Abstract
Background
Clinical trials provide access to innovative, quality cancer treatment. Simultaneously, broad access helps ensure trial inclusion of heterogeneous patient populations, which improves generalizability of findings and development of interventions that are effective for diverse populations. We provide updated data describing enrollment into cancer treatment trials in North Carolina.
Methods
For 1996 to 2009, person-level data regarding cancer clinical trial enrollment and cancer incidence were obtained from the North Carolina Central Cancer Registry and the National Cancer Institute (NCI). Enrollment rates were estimated as the ratio of trial enrollment to cancer incidence for race, gender, and year for each county, Area Health Education Center (AHEC) region, and the state overall. Enrollment rates for common cancers are presented.
Results
From 1996 to 2009, North Carolina NCI treatment trial enrollment rate was 2.4% and 2.2% for whites and minorities, respectively. From 2007 to 2009, rates were 3.8% for white females, 3.5% for minority females, 1.3% for white men, and 1.0% for minority men, with greater enrollment among more urban populations (2.4%) than the most rural populations (1.5%).
Limitations
This study is limited to NCI-sponsored treatment trials in North Carolina. Policies governing collection of original data necessitate a delay in data availability.
Conclusions
Effort is needed to ensure trial access and enrollment among all North Carolina populations. Specifically, we identified racial and gender disparities, particularly for certain cancers (e.g., breast). Programs in North Carolina and across the nation can use the methods we employ to assess their success in broadening clinical trials enrollment for diverse populations.
Keywords: cancer, surveillance, clinical trials enrollment, disparities, North Carolina
Background
Randomized controlled clinical trials are a critical tool in improving the science of cancer care. With rapid advancement of basic clinical sciences, such as genomics and proteomics, trials provide the primary forum for translating advances in basic sciences into cutting edge, innovative clinical care for patients. As treatments are increasingly tailored to specific genotypes, treatment efficacy may vary across diverse patient groups [1]. To evaluate the efficacy of new treatments, it is important that trials enroll heterogeneous patients that are representative of the underlying cancer population. Despite the importance of this diversity, certain groups – the elderly, men, and patients of minority races – have historically been underrepresented in cancer clinical trials [2]. As a result, new discoveries tested only within a homogenous group may not be widely translatable in the clinical setting with “real” patients.
With the goal of improving access to trials and diversity of enrollees, the National Cancer Institute (NCI) developed two cancer-focused practice-based research networks (PBRN) – Community Clinical Oncology Program (CCOP) in the early 1980s, and the National Community Cancer Centers Program (NCCCP) in the 2000s [1, 3, 4]. NCCCP aimed to improve the quality of cancer care delivered in community settings [1, 5] and the CCOP program engaged community physicians in NCI clinical trials [1, 6]. The promise of these programs is to enhance access to cancer trials – NCI Cooperative Group trials in particular – and enrollment of diverse patients, and thus contribute to broad improvement in future treatment effectiveness and outcomes.
Many states have embraced cancer-related PBRNs as a mechanism for achieving diversity in clinical trial enrollment and supporting diffusion of innovation, which are critical to developing interventions that are effective in all populations and that actually reach the field [1]. Moreover, there is growing evidence of their effectiveness in facilitating the diffusion of trial-proven interventions to broader populations. For example, in the context of colorectal cancer, PBRNs have been associated with patients having a greater probability of receiving innovative cancer care [7, 8]. We previously reported on a novel statewide system for ongoing monitoring and public reporting of NCI clinical trial enrollment, developed to extend clinical trials access to broader patient populations [3]. In this research brief, we now present updated enrollment data from this surveillance system. In addition to illustrating utility of the surveillance system and providing information regarding possible changes in patterns of enrollment, this study provides relevant insight that will inform North Carolina as it seeks to enhance access to clinical trials and quality care vis-à-vis the broad use of trial-proven interventions. It also informs the NCI as it seeks to further promote clinical research in the community, by reorganizing its NCCCP and CCOP programs into the NCI Community Oncology Research Program (NCORP) [9].
Methods
Methods have been previously described in detail [3]. In brief, person-level cancer incidence data were obtained from the North Carolina Central Cancer Registry (NCCCR) for years 1996–2009. Person-level NCI clinical treatment trial accrual data were obtained from the NCI Cancer Therapy Evaluation Program (CTEP). This data set includes information on enrollment into NCI-sponsored cancer clinical treatment trials (i.e., those funded by NCI and managed primarily through NCI Cooperative Groups). These datasets included age at diagnosis and enrollment, gender, race, county of residence, and primary cancer type (e.g., breast, colon, etc.), but were not sufficiently granular to characterize cancer sub-type. Because clinical treatment trials and their relevant populations differ substantially from those of cancer prevention and control, this analysis focused on cancer treatment trials [5]. Select socioeconomic and regional healthcare organization data were obtained from Area Resource Files (ARFs). Counties were categorized as urban or rural on a scale from 1 (Metropolitan area core) to 10 (Rural area core) based on the U.S. Department of Agriculture's rural-urban commuting area (RUCA) codes. To differentiate truly rural counties from those that might include “sprawling suburbia” with greater healthcare access, a conservative approach was used to define counties as rural when RUCA equaled 9 or 10. We also evaluated county-level characteristics such as whether the county hosted a CCOP or medical school.
The primary goal was to provide an updated report of trial enrollment across North Carolina; thus, analysis was primarily descriptive and focused on characteristics of adults (aged 21 years and older) who enrolled in a cancer treatment clinical trial. Due to regulations governing data access and data use, there was insufficient identifying information to enable person-level linkages across datasets. The data were pooled and analyzed first at the county level then aggregated and analyzed at the Area Health Education Center (AHEC) level or state level. As in our initial analysis [3], trial accrual rate estimates were calculated by dividing the count of annual enrollment by the count of the newly incident cases for each race, gender, county, and year combination. We used Pearson's chi-square tests to evaluate differences in categorical variables. Three-year averages were used to mitigate spurious fluctuations resulting from sparse data for several counties and race–gender combinations. Because the data were structured in this way, a repeated measures approach (Proc Glimmix, SAS 9.2) was used to fit the logistic model and calculate odds ratios (OR) to examine enrollment rate trends for race and gender.
Results
From 1996 to 2009 the estimated overall adult enrollment rate for NCI-sponsored cancer treatment trials in North Carolina was 2.3%. Between 2007 and 2009, a total of 154,565 adults were diagnosed with cancer in North Carolina; 3,771 adults were enrolled in NCI-sponsored treatment trials, yielding an estimated overall enrollment rate of 2.4% (Table 1), slightly lower than the nationally estimated 3-5% enrollment rate [10]. Mean age at diagnosis during this time was 64.7 years, and mean age at enrollment for those enrolling in trials was 57.4 years. Examining all years (1996-2009) and the most recent period of 2007-2009, substantially more women than men enrolled in trials (3.3% women vs. 1.5% men in all years [OR 2.26, 95% CI 2.18 – 2.34]; 3.7% vs. 1.2% in recent years [OR 3.08, 95% CI 2.86-3.32]).
Table 1.
Cancer incidence and clinical trials accrual in North Carolina (1996-2009)
| Overall (1996-2009) |
Recent (2007-2009) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cancer Incidence | Number Enrolled | Est. Enrollment Rate | OR | 95% CI | Cancer Incidence | Number Enrolled | Est. Enrollment Rate | OR | 95% CI | |||
| Overall | 588,317 | 13,795 | 2.34% | 154,565 | 3,771 | 2.44% | ||||||
| Gender | ||||||||||||
| Male | 298,172 | 4,364 | 1.46% | 1.00 | 78,361 | 961 | 1.23% | 1.00 | ||||
| Female | 290,131 | 9,418 | 3.25% | 2.26 | (2.18,2.34) | *** | 76,201 | 2,808 | 3.68% | 3.08 | (2.86,3.32) | *** |
| Unknown/not documented | 14 | 13 | - | 3 | 2 | - | ||||||
| Race | ||||||||||||
| White | 473,812 | 11,208 | 2.37% | 1.00 | 123,566 | 3,084 | 2.50% | 1.00 | ||||
| Minority | 110,467 | 2,445 | 2.21% | 0.90 | (0.86,0.94) | *** | 29,434 | 652 | 2.22% | 0.89 | (0.81, 0.96) | ** |
| Unknown/not documented | 4,038 | 142 | - | 1,565 | 35 | - | ||||||
| Gender * Race | ||||||||||||
| White Males | 239,164 | 3,579 | 1.50% | 1.00 | 62,619 | 796 | 1.27% | |||||
| White Females | 234,648 | 7,622 | 3.25% | 2.21 | (2.12,2.30) | *** | 60,946 | 2,286 | 3.75% | 3.03 | (2.79,3.28) | *** |
| Minority Males | 57,052 | 725 | 1.27% | 0.85 | (0.78,0.92) | *** | 14,980 | 153 | 1.02% | 0.80 | (0.67,0.95) | ** |
| Minority Females | 53,415 | 1,719 | 3.22% | 2.19 | (2.06,2.32) | *** | 14,453 | 499 | 3.45% | 2.78 | (2.48,3.11) | *** |
| Unknown/not documented | 4,038 | 150 | - | 1,567 | 37 | - | ||||||
| Age | ||||||||||||
| Mean age: Diagnosis, Enrollment (SD) | 64.7 (13.9) | 57.8 (12.9) | 64.3 (13.9) | 57.4 (12.8) | ||||||||
| AHEC Region (# Counties) | ||||||||||||
| AHEC Area L (5) | 22,500 | 437 | 1.94% | 0.72 | (0.65,0.80) | *** | 5,668 | 112 | 1.98% | 0.72 | (0.59,0.88) | *** |
| AHEC Charlotte (8) | 91,673 | 2,005 | 2.19% | 0.81 | (0.77,0.86) | *** | 25,175 | 539 | 2.14% | 0.78 | (0.70,0.87) | *** |
| AHEC Eastern (23) | 67,129 | 1,683 | 2.51% | 0.94 | (0.88,0.99) | * | 16,938 | 451 | 2.66% | 0.97 | (0.86,1.09) | |
| AHEC Greensboro (8) | 76,371 | 1,976 | 2.59% | 0.97 | (0.91,1.02) | 19,807 | 600 | 3.03% | 1.11 | (1.00,1.24) | * | |
| AHEC Mountain (16) | 61,806 | 1,391 | 2.25% | 0.84 | (0.78,0.89) | *** | 16,349 | 452 | 2.76% | 1.01 | (0.90,1.14) | |
| AHEC Wake (9) | 78,569 | 1,505 | 1.92% | 0.71 | (0.67,0.76) | *** | 22,343 | 393 | 1.98% | 0.72 | (0.64,0.81) | *** |
| AHEC Southeast (5) | 30,533 | 573 | 1.88% | 0.70 | (0.64,0.76) | *** | 7,865 | 148 | 1.88% | 0.68 | (0.57,0.81) | *** |
| AHEC Southern (9) | 50,841 | 1,151 | 2.26% | 0.84 | (0.79,0.90) | *** | 12,584 | 240 | 1.91% | 0.69 | (0.60,0.80) | *** |
| AHEC Northwest (17) | 107,515 | 2,878 | 2.68% | 1.00 | 27,707 | 758 | 2.74% | |||||
| Unknown/not documented | 1,380 | 196 | - | 129 | 78 | - | ||||||
| CCOP in County? | ||||||||||||
| No | 376,921 | 8,002 | 2.12% | 1.00 | 56,830 | 1,995 | 3.51% | 1.00 | ||||
| Yes | 210,016 | 5,597 | 2.67% | 1.26 | (1.22,1.31) | *** | 97,606 | 1,698 | 1.74% | 0.49 | (0.46,0.52) | *** |
| Medical School in County? | ||||||||||||
| No (n=96) | 530,976 | 11,947 | 2.25% | 1.00 | 140,275 | 3,213 | 2.29% | 1.00 | ||||
| Yes (n=4) | 55,961 | 1,652 | 2.95% | 1.32 | (1.25,1.39) | *** | 14,161 | 480 | 3.39% | 1.50 | (1.36,1.65) | *** |
| Percentile uninsured (county-level) | ||||||||||||
| Quartile 1 (Fewest uninsured) | 274,330 | 6,094 | 2.22% | 1.00 | 74,309 | 1,720 | 2.31% | 1.00 | ||||
| Quartile 2 | 110,291 | 2,683 | 2.43% | 1.10 | (1.05,1.15) | *** | 28,644 | 768 | 2.68% | 1.16 | (1.07,1.27) | *** |
| Quartile 3 | 135,417 | 3,376 | 2.49% | 1.13 | (1.08,1.17) | *** | 34,973 | 887 | 2.54% | 1.10 | (1.01,1.19) | * |
| Quartile 4 (Most uninsured) | 66,899 | 1,446 | 2.16% | 0.97 | (0.92,1.03) | 16,510 | 318 | 1.93% | 0.83 | (0.73,0.94) | ** | |
| Unknown/not documented | 1,380 | 196 | - | 129 | 78 | - | ||||||
| Years | ||||||||||||
| 1996-1998 | 99,872 | 1,776 | 1.78% | 1.00 | - | - | - | |||||
| 1999-2001 | 113,138 | 2,894 | 2.56% | 1.45 | (1.37,1.54) | *** | - | - | - | |||
| 2002-2004 | 125,370 | 2,729 | 2.18% | 1.23 | (1.16,1.31) | *** | - | - | - | |||
| 2005-2007 | 145,832 | 3,912 | 2.68% | 1.52 | (1.44,1.61) | *** | - | - | - | |||
| 2007-2009 | 154,565 | 3,771 | 2.44% | 1.38 | (1.30,1.46) | *** | ||||||
| Geography Type | ||||||||||||
| Urban/ Metropolitan | 555,265 | 13,128 | 2.36% | 1.00 | 146,340 | 3,571 | 2.44% | 1.00 | ||||
| Rural | 31,672 | 471 | 1.49% | 0.62 | (0.57,0.68) | *** | 8,096 | 122 | 1.51% | 0.61 | (0.51,0.73) | *** |
| Selected Common Cancer Types | ||||||||||||
| Lung | 87,352 | 1,499 | 1.72% | 1.00 | 21,729 | 244 | 1.12% | 1.00 | ||||
| Colorectal | 61,227 | 680 | 1.11% | 0.64 | (0.59,0.70) | *** | 13,464 | 102 | 0.76% | 0.67 | (0.53,0.85) | *** |
| Breast | 98,655 | 4,274 | 4.33% | 2.59 | (2.44,2.75) | *** | 24,911 | 1,482 | 5.95% | 5.57 | (4.86,6.39) | *** |
| Prostate | 82,153 | 640 | 0.78% | 0.45 | (0.41,0.49) | *** | 20,817 | 205 | 0.98% | 0.88 | (0.73,1.06) | |
Statistical Significance:
0.001
0.01
0.1
Note: For the period from 1996-2009, county information was missing for CA incidence (n=1,380) and enrolled (n=196). For the period from 2007-2009, county information was missing for CA incidence (n=129) and enrolled (n= 78).
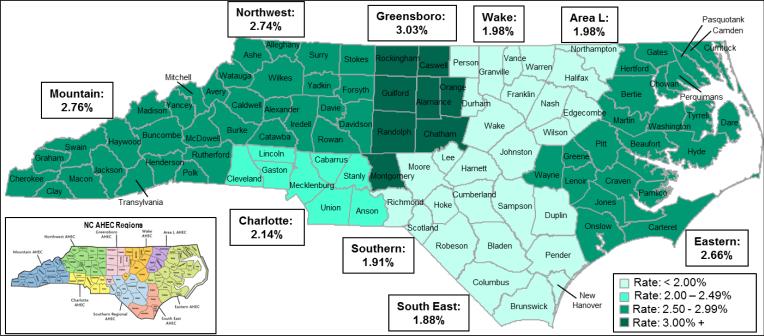
Enrollment rates were significantly lower among minority patients compared to whites. This was true across all years (2.2% minority vs. 2.4% white [OR 0.90, 95% CI 0.86 – 0.94]) and between 2007 and 2009 (2.2% minority vs. 2.5% white [OR 0.89, 95% CI 0.81 – 0.96]). White women had the highest enrollment rate (3.3% across all years [OR 2.21, 95% CI 2.12 – 2.30; referent: white males]; and 3.8% between 2007 and 2009 [OR 3.03, 95% CI 2.79-3.28]; referent: white males). Minority men had the lowest enrollment rate (1.3% across all years [OR 0.85, 95% CI 0.78 – 0.92; referent: white males]; and 1.0% between 2007 and 2009 [OR 0.80, 95% CI 0.67 – 0.95]; referent: white males). Because NCI-sponsored trials emphasize reaching community-based settings (i.e., outside of academic medical centers alone), we also examined geographic differences in enrollment (Table 1, Figure 4). Consistent with prior years, for 2007 to 2009 the treatment trial enrollment rate was only 1.5% (122 enrolled of 8,096 with cancer, in recent years) among those residing in the most rural counties (OR 0.61, 95% CI 0.51 – 0.73).
Figure 4.
Enrollment in NCI Cancer Treatment Clinical Trials, by Area Health Education Center region, 2007-2009.
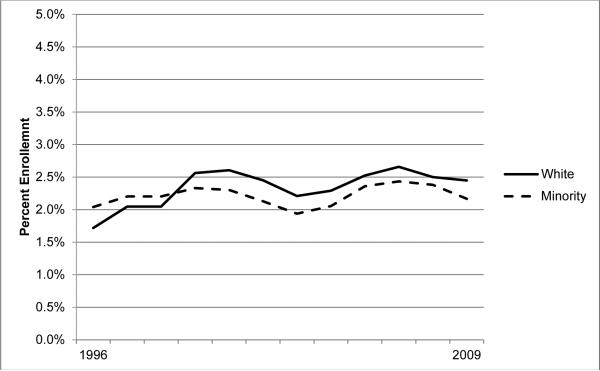
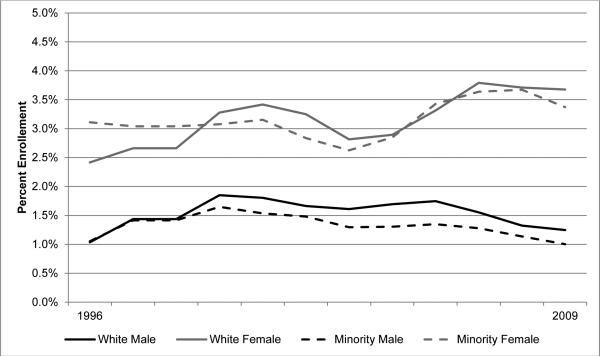
In general, enrollment trends appear to be remaining steady overall (Figure 1). However, closer analysis reveals that enrollment among white patients is increasing overall (trend p<0.001), though this trend is not present among minorities (trend p=0.278). Figure 2 depicts NC treatment trial enrollment trends by race and gender spanning 1996 to 2009, and reflect a gender disparity; enrollment is increasing among women (p<0.001), a trend which is not present among men (p=0.520). Among women, there is no apparent racial disparity in enrollment rates when examining all cancers together, nor evidence of a trend towards one (trend p=0.133). However, among men the racial disparity appears to be significant and widening (trend p=0.002).
Figure 1.
Trends in NCI treatment trial enrollment rates in North Carolina, by race, 3-year averages, 1996–2009.
Figure 2.
Trends in NCI treatment trial enrollment rates in North Carolina, by gender and race, 3-year averages, 1996–2009.
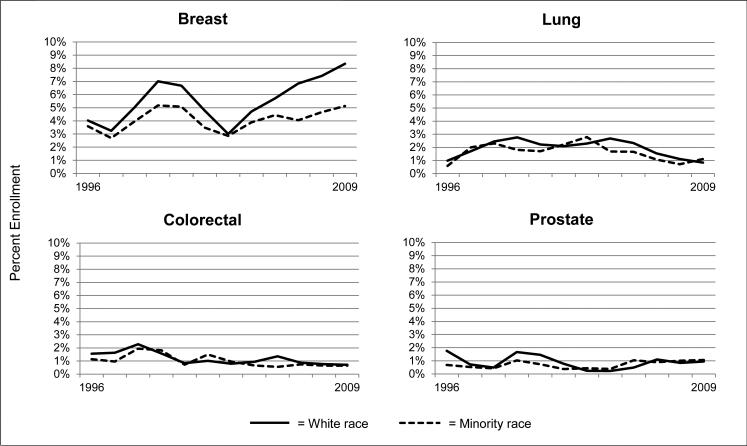
Among the most commonly diagnosed cancers (breast, lung, colorectal and prostate cancers), breast cancer treatment trials had the highest enrollment rate overall and in the most recent 2007 to 2009 time period (breast cancer enrollment 5.95%, OR 5.57, 95% CI, 4.86 – 6.39; colorectal cancer enrollment 0.76%, OR 0.67, 95% CI 0.53 – 0.85; prostate cancer enrollment 0.98%, OR 0.88, 95% CI 0.73 – 1.06, all vs. lung [referent]). Simultaneously, breast cancer also demonstrated the greatest racial disparity in enrollment, with a trend that suggests the disparity is worsening (p<0.001) (Figure 3). Across all races, enrollment in breast cancer trials has increased in recent years, but the upsurge is most dramatic for white patients. Enrollment into lung, colorectal, and prostate cancer trials is essentially unchanged over time but comparable across racial groups.
Figure 3.
Trends in NCI treatment trial enrollment rates in North Carolina, by type of cancer, 3-year averages, 1996–2009.
Discussion
The surveillance system described previously [3] can be used for monitoring, public reporting, and potentially improving minority access to cancer clinical trials. When implemented at regular intervals, the surveillance system can provide an updated understanding of cancer trial access and enrollment patterns as they correspond to regional and racial variation in the burden of cancer. For example, it could be incorporated into a kind of “alert system” indicating potential populations and regions for which clinical trial enrollment is low, thus informing efforts to open select clinical trials or implement interventions to increase awareness of them. Ongoing monitoring of trial enrollment is critical to maintain a pulse on both current enrollment rates and longitudinal trends. There is variation in adult enrollment into cancer treatment trials, which appears to have reached a plateau overall.
Given the flattening of the National Institutes of Health (NIH) budget in recent years and the Sequestration that cut the NIH budget by 5% [11], this report is timely. This budget change places a risk of downward pressure on the ability to enroll patients in treatment trials. There is also risk of aggravating the existing problem of under-representing diverse populations; enrollment counts are stable, though cancer incidence is growing, as is the population overall. Due to structural components of the reporting systems for both data sets (i.e., cancer registry and clinical trials), there is a lag in their availability for these analyses. As such, repeating this analysis in the future through this monitoring system may shed light on the impact of these budget cuts and further inform our understanding of the health implications for North Carolinians.
Our analysis showed ongoing racial disparities in trial enrollment. This racial difference is troublesome for several reasons, and the case of breast cancer sub-types provides a good example as to why. Recent research has documented how different sub-types of breast cancer may have different morbidity profiles and be more aggressive among specific subpopulations, including African Americans [12-16]. Data were not available regarding the cancer subtype for either the broader incident cancer population or the population enrolled in clinical trials. However, the observed under-enrollment of African American women is likely at least partially a function of (1) the two- to three-fold greater incidence of a specific breast cancer subtype (“triple-negative” disease) among African American women compared to Caucasian women, coupled with (2) the greater scientific progress and correspondingly greater prevalence of clinical trials for other, non-triple-negative breast cancer subtypes, historically. Going forward, access to more refined data would allow important insight into how these factors explain the overall under-enrollment of African American women in breast cancer trials, despite comparable enrollment rates in cancer trials overall. Moreover, an empirical connection between these issues would contribute important evidence supporting the call for additional basic science and clinical trial research in triple-negative breast cancer, which has been clearly linked to racial disparities in breast cancer recurrence and mortality. The case of breast cancer exemplifies why enrolling a diverse cohort is imperative to identifying effective therapies for all cancer sub-types among all populations in terms of not only survival, but also side effects and treatment sequelae.
Of important note, racial disparities often present as geographic variation in enrollment, which our examination also found, for example, with lower enrollment in the most rural areas. Although beyond the scope of this analysis, this finding suggests that, despite the CCOP program and many ongoing initiatives, access barriers may still be a problem in many rural communities and those not proximal to an academic medical center, and this may disparately impact racial minorities.
This study and the ongoing examination of these data can inform our understanding of the effectiveness of population-based efforts to address these challenges, and programs that seek to reduce cancer health disparities. For example, in North Carolina, the NCI-funded Carolina Community Network to Reduce Cancer Health Disparities (CCN) has addressed these issues through a multifaceted approach, including seeking to increase minority participation in clinical trials by first understanding their perceptions of clinical trials, as well as through its community outreach and training programs. CCN has advanced our knowledge of how some groups misunderstand the purpose and/or intent of clinical trials, which has contributed to minorities’ greater refusal to participate compared to Caucasians [16-18]. Together with the results of this study, we now have a stronger understanding of how interventions to improve minority enrollment must not only increase geographic accessibility, advancing science and opening trials that are most relevant to under-represented populations (e.g. triple-negative breast cancer; prostate cancer), and also establishing trial eligibility criteria that are most likely to be inclusive of minority populations, while building trust and targeting underlying social constructs precluding research participation. This study may also inform other NCI programs in North Carolina and nationally, such as the NCORP and other programs, which continue to take strides to extend cancer treatment trials into the community setting. This has been through broadening research access points as well as addressing broader structural challenges, such as that of eligibility criteria that can be unintentionally restrictive for minority populations [19-21].
This analysis has several limitations. First, this study focuses on enrollment in NCI-sponsored cancer treatment clinical trials in NC, and does not incorporate data from other clinical trials such as investigator-initiated trials or industry-sponsored trials. Therefore, our findings do not comprehensively reflect the total cancer clinical trials experience, and may not be generalizable to non-treatment trials or other geographic settings. This said, data such as these have been consistently used to assess the state of clinical treatment trial enrollment, and represent a large portion of the clinical treatment trial population, if not the majority of it [1-3]. Second, policy restrictions precluded the ability to make person-level data linkages. Instead, data were pooled and analyzed at the county, AHEC, or state levels, which limits the granularity of the examination. Finally, although this analysis used the most recent data available, it is still a retrospective analysis. Obtaining real-time information would be an asset to improving our understanding of trial enrollment. Despite these limitations, this analysis provides important insight into cancer treatment clinical trial enrollment in NC.
In summary, while rates of enrollment into NCI-sponsored cancer treatment clinical trials in North Carolina are generally stable, racial, gender, and regional differences indicate that effort is needed to ensure broad access to trials and heterogeneity of the population enrolled. We identified important gender and racial disparities, as men and racial minorities experience an equal if not greater burden of cancer in North Carolina, yet comprise a comparatively smaller and apparently declining proportion of the clinical trials population. The approach used in this study can be used again in the future to examine whether current and future interventions to resolve these disparities are effective. Programs such as the NCI NCORP program, the Carolina Community Network to Reduce Cancer Health Disparities, and other local and national programs continue to work to improve trial access for diverse populations. These and other programs may use the methods we describe to examine their programs’ effectiveness in achieving these goals not only in North Carolina, but also in other states across the nation as they seek to ensure program stability, growth, and enhanced access to community-based trials.
Acknowledgments
Portions of this work were supported by the Carolina Community Network through a grant from the National Cancer Institute [grant number U01 CA114629]; training grants from the National Cancer Institute [grants number R25T CA 57726 and 5R25 CA 116339]; and the Integrated Cancer Information and Surveillance System (ICISS), UNC Lineberger Comprehensive Cancer Center with funding provided by the University Cancer Research Fund (UCRF) via the State of North Carolina. Dr. Zullig is supported by a VA Health Services Research and Development (HSR&D) Career Development Award (CDA 13-025). The views expressed in this article are those of the author(s) and do not necessarily represent the views of the Department of Veterans Affairs.
Footnotes
The authors have no relevant conflicts of interest to report.
References
- 1.Minasian LM, Carpenter WR, Weiner BJ, Anderson DE, McCaskill-Stevens W, Nelson S, Whitman C, Kelaghan J, O'Mara AM, Kaluzny AD. Translating research into evidence-based practice: the National Cancer Institute Community Clinical Oncology Program. Cancer. 2010;116(19):4440–4449. doi: 10.1002/cncr.25248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA : the journal of the American Medical Association. 2004;291(22):2720–2726. doi: 10.1001/jama.291.22.2720. [DOI] [PubMed] [Google Scholar]
- 3.Carpenter WR, Tyree S, Wu Y, Meyer AM, DiMartino L, Zullig L, Godley PA. A surveillance system for monitoring, public reporting, and improving minority access to cancer clinical trials. Clinical trials (London, England) 2012;9(4):426–435. doi: 10.1177/1740774512449531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clauser SB, Johnson MR, O'Brien DM, Beveridge JM, Fennell ML, Kaluzny AD. Improving clinical research and cancer care delivery in community settings: evaluating the NCI community cancer centers program. Implementation science : IS. 2009;4:63. doi: 10.1186/1748-5908-4-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.NCI Community Cancer Centers Program. [ http://ncccp.cancer.gov]
- 6.Community Clinical Oncology Program. [ http://ccop.cancer.gov]
- 7.Carpenter WR, Meyer AM, Wu Y, Qaqish B, Sanoff HK, Goldberg RM, Weiner BJ. Translating research into practice: the role of provider-based research networks in the diffusion of an evidence-based colon cancer treatment innovation. Medical care. 2012;50(8):737–748. doi: 10.1097/MLR.0b013e31824ebe13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meyer AM, Reeder-Hayes KE, Liu H, Wheeler SB, Penn D, Weiner BJ, Carpenter WR. Differential receipt of sentinel lymph node biopsy within practice-based research networks. Medical care. 2013;51(9):812–818. doi: 10.1097/MLR.0b013e31829c8ca4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCaskill-Stevens W, Lyss AP, Good M, Marsland T, Lilenbaum R. The NCI Community Oncology Research Program. American Society of Clinical Oncology educational book / ASCO American Society of Clinical Oncology Meeting. 2013;2013:84–89. doi: 10.14694/EdBook_AM.2013.33.e84. [DOI] [PubMed] [Google Scholar]
- 10.Institute of Medicine . A National Cancer Clinical Trials System for the 21st Century: Reinvigorating the NCI Cooperative Group Program. National Academies Press; Washington DC: 2010. [PubMed] [Google Scholar]
- 11.Fact sheet: Impact of Sequestration on the National Institutes of Health [ http://www.nih.gov/news/health/jun2013/nih-03.htm]
- 12.Ashktorab H, Smoot DT, Carethers JM, Rahmanian M, Kittles R, Vosganian G, Doura M, Nidhiry E, Naab T, Momen B, et al. High incidence of microsatellite instability in colorectal cancer from African Americans. Clinical cancer research : an official journal of the American Association for Cancer Research. 2003;9(3):1112–1117. [PubMed] [Google Scholar]
- 13.Kauh J, Brawley OW, Berger M. Racial disparities in colorectal cancer. Current problems in cancer. 2007;31(3):123–133. doi: 10.1016/j.currproblcancer.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Woodward WA, Huang EH, McNeese MD, Perkins GH, Tucker SL, Strom EA, Middleton L, Hahn K, Hortobagyi GN, Buchholz TA. African-American race is associated with a poorer overall survival rate for breast cancer patients treated with mastectomy and doxorubicin-based chemotherapy. Cancer. 2006;107(11):2662–2668. doi: 10.1002/cncr.22281. [DOI] [PubMed] [Google Scholar]
- 15.Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, Karaca G, Troester MA, Tse CK, Edmiston S, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA : the journal of the American Medical Association. 2006;295(21):2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 16.Haynes-Maslow L, Godley P, Dimartino L, White B, Odom J, Richmond A, Carpenter W. African American women's perceptions of cancer clinical trials. Cancer medicine. 2014 doi: 10.1002/cam4.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corbie-Smith G, Thomas SB, Williams MV, Moody-Ayers S. Attitudes and beliefs of African Americans toward participation in medical research. Journal of general internal medicine. 1999;14(9):537–546. doi: 10.1046/j.1525-1497.1999.07048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mouton CP, Harris S, Rovi S, Solorzano P, Johnson MS. Barriers to black women's participation in cancer clinical trials. Journal of the National Medical Association. 1997;89(11):721–727. [PMC free article] [PubMed] [Google Scholar]
- 19.BeLue R, Taylor-Richardson KD, Lin J, Rivera AT, Grandison D. African Americans and participation in clinical trials: differences in beliefs and attitudes by gender. Contemporary clinical trials. 2006;27(6):498–505. doi: 10.1016/j.cct.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Gross CP, Filardo G, Mayne ST, Krumholz HM. The impact of socioeconomic status and race on trial participation for older women with breast cancer. Cancer. 2005;103(3):483–491. doi: 10.1002/cncr.20792. [DOI] [PubMed] [Google Scholar]
- 21.Penberthy L, Brown R, Wilson-Genderson M, Dahman B, Ginder G, Siminoff LA. Barriers to therapeutic clinical trials enrollment: differences between African-American and white cancer patients identified at the time of eligibility assessment. Clinical trials (London, England) 2012;9(6):788–797. doi: 10.1177/1740774512458992. [DOI] [PMC free article] [PubMed] [Google Scholar]