(3):371–377. doi: 10.1016/j.lungcan.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 3.Non-Small Cell Lung Cancer. [Accessed December 16, 2014];NCCN Guidelines Version 2.2015. http://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf.
- 4.Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1N0 non-small cell lung cancer. Ann Thorac Surg. 1995;60(3):615–23. doi: 10.1016/0003-4975(95)00537-u. [DOI] [PubMed] [Google Scholar]
- 5.Shields TW. The “incomplete” resection. Ann Thorac Surg. 1989;47(4):487–8. doi: 10.1016/0003-4975(89)90419-0. [DOI] [PubMed] [Google Scholar]
- 6.Lacasse Y, Bucher HC, Wong E, et al. “Incomplete resection” in non-small cell lung cancer: need for a new definition. Ann Thorac Surg. 1998;65(1):220–6. doi: 10.1016/s0003-4975(97)01190-9. [DOI] [PubMed] [Google Scholar]
- 7.Rami-Porta R, Wittekind C, Goldstraw P. Complete resection in lung cancer surgery: proposed definition. Lung Cancer. 2005;49(1):25–33. doi: 10.1016/j.lungcan.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Osarogiagbon RU, Yu X. Mediastinal lymph node examination and survival in resected early stage non-small cell lung cancer in the Surveillance, Epidemiology and End Results Program database. J Thorac Oncol. 2012;7(12):1798–1806. doi: 10.1097/JTO.0b013e31827457db. [DOI] [PubMed] [Google Scholar]
- 9.Osarogiagbon RU, Yu X. Nonexamination of lymph nodes and survival after resection of non-small cell lung cancer. Ann Thorac Surg. 2013;96(4):1178–89. doi: 10.1016/j.athoracsur.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 10.Lequaglie C, Conti B, Brega Massone PP, et al. Unsuspected residual disease at the resection margin after surgery for lung cancer: fate of patients after long-term follow up. Eur J Cardiothorac Surg. 2003;23(2):229–32. doi: 10.1016/s1010-7940(02)00750-9. [DOI] [PubMed] [Google Scholar]
- 11.Wind J, Smit EJ, Senan S, et al. Residual disease at the bronchial stump after curative resection for lung cancer. Eur J Cardiothorac Surg. 2007;32(1):29–34. doi: 10.1016/j.ejcts.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 12.PORT Meta-analysis Trialist Group. Postoperative radiotherapy in non-small-cell lung cancer: systematic review and meta-analysis of individual patient data from nine randomized controlled trials. Lancet. 1998;352(9124):267–63. [PubMed] [Google Scholar]
- 13.The International Adjuvant Lung Cancer Trial Collaborative Group. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. 2004;350(4):351–60. doi: 10.1056/NEJMoa031644. [DOI] [PubMed] [Google Scholar]
- 14.Winton T, Livingston R, Johnson D, et al. Vinorelbine plus Cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med. 2005;352(25):2589–97. doi: 10.1056/NEJMoa043623. [DOI] [PubMed] [Google Scholar]
- 15.Law MR, Hodson ME, Lennox SC. Implications of histologically reported residual tumour on the bronchial margin after resection for bronchial carcinoma. Thorax. 1982;37(7):492–95. doi: 10.1136/thx.37.7.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaiser LR, Fleshner P, Keller S, et al. Significance of extramucosal residual tumor at the bronchial resection margin. Ann Thorac Surg. 1989;47(2):265–9. doi: 10.1016/0003-4975(89)90284-1. [DOI] [PubMed] [Google Scholar]
- 17.Gebitekin C, Gupta NK, Satur CMR, et al. Fate of patients with residual tumour at the bronchial resection margin. E J Cardio-thoracic Surg. 1995;8(7):339–44. doi: 10.1016/1010-7940(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 18.Snijder RJ, de la Riviere AB, Elbers HJJ, et al. Survival in resected stage I lung cancer with residual tumor at the bronchial resection margin. Ann Thorac Surg. 1998;65(1):212–6. doi: 10.1016/s0003-4975(97)01114-4. [DOI] [PubMed] [Google Scholar]
- 19.Ghiribelli C, Voltolini L, Paladinin P, et al. Treatment and survival after lung resection for non-small cell lung cancer in patients with microscopic residual disease at the bronchial stump. E J Cardio-thorac Surg. 1999;16(5):555–9. doi: 10.1016/s1010-7940(99)00310-3. [DOI] [PubMed] [Google Scholar]
- 20.Massard G, Doddoli C, Gasser B, et al. Prognostic implications of a positive bronchial resection margin. E J Cardio-thorac Surg. 2000;17(5):557–65. doi: 10.1016/s1010-7940(00)00384-5. [DOI] [PubMed] [Google Scholar]
- 21.Passlick B, Sitar I, Sienel W, et al. Significance of lymphangiosis carcinomatosa at the bronchial resection margin in patients with non-small cell lung cancer. Ann Thorac Surg. 2001;72(4):1160–4. doi: 10.1016/s0003-4975(01)03067-3. [DOI] [PubMed] [Google Scholar]
- 22.Riquet M, Achour K, Foucault C, et al. Microscopic residual disease after resection for lung cancer: a multifaceted but poor factor of prognosis. Ann Thorac Surg. 2010;89(3):870–5. doi: 10.1016/j.athoracsur.2009.11.052. [DOI] [PubMed] [Google Scholar]
- 23. [Accessed March 15, 2015];National Cancer Data Base. https://www.facs.org/quality%20programs/cancer/ncdb.
- 24.Massarweh NN, Hu C, You YN, et al. Risk-adjusted pathologic margin positivity rate as a quality indicator in rectal cancer surgery. J Clin Oncol. 2014;32(27):2967–74. doi: 10.1200/JCO.2014.55.5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hughes RK, Tildon TT. Prognosis of residual invasive cancer at the margin of bronchial resection. Ann Thorac Surg. 1966;2(1):102–5. doi: 10.1016/s0003-4975(10)66545-9. [DOI] [PubMed] [Google Scholar]
- 26.Soorae AS, Stevenson HM. Survival with residual tumor on the bronchial margin after resection for bronchogenic carcinoma. J Thorac Cardovasc Surg. 1979;78(2):175–80. [PubMed] [Google Scholar]
- 27.Liewald F, Hatz RA, Dienemann H, et al. J Thorac Cardiovasc Surg. 1992;104(2):408–12. [PubMed] [Google Scholar]
- 28.Miller DL, McManus KG, Allen MS, et al. Results of surgical resection in patients with N2 non-small cell lung cancer. Ann Thorac Surg. 1994;57(5):1095–100. doi: 10.1016/0003-4975(94)91335-8. [DOI] [PubMed] [Google Scholar]
- 29.Tan KK, Kennedy MM, Kerr KM, et al. Patient survival and bronchial resection line status in primary lung carcinoma. Thorax. 1995;50:437P. [Google Scholar]
- 30.Dienemann H, Trainer C, Hoffmann H, et al. Incomplete resection of lung cancer: morbidity and prognosis. Chirurg. 1997;68(10):1014–19. doi: 10.1007/s001040050313. [DOI] [PubMed] [Google Scholar]
- 31.Fang D, Zhang D, Huang G, et al. Results of surgical resection of patients with primary lung cancer: a retrospective analysis of 1,905 cases. Ann Thorac Surg. 2001;72(4):1155–9. doi: 10.1016/s0003-4975(01)02932-0. [DOI] [PubMed] [Google Scholar]
- 32.Hofmann HS, Taege C, Lautenschlager C, et al. Microscopic (R1) and macroscopic (R2) residual disease in patients with resected non-small cell lung cancer. E J Cardio-thorac Surg. 2002;21(4):606–10. doi: 10.1016/s1010-7940(02)00030-1. [DOI] [PubMed] [Google Scholar]
- 33.Rena O, Oliaro A, Cavallo A, et al. Stage I non-small cell lung carcinoma: really an early stage? Eur J Cardiothorac Surg. 2002;21(3):514–19. doi: 10.1016/s1010-7940(01)01153-8. [DOI] [PubMed] [Google Scholar]
- 34.Jeffery RM. Survival in bronchial carcinoma. Tumor remaining in the bronchial stump following resection. Ann Roy Coll Surg Engl. 1972;51(1):55–59. [PMC free article] [PubMed] [Google Scholar]
- 35.Heikkila L, Harjula A, Suomalainen RJ, et al. Residual carcinoma in bronchial resection line. Annales Chirurgiae et Gynaecologiae. 1986;75(3):151–54. [PubMed] [Google Scholar]
- 36.Whyte RI, Kapalan DK, Sharpe JA, et al. Carcinoma of the bronchus with unsuspected microscopic resection-line involvement. Cancer. 1988;62(5):1014–6. doi: 10.1002/1097-0142(19880901)62:5<1014::aid-cncr2820620529>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 37.Cotton RE. The bronchial spread of lung cancer. Brit J Dis Chest. 1959;53(2):142–150. doi: 10.1016/s0007-0971(59)80004-8. [DOI] [PubMed] [Google Scholar]
- 38.Kayser K, Anyanwu E, Bauer HG, et al. Tumor presence at resection boundaries and lymph-node metastasis in bronchial carcinoma patients. Thorac Cardiovasc Surgeon. 1993;41(5):308–11. doi: 10.1055/s-2007-1013878. [DOI] [PubMed] [Google Scholar]
- 39.Donabedian A. The quality of care. How can it be assessed? JAMA. 1988;260(12):1743–8. doi: 10.1001/jama.260.12.1743. [DOI] [PubMed] [Google Scholar]
- 40.Bach PB, Pham HH, Schrag D, et al. Primary care physicians who treat blacks and whites. N Engl J Med. 2004;351(6):575–84. doi: 10.1056/NEJMsa040609. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental digital content Table 1: Sensitivity analysis excluding R2 or undetermined R patients in predicting the likelihood of margin positive
Supplemental digital content Table 2. Survival rates of patients with complete and incomplete lung cancer resections at 1, 3, and 5 years.
Supplemental digital content Table 3: sensitivity analysis. Survival rates of patients with complete and incomplete lung cancer resections separated by R1, R2+R1/R2 unknown status at 1, 3, 5 years.
Supplemental digital content Table 4: sensitivity analysis. Impact of limiting analysis to patients surviving past post-operative day 30, 60, and 90 on 1, 3, and 5 year overall survival rates of patients with and without positive resection margins unstratified cohort. The proportion of patients with positive margins in the 30, 60, or 90-day post-operative survival cohorts was 4.76%, 4.72%, and 4.65%, respectively.
Supplemental Table 5: sensitivity analysis. Five-year overall survival rates after incomplete lung cancer resection with R1 cases analyzed separately from cases with R2+R1/R2 unknown status. Data stratified by stage and post-operative treatment.
Supplemental digital content Table 6: sensitivity analysis. Impact of limiting analysis to patients surviving past post-operative day 30, 60, and 90 on the five-year overall survival rates after incomplete lung cancer resection, stratified by stage and post-operative treatment.