Abstract
The discoveries of rapid, membrane-initiated steroid actions and central nervous system steroidogenesis have changed our understanding of the neuroendocrinology of reproduction. Classical nuclear actions of estradiol and progesterone steroids affecting transcription are essential. However, with the discoveries of membrane-associated steroid receptors, it is becoming clear that estradiol and progesterone have neurotransmitter-like actions activating intracellular events. Ultimately, membrane-initiated actions can influence transcription. Estradiol membrane-initiated signaling (EMS) modulates female sexual receptivity and estrogen feedback regulating the luteinizing hormone (LH) surge. In the arcuate nucleus, EMS activates a lordosis-regulating circuit that extends to the medial preoptic nucleus and subsequently to the ventromedial nucleus (VMH)—the output from the limbic and hypothalamic regions. Here, we discuss how EMS leads to an active inhibition of lordosis behavior. To stimulate ovulation, EMS facilitates astrocyte synthesis of progesterone (neuroP) in the hypothalamus. Regulation of GnRH release driving the LH surge is dependent on estradiol-sensitive kisspeptin (Kiss1) expression in the rostral periventricular nucleus of the third ventricle (RP3V). NeuroP activation of the LH surge depends on Kiss1, but the specifics of signaling have not been well elucidated. RP3V Kiss1 neurons appear to integrate estradiol and progesterone information which feeds back onto GnRH neurons to stimulate the LH surge. In a second population of Kiss1 neurons, estradiol suppresses the surge but maintains tonic LH release, another critical component of the estrous cycle. Together, evidence suggests that regulation of reproduction involves membrane action of steroids, some of which are synthesized in the brain.
Introduction
Over the years, it has become clear that steroids have global effects and that the brain produces neurosteroids synthesized de novo in both glia and neurons [reviewed in (99, 126, 181)]. Coincident with these discoveries was the finding that steroid signaling is not restricted to the regulation of transcription, but steroids can also rapidly elicit intracellular changes [e.g., (18, 24, 115, 161)]. These observations have radically changed our understanding of the sex steroid modulation of central nervous system circuits including those that that mediate reproductive physiology and behavior. While there is no doubt that sex steroids can and do behave like traditional steroid hormones, the ability of the brain to synthesize and rapidly respond to steroids strongly suggests that sex steroids can also signal like neurotransmitters. Conceptually, neurosteroids may be considered fourth generation (fourth Gen) transmitters (74, 120), whose members include nitric oxide (NO), prostaglandins, and endocanabinoids [reviewed in (6, 95, 157)]. A hallmark of fourth Gen transmitters is that unlike cholinergic, amino acid, adrenergic, or neuropeptide transmitters, they are regulated at the point of synthesis rather than release. Once synthesized, these molecules diffuse out of the cell to activate nearby receptors, which couple to a wide array of signaling cascades (e.g., cAMP, MAPK, Akt, Src, and Ca2+), and lead to either excitation or inhibition of “postsynaptic” neurons.
Sex steroids, for decades, were considered to be classic hormones whose receptors acted as ligand-gated transcription factors at specific sites on DNA [estrogen response element, ERE, the AP-1 site (209) or at the Sp1 site (82, 175)]. Both physiological and anatomical studies were tailored to examine this nuclear role of steroid hormones in the brain. For example, when examining the expression of neuropeptides or receptors, steroids were given for an extended time, from 48 h to weeks. Similarly, autoradiographic studies to identify steroid-receptive cells were quantified by examining the accumulation of exposed silver grains marking the location of radiolabeled estradiol (or testosterone or progesterone). A steroid-receptive cell was identified as a cell with silver grains concentrated over the nucleus—the apparent site of steroid action (152, 206). Interestingly, sites in the brain that had accumulations of silver grains over cell nuclei also had higher “background” compared with nonsteroid sensitive regions (Fig. 1). This was usually not discussed in formal reports, but it was debated among autoradiographers who proposed that high “background” might imply the presence of steroid receptors on the membrane.
Figure 1.
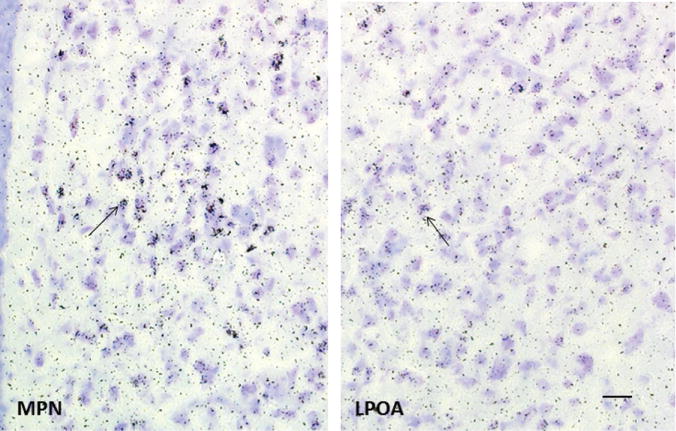
Female rats were anesthetized with pentobarbital and injection (i.v.) with 100 μCi/100 g body weight of 2,4,6,7,16,17-[3H]estradiol (specific activity 130 Ci/mmol, New England Nuclear). At 2 h after injection of the isotope, animals were killed, brains removed and cryosectioned (10 μm) through the medial preoptic nucleus (MPN) and lateral preoptic area (LPOA). Sections were collected under safe-light conditions and thaw-mounted onto slides which had been coated with nuclear track emulsion and exposed at −70°C for 28 weeks, then photodeveloped. The MPN contains many cells that concentrate 3H-estradiol as indicated by the accumulation of silver grains over nuclei (arrow). In the LPOA, some cells have accumulations of silver grains (arrow) but levels are much lower in both the estradiol-accumulating cells and the surrounding background compared with the MPN. Bar in LPOA is 20 μm and applies to both images. Sections are adapted, with permission, from a study by Akesson TR and Micevych PE (4).
In fact, over the years, there had been indications that not all steroid action could be explained by nuclear receptors. In the periphery, the seminal work of Clair Szego and coworkers demonstrated the accumulation of cAMP within the uterus just minutes after exposure to estradiol (200). In the brain, initial clues about the rapid action of estradiol were reported by Martin Kelly and co-workers [(76, 77); reviewed in (75)]; however, the prevailing idea at the time was that steroids (estradiol in particular) acted in the nucleus to modulate transcription of genes.
Indeed, it is a well-established fact that estradiol, acting as a hormone, affects gene transcription, and can thus affect physiology and behavior. For example, the expression of a large number of neuropeptides and receptors implicated in reproductive function are regulated by estradiol [reviewed in (119)]. However, in some cases, colocalization of nuclear ER and estrogen-sensitive neuropeptides could not be demonstrated (5), suggesting that other mechanisms were involved. One possibility was that steroids act indirectly, on afferent neurons whose neurotransmitter(s) modulate protein expression in target neurons. The most famous example of this is the GnRH neuron, which does not express the steroid receptors critical for neural control of ovulation, but whose activity and GnRH expression are regulated by estradiol and progesterone [(187); reviewed in (34)].
Reports of membrane actions of estradiol increased in the 1990s [e.g., (115)], but it was the demonstration that nuclear ERs were trafficked to the plasma membrane by Ellis Levin’s group (166) that changed the way we viewed and understood membrane-initiated steroid signaling. Prior to this discovery, it was assumed that the membrane ER would be a different receptor from the nuclear ER. There are now several such candidates for the cell-surface membrane ERs. One such candidate is GPR30/GPER, a seven transmembrane receptor first described in breast cancer cells [(22,169); reviewed in (159)]. However, in hypothalamic cells, GPR30/GPER has not been localized to the cell surface (15, 48, 58), but appears to be present in another subcellular compartment, the smooth endoplasmic reticulum. As estradiol can freely diffuse into the cell, GPR30/GPER in this compartment may be readily activated (88). The precise role for GPR30/GPER is unknown, as either disruption of the coding region of GPR30/GPER or knockdown of the gene does not appear to influence reproduction (70, 144). It has been proposed that GPR30/GPER interacts with ERα at the membrane to organize downstream signaling although the details of such a role have not been elucidated (96). Another potential membrane ER is Gq-mER, which is activated by a tamoxifen derivation, STX (161). Gq-mER, which is blocked by the universal ER antagonist faslodex (ICI 182,780), activates kinase pathways and retains its activity in ERα, ERβ, and GPR30/GPER knockout mice [reviewed in (128,170)]. STX activation of Gq-mER in the arcuate nucleus (ARH) potentiates sexual receptivity, suggesting a role in mediating reproductive behavior (31). At present, it is difficult to sort which one (or more) of these putative receptors are responsible for estradiol actions at the membrane. The predominance of evidence suggests that ERα mediates the critical actions of estradiol for the central control of reproduction (128,129). Found in the cell nucleus and membrane, the same receptor, ERα, mediates estradiol’s slow, hormone-like actions, and more rapid, neurotransmitter-like effects.
This review discusses the estradiol activation of female sexual receptive behavior and the control of gonadotropin release by the hypothalamus. Both of these events require the hormonal (direct nuclear) and the neurotransmitter (membrane) actions for a coordinated response.
Sexual Receptivity
Estradiol is essential for the eventual induction of sexual receptivity, which in rodents is expressed as lordosis behavior [reviewed in (116)]. However, immediately after systemic estradiol treatment, ovariectomized (ovx) rats are not sexually receptive (11). Explanations have ranged from the ideas that transcription and the formation of active dendritic spines take days (29) to the idea that estradiol activates inhibitory circuits (192, 194). In fact, all of these processes have been shown to be necessary. Under the rubric of longer timescale events, estradiol induces a plethora of protein expression throughout the nuclei of the limbic-hypothalamic circuit including the medial preoptic area (MPN), the bed nucleus of the stria terminalis (BST), posteriodorsal part of the medial nucleus of the amygdala (MeApd) and ventromedial nucleus of the hypothalamus (VMH). Indeed, this was convincingly demonstrated in a classic study, in which blocking protein synthesis in the hypothalamus prevented the display of lordosis behavior (150). This estrogenic transcriptional regulation is widespread including neuropeptides [e.g., CCK, enkephalins (123,127,158)], transmitter-synthesizing enzymes [e.g., GAD (139)] and receptors [e.g., opioid receptors, CCK receptors (151, 156, 162)]. There are also complexities in estradiol’s influence on peptide expression that include the opioid regulation of estrogen-induced CCK expression (52). Arguably the most critical expression for regulation of sexual receptivity (and perhaps reproduction) is the estradiol-induction of progesterone receptors [(12, 13); reviewed in (107)]. While estradiol-only induced sexual receptivity is not dependent on progesterone receptors, estradiol + progesterone induced behavior (the natural in vivo condition) requires activation of progesterone receptors (108). In addition to these more classically hormonal actions, lordosis behavior is also dependent on estrogen membrane-initiated signaling (EMS).
Estradiol membrane-initiated signaling
Within minutes of treatment with estradiol, an active inhibition of lordosis is initiated in the ARH (Fig. 2). Here, estradiol induces the release of neuropeptide Y (NPY) onto proopiomelanocortin (POMC) neurons expressing NPY-Y1 receptors and GABAB receptors (134) (190). These neurons project to the MPN where released β-endorphin (β-END) activates and induces internalization of μ-opioid receptors (MOR) (53). The MOR neurons in turn innervate VMH neurons regulating the descending output of the hypothalamus that controls lordosis behavior [for review, see (126)]. For approximately 20 h, this activation of MOR prevents the display of lordosis behavior. In the gonadally intact female, progesterone reverses the estradiol inhibition—allowing for lordosis (191).
Figure 2.
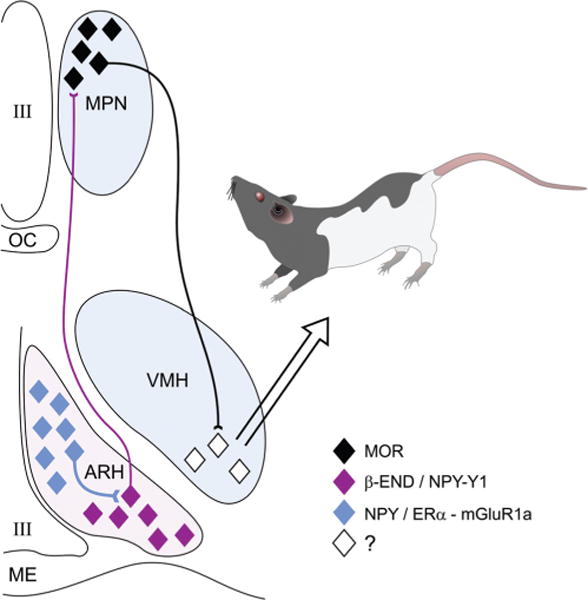
The estradiol (E2) induction of sexual receptivity in the female rat is indicated by lordosis behavior. The CNS regulation of this global response to hormonal and sensory input is regulated by a diffuse circuit that extends from the limbic system to the spinal cord. Within this lordosis regulating circuit, E2 acts rapidly through estrogen membrane signaling (EMS) to release neuropeptide Y (NPY) in the arcuate nucleus of the hypothalamus (ARH), which activates β-endorphin (β-END) projection neurons that extend to the medial preoptic nucleus (MPN). The MPN is an important integrative node receiving accessory olfactory and limbic input. β-END activates μ-opioid receptors (MOR), producing a transient inhibition of the MPN which is relieved by progesterone in the cycling female. The MPN MOR neurons in turn project to the ventromedial nucleus of the hypothalamus (VMH), the final common output of the hypothalamus. The integrated hypothalamic output is modified by inputs from the periaquaductal gray, and the vestibular complex on its way to the motoneurons mediating lordosis behavior. The EMS that mediates this activation of the circuit requires the transactivation of metabotropic glutamate receptor-1a (mGluR1a), which leads to the phosphorylation of PKCθ and the release of NPY and activation of the Y1 receptor on β-END projection cells. The EMS and resulting transient inhibition is necessary for the full expression of lordosis behavior in the rat [adapted, with permission, from (116)].
The time course of estradiol-induced MOR activation along with the ability of a membrane-impermeable estradiol (E-biotin) introduced into the ARH to activate MOR indicated an EMS action (44). These results are consistent with the rapid estradiol inhibition of MPN neuronal firing (83) and the necessity of an opioid inhibition for full lordosis behavior (194, 207, 208). Thus, the induction of sexual receptivity requires both relatively rapid EMS and nuclear-initiated increase in protein expression. Such cooperation between EMS and nuclear estradiol signaling was demonstrated using estradiol in conjunction with E-BSA, another membrane-impermeable estradiol (84). In these experiments, pretreatment with E-BSA augmented the estradiol benzoate (EB)-induced lordosis behavior in ovx rats, which is consistent with the activation of a transient inhibitory circuit that allows for the full display of sexual receptivity behavior.
EMS-mediated lordosis behavior depends on ERα, which induces PKCθ phosphorylation in ARH and increases free cytoplasmic Ca2+ ([Ca2+]i) in immortalized NPY neurons (45, 46, 130). Most putative membrane ERs implicated in membrane-initiated signaling share the ability to active G protein-coupled cascades (117, 128). For ERα on the membrane (mERα), this requires a transactivation of metabotropic glutamate receptor-1a (mGluR1a) (18), which initiates an intracellular signaling cascade involving Ca2+ release and a series of kinases, which can also ultimately modulate gene transcription (2, 27, 45, 89, 112, 142, 167, 185). Indeed, ERα transactivation of mGluR1a in the ARH is needed for the estradiol activation of MOR in the MPN and eventual lordosis behavior. Direct stimulation of mGluR1a, without mERα activation also results in MOR internalization and facilitation of lordosis behavior, further supporting a downstream role for mGluR1a in mERα-initiated signaling (44). Similarly, further evidence of this rapid estradiol signaling is that PKC stimulation in the ARH can overcome both ER and mGluR1a antagonism to stimulate lordosis behavior (45). This set of experiments show that lordosis behavior, a classical assay of estradiol action, has an important EMS component and underscores the fundamental importance of ER-mGluR signaling in explaining estradiol actions in the neuraxis.
Though not within the scope of this review, it is worth noting that estradiol actions in ARH neurons may directly impact energy balance—a hypothalamic function with which NPY and POMC neurons are more commonly associated. Sex steroid modulation of appetite, satiety, and energy expenditure is incredibly complex, though estrogens have been shown to increase activity and depress appetite. Withdrawal of ovarian steroids is associated with increased body weight and early work demonstrated this to be associated with increased meal size (14). Estradiol acting through ERα appears to be the dominant player in ovarian steroid control of body weight, as ERα knockout mice have increased adiposity (65). The critical site for ERα activation in maintenance of body weight has not been determined, but is very likely to involve NPY and POMC neurons [see (20) for an in depth review of sex hormones and energy balance]. However, tract tracing studies indicate that ~10% of the ARH POMC neurons project to any single target (79). Our own observations indicate that the MPN-projecting POMC neurons are not the same as those that project to the paraventricular nucleus and lateral hypothalamus (unpublished observations, Dong and Micevych).
Dynamics of membrane ERα: Trafficking and internalization
Receptor levels on the cell membrane are not static. Levels of mERα are modified by the counteracting processes of trafficking to the membrane and removal from the membrane or internalization. To be trafficked to the cell membrane, ERα, a classic ligand-gated nuclear receptor, must be palmitoylated and complexed with caveolin-1 [CAV1 (111, 165)]. In the ARH, CAV1 knockdown does not affect intracellular levels of ERα, but significantly reduces levels of membrane ERα confirming that CAV1 is important for the trafficking of ERα to the membrane (30). Significantly, in animals with reduced mERα in the ARH, estradiol-induced lordosis behavior is attenuated, confirming that EMS is an important component of the estradiol actions in the ARH to induce sexual receptivity. To further study membrane ERα, we utilized both primary embryonic hypothalamic neuronal cultures and immortalized hypothalamic NPY expressing neurons (N-38). Interestingly, both the primary cultured neurons and the N-38s expressed full length membrane ERα (66 kDa) and a form of ERα translated from an alternatively spliced mRNA that is missing exon 4 (ERαΔ4) (46, 48). While the function of ERαΔ4 remains to be elucidated, it is the full-length ERα that interacts with mGluR1a to induce PKCθ phosphorylation in the N-38s. This is the same PKC isoform that is activated by EMS in the ARH, and on which EB-induced lordosis depends (45). Interestingly, estradiol-induced trafficking of ERα and ERαΔ4 to the membrane requires PKC activation (46). These results demonstrate the ability of estradiol to regulate its receptors on the membrane through which estradiol modulates its own signaling.
Internalization, the process opposing trafficking, can limit cellular responses initiated by agonist stimulation or adaptation to a persistent stimulus (i.e., desensitization). Internalization removes ligand-bound receptors from the cell membrane and sequesters them into early endosomes, where the receptor releases its ligand. We reasoned that such a mechanism was critical to constrain estradiol signaling in the face of long lasting estradiol stimulation. The internalization mechanism involves activation of G protein-coupled receptor kinases (GRKs), which in turn phosphorylate membrane receptors, including mERα (37, 47). Subsequently, arrestins bind to the phosphorylated receptors, uncouple G proteins and link receptors to clathrin-dependent internalization pathways (32,101,132,217). Estradiol treatment of N-38 neurons results in rapid ERα and ERαΔ4 internalization that is counterbalanced by their trafficking to the membrane, but within several hours, mERα levels are depleted indicating a downregulation of this receptor. To establish that the initial internalization is due to arrestins, we first determined that both N-38 and the ARH have β-arrestin-1 (Arrb1) as the predominate arrestin. Then, we used siRNA to knock down Arrb1 and found that mERα and ERαΔ4 internalization was significantly attenuated. In fact, membrane levels were increased indicating that trafficking to the membrane was not compromised, only internalization, which we measured by tracking surface-biotinylated mERs (211).
In vitro, cell surface ERα and ERαΔ4 are downregulated by 2 h of estradiol treatment while in vivo, EMS is prolonged, lasting for more than 30 h. If internalization abrogates EMS, then the time course of internalization does not appear to match action of estradiol in the ARH to MPN circuit (191). A possible explanation is that internalized ERs continue signaling. This has been described for other sequestered membrane receptors where arrestins organize intracellular signaling through a scaffolding action to bind and organize downstream signaling molecules (e.g., Ras/Raf/MEK) (57, 92, 113, 132). To test whether EMS involved Arrb1 and whether internalized ERs continue to signal we tracked estradiol induced MAPK (ERK1/2) phosphorylation, in vitro. As suggested by the in vivo results, this signaling continued after mERα was internalized (211). Direct demonstration of the role of Arrb1 was obtained by siRNA knockdown that prevented both the initial EMS and endosomal signaling in vitro, and in vivo where Arrb1 knockdown in the ARH prevented estradiol-induced lordosis behavior (211). These results provide a mechanistic explanation of estradiol-only activation of female sexual receptivity. Physiological doses of EB require progesterone treatment to stimulate lordosis (103). These levels of EB maintain EMS, even after membrane levels of ERs are reduced. However, our results that internalized mER continues to signal suggests that estradiol continues to activate the opioid circuitry inhibiting sexual receptivity even after the receptors are missing from the cell membrane.
We still do not understand precisely how progesterone activation of neural signaling interacts with direct nuclear estradiol action and EMS, to modulate sexual receptivity. The importance of progesterone to sexual receptivity has been known for decades, and this was formally established by breeding mice with a null mutation in the progesterone receptor gene (100). These null PR mice were not able to show lordosis behavior following estradiol plus progesterone stimulation. Within the brain, progesterone actions are complex including their activation by dopamine through the D1 receptor (106). There is now intriguing evidence that progesterone activates a separate neural circuit to facilitate lordosis behavior in distinction to the circuit activated by estradiol-only facilitation of lordosis behavior. Following the initial inhibition, estradiol activates an opioid circuit that involves orphanin FQ/nociceptin (193). However, in estradiol primed females, progesterone activates another circuit that coexists in the ARH and interacts with the lordosis inhibitory elements (176, 177, 189). Interestingly, progesterone facilitates sexual receptivity by suppressing activity of β-END neurons (183), but whether progesterone acts presynaptically or directly on these neurons has not been resolved. What is clear is that the progesterone activation of lordosis behavior requires activation of progesterone receptor isoform B and Src kinase, a member of the nonreceptor tyrosine kinase family. Within 30 minutes, progesterone infusions into the ARH facilitate lordosis behavior. These relatively rapid actions are blocked by antagonism of Src family kinases (183). At present, coimmunoprecipitation suggests a direct interaction of membrane-associated progesterone receptor-B with Src (153). Thus, the actions of estradiol and progesterone are relatively rapid, involving membrane-initiated signaling in different ARH circuits that converge on β-END neurons.
Control of Estrogen Negative Feedback
In addition to its role in female sexual receptivity, estradiol signaling is also important in regulation of gonadotropin release. During much of the estrous cycle, estradiol inhibits surge release of gonadotropin releasing hormone (GnRH) and luteinizing hormone (LH) and maintains a tonic pattern of release from the hypothalamus and pituitary. Throughout most of the estrous cycle, LH levels are low but have an oscillatory pattern [e.g., (213)], due to the negative feedback of estrogens. Withdrawal of this feedback (e.g., following ovx) is marked by high LH levels. Estradiol negative feedback is dependent on ERα (49, 214), which is not expressed in GnRH neurons. Mounting evidence implicates neurons in the ARH, coexpressing ERα, kisspeptin (Kiss1), neurokinin B, and dynorphin [known as kisspeptin/neurokinin B/dynorphin (KNDy) neurons], as mediators of estrogen negative feedback [(28,114,135,137); reviewed in (93)].
Kisspeptin/neurokinin B/dynorphin neurons and estrogen negative feedback
KNDy neurons exhibit many features consistent with a role in mediating estrogen negative feedback. Importantly, KNDy neurons express ERα and project to GnRH neurons, criteria that are necessary for a population of neurons mediating estrogen negative feedback. GnRH neurons express GPR54 and NK3R (85,204), the dominant receptors for kisspeptin and neurokinin B, respectively, providing a framework for communication between KNDy and GnRH neuronal populations. Two of the KNDy peptides, Kiss1 and neurokinin B, are critical for reproduction. In humans, mutations in the genes encoding Kiss1, neurokinin B, or their respective receptors, lead to hypogonadotrophic hypogonadism (41,182,205). This disorder is marked by infertility and failure to reach sexual maturation. Animals with impaired Kiss1 or neurokinin B signaling are also subfertile [(212); reviewed in (51)]. KNDy neurons are the only cells that coexpress these two neuropeptides, further implicating these neurons in reproductive function. Infusions of Kiss1 or NK3R agonists modulate LH release although the direction of the change is not always consistent: while Kiss1 is reliably stimulatory, NK3R agonists have variable effects (36, 56, 140, 179). These disparate data suggest that timing and quantities of neuropeptide release are critical factors for central control of reproductive physiology. Timing may be particularly critical in studies examining effects of NK3R agonism because KNDy neurons, themselves, express NK3R. Therefore, NK3R agonists may affect inter-KNDy neuron communication in addition to their effects on GnRH neurons, which may explain why Kiss1 (whose receptor has not been demonstrated in KNDy neurons) seems to consistently stimulate LH release: Kiss1 does not appear to affect KNDy neurons, whereas NK3R agonists do (39, 62, 174). Indeed, factors such as physiological concentration, location, and precise timing of steroid or neuropeptide release are difficult to mirror using pharmacological approaches. Regardless, it is clear that Kiss1 and NKB play important roles in both tonic and surge LH release.
Congruent with their role as negative feedback mediators, estradiol suppresses Kiss1 and NKB gene expression in ARH KNDy neurons (1, 3, 38, 163, 164, 171, 196). Currently, the type of estradiol signaling (nuclear or EMS) regulating KNDy neurons has not been resolved, though evidence indicates that EMS may also be responsible for estrogen feedback in KNDy neurons (61). Previous studies have examined tissue from pre- versus postmenopausal women or ovx animals with prolonged exposure to estradiol. The long time course and the effect on transcription might lead one to assume that actions on Kiss1 and NKB are through classic nuclear signaling. However, these results do not exclude the possibility of membrane-to-nuclear estrogen signaling, since EMS activates CREB (2, 18). Clearly, more studies are needed to uncover these signaling pathways. The electrophysiological effects of steroids on KNDy neurons have provided some clues [reviewed in (155)]; however, the results are inconsistent. Some data show no increase in spontaneous firing rates in KNDy neurons following gonadectomy (28, 55, 174), while others do report a change (40). In ovx females, estradiol appears to suppress evoked excitability of KNDy neurons [(28) but see (7)]. The source of the variability between studies may be due to sex differences or the hormonal environment (ovx and ovx + estradiol vs. gonadally intact mice). Studies to date have examined the effects of long-term steroid exposure (intact or replaced) as opposed to acute stimulation with steroids. Whether estradiol has more acute, membrane-initiated effects in these neurons remains to be shown. Therefore, while it seems likely that KNDy neurons mediate estradiol negative feedback, much needs to be worked out regarding the nature of estradiol signaling in these neurons.
Control of Estrogen Positive Feedback
As reviewed recently, EMS plays an important role in the CNS synthesis of progesterone (neuroP) needed for estrogen positive feedback of the LH surge (90, 131). The preovulatory rise in circulating estradiol is essential for stimulating gonadotropin release (19, 54, 91), but preovulatory progesterone is also necessary for the LH surge (42,68,104,121,168). This is contrasted with the high levels of ovarian progesterone that follow ovulation, which participate in negative feedback (72). In ovx rats, exogenous estradiol alone will cause LH release (43). However, blocking progesterone receptors (PR) or progesterone synthesis prevents the estradiol-induced GnRH and LH surges in ovx rats (25), and arrests the estrous cycle in intact female rats [reviewed in (131)]. Therefore, it is clear that both estradiol and progesterone are necessary for surge release of LH.
Although the adrenal has been proposed as the source of preovulatory progesterone (21, 105, 160, 184), it is becoming clear that it is the locally synthesized progesterone that affects hypothalamic control of LH release. In ovx and adrenalectomized (ovx/adx) rats, the estradiol-induced LH surge is blunted but not blocked [(110, 121), but see (109)], supporting a role for alternate sources of progesterone. Treatment with PR antagonist RU486 or P4 synthesis inhibitors (trilostane or epostane) blocks the estradiol-induced LH surge (42,68,104,121,178,198). In gonadally intact rats, hypothalamic neuroP synthesis is critical for initiating the LH surge [(121, 125, 133); reviewed in (129)]. When neuroP synthesis in the hypothalamus is blocked, the estradiol-induced LH surge is eliminated in ovx/adx rats, but the surge can be restored with exogenous progesterone (121, 146), suggesting that neuroP plays an important role downstream of estradiol.
Regulation of neuroP synthesis in astrocytes
Hypothalamic astrocytes locally synthesize neuroP, representing a critical step of estradiol-positive feedback on gonadotropin release. Astrocytes have the steroidogenic enzymes and transport proteins required to convert cholesterol to neuroP (180). In fact, astrocytes are the most steroidogenic cells in the CNS (220). Only in females and female-derived astrocytes will estradiol stimulate neuroP biosynthesis (87, 118). The rapid neuroP synthesis is not regulated by transcription; however, it is dependent on estradiol-induced [Ca2+]i increase [(87–89, 122) but see (199)]. Using primary cultures of adult female hypothalamic astrocytes, we mapped the signaling pathway through which estradiol acts at the membrane to regulate neuroP synthesis (27). Like neurons, astrocytes express ERα, ERαΔ4, and mGluR1a on the cell membrane (15, 23, 89). Estradiol stimulation induces transactivation of mGluR1a leading to the production of inositol trisphosphate (IP3), which stimulates the release of intracellular Ca2+ stores. Stimulating [Ca2+]i even without estrogen treatment can stimulate neuroP synthesis (23, 124).
In contrast to the activation of PKC in neurons, estradiol activates protein kinase A (PKA) in astrocytes (27). The estradiol increase of [Ca2+]i activates a Ca2+-sensitive adenylyl cyclase-1 (AC1) producing cyclic AMP (cAMP) needed for phosphorylation of protein kinase A. This PKA mediates the rate-limiting step of steroid biosynthesis—cholesterol transport into the mitochondrial matrix through the activation of steroid acute regulatory protein (StAR) and translocator protein [TSPO (71,148,188)]. In the mitochondrion, cholesterol is converted to pregnenolone by the cholesterol side-chain cleavage enzyme, P450scc (CYP11A1). The resulting pregnenolone is converted by 3-β-hydroxysteroid dehydrogenase (3-βHSD) to neuroP and secreted. Inhibition of any step of this pathway via antagonists to mERα, mGluR1a, IP3 receptors, AC1, or PKA inhibits neuroprogesterone synthesis (27,88,89) (See Fig. 3).
Figure 3.
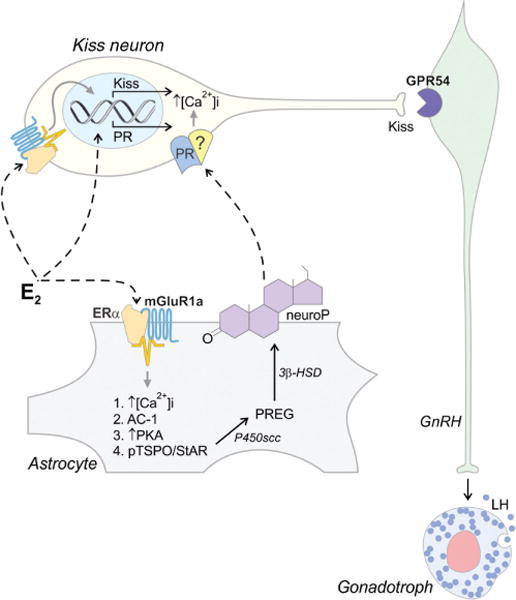
A model showing proposed estradiol (E2) actions on hypothalamic cells. In Kiss1 neurons, E2 acts at both membrane and nuclear estrogen receptors. During diestrus, classical nuclear E2 signaling induces PR expression in kisspeptin (Kiss1) neurons in the RP3V. On proestrus, rising E2 leads to transactivation of mGluR1a in astrocytes, which increases [Ca2+]i leading to stimulation of a kinase cascade resulting in the activation of translocator protein (TSPO) and steroid acute regulatory protein (StAR), which results in an increase of cholesterol import into the mitochondrion—the rate limiting step in steroidogenesis. The resulting pregnenolone (PREG) is converted to progesterone (neuroP) by 3β-HSD. Simultaneously, E2 activates an ERα-mGluR1a complex in neurons leading to the expression of Kiss1. Newly synthesized neuroP diffuses out of the astrocytes and activates E2-induced PR, which have been trafficked to the Kiss1 neuronal membrane. This leads to a series of events culminating in Kiss1 secretion onto GPR54 expressing GnRH neurons. We hypothesize that signaling through the membrane PR involves transactivation of another receptor (indicated by [?]) to stimulate [Ca2+]i, and induce Kiss1 release, activating GnRH neurons and triggering the E2-induced LH surge from anterior pituitary gonadotrophs.
RP3V Kiss1 neurons: Integrators of steroid input to the GnRH neuron
In addition to the ARH, Kiss1 expressing neurons are also found in the rostral periventricular nucleus, RP3V (an area including the anterior periventricular nucleus and AVPV). These neurons are prime candidates to integrate estradiol and progesterone information that regulates GnRH secretion underlying the LH surge. Although most of the work on steroid regulation of Kiss1 and its gene, KiSS-1 has focused on estradiol (80,197), there are interesting results that suggest estradiol and neuroP may interact to stimulate Kiss1 in the RP3V (136). First, both ERα and PR are needed for positive feedback of the LH surge (25, 26, 210), and both have been localized in RP3V Kiss1 neurons, but neither are found in GnRH neurons (67, 94, 186, 196, 218). A substantial number of Kiss1 neurons in RP3V express PR after estradiol treatment (33, 195, 197, 218). During proestrus, rising estradiol levels induce neuroP synthesis, and Kiss1 expression in the RP3V. Kiss1 neurons innervate GnRH neurons that express the Kiss1 receptor, GPR54 (17, 35, 63, 66, 69, 81, 97, 143, 149, 172, 210) and Kiss1 is the most potent activator of GnRH neurons (33, 50, 63, 98, 154, 196, 216). Together, these findings indicate that Kiss1 neurons are presynaptic to GnRH neurons and are the primary modulators of GnRH neuronal activity [as reviewed in (33, 78)].
In a continuation of these studies, we showed that neuroP acts though Kiss1 to mediate estrogen positive feedback (see Fig. 3). Preliminary experiments demonstrate that exogenous progesterone rescues the LH surge in females where hypothalamic steroidogenesis was blocked with the CYP11A1 inhibitor aminoglutethimide [AGT (145, 146)]. Further, infusions of Kiss1 into the diagonal band of Broca (DBB) in AGT-treated animals induced an LH surge, confirming that Kiss1 operates downstream of neuroP. Finally, Kiss1 knockdown in the RP3V prevented the estradiol-induced LH surge. The nature of progesterone signaling in Kiss1 neurons remains to be elucidated. In addition to classical nuclear PR, there are intriguing suggestions that Kiss1 neurons may have membrane progesterone receptors, especially mPRβ (136, 219). The mPRs are 7-transmembrane domain proteins that belong to the progestin and adipoQ receptor (PAQR) family, not the classic G protein-coupled receptor (GPCR) family (138,201,203). PAQRs can signal through MAPK activation by increasing [Ca2+]i [(9, 10, 64, 73, 138, 147, 202, 215); but see (86)]. It will be interesting to determine the role of these mPRs in reproduction in light of the demonstrated importance of classical PRs.
Sexual differentiation of neuroP synthesis in astrocytes
In rodents, the ability to have estrogen positive feedback is highly sexually differentiated, with males losing this ability upon exposure to testosterone during development [(60, 102, 141, 173) reviewed in (59) and (16)]. While both male and female rodents synthesize neuroP, estradiol-stimulated neuroP is specific to females in vivo (121). To determine whether hypothalamic astrocytes are sexually differentiated and whether gonadal steroid or chromosomal actions are responsible, astrocytes were isolated and cultured from wild-type male and female mice as well as from “Four Core Genotype” mice in which the chromosomal sex and the gonadal sex are uncoupled through the deletion of the sex determining region of the Y chromosome (Sry) from the Y chromosome and insertion onto an autosome [reviewed in (8)]. Regardless of the chromosomal sex, astrocytes from mice with testes did not respond to estradiol with an increase on neuroP synthesis, a finding consistent with our idea that astrocytes provide the necessary neuroP needed for positive feedback of the LH surge. While the mechanism through which testosterone blocks estradiol action in astrocytes has not been characterized, we have observed that only in astrocytes from gonadal females does estradiol increase mERα levels, which results in the [Ca2+]i signaling required for neuroP synthesis. This indicates that sexual differentiation of astrocytes is mediated by sex steroid hormones and suggests that early exposure to testosterone affects the mechanism responsible for trafficking ERα to the cell membrane.
Conclusion
The studies reviewed here demonstrate that estradiol acts as both a classical steroid hormone and as a neurotransmitter in the CNS to regulate reproduction. It is clear that EMS is responsible for the estradiol stimulation of neuroP synthesis— a step needed for estrogen positive feedback. Interestingly, neuroP does not appear to affect sexually receptive behavior but it does stimulate proceptivity in rats (122). Specifics of estradiol signaling in both positive and negative feedback governing LH release remain to be elucidated. Further, the picture of how estrogens and progestins interact has recently grown more complicated with the discovery of membrane and membrane to nucleus signaling for estradiol and for progesterone. While much has been learned, much remains to be learned, especially about the actions of progesterone both in reproductive behavior and control of the LH surge.
References
- 1.Abel TW, Voytko ML, Rance NE. The effects of hormone replacement therapy on hypothalamic neuropeptide gene expression in a primate model of menopause. J Clin Endocrinol Metab. 1999;84:2111–2118. doi: 10.1210/jcem.84.6.5689. [DOI] [PubMed] [Google Scholar]
- 2.Abraham IM, Todman MG, Korach KS, Herbison AE. Critical in vivo roles for classical estrogen receptors in rapid estrogen actions on intracellular signaling in mouse brain. Endocrinology. 2004;145:3055–3061. doi: 10.1210/en.2003-1676. [DOI] [PubMed] [Google Scholar]
- 3.Adachi S, Yamada S, Takatsu Y, Matsui H, Kinoshita M, Takase K, Sugiura H, Ohtaki T, Matsumoto H, Uenoyama Y, Tsukamura H, Inoue K, Maeda K. Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J Reprod Dev. 2007;53:367–378. doi: 10.1262/jrd.18146. [DOI] [PubMed] [Google Scholar]
- 4.Akesson TR, Micevych PE. Endogenous opioid-immunoreactive neurons of the ventromedial hypothalamic nucleus concentrate estrogen in male and female rats. J Neurosci Res. 1991;28:359–366. doi: 10.1002/jnr.490280307. [DOI] [PubMed] [Google Scholar]
- 5.Akesson TR, Micevych PE. Evidence for an absence of estrogen concentration by CCK-immunoreactive neurons in the hypothalamus of the female rat. J Neurobiol. 1988;19:3–16. doi: 10.1002/neu.480190103. [DOI] [PubMed] [Google Scholar]
- 6.Alger BE. Retrograde signaling in the regulation of synaptic transmission: Focus on endocannabinoids. Prog Neurobiol. 2002;68:247–286. doi: 10.1016/s0301-0082(02)00080-1. [DOI] [PubMed] [Google Scholar]
- 7.Alreja M. Electrophysiology of kisspeptin neurons. Adv Exp Med Biol. 2013;784:349–362. doi: 10.1007/978-1-4614-6199-9_16. [DOI] [PubMed] [Google Scholar]
- 8.Arnold AP, Burgoyne PS. Are XX and XY brain cells intrinsically different? Trends Endocrinol Metab. 2004;15:6–11. doi: 10.1016/j.tem.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Ashley RL, Clay CM, Farmerie TA, Niswender GD, Nett TM. Cloning and characterization of an ovine intracellular seven transmembrane receptor for progesterone that mediates calcium mobilization. Endocrinology. 2006;147:4151–4159. doi: 10.1210/en.2006-0002. [DOI] [PubMed] [Google Scholar]
- 10.Bashour NM, Wray S. Progesterone directly and rapidly inhibits GnRH neuronal activity via progesterone receptor membrane component 1. Endocrinology. 2012;153:4457–4469. doi: 10.1210/en.2012-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beach FA. Hormones and Behavior. New York: Paul B Hoeber; 1948. [Google Scholar]
- 12.Blaustein JD, Feder HH. Cytoplasmic progestin receptors in female guinea pig brain and their relationship to refractoriness in expression of female sexual behavior. Brain Res. 1979;177:489–498. doi: 10.1016/0006-8993(79)90466-9. [DOI] [PubMed] [Google Scholar]
- 13.Blaustein JD, Feder HH. Nuclear progestin receptors in guinea pig brain measured by an in vitro exchange assay after hormonal treatments that affect lordosis. Endocrinol. 1980;106:1061–1069. doi: 10.1210/endo-106-4-1061. [DOI] [PubMed] [Google Scholar]
- 14.Blaustein JD, Wade GN. Ovarian influences on the meal patterns of female rats. Physiol Behav. 1976;17:201–208. doi: 10.1016/0031-9384(76)90064-0. [DOI] [PubMed] [Google Scholar]
- 15.Bondar G, Kuo J, Hamid N, Micevych P. Estradiol-induced estrogen receptor-alpha trafficking. J Neurosci. 2009;29:15323–15330. doi: 10.1523/JNEUROSCI.2107-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Booth JE. Sexual differentiation of the brain. In: Finn CA, editor. Oxford Reviews of Reproductive Biology. Oxford: Clarendon Press; 1979. pp. 58–158. [Google Scholar]
- 17.Bosch MA, Tonsfeldt KJ, Ronnekleiv OK. mRNA expression of ion channels in GnRH neurons: Subtype-specific regulation by 17beta-estradiol. Mol Cell Endocrinol. 2013;367:85–97. doi: 10.1016/j.mce.2012.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, Mermelstein PG. Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. J Neurosci. 2005;25:5066–5078. doi: 10.1523/JNEUROSCI.1427-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brom GM, Schwartz NB. Acute changes in the estrous cycle following ovariectomy in the golden hamster. Neuroendocrinology. 1968;3:366–377. doi: 10.1159/000121725. [DOI] [PubMed] [Google Scholar]
- 20.Brown LM, Gent L, Davis K, Clegg DJ. Metabolic impact of sex hormones on obesity. Brain Res. 2010;1350:77–85. doi: 10.1016/j.brainres.2010.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buckingham JC, Deohler KD, Wilson CA. Activity of the pituitary-adrenocortical system and thyroid gland during the oestrous cycle of the rat. J Endocrinol. 1978;78:359–366. doi: 10.1677/joe.0.0780359. [DOI] [PubMed] [Google Scholar]
- 22.Carmeci C, Thompson DA, Ring HZ, Francke U, Weigel RJ. Identification of a gene (GPR30) with homology to the G-protein-coupled receptor superfamily associated with estrogen receptor expression in breast cancer. Genomics. 1997;45:607–617. doi: 10.1006/geno.1997.4972. [DOI] [PubMed] [Google Scholar]
- 23.Chaban VV, Lakhter AJ, Micevych P. A membrane estrogen receptor mediates intracellular calcium release in astrocytes. Endocrinology. 2004;145:3788–3795. doi: 10.1210/en.2004-0149. [DOI] [PubMed] [Google Scholar]
- 24.Chaban VV, Mayer EA, Ennes HS, Micevych PE. Estradiol inhibits atp-induced intracellular calcium concentration increase in dorsal root ganglia neurons. Neuroscience. 2003;118:941–948. doi: 10.1016/s0306-4522(02)00915-6. [DOI] [PubMed] [Google Scholar]
- 25.Chappell PE, Levine JE. Stimulation of gonadotropin-releasing hormone surges by estrogen. I. Role of hypothalamic progesterone receptors. Endocrinology. 2000;141:1477–1485. doi: 10.1210/endo.141.4.7428. [DOI] [PubMed] [Google Scholar]
- 26.Chappell PE, Schneider JS, Kim P, Xu M, Lydon JP, O’Malley BW, Levine JE. Absence of gonadotropin surges and gonadotropin-releasing hormone self- priming in ovariectomized (OVX), estrogen (E2)-treated, progesterone receptor knockout (PRKO) mice. Endocrinology. 1999;140:3653–3658. doi: 10.1210/endo.140.8.6895. [DOI] [PubMed] [Google Scholar]
- 27.Chen C, Kuo J, Wong A, Micevych P. Estradiol modulates translocator protein (TSPO) and steroid acute regulatory protein (StAR) via protein kinase A (PKA) signaling in hypothalamic astrocytes. Endocrinology. 2014;155:2976–2985. doi: 10.1210/en.2013-1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cholanian M, Krajewski-Hall SJ, Levine RB, McMullen NT, Rance NE. Electrophysiology of arcuate neurokinin B neurons in female Tac2-EGFP transgenic mice. Endocrinology. 2014;155:2555–2565. doi: 10.1210/en.2014-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Christensen A, Dewing P, Micevych P. Membrane-initiated estradiol signaling induces spinogenesis required for female sexual receptivity. J Neurosci. 2011;31:17583–17589. doi: 10.1523/JNEUROSCI.3030-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Christensen A, Micevych P. CAV1 siRNA reduces membrane estrogen receptor-alpha levels and attenuates sexual receptivity. Endocrinology. 2012;153:3872–3877. doi: 10.1210/en.2012-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Christensen A, Micevych P. A novel membrane estrogen receptor activated by STX induces female sexual receptivity through an interacation with mGluR1a. Neuroendocrinology. 2013;97:363–368. doi: 10.1159/000351077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Claing A, Laporte SA, Caron MG, Lefkowitz RJ. Endocytosis of G protein-coupled receptors: Roles of G protein-coupled receptor kinases and beta-arrestin proteins. Prog Neurobiol. 2002;66:61–79. doi: 10.1016/s0301-0082(01)00023-5. [DOI] [PubMed] [Google Scholar]
- 33.Clarkson J, d’Anglemont de Tassigny X, Moreno AS, Colledge WH, Herbison AE. Kisspeptin-GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. J Neurosci. 2008;28:8691–8697. doi: 10.1523/JNEUROSCI.1775-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clarkson J, Herbison AE. Oestrogen, kisspeptin, GPR54 and the pre-ovulatory luteinising hormone surge. J Neuroendocrinol. 2009;21:305–311. doi: 10.1111/j.1365-2826.2009.01835.x. [DOI] [PubMed] [Google Scholar]
- 35.Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology. 2006;147:5817–5825. doi: 10.1210/en.2006-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Corander MP, Challis BG, Thompson EL, Jovanovic Z, Loraine Tung YC, Rimmington D, Huhtaniemi IT, Murphy KG, Topaloglu AK, Yeo GS, O’Rahilly S, Dhillo WS, Semple RK, Coll AP. The effects of neurokinin B upon gonadotrophin release in male rodents. J Neuroen-docrinol. 2010;22:181–187. doi: 10.1111/j.1365-2826.2009.01951.x. [DOI] [PubMed] [Google Scholar]
- 37.Daaka Y, Pitcher JA, Richardson M, Stoffel RH, Robishaw JD, Lefkowitz RJ. Receptor and G betagamma isoform-specific interactions with G protein-coupled receptor kinases. Proc Natl Acad Sci U S A. 1997;94:2180–2185. doi: 10.1073/pnas.94.6.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Danzer SC, Price RO, McMullen NT, Rance NE. Sex steroid modulation of neurokinin B gene expression in the arcuate nucleus of adult male rats. Brain Res Mol Brain Res. 1999;66:200–204. doi: 10.1016/s0169-328x(99)00024-8. [DOI] [PubMed] [Google Scholar]
- 39.de Croft S, Boehm U, Herbison AE. Neurokinin B activates arcuate kisspeptin neurons through multiple tachykinin receptors in the male mouse. Endocrinology. 2013;154:2750–2760. doi: 10.1210/en.2013-1231. [DOI] [PubMed] [Google Scholar]
- 40.de Croft S, Piet R, Mayer C, Mai O, Boehm U, Herbison AE. Spontaneous kisspeptin neuron firing in the adult mouse reveals marked sex and brain region differences but no support for a direct role in negative feedback. Endocrinology. 2012;153:5384–5393. doi: 10.1210/en.2012-1616. [DOI] [PubMed] [Google Scholar]
- 41.de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci U S A. 2003;100:10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DePaolo LV. Attenuation of preovulatory gonadotrophin surges by epostane: A new inhibitor of 3 beta-hydroxysteroid dehydrogenase. J Endocrinol. 1988;118:59–68. doi: 10.1677/joe.0.1180059. [DOI] [PubMed] [Google Scholar]
- 43.DePaolo LV, Barraclough CA. Dose dependent effects of progesterone on the facilitation and inhibition of spontaneous gonadotropin surges in estrogen treated ovariectomized rats. BiolReprod. 1979;21:1015–1023. doi: 10.1095/biolreprod21.4.1015. [DOI] [PubMed] [Google Scholar]
- 44.Dewing P, Boulware MI, Sinchak K, Christensen A, Mermelstein PG, Micevych P. Membrane estrogen receptor-alpha interactions with metabotropic glutamate receptor 1a modulate female sexual receptivity in rats. J Neurosci. 2007;27:9294–9300. doi: 10.1523/JNEUROSCI.0592-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dewing P, Christensen A, Bondar G, Micevych P. Protein kinase C signaling in the hypothalamic arcuate nucleus regulates sexual receptivity in female rats. Endocrinology. 2008;149:5934–5942. doi: 10.1210/en.2008-0847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dominguez R, Dewing P, Kuo J, Micevych P. Membrane-initiated estradiol signaling in immortalized hypothalamic N-38 neurons. Steroids. 2013;78:607–613. doi: 10.1016/j.steroids.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dominguez R, Hu E, Zhou M, Baudry M. 17Beta-estradiol-mediated neuroprotection and ERK activation require a pertussis toxin-sensitive mechanism involving GRK2 and beta-arrestin-1. J Neurosci. 2009;29:4228–4238. doi: 10.1523/JNEUROSCI.0550-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dominguez R, Micevych P. Estradiol rapidly regulates membrane estrogen receptor alpha levels in hypothalamic neurons. J Neurosci. 2010;30:12589–12596. doi: 10.1523/JNEUROSCI.1038-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dorling AA, Todman MG, Korach KS, Herbison AE. Critical role for estrogen receptor alpha in negative feedback regulation of gonadotropin-releasing hormone mRNA expression in the female mouse. Neuroendocrinology. 2003;78:204–209. doi: 10.1159/000073703. [DOI] [PubMed] [Google Scholar]
- 50.Dumalska I, Wu M, Morozova E, Liu R, van den Pol A, Alreja M. Excitatory effects of the puberty-initiating peptide kisspeptin and group I metabotropic glutamate receptor agonists differentiate two distinct subpopulations of gonadotropin-releasing hormone neurons. J Neurosci. 2008;28:8003–8013. doi: 10.1523/JNEUROSCI.1225-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dungan Lemko HM, Elias CF. Kiss of the mutant mouse: How genetically altered mice advanced our understanding of kisspeptin’s role in reproductive physiology. Endocrinology. 2012;153:5119–5129. doi: 10.1210/en.2012-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eckersell CB, Micevych PE. Opiate receptors modulate estrogen-induced cholecystokinin and tachykinin but not enkephalin mRNA levels in the limbic system and hypothalamus. Neuroscience. 1997;80:473–485. doi: 10.1016/s0306-4522(97)00072-9. [DOI] [PubMed] [Google Scholar]
- 53.Eckersell CB, Popper P, Micevych PE. Estrogen-induced alteration of mu-opioid receptor immunoreactivity in the medial preoptic nucleus and medial amygdala. J Neurosci. 1998;18:3967–3976. doi: 10.1523/JNEUROSCI.18-10-03967.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ferin M, Tempone A, Zimmering PE, Van de Wiele RL. Effect of antibodies to 17beta-estradiol and progesterone on the estrous cycle of the rat. Endocrinology. 1969;85:1070–1078. doi: 10.1210/endo-85-6-1070. [DOI] [PubMed] [Google Scholar]
- 55.Frazao R, Cravo RM, Donato J, Jr, Ratra DV, Clegg DJ, Elmquist JK, Zigman JM, Williams KW, Elias CF. Shift in Kiss1 cell activity requires estrogen receptor alpha. J Neurosci. 2013;33:2807–2820. doi: 10.1523/JNEUROSCI.1610-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gaskins GT, Glanowska KM, Moenter SM. Activation of neurokinin 3 receptors stimulates GnRH release in a location-dependent but kisspeptin-independent manner in adult mice. Endocrinology. 2013;154:3984–3989. doi: 10.1210/en.2013-1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ge L, Ly Y, Hollenberg M, De Fea K. A beta-arrestin-dependent scaffold is associated with prolonged MAPK activation in pseudopodia during protease-activated receptor-2-induced chemotaxis. J Biol Chem. 2003;278:34418–34426. doi: 10.1074/jbc.M300573200. [DOI] [PubMed] [Google Scholar]
- 58.Gorosito SV, Lorenzo AG, Cambiasso MJ. Estrogen receptor alpha is expressed on the cell-surface of embryonic hypothalamic neurons. Neuroscience. 2008;154:1173–1177. doi: 10.1016/j.neuroscience.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 59.Gorski RA. Sexual differentiation of the endocrine brain and its control. In: Motta M, editor. Brain Endocrinology. 2nd. New York: Raven Press; 1991. pp. 71–104. [Google Scholar]
- 60.Gorski RA. Sexual dimorphisms of the brain. J Anim Sci. 1985;61(Suppl 3):38–61. doi: 10.1093/ansci/61.supplement_3.38. [DOI] [PubMed] [Google Scholar]
- 61.Gottsch ML, Navarro VM, Zhao Z, Glidewell-Kenney C, Weiss J, Jameson JL, Clifton DK, Levine JE, Steiner RA. Regulation of Kiss1 and dynorphin gene expression in the murine brain by classical and nonclassical estrogen receptor pathways. J Neurosci. 2009;29:9390–9395. doi: 10.1523/JNEUROSCI.0763-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gottsch ML, Popa SM, Lawhorn JK, Qiu J, Tonsfeldt KJ, Bosch MA, Kelly MJ, Ronnekleiv OK, Sanz E, McKnight GS, Clifton DK, Palmiter RD, Steiner RA. Molecular properties of Kiss1 neurons in the arcuate nucleus of the mouse. Endocrinology. 2011;152:4298–4309. doi: 10.1210/en.2011-1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25:11349–11356. doi: 10.1523/JNEUROSCI.3328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hanna R, Pang Y, Thomas P, Zhu Y. Cell-surface expression, progestin binding, and rapid nongenomic signaling of zebrafish membrane progestin receptors alpha and beta in transfected cells. J Endocrinol. 2006;190:247–260. doi: 10.1677/joe.1.06694. [DOI] [PubMed] [Google Scholar]
- 65.Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc Natl Acad Sci U S A. 2000;97:12729–12734. doi: 10.1073/pnas.97.23.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Herbison AE, de Tassigny X, Doran J, Colledge WH. Distribution and postnatal development of Gpr54 gene expression in mouse brain and gonadotropin-releasing hormone neurons. Endocrinology. 2010;151:312–321. doi: 10.1210/en.2009-0552. [DOI] [PubMed] [Google Scholar]
- 67.Herbison AE, Theodosis DT. Localization of oestrogen receptors in preoptic neurons containing neurotensin but not tyrosine hydroxylase, cholecystokinin or luteinizing hormone-releasing hormone in the male and female rat. Neuroscience. 1992;50:283–298. doi: 10.1016/0306-4522(92)90423-y. [DOI] [PubMed] [Google Scholar]
- 68.Hibbert ML, Stouffer RL, Wolf DP, Zelinski-Wooten MB. Midcycle administration of a progesterone synthesis inhibitor prevents ovulation in primates. Proc Natl Acad Sci U S A. 1996;93:1897–1901. doi: 10.1073/pnas.93.5.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology. 2004;80:264–272. doi: 10.1159/000083140. [DOI] [PubMed] [Google Scholar]
- 70.Isensee J, Meoli L, Zazzu V, Nabzdyk C, Witt H, Soewarto D, Effertz K, Fuchs H, Gailus-Durner V, Busch D, Adler T, de Angelis MH, Irgang M, Otto C, Noppinger PR. Expression pattern of G protein-coupled receptor 30 in LacZ reporter mice. Endocrinology. 2009;150:1722–1730. doi: 10.1210/en.2008-1488. [DOI] [PubMed] [Google Scholar]
- 71.Itzhak Y, Roig-Cantisano A, Norenberg MD. Ontogeny of peripheral-type benzodiazepine receptors in cultured astrocytes and brain from rat. Brain Res Dev Brain Res. 1995;84:62–66. doi: 10.1016/0165-3806(94)00163-t. [DOI] [PubMed] [Google Scholar]
- 72.Karsch FJ, Legan SJ, Hauger RL, Foster DL. Negative feedback action of progesterone on tonic luteinizing hormone secretion in the ewe: Dependence on the ovaries. Endocrinology. 1977;101:800–806. doi: 10.1210/endo-101-3-800. [DOI] [PubMed] [Google Scholar]
- 73.Karteris E, Zervou S, Pang Y, Dong J, Hillhouse EW, Randeva HS, Thomas P. Progesterone signaling in human myometrium through two novel membrane G protein-coupled receptors: Potential role in functional progesterone withdrawal at term. Mol Endocrinol. 2006;20:1519–1534. doi: 10.1210/me.2005-0243. [DOI] [PubMed] [Google Scholar]
- 74.Kawato S, Yamada M, Kimoto T. Brain neurosteroids are 4th generation neuromessengers in the brain: Cell biophysical analysis of steroid signal transduction. Adv Biophys. 2003;37:1–48. doi: 10.1016/s0065-227x(03)80002-3. [DOI] [PubMed] [Google Scholar]
- 75.Kelly M, Wagner E. Estrogen modulation of G-protein-coupled receptors. Trends Endocrinol Metab. 1999;10:369–374. doi: 10.1016/s1043-2760(99)00190-3. [DOI] [PubMed] [Google Scholar]
- 76.Kelly MJ, Moss RL, Dudley CA. Differential sensitivity of preoptic-septal neurons to microelectrophoresed estrogen during the estrous cycle. Brain Res. 1976;114:152–157. doi: 10.1016/0006-8993(76)91017-9. [DOI] [PubMed] [Google Scholar]
- 77.Kelly MJ, Moss RL, Dudley CA. The effects of microelectrophoretically applied estrogen, cortisol and acetylcholine on medial preopticseptal unit activity throughout the estrous cycle of the female rat. Exp Brain Res. 1977;30:53–64. doi: 10.1007/BF00237858. [DOI] [PubMed] [Google Scholar]
- 78.Kelly MJ, Zhang C, Qiu J, Ronnekleiv OK. Pacemaking kisspeptin neurons. Exp Physiol. 2013;98:1535–1543. doi: 10.1113/expphysiol.2013.074559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.King CM, Hentges ST. Relative number and distribution of murine hypothalamic proopiomelanocortin neurons innervating distinct target sites. PLoS One. 2011;6:e25864. doi: 10.1371/journal.pone.0025864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kinoshita M, Tsukamura H, Adachi S, Matsui H, Uenoyama Y, Iwata K, Yamada S, Inoue K, Ohtaki T, Matsumoto H, Maeda K. Involvement of central metastin in the regulation of preovulatory luteinizing hormone surge and estrous cyclicity in female rats. Endocrinology. 2005;146:4431–4436. doi: 10.1210/en.2005-0195. [DOI] [PubMed] [Google Scholar]
- 81.Kirilov M, Clarkson J, Liu X, Roa J, Campos P, Porteous R, Schutz G, Herbison AE. Dependence of fertility on kisspeptin-Gpr54 signaling at the GnRH neuron. Nat Commun. 2013;4:2492. doi: 10.1038/ncomms3492. [DOI] [PubMed] [Google Scholar]
- 82.Klinge CM. Estrogen receptor interaction with estrogen response elements. Nucleic Acids Res. 2001;29:2905–2919. doi: 10.1093/nar/29.14.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kow LM, Mobbs CV, Pfaff DW. Roles of second-messenger systems and neuronal activity in the regulation of lordosis by neurotransmitters, neuropeptides, and estrogen: A review. Neurosci Biobehav Rev. 1994;18:251–268. doi: 10.1016/0149-7634(94)90028-0. [DOI] [PubMed] [Google Scholar]
- 84.Kow LM, Pfaff DW. The membrane actions of estrogens can potentiate their lordosis behavior-facilitating genomic actions. Proc Natl Acad Sci U S A. 2004;101:12354–12357. doi: 10.1073/pnas.0404889101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Krajewski SJ, Burke MC, Anderson MJ, McMullen NT, Rance NE. Forebrain projections of arcuate neurokinin B neurons demonstrated by anterograde tract-tracing and monosodium glutamate lesions in the rat. Neuroscience. 2010;166:680–697. doi: 10.1016/j.neuroscience.2009.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Krietsch T, Fernandes MS, Kero J, Losel R, Heyens M, Lam EW, Huhtaniemi I, Brosens JJ, Gellersen B. Human homologs of the putative G protein-coupled membrane progestin receptors (mPRalpha, beta, and gamma) localize to the endoplasmic reticulum and are not activated by progesterone. Mol Endocrinol. 2006;20:3146–3164. doi: 10.1210/me.2006-0129. [DOI] [PubMed] [Google Scholar]
- 87.Kuo J, Hamid N, Bondar G, Dewing P, Clarkson J, Micevych P. Sex differences in hypothalamic astrocyte response to estradiol stimulation. Biol Sex Differ. 2010;1:7. doi: 10.1186/2042-6410-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kuo J, Hamid N, Bondar G, Prossnitz ER, Micevych P. Membrane estrogen receptors stimulate intracellular calcium release and progesterone synthesis in hypothalamic astrocytes. J Neurosci. 2010;30:12950–12957. doi: 10.1523/JNEUROSCI.1158-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kuo J, Hariri OR, Bondar G, Ogi J, Micevych P. Membrane estrogen receptor-alpha interacts with metabotropic glutamate receptor type 1a to mobilize intracellular calcium in hypothalamic astrocytes. Endocrinology. 2009;150:1369–1376. doi: 10.1210/en.2008-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kuo J, Micevych P. Neurosteroids, trigger of the LH surge. J Steroid Biochem Mol Biol. 2012;131:57–65. doi: 10.1016/j.jsbmb.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Labhsetwar AP. Role of estrogens in ovulation: A study using the estrogen-antagonist, I.C.I. 46,474. Endocrinology. 1970;87:542–551. doi: 10.1210/endo-87-3-542. [DOI] [PubMed] [Google Scholar]
- 92.Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by beta-arrestins. Science. 2005;308:512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- 93.Lehman MN, Coolen LM, Goodman RL. Minireview: Kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: A central node in the control of gonadotropin-releasing hormone secretion. Endocrinology. 2010;151:3479–3489. doi: 10.1210/en.2010-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lehman MN, Merkley CM, Coolen LM, Goodman RL. Anatomy of the kisspeptin neural network in mammals. Brain Res. 2010;1364:90–102. doi: 10.1016/j.brainres.2010.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Leonelli M, Torrao AS, Britto LR. Unconventional neurotransmitters, neurodegeneration and neuroprotection. Braz J Med Biol Res. 2009;42:68–75. doi: 10.1590/s0100-879x2009000100011. [DOI] [PubMed] [Google Scholar]
- 96.Levin ER. G protein-coupled receptor 30: Estrogen receptor or collaborator? Endocrinology. 2009;150:1563–1565. doi: 10.1210/en.2008-1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li XF, Kinsey-Jones JS, Cheng Y, Knox AM, Lin Y, Petrou NA, Roseweir A, Lightman SL, Milligan SR, Millar RP, O’Byrne KT. Kisspeptin signalling in the hypothalamic arcuate nucleus regulates GnRH pulse generator frequency in the rat. PLoS One. 2009;4:e8334. doi: 10.1371/journal.pone.0008334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu X, Lee K, Herbison AE. Kisspeptin excites gonadotropin-releasing hormone neurons through a phospholipase C/calcium-dependent pathway regulating multiple ion channels. Endocrinology. 2008;149:4605–4614. doi: 10.1210/en.2008-0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.London SE, Monks DA, Wade J, Schlinger BA. Widespread capacity for steroid synthesis in the avian brain and song system. Endocrinology. 2006;147:5975–5987. doi: 10.1210/en.2006-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA, Jr, Shyamala G, Conneely OM, O’Malley BW. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9:2266–2278. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- 101.Ma L, Pei G. Beta-arrestin signaling and regulation of transcription. J Cell Sci. 2007;120:213–218. doi: 10.1242/jcs.03338. [DOI] [PubMed] [Google Scholar]
- 102.MacLusky NJ, Naftolin F. Sexual differentiation of the central nervous system. Science. 1981;211:1294–1302. doi: 10.1126/science.6163211. [DOI] [PubMed] [Google Scholar]
- 103.Mahavongtrakul M, Kanjiya MP, Maciel M, Kanjiya S, Sinchak K. Estradiol dose-dependent regulation of membrane estrogen receptor-alpha, metabotropic glutamate receptor-1a, and their complexes in the arcuate nucleus of the hypothalamus in female rats. Endocrinology. 2013;154:3251–3260. doi: 10.1210/en.2013-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mahesh VB, Brann DW. Interaction between ovarian and adrenal steroids in the regulation of gonadotropin secretion. J Steroid Biochem Mol Biol. 1992;41:495–513. doi: 10.1016/0960-0760(92)90375-s. [DOI] [PubMed] [Google Scholar]
- 105.Mahesh VB, Brann DW. Neuroendocrine mechanisms underlying the control of gonadotropin secretion by steroids. Steroids. 1998;63:252–256. doi: 10.1016/s0039-128x(98)00031-2. [DOI] [PubMed] [Google Scholar]
- 106.Mani SK, Allen JM, Clark JH, Blaustein JD, O’Malley BW. Convergent pathways for steroid hormone- and neurotransmitter-induced rat sexual behavior. Science. 1994;265:1246–1249. doi: 10.1126/science.7915049. [DOI] [PubMed] [Google Scholar]
- 107.Mani SK, Blaustein JD. Neural progestin receptors and female sexual behavior. Neuroendocrinology. 2012;96:152–161. doi: 10.1159/000338668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mani SK, Blaustein JD, Allen JM, Law SW, O’Malley BW, Clark JH. Inhibition of rat sexual behavior by antisense oligonucleotides to the progesterone receptor. Endocrinology. 1994;135:1409–1414. doi: 10.1210/endo.135.4.7925102. [DOI] [PubMed] [Google Scholar]
- 109.Mann DR, Barraclough CA. Role of estrogen and progesterone in facilitating LH release in 4-day cyclic rats. Endocrinol. 1973;93:694–699. doi: 10.1210/endo-93-3-694. [DOI] [PubMed] [Google Scholar]
- 110.Mann DR, Korowitz CD, Barraclough CA. Adrenal gland involvement in synchronizing the preovulatory release of LH in rats. Proc Soc Exp Biol Med. 1975;150:115–120. doi: 10.3181/00379727-150-38985. [DOI] [PubMed] [Google Scholar]
- 111.Meitzen J, Luoma JI, Boulware MI, Hedges VL, Peterson BM, Tuomela K, Britson KA, Mermelstein PG. Palmitoylation of estrogen receptors is essential for neuronal membrane signaling. Endocrinology. 2013;154:4293–4304. doi: 10.1210/en.2013-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Meitzen J, Mermelstein PG. Estrogen receptors stimulate brain region specific metabotropic glutamate receptors to rapidly initiate signal transduction pathways. J Chem Neuroanat. 2011;42:236–241. doi: 10.1016/j.jchemneu.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Meng D, Lynch MJ, Huston E, Beyermann M, Eichhorst J, Adams DR, Klussmann E, Houslay MD, Baillie GS. MEK1 binds directly to betaarrestin1, influencing both its phosphorylation by ERK and the timing of its isoprenaline-stimulated internalization. J Biol Chem. 2009;284:11425–11435. doi: 10.1074/jbc.M806395200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Merkley CM, Porter KL, Coolen LM, Hileman SM, Billings HJ, Drews S, Goodman RL, Lehman MN. KNDy (kisspeptin/neurokinin B/dynorphin) neurons are activated during both pulsatile and surge secretion of LH in the ewe. Endocrinology. 2012;153:5406–5414. doi: 10.1210/en.2012-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mermelstein P, Becker J, Surmeister D. Estradiol reduces calcium currents in rat neostriatal neurons via a membrane receptor. J Neurosci. 1996;16:595–604. doi: 10.1523/JNEUROSCI.16-02-00595.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Micevych P, Christensen A. Membrane-initiated estradiol actions mediate structural plasticity and reproduction. Front Neuroendocrinol. 2012;33:331–341. doi: 10.1016/j.yfrne.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Micevych P, Dominguez R. Membrane estradiol signaling in the brain. Front Neuroendocrinol. 2009;30:315–327. doi: 10.1016/j.yfrne.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Micevych P, Mills R, Sinchak K, Lapolt P, Chen J, Tao L, Lu J. Soc Neurosci Abstr. San Diego, CA: 2001. Estrogen stimulates progesterone synthesis in rat hypothalamus. [Google Scholar]
- 119.Micevych P, Sinchak K. The neurochemistry of limbic-hypothalamic circuits regulating sexual receptivity. In: Lajtha A, editor. Handbook of Neurochemistry and Molecular Neurobiology. 3rd. New York: Springer; 2007. pp. 151–193. [Google Scholar]
- 120.Micevych P, Sinchak K. Estradiol regulation of progesterone synthesis in the brain. Mol Cell Endocrinol. 2008;290:44–50. doi: 10.1016/j.mce.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Micevych P, Sinchak K, Mills RH, Tao L, LaPolt P, Lu JK. The luteinizing hormone surge is preceded by an estrogen-induced increase of hypothalamic progesterone in ovariectomized and adrenalectomized rats. Neuroendocrinology. 2003;78:29–35. doi: 10.1159/000071703. [DOI] [PubMed] [Google Scholar]
- 122.Micevych P, Soma KK, Sinchak K. Neuroprogesterone: Key to estrogen positive feedback? Brain Res Rev. 2008;57:470–480. doi: 10.1016/j.brainresrev.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Micevych PE, Abelson L, Fok H, Ulibarri C, Priest CA. Gonadal steroid control of preproenkephalin mRNA expression in the limbic-hypothalamic circuit: Comparison of adult with neonatal steroid treatments. J of Neurosci Res. 1994;38:386–398. doi: 10.1002/jnr.490380404. [DOI] [PubMed] [Google Scholar]
- 124.Micevych PE, Chaban V, Ogi J, Dewing P, Lu JK, Sinchak K. Estradiol stimulates progesterone synthesis in hypothalamic astrocyte cultures. Endocrinology. 2007;148:782–789. doi: 10.1210/en.2006-0774. [DOI] [PubMed] [Google Scholar]
- 125.Micevych PE, Chaban V, Ogi J, Lakhter A, Lu JKH, Sinchak K. Estradiol stimulates progesterone synthesis in hypothalamic astrocyte cultures. Endocrinology. 2007;148:782–789. doi: 10.1210/en.2006-0774. [DOI] [PubMed] [Google Scholar]
- 126.Micevych PE, Dewing P. Membrane-initiated estradiol signaling regulating sexual receptivity. Front Endocrinol. 2011;2:26. doi: 10.3389/fendo.2011.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Micevych PE, Eckersell CB, Holland KL, Smith A. Induction of CCK mRNA levels in the limbic-hypothalamic circuit: Time course and site-specific effects of estrogen. J Neurobiol. 1996;30:465–479. doi: 10.1002/(SICI)1097-4695(199608)30:4<465::AID-NEU3>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 128.Micevych PE, Kelly MJ. Membrane estrogen receptor regulation of hypothalamic function. Neuroendocrinology. 2012;96:103–110. doi: 10.1159/000338400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Micevych PE, Mermelstein PG. Membrane estrogen receptors acting through metabotropic glutamate receptors: An emerging mechanism of estrogen action in brain. Mol Neurobiol. 2008;38:66–77. doi: 10.1007/s12035-008-8034-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Micevych PE, Rissman EF, Gustafsson JA, Sinchak K. Estrogen receptor-alpha is required for estrogen-induced mu-opioid receptor internalization. J Neurosci Res. 2003;71:802–810. doi: 10.1002/jnr.10526. [DOI] [PubMed] [Google Scholar]
- 131.Micevych PE, Sinchak K. The neurosteroid progesterone underlies estrogen positive feedback of the LH surge. Front Endocrinol. 2011;2 doi: 10.3389/fendo.2011.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Miller WE, Lefkowitz RJ. Expanding roles for beta-arrestins as scaffolds and adapters in GPCR signaling and trafficking. Curr Opin Cell Biol. 2001;13:139–145. doi: 10.1016/s0955-0674(00)00190-3. [DOI] [PubMed] [Google Scholar]
- 133.Mills R, Chiang I, Abdian P, Sinchak K, Romeo H, Lu J, Micevych P. Soc Neurosci Abstr. Orlando, FL: 2002. Reproductive aging is associated with loss of hypothalamic progesterone synthesis but not decreased estrogen and progesterone receptor mRNA expression. [Google Scholar]
- 134.Mills RH, Sohn RK, Micevych PE. Estrogen-induced mu-opioid receptor internalization in the medial preoptic nucleus is mediated via neuropeptide Y-Y1 receptor activation in the arcuate nucleus of female rats. J Neurosci. 2004;24:947–955. doi: 10.1523/JNEUROSCI.1366-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mittelman-Smith MA, Williams H, Krajewski-Hall SJ, Lai J, Ciofi P, McMullen NT, Rance NE. Arcuate kisspeptin/neurokinin B/dynorphin (KNDy) neurons mediate the estrogen suppression of gonadotropin secretion and body weight. Endocrinology. 2012;153:2800–2812. doi: 10.1210/en.2012-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mittelman-Smith MA, Wong AM, Kathiresan AQ, Micevych PE. Classical and membrane-initiated estrogen signaling in an in vitro model of anterior hypothalamic kisspeptin neurons. Endocrinology. doi: 10.1210/en.2014-1803. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mostari P, Ieda N, Deura C, Minabe S, Yamada S, Uenoyama Y, Maeda K, Tsukamura H. dynorphin-kappa opioid receptor signaling partly mediates estrogen negative feedback effect on LH pulses in female rats. J Reprod Dev. 2013;59:266–272. doi: 10.1262/jrd.2012-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Moussatche P, Lyons TJ. Non-genomic progesterone signalling and its non-canonical receptor. Biochem Soc Trans. 2012;40:200–204. doi: 10.1042/BST20110638. [DOI] [PubMed] [Google Scholar]
- 139.Nakamura NH, Rosell DR, Akama KT, McEwen BS. Estrogen and ovariectomy regulate mRNA and protein of glutamic acid decarboxylases and cation-chloride cotransporters in the adult rat hippocampus. Neuroendocrinology. 2004;80:308–323. doi: 10.1159/000083657. [DOI] [PubMed] [Google Scholar]
- 140.Navarro VM, Castellano JM, McConkey SM, Pineda R, Ruiz-Pino F, Pinilla L, Clifton DK, Tena-Sempere M, Steiner RA. Interactions between kisspeptin and neurokinin B in the control of GnRH secretion in the female rat. Am J Physiol Endocrinol Metab. 2011;300:E202–210. doi: 10.1152/ajpendo.00517.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Neill JD. Sexual differences in the hypothalamic regulation of prolactin secretion. Endocrinology. 1972;90:1154–1159. doi: 10.1210/endo-90-5-1154. [DOI] [PubMed] [Google Scholar]
- 142.Neves SR, Ram PT, Iyengar R. G protein pathways. Science. 2002;296:1636–1639. doi: 10.1126/science.1071550. [DOI] [PubMed] [Google Scholar]
- 143.Novaira HJ, Sonko ML, Hoffman G, Koo Y, Ko C, Wolfe A, Radovick S. Disrupted kisspeptin signaling in GnRH neurons leads to hypogonadotrophic hypogonadism. Mol Endocrinol. 2014;28:225–238. doi: 10.1210/me.2013-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Otto C, Fuchs I, Kauselmann G, Kern H, Zevnik B, Andreasen P, Schwarz G, Altmann H, Klewer M, Schoor M, Vonk R, Fritzemeier KH. GPR30 does not mediate estrogenic responses in reproductive organs in mice. Biol Reprod. 2009;80:34–41. doi: 10.1095/biolreprod.108.071175. [DOI] [PubMed] [Google Scholar]
- 145.Paaske LK, Chuon T, Micevych P, Sinchak K. Soc Neurosci Abstr. Washington, DC: 2014. AVPV kisspeptin neurons mediate neuroprogesterone induction of the leuteinizing hormone surge. [Google Scholar]
- 146.Paaske LK, Micevych PE, Sinchak K. Kisspeptin mediates neuroprogesterone induction of the luteinizing hormone surge. The Endocrine Society’s 95th Annual Meeting & Expo; San Francisco, CA, USA. 2013. [Google Scholar]
- 147.Pang Y, Dong J, Thomas P. Characterization, neurosteroid binding and brain distribution of human membrane progesterone receptors delta and {epsilon} (mPRdelta and mPR neurosteroid {epsilon}) and mPRdelta involvement in inhibition of apoptosis. Endocrinology. 2013;154:283–295. doi: 10.1210/en.2012-1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Papadopoulos V, Baraldi M, Guilarte TR, Knudsen TB, Lacapere JJ, Lindemann P, Norenberg MD, Nutt D, Weizman A, Zhang MR, Gavish M. Translocator protein (18 kDa): New nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol Sci. 2006;27:402–409. doi: 10.1016/j.tips.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 149.Parhar IS, Ogawa S, Sakuma Y. Laser-captured single digoxigenin-labeled neurons of gonadotropin-releasing hormone types reveal a novel G protein-coupled receptor (Gpr54) during maturation in cichlid fish. Endocrinology. 2004;145:3613–3618. doi: 10.1210/en.2004-0395. [DOI] [PubMed] [Google Scholar]
- 150.Parsons B, Rainbow TC, Pfaff DW, McEwen BS. Hypothalamic protein synthesis essential for the activation of the lordosis reflex in the female rat. Endocrinology. 1982;110:620–624. doi: 10.1210/endo-110-2-620. [DOI] [PubMed] [Google Scholar]
- 151.Petersen SL, LaFlamme KD. Progesterone increases levels of μ-opioid receptor mRNA in the preoptic area and arcuate nucleus of ovariectomized, estradiol-treated female rats. Brain Res Mol Brain Res. 1997;52:32–37. doi: 10.1016/s0169-328x(97)00194-0. [DOI] [PubMed] [Google Scholar]
- 152.Pfaff DW. Uptake of 3H-estradiol by the female rat brain. An autoradiographic study. Endocrinology. 1968;82:1149–1155. doi: 10.1210/endo-82-6-1149. [DOI] [PubMed] [Google Scholar]
- 153.Phan J, Mahavongtrakul M, Sinchak K. Progesterone receptor-B and Src kinase complex in the plasma membrane of the arcuate nucleus of the hypothalamus of female rats. Soc Neurosci Abstr. 2014 [Google Scholar]
- 154.Pielecka-Fortuna J, Chu Z, Moenter SM. Kisspeptin acts directly and indirectly to increase gonadotropin-releasing hormone neuron activity and its effects are modulated by estradiol. Endocrinology. 2008;149:1979–1986. doi: 10.1210/en.2007-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Piet R, de Croft S, Liu X, Herbison AE. Electrical properties of kisspeptin neurons and their regulation of GnRH neurons. Front Neuroendocrinol. 2014 doi: 10.1016/j.yfrne.2014.05.006. pii: S0091-3022(14)00053-3. [DOI] [PubMed] [Google Scholar]
- 156.Popper P, Priest CA, Micevych PE. Regulation of cholecystokinin receptors in the ventromedial nucleus of the hypothalamus: Sex steroid hormone regulation. Brain Res. 1996;715:335–339. doi: 10.1016/0006-8993(95)01561-2. [DOI] [PubMed] [Google Scholar]
- 157.Prast H, Philippu A. Nitric oxide as modulator of neuronal function. Prog Neurobiol. 2001;64:51–68. doi: 10.1016/s0301-0082(00)00044-7. [DOI] [PubMed] [Google Scholar]
- 158.Priest CA, Vink KL, Micevych PE. Temporal regulation by estrogen of beta-preprotachykinin mRNA expression in the rat ventromedial nucleus of the hypothalamus. Brain Res Mol Brain Res. 1995;28:61–71. doi: 10.1016/0169-328x(94)00184-g. [DOI] [PubMed] [Google Scholar]
- 159.Prossnitz ER, Barton M. The G-protein-coupled estrogen receptor GPER in health and disease. Nat Rev Endocrinol. 2011;7:715–726. doi: 10.1038/nrendo.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Putnam CD, Brann DW, Mahesh VB. Acute activation of the adrenocorticotropin-adrenal axis: Effect on gonadotropin and prolactin secretion in the female rat. Endocrinology. 1991;128:2558–2566. doi: 10.1210/endo-128-5-2558. [DOI] [PubMed] [Google Scholar]
- 161.Qiu J, Bosch MA, Tobias SC, Grandy DK, Scanlan TS, Ronnekleiv OK, Kelly MJ. Rapid signaling of estrogen in hypothalamic neurons involves a novel G-protein-coupled estrogen receptor that activates protein kinase C. J Neurosci. 2003;23:9529–9540. doi: 10.1523/JNEUROSCI.23-29-09529.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Quinones-Jenab V, Jenab S, Ogawa S, Inturrisi C, Pfaff DW. Estrogen regulation of mu-opioid receptor mRNA in the forebrain of female rats. Mol Brain Res. 1997;47:134–138. doi: 10.1016/s0169-328x(97)00041-7. [DOI] [PubMed] [Google Scholar]
- 163.Rance NE, Bruce TR. Neurokinin B gene expression is increased in the arcuate nucleus of ovariectomized rats. Neuroendocrinology. 1994;60:337–345. doi: 10.1159/000126768. [DOI] [PubMed] [Google Scholar]
- 164.Rance NE, Young WS., 3rd Hypertrophy and increased gene expression of neurons containing neurokinin-B and substance-P messenger ribonucleic acids in the hypothalami of postmenopausal women. Endocrinology. 1991;128:2239–2247. doi: 10.1210/endo-128-5-2239. [DOI] [PubMed] [Google Scholar]
- 165.Razandi M, Oh P, Pedram A, Schnitzer J, Levin ER. ERs associate with and regulate the production of caveolin: Implications for signaling and cellular actions. Mol Endocrinol. 2002;16:100–115. doi: 10.1210/mend.16.1.0757. [DOI] [PubMed] [Google Scholar]
- 166.Razandi M, Pedram A, Greene G, Levin E. Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: Studies of ERalpha and ERbeta expressed in Chinese hamster ovary cells. Mol Endocrinol. 1999;13:307–319. doi: 10.1210/mend.13.2.0239. [DOI] [PubMed] [Google Scholar]
- 167.Razandi M, Pedram A, Park ST, Levin ER. Proximal events in signaling by plasma membrane estrogen receptors. J Biol Chem. 2003;278:2701–2712. doi: 10.1074/jbc.M205692200. [DOI] [PubMed] [Google Scholar]
- 168.Remohi J, Balmaceda JP, Rojas FJ, Asch RH. The role of pre-ovulatory progesterone in the midcycle gonadotrophin surge, ovulation and subsequent luteal phase: Studies with RU486 in rhesus monkeys. Hum Reprod. 1988;3:431–435. doi: 10.1093/oxfordjournals.humrep.a136722. [DOI] [PubMed] [Google Scholar]
- 169.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 170.Roepke TA, Qiu J, Bosch MA, Ronnekleiv OK, Kelly MJ. Cross-talk between membrane-initiated and nuclear-initiated oestrogen signalling in the hypothalamus. J Neuroendocrinol. 2009;21:263–270. doi: 10.1111/j.1365-2826.2009.01846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Rometo AM, Krajewski SJ, Voytko ML, Rance NE. Hypertrophy and increased kisspeptin gene expression in the hypothalamic infundibular nucleus of postmenopausal women and ovariectomized monkeys. J Clin Endocrinol Metab. 2007;92:2744–2750. doi: 10.1210/jc.2007-0553. [DOI] [PubMed] [Google Scholar]
- 172.Roseweir AK, Kauffman AS, Smith JT, Guerriero KA, Morgan K, Pielecka-Fortuna J, Pineda R, Gottsch ML, Tena-Sempere M, Moenter SM, Terasawa E, Clarke IJ, Steiner RA, Millar RP. Discovery of potent kisspeptin antagonists delineate physiological mechanisms of gonadotropin regulation. J Neurosci. 2009;29:3920–3929. doi: 10.1523/JNEUROSCI.5740-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Rudd CD, Short RV, McFarlane JR, Renfree MB. Sexual differentiation of oestradiol-LH positive feedback in a marsupial. J Reprod Fertil. 1999;115:269–274. doi: 10.1530/jrf.0.1150269. [DOI] [PubMed] [Google Scholar]
- 174.Ruka KA, Burger LL, Moenter SM. Regulation of arcuate neurons coexpressing kisspeptin, neurokinin B, and dynorphin by modulators of neurokinin 3 and kappa-opioid receptors in adult male mice. Endocrinology. 2013;154:2761–2771. doi: 10.1210/en.2013-1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Safe S. Transcriptional activation of genes by 17 beta-estradiol through estrogen receptor-Sp1 interactions. Vitam Horm. 2001;62:231–252. doi: 10.1016/s0083-6729(01)62006-5. [DOI] [PubMed] [Google Scholar]
- 176.Sanathara NM, Moraes J, Kanjiya S, Sinchak K. Orphanin FQ in the mediobasal hypothalamus facilitates sexual receptivity through the deactivation of medial preoptic nucleus mu-opioid receptors. Horm Behav. 2011;60:540–548. doi: 10.1016/j.yhbeh.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sanathara NM, Moraes J, Mahavongtrakul M, Sinchak K. Estradiol upregulates progesterone receptor and orphanin FQ colocalization in arcuate nucleus neurons and opioid receptor-like receptor-1 expression in proopiomelanocortin neurons that project to the medial preoptic nucleus in the female rat. Neuroendocrinology. 2014 doi: 10.1159/000363324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Sanchez-Criado JE, Hernandez G, Bellido C, Gonzalez D, Tebar M, Diaz-Cruz MA, Alonso R. Periovulatory LHRH, LH and FSH secretion in cyclic rats treated with RU486: Effects of exogenous LHRH and LHRH antagonist on LH and FSH secretion at early oestrus. J Endocrinol. 1994;141:7–14. doi: 10.1677/joe.0.1410007. [DOI] [PubMed] [Google Scholar]
- 179.Sandoval-Guzman T, Rance NE. Central injection of senktide, an NK3 receptor agonist, or neuropeptide Y inhibits LH secretion and induces different patterns of Fos expression in the rat hypothalamus. Brain Res. 2004;1026:307–312. doi: 10.1016/j.brainres.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 180.Schumacher M, Guennoun R, Robert F, Carelli C, Gago N, Ghoumari A, Gonzalez Deniselle MC, Gonzalez SL, Ibanez C, Labombarda F, Coirini H, Baulieu EE, De Nicola AF. Local synthesis and dual actions of progesterone in the nervous system: Neuroprotection and myelination. Growth Horm IGF Res. 2004;14(Suppl A):S18–33. doi: 10.1016/j.ghir.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 181.Schumacher M, Hussain R, Gago N, Oudinet JP, Mattern C, Ghoumari AM. Progesterone synthesis in the nervous system: Implications for myelination and myelin repair. Front Neurosci. 2012;6:10. doi: 10.3389/fnins.2012.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O’Rahilly S, Carlton MB, Crowley WF, Jr, Aparicio SA, Colledge WH. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 183.Serey CS, Huss B, Chuon T, Mahavongtrakul M, Sinchak K. Soc Neurosci Abstr. Washington, DC: 2014. The signaling pathways of progesterone receptor, Src kinase, and dopamine D1 receptor converge in the arcuate nucleus of the hypothalamus to facilitate lordosis. [Google Scholar]
- 184.Shaikh AA, Shaikh SA. Adrenal and ovarian steroid secretion. Endocrinology. 1975;96:37–44. doi: 10.1210/endo-96-1-37. [DOI] [PubMed] [Google Scholar]
- 185.Shaywitz AJ, Greenberg ME. CREB: A stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu Rev Biochem. 1999;68:821–861. doi: 10.1146/annurev.biochem.68.1.821. [DOI] [PubMed] [Google Scholar]
- 186.Shivers BD, Harlan RE, Morrell JI, Pfaff DW. Absence of oestradiol concentration in cell nuclei of LHRH-immunoreactive neurones. Nature. 1983;304:345–347. doi: 10.1038/304345a0. [DOI] [PubMed] [Google Scholar]
- 187.Shivers BD, Harlan RE, Morrell JI, Pfaff DW. Immunocytochemical localization of luteinizing hormone-releasing hormone in male and female rat brains. Quantitative studies on the effect of gonadal steroids. Neuroendocrinology. 1983;36:1–12. doi: 10.1159/000123522. [DOI] [PubMed] [Google Scholar]
- 188.Simpson ER, Waterman MR. Regulation by ACTH of steroid hormone biosynthesis in the adrenal cortex. Can J Biochem Cell Biol. 1983;61:692–707. doi: 10.1139/o83-088. [DOI] [PubMed] [Google Scholar]
- 189.Sinchak K, Dewing P, Cook M, Micevych P. Release of orphanin FQ/nociceptin in the medial preoptic nucleus and ventromedial nucleus of the hypothalamus facilitates lordosis. Horm Behav. 2007;51:406–412. doi: 10.1016/j.yhbeh.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Sinchak K, Dewing P, Ponce L, Gomez L, Christensen A, Berger M, Micevych P. Modulation of the arcuate nucleus-medial preoptic nucleus lordosis regulating circuit: A role for GABAB receptors. Horm Behav. 2013;64:136–143. doi: 10.1016/j.yhbeh.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Sinchak K, Micevych PE. Progesterone blockade of estrogen activation of μ-opioid receptors regulates reproductive behavior. J Neurosci. 2001;21:5723–5729. doi: 10.1523/JNEUROSCI.21-15-05723.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Sinchak K, Mills RH, Eckersell CB, Micevych PE. Medial preoptic area delta-opioid receptors inhibit lordosis. Behav Brain Res. 2004;155:301–306. doi: 10.1016/j.bbr.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 193.Sinchak K, Romeo HE, Micevych PE. Site-specific estrogen and progestin regulation of orphanin FQ/nociceptin and nociceptin opioid receptor mRNA expression in the female rat limbic hypothalamic system. J Comp Neurol. 2006;496:252–268. doi: 10.1002/cne.20949. [DOI] [PubMed] [Google Scholar]
- 194.Sinchak K, Shahedi K, Dewing P, Micevych P. Sexual receptivity is reduced in the female mu-opioid receptor knockout mouse. Neuroreport. 2005;16:1697–1700. doi: 10.1097/01.wnr.0000181585.49130.93. [DOI] [PubMed] [Google Scholar]
- 195.Smith JT, Clay CM, Caraty A, Clarke IJ. KiSS-1 messenger ribonucleic acid expression in the hypothalamus of the ewe is regulated by sex steroids and season. Endocrinology. 2007;148:1150–1157. doi: 10.1210/en.2006-1435. [DOI] [PubMed] [Google Scholar]
- 196.Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005;146:3686–3692. doi: 10.1210/en.2005-0488. [DOI] [PubMed] [Google Scholar]
- 197.Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA. Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J Neurosci. 2006;26:6687–6694. doi: 10.1523/JNEUROSCI.1618-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Snyder BW, Beecham GD, Schane HP. Inhibition of ovulation in rats with epostane, an inhibitor of 3 beta-hydroxysteroid dehydrogenase. Proc Soc Exp Biol Med. 1984;176:238–242. doi: 10.3181/00379727-176-41865. [DOI] [PubMed] [Google Scholar]
- 199.Soma KK, Sinchak K, Lakhter A, Schlinger BA, Micevych PE. Neurosteroids and female reproduction: Estrogen increases 3beta-HSD mRNA and activity in rat hypothalamus. Endocrinology. 2005;146:4386–4390. doi: 10.1210/en.2005-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Szego CM, Davis JS. Adenosine 3′,5′-monophosphate in rat uterus: Acute elevation by estrogen. Proc Natl Acad Sci U S A. 1967;58:1711–1718. doi: 10.1073/pnas.58.4.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Tang YT, Hu T, Arterburn M, Boyle B, Bright JM, Emtage PC, Funk WD. PAQR proteins: A novel membrane receptor family defined by an ancient 7-transmembrane pass motif. J Mol Evol. 2005;61:372–380. doi: 10.1007/s00239-004-0375-2. [DOI] [PubMed] [Google Scholar]
- 202.Thomas P, Pang Y. Membrane progesterone receptors: Evidence for neuroprotective, neurosteroid signaling and neuroendocrine functions in neuronal cells. Neuroendocrinology. 2012;96:162–171. doi: 10.1159/000339822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Thomas P, Pang Y, Dong J, Groenen P, Kelder J, de Vlieg J, Zhu Y, Tubbs C. Steroid and G protein binding characteristics of the seatrout and human progestin membrane receptor alpha subtypes and their evolutionary origins. Endocrinology. 2007;148:705–718. doi: 10.1210/en.2006-0974. [DOI] [PubMed] [Google Scholar]
- 204.Todman MG, Han SK, Herbison AE. Profiling neurotransmitter receptor expression in mouse gonadotropin-releasing hormone neurons using green fluorescent protein-promoter transgenics and microarrays. Neuroscience. 2005;132:703–712. doi: 10.1016/j.neuroscience.2005.01.035. [DOI] [PubMed] [Google Scholar]
- 205.Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, Serin A, Mungan NO, Cook JR, Ozbek MN, Imamoglu S, Akalin NS, Yuksel B, O’Rahilly S, Semple RK. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for Neurokinin B in the central control of reproduction. Nat Genet. 2009;41:354–358. doi: 10.1038/ng.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Toran-Allerand CD, Gerlach JL, McEwen BS. Autoradiographic localization of [3H]estradiol related to steroid responsiveness in cultures of the newborn mouse hypothalamus and preoptic area. Brain Res. 1980;184:517–522. doi: 10.1016/0006-8993(80)90820-3. [DOI] [PubMed] [Google Scholar]
- 207.Torii M, Kubo K, Sasaki T. Influence of opioid peptides on the priming action of estrogen on lordosis in ovariectomized rats. Neurosci Lett. 1996;212:68–70. doi: 10.1016/0304-3940(96)12763-4. [DOI] [PubMed] [Google Scholar]
- 208.Torii M, Kubo K, Sasaki T. Naloxone and initial estrogen action to induce lordosis in ovariectomized rats: The effect of a cut between the septum and preoptic area. Neurosci Lett. 1995;195:167–170. doi: 10.1016/0304-3940(95)11809-b. [DOI] [PubMed] [Google Scholar]
- 209.Webb P, Nguyen P, Valentine C, Lopez GN, Kwok GR, McInerney E, Katzenellenbogen BS, Enmark E, Gustafsson JA, Nilsson S, Kushner PJ. The estrogen receptor enhances AP-1 activity by two distinct mechanisms with different requirements for receptor transactivation functions. Mol Endocrinol. 1999;13:1672–1685. doi: 10.1210/mend.13.10.0357. [DOI] [PubMed] [Google Scholar]
- 210.Wintermantel TM, Campbell RE, Porteous R, Bock D, Grone HJ, Tod-man MG, Korach KS, Greiner E, Perez CA, Schutz G, Herbison AE. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron. 2006;52:271–280. doi: 10.1016/j.neuron.2006.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Wong AM, Abrams MC, Micevych P. Beta-Arrestin-1 regulates estrogen membrane signaling in hypothalamic neurons. PLoS One. 2015 doi: 10.1371/journal.pone.0120530. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Yang JJ, Caligioni CS, Chan YM, Seminara SB. Uncovering novel reproductive defects in neurokinin B receptor null mice: Closing the gap between mice and men. Endocrinology. 2012;153:1498–1508. doi: 10.1210/en.2011-1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Yen SS, Tsai CC. Acute gonadotropin release induced by exogenous estradiol during the mid-follicular phase of the menstrual cycle. J Clin Endocrinol Metab. 1972;34:298–305. doi: 10.1210/jcem-34-2-298. [DOI] [PubMed] [Google Scholar]
- 214.Yeo SH, Herbison AE. Estrogen-negative feedback and estrous cyclicity are critically dependent upon estrogen receptor-alpha expression in the arcuate nucleus of adult female mice. Endocrinology. 2014;155:2986–2995. doi: 10.1210/en.2014-1128. [DOI] [PubMed] [Google Scholar]
- 215.Yoshikuni M, Nagahama Y. Involvement of an inhibitory G-protein in the signal transduction pathway of maturation-inducing hormone (17 alpha,20 beta-dihydroxy-4-pregnen-3-one) action in rainbow trout (Oncorhynchus mykiss) oocytes. Dev Biol. 1994;166:615–622. doi: 10.1006/dbio.1994.1341. [DOI] [PubMed] [Google Scholar]
- 216.Zhang C, Roepke TA, Kelly MJ, Ronnekleiv OK. Kisspeptin depolarizes gonadotropin-releasing hormone neurons through activation of TRPC-like cationic channels. J Neurosci. 2008;28:4423–4434. doi: 10.1523/JNEUROSCI.5352-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Zhang J, Barak LS, Winkler KE, Caron MG, Ferguson SS. A central role for beta-arrestins and clathrin-coated vesicle-mediated endocytosis in beta2-adrenergic receptor resensitization. Differential regulation of receptor resensitization in two distinct cell types. J Biol Chem. 1997;272:27005–27014. doi: 10.1074/jbc.272.43.27005. [DOI] [PubMed] [Google Scholar]
- 218.Zhang J, Yang L, Lin N, Pan X, Zhu Y, Chen X. Aging-related changes in RP3V kisspeptin neurons predate the reduced activation of GnRH neurons during the early reproductive decline in female mice. Neurobiol Aging. 2014;35:655–668. doi: 10.1016/j.neurobiolaging.2013.08.038. [DOI] [PubMed] [Google Scholar]
- 219.Zuloaga DG, Yahn SL, Pang Y, Quihuis AM, Oyola MG, Reyna A, Thomas P, Handa RJ, Mani SK. Distribution and estrogen regulation of membrane progesterone receptor-beta in the female rat brain. Endocrinology. 2012;153:4432–4443. doi: 10.1210/en.2012-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Zwain IH, Yen SS. Neurosteroidogenesis in astrocytes, oligodendrocytes, and neurons of cerebral cortex of rat brain. Endocrinol. 1999;140:3843–3852. doi: 10.1210/endo.140.8.6907. [DOI] [PubMed] [Google Scholar]


