Abstract
Brain edema is an important complication of acute hepatic encephalopathy (AHE), and astrocyte swelling is largely responsible for its development. Elevated blood and brain ammonia levels have been considered as major etiological factors in this edema. In addition to ammonia, recent studies have suggested that systemic infection, inflammation (and associated cytokines), as well as endotoxin (lipopolysaccharide, LPS) are also involved in AHE-associated brain edema. As endothelial cells (ECs) are the first resident brain cells exposed to blood-borne “noxious agents” (i.e., ammonia, cytokines, LPS) that are present in AHE, these cells may be in a critical position to react to these agents and trigger a process resulting in astrocyte swelling/brain edema. We therefore examined the effect of conditioned media (CM) from ammonia, LPS and cytokine-treated cultured brain ECs on cell swelling in cultured astrocytes. CM from ammonia-treated ECs when added to astrocytes caused significant cell swelling, and such swelling was potentiated when astrocytes were exposed to CM from ECs-treated with a combination of ammonia, LPS and CKs. We also found an additive effect when astrocytes were exposed to ammonia along with CM from ammonia-treated ECs. Additionally, ECs treated with ammonia showed a significant increase in the production of oxy-radicals, nitric oxide, as well as evidence of oxidative/nitrative stress and activation of the transcription factor NF-κ B. CM derived from ECs treated with ammonia, along with antioxidants or the NF-κB inhibitor BAY 11-7082, when added to astrocytes resulted in a significant reduction in cell swelling, as compared to the effect of CM from ECs-treated only with ammonia. We also identified increased nuclear NF-κB expression in rat brain cortical ECs in the thioacetamide model of AHE. These studies suggest that endothelial cells significantly contribute to the astrocyte swelling/brain edema in AHE, likely as a consequence of oxidative/nitrative stress and activation of NF-κB.
Keywords: Acute hepatic encephalopathy, astrocyte swelling, brain endothelial cells, inflammation, lipopolysaccharide, oxidative/nitrative stress
1. Introduction
Brain edema is the major neurological complication of acute hepatic encephalopathy (AHE), and astrocyte swelling appears to play a major role in this process (Martinez, 1968; Norenberg, 1977, 2001; Traber et al., 1987; Kato et al., 1992). While the pathogenesis of brain edema in AHE is incompletely understood, ammonia has been strongly implicated in its development as ammonia has been shown to induce cell swelling in cultured astrocytes (Gregorios et al., 1985a, 1985b; Norenberg et al., 1991; Zwingmann et al., 2000), brain slices (Ganz et al., 1989; Zielinska et al., 2003), as well as in vivo (Voorhies et al., 1983; Norenberg, 1977; Blei et al., 1994; Willard-Mack et al., 1996).
In addition to ammonia, recent studies suggest the involvement of sepsis and inflammation in the mechanism of the brain edema in AHE (Rolando et al., 2000). Induction of endotoxemia by the administration of lipopolysaccharide (LPS) was shown to exacerbate the brain edema in an experimental model of AHE (Wright et al., 2007), suggesting that infection and inflammation (and associated cytokines) also contribute to the astrocyte swelling/brain edema in AHE. As ECs are the first resident brain cells exposed to blood-borne “noxious agents” (i.e., ammonia, cytokines, LPS), it is possible that all of these agents trigger a process in ECs that ultimately results in astrocyte swelling/brain edema in AHE. Moreover, as LPS does not cross the blood-brain barrier (Chung et al., 2010), the means by which LPS exerts its effect on brain edema likely occurs through an influence on brain endothelial cells (ECs).
While not generally thought of as “inflammatory” cells, ECs are indeed capable of activating a plethora of inflammatory factors [inducible nitric oxide synthase (iNOS), NADPH oxidase (NOX), phospholipase A2 (PLA2), cyclooxygenase-2 (COX2), as well as the transcription factor nuclear factor kappa B (NF-κB)]. Activation of these factors results in the production of “cell swelling mediators”, including arachidonic acid (AA), reactive oxygen/nitrogen species (RONS), prostaglandins, cytokines (CKs), and chemokines (for review, see Feuerstein et al., 1998). iNOS gene expression and NO production are particularly important events in brain ECs during systemic inflammation (Trickler et al., 2005; see also Feuerstein et al., 1998 for review), that could potentiate the direct effect of ammonia on astrocyte swelling/brain edema in AHE. Additionally, receptors for CKs such as IL-1β and TNFα, have been identified in brain ECs in experimental models of sepsis (Hashimoto et al., 1991), and activation of these receptors may further stimulate the synthesis and release of additional CKs (Matsumura and Kobayashi, 2004) that may exacerbate the brain edema in AHE.
While LPS and CKs are known to influence brain ECs resulting in the production of inflammatory factors and potential cell swelling mediators, there is a dearth of information regarding the effect of ammonia on ECs in AHE. Alterations in amino acid transport, inhibition of cGMP and Ca2+ accumulation have been documented in ammonia-treated brain ECs (James et al., 1978; Zanchin et al., 1979; Cardelli-Cangiano et al., 1984; Hilgier et al., 1992; Konopacka et al., 2008). However, there is currently no evidence pertaining to a role of brain ECs in the mechanism of astrocyte swelling/brain edema in AHE. This study therefore examined the potential involvement of brain ECs on astrocyte swelling in response to ammonia, cytokines and LPS.
2. Materials and Methods
2.1 Astrocyte cultures
Primary cultures of cortical astrocytes were prepared as described previously (Ducis et al. 1990). Briefly, neonatal (1–2 day old rat pups) brain cortices were minced in ice-cold Hank’s balanced salt solution with 15% fetal bovine serum. The tissue suspension was vortexed for 25 seconds; passed through 75 μm and 10 μm filters and centrifuged for 5 min at 1,500 rpm. The supernatants were removed and the cells were resuspended in Dulbecco’s modified Eagle’s medium containing 15% FBS, penicillin/streptomycin and Fungizone. Cells were grown on 35-mm culture dishes and maintained at 37°C in a humidified 5% CO2 incubator. Culture media was changed twice weekly. On day 10 post-seeding, FBS was replaced with 10% horse serum. After 14 days, cultures were treated with 0.5 mM dibutyryl cAMP to enhance cellular differentiation (Juurlink and Hertz, 1985). Cultures consisted of at least 95% astrocytes as determined by glial fibrillary acidic protein (GFAP) immunohistochemistry. All cultures used were 25–28 days old. All animal procedures followed guidelines established by the National Institute of Health Guide for the Care and Use of Laboratory Animals and were approved by our Institutional Animal Care and Use Committee (IACUC).
The use of ammonia-treated cultured astrocytes as a model for hepatic encephalopathy is highly appropriate since substantial evidence invokes the role of ammonia in the pathogenesis of hepatic encephalopathy (Albrecht and Jones, 1999; Hazell and Butterworth, 1999), and astrocytes are the principal cells affected in this condition (Norenberg, 1981, 1992, 1998). Moreover, many of the findings occurring in hepatic encephalopathy in vivo are also observed in these cultures, including characteristic morphologic changes, cell swelling, defects in glutamate transport, up-regulation of the peripheral benzodiazepine receptor (recently renamed the 18-kDa translocator protein), reduction in levels of glial fibrillary acidic protein and myo-inositol, disturbance in energy metabolism, and evidence of oxidative/nitrosative stress (for review, see Norenberg et al., 2009).
2.2. Endothelial cell cultures
Primary cultures of brain endothelial cells (ECs) were prepared from adult (300 gm) male Wistar rats following the method of Bowman et al. (1983). Briefly, cerebral cortices were removed from rat brain, finely minced at room temperature and incubated in Dispase solution (0.8% Dispase in DMEM/F12 containing antibiotics) for 2 h at 37°C in a shaking water bath. Following incubation, cells were gently triturated and centrifuged at 5000 g for 30 min. The pellet containing the microvessels were washed once in DMEM/F12, transferred onto a 30% dextran gradient and centrifuged at 6000 g for 30 min. The pellet was digested for another 2.5 h at 37°C with collagenase-Dispase solution (1 mg/ml in DMEM/F12 containing antibiotics). The cell suspension was carefully layered on a continuous 50% Percoll gradient and centrifuged at 1000 g for 10 min at RT. The band of endothelial cells was aspirated, washed twice in DMEM/F12, and then plated onto rat-tail collagen-coated 60-mm plastic dishes (Fisher Scientific, Springfield, NJ). Cultures were used for experimentation at day 14. The purity of the cultures was determined by immunocytochemistry using the endothelial marker von Willebrand factor (Factor VIII). Cultures consisted of at least 97% endothelial cells.
2.3. Cell volume measurement
Astrocyte cell volume (intracellular water space) was determined using 3-O-methyl-[3H]-glucose (OMG) equilibration method (Kletzein et al., 1975), as modified for astrocyte cultures by Bender and Norenberg (1998). Briefly, cultures were incubated with [3H]OMG (NEN, Life sciences, Boston MA) (1 mM containing 1 μCi of radioactive OMG) for 12 h, and at the end of incubation a small aliquot of medium was saved for specific activity determination. Cultures were quickly washed five times with ice-cold buffer containing 290 mM sucrose, 1 mM Tris-nitrate (pH 7.4), 0.5 mM calcium nitrate, and 0.1 mM phloretin. Cells were then lysed in 0.5 ml of 1 N sodium hydroxide. Radioactivity was converted to intracellular water space and expressed as μl/mg protein. Protein content was determined employing the bicinchoninic acid assay (Pierce, Rockford, IL, USA).
2.4. Measurement of reactive oxygen species (ROS)
ROS production was measured using the fluorescent probe dihydrorhodamine 123 (DHR) as described previously (Grzelak et al., (2001). Briefly, ECs were treated with ammonia and at different time points after treatment, 20 μl of culture medium was added to 1 ml phosphate buffered saline and mixed well with 20 μM DHR and incubated for 15 min at 37°C. At the end of each incubation, the fluorescence in the culture media was measured at an excitation wavelength of 485 nm and emission wavelength of 535 nm.
2.5. Measurement of nitric oxide (NO)
NO released into the culture medium was measured by the Griess reaction (which detect total nitrite in the sample) using 1% sulfanilamide/0.l% naphthyethylenediamine dihydrochloride/2.5% phosphoric acid (H3PO4), as described previously (Grisham et al., 1996).
2.6. Determination of oxidized and nitrated proteins in ammonia-treated ECs
Oxy/nitro protein adducts (an index of protein oxidation and nitration) were determined by using the OxyBlot™ protein oxidation detection kit (S7150; Chemicon International) as previously described (Jayakumar et al., 2008). Briefly, equal amounts of cell lysates were subjected to gel electrophoresis, and immunoblotting was performed as previously described (Jayakumar et al., 2008). DNP antibody (1:350 dilution, OxyBlot™ protein oxidation detection kit) was used to detect oxidized proteins, and anti-nitrotyrosine (mouse monoclonal antibody, 1:1000 dilution, catalog number 487923; Calbiochem) was used to detect nitrated proteins.
2.7. Heme oxygenase-1 (HO-1) determination
HO-1 expression (a marker of oxidative stress) was measured as previously described (Méthy et al., 2004). Briefly, equal amounts of protein were subjected to gel electrophoresis and the proteins were transferred to polyvinylidene difluoride membranes. After blocking with bovine serum albumin, the membranes were incubated with rabbit anti-heme oxygenase-1 (1:2000, Millipore, CA) and mouse anti–α-tubulin antibodies (1:2000, Oncogene, CA). Anti-rabbit and anti-mouse horseradish peroxidase-conjugated secondary antibodies (Vector Laboratories) were used at 1:1000. The peroxidase activity was detected by chemiluminescence using the ECL detection system (Amersham, Arlington Heights, IL). Optical density of the bands was determined with the Chemi-Imager (Alpha Innotech Corp, San Leandro, CA) digital imaging system, and the results were quantified with the Sigma Scan Pro (Jandell Scientific, San Jose, CA) program as a proportion of the signal of a housekeeping protein band (α-tubulin).
2.8. Nuclear translocation (activation) of NF-κB
The nuclear extract was prepared as previously described (Sinke et al., 2008). In brief, after treatment with ammonia, ECs were harvested in 1 ml PBS and centrifuged at 735 g for 3 min at 4°C. The cell pellet was suspended in a buffer containing 10 mM HEPES (pH 7.9), 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 μM dithiothreitol and a complete protease inhibitor cocktail (Roche, Mannheim, Germany); incubated on ice for 15 min; 15 μl of 10% NP-40 (Roche Diagnostics Corp., Indianapolis, IN) was added and the sample vortexed thoroughly for 40 s and centrifuged at 735 g for 3 min at 4°C. The resulting nuclear pellet was resuspended in a buffer containing 20 mM HEPES (pH 7.9), 0.4 M NaCl, 1 mM EDTA, 1 mM EGTA, 1 μM dithiothreitol and protease inhibitors and centrifuged at 16,000 g for 5 min at 4°C. The supernatant was loaded onto SDS polyacrylamide gels and Western blots were performed as noted above.
Antibodies against NF-κB-p65 (H-286) (Santa Cruz Biotechnology Inc, Santa Cruz, CA) and the nuclear marker lamin (Cell Signaling Technology, Beverly, MA) were used at 1:750 and 1:1000 dilutions, respectively. Horseradish peroxidase-conjugated anti-rabbit secondary antibody (1:1000) (Vector Laboratories, Burlingame, CA) was used. Membranes were visualized using an enhanced chemiluminescence reagent. The optical density of the bands was measured with the Chemi-Imager digital imaging system as noted above, as a proportion of the signal of a housekeeping protein band (lamin A/C).
2.9. AHE model
The thioacetamide (TAA)-treated rat model of AHE was used in this study (Hilgier et al., 1983; Zimmermann et al., 1989; Rama Rao et al., 2010c; Jayakumar et al., 2011a). This model has been well established relative to the clinical status, liver function, blood–brain barrier integrity, and brain edema development (Zimmermann et al., 1989; Hilgier et al., 1996; Rama Rao et al., 2010c; Jayakumar et al., 2011b). In brief, rats were given TAA (300 mg/kg bwt) for 3 consecutive days. Animals were sacrificed 4 h after the last injection. To prevent hypoglycemia and dehydration, rats were given 12.5 ml/kg of fluid therapy (5% dextrose and 0.45% saline with 20 mEq/L of potassium chloride) every 12 hours, s.c. Normal controls received saline (vehicle used for TAA). Rats were clinically monitored at least twice a day and stages of encephalopathy were graded according to the criteria of Gammal et al. (1990).
2.10. Immunohistochemistry of nuclear NF-κB in a rat model of AHE
Control and TAA-treated rats (four animals each) were anesthetized at the end of TAA treatment and then transcardially perfused with heparinized saline for 1 min, followed by fixation in 4% paraformaldehyde for 15 min. After decapitation, heads were left in the same fixative for an additional 24 h. Brains were cryoprotected (12–24 h) in 30% sucrose, and 20 micron-thick cortical sections obtained with a cryostat were blocked with 10% goat serum and incubated overnight at 4°C with specific antibodies to the p65 subunit of NF-κB (1:200 dilution; Santa Cruz Biotech. Inc, Santa Cruz, CA) and Factor VIII (endothelial marker, 1:150 dilution Abcam, Cambridge, MA). Following incubation with primary antibodies, sections were washed with TBS containing 1% Tween-20 (TBS-T) and incubated with fluorescent HRP-conjugated secondary antibodies (1:500; AlexaFlour-Rhodamine for GFAP and Factor-VIII; Alexa Flour-FITC for NF-κB) for 2 h. Sections were then mounted with commercial mounting media (Vector Labs., Burlingame, CA) containing DAPI (nuclear stain). Immunofluorescent images were acquired with a Zeiss LSM510/UV Axiovert 200M confocal microscope with a plan apochromat 40x objective lens, and a 2x zoom resulting in images 125×125 μm in area and 1.0 μm optical slice thickness (1.0 Airy units for Alexa Fluor 546 or 568 emission channel). Random collection of images from sections of control and TAA-treated rats was achieved by systematically capturing each image in a blinded manner by moving the microscope stage approximately 5 mm in four different directions. At least 15 fluorescent images were captured per rat, and the images were merged to localize endothelial nuclear NF-κ B expression.
2.11. Statistical analysis
All experiments were repeated four to seven times using cells derived from different batches of astrocyte and endothelial cultures. The number of individual culture plates in each experimental group was five for astrocyte swelling, and six to eight plates for free radical measurement. Data of all experiments were subjected to ANOVA followed by Tukey’s post hoc comparisons. A value of p < 0.05 was considered significant. Error bars represent mean ± SEM.
3. Results
3.1. Conditioned media (CM) from ammonia-treated endothelial cells (ECs) increases cell swelling in cultured astrocytes
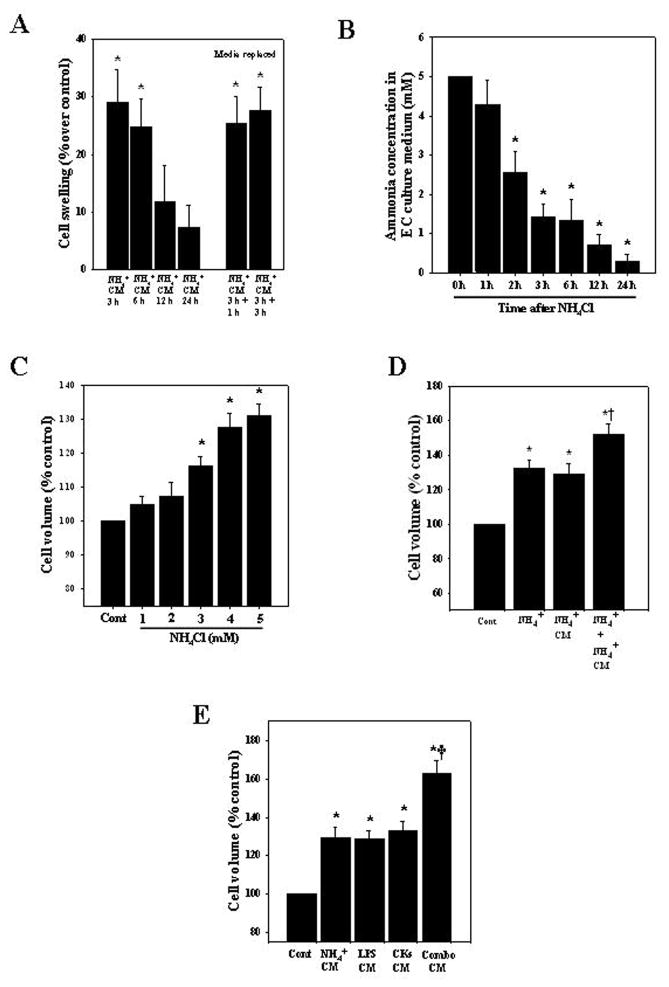
ECs were treated with ammonia (5 mM NH4Cl) for different time points (3, 6, 12 and 24 h) as this concentration has been observed in brains of experimental animals with AHE (Mans et al., 1979; Swain et al., 1992; Rama Rao et al., 2010a, 2010c). At the end of treatment, 2 ml of CM from ammonia-treated ECs was added to astrocyte cultures and cell volume was determined 24 h later. CM from ECs that were treated with ammonia for 3 and 6 h significantly increased cell volume by 29.1 and 24.7%, respectively (Fig. 1A). Astrocytes exposed to CM from untreated (control) ECs had no effect on cell volume.
Figure 1.
Cell swelling in cultured astrocytes after exposure to conditioned media (CM) from ammonia-treated endothelial cells (ECs). A. Cultured brain ECs were exposed to 5 mM NH4Cl for different time periods (3–24 h), the CM was added to astrocyte cultures for 24 h, and cell volume was determined. To exclude a potential role of any retained ammonia in the CM that might have contributed to the astrocyte swelling, ECs were treated with ammonia for 3 h, after which the ammonia-containing media was completely removed, and fresh media added to the ECs. At 1 and 3 h later the conditioned media from ECs was added to astrocytes and cell volume determined 24 h later (5th and 6th bars, media replaced). B. Ammonia concentration at different time points after ECs were treated with 5 mM NH4Cl. C. Time course of cell volume in ammonia-treated astrocyte cultures. D. Direct exposure of astrocyte cultures to ammonia, or to CM derived from ammonia-treated ECs (3 h) showed a significant increase in cell swelling, while a combination of these two treatments displayed a potentiation in cell swelling. E. ECs were treated with ammonia, LPS or with a mixture of cytokines (IL-1, IL-6, TNF-α, IFN-γ), alone or in combination (combo). CM from ammonia, LPS or mixture of cytokine-treated ECs when added to astrocytes caused a significant increase in astrocyte swelling, while CM from ECs treated with a combination of these agents resulted in a potentiation of astrocyte swelling. Data were subjected to ANOVA (n=7 for A; n=5 for B; n=4 for C; n=6 for D; n=5 for E). *p<0.05 vs. control. †p<0.05 vs. ammonia alone or with CM from ammonia-treated ECs in D; †p<0.05 versus ammonia, LPS or cytokines in E. Error bars, mean ± S.E. NH4+, ammonium chloride; LPS, lipopolysaccharide; CKs, a mixture of cytokines.
We chose a 3–24 h ammonia exposure to ECs to examine their effect on astrocyte swelling, since during these time periods ammonia levels in EC conditioned culture media were reduced to 1.4 to 0.25 mM after 3–24 h (Figure 1B), concentrations that do not cause cell swelling (Figure 1C) (i.e., the amount of ammonia remaining in the CM at these time intervals cannot contribute to the astrocyte swelling). To further exclude a potential role of any retained ammonia in the CM that might have contributed to the astrocyte swelling, we treated ECs with 5 mM ammonia for 3 h, after which the ammonia-containing media was completely removed, cells washed twice and fresh media added to the ECs. At 1–3 h later the conditioned media from ECs was added to astrocytes and cell volume determined 24 h later. This procedure also resulted in a similar degree of cell swelling (25.4% and 27.6 and at 1 and 3 h, respectively) (Figure 1A), indicating that the astrocyte swelling was derived from factors released by ammonia-treated ECs and not from any ammonia that may have been retained in the CM. A similar strategy was employed with regard to LPS and cytokines and comparable results were obtained as with ammonia (data not shown).
3.2. Effect of ammonia on astrocyte swelling in the presence of CM from ammonia-treated ECs
Ammonia is known to cause cell swelling in cultured astrocytes (Norenberg et al., 1991). Here we examined whether such swelling can be additionally influenced by CM derived from ammonia-treated ECs. Astrocyte cultures exposed to ammonia for 24 h showed a 32.1% increase in cell swelling, while adding the CM from ammonia-treated ECs for 3 h to astrocytes caused a 29.1% increase in cell swelling at 24 h. When both ammonia and CM from ammonia-treated ECs were added to astrocytes for 24 h, a potentiation of cell swelling was observed (51.86%, p<0.05) (Fig. 1D).
3.3. Effect of CM from ammonia, LPS and cytokine treated ECs on astrocyte swelling
Since LPS and cytokines are well known inflammatory factors that can influence brain ECs (Verma et al., 2006; Durieu-Trautmann et al., 1993; Collins-Underwood et al., 2006; Vigne and Frelin, 1994; Trickler et al., 2005), we examined whether LPS- and cytokine-treated ECs are also involved in astrocyte swelling. ECs were treated with ammonia (5 mM), LPS (10 ng/ml) or with a mixture of cytokines (IL-1, IL-6, TNF-α, IFN-γ, each at 10 ng/ml), alone or in combination, for 3 h. At the end of treatment, 2 ml of CM was added to astrocyte cultures, and cell volume determined 24 h later. CM from ECs treated with ammonia, LPS or cytokines for 3 h when added to astrocytes caused 29.1, 28.6 and 32.9% cell swelling, respectively. Such swelling was potentiated (62.7%) when astrocytes were exposed to CM from ECs that were treated with a combination of these agents (Fig. 1E).
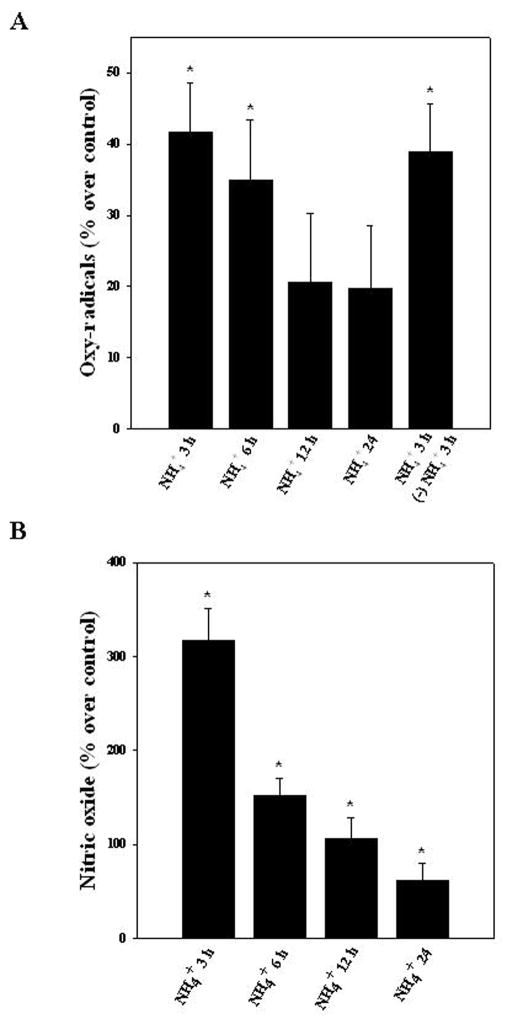
3.4. Oxy-radicals and nitric oxide (NO) release from ammonia-treated ECs
Cultured ECs were exposed to ammonia (5 mM) for different time periods (3, 6, 12 and 24 h) and levels of oxy-radicals and NO in the culture medium were measured. A significant increase in oxy-radicals was observed only at 3 and 6 h after ammonia treatment (41.7 and 34.9%, respectively) (Fig. 2A). On the other hand, a significant increase in NO was observed in endothelial cell culture medium at all time points after ammonia treatment (316.4, 152.1, 106.7 and 61.8%) at 3, 6, 12 and 24 h, respectively (Fig. 2B). Noteworthy, the time course of oxy-radicals and nitric oxide released from ammonia-treated ECs (peak release, 3 and 6 h) corresponded well with the time course of astrocyte swelling produced by CM from ammonia-treated ECs (Fig. 1A).
Figure 2.
Time-dependent changes in free radical production in ECs following treatment with ammonia. A. Cultured ECs were exposed to ammonia (5 mM) for different time periods (3, 6, 12 and 24 h) and levels of free radicals in the culture medium were measured. A significant increase in oxy-radicals was observed at 3 and 6 h. Additionally, to establish whether free radicals (which might contribute to astrocyte swelling) can be identified after replacing the ammonia-containing media with fresh culture media, we measured free radicals in the freshly added cell culture media after 3 h. Free radicals were identified in the freshly added culture media (5th bar). B. A significant increase in nitric oxide was observed after ammonia treatment at all time points, with a peak at 3 h. Data were subjected to ANOVA (n=4). *p<0.05 vs. control. Error bars, mean ± S.E.
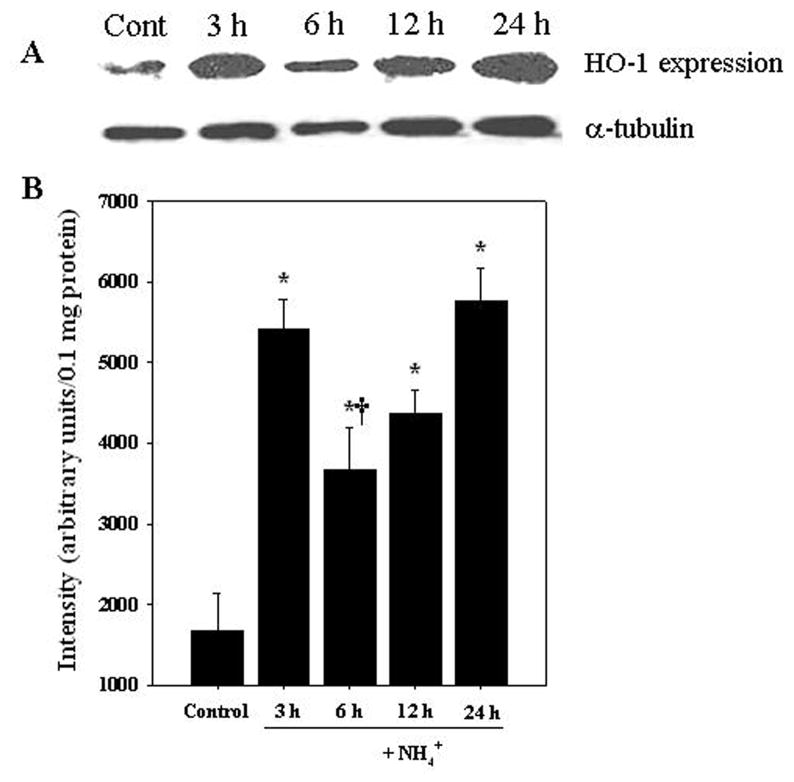
3.5. Heme oxygenase (HO-1) in ammonia-treated ECs
Cultured ECs were exposed to ammonia (5 mM) for different time periods (3, 6, 12 and 24 h) and at the end of treatment, heme oxygenase (HO-1), a commonly used marker of oxidative stress (Sandau et al., 1998; Méthy et al., 2004), was measured in the cell extract. Ammonia significantly increased the level of HO-1 expression (218.5–342.4%) at all time points examined (Fig. 3).
Figure 3.
Heme oxygenase-1 (HO-1) protein expression in ECs following ammonia treatement. A. Representative western blots show a significant increase in HO-1 protein level when endothelial cultures were exposed to 5 mM NH4Cl. B. Quantification of NH4Cl-induced changes in HO-1 protein expression. HO-1 levels were normalized against α-tubulin. Data were subjected to ANOVA (n=5). *p<0.05 vs. control. †p<0.05 versus 3 and 24 h. Error bars, mean ± S.E.
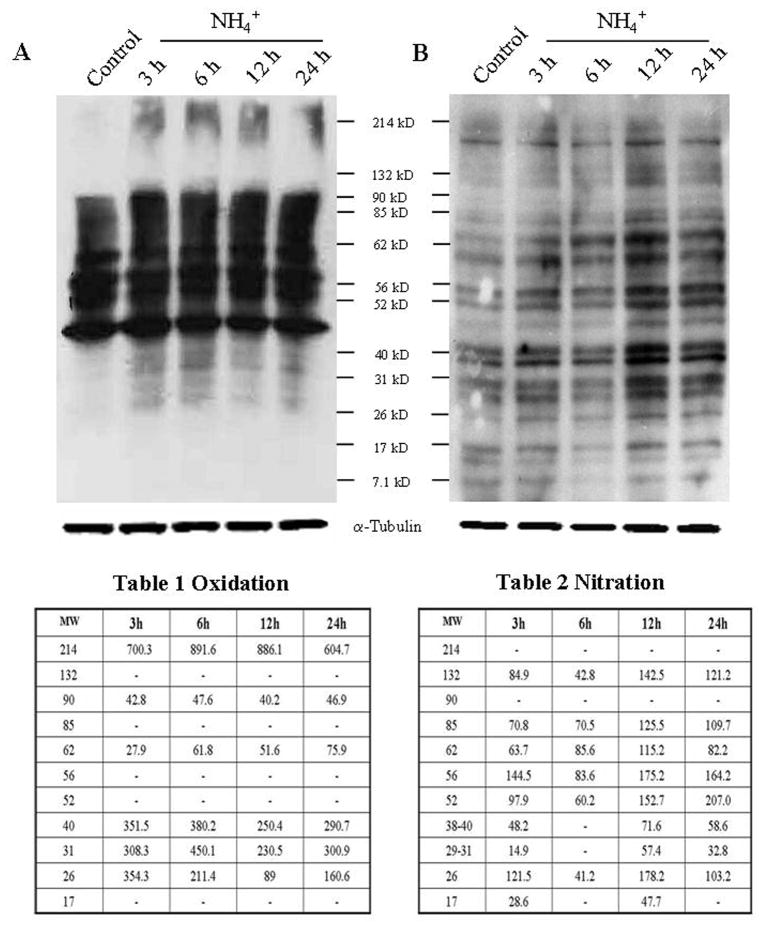
3.6. Oxy/nitro protein adducts after ammonia treatment in cultured ECs
Cultured ECs were exposed to ammonia (5 mM) for different time periods (3, 6, 12 and 24 h), and at the end of treatment the extent of protein oxidation and nitration was determined in cell extracts. Ammonia significantly increased protein oxidation. Proteins of molecular weight 26–40 kD, 62, 90 and 214 kD were found to be oxidized at all time points (Fig. 4A, Table 1). We also found increased protein nitration. Proteins of molecular weight 26, 52–85 kD and 132 kD were found to be nitrated at all time points (Fig. 4B, Table 2). Proteins of molecular weight 17 and 29–40 kD were nitrated at 3, 12 and 24 h after ammonia exposure (Fig. 4B, Table 2), while the extent of nitration was of lower intensity at 3 h.
Figure 4.
Protein oxidation/nitration in ammonia-treated ECs. A. Oxidized proteins were detected by Western blot analysis with 2-DNPH. Proteins ranging in molecular weight from 26–40 kD as well as 62, 90 and 214 kD were found to be oxidized at all time points. Table 1, quantification of NH4Cl-induced changes in oxidized protein in ECs. B. Protein tyrosine nitration was also detected by Western blot analysis with an antibody raised against 3-nitrotyrosine. Proteins of molecular weight 26, 52–85 kD and 132 kD were found to be nitrated at all time points. Proteins of molecular weight 17 and 29–40 were nitrated at 3, 12 and 24 h after ammonia exposure (n=4). Table 2, quantification of NH4Cl-induced changes in nitrated protein in ECs. Oxidized and nitrated protein levels were normalized against α-tubulin.
3.7. Astrocytes exposed to CM from ammonia plus antioxidant treated ECs show less cell swelling
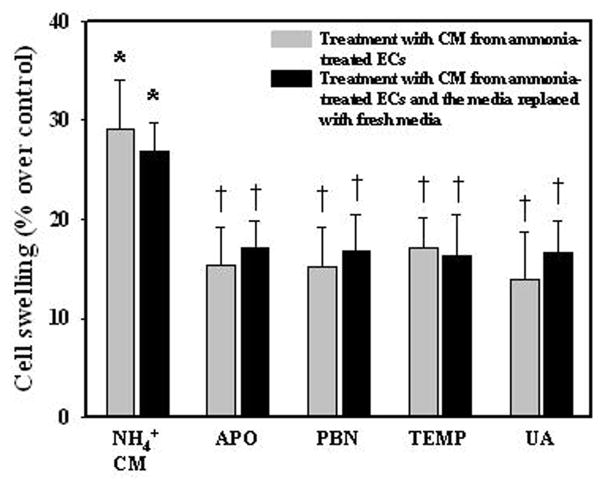
Since ammonia-treated ECs increases oxy-radical and nitric oxide (NO) production, we examined the potential role of endothelial-derived free radicals on astrocyte swelling. ECs were treated with the membrane-permeable antioxidants, the NADPH oxidase inhibitor apocynin (APO, 300 μM), the spin trapping agent N-tert-butyl-α-phenylnitrone (PBN, 100 μM), superoxide scavenger Tempol (10 μM), or the peroxynitrite scavenger uric acid (UA, 500 μM), along with ammonia for 3 h. CM derived from these antioxidant-treated endothelial cells were added to astrocyte cultures and cell swelling measured 24 h later. CM derived from ammonia-treated ECs showed 29.1% astrocyte swelling, and such effect was diminished by apocynin, PBN, Tempol, or uric acid (47.21, 47.52, 40.89, and 51.85% inhibition, respectively) (Fig. 5 gray bars). We chose the 3 h time point to examine the effect of CM from antioxidants (AOs) plus ammonia-treated ECs on astrocyte swelling, since the peak increase in free radical generation was detected at 3 h after ammonia-treatment in ECs (Fig. 2). To further exclude a direct effect of AOs on astrocyte swelling, we treated ECs with ammonia plus AOs for 3 h, after which the ammonia- and AO-containing media was removed, cells washed two-times and fresh media added to the ECs. At 3 h later the conditioned media from ECs was added to astrocyte cultures and cell volume determined 24 h later. This procedure also resulted in a comparable decrease in cell swelling (Fig. 5 dark bars). Additionally, ECs were treated with ammonia (3 h), after which the ammonia-containing media was removed; replaced with fresh media and the extent of free radical release was measured 3 h later. This procedure also resulted in a significant increase in free radical release (Figure 2A). Further, direct exposure of normal (untreated) astrocytes to these AOs were previously shown to have no effect on cell volume (Jayakumar et al., 2006). These studies collectively suggest that free radicals released from ECs in response to ammonia contribute to astrocyte swelling.
Figure 5.
Effect of CM from ECs treated with ammonia, in the presence or absence of antioxidants on astrocyte swelling. Astrocytes were exposed to CM derived from ammonia or ammonia plus antioxidants-treated ECs for 3 h. Ammonia plus antioxidant-treated ECs displayed a lesser degree of cell swelling as compared to astrocytes exposed to CM from only ammonia-treated ECs (gray bars). To rule-out the possibility that AOs in endothelial CM may have directly influenced the astrocyte swelling, ECs were treated with ammonia plus AOs for 3 h, after which the ammonia- and AO-containing media was removed, and fresh media added to the ECs. After 3 h the conditioned media from ECs was added to astrocyte cultures and cell volume determined 24 h later. This procedure also resulted in a comparable decrease in cell swelling (dark bars). Data were subjected to ANOVA (n=5 for gray bars and n=4 for dark bars). *p<0.05 vs. control. †p<0.05 vs. ammonia. Error bars, mean ± S.E. PBN, N-tert-butyl-α-phenylnitrone; APO, apocynin; TEMP, Tempol; UA, uric acid.
3.8. NF-κB nuclear translocation (activation) in ammonia-treated ECs
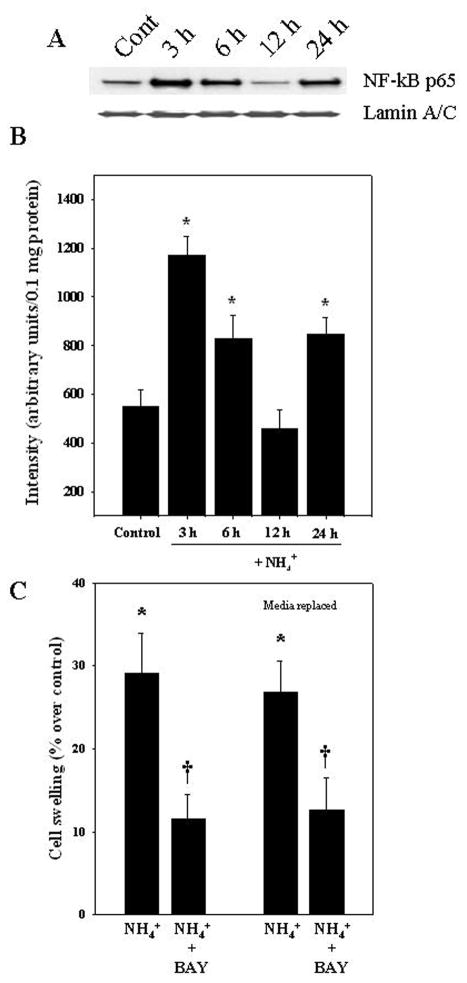
One potential factor known to be activated in ECs exposed to blood-derived noxious agents (e.g., LPS, cytokines) is the transcription factor NF-κ B (Trickler et al., 2005), and such activation was shown to stimulate the production and release of various agents, including pro-inflammatory cytokines, reactive oxygen/nitrogen species, arachidonic acid and prostaglandins (Verma et al., 2006; Durieu-Trautmann et al., 1993; Collins-Underwood et al., 2006; Vigne and Frelin, 1994; Trickler et al., 2005), all of which may impact on astrocytes and result in their swelling (Chan et al., 1982, 1989). Cultured ECs were exposed to ammonia (5 mM) for different time periods (3, 6, 12 and 24 h) and at the end of treatment the level of nuclear NF-κ B translocation was determined as previously described (Sinke et al., 2008). ECs exposed to ammonia showed a significant increase in nuclear NF-κ B expression at 3, 6, and 24 h (113.6, 51.2, and 54.5%, respectively). Ammonia had no effect on NF-κ B activation at 12 h (Figs. 6A,B).
Figure 6.
Nuclear translocation (activation) of NF-κ B in ammonia-treated ECs. Increased NF-κ B was identified in the nuclear fraction of cultured ECs that were treated with 5 mM NH4Cl. A. Western blots of NF-κ B (p65) in nuclear fraction. B. Quantification of Western blots. Ammonia caused an increase in nuclear NF-κ B at 3, 6, and 24 h, but had no effect at 12 h. NF-κ B content was normalized against lamin A/C. C. ECs were treated with BAY 11-7082, an inhibitor of NF-κ B, along with ammonia and the CM was added to astrocytes. Such treatment resulted in a lesser degree of cell swelling as compared to CM from ECs treated with ammonia alone. To exclude a direct effect of BAY 11-7082 on astrocyte swelling, ECs were treated with ammonia plus BAY 11-7082 for 3 h, after which the ammonia- and BAY 11-7082-containing media was removed, and fresh media added to the ECs. After 3 h the conditioned media from ECs was added to astrocyte cultures and cell volume determined 24 h later. This procedure also resulted in a comparable decrease in cell swelling (media replaced). ANOVA (n=4) for NF-κ B expression and n=5 for cell swelling studies. *p<0.05 versus control. †p<0.05 vs. ammonia. Error bars, mean ± S.E. BAY, BAY 11-7082.
We next examined the role of endothelial NF-κ B on astrocyte swelling by exposing astrocytes to CM from ECs in which the activation of NF-κ B was inhibited with BAY 11-7082 (Sinke et al., 2008). ECs were treated with ammonia plus BAY 11-7082 (5 μM) for 3 h. CM derived from these ECs were then added to astrocyte cultures and cell swelling was measured 24 h later. CM derived from ammonia-treated ECs showed 29.1% increase in astrocyte swelling, and such effect was diminished by 60.1% when astrocytes were exposed to endothelial CM to which ammonia plus BAY 11-7082 had been added (Fig. 6C). We chose the 3 h time point to examine the effect of BAY 11-7082 since the peak increase in NF-κ B activation was detected at 3 h after ammonia-treatment in ECs (Figs. 6A,B). Direct exposure of normal astrocytes to the NF-κB inhibitor BAY 11-7082 was previously shown to have no effect on cell volume (Sinke et al., 2008). To exclude a direct effect of BAY 11-7082 on astrocyte swelling, we treated ECs with ammonia plus BAY 11-7082 for 3 h, after which the ammonia- and BAY 11-7082-containing media were removed, cells washed two-times and fresh media added to the ECs. After 3 h the conditioned media from ECs was added to astrocyte cultures and cell volume determined 24 h later. This procedure also resulted in a comparable decrease in cell swelling (Fig. 6C).
3.9. Activation of endothelial NF-κB in cerebral cortex of rats with AHE following the administration of thioacetamide (TAA)
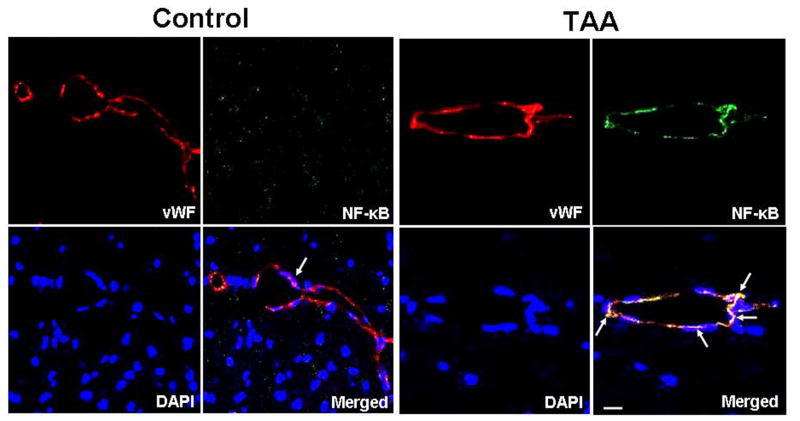
Since inflammation plays a major role in AHE (Rolando et al., 2000, and considering that cerebral endothelial cells are likely to contribute to the inflammatory process (see Introduction), we examined whether activation of NF-κ B also occurs in cerebral endothelial cells in an experimental model of AHE (thioacetamide-treated rats). Cortical sections were co-immunostained with NF-κ B-p65, von Willebrand factor (endothelial marker) and DAPI (nuclear stain). TAA-treated rats exhibited increased NF-κB nuclear staining (activation) in endothelial cells as compared to control animals (Fig. 7).
Figure 7.
Activation of NF-κ B in endothelial cells from cerebral cortex of rats with acute hepatic encephalopathy following the administration of the hepatotoxin thioacetamide (TAA) for 3 days. Control (normal) brain: von Willebrand Factor (vWF, red, endothelial cells), NF-κ B (green), and DAPI (blue, nuclei). The co-localization (merged) of NF-κ B, von Willebrand factor and DAPI illustrates that NF-κ B is not present in nuclei of von Willebrand factor-positive cells (arrows). TAA-treated rat brain: note the co-localization of NF-κ B and DAPI in vWF-positive cells, indicating nuclear localization (activation) of NF-κ B in endothelial cells (arrows). Scale bar =15 μm.
Beside ECs, increased fluorescent signal in other neural cells were also observed. We previously documented that ammonia-treated astrocyte cultures exhibit NF-κ B activation (Sinke et al. 2008), and a similar activation was also observed in microglia of TAA-treated rat brain (unpublished observations). However, as the focus of this study was endothelial cells, the status of NF-κ B activation in other neural cells was not examined.
4. Discussion
This study demonstrates for the first time that astrocyte cultures exposed to conditioned media (CM) from ammonia-treated brain endothelial cells (ECs) caused cell swelling, and that such swelling was enhanced by the direct addition of ammonia to astrocytes. ECs exposed to ammonia showed a significant increase in free radical production and activation of NF-κ B, and blocking these events resulted in a lesser degree of astrocyte swelling. Further, CM from ECs treated with a combination of ammonia, LPS and a mixture of cytokines (IL-1, IL-6, TNF-α, IFN-γ) resulted in a significantly greater degree of cell swelling as compared to the effect of CM from ECs treated only with ammonia. We also identified increased nuclear NF-κ B expression (activation) in rat brain cortical ECs in the thioacetamide model of acute hepatic encephalopathy (AHE). A preliminary account of these findings has been presented (Norenberg et al., 2011).
Ammonia is known to cause astrocyte swelling/brain edema (see Introduction). While the precise means by which ammonia brings about these changes are not completely understood, recent studies have shown the potential involvement of increased intracellular calcium, oxidative/nitrosative stress, the mitochondrial permeability transition and the subsequent activation of mitogen-activated protein kinases (MAPKs), as well as the transcription factors p53 and NF-κ B (for review, see Norenberg et al., 2009). Activation of NF-κ B then upregulates the ion transporter, Na+, K+, Cl− cotransporter-1 (NKCC1), as well as the water channel, aquaporin-4 (AQP4); the latter two events representing crucial penultimate phenomena in the process of astrocyte swelling by ammonia (Norenberg et al., 2009).
In addition to the direct ammonia effect on astrocytes, it is also possible that ammonia may impact other brain cells, in particular ECs, that may ultimately affect astrocytes. This view is plausible as astrocyte processes are juxtaposed to ECs and completely encircle these cells (Mathiisen et al., 2010). Brain ECs are dynamic cells that perform a variety of functions, including the release of neuroprotective growth factors, prevention of thrombosis, provision of a barrier against potentially noxious substances, transport of nutrients (e.g., amino acids, glucose, oxygen) and leukocyte trafficking (for reviews, see Wolburg et al., 2009; Abbott et al., 2010). When endothelial and glial cells are grown together there is a mutual up-regulation of many proteins, including AQP4 and antioxidant enzymes, such that the endothelial/glial relationship may be better able to cope with oxidative and osmotic stress (for review, see Abbott and Hansson, 2006). Thus signals between endothelium and glia (glio-vascular unit) seem to be critical for the maintenance of central nervous system (CNS) homeostasis, and a disturbance of this unit may negatively impact the CNS resulting in the development of brain edema.
The means by which ammonia-treated ECs contribute to astrocyte swelling, however, is not clear. Literature evidence, however, indicates that activation of NF-κB is likely a major event in ECs in response to various extracellular stimuli (Trickler et al., 2005; for review, see Baldwin, 1996). In the present study we observed the nuclear translocation (activation) of NF-κB when ECs were treated with ammonia. Additionally, exposing astrocytes to CM from ECs that were treated with ammonia plus BAY 11-7082 (an inhibitor of NF-κ B) showed a lesser degree of cell swelling as compared to CM from ECs treated only with ammonia, strongly implicating NF-κ B in the mechanism of astrocyte swelling. Consistent with these observations, we also found increased nuclear NF-κB expression in rat brain cortical endothelial cells in the thioacetamide model of AHE.
Ammonia caused an increase in NF-κB activation in cultured ECs in a biphasic manner, with an initial increase at 3 h, followed by a decline at 6 and 12 h, followed by a second rise at 24 h (Fig. 6). The reason for the decline in NF-κB activation at 6 and 12 h is not known. It is possible that failure of proteasome-mediated degradation of IkB-α may be impaired resulting in an inhibition of NF-κB (for review, see Verma et al., 1995). Similarly, we also observed a biphasic effect of ammonia on HO-1 expression in ECs (Fig. 3). Comparable findings have also been reported in macrophages when stimulated with LPS (Srisook and Cha, 2004). These authors suggested that the biphasic induction of heme oxygenase-1 following treatement with LPS was due to a suppression of free radical and nitric oxide production by elevated HO-1 through an auto-regulatory mechanism.
One consequence of NF-κ B activation in response to various stress stimuli in brain ECs is the production and release of reactive oxygen/nitrogen species (Durieu-Trautmann et al., 1993; Xu et al., 1997; Collins-Underwood et al., 2008), agents known to cause astrocyte swelling (Jayakumar et al., 2006; Sinke et al., 2008). Activation of NF-κ B in brain ECs is also known to produce and release other factors capable of inducing cell swelling, including arachidonic acid (AA) (Vigne and Frelin, 1994) and prostaglandins (Trickler et al., 2005) through the activation of phospholipase A2, cyclooxygenase-2, respectively (Trickler et al., 2005). Consistent with findings that ECs produce and release free radicals in response to various stimuli, we observed an increase in reactive oxygen/nitrogen species in primary endothelial cell culture medium when exposed to ammonia. Additionally, treating astrocytes with CM from ECs that were given ammonia plus antioxidants or a peroxynitrite scavenger showed a lesser degree of cell swelling, as compared to CM in which antioxidants were omitted, implicating free radicals in the mechanism of astrocyte swelling caused by CM from ammonia-treated ECs. Increased free radical production has recently been reported in a brain endothelial cell line (RBE-4) after exposure to ammonia (Skowronska et al., 2012).
Recent studies have shown that sepsis and inflammation also play major roles in the mechanism of brain edema formation in AHE. A high incidence of systemic infection and sepsis was shown to correlate with the severity of encephalopathy and brain edema in AHE (Rolando et al., 2000), and induction of endotoxemia by the administration of lipopolysaccharide (LPS) was shown to exacerbate the brain edema in an experimental model of AHE (Wright et al., 2007). Additionally, elevated blood cytokine levels have been identified in patients and experimental animals with AHE (Odeh et al., 2005), possibly as a consequence of liver necrosis, as well as sepsis. Increases in blood cytokine levels may result in their levels being increased in brain, as cytokines are known to cross the blood-brain barrier (Banks et al., 1995). High ammonia levels in brain may also contribute to the production of cytokines as a recent study showed increased levels of IL-1β, and other inflammatory mediators in brains of hyperammonemic rats (Rodrigo et al., 2010). Additionally, transgenic mice deficient in TNFα, IL1β and IL6 receptors were shown to exhibit a lesser degree of brain edema after the administration of the liver toxin, azoxymethane, as compared to wild-type mice (Bémeur et al., 2010). Similarly, we recently reported that TNF-α, IL-1β, IL-6 and IFN-γ resulted in significant cell swelling in cultured astrocytes (Rama Rao et al., 2010b).
In addition to cytokines directly influencing astrocyte swelling (Rama Rao et al., 2010b), cytokines may also stimulate brain ECs resulting in the synthesis and release of arachidonic acid, prostaglandins and the activation of phospholipase A2, cyclooxygenase-2, as well as NF-κ B (Vallieres and Rivest, 1997; Matsumura and Kobayashi, 2004; Rizzo and Leaver, 2010), all of which can lead to cell swelling (for review, see Norenberg et al., 2009). Cytokines can further promote the production of additional cytokines in ECs through the activation of NF-κ B (Vallieres and Rivest, 1997; Zheng and Yenari, 2004) thereby amplifying the direct effect of cytokines on astrocyte swelling. Consistent with these observations, we found that astrocytes exposed to CM from ECs treated with a combination of cytokines (TNF-α, IL-1β, IL-6 and IFN-γ) caused cell swelling in cultured astrocytes and such effect was potentiated by CM from ammonia-treated ECs (Fig. 1C).
As noted above, sepsis also plays a major role in the mechanism of brain edema in AHE. Lipopolysaccharide (LPS), a cell-wall component of gram-negative bacteria, contributes to tissue injury and pathophysiological changes associated with sepsis (Holst et al., 1996). Like cytokines, LPS was also shown to stimulate a number of signaling systems in brain ECs. LPS is known to bind to toll-like receptor-4 (TLR-4) on endothelial cell membranes (Maslinska et al., 2004) thus initiating various signaling events, including the activation of NF-κB (Singh and Jiang, 2000; Bannerman and Goldblum, 2003; Dauphinee and Karsan, 2006)). This results in the production of factors comparable to those generated by cytokines (e.g., ROS, NO) (Verma et al., 2006; Durieu-Trautmann et al., 1993; Collins-Underwood et al., 2006; Vigne and Frelin, 1994; Trickler et al., 2005), which are known to contribute to cell swelling/brain edema in other neurological conditions (Katayama et al., 1990; Norenberg et al., 2009; Rama Rao et al., 2010b). Consistent with these observations, we found that CM from ECs treated with LPS when added to astrocyte cultures resulted in a significant increase in cell swelling, and such swelling was enhanced when astrocytes were exposed to CM from ECs treated with ammonia and cytokines (Fig. 1C).
In summary, CM from ammonia-treated brain ECs caused cell swelling in cultured astrocytes and such effect was potentiated by the direct addition of ammonia to astrocytes. Ammonia-induced free radical production and the activation of NF-κ B by ECs are likely involved in the mediation of such cell swelling. Additionally, CM from LPS and cytokines-treated ECs potentiated the effect of CM from ammonia-treated ECs on astrocyte swelling. We anticipate that studies on the involvement of endothelial cells in the mechanism of astrocyte swelling/brain edema in AHE will translate into novel therapeutic modalities for the brain edema associated with AHE and other hyperammonemic conditions.
Highlights.
Ammonia stimulates endothelial cells to release astrocyte swelling factors
Cultured ECs release free radicals and activate NF-κ B when exposed to ammonia
CM from ammonia, LPS and cytokine-treated ECs potentiates astrocyte swelling
NF-κ B activation is increased in brain endothelial cells (ECs) in acute liver failure
Acknowledgments
This work was supported by a Merit Review from the Department of Veterans Affairs and by National Institutes of Health grants DK063311. ARJ is supported by a Stanley J. Glaser Foundation grant. We thank Alina Fernandez-Revuelta for the preparation of cell cultures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- Abbott NJ, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- Albrecht J, Jones EA. Hepatic encephalopathy: molecular mechanisms underlying the clinical syndrome. J Neurol Sci. 1999;170:138–146. doi: 10.1016/s0022-510x(99)00169-0. [DOI] [PubMed] [Google Scholar]
- Baldwin AS., Jr The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ, Broadwell RD. Passage of cytokines across the blood-brain barrier. Neuroimmunomodulation. 1995;2:241–248. doi: 10.1159/000097202. [DOI] [PubMed] [Google Scholar]
- Bannerman DD, Goldblum SE. Mechanisms of bacterial lipopolysaccharide-induced endothelial apoptosis. Am J Physiol Lung Cell Mol Physiol. 2003;284:L899–L914. doi: 10.1152/ajplung.00338.2002. [DOI] [PubMed] [Google Scholar]
- Bémeur C, Qu H, Desjardins P, Butterworth RF. IL-1 or TNF receptor gene deletion delays onset of encephalopathy and attenuates brain edema in experimental acute liver failure. Neurochem Int. 2010;56:213–215. doi: 10.1016/j.neuint.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Bender AS, Norenberg MD. Effect of benzodiazepines and neurosteroids on ammonia-induced swelling in cultured astrocytes. J Neurosci Res. 1998;54:673–680. doi: 10.1002/(SICI)1097-4547(19981201)54:5<673::AID-JNR12>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Blei AT, Olafsson S, Therrien G, Butterworth RF. Ammonia-induced brain edema and intracranial hypertension in rats after portacaval anastomosis. Hepatology. 1994;19:1437–1444. [PubMed] [Google Scholar]
- Bowman PD, Ennis SR, Rarey KE, Betz AL, Goldstein GW. Brain microvessel endothelial cells in tissue culture: a model for study of blood-brain barrier permeability. Ann Neurol. 1983;14:396–402. doi: 10.1002/ana.410140403. [DOI] [PubMed] [Google Scholar]
- Cardelli-Cangiano P, Cangiano C, James JH, Ceci F, Fischer JE, Strom R. Effect of ammonia on amino acid uptake by brain microvessels. J Biol Chem. 1984;259:5295–5300. [PubMed] [Google Scholar]
- Chan PH, Yurko M, Fishman RA. Phospholipid degradation and cellular edema induced by free radicals in brain cortical slices. J Neurochem. 1982;38:525–531. doi: 10.1111/j.1471-4159.1982.tb08659.x. [DOI] [PubMed] [Google Scholar]
- Chan PH, Longar S, Chen S, Yu AC, Hillered L, et al. The role of arachidonic acid and oxygen radical metabolites in the pathogenesis of vasogenic brain edema and astrocytic swelling. Ann N Y Acad Sci. 1989;559:237–247. doi: 10.1111/j.1749-6632.1989.tb22612.x. [DOI] [PubMed] [Google Scholar]
- Chung DW, Yoo KY, Hwang IK, Kim DW, Chung JY, Lee CH. Systemic administration of lipopolysaccharide induces cyclooxygenase-2 immunoreactivity in endothelium and increases microglia in the mouse hippocampus. Cell Mol Neurobiol. 2010;30:531–541. doi: 10.1007/s10571-009-9477-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins-Underwood JR, Zhao W, Sharpe JG, Robbins ME. NADPH oxidase mediates radiation-induced oxidative stress in rat brain microvascular endothelial cells. Free Rad Biol Med. 2008;45:929–938. doi: 10.1016/j.freeradbiomed.2008.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauphinee SM, Karsan A. Lipopolysaccharide signaling in endothelial cells. Lab Invest. 2006;86:9–22. doi: 10.1038/labinvest.3700366. [DOI] [PubMed] [Google Scholar]
- Ducis I, Norenberg LO, Norenberg MD. The benzodiazepine receptor in cultured astrocytes from genetically epilepsy-prone rats. Brain Res. 1990;531:318–321. doi: 10.1016/0006-8993(90)90793-b. [DOI] [PubMed] [Google Scholar]
- Durieu-Trautmann O, Federici C, Roux F, Claire M. Nitric oxide and endothelin secretion by brain microvessel endothelial cells: regulation by cyclic nucleotides. J Cell Physiol. 1993;155:104–111. doi: 10.1002/jcp.1041550114. [DOI] [PubMed] [Google Scholar]
- Feuerstein GZ, Wang X, Barone FC. The role of cytokines in the neuropathology of stroke and neurotrauma. Neuroimmunomodulation. 1998;5:143–159. doi: 10.1159/000026331. [DOI] [PubMed] [Google Scholar]
- Gammal SH, Basile AS, Geller D, et al. Reversal of the behavioral and electrophysiological abnormalities of an animal model of hepatic encephalopathy by benzodiazepine receptor ligands. Hepatology. 1990;11:371–378. doi: 10.1002/hep.1840110307. [DOI] [PubMed] [Google Scholar]
- Ganz R, Swain M, Traber P, DalCanto M, Butterworth RF, et al. Ammonia-induced swelling of rat cerebral cortical slices: implications for the pathogenesis of brain edema in acute hepatic failure. Metab Brain Dis. 1989;4:213–223. doi: 10.1007/BF01000297. [DOI] [PubMed] [Google Scholar]
- Gregorios JB, Mozes LW, Norenberg LOB, Norenberg MD. Morphologic effects of ammonia on primary astrocyte cultures. I: Light microscopic studies. J Neuropathol Exp Neurol. 1985a;44:397–403. doi: 10.1097/00005072-198507000-00003. [DOI] [PubMed] [Google Scholar]
- Gregorios JB, Mozes LW, Norenberg MD. Morphologic effects of ammonia on primary astrocyte cultures. II: Electron microscopic studies. J Neuropathol Exp Neurol. 1985b;44:404–414. doi: 10.1097/00005072-198507000-00004. [DOI] [PubMed] [Google Scholar]
- Grisham MB, Johnson GG, Lancaster JR. Quantitation of nitrate and nitrite in extracellular fluids. Methods Enzymol. 1996;268:237–246. doi: 10.1016/s0076-6879(96)68026-4. [DOI] [PubMed] [Google Scholar]
- Grzelak A, Rychlik B, Bartosz G. Light-dependent generation of reactive oxygen species in cell culture media. Free Radic Biol Med. 2001;30:1418–425. doi: 10.1016/s0891-5849(01)00545-7. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Ishikawa Y, Yokota S, Goto F, Bando T, Sakakibara Y. Action site of circulating interleukin-1 on the rabbit brain. Brain Res. 1991;540(1–2):217–223. doi: 10.1016/0006-8993(91)90510-3. [DOI] [PubMed] [Google Scholar]
- Hazell AS, Butterworth RF. Hepatic encephalopathy: an update of pathophysiologic mechanisms. Proc Soc Exp Biol Med. 1999;222:99–112. doi: 10.1046/j.1525-1373.1999.d01-120.x. [DOI] [PubMed] [Google Scholar]
- Hilgier W, Albrecht J, Kraśnicka Z. Thioacetamide-induced hepatic encephalopathy in the rat. I. Preliminary morphological and biochemical observations. Neuropatol Pol. 1983;21:487–94. [PubMed] [Google Scholar]
- Hilgier W, Olson JE, Albrecht J. Relation of taurine transport and brain edema in rats with simple hyperammonemia or liver failure. J Neurosci Res. 1996;45:69–74. doi: 10.1002/(SICI)1097-4547(19960701)45:1<69::AID-JNR6>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Hilgier W, Puka M, Albrecht J. Characteristics of large neutral amino acid-induced release of preloaded L-glutamine from rat cerebral capillaries in vitro: effects of ammonia, hepatic encephalopathy, and gamma-glutamyl transpeptidase inhibitors. J Neurosci Res. 1992;32:221–226. doi: 10.1002/jnr.490320211. [DOI] [PubMed] [Google Scholar]
- Holst O, Ulmer AJ, Brade H, Flad HD, Rietschel ET. Biochemistry and cell biology of bacterial endotoxins. FEMS Immunol Med Microbiol. 1996;16:83–104. doi: 10.1111/j.1574-695X.1996.tb00126.x. [DOI] [PubMed] [Google Scholar]
- James JH, Escourrou J, Fischer JE. Blood-brain neutral amino acid transport activity is increased after portacaval anastomosis. Science. 1978;200:1395–1397. doi: 10.1126/science.663619. [DOI] [PubMed] [Google Scholar]
- Jayakumar AR, Liu M, Moriyama M, Ramakrishnan R, Forbush B, 3rd, et al. Na–K–Cl Cotransporter-1 in the mechanism of ammonia-induced astrocyte swelling. J Biol Chem. 2008;283:33874–33882. doi: 10.1074/jbc.M804016200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayakumar AR, Panickar KS, Murthy ChR, Norenberg MD. Oxidative stress and mitogen-activated protein kinase phosphorylation mediate ammonia-induced cell swelling and glutamate uptake inhibition in cultured astrocytes. J Neurosci. 2006;26:4774–4784. doi: 10.1523/JNEUROSCI.0120-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayakumar AR, Valdes V, Norenberg MD. The Na-K-Cl cotransporter in the brain edema of acute liver failure. J Hepatol. 2011a;54:272–278. doi: 10.1016/j.jhep.2010.06.041. [DOI] [PubMed] [Google Scholar]
- Jayakumar AR, Bethea JR, Tong XY, Gomez J, Norenberg MD. NF-κB in the mechanism of brain edema in acute liver failure: studies in transgenic mice. Neurobiol Dis. 2011b;41:498–507. doi: 10.1016/j.nbd.2010.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juurlink BH, Hertz L. Plasticity of astrocytes in primary cultures: an experimental tool and a reason for methodological caution. Dev Neurosci. 1985;7:263–277. doi: 10.1159/000112295. [DOI] [PubMed] [Google Scholar]
- Katayama Y, Shimizu J, Suzuki S, Memezawa H, Kashiwagi F, et al. Role of arachidonic acid metabolism on ischemic brain edema and metabolism. Adv Neurol. 1990;52:105–108. [PubMed] [Google Scholar]
- Kato M, Hughes RD, Keays RT, Williams R. Electron microscopic study of brain capillaries in cerebral edema from fulminant hepatic failure. Hepatology. 1992;15:1060–1066. doi: 10.1002/hep.1840150615. [DOI] [PubMed] [Google Scholar]
- Kletzien RF, Pariza MW, Becker JE, Potter VR. A method using 3-O-methyl-D-glucose and phloretin for the determination of intracellular water space of cells in monolayer culture. Ann Biochem. 1975;68:537–544. doi: 10.1016/0003-2697(75)90649-1. [DOI] [PubMed] [Google Scholar]
- Konopacka A, Zielińska M, Albrecht J. Ammonia inhibits the C-type natriuretic peptide-dependent cyclic GMP synthesis and calcium accumulation in a rat brain endothelial cell line. Neurochem Int. 2008;52:1160–6. doi: 10.1016/j.neuint.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Maslinska D, Laure-Kamionowska M, Maslinski S. Toll-like receptors in rat brains injured by hypoxic-ischaemia or exposed to staphylococcal alpha-toxin. Folia Neuropathol. 2004;42:125–132. [PubMed] [Google Scholar]
- Mans AM, Saunders SJ, Kirsch RE, Biebuyck JF. Correlation of plasma and brain amino acid and putative neurotransmitter alterations during acute hepatic coma in the rat. J Neurochem. 1979;32:285–292. doi: 10.1111/j.1471-4159.1979.tb00350.x. [DOI] [PubMed] [Google Scholar]
- Mathiisen TM, Lehre KP, Danbolt NC, Ottersen OP. The perivascular astroglial sheath provides a complete covering of the brain microvessels: an electron microscopic 3D reconstruction. Glia. 2010;58:1094–1103. doi: 10.1002/glia.20990. [DOI] [PubMed] [Google Scholar]
- Matsumura K, Kobayashi S. Signaling the brain in inflammation: the role of endothelial cells. Front Biosci. 2004;9:2819–2826. doi: 10.2741/1439. [DOI] [PubMed] [Google Scholar]
- Martinez A. Electron microscopy in human hepatic encephalopathy. Acta Neuropathol. 1968;11:82–86. doi: 10.1007/BF00692797. [DOI] [PubMed] [Google Scholar]
- Méthy D, Bertrand N, Prigent-Tessier A, Stanimirovic D, Beley A, et al. Differential MnSOD and HO-1 expression in cerebral endothelial cells in response to sublethal oxidative stress. Brain Res. 2004;1003:151–158. doi: 10.1016/j.brainres.2003.12.031. [DOI] [PubMed] [Google Scholar]
- Norenberg MD. A light and electron microscopic study of experimental portal-systemic (ammonia) encephalopathy. Progression and reversal of the disorder. Lab Invest. 1977;36:618–627. [PubMed] [Google Scholar]
- Norenberg MD. The astrocyte in liver disease. In: Fedoroff S, Hertz L, editors. Advances in Cellular Neurobiology. Vol. 2. Academic Press; New York: 1981. pp. 303–352. [Google Scholar]
- Norenberg MD. Astroglial dysfunction in hepatic encephalopathy. Metab Brain Dis. 1998;13:319–335. doi: 10.1023/a:1020688925901. [DOI] [PubMed] [Google Scholar]
- Norenberg MD. Astrocytes and ammonia in hepatic encephalopathy. In: de Vellis J, editor. Astrocytes in the Aging Brain. Humana Press; Totowa, NJ: 2001. pp. 477–496. [Google Scholar]
- Norenberg MD, Baker L, Norenberg LOB, Blicharska J, Bruce-Gregorios JH, et al. Ammonia-induced astrocyte swelling in primary culture. Neurochem Res. 1991;16:833–836. doi: 10.1007/BF00965694. [DOI] [PubMed] [Google Scholar]
- Norenberg MD, Jayakumar AR, Tong XT, et al. Role of brain endothelial cells on astrocyte swelling associated with ammonia toxicity. Soc Neurosci. 2011;240:E8. doi: 10.1016/j.neuroscience.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norenberg MD, Neary JT, Bender AS, Dombro RS. Hepatic encephalopathy: a disorder in glial-neuronal communication. In: Yu ACH, Hertz L, Norenberg MD, Sykova E, Waxman SG, editors. Neuronal-Astrocytic Interactions: Pathological Implications. Elsevire; Amsterdam: 1992. pp. 261–269. [Google Scholar]
- Norenberg MD, Rama Rao KV, Jayakumar AR. Signaling factors in the mechanism of ammonia neurotoxicity. Metab Brain Dis. 2009;24:103–117. doi: 10.1007/s11011-008-9113-6. [DOI] [PubMed] [Google Scholar]
- Odeh M, Sabo E, Srugo I, Oliven A. Relationship between tumor necrosis factor-alpha and ammonia in patients with hepatic encephalopathy due to chronic liver failure. Ann Med. 2005;37:603–612. doi: 10.1080/07853890500317414. [DOI] [PubMed] [Google Scholar]
- Rama Rao KV, Jayakumar AR, Tong X, Curtis KM, Norenberg MD. Brain aquaporin-4 in experimental acute liver failure. J Neuropathol Exp Neurol. 2010a;69:869–879. doi: 10.1097/NEN.0b013e3181ebe581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rama Rao KV, Jayakumar AR, Tong X, Alvarez VM, Norenberg MD. Marked potentiation of cell swelling by cytokines in ammonia-sensitized cultured astrocytes. J Neuroinflammation. 2010b;7:66–73. doi: 10.1186/1742-2094-7-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rama Rao KV, Reddy PV, et al. Brain edema in acute liver failure: inhibition by L-histidine. Am J Pathol. 2010c;176:1400–1408. doi: 10.2353/ajpath.2010.090756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo MT, Leaver HA. Brain endothelial cell death: modes, signaling pathways, and relevance to neural development, homeostasis, and disease. Mol Neurobiol. 2010;42:52–63. doi: 10.1007/s12035-010-8132-6. [DOI] [PubMed] [Google Scholar]
- Rodrigo R, Cauli O, Gomez-Pinedo U, Agusti A, Hernandez-Rabaza V, et al. Hyperammonemia induces neuroinflammation that contributes to cognitive impairment in rats with hepatic encephalopathy. Gastroenterology. 2010;139:675–684. doi: 10.1053/j.gastro.2010.03.040. [DOI] [PubMed] [Google Scholar]
- Rolando N, Wade J, Davalos M, Wendon J, Philpott-Howard J, et al. The systemic inflammatory response syndrome in acute liver failure. Hepatology. 2000;32:734–739. doi: 10.1053/jhep.2000.17687. [DOI] [PubMed] [Google Scholar]
- Sandau K, Pfeilschifter J, Brüne B. Nitrosative and oxidative stress induced heme oxygenase-1 accumulation in rat mesangial cells. Eur J Pharmacol. 1998;342:77–84. doi: 10.1016/s0014-2999(97)01321-6. [DOI] [PubMed] [Google Scholar]
- Singh AK, Jiang Y. How does peripheral lipopolysaccharide induce gene expression in the brain of rats? Toxicology. 2000;201:197–207. doi: 10.1016/j.tox.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Sinke AP, Jayakumar AR, Panickar KS, Moriyama M, Reddy PV, et al. NFkappaB in the mechanism of ammonia-induced astrocyte swelling in culture. J Neurochem. 2008;106:2302–2311. doi: 10.1111/j.1471-4159.2008.05549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skowrońska M, Zielińska M, Wójcik-Stanaszek L, et al. Ammonia increases paracellular permeability of rat brain endothelial cells by a mechanism encompassing oxidative/nitrosative stress and activation of matrix metalloproteinases. J Neurochem. 2012;121:125–134. doi: 10.1111/j.1471-4159.2012.07669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srisook K, Cha YN. Biphasic induction of heme oxygenase-1 expression in macrophages stimulated with lipopolysaccharide. Biochem Pharmacol. 2004;68:1709–1720. doi: 10.1016/j.bcp.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Swain M, Butterworth RF, Blei AT. Ammonia and related amino acids in the pathogenesis of brain edema in acute ischemic liver failure in rats. Hepatology. 1992;15:449–453. doi: 10.1002/hep.1840150316. [DOI] [PubMed] [Google Scholar]
- Traber PG, Dal Canto M, Ganger DR, Blei AT. Electron microscopic evaluation of brain edema in rabbits with galactosamine-induced fulminant hepatic failure: ultrastructure and integrity of the blood-brain barrier. Hepatology. 1987;7:1272–1277. doi: 10.1002/hep.1840070616. [DOI] [PubMed] [Google Scholar]
- Trickler WJ, Mayhan WG, Miller DW. Brain microvessel endothelial cell responses to tumor necrosis factor-alpha involve a nuclear factor kappa B signal transduction pathway. Brain Res. 2005;1048:24–31. doi: 10.1016/j.brainres.2005.04.028. [DOI] [PubMed] [Google Scholar]
- Vallieres L, Rivest S. Regulation of the genes encoding interleukin-6, its receptor, and gp130 in the rat brain in response to the immune activator lipopolysaccharide and the proinflammatory cytokine interleukin-1beta. J Neurochem. 1997;69:1668–1683. doi: 10.1046/j.1471-4159.1997.69041668.x. [DOI] [PubMed] [Google Scholar]
- Verma S, Nakaoke R, Dohgu S, Banks WA. Release of cytokines by brain endothelial cells: A polarized response to lipopolysaccharide. Brain Behavior Immun. 2006;20:449–455. doi: 10.1016/j.bbi.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Verma IM, Stevenson JK, Schwarz EM, Van Antwerp D, Miyamoto S. Rel/NF-kB/IkB family: intimate tales of association and dissociation. Genes Dev. 1995;9:2723–2735. doi: 10.1101/gad.9.22.2723. [DOI] [PubMed] [Google Scholar]
- Vigne P, Frelin C. Endothelins activate phospholipase A2 in brain capillary endothelial cells. Brain Res. 1994;651:342–344. doi: 10.1016/0006-8993(94)90716-1. [DOI] [PubMed] [Google Scholar]
- Voorhies TM, Ehrlich ME, Duffy TE, Petito CK, Plum F. Acute hyperammonemia in the young primate. Physiologic and neuropathological correlates. Pediatr Res. 1983;17:970–975. doi: 10.1203/00006450-198312000-00009. [DOI] [PubMed] [Google Scholar]
- Willard-Mack CL, Koehler RC, Hirata T, Cork LC, Takahashi H, et al. Inhibition of glutamine synthetase reduces ammonia-induced astrocyte swelling in rat. Neuroscience. 1996;71:589–599. doi: 10.1016/0306-4522(95)00462-9. [DOI] [PubMed] [Google Scholar]
- Wolburg H, Noell S, Mack A, Wolburg-Buchholz K, Fallier-Becker P. Brain endothelial cells and the gliovascular complex. Cell Tissue Res. 2009;335:75–96. doi: 10.1007/s00441-008-0658-9. [DOI] [PubMed] [Google Scholar]
- Wright G, Davies NA, Shawcross DL, Hodges SJ, Zwingmann C, et al. Endotoxemia produces coma and brain swelling in bile duct ligated rats. Hepatology. 2007;45:1517–1526. doi: 10.1002/hep.21599. [DOI] [PubMed] [Google Scholar]
- Xu J, Wu Y, He L, Yang Y, Moore SA, et al. Regulation of cytokine-induced iNOS expression by a hairpin oligonucleotide in murine cerebral endothelial cells. Biochem Biophys Res Commun. 1997;235:394–7. doi: 10.1006/bbrc.1997.6800. [DOI] [PubMed] [Google Scholar]
- Zanchin G, Rigotti P, Dussini N, Vassanelli P, Battistin L. Cerebral amino acid levels and uptake in rats after portocaval anastomosis: II. Regional studies in vivo. J Neurosci Res. 1979;4:301–310. doi: 10.1002/jnr.490040407. [DOI] [PubMed] [Google Scholar]
- Zheng Z, Yenari MA. Post-ischemic inflammation: molecular mechanisms and therapeutic implications. Neurol Res. 2004;26:884–892. doi: 10.1179/016164104X2357. [DOI] [PubMed] [Google Scholar]
- Zielinska M, Law RO, Albrecht J. Excitotoxic mechanism of cell swelling in rat cerebral cortical slices treated acutely with ammonia. Neurochem Int. 2003;43:299–303. doi: 10.1016/s0197-0186(03)00015-9. [DOI] [PubMed] [Google Scholar]
- Zimmermann Z, Ferenci P, et al. Hepatic encephalopathy in thioacetamide induced acute liver failure in rats: characterization an improved model and study of amino acid-ergic neurotransmission. Hepatology. 1989;9:594–601. doi: 10.1002/hep.1840090414. [DOI] [PubMed] [Google Scholar]
- Zwingmann C, Flogel U, Pfeuffer J, Leibfritz D. Effects of ammonia exposition on glioma cells: Changes in cell volume and organic osmolytes studied by diffusion-weighted and high-resolution NMR spectroscopy. Dev Neurosci. 2000;22:463–471. doi: 10.1159/000017476. [DOI] [PubMed] [Google Scholar]