Abstract
One proposed mechanism for acute and chronic hepatic encephalopathy (HE) is a disturbance in cerebral energy metabolism. This article reviews the current status of this mechanism in both acute and chronic HE, as well as in other hyperammonemic disorders. This article reviews abnormalities in glycolysis, lactate metabolism, citric acid cycle, and oxidative phosphorylation and associated energy impairment. Additionally, the role of mitochondrial permeability transition (mPT), a recently established factor in the pathogenesis of HE and hyperammonemia, is emphasized. Energy failure appears to be an important pathogenetic component of both acute and chronic HE and a potential target for therapy.
Introduction
Hepatic encephalopathy (HE) is the major neurological disorder occurring in patients with severe liver disease which presents in chronic and acute forms (Williams, 1973). Chronic HE is a neuropsychiatric disorder which commonly occurs in the setting of alcoholic cirrhosis and is often associated with changes in personality, altered mood, decline in intellectual capacity and abnormal muscle tone (Jones and Weissenborn, 1997). Acute HE (acute liver failure; ALF) has a high mortality rate (80–90%) due to the development of brain edema and increased intracranial pressure, and often presents with the abrupt onset of delirium, seizures and coma (Capocaccia and Angelico, 1991). ALF usually occurs following viral-mediated hepatitis, acetaminophen toxicity, and exposure to other hepatotoxins (Lee, 1994).
Elevated blood and brain ammonia levels have been strongly implicated in the pathogenesis of HE (Butterworth, 2002). Ammonia is also an etiological factor in inborn errors of the urea cycle, Reye’s syndrome, valproate toxicity, idiopathic hyperammonemia and other conditions (Hindfelt, 1972; Brusilow, 2002). Astrocytes are the cells in brain that are most affected in both acute and chronic HE (Norenberg, 1987), likely due to the fact that ammonia is primarily metabolized in these cells by glutamine synthetase (Martinez-Hernandez et al., 1977).
Pathogenetic mechanisms in acute and chronic HE/hyperammonemia are not clear. One proposed mechanism is a disturbance in cerebral energy metabolism (Bessman and Bessman, 1955; Hindfelt and Siesjo, 1970). Subsequent studies carried out in various animal models of acute and chronic HE and hyperammonemia have reported altered glucose utilization, increased glycolysis, and reduction in the operational rate of the citric acid cycle (TCA cycle) by the removal of α-ketoglutarate (α-KG) for the purpose of ammonia detoxification have all been identified in HE and hyperammonemia. An impairment in the malate-aspartate shuttle, a process that leads to an interference in the transfer of reducing equivalents from the cytosol to mitochondria, has also been reported (Hindfelt et al., 1977).
This article reviews the current status of cerebral energy metabolic changes in acute and chronic hepatic encephalopathy and other hyperammonemic disorders, including changes in glycolysis, TCA cycle, and the electron transport chain. Additionally, updates on oxidative/nitrosative stress (ONS), a well established factor in the pathogenesis of acute and chronic HE and hyperammonemia relative to energy metabolism, is also assessed. Lastly, the mitochondrial permeability transition (mPT), a recently established factor in the pathogenesis of HE and hyperammonemia, is emphasized.
Glucose Utilization
The cerebral metabolic rate for glucose (CMRglc) has consistently been reported to be decreased in patients with chronic HE (for review, see Hazell and Butterworth, 1999), suggesting that hypometabolism contributes to the neuropsychiatric symptoms commonly observed in HE. However, studies in animal models of HE are equivocal. Whereas a decrease in CMRglc was reported in portacaval-shunted rats (Hawkins and Jessy, 1991), other studies documented either an increase in CMRglc in the same animal model (Cruz and Duffy, 1983) or no change in hyperammonemic rats (Cruz and Dienel, 1994). Additionally, increased cerebral glucose levels were reported in the sparse-fur (spf) mouse model of congenital hyperammonemia (Ratnakumari et al., 1992), suggesting a decrease in glucose utilization. Additionally, regional heterogeneity in CMRglc was observed by Lockwood et al. (1986) in the portacaval-shunt model of HE, wherein the CMRglc was observed to be increased in the thalamus and hypothalamus, but was found to be decreased in cerebral cortex. Recent studies using NMR spectroscopy showed increased glucose utilization in brains of rats with acute liver failure (hepatic devascularization) and in chronic HE (portacaval-shunted rats) (Chatauret et al., 2003); (Zwingmann, 2007). While the reasons for discrepancies among several studies on changes in CMRglc are not known, the use of different animal models (portacaval-shunted rats vs. acute/chronic hyperammonemic rats and mice) may, in part, be responsible for the conflicting results. Nevertheless, in an animal model of acute liver failure induced in rats by hepatic devascularization, whole brain cerebral glucose utilization was shown to be reduced (Mans et al., 1994). This study also demonstrated a lack of correlation between changes in glucose metabolism and the degree of encephalopathy.
A recent study showed increased glucose utilization in cultured neurons and co-cultures of neurons and astrocytes treated with ammonia which led to an increase in the production of alanine (via pyruvate) (Leke et al. 2011). These authors suggested that in addition to the traditional process of ammonia being metabolized predominantly in astrocytes, increased production of alanine (derived from increased glucose utilization) might represent an alternate pathway for ammonia detoxification in neurons.
Glycolysis
Increase in the rate of glycolysis is a well-known phenomenon in acute and chronic HE and hyperammonemia. It was initially reported that ammonia stimulates glycolysis in brain homogenates of normal rats (Muntz and Hurwitz, 1951), possibly by enhancing the activity of phosphofructokinase, a rate-limiting enzyme in glycolysis (Abrahams and Younathan, 1971). Subsequent studies using a rat model of hyperammonemia showed increased brain levels of glycolytic intermediates, along with significant increases in the activities of several glycolytic enzymes, including phosphofructokinase, aldolase, glyceraldehyde-3-phosphate dehydrogenase and pyruvate kinase (Ratnakumari and Murthy, 1992, 1993). Increased rate of glycolysis in brain was also observed in the spf-mouse model of congenital chronic hyperammonemia (Ratnakumari et al., 1992). While an increased rate of glycolysis was expected to enhance the operational rate of the TCA cycle in HE and hyperammonemia, this did not occur as the pyruvate generated in glycolysis was shown to be converted to lactate, instead of being channeled into the TCA cycle (see also section on the TCA cycle below).
Lactate metabolism
Increased lactate levels in blood and brain has traditionally been considered a marker of energy impairment (Siesjo and Plum, 1971), and increased blood and brain lactate levels have been documented in patients with HE and hyperammonemia (Walsh et al., 1999). Increased lactate levels were also observed in blood and brains of pigs with ALF created by hepatic devascularization (Rose et al., 2007); and in rats with ALF induced by hepatic devascularization (Zwingmann et al., 2003), as well as in rats with acute hyperammonemia (Fitzpatrick et al., 1989) consistent with a disturbance in cerebral energy metabolism. Results from these studies, in conjunction with the observation of increased blood and brain lactate levels in patients with ALF, suggested the possibility that a rise in lactate is a prognostic marker for HE outcome (Bernal et al., 2002; Schmidt and Larsen, 2006).
A decrease in the levels of pyruvate and increase in lactate levels were observed in brains of congenitally hyperammonemic spf-mice, which ultimately resulted in a decreased pyruvate/lactate ratio (Ratnakumari et al., 1992). Similarly, cultured astrocytes treated with ammonia (3–5 mM NH4Cl) showed a significant increase in lactate, which also resulted in a decreased pyruvate/lactate ratio (Kala and Hertz, 2005). A decrease in pyruvate levels and a concomitant rise in lactate concentration can lead to a disturbance in operational rates of TCA cycle (reduced oxaloacetate production), as well as in the rate of electron transport chain due to a diminished availability of reducing equivalents (NAD and NADH).
Mechanisms responsible for increased brain lactate levels in HE and hyperammonemia are not completely clear. While studies suggest that increased brain lactate reflects a compromised energy metabolic state due to a reduction in the production of pyruvate and the subsequent decrease in the operational rate of the TCA cycle (for review, see (Zwingmann et al., 2003), a recent study using a pig model of ALF suggested that increased brain lactate levels might be due to decreased utilization of lactate, presumably by neurons (Rose et al., 2007). This view was based on studies demonstrating that albumin dialysis in pigs with ALF, which resulted in a significant clinical improvement, was associated with reduced brain extracellular levels of lactate without a commensurate reduction in the activity of lactate dehydrogenase (the lactate synthesizing enzyme).
Lactate in brain is mainly synthesized in astrocytes and released into the extracellular space where it may be transported to neurons for further utilization (astrocyte-neuron lactate shuttle) (Pellerin et al., 1998). It is also well known that neurons can utilize lactate as an energy substrate in brain (Schurr et al., 1997). However, whether or not neuronal utilization of lactate is defective in HE and hyperammonemia remains to be determined.
It is also possible that the increase in lactate in HE and hyperammonemia could be due to increased production of pyruvate through glycolysis and the subsequent failure of pyruvate to enter into the TCA cycle as acetyl CoA, a reaction mediated by pyruvate dehydrogenase (PDH). In support of this mechanism, one study demonstrated that ammonia inhibits PDH (Katanuma et al., 1966). Similarly, inhibition of PDH was also shown in a rat model of ALF induced by hepatic devascularization (Zwingmann et al., 2003). Such inhibition of PDH could result in decreased operational rates of the TCA cycle which may negatively impact on the electron transport chain and the subsequent production of ATP.
Increased brain levels of lactate have recently been suggested to play a role in the development of brain edema and the associated increase in intracranial pressure (ICP), the major neurological complication of ALF (Tofteng and Larsen, 2004). Likewise, a parallel increase in extracellular levels of lactate with the severity of edema was detected in brains of rats and pigs with ALF created by hepatic devascularization· (Chatauret et al., 2003). These studies provide a nexus between compromised energy metabolism and the subsequent development of brain edema in ALF.
The proposition that lactate may contribute to the brain edema in ALF was largely derived from previous observations that treatment of cultured astrocytes with lactic acid resulted in many adverse effects (Norenberg et al., 1987) including cell swelling (Lomneth et al., 1990; Staub et al., 1990; Andersson et al., 2009). However, these in vitro studies required high concentrations (>20 mM) of lactic acid to induce astrocyte swelling, whereas brain extracellular levels of lactate in ALF are usually in the 4–6 mM range (Tofteng and Larsen, 2002, 2004; Ott et al., 2005; Rose et al., 2007). While there may be an association between blood/brain lactate levels and brain edema, a cause and effect relationship remains to be established.
Mitochondrial dysfunction and bioenergetic changes
TCA cycle
An early proposal by Bessman and Bessman (Bessman and Bessman, 1955) postulated that the removal of α-ketoglutarate (α-KG), a substrate required to generate glutamate, contributes to the pathogenesis of HE. This proposition was based on findings that brain utilizes the majority of free ammonia derived from blood to generate glutamate (by reductive amination mediated by glutamate dehydrogenase). However, a subsequent report suggested that inhibition of α-ketoglutarate dehydrogenase (α-KGDH) activity was responsible for increased α-KG levels in the CSF in rats with portacaval shunts (Shorey et al., 1967). Further studies showed that ammonia inhibited α-ketoglutarate dehydrogenase (α-KGDH) in mitochondria isolated from cerebral cortex (Lai and Cooper, 1991). Additionally, a recent NMR study showing increased α-KG levels in brain of rats with ALF induced by hepatic devascularization is consistent with the inhibition of α-KGDH in ALF (Zwingmann et al., 2003). Such inhibition of α-KGDH and the subsequent depletion of α-KG levels could profoundly affect the operational rate of TCA cycle and subsequently the electron transport chain. Taken together, it appears that a reduction in α-KGDH activity, the rate limiting enzyme in the TCA cycle, may adversely affect cerebral bioenergetics in acute and chronic HE.
In addition to α-KGDH, other TCA cycle enzymes, including pyruvate dehydrogenase and isocitrate dehydrogenase, were found to be inhibited by ammonia (Katanuma, 1966) (Zwingmann et al., 2003). In contrast, levels of α-KG and malate were shown to be unaltered in portacaval-shunted rats (model chronic HE) (Hawkins and Mans, 1990). This is consistent with reports showing unchanged activity of α-KGDH in brain tissue of autopsied patients with chronic HE (Lavoie et al., 1987). While the reason for discrepancies regarding the activity of α-KGDH and the changes in the levels of TCA cycle intermediates (α-KG, citrate and oxaloacetate) are not known, the differences could be due to dissimilar models of HE employed (acute vs. chronic), as well as the length of the experimental period.
The malate-aspartate shuttle (MAS) is the principal mechanism for the transfer of reducing equivalents (NAD, NADH) from the cytosol into mitochondria for oxidative phosphorylation (Hindfelt et al., 1977; Fitzpatrick et al., 1988; Ratnakumari and Murthy, 1989). Components of this shuttle include cytosolic and mitochondrial malate dehydrogenase and aspartate aminotransferase, along with the translocator of malate-α-ketoglutarate and glutamate-aspartate. A dysfunction of MAS was proposed as an explanation for altered energy status in acute HE/hyperammonemia (Hindfelt et al., 1977). It was also suggested that depletion of cerebral glutamate pools by ammonia might be responsible for a disruption of the MAS observed in portacaval-shunted rats (Hindfelt et al., 1977). A dysfunction in MAS could bring about a reduction in the availability of reducing equivalents at the inner mitochondrial membrane which are required for the normal operation of the electron transport chain (Hindfelt et al., 1977).
Oxidative phosphorylation
Studies have shown that the addition of ammonia to mitochondria isolated from cerebral cortex of normal rats results in an inhibition of State III respiration (Walshe et al., 1958; McKhann and Tower, 1961; Baraona et al., 1965). Consistent with these findings, Kosenko et al, (1997) reported the inhibition of State III respiration in brain mitochondria isolated from acute ammonia-intoxicated rats. In congenitally chronic hyperammonemic spf-mice a progressive inhibition of cytochrome C oxidase (complex IV of electron transport chain, ETC) activity and mRNA levels of its subunit II were observed (Rao et al., 1997). Additionally, a higher reduction of other ETC enzymes (complexes II and III) was observed in synaptosomes compared to non-synaptic mitochondria in spf-mice (Qureshi et al., 1998).
In addition to chronic HE and hyperammonemia, a recent study has shown that ALF induced in rats by carbon tetrachloride (CCl4) also results inhibition of mitochondrial respiratory chain complexes (I, III and IV) in cerebellum and cerebral cortex (Boer et al., 2009). Another study employing two different models of ALF, one induced by carbon tetrachloride and the other by acetaminophen, showed an inhibition of creatine kinase (CK) activity in different brain regions (Pacheco et al., 2009). Such inhibition was identified in different regions among these two models: CK activity was reduced in cerebellum but not in hippocampus in CCl4 treated rats, whereas acetaminophen-induced ALF resulted in inhibition of CK activity in both cerebellum and hippocampus. Such inhibition of CK activity could profoundly reduce the levels of phosphocreatine in ALF (see below).
High energy metabolites
Decreased levels of ATP and phosphocreatine were observed in brains of portacaval-shunted rats infused with ammonia (Hindfelt et al., 1977) by biochemical as well as by NMR methods in rats with chronic HE (Astore and Boicelli, 2000). Reduced brain ATP levels were likewise reported in rats with acute hyperammonemia (McCandless and Schenker, 1981), as well as in brains of the spf-mice model of chronic hyperammonemia (Rao et al., 1997). Further, decreased levels of ATP were observed in cultured astrocytes treated with ammonium chloride (Haghighat et al., 2000). Recent studies have also indicated reduced levels of AMP and ADP in rats with acute hyperammonemia, and such decrease was found to be due to increased activity of AMP deaminase and adenosine deaminase (Kaminsky and Kosenko, 2010; Kosenko and Kaminsky, 2010). In contrast, a few reports have shown no change in ATP and AMP levels in acute hyperammonemia (Hindfelt and Siesjo, 1971; Hawkins et al., 1973; Lin and Raabe, 1985; Ratnakumari et al., 1992). The reason for the failure to observe ATP reduction in some studies may be partly explained by differences in the duration of ammonia exposure.
Reduced ATP levels were also reported in rats with ALF induced by CCl4 (Bates et al., 1989). By contrast, employing rats with ALF induced by hepatic devascularization, Mans et al (1994), reported that levels of ATP and other high energy phosphates were unchanged. One possible explanation for this discrepancy is the severity and timing of clinical symptoms. The hepatic devascularization model represents a hyper-acute model of ALF resulting in total liver necrosis with coma occurring as early as 6 h following experimental procedure. On the other hand, the CCl4 model in rats exhibits a slower rate of hepatocellular necrosis with a delay in the appearance of clinical symptoms which begins at 24 h and peaks at 48 h after CCl4 administration) (Bates et al., 1989). It appears that early phase of the hyper-acute model of ALF is associated with an encephalopathy that is independent of energy failure.
While a reduction in brain ATP levels observed in chronic HE and acute hyperammonemia could be due to inhibition of oxidative phosphorylation, it could also be due to its increased consumption. Studies have indeed shown increased activity of Na+-K+-ATPase in brains of rats with acute ammonia intoxication (Kosenko et al., 1994), and more recently in cultured astrocytes treated with ammonia (Xue et al. 2010).
The mitochondrial permeability transition (mPT)
Another possible mechanism for impaired energy metabolism in HE and hyperammonemia is the mitochondrial permeability transition (mPT). The mPT is characterized by a sudden increase in the permeability of the inner mitochondrial membrane to small solutes (ions and other molecules <1500 Da). The mPT is due to the opening of the permeability transition pore (PTP) in the inner mitochondrial membrane, usually in response to an increase in mitochondrial Ca2+ levels. This leads to a collapse of the mitochondrial inner membrane potential that is created by the pumping out of protons by the electron transport chain. Loss of the membrane potential leads to colloid osmotic swelling of the mitochondrial matrix, movement of metabolites across the inner membrane (e.g., Ca2+, Mg2+, glutathione, NADPH), defective oxidative phosphorylation, cessation of ATP synthesis, and the generation of reactive oxygen species (ROS). The latter acts to further aggravate the mPT (for reviews, see Zoratti and Szabo, 1995; Bernardi et al., 1998; Norenberg and Rao, 2007).
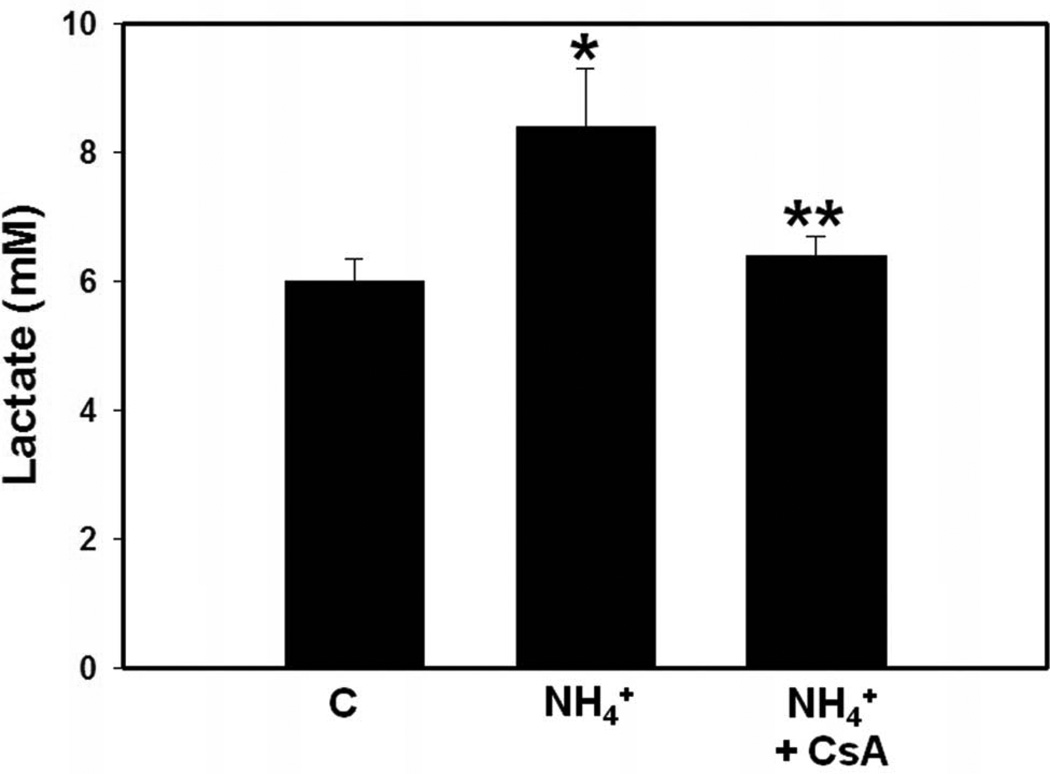
We previously documented that treatment of cultured astrocytes with a pathophysiological level of ammonia (5 mM NH4Cl) results in the induction of the mPT (Bai et al., 2001). More recently we observed the induction of the mPT in brains of rats with ALF induced by hepatotoxin thioacetamide (RamaRao et al., 2010). Cyclosporin A, an inhibitor of the mPT, was shown to block the reduction in ATP levels in ammonia-treated cultured astrocytes (Pichili et al., 2007), suggesting an important role of the mPT in cerebral energy metabolic disturbances. Additionally, we found that CsA significantly attenuated ammonia-induced release of lactate from cultured astrocytes (Figure 1). Further, treatment of cultured astrocytes with CsA also diminished ammonia-induced astrocyte swelling (Rama Rao et al., 2003a). CsA also reduced brain edema (30%) in rats with ALF induced by the hepatotoxin thioacetamide (TAA), although CsA resulted in a severe systemic toxicity and worsening of the clinical outcome (unpublished observations).
Figure 1.
Effect of cyclosporin A (CsA, 5 µM), an inhibitor of the mPT, on ammonia-induced release of lactate in cultured astrocytes. Following treatment of ammonia (5 mM NH4Cl, 24 h), culture medium was collected and lactate levels in the media were determined by an enzymatic assay employing lactate dehydrogenase that converts lactate to pyruvate (Kala and Hertz, 2005). Values are mean ± SEM of 10 individual plates in each experimental group obtained from two different seedings. * vs. control, p<0.01; ** vs. ammonia (NH4), p<0.01.
Notably, the mPT was not observed in cultured cortical neurons treated with the same concentration of ammonia (Bai et al., 2001). The absence of the mPT in neurons was also reported in cerebellar granule cells exposed to low levels (0.1–0.5 mM) of ammonia (Llansola et al., 2003). While the reason for the absence of the mPT in neurons is not clear, one possibility could be differences in the susceptibility of neuronal and astrocytic mitochondria to the induction of the mPT. Alternatively, the inability of neurons to synthesize glutamine may preclude these cells from developing the mPT after treatment with ammonia (see below).
Ca2+ is a well known inducer of the mPT (Kristal and Dubinsky, 1997). Significantly, increased intracellular Ca2+ is one of the earliest events occurring in cultured astrocytes exposed to ammonia (Norenberg MD, 2003a; Rose, 2006). Such early rise in intracellular Ca2+ appears to be responsible for the ammonia-induced mPT as pre-treatment of cultures with BAPTA-AM, a chelator of intracellular Ca2+, resulted in a complete blockade of the ammonia-induced mPT as well as astrocyte swelling (Jayakumar et al., 2009).
Oxidative/nitrosative stress (ONS), a major factor in the pathogenesis of acute and chronic HE (for reviews, see Norenberg et al., 2004 Jayakumar and Norenberg, 2011) is also a major inducer of the mPT (Castilho et al., 1995; Halestrap et al., 1997; Canevari et al., 2004). Antioxidants have been shown to diminish the induction of the mPT in cultured astrocytes exposed to beta-amyloid peptide (Abramov et al., 2004), as well as in hepatocytes exposed to ethanol (Higuchi et al., 2001). Additionally, various antioxidants diminished the ammonia-induced mPT in cultured astrocytes (Rama Rao et al., 2005b).
Glutamine, whose levels in brain are elevated in HE and hyperammonemia (Hourani et al., 1971; Record et al., 1976; McConnell et al., 1995), was also recently implicated in the induction of the mPT in cultured astrocytes (Rama Rao et al., 2003b). The mechanism by which glutamine induces the mPT appears to be mediated by its transport into mitochondria and its subsequent hydrolysis in that organelle through the action of phosphate-activated glutaminase (PAG), thereby resulting in the generation of high levels of ammonia in mitochondria, and in the production of free radicals (the Trojan horse hypothesis; (Albrecht and Norenberg, 2006). Consistent with this hypothesis, recent studies showed that histidine, an inhibitor of mitochondrial glutamine transport, as well as 6-diazo-5-oxy-norleucine (DON), an inhibitor of phosphate-activated glutaminase, significantly blocked the ammonia-induced mPT in cultured astrocytes (Rama Rao et al., 2005a). L-histidine was recently shown to also completely block the induction of the mPT in brains of rats with thioacetamide-induced ALF, and such blockade was associated with a marked reduction in brain edema (RamaRao et al., 2010).
The validity of this hypothesis, however, has recently been questioned (Häussinger and Schliess, 2011). The major concern expressed was a discordance in the time course of free radical production (2 min following treatment with ammonia) (Murthy et al., 2001; Reinehr et al., 2007) and the occurrence of astrocyte swelling (<5% occurring as early as 3 min after ammonia exposure) with that of mPT induction (occurring at 4–24 h) in cultured astrocytes by ammonia/glutamine. While the mPT does not account for the early transient rise in free radical levels and the small degree of early astrocyte swelling caused by ammonia, it should be emphasized that the robust astrocyte swelling (>50% increase in cell volume) and the persistent production of free radicals continue to occur (peaks at 12–24 h) at the time mPT was also induced (4–24 h) PEAK?. Additionally, as noted above, histidine, an inhibitor of glutamine transport into the mitochondria, as well DON, an inhibitor of phosphate activated glutaminase, significantly blocked free radical production, mPT induction and astrocyte swelling. The chronology of events explains the divergent views on Trojan horse hypothesis.
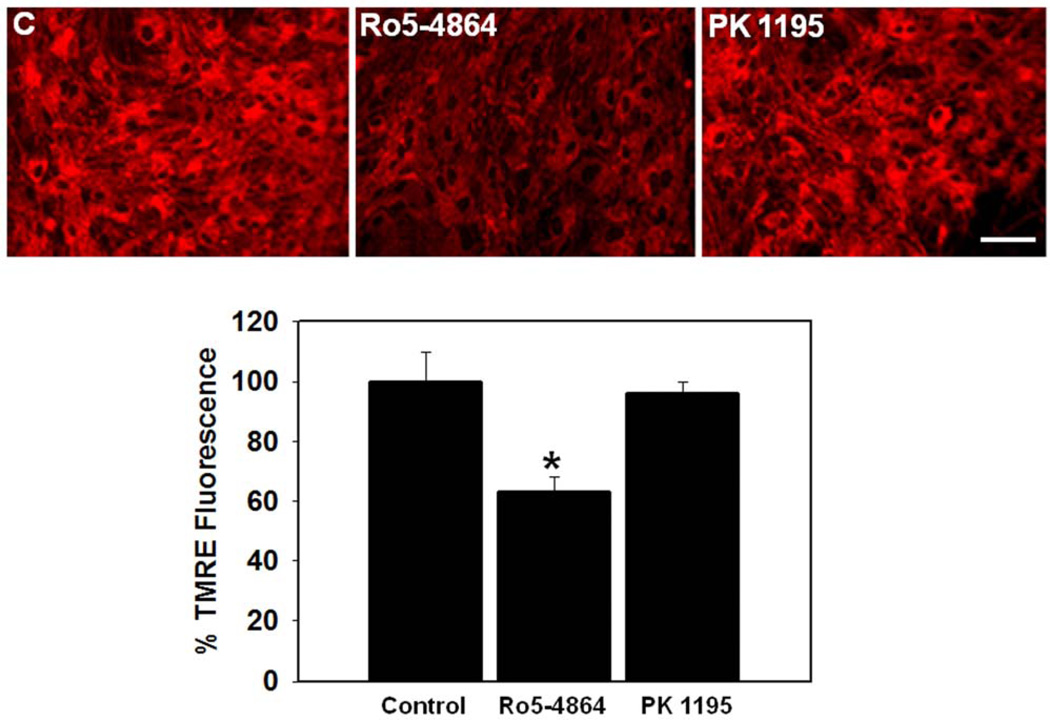
In addition to glutamine, the peripheral benzodiazepine receptor (PBR; recently renamed as translocator protein of 18 Kda; TSPO) also been shown to be involved in the induction of the mPT as well as in the pathogenesis of HE and ammonia toxicity (Norenberg et al., 2004b). TSPO is an 18 kDa protein that is present in the outer mitochondrial membrane of astrocytes and microglia (Snyder et al., 1990). TSPO is known to be upregulated in HE and hyperammonemia, as well as in cultured astrocytes exposed to ammonia (Itzhak and Norenberg, 1994; Norenberg et al., 2006). Further, TSPO ligands were shown to induce the production of free radicals (Jayakumar et al., 2002) and to promote ONS (Häussinger et al., 2005). In support of these findings, we recently documented that down-regulation of TSPO in cultured astrocytes by antisense oligonucleotides caused a reduction in the ammonia-induced mPT (Panickar et al., 2007). Additionally, treatment of cultured astrocytes with Ro 5–4864, an agonist of TSPO, induced the mPT, whereas the TSPO antagonist PK 11195 had no effect (Figures 2 A and B).
Figure 2.
A. Effect of Ro5–4864 (10 nM) and PK11195 (10 nM), agonist and antagonist of TSPO, respectively, on the dissipation of inner mitochondrial membrane potential (ΔΨm) (24 h) in cultured astrocytes. The ΔΨm was measured by incubating (15 min) cultures with a tetramethyl-rhodamine ethyl-ester (TMRE, 25 nM), a potentiometric fluorescent dye commonly employed to determine ΔΨm (Rama Rao et al, 2005a; 2005b). Treatment with Ro5–4864 (Ro5) significantly dissipated the ΔΨm as shown by a reduction in TMRE fluorescence, whereas PK 11195 (PK) had no effect. Scale bar = 100 µm. B. Quantitation of TMRE fluorescence. Values are mean ± SEM of the total number of mean pixel values in each experimental group. * vs. control (C), p<0.01.
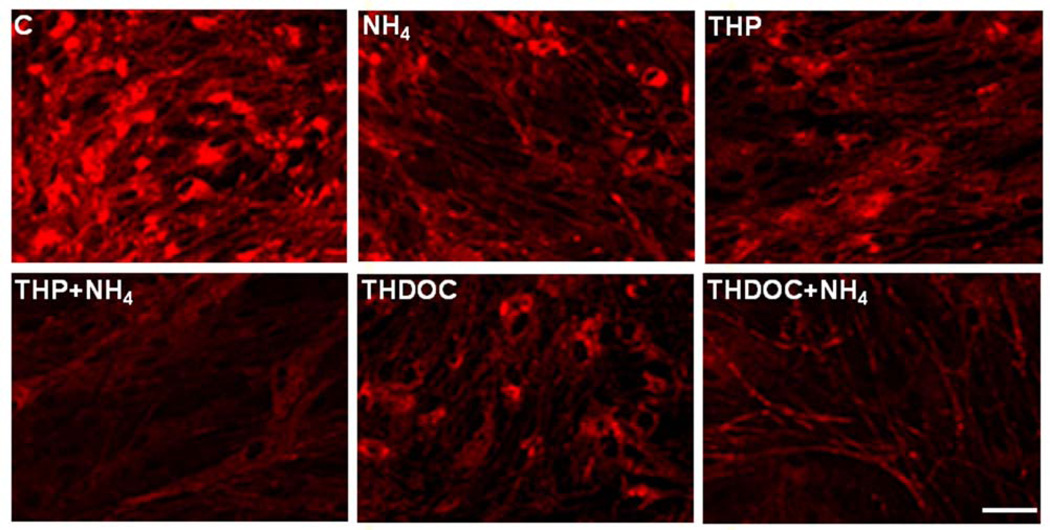
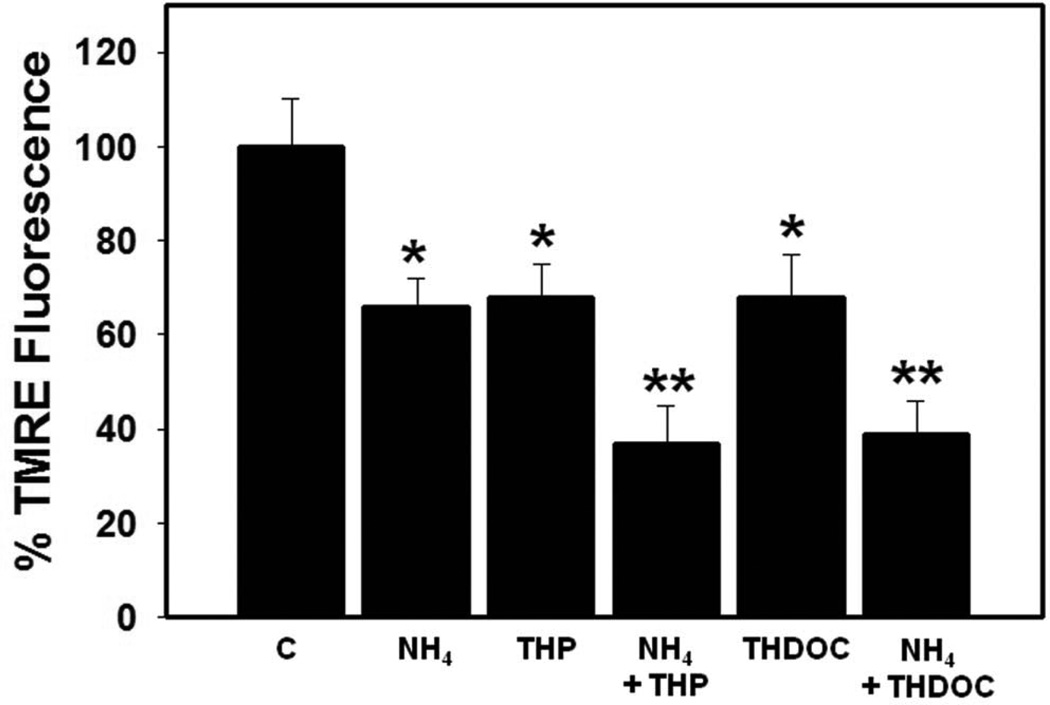
Another factor also implicated in the induction of the mPT in astrocytes are neurosteroids, a consequence of TSPO activation (Papadopoulos et al., 1992). Neurosteroids have been implicated in the pathogenesis of HE by exerting a positive modulatory effect on GABAA receptor. (An updated account of neurosteroids in the pathogenesis of HE is included in this special issue by Ahboucha et al.). The neurosteroids tetrahydroprogesterone (THP, allopregnanolone) and tetrahydrodeoxycorticosterone (THDOC) have recently been shown to increase free radical production (Norenberg et al., 2006). Further, treatment of cultured astrocytes with THP or THDOC (10 nM) significantly dissipated the mitochondrial membrane potential ΔΨm, as measured by a decrease in TMRE fluorescence (Figures 3 A and B). In addition, both THP THDOC significantly potentiated the ammonia-induced reduction of the ΔΨm (Figures 3 A and B). These studies indicate that neurosteroids, in addition to influencing GABAergic tone, are also involved in the pathogenesis of HE by inducing the mPT through a process that is potentiated by ammonia.
Figure 3.
A. Effect of tetrahydroprogesterone (THP; 10 nM) and tetrahydrodeoxycorticosterone (THDOC; 10 nM), alone or in combination with ammonia (5 mM NH4Cl), on the dissipation of inner mitochondrial membrane potential (ΔΨm) (24 h) in cultured astrocytes as measured by TMRE fluorescence. Both THP and THDOC significantly dissipated the ΔΨm and such dissipation was potentiated in the presence of ammonia. Scale bar = 100 µm. B. Quantitation of TMRE fluorescence. Values are expressed as mean ± standard error of mean (SEM) of the total number of mean pixel values derived from 15 randomly captured image fields from each culture plate (total of 6 plates) obtained from 2 separate seedings. * vs. control (C), p<0.01; ** vs. THP and THDOC, p<0.05.
Recent studies suggest that proinflammatory cytokines also play a role in the pathogenesis of HE and hyperammonemia, as well as in ALF, including the development of cytotoxic brain edema/astrocyte swelling (O'Grady and Williams, 1986; Jalan and Williams, 2001; Jalan et al., 2004; Shawcross and Jalan, 2005; O'Beirne et al., 2006). The generation of cytokines is a consequence of extensive liver necrosis as well as infection/sepsis, a frequent complication of ALF (Williams and Smith, 1972; Wilkinson et al., 1974). Increased brain levels of TNF-α, IL-1β, and IL-6 were reported in experimental model of ALF (Jiang et al., 2009). We recently found that TNF- α, IL-1β, and IL-6 and IFN-γ significantly induced the mPT in cultured astrocytes. Additionally, a combination of ammonia and a mixture of cytokines exerted a marked additive effect on the induction of the mPT (Alvarez et al., 2011). The mechanism for such additive effects on the mPT appears to involve ONS as ammonia and cytokines together caused a synergistic upregulation in hemeoxygenase-1 (HO-1), a marker of ONS, as antioxidants significantly blocked the cytokine plus ammonia-induced mPT as well as the upregulation of HO-1 protein expression.
Oxidative/nitrosative stress (ONS)
As noted above, ONS is an established factor in the pathogenesis of in HE and hyperammonemia (for reviews, see (Norenberg, 2003b; Norenberg et al., 2004a; Häussinger et al., 2005; Schliess et al., 2006). A critical pathogenetic linkage between ONS and disturbed cerebral energy metabolism appears to be the mPT. Additionally, ONS can cause mitochondrial injury, including oxidation of membrane phospholipids and various enzymes involved in energy metabolism (Gutierrez et al., 2006). In support of this view, recent studies found that N-acetyl-cysteine and the iron chelating agent desferroximine significantly attenuated the inhibition of electron transport chain enzyme activities, as well as the activity of creatine kinase, in rats with ALF induced by CCl4 (Boer et al, 2009; Bates et al, 1989). Similar to free radicals, nitric oxide (NO), which has been shown to be increased in HE and hyperammonemia (Rao, 2002) (Jekabsone et al., 2003), may adversely affect mitochondria as NO was shown to inhibit cytochrome C oxidase (Bolanos et al., 1994). Alternatively, ONS can also result from mitochondrial dysfunction as a consequence of the mPT (Votyakova and Reynolds, 2005; Zorov et al., 2006).
Cerebral energy metabolic failure in ALF
The principal feature of ALF is the development of brain edema and the associated increase in intracranial pressure. Such edema in ALF is mainly cytotoxic due to swelling of astrocytes (Martinez, 1968; Kato et al., 1989; Traber et al., 1989). While altered brain energy metabolism likely contributes to the pathogenesis of chronic HE and hyperammonemia, a direct role of energy impairment in the development of brain edema in ALF is not firmly established.
A depressed cerebral metabolic rate for oxygen in patients with ALF has been reported, which was suggested to contribute to increased lactate production (Strauss et al., 2003). Subsequent studies also found a correlation between increased cerebral lactate levels and the development of brain edema (Strauss et al., 2003; Tofteng and Larsen, 2004). Additionally, reduced cerebral metabolic rate for glucose was found in patients with ALF before the onset of brain edema, suggesting that hypometabolism of glucose could be one factor in the development of brain edema (Strauss et al., 2003).
As noted in the above sections dealing with glycolysis, TCA cycle and oxidative phosphorylation, various animal models of ALF have been used to examine bioenergetic events in ALF. These studies described several abnormalities in cerebral energy metabolism, including glucose utilization (Mans et al., 1994), reduction in TCA cycle enzyme activity (Zwingmann et al., 2003), decreased rate of respiratory chain activity (Boer et al., 2009), inhibition of creatine kinase activity (Pacheco et al., 2009), and reduced levels of ATP (Bates et al., 1989).
We recently examined levels of ATP in brains of rats with ALF induced by thioacetamide (TAA). Briefly, TAA (300 mg/kg wt, i.p.) was administered to Fisher 344 rats (200–250 gm) daily for 3 days as described previously (Rama Rao et al, 2010). Animals were killed 4 –6 h after the third injection of TAA, at which time the animals exhibited the symptoms of Grade IV/V encephalopathy. Brain ATP levels were determined by colorimetric method (BioVision Mountain View, CA). We found a 32% reduction in ATP levels in brain of TAA-treated rats as compared to controls (3.2 vs. 2.2 µmoles/gm wet wt in TAA)
It is possible that some of the cerebral energy metabolic disturbances in ALF including the reduction in brain ATP levels are a consequence of the mPT, since our recent studies demonstrate induction of the mPT in brains of rats with ALF (Grade IV/V encephalopathy) induced by administration of TAA along with a commensurate reduction in brain levels of ATP (noted just above).
Moreover, our recent unpublished observation shows that CsA, an inhibitor of the mPT, partially diminished brain edema in rats with ALF induced by hepatotoxin thioacetamide strongly suggesting that cerebral energy metabolic failure contributes to the brain edema in ALF. The involvement of energy failure in the development of brain edema is not surprising as cell volume regulation is an energy-dependent process due to operation of Na+-K+-Cl− co-transporters Na+/H+ exchangers, and extrusion of osmotically active amino acids (Kimelberg and Mongin, 1998) all of which require energy (Kimelberg, 1995). Studies have indeed demonstrated a close correlation between energy metabolism and cell volume regulation in cultured astrocytes (Olson et al., 1992). It is likely that mitochondrial dysfunction resulting from the mPT represents a critical factor in the development of brain edema in ALF. It is possible “energy enhancing” compounds (e.g. L-carnitine; acetyl-L-carnitine, and creatine), that have been shown to improve neurological functions in chronic HE and hyperammonemia (see Discussion below), may also mitigate the brain edema in ALF.
Concluding remarks/perspectives
Studies performed over the past four decades utilizing in vitro and in vivo models of hepatic encephalopathy and hyperammonemia have disclosed disturbances in various pathways of cerebral energy metabolism. Accordingly, cerebral metabolic rate for glucose was shown to be increased in animal models of HE and hyperammonemia. Likewise, increased rate of glycolysis by ammonia was reported to be due to activation of phosphofructokinase, aldolase, pyruvate kinase and glyceraldehydes-3-phosphate dehydrogenase. While increased glycolysis in HE and hyperammonemia was predicted to enhance the operational rate of TCA cycle, this did not occur as the pyruvate generated from glycolysis was converted to lactate. This conversion was due to the inability of pyruvate to be channeled into the TCA cycle as PDH was shown to be inhibited by ammonia. Additionally, ammonia can inhibit α-KGDH and malate dehydrogenase activities, which might interfere with the transfer of reducing equivalents through malate-aspartate shuttle. Inhibition of the TCA cycle by ammonia likely leads to decreased mitochondrial oxidative phosphorylation resulting in a depletion of ATP and other high energy metabolites in hyperammonemic animals.
It must be noted, however, that inconsistencies exist with regard to changes in energy metabolism in HE and hyperammonemia. As summarized in the Table, conflicting results were reported with regard to CMRglc, glycolysis, TCA cycle, as well as with regard to the status of high energy metabolites in HE and hyperammonemia. While reasons for inconsistent results reported are not completely clear, various studies examined different brain regions for changes in energy metabolism. The interpretation of these findings is thus complicated by regional heterogeneity in energy metabolism observed in HE and hyperammonemia. Additionally, since the majority of studies were performed in vivo employing different animal models of HE and hyperammonemia, the precise neural cell vulnerable to changes in the energy metabolism in these studies, for the most part, is unknown. Resolution of these inconsistencies will require an analysis of various pathways of energy metabolism, principally by the use of primary cultures of different neural cells.
Studies reporting that “energy enhancing” agents improve the clinical status, neuropsychiatric and behavioral abnormalities in patients and experimental animals with HE and hyperammonemia potentially highlights the importance of cerebral energy metabolism in the pathogenesis of HE and other hyperammonemic disorders. Thus, L-carnitine (L-3-hydroxy-4-aminobutyrobetaine), a naturally occurring amino acid derivative, completely blocked the mortality in mice with acute hyperammonemia (O'Connor et al., 1984b), and diminished the rise in cerebral lactate levels as well as enhanced pyruvate concentrations in mice with acute hyperammonemia (O'Connor et al., 1984a). Subsequent studies showed that L-carnitine significantly improved cerebral energy metabolism in spf-mice by mitigating the reduction in the levels of pyruvate, free coenzyme A, ADP and ATP (Ratnakumari et al., 1993). Acetyl-L-carnitine (ALC), an acetylated derivative of carnitine, has also been shown to improve cerebral energy metabolism by activating enzymes of the electron transport chain, and by enhancing brain levels of ATP (Rao et al., 1997; Qureshi et al., 1998) in spf-mice. Consistent with these findings, recent studies have reported that treatment with L-carnitine or ALC improved cognitive functions and reduced behavioral abnormalities in patients with HE (Malaguarnera et al., 2008; Malaguarnera et al. 2010a; Malaguarnera et al. 2010b). In addition to improving the energy metabolism, carnitine inhibited the activation of the NMDA sub-type of glutamate receptor in rats with acute hyperammonemia and in rats with HE created by portacaval shunts (Felipo et al., 1993; Therrien et al., 1997), as well as in spf-mice (Rao and Qureshi, 1999).
In summary, in vivo studies employing various animal models, along with limited in vitro studies using cultured neurons and astrocytes treated with ammonia, demonstrates that cerebral energy metabolism is altered in both acute and chronic models of HE and hyperammonemia. Studies showing the induction of the mPT in ammonia-treated cultured astrocytes, as well as in brains of rats with ALF suggest that the mPT plays a crucial role in the bioenergetic failure associated with HE and hyperammonemia. Consistent with this view, recent studies have shown that the mPT contributes to astrocyte swelling/brain edema in ALF. Additionally, oxidative/nitrosative stress (ONS), a cause and consequence of bioenergetic failure, has also been strongly implicated in astrocyte swelling. Targeting bioenergetic failure represents a potentially useful approach for the treatment of HE and other hyperammonemic disorders.
Research Highlights.
Changes in brain energy metabolism in HE and hyperammonemia have been highlighted in this review.
The role of mitochondrial permeability transition in the pathogenesis of HE has been emphasized.
Impact of altered bioenergetics on brain edema in acute liver failure has also been discussed.
Acknowledgements
This work was supported by grants from the Department of Veterans Affairs Merit review and the NIH (DK063311).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrahams SL, Younathan ES. Modulation of the kinetic properties of phosphofructokinase by ammonium ions. J Biol Chem. 1971;246:2464–2467. [PubMed] [Google Scholar]
- Abramov AY, Canevari L, Duchen MR. Beta-amyloid peptides induce mitochondrial dysfunction and oxidative stress in astrocytes and death of neurons through activation of NADPH oxidase. J Neurosci. 2004;24:565–575. doi: 10.1523/JNEUROSCI.4042-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht J, Norenberg MD. Glutamine: a Trojan horse in ammonia neurotoxicity. Hepatology. 2006;44:788–794. doi: 10.1002/hep.21357. [DOI] [PubMed] [Google Scholar]
- Alvarez-Leefmans FJ, Herrera-Perez JJ, Marquez MS, Blanco VM. Simultaneous measurement of water volume and pH in single cells using BCECF and fluorescence imaging microscopy. Biophys J. 2006;90:608–618. doi: 10.1529/biophysj.105.069450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez VM, Rama Rao KV, Brahmbhatt M, Norenberg MD. Interaction between cytokines and ammonia in the mitochondrial permeability transition in cultured astrocytes. J Neurosci Res. 2011 doi: 10.1002/jnr.22708. (in press) [DOI] [PubMed] [Google Scholar]
- Andersson AK, Adermark L, Persson M, Westerlund A, Olsson T, Hansson E. Lactate contributes to ammonia-mediated astroglial dysfunction during hyperammonemia. Neurochem Res. 2009;34:556–565. doi: 10.1007/s11064-008-9819-1. [DOI] [PubMed] [Google Scholar]
- Astore D, Boicelli CA. Hyperammonemia and chronic hepatic encephalopathy: an in vivo PMRS study of the rat brain. MAGMA. 2000;10:160–166. doi: 10.1007/BF02590641. [DOI] [PubMed] [Google Scholar]
- Bai G, Rama Rao KV, Murthy CR, Panickar KS, Jayakumar AR, Norenberg MD. Ammonia induces the mitochondrial permeability transition in primary cultures of rat astrocytes. J Neurosci Res. 2001;66:981–991. doi: 10.1002/jnr.10056. [DOI] [PubMed] [Google Scholar]
- Baraona E, Salinas A, Navia E, Orrego H. Alterations of Ammonia Metabolism in the Cerebral Cortex of Rats with Hepatic Damage Induced by Carbon Tetrachloride. Clin Sci. 1965;28:201–208. [PubMed] [Google Scholar]
- Bates TE, Williams SR, Kauppinen RA, Gadian DG. Observation of cerebral metabolites in an animal model of acute liver failure in vivo: a 1H and 31P nuclear magnetic resonance study. J Neurochem. 1989;53:102–110. doi: 10.1111/j.1471-4159.1989.tb07300.x. [DOI] [PubMed] [Google Scholar]
- Bernal W, Donaldson N, Wyncoll D, Wendon J. Blood lactate as an early predictor of outcome in paracetamol-induced acute liver failure: a cohort study. Lancet. 2002;359:558–563. doi: 10.1016/S0140-6736(02)07743-7. [DOI] [PubMed] [Google Scholar]
- Bernardi P, Colonna R, Costantini P, Eriksson O, Fontaine E, Ichas F, Massari S, Nicolli A, Petronilli V, Scorrano L. The mitochondrial permeability transition. Biofactors. 1998;8:273–281. doi: 10.1002/biof.5520080315. [DOI] [PubMed] [Google Scholar]
- Bessman SP, Bessman AN. The cerebral and peripheral uptake of ammonia in liver disease with an hypothesis for the mechanism of hepatic coma. J Clin Invest. 1955;34:622–628. doi: 10.1172/JCI103111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boer LA, Panatto JP, Fagundes DA, Bassani C, Jeremias IC, Daufenbach JF, Rezin GT, Constantino L, Dal-Pizzol F, Streck EL. Inhibition of mitochondrial respiratory chain in the brain of rats after hepatic failure induced by carbon tetrachloride is reversed by antioxidants. Brain Res Bull. 2009;80:75–78. doi: 10.1016/j.brainresbull.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Bolãnos JP, Peuchen S, Heales SJ, Land JM, Clark JB. Nitric oxide-mediated inhibition of the mitochondrial respiratory chain in cultured astrocytes. J Neurochem. 1994;63:910–916. doi: 10.1046/j.1471-4159.1994.63030910.x. [DOI] [PubMed] [Google Scholar]
- Brusilow SW. Hyperammonemic encephalopathy. Medicine (Baltimore) 2002;81:240–249. doi: 10.1097/00005792-200205000-00007. [DOI] [PubMed] [Google Scholar]
- Butterworth RF. Pathophysiology of hepatic encephalopathy: a new look at ammonia. Metab Brain Dis. 2002;17:221–227. doi: 10.1023/a:1021989230535. [DOI] [PubMed] [Google Scholar]
- Canevari L, Abramov AY, Duchen MR. Toxicity of amyloid beta peptide: tales of calcium, mitochondria, and oxidative stress. Neurochem Res. 2004;29:637–650. doi: 10.1023/b:nere.0000014834.06405.af. [DOI] [PubMed] [Google Scholar]
- Capocaccia L, Angelico M. Fulminant hepatic failure. Clinical features, etiology, epidemiology, and current management. Dig Dis Sci. 1991;36:775–779. doi: 10.1007/BF01311236. [DOI] [PubMed] [Google Scholar]
- Castilho RF, Kowaltowski AJ, Meinicke AR, Bechara EJ, Vercesi AE. Permeabilization of the inner mitochondrial membrane by Ca2+ ions is stimulated by t-butyl hydroperoxide and mediated by reactive oxygen species generated by mitochondria. Free Radic Biol Med. 1995;18:479–486. doi: 10.1016/0891-5849(94)00166-h. [DOI] [PubMed] [Google Scholar]
- Chatauret N, Zwingmann C, Rose C, Leibfritz D, Butterworth RF. Effects of hypothermia on brain glucose metabolism in acute liver failure: a H/C-nuclear magnetic resonance study. Gastroenterology. 2003;125:815–824. doi: 10.1016/s0016-5085(03)01054-0. [DOI] [PubMed] [Google Scholar]
- Cruz NF, Dienel GA. Brain glucose levels in portacaval-shunted rats with chronic, moderate hyperammonemia: implications for determination of local cerebral glucose utilization. J Cereb Blood Flow Metab. 1994;14:113–124. doi: 10.1038/jcbfm.1994.16. [DOI] [PubMed] [Google Scholar]
- Cruz NF, Duffy TE. Local cerebral glucose metabolism in rats with chronic portacaval shunts. J Cereb Blood Flow Metab. 1983;3:311–320. doi: 10.1038/jcbfm.1983.46. [DOI] [PubMed] [Google Scholar]
- Felipo V, Grau E, Minana MD, Grisolia S. Ammonium injection induces an N-methyl-D-aspartate receptor-mediated proteolysis of the microtubule-associated protein MAP-2. J Neurochem. 1993;60:1626–1630. doi: 10.1111/j.1471-4159.1993.tb13384.x. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick SM, Cooper AJ, Hertz L. Effects of ammonia and beta-methylene-DL-aspartate on the oxidation of glucose and pyruvate by neurons and astrocytes in primary culture. J Neurochem. 1988;51:1197–1203. doi: 10.1111/j.1471-4159.1988.tb03087.x. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick SM, Hetherington HP, Behar KL, Shulman RG. Effects of acute hyperammonemia on cerebral amino acid metabolism and pHi in vivo, measured by 1H and 31P nuclear magnetic resonance. J Neurochem. 1989;52:741–749. doi: 10.1111/j.1471-4159.1989.tb02517.x. [DOI] [PubMed] [Google Scholar]
- Gutierrez J, Ballinger SW, Darley-Usmar VM, Landar A. Free radicals, mitochondria, and oxidized lipids: the emerging role in signal transduction in vascular cells. Circ Res. 2006;99:924–932. doi: 10.1161/01.RES.0000248212.86638.e9. [DOI] [PubMed] [Google Scholar]
- Haghighat N, McCandless DW, Geraminegad P. The effect of ammonium chloride on metabolism of primary neurons and neuroblastoma cells in vitro. Metab Brain Dis. 2000;15:151–162. doi: 10.1007/BF02679981. [DOI] [PubMed] [Google Scholar]
- Halestrap AP, Woodfield KY, Connern CP. Oxidative stress, thiol reagents, and membrane potential modulate the mitochondrial permeability transition by affecting nucleotide binding to the adenine nucleotide translocase. J Biol Chem. 1997;272:3346–3354. doi: 10.1074/jbc.272.6.3346. [DOI] [PubMed] [Google Scholar]
- Häussinger D, Görg B, Reinehr R, Schliess F. Protein tyrosine nitration in hyperammonemia and hepatic encephalopathy. Metab Brain Dis. 2005;20:285–294. doi: 10.1007/s11011-005-7908-2. [DOI] [PubMed] [Google Scholar]
- Häussinger D SF. Pathogenetic mechanisms of hepatic encephalopathy. Gut. 2011;57:1156–1165. doi: 10.1136/gut.2007.122176. [DOI] [PubMed] [Google Scholar]
- Hawkins RA, Jessy J. Hyperammonaemia does not impair brain function in the absence of net glutamine synthesis. Biochem J. 1991;277:697–703. doi: 10.1042/bj2770697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins RA, Mans AM. Cerebral function in hepatic encephalopathy. Adv Exp Med Biol. 1990;272:1–22. doi: 10.1007/978-1-4684-5826-8_1. [DOI] [PubMed] [Google Scholar]
- Hawkins RA, Miller AL, Nielsen RC, Veech RL. The acute action of ammonia on rat brain metabolism in vivo. Biochem J. 1973;134:1001–1008. doi: 10.1042/bj1341001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazell AS, Butterworth RF. Hepatic encephalopathy: An update of pathophysiologic mechanisms. Proc Soc Exp Biol Med. 1999;222:99–112. doi: 10.1046/j.1525-1373.1999.d01-120.x. [DOI] [PubMed] [Google Scholar]
- Higuchi H, Adachi M, Miura S, Gores GJ, Ishii H. The mitochondrial permeability transition contributes to acute ethanol-induced apoptosis in rat hepatocytes. Hepatology. 2001;34:320–328. doi: 10.1053/jhep.2001.26380. [DOI] [PubMed] [Google Scholar]
- Hindfelt B. The effect of sustained hyperammonemia upon the metabolic state of the brain. Scand J Clin Lab Invest. 1972;30:245–255. doi: 10.3109/00365517209084286. [DOI] [PubMed] [Google Scholar]
- Hindfelt B, Plum F, Duffy TE. Effect of acute ammonia intoxication on cerebral metabolism in rats with portacaval shunts. J Clin Invest. 1977;59:386–396. doi: 10.1172/JCI108651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindfelt B, Siesjo BK. The effect of ammonia on the energy metabolism of the rat brain. Life Sci II. 1970;9:1021–1028. doi: 10.1016/0024-3205(70)90010-x. [DOI] [PubMed] [Google Scholar]
- Hindfelt B, Siesjo BK. Cerebral effects of acute ammonia intoxication. II. The effect upon energy metabolism. Scand J Clin Lab Invest. 1971;28:365–374. doi: 10.3109/00365517109095711. [DOI] [PubMed] [Google Scholar]
- Hourani BT, Hamlin EM, Reynolds TB. Cerebrospinal fluid glutamine as a measure of hepatic encephalopathy. Arch Intern Med. 1971;127:1033–1036. [PubMed] [Google Scholar]
- Ichas F, Jouaville LS, Sidash SS, Mazat JP, Holmuhamedov EL. Mitochondrial calcium spiking: a transduction mechanism based on calcium-induced permeability transition involved in cell calcium signalling. FEBS Lett. 1994;348:211–215. doi: 10.1016/0014-5793(94)00615-6. [DOI] [PubMed] [Google Scholar]
- Itzhak Y, Norenberg MD. Ammonia-induced upregulation of peripheral-type benzodiazepine receptors in cultured astrocytes labeled with [3H]PK 11195. Neurosci Lett. 1994;177:35–38. doi: 10.1016/0304-3940(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Jalan R, Olde Damink SW, Hayes PC, Deutz NE, Lee A. Pathogenesis of intracranial hypertension in acute liver failure: inflammation, ammonia and cerebral blood flow. J Hepatol. 2004;41:613–620. doi: 10.1016/j.jhep.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Jalan R, Williams R. The inflammatory basis of intracranial hypertension in acute liver failure. J Hepatol. 2001;34:940–942. doi: 10.1016/s0168-8278(01)00038-1. [DOI] [PubMed] [Google Scholar]
- Jayakumar AR, Panickar KS, Norenberg MD. Effects on free radical generation by ligands of the peripheral benzodiazepine receptor in cultured neural cells. J Neurochem. 2002;83:1226–1234. doi: 10.1046/j.1471-4159.2002.01261.x. [DOI] [PubMed] [Google Scholar]
- Jayakumar AR, Rama Rao KV, Tong XY, Norenberg MD. Calcium in the mechanism of ammonia-induced astrocyte swelling. J Neurochem. 2009;109(Suppl 1):252–257. doi: 10.1111/j.1471-4159.2009.05842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayakumar AR, Norenberg MD. Oxidative/nitrative stress in hepatic encephalopathy. In: Mullen K PR, editor. Hepatic Encephalopathy. Springer; 2011. (in press) [Google Scholar]
- Jiang W, Desjardins P, Butterworth RF. Direct evidence for central proinflammatory mechanisms in rats with experimental acute liver failure: protective effect of hypothermia. J Cereb Blood Flow Metab. 2009;29:944–952. doi: 10.1038/jcbfm.2009.18. [DOI] [PubMed] [Google Scholar]
- Jones EA, Weissenborn K. Neurology and the liver. J Neurol Neurosurg Psychiatry. 1997;63:279–293. doi: 10.1136/jnnp.63.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kala G, Hertz L. Ammonia effects on pyruvate/lactate production in astrocytes interaction with glutamate. Neurochem Int. 2005;47:4–12. doi: 10.1016/j.neuint.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Kaminsky Y, Kosenko E. AMP deaminase and adenosine deaminase activities in liver and brain regions in acute ammonia intoxication and subacute toxic hepatitis. Brain Res. 2010;1311:175–181. doi: 10.1016/j.brainres.2009.10.073. [DOI] [PubMed] [Google Scholar]
- Katanuma NO, M Nishii Y. Regulation of urea cycle and TCA cycle by ammonia. Advances in Enzyme Regulation. 1966;Vol. 4:317–335. doi: 10.1016/0065-2571(66)90025-2. [DOI] [PubMed] [Google Scholar]
- Kato M, Sugihara J, Nakamura T, Muto Y. Electron microscopic study of the blood-brain barrier in rats with brain edema and encephalopathy due to acute hepatic failure. Gastroenterol Jpn. 1989;24:135–142. doi: 10.1007/BF02774187. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK. Current concepts of brain edema. Review of laboratory investigations. J Neurosurg. 1995;83:1051–1059. doi: 10.3171/jns.1995.83.6.1051. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK, Mongin AA. Swelling-activated release of excitatory amino acids in the brain: relevance for pathophysiology. Contrib Nephrol. 1998;123:240–257. doi: 10.1159/000059916. [DOI] [PubMed] [Google Scholar]
- Kosenko E, Felipo V, Montoliu C, Grisolia S, Kaminsky Y. Effects of acute hyperammonemia in vivo on oxidative metabolism in nonsynaptic rat brain mitochondria. Metab Brain Dis. 1997;12:69–82. doi: 10.1007/BF02676355. [DOI] [PubMed] [Google Scholar]
- Kosenko E, Kaminsky Y, Grau E, Minana MD, Marcaida G, Grisolia S, Felipo V. Brain ATP depletion induced by acute ammonia intoxication in rats is mediated by activation of the NMDA receptor and Na+,K+-ATPase. J Neurochem. 1994;63:2172–2178. doi: 10.1046/j.1471-4159.1994.63062172.x. [DOI] [PubMed] [Google Scholar]
- Kosenko EA, Kaminsky YG. Activation of AMP deaminase and adenosine deaminase in the liver during ammonia poisoning and hepatitis. Bull Exp Biol Med. 2011;150:36–38. doi: 10.1007/s10517-010-1061-6. [DOI] [PubMed] [Google Scholar]
- Kristal BS, Dubinsky JM. Mitochondrial permeability transition in the central nervous system: induction by calcium cycling-dependent and -independent pathways. J Neurochem. 1997;69:524–538. doi: 10.1046/j.1471-4159.1997.69020524.x. [DOI] [PubMed] [Google Scholar]
- Lai JC, Cooper AJ. Neurotoxicity of ammonia and fatty acids: differential inhibition of mitochondrial dehydrogenases by ammonia and fatty acyl coenzyme A derivatives. Neurochem Res. 1991;16:795–803. doi: 10.1007/BF00965689. [DOI] [PubMed] [Google Scholar]
- Lavoie J, Giguere JF, Layrargues GP, Butterworth RF. Activities of neuronal and astrocytic marker enzymes in autopsied brain tissue from patients with hepatic encephalopathy. Metab Brain Dis. 1987;2:283–290. doi: 10.1007/BF00999698. [DOI] [PubMed] [Google Scholar]
- Lee WM. Acute liver failure. Am J Med. 1994;96:3S–9S. doi: 10.1016/0002-9343(94)90183-x. [DOI] [PubMed] [Google Scholar]
- Leke R, Bak LK, Anker M, Melo TM, Sorensen M, Keiding S, Vilstrup H, Ott P, Portela LV, Sonnewald U, Schousboe A, Waagepetersen HS. Detoxification of ammonia in mouse cortical GABAergic cell cultures increases neuronal oxidative metabolism and reveals an emerging role for release of glucose-derived alanine. Neurotox Res. 2011;19:496–510. doi: 10.1007/s12640-010-9198-7. [DOI] [PubMed] [Google Scholar]
- Lin S, Raabe W. Ammonia intoxication: effects on cerebral cortex and spinal cord. J Neurochem. 1985;44:1252–1258. doi: 10.1111/j.1471-4159.1985.tb08751.x. [DOI] [PubMed] [Google Scholar]
- Llansola M, Bosca L, Felipo V, Hortelano S. Ammonia prevents glutamate-induced but not low K+-induced apoptosis in cerebellar neurons in culture. Neuroscience. 2003;117:899–907. doi: 10.1016/s0306-4522(02)00957-0. [DOI] [PubMed] [Google Scholar]
- Lockwood AH, Ginsberg MD, Rhoades HM, Gutierrez MT. Cerebral glucose metabolism after portacaval shunting in the rat. Patterns of metabolism and implications for the pathogenesis of hepatic encephalopathy. J Clin Invest. 1986;78:86–95. doi: 10.1172/JCI112578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomneth R, Medrano S, Gruenstein EI. The role of transmembrane pH gradients in the lactic acid induced swelling of astrocytes. Brain Res. 1990;523:69–77. doi: 10.1016/0006-8993(90)91636-u. [DOI] [PubMed] [Google Scholar]
- Malaguarnera M, Bella R, Vacante M, Giordano M, Malaguarnera G, Gargante MP, Motta M, Mistretta A, Rampello L, Pennisi G. Acetyl-l-carnitine reduces depression and improves quality of life in patients with minimal hepatic encephalopathy. Scand J Gastroenterol. 2011;46:750–759. doi: 10.3109/00365521.2011.565067. [DOI] [PubMed] [Google Scholar]
- Malaguarnera M, Gargante MP, Cristaldi E, Vacante M, Risino C, Cammalleri L, Pennisi G, Rampello L. Acetyl-L-carnitine treatment in minimal hepatic encephalopathy. Dig Dis Sci. 2008;53:3018–3025. doi: 10.1007/s10620-008-0238-6. [DOI] [PubMed] [Google Scholar]
- Malaguarnera M, Vacante M, Giordano M, Pennisi G, Bella R, Rampello L, Malaguarnera M, Li Volti G, Galvano F. Oral acetyl-L-carnitine therapy reduces fatigue in overt hepatic encephalopathy: a randomized, double-blind, placebo-controlled study. Am J Clin Nutr. 2011;93:799–808. doi: 10.3945/ajcn.110.007393. [DOI] [PubMed] [Google Scholar]
- Mans AM, DeJoseph MR, Hawkins RA. Metabolic abnormalities and grade of encephalopathy in acute hepatic failure. J Neurochem. 1994;63:1829–1838. doi: 10.1046/j.1471-4159.1994.63051829.x. [DOI] [PubMed] [Google Scholar]
- Martinez-Hernandez A, Bell KP, Norenberg MD. Glutamine synthetase: glial localization in brain. Science. 1977;195:1356–1358. doi: 10.1126/science.14400. [DOI] [PubMed] [Google Scholar]
- Martinez A. Electron microscopy in human hepatic encephalopathy. Acta Neuropathol (Berl) 1968;11:82–86. doi: 10.1007/BF00692797. [DOI] [PubMed] [Google Scholar]
- McCandless DW, Schenker S. Effect of acute ammonia intoxication on energy stores in the cerebral reticular activating system. Exp Brain Res. 1981;44:325–330. doi: 10.1007/BF00236570. [DOI] [PubMed] [Google Scholar]
- McConnell JR, Antonson DL, Ong CS, Chu WK, Fox IJ, Heffron TG, Langnas AN, Shaw BW., Jr Proton spectroscopy of brain glutamine in acute liver failure. Hepatology. 1995;22:69–74. [PubMed] [Google Scholar]
- McKhann GM, Tower DB. Ammonia toxicity and cerebral oxidative metabolism. Am J Physiol. 1961;200:420–424. doi: 10.1152/ajplegacy.1961.200.3.420. [DOI] [PubMed] [Google Scholar]
- Muntz JA, Hurwitz J. Effect of potassium and ammonium ions upon glycolysis catalyzed by an extract of rat brain. Arch Biochem Biophys. 1951;32:124–136. doi: 10.1016/0003-9861(51)90246-9. [DOI] [PubMed] [Google Scholar]
- Murthy CR, Rama Rao KV, Bai G, Norenberg MD. Ammonia-induced production of free radicals in primary cultures of rat astrocytes. J Neurosci Res. 2001;66:282–288. doi: 10.1002/jnr.1222. [DOI] [PubMed] [Google Scholar]
- Norenberg MD. The role of astrocytes in hepatic encephalopathy. Neurochem Pathol. 1987;6:13–33. doi: 10.1007/BF02833599. [DOI] [PubMed] [Google Scholar]
- Norenberg MD, Rama Rao KV, Jayakumar AR. The mitochondrial permeability transition in ammonia neurotoxicity. In: Jones E, Meijer AJ, Chamuleau AF, editors. Encephalopathy and Nitrogen Metabolism in Liver Failure. Dordrecht: Kluwer; 2003a. pp. 267–286. [Google Scholar]
- Norenberg MD. Oxidative and nitrosative stress in ammonia neurotoxicity. Hepatology. 2003b;37:245–248. doi: 10.1053/jhep.2003.50087. [DOI] [PubMed] [Google Scholar]
- Norenberg MD, Jayakumar AR, Rama Rao KV, Panickar KS. The peripheral benzodiazepine receptor and neurosteroids in the pathogenesis of hepatic encephalopathy and ammonia neurotoxicity. In: Häussinger D, KIrcheis G, Schliess F, editors. Hepatic Encephalopathy and Nitrogen Metabolism. Dordtrecht: Springer; 2006. pp. 143–160. [Google Scholar]
- Norenberg MD, Jayakumar AR, Rama Rao KV. Oxidative stress in the pathogenesis of hepatic encephalopathy. Metab Brain Dis. 2004a;19:313–329. doi: 10.1023/b:mebr.0000043978.91675.79. [DOI] [PubMed] [Google Scholar]
- Norenberg MD, Mozes LW, Gregorios JB, Norenberg LO. Effects of lactic acid on astrocytes in primary culture. J Neuropathol Exp Neurol. 1987;46:154–166. doi: 10.1097/00005072-198703000-00004. [DOI] [PubMed] [Google Scholar]
- Norenberg MD, Rao KV. The mitochondrial permeability transition in neurologic disease. Neurochem Int. 2007;50:983–997. doi: 10.1016/j.neuint.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Beirne JP, Chouhan M, Hughes RD. The role of infection and inflammation in the pathogenesis of hepatic encephalopathy and cerebral edema in acute liver failure. Nat Clin Pract Gastroenterol Hepatol. 2006;3:118–119. doi: 10.1038/ncpgasthep0417. [DOI] [PubMed] [Google Scholar]
- O'Connor JE, Costell M, Grisolia S. Prevention of ammonia toxicity by L-carnitine: metabolic changes in brain. Neurochem Res. 1984a;9:563–570. doi: 10.1007/BF00964383. [DOI] [PubMed] [Google Scholar]
- O'Connor JE, Costell M, Grisolia S. Protective effect of L-carnitine on hyperammonemia. FEBS Lett. 1984b;166:331–334. doi: 10.1016/0014-5793(84)80106-4. [DOI] [PubMed] [Google Scholar]
- O'Grady JG, Williams R. Management of acute liver failure. Schweiz Med Wochenschr. 1986;116:541–544. [PubMed] [Google Scholar]
- Olson JE, Evers JA, Holtzman D. Astrocyte volume regulation and ATP and phosphocreatine concentrations after exposure to salicylate, ammonium, and fatty acids. Metab Brain Dis. 1992;7:183–196. doi: 10.1007/BF01000245. [DOI] [PubMed] [Google Scholar]
- Ott P, Clemmesen O, Larsen FS. Cerebral metabolic disturbances in the brain during acute liver failure: from hyperammonemia to energy failure and proteolysis. Neurochem Int. 2005;47:13–18. doi: 10.1016/j.neuint.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Pacheco GS, Panatto JP, Fagundes DA, Scaini G, Bassani C, Jeremias IC, Rezin GT, Constantino L, Dal-Pizzol F, Streck EL. Brain creatine kinase activity is inhibited after hepatic failure induced by carbon tetrachloride or acetaminophen. Metab Brain Dis. 2009;24:383–394. doi: 10.1007/s11011-009-9143-8. [DOI] [PubMed] [Google Scholar]
- Panickar KS, Jayakumar AR, Rama Rao KV, Norenberg MD. Downregulation of the 18-kDa translocator protein: effects on the ammonia-induced mitochondrial permeability transition and cell swelling in cultured astrocytes. Glia. 2007;55:1720–1727. doi: 10.1002/glia.20584. [DOI] [PubMed] [Google Scholar]
- Papadopoulos V, Guarneri P, Kreuger KE, Guidotti A, Costa E. Pregnenolone biosynthesis in C6-2B glioma cell mitochondria: regulation by a mitochondrial diazepam binding inhibitor receptor. Proc Natl Acad Sci U S A. 1992;89:5113–5117. doi: 10.1073/pnas.89.11.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellerin L, Pellegri G, Bittar PG, Charnay Y, Bouras C, Martin JL, Stella N, Magistretti PJ. Evidence supporting the existence of an activity-dependent astrocyte-neuron lactate shuttle. Dev Neurosci. 1998;20:291–299. doi: 10.1159/000017324. [DOI] [PubMed] [Google Scholar]
- Qureshi K, Rao KV, Qureshi IA. Differential inhibition by hyperammonemia of the electron transport chain enzymes in synaptosomes and non-synaptic mitochondria in ornithine transcarbamylase-deficient spf-mice: restoration by acetyl-L-carnitine. Neurochem Res. 1998;23:855–861. doi: 10.1023/a:1022406911604. [DOI] [PubMed] [Google Scholar]
- Rama Rao KV, Chen M, Simard JM, Norenberg MD. Suppression of ammonia-induced astrocyte swelling by cyclosporin A. J Neurosci Res. 2003a;74:891–897. doi: 10.1002/jnr.10755. [DOI] [PubMed] [Google Scholar]
- Rama Rao KV, Jayakumar AR, Norenberg MD. Induction of the mitochondrial permeability transition in cultured astrocytes by glutamine. Neurochem Int. 2003b;43:517–523. doi: 10.1016/s0197-0186(03)00042-1. [DOI] [PubMed] [Google Scholar]
- Rama Rao KV, Jayakumar AR, Norenberg MD. Differential response of glutamine in cultured neurons and astrocytes. J Neurosci Res. 2005a;79:193–199. doi: 10.1002/jnr.20295. [DOI] [PubMed] [Google Scholar]
- Rama Rao KV, Jayakumar AR, Norenberg MD. Role of oxidative stress in the ammonia-induced mitochondrial permeability transition in cultured astrocytes. Neurochem Int. 2005b;47:31–38. doi: 10.1016/j.neuint.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Rama Rao KV, Reddy PVB, Tong X, Norenberg MD. Brain edema in acute liver failure: inhibition by L-histidine. Am J Pathol. 2010;176:1400–1408. doi: 10.2353/ajpath.2010.090756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao KV, Mawal YR, Qureshi IA. Progressive decrease of cerebral cytochrome C oxidase activity in sparse-fur mice: role of acetyl-L-carnitine in restoring the ammonia-induced cerebral energy depletion. Neurosci Lett. 1997;224:83–86. doi: 10.1016/s0304-3940(97)13476-0. [DOI] [PubMed] [Google Scholar]
- Rao KV, Qureshi IA. Reduction in the MK-801 binding sites of the NMDA sub-type of glutamate receptor in a mouse model of congenital hyperammonemia: prevention by acetyl-L-carnitine. Neuropharmacology. 1999;38:383–394. doi: 10.1016/s0028-3908(98)00160-9. [DOI] [PubMed] [Google Scholar]
- Rao VL. Nitric oxide in hepatic encephalopathy and hyperammonemia. Neurochem Int. 2002;41:161–170. doi: 10.1016/s0197-0186(02)00038-4. [DOI] [PubMed] [Google Scholar]
- Ratnakumari L, Murthy CR. Activities of pyruvate dehydrogenase, enzymes of citric acid cycle, and aminotransferases in the subcellular fractions of cerebral cortex in normal and hyperammonemic rats. Neurochem Res. 1989;14:221–228. doi: 10.1007/BF00971314. [DOI] [PubMed] [Google Scholar]
- Ratnakumari L, Murthy CR. In vitro and in vivo effects of ammonia on glucose metabolism in the astrocytes of rat cerebral cortex. Neurosci Lett. 1992;148:85–88. doi: 10.1016/0304-3940(92)90810-t. [DOI] [PubMed] [Google Scholar]
- Ratnakumari L, Murthy CR. Response of rat cerebral glycolytic enzymes to hyperammonemic states. Neurosci Lett. 1993;161:37–40. doi: 10.1016/0304-3940(93)90134-7. [DOI] [PubMed] [Google Scholar]
- Ratnakumari L, Qureshi IA, Butterworth RF. Effects of congenital hyperammonemia on the cerebral and hepatic levels of the intermediates of energy metabolism in spf mice. Biochem Biophys Res Commun. 1992;184:746–751. doi: 10.1016/0006-291x(92)90653-3. [DOI] [PubMed] [Google Scholar]
- Ratnakumari L, Qureshi IA, Butterworth RF. Effect of L-carnitine on cerebral and hepatic energy metabolites in congenitally hyperammonemic sparse-fur mice and its role during benzoate therapy. Metabolism. 1993;42:1039–1046. doi: 10.1016/0026-0495(93)90020-o. [DOI] [PubMed] [Google Scholar]
- Record CO, Buxton B, Chase RA, Curzon G, Murray-Lyon IM, Williams R. Plasma and brain amino acids in fulminant hepatic failure and their relationship to hepatic encephalopathy. Eur J Clin Invest. 1976;6:387–394. doi: 10.1111/j.1365-2362.1976.tb00533.x. [DOI] [PubMed] [Google Scholar]
- Reinehr R, Gorg B, Becker S, Qvartskhava N, Bidmon HJ, Selbach O, Haas HL, Schliess F, Haussinger D. Hypoosmotic swelling and ammonia increase oxidative stress by NADPH oxidase in cultured astrocytes and vital brain slices. Glia. 2007;55:758–771. doi: 10.1002/glia.20504. [DOI] [PubMed] [Google Scholar]
- Rose C. Effect of ammonia on astrocytic glutamate uptake/release mechanisms. J Neurochem. 2006;97(Suppl 1):11–15. doi: 10.1111/j.1471-4159.2006.03796.x. [DOI] [PubMed] [Google Scholar]
- Rose C, Ytrebo LM, Davies NA, Sen S, Nedredal GI, Belanger M, Revhaug A, Jalan R. Association of reduced extracellular brain ammonia, lactate, and intracranial pressure in pigs with acute liver failure. Hepatology. 2007;46:1883–1892. doi: 10.1002/hep.21877. [DOI] [PubMed] [Google Scholar]
- Schliess F, Görg B, Häussinger D. Pathogenetic interplay between osmotic and oxidative stress: the hepatic encephalopathy paradigm. Biol Chem. 2006;387:1363–1370. doi: 10.1515/BC.2006.171. [DOI] [PubMed] [Google Scholar]
- Schmidt LE, Larsen FS. Prognostic implications of hyperlactatemia, multiple organ failure, and systemic inflammatory response syndrome in patients with acetaminophen-induced acute liver failure. Crit Care Med. 2006;34:337–343. doi: 10.1097/01.ccm.0000194724.70031.b6. [DOI] [PubMed] [Google Scholar]
- Schurr A, Payne RS, Miller JJ, Rigor BM. Brain lactate is an obligatory aerobic energy substrate for functional recovery after hypoxia: further in vitro validation. J Neurochem. 1997;69:423–426. doi: 10.1046/j.1471-4159.1997.69010423.x. [DOI] [PubMed] [Google Scholar]
- Shawcross D, Jalan R. The pathophysiologic basis of hepatic encephalopathy: central role for ammonia and inflammation. Cell Mol Life Sci. 2005;62:2295–2304. doi: 10.1007/s00018-005-5089-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorey J, McCandless DW, Schenker S. Cerebral alpha-ketoglutarate in ammonia intoxication. Gastroenterology. 1967;53:706–711. [PubMed] [Google Scholar]
- Siesjo BK, Plum F. Cerebral energy metabolism in normoxia and in hypoxia. Acta Anaesthesiol Scand Suppl. 1971;45:81–101. doi: 10.1111/j.1399-6576.1971.tb00662.x. [DOI] [PubMed] [Google Scholar]
- Snyder SH, McEnery MW, Verma A. Molecular mechanisms of peripheral benzodiazepine receptors. Neurochem Res. 1990;15:119–123. doi: 10.1007/BF00972201. [DOI] [PubMed] [Google Scholar]
- Staub F, Baethmann A, Peters J, Weigt H, Kempski O. Effects of lactacidosis on glial cell volume and viability. J Cereb Blood Flow Metab. 1990;10:866–876. doi: 10.1038/jcbfm.1990.143. [DOI] [PubMed] [Google Scholar]
- Strauss GI, Moller K, Larsen FS, Kondrup J, Knudsen GM. Cerebral glucose and oxygen metabolism in patients with fulminant hepatic failure. Liver Transpl. 2003;9:1244–1252. doi: 10.1016/j.lts.2003.09.020. [DOI] [PubMed] [Google Scholar]
- Therrien G, Rose C, Butterworth J, Butterworth RF. Protective effect of L-carnitine in ammonia-precipitated encephalopathy in the portacaval shunted rat. Hepatology. 1997;25:551–556. doi: 10.1002/hep.510250310. [DOI] [PubMed] [Google Scholar]
- Tofteng F, Larsen FS. Monitoring extracellular concentrations of lactate, glutamate, and glycerol by in vivo microdialysis in the brain during liver transplantation in acute liver failure. Liver Transpl. 2002;8:302–305. doi: 10.1053/jlts.2002.32283. [DOI] [PubMed] [Google Scholar]
- Tofteng F, Larsen FS. Management of patients with fulminant hepatic failure and brain edema. Metab Brain Dis. 2004;19:207–214. doi: 10.1023/b:mebr.0000043970.34533.04. [DOI] [PubMed] [Google Scholar]
- Traber P, DalCanto M, Ganger D, Blei AT. Effect of body temperature on brain edema and encephalopathy in the rat after hepatic devascularization. Gastroenterology. 1989;96:885–891. [PubMed] [Google Scholar]
- Votyakova TV, Reynolds IJ. Ca2+-induced permeabilization promotes free radical release from rat brain mitochondria with partially inhibited complex I. J Neurochem. 2005;93:526–537. doi: 10.1111/j.1471-4159.2005.03042.x. [DOI] [PubMed] [Google Scholar]
- Walsh TS, McLellan S, Mackenzie SJ, Lee A. Hyperlactatemia and pulmonary lactate production in patients with fulminant hepatic failure. Chest. 1999;116:471–476. doi: 10.1378/chest.116.2.471. [DOI] [PubMed] [Google Scholar]
- Walshe JM, De Carli L, Davidson CS. Some factors influencing cerebral oxidation in relation to hepatic coma. Clin Sci (Lond) 1958;17:11–25. [PubMed] [Google Scholar]
- Wilkinson SP, Arroyo V, Moodie H, Williams R. Proceedings: Endotoxaemia in fulminant hepatic failure. Clin Sci Mol Med. 1974;46:30P–31P. doi: 10.1042/cs046030pb. [DOI] [PubMed] [Google Scholar]
- Williams R. Hepatic encephalopathy. J R Coll Physicians Lond. 1973;8:63–74. [PMC free article] [PubMed] [Google Scholar]
- Williams R, Smith MG. Liver transplantation: a clinical and immunological appraisal. Prog Liver Dis. 1972;4:433–446. [PubMed] [Google Scholar]
- Xue Z, Li B, Gu L, Hu X, Li M, Butterworth RF, Peng L. Increased, Na K-ATPase alpha2 isoform gene expression by ammonia in astrocytes and in brain in vivo. Neurochem Int. 2010;57:395–403. doi: 10.1016/j.neuint.2010.04.014. [DOI] [PubMed] [Google Scholar]
- Zoratti M, Szabo I. The mitochondrial permeability transition. Biochim Biophys Acta. 1995;1241:139–176. doi: 10.1016/0304-4157(95)00003-a. [DOI] [PubMed] [Google Scholar]
- Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial ROS-induced ROS release: an update and review. Biochim Biophys Acta. 2006;1757:509–517. doi: 10.1016/j.bbabio.2006.04.029. [DOI] [PubMed] [Google Scholar]
- Zwingmann C. The anaplerotic flux and ammonia detoxification in hepatic encephalopathy. Metab Brain Dis. 2007;22:235–249. doi: 10.1007/s11011-007-9069-y. [DOI] [PubMed] [Google Scholar]
- Zwingmann C, Chatauret N, Leibfritz D, Butterworth RF. Selective increase of brain lactate synthesis in experimental acute liver failure: results of a [H-C] nuclear magnetic resonance study. Hepatology. 2003a;37:420–428. doi: 10.1053/jhep.2003.50052. [DOI] [PubMed] [Google Scholar]