Abstract
A number of studies have found an association between attentional bias for negative stimuli and variation in the serotonin transporter promoter region polymorphism (5-HTTLPR). The current project examined whether a positive social environment mitigates this association. More specifically, we examined the relationship between attentional bias on the dot-probe task, variation in the 5-HTTLPR and current social support among a community sample of adults (N=216). Consistent with prior research, the S/LG homozygotes were more likely than the other genotype groups to have a negative attention bias. However, social support moderated the association between 5-HTTLPR variation and attentional bias. The S/LG homozygote group was particularly likely to exhibit greater attentional bias towards negative stimuli at low levels of social support. However, as social support improved, negative attention bias decreased. Findings suggest that supportive environments may attenuate genetic associations with negative attention bias.
Keywords: Depression, Environmental Effects, Genetics, Attention, Cognitive Processes
Introduction
The cognitive model of depression (Beck, 1967) posits that attentional bias towards negative stimuli plays an important role in the development of depression. A negative attentional bias occurs when an individual disproportionately attends to negative stimuli over neutral or positive stimuli. Negative attentional bias has been implicated in the onset of depression (Beevers, Lee, Wells, Ellis, & Telch, 2011), has been linked to prolonged negative affect (Clasen, Wells, Ellis, & Beevers, 2013; Sanchez, Vazquez, Marker, LeMoult, & Joormann, 2013), and increases in stress responsivity (Fox, Cahill, & Zougkou, 2010). Although depression-vulnerable individuals often show a negative attentional bias compared to controls (Joormann & Gotlib, 2007), not all vulnerable individuals shows this bias. Thus, individual differences may play a critical role in determining who is likely to possess a negative attentional bias.
Serotonergic function is one individual difference that has been associated with the pathogenesis of depression (Olfson et al., 2002; Stockmeier et al., 1998), although the exact mechanisms behind this association are not yet completely understood (Lacasse & Leo, 2005). In recent years, serotonergic genetic variation has been intensely studied in relation to depression. The serotonin transporter linked polymorphic region polymorphism (5-HTTLPR) in the promoter region of the serotonin transporter gene (SLC6A4) has been of particular interest to researchers, as it putatively influences transcriptional activity and availability of serotonin (Collier et al., 1996). Of note, a recent meta-analysis suggests that individuals with two copies of the 5-HTTLPR S/LG variants are more likely to have a negative attentional bias than carriers of other variants (Pergamin-Hight, Bakermans-Kranenburg, van Ijzendoorn, & Bar-Haim, 2012). Thus, variation in the 5-HTTLPR appears to be reliably associated with negatively biased attention.
The current study aims to determine whether social support moderates the association between 5-HTTLPR variation and negative attention bias. We focus on this possibility for the following reasons. First, environmental adversity is an important risk factor for the onset of depression (Mazure, 1998), although it seems unlikely that all environmental adversity is created equally. Social support may be especially important in the context of depression. For example, low social support prospectively predicted the emergence of depressive symptoms (George, Blazer, Hughes, & Fowler, 1989), while high social support can buffer the deleterious effects of stressors and attenuate the onset of depression (Cohen & Hoberman, 1983). The presence of social support also influences the course of depression. For example, low social support increases the risk of suicidal behavior (Suresh Kumar & George, 2013) and is associated with depression persistence (Lara, Leader, & Klein, 1997).
Second, earlier work suggests that carriers of at least one short allele of the 5-HTTLPR are at increased likelihood for developing depression when they encounter environmental adversity (Caspi et al., 2003), although this finding remains somewhat controversial as a number of conflicting meta-analytic results have been observed (Risch et al. 2009; Karg K, Burmeister M, Shedden K, & Sen S, 2011). Nevertheless, and most importantly for the current study, social stressors have specifically been shown to interact with 5-HTTLPR variation to predict MDD onset (Vrshek-Schallhorn et al., 2013). Notably, non-social stressors did not have a similar association, suggesting that social stressors are particularly important for the development of depression. Furthermore, the short allele of 5-HTTLPR has been shown to predispose individuals to depression in the context of environmental adversity and low social support (Kilpatrick et al., 2007). Thus, it appears likely that the social environment could also impact the association between 5-HTTLPR variation and other depression-related phenotypes, such as a negative attention bias.
The aim of this study was twofold. First, we examined whether we could replicate prior research that suggests S or LG 5-HTTLPR homozygotes have a greater negative attentional bias than other 5-HTTLPR genotype groups. Second, we examined if the social environment moderated the relationship between 5-HTTLPR variation and negative attentional bias. We hypothesized that S or LG 5-HTTLPR homozygotes would show more negative attentional bias, particularly under conditions of low social support. In contrast, the association between 5-HTTLPR variation and negative attentional bias would be attenuated when high social support is present.
Methods
Participants
Participating community members (N=216, Mean age= 25, SD= 4.28, 58% female) were 61% Caucasian, 20% Asian, 5% African American, 5% more than one race, and 1% Hawaiian/Pacific Islander, with 8% not endorsing race. Participants did not meet criteria for serious current psychopathology, as determined by the Mini International Neuropsychiatric Interview (MINI;Sheehan et al., 1998). Healthy individuals with no past or current psychopathology were recruited in an effort to isolate the genetic and environmental contributions to the attentional bias while removing third variable explanations, such as the presence of psychopathology, which may be related to both social support and variation within the selected gene.
Measures
Genotyping
Genomic DNA was isolated from buccal cells and saliva using a modification of published methods (Freeman et al., 1997; Lench, Stanier, & Williamson, 1988; Meulenbelt, Droog, Trommelen, Boomsma, & Slagboom, 1995; Spitz et al., 1996). Participants expectorated 2 ml of saliva into a 50ml tube. Swabs previously impregnated and dried with lysis buffer (500 μl of 1 MTris–HCl; pH 8.0) 500 μl of 10% sodium docecyl sulfate; and 100 μl of 5 M sodium chloride were then added to the 50ml tube. Samples were stored at 4 °C until the DNA was extracted.
The assay for 5-HTTLPR was a modification of that used by Lesch and colleagues (Lesch et al., 1996). The primer sequences are forward, 5′-GGCGTTGCCGCTCTGAATGC-3′ (fluorescently labeled), and reverse, 5′-GAGGGACTGAGCTGGACAACCAC-3′ with yield products of 484 or 528 bp. To distinguish between the S, LA, and LG fragments, the PCR fragment was digested with MspI by methods described in Wigg et al. 2006. Allele sizes are scored by two investigators independently and inconsistencies were reviewed and rerun when necessary. An exact test for multiallelic Hardy Weinberg Equilibrium suggested that the sample deviated significantly from Hardy Weinberg Equilibrium (p = 3.5 × 10 −5) as there were an excess of homozygotes.
Previous research (Hu et al., 2005; Zalsman et al., 2006) suggests that the LG allele and the S allele are similar in terms of transcriptional activity. Therefore, the S and LG alleles were treated as equivalents. For the sake of brevity and ease, LG will hereafter be referred to as S.
Interpersonal Support Evaluation List
Quality of social support was measured by the Interpersonal Support Evaluation List (ISEL:Cohen & Hoberman, 1983). The ISEL consists of 40-items and is made up of 4 scales; Appraisal support, Belonging support, Tangible support and Self-esteem support, which consist of 10 items each. In this study only total ISEL scores were considered. Items are scored on a four-point Likert scale ranging from “probably false” to “definitely true”. Internal and test-retest reliability are satisfactory, and the scale has demonstrated convergent validity (Cohen, Mermelstein, Kamarck, & Hoberman, 1985).
Dot-probe task
Attentional bias was measured with the dot-probe task. The task was presented on a 20-inch computer monitor. A fixation cross was presented for 500 ms, followed by a pair of stimuli depicting an emotional or neutral facial expression that was presented for 1000ms. The stimuli were selected from the KDEF stimulus set (REF) and presented on the left and right side of visual field. Location of the emotion and neutral stimulus varied randomly. The stimuli pairs consisted of an emotionally valenced facial expression (e.g., sad or happy) and a neutral facial expression. Following offset of the stimuli pair, a target, consisting of the letter O or Q, appeared in the place of either stimulus. The target remained on screen until participants responded by classifying the target as O or Q. The latency and accuracy of their responses was recorded. Incorrect responses were excluded from the analyses. A total of 12 sad, 12 happy, and 24 neutral facial expressions were presented four times each in two different blocks for a total of 192 trials. Stimuli were matched for actor so that the only difference between stimuli pairs was emotion expression.
As described elsewhere (Gotlib, Krasnoperova, Yue, & Joormann, 2004), attentional bias scores were calculated using the following formula:
where R = right position, L = left position, t = target and e = emotional word stimulus. A positive bias score reflects a bias toward the emotional stimuli while a negative bias score reflects a bias away from the emotional stimuli.
Center for Epidemiological studies Depression scale
The Center for Epidemiological studies Depression scale (CESD; Radloff, 1977) was used to measure severity of depressive symptoms. The CESD has 20 items; a sample item is “I thought my life had been a failure”. The presence of symptoms in the last week is rated on a four-point Likert scale ranging from “Not at all” to “A lot”. The CESD has demonstrated reliability and validity (Radloff, 1977).
Procedure
All study procedures were completed at the University of Texas. Informed consent was obtained and eligibility criteria were confirmed by structured clinical interview. The self-report measures and a saliva sample for genetic analysis were collected during a one-time laboratory assessment. This study was approved by the Institutional Review Board at the University of Texas at Austin.
Statistical Analysis
All analyses were performed in Stata 12 (StataCorp, 2013). The assumptions underlying regression were tested and confirmed for all analyses. Standardized residuals following statistical modeling were examined for outliers using the Grubbs test. No outliers were detected.
Results
Linear regressions were used to examine the relationship between 5-HTTLPR genotype, social support and attentional bias. Social support and attentional bias were modeled as continuous variables and 5-HTTLPR genotype was modeled as a group variable. Depression severity (as measured by the CESD) was entered into all models as a covariate. The correlation between 5-HTTLPR genotype and the social environment (as measured by the ISEL) was non-significant (r = −0.0899, p = 0.1870), thus ruling out substantial gene-environment correlation. All reported results were highly consistent when race was accounted for, reducing the likelihood that the results were the result of population stratification.
Main effects
There was a significant main effect of 5-HTTLPR genotype on negative attentional bias (F(2,212) = 4.01, p=0.02, R2= 0.04). Individuals with the SS genotype had significantly more negative attentional bias than individuals with the SL and LL genotype (see Figure 1). In a separate model, there was no significant main effect of level of social support on negative attentional bias (F(2,213) = 0.98, p=0.4, R2= 0.01). To examine the specificity of these results to negative attentional bias, we also examined the main effects of 5-HTTLPR genotype and social support with positive attentional bias. There was no main effect of 5-HTTLPR genotype on positive attention bias (F(2,212) = 1.24, p=0.29), nor a main effect of social support (F(2,213) = 0.96, p=0.4, R2= 0.01).
Figure 1.
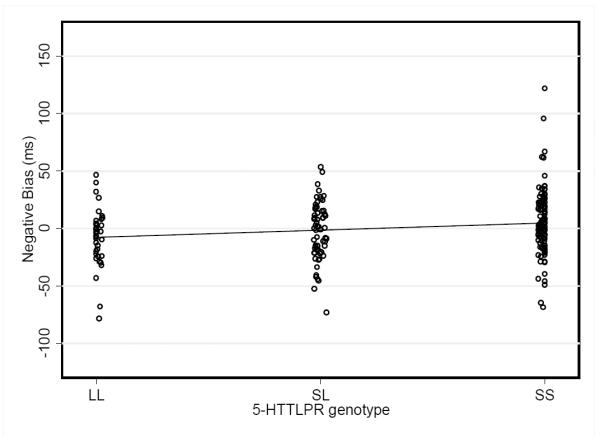
Relationship between 5-HTTLPR genotype and negative attentional bias.
5-HTTLPR x ISEL interaction
We then examined the interaction between ISEL scores and 5-HTTLPR: this interaction significantly predicted negative attentional bias (F(2,206) = 3.96, p=0.02, R2(model)= 0.095; see Figure 2). To clarify this interaction we first examined which slopes significantly differed from 0. The slope for the SS group (b = −.48, SE = .19, p = 0.01) was significantly different from 0, but the slopes of the SL (b = .39, SE = .30, p = 0.2) and LL (b = .68, SE = .34, p = 0.5) groups were not. This indicates that negative attention bias decreased as ISEL scores increased, but only in the SS group.
Figure 2.
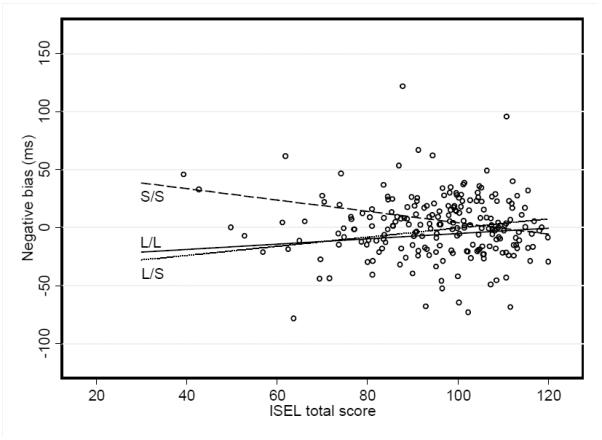
Relationship between negative attentional bias and social support (ISEL total scores) presented as function of 5-HTTLPR genotype.
Next, we examined if the slopes for each genotype were significantly different from each other. The slope of the SS group was marginally different from the slope of the LL group (F(1,206) = 3.61, p=0.06) and significantly different from the slope of the SL group (F(1,206) = 6.11, p=0.01). Regions of significance testing for the LL versus SL groups indicated no region where the slopes differed from each other; however, the slope for the SS group differed from the slope of the LL group at an ISEL total < 100 (p = .009) and the SS and SL slopes differed at an ISEL < 96 (p=0.046). These cut-points were just above the ISEL sample mean (M = 95.85, SD = 14.50).
Again, to establish the specificity of these results, we examined the interaction of 5-HTTLPR genotype and social support on positive attentional bias. This interaction was not significant (F(2,206) = 1.06, p=0.35).
Conclusion
In the current study we examined the relationship between 5-HTTLPR genotype, social support and negative attentional bias. We first replicated previous findings that S/LG homozygotes were more likely to have a negative attentional bias than the other 5-HTTLPR genotype groups (Pergamin-Hight et al., 2012). There are now a large number of studies that have found this association, which is notable for genetic association studies that have often been quite inconsistent (Kruijt, Putman, & Van der Does, 2014; Naudts, Azevedo, David, van Heeringen, & Gibbs, 2012).
However, we build upon this prior work by demonstrating for the first time that the relationship between 5-HTTLPR genotype and negative attentional bias may be moderated by the social environment. More specifically, negative attention biases were present among S allele homozygotes with adverse social circumstances (i.e., low social support). Furthermore, the model that included social environment as a moderating variable explained more of the variance than the model examining the main effect of 5-HTTLPR only (9.5% vs 4%).
These findings have several implications. First, when examining genetic contributions to negative attentional bias it may also be important to consider the role of the environment in order to maximize explanatory power. Second, these findings support earlier work (Slavich, O'Donovan, Epel, & Kemeny, 2010; Vrshek-Schallhorn et al., 2013) suggesting that the social environment may be especially important in the etiology of depression. Going forward, investigators might distinguish between different types of environmental stressors—the so-called candidate environment—since it seems unlikely that all environmental stressors influence depression (or related phenotypes) to the same degree.
There are several limitations to this study that should be noted. The sample size (N = 216) is relatively small for a genetic association study (particularly given our interest in the interaction between social support and genetic variation), so these results need to be replicated in a larger sample before we can be highly confident in the reliability of these findings. In this study no measures of non-social environments were included, therefore we cannot conclude that that the moderating effect of the environment is specific to the social environment. We also relied on self-report measures of environment, which are prone to bias (Monroe & Reid, 2008). Including interview-based measures that cover a broad range of environment types (e.g., Adrian & Hammen, 1993; Hammen, 1991) would strengthen the methodology. Future work may also examine the severity and timing of environmental stressors. More severe stressors (Vrshek-Schallhorn et al., 2013) and stressors that occur early in life (Heim, Plotsky, & Nemeroff, 2004) may be especially important in the context of a genetic vulnerability. Since it is impossible to assign individuals to genotypes, we cannot exclude that unmeasured third variables (e.g., linkage disequilibrium or population stratification) are driving these results. Lastly, attentional bias is likely influenced by many genes, therefore using methods that model the contribution of multiple genes to attentional bias might explain more of the variance (Lubke et al., 2012).
Despite these limitations, to our knowledge this study is the first to examine the relationship between 5-HTTLPR genotype, social environment and negative attentional bias. These findings suggest that individual differences in attentional bias might be influenced by genetic variation and social support, suggesting a pathway through which genetic and environmental vulnerability might influence the onset of depression.
Acknowledgements
This study was funded by a NIDA grant DA032457 to WTM and CGB, as well as 1S10RR023457-01A1 and Shared equipment grants (ShEEP) from the Medical Research Service of the Department of Veteran Affairs awarded to JEM. We would like to thank Alex Kline, Seth Koslov and the rest of the MaddoxLab RAs for their help with data collection. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
Footnotes
The authors declare no conflict of interest.
References
- Adrian C, Hammen C. Stress exposure and stress generation in children of depressed mothers. Journal of Consulting and Clinical Psychology. 1993;61(2):354–359. doi: 10.1037//0022-006x.61.2.354. doi:10.1037/0022-006X.61.2.354. [DOI] [PubMed] [Google Scholar]
- Beck AT. Depression: Clinical, Experimental, and Theoretical Aspects. University of Pennsylvania Press; 1967. [Google Scholar]
- Beevers CG, Lee H-J, Wells TT, Ellis AJ, Telch MJ. Association of Predeployment Gaze Bias for Emotion Stimuli With Later Symptoms of PTSD and Depression in Soldiers Deployed in Iraq. American Journal of Psychiatry. 2011;168(7):735–741. doi: 10.1176/appi.ajp.2011.10091309. doi:10.1176/appi.ajp.2011.10091309. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, Poulton R. Influence of Life Stress on Depression: Moderation by a Polymorphism in the 5-HTT Gene. Science. 2003;301(5631):386–389. doi: 10.1126/science.1083968. doi:10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Clasen PC, Wells TT, Ellis AJ, Beevers CG. Attentional biases and the persistence of sad mood in major depressive disorder. Journal of Abnormal Psychology. 2013;122(1):74–85. doi: 10.1037/a0029211. doi:10.1037/a0029211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Hoberman HM. Positive Events and Social Supports as Buffers of Life Change Stress1. Journal of Applied Social Psychology. 1983;13(2):99–125. doi:10.1111/j.1559-1816.1983.tb02325.x. [Google Scholar]
- Cohen S, Mermelstein R, Kamarck T, Hoberman HM. Measuring the Functional Components of Social Support. In: Sarason IG, Sarason BR, editors. Social Support: Theory, Research and Applications. Springer; Netherlands: 1985. pp. 73–94. Retrieved from http://link.springer.com/chapter/10.1007/978-94-009-5115-0_5. [Google Scholar]
- Collier DA, Stöber G, Li T, Heils A, Catalano M, Di Bella D, Lesch KP. A novel functional polymorphism within the promoter of the serotonin transporter gene: Possible role in susceptibility to affective disorders. Molecular Psychiatry. 1996;1(6):453–460. [PubMed] [Google Scholar]
- Fox E, Cahill S, Zougkou K. Preconscious processing biases predict emotional reactivity to stress. Biological Psychiatry. 2010;67(4):371–377. doi: 10.1016/j.biopsych.2009.11.018. doi:10.1016/j.biopsych.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman B, Powell J, Ball D, Hill L, Craig I, Plomin R. DNA by mail: an inexpensive and noninvasive method for collecting DNA samples from widely dispersed populations. Behavior Genetics. 1997;27(3):251–257. doi: 10.1023/a:1025614231190. [DOI] [PubMed] [Google Scholar]
- George LK, Blazer DG, Hughes DC, Fowler N. Social support and the outcome of major depression. The British Journal of Psychiatry. 1989;154(4):478–485. doi: 10.1192/bjp.154.4.478. doi:10.1192/bjp.154.4.478. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Krasnoperova E, Yue DN, Joormann J. Attentional Biases for Negative Interpersonal Stimuli in Clinical Depression. Journal of Abnormal Psychology. 2004;113(1):127–135. doi: 10.1037/0021-843X.113.1.121. doi:10.1037/0021-843X.113.1.121. [DOI] [PubMed] [Google Scholar]
- Hammen C. Generation of stress in the course of unipolar depression. Journal of Abnormal Psychology. 1991;100(4):555–561. doi: 10.1037//0021-843x.100.4.555. [DOI] [PubMed] [Google Scholar]
- Heim C, Plotsky PM, Nemeroff CB. Importance of studying the contributions of early adverse experience to neurobiological findings in depression. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2004;29(4):641–648. doi: 10.1038/sj.npp.1300397. doi:10.1038/sj.npp.1300397. [DOI] [PubMed] [Google Scholar]
- Hu X, Oroszi G, Chun J, Smith TL, Goldman D, Schuckit MA. An expanded evaluation of the relationship of four alleles to the level of response to alcohol and the alcoholism risk. Alcoholism, Clinical and Experimental Research. 2005;29(1):8–16. doi: 10.1097/01.alc.0000150008.68473.62. [DOI] [PubMed] [Google Scholar]
- Joormann J, Gotlib IH. Selective attention to emotional faces following recovery from depression. Journal of Abnormal Psychology. 2007;116(1):80–85. doi: 10.1037/0021-843X.116.1.80. doi:10.1037/0021-843X.116.1.80. [DOI] [PubMed] [Google Scholar]
- Karg K, Burmeister M, Shedden K, Sen S. The serotonin transporter promoter variant (5-httlpr), stress, and depression meta-analysis revisited: Evidence of genetic moderation. Archives of General Psychiatry. 2011;68(5):444–454. doi: 10.1001/archgenpsychiatry.2010.189. doi:10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick PD, Dean, Koenen PD, Karestan, Ruggiero PD, Kenneth, Acierno PD, Ron, Galea MD, Dr.P.H., Sandro, Resnick PD, Heidi, Gelernter MD, Joel The Serotonin Transporter Genotype and Social Support and Moderation of Posttraumatic Stress Disorder and Depression in Hurricane-Exposed Adults. American Journal of Psychiatry. 2007;164(11):1693–1699. doi: 10.1176/appi.ajp.2007.06122007. doi:10.1176/appi.ajp.2007.06122007. [DOI] [PubMed] [Google Scholar]
- Kruijt A-W, Putman P, Van der Does W. The 5-HTTLPR polymorphism, early and recent life stress, and cognitive endophenotypes of depression. Cognition & Emotion. 2014;28(7):1149–1163. doi: 10.1080/02699931.2013.873018. doi:10.1080/02699931.2013.873018. [DOI] [PubMed] [Google Scholar]
- Lacasse JR, Leo J. Serotonin and Depression: A Disconnect between the Advertisements and the Scientific Literature. PLoS Med. 2005;2(12):e392. doi: 10.1371/journal.pmed.0020392. doi:10.1371/journal.pmed.0020392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara ME, Leader J, Klein DN. The association between social support and course of depression: Is it confounded with personality? Journal of Abnormal Psychology. 1997;106(3):478–482. doi: 10.1037//0021-843x.106.3.478. doi:10.1037/0021-843X.106.3.478. [DOI] [PubMed] [Google Scholar]
- Lench N, Stanier P, Williamson R. Simple non-invasive method to obtain DNA for gene analysis. Lancet. 1988;1(8599):1356–1358. doi: 10.1016/s0140-6736(88)92178-2. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science (New York, N.Y.) 1996;274(5292):1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Lubke GH, Hottenga JJ, Walters R, Laurin C, de Geus EJC, Willemsen G, Boomsma DI. Estimating the Genetic Variance of Major Depressive Disorder Due to All Single Nucleotide Polymorphisms. Biological Psychiatry. 2012;72(8):707–709. doi: 10.1016/j.biopsych.2012.03.011. doi:10.1016/j.biopsych.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazure CM. Life Stressors as Risk Factors in Depression. Clinical Psychology: Science and Practice. 1998;5(3):291–313. doi:10.1111/j.1468-2850.1998.tb00151.x. [Google Scholar]
- Meulenbelt I, Droog S, Trommelen GJ, Boomsma DI, Slagboom PE. High-yield noninvasive human genomic DNA isolation method for genetic studies in geographically dispersed families and populations. American Journal of Human Genetics. 1995;57(5):1252–1254. [PMC free article] [PubMed] [Google Scholar]
- Monroe SM, Reid MW. Gene-Environment Interactions in Depression Research Genetic Polymorphisms and Life-Stress Polyprocedures. Psychological Science. 2008;19(10):947–956. doi: 10.1111/j.1467-9280.2008.02181.x. doi:10.1111/j.1467-9280.2008.02181.x. [DOI] [PubMed] [Google Scholar]
- Naudts KH, Azevedo RT, David AS, van Heeringen K, Gibbs AA. Epistasis between 5-HTTLPR and ADRA2B polymorphisms influences attentional bias for emotional information in healthy volunteers. The International Journal of Neuropsychopharmacology / Official Scientific Journal of the Collegium Internationale Neuropsychopharmacologicum (CINP) 2012;15(8):1027–1036. doi: 10.1017/S1461145711001295. doi:10.1017/S1461145711001295. [DOI] [PubMed] [Google Scholar]
- Olfson M, Marcus SC, Druss B, Elinson L, Tanielian T, Pincus HA. National trends in the outpatient treatment of depression. JAMA: The Journal of the American Medical Association. 2002;287(2):203–209. doi: 10.1001/jama.287.2.203. [DOI] [PubMed] [Google Scholar]
- Pergamin-Hight L, Bakermans-Kranenburg MJ, van Ijzendoorn MH, Bar-Haim Y. Variations in the promoter region of the serotonin transporter gene and biased attention for emotional information: a meta-analysis. Biological Psychiatry. 2012;71(4):373–379. doi: 10.1016/j.biopsych.2011.10.030. doi:10.1016/j.biopsych.2011.10.030. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale A Self-Report Depression Scale for Research in the General Population. Applied Psychological Measurement. 1977;1(3):385–401. doi:10.1177/014662167700100306. [Google Scholar]
- Sanchez A, Vazquez C, Marker C, LeMoult J, Joormann J. Attentional disengagement predicts stress recovery in depression: An eye-tracking study. Journal of Abnormal Psychology. 2013;122(2):303–313. doi: 10.1037/a0031529. doi:10.1037/a0031529. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. The Journal of Clinical Psychiatry. 1998;59(Suppl 20):22–33. quiz 34–57. [PubMed] [Google Scholar]
- Slavich GM, O'Donovan A, Epel ES, Kemeny ME. Black sheep get the blues: A psychobiological model of social rejection and depression. Neuroscience & Biobehavioral Reviews. 2010;35(1):39–45. doi: 10.1016/j.neubiorev.2010.01.003. doi:10.1016/j.neubiorev.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitz E, Moutier R, Reed T, Busnel MC, Marchaland C, Roubertoux PL, Carlier M. Comparative diagnoses of twin zygosity by SSLP variant analysis, questionnaire, and dermatoglyphic analysis. Behavior Genetics. 1996;26(1):55–63. doi: 10.1007/BF02361159. [DOI] [PubMed] [Google Scholar]
- Stockmeier CA, Shapiro LA, Dilley GE, Kolli TN, Friedman L, Rajkowska G. Increase in Serotonin-1A Autoreceptors in the Midbrain of Suicide Victims with Major Depression—Postmortem Evidence for Decreased Serotonin Activity. The Journal of Neuroscience. 1998;18(18):7394–7401. doi: 10.1523/JNEUROSCI.18-18-07394.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrshek-Schallhorn S, Mineka S, Zinbarg RE, Craske MG, Griffith JW, Sutton J, Adam EK. Clinical Psychological Science. 2013. Refining the Candidate Environment Interpersonal Stress, the Serotonin Transporter Polymorphism, and Gene-Environment Interactions in Major Depression. 2167702613499329. doi:10.1177/2167702613499329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalsman MD, Gil, Huang MS, Yung-yu, Oquendo MD, Maria, Burke PD, Ainsley, Hu M, Ph.D., Xian-zhang, Brent MD, David, Mann MD, J. Association of a Triallelic Serotonin Transporter Gene Promoter Region (5-HTTLPR) Polymorphism With Stressful Life Events and Severity of Depression. American Journal of Psychiatry. 2006;163(9):1588–1593. doi: 10.1176/ajp.2006.163.9.1588. doi:10.1176/appi.ajp.163.9.1588. [DOI] [PubMed] [Google Scholar]


