Abstract
Bang sensitive (BS) Drosophila mutants display characteristic seizure-like phenotypes resembling, in some aspects, those of human seizure disorders such as epilepsy. The BS mutant parabss1, caused by a gain-of-function mutation of the voltage-gated Na+ channel gene, is extremely seizure-sensitive with phenotypes that have proven difficult to ameliorate by anti-epileptic drug feeding or by seizure-suppressor mutation. It has been presented as a model for intractable human epilepsy. Here we show that cacophony (cacTS2), a mutation of the Drosophila presynaptic Ca++ channel α1 subunit gene, is a particularly potent seizure-suppressor mutation, reverting seizure-like phenotypes for parabss1 and other BS mutants. Seizure-like phenotypes for parabss1 may be suppressed by as much as 90% in double mutant combinations with cacTS2. Unexpectedly, we find that parabss1 also reciprocally suppresses cacTS2 seizure-like phenotypes. The cacTS2 mutant displays these seizure-like behaviors and spontaneous high-frequency action potential firing transiently after exposure to high temperature. We find that this seizure-like behavior in cacTS2 is ameliorated by 85% in double mutant combinations with parabss1.
Author Summary
Seizure disorders, such as epilepsy, are a serious health concern because of the large number of patients affected and a limited availability of treatment options. About 10% of the population will have at least one seizure during their lifetime, and 1% will experience persistent, recurrent epileptic seizures. Moreover, for about one-third of patients, epilepsy is intractable with seizures that are not controlled with any available drugs. Genetic seizure suppressors are modifier mutations that are capable of reverting seizure susceptibility to wild type levels when combined with seizure-prone mutants in double mutant individuals. Suppressors are valuable in providing an experimental approach that can provide insight into mechanisms underlying seizure susceptibility. Also, they identify novel gene products that may be targets for therapeutic drug development. In the present study we show that a severe seizure phenotype of the Drosophila paralyticbss1 (parabss1) mutant is 90% suppressed by the N-type calcium channel mutation cacophonyTS2 (cacTS2). The effect of suppression is not restricted to parabss1, but cacTS2 can also revert seizure-like phenotypes of other Drosophila mutants like easily-shocked (eas) and slamdance (sda). Thus, cacTS2 acts as a highly potent, general seizure suppressor mutation. A surprising finding in this study is co-suppression: parabss1 also suppresses a seizure phenotype in cacTS2 mutants induced by elevated temperature. A current view of complex diseases such as epilepsy, is that multiple genes and environmental factors can each contribute small, additive effects that can summate to produce a disease state when some threshold value is exceeded. Our findings indicate that different pathogenic ion channel mutations can sometimes form therapeutic combinations with effects that mask one another.
Introduction
Human seizure disorders are a substantial neurological health problem because of the large number of affected individuals, and heterogeneity underlying the many syndromes. An estimated 1% of the U.S. population, nearly 3 million Americans, is affected by the more than 40 different syndromes that comprise the epilepsies [1,2]. Seizures occur because of an imbalance in excitation and inhibition: excitation can be excessive, inhibition can be inadequate, or both. The resulting seizure activity involves large numbers of neurons firing uncontrollably and synchronously, usually in a rhythmic pattern. Multiple and different molecular aspects of electrical signaling appear to be responsible for the triggering of seizures at the site of initiation or focus, their subsequent spread from the focus to adjacent regions of nervous tissue, and their eventual termination.
In this study, we examine the contribution of basic synaptic transmission to seizure-susceptibility in a Drosophila model using mutations of the cacophony (cac) gene, responsible for neurotransmitter release. The cac gene encodes the α1 subunit of the Drosophila voltage-gated presynaptic Ca++ channel, homologous to the mammalian N-type channel [3–7]. The allele used here, cacTS2, shows conditional and reversible phenotypes dependent on temperature: a behavioral paralysis phenotype and a loss of neurotransmitter release phenotype [5–7]. At restrictive high temperatures, evoked synaptic currents are markedly reduced in cacTS2 mutants, returning to wild-type levels when temperature is lowered to permissive temperatures.
We report here that the cacTS2 mutation affects seizure susceptibility in complex ways including seizure-sensitivity and seizure-resistance, under different conditions. As reported [8], cac temperature-sensitive mutants display spontaneous seizure-like activity when shifted to restrictive temperature. We find here that at permissive temperature, cacTS2 is a seizure-resistant mutation and a potent seizure-suppressor. In double mutant combinations with bang-sensitive (BS) seizure-sensitive mutants, cacTS2 is one of the strongest seizure-suppressors that we have identified in the fly, to date. In particular, the cacTS2 mutation is found to ameliorate seizure-like phenotypes in homozygous parabss1, a Na+ channel gain-of-function mutation, the most severe of Drosophila seizure-sensitive mutations [9,10] and resembling, in some aspects, Na+ channel loss-of-function mutations responsible for intractable epilepsy [11,12]. The cacTS2 mutation is a good suppressor of parabss1 phenotypes comparable to malelessnapts and stronger than heat-treated shibirets1 and gilgamesh (Table 1)[13,14,15,16].
Table 1. Suppression of behavioral bang-sensitive paralytic phenotypes by cacTS2 and cacRNAi.
Flies of the appropriate genotype (n ≥ 100) were stimulated by mechanical stimulation delivered by a vortex mixer (10 sec at maximum speed). The number of flies undergoing paralysis was counted to determine the percent paralysis and the percent suppressed (unparalyzed).
| Genotype | % BS | % Suppression |
|---|---|---|
| sda (RT) | 100 | 0 |
| sda (HS) | 100 | 0 |
| eas (RT) | 100 | 0 |
| eas (HS) | 100 | 0 |
| parabss1 (RT) | 100 | 0 |
| parabss1 (HS) | 100 | 0 |
| parabss1/+ (RT) | 62 | 38 |
| cacTS2/Y; sda (RT) | 12 | 88 |
| cacTS2/Y; sda (HS) | 0 | 100 |
| eas cacTS2/Y (RT) | 90 | 10 |
| eas cacTS2/Y (HS) | 54 | 46 |
| parabss1 cacTS2/Y (RT) | 36 | 64 |
| parabss1 cacTS2/Y (HS) | 13 | 87 |
| parabss1 cacTS2 (RT) | 23 | 77 |
| parabss1 cacTS2 (HS) | 8 | 92 |
| parabss1 cacTS2/para+ cacTS2 (RT) | 2 | 98 |
| elavc155-GAL4 parabss1/Y;UAS-cacRNAi/+) (RT) | 64 | 36 |
| elavc155-Gal4 eas/Y;UAS-cacRNAi/+) (RT) | 15 | 85 |
At restrictive temperatures, cacTS2 exhibits complex phenotypes including TS seizure-like activity, synaptic failure and paralysis. We found that all cacTS2 phenotypes are reciprocally suppressed in double mutant combination with parabss1. Suppression of TS seizure-like behaviors in cacTS2 by a Na+ channel mutation indicates that the combination of two ion channel alleles involved in epilepsy can have beneficial clinical effects when present in the same individual organism: that is, each of the two mutations co-suppresses seizures caused by the other, similar to observations reported for mouse [17].
Results
The cacTS2 mutant is seizure-resistant at room temperature; seizure-sensitive at high temperature
The behaviors of cacTS2 mutants are unexceptional at room temperature (24°C): feeding, grooming, and mating behaviors appear normal. Overall activity levels are unaltered: flies are neither sluggish nor hyperactive. The cacTS2 mutants show no bang-sensitive (BS) behavioral paralysis phenotype and are unaffected by mechanical stimulation. Using the adult giant fiber (GF) neurocircuit as a proxy for holo-nervous system function, the electrophysiology phenotype for cacTS2 at room temperature generally resembles wild-type [18]. Thus, single pulse stimulation of the GF produces evoked potentials and synaptic currents in the dorsal longitudinal muscle (DLM) that are normal in appearance (S1 Fig) [5], have a threshold of 0.96 ± 0.12 V (mean ± s.e.m., n = 9) and a latency of 1.1 ± 0.04 msec (mean ± s.e.m., n = 5).
Seizure-like electrical activity in cacTS2 mutants can be evoked with high-frequency stimuli (HFS; 0.5 msec stimuli at 200 Hz for 300 msec; Fig 1A), similar to discharges observed for other Drosophila mutants [19,20]. Large HFS voltages were characteristically required to evoke seizures at room temperature indicating that cacTS2 behaves as a seizure-resistant mutant. Seizure threshold for cacTS2 was (58.3 ± 1.0 V HFS, mean ± s.e.m., n = 13), nearly twice that of Canton-Special wild type flies (24.64 ± 2.83 HFS, mean ± s.e.m., n = 10; Fig 1A–1C). The seizure threshold for cacTS2 is comparable to previously-reported seizure-resistant mutants such as paralyticts1, ShakerKS133 and shakingB2 that have high seizure thresholds and can also act as seizure-suppressor mutations in double mutant combinations with BS mutants [19].
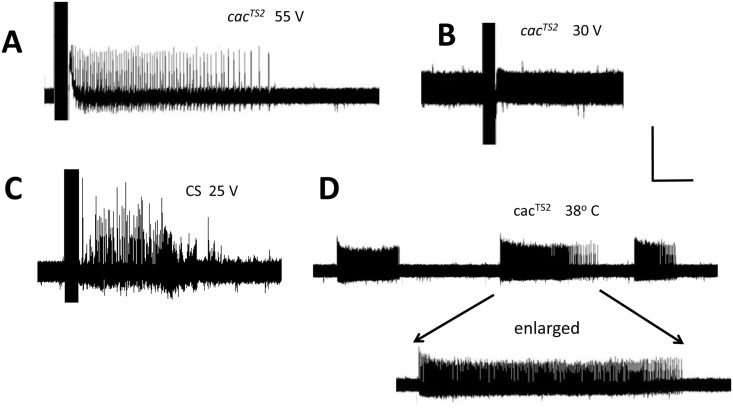
Fig 1. Drosophila cacTS2 electrophysiology.
A. Electrical recording from a cacTS2 DLM fiber following delivery of 55 V HFS stimulation (0.5 msec stimuli at 200 Hz for 300 msec) at room temperature. The stimulation evokes seizure-like electrical activity, indicating that the voltage is at or above seizure threshold. B. Recording from a cacTS2 DLM fiber following delivery of a 30 V HFS stimulus showing that this stimulation voltage fails to evoke seizure-like activity. This stimulation voltage, near the wild-range, is below seizure threshold for cacTS2. C. Recording from a wild type Canton Special (CS) DLM fiber following delivery of a 25 V HFS stimulus that is effective in evoking seizure-like activity. D. Recording from a cacTS2 DLM fiber following a temperature shift from permissive room temperature to restrictive 38°C temperature. Spontaneous seizure-like electrical activity is observed in the DLM fiber, indicating that the mutant is seizure-sensitive at restrictive temperatures. Recording shows a representative example of three spontaneous seizure-like discharges. Horizontal calibration is 1.0 sec for A, B, and C; 4 sec for D and 1.5 sec for D (enlarged). Vertical calibration is 20 mV.
Several cac alleles, especially cacTS2 and cacNT27, are notable for their temperature-sensitivity (TS) with essentially normal behavior and neurology at room temperature which is permissive; and displaying complicated neurological phenotypes at high temperature (>38°C) which is restrictive [5,8]. The shift from permissive to restrictive temperature, causes a transient period of nervous system hyperexcitability lasting several seconds [8], followed by a prolonged period of hypoexcitability with synaptic failure and behavioral paralysis [5]. The hyperactive period is characterized by spontaneous seizure-like behaviors: leg-shaking, abdominal twitching, wing scissoring, and proboscis extensions. These are accompanied by spontaneous seizure-like firing of the DLM motor neurons in electrophysiology recordings (Fig 1D), similar to that described previously [8]. Seizure-like activity for cacTS2 at elevated temperature is interesting considering the seizure-resistant phenotype observed at room temperature. The spontaneous seizure-like DLM activity generally resembles that observed during evoked seizure-like activity (Fig 1). We were unable to determine a reliable evoked seizure-threshold for cacTS2 at restrictive temperature. Immediately following the shift to restrictive temperature, the seizure-threshold is still high, resembling the threshold of cacTS2 at room temperature. Spontaneous seizure-like activity ensued soon after the shift to restrictive temperature and their nearly continuous occurences made it difficult to distinguish them from stimulus-evoked seizures. Taken together, these findings indicate that cacTS2 is apparently a seizure-resistant mutant at room temperature, changing to a seizure-sensitive mutant after shift to restrictive temperature.
The cacTS2 mutation suppresses BS paralysis in sda and eas mutants
In order to test for genetic interactions, we constructed double mutants between cacTS2 and different BS mutants. This resulted in suppression of the BS phenotype as exemplified by hemizygous double mutant male flies cacTS2/Y;;sda that showed 12% behavioral BS paralysis (indicating 88% phenotypic suppression, n = 147; P < 0.0001, chi- square test; Fig 2A; Table 1) at room temperature (24°C), compared to the sda single mutant control flies which showed 100% BS paralysis (P < 0.0001). This finding of suppression at room temperature was a little unexpected since cacTS2 has previously been described as a temperature-sensitive mutation and permissive temperature phenotypes have not been reported. This result may be related to the observation above that cacTS2 is seizure-resistant at room temperature; heretofore the only other difference from wild type that we have seen at room temperature.
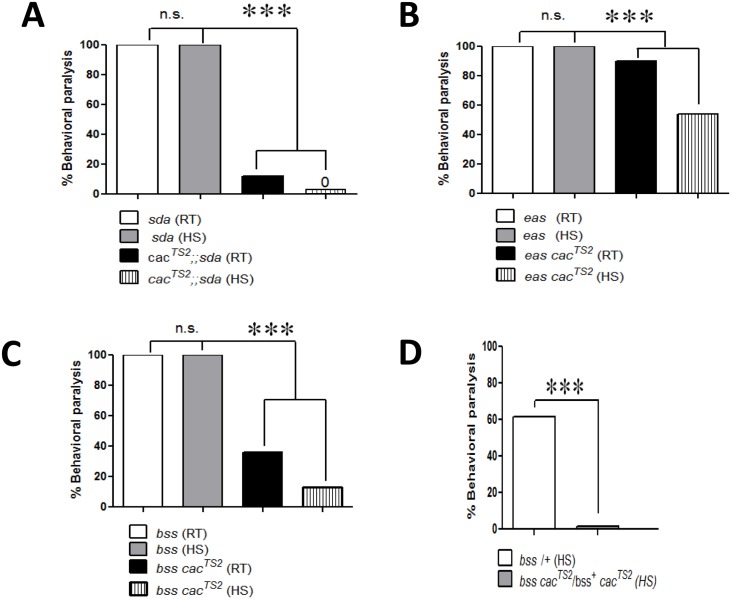
Fig 2. Suppression of bang sensitive behavioral phenotypes by cacTS2.
A. Behavioral paralysis in sda hemizygotes (white bar) is suppressed in hemizygous double mutants cacTS2/Y;;sda at room temperature (RT) by about a factor of 10 (black bar; n = 147). Following a brief heat shock (HS = 3 min at 30°C), complete suppression of behavioral paralysis is observed in hemizygous double mutants cacTS2/Y;;sda (striped bar; n = 93). B. Behavioral paralysis in hemizygous eas cacTS2/Y double mutants at RT (black bar, n = 147) shows a small reduction compared to hemizygous eas/Y (white bar). Following a brief heat shock (HS = 3 min at 30°C), suppression is by about a factor of 2 (striped bar; n = 93). C. Behavioral paralysis in parabss1 hemizygotes (white bar) is suppressed in hemizygous parabss1 cacTS2 double mutants at RT by about a factor of 3 (black bar; 64% suppression; n = 658). Following a brief HS, suppression is increased in the parabss1 cacTS2 double mutants (striped bar; 87% suppression; n = 650). D. Following a brief HS, cacTS2 completely suppresses behavioral paralysis in parabss1/+ heterozygous females (n = 60). *P < 0.01, **P = 0.001, ***P < 0.0001, chi-square test.
In order to examine if temperature has an effect on BS suppression, we examined double mutants at elevated temperatures within the nominally permissive range, that is below 38°C, to avoid cacTS2 behavioral paralysis. We found that seizure-suppression by cacTS2 is increased at elevated temperatures. In hemizygous double mutant flies cacTS2/Y;;sda, a brief heat shock (HS; 3 min at 30°C), completely suppressed all sda bang-sensitivity (0% paralysis, 100% suppression, n = 93; P < 0.0001, chi-square test; Fig 2A; Table 1). A similar brief HS delivered to control single mutant sda flies had no effect on bang-sensitivity: 100% of control flies continued to show BS paralysis (P < 0.0001).
The cacTS2 mutation is a general seizure-suppressor, not limited to suppression of BS phenotypes in sda mutants: modest seizure-suppression is also observed for eas mutants. In hemizygous double mutant male flies eas cacTS2/Y, BS was 90% (10% suppression, n = 147; P = 0.0002, chi- square test; Fig 2B; Table 1) at room temperature compared to 100% BS in eas single mutant controls. In eas, suppression by cacTS2 was also increased with exposure to elevated temperature. In hemizygous double mutant flies eas cacTS2/Y, bang-sensitivity was 54% (46% suppression, n = 93; P = < 0.0001, chi-square test; Fig 2B; Table 1) following a brief HS (3 min at 30°C). HS had no effect on the bang sensitivity of eas single mutant control flies: 100% of the control flies showed BS paralysis (P < 0.0001). Thus, cacTS2 acts as a general suppressor of BS behavior, reverting phenotypes of both sda and eas BS mutants in double mutant combinations. Some suppression occurs at room temperature, although suppression increases with increases in temperature within the permissive temperature range. Previous studies have also shown that BS phenotypes in sda mutants are easier to suppress than for eas mutants [21,22].
The cacTS2 mutation suppresses BS paralysis in parabss1 mutants
We investigated genetic interactions between cacTS2 and parabss1 by constructing the appropriate double mutant combinations. Previous studies have found that seizure-like phenotypes are difficult to suppress in parabss1 mutants, that carry a gain-of-function voltage-gated Na+ channel defect [10 (Table 2)]. We find here that cacTS2 is an effective suppressor of parabss1 behavioral phenotypes. For hemizygous double mutant males (genotype: parabss1 cacTS2/Y), BS paralysis was 36% (64% suppression; n = 658; P < 0.0001, chi-square test) at room temperature (Fig 2C; Table 1). Suppression by cacTS2 was also increased at elevated temperature. After a brief HS, BS paralysis decreased to 13% (87% suppression; n = 650; P < 0.0001, chi-square test) in hemizygous double mutants (Fig 2C; Table 2). Homozygous double mutant females (genotype: parabss1 cacTS2) showed 23% BS paralysis at room temperature (77% suppression) which decreased to 8% (92% suppression) following HS (Table 2). In control parabss1 flies, there was no effect of HS: 100% of flies showed BS paralysis (P < 0.0001; Table 2). The cacTS2 mutation is an especially effective suppressor of parabss1/+ heterozygote behavioral phenotypes. In double mutant flies (genotype: parabss1 cacTS2/para+ cacTS2) BS paralysis was 2% (98% suppression) at room temperature, compared to 62% BS paralysis seen in control parabss1/+ heterozygotes without the cacTS2 suppressor (n = 60; P < 0.0001, chi-square test; Fig 2D; Table 2).
Table 2. Seizure suppression of parabss1 by cacTS2 and other suppressors.
The table lists four mutations that have been reported to revert parabss1 phenotypes in double mutant combinations. An additional 12 seizure-suppressor mutations have been reported that are ineffective at suppressing parabss1 [22].
| Suppressor | Genotype | Threshold (HFS V) | Effect | Reference |
|---|---|---|---|---|
| cacTS2 | parabss1 cacTS2 | 51.6 ± 1.2 | Homozygous, hemizyous, and heterozygous parabss1 flies are all suppressed. | Current paper |
| mlenapts | parabss1; mlenapts | 29 ± 4.7 | Suppresses homozygous parabss1 flies | 13, 14 |
| shits1 | shits1 parabss1 | 3.5 HFS V | Behavioral suppression at elevated temperatures. Minimal effect on seizure threshold. | 15 |
| gish | parabss1/+;gish04895/+ | 15.6 ± 2.4 | Specific supppressor of heterozygous parabss1/+ flies. Does not suppress parabss1 homozygotes and hemizygotes. Does not suppress sda or eas. | 16 |
The salient consequence of cacTS2 suppression is the increased percentage of flies escaping BS paralysis, but flies that undergo paralysis are also influenced by the suppressor: observed as a reduction in the time required for recovery. Control parabss1/Y mutant males when paralyzed ordinarily have a long recovery time 195 sec. In contrast, paralyzed flies carrying the suppressor (genotype: parabss1 cacTS2/Y) have about a four-fold reduction in the time to recovery 46 sec (n = 45; P < 0.0001, unpaired student t-test). Moreover, cacTS2 reduced the refractory time period for hemizygous double mutant male flies parabss1 cacTS2/Y. Double mutants show a shorter refractory time period of 17 min, compared to 25 min for parabss1 single mutant flies (n = 34; P = 0.0005, unpaired student t-test).
The electrophysiology of cacTS2 seizure-suppression
Seizure suppression by cacTS2 is also observed in evoked seizure-like neuronal activity recorded electrophysiologically. This analysis shows an unusual seizure-suppression of parabss1 by cacTS2. Immediately following HS, there is considerable suppression of parabss1 (Fig 3A–3D), but this suppression is transient and short-lasting (Fig 3D). Thus, immediately following the HS (3 min at 30°C), seizure-threshold for parabss1 cacTS2 double mutants is high (51.6 ± 1.2 V HFS, mean ± s.e.m., n = 7; P < 0.0001, ANOVA test; Fig 3D). This is greater than the seizure threshold for the parabss1 by about a factor of ten, and greater than wild type seizure-threshold, by nearly a factor of two; flies with seizure-thresholds in this range are seizure-resistant mutants (Table 3) [14]. Seizure-threshold quickly decreases when maintained at room temperature and the steady-state seizure-threshold of parabss1 cacTS2 double mutants at room temperature is (3.82 ± 0.2 V HFS, mean ± s.e.m., n = 10), similar to the parabss1 single mutant (3.2 ± 0.1 V HFS, mean ± s.e.m., n = 8; Fig 3A). The time course of the threshold change is difficult for us to determine with our present electrophysiology protocols, but it appears similar to changes in BS behavior following HS, about 5–7 min.
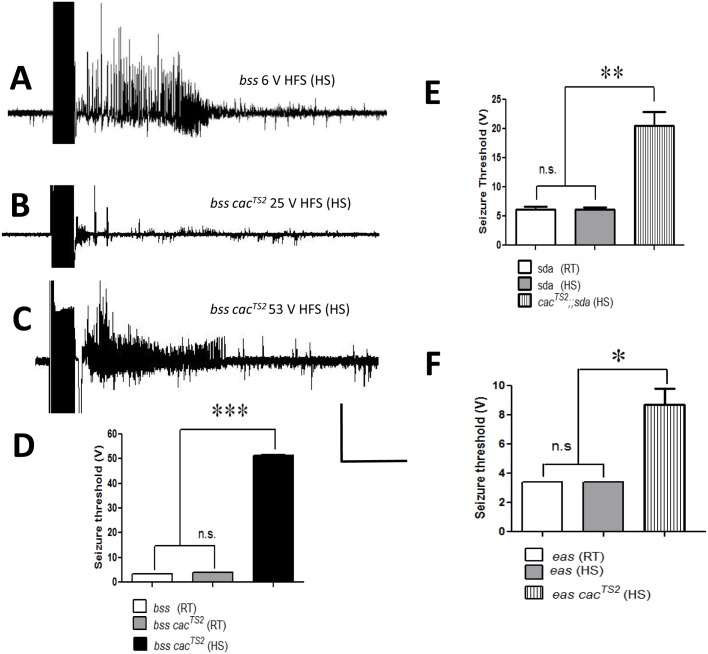
Fig 3. Electrophysiology of cacTS2 suppression.
A. Electrical recording from a parabss1 DLM fiber showing seizure-like activity evoked by a 6 V HFS stimulation, showing that the single BS mutant has a low seizure threshold. B. Electrical recording from a parabss1 cacTS2 DLM fiber showing that stimulation at 25 V HFS is below threshold and fails to evoke a seizure event in the double mutant indicating suppression by cacTS2. C. Electrical recording from a parabss1 cacTS2 DLM fiber showing that a high voltage stimulation at 53 V HFS is above threshold and evokes a seizure-like event in the double mutant. For the traces depicted in A-C, the HFS stimulus is delivered immediately after HS (3 min at 30°C). D. At room temperature (RT; steady state), seizure threshold for the parabss1 cacTS2/Y double mutant (gray bar) is similar to the parabss1/Y single mutant (white bar). Immediately following a HS, seizure threshold for parabss1 cacTS2/Y is transiently high indicating suppression (black bar; n = 7). E. Average seizure threshold is increased by about a factor of 4 in cacTS2/Y;;sda double mutants following a brief HS (striped bar; 20.5 ± 2.42 V HFS; n = 7) compared to hemizygous sda single mutant (white and gray bars). F. Average seizure threshold is increased by about a factor of 3 in hemizygous double mutant eas cacTS2/Y following a brief HS (striped bar; 8.7 ± 1.1 V HFS; n = 11) compared to the eas single mutant (white and gray bars). Quantitative data are represented as mean ± s.e.m. *P < 0.01, **P = 0.001, ***P < 0.0001, (D-F) chi-square test. Horizontal calibration: 800 msec for A-C; Vertical calibration: 20 mV for A-C.
Table 3. Seizure thresholds for different single mutant and double mutant genotypes.
Seizure-like activity is evoked by high frequency (HF) stimuli delivered to the brain (0.5 ms pulses at 200 Hz for 300 ms). Stimulation voltage was gradually increased and seizure threshold, a measure of seizure-susceptibility, was defined as the minimum voltage required for HF stimulation to become an electroconvulsive shock; that is, to induce seizure activity. Each genotype has a characteristic seizure threshold. Values of seizure threshold are presented as mean V HFS ± s. e. m. with n ≥ 5.
| Genotype | Threshold (V HFS) |
|---|---|
| bss (RT) | 3.2 ± 0.10 |
| bss (HS) | 6.9 ± 0.35 |
| eas/Y (RT) | 3.8 ± 0.11 |
| eas/Y (HS) | 3.7 ± 0.11 |
| sda (RT) | 6.2 ± 0.30 |
| sda (HS) | 6.8 ± 0.23 |
| CS/Y (RT) | 24.6 ± 2.83 |
| CS/Y (HS) | 24.3 ± 3.9 |
| eas cacTS2/Y(HS) | 8.7 ± 1.12 |
| cacTS2;;sda (HS) | 20.5 ± 2.42 |
| bss cacTS2 (RT) | 3.8 ± 0.2 |
| bss cacTS2 (HS) | 51.6 ± 1.22 |
| cacTS2 (RT) | 58.3 ± 1.00 |
Electrophysiology analysis also shows cacTS2 suppression of other BS mutants. The cacTS2;;sda double mutant has a seizure-threshold of (20.5 ± 2.42 V HFS, mean ± s.e.m., n = 7) following HS and tested at room temperature, about 3-fold higher than the threshold of the sda single mutant (6.2 ± 0.3 V HFS, mean ± s.e.m., n = 5, P < 0.0001, unpaired student t-test; Fig 3E). That is, in the double mutant, there is a reversion of BS electrophysiology by cacTS2 to nearly the wild-type range of seizure-threshold. The cacTS2 mutation also suppresses eas seizure-like activity. Hemizygous double mutant flies eas cacTS2/Y have a seizure-threshold of (8.7 ± 1.1 V HFS, mean ± s.e.m., n = 11) following HS, about two-fold higher than the low threshold of the eas single mutant (3.8 ± 0.1 V HFS, mean ± s.e.m., n = 6; P < 0.01; unpaired student t-test; Fig 3D; Table 3).
Seizure-suppression by cacRNAi
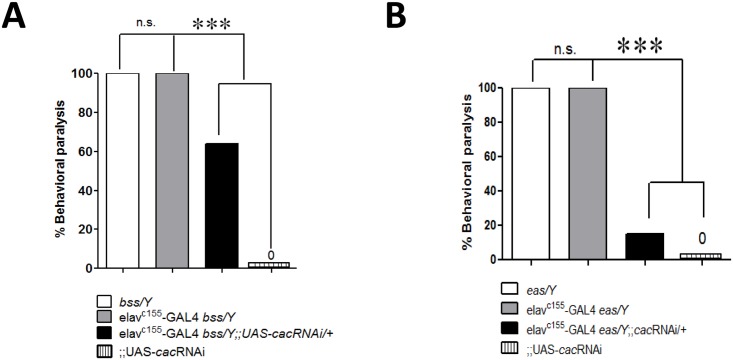
To further study cac seizure-suppression, we generated loss-of-function cac genotypes using cacRNAi to knockdown cac expression; these were tested for BS mutant suppression. Flies utilizing a pan-neuronal GAL4 driver and one copy of cacRNAi were viable and had largely normal behavior. Similar to the cacTS2 mutation, cacRNAi suppressed BS behavioral phenotypes in double mutant male parabss1 flies. Males (genotype: elavc155-GAL4 parabss1/Y;;UAS-cacRNAi/+) showed BS paralysis in 64% of flies (36% suppression; n = 212; P < 0.0001, chi-square test; Fig 4A) at room temperature. Suppression of BS by cacRNAi was more effective in eas mutants. Males (genotype: elavc155-Gal4 eas/Y;;UAS-cacRNAi/+) showed BS paralysis in 15% of flies (85% suppression, n = 74; P < 0.0001, chi- square test; Fig 4B). Although cacRNAi was effective at suppressing BS phenotypes, in other respects it was different than cacTS2 mutations because it did not cause temperature-sensitive phenotypes. Thus, male cacRNAi flies (genotype: elavc155-GAL4/Y;;UAS-cacRNAi/+) at 38°C showed netiher spontaneous seizure-like behaviors no behavioral paralysis.
Fig 4. Suppression of parabss1 and eas behavioral phenotypes by cacRNAi at room temperature.
A. Behavioral paralysis in parabss1 hemizygotes (white bar) is suppressed by cacRNAi when expressed by a pan-neuronal driver (black bar; genotype: parabss1 elavc155-GAL4/Y;;UAS-cacRNAi). B. Behavioral paralysis in eas hemizygotes (white bar) is suppressed by cacRNAi when expressed with a pan-neuronal driver (black bar; genotype: eas elavc155-GAL4/Y;;UAS-cacRNAi). ***P < 0.0001, chi-square test (n = 212 and 74 for panels A and B, respectively).
Suppression of cacTS2 seizure phenotypes by the parabss1 mutation
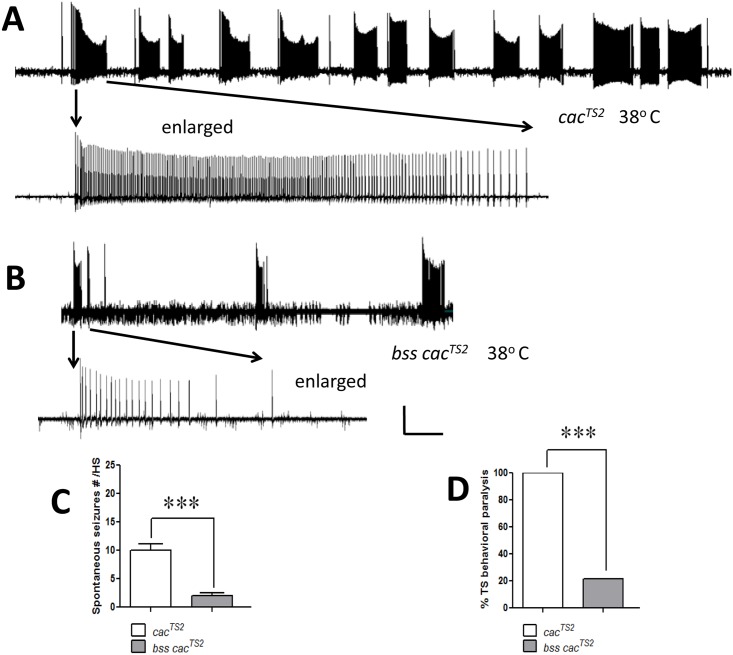
Double mutant parabss1 cacTS2 flies were examined following a temperature shift from room temperature to 38°C, the restrictive temperature for cacTS2. Interestingly, some temperature-sensitive phenotypes, prominent in the cacTS2 single mutant, were reduced in the double mutant, apparently suppressed by the presence of parabss1 in the double mutant combination. In parabss1 cacTS2 flies the TS spontaneous seizure-like electrophysiological phenotype was greatly reduced (Fig 5A and 5B). Electrophysiological recordings from cacTS2 single mutants show the number of spontaneous seizure-like discharges was 10 ± 1 spontaneous discharges/HS (mean ± s.e.m., n = 10; HS = 3 min at 38°C. Fig 5A and 5C). In contrast, recordings from parabss1 cacTS2 double mutants show 2.5 ± 0.42 spontaneous discharges/HS (mean ± s.e.m., n = 20, P = 0.0003, unpaired student-t test; Fig 5B and 5C). In addition to the number of spontaneous discharges being reduced, there also appeared to be a reduction in discharge duration (Fig 5A and 5B). The temperature-sensitive behavioral paralysis phenotype of cacTS2 was also suppressed by parabss1 (Fig 5D). For the cacTS2 single mutant, 100% of flies undergo paralysis when the temperature is increased from room temperature to 38°C, as described in previously [5]. In contrast, for parabss1 cacTS2 double mutants, only 20% of flies are paralyzed at 38°C (80% suppression, n = 76; P < 0.001, chi-square test, Fig 5D).
Fig 5. Suppression of cacTS2 temperature-sensitive seizure-like activity by parabss1.
A. Spontaneous seizure-like activity observed in a cacTS2 single mutant when the temperature is increased from room temperature to 38°C, indicating that the mutant is seizure-sensitive at restrictive temperature. Recording shows a representative example of 13 spontaneous seizure-like discharges during HS. Enlargement (lower trace) shows one of the spontaneous discharges at a higher sweep speed. B. Spontaneous seizure-like activity is decreased in double mutant homozygous parabss1 cacTS2 at restrictive temperature. C. Number of spontaneous seizure-like activity evoked due to restrictive temperature and measured by electrophysiology. Compared to cacTS2, in homozygous parabss1 cacTS2 double mutant flies number of TS seizure-like activity is reduced by about a factor of 5 (white bar: 10 ± 1.2 events/3 min HS; n = 10; compared to gray bar: 2.5 ± 0.42 events/3 min HS; n = 20). D. Behavioral seizure-like activity is decreased in homozygous double mutant parabss1 cacTS2 flies at restrictive temperature. Compared to cacTS2 single mutants (white bar), parabss1 cacTS2 double mutants (gray bar) exhibit 5 fold decrease in TS seizure-like behaviors. Quantitative data are represented as mean ± s.e.m. ***P < 0.0001, based on unpaired student-t test (C) and chi-square test (D). Horizontal calibration: A. 15 sec, A (enlarged) 1 sec, B. 15 sec, B (enlarged) 1.5 sec. Vertical calibration: 10 mV.
Discussion
We find that cacTS2 is a general seizure-suppressor mutation, reverting neurological phenotypes of several BS mutants: sda, eas, and parabss1. Suppression of parabss1 is especially notable because it is a BS mutant that has previously been difficult to modify by suppressor mutation [21,22] or antiepileptic drug [23–27]. Recently, directed efforts to target parabss1 by suppressors have identified two: gilgamesh (gish) and shibirets (shits), although both appear somewhat weaker than cacTS2 [15–16 (Table 1)]. Suppression by gish is unusual because it is selectively effective against parabss1/+ heterozygotes; gish does not suppress homozygous parabss1. Also, gish does not suppress other BS mutants, such as sda or eas. [16]. For shits, suppression of parabss1 is not evident at room temperature, but occurs with increased temperature that causes interference with endocytosis during synaptic vesicle recycling [15]. BS suppression reported here for cacTS2 is comparable or better than for gish and shits.
The major questions arising from this study are: how does cacTS2 suppression work? And what is it about the cacTS2 mutation that makes it such an effective suppressor of parabss1 primary phenotypes, BS behavior and seizure threshold? A complete answer to these questions remains unclear from the experiments we are able to perform here, but leads to speculation about mechanisms of seizure, and about how seizure-suppression might be accomplished. The cacTS2 allele behaves as a recessive loss-of-function mutation with reduced neurotransmitter release at the neuromuscular junction and paralysis at high temperature [5]. Also at high temperature, the mutant displays considerable spontaneous seizure-like activity seen in muscle fiber recordings [8]. At first, it might appear that this seizure-like activity is inconsistent with the cacTS2 phenotype of reduced transmitter release, especially if this reflects an overall reduction in excitability. The cacTS2 seizure-like activity must be due to spontaneous action potential bursting in adult and larval motor neurons; the activity recorded in the muscle fiber is reflecting seizure-like motor neuron firing. We suggest that this motor neuron firing may be due to a loss of inhibitory synaptic activity impinging on them, possibly causing some type of post-inhibitory rebound excitation within the motor neurons. That is, as excitatory transmitter reduction by temperature is observed at the neuromuscular junction, synaptic inhibition that ordinarily limits motor neuron firing is concurrently reduced leading to spontaneous seizure-like activity observed in muscle.
About fifteen mutations have been identified previously as seizure-suppressors [reviewed in 22]. Some of these suppressors encode well-studied gene products that have not heretofore been associated with neuronal signaling or membrane excitability such as the de-ubiquitinase USP9X [28] and DNA topoisomerase I [29]. Some of the seizure-suppressor genes encode neuronal signaling molecules that have allowed us to consider previously three likely mechanisms for how seizure-like activity might be suppressed by second-site mutations; here, suppression by cacTS2 suggests to us a fourth mechanism. Previously, we found that:
Seizure-suppression can occur by limiting high frequency action potential firing. The K+ channel mutation ShKS133, increases action potential duration, leading to long refractory periods [14,19,30]. Action potentials cannot be generated during the refractory period, apparently leading to seizure-suppression by limiting axons to low action potential firing frequencies. Thus, despite ShKS133 generally causing nervous system hyperexcitability, the mutation is also a seizure-suppressor because the high-frequency action potential firing required for seizure-like activity is not supported by axons in these mutants.
Seizure-suppression can occur by opposing effects on nerve excitability [9,14,19,31,32]. The paraST76, paraJS and mlenapts mutations reduce nerve excitability by Na+ channel loss-of-function. Hypoexcitability from these mutations can suppress seizure-like activity in BS mutations causing hyperexcitability such as eas and sda.
Seizure-suppression can occur by preventing synchronized firing. The ShakB2, a mutation of the gap junction innexin channel, causes a defect in electrical synaptic transmission. Seizure-like activity is suppressed because electrical synaptic transmission appears to be critical for synchronizing the activity of populations of firing neurons and for the spread of seizure-like excitation [14,33].
The signaling molecules responsible for the process of chemical synaptic transmission are a potentially rich source for identifying seizure-suppressor mutations. Seizure-suppressors are most logically expected from among mutations enhancing inhibitory GABAergic synaptic transmission or mutations diminishing excitatory cholinergic transmission. Some other mutations are most logically expected to enhance seizure phenotypes such as mutations decreasing GABAergic function or enhancing cholinergic transmission. It is more difficult to anticipate the effect on seizures of mutations affecting general synaptic transmission properties, that is, molecules that are common to both excitatory and inhibitory synaptic processes. The cacTS2 mutation examined here is such a mutation, the cac gene encodes the α1, primary structural subunit of the voltage-gated Ca++ channel responsible for triggering regulated synaptic vesicle release at both excitatory and inhibitory synapses [4,34]. Thus, it was surprising that cacTS2 was not only a seizure-suppressor mutation, it was one of the most effective suppressors that we have identified. Because of this, we propose that cacTS2 suppression may work via a somewhat different mechanism than we have observed previously, generally, using neurocircuitry to cause seizure suppression. We presume that the suppression works by interfering with chemical synaptic transmission in many or most circuits in the fly. Modest interference in synaptic transmission at room temperature is sufficient to suppress weak BS mutants, such as sda. Stronger disabling of synaptic transmission following a heat pulse is necessary to suppress the stronger BS parabss1.
We thought it possible to identify specific circuits responsible for suppression by the differential GAL4/UAS expression of cacRNAi. Our initial attempts expressed cacRNAi selectively in excitatory interneurons (cha-GAL4 driver), or inhibitory interneurons (GAD-GAL4 driver). Expression of cacRNAi in different interneuronal populations was a little less effective than pan-neuronal expression, but differences were small (S2 Fig). From this limited investigation, we do not find indications for specific circuits suppressing seizures or, if they exist, how we might go about discovering them. We entertain the interesting possibility that cac suppression may not be due to the disabling of particular circuits, but is a general block of seizure-like activity by an overall poorly-transmitting nervous system. It remains surprising that such a putative mechanism of seizure-suppression would be especially effective at reverting parabss1 seizure phenotypes which are severe.
The cac gene is one of the most interesting Drosophila neurological genes. The gene is predicted to encode 15 annotated transcripts and 14 unique polypeptides. Numerous mutations have been identified (72 alleles) and functions ascribed to different subsets of cac mutations [35–37]. Male courtship song alteration is one of the canonical phenotypes of cac exemplified by the original cacS mutation. Subsequently cacTS2 and cacNT27 were also shown to have alterations in courtship song [37]. All of the mutations in this subset show motor defects, seizure-like activity, and behavioral paralysis. These mutations and several other cac alleles in this subset all fail to complement each other. The cacTS2 mutation is due to a mis-sense mutation that is thought to alter Ca++-dependent inactivation [38]. Thus, although cacTS2 is recessive, it could behave as a gain-of-function mutation. Nevertheless, RNAi experiments presented here show that cac loss-of-function can cause seizure-suppression. However, flies carrying cacRNAi show neither seizure-like activity nor paralysis, suggesting these phenotypes could be due to gain-of-function phenotypes of cacTS2. These issues remain to be determined in future experiments.
Another interesting finding in this study is the co-suppression by parabss1 of the cacTS2 spontaneous seizure-like phenotype induced by high temperature. We presume that this must be due to a loss of spontaneous motoneuron spiking, since activity in the DLM muscle fiber reflects post-synaptic potentials from neuromuscular transmission. The mechanism responsible for this loss of motoneuron spiking is unclear; there are not previously described functions of parabss1 that easily account for it. The parabss1 sodium channel mutation causes gain-of-function phenotypes and leads to hyper-excitability in neurons. It is this hyper-excitability that makes parabss1 mutants more prone to seizures. The cacTS2 mutation causes a less functional Ca2+ channel and hence a decrease in release of neurotransmitter. So, bringing two defective ion channels with different effects on membrane excitability effects leads to the suppression of epilepsy. This Drosophila suppression resembles seizure-suppression findings in mice [17]. Double mutant mice carrying mutations in two epilepsy genes, Cacna1and Kcna1a showed improvement in both absence epilepsy and limbic seizure phenotypes caused by these mutations [17].
Materials and Methods
Fly stocks
Drosophila strains were maintained on standard cornmeal-molasses agar medium at room temperature (24°C). The cacophony (cac) gene is located on the X chromosome at 10F-11A on the cytological map and encodes a voltage-gated Ca++ channel α1 subunit implicated in neurotransmitter release [3–8]. The cacTS2 allele is a recessive mutation caused by a substitution (P1385S) at the C-terminus [4]. This position is adjacent to an EF hand motif thought to be involved in calcium dependent inactivation. The cacTS2 mutation causes temperature-sensitive paralysis: apparently due to a reduction, and then loss of synaptic current as the temperature is raised from permissive to restrictive values [4]. The paralytic (para) gene is located at map position 1–53.5 and encodes a voltage-gated Na+ channel [39–40]. The allele use here is a bang-sensitive (BS) paralytic mutation, parabss1, previously named bss1[14] It is the most seizure-sensitive of fly mutants, the most difficult to suppress by mutation and by drug, and has been proposed as a model for human intractable epilepsy [10]. The parabss1 allele is a gain-of-function mutation caused by the substitution (L1699F) of a highly conserved residue in the third membrane-spanning segment (S3b) of homology domain IV [10]. The easily shocked (eas) gene is located at 14B on the cytological map and encodes an ethanolamine kinase [41]. The BS allele used in this study is easPC80, which is caused by a 2-bp deletion that introduces a frame shift; the resulting truncated protein lacks a kinase domain and abolishes all enzymatic activity [22]. The slamdance (sda) gene is located at 97D and encodes an aminopeptidase N. The allele used in this study is sdais07.8 caused by a 2-bp insertion in the 5’ untranslated region [42]. The UAS-cacRNAi line was obtained from Bloomington Drosophila Stock Center. The insert for UAS-cacRNAi is located on the 3rd chromosome.
Double mutants
The double mutants used in this study were constructed by standard genetic crosses and then verified for the presence of both the BS mutation (sda, eas or parabss1), as well as cacTS2. The presence of cacTS2 in the homozygous double mutant stock was verified by testing for behavioral paralysis after heat shock (37°C for 5 min), which is characteristic for this mutation; BS mutants do not paralyze under such conditions. The presence of the homozygous BS mutation in the double-mutant stocks was verified by backcrossing each double mutant stock to females of the appropriate BS genotype. The progeny from those crosses, which should be homozygous for the BS mutation and heterozygous for cacTS2, were then tested for the BS behavioral phenotype. All of the genotypes arising from the back cross phenotypically resembled BS homozygous flies. The lack of any obvious effects among the different genetic backgrounds tested also indicated the alterations in seizure-sensitivity reported here were due to the homozygous presence of cacTS2 in the double mutant combinations and not likely due to nonspecific genetic background differences.
BS behavior and heat shock
Behavioral testing for BS paralysis was performed on flies 3d after eclosion, as described previously [16]. Flies were anesthetized with CO2 before collection and tested the following day. For testing, 10 flies were placed in a clean food vial and stimulated mechanically with a VWR vortex mixer at maximum speed for 10 s. The parabss1, eas, and sda mutants ordinarily show 100% penetrance of BS paralytic behavior with this test. Suppression by cacTS2 was initially manifest as a reduced percentage of BS behavioral paralysis in the double mutant compared to the single BS mutant. Recovery from BS paralysis was determined by counting the number of flies standing at different intervals following stimulation. Recovery time was the time at which 50% of flies had recovered from paralysis. For genotypes that display partial penetrance of BS paralysis, only those flies that displayed paralysis were used for recovery time analysis. For BS behavioral analysis, pools of flies are combined for each genotype from among the separate trials (in total, n ≈ 100 for each genotype). For analyses using heat shock (HS), a single fly was placed in a clean food vial and tested the following day. The vial was submerged in a water bath (30°C for 3 min), rested at room temperature (24°C for 30 seconds), and then tested for BS behavioral paralysis. For construction of double mutant stocks, flies were tested similarly for the presence of the cacTS2 mutation except that water bath temperature was 37°C, and the assay was temperature-sensitive behavioral paralysis.
Electrophysiology
In vivo recording of seizure-like activity and seizure threshold determination in adult flies was performed as described previously [16]. Flies 2–3 days post-eclosion were mounted in wax on a glass slide, leaving the dorsal head, thorax, and abdomen exposed. Stimulating, recording, and ground metal electrodes were made of uninsulated tungsten. Seizure-like activity was evoked by high-frequency electrical brain stimulation (0.5 msec pulses at 200 Hz for 300 msec) and monitored by dorsal longitudinal muscle (DLM) recording. During the course of each experiment, the giant fiber (GF) circuit was stimulated by single-pulse electrical brain stimulation and monitored continuously as a proxy for holobrain function. For each genotype tested n ≥ 10.
Data analysis
Chi-square tests were used to compare the penetrance of seizures. Student’s t-test and ANOVA were used to compare recovery times and seizure thresholds across genotypes, as appropriate. For ANOVA analysis, where the null hypothesis was rejected by the overall F ratio, multiple comparisons of data sets were performed by Fisher’s least significant difference with t-test significance set at P < 0.05. For Figures (1–3 and 5) error bars represents standard error of the mean, and statistical significance is indicated by * P < 0.01, ** P < 0.001 and *** P < 0.0001.
Supporting Information
Single pulse electrical stimuli are delivered to the brain (0.2 msec in duration, 0.8 Hz) to activate the giant fiber (GF) neurocircuit. A. GF stimulation comparing DLM responses of parabss1 and cacTS2 single mutants, and a parabss1 cacTS2 double mutant. GF responses are all similar and resemble the wild type GF response. B. GF responses from stimuli delivered at 73 Hz. Upper trace: GF responses in a sda mutant occur after each stimulus showing that the GF circuit responds reliably at this frequency. Upper trace: GF responses in a cacTS2 mutant show failures at this stimulation frequency. Arrows show examples of response failures. C. Quantification of synaptic responses shows that sda GF responses show 73 responses without at failure (100% successful GF stimulations at 73 Hz). For cacTS2, only 31 GF responses were elicited by 73 stimulations at 73 Hz (42% successful GF stimulations at 73 Hz). Horizontal calibration: 500 msec for A, 10 msec for B. Vertical calibration: 20 msec for A and B.
(PDF)
In eas/Y hemizygous males without cac-RNAi (white bar), all flies are completely paralyzed by mechanical stimulation. Behavioral paralysis is suppressed using a pan-neuronal GAL4 driver to express cacRNAi (gray bar; genotype: elavC155-GAL4 eas/Y;; UAS-cacRNAi/+). Less effective suppression is observed when cacRNAi is expressed only in GABAergic inhibitory interneurons (black bar; genotype: eas/Y; GAD-GAL4/+; UAS-cacRNAi/+) or only in excitatory cholinergic interneurons (striped bar; genotype: eas/Y; Cha-GAL4/+; UAS-cacRNAi/+).
(PDF)
Acknowledgments
We thank the members of the Tanouye lab for helpful discussions throughout the project.
Data Availability
Data are from the 'Seizure suppression by cac' study whose authors may be contacted at sarasarunesh@gmail.com
Funding Statement
This work was supported by the McKnight Foundation and the NIH (NS31231). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Proposal for revised classification of epilepsies and epileptic syndromes. Commission on classification and terminology of the International League against Epilepsy. Epilepsia. 1989; 30(4): 389–399. [DOI] [PubMed] [Google Scholar]
- 2.Shneker B F, Fountain N B. Epilepsy. Dis Mon. 2003; 49(7): 426–478. [DOI] [PubMed] [Google Scholar]
- 3.Kulkarni S J, Hall J C. Behavioral and cytogenetic analysis of the cacophony courtship song mutant and interacting genetic variants in Drosophila melanogaster. Genetics. 1987; 115(3): 461–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith L A, Wang X, Peixoto A A, Neumann E K, Hall L M, Hall J C. A Drosophila calcium channel alpha1 subunit gene maps to a genetic locus associated with behavioral and visual defects. J Neurosci. 1996; 16(24): 7868–7879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawasaki F, Felling R, Ordway R W. A temperature-sensitive paralytic mutant defines a primary synaptic calcium channel in Drosophila. J Neurosci. 2000; 20(13): 4885–4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dellinger B, Felling R, Ordway R W. Genetic modifiers of the Drosophila NSF mutant, comatose, include a temperature-sensitive paralytic allele of the calcium channel alpha1-subunit gene, cacophony. Genetics. 2000; 155(1): 203–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuromi H, Honda A, Kidokoro Y. Ca2+ influx through distinct routes controls exocytosis and endocytosis at Drosophila presynaptic terminals. Neuron. 2004; 41(1): 101–111. [DOI] [PubMed] [Google Scholar]
- 8.Rieckhof G E, Yoshihara M, Guan Z, Littleton J T. Presynaptic N-type calcium channels regulate synaptic growth. J Biol Chem. 2003; 278(2): 41099–41108. [DOI] [PubMed] [Google Scholar]
- 9.Ganetzky B, Wu C F. Indirect suppression involving behavioral mutants with altered nerve excitability in Drosophila melanogaster. Genetics. 1982; 100(4): 597–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parker L, Padilla M, Du Y, Dong K, Tanouye M A. Drosophila as a model for epilepsy: bss is a gain-of-function mutation in the para sodium channel gene that leads to seizures. Genetics. 2011; 187(2): 523–534. 10.1534/genetics.110.123299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mulley J C, Scheffer I E, Petrou S, Dibbens L M, Berkovic S F, Harkin L A. SCN1A mutations and epilepsy. Hum Mutat. 2005; 25(6): 535–542. [DOI] [PubMed] [Google Scholar]
- 12.Lossin C. A catalog of SCN1A variants. Brain Dev. 2009; 31(2): 114–130. 10.1016/j.braindev.2008.07.011 [DOI] [PubMed] [Google Scholar]
- 13.Pavlidis P, Tanouye M A. Seizures and failures in the giant fiber pathway of Drosophila bang-sensitive paralytic mutants. J Neurosci. 1995; 15(8): 5810–5819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuebler D, Zhang H, Ren X, Tanouye M A. Genetic suppression of seizure susceptibility in Drosophila. J Neurophysiol. 2001; 86(3): 1211–25. [DOI] [PubMed] [Google Scholar]
- 15.Kroll J R, Wong K G, Siddiqui F M, Tanouye M A. Disruption of endocytosis with the dynamin mutant shibirets1 suppresses seizures in Drosophila. Genetics. 2015. pii: genetics.115.177600. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Howlett I C, Rusan Z M, Parker L, Tanouye M A. Drosophila as a model for intractable epilepsy: gilgamesh suppresses seizures in parabss1 heterozygote flies. G3 (Bethesda). 2013; 3(8): 1399–1407. 10.1534/g3.113.006130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glasscock E, Qian J, Yoo J W, Noebels J L. Masking epilepsy by combining two epilepsy genes. Nat Neurosci. 2007; 10(12): 1554–1558. [DOI] [PubMed] [Google Scholar]
- 18.Tanouye M A, Wyman R J. Motor outputs of giant nerve fiber in Drosophila. J. Neurophysiol. 1980; 44(2): 405–21. [DOI] [PubMed] [Google Scholar]
- 19.Kuebler D, Tanouye M A. Modifications of seizure susceptibility in Drosophila. J Neurophysiol. 2000; 83(2): 998–1009. [DOI] [PubMed] [Google Scholar]
- 20.Lee J, Wu C F. Electroconvulsive seizure behavior in Drosophila: analysis of the physiological repertoire underlying a stereotyped action pattern in bang-sensitive mutants. J Neurosci. 2002; 22(24): 11065–11079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song J, Tanouye M A. From bench to drug: human seizure modeling using Drosophila. Prog. Neurobiol. 2008; 84(2): 182–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parker L, Howlett I C, Rusan Z M, Tanouye M A. Seizure and epilepsy: studies of seizure disorders in Drosophila. Int Rev Neurobiol. 2011; 99: 1–21. 10.1016/B978-0-12-387003-2.00001-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuebler D, Tanouye M A. The anticonvulsant sodium valproate reduces seizure-susceptibility in mutant Drosophila. Brain Res. 2002; 958(1): 36–42. [DOI] [PubMed] [Google Scholar]
- 24.Reynolds E R, Stauffer E A, Feeney L, Rojahn E, Jacobs B, McKeever C. Treatment with the antiepileptic drugs phenytoin and gabapentin ameliorates seizure and paralysis of Drosophila bang-sensitive mutants. J Neurobiol. 2003; 58(4): 503–513. [DOI] [PubMed] [Google Scholar]
- 25.Tan J S, Lin F, Tanouye M A. Potassium bromide, an anticonvulsant, is effective at alleviating seizures in the Drosophila bang-sensitive mutant bang senseless. Brain Res. 2003; 1020(1–2): 45–52. [DOI] [PubMed] [Google Scholar]
- 26.Song J, Parker L, Hormozi L, Tanouye M A. DNA topoisomerase I inhibitors ameliorate seizure-like behaviors and paralysis in a Drosophila model of epilepsy. Neuroscience. 2008; 156(3): 722–728. 10.1016/j.neuroscience.2008.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Howlett I C, Tanouye M A. Seizure-sensitivity in Drosophila is ameliorated by dorsal vessel injection of the antiepileptic drug valproate. J Neurogenet. 2013; 27(4): 143–150. 10.3109/01677063.2013.817574 [DOI] [PubMed] [Google Scholar]
- 28.Paemka L, Mahajan V B, Ehaideb S N, Skeie J M, Tan M C, Wu S, Cox A J, Sowers L P, Gecz J, Jolly L, Ferguson P J, Darbro B, Schneider A, Scheffer I E, Carvill G L, Mefford H C, El-Shanti H, Wood S A, Manak J R, Bassuk A G. Seizures are regulated by ubiquitin-specific peptidase 9 X-linked (USP9X), a de-ubiquitinase. PLoS Genet. 2015; March 12;11(3):e1005022 10.1371/journal.pgen.1005022 eCollection 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song J, Hu J, Tanouye M. Seizure suppression by top1 mutations in Drosophila. J Neurosci. 2007; 27(11): 2927–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanouye M A, Ferrus A, Fujita S C. Abnormal action potentials associated with the Shaker complex locus of Drosophila. Proc Natl Acad Sci USA. 1981; 78(10): 6548–6552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ganetzky B, Wu C F. Drosophila mutants with opposing effects on nerve excitability: genetic and spatial interactions in repetitive firing. J Neurophysiol. 1982b; 47(3): 501–514. [DOI] [PubMed] [Google Scholar]
- 32.Song J, Tanouye M A. Role for para sodium channel gene 3' UTR in the modification of Drosophila seizure susceptibility. Dev Neurobiol. 2007; 67(14): 1944–1956. [DOI] [PubMed] [Google Scholar]
- 33.Song J, Tanouye M A. Seizure suppression by shakB2, a gap junction mutation in Drosophila. J Neurophysiol. 2006; 95(2): 627–35. [DOI] [PubMed] [Google Scholar]
- 34.Littleton J T, Ganetzky B. Ion channels and synaptic organization: analysis of the Drosophila genome. Neuron. 2000; 26(1): 35–43. [DOI] [PubMed] [Google Scholar]
- 35.Flybase Gene Report Dmel/cac. Flybase. 2015; FB2015_05. http://flybase.org/reports/FBgn0263111.html
- 36.Smith L A, Peixoto A A, Kramer E M, Villella A, Hall J C. Courtship and visual defects of cacophony mutants reveal functional complexity of a calcium-channel alpha1 subunit in Drosophila. Genetics. 1998; 149(3): 1407–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chan B, Villella A, Funes P, Hall JC. Courtship and other behaviors affected by a heat-sensitive, molecularly novel mutation in the cacophony calcium-channel gene of Drosophila. Genetics. 2002; 162(1): 135–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kawasaki F, Collins SC, Ordway RW. Synaptic calcium-channel function in Drosophila: analysis and transformation rescue of temperature-sensitive paralytic and lethal mutations of cacophony. J Neurosci. 2002. July 15;22(14): 5856–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Loughney K, Kreber R, Ganetzky B. Molecular analysis of the para locus, a sodium channel gene in Drosophila. Cell. 1989; 58(6): 1143–1154. [DOI] [PubMed] [Google Scholar]
- 40.Ramaswami M, Tanouye M A. Two sodium channel genes in Drosophila: implications for channel diversity. Proc Natl Acad Sci USA. 1989; 86(6): 2079–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pavlidis P, Ramaswami M, Tanouye M A. The Drosophila easily shocked gene: a mutation in a phospholipid synthetic pathway causes seizure, neuronal failure, and paralysis. Cell. 1994; 79(1): 23–33. [DOI] [PubMed] [Google Scholar]
- 42.Zhang H, Tan J, Reynolds E, Kuebler D, Faulhaber S, Tanouye M A. The Drosophila slamdance gene: a mutation in an aminopeptidase can cause seizure, paralysis and neuronal failure. Genetics. 2002; 162(3):1283–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Single pulse electrical stimuli are delivered to the brain (0.2 msec in duration, 0.8 Hz) to activate the giant fiber (GF) neurocircuit. A. GF stimulation comparing DLM responses of parabss1 and cacTS2 single mutants, and a parabss1 cacTS2 double mutant. GF responses are all similar and resemble the wild type GF response. B. GF responses from stimuli delivered at 73 Hz. Upper trace: GF responses in a sda mutant occur after each stimulus showing that the GF circuit responds reliably at this frequency. Upper trace: GF responses in a cacTS2 mutant show failures at this stimulation frequency. Arrows show examples of response failures. C. Quantification of synaptic responses shows that sda GF responses show 73 responses without at failure (100% successful GF stimulations at 73 Hz). For cacTS2, only 31 GF responses were elicited by 73 stimulations at 73 Hz (42% successful GF stimulations at 73 Hz). Horizontal calibration: 500 msec for A, 10 msec for B. Vertical calibration: 20 msec for A and B.
(PDF)
In eas/Y hemizygous males without cac-RNAi (white bar), all flies are completely paralyzed by mechanical stimulation. Behavioral paralysis is suppressed using a pan-neuronal GAL4 driver to express cacRNAi (gray bar; genotype: elavC155-GAL4 eas/Y;; UAS-cacRNAi/+). Less effective suppression is observed when cacRNAi is expressed only in GABAergic inhibitory interneurons (black bar; genotype: eas/Y; GAD-GAL4/+; UAS-cacRNAi/+) or only in excitatory cholinergic interneurons (striped bar; genotype: eas/Y; Cha-GAL4/+; UAS-cacRNAi/+).
(PDF)
Data Availability Statement
Data are from the 'Seizure suppression by cac' study whose authors may be contacted at sarasarunesh@gmail.com