Abstract
Objective:
Adolescence is a period of neuromaturation concomitant with increased substance involvement. Most substance use studies of adolescents have focused on categorical classifications (e.g., dependent vs. nondependent), but little is known about the influence of specific substance use behaviors on cognitive functioning in youth.
Method:
This study prospectively evaluated the quantitative effects of different substance use behaviors on neuropsychological functioning. A cognitive test battery was administered at baseline (ages 12–14 years), before substance use initiation, and at follow-up (M = 4.0 years, SD = 2.0) to evaluate changes in verbal memory, visuospatial ability, psychomotor speed, processing speed, and working memory. Robust regressions examined substance use behaviors as predictors of neuropsychological functioning (N = 234).
Results:
Several substance use behaviors predicted follow-up neuropsychological functioning above and beyond effects of baseline performance on the same measure (ps < .05). Specifically, more alcohol use days predicted worse verbal memory ( = -.15) and visuospatial ability (
= -.15) and visuospatial ability ( = -.19). More postdrinking effects (
= -.19). More postdrinking effects ( = -.15) and greater drug use (
= -.15) and greater drug use ( = -.11) predicted worse psychomotor speed. Processing speed was not predicted by substance involvement (ps > .05). Unexpectedly, more alcohol use predicted better working memory performance (
= -.11) predicted worse psychomotor speed. Processing speed was not predicted by substance involvement (ps > .05). Unexpectedly, more alcohol use predicted better working memory performance ( = .12).
= .12).
Conclusions:
The frequency and intensity of adolescent alcohol use may be more intricately linked to neuropsychological outcomes than previously considered. The low prevalence of substance use disorder in the sample suggests that subdiagnostic users may still experience adverse effects to verbal memory, visuospatial functioning, and psychomotor speed after initiating intense or frequent alcohol use.
Adolescence is a unique developmental period characterized by major physiological, psychological, and neurodevelopmental changes. These changes typically coincide with an escalation in alcohol consumption and other drug use, which continues into early adulthood (Sartor et al., 2007). The comorbid use of alcohol and marijuana among teens continues to rise as perception of harm declines. Alcohol and marijuana are the two most commonly used substances among adolescents, with 70% and 36% of 12th graders reporting past-year use, respectively (Johnston et al., 2014a, 2014b). In 2013, 45% of 12th graders had tried marijuana at least once in their lifetime. By the end of high school, almost 7% reported daily marijuana use within the past 30 days (Johnston et al., 2014b). Of particular concern is the increase in heavy episodic drinking (i.e., consuming five or more drinks on at least one occasion in the prior 2 weeks), due to the acute negative consequences (Hingson & Zha, 2009) and potential long-term consequences on health and development (Crews et al., 2007).
Cross-sectional studies suggest that compared with light drinkers and nondrinkers, heavy drinking adolescents show neuropsychological decrements in various cognitive domains, including learning and memory (Brown et al., 2000; Green et al., 2010; Sneider et al., 2013), visuospatial functioning (Tapert et al., 2002; Squeglia et al., 2009; Tapert & Brown, 1999), executive function (Giancola et al., 1998; Parada et al., 2012), attention and information processing (Tapert et al., 2002; Tarter et al., 1995), and language skills (Moss et al., 1994). In relation to substance-naive controls, marijuana-using youths have been found to perform worse on measures of nonverbal memory and learning (Harvey et al., 2007), attention, and problem solving (Lane et al., 2007). Adolescents who use both alcohol and marijuana show poorer performance than non–marijuana-using controls on tasks of learning and memory (Medina et al., 2007) and visuospatial skills (Winward et al., 2014). Results from prospective studies appear to support those found in the above cross-sectional designs. Adolescents who transitioned to marijuana use continued to show decrements in verbal learning and memory over 10 years compared with nonusers (Hanson et al., 2011). Further, marijuana users continued to perform poorer on verbal learning and memory even after 3 weeks of abstinence (Hanson et al., 2010). Collectively, these neuropsychological data illustrate the concern that adolescent use of alcohol, marijuana, and other substances may have a detrimental effect on the maturing brain. As such, a prospective investigation will help clarify cognitive changes associated with adolescent use by taking into account pre-drinking neurocognitive functioning.
Much of the literature has used categorical classifications of alcohol and other drug use behaviors in studies of adolescent substance use. Such categories include users versus nonusers, heavy episodic drinkers versus nondrinkers, and dependent users versus controls. Ryback (1971) proposed a shift of focus from the examination of individuals with severe and maladjusted alcohol use to the consideration of drinking behaviors as a continuum that ranged from social drinkers to alcoholics and Korsakoff syndrome patients. Ryback’s Continuity Hypothesis suggested that the influences of alcohol use on cognitive and neurological functions, such as memory, should also manifest as a continuum, such that more severe alcohol use linearly predicts worse functioning. This hypothesis was later expanded by Ryan and Butters (1980) to include other neuropsychological domains (e.g., attention and language abilities) to show a range of impairment among alcohol-dependent patients as a continuous function of drinking severity. Cross-sectional studies that have explored the quantitative relationship between use behaviors and cognition have found null or mixed results (Day et al., 2013; Green et al., 2010; Hannon et al., 1983; Sinha et al., 1989; Thoma et al., 2011). These unexpected findings in the literature may be influenced in part by a small sample size, the lack of statistical control for pre-drinking cognitive functioning, or no true underlying effect in the samples considered.
It is possible and has been suggested (e.g., Squeglia et al., 2009) that youths who may not meet diagnostic criteria for a substance use disorder (SUD) could develop subtle neuropsychological changes after substance use begins and escalates. This may be important, as in 2012, 43% of youths ages 12–25 years reported current use of alcohol, but only 9% met the criteria for SUD (Substance Abuse and Mental Health Services Administration [SAMHSA], 2013). Similarly, 15% of youths reported current illicit drug use, yet only 8% met diagnostic criteria (SAMHSA, 2013).
Detrimental effects of alcohol use on cognitive functioning in adolescents are not limited to severe, long-term drinking behaviors and can be seen in dose-dependent episodic short-term drinking. Acute alcohol intoxication at 0.8 g/kg blood alcohol concentration negatively affects planning abilities, response time, inhibition, and spatial working memory (Caswell et al., 2013; Field et al., 2010; Fillmore, 2007; Weissenborn & Duka, 2003), acting as a global central nervous system depressant. Over the long term, persistent alcohol use impairs learning and memory (e.g., blackout) through interaction with glutamate neurotransmitter receptors in the brain. Evidence suggests that excessive drinking and resulting withdrawal symptoms further dysregulate glutamine receptor activity, leading to degeneration and death of neurons. These sequelae of neurotoxic events may be detected through behavioral cognitive impairments in neuropsychological assessments (for a review, see Zeigler et al., 2005). Thus, it is important to consider substance use behaviors, such as postdrinking effects, beyond quantity and frequency.
The current study prospectively examined the effects of specific alcohol, marijuana, and other drug involvement behaviors on neuropsychological performance in adolescents across five domains. Substance use behaviors were examined as continuous quantitative variables. More severe drinking and other drug use behaviors were hypothesized to be associated with worse cognitive functioning in a linear fashion, above and beyond baseline neuropsychological functioning before use initiation.
Method
Participants
The current study is part of a larger ongoing longitudinal substance use and neuroimaging project. At baseline, participants were healthy 12- to 14-year-olds (42% female) with very little to no experience with alcohol and other drug use, recruited through flyers sent to households of students attending public middle schools in the San Diego area (Squeglia et al., 2009, 2011). Data analysis for the current study and the work of Squeglia and colleagues (2009) shared eight participants from the same follow-up years. Baseline exclusionary criteria for the larger study included any report of prenatal alcohol (more than two drinks during a given week) or illicit drug exposure; premature birth, before the 35th gestational week; a history of any neurological or Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV; American Psychiatric Association, 1994), Axis I disorder; head trauma or loss of consciousness (>2 minutes); chronic medical illness; learning disability or mental retardation; psychoactive medication use; experience with alcohol or other drugs, defined as more than 10 total lifetime drinking days, or more than two drinks per week; more than three lifetime experiences with marijuana and use in the past 3 months; more than five lifetime cigarette uses; a history of other intoxicant use (Squeglia et al., 2009, 2011); inadequate English comprehension; and noncorrectable sensory problems. Follow-up exclusion criteria included emergent Axis I disorder as measured by a structured diagnostic interview (Shaffer et al., 2000). All participants were asked not to use alcohol and other recreational drugs for at least 24 hours before the study, confirmed with breath alcohol concentration and urine drug screen in the laboratory. The study protocol and procedures were approved by the University of California San Diego Human Research Protections Program.
Measures
Substance use measures.
At baseline and follow-up, the Customary Drinking and Drug Use Record (Brown et al., 1998) was administered to assess the pattern and severity of alcohol, marijuana, and other drug use. The Timeline Followback was used to examine the frequency of substance use during the past 30 days (Medina et al., 2007; Sobell et al., 1979; Sobell & Sobell, 1992). Parental or informant (sibling, friend, roommate) reports of youth substance use were collected to confirm youth self-report data. In cases of discrepant reports, participants were classified as users if information from either the self-report or parental/informant report indicated use.
Demographics.
The Family History Assessment Module (Rice et al., 1995) was administered to assess family history (FH) of SUD. Participants were classified either as FH negative, mild, or positive. FH negative was defined as having no relative with a history of SUD; FH mild was defined as having one second-degree relative with a history of SUD; FH positive was defined as having at least one parent or at least two second-degree relatives with a history of SUD.
The Hollingshead Index of Social Position score (Hollingshead, 1965), an index of socioeconomic status (SES), was calculated for each subject using parental socioeconomic background information (i.e., educational attainment, occupation, and salary of each parent) to characterize the youth’s rearing environment. Higher values indicate lower SES.
Psychopathology.
Youths were administered the computerized Diagnostic Interview Schedule for Children Predictive Scale (Shaffer et al., 2000) by a trained psychometrist to determine the presence of psychiatric disorders. Parents were separately administered the parental version.
Neuropsychological test measures.
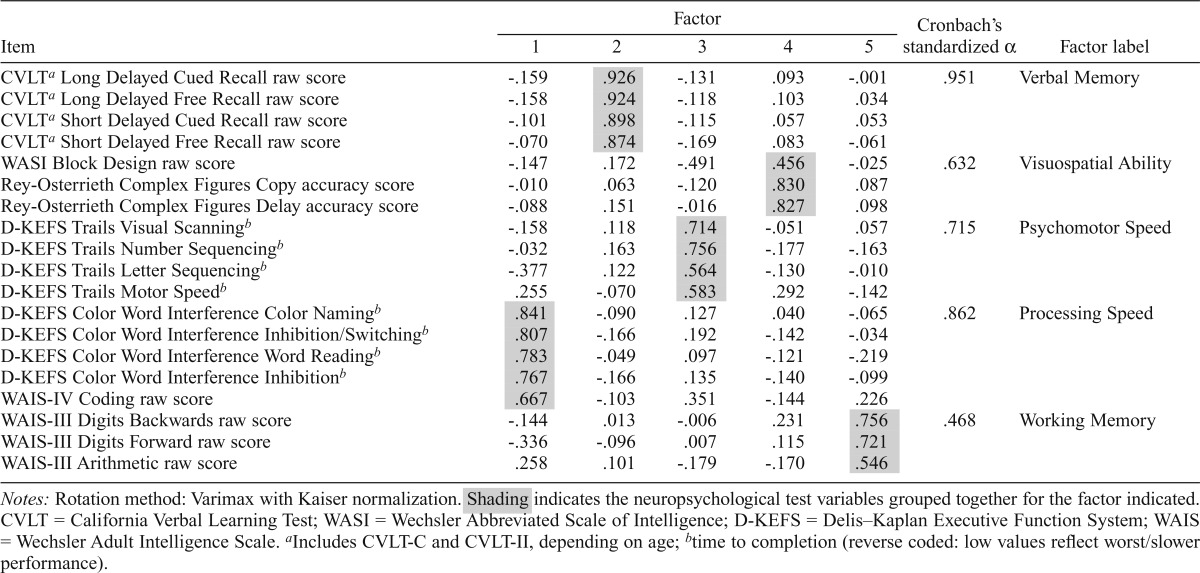
A comprehensive neuropsychological battery was administered at baseline and follow-up to assess cognitive functioning. At baseline, the assessment included (Table 3) the Delis–Kaplan Executive Function System (D-KEFS; Delis et al., 2001) Color-Word Interference and Trails subtests; California Verbal Learning Test-Children’s Version (CVLT-Children; Delis et al., 1994); Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, 1999) Block Design subtest; Wechsler Intelligence Scale for Children, 3rd edition (Wechsler, 1991) Coding and Digit Span subtests; and the Rey-Osterrieth Complex Figure task (Rey & Osterrieth, 1993). At follow-up, participants ages 18 years and older were administered the adult versions of the CVLT (CVLT-II; Delis et al., 2000) and Wechsler Coding/Digit Symbol and Digit Span subtests (Wechsler Adult Intelligence Scale [WAIS-III]; Wechsler, 1997).
Table 3.
Principal component analysis loading structure of follow-up neuropsychological test variables
| Item | Factor |
Cronbach’s standardized α | Factor label | ||||
| 1 | 2 | 3 | 4 | 5 | |||
| CVLPa Long Delayed Cued Recall raw score | -.159 | .926 | -.131 | .093 | -.001 | .951 | Verbal Memory |
| CVLPa Long Delayed Free Recall raw score | -.158 | .924 | -.118 | .103 | .034 | ||
| CVLPa Short Delayed Cued Recall raw score | -.101 | .898 | -.115 | .057 | .053 | ||
| CVLPa Short Delayed Free Recall raw score | -.070 | .874 | -.169 | .083 | -.061 | ||
| WASI Block Design raw score | -.147 | .172 | -.491 | .456 | -.025 | .632 | Visuospatial Ability |
| Rey-Osterrieth Complex Figures Copy accuracy score | -.010 | .063 | -.120 | .830 | .087 | ||
| Rey-Osterrieth Complex Figures Delay accuracy score | -.088 | .151 | -.016 | .827 | .098 | ||
| D-KEFS Trails Visual Scanningb | -.158 | .118 | .714 | -.051 | .057 | .715 | Psychomotor Speed |
| D-KEFS Trails Number Sequencingb | -.032 | .163 | .756 | -.177 | -.163 | ||
| D-KEFS Trails Letter Sequencingb | -.377 | .122 | .564 | -.130 | -.010 | ||
| D-KEFS Trails Motor Speedb | .255 | -.070 | .583 | .292 | -.142 | ||
| D-KEFS Color Word Interference Color Namingb | .841 | -.090 | .127 | .040 | -.065 | .862 | Processing Speed |
| D-KEFS Color Word Interference Inhibition/Switchingb | .807 | -.166 | .192 | -.142 | -.034 | ||
| D-KEFS Color Word Interference Word Readingb | .783 | -.049 | .097 | -.121 | -.219 | ||
| D-KEFS Color Word Interference Inhibitionb | .767 | -.166 | .135 | -.140 | -.099 | ||
| WAIS-IV Coding raw score | .667 | -.103 | .351 | -.144 | .226 | ||
| WAIS-III Digits Backwards raw score | -.144 | .013 | -.006 | .231 | .756 | .468 | Working Memory |
| WAIS-III Digits Forward raw score | -.336 | -.096 | .007 | .115 | .721 | ||
| WAIS-III Arithmetic raw score | .258 | .101 | -.179 | -.170 | .546 | ||
Notes: Rotation method: Varimax with Kaiser normalization. Shading indicates the neuropsychological test variables grouped together for the factor indicated. CVLT = California Verbal Learning Test; WASI = Wechsler Abbreviated Scale of Intelligence; D-KEFS = Delis–Kaplan Executive Function System; WAIS = Wechsler Adult Intelligence Scale.
Includes CVLT-C and CVLT-II, depending on age;
time to completion (reverse coded: low values reflect worst/slower performance).
Procedures
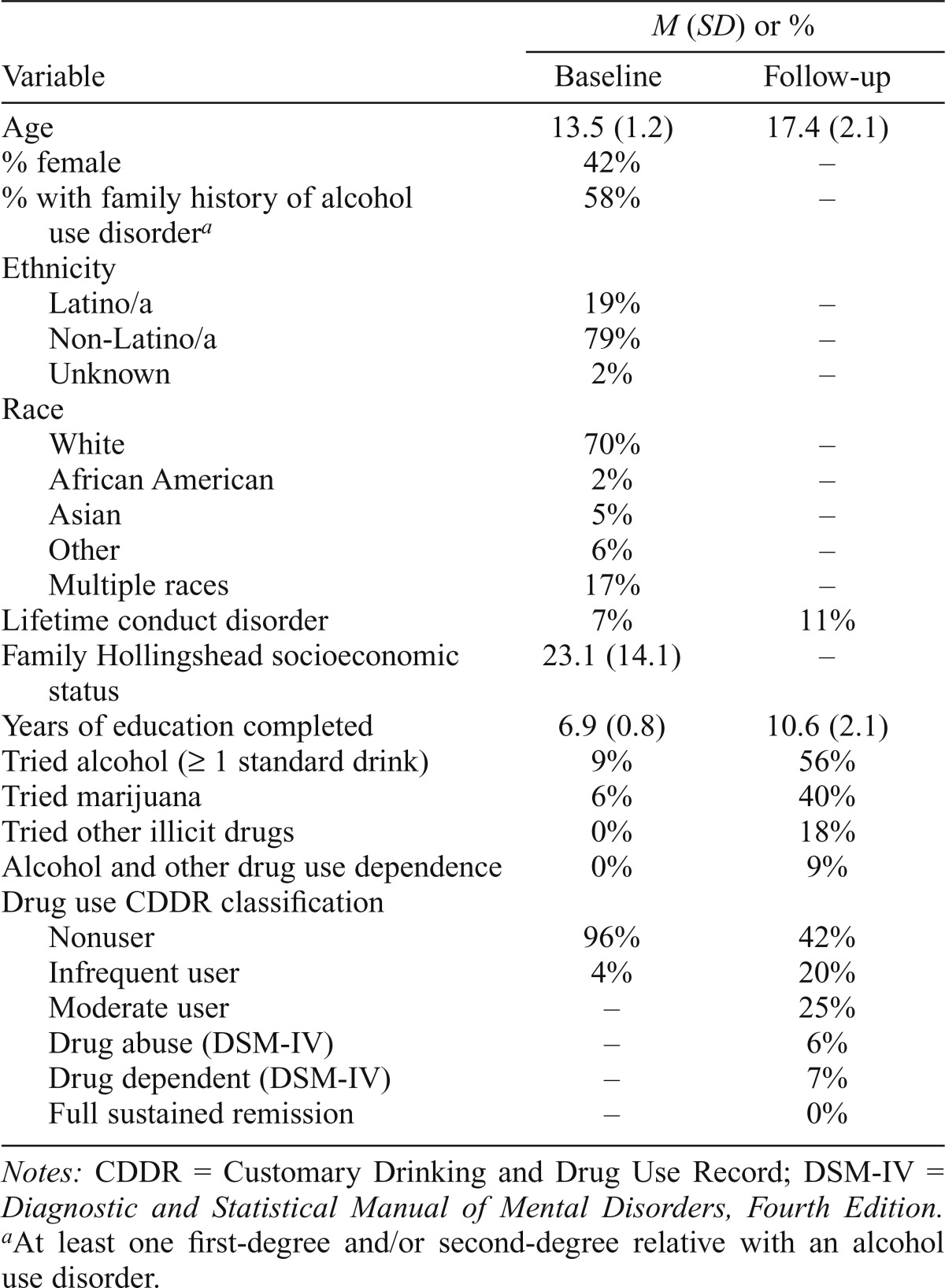
At baseline, potential participants were screened by trained bachelors- and masters-level psychometrists and consent from a parent/guardian and assent from youths were obtained. Eligible youths were administered comprehensive interviews assessing FH, alcohol and other drug use, psychopathology, and general background information (Kleschinsky et al., 2009; Squeglia et al., 2009; Twitchell et al., 1992). A different psychometrist interviewed one informant (most commonly a biological parent, but in some cases a close relative) on background and FH. Participants were ensured that substance use self-report data would not be shared with parents, informants, or schools. After an average of 4.0 (SD = 2.0; range: 1–9) years after baseline, youths were brought back into the laboratory for a repeat neuropsychological assessment and interview. To maintain a high follow-up rate, quarterly brief interviews were conducted and annual birthday cards and semiannual informational newsletters were disbursed to participants (Twitchell et al., 1992). Follow-up rates exceeded 95% in this study. Two participants (not described in this study) were excluded from data analysis because of the presence of psychopathology at follow-up, for a final sample size of N = 234. Seventy percent of the sample was White, and 19% was Latino. Forty-two percent was female and 42% reported no FH of alcohol use disorder. Participants were, on average, 13.5 years old at project entry and 17.4 years at follow-up (Table 1).
Table 1.
Demographic variables at baseline and follow-up (N = 234)
| M (SD) or % |
||
| Variable | Baseline | Follow-up |
| Age | 13.5 (1.2) | 17.4 (2.1) |
| % female | 42% | – |
| % with family history of alcohol use disordera | 58% | – |
| Ethnicity | ||
| Latino/a | 19% | – |
| Non-Latino/a | 79% | – |
| Unknown | 2% | – |
| Race | ||
| White | 70% | – |
| African American | 2% | – |
| Asian | 5% | – |
| Other | 6% | – |
| Multiple races | 17% | – |
| Lifetime conduct disorder | 7% | 11% |
| Family Hollingshead socioeconomic status | 23.1 (14.1) | – |
| Years of education completed | 6.9 (0.8) | 10.6 (2.1) |
| Tried alcohol (> 1 standard drink) | 9% | 56% |
| Tried marijuana | 6% | 40% |
| Tried other illicit drugs | 0% | 18% |
| Alcohol and other drug use dependence | 0% | 9% |
| Drug use CDDR classification | ||
| Nonuser | 96% | 42% |
| Infrequent user | 4% | 20% |
| Moderate user | – | 25% |
| Drug abuse (DSM-IV) | – | 6% |
| Drug dependent (DSM-IV) | – | 7% |
| Full sustained remission | – | 0% |
Notes: CDDR = Customary Drinking and Drug Use Record; DSM-IV = Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition.
At least one first-degree and/or second-degree relative with an alcohol use disorder.
Data analyses
Data reduction of substance use variables.
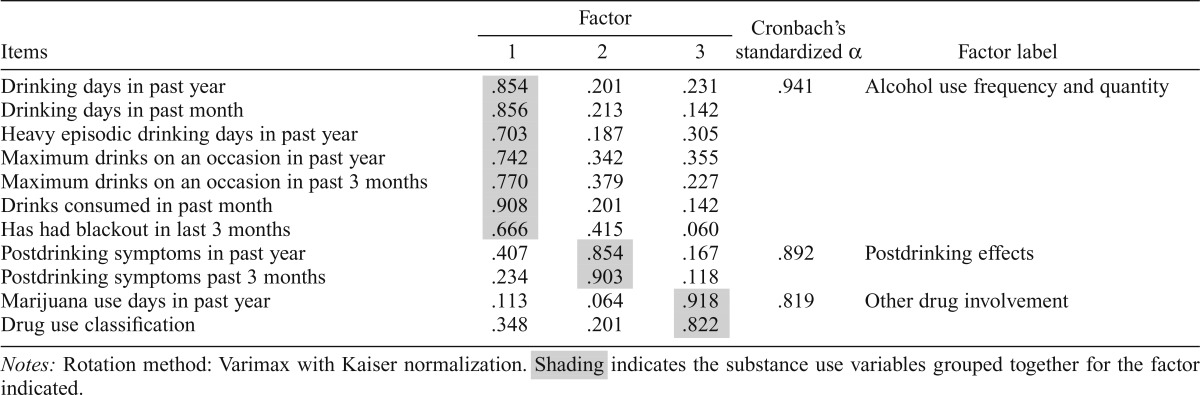
To control for type I error and to avoid redundancy among explanatory variables and associated collinearity problems, follow-up alcohol and other drug use variables were subjected to a principle components analysis using varimax rotation with Kaiser normalization to obtain orthogonal factors (identical factor structure was confirmed with oblique [direct oblimin] rotation). Three factors with eigenvalues greater than 1.0 were retained (Table 2); this three-factor solution accounted for 80.2% of the total variance. Seven variables loaded onto Factor 1, Alcohol Use Frequency and Quantity, as follows: (a) number of drinking days in the past year and (b) in the past month, (c) number of heavy episodic drinking days in the past year, (d) maximum number of drinks consumed on one occasion in the past year and (e) in the past 3 months, (f) number of drinks consumed in the past month, and (g) having experienced a blackout in the past 3 months. Factor 2, Postdrinking Effects, consisted of (a) the number of withdrawal or hangover symptoms in the past year and (b) in the past 3 months. Factor 3, Other Drug Involvement, consisted of (a) the number of marijuana use occasions in the past year and (b) drug use severity classification (nonuser, infrequent/light user, moderate/social user, DSM-IV drug abuse, DSM-IV drug dependent, or full sustained remission; Table 1). Higher scores on each of these three factors indicated greater substance involvement.
Table 2.
Principle component analysis loading structure of follow-up substance use variables
| Items | Factor |
Cronbach’s standardized α | Factor label | ||
| 1 | 2 | 3 | |||
| Drinking days in past year | .854 | .201 | .231 | .941 | Alcohol use frequency and quantity |
| Drinking days in past month | .856 | .213 | .142 | ||
| Heavy episodic drinking days in past year | .703 | .187 | .305 | ||
| Maximum drinks on an occasion in past year | .742 | .342 | .355 | ||
| Maximum drinks on an occasion in past 3 months | .770 | .379 | .227 | ||
| Drinks consumed in past month | .908 | .201 | .142 | ||
| Has had blackout in last 3 months | .666 | .415 | .060 | ||
| Postdrinking symptoms in past year | .407 | .854 | .167 | .892 | Postdrinking effects |
| Postdrinking symptoms past 3 months | .234 | .903 | .118 | ||
| Marijuana use days in past year | .113 | .064 | .918 | .819 | Other drug involvement |
| Drug use classification | .348 | .201 | .822 | ||
Notes: Rotation method: Varimax with Kaiser normalization. Shading indicates the substance use variables grouped together for the factor indicated.
Data reduction of neuropsychological test variables.
At baseline and follow-up, 19 neuropsychological test variables were subjected to a principle components analysis using varimax rotation with Kaiser normalization (identical factor structure was confirmed with oblique [direct oblimin] rotation), yielding five cognitive domains that showed consistent factor loadings at baseline and follow-up: (a) verbal memory, (b) visuospatial ability, (c) psychomotor speed, (d) processing speed, and (e) working memory (Table 3). Internal consistency was assessed with Cronbach’s a coefficients (Table 3). All time-to-complete measures (i.e., D-KEFS Color-Word Interference and Trails tasks) were reverse-coded so that higher scores indicated better performance. Principle components analyses were carried out in SPSS Version 18.0 (SPSS Inc., Chicago, IL).
Hypothesis testing.
Examination of the relationship between substance use and neuropsychological functioning was conducted using Hubert- and bi-weighted robust regressions (Møller et al., 2005) with the “rreg” command. Robust regression effect sizes (R2 and R2Δ) were obtained using the “rregfit” command in Stata Version 12 (StataCorp LP, College Station, TX). Each neuropsychological domain was analyzed in separate multivariable (i.e., multiple explanatory variables) robust regressions, controlling for age, SES, baseline functioning, gender, and abstinence. Early to late adolescence represents a crucial period for neuromaturation (Giedd, 2004), and age was associated with follow-up performance in all neuropsychological domains (ps < .05) and, therefore, was controlled for in all analyses. Some research suggests a correlation between performance on cognitive tests and SES (Raizada & Kishiyama, 2010; Roberts et al., 1999), so SES was included as a covariate. In this sample, SES was associated with verbal memory but not other domains (p < .05).
To understand the effects of substance use independent of baseline neurocognitive functioning, domain-specific performance before substance use initiation served as a covariate in all analyses. Baseline performance was significantly associated with follow-up performance for all five domains of functioning. Using methodology described by Cohen and Cohen (1983) for missing data, lifetime abstinent participants (i.e., never consumed more than several sips of alcohol) were coded as a dummy variable (drinkers = 0; never drinkers = 1); outcome alcohol use for these participants was recorded as “0.” This dichotomous variable was entered as a covariate in robust regression analyses to examine the linear relationship between alcohol use and neuropsychological functioning above and beyond the effect of alcohol versus no alcohol use; further, this method allowed for effective consideration of excess zeros in the data. This variable was not a significant predictor of neuropsychological functioning but was included as a covariate to account for potential variance attributable to the relatively large number of participants who did not transition into substance use. Boys and girls show differential neurodevelopmental trajectories (Lenroot & Giedd, 2006) that may affect neuropsychological performance; thus, gender was included in the same regression model as a moderator to examine whether the effects of substance use on cognition differed in boys (coded 2) and girls (coded 1). Significant findings for the factor scores were followed up using robust regressions and semipartial correlations (sr), in which the dependent variable was an individual neuropsychological test and the independent variable was a specific substance use behavior, and the same four covariates and one moderator (gender) were included as above. All reported regression coefficients are standardized betas ( ), unless otherwise noted.
), unless otherwise noted.
Results
Description of sample
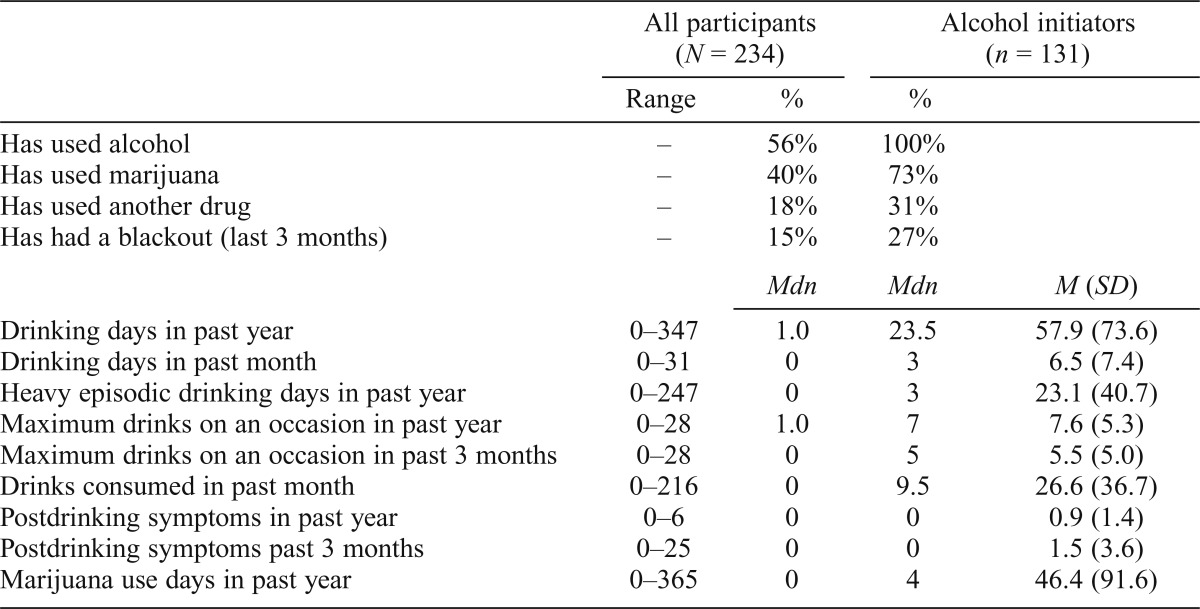
At baseline, 91% of participants were completely substance naive, and at follow-up, 44% remained so (Table 1). By follow-up, 56% had consumed at least one standard drink of alcohol, 40% had tried marijuana, and 18% had tried another illicit drug at least once, consistent with epidemiological data (Johnston et al., 2014a). Participants displayed a wide range of substance use behaviors (Table 4). Among those who initiated drinking during the follow-up period, the number of past-year drinking days ranged from 0 to 347, and the maximum number of drinks consumed on one occasion (within a 24-hour period) in the past 3 months ranged from 0 to 28. The number of days since last alcohol use before the follow-up neuropsychological testing session ranged from 1 to 987 days (Mdn = 14; M = 78, SD = 175). Fifteen percent of the sample reported having had at least one blackout from drinking within the past 3 months. The number of past-year marijuana use days ranged from none to daily.
Table 4.
Range, median, mean, and standard deviations of follow-up substance use variables
| All participants (N = 234) |
Alcohol initiators (n = 131) |
|||
| Range | % | % | ||
| Has used alcohol | – | 56% | 100% | |
| Has used marijuana | – | 40% | 73% | |
| Has used another drug | – | 18% | 31% | |
| Has had a blackout (last 3 months) | – | 15% | 27% | |
| Mdn | Mdn | M (SD) | ||
| Drinking days in past year | 0–347 | 1.0 | 23.5 | 57.9 (73.6) |
| Drinking days in past month | 0–31 | 0 | 3 | 6.5 (7.4) |
| Heavy episodic drinking days in past year | 0–247 | 0 | 3 | 23.1 (40.7) |
| Maximum drinks on an occasion in past year | 0–28 | 1.0 | 7 | 7.6 (5.3) |
| Maximum drinks on an occasion in past 3 months | 0–28 | 0 | 5 | 5.5 (5.0) |
| Drinks consumed in past month | 0–216 | 0 | 9.5 | 26.6 (36.7) |
| Postdrinking symptoms in past year | 0–6 | 0 | 0 | 0.9 (1.4) |
| Postdrinking symptoms past 3 months | 0–25 | 0 | 0 | 1.5 (3.6) |
| Marijuana use days in past year | 0–365 | 0 | 4 | 46.4 (91.6) |
Hypothesis testing
Gender influence.
Tests of gender as an independent predictor of neuropsychological outcome showed that boys performed better than girls on working memory at followup, F(1, 202) = 7.01,  = .12, p = .008, after controlling for baseline performance and other covariates. No Gender × Substance Use or Gender × Baseline Working Memory interaction was detected. Gender was not a significant predictor in other domains of neuropsychological performance.
= .12, p = .008, after controlling for baseline performance and other covariates. No Gender × Substance Use or Gender × Baseline Working Memory interaction was detected. Gender was not a significant predictor in other domains of neuropsychological performance.
Verbal memory.
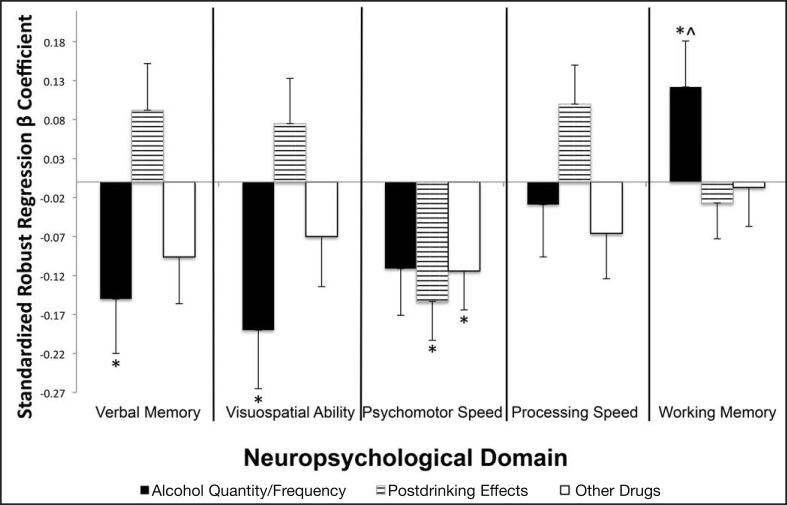
Higher Alcohol Use Quantity and Frequency values predicted worse verbal memory functioning at follow-up, F(1, 200) = 4.40,  = -.15, p = .037, above and beyond age, SES, abstinence, gender, and baseline performance (Figure 1 and Table 5). Follow-up analyses showed that more heavy episodic drinking occasions in the past year predicted worse scores on CVLT short delay free recall, F(1, 205) = 7.65,
= -.15, p = .037, above and beyond age, SES, abstinence, gender, and baseline performance (Figure 1 and Table 5). Follow-up analyses showed that more heavy episodic drinking occasions in the past year predicted worse scores on CVLT short delay free recall, F(1, 205) = 7.65,  = -.17, p = .006, and more drinking days in the past year predicted worse scores on CVLT long delay free recall, F(1, 214) = 4.28,
= -.17, p = .006, and more drinking days in the past year predicted worse scores on CVLT long delay free recall, F(1, 214) = 4.28,  = -.15, p = .040, above and beyond all five covariates. Follow-up semipartial correlations showed that larger maximum alcohol quantities consumed on one occasion in the past year corresponded with worse scores on CVLT short delay free recall (sr2 = .028, p = .012).
= -.15, p = .040, above and beyond all five covariates. Follow-up semipartial correlations showed that larger maximum alcohol quantities consumed on one occasion in the past year corresponded with worse scores on CVLT short delay free recall (sr2 = .028, p = .012).
Figure 1.
Association between neuropsychological performance and substance use, above and beyond baseline neuropsychological performance, age, socioeconomic status, gender and abstinence status. Bar length indicates strength of association; bar direction indicates negative (i.e., more substance use linked to worse neuropsychological performance) versus positive (i.e., more substance use linked to better neuropsychological performance) relationship. indicates unexpected directionality; *p < .05.
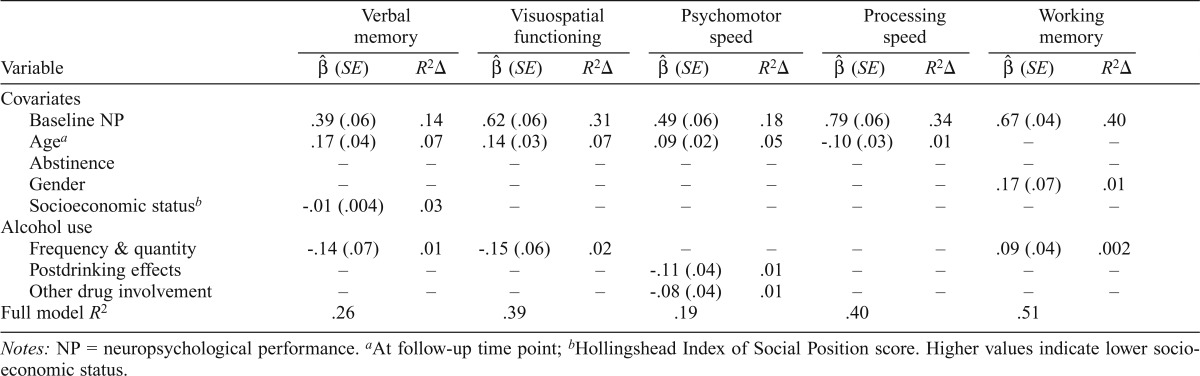
Table 5.
Robust regression unstandardized coefficients ( ), standard errors (SE), and proportion of variances (R2Δ) in neuropsychological functioning accounted for by significant predictors
), standard errors (SE), and proportion of variances (R2Δ) in neuropsychological functioning accounted for by significant predictors
| Variable | Variable memory |
Visuospatial functioning |
Psychomotor speed |
Processing speed |
Working memory |
|||||
| β (SE) | R2 Δ | β (SE) | R2 Δ | β (SE) | R2 Δ | β (SE) | R2 Δ | β (SE) | R2 Δ | |
| Covariates | ||||||||||
| Baseline NP | .39 (.06) | .14 | .62 (.06) | .31 | .49 (.06) | .18 | .79 (.06) | .34 | .67 (.04) | .40 |
| Agea | .17 (.04) | .07 | .14 (.03) | .07 | .09 (.02) | .05 | -.10 (.03) | .01 | – | – |
| Abstinence | – | – | – | – | – | – | – | – | – | – |
| Gender | – | – | – | – | – | – | – | – | .17 (.07) | .01 |
| Socioeconomic statusb | -.01 (.004) | .03 | – | – | – | – | – | – | – | – |
| Alcohol use | ||||||||||
| Frequency & quantity | -.14 (.07) | .01 | -.15 (.06) | .02 | – | – | – | – | .09 (.04) | .002 |
| Postdrinking effects | – | – | – | – | -.11 (.04) | .01 | – | – | – | – |
| Other drug involvement | – | – | – | – | -.08 (.04) | .01 | – | – | – | – |
| Full model R2 | .26 | .39 | .19 | .40 | .51 | |||||
Notes: NP = neuropsychological performance.
At follow-up time point;
Hollingshead Index of Social Position score. Higher values indicate lower socioeconomic status.
Visuospatial functioning.
Higher scores on Alcohol Use Quantity and Frequency predicted worse visuospatial ability, F(1, 200) = 6.26,  = -.19, p = .013, above and beyond age, SES, abstinence, gender, and baseline performance (Figure 1). Follow-up analyses showed that more drinking days in the past month predicted poorer functioning on the WASI Block Design task, F(1, 223) = 4.71,
= -.19, p = .013, above and beyond age, SES, abstinence, gender, and baseline performance (Figure 1). Follow-up analyses showed that more drinking days in the past month predicted poorer functioning on the WASI Block Design task, F(1, 223) = 4.71,  = -.15, p = .031.
= -.15, p = .031.
Psychomotor speed.
Higher scores on Postdrinking Effects and higher scores on Other Drug Involvement predicted worse psychomotor speed, F(2, 200) = 5.44, p = .005, above and beyond age, SES, abstinence, gender, and baseline performance (Figure 1). More instances of postdrinking symptoms in the past 3 months predicted worse (slower) performances on D-KEFS Trails Motor Speed, F(1, 215) = 5.19,  = -.15, p = .024; Number Sequencing, F(1, 215) = 11.77,
= -.15, p = .024; Number Sequencing, F(1, 215) = 11.77,  = -.19, p = .0007; and Letter Sequencing, F(1, 215) = 4.48,
= -.19, p = .0007; and Letter Sequencing, F(1, 215) = 4.48,  = -.08, p = .036. More instances of postdrinking symptoms in the past year predicted worse performance on D-KEFS Trails Number Sequencing, F(1, 215) = 5.23,
= -.08, p = .036. More instances of postdrinking symptoms in the past year predicted worse performance on D-KEFS Trails Number Sequencing, F(1, 215) = 5.23,  = -.14, p = .022. For the Other Drug Involvement factor, more occasions of marijuana use in the past year, F(1, 215) = 5.03,
= -.14, p = .022. For the Other Drug Involvement factor, more occasions of marijuana use in the past year, F(1, 215) = 5.03,  = -.15, p = .026, and more drug use, F(1, 215) = 7.32,
= -.15, p = .026, and more drug use, F(1, 215) = 7.32,  = -.20,
= -.20,  = .0007, independently predicted worse performance on D-KEFS Trails Motor Speed. Follow-up semipartial correlations showed similar results as follow-up robust regressions, such that more postdrinking symptoms in the past 3 months correlated with worse functioning on D-KEFS Trails Motor Speed (sr2 = .018, p = .035) and Number Sequencing (sr2 = .030, p = .003). A greater number of postdrinking symptoms in the past year correlated with worse performance on D-KEFS Trails Number Sequencing (sr2 = .017, p = .027). More drug use (sr2 = .028, p = .009) and more occasions of marijuana use (sr2 = .018, p = .037) in the past year correlated with worse performance on D-KEFS Trails Motor Speed.
= .0007, independently predicted worse performance on D-KEFS Trails Motor Speed. Follow-up semipartial correlations showed similar results as follow-up robust regressions, such that more postdrinking symptoms in the past 3 months correlated with worse functioning on D-KEFS Trails Motor Speed (sr2 = .018, p = .035) and Number Sequencing (sr2 = .030, p = .003). A greater number of postdrinking symptoms in the past year correlated with worse performance on D-KEFS Trails Number Sequencing (sr2 = .017, p = .027). More drug use (sr2 = .028, p = .009) and more occasions of marijuana use (sr2 = .018, p = .037) in the past year correlated with worse performance on D-KEFS Trails Motor Speed.
Processing speed.
No significant associations between substance use and processing speed were detected (p > .05).
Working memory.
Alcohol Use Quantity and Frequency and gender each predicted working memory performance at follow-up, F(2, 202) = 5.52, p > .005, above and beyond age, SES, abstinence, and baseline performance. After we controlled for gender in addition to other covariates, higher values on Alcohol Use Quantity and Frequency significantly predicted better working memory, F(1, 202) = 4.30,  = .12, p = .039 (Figure 1). Follow-up analyses showed that this effect appeared largely due to having had a blackout in the past 3 months being linked to better performance on the Digits Forward subtest, F(1, 218) = 5.36,
= .12, p = .039 (Figure 1). Follow-up analyses showed that this effect appeared largely due to having had a blackout in the past 3 months being linked to better performance on the Digits Forward subtest, F(1, 218) = 5.36,  = .15, p = .007, after controlling for age, SES, abstinence, gender, and baseline performance. When we did not control for gender and other covariates, no relationship between working memory and Alcohol Use Quantity and Frequency was found, F(1, 214) = 2.77,
= .15, p = .007, after controlling for age, SES, abstinence, gender, and baseline performance. When we did not control for gender and other covariates, no relationship between working memory and Alcohol Use Quantity and Frequency was found, F(1, 214) = 2.77,  = .12, p = .10. No other follow-up test supported this unexpected finding.
= .12, p = .10. No other follow-up test supported this unexpected finding.
For all omnibus regression analyses in the five neuropsychological domains, results and directionality remained intact after controlling for the additional potential covariates of parental income, education, race, ethnicity, and FH of alcoholism. Tests of nonlinear and polynomial effects between the neuropsychological domains and substance use domains were not significant (p > .05), and no Gender × Substance Use interactions were detected.
Discussion
The current study investigated the effects of specific drinking and other drug use behaviors on neuropsychological performance in adolescents. The hypothesis that more severe substance use behaviors would predict worse neuropsychological performance was partially confirmed. In 15 regressions analyzing five domains of neurocognition and three domains of substance use, 4 met the hypothesis, 1 was counter to the hypothesis, and 10 were nonsignificant. Among the three factor-analyzed substance use domains, greater Alcohol Use Quantity and Frequency predicted poorer follow-up verbal memory and visuospatial performance, even after controlling for age, SES, abstinence, gender, and baseline neuropsychological performance. Postdrinking Effects as well as Other Drug Involvement similarly predicted poorer follow-up psychomotor speed, above and beyond age, SES, abstinence, gender, and baseline psychomotor speed. Greater Alcohol Use Quantity and Frequency predicted better working memory performance, driven largely by an unexpected relationship between blackout history and auditory attention scores.
This study is among the first to prospectively examine the linear quantitative effects of substance use behaviors on cognition in adolescents while accounting for neuropsychological performance before the onset of substance use (Squeglia et al., 2009). Using a similar longitudinal design, Squeglia and colleagues (2009) found that more drinking days in the past 3 months (regardless of gender) and past year (for girls only) significantly correlated with decreased performances in the Complex Figures visuospatial functioning task. Although the overall pattern of decreased visuospatial (i.e., Block Design) ability with increased alcohol use was replicated in the current study, no effect of gender or relationship with Complex Figures was detected. Several methodological considerations may account for these differences. To address significant deviations from a normal distribution seen in the substance use self-report data, we opted to use robust regressions and not ordinary least squares (OLS) regression. To better examine the continuous linear relationship between alcohol use and neuropsychological functioning, all participants were included in regression analyses without the use of categorical “controls” versus “drinkers.” Further, the current study benefits from a larger sample size. Overall, results from both studies substantiated the inverse correlation between alcohol use and cognitive functioning. However, additional research is needed to understand the impact of drinking on individual neuropsychological tasks in adolescents. Consistent with previous studies, we found that the quantity and frequency of alcohol use were significant predictors of poorer verbal memory and visuospatial functioning; however, no single substance use behavior consistently drove these findings. Because participants had not yet engaged in consistent substance use at project intake, this allowed for control of preexisting, non–substance-related neuropsychological functioning differences between subjects. Further, previous studies have focused on a smaller subset of drinking and other drug use behaviors, with primary emphasis on quantity and frequency. In this study, substance use behaviors were extended to postdrinking effects and nonalcohol substance involvement. Participants in the current study were physically and psychiatrically healthy adolescents recruited from the community with relatively high SES families. Few youths transitioned into SUD, further supporting the notion that substance users who do not meet the criteria for SUD may exhibit subtle, but detectable, neuropsychological disadvantages compared with youths who did not initiate substance use (McQueeny et al., 2009).
Follow-up robust regressions and semipartial correlations suggested that individual drinking and other drug use behaviors may influence each cognitive domain in different ways. For example, greater instances of heavy episodic drinking may specifically affect verbal memory, perhaps via impact to memory-encoding systems (Beresford et al., 2006; De Bellis et al., 2000). Another possible mechanism is the selective disruption of hippocampal memory consolidation through consumption of large quantities of alcohol over a short period (Verster et al., 2003). On the other hand, repeated recent drinking may influence more complex multinetwork functions through widespread neurotoxicity that contributes to enhanced frontal cortical atrophy and ventricular enlargements. This relationship may not be identified until after a prolonged period of drinking patterns leading to alcohol dependence-like symptoms (for a review, see Moselhy et al., 2001). These results highlight the complex interplay between substance use and cognition, providing preliminary evidence for the importance of understanding drinking and other drug use behaviors beyond mere quantity and frequency.
The results of the current study are consistent with the literature indicating deleterious continuous effects of substance use on neuropsychological performance (Day et al., 2013; Hannon et al., 1983). However, much evidence on the relationship between adolescent alcohol use and neuropsychological functioning has been derived largely through cross-sectional studies of matched cases (e.g., heavy drinkers) versus controls (e.g., light drinkers or nondrinkers). The prospective design of the current study controlled for the effects of preexisting interindividual differences in biology (e.g., gender and age), SES, and importantly, baseline neuropsychological functioning in the absence of the influence of alcohol. This provided further confidence that the reported results are less likely to be confounded by baseline differences that may be present in cross-sectional designs. Similar findings on the prospective effects of alcohol on neurocognition have been reported by Squeglia et al. (2009), in which the authors found reduced visuospatial functioning with an increasing number of drinking days in girls and increasing hangover symptoms in boys, after controlling for baseline cognitive functioning.
Unexpected findings in the present study are in line with previous reports of nonsignificant, negative, and positive associations between substance use and cognition (Green et al., 2010; Hannon et al., 1983; Sinha et al., 1989). Green and colleagues (2010) speculated that nonsignificant and nonintuitive correlation findings may be attributable to the interplay between cognitive functioning and complex variables not considered in analyses, such as familial density of alcoholism and biological alcohol metabolism. Preliminary analyses (not reported) with the current study showed that parental income, education, race, ethnicity, and FH of alcoholism did not affect the strength and directionality of the results. Although genetics and alcohol metabolism were not considered in this analysis series, a longitudinal design allowed for the control of biologically related predrinking differences.
Another suggested reason for unexpected results is the unreliability of self-report data. Although this possibility exists, careful measures were taken in the current study to increase the accuracy of participant data, including the use of informants for corroborating information, different interviewers for informants and subjects, toxicology screening, and quarterly (i.e., four times yearly) Timeline Followback data in addition to detailed yearly interviews to reduce demand on participant recall. To increase self-report accuracy, careful measures were taken to ensure subjects were aware that substance use self-report data would not be shared with parents or schools. It may also be possible that no underlying relationship exists between certain neuropsychological domains and alcohol use, no effects were detected because of inadequate power, or the severity of alcohol use in this sample may not have reached the threshold in which neuropsychological impairments can be detected. Further evidence is needed to understand the nature of the reported unexpected finding.
Limitations of the current study included the relatively small number of other drug use variables compared with alcohol use variables. Only two drug use behaviors were considered: number of marijuana use times last year and DSM-based classification of drug use. This was, in part, because of the lower prevalence of consistent illicit substance use within the sample. Although results showed that drug use behaviors negatively affect memory and psychomotor speed, a sample with more drug users is needed to further understand the direct relationship between drug use and neuropsychological performance.
Robust regressions were used in place of OLS models to account for outliers and departures from normality in the data without a reduction of power, thereby strengthening the generalizability of the present findings (Wilcox, 2012). However, the calculation of effect sizes (R2) for robust regressions departs from that of OLS regressions. Robust regressions allow for better predictive power by avoiding artificially inflated standard error, in turn often producing smaller R2 values than equivalent OLS regressions, which may reach a nontrivial 10% difference, corresponding to a difference in R2 of .10 in robust versus OLS regressions (Brossart et. al., 2011). The corresponding effect sizes in robust regressions when compared with OLS regressions are currently unknown. Thus, an important limitation of this statistical methodology is that although it was used in the current study to decrease type I error and increase power, the proportion of variance accounted for in robust regressions cannot be easily characterized based on conventional methods, such as Cohen’s small, medium, and large effect size cutoffs (Cohen, 1992). This caveat should be considered when interpreting the reported R2 values of the current study (Table 5).
Findings from this study highlight the importance of examining the quantitative effects of substance use. The impact of substance use on neuropsychological functioning varies based on the specific substance use behavior and neurocognitive domain under consideration. This introduces the possibility of prevention and treatment of specific cognitive impairments based on use patterns and behaviors. Examination of substance-related behaviors on a continuous spectrum may allow for more personalized rather than category-based (e.g., users vs. nonusers, dependence vs. abuse) treatment recommendations.
Acknowledgments
The authors thank the participants, families, and other informants; participating San Diego schools; and the following individuals: Lotte Berk, Sonja Eberson, Erick Idy, Alejandra Infante, Ashley Tracas, Dr. M. J. Meloy, Dr. Sandra A. Brown, Dr. Mark Myers, and Dr. Sarah Mattson.
Footnotes
This study was supported by National Institute on Alcohol Abuse and Alcoholism Grants R01 AA13419, U01 AA021695 (principal investigator: Susan F. Tapert), and T32 AA013525 (principal investigator: Edward P. Riley, Department of Psychology, San Diego State University, San Diego, CA). Portions of this study were presented at the 2014 meeting of the Research Society on Alcoholism. This work represents the master’s thesis of the first author.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: Author; 1994. [Google Scholar]
- Beresford T. P., Arciniegas D. B., Alfers J., Clapp L., Martin B., Du Y., Davatzikos C. Hippocampus volume loss due to chronic heavy drinking. Alcoholism: Clinical and Experimental Research. 2006;30:1866–1870. doi: 10.1111/j.1530-0277.2006.00223.x. doi:10.1111/j.1530-0277.2006.00223.x. [DOI] [PubMed] [Google Scholar]
- Brossart D. F., Parker R. I., Castillo L. G. Robust regression for single-case data analysis: How can it help? Behavior Research Methods. 2011;43:710–719. doi: 10.3758/s13428-011-0079-7. doi:10.3758/s13428-011-0079-7. [DOI] [PubMed] [Google Scholar]
- Brown S. A., Myers M. G., Lippke L., Tapert S. F., Stewart D. G., Vik P. W. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): A measure of adolescent alcohol and drug involvement. Journal of Studies on Alcohol. 1998;59:427–438. doi: 10.15288/jsa.1998.59.427. doi:10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- Brown S. A., Tapert S. F., Granholm E., Delis D. C. Neurocognitive functioning of adolescents: Effects of protracted alcohol use. Alcoholism: Clinical and Experimental Research. 2000;24:164–171. doi:10.1111/j.1530-0277.2000.tb04586.x. [PubMed] [Google Scholar]
- Caswell A. J., Morgan M. J., Duka T. Acute alcohol effects on subtypes of impulsivity and the role of alcohol-outcome expectancies. Psychopharmacology. 2013;229:21–30. doi: 10.1007/s00213-013-3079-8. doi:10.1007/s00213-013-3079-8. [DOI] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychological Bulletin. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. doi:10.1037/0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Cohen J., Cohen P. Applied multiple regression/correlation analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: Erlbaum; 1983. [Google Scholar]
- Crews F., He J., Hodge C. Adolescent cortical development: A critical period of vulnerability for addiction. Pharmacology, Biochemistry, and Behavior. 2007;86:189–199. doi: 10.1016/j.pbb.2006.12.001. doi:10.1016/j.pbb.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day A. M., Celio M. A., Lisman S. A., Johansen G. E., Spear L. P. Acute and chronic effects of alcohol on trail making test performance among underage drinkers in a field setting. Journal of Studies on Alcohol and Drugs. 2013;74:635–641. doi: 10.15288/jsad.2013.74.635. doi:10.15288/jsad.2013.74.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis M. D., Clark D. B., Beers S. R., Soloff P. H., Boring A. M., Hall J, Keshavan M. S. Hippocampal volume in adolescent-onset alcohol use disorders. American Journal of Psychiatry. 2000;157:737–744. doi: 10.1176/appi.ajp.157.5.737. doi:10.1176/appi.ajp.157.5.737. [DOI] [PubMed] [Google Scholar]
- Delis D. C., Kaplan E., Kramer J. H. The Delis–Kaplan Executive Function System: Examiner’s manual. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- Delis D. C., Kramer J. H., Kaplan E., Ober B. A. Manual for the California Verbal Learning Test–Children’s Version. San Antonio, TX: The Psychological Corporation; 1994. [Google Scholar]
- Delis D. C., Kramer J. H., Kaplan E., Ober B. A. California Verbal Learning Test. San Antonio, TX: The Psychological Corporation; 2000. [Google Scholar]
- Field M., Wiers R. W., Christiansen P, Fillmore M. T., Verster J. C. Acute alcohol effects on inhibitory control and implicit cognition: Implications for loss of control over drinking. Alcoholism: Clinical and Experimental Research. 2010;34:1346–1352. doi: 10.1111/j.1530-0277.2010.01218.x. doi:10.1111/j.1530-0277.2010.01218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillmore M. T. Acute alcohol-induced impairment of cognitive functions: Past and present findings. International Journal on Disability and Human Development. 2007;6:115–125. doi:10.1515/IJDHD.2007.6.2.115. [Google Scholar]
- Giancola P. R., Mezzich A. C., Tarter R. E. Disruptive, delinquent and aggressive behavior in female adolescents with a psychoactive substance use disorder: Relation to executive cognitive functioning. Journal of Studies on Alcohol. 1998;59:560–567. doi: 10.15288/jsa.1998.59.560. doi:10.15288/jsa.1998.59.560. [DOI] [PubMed] [Google Scholar]
- Giedd J. N. Structural magnetic resonance imaging of the adolescent brain. Annals of the New York Academy of Sciences. 2004;1021:77–85. doi: 10.1196/annals.1308.009. doi:10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Green A., Garrick T., Sheedy D., Blake H., Shores E. A., Harper C. The effect of moderate to heavy alcohol consumption on neuropsychological performance as measured by the repeatable battery for the assessment of neuropsychological status. Alcoholism: Clinical and Experimental Research. 2010;34:443–450. doi: 10.1111/j.1530-0277.2009.01108.x. doi:10.1111/j.1530-0277.2009.01108.x. [DOI] [PubMed] [Google Scholar]
- Hannon R., Day C. L., Butler A. M., Larson A. J., Casey M. Alcohol consumption and cognitive functioning in college students. Journal of Studies on Alcohol. 1983;44:283–298. doi: 10.15288/jsa.1983.44.283. doi:10.15288/jsa.1983.44.283. [DOI] [PubMed] [Google Scholar]
- Hanson K. L., Cummins K., Tapert S. F., Brown S. A. Changes in neuropsychological functioning over 10 years following adolescent substance abuse treatment. Psychology of Addictive Behaviors. 2011;25:127–142. doi: 10.1037/a0022350. doi:10.1037/a0022350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson K. L., Winward J. L., Schweinsburg A. D., Medina K. L., Brown S. A., Tapert S. F. Longitudinal study of cognition among adolescent marijuana users over three weeks of abstinence. Addictive Behaviors. 2010;35:970–976. doi: 10.1016/j.addbeh.2010.06.012. doi:10.1016/j.addbeh.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey M. A., Sellman J. D., Porter R. J., Frampton C. M. The relationship between non-acute adolescent cannabis use and cognition. Drug and Alcohol Review. 2007;26:309–319. doi: 10.1080/09595230701247772. doi:10.1080/09595230701247772. [DOI] [PubMed] [Google Scholar]
- Hingson R. W., Zha W. Age of drinking onset, alcohol use disorders, frequent heavy drinking, and unintentionally injuring oneself and others after drinking. Pediatrics. 2009;123:1477–1484. doi: 10.1542/peds.2008-2176. doi:10.1542/peds.2008-2176. [DOI] [PubMed] [Google Scholar]
- Hollingshead A. B. Two-factor index of social position. New Haven, CT: Yale University Press; 1965. [Google Scholar]
- Johnston L. D., O’Malley P. M., Bachman J. G., Schulenberg J. E. Monitoring the Future national survey results on drug use: 1975-2013: Overview, key findings on adolescent drug use. Ann Arbor, MI: Institute for Social Research, The University of Michigan; 2014a. [Google Scholar]
- Johnston L. D., O’Malley P. M., Miech R. A., Bachman J. G., Schulenberg J. E. 2013 Overview: Key findings on adolescent drug use. Ann Arbor, MI: Institute for Social Research, The University of Michigan; 2014b. [Google Scholar]
- Kleschinsky J. H., Bosworth L. B., Nelson S. E., Walsh E. K., Shaffer H. J. Persistence pays off: Follow-up methods for difficult-to-track longitudinal samples. Journal of Studies on Alcohol and Drugs. 2009;70:751–761. doi: 10.15288/jsad.2009.70.751. doi:10.15288/jsad.2009.70.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane S. D., Cherek D. R., Tcheremissine O. V, Steinberg J. L., Sharon J. L. Response perseveration and adaptation in heavy marijuanasmoking adolescents. Addictive Behaviors. 2007;32:977–990. doi: 10.1016/j.addbeh.2006.07.007. doi:10.1016/j.addbeh.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Lenroot R. K., Giedd J. N. Brain development in children and adolescents: Insights from anatomical magnetic resonance imaging. Neuroscience and Biobehavioral Reviews. 2006;30:718–729. doi: 10.1016/j.neubiorev.2006.06.001. doi:10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- McQueeny T., Schweinsburg B. C., Schweinsburg A. D., Jacobus J., Bava S., Frank L. R., Tapert S. F. Altered white matter integrity in adolescent binge drinkers. Alcoholism, Clinical and Experimental Research. 2009;33:1278–1285. doi: 10.1111/j.1530-0277.2009.00953.x. doi:10.1111/j.1530-0277.2009.00953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina K. L., Nagel B. J., Park A., McQueeny T., Tapert S. F. Depressive symptoms in adolescents: Associations with white matter volume and marijuana use. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2007;48:592–600. doi: 10.1111/j.1469-7610.2007.01728.x. doi:10.1111/j.1469-7610.2007.01728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller S. F., von Frese J., Bro R. Robust methods for multivariate data analysis. Journal of Chemometrics. 2005;19:549–563. doi:10.1002/cem.962. [Google Scholar]
- Moselhy H. F, Georgiou G., Kahn A. Frontal lobe changes in alcoholism: A review of the literature. Alcohol and Alcoholism. 2001;36:357–368. doi: 10.1093/alcalc/36.5.357. doi:10.1093/alcalc/36.5.357. [DOI] [PubMed] [Google Scholar]
- Moss H. B., Kirisci L., Gordon H. W., Tarter R. E. A neuropsychologic profile of adolescent alcoholics. Alcoholism: Clinical and Experimental Research. 1994;18:159–163. doi: 10.1111/j.1530-0277.1994.tb00897.x. doi:10.1111/j.1530-0277.1994.tb00897.x. [DOI] [PubMed] [Google Scholar]
- Parada M., Corral M., Mota N., Crego A., Rodriguez Holguin S., Cadaveira F. Executive functioning and alcohol binge drinking in university students. Addictive Behaviors. 2012;37:167–172. doi: 10.1016/j.addbeh.2011.09.015. doi:10.1016/j.addbeh.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Raizada R. D., Kishiyama M. M. Effects of socioeconomic status on brain development, and how cognitive neuroscience may contribute to leveling the playing field. Frontiers in Human Neuroscience. 2010;4:3. doi: 10.3389/neuro.09.003.2010. doi:10.3389/neuro.09.003.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey A., Osterrieth P. A. Translations of excerpts from Andre Rey’s “Psychological examination of traumatic encephalopathy” and PA. Osterrieth’s “The complex figure copy test” (J. Corwin & F. W. Bylsma, Trans. Clinical Neuropsychologist. 1993;7:3–21. [Google Scholar]
- Rice J. P, Reich T., Bucholz K. K., Neuman R. J., Fishman R., Rochberg N., Begleiter H. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcoholism: Clinical and Experimental Research. 1995;19:1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x. doi:10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Roberts E., Bornstein M. H., Slater A. M., Barrett J. Early cognitive development and parental education. Infant and Child Development. 1999;8:49–62. doi:10.1002/(SICI)1522-7219(199903)8:1<49::AID-ICD188>3.0.CO;2–1. [Google Scholar]
- Ryan C., Butters N. Further evidence for a continuum-of-impairment encompassing male alcoholic Korsakoff patients and chronic alcoholic men. Alcoholism: Clinical and Experimental Research. 1980;4:190–198. doi: 10.1111/j.1530-0277.1980.tb05634.x. doi:10.1111/j.1530-0277.1980.tb05634.x. [DOI] [PubMed] [Google Scholar]
- Ryback R. S. The continuum and specificity of the effects of alcohol on memory. A review. Quarterly Journal of Studies on Alcohol. 1971;32:995–1016. [PubMed] [Google Scholar]
- Sartor C. E., Lynskey M. T., Heath A. C., Jacob T., True W. The role of childhood risk factors in initiation of alcohol use and progression to alcohol dependence. Addiction. 2007;102:216–225. doi: 10.1111/j.1360-0443.2006.01661.x. doi:10.1111/j.1360-0443.2006.01661.x. [DOI] [PubMed] [Google Scholar]
- Shaffer D., Fisher P, Lucas C. P, Dulcan M. K., Schwab-Stone M.E. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): Description, differences from previous versions, and reliability of some common diagnoses. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. doi:10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Sinha R., Parsons O. A., Glenn S. W. Drinking variables, affective measures and neuropsychological performance: Familial alcoholism and gender correlates. Alcohol. 1989;6:77–85. doi: 10.1016/0741-8329(89)90077-3. doi:10.1016/0741-8329(89)90077-3. [DOI] [PubMed] [Google Scholar]
- Sneider J. T., Cohen-Gilbert J. E., Crowley D. J., Paul M. D., Silveri M. M. Differential effects of binge drinking on learning and memory in emerging adults. Journal of Addiction Research & Therapy, Supplement. 2013;7 doi: 10.4172/2155-6105.S7-006. doi:10.4172/2155–6105.s7-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell L. C., Maisto S. A., Sobell M. B., Cooper A. M. Reliability of alcohol abusers’ self-reports of drinking behavior. Behaviour Research and Therapy. 1979;17:157–160. doi: 10.1016/0005-7967(79)90025-1. doi:10.1016/0005-7967(79)90025-1. [DOI] [PubMed] [Google Scholar]
- Sobell L., Sobell M. Timeline Follow-Back. In: Litten R., Allen J., editors. Measuring alcohol consumption. Totowa, NJ: Humana Press; 1992. pp. 41–72. [Google Scholar]
- Squeglia L. M., Schweinsburg A. D., Pulido C., Tapert S. F. Adolescent binge drinking linked to abnormal spatial working memory brain activation: Differential gender effects. Alcoholism: Clinical and Experimental Research. 2011;35:1831–1841. doi: 10.1111/j.1530-0277.2011.01527.x. doi:10.1111/j.1530-0277.2011.01527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia L. M., Spadoni A. D., Infante M. A., Myers M. G., Tapert S. F. Initiating moderate to heavy alcohol use predicts changes in neuropsychological functioning for adolescent girls and boys. Psychology of Addictive Behaviors. 2009;23:715–722. doi: 10.1037/a0016516. doi:10.1037/a0016516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: Author; 2013. NSDUH Series H-46, HHS Publication No. (SMA) 13–4795. [Google Scholar]
- Tapert S. F., Brown S. A. Neuropsychological correlates of adolescent substance abuse: Four-year outcomes. Journal of the International Neuropsychological Society. 1999;5:481–493. doi: 10.1017/s1355617799566010. doi:10.1017/S1355617799566010. [DOI] [PubMed] [Google Scholar]
- Tapert S. F., Granholm E., Leedy N. G., Brown S. A. Substance use and withdrawal: Neuropsychological functioning over 8 years in youth. Journal of the International Neuropsychological Society. 2002;8:873–883. doi: 10.1017/s1355617702870011. doi:10.1017/S1355617702870011. [DOI] [PubMed] [Google Scholar]
- Tarter R. E., Mezzich A. C., Hsieh Y. C., Parks S. M. Cognitive capacity in female adolescent substance abusers. Drug and Alcohol Dependence. 1995;39:15–21. doi: 10.1016/0376-8716(95)01129-m. doi:10.1016/0376-8716(95)01129-M. [DOI] [PubMed] [Google Scholar]
- Thoma R. J., Monnig M. A., Lysne P. A., Ruhl D. A., Pommy J. A., Bogenschutz M., Yeo R. A. Adolescent substance abuse: the effects of alcohol and marijuana on neuropsychological performance. Alcoholism, Clinical and Experimental Research. 2011;35:39–46. doi: 10.1111/j.1530-0277.2010.01320.x. doi:10.1111/j.1530-0277.2010.01320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twitchell G. R., Hertzog C. A., Klein J. L., Schuckit M. A. The anatomy of a follow-up. British Journal of Addiction. 1992;87:1327–1333. doi: 10.1111/j.1360-0443.1992.tb02741.x. doi:10.1111/j.1360-0443.1992.tb02741.x. [DOI] [PubMed] [Google Scholar]
- Verster J. C., van Duin D., Volkerts E. R., Schreuder A. H., Verbaten M. N. Alcohol hangover effects on memory functioning and vigilance performance after an evening of binge drinking. Neuropsychopharmacology. 2003;28:740–746. doi: 10.1038/sj.npp.1300090. doi:10.1038/sj.npp.1300090. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children. 3rd ed. New York, NY: Psychological Corporation; 1991. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. 3rd ed. New York, NY: Psychological Corporation; 1997. [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- Weissenborn R., Duka T. Acute alcohol effects on cognitive function in social drinkers: Their relationship to drinking habits. Psychopharmacology. 2003;165:306–312. doi: 10.1007/s00213-002-1281-1. doi:10.1007/s00213-002-1281-1. [DOI] [PubMed] [Google Scholar]
- Wilcox R. R. Introduction to robust estimation and hypothesis testing. Waltham, MA: Academic Press; 2012. [Google Scholar]
- Winward J. L., Hanson K. L., Tapert S. F., Brown S. A. Heavy alcohol use, marijuana use, and concomitant use by adolescents are associated with unique and shared cognitive decrements. Journal of the International Neuropsychological Society. 2014;20:784–795. doi: 10.1017/S1355617714000666. doi:10.1017/S1355617714000666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeigler D. W., Wang C. C., Yoast R. A., Dickinson B. D., McCaffree M. A., Robinowitz C. B., Sterling M. L the Council on Scientific Affairs, American Medical Association. The neurocognitive effects of alcohol on adolescents and college students. Preventive Medicine. 2005;40:23–32. doi: 10.1016/j.ypmed.2004.04.044. doi:10.1016/j.ypmed.2004.04.044. [DOI] [PubMed] [Google Scholar]