Abstract
Purpose
Intravenous contrast media (ICM) administration is recommended as part of radiation therapy (RT) simulation in a variety of clinical scenarios, but can cause adverse events. We sought to assess radiation oncology resident knowledge about ICM, and to determine if an educational intervention (EI) could improve this level of knowledge. In conjunction, we retrospectively analyzed risk factors and adverse events related to ICM use before and after the EI to determine whether any improvements in patient outcomes could be realized.
Methods
Over 2 years, 21 residents in radiation oncology at Memorial Sloan-Kettering Cancer Center (MSKCC) participated in a pretest-EI-posttest study based on the ACR’s Manual on Contrast Media. Medical and RT records were reviewed, and ICM use, risk factors and adverse events were recorded.
Results
There was no significant difference in resident understanding of ICM use in residents of different years of training (p=0.85). Understanding of ICM use increased in residents that attended the EI (p<0.05), but this was not sustained 1 year after the EI (p=0.48). Of the 6852 RT simulations that were performed at MSKCC, 1350 (19.7%) involved ICM. Mild adverse events occurred in a few patients (<5%) simulated with ICM, but there was no difference in the number of risk factors or adverse events before and after the EI.
Conclusions
The EI effectively improved short-term understanding of ICM use. However, the effect was not sustained. The frequency of adverse events related to ICM use was small and not significantly impacted by the EI.
Keywords: radiation oncology, resident, educational intervention, contrast media, adverse event
Introduction
Contrast media are radiopaque substances administered to patients during medical imaging to enhance the visualization of normal and pathologic anatomy. Used in diagnostic radiology for over a century (1), contrast media has long been considered a valuable aid during radiation therapy simulation, which involves the acquisition of images for treatment planning purposes. For example, as part of radiation therapy simulation using plain film radiographs, contrast media were often administered by mouth, urethra, vagina, and rectum, to help delineate radiotherapeutic targets and avoidance structures (2). In the 1980s, the development of intravenous contrast-enhanced computed tomography (CT) allowed the acquisition of more sophisticated images at the time of simulation, which have in turn facilitated the application of more advanced radiation therapy techniques (3). The widespread adoption of advanced imaging as part of simulation was demonstrated by a recent survey which revealed that 95% of radiation oncologists in the United States use some form of advanced imaging as part of the radiation therapy simulation; however, the use of contrast media as a component of advanced imaging was not specified (4).
Several organizations have indicated that using intravenous contrast media (ICM) as part of the radiation therapy simulation is appropriate for patients without contraindications. The National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology™ suggest using ICM as part of radiation therapy planning for esophageal (5), gastric (6), and non-small cell lung cancers (7). The Radiation Therapy Oncology Group (RTOG) used ICM when creating expert consensus guidelines for the delineation of target volumes for treatment of cancers of the uterus (8), prostate (9), anus and rectum (10), the latter guideline stating that “although not mandatory… many patients could also benefit from intravenous contrast at the time of simulation.” The European Organization for Research and Treatment of Cancer (EORTC) recommends ICM during the radiation therapy simulation for centrally located lung cancers (11). The Royal College of Radiology (RCR) recommends simulation imaging with ICM when planning radiation therapy for nodal disease in the neck, and cancers of the lung, esophagus, stomach, pancreas, kidneys, biliary tract (cholangiocarcinoma) and liver; consideration of using ICM for at least 12 other types of tumors is also mentioned in their recommendations (12).
The clinical aspects of ICM use in radiation oncology have probably not been well studied because the majority of ICM use occurs in diagnostic radiology departments. The development of safer, less-toxic ICM agents (i.e., low osmolarity agents), has resulted in lower rates of adverse events when using these substances during diagnostic and therapeutic imaging procedures. However, the risk of adverse reactions exists, and in rare situations, can be fatal. Importantly, the skills required to use ICM safely and effectively may not be taught or assessed as part of radiation oncology training. For this reason, and in accordance with the Accreditation Council for Graduate Medical Education (ACGME) radiation oncology residency program requirements, we designed a quality improvement project to study the use of contrast media in our radiation oncology department to answer several questions:
What do radiation oncology residents know about using ICM
Could an educational intervention (EI) improve resident understanding of ICM use?
How often is ICM administered during CT simulation for external beam radiation therapy in our department, and how often do adverse events occur?
Could an EI on ICM use alter patterns of use or reduce adverse events in our department?
This report describes the result of our experience with this quality improvement project.
Methods and Materials
Educational intervention participants
Resident physicians in the ACGME-accredited training program in radiation oncology at Memorial Sloan-Kettering Cancer Center (MSKCC) during the 2 academic years from 2008–2010 were eligible for participation in this study. All residents participated on an anonymous, voluntary basis. The primary investigator (C.A.B.) facilitated the EI, and therefore did not participate. The study was conducted as part of the MSKCC Quality of Care Initiative, and integrated into the radiation oncology resident teaching curriculum, and was therefore deemed exempt from local Institutional Review Board (IRB) approval.
Knowledge assessment
A non-validated 21-item multiple choice test was designed to assess resident knowledge regarding the use of contrast media (see Appendix 1). The test was based on the American College of Radiology (ACR) Manual on Contrast Media (13), and specifically covered domains related to oral and intravenous contrast media use, adverse reactions and management. The assessment specifically excluded information regarding use or complications from gadolinium-based contrast media or administration in pregnant or breast-feeding women, as these situations are unlikely to be encountered in a radiation oncology department. Two different versions of the test, which assessed the same body of knowledge, were designed. One was completed before the EI (pretest) and the other was completed after the EI (posttest).
All residents that participated in the study completed the pretest and posttest. Residents that completed the pretest, EI and posttest in the appropriate sequence served as the Educational Intervention Group. Based on the hypothesis that the EI would increase posttest scores (compared to pretest scores) in the Educational Intervention Group, residents that did not complete the pretest, EI and posttest in the appropriate sequence served as the Control Group. The residents in the Control Group included those that did not attend the EI (but completed the pretest and posttest anyway) and those who did not complete the pretest before the EI (but completed both the pretest and posttest after attending the EI).
Educational intervention (EI)
A lecture about the use of contrast media, based on the ACR Manual on Contrast Media (13) and MSKCC departmental procedures and policies was designed and delivered to residents that chose to attend the EI on 12/5/08. At the end of the presentation, problem-based scenarios were presented and discussed. After attending the EI, residents completed the posttest.
Long-term follow-up assessment
One year after conducting the EI, a follow up program was conducted using the same pretests, EI (given on 12/11/09), and posttests, as described above. During that time (year 2 of the study), 4 residents completed the residency program, and 5 new residents began their training. Residents that completed the pretest before the EI in year 1, attended the EI in year 1, and completed the pretest prior to the EI in year 2, comprised the Long-term Educational Intervention Group. New residents, who were not present for the EI in 2008, had data collected as described above.
Intravenous contrast media use, risk factors, and adverse event frequency
External beam radiation therapy simulations occurring over the 2 years between 12/4/07 and 12/4/09 were identified in log books and billing databases of the Department of Radiation Oncology at the main campus of MSKCC. Date of simulation and use of ICM was recorded. The MSKCC “Reporting to Improve Safety and Quality (RISQ)” database and electronic clinical records were searched for allergic-like and nephropathic risk factors and adverse events related to ICM administration occurring in the Department of Radiation Oncology. Allergic-like events were determined by clinicians at MSKCC. Nephropathy was determined through assessment of serum creatinine in the MSKCC clinical laboratories 6 weeks before or 2 weeks after simulation.
Patients with risk factors for adverse events from ICM were defined as patients with history of allergic-like reaction to ICM (prior to simulation), and those with pre-existing nephropathy (serum creatinine greater than the upper limit of normal 6 weeks or less prior to simulation). Adverse events from ICM administration during the radiation therapy simulation were defined as allergic-like events occurring within 7 days of simulation, and nephropathic events (serum creatinine greater than the upper limit of normal 2 weeks or less after simulation).
Severity of allergic-like reaction and azotemia was graded using Common Terminology Criteria for Adverse Events, version 3.0. Retrospective medical record review was conducted with permission of the IRB.
Statistical analysis
A Kruskal-Wallis test was performed to determine if there was any difference in median score (% correct on multiple choice test) between residents in different post-graduate years (PGY) of training. A one-sided Wilcoxon rank sum test was used to determine whether the difference between the posttest scores and the pretest scores in the EI group was significantly greater than the difference between the scores in the Control Group. A Wilcoxon signed-rank test was used to determine whether pretest scores in year 2 were significantly higher than pretest scores in year 1 in the Long-term Educational Intervention Group. Scores are presented as mean percent correct, ± standard deviation, unless otherwise noted. Two-sided, two-sample proportionality tests with continuity correction were used to determine if any significant differences existed in the frequency of risk factors or adverse events in patients receiving ICM in years 1 and 2 of the study.
Results
Resident participation
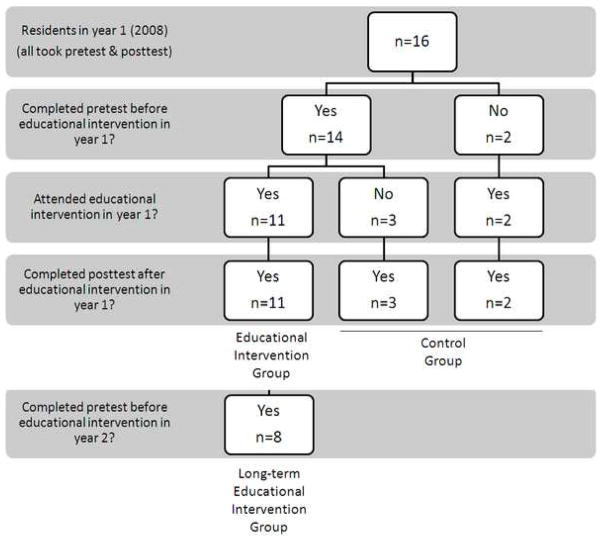
Twelve of the participants were male, and 9 were female. Median age of participants was 31 years, with a range of 27 – 38 years. Figure 1 demonstrates the participation of residents during year 1 of the study. Eight participants (50%) in year 1 completed the pretest and EI in appropriate sequence, and were available for follow up evaluation as the Long-term Educational Intervention Group.
Figure 1.
Resident participation in the educational intervention in year 1 of the study. In 2008, there were 16 resident physicians in the Department of Radiation Oncology at Memorial Sloan-Kettering Cancer Center. All residents completed the pretest and posttest. Resident participation in various phases of the first year of the study determined their group assignment. During year 2 of the study, 4 residents graduated, and 5 new residents started their training.
Knowledge assessment
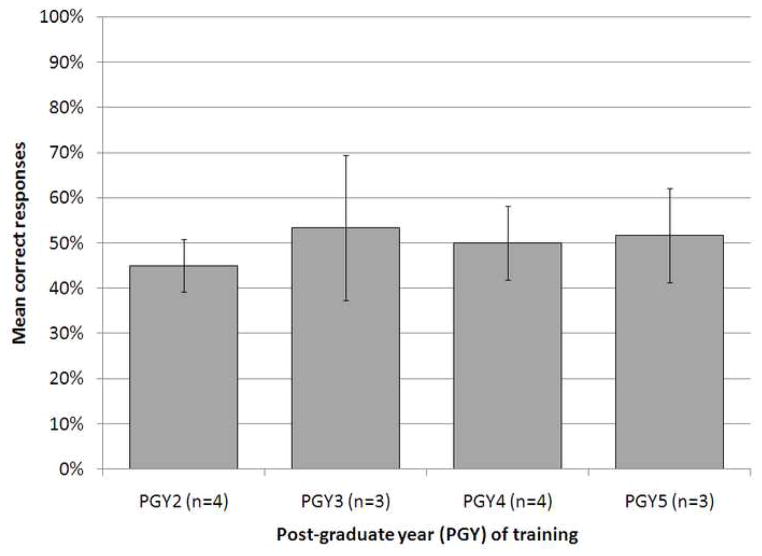
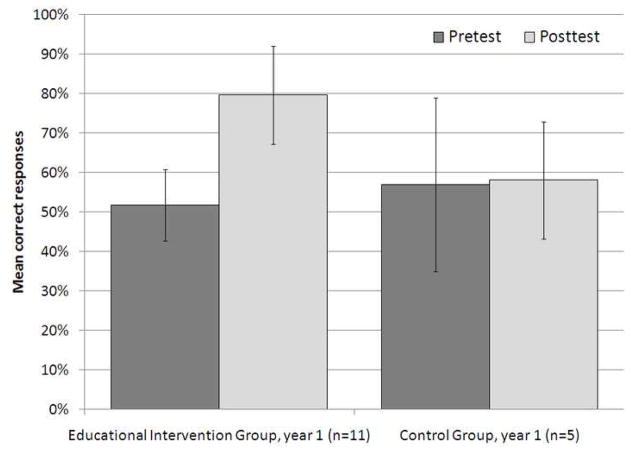
There was no statistically significant difference in pretest score across the different years of post-graduate training, with mean scores of 45±6%, 53±16%, 50±8%, and 52±10% for residents in the 2nd, 3rd, 4th and 5th year of post-graduate training who completed the pretest before the EI (p=0.85) (Figure 2). Participants in the Control Group did not demonstrate a significant difference in pretest and posttest scores (57±22% and 58±15%, respectively, p=1.0). Participants that completed the EI demonstrated a 28% increase in mean test score, in comparison to a 1% increase in residents that did not complete the EI. The difference was statistically significant (p<0.05, Figure 3).
Figure 2.
Mean percent correct responses on the pretest in year 1 of the study, by resident postgraduate training year (PGY). Scores of residents who completed the pretest prior to the educational intervention are included. There was no statistically significant association between year of post-graduate training and pretest score (p=0.85). Error bars represent standard deviation of the mean. PGY, post-graduate year.
Figure 3.
Mean percent correct responses on the pretest and posttest in year 1 of the study, by group. A statistically significant improvement in score was noted in the Educational Intervention Group, but not the Control Group (p<0.05). Error bars represent standard deviation of the mean.
Long-term follow up assessment
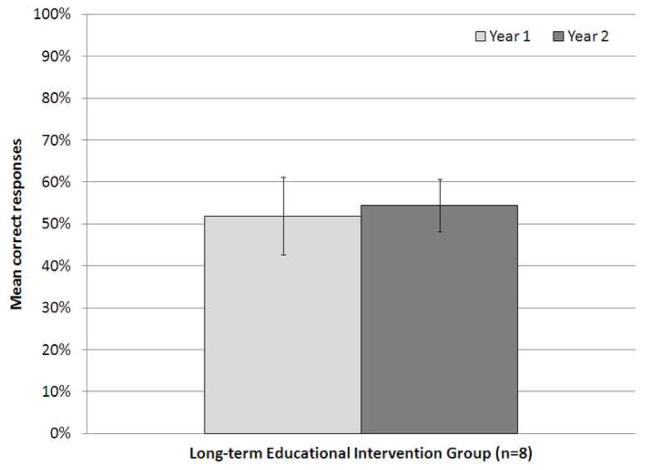
The Long-term Educational Intervention Group demonstrated no significant difference in pretest score in years 1 and 2 (52±9% and 54±6%, respectively, p=0.48, Figure 4). A significantly higher score on the posttest was again noted in participants who attended the EI in year 2 (pretest 51±9%, posttest 78±11%, p<0.05), even among those who comprised the Long-term Educational Intervention Group (pretest 54±6%, posttest 76±15%, p<0.05).
Figure 4.
Mean percent correct responses on the pretest in year 1 and 2 of the study, in the Long-term Education Intervention Group. There was no statistically significant difference in pretest score between year 1 and year 2 in the residents that attended the educational intervention in year 1 (p=0.48). Error bars represent standard deviation of the mean.
Intravenous contrast media use, risk factors, and adverse events
During the year prior to the first EI (year 1; 12/4/07 to 12/4/08), 3374 patients underwent simulation for external beam radiation therapy at main campus of MSKCC; 669 (19.8%) of these patients received ICM as part of the simulation (see Table 1). Twelve patients (1.8%) with history of allergic-like reactions to ICM underwent simulation with ICM after being premedicated. Prior to simulation with ICM, 477 (71.3%) patients underwent renal assessment, through determination of serum creatinine; 17 (3.6%) of these patients had grade 1 azotemia, but still underwent simulation with ICM. Overall, no patients experienced allergic-like reactions after simulation with ICM during year 1 of the study. Three-hundred twenty (47.8%) patients that underwent simulation with ICM underwent renal assessment within 2 weeks of simulation during year 1; 14 (4.4%) of these patients were noted to have grade 1 azotemia. Two-hundred fifty-five (38.1%) patients underwent renal evaluation before and after simulation; 7 (2.7%) of these patients, who had normal renal function prior to simulation, developed grade 1 azotemia after simulation. No grade 2 or worse azotemia was noted.
Table 1. Characteristics of patients simulated for external beam radiation therapy.
Patient characteristics and outcomes during the 2 years of the study. There were no statistically significant differences in the use of intravenous contrast media, frequency of risk factors or adverse events over the 2 years of the study. ICM, intravenous contrast media.
| Year of study | p-value | ||
|---|---|---|---|
| Number of patients: | 1 (12/4/07-12/4/08) | 2 (12/5/08-12/5/09) | |
| simulated for external beam radiation therapy | 3374 | 3478 | |
| - with ICM (%) | 669 (19.8%) | 681 (19.6%) | 0.82 |
| - - with history of allergic-like reaction to ICM (risk factor, %) | 12 (1.8%) | 19 (2.8%) | 0.30 |
| - - that developed mild allergic-like reaction (adverse event, %) | 0 (0.0%) | 2 (0.3%) | 0.49 |
| - - that had renal assessment <6 weeks before simulation (%) | 477 (71.3%) | 492 (72.2%) | 0.74 |
| - - - found to have grade 1 azotemia before simulation (risk factor, %) | 17 (3.6%) | 9 (1.8%) | 0.14 |
| - - that had renal assessment <2 weeks after simulation (%) | 320 (47.8%) | 336 (49.3%) | 0.62 |
| - - - found to have grade 1 azotemia after simulation (%) | 14 (4.4%) | 13 (3.9%) | 0.90 |
| - - that had renal assessment before and after simulation (%) | 255 (38.1%) | 273 (40.1%) | 0.49 |
| - - - found to have new grade 1 azotemia after simulation (adverse event, %) | 7 (2.7%) | 10 (3.7%) | 0.73 |
In the year after the first EI (year 2; 12/5/08 to 12/5/09), 3478 patients underwent simulation for external beam radiation therapy; 681 (19.6%) of these patients received ICM. Table 1 demonstrates the number of patients with history of prior allergic-like reaction to ICM, was not significantly different in year 2, compared to year 1. Fewer patients with mild azotemia underwent simulation with ICM after the EI (during the year 2 of the study), although this was not statistically significant (p=0.14). Two patients developed grade 1 allergic-like cutaneous reactions after simulation with ICM. No allergic-like reactions of a moderate or severe nature were noted. No significant difference in azotemia after simulation with ICM was noted in year 1 and 2 of the study (see Table 1). No grade 2 or worse azotemia was noted.
Conclusions
In 1981, at a multidisciplinary conference about the role of CT in radiation therapy, Goitein concluded that “[contrast media is] another ingredient of the CT recipe… the delineation of tissues is, as repeatedly emphasized, extremely important for the design of the treatment field and should receive the highest priority in treatment planning scans” (14). Despite the countless advances in imaging and radiation therapy that have been made since this claim was made 3 decades ago, it still holds true. But how is contrast media being used by radiation oncologists? A recent study surveying managers from 50 radiation oncology centers in the United Kingdom concluded that ICM use was “suboptimal,” as 24% of centers never performed a CT simulation with ICM. Moreover, none of the radiation oncology centers used ICM for all of the disease sites that the RCR recommended implementing a CT simulation with ICM. Nine radiation oncology centers reported not administering ICM because of a lack of trained personnel (15). In fact, 25% of the centers that did not administer ICM at all cited a lack of trained personnel (A. Saleem, PhD, FRCR, personal communication 1/2010). This suggests that radiation oncology staff and physicians alike could benefit from the education on use of contrast media.
This study represents the first educational research study addressing the subject of contrast media use by radiation oncology residents. We found no association of pretest scores and the number of years of post-graduate training of residents, suggesting that education in this skill has not been occurring in our training program. Importantly, we found that a brief presentation significantly improved resident knowledge on the use of contrast media, reflected as an increase in mean test score after the EI. Unfortunately, this gain in knowledge did not appear to be sustained, suggesting the need for a better EI. Moreover, there did not appear to be a statistically significant impact on the number of adverse events that occurred in our department. The latter finding is not surprising, given that the rarity of these adverse events (<5%). Nevertheless, approximately half the number of patients (n=17) with mild azotemia in year 1 of the study underwent simulation with ICM during year 2 of the study (n=9), although this difference was not statistically significant (p=0.14). This finding suggests a possible change in the pattern of ICM use brought about by the EI. It is likely that the ability of the EI to improve patient outcomes was limited due to academic turnover and suboptimal attendance (the EI was only completed by 53% of the residents who were present for the entire duration of year 2 of the study). Moreover, attending physicians and nursing staff, who are also actively involved in medical decision making and management of patients, did not participate in the EI.
Similar findings were reported by Echenique et al. from the University of Miami, who also conducted a pretest-EI-posttest study of new and seasoned radiology department staff (nurses and physicians). In this study, the EI was a 3 day course that consisted of 3 hours of didactic lectures and a hands-on scenario on recognizing and treating reactions to contrast media. The pretest and posttest tools used were very similar to those used in our study. The authors found a statistically significant improvement in the mean score of those who attended the intervention (n=29), from 46.7% (pretest) to 77.2% (posttest), which closely paralleled our findings. Interestingly, the experienced course participants (senior residents and faculty) also demonstrated an improvement in mean score, from 49.2% (pretest) to 80.9% (posttest). This finding is consistent with our finding that baseline knowledge, as quantified by the pretest, was not significantly different amongst junior and senior residents. Moreover, it suggests that even more senior members of the radiation oncology department could benefit from additional training in contrast media use. Unfortunately, in the Miami study, no control group was used to compare results in those individuals who did not attend the intervention, to demonstrate its effectiveness, and no long term follow-up or effect on patient outcomes have been reported (16).
To our knowledge, this is the first study reporting the frequency of adverse reactions to contrast media in a radiation oncology department. We found that 2.4% of patients that were simulated for radiation therapy with ICM had history of, or developed, mild allergic-like reactions. This is entirely consistent with the frequency of adverse events from contrast media reported in the diagnostic radiology literature (<5% for mild reactions). The paucity of events associated with simulation suggests that most patients will develop allergic-like reactions during diagnostic work-up for their cancer, but a small number (0.15% over the 2 years of this study), may develop an allergic-like reaction associated with simulation. Mild nephropathic events, reflected as grade 1 azotemia, were likewise rare (3.2% over the 2 years of this study), but probably represent a more common finding in the cancer patient population, given the prevalence of nephrotoxic antineoplastic therapies.
As adverse reactions to contrast media are rare events, several studies have used human patient simulators (not to be confused with radiation therapy simulators) to train residents in their management. Sica and colleagues at Brigham and Women’s Hospital were the first to implement a computer-based patient simulator to assess and improve resident management of emergencies in a radiology department, such as an adverse reaction to contrast media (17). Gaca and colleagues at Duke University later used a mannequin-based patient simulator activity to assess the value of a resuscitation-aid for pediatric anaphylactic contrast media reactions (18). The same group recently reported that a computer-based resuscitation tool improved radiology resident performance in simulated emergencies occurring in children (19). Tubbs and colleagues recently demonstrated that high-fidelity medical simulation of an acute adverse reaction to contrast media was able to improve scores on a multiple-choice test (20). While it seems intuitive that simulator-based training activities would be beneficial for infrequently occurring events (21), problem-based discussions, such as those used in our EI, have been shown to yield equivalent outcomes, and are not associated with the cost of procuring and operating a patient simulator (22). Morbidity and mortality conferences are a known method of presenting and learning about adverse events as they happen, and are probably be an ideal forum discussing complications that may arise from ICM (23). For this reason, the optimal method to train radiation oncologists in the safe use of contrast media is unclear, and further research is necessary.
This study has several limitations worthy of note. While we attempted to demonstrate an improvement the quality of patient care, by demonstrating fewer adverse events related to ICM use after an EI, too few events occurred to demonstrate this conclusively. As MSKCC is one of the largest radiation oncology centers in the world, a multi-institutional effort, or much longer period of study would have been necessary to overcome this problem. In addition, this study dealt with a small number of participants (n=21) from a single institution, therefore it may not be possible to generalize the results. Furthermore, less common types of adverse events related to ICM, such as cardiac toxicity, and toxicities of oral contrast media would not have been captured in our data analysis, and the adverse event data collection was retrospective, possibly leading to under-reporting. Nevertheless, we hope that other residency programs will institute a contrast media training initiative that conforms to the philosophies and ideals of their respective institutions.
Is it important to train radiation oncologists in the safe use of contrast media? Although the patient outcome data in our study might not support an affirmative response to this question, we still believe the answer is “yes”. The Agency for Healthcare Research and Quality’s report, “Making Health Care Safer: A Critical Analysis of Patient Safety Practices,” clearly indicates that preventing adverse events from the use of contrast media is a national priority. Moreover, the report suggests that “academic detailing” may benefit providers, such as radiation oncology residents. While the report suggests that patient simulator-based activities may be a viable means of training, little data support their use at this time (24).
Delaney and colleagues estimated that 52.3% of patients with cancer should receive external beam radiation therapy as part of optimal care (25). The American Cancer Society estimates that 1.47 million people in the United States were diagnosed with cancer in 2009 (26). Assuming all patients treated with external beam radiation therapy are simulated, and patterns of ICM use at our institution are generalizable, approximately 150,000 patients received ICM in a radiation oncology department in 2009. Using the frequency of adverse events reported in the diagnostic radiology literature (0.2 – 5% for mild events, 0.0005% for fatal events), it can therefore be estimated that in 2009 alone, between 300 and 7500 patients might experience a mild adverse reaction and 1 patient might die as a result of ICM use in a radiation oncology department. Given these facts, we believe that training radiation oncologists to safely and effectively use contrast media is necessary.
Acknowledgments
The authors acknowledge the tireless efforts of the radiation oncology house staff at MSKCC for their commitment to patient care. The authors also thank Nikki Barker, Shari Damast, Benjamin Lok, Joanne Kelvin, Michael Lovelock, Jeremy Miransky, Michael Sullivan, and the radiation therapy simulation staff for their assistance with this project.
Footnotes
The authors have no conflicts of interest relevant to the content of this article.
Presented in part at the 95th annual meeting of the Radiologic Society of North America, December 1, 2009 in Chicago, Illinois.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Grainger RG, Thomas AMK. History of the development of radiological contrast media (1895–1996) In: Dawson P, Cosgrove DO, Grainger RG, editors. Textbook of Contrast Media. Oxford: Isis Medical Media, Ltd; 1999. pp. 3–14. [Google Scholar]
- 2.Campostrini F, Garusi G, Donati E. A Practical Technique for Conformal Simulation in Radiation-Therapy of Pelvic Tumors. International Journal of Radiation Oncology Biology Physics. 1995;32:355–365. doi: 10.1016/0360-3016(94)00448-T. [DOI] [PubMed] [Google Scholar]
- 3.Lichter AS, Lawrence TS. Medical Progress - Recent Advances in Radiation Oncology. New England Journal of Medicine. 1995;332:371–379. doi: 10.1056/NEJM199502093320607. [DOI] [PubMed] [Google Scholar]
- 4.Simpson DR, Lawson JD, Nath SK, et al. Utilization of advanced imaging technologies for target delineation in radiation oncology. J Am Coll Radiol. 2009;6:876–883. doi: 10.1016/j.jacr.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 5.The NCCN Clinical Practice Guidelines in Oncology™ Esophageal Cancer (Version 1.2010) Vol. 2009. National Comprehensive Cancer Network, Inc; 2010. [Google Scholar]
- 6.The NCCN Clinical Practice Guidelines in Oncology™ Gastric Cancer (Version 1.2010) Vol. 2009. National Comprehensive Cancer Network, Inc; 2010. [Google Scholar]
- 7.The NCCN Clinical Practice Guidelines in Oncology™ Non-Small Cell Lung Cancer (Version 2.2010) Vol. 2009. National Comprehensive Cancer Network, Inc; 2010. [Google Scholar]
- 8.Small W, Mell LK, Anderson P, et al. Consensus guidelines for delineation of clinical target volume for intensity-modulated pelvic radiotherapy in postoperative treatment of endometrial and cervical cancer. International Journal of Radiation Oncology Biology Physics. 2008;71:428–434. doi: 10.1016/j.ijrobp.2007.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lawton CAF, Michalski J, El-Naqa I, et al. RTOG GU Radiation Oncology Specialists Reach Consensus on Pelvic Lymph Node Volumes for High-Risk Prostate Cancer. International Journal of Radiation Oncology Biology Physics. 2009;74:383–387. doi: 10.1016/j.ijrobp.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Myerson RJ, Garofalo MC, El Naqa I, et al. Elective Clinical Target Volumes for Conformal Therapy in Anorectal Cancer: A Radiation Therapy Oncology Group Consensus Panel Contouring Atlas. International Journal of Radiation Oncology Biology Physics. 2009;74:824–830. doi: 10.1016/j.ijrobp.2008.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Senan S, De Ruysscher D, Giraud P, et al. Literature-based recommendations for treatment planning and execution in high-dose radiotherapy for lung cancer. Radiotherapy and Oncology. 2004;71:139–146. doi: 10.1016/j.radonc.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 12.Imaging for Oncology: Collaboration between Clinical Radiologists and Clinical Oncologists in Diagnosis, Staging, and Radiotherapy Planning. London: Royal College of Radiologists; 2004. [Google Scholar]
- 13.Radiology ACo, editor. Manual on Contrast Media, Version 6. American College of Radiology; 2008. [Google Scholar]
- 14.Goitein M. Patient Positioning During CT Scanning. In: Ling CC, Rogers CC, Morton RJ, et al., editors. Computed tomography in radiation therapy. New York: Raven Press; 1983. pp. 147–153. [Google Scholar]
- 15.Kim S, Russell W, Price P, et al. Suboptimal use of intravenous contrast during radiotherapy planning in the UK. British Journal of Radiology. 2008;81:963–969. doi: 10.1259/bjr/24432468. [DOI] [PubMed] [Google Scholar]
- 16.Echenique AM, Joseph R, Casillas VJ. Recognition and treatment of reactions to contrast media: A model for resident and faculty education employing lectures and case scenario workshops. Academic Radiology. 1997;4:230–234. doi: 10.1016/s1076-6332(05)80296-x. [DOI] [PubMed] [Google Scholar]
- 17.Sica GT, Barron DM, Blum R, et al. Computerized realistic simulation: A teaching module for crisis management in radiology. American Journal of Roentgenology. 1999;172:301–304. doi: 10.2214/ajr.172.2.9930771. [DOI] [PubMed] [Google Scholar]
- 18.Gaca AM, Frush DP, Hohenhaus SM, et al. Enhancing pediatric safety: Using simulation to assess radiology resident preparedness for anaphylaxis from intravenous contrast media. Radiology. 2007;245:236–244. doi: 10.1148/radiol.2451061381. [DOI] [PubMed] [Google Scholar]
- 19.Lerner C, Gaca AM, Frush DP, et al. Enhancing pediatric safety: assessing and improving resident competency in life-threatening events with a computer-based interactive resuscitation tool. Pediatric Radiology. 2009;39:703–709. doi: 10.1007/s00247-009-1265-y. [DOI] [PubMed] [Google Scholar]
- 20.Tubbs RJ, Murphy B, Mainiero MB, et al. High-fidelity medical simulation as an assessment tool for radiology residents' acute contrast reaction management skills. J Am Coll Radiol. 2009;6:582–587. doi: 10.1016/j.jacr.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 21.Becker GJ. Simulation and the coming transformation of medical education and training. Radiology. 2007;245:7–9. doi: 10.1148/radiol.2451070674. [DOI] [PubMed] [Google Scholar]
- 22.Wenk M, Waurick R, Schotes D, et al. Simulation-based medical education is no better than problem-based discussions and induces misjudgment in self-assessment. Advances in Health Sciences Education. 2009;14:159–171. doi: 10.1007/s10459-008-9098-2. [DOI] [PubMed] [Google Scholar]
- 23.Pierluissi E, Fischer MA, Campbell AR, Landefeld CS. Discussion of medical errors in morbidity and mortality conferences. JAMA. 2003 Dec 3;290(21):2838–2842. doi: 10.1001/jama.290.21.2838. [DOI] [PubMed] [Google Scholar]
- 24.Shojania KG, Duncan BW, McDonald KM, et al., editors. Making Health Care Safer: A Critical Analysis of Patient Safety Practices. Agency for Healthcare Research and Quality; 2001. [PMC free article] [PubMed] [Google Scholar]
- 25.Delaney G, Jacob S, Featherstone C, et al. The role of radiotherapy in cancer treatment: estimating optimal utilization from a review of evidence-based clinical guidelines. Cancer. 2005;104:1129–1137. doi: 10.1002/cncr.21324. [DOI] [PubMed] [Google Scholar]
- 26.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]