Abstract
Ammonia is a major neurotoxin implicated in hepatic encephalopathy (HE). Here we discuss evidence that many aspects of ammonia toxicity in HE-affected brain are mediated by glutamine (Gln), synthesized in excess from ammonia and glutamate by glutamine synthetase (GS), an astrocytic enzyme. The degree to which Gln is increased in brains of patients with HE was found to positively correlate with the grade of HE. In animals with HE, a GS inhibitor, methionine sulfoximine (MSO), reversed a spectrum of manifestations of ammonia toxicity, including brain edema and increased intracranial pressure, even though MSO itself increased brain ammonia levels. MSO inhibited, while incubation with Gln reproduced the oxidative stress and cell swelling observed in ammonia-exposed cultured astrocytes. Recent studies have shown that astrocytes swell subsequent to Gln transport into mitochondria and its degradation back to ammonia, which then generates reactive oxygen species and the mitochondrial permeability transition. This sequence of events led to the formulation of the “Trojan Horse” hypothesis. Further verification of the role of Gln in the pathogenesis of HE will have to account for: 1) modification of the effects of Gln by interaction of astrocytes with other CNS cells; and 2) direct effects of Gln on these cells. Recent studies have demonstrated a “Trojan Horse”-like effect of Gln in microglia, as well as an interference by Gln with the activation of the NMDA/NO/cGMP pathway by ammonia as measured in whole brain, a process that likely also involves neurons.
Keywords: Hepatic encephalopathy; Ammonia, Glutamine; “Trojan Horse” hypothesis; Astrocytes; Mitochondria; Microglia; NO/cGMP pathway
1. Introduction
Hepatic encephalopathy (HE) is a complex neuropsychiatric disorder associated with liver dysfunction. The cellular and molecular mechanisms underlying HE are complex, but there is a consensus that an excessive accumulation of ammonia in brain is a primary causative factor (for reviews, see [1, 2]). Ammonia mainly affects the metabolism and function of astrocytes, leading to impaired astrocytic-neuronal interactions and the ensuing imbalance of neural transmission [3]. Ammonia induces astrocytic swelling [4] which is the primary cause of cerebral edema, resulting in increased intracranial pressure and brain herniation, a sequence of events often leading to death in patients with HE [5]. Increase of blood ammonia level, along with EEG abnormalities and impaired psychometric scores, are among key parameters used in the clinical grading of patients with HE [6].
Since in brain ammonia is initially metabolized to glutamine (Gln), it is not unexpected that HE in humans and experimental animals is usually associated with increased brain and cerebrospinal fluid Gln content [7, 8, 9]. This increase is particularly pronounced in acute hyperammonemic conditions where brain Gln levels reaches 10 mM concentration in the extracellular space in comatose patients with HE; i.e., a ~20-fold increase above the control levels [10]. Gln is an essential precursor of the neurotransmitter amino acids glutamate and GABA, as well as an important energy metabolite in brain [for review, see 11].
For decades the formation of Gln was generally viewed as the principal means of ammonia detoxification. However, in recent years a more sinister aspect of Gln was uncovered; namely, that when synthesized in excess in brain it appears to mediate key aspects of ammonia neurotoxicity. Here we provide supportive evidence in favor of this view. We focus on astrocytic mitochondria as a primary target of Gln, but also hypothesize on the potential role of Gln fluxes to other compartments of the CNS, and speculate how ammonia could affect critical steps in these fluxes. We also discuss recent data indicating that Gln may contribute to ammonia toxicity in other neural cells, e.g., neurons and microglia.
2. Glutamine as a mediator of ammonia neurotoxicity: experimental demonstration of the phenomenon
In the pioneering study of Warren and Schenker [12], the glutamine synthetase inhibitor methionine sulfoximine (MSO) was shown to increase the survival of rats acutely exposed to toxic concentrations of ammonia. Unexpectedly, the beneficial effect of MSO became apparent despite the concomitant increase of blood and/or brain ammonia. In hyperammonemic rats or in rats with HE, MSO reversed the biochemical manifestations of ammonia toxicity such as depressed glucose consumption, increased blood-brain barrier transport of aromatic amino acids and other abnormalities (Table 1). MSO administration also attenuated ammonia-induced brain swelling, as manifested by a return of the brain water content to normal (Table 1). In the aforementioned studies, MSO not only decreased cerebral Gln content but it also substantially raised blood and brain ammonia levels. At the cellular level, MSO reduced swelling of astrocytes and pericytes in vivo [13, 14], and decreased ammonia-induced swelling of cortical astrocytes in culture [15]. Pathophysiological manifestations of HE, including an increase of cerebral blood flow and intracranial pressure [16], excessive potassium accumulation in the intracellular space [17], and impaired vascular reactivity to CO2 [18] were also corrected by MSO.
Table 1.
Effects of MSO on the major HE parameters in hyperammonemic (HA) or portacaval shunted (PCS) rats.
| Control | HA | PCS | HA + MSO | PCS+MSO | |
|---|---|---|---|---|---|
|
| |||||
| Ammonia (blood) | |||||
| (μM)1 | 29 ±31 | 601 ± 381 | 908 ± 1961 | ||
| (nmol/l)2 | 97 ± 82 | 227 ± 152 | 265 ± 272 | ||
|
| |||||
| Ammonia (brain) | |||||
| (nmol/g)1 | 146 ± 61 | 384 ± 221 | 424 ± 461 | ||
|
| |||||
| GS (brain) (IU/g)1 | 38.8 ± 0.81 | 39.9 ± 0.31 | 14.1 ± 0.21 | ||
|
| |||||
| Gln (brain) | |||||
| (mmol/kg)1 | 5.6 ± 0.41 | 18.8 ± 0.41 | 8780 ± 5702 | 2.6 ± 0.41 | 5180 ± 5402 |
| (nmol/g)2 | 5580 ± 3602 | ||||
|
| |||||
| Glu (brain) | |||||
| (mmol/kg)1 | 10.1 ± 1.01 | 6.6 ± 0.41 | 9.5 ± 0.61 | ||
|
| |||||
| Spec. gravity (brain)1 | 1.04521 | 1.0424 1 | 1.04461 | ||
|
| |||||
| Water content (brain) (%)1 | 0.7831 | 0.804 1 | 0.7841 | ||
|
| |||||
| Tryptophan (brain) | |||||
| (nmol/g)2 | 17 ± 12 | 45 ± 32 | 25 ± 22 | ||
|
| |||||
| Glucose consumption (brain) | 861 ± 532 | 614 ± 392 | 733 ± 872 | ||
| (nmol/min/g)2 | |||||
|
| |||||
| BBB transport of tryptophan | 18 ± 22 | 31 ± 52 | 15 ± 32 | ||
| (μl/min/g)2 | |||||
3. Brain Gln content correlates with advancement of symptoms in patients with HE
The development of the NMR technique led in 1990 to the first demonstration of increased Gln content in the brain of a symptomatic HE patient [19]. It was later demonstrated that the cerebral Gln content positively correlated with the HE grade [20]. More recently, a bedside brain microdialysis study revealed that in patients with acute HE awaiting liver transplantation due to cirrhosis, cerebral (microdialysate) Gln correlated well with blood ammonia, and with the increased intracranial pressure observed in these patients [10]. Altogether, these findings strongly implicate Gln in the pathogenesis of HE.
4. Toxic effects of glutamine in the CNS are triggered by its accumulation in astrocytic mitochondria
In 1986 Brusilow and Traystman [21] proposed an interesting hypothesis regarding the potential role of Gln in the mechanism of brain edema in HE/hyperammonemia. These investigators posited that by acting as an osmolyte Gln caused an increase in osmotic pressure in astrocytes which resulted in their swelling. This interpretation assumed the predominant localization of the newly synthesized Gln in astrocytes. The authors considered that as astrocytes account for ~30% of cortical volume, a concentration as high as 30 mM of Gln could be reached in these cells. While an attractive possibility, the available data, however, make this concept difficult to accept. First, Gln accounts for not more than 1.5% of the sum of osmolytes (ions plus small-and medium size soluble molecules) residing in the brain [22]. Second, a significant proportion of newly synthesized Gln rapidly leaves astrocytes, both by diffusion and by specific Gln transporters (mainly the N-system transporter SNAT3 [23] which reduces Gln overload in the cells. Furthermore, an increase of brain Gln during HE is accompanied by loss of different low MW osmolytes, including myo-inositol, taurine and betaine [9]. Perhaps the strongest argument against the osmolyte hypothesis comes from a study demonstrating a negative correlation between astrocyte glutamine levels after ammonia treatment and the extent of cell swelling [24]. Regardless, the notion that glutamine plays a key role in the mechanism of brain edema in acute liver failure represents an important concept put forward by Brusilow and colleagues.
The current interpretation is that Gln impairs mitochondrial function secondary to its excessive accumulation in mitochondria. In cultured astrocytes, inhibition of Gln synthesis by MSO suppressed ammonia-induced formation of reactive oxygen species (ROS) [25]. When added directly to a non-synaptic mitochondrial preparation [26] or cultured astrocytes [27], Gln induced mitochondrial permeability transition (mPT) and mitochondrial swelling. In either case, this effect was blocked by the mPT inhibitor cyclosporine A (CsA), and by an inhibitor of mitochondrial Gln uptake, histidine. Recently, the finding with histidine was verified in an in vivo setting in that histidine administered i.p. prevented the brain edema in rats with thioacetamide-induced liver failure [28]. In unpublished observations we found that histidine completely blocked i)- the overexpression of astrocytic aquaporin-4 in brains of rats with thioacetamide-induced acute liver failure and ii) - the ammonia-induced activation of NF-kB and MAPKs - factors that are activated by ammonia and whose activation contribute to cell swelling/brain edema [24,29]. Gln added in the absence of ammonia dose-dependently increased ROS synthesis in astrocytes, and this effect was suppressed by CsA [30]. CsA also attenuated Gln-induced swelling of non-synaptic mitochondria [26].
In contrast to Gln, ammonia did not induce mPT or mitochondrial swelling indicating that its direct entry to the mitochondria may not be efficient enough to exert a deleterious effect [26]. However, Gln uptake to mitochondria was increased in the presence of elevated ammonia levels [31]. This indicates that in hyperammonemic conditions, ammonia accumulating in excess in extramitochondrial cell compartments may potentiate Gln toxicity by facilitating its entry to mitochondria.
5. Intra-mitochondrial degradation of Gln by phosphate-activated glutaminase (PAG) is a prerequisite for its gliotoxic action: the Trojan Horse hypothesis
Maximum cell volume increase in ammonia-treated astrocytes occurred at a time when their Gln content had already returned to control values [29], implicating a Gln-dependent process (likely ROS and/or the mPT) in the mediation of astrocyte swelling rather than the Gln molecule per se. In support of this mechanism, swelling of ammonia-treated astrocytes was prevented by the glutaminase inhibitor (6-diazo-5-oxo-L-norleucine; DON) [29]. Of note, inhibition of mitochondrial Gln transport by histidine, which ameliorated Gln toxicity in different experimental settings (see above section), specifically involves a Gln pool of an as yet undefined origin and location which is easily accessible to intramitochondrial deamidation [32]. On the basis of these results, a “Trojan Horse” hypothesis was formulated [33]; excess Gln accumulating in astrocytic cytoplasm (the Trojan Horse) crosses mitochondrial membrane and unloads in mitochondrial interior excess ammonia. The Gln-derived ammonia triggers mitochondrial impairment, and an ensuing chain of deleterious events leading to astrocytic dysfunction, swelling and brain edema. Current evidence suggests that the tricarboxylic cycle and the malate-aspartate shuttle enzymes are the primary astroglia-specific mitochondrial targets of ammonia [34–37].
6. Gln fluxes from astrocytes to other CNS compartments as potential target of ammonia
In situ the availability of Gln for entry to astrocytic mitochondria will not only depend on its excessive accumulation in that organelle, but also upon the rate at which it is cleared from the cells. Only a fraction of newly synthesized Gln will be transported to astrocytic mitochondria as a significant proportion will exit astrocytes and enter neurons, to give rise to the amino acid neurotransmitters glutamate (Glu) and GABA, reflecting one component of the glutamate Glu/Gln cycle [11]. The direct effect of ammonia on Gln efflux from astrocytes, or its uptake by neurons, have not as yet been investigated. Of note, MSO – the agent used in the aforementioned studies to inhibit GS, induces a massive efflux of Gln from astrocytes in an in vitro system [38]. To what extent this phenomenon affects the interpretation of the Trojan Horse mechanism is unknown.
A certain proportion of astrocyte-derived Gln will leave the CNS via the cerebral capillary endothelial cells forming the blood-brain barrier [39]. Increased Gln transport has been demonstrated in cerebral microvessels incubated with ammonia [40], or microvessels derived from rats with HE [41]. Increased Gln efflux across the blood-brain barrier could favor the efflux of Gln from astrocytes and thereby alleviate the toxicity of Gln on astrocytic mitochondria.
Gln transport in CNS cells participating in the different components of the Gln/Glu cycle is mediated by multiple carriers, some of which are either cell-specific or dominate in a given cell type. Gln uptake to, and efflux from, astrocytes occurs mainly via the system N carrier, SN1 (SNAT3) [23], whereas Gln uptake to neurons involves the system A carriers SAT1 and SAT2 (SNAT2, SNAT1) [42]. Gln transport from brain to blood is mediated by carriers belonging to systems A, N and ASC [39, 43]. Effects of ammonia and HE on the expression and/or activity of astrocytic or neuronal Gln transporters has not been investigated as yet. Stimulation of Gln efflux from cerebral capillary endothelial cells occurs in exchange for tryptophan and other large neutral amino acids and as such has been suspected to involve system L [40, 44]. However, the effect on the expression of system L isoforms has not been investigated, and the role of system N has not been examined.
The activity of the N system-mediated Gln efflux from astrocytes is controlled by Glu released from neurons [45]. Hyperammonemia or HE are invariably associated with increased Glu accumulation in the extracellular space of the CNS [44, 46, 47, 48], an effect related to decreased astrocytic Glu uptake [49, 50, 51]. This increased extracellular Glu may promote increased astrocytic Gln efflux. In favor of this concept, extracellular brain Gln content is often found increased in hyperammonemia or in animal models of HE [44, 52] or humans [10]. Alternatively or additionally, the increase of extracellular Gln may be a direct result of increased GS activity, which is strongly pronounced at the early stages of HE [see 53 for review], and may be promoted by increased extracellular Glu [54].
While intracellular His is a well documented inhibitor of mitochondrial Gln transport [26, 55], extracellular His competes with Gln for the cell membrane N system transport in astrocytes [23]. Since in astrocytes the N system carriers mediate both Gln efflux and influx, part of the cerebral edema-ameliorating effect of His during HE [28] could have been due to inhibition of Gln re-entry to astrocytes. The above considerations further substantiate the need to analyze the response of astrocytic Gln carriers, in particular N system carriers, to ammonia exposure in situ and in vitro.
7. Aspects of ammonia neurotoxicity that appear not to correlate with increased brain Gln levels
A few studies have suggested that the accumulation of Gln accumulation may not be critical in the development of cerebral edema and other manifestations of acute HE. Close analysis of the evidence indicates that this may not be the case. Two studies explored the utility of mild hypothermia as a treatment modality for the brain edema associated with HE. The first study found that hypothermia delayed the development of brain edema in portacaval shunted rats infused with ammonia, without similarly correcting the ammonia-induced rise in cerebral glutamine content [9]. A later study using a combined 1H and 13C NMR spectroscopy demonstrated that while the cerebral edema in rats with acute liver failure was associated with an increase in Gln brain content and glucose-derived products (lactate and alanine), the amelioration of the edema by mild hypothermia was associated with reduction in brain alanine and lactate levels, but not Gln levels [60]. It must be noted, however, that hypothermia decreases brain metabolism and more importantly, decreases CBF which would impact on the severity of intracranial pressure (it would decrease it) [57]. Also, the Gln concentration provided does not define in what compartment the Gln is located (intra- vs extracellular), nor its distribution within cells of the same type. Hypothermia has many effects on the CNS and its failure to influence Gln levels cannot be taken as evidence that glutamine does not play a pivotal role in the pathogenesis of the brain edema in ALF.
Ammonia-induced swelling of cerebral cortical slices was prevented by NMDA receptor antagonists and scavengers of oxygen and nitrogen free radicals, supporting the role of oxidative/nitrosative stress in brain edema; however, improvement occurred despite the Gln content in the slices remaining elevated [58]. Likewise, (1) Gln may not exclusively reside in astrocytes, and (2) attenuation of oxidative/nitrosative stress (ONS) could have directly decreased energy metabolism, making brain tissue less sensitive to the mitochondrial dysfunction exerted by Gln. Analogous to the effects of hypothermia, reports on the amelioration of ONS are not to be viewed as contradictory to the Gln/Trojan Horse theory.
In ammonium acetate-treated rats, the transition of behavioral manifestations of HE from moderate to precomatous stage (stage III) or to coma (stage IV) was not prevented by inhibition of Gln synthesis with MSO [63]. The following factors may explain these apparently contradictory findings: (1) Neurobehavioral manifestations of advanced HE develop in part independently of brain edema [60]. (2) Instant elevation of ammonia by MSO treatment could have superimposed a component of ammonia toxicity on the pre-existing mitochondrial dysfunction caused by Gln. (3) Impairment of mitochondrial function by Gln at moderate levels of HE could have sensitized the brain to MSO, effects independent of its inhibition of GS. Moreover, MSO is a convulsing agent frequently used to model epileptic discharges in animals [61], which at the cellular level is associated with increased glycogen synthesis in astrocytes [62], a reaction also directly evoked by astrocytic swelling [63]. As judged from studies in in vitro systems, MSO inhibits Gln uptake in one or more CNS cell types in brain slices [64], and induces a massive Gln efflux from astrocytes [42]. Taken together, caution must be exercised when attempting to implicate a role of Gln solely on the basis of the effects of MSO, which under certain experimental conditions may overemphasize Gln-unrelated manifestations of ammonia neurotoxicity. It will be worthwhile to use in the future studies other, potentially less toxic interventions to manipulate GS activity; of these GS knockdown by siRNA would appear to be the method of choice.
8. Effects of Gln at targets other than astrocytic mitochondria
8.1. Attenuation of the NO/cGMP pathway activity
Alterations in NO and cGMP levels play varied and independent roles in the pathogenesis of HE. In acute HE, increased NO synthesis, often associated with the activation of NMDA receptors, contributes to oxidative/nitrosative stress (reviewed by [65, 66], and elevated cGMP level serves as marker of this activation [47, 67]. A recent microdialysis study showed that increasing extracellular Gln in the brain to levels found in patients with acute HE, caused a decrease in NO and cGMP production in both normal and ammonia-exposed brain. This occurred by limiting the transport of the NO precursor, arginine (Arg), to most likely via the hybrid y+LAT2 system for which Gln and Arg compete [68]. Whether interference of the NO/cGMP pathway by Gln is beneficial or detrimental for the HE-affected brain is unknown. The implications may differ in different stages of the disease. In acute HE, decreased NO synthesis might ameliorate symptoms by decreasing the severity of oxidative/nitrosative stress, including astrocytic swelling, an event to which protein kinase G activation contributes [69]. Chronic HE is associated with a deficit in cGMP, which may be responsible for cognitive and memory impairments [70]. Whether in chronic HE Gln inhibits cGMP synthesis in neurons remains to be investigated. If it does, it will add a role for Gln in the cGMP-dependent impairment in memory and cognition. Clarification of whether Gln interferes with Arg transport in neurons, astrocytes or both is needed to better understand its pathophysiological impact. The NMDA receptor-mediated activation of the NO/cGMP pathway by ammonia was originally thought to occur mainly, if not exclusively in neurons. However, evidence indicates that this pathway also occurs in astrocytes [71], where it likewise contributes to oxidative/nitrosative stress [72, and references therein]. The issue is further complicated by the paucity and inconclusiveness of data regarding y+ LAT2 expression in the different neural cells [73, 74].
8.2. Gln as a Trojan Horse in microglia?
A recent study documented that exogenously added Gln causes apoptosis in a microglial cell line and that this effect was prevented upon inhibition of Gln degradation by the PAG inhibitor, DON [75]. Also, Gln mimicked ammonia in producing the oxidative stress response in these cells. However, the findings remain to be confirmed with native microglial cells where GS activity has not been definitively demonstrated. These findings also require their validation in an in vivo HE model. More work is needed to interpret the effect observed in the microglial cell line as another pathologic manifestation ammonia leading to the “Trojan Horse” pathway as reported for astrocytes.
9. Concluding remarks
The available evidence suggests that the most profound effect of excess of Gln accumulating in the CNS during HE is an impairment of mitochondrial function in astrocytes. This results in the intramitochondrial liberation of ammonia, induction of the mPT and the generation of free radicals resulting in astrocytic swelling and brain edema. Deregulation of the NO/cGMP pathway by Gln due to interference with Arg transport across the cell membrane and the promotion of microglial apoptosis represent potential new factors in the mechanism of HE, but whose physiological significance requires further investigation. The actions of Gln at different targets are likely to be operative in the pathogenesis of HE, and this in turn may be complicated by the effects of ammonia or Gln itself on the transfer of newly synthesized astrocytic Gln to other compartments of the CNS (Fig. 1). Influencing the synthesis of glutamine, its metabolism, and intra- extracellular transport may yield promising novel therapeutic approaches for HE and other hyperammonemic states.
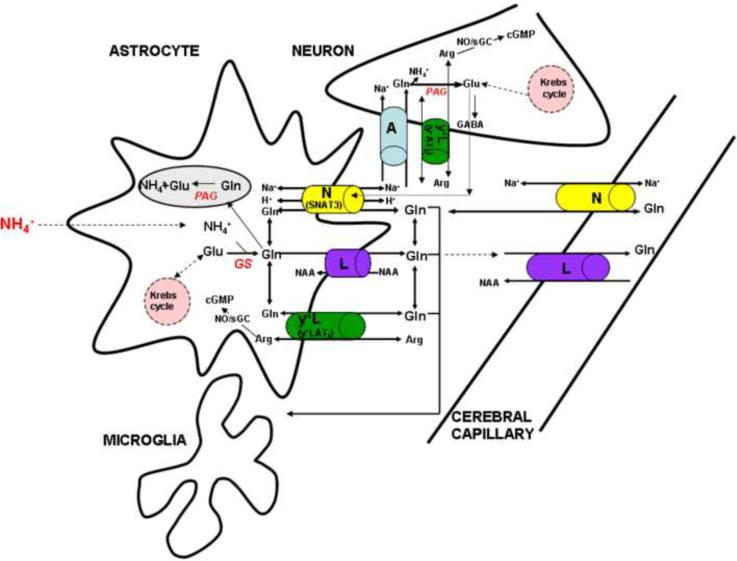
Fig. 1.
Major steps of Gln metabolism and transport in the CNS which may contribute to, or modulate, Gln-mediated ammonia toxicity. Ammonia (NH4+) is incorporated (“detoxified”) into glutamine (Gln) in astrocytes (and perhaps microglia) by the amidation of glutamate (Glu) to glutamine (Gln), a process catalyzed by glutamine synthase (GS). Gln accumulated in astrocytes i) crosses the mitochondrial membrane and is hydrolyzed by phosphate-activated glutaminase (PAG) to glutamate (Glu) and NH4+, and ii) exits astrocytes preferably by the system N (the SNAT3 transporter) which is controlled by extracellular Glu, and partly by system L in exchange for large neutral amino acids (NAA). The Gln that leaves astrocytes enters neurons mainly via system A to be converted to the amino acid neurotransmitters Glu and GABA, and possibly by system y+L (the y+LAT2 transporter) in exchange of Arg, which serves the purpose of modulating the availability of Arg for NO synthesis and thus may influence the rate of the NO/cGMP pathway. A portion of Gln derived from astrocytes leaves the CNS via the L and N systems that are localized in capillary endothelial cells that forms the blood-brain barrier, some of the Gln may enter the microglial cells and modulate their function.
Acknowledgments
Supported by the Ministry of Science and Education of Poland, grants no S 005/P-N/2007/01 (JA) and NN 401 0550 33 (MZ), and by a Department of Veterans Affairs Merit Review Award and NIH grant DK06331 (MDN).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Albrecht J, Jones EA. Hepatic encephalopathy: molecular mechanisms underlying the clinical syndrome. J Neurol Sci. 1999;170:138–46. doi: 10.1016/s0022-510x(99)00169-0. [DOI] [PubMed] [Google Scholar]
- [2].Felipo V, Butterworth RF. Neurobiology of ammonia. Prog Neurobiol. 2002;67:259–69. doi: 10.1016/s0301-0082(02)00019-9. [DOI] [PubMed] [Google Scholar]
- [3].Norenberg MD. Astrocytic-ammonia interactions in hepatic encephalopathy. Semin Liver Dis. 1996;16:245–53. doi: 10.1055/s-2007-1007237. [DOI] [PubMed] [Google Scholar]
- [4].Norenberg MD, Baker L, Norenberg LO, Blicharska J, Bruce-Gregorios JH, Neary JT. Ammonia-induced astrocyte swelling in primary culture. Neurochem Res. 1991;16:833–6. doi: 10.1007/BF00965694. [DOI] [PubMed] [Google Scholar]
- [5].Blei AT. Brain edema in acute liver failure: can it be prevented? Can it be treated? J Hepatol. 2007;46:564–69. doi: 10.1016/j.jhep.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Conn HO, Bircher J. Hepatic encephalopathy: Syndromes and therapies. Medi-Ed Press; Bloomington: 1994. [Google Scholar]
- [7].Hourani BT, Hamlin EM, Reynolds TB. Cerebrospinal fluid glutamine as a measure of hepatic encephalopathy. Arch Intern Med. 1971;127:1033–36. [PubMed] [Google Scholar]
- [8].Swain M, Butterworth RF, Blei AT. Ammonia and related amino acids in the pathogenesis of brain edema in acute ischemic liver failure in rats. Hepatology. 1992;15:449–53. doi: 10.1002/hep.1840150316. [DOI] [PubMed] [Google Scholar]
- [9].Cordoba J, Gottstein J, Blei AT. Glutamine, myo-inositol, and organic brain osmolytes after portocaval anastomosis in the rat: implications for ammonia-induced brain edema. Hepatology. 1996;24:919–23. doi: 10.1002/hep.510240427. [DOI] [PubMed] [Google Scholar]
- [10].Tofteng F, Hauerberg J, Hansen BA, Pedersen CB, Jørgensen L, Larsen FS. Persistent arterial hyperammonemia increases the concentration of glutamine and alanine in the brain and correlates with intracranial pressure in patients with fulminant hepatic failure. J Cereb Blood Flow Metab. 2006;26:21–27. doi: 10.1038/sj.jcbfm.9600168. [DOI] [PubMed] [Google Scholar]
- [11].Albrecht J, Sonnewald U, Waagepetersen HS, Schousboe A. Glutamine in the central nervous system: function and dysfunction. Front Biosci. 2007;12:332–43. doi: 10.2741/2067. [DOI] [PubMed] [Google Scholar]
- [12].Warren KS, Schenker S. Effect of an inhibitor of glutamine synthesis (methionine sulfoximine) on ammonia toxicity and metabolism. J Lab Clin Med. 1964;64:442–49. [PubMed] [Google Scholar]
- [13].Willard-Mack CL, Koehler RC, Hirata T, Cork LC, Takahashi H, Traystman RJ, Brusilow SW. Inhibition of glutamine synthetase reduces ammonia-induced astrocyte swelling in rat. Neuroscience. 1996;71:589–99. doi: 10.1016/0306-4522(95)00462-9. [DOI] [PubMed] [Google Scholar]
- [14].Tanigami H, Rebel A, Martin LJ, Chen TY, Brusilow SW, Traystman RJ, et al. Effect of glutamine synthetase inhibition on astrocyte swelling and altered astroglial protein expression during hyperammonemia in rats. Neuroscience. 2005;131:437–49. doi: 10.1016/j.neuroscience.2004.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bender AS, Norenberg MD. Effects of ammonia on L-glutamate uptake in cultured astrocytes. Neurochem Res. 1996;21:567–73. doi: 10.1007/BF02527755. [DOI] [PubMed] [Google Scholar]
- [16].Master S, Gottstein J, Blei AT. Cerebral blood flow and the development of ammonia-induced brain edema in rats after portacaval anastomosis. Hepatology. 1999;30:876–80. doi: 10.1002/hep.510300428. [DOI] [PubMed] [Google Scholar]
- [17].Sugimoto H, Koehler RC, Wilson DA, Brusilow SW, Traystman RJ. Methionine sulfoximine, a glutamine synthetase inhibitor, attenuates increased extracellular potassium activity during acute hyperammonemia. J Cereb Blood Flow Metab. 1997;17:44–49. doi: 10.1097/00004647-199701000-00006. [DOI] [PubMed] [Google Scholar]
- [18].Takahashi H, Koehler RC, Brusilow SW, Traystman RJ. Restoration of cerebrovascular CO2 responsivity by glutamine synthesis inhibition in hyperammonemic rats. Circ Res. 1992;71:1220–30. doi: 10.1161/01.res.71.5.1220. [DOI] [PubMed] [Google Scholar]
- [19].Kreis R, Farrow N, Ross BD. Diagnosis of hepatic encephalopathy by proton magnetic resonance spectroscopy. Lancet. 1990;336:635–36. doi: 10.1016/0140-6736(90)93439-v. [DOI] [PubMed] [Google Scholar]
- [20].Laubenberger J, Häussinger D, Bayer S, Gufler H, Hennig J, Langer M. Proton magnetic resonance spectroscopy of the brain in symptomatic and asymptomatic patients with liver cirrhosis. Gastroenterology. 1997;112:1610–16. doi: 10.1016/s0016-5085(97)70043-x. [DOI] [PubMed] [Google Scholar]
- [21].Brusilow SW, Traystman R. Letter to the editor. N Engl J Med. 1986;314:786–97. [PubMed] [Google Scholar]
- [22].Pasantes-Morales H, Franco R, Ordaz B, Ochoa LD. Mechanisms counteracting swelling in brain cells during hyponatremia. Archives of Medical Research. 2002;33:237–44. doi: 10.1016/s0188-4409(02)00353-3. [DOI] [PubMed] [Google Scholar]
- [23].Chaudhry FA, Reimer RJ, Krizaj D, Barber D, Storm-Mathisen J, Copenhagen DR, et al. Molecular analysis of system N suggests novel physiological roles in nitrogen metabolism and synaptic transmission. Cell. 1999;99:769–80. doi: 10.1016/s0092-8674(00)81674-8. [DOI] [PubMed] [Google Scholar]
- [24].Jayakumar AR, Panickar KS, Murthy ChR, Norenberg MD. Oxidative stress and mitogen-activated protein kinase phosphorylation mediate ammonia-induced cell swelling and glutamate uptake inhibition in cultured astrocytes. J Neurosci. 2006;26:4774–84. doi: 10.1523/JNEUROSCI.0120-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Murthy CR, Rama Rao KV, Bai G, Norenberg MD. Ammonia-induced production of free radicals in primary cultures of rat astrocytes. J Neurosci Res. 2001;66:282–88. doi: 10.1002/jnr.1222. [DOI] [PubMed] [Google Scholar]
- [26].Ziemińska E, Dolińska M, Lazarewicz JW, Albrecht J. Induction of permeability transition and swelling of rat brain mitochondria by glutamine. Neurotoxicology. 2000;21:295–300. [PubMed] [Google Scholar]
- [27].Pichili VB, Rao KV, Jayakumar AR, Norenberg MD. Inhibition of glutamine ransport into mitochondria protects astrocytes from ammonia toxicity. Glia. 2007;55:801–09. doi: 10.1002/glia.20499. [DOI] [PubMed] [Google Scholar]
- [28].Rama Rao KV, Reddy PV, Tong X, Norenberg MD. Brain edema in acute liver failure: inhibition by L-histidine. Am J Pathol. 2010;176:1400–08. doi: 10.2353/ajpath.2010.090756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sinke AP, Jayakumar AR, Panickar KS, Moriyama M, et al. NFkappaB in the mechanism of ammonia-induced astrocyte swelling in culture. J Neurochem. 2008;106:2302–11. doi: 10.1111/j.1471-4159.2008.05549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Jayakumar Rao KV, Murthy ChR, Norenberg MD. Glutamine in the mechanism of ammonia-induced astrocyte swelling. Neurochem Int. 2004;48:623–28. doi: 10.1016/j.neuint.2005.11.017. [DOI] [PubMed] [Google Scholar]
- [31].Dolińska M, Hilgier W, Albrecht J. Ammonia stimulates glutamine uptake to the cerebral non-synaptic mitochondria of the rat. Neurosci Lett. 1996;213:45–48. doi: 10.1016/0304-3940(96)12827-5. [DOI] [PubMed] [Google Scholar]
- [32].Ziemińska E, Hilgier W, Waagepetersen HS, Hertz L, Sonnewald U, Schousboe A, Albrecht J. Analysis of glutamine accumulation in rat brain mitochondria in the presence of a glutamine uptake inhibitor, histidine, reveals glutamine pools with a distinct access to deamidation. Neurochem Res. 2004;29:2121–23. doi: 10.1007/s11064-004-6885-x. [DOI] [PubMed] [Google Scholar]
- [33].Albrecht J. Norenberg MD Glutamine: a Trojan horse in ammonia neurotoxicity. Hepatology. 2006;44:788–94. doi: 10.1002/hep.21357. [DOI] [PubMed] [Google Scholar]
- [34].Faff-Michalak L, Albrecht J. The two catalytic components of the 2-oxoglutarate dehydrogenase complex in rat cerebral synaptic and nonsynaptic mitochondria: comparison of the response to in vitro treatment with ammonia, hyperammonemia, and hepatic encephalopathy. Neurochem Res. 1993;18:119–23. doi: 10.1007/BF01474673. [DOI] [PubMed] [Google Scholar]
- [35].Faff-Michalak L, Albrecht J. Aspartate aminotransferase, malate dehydrogenase, and pyruvate carboxylase activities in rat cerebral synaptic and nonsynaptic mitochondria: effects of in vitro treatment with ammonia, hyperammonemia and hepatic encephalopathy. Metab Brain Dis. 1991;6:187–97. doi: 10.1007/BF00996918. [DOI] [PubMed] [Google Scholar]
- [36].Hertz L, Kala G. Energy metabolism in brain cells: effects of elevated ammonia concentrations. Metab Brain Dis. 2007;22:199–218. doi: 10.1007/s11011-007-9068-z. [DOI] [PubMed] [Google Scholar]
- [37].Hertz L, Yu AC, Kala G, Schousboe A. Neuronal-astrocytic and cytosolic-mitochondrial metabolite trafficking during brain activation, hyperammonemia and energy deprivation. Neurochem Int. 2000;37:83–102. doi: 10.1016/s0197-0186(00)00012-7. [DOI] [PubMed] [Google Scholar]
- [38].Albrecht J, Norenberg MD. L-methionine-DL-sulfoximine induces massive efflux of glutamine from cortical astrocytes in primary culture. Eur J Pharmacol. 1990;182:587–89. doi: 10.1016/0014-2999(90)90061-a. [DOI] [PubMed] [Google Scholar]
- [39].Lee WJ, Hawkins RA, Viña JR, Peterson DR. Glutamine transport by the blood-brain barrier: a possible mechanism for nitrogen removal. Am J Physiol. 1998;274:C1101–07. doi: 10.1152/ajpcell.1998.274.4.C1101. [DOI] [PubMed] [Google Scholar]
- [40].Cangiano C, Cardelli-Cangiano P, James JH, Rossi-Fanelli F, Patrizi MA, Brackett KA, Strom R, Fischer JE. Brain microvessels take up large neutral amino acids in exchange for glutamine. Cooperative role of Na+-dependent and Na+-independent systems. J Biol Chem. 1983;258:8949–54. [PubMed] [Google Scholar]
- [41].Hilgier W, Puka M, Albrecht J. Characteristics of large neutral amino acid-induced release of preloaded L-glutamine from rat cerebral capillaries in vitro: effects of ammonia, hepatic encephalopathy, and gamma-glutamyl transpeptidase inhibitors. J Neurosci Res. 1992;32:221–26. doi: 10.1002/jnr.490320211. [DOI] [PubMed] [Google Scholar]
- [42].Chaudhry FA, Schmitz D, Reimer RJ, Larsson P, Gray AT, Nicoll R, et al. Glutamine uptake by neurons: interaction of protons with system a transporters. J Neurosci. 2002;22:62–72. doi: 10.1523/JNEUROSCI.22-01-00062.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].O'Kane RL, Viña JR, Simpson I, Hawkins RA. Na+ -dependent neutral amino acid transporters A, ASC, and N of the blood-brain barrier: mechanisms for neutral amino acid removal. Am J Physiol. 2004;287:E622–E29. doi: 10.1152/ajpendo.00187.2004. [DOI] [PubMed] [Google Scholar]
- [44].Hilgier W, Zielinska M, Borkowska HD, Gadamski R, Walski M, Oja SS, Saransaari P, Albrecht J. Changes in the extracellular profiles of neuroactive amino acids in the rat striatum at the asymptomatic stage of hepatic failure. J Neurosci Res. 1999;56:76–84. doi: 10.1002/(SICI)1097-4547(19990401)56:1<76::AID-JNR10>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- [45].Bröer A, Deitmer JW, Bröer S. Astroglial glutamine transport by system N is upregulated by glutamate. Glia. 2004;48:298–10. doi: 10.1002/glia.20081. [DOI] [PubMed] [Google Scholar]
- [46].Michalak A, Rose C, Butterworth J, Butterworth RF. Neuroactive amino acids and glutamate (NMDA) receptors in frontal cortex of rats with experimental acute liver failure. Hepatology. 1996;24:908–13. doi: 10.1002/hep.510240425. [DOI] [PubMed] [Google Scholar]
- [47].Hermenegildo C, Monfort P, Felipo V. Activation of N-methyl-D-aspartate receptors in rat brain in vivo following acute ammonia intoxication: characterization by in vivo brain microdialysis. Hepatology. 2000;31:709–15. doi: 10.1002/hep.510310322. [DOI] [PubMed] [Google Scholar]
- [48].Zielińska M, Hilgier W, Borkowska HD, Oja SS, Saransaari P, Goryński P, Albrecht J. Ammonia-induced extracellular accumulation of taurine in the rat striatum in vivo: role of ionotropic glutamate receptors. Neurochem Res. 2002;27:37–42. doi: 10.1023/a:1014894320421. [DOI] [PubMed] [Google Scholar]
- [49].Albrecht J, Hilgier W, Łazarewicz JW, Rafałowska U, Wysmyk-Cybula U. In: Astrocytes in acute hepatic encephalopathy: Metabolic properties and transport functions in Biochemical Pathology of Astrocytes. MD Norenberg, et al., editors. Liss R; New York: 1988. pp. 465–476. [Google Scholar]
- [50].Knecht K, Michalak A, Rose C, Rothstein JD, Butterworth RF. Decreased glutamate transporter (GLT-1) expression in frontal cortex of rats with acute liver failure. Neurosci Lett. 1997;229:201–03. doi: 10.1016/s0304-3940(97)00444-8. [DOI] [PubMed] [Google Scholar]
- [51].Norenberg MD, Huo Z, Neary JT, Roig-Cantesano A. The glial glutamate transporter in hyperammonemia and hepatic encephalopathy: relation to energy metabolism and glutamatergic neurotransmission. Glia. 1997;21:124–33. [PubMed] [Google Scholar]
- [52].Vogels BA, Maas MA, Daalhuisen J, Quack G, Chamuleau RA. Memantine, a noncompetitive NMDA receptor antagonist improves hyperammonemia-induced encephalopathy and acute hepatic encephalopathy in rats. Hepatology. 1997;25:820–27. doi: 10.1002/hep.510250406. [DOI] [PubMed] [Google Scholar]
- [53].Suárez I, Bodega G, Fernández B. Glutamine synthetase in brain: effect of ammonia. Neurochem Int. 2002;41:123–42. doi: 10.1016/s0197-0186(02)00033-5. [DOI] [PubMed] [Google Scholar]
- [54].Lehmann C, Bette S, Engele J. High extracellular glutamate modulates expression of glutamate transporters and glutamine synthetase in cultured astrocytes. Brain Res. 2009;1297:1–8. doi: 10.1016/j.brainres.2009.08.070. [DOI] [PubMed] [Google Scholar]
- [55].Albrecht J, Dolińska M, Hilgier W, Lipkowski AW, Nowacki J. Modulation of glutamine uptake and phosphate-activated glutaminase activity in rat brain mitochondria by amino acids and their synthetic analogues. Neurochem Int. 2000;36:341–47. doi: 10.1016/s0197-0186(99)00142-4. [DOI] [PubMed] [Google Scholar]
- [56].Chatauret N, Zwingmann C, Rose C, Leibfritz D, Butterworth RF. Effects of hypothermia on brain glucose metabolism in acute liver failure: a H/C-nuclear magnetic resonance study. Gastroenterology. 2003;125:815–24. doi: 10.1016/s0016-5085(03)01054-0. [DOI] [PubMed] [Google Scholar]
- [57].Stravitz RT, Larsen FS. Therapeutic hypothermia for acute liver failure. Crit Care Med. 2009;37(7 Suppl):S258–S264. doi: 10.1097/CCM.0b013e3181aa5fb8. [DOI] [PubMed] [Google Scholar]
- [58].Zielińska M, Law RO, Albrecht J. Excitotoxic mechanism of cell swelling in rat cerebral cortical slices treated acutely with ammonia. Neurochem Int. 2003;43:299–03. doi: 10.1016/s0197-0186(03)00015-9. [DOI] [PubMed] [Google Scholar]
- [59].Kanamori K, Ross BD, Chung JC, Kuo EL. Severity of hyperammonemic encephalopathy correlates with brain ammonia level and saturation of glutamine synthetase in vivo. J Neurochem. 1996;67:1584–94. doi: 10.1046/j.1471-4159.1996.67041584.x. [DOI] [PubMed] [Google Scholar]
- [60].Scorticati C, Prestifilippo JP, Eizayaga FX, Castro JL, Romay S, Fernández MA, et al. Hyperammonemia, brain edema and blood-brain barrier alterations in prehepatic portal hypertensive rats and paracetamol intoxication. World J Gastroenterol. 2004;10:1321–24. doi: 10.3748/wjg.v10.i9.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Peters EL, Tower DB. Glutamic acid and glutamine metabolismin cerebral cortex after seizures induced by methionine sulphoximine. J Neurochem. 1959;5:80–90. doi: 10.1111/j.1471-4159.1959.tb13336.x. [DOI] [PubMed] [Google Scholar]
- [62].Cloix J-F, Hévor T. Epilepsy, Regulation of brain energy metabolism and neurotransmission. Curr Med Chem. 2009;16:841–53. doi: 10.2174/092986709787549316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Dombro RS, Bender AS, Norenberg MD. Association between cell swelling and glycogen content in cultured astrocytes. Int J Dev Neurosci. 2000;18:161–69. doi: 10.1016/s0736-5748(99)00084-2. [DOI] [PubMed] [Google Scholar]
- [64].Zielińska M, Stafiej A, Law RO, Albrecht J. Effects of methionine sulfoximine on the glutamine and glutamate content and cell volume in rat cerebral cortical slices: involvement of mechanisms not related to inhibition of glutamine synthesis. Neurotoxicology. 2004;25:443–49. doi: 10.1016/j.neuro.2003.10.003. [DOI] [PubMed] [Google Scholar]
- [65].Norenberg MD. Oxidative and nitrosative stress in ammonia neurotoxicity. Hepatology. 2003;37:245–48. doi: 10.1053/jhep.2003.50087. [DOI] [PubMed] [Google Scholar]
- [66].Norenberg MD, Jayakumar AR, Rama Rao KV. Oxidative stress in the pathogenesis of hepatic encephalopathy. Metab Brain Dis. 2004;19:313–25. doi: 10.1023/b:mebr.0000043978.91675.79. [DOI] [PubMed] [Google Scholar]
- [67].Hilgier W, Oja SS, Saransaari P, Albrecht J. A novel glycine site-specific N-methyl-D-aspartate receptor antagonist prevents activation of the NMDA/NO/CGMP pathway by ammonia. Brain Res. 2004;1015:186–88. doi: 10.1016/j.brainres.2004.05.014. [DOI] [PubMed] [Google Scholar]
- [68].Hilgier W, Freśko I, Klemenska E, Beresewicz A, Oja SS, Saransaari P, et al. Glutamine inhibits ammonia-induced accumulation of cGMP in rat striatum limiting arginine supply for NO synthesis. Neurobiol Dis. 2009;35:75–81. doi: 10.1016/j.nbd.2009.04.004. [DOI] [PubMed] [Google Scholar]
- [69].Konopacka A, Konopacki F, Albrecht J. Protein kinase G is involved in ammonia-induced swelling of astrocytes. J Neurochem. 2009;109(Suppl 1):246–51. doi: 10.1111/j.1471-4159.2009.05802.x. [DOI] [PubMed] [Google Scholar]
- [70].Monfort P, Erceg S, Piedrafita B, Llansola M, Felipo V. Chronic liver failure in rats impairs glutamatergic synaptic transmission and long-term potentiation in hippocampus and learning ability. Eur J Neurosci. 2007;25:2103–11. doi: 10.1111/j.1460-9568.2007.05444.x. [DOI] [PubMed] [Google Scholar]
- [71].Schliess F, Görg B, Fischer R, Desjardins P, Bidmon HJ, Herrmann A, et al. Ammonia induces MK-801-sensitive nitration and phosphorylation of protein tyrosine residues in rat astrocytes. FASEB. 2002;J 16:739–41. doi: 10.1096/fj.01-0862fje. [DOI] [PubMed] [Google Scholar]
- [72].Häussinger D, Görg B. Interaction of oxidative stress, astrocyte swelling and cerebral ammonia toxicity. Curr Opin Clin Nutr Metab. 2010;13:87–92. doi: 10.1097/MCO.0b013e328333b829. [DOI] [PubMed] [Google Scholar]
- [73].Bröer A, Wagner CA, Lang F, Bröer S. The heterodimeric amino acid transporter 4F2hc/y+LAT2 mediates arginine efflux in exchange with glutamine. Biochem J. 2000;349:787–95. doi: 10.1042/bj3490787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Bae SY, Xu Q, Hutchinson D, Colton CA. Y+ and y+ L arginine transporters in neuronal cells expressing tyrosine hydroxylase. Biochim Biophys Acta. 2005;1745:65–73. doi: 10.1016/j.bbamcr.2004.12.006. [DOI] [PubMed] [Google Scholar]
- [75].Svoboda N, Kerschbaum HH. L-Glutamine-induced apoptosis in microglia is mediated by mitochondrial dysfunction. Eur J Neuroscience. 2009;30:196–06. doi: 10.1111/j.1460-9568.2009.06828.x. [DOI] [PubMed] [Google Scholar]
- [76].Hawkins RA, Jessy J, Mans AM, De Joseph MR. Effect of reducing brain glutamine synthesis on metabolic symptoms of hepatic encephalopathy. J Neurochem. 1993;60:1000–06. doi: 10.1111/j.1471-4159.1993.tb03247.x. [DOI] [PubMed] [Google Scholar]