Abstract
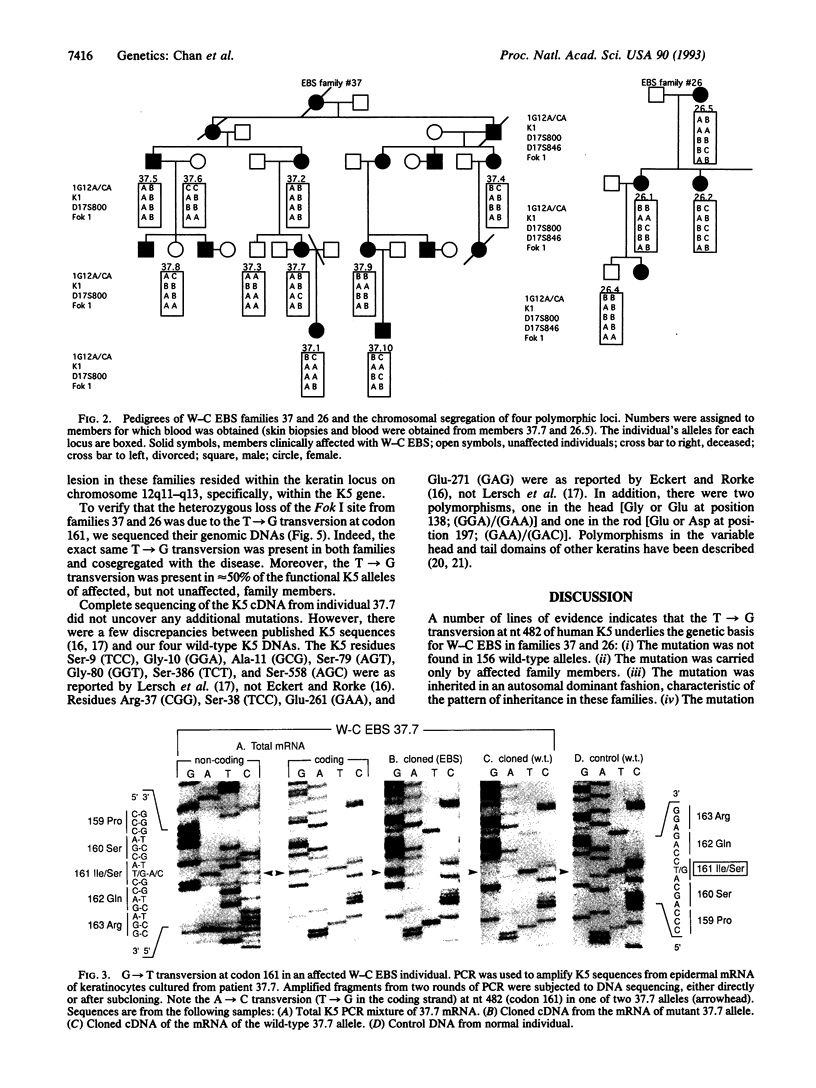
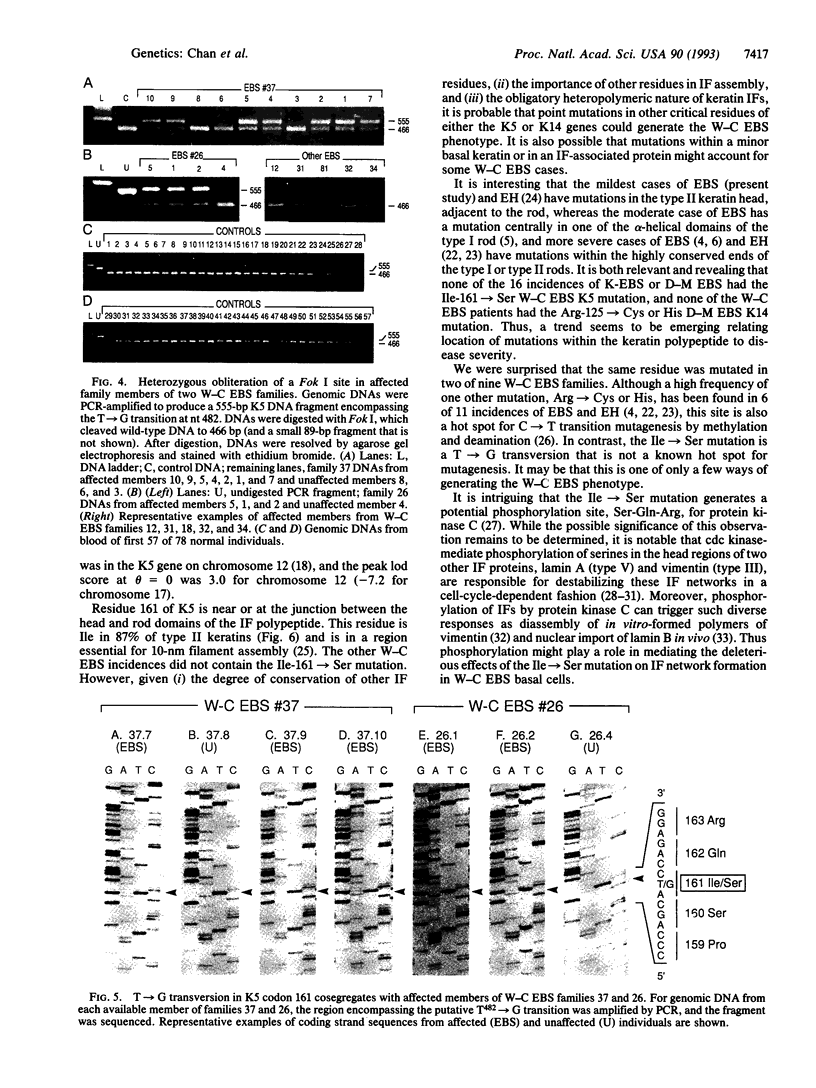
Epidermolysis bullosa simplex (EBS) is a group of autosomal dominant skin diseases characterized by blistering, due to mechanical-stress-induced degeneration of basal epidermal cells. Recently, it was discovered that the more severe types, Dowling-Meara and Koebner, are genetic disorders of the basal epidermal keratins, keratin 5 (K5) and keratin 14 (K14). Here, we show that the mildest type of EBS, Weber-Cockayne, is also a disorder of these keratins. Affected members of two unrelated families with Weber-Cockayne EBS had a T-->G point mutation in the second base position of codon 161 of one of two K5 alleles, leading to an Ile-->Ser mutation. This mutation was not present in unaffected members or in 156 alleles from normal individuals. Linkage analyses mapped the defect to the type II keratin gene cluster on chromosome 12q11-q13 (peak logarithm of odds score at theta = 0 of 3.0), providing strong additional evidence that this mutation is responsible for the Weber-Cockayne EBS phenotype. Conserved among type II keratins, Ile-161 is in the nonhelical head domain of K5, a region previously shown to be important for 10-nm filament assembly. The mutation generates a potential substrate site for protein kinase C, which could influence intermediate filament architecture, perhaps leading to the intrafilament association seen ultrastructurally in patients with the mutation.
Full text
PDF

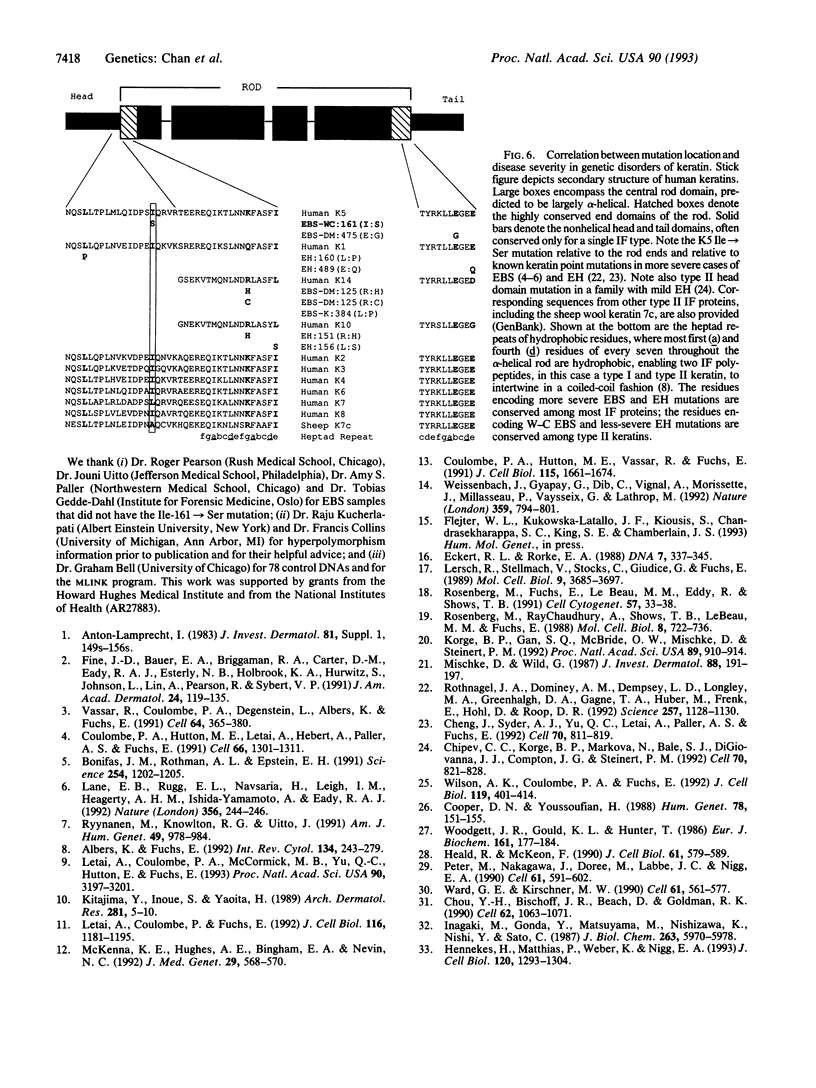
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albers K., Fuchs E. The molecular biology of intermediate filament proteins. Int Rev Cytol. 1992;134:243–279. doi: 10.1016/s0074-7696(08)62030-6. [DOI] [PubMed] [Google Scholar]
- Anton-Lamprecht I. Genetically induced abnormalities of epidermal differentiation and ultrastructure in ichthyoses and epidermolyses: pathogenesis, heterogeneity, fetal manifestation, and prenatal diagnosis. J Invest Dermatol. 1983 Jul;81(1 Suppl):149s–156s. doi: 10.1111/1523-1747.ep12540961. [DOI] [PubMed] [Google Scholar]
- Bonifas J. M., Rothman A. L., Epstein E. H., Jr Epidermolysis bullosa simplex: evidence in two families for keratin gene abnormalities. Science. 1991 Nov 22;254(5035):1202–1205. doi: 10.1126/science.1720261. [DOI] [PubMed] [Google Scholar]
- Cheng J., Syder A. J., Yu Q. C., Letai A., Paller A. S., Fuchs E. The genetic basis of epidermolytic hyperkeratosis: a disorder of differentiation-specific epidermal keratin genes. Cell. 1992 Sep 4;70(5):811–819. doi: 10.1016/0092-8674(92)90314-3. [DOI] [PubMed] [Google Scholar]
- Chipev C. C., Korge B. P., Markova N., Bale S. J., DiGiovanna J. J., Compton J. G., Steinert P. M. A leucine----proline mutation in the H1 subdomain of keratin 1 causes epidermolytic hyperkeratosis. Cell. 1992 Sep 4;70(5):821–828. doi: 10.1016/0092-8674(92)90315-4. [DOI] [PubMed] [Google Scholar]
- Chou Y. H., Bischoff J. R., Beach D., Goldman R. D. Intermediate filament reorganization during mitosis is mediated by p34cdc2 phosphorylation of vimentin. Cell. 1990 Sep 21;62(6):1063–1071. doi: 10.1016/0092-8674(90)90384-q. [DOI] [PubMed] [Google Scholar]
- Cooper D. N., Youssoufian H. The CpG dinucleotide and human genetic disease. Hum Genet. 1988 Feb;78(2):151–155. doi: 10.1007/BF00278187. [DOI] [PubMed] [Google Scholar]
- Coulombe P. A., Hutton M. E., Letai A., Hebert A., Paller A. S., Fuchs E. Point mutations in human keratin 14 genes of epidermolysis bullosa simplex patients: genetic and functional analyses. Cell. 1991 Sep 20;66(6):1301–1311. doi: 10.1016/0092-8674(91)90051-y. [DOI] [PubMed] [Google Scholar]
- Coulombe P. A., Hutton M. E., Vassar R., Fuchs E. A function for keratins and a common thread among different types of epidermolysis bullosa simplex diseases. J Cell Biol. 1991 Dec;115(6):1661–1674. doi: 10.1083/jcb.115.6.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert R. L., Rorke E. A. The sequence of the human epidermal 58-kD (#5) type II keratin reveals an absence of 5' upstream sequence conservation between coexpressed epidermal keratins. DNA. 1988 Jun;7(5):337–345. doi: 10.1089/dna.1.1988.7.337. [DOI] [PubMed] [Google Scholar]
- Fine J. D., Bauer E. A., Briggaman R. A., Carter D. M., Eady R. A., Esterly N. B., Holbrook K. A., Hurwitz S., Johnson L., Lin A. Revised clinical and laboratory criteria for subtypes of inherited epidermolysis bullosa. A consensus report by the Subcommittee on Diagnosis and Classification of the National Epidermolysis Bullosa Registry. J Am Acad Dermatol. 1991 Jan;24(1):119–135. doi: 10.1016/0190-9622(91)70021-s. [DOI] [PubMed] [Google Scholar]
- Heald R., McKeon F. Mutations of phosphorylation sites in lamin A that prevent nuclear lamina disassembly in mitosis. Cell. 1990 May 18;61(4):579–589. doi: 10.1016/0092-8674(90)90470-y. [DOI] [PubMed] [Google Scholar]
- Hennekes H., Peter M., Weber K., Nigg E. A. Phosphorylation on protein kinase C sites inhibits nuclear import of lamin B2. J Cell Biol. 1993 Mar;120(6):1293–1304. doi: 10.1083/jcb.120.6.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki M., Gonda Y., Matsuyama M., Nishizawa K., Nishi Y., Sato C. Intermediate filament reconstitution in vitro. The role of phosphorylation on the assembly-disassembly of desmin. J Biol Chem. 1988 Apr 25;263(12):5970–5978. [PubMed] [Google Scholar]
- Kitajima Y., Inoue S., Yaoita H. Abnormal organization of keratin intermediate filaments in cultured keratinocytes of epidermolysis bullosa simplex. Arch Dermatol Res. 1989;281(1):5–10. doi: 10.1007/BF00424265. [DOI] [PubMed] [Google Scholar]
- Korge B. P., Gan S. Q., McBride O. W., Mischke D., Steinert P. M. Extensive size polymorphism of the human keratin 10 chain resides in the C-terminal V2 subdomain due to variable numbers and sizes of glycine loops. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):910–914. doi: 10.1073/pnas.89.3.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane E. B., Rugg E. L., Navsaria H., Leigh I. M., Heagerty A. H., Ishida-Yamamoto A., Eady R. A. A mutation in the conserved helix termination peptide of keratin 5 in hereditary skin blistering. Nature. 1992 Mar 19;356(6366):244–246. doi: 10.1038/356244a0. [DOI] [PubMed] [Google Scholar]
- Lersch R., Stellmach V., Stocks C., Giudice G., Fuchs E. Isolation, sequence, and expression of a human keratin K5 gene: transcriptional regulation of keratins and insights into pairwise control. Mol Cell Biol. 1989 Sep;9(9):3685–3697. doi: 10.1128/mcb.9.9.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letai A., Coulombe P. A., Fuchs E. Do the ends justify the mean? Proline mutations at the ends of the keratin coiled-coil rod segment are more disruptive than internal mutations. J Cell Biol. 1992 Mar;116(5):1181–1195. doi: 10.1083/jcb.116.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letai A., Coulombe P. A., McCormick M. B., Yu Q. C., Hutton E., Fuchs E. Disease severity correlates with position of keratin point mutations in patients with epidermolysis bullosa simplex. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3197–3201. doi: 10.1073/pnas.90.8.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna K. E., Hughes A. E., Bingham E. A., Nevin N. C. Linkage of epidermolysis bullosa simplex to keratin gene loci. J Med Genet. 1992 Aug;29(8):568–570. doi: 10.1136/jmg.29.8.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mischke D., Wild G. Polymorphic keratins in human epidermis. J Invest Dermatol. 1987 Feb;88(2):191–197. doi: 10.1111/1523-1747.ep12525329. [DOI] [PubMed] [Google Scholar]
- Peter M., Nakagawa J., Dorée M., Labbé J. C., Nigg E. A. In vitro disassembly of the nuclear lamina and M phase-specific phosphorylation of lamins by cdc2 kinase. Cell. 1990 May 18;61(4):591–602. doi: 10.1016/0092-8674(90)90471-p. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Fuchs E., Le Beau M. M., Eddy R. L., Shows T. B. Three epidermal and one simple epithelial type II keratin genes map to human chromosome 12. Cytogenet Cell Genet. 1991;57(1):33–38. doi: 10.1159/000133109. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., RayChaudhury A., Shows T. B., Le Beau M. M., Fuchs E. A group of type I keratin genes on human chromosome 17: characterization and expression. Mol Cell Biol. 1988 Feb;8(2):722–736. doi: 10.1128/mcb.8.2.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothnagel J. A., Dominey A. M., Dempsey L. D., Longley M. A., Greenhalgh D. A., Gagne T. A., Huber M., Frenk E., Hohl D., Roop D. R. Mutations in the rod domains of keratins 1 and 10 in epidermolytic hyperkeratosis. Science. 1992 Aug 21;257(5073):1128–1130. doi: 10.1126/science.257.5073.1128. [DOI] [PubMed] [Google Scholar]
- Ryynänen M., Knowlton R. G., Uitto J. Mapping of epidermolysis bullosa simplex mutation to chromosome 12. Am J Hum Genet. 1991 Nov;49(5):978–984. [PMC free article] [PubMed] [Google Scholar]
- Vassar R., Coulombe P. A., Degenstein L., Albers K., Fuchs E. Mutant keratin expression in transgenic mice causes marked abnormalities resembling a human genetic skin disease. Cell. 1991 Jan 25;64(2):365–380. doi: 10.1016/0092-8674(91)90645-f. [DOI] [PubMed] [Google Scholar]
- Ward G. E., Kirschner M. W. Identification of cell cycle-regulated phosphorylation sites on nuclear lamin C. Cell. 1990 May 18;61(4):561–577. doi: 10.1016/0092-8674(90)90469-u. [DOI] [PubMed] [Google Scholar]
- Weissenbach J., Gyapay G., Dib C., Vignal A., Morissette J., Millasseau P., Vaysseix G., Lathrop M. A second-generation linkage map of the human genome. Nature. 1992 Oct 29;359(6398):794–801. doi: 10.1038/359794a0. [DOI] [PubMed] [Google Scholar]
- Wilson A. K., Coulombe P. A., Fuchs E. The roles of K5 and K14 head, tail, and R/K L L E G E domains in keratin filament assembly in vitro. J Cell Biol. 1992 Oct;119(2):401–414. doi: 10.1083/jcb.119.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodgett J. R., Gould K. L., Hunter T. Substrate specificity of protein kinase C. Use of synthetic peptides corresponding to physiological sites as probes for substrate recognition requirements. Eur J Biochem. 1986 Nov 17;161(1):177–184. doi: 10.1111/j.1432-1033.1986.tb10139.x. [DOI] [PubMed] [Google Scholar]