Abstract
Central line-associated bloodstream infections (CLABSIs) are not easily treated and many catheters (e.g., hemodialysis catheters) are not easily replaced. Biofilms (the source of infection) on catheter surfaces are notoriously difficult to eradicate. We have recently demonstrated that modest elevations of temperature lead to increased staphylococcal susceptibility to vancomycin and significantly soften the biofilm matrix. In this study, using a combination of microbiological, computational, and experimental studies, we demonstrate the efficacy, feasibility, and safety of using heat as an adjuvant treatment for infected hemodialysis catheters. Specifically, we show that treating with heat in the presence of antibiotics led to additive killing of Staphylococcus epidermidis with similar trends seen for Staphylococcus aureus and Klebsiella pneumoniae. The magnitude of temperature elevation required is relatively modest (45-50°C) and similar to that used as an adjuvant to traditional cancer therapy. Using a custom-designed benchtop model of a hemodialysis catheter positioned with tip in the human vena cava as well as computational fluid dynamic simulations we demonstrate that these temperature elevations are likely achievable in situ with minimal increased in overall blood temperature.
Keywords: Catheter associated blood stream infection, Staphylococcus epidermidis, Computational fluid dynamics
INTRODUCTION
Device-related bloodstream infections present a tremendous challenge in delivering complex medical care. Central line-associated bloodstream infections (CLABSIs) are not easily treated and many devices implicated in these infections (e.g., hemodialysis catheters) are not easily replaced. One strategy aimed to reduce the frequency of CLABSIs is the application of antimicrobial agents onto the catheter surface. However, these engineering advancements have not yielded clear clinical benefit.1, 2 Although advancements in surgical insertion procedures and processes have reduced their incidence, CLABSIs remain one of the leading causes of bloodstream infections in the US with over 250,000 infected catheters annually.3, 4 Importantly, the Centers for Disease Control estimate an attributable mortality of 12%-25% for each infection.5 The average associated healthcare cost for a CLABSI is $45,000, contributing to the financial burden on the healthcare system.6 Infected dialysis catheters are a particularly important subset of CLABSI in that these devices are truly life-sustaining and can be difficult to replace, especially in patients that have received multiple prior catheters.
Bacteria associated into sessile communities encapsulated in an extracellular matrix (i.e., a biofilm) on catheter surfaces is at the root of the problem.7 This material is notoriously difficult to eradicate. The immune system has difficulty penetrating the matrix of bacterial and host elements that form these communities, and antibiotic treatment of biofilm-associated bacteria can increase their antibiotic resistance without eradicating the biofilm.8 In some instances, infections can be suppressed but not resolved in situ with long-term antibiotic treatment; ultimately most infected catheters must be removed.9
We have recently become interested in the use of elevated temperature to facilitate the in situ treatment of biofilms. Whole body, regional, and localized hyperthermia (39-45°C) have been effectively used as an adjuvant to chemotherapy and radiation in the treatment of malignancy since the 1970's.10 The primary mechanism for radiofrequency (RF) catheter ablation for the treatment of supraventricular tachyarrhythmia is thermally mediated irreversible tissue injury, which occurs at temperatures greater than 50-55°C11. However, in many circumstances the local temperature near an RF catheter may transiently exceed 70°C.12 These therapies, currently in use clinically, provide a temperature range for the safe use of heat in the treatment of biofilm based infections.
At temperatures comparable to those used adjunctively in the treatment of some malignancies10, 13, staphylococcus species (the most common pathogen associated with CLABSI) show increased susceptibility to vancomycin.14 Additionally, elevated temperature significantly softens the staphylococcal biofilm matrix, raising the possibility that thermal treatment might facilitate mechanical clearing of biofilms without device removal.15, 16 Mechanical debulking of the temperature-softened biofilm also offers the possibility of shortening the diffusion path of drugs and host immune effectors into infected materials, thereby inducing more effective killing.
There are significant technical hurdles to be overcome before elevated temperature might be employed as an adjunct therapy in the treatment of infected dialysis catheters. For instance, the specific location of the biofilm (source of infection) on a dialysis catheter could be the intraluminal surface (typically inoculated from the connecting hub or contaminated fluids) or the extraluminal surface (typically inoculated from the skin penetrating site).17 These two regions are thermodynamically distinct and the methods to provide elevated temperatures to them may be quite different. Among the first ‘proof of concept’ issues to be addressed – and those addressed in the current report -- are to develop a better understanding of the heat transfer characteristics of indwelling catheters and to establish the temperatures necessary to augment antibiotic-driven bacterial killing. Using a combination of computational and experimental fluid dynamics as well as microbiology techniques, we present a case for the efficacy, feasibility, and safety of thermal strategies for the in situ treatment of hemodialysis catheter-associated blood stream infections.
METHODS
Microbiology
Catheter-derived isolates of Staphylococcus epidermidis RP62a, and Staphylococcus aureus ATCC27660 were obtained from American Type Culture Collection. A clinical isolate of Klebsiella pneumonia LM21 was kindly provided by Christiane Forestier18. On the day of experimentation, early-log growth cultures were grown in tryptic soy broth at 37°C from cryopreserved stocks. Bacteria were treated with 2 hours of temperatures from 37°C to 55°C. Near 100% killing was observed at 60°C and therefore this condition was used as a positive control. To look for synergy between elevated temperature and usual antibiotic therapy, vancomycin was added to some replicates of the staphylococcal species and ciprofloxacin was added to some replicates of K. pneumoniae. To establish the appropriate antibiotic concentration, dose responses were established at concentrations spanning the minimum inhibitory concentration (MIC) value for each strain up to 8-fold higher concentration (Supplemental Figure S1). Based on these results, 4, 1, and 0.6 ug/ml were used for S. epidermidis, S. aureus, and K. pneumonia respectively, for heat-antibiotic synergy testing, as at 37°C these concentrations produced only modest killing after 2 hours of exposure.
Cellular viability following heat treatment was assessed using flow cytometry (BAcLight Bacterial Viability and Counting Kit for flow cytometry, Life Technologies). This method uses two nucleic acid dyes to assess total bacterial number (via Syto-9) and number of dead organisms (via propidium iodide, PI). A MoFlo Astrios (Beckman Coulter) cytometer was used according to manufacturer's instructions, and live- and dead gates were established using healthy bacteria grown at 37°C and heat killed (2 hours at 60°C) bacteria. Results were expressed as the percent of live (i.e., PI-negative) cells. At least 10,000 events were collected for every replicate of every condition, and at least 3 between-day replicates were performed for every experiment.
Computational catheter modeling
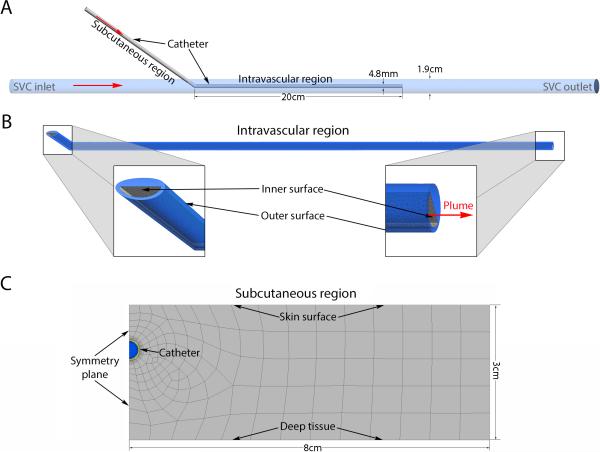
For the purposes of modeling, we considered a standard double-lumen, tunneled hemodialysis catheter positioned with the tip in the superior vena cava (SVC). The catheter was assumed to have two distinct thermal domains, the intravascular region and the tunneled subcutaneous region (Figure 1A). For the intravascular region, steady state 3D temperature profiles were estimated using the computational fluid dynamics (CFD) solver Fluent (Ansys, v15.0, Canonsburg, PA). A simplified geometric model based on the Palindrome hemodialysis catheter (Covidien, Inc.) was designed in AutoCAD (2014, Mill Valley, CA), imported into Ansys Workbench and discretized. Multiple mesh densities were evaluated to confirm mesh independence. Note that only one of the catheter lumens was modeled as a fluid. The other was modeled as solid polyurethane to approximate our bench top experimental validation model where one of the lumens was completely occluded by thermocouples (see below). The SVC inlet boundary condition was a uniform velocity profile corresponding to a flow rate of 2 L/min19 with temperature 37°C. A parametric analysis was performed for a combination of infusion rates (3.5 to 80 ml/min) and infusate temperatures (50° to 70°C) at the catheter inlet. The velocity profile at the catheter inlet was assumed to be uniform. Greater than 10x diameter flow extensions were added to the SVC and catheter entrance to ensure fully developed flow. The SVC outlet boundary was a zero pressure outlet. The working fluid for both the catheter and the SVC was liquid water. The wall of the SVC was considered a nonslip wall with fixed temperature of 37°C. The walls of the catheter were also considered nonslip boundaries. The outer wall of the catheter prior to entering the SVC had a fixed temperature of 37°C. All solid surfaces in the model were considered rigid. The material properties for polyurethane were assumed to be density=1200kg/m3, specific heat=1800J/kg-°C, and thermal conductivity=0.02W/m-°C. Convergence criteria included residuals of continuity, velocity, and energy as well as the average, minimum and maximum temperatures of the outer and inner catheter surface; the average velocity on a midpoint cross section of the model; the maximum temperature of fluid exiting the catheter (plume); and the average temperature of the fluid exiting the SVC outlet (Figure 1B).
Figure 1.
(A) Geometry representation of the computational model. Red arrows indicate direction of flow. (B) Close-up view of intravascular region demonstrating some of the computational mesh and zoomed-in views of specific regions of interest. (C) Close-up view of computational mesh for the subcutaneous region demonstrating catheter in between skin surface and deep tissue.
To estimate the steady state temperature profile of the subcutaneous tissue surrounding a heated, tunneled, dialysis catheter, we took advantage of a midline symmetry plane. The geometry was generated in the Ansys Workbench and discretized for the Fluent CFD solver (Figure 1C). Again multiple mesh densities were tested to confirm mesh independence. The working fluid was water. The outlet had a zero pressure boundary condition. The apparent heat convection coefficient due to natural convection and radiation at the skin surface was assumed to be 10W/m2-°C with surrounding bulk air temperature of 25°C.20 The boundary 3cm deep to the skin was considered core body temperature and was set to a constant 37°C. This assumption has previously been demonstrated to be valid21. The vertical boundaries of the tissue were considered to be sufficiently distant from the region of interest to have zero heat flux. Similar boundary conditions have previously been applied to modeling heat transfer of biological tissue.20 The subcutaneous tissue was modeled as a solid with density=1000kg/m3, specific heat 4200J/kg-°C and thermal conductivity 0.5W/m-°C (ref). To account for heat transfer due to capillary perfusion of the subcutaneous tissue, a heat source term (Q) was added according to:
| (1) |
where ω is the capillary perfusion (0.0005ml/s/ml), ρ is the density of blood (1000kg/m3), c is the specific heat of blood (4200J/kg-°C), Ta is the arterial blood temperature and T is the location specific subcutaneous tissue temperature.22 In this model T will always be greater than or equal to Ta generating a negative heat source term indicating the capacity for capillary perfusion to remove heat. Convergence criteria included residuals of continuity, velocity, and energy as well as average and maximum catheter outer surface temperature.
Experimental Model
A fluid dynamically and thermally representative model of the SVC was developed using an acrylic tube with a diameter of 19.05mm. A centrifugal pump perfused 2.0 L/min of water at 37°C through the model to mimic blood flow through the SVC in an adult.19 The acrylic tube was extended ten times the diameter on both sides of the catheter to ensure fully developed flow in the test region. The catheter was stabilized in the center of the tube with plastic guides. A 14.5 Fr Covidien Palindrome catheter was heated by pumping water at variable volumes (1-100 ml/min) and temperatures (50-65°C) through one of the lumens of the catheter using a peristaltic pump. The pump was controlled with voltage commands through LabVIEW's (National Instruments, 2014, Austin, TX) native proportional-integral-derivative (PID) controller. Temperature readings were acquired by four Heraeus C220 resistance temperature detectors, one of which was free in the SVC while the other three were embedded at equally spaced intervals within the dry lumen of the catheter. SVC temperature, catheter temperatures, and catheter flow volumes were recorded at 1 Hz using National Instruments compactDAQ hardware.
RESULTS
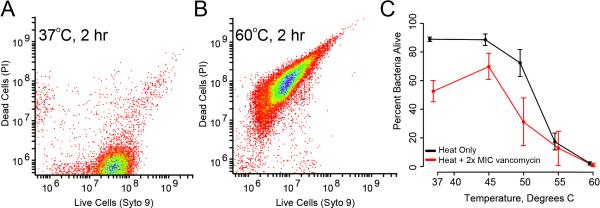
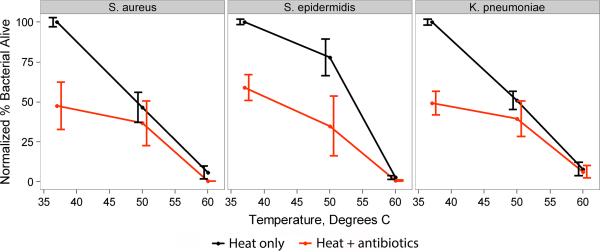
Staphylococcus epidermidis demonstrated progressive susceptibility to two hours of elevated temperature, with nearly complete killing of organisms at temperatures above 55°C (Figure 2). Treating with heat in the presence of vancomycin at 4-fold its MIC for this strain showed additive killing, with both heat and antibiotics statistically significant to p < 0.001 by two-factor ANOVA. Similar trends were seen with S. aureus and K. pneumoniae (Figure 3). However, when compared to S. epidermidis, these species were more susceptible to elevated temperature at 50°C but had less additive killing by antibiotics.
Figure 2.
(A) Flow cytometric signature of a healthy population of S. epidermidis. Bacteria were stained with complementary fluorophores indicating living cells (via the nucleic acid stain Syto 9) and cells in which membrane permeability has been lost (an early finding in dead bacteria, via propidium iodide, PI). The clustering of cells is characteristic of rapidly growing populations. (B) A similar signature of recently killed bacteria, where Syto 9 staining is diminished and PI staining is significantly augmented. In this case, cells were treated at 60C for 2 hours. In both (A) and (B), distributions were gathered with analysis of greater than 10,000 cells. (C) Interactions between vancomycin and temperature derived from flow cytometric measurement of treated bacteria. Cells were treated with 4-fold MIC values of vancomycin and temperatures ranging from 37 to 60°C. Shown are standard error bars, with at least 5 replicate experiments per condition (n = 130 for entire series). Heat treatment increased bacterial killing over vancomycin alone for all temperatures up to and including 50°C (p < 0.01 by 2-way ANOVA).
Figure 3.
Interactions between antibiotics and temperature derived from flow cytometric measurement of treated bacteria. S. aureus and S. epidermidis were treated with 4 and 1 μg/ml of vancomycin while K. pneumoniae was treated with 0.6 μg/ml of ciprofloxaxin. Bacteria were also exposed to 37, 50, and 60°C. Shown are standard error bars, with at least 5 replicate experiments per condition.
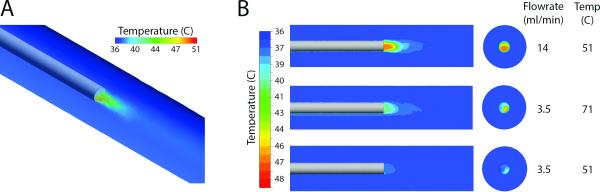
Generation of a CFD model of a dialysis catheter with a heated infusate allowed for rapid, iterative evaluation of the effects of both infusion flow rate and temperature (Figure 4A). Cross sectional views of the SVC-catheter model reveal that polyurethane is a good thermal-insulator and therefore maintains the temperature of the infusate over the length of the catheter, without significant heating of the outer surface. Views of the catheter outlet flow (plume) show that the high volumetric flow rate of the SVC leads to rapid cooling of the infusate plume to near baseline blood temperature (Figure 4B).
Figure 4.
(A) Volume rendering of catheter outflow temperature contours from CFD simulation demonstrating rapid cooling of the infusate plume to near baseline blood temperature. (B) Temperature contours for longitudinal and cross sections at the catheter outlet re-demonstrate rapid plume cooling but also show minimal elevation of the temperature of blood surrounding the catheter.
Parametric analysis was performed to study the effects of variable flow rates and infusate temperatures on the inner and outer catheter surfaces, plume, and SVC outlet temperatures respectively. Inner surface and plume temperatures both increase with increases in flow rate but begin to level off at high flow rates(Figure 5A & C). Changes in the infusate temperature allow for additional control for both the inner surface and plume temperatures. The outer surface of the catheter had minimal increase in average temperature at all flow rates and infusate temperatures (Figure 5B). This is expected given the low thermal conductivity of polyurethane. The range of temperatures on the outer surface however was quite large with a few high temperature outliers. Interrogation of the simulation results demonstrated that these high maximum temperatures are located at the vertical face of the outlet of the catheter where the infusate plume is exiting the catheter (recall Figure 1B). Importantly, the average temperature of the fluid exiting the model SVC (representing the increase in blood temperature due to warm infusate) never increased by more than 0.5°C at all flowrates and temperatures. (Figure 5D).
Figure 5.
Parametric analysis for various catheter inlet flow rates and temperatures demonstrating the average temperature of the (A) inner surface can be increased to a range that will augment bacterial killing without causing thermal injury. The insulating properties of polyurethane, however cause minimal temperature elevation of the (B) outer surface of the intravascular region of the catheter. Shaded regions represent the range of temperatures across all faces in the mesh for that surface. Also shown are the maximum temperature of the (C) plume from the catheter and the average temperature exiting the (D) SVC outlet. In all panels the dotted, dashed and solid lines represent physiologic temperature (37°C), bacterial inhibition temperature (45°C), and the thermal injury temperature (50°C).
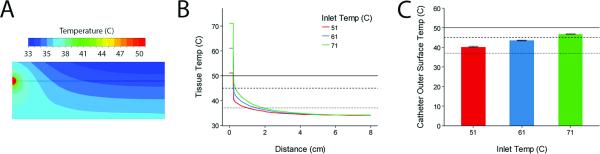
For the subcutaneous portion of a tunneled dialysis catheter, we generated contour plots of temperature to demonstrate the transfer of heat from the catheter to subcutaneous tissue(Figure 6A). The temperature profile shows that the tissue temperature returns to baseline only 2cm in radius from catheter but parallel to the skin surface (Figure 6B). Again, the majority of the temperature decrease occurs across the thickness of the catheter wall due to the low thermal conductivity of polyurethane. Finally, under the range of infusate temperatures tested, the temperature of the outer surface of the subcutaneous segment of the catheter can be increased into a range that will augment antibiotic killing (45-50°C) but will not lead to tissue thermal injury (>50°C)11 and protein denaturation (>60°C)23 (Figure 6C).
Figure 6.
(A) Contours of temperature in tissue surrounding the subcutaneous region of the catheter (see Figure 1C) with infusate temperature of 51°C and flowrate of 40ml/min. (B) Temperature profile along horizontal line in (A) for different inlet temperatures at 40ml/min. Note that for all inlet temperatures the surrounding tissue falls below 37°C around 2cm radially from the catheter. (C) Average outer surface temperature for the subcutaneous region of the catheter for different inlet temperatures at 40ml/min. Error bars represent range of surface temperatures. Dotted, dashed and solid lines represent physiologic temperature (37°C), bacterial inhibition temperature (45°C), and the thermal injury temperature (50°C).
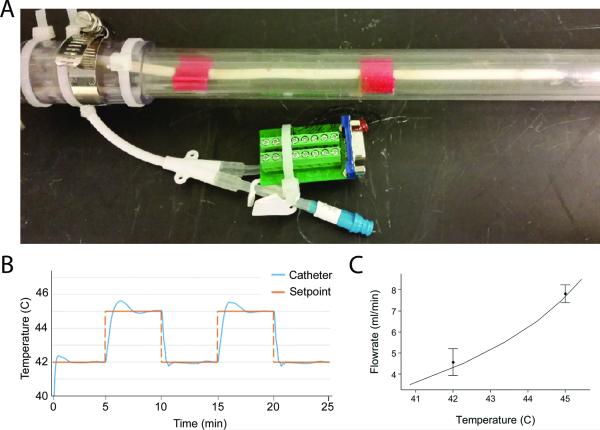
A physical model was created to allow for confirmatory testing of the SVC-catheter system and to experimentally validate the computational model (Figure 7A). The system showed fast and accurate adherence to programmed set-points, requiring less than 100 seconds to achieve steady state (set-point temperature ± 1%) control with Proportional-Integral-Derivative parameters of P=1.3, I=0.15, and D=0.1 (Figure 7B). Infusate temperatures and flow rates required to maintain steady-state defined catheter temperatures were similar for both the CFD and physical models (Figure 7C).
Figure 7.
(A) Photograph of the benchtop SVC-catheter model used for experimental validation of the computational model. The catheter was positioned centrally in the model SVC using two guides (red). (B) The thermal response of the catheter due to programmed set point changes in the PID controller (inlet temperature 56°C). (C) A comparison of required flowrates to achieve steady-state heating in the computational model (solid line) and benchtop (points) experiments validates the computational. Error bars represent standard deviation for replicate experiments (n=3).
DISCUSSION
We present preclinical data in support of the use of localized elevated temperature as an adjuvant to antibiotics in the treatment of hemodialysis catheter-based CLABSI. Extending observations made previously by our group of thermal susceptibility of both planktonic and biofilm-associated Staphylococcus, we show here the additive utility of antibiotic therapy and elevated temperature against biofilm derived strains of the most common catheter-infecting microorganisms, S. epidermidis and S. aureus14, 16 as well as a commonly isolated Gram-negative organism, K. pneumonia. It should be noted that the working fluid in this case was water, which is the most common solvent for antibiotics, and therefore would allow for simultaneous administration of antibiotics and heat. That is, the antibiotic infusion could be warmed to the target temperature. Our data also indicate that the magnitude of temperature elevation required is relatively modest and, based on both an experimental flow apparatus as well as on CFD modeling, is not only achievable in situ in an adult human vena cava but likely safe. Specifically, infusion of 51°C water at 40ml/min would result in temperatures on the entire intraluminal surface as well as the subcutaneous portion of the extraluminal surface >45°C. However, the maximal temperature attained within the blood is never >50°C and less than 0.003% of the total volume of blood passing the catheter has a temperature >45°C. Other combinations of higher flowrates and lower infusate temperatures also generate temperatures that are both efficacious and safe.
Hyperthermia of various intensities has become standard practice in numerous clinical settings. Modest elevations in temperature (e.g, 42-45°C) applied locally or regionally are currently used as adjuvants to traditional cancer therapy and can be delivered safely.13 The intent in these circumstances is to exploit differential susceptibility to thermal stress between healthy and unhealthy tissue. In the setting of intravascular device infection, temperature induced protein denaturation and blood coagulation place an upper limit on the temperature that can be applied in the blood stream. Thermal denaturation of serum proteins has been evaluated and shown to occur around 60°C23. Whole body hyperthermia (42-47°C), as in heat stroke, leads to activation of inflammation and coagulation systems24, 25. However, in heat stroke the entire blood volume has increased temperature. In the application described here, the heat is highly localized with minimal increase in total blood temperature. A more closely analogous example would be heat generated during cardiac radiofrequency ablation (RFA). It is well known that thermal injury of cardiomyocytes is the mechanism by which RFA works to treat many atrial tachyarrythmias. Nath et al. showed that irreversible injury of cardiomyocytes occurs above 50°C11. However, the endocardium in contact with the RFA probe can reach temperatures that approach 100°C26. Indeed, at temperatures between 95-100°C a coagulum will form on the RFA probe tip26. Thrombus formation has been seen at temperatures as low as 73°C27. Therefore, we chose 50°C as a conservative upper limit understanding that the time required for bacterial killing is significantly longer than the 60 seconds used in RFA.
Our data indicate that simultaneously applied antibiotics may be a means of maximizing the difference in thermotolerance between host tissue and biofilm-based microorganisms. Although the exact clinically optimal temperature remains to be seen, from a microbiology standpoint, temperatures between 45-50°C lead to significant bacterial killing that is further augmented by antibiotics (Figure 1). In addition, this temperature appears to be in a range that is safe in regards to protein denaturation, tissue injury, and coagulation as described above. By varying infusate temperature and flow rate these temperatures are achievable on the interior of the catheter (Figure 5) as well as the exterior of the subcutaneous portion of the catheter (Figure 6). Finally the PID controller designed on the bench top model was capable of maintaining specific set point temperatures within 1% (Figure 7). As a result, we believe that adjuvant thermal treatment may provide a way forward beyond the typical need to remove and replace infected devices.
An obvious limitation of this application demonstrated here was related to the insulating properties of polyurethane and the high heat capacity of blood. Our computational modeling demonstrated that these properties significant impede the warming of the exterior, intravascular surface of the catheter by warm infusion. This is further exacerbated by the fact that clinically, many long-term dialysis catheters develop a fibrin sheath that not only provides additional insulation but can itself become infected. One can easily imagine other mechanisms for applying heat to the exterior surfaces (including the fibrin sheath) such as by RFA probe. However, redesigning the probe geometry to apply more diffuse heat over the entire surface and the actual procedure to bring these two devices in close contact are not trivial and require significant future basic research.
While heat treatment might be employed for many different types of implantable devices, hemodialysis catheters represent an important early test bed. Hemodialysis catheters have the highest rate of infection of any implantable catheter. Due in part to their large diameter, hemodialysis catheters are frequently associated with the development of venous stenosis or occlusion, limiting the options for repeated replacement and driving a desire to treat infections ‘in place.’ In addition, hemodialysis catheters have an inherent design feature making them ideal candidates for thermal treatment: they are designed to interface to high-flow fluidic systems (i.e., hemodialysis machines) and can easily be integrated with heating systems.
There are definitely challenges to implementing this technique. Using a dialysis machine to overheat blood is certainly an elegant solution but given the flow rates and volumes of blood passing through the circuit this may lead to elevation of the temperature of the entire blood volume leading to comorbidities associated with hyperthermia. On the other hand, simple infusion of warmed fluids in a patient off dialysis may lead to volume overload. Another potential application may be to cycle a small volume of heated fluid in and out of the catheter. This would prevent large volume infusion for a dialysis patient. Future work would be required to determine the exact duty cycle needed to ensure adequate bacterial killing.
In summary we have shown that modest elevations of temperature augment antibiotic therapy for the most common pathogen causing CLABSI. These temperature elevations can be achieved on the inner surface and the subcutaneous portion of the outer surface of the catheter via the infusion of warmed fluids through the catheter without reaching temperatures known to cause denaturation or thermal injury. This combination of microbiological, computational, and experimental studies, demonstrates the efficacy, feasibility, and safety of using heat as an adjuvant treatment for infected hemodialysis catheters. In our view, the next steps are to first better understand experimentally, rather than computationally, the heat transfer properties of implanted hemodialysis catheters. There are at least three thermally distinct regions in a catheter in place, the fully external catheter where heat is lost to the environment, the tunnel where heat transfer is slower and the potential for tissue injury is most pronounced, and the intravascular region, where rapid transfer of heat to flowing blood may make it difficult to maintain elevated temperatures. In addition, the insulating properties of PDMS or polyurethane catheters make the intraluminal and extraluminal surfaces relatively distinct. Once these regions have been better evaluated in terms of their thermal characteristics, engineering approaches for delivering, and monitoring, the required thermal energy can more effectively be developed. This will provide the necessary data to begin in vivo safety and efficacy testing.
Supplementary Material
Acknowledgments
Funding Support:
University of Michigan M-Cubed Grant (JGY, MH, and MJS)
NIGMS R01 GM081702 Biomechanics of Bloodstream Infection (JGY and MJS)
SAEM Research Fellowship Award (JSV)
Footnotes
COI: The authors have no conflict of interest to report
REFERENCES
- 1.Cox EG, Knoderer CA, Jennings A, Brown JW, Rodefeld MD, Walker SG, et al. A Randomized, Controlled Trial of Catheter-Related Infectious Event Rates Using Antibiotic-Impregnated Catheters Versus Conventional Catheters in Pediatric Cardiovascular Surgery Patients. Journal of the Pediatric Infectious Diseases Society. 2013;2(1):67–70. doi: 10.1093/jpids/pis066. doi: 10.1093/jpids/pis066. [DOI] [PubMed] [Google Scholar]
- 2.Darouiche R. In: Antimicrobial-Modified Vascular Catheters. Moriarty TF, Zaat SAJ, Busscher HJ, editors. Biomaterials Associated Infection; Springer New York: 2013. pp. 485–503. [Google Scholar]
- 3.Diekema DJ, Beekmann SE, Chapin KC, Morel KA, Munson E, Doern GV. Epidemiology and Outcome of Nosocomial and Community-Onset Bloodstream Infection. Journal of Clinical Microbiology. 2003;41(8):3655–60. doi: 10.1128/JCM.41.8.3655-3660.2003. doi: 10.1128/JCM.41.8.3655-3660.2003. PubMed PMID: PMC179863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maki DG, Kluger DM, Crnich CJ. The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clinic proceedings. 2006;81(9):1159–71. doi: 10.4065/81.9.1159. Epub 2006/09/15. doi: 10.4065/81.9.1159. PubMed PMID: 16970212. [DOI] [PubMed] [Google Scholar]
- 5.Muto C, Herbert C, Harrison E, Edwards JR, Horan T, Andrus M, et al. Reduction in central line-associated bloodstream infections among patients in intensive care units - Pennsylvania, April 2001-March 2005 (Reprinted from MMWR, vol 54, pg 1013-1016, 2005). Jama-J Am Med Assoc. 2006;295(3):269–70. PubMed PMID: WOS:000234684500010. [Google Scholar]
- 6.O'Grady NP, Alexander M, Dellinger EP, Gerberding JL, Heard SO, Maki DG, et al. Guidelines for the prevention of intravascular catheter-related infections. Am J Infect Control. 2002;30(8):476–89. doi: 10.1067/mic.2002.129427. doi: DOI 10.1067/mic.2002.129427. PubMed PMID: WOS:000179930400004. [DOI] [PubMed] [Google Scholar]
- 7.Christensen GD, Baldassari L, Simpson WA. Colonization of medical devices by coagulase-negative staphylococci. In: Bisno AL, F.A. W, editors. Infections associated with indwelling medical devices. Second ed. American Society of Microbiology; Washington, DC: 1994. pp. 45–78. [Google Scholar]
- 8.Marr KA, Sexton DJ, Conlon PJ, Corey GR, Schwab SJ, Kirkland KB. Catheter-related bacteremia and outcome of attempted catheter salvage in patients undergoing hemodialysis. Annals of internal medicine. 1997;127(4):275–80. doi: 10.7326/0003-4819-127-4-199708150-00003. Epub 1997/08/15. PubMed PMID: 9265426. [DOI] [PubMed] [Google Scholar]
- 9.Pronovost P, Needham D, Berenholtz S, Sinopoli D, Chu H, Cosgrove S, et al. An Intervention to Decrease Catheter-Related Bloodstream Infections in the ICU. New England Journal of Medicine. 2006;355(26):2725–32. doi: 10.1056/NEJMoa061115. doi: doi:10.1056/NEJMoa061115. PubMed PMID: 17192537. [DOI] [PubMed] [Google Scholar]
- 10.Hurwitz M, Stauffer P. Hyperthermia, radiation and chemotherapy: the role of heat in multidisciplinary cancer care. Seminars in oncology. 2014;41(6):714–29. doi: 10.1053/j.seminoncol.2014.09.014. Epub 2014/12/17. doi: 10.1053/j.seminoncol.2014.09.014. PubMed PMID: 25499632. [DOI] [PubMed] [Google Scholar]
- 11.Nath S, Lynch C, Whayne JG, Haines DE. Cellular electrophysiological effects of hyperthermia on isolated guinea pig papillary muscle. Implications for catheter ablation. Circulation. 1993;88(4):1826–31. doi: 10.1161/01.cir.88.4.1826. doi: 10.1161/01.cir.88.4.1826. [DOI] [PubMed] [Google Scholar]
- 12.Eick OJ. Temperature Controlled Radiofrequency Ablation. Indian Pacing and Electrophysiology Journal. 2002;2(3):66–73. PubMed PMID: PMC1564057. [PMC free article] [PubMed] [Google Scholar]
- 13.U R, Noell KT, Woodward KT, Worde BT, Fishburn RI, Miller LS. Microwave-induced local hyperthermia in combination with radiotherapy of human malignant tumors. Cancer. 1980;45(4):638–46. doi: 10.1002/1097-0142(19800215)45:4<638::aid-cncr2820450404>3.0.co;2-f. Epub 1980/02/15. PubMed PMID: 6766789. [DOI] [PubMed] [Google Scholar]
- 14.Sturtevant RA, Sharma P, Pavlovsky L, Stewart EJ, Solomon MJ, Younger JG. Thermal Augmentation of Vancomycin against Staphylococcal Biofilms. Shock (Augusta, Ga) 2015 doi: 10.1097/SHK.0000000000000369. Epub 2015/03/19. doi: 10.1097/shk.0000000000000369. PubMed PMID: 25784524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pavlovsky L, Younger JG, Solomon MJ. In situ rheology of Staphylococcus epidermidis bacterial biofilms. Soft matter. 2013;9(1):122–31. doi: 10.1039/C2SM27005F. Epub 2013/01/07. doi: 10.1039/c2sm27005f. PubMed PMID: 25544855; PubMed Central PMCID: PMCPMC4276346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pavlovsky L, Sturtevant RA, Younger JG, Solomon MJ. Effects of temperature on the morphological, polymeric, and mechanical properties of Staphylococcus epidermidis bacterial biofilms. Langmuir : the ACS journal of surfaces and colloids. 2015;31(6):2036–42. doi: 10.1021/la5044156. Epub 2015/01/21. doi: 10.1021/la5044156. PubMed PMID: 25602470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mermel LA. What Is The Predominant Source of Intravascular Catheter Infections? Clinical Infectious Diseases. 2011;52(2):211–2. doi: 10.1093/cid/ciq108. doi: 10.1093/cid/ciq108. [DOI] [PubMed] [Google Scholar]
- 18.Favre-Bonte S, Joly B, Forestier C. Consequences of Reduction of Klebsiella pneumoniae Capsule Expression on Interactions of This Bacterium with Epithelial Cells. Infection and Immunity. 1999;67(2):554–61. doi: 10.1128/iai.67.2.554-561.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuzo RS, Pooley RA, Crook JE, Heckman MG, Gerber TC. Measurement of Caval Blood Flow with MRI During Respiratory Maneuvers: Implications for Vascular Contrast Opacification on Pulmonary CT Angiographic Studies. American Journal of Roentgenology. 2007;188(3):839–42. doi: 10.2214/AJR.06.5035. doi: 10.2214/AJR.06.5035. [DOI] [PubMed] [Google Scholar]
- 20.Deng ZS, Liu J. Analytical study on bioheat transfer problems with spatial or transient heating on skin surface or inside biological bodies. Journal of biomechanical engineering. 2002;124(6):638–49. doi: 10.1115/1.1516810. Epub 2003/02/25. PubMed PMID: 12596630. [DOI] [PubMed] [Google Scholar]
- 21.Liu J, Xu LX. Estimation of blood perfusion using phase shift in temperature response to sinusoidal heating at the skin surface. IEEE transactions on bio-medical engineering. 1999;46(9):1037–43. doi: 10.1109/10.784134. Epub 1999/09/24. PubMed PMID: 10493066. [DOI] [PubMed] [Google Scholar]
- 22.Pennes HH. Analysis of tissue and arterial blood temperatures in the resting human forearm. Journal of applied physiology. 1948;1(2):93–122. doi: 10.1152/jappl.1948.1.2.93. Epub 1948/08/01. PubMed PMID: 18887578. [DOI] [PubMed] [Google Scholar]
- 23.Michnik A, Drzazga Z. Thermal denaturation of mixtures of human serum proteins. J Therm Anal Calorim. 2010;101(2):513–8. doi: 10.1007/s10973-010-0826-5. [Google Scholar]
- 24.Roberts GT, Ghebeh H, Chishti MA, Al-Mohanna F, El-Sayed R, Al-Mohanna F, et al. Microvascular injury, thrombosis, inflammation, and apoptosis in the pathogenesis of heatstroke: a study in baboon model. Arteriosclerosis, thrombosis, and vascular biology. 2008;28(6):1130–6. doi: 10.1161/ATVBAHA.107.158709. Epub 2008/04/05. doi: 10.1161/atvbaha.107.158709. PubMed PMID: 18388331. [DOI] [PubMed] [Google Scholar]
- 25.Bouchama A, Al-Mohanna F, Assad L, Baturcam E, Eldali A, Owaidah T, et al. Tissue factor/factor VIIa pathway mediates coagulation activation in induced-heat stroke in the baboon. Critical care medicine. 2012;40(4):1229–36. doi: 10.1097/CCM.0b013e3182387bef. Epub 2011/11/15. doi: 10.1097/CCM.0b013e3182387bef. PubMed PMID: 22080642. [DOI] [PubMed] [Google Scholar]
- 26.Langberg JJ, Calkins H, el-Atassi R, Borganelli M, Leon A, Kalbfleisch SJ, et al. Temperature monitoring during radiofrequency catheter ablation of accessory pathways. Circulation. 1992;86(5):1469–74. doi: 10.1161/01.cir.86.5.1469. doi: 10.1161/01.cir.86.5.1469. [DOI] [PubMed] [Google Scholar]
- 27.Matsudaira K, Nakagawa H, Wittkampf FH, Yamanashi WS, Imai S, Pitha JV, et al. High incidence of thrombus formation without impedance rise during radiofrequency ablation using electrode temperature control. Pacing and clinical electrophysiology : PACE. 2003;26(5):1227–37. doi: 10.1046/j.1460-9592.2003.t01-1-00173.x. Epub 2003/05/27. PubMed PMID: 12765451. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.