Abstract
Aging in female rats is characterized by a state called “constant estrous” in which rats are unable to ovulate, have polycystic ovaries and moderately elevated estrogen levels. We hypothesized that chronic exposure of young animals to low levels of E2 can produce reproductive changes similar to that seen in aging animals. Adult female rats were sham-implanted (control) or implanted with slow-release E2 (20 ng/day) pellets for 30, 60, or 90 days. Old constant estrous (OCE) rats were used for comparison. Estrous cyclicity was monitored periodically. At the end of treatment, animals were sacrificed, trunk blood was collected for hormone measurements and ovaries for immunohistochemistry. Young animals became acyclic with increasing duration of E2 exposure while OCE rats were in a state of acyclicity. Ovaries became increasingly more cystic with E2 exposure, and were comparable to OCE rats; however, there was a marked reduction in interstitial tissue with exogenous E2 treatment. Exogenous E2 also decreased Mullerian inhibiting substance expression, increased infiltration of macrophages without much impact on apoptosis in the ovaries. Serum testosterone levels decreased in E2-treated young animals, while it increased significantly in OCE rats. There was a marked reduction in LH but not FSH levels with E2 exposure in both young and old animals. These results indicate that even very low doses of E2 are capable of inducing aging-like changes in young animals.
Keywords: estrogen, follicular cysts, ovary, TUNEL, CD68, Mullerian inhibiting substance
Introduction
Women are chronically exposed to low levels of estrogen in the form of oral contraceptives1, hormone replacement therapy2 and estrogenic environmental endocrine disruptors3. Women of reproductive age are also exposed to endogenous estrogens for approximately 40 years during the pre- and peri-menopausal stages of their lives4. The main endogenous estrogen is estradiol-17β (E2). As in humans, E2 is produced mainly by the ovaries in the female rat, and is also synthesized by the adrenal gland and fat5.
In the ovary, granulosa cells of the ovarian follicle secrete E26. There is a gradual increase in circulating E2 levels as the follicle matures and levels peak during the afternoon of proestrus reaching about 35–50 pg/ml7. This increase in E2 is important for triggering GnRH secretion from the hypothalamus and LH secretion from the pituitary7,8. This results in ovulation and contributes to the progression of the estrous cycle9. On the other hand, repeated exposures to periodic increases in E2 during the course of normal reproductive cycles probably contribute to a reduction in hypothalamic catecholamines that causes a decrease in LH secretion and failure of ovulation leading to reproductive senescence10. We have found that a similar state of reproductive senescence can be induced in young animals that are exposed to low levels of E2 on a daily basis for 60 or 90 days11. Ovaries from old constant estrous (OCE) rats that are exposed to endogenous E2 during the course of their estrous cycles appear to be cystic and very similar to ovaries from young, E2-exposed rats. However the effects of chronic E2 exposure on other ovarian and hormonal parameters have not been studied in detail. Therefore, in this study, we used two exposure paradigms, one that involves treating young animals to exogenous E2 for extended periods of time and the other that involves exposure to endogenous E2 as in old constant estrous (OCE) rats.
The dose of E2 that was used in young animals was lower than previously reported studies. It is important to study the effects of this dose because women use low doses of estrogens as oral contraceptives and hormonal therapy for prolonged periods of time. Oral contraceptives such as ethinyl estradiol are capable of contaminating waste water12. Moreover, E2, along with oral contraceptives is recognized as one of the major estrogenic EDCs that need to be controlled in municipal sewage plants13. Therefore it is important to understand if chronic exposures to these estrogens can induce degenerative changes in the ovaries and if they are similar to what is seen during normal aging.
Several hormones change during reproductive aging. Luteinizing hormone (LH) and follicle stimulating hormone (FSH) decline, while there is a progressive increase in testosterone14. Moreover, increases in testosterone and LH levels are characteristic of polycystic ovarian syndrome15, a common condition in women of reproductive age16. In these women, the expression of Mullerian inhibiting substance (MIS) in the ovarian follicles persists and is believed to contribute to ovarian failure17. It is not clear if E2 exposures could produce hormonal changes similar to that seen in PCOS to cause ovarian failure. It could also result in hormonal changes that lead to premature aging. On the other hand, E2 could also directly impact the ovary by causing follicular apoptosis, inflammation and degeneration, but this effect has not been studied before.
Therefore, in the present study, we measured testosterone, LH and FSH in the serum of OCE and young rats chronically exposed to E2. LH and FSH are important for follicular growth in the ovaries and ovulation9. We also measured Mullerian inhibiting substance (MIS) expression, apoptosis and infiltration of macrophages to determine if they played a role in ovarian failure in this model..
Results
Estrous cyclicity
Effects of exogenous and endogenous E2 exposure on estrous cyclicity are shown in Fig. 1 A. Ninety four percent of animals in the sham-implanted control group exhibited regular estrous cycles. In the E30 group, 81% of the animals showed regular estrous cycles. In contrast, only 32% of the animals in E60 and 18% of the animals in E90 had regular estrous cycles. None of the animals in the OCE group had regular estrous cycles. These findings are comparable to that observed in our previous study11. Fig 1 B shows serum estradiol levels in the different treatment groups. Serum estradiol levels increased gradually with increasing duration of estrogen exposure. Levels in the E-90 group were comparable to that in OCE rats.
Figure 1.
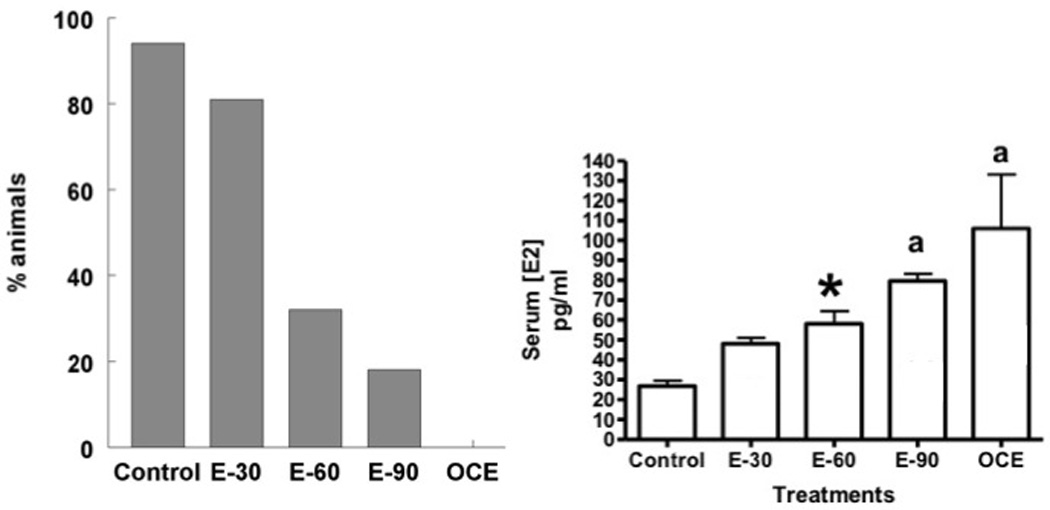
(A) Estrous cyclicity in control and E2-treated animals. Estrous cycles were determined by daily vaginal cytology. Graph represents percentage of animals demonstrating regular estrous cycles. (B) Serum estradiol levels in the control and treatment groups. * indicates p<0.05 when compared to control rats. “a” indicates significant difference from both control and E-30 groups.
Ovarian Histology
Photomicrographs of representative ovarian sections from control, E2 treated and OCE animals are provided in Fig. 2A. Exogenous administration of E2 to young animals, produced an exposure-dependent increase in the size of mature follicles. Cystic follicles were particularly evident in the E90 group (panel D) and were comparable to the OCE group (panel E). The percentage of tertiary follicles increased in an E2 exposure-dependent manner in young animals (Fig 2B), but the percentage in the E30 and E60 groups were not significantly different from that in the controls. The percentage of tertiary follicles (mean±SE; %) in the E90 group (69.1±11.5) was significantly higher compared to the E30 (39.0±6.7) and E60 (44.8±8.2) groups (p<0.05) and comparable to that in OCE rats (71.8±7).
Figure 2.
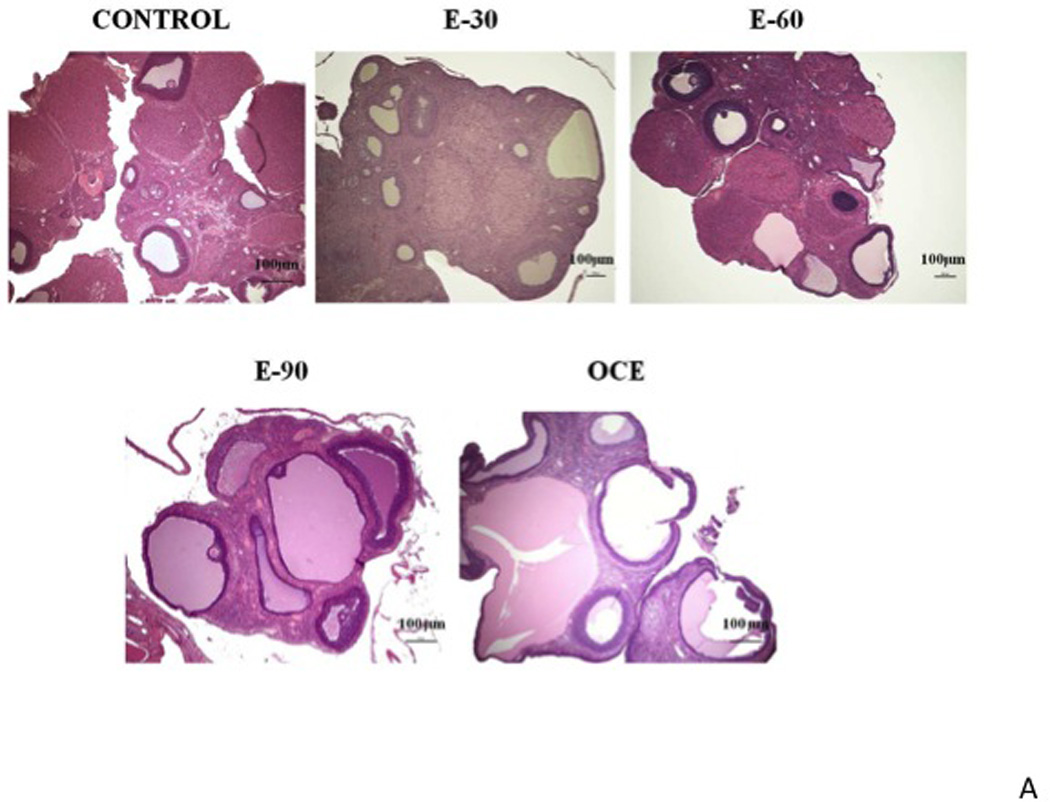
(A) Representative histological sections (H&E staining) of ovaries from different groups. Control (panel 1); E30 (panel 2), E60 (panel 3), E90 (panel 4) and OCE (panel 5). X represents fresh CL and # represents old CL. (B) Total number of tertiary follicles in the different treatment groups. * indicates p<0.05 when compared to the E30 and E60 groups. (C) Total number of fresh and old CLs in the ovaries of different treatment groups. * indicates p<0.05; ** indicates p<0.0001.
Chronic E2 exposure decreased ovulation rate in a duration-dependent manner. There was a marked decrease in the number of fresh CL (mean±SE) in the E30 group (0.2±0.2) and was completely absent in the other E2-treated groups and the OCE group, compared to the controls (1.3±0.4) (p<0.0001). Furthermore, the number of old CLs was also significantly reduced in the E90 group (1.7±1.5) and were completely absent in the OCE group compared to the control (6.5±0.9) rats (p<0.0001), suggesting that there was ovulation failure for a prolonged period of time in the E90 and OCE groups (Fig. 2C).
Degenerative changes in the ovaries
Histological evidence of follicular degeneration and necrosis was present in the ovaries of E2-treated animals (Fig.3, panels A–D). The degree of degeneration/necrosis increased in severity with the duration of E2 exposure. Panel A has a representative section containing a Graafian follicle demonstrating nuclear pyknosis of granulosa cells (GC). GC dispersion with loss of polarity, multifocal to focally transmural accumulations of neutrophils in the GC layers are apparent in panels B and C. Panel D shows oocyte degeneration with loss of cell architecture, antral fluid basophilia with globular formations and accumulation of cellular debris. Mineralization, hydropic degeneration of GCs, and infiltration of foamy macrophages are apparent in panel C. Aggregates of foamy macrophages were also present in the interstitium (Panel B). Moreover, there was a marked loss of interstitial tissue in the E-90 group compared to the other E2 treated groups and the OCE group that was visibly apparent.
Figure 3.
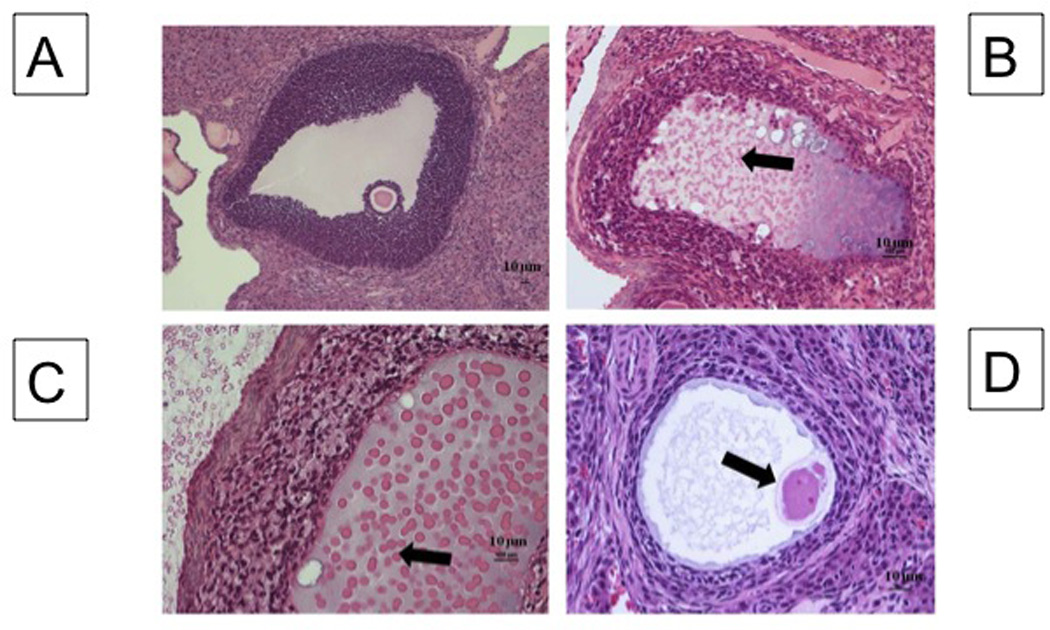
Degenerating follicular elements with E2 treatment. Tertiary follicle in a Control ovary (A). Follicle from E90 ovary (B): Note granulosa cell (GC) nuclear pyknosis, cell dispersion with loss of polarity, antral fluid basophilia (block arrow) and mineralization with globular formation and accumulation of cellular debris, and follicular wall neutrophilic infiltration. Follicle from E90 ovary (C): Note marked accumulation of foamy macrophages. The antral fluid is degenerate and characterized by basophilia and accumulation of homogenous eosinophilic, globular debris (protein) (block arrow). (D) E90 follicle undergoing degeneration and a degenerate oocyte (block arrow) can be observed, along with fibrillar antral fluid, GC nuclear pyknosis, and GC dispersion.
TUNEL staining
The percentage of follicles expressing TUNEL in all the ovaries from control and E2-treated animals was remarkably low. There was also no significant difference in TUNEL staining of follicles in control and OCE ovaries (data not shown).
MIS expression
MIS expression was present in the developing follicles of control ovaries but not in large tertiary follicles (Fig. 4A, panel A). There was no MIS expression in E-90 and OCE ovaries as well (Panels B and C). Percentage of follicles expressing MIS (mean±SE; %) declined in the E-30 group (63.5±6) compared to control (80.3±7) and was completely absent in the E60 and E-90 groups (Fig 4B).
Figure 4.
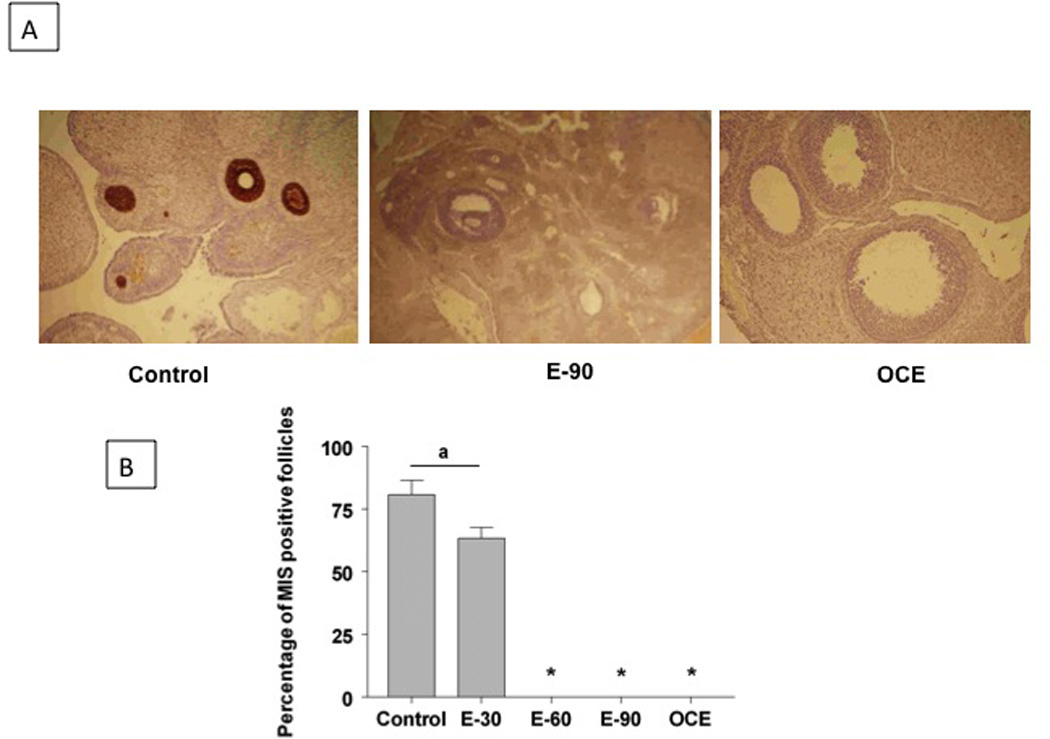
(A) MIS immunohistochemistry in ovaries from control and E2 treated animals. Panel 1: Section from a control ovary. There was strong MIS immunoreactivity in primary, secondary, and small tertiary follicles. Panel 2 and 3: Sections from a E90 ovary and OCE ovary respectively. (B) Effect of E2 treatment on the percentage of follicles expressing MIS immunoreactivity. Total number of follicles counted ranged from 65–85 in the different groups * indicates significant decrease from control. p<0.05.
CD68 Expression
CD68 is specifically expressed by monocytes and macrophages. Fig 5A has representative sections from a control, E90 and OCE ovary that were immunohistochemically labelled with anti-CD68 antibody. The percentage of follicles that contained CD68 positive cells (%; Mean±SE) was highest in the E90 (39.85±9.04) and OCE (52.27±6.73) groups and they were significantly different from the control groups (13.89±9.04) (Fig 5B). The number of cells per follicle that were positive for CD68 (Mean±SE) were more in the OCE group (34.8±5.17) but not in the exogenous E2 treated groups when compared to the control group (10.67±0.88) (Fig 5C).
Figure 5.
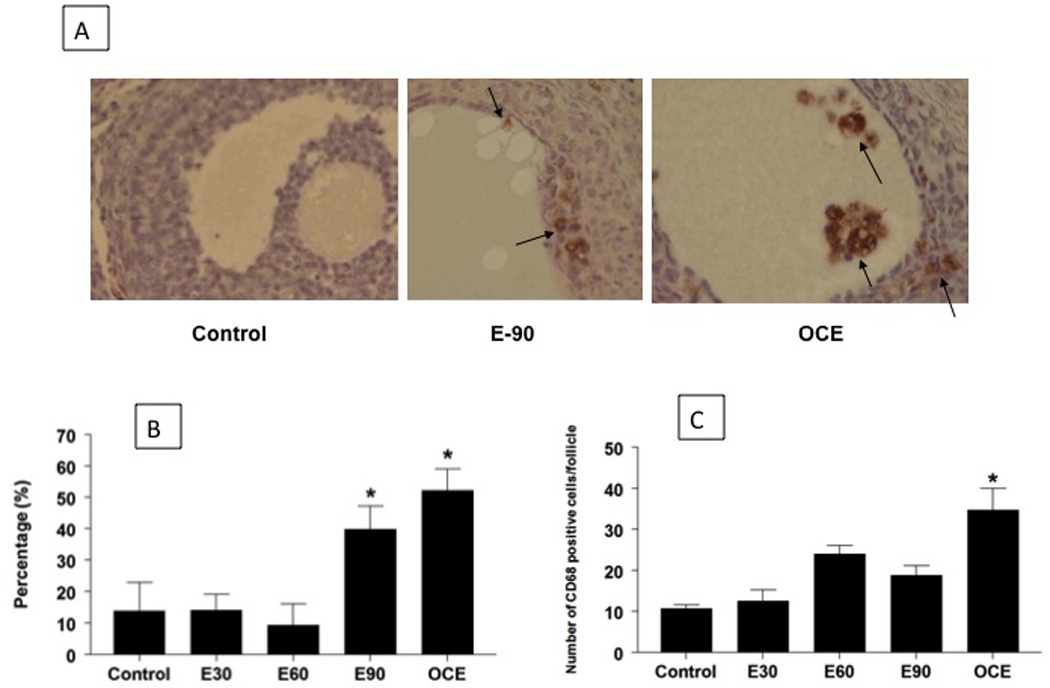
(A) Effect of E2 exposure in young and old (OCE) rats on CD68 expression. Arrows indicate cells in the follicles staining positive for CD68. (B) Effect of E2 exposure on follicles staining positive for CD68. The percentage of CD68 positive follicles increased significantly in the E90 and OCE groups compared to the rest of the groups (p<0.05) Total number of follicles counted ranged from 65-85 in the different groups. (C) Effect of E2 exposure on average number of CD38 positive cells per follicle. * indicates p<0.05.
Serum Testosterone, LH and FSH
Serum testosterone levels (mean±SE; pg/ml) are shown in Fig. 6A. Serum testosterone levels decreased significantly in E90 animals (64.1±6.7) compared to control rats (193.7±28.8; p<0.05). In contrast, serum testosterone levels increased significantly in OCE animals (335.7±112.5) compared to the control group (p<0.05). LH levels were dramatically reduced in all E2-treated groups and in OCE rats compared to controls (Fig 6B). However, FSH levels were unaffected by both exogenous and endogenous E2 exposure (Fig 6C).
Figure 6.
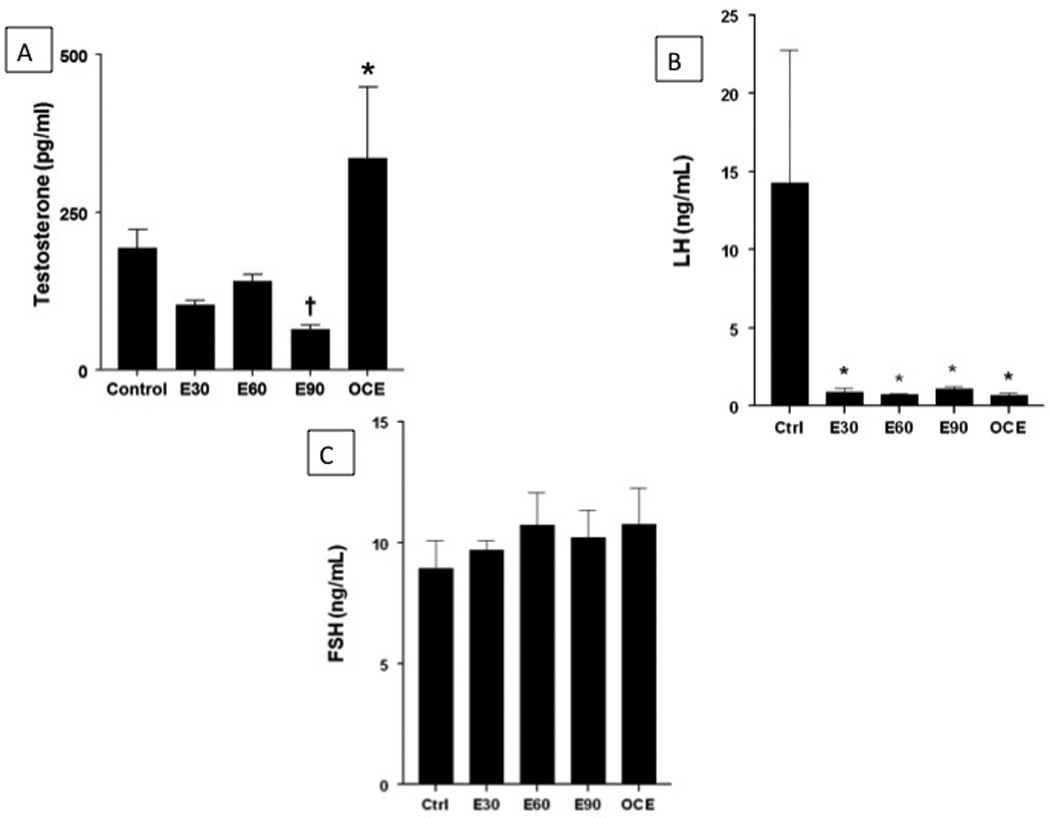
(A) Effect of E2 exposure on serum testosterone levels. * indicates significant difference from the rest of the groups. † indicates significant decrease from control and OCE groups. p<0.05. (B) Effect of E2 exposure on serum LH levels. * indicates significant difference from control. (C) Effect of chronic E2 exposure on serum FSH levels.
Discussion
The results from the present study provide evidence that chronic exposure to low levels of E2 results in cystic ovarian morphology which is similar to that observed in aging female rats. There was also a marked reduction in MIS expression, and an increase in CD68 expression, suggesting functional and inflammatory changes both in E2 treated and OCE rats. There was histomorphologic evidence of degeneration and necrosis in E2-treated and OCE rats with minimal TUNEL expression, suggesting these changes were not associated with apoptosis. Taken together, E2-induced ovarian and hormonal changes probably resulted in the loss of ovulation in young E2-treated rats making them acyclic, similar to OCE rats. This suggests that chronic exposures to low levels of E2 as described in this study are capable of accelerating reproductive aging.
A larger number of young women are on oral contraceptives now compared to 2 decades ago18. It is therefore important to understand how prolonged use of these estrogenic compounds can affect ovarian function. The dose of E2 used in this study (20ng/day or 20ppt) is lower than the current no observed adverse effect level (NOAEL) for estradiol for humans which is 5 µg/kg/day or 5 ppb19. The acceptable daily intake for E2 as recommended by the FAO/WHO expert committee is 50ng/kg BW20161514. In the present study, we were able to observe marked effects on the ovary with doses of E2 lower than the NOAEL. Moreover, these effects were comparable to the effects seen in aging animals.
Chronic E2 exposure caused polycystic ovarian morphology that was dependent on the duration of exposure. A variety of estrogenic compounds have been shown to cause cystic ovarian morphology21–23 possibly due to a direct effect on the ovary. Persistent organic pollutants are known to be cytotoxic to the oocyte and cause ovulation failure, thus resulting in cystic ovaries24. On the other hand, this could be due to the effects of E2 on the hypothalamo pituitary gonadal axis that regulates reproductive function. In fact, similar exposure to E2, in terms of dose and duration, has been shown to cause a duration dependent reduction in hypothalamic monoamines, LH and cause acyclicity11. The lack of sufficient levels of LH results in failure of ovulation and anovulated follicles give the ovaries a cystic appearance. A similar phenomenon is observed in aging animals and rats treated with tamoxifen25.
Besides inducing a cystic morphology, exogenous E2 exposure caused a variety of degenerative changes ranging from degeneration of the oocyte, mineralization of follicles, infiltration of leukocytes and pyknosis of GCs. There was an increase in CD68 expression in the follicles of E2 treated rats that indicates an increase in the infiltration of macrophages. Macrophages are capable of recognizing apoptotic and necrotic cells and phagocytizing them. The E2-induced degeneration of GCs probably triggers the infiltration of macrophages in an attempt to remove them from the follicle26,27,28. Moreover, macrophages are known to secrete cytokines and other factors and contribute to restructuring and remodeling within the ovaries29. Similar degenerative changes were observed in OCE rats as well and this could be attributed to the elevated endogenous E2 levels in these animals. However, there were minimal apoptotic changes in both E2 treated and OCE rats suggesting that these degenerative changes are inflammatory rather than apoptotic in nature.
The other functional ovarian parameter that was assessed was the follicular expression of MIS. MIS (also known as anti-Mullerian hormone) is produced predominantly by GCs of primary and secondary follicles. It is down-regulated in tertiary follicles as the follicle matures and is minimally expressed in preovulatory follicles30,31,32. The exact function and mechanism of action of MIS in the ovary is not fully understood, but it has been suggested to be a key regulator of folliculogenesis and dominant follicle selection30,33,31,34,32,35. In the present study, we provide evidence for the first time that chronic exposure to E2 for 60 and 90 days, results in the complete loss of MIS expression in the ovaries. This is important because MIS is known to be involved in protecting primordial follicles. In fact, in MIS-deficient mice, primordial follicles are prematurely recruited and depleted followed by subsequent premature cessation of the estrous cycle36. Therefore, it is possible that E2-induced down-regulation of ovarian MIS may result in premature primordial follicle depletion, thereby causing early reproductive senescence.
Besides producing inflammatory changes in the ovary and decreasing MIS expression, chronic E2 exposure also affects hormonal levels. Consistent with our previous study11 there was a significant reduction in LH levels in the E2 treated groups and the OCE group. This is most likely due to the reduction in hypothalamic monoamines that are stimulatory to the secretion of this hormone. FSH levels were unaltered with E2 exposure and during aging. This could be attributed to the negative feedback inhibition by elevated E2 levels in these animals. In contrast to FSH and LH, there was a marked increase in testosterone levels in OCE animals, consistent with previously reported studies37,38. However, there was a significant decrease in testosterone levels in E90 animals compared to controls. The exact cause of these differences in testosterone levels is still unclear. Since testosterone is converted to E2 by the enzyme aromatase, it is possible that aromatase activity is reduced in older animals but augmented in E2-treated younger animals. This needs to be investigated further.
Overall, our study demonstrates that chronic exposure to low-doses of E2 can be detrimental to the ovaries. It induces cystic follicles, causes follicular degeneration and by down-regulating MIS expression, probably promotes early follicular recruitment and depletion. Chronic E2 exposure also produced marked decrease in serum testosterone, LH and FSH levels in young animals. The only difference between E2 treated young and OCE rats was in the levels of testosterone. Since most ovarian and hormonal changes after E2 exposure were similar between young and OCE rats, it is safe to state that chronic exposures to low levels of E2 accelerates reproductive aging in rats. The point to be noted is that the dose of E2 that was used in this study corresponds to the acceptable daily intake suggested by the FAO/WHO and is less than the NOAEL for E2. We therefore need to re-evaluate acceptable exposure levels for E2.
Materials and Methods
Animals
Adult, 3 months old, female Sprague-Dawley rats and retired breeders (approximately 10 months of age) were obtained from Harlan Inc. (Indianapolis, IN, USA). Housing was light-controlled (lights on from 0700–1900h) and air-conditioned (23±2°C) and the rats were fed rat chow and water ad libitum. Animals were used in accordance with the NIH guide for the care and use of laboratory animals and were approved by the Institutional Animal Care and Use Committee at Michigan State University.
Treatment
Animals (n=8) were sham-implanted (controls) or implanted with slow-release E2 pellets (20ng/day; Innovative Research America, Sarasota, FL, http://www.innovrsrch.com) for 30 (E30 group), 60 (E60 group), or 90 (E90 group) days and is described by Kasturi et al11. Retired breeders (n=8) were aged in our facility to 12–16 months of age and used as old constant estrous (OCE) rats for comparison. Estrous cyclicity was monitored by vaginal cytology periodically as described previously11. Since control animals from the different age groups had no differences in estrous cycles, or hypothalamic neurotransmitters11, animals from these groups were pooled together. At the end of the experiment, control and E30 animals that had regular estrous cycles were sacrificed by rapid decapitation on the day of estrous at 1200 h. Animals in the E60, E90 and OCE groups were in a state of constant estrous. Trunk blood was collected and serum and plasma were stored at −80°C. Brains from this study was used in the study by Kasturi et al11. Ovaries from that study were collected and placed in 10% neutral buffered formalin for routine histopathology.
Histological evaluation of ovaries
Four sections from the ovaries (4 µm thick, 20 µm apart) were obtained and stained with hematoxylin and eosin. The slides were histologically examined for the effects of chronic E2 exposure using a Nikon microscope (Melville, NY). The follicles were characterized as primordial (oocyte surrounded by a single layer of squamous granulosa cells), primary (oocyte surrounded by a single layer of cuboidal granulosa cells), secondary (oocyte surrounded by multiple layers of cuboidal granulosa cells with or without antral space development), and tertiary (oocyte situated on cumulus oophorus with multiple layers of granulosa cells and a large confluent antral space) according to Myers et al.39. Histopathologic alterations including inflammation, degeneration etc. were determined by assessing the extent of infiltration of macrophages (staining with CD68), presence of pyknosis and TUNEL staining. Morphological analysis was done using NIS Elements BR 3.00, SP7, Hotfix8 (Build 548) Copyright ©1991–2009 Laboratory Imaging Hasp ID: 5665130E.
Immunohistochemistry for MIS and CD68
Ovarian sections were stained for MIS and CD68 by immunohistochemistry as follows: Four µm sections were placed on slides coated with 2% 3-Aminopropyltriethoxysilane, dried at 56°C overnight, deparaffinized in xylene, and hydrated through descending grades of ethanol to distilled water. Sections of mouse ovary were used as positive controls for MIS and sections of the spleen were used as positive controls for CD68. Slides were placed in Tris Buffered Saline (TBS) pH 7.5 for 5 minutes. The antigen was retrieved using citrate buffer pH 6.0 for 30 minutes at 100°C, allowed to cool at room temperature for 10 minutes, and rinsed in several changes of distilled water. Endogenous peroxidase was blocked utilizing 3% hydrogen peroxide/methanol bath for 30 minutes. After blocking for non specific protein with Normal Horse Serum (Vector Labs, Burlingame, CA, https://www.vectorlabs.com) for 30 minutes; sections were incubated with Avidin / Biotin blocking system for 15 minutes (Avidin from Vector Labs, Burlingame, CA, https://www.vectorlabs.com; Biotin from Sigma Chemical, St. Louis, MO, http://www.sigmaaldrich.com/united-states.html). Slides were incubated for 60 minutes with the polyclonal goat MIS antibody (C20) or CD68 antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA, http://www.scbt.com/datasheet-6886-mis-c-20-antibody.html), diluted 1:150. After rinsing, slides were incubated in Biotinylated Horse anti-Goat IgG H+L (Vector Labs, Burlingame, CA, USA, https://www.vectorlabs.com/catalog.aspx?prodID=531) in NAD diluted at 11 g/ml for 30 minutes. This was followed by application of Vectastain Elite ABC Reagent (Vector Labs, Burlingame, CA, https://www.vectorlabs.com/catalog.aspx?prodID=395) for 30 minutes. The slides were developed using Nova Red Peroxidase substrate kit (Vector Labs, Burlingame, CA, https://www.vectorlabs.com/catalog.aspx?prodID=37) for 15 minutes. Finally, the slides were counterstained using Gill 2 Hematoxylin (ThermoFisher, Pittsburgh, PA, https://www.thermofisher.com/global/en/home.asp) for 1 minute, differentiated in 1% Aqueous Glacial Acetic Acid, and rinsed. Slides were then dehydrated through ascending grades of ethanol, cleared through several changes of xylene, and cover slipped using Flotex permanent mounting media. The number of follicles that stained positive for MIS and CD68 were counted using a Nikon microscope and the NIS software. Follicles that were counted were marked in order to avoid duplicate counting. The number of CD68 positive cells were also counted within each follicle.
TUNEL Labeling
Terminal deoxynucleotidyl transferase mediated X-dUTP nick end labeling (TUNEL) staining was performed on a representative section of ovary from each group. TUNEL is a diagnostic tool that labels DNA breaks in nuclei and is used to identify apoptotic cells. Ovarian sections were exposed to proteolytic treatment that results in exposure of nuclear DNA. Then, the DNA polymerase terminal deoxynucleotidyl transferase (TdT) is used to incorporate biotinylated deoxyuridine at sites of DNA breaks. The signal is amplified by avidin-peroxidase and visualized by light microscopy. Apoptotic cells in the ovaries were detected using the TACS-XL basic in situ apoptosis detection kit (Trevigen, Inc., Gaithersburg, MD, http://www.trevigen.com/item.php?itemsKey=536). The percentage of TUNEL positive follicles and the number of positive cells within each TUNEL positive follicle were also identified and counted.
Serum Testosterone and Estradiol
Serum estradiol was measured using a Coat-a-count RIA kit (Siemens, Malvern, PA). Serum testosterone levels were measured using a Testosterone EIA kit (Cayman Chemical, Ann Arbor, MI, as provided in the link below. The samples were assayed in duplicate according to the manufacturer’s protocol with one exception that samples were used undiluted. The sensitivity of the testosterone assay was 32pg/ml and that of the estradiol assay was 8pg/ml. The intra-assay variability was less than 5%. https://www.caymanchem.com/app/template/Product.vm/catalog/582701).
Serum LH and FSH
Serum samples were analyzed for LH and FSH levels by double antibody radioimmunoassay by Dr. A. F. Parlow, National Hormone and Pituitary Program. Samples were assayed in duplicate. The reference preparation used for FSH was NIDDK-rFSH-RP-2. Anti-rrFSH AFP9305A antibody was used at a final dilution of 1:16,000. The reference preparation used for LH was NIDDK-rLH-RP-3. NIDDK-Anti-rLH-S-10 antibody was used at a final dilution of 1:200,000. The sensitivity of the LH assay was 0.04ng/ml and for FSH it was 1.5 ng/ml. Samples were run in duplicate in a single assay. Intra-assay variability was less than 5%.
Statistical Analysis
Changes in estrous cycles were analyzed using Kruskal-Wallis non-parametric test. Changes in all other parameters were analyzed using one-way ANOVA followed by posthoc Fisher’s LSD test. p<0.05 was considered to be significant.
Table 1.
Effects of chronic E2 exposures on ovarian follicles. Follicle numbers were averaged over 4 sections from the same ovaries.
| Type of Follicle |
Treatment groups | Sig. | ||||
|---|---|---|---|---|---|---|
| Control | E-30 | E-60 | E-90 | OCE | ||
| Primordial | 0.67±0.3 | 3.8±0.9* | 1.18±0.4 | 1.3±0.8 | 1.2±0.7 | p<0.05 |
| Primary | 4±1.4 | 2.9±1.0 | 4.27±0.8 | 2.67±1.4 | 3±1.1 | NS |
| Secondary | 2.5±0.9 | 2.5±0.6 | 2.64±0.7 | 1±0.6 | 0.57±0.4 | NS |
| Tertiary | 8.67±1.8 | 5.1±1.4 | 6.81±1.4 | 6.17±1.0 | 9.43±1.0 | NS |
| % Tertiary follicles | 51.2±7.9 | 39±6.7 | 44.8±8.2 | 69.1±11.5* | 71.8±7* | p<0.05 |
| Tertiary follicle size (µm) | 492.4±15.3 | 479.4±20.0 | 512.0±53.2 | 621.6±47.6* | 614.0±30.0* | p<0.05 |
Acknowledgements
We would like to thank Dr. A. F. Parlow at the National Hormone and Peptide Program (Torrance, CA) for sample analysis of serum for FSH and LH RIA. Sincere gratitude is extended to Amy Porter, Investigative Histopathology Laboratory, Michigan State University for histological and immunohistochemical processing of the tissues. We also thank Ms. Katrina Linning for her technical assistance.
References
- 1.Spencer AL, Bonnema R, McNamara MC. Helping women choose appropriate hormonal contraception: update on risks, benefits, and indications. Am J Med. 2009 Jun;122(6):497–506. doi: 10.1016/j.amjmed.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 2.Brett KM, Reuben CA. Prevalence of estrogen or estrogen-progestin hormone therapy use. Obstet Gynecol. 2003 Dec;102(6):1240–1249. doi: 10.1016/j.obstetgynecol.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 3.Goodman A, Schorge J, Greene MF. The long-term effects of in utero exposures--the DES story. N Engl J Med. 2011 Jun 2;364(22):2083–2084. doi: 10.1056/NEJMp1104409. [DOI] [PubMed] [Google Scholar]
- 4.O'Connor KA, Ferrell RJ, Brindle E, et al. Total and unopposed estrogen exposure across stages of the transition to menopause. Cancer Epidemiol Biomarkers Prev. 2009 Mar;18(3):828–836. doi: 10.1158/1055-9965.EPI-08-0996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell CS, Schwartz NB, Firlit MG. The role of adrenal and ovarian steroids in the control of serum LH and FSH. Endocrinology. 1977 Jul;101(1):162–172. doi: 10.1210/endo-101-1-162. [DOI] [PubMed] [Google Scholar]
- 6.Valdez KE, Cuneo SP, Gorden PJ, Turzillo AM. The role of thecal androgen production in the regulation of estradiol biosynthesis by dominant bovine follicles during the first follicular wave. J Anim Sci. 2005 Mar;83(3):597–603. doi: 10.2527/2005.833597x. [DOI] [PubMed] [Google Scholar]
- 7.Kalra SP, Kalra PS. Effects of circulating estradiol during rat estrous cycle on LH release following electrochemical stimulation of preoptic brain or administration of synthetic LRF. Endocrinology. 1974 Mar;94(3):845–851. doi: 10.1210/endo-94-3-845. [DOI] [PubMed] [Google Scholar]
- 8.Radovick S, Levine JE, Wolfe A. Estrogenic regulation of the GnRH neuron. Front Endocrinol (Lausanne) 2012;3:52. doi: 10.3389/fendo.2012.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mueller EE, Nistico G. Brain Messengers and the Pituitary. New York: Academic Press; 1989. [Google Scholar]
- 10.Mohankumar PS, Thyagarajan S, Quadri SK. Correlations of catecholamine release in the medial preoptic area with proestrous surges of luteinizing hormone and prolactin: effects of aging. Endocrinology. 1994 Jul;135(1):119–126. doi: 10.1210/endo.135.1.8013343. [DOI] [PubMed] [Google Scholar]
- 11.Kasturi BS, MohanKumar SM, Sirivelu MP, MohanKumar PS. Chronic exposure to low levels of oestradiol-17beta affects oestrous cyclicity, hypothalamic norepinephrine and serum luteinising hormone in young intact rats. J Neuroendocrinol. 2009 Jun;21(6):568–577. doi: 10.1111/j.1365-2826.2009.01873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mastrup M, Schafer AI, Khan SJ. Predicting fate of the contraceptive pill in wastewater treatment and discharge. Water Sci Technol. 2005;52(8):279–286. [PubMed] [Google Scholar]
- 13.Sun Y, Huang H, Wang C, et al. Ecological risk of estrogenic endocrine disrupting chemicals in sewage plant effluent and reclaimed water. Environ Pollut. 2013 Sep;180:339–344. doi: 10.1016/j.envpol.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 14.Steger RW, Peluso JJ. Effects of age on hormone levels and in vitro steroidogenesis by rat ovary and adrenal. Exp Aging Res. 1982 Fall-Winter;8(3–4):203–208. doi: 10.1080/03610738208260367. [DOI] [PubMed] [Google Scholar]
- 15.Kasuga Y. Ovarian steroidogenesis in Japanese patients with polycystic ovary syndrome. Endocrinol Jpn. 1980 Oct;27(5):541–550. doi: 10.1507/endocrj1954.27.541. [DOI] [PubMed] [Google Scholar]
- 16.Balen AH, Conway GS, Kaltsas G, et al. Polycystic ovary syndrome: the spectrum of the disorder in 1741 patients. Hum Reprod. 1995 Aug;10(8):2107–2111. doi: 10.1093/oxfordjournals.humrep.a136243. [DOI] [PubMed] [Google Scholar]
- 17.Wang HQ, Takakura K, Takebayashi K, Noda Y. Mutational analysis of the mullerian-inhibiting substance gene and its receptor gene in Japanese women with polycystic ovary syndrome and premature ovarian failure. Fertil Steril. 2002 Dec;78(6):1329–1330. doi: 10.1016/s0015-0282(02)04351-0. [DOI] [PubMed] [Google Scholar]
- 18.Mosher WD, Jones J. Use of contraception in the United States: 1982–2008. Vital Health Stat. 2010 Aug;23(29):1–44. [PubMed] [Google Scholar]
- 19.Snyder S, Trenholm RA, Snyder EM, Bruce GM, Pleus RC, Hemming JDC. Toxicological relevance of EDCs and pharmaceuticals in drinking water. Denver, Colorado: American Water Works Association Research Foundation; 2008. [Google Scholar]
- 20.Evaluation of certain veterinary drug residues in food. World Health Organ Tech Rep Ser. 2000;893:i–viii. 1–102. [PubMed] [Google Scholar]
- 21.Quandt LM, Hutz RJ. Induction by estradiol-17 beta of polycystic ovaries in the guinea pig. Biol Reprod. 1993 May;48(5):1088–1094. doi: 10.1095/biolreprod48.5.1088. [DOI] [PubMed] [Google Scholar]
- 22.Luza SM, Lizama L, Burgos RA, Lara HE. Hypothalamic changes in norepinephrine release in rats with estradiol valerate-induced polycystic ovaries. Biol Reprod. 1995 Feb;52(2):398–404. doi: 10.1095/biolreprod52.2.398. [DOI] [PubMed] [Google Scholar]
- 23.Chapman JC, Min SH, Freeh SM, Michael SD. The estrogen-injected female mouse: new insight into the etiology of PCOS. Reprod Biol Endocrinol. 2009;7:47. doi: 10.1186/1477-7827-7-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pocar P, Augustin R, Gandolfi F, Fischer B. Toxic effects of in vitro exposure to p-tert-octylphenol on bovine oocyte maturation and developmental competence. Biol Reprod. 2003 Aug;69(2):462–468. doi: 10.1095/biolreprod.102.010355. [DOI] [PubMed] [Google Scholar]
- 25.Tsujioka S, Ban Y, Wise LD, et al. Collaborative work on evaluation of ovarian toxicity. 3) Effects of 2- or 4- week repeated-dose toxicity and fertility studies with tamoxifen in female rats. J Toxicol Sci. 2009;34(Suppl 1):SP43–SP51. doi: 10.2131/jts.34.s43. [DOI] [PubMed] [Google Scholar]
- 26.Paavola LG. Cellular mechanisms involved in luteolysis. Adv Exp Med Biol. 1979;112:527–533. doi: 10.1007/978-1-4684-3474-3_59. [DOI] [PubMed] [Google Scholar]
- 27.Kuryszko J, Adamski RT. Macrophages in atretic process of maturing ovarian follicles in mouse. Z Mikrosk Anat Forsch. 1987;101(2):212–220. [PubMed] [Google Scholar]
- 28.Kasuya K. Elimination of apoptotic granulosa cells by intact granulosa cells and macrophages in atretic mature follicles of the guinea pig ovary. Arch Histol Cytol. 1997 Jun;60(2):175–184. doi: 10.1679/aohc.60.175. [DOI] [PubMed] [Google Scholar]
- 29.Wu R, Van der Hoek KH, Ryan NK, Norman RJ, Robker RL. Macrophage contributions to ovarian function. Hum Reprod Update. 2004 Mar-Apr;10(2):119–133. doi: 10.1093/humupd/dmh011. [DOI] [PubMed] [Google Scholar]
- 30.Hirobe S, He WW, Gustafson ML, MacLaughlin DT, Donahoe PK. Mullerian inhibiting substance gene expression in the cycling rat ovary correlates with recruited or graafian follicle selection. Biol Reprod. 1994 Jun;50(6):1238–1243. doi: 10.1095/biolreprod50.6.1238. [DOI] [PubMed] [Google Scholar]
- 31.Weenen C, Laven JS, Von Bergh AR, et al. Anti-Mullerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod. 2004 Feb;10(2):77–83. doi: 10.1093/molehr/gah015. [DOI] [PubMed] [Google Scholar]
- 32.Modi D, Bhartiya D, Puri C. Developmental expression and cellular distribution of Mullerian inhibiting substance in the primate ovary. Reproduction. 2006 Sep;132(3):443–453. doi: 10.1530/rep.1.01178. [DOI] [PubMed] [Google Scholar]
- 33.Durlinger AL, Visser JA, Themmen AP. Regulation of ovarian function: the role of anti-Mullerian hormone. Reproduction. 2002 Nov;124(5):601–609. doi: 10.1530/rep.0.1240601. [DOI] [PubMed] [Google Scholar]
- 34.Fleming R, Harborne L, MacLaughlin DT, et al. Metformin reduces serum mullerian-inhibiting substance levels in women with polycystic ovary syndrome after protracted treatment. Fertil Steril. 2005 Jan;83(1):130–136. doi: 10.1016/j.fertnstert.2004.05.098. [DOI] [PubMed] [Google Scholar]
- 35.Visser JA, de Jong FH, Laven JS, Themmen AP. Anti-Mullerian hormone: a new marker for ovarian function. Reproduction. 2006 Jan;131(1):1–9. doi: 10.1530/rep.1.00529. [DOI] [PubMed] [Google Scholar]
- 36.Durlinger AL, Kramer P, Karels B, et al. Control of primordial follicle recruitment by anti-Mullerian hormone in the mouse ovary. Endocrinology. 1999 Dec;140(12):5789–5796. doi: 10.1210/endo.140.12.7204. [DOI] [PubMed] [Google Scholar]
- 37.Chan SW, Leathem JH. Aging and ovarian steroidogenesis in the rat. J Gerontol. 1977 Jul;32(4):395–401. doi: 10.1093/geronj/32.4.395. [DOI] [PubMed] [Google Scholar]
- 38.Peluso JJ, Steger RW, Huang H, Meites J. Pattern of follicular growth and steroidogenesis in the ovary of aging cycling rats. Exp Aging Res. 1979 Aug;5(4):319–333. doi: 10.1080/03610737908257208. [DOI] [PubMed] [Google Scholar]
- 39.Myers M, Britt KL, Wreford NG, Ebling FJ, Kerr JB. Methods for quantifying follicular numbers within the mouse ovary. Reproduction. 2004 May;127(5):569–580. doi: 10.1530/rep.1.00095. [DOI] [PubMed] [Google Scholar]


