Abstract
Licorice root extracts are often consumed as botanical dietary supplements by menopausal women as a natural alternative to pharmaceutical hormone replacement therapy. In addition to their components liquiritigenin (Liq) and isoliquiritigenin (Iso-Liq), known to have estrogenic activity, licorice root extracts also contain a number of other flavonoids, isoflavonoids, and chalcones. We have investigated the estrogenic activity of 7 of these components, obtained from an extract of Glycyrrhiza glabra powder, namely Glabridin (L1), Calycosin (L2), Methoxychalcone (L3), Vestitol (L4), Glyasperin C (L5), Glycycoumarin (L6), and Glicoricone (L7), and compared them with Liq, Iso-Liq, and estradiol (E2). All components, including Liq and Iso-Liq, have low binding affinity for estrogen receptors (ERs). Their potency and efficacy in stimulating the expression of estrogen-regulated genes reveal that Liq and Iso-Liq and L2, L3, L4, and L6 are estrogen agonists. Interestingly, L3 and L4 have an efficacy nearly equivalent to E2 but with a potency ca. 10,000-fold less. The other components, L1, L5 and L7, acted as partial estrogen antagonists. All agonist activities were reversed by the antiestrogen, ICI 182,780, or by knockdown of ERα with siRNA, indicating that they are ER dependent. In HepG2 hepatoma cells stably expressing ERα, only Liq, Iso-Liq, and L3 stimulated estrogen-regulated gene expression, and in all cases gene stimulation did not occur in HepG2 cells lacking ERα. Collectively, these findings classify the components of licorice root extracts as low potency, mixed ER agonists and antagonists, having a character akin to that of selective estrogen receptor modulators or SERMs.
Keywords: Estrogen receptor, selective estrogen receptor modulator, botanical estrogens, licorice root estrogens
INTRODUCTION
Estrogens working through estrogen receptors (ERs) exert effects on diverse target cells and tissues, so that the sharp decline in ovarian estrogen production at menopause broadly impacts the health of women [1–3]. Many menopausal women take botanical dietary supplements as an alternative to pharmaceutical hormone replacement therapy (HRT), to achieve relief from menopausal symptoms that include hot flushes, vaginal atrophy, bone loss, and changes in cardiovascular and metabolic function. While pharmaceutical estrogens used in HRT are effective in alleviating vasomotor symptoms and have beneficial effects on bone, cardiovascular and metabolic health, they may pose risks by exacerbating breast and uterine cancer development and/or progression.
Licorice root extracts are frequently used in dietary supplements and are believed to contain estrogenic components that might, in principle, provide a spectrum of beneficial effects with reduced stimulation of the breast and uterus compared with that of the endogenous hormone estradiol (E2) or the pharmaceutical estrogens used in HRT [4, 5]. However, much is still not known about the mechanisms by which licorice root components act and whether they have the same or a different spectrum of activity from that of ovarian or pharmaceutical estrogens. Also of importance is whether any of the estrogenic components in licorice extracts are of sufficient potency that they would be efficacious at the doses typically provided by licorice botanical supplements consumed by women.
Recent studies by us and others have characterized the estrogenic activity of a major licorice root component, liquiritigenin (Liq) [6, 7]. Our whole genome RNA-seq gene expression and cell proliferation studies comparing E2 and Liq revealed that the estrogenic activity of Liq was weaker than that of E2 and that some genes showed distinctly different regulation of expression (stimulation and/or suppression) by Liq versus E2 [7]. Licorice root extracts, as used in dietary supplements, contain variable amounts of a number of compounds in addition to Liq that could have estrogenic and/or anti-estrogenic activities, and might contribute in a differential manner to the overall potency and activity of these dietary supplements. Therefore, we have characterized chemically and biologically the activity of these components in ER-containing breast cancer cells (MCF7) and liver hepatoma (HepG2-ER) cells, these being representative of two target tissues in which estrogens are known to elicit important physiological effects, including enhanced breast cancer cell proliferation (MCF7) and beneficial changes in cell metabolism (liver cells).
In this study, we report on the estrogenic and anti-estrogenic activities of seven components of licorice root, and we compare these with each other and with E2, as well as with Liq and Iso-Liq, the two most widely studied components in licorice root extracts. Our findings reveal that four of the seven components have significant estrogen agonist activity that can contribute, along with Liq and Iso-Liq, to the overall composite estrogen activity of licorice root extracts. Of interest, three of the components are largely ER antagonists. Our observations reveal the biological activities present in multi-component dietary supplements such as licorice root extracts. Furthermore, they highlight the need to standardize the preparation of licorice root extracts in dietary supplements, because the manner in which supplements are prepared could greatly impact the relative proportions of the different components and hence the spectrum of biological activities in different licorice root dietary supplement preparations.
EXPERIMENTAL
Ligands and Cell Culture
Estradiol was from Sigma (St Louis, MO). Liquiritigenin and iso-liquiritigenin, and the antiestrogen ICI 182,780 were from Tocris Bioscience (Bristol, UK). All compounds were checked for identity and purity by mass spectrometry and NMR. MCF-7 cells (ATCC) were cultured in DMEM, Dulbecco’s Minimal Essential Medium (Gibco/Life Technologies, Grand Island, NY), supplemented with 5% calf serum (HyClone, Logan, UT) and 100 μg/ml penicillin/streptomycin (Invitrogen, Carlsbad, CA), as previously described [7, 8]. For estrogen-free experiments, the cells were seeded in phenol red-free DMEM (Gibco/Life Technologies) plus 5% charcoal-dextran-treated calf serum for 3 days before treatments with compounds were initiated. HepG2 cells (ATCC) and HepG2 cells stably expressing ERα (HepG2+ERα cells, kindly provided by David J. Shapiro, University of Illinois at Urbana-Champaign) were grown as described [9, 10]. The siRNA experiments for knockdown of the endogenous ERα in MCF-7 cells were performed as previously described and resulted in knockdown of ERα mRNA and protein by greater than 95% [11]. siERα sequences (Dharmacon, Lafayette, CO) were forward, 5′-UCAUCGCAUUCCUUGCAAAdTdT-3′, and reverse, 5′-UUUGCAAGGAAUGCGAUGAdTdT-3′ [11].
Preparation of Licorice Root Extract and Isolation and Purification of Licorice Root Components
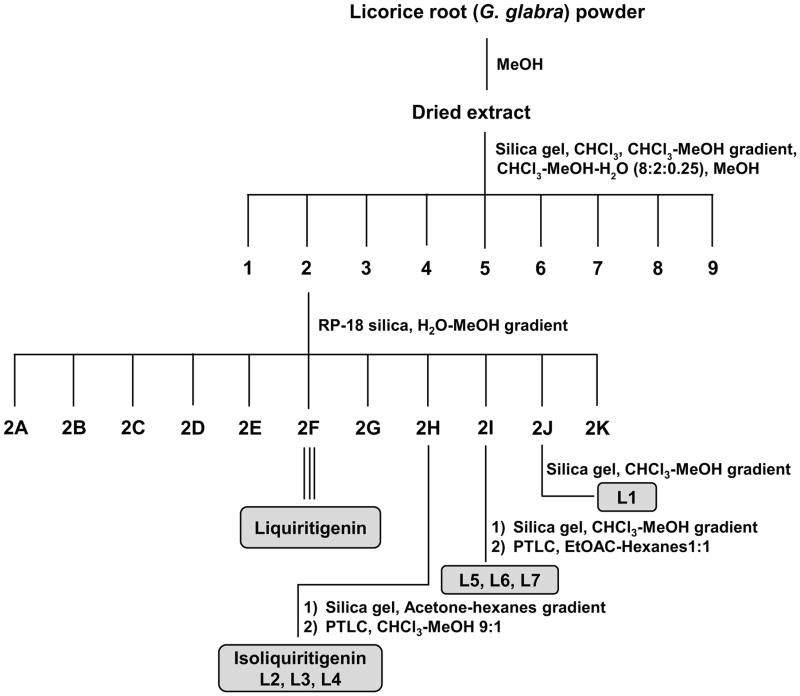
Licorice root (G. glabra) powder (750 g) was extracted with methanol (3 × 3L for 20h) using a percolator. The solvent was removed under reduced pressure at 48 °C to yield the dried extract (107 g). The pure compounds, Liq (0.378% yield), Isoliq (0.23%), L1 (0.514%), L2 (0.006%), L3 (0.027%), L4 (0.027%), L5 (0.021%), L6 (0.014%), and L7 (0.005%) were obtained from the methanolic extract of licorice by column chromatography over normal silica gel, reversed phase silica (RP-18), and Sephadex LH-20, as well as preparative thin layer chromatography (PTLC). Product integrity and purity of all 9 flavonoids were characterized using various analytical techniques such as NMR and mass spectrometry.
Relative binding affinity assays
Relative binding affinities were determined by a competitive radiometric binding assay as previously described [12, 13], using 2 nM [3H]-estradiol as tracer ([2,4,6,7-3H]-estra-1,3,5(10)-triene-3,17β-diol, 70–115 Ci/mmol, Perkin Elmer, Waltham, MA), and purified, full-length, human ERα and ERβ purchased from PanVera/Invitrogen (Carlsbad, CA). Incubations were for 18–24 h at 0 °C. Hydroxyapatite (BioRad, Hercules, CA) was used to absorb the receptor-ligand complexes, and free ligand was washed away. The binding affinities are expressed as relative binding affinity (RBA) values with the RBA of estradiol set to 100%. The values given are the average ± range or SD of two to three independent determinations. Estradiol binds to ERα with a Kd of 0.2 nM and to ERβ with a Kd of 0.5 nM, as previously determined [12].
RNA isolation and real-time PCR
Total RNA was isolated from cells using TRIzol (Invitrogen), RNA samples were reverse transcribed by MMTV reverse transcriptase (New England Biolabs, Ipswitch, MA), and real-time PCR was carried out on the ABI Prism 7900HT instrument using SYBR Green PCR Master Mix (Roche), as described previously [14].
Statistical Analysis
Statistical analyses used one-way ANOVA with Bonferroni’s Multiple Comparison Test or two-way ANOVA with Bonferroni posttest and used GraphPad Prism version 5.00 (GraphPad Software, San Diego, CA). Data are expressed as mean ± SD and p <0.05 was assigned as statistically significant.
RESULTS
Preparation of licorice root extracts and characterization of chemical components and their binding affinities for estrogen receptors
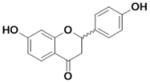
An overview of the extraction and purification protocol is presented in Figure 1 and described in the Methods section. All compounds were checked for purity and identity by mass spectrometry and nuclear magnetic resonance (NMR). As shown in Figure 1, we identified seven components in licorice root, in addition to liquiritigenin (Liq) and isoliquiritigenin (Iso-Liq). Structures of the compounds are shown in Table 1. All of the components are non-steroidal compounds that belong to the flavonoid, isoflavonoid, or chalcone classes, with some of them being prenylated (L1, L5, L6, and L7).
Figure 1.
Schematic overview of the extraction of licorice root components from licorice root and their purification and identification by mass spectrometry and nuclear magnetic resonance (NMR) spectrometry.
Table 1.
Structures and Relative Estrogen Receptor Binding Affinities (RBA) of Licorice Root Components
| Compound Name | Structure | RBA (mean ± SD) | ||
|---|---|---|---|---|
| ERα | ERβ | ERβ/ERα ratio | ||
| Liquiritigenin (Liq) |
|
<0.001 | 0.013 ±0.003 | >13 |
| Iso-liquiritigenin (Iso-Liq) |
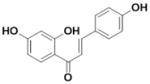
|
0.005 ± 0 | 0.013±0.004 | 2.6 |
| Glabridin (L1) |
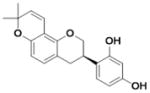
|
0.075 ± 0.015 | 0.091 ± 0.001 | 1.2 |
| Calycosin (L2) |
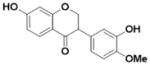
|
0.010 ± 0.004 | 0.027 ± 0.004 | 2.7 |
| Methoxychalcone (L3) |
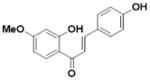
|
0.032 ± 0.008 | 0.035 ± 0.001 | 1.1 |
| Vestitol (L4) |
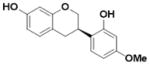
|
0.062 ± 0.008 | 0.039 ± 0.011 | 0.6 |
| Glyasperin C (L5) |
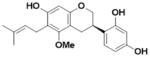
|
0.010 ± 0.001 | 0.111 ± 0.004 | 11.1 |
| Glycycoumarin (L6) |
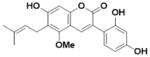
|
0.017 ± 0.001 | 0.040 ± 0.001 | 2.4 |
| Glicoricone (L7) |
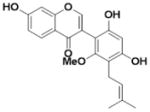
|
0.008 ± 0.001 | 0.019 ± 0.004 | 2.4 |
Relative binding affinities of each licorice component for estrogen receptors ERα and ERβ were determined by competitive radiometric binding assays using tritiated estradiol as tracer. Affinities are expressed as relative binding affinity values, where the affinity of E2 for ERα or ERβ is set at 100% (Table 1). Of the 9 components, all bound with approximately equal affinity to ERα and ERβ (beta/alpha ratio approximately 1), except for Liq and L5, which had a greater than 10-fold preference for ERβ. All of the licorice root components had binding affinities for ERs at least 1000-times lower than that of E2.
Licorice root components differ in their ability to activate estrogen target gene expression
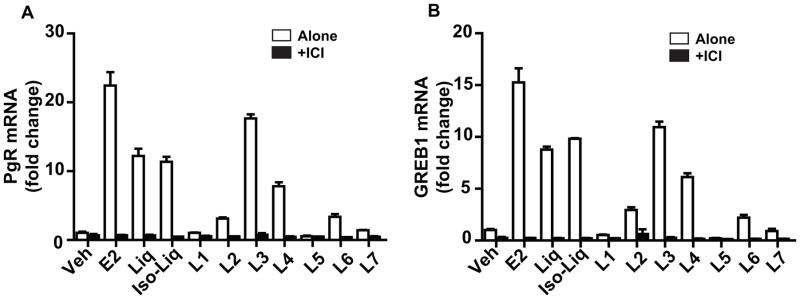
We next evaluated the activity of the licorice root components in regulating the expression of estrogen-responsive genes in MCF7 breast cancer cells. As shown in Figure 2, where expression of the progesterone receptor (PgR) and the GREB1 gene was examined, we found that E2, Liq, and Iso-Liq markedly increased the RNA level for both of these genes, and components L2, L3, L4 and L6 were also effective in increasing PgR and GREB1 expression, particularly L3 and L4. By contrast, components L1, L5 and L7 elicited little or no change in gene expression (Fig. 2A and 2B). In addition, gene stimulation by the licorice components and by E2 was completely suppressed by cell cotreatment with an excess of the antiestrogen ICI 182,780 (ICI, Fulvestrant) (Fig. 2A, B), confirming that activation of estrogen- responsive gene expression by the licorice components requires ERα.
Figure 2.
Effects of estradiol (E2) and licorice root components on gene regulation in MCF-7 cells and suppression of gene stimulation by cotreatment with the antiestrogen ICI 182,780. (A, B) Effects of E2, liquiritigenin (Liq), Isoliqiritigenin (Iso-Liq), and licorice components L1–L7 on progesterone receptor (PgR) or GREB1gene expression. Cells were treated with control vehicle, 10−8 M E2, 10−6 M Liq or Iso-Liq, or 3×10−6 M L1–L7 alone or with cotreatment with 10−6 M antiestrogen ICI for 24 h. RNA was then harvested from cells and PgR or GREB1 RNA monitored by qPCR.
Licorice root components that do not activate, or only weakly activate, gene expression through ERα act as antiestrogens and suppress E2-stimulated ERα activity
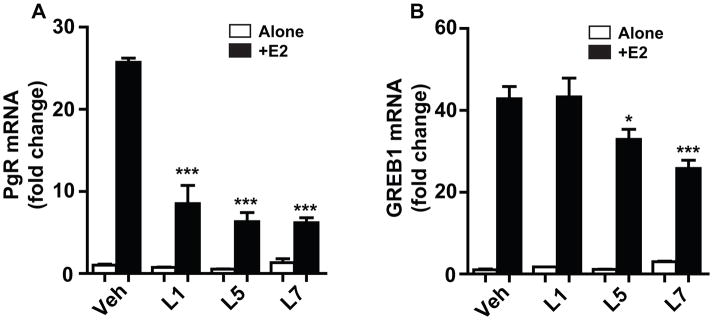
Licorice components L1, L5, and L7 stimulated estrogen responsive gene expression only weakly if at all, yet they bound to ERα with affinities similar to those of components L3 and L4 that activated gene transcription. Consequently, we asked whether these three components might be acting as antagonists, binding to ER but inhibiting the activation of gene expression by E2. As seen in Fig. 3, components L1, L5 and L7 did not stimulate expression of PgR or GREB1, but did block the E2-stimulated increased expression of these genes, either almost fully (PgR, Fig. 3A) or only partially (GREB1, Fig. 3B). Of interest, the three licorice components L1, L5, and L7 were approximately equally inhibitory of E2 activity on PgR, whereas they differed in their efficacy in inhibiting GREB1; of the three components, L7 was the most effective antagonist of GREB1 stimulation by E2.
Figure 3.
Assessment of the ability of licorice root components L1, L5, and L7 to inhibit gene stimulation by E2. MCF-7 cells were treated with control vehicle, or 3×10−6 M L1, L5, or L7 alone or along with 10−9 M E2 for 24 h, and stimulation of PgR (A) and GREB1 (B) gene expression was monitored by qPCR. *, P<0.05; ***, P<0.001.
Licorice root components Liquiritigenin and L3 can effectively stimulate estrogen-responsive gene expression at high concentrations
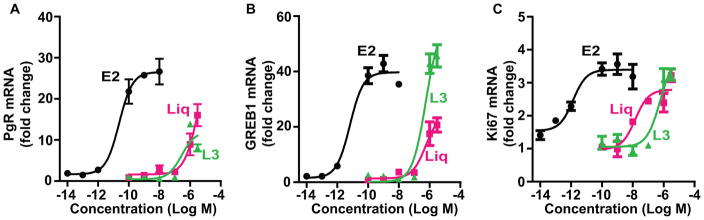
The dose-response profiles shown in Fig. 4 reveal that Liq and component L3 show a similar dose-dependent stimulation of these ERα target genes. Gene activation of a magnitude similar to that elicited by estradiol was reached when high concentrations of Liq and L3 were used, these concentrations being approximately 10,000 times higher than the concentrations needed with E2. The dose-response findings show that the low potency of Liq and L3 in terms of activation of gene expression reflects the low binding affinity of L3 and Liq for ERα, compared with that of the endogenous hormone E2.
Figure 4.
Dose-response effects of E2, Liquiritigenin, and licorice root component L3 on the expression of three estrogen target genes (PgR, GREB1, and Ki67) in MCF-7 cells. The RNA level of the three genes was monitored by qPCR after 24 h exposure of cells to different concentrations of the compounds, as indicated.
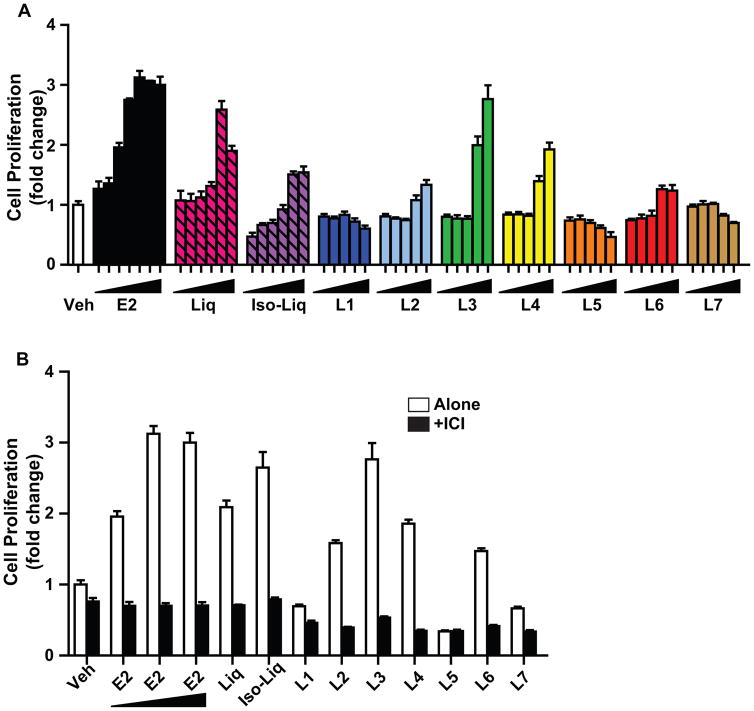
Licorice root components vary in their effectiveness in promoting cell proliferative activity through ERα
We examined effects of the licorice root components on the proliferation of MCF7 breast cancer cells (Figure 5A). Liq, Iso-Liq, and components L3 and L4 stimulated cell proliferation quite strongly at the two highest concentrations tested, 10−6 and 3×10−6 M. L3 and Liq gave the highest level of proliferation stimulation, reaching levels similar to that evoked by E2. E2-stimulated cell proliferation, however, began at very low concentrations (10−12 M) and reached maximal stimulation at 10−10 M, whereas 10,000-fold higher concentrations of Liq and L3 were required to reach similar levels of cell proliferation, consistent with the markedly lower ER binding affinity of Liq and L3 compared to E2.
Figure 5.
Dose-response effects of E2 and licorice root components on proliferation of MCF-7 cells. (A) Proliferation was monitored after 6 days of exposure to the indicated compounds. Concentrations of E2 used in 10-fold increments were 10−14 M through 10−8 M; Liq and Iso-Liq were 10−10 M through 10−6 M and 3×10−6 M; and L1–L7 were 10−9 M through 10−6 M and 3×10−6 M (B) Proliferation was monitored after 6 days of exposure to compounds alone (control vehicle; 10−12 M, 10−10 M, or 10−8 M E2; 10−6 M Liq or Iso-Liq; or 3×10−6 M L1–L7) or compounds plus cotreatment with 10−6 M antiestrogen ICI 182,780.
Furthermore, as shown in Fig. 5B, the stimulation of cell proliferation by all compounds, as monitored by cell number after 6 days of treatment, was fully eliminated upon cotreatment with the antiestrogen ICI 182,780, confirming that the proliferative activity by E2 and all of the stimulatory licorice root components was mediated through ERα. Again, as observed in the regulation of gene expression, components L1, L5 and L7 were inactive in eliciting elevation of cell proliferation.
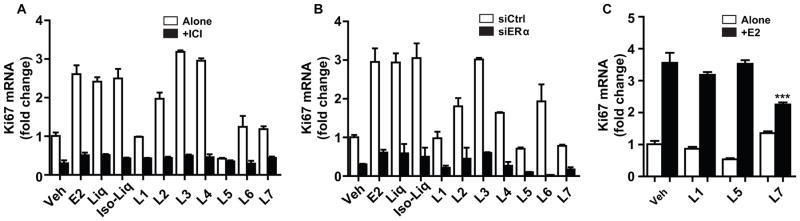
We also examined the effects of all compounds on expression of the proliferation-associated gene Ki67. As shown in Figure 6A, all compounds except for L1, L5, L6 and L7 increased Ki67 RNA expression, with these increases being eliminated upon cotreatment with the antiestrogen ICI (Fig. 6A) or following knockdown of ERα with siRNA (Fig. 6B), again implying mediation by ERα. Of interest, L7 did not stimulate Ki67 expression but partially suppressed the increase in Ki67 mRNA elicited by E2 (Fig. 6C). By contrast, L1 and L5 did not stimulate Ki67 gene expression and were not effective in suppressing stimulation of this gene expression by E2.
Figure 6.
Effects of E2 and licorice root components on expression of the proliferation-related gene Ki67 in MCF-7 cells. (A) Effect of cotreatment with the pure antiestrogen 10−6 M ICI 182,780 or (B) depletion of ERα with receptor-specific siRNA vs. control siRNA. Cells were treated with control vehicle, 10−8 M E2, or with 10−6 M Liq or Iso-Liq, or 3×10−6 M L1–L7 with or without ICI 182,780 for 24 h, or with licorice root components, E2, or control vehicle (0.1% ethanol) after exposure to siRNA for 24 h. RNA was harvested from cells after treatments and gene RNA level determined by qPCR. (C) Assessment of the ability of licorice components (L1, L5, or L7) to alter Ki67 gene expression when given alone, or to reduce Ki67 gene expression stimulation by E2. Cells were treated with control vehicle or 3×10−6 M L1, L5, or L7 alone or in the presence of 10−9 M E2 for 24 h prior to RNA harvest and qPCR analysis for Ki67 RNA. ***, P<0.001.
Effects of Licorice Components on Estrogen-Responsive Gene Expression in Hepatoma Cells Containing ERα
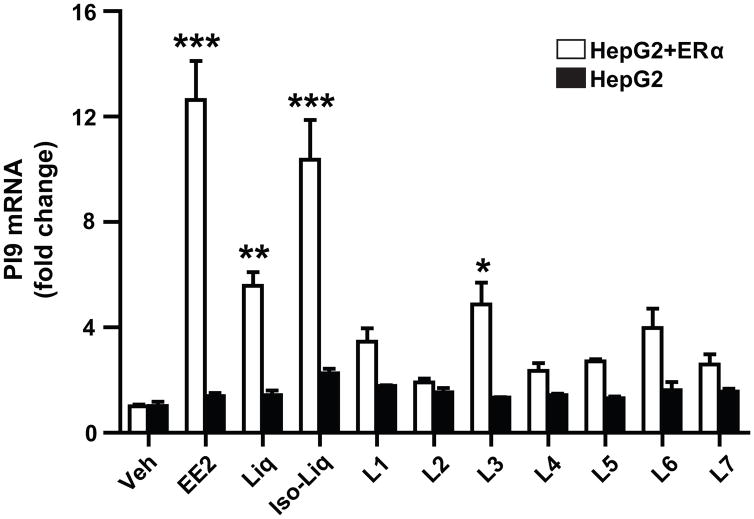
For comparison, we also examined effects of the licorice components in a different estrogen target cell. For the studies shown in Figure 7, we utilized liver cancer cells stably expressing ERα (HepG2 + ERα) or parental HepG2 cells that lack ERα. As seen in Fig. 7, ethinyl estradiol (EE2), Liq, Iso-Liq, and only component L3 stimulated expression of proteinase inhibitor 9 (PI9), an estrogen responsive gene that encodes an inhibitor of granzyme B-mediated apoptosis [9, 10] in HepG2 + ERα cells. Because of possible metabolism of compounds by the liver cells, we used EE2, a less readily metabolized estrogen in place of E2 in these cell treatments, and we tested all licorice components at 3×10−6 M. No gene stimulation by the compounds was obtained in cells lacking ERα (Fig. 7), implying that the gene stimulations were dependent on presence of the estrogen receptor.
Figure 7.
Effects of licorice root components on expression of the estrogen-responsive gene, proteinase inhibitor 9 (PI9), in hepatoma HepG2 cells containing ERα or lacking ERα. Cells were treated with control 0.1% ethanol vehicle, or with 10−8 M ethinyl E2 (EE2), or 3 × 10−6 M Liq, Iso-Liq, or L1–L7 for 24 h. RNA was then harvested from cells and PI9 RNA level determined by qPCR. *, P<0.05; **, P<0.01, ***, P<0.001.
DISCUSSION
Flavonoid components of licorice root extract have SERM-like estrogen and antiestrogen activity
To more fully understand the estrogenic nature of licorice-containing dietary supplements, beyond that of the most widely studied components, Liq and Iso-Liq, we have evaluated 7 isoflavone and chalcone components of an extract of licorice root powder in terms of their affinity for binding to the estrogen receptors and their estrogen agonist and antagonist activity in breast cancer and hepatoma cells. These seven licorice root components have affinities for both ERs that are greater than those of Liq and Iso-Liq.
The best estrogen agonists were methoxychalcone (L3), Liq, and Iso-Liq, followed by Calycosin (L2), Verstitol (L4) and Glycycoumarin (L6). Interestingly, the best of these agonists had intrinsic activities equivalent to that of E2 in terms of activation of estrogen-regulated genes and stimulation of proliferation; they reached this level of activity, however, only at concentrations much greater than E2. Glicoricone (L7) was not an agonist, but it binds to ER and showed estrogen antagonist activity. Finally, Glabridin (L1) and Glyasperin (L5) were very weak agonists and showed some antagonist activity that was dependent on the particular gene. These estrogenic activities were all shown to be dependent on ER. Of interest, previous studies have reported on the estrogenicity of Glabridin in endometrial cancer cells [15] and osteoblast cells [16] at high concentrations and on the anti-obesity effects of Glabridin in high fat diet-fed obese mice [17–19].
Among these compounds, there are some structural features that appear to denote significant agonist activity: The most agonistic components, Liq, Iso-Liq, and L3, are either a flavan (Liq), or its corresponding chalcone (Iso-Liq) and the chalcone methyl ether (L3). All of the other components are isoflavonoids, but beyond this, there are no clear structural features that connote substantial agonist activity (L2, L4, and L6) vs. very low agonist and antagonist activity (L1, L5, and L7). Binding affinity for either ER also fails to distinguish either potency or efficacy, with two of the best binders (L1 and L5) actually having the least pronounced agonist or antagonist activity.
Our findings highlight that the multiple components in licorice root extracts have selective estrogen receptor modulator or SERM-like activity and function as mixed estrogens and antiestrogens to different degrees in different target cells. That these botanical estrogens behave as SERMs suggest that they likely can induce distinct conformations of the estrogen receptors (ERs) that recruit different sets of coregulators to generate distinct receptor multicomponent complexes known to bring about different biological activities, as observed for other SERMs such as tamoxifen and raloxifene [20–22]. Because ERβ and GPR30 are present at only very low levels in the two cell types we have studied, we think it unlikely that either of these receptors play a role in the responses we report herein [8, 23].
Of note, the potencies of the most active components are several orders of magnitude less than that of E2 and other pharmaceutical estrogens used in hormone replacement therapies. It is well known that isoliquiritigenin and its isomer liquiritigenin are in dynamic equilibrium [24], but blood levels of free (unconjugated) compounds after oral consumption of licorice-containing dietary supplements by humans have not been characterized.
Estrogens exert effects on diverse target cells and tissues
Estrogens have effects on a wide range of cells and tissues, including those in the breast and liver, the two cell types investigated here. Our findings illustrate that the multiple flavonoid components in licorice root extracts have selective estrogen receptor modulator or SERM-like activity that could function as mixed estrogens and antiestrogens to different degrees in different target tissues. Their SERM-like activities and the differences we have observed among the components in their actions and efficacy in breast and liver cells likely underlie and help in understanding the mechanistic basis for reported differences in their impact on different estrogen target cells and tissues. Thus, while there are reports that various preparations of licorice root may not be effective in moderating the vasomotor symptoms of hot flush [25, 26], there are reports that botanical supplements containing licorice root or licorice root components may provide estrogen-like benefits to other estrogen target tissues, notably to the cardiovascular system [27] and the metabolic profile [15, 17, 19, 28, 29], consistent with their tissue-selective SERM-like activity observed in our studies.
HIGHLIGHTS.
Menopausal women often consume licorice root botanical dietary supplements as a natural alternative to pharmaceutical hormone replacement therapy.
Characterization of the estrogen receptor (ER) activity of 9 components from licorice root extracts reveal that they have estrogen agonist and/or antagonist activity, akin to selective estrogen receptor modulators (SERMs).
The 9 components, which are flavonoids, isoflavonoids, and chalcones, bind to ERs with low affinity.
All agonist or antagonist activities were ER dependent.
Acknowledgments
This study was made possible by NIH Grant Number P50AT006268 (to WGH, IK, DD, JAK and BSK) from the National Center for Complementary and Integrative Health (NCCIH), the Office of Dietary Supplements (ODS) and the National Cancer Institute (NCI). Financial support from the Thailand Research Fund through the Royal Golden Jubilee Ph.D. Program (Grant No. PHD/0170/2552) to PP and NB, and from NIH DK015556 (to JAK) is gratefully acknowledged.
ABBREVIATIONS
- E2
estradiol
- Iso-Liq
isoliquiritigenin
- Liq
liquiritigenin
- RBA
relative binding affinity
- SERM
selective estrogen receptor modulator
Footnotes
The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the NCCIH, ODS, NCI, the National Institutes of Health, or the U.S. Food and Drug Administration.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Burns KA, Korach KS. Estrogen receptors and human disease: an update. Archives of toxicology. 2012;86:1491–504. doi: 10.1007/s00204-012-0868-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deroo BJ, Korach KS. Estrogen receptors and human disease. J Clin Invest. 2006;116:561–70. doi: 10.1172/JCI27987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dahlman-Wright K, Cavailles V, Fuqua SA, Jordan VC, Katzenellenbogen JA, Korach KS, et al. International Union of Pharmacology. LXIV. Estrogen receptors. Pharmacological reviews. 2006;58:773–81. doi: 10.1124/pr.58.4.8. [DOI] [PubMed] [Google Scholar]
- 4.Hajirahimkhan A, Simmler C, Yuan Y, Anderson JR, Chen SN, Nikolic D, et al. Evaluation of estrogenic activity of licorice species in comparison with hops used in botanicals for menopausal symptoms. PLoS One. 2013;8:e67947. doi: 10.1371/journal.pone.0067947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kao TC, Wu CH, Yen GC. Bioactivity and potential health benefits of licorice. J Agric Food Chem. 2014;62:542–53. doi: 10.1021/jf404939f. [DOI] [PubMed] [Google Scholar]
- 6.Hajirahimkhan A, Dietz BM, Bolton JL. Botanical modulation of menopausal symptoms: mechanisms of action? Planta Med. 2013;79:538–53. doi: 10.1055/s-0032-1328187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang Y, Gong P, Madak-Erdogan Z, Martin T, Jeyakumar M, Carlson K, et al. Mechanisms enforcing the estrogen receptor beta selectivity of botanical estrogens. Faseb J. 2013;27:4406–18. doi: 10.1096/fj.13-234617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Madak-Erdogan Z, Charn TH, Jiang Y, Liu ET, Katzenellenbogen JA, Katzenellenbogen BS. Integrative genomics of gene and metabolic regulation by estrogen receptors alpha and beta, and their coregulators. Molecular systems biology. 2013;9:676. doi: 10.1038/msb.2013.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krieg AJ, Krieg SA, Ahn BS, Shapiro DJ. Interplay between estrogen response element sequence and ligands controls in vivo binding of estrogen receptor to regulated genes. J Biol Chem. 2004;279:5025–34. doi: 10.1074/jbc.M307076200. [DOI] [PubMed] [Google Scholar]
- 10.Kanamori H, Krieg S, Mao C, Di Pippo VA, Wang S, Zajchowski DA, et al. Proteinase inhibitor 9, an inhibitor of granzyme B-mediated apoptosis, is a primary estrogen-inducible gene in human liver cells. J Biol Chem. 2000;275:5867–73. doi: 10.1074/jbc.275.8.5867. [DOI] [PubMed] [Google Scholar]
- 11.Chang EC, Charn TH, Park SH, Helferich WG, Komm B, Katzenellenbogen JA, et al. Estrogen Receptors alpha and beta as determinants of gene expression: influence of ligand, dose, and chromatin binding. Mol Endocrinol. 2008;22:1032–43. doi: 10.1210/me.2007-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carlson KE, Choi I, Gee A, Katzenellenbogen BS, Katzenellenbogen JA. Altered ligand binding properties and enhanced stability of a constitutively active estrogen receptor: evidence that an open pocket conformation is required for ligand interaction. Biochemistry. 1997;36:14897–905. doi: 10.1021/bi971746l. [DOI] [PubMed] [Google Scholar]
- 13.Katzenellenbogen JA, Johnson HJ, Jr, Carlson KE. Studies on the uterine, cytoplasmic estrogen binding protein. Thermal stability and ligand dissociation rate. An assay of empty and filled sites by exchange. Biochemistry. 1973;12:4092–9. doi: 10.1021/bi00745a011. [DOI] [PubMed] [Google Scholar]
- 14.Frasor J, Chang EC, Komm B, Lin CY, Vega VB, Liu ET, et al. Gene expression preferentially regulated by tamoxifen in breast cancer cells and correlations with clinical outcome. Cancer Res. 2006;66:7334–40. doi: 10.1158/0008-5472.CAN-05-4269. [DOI] [PubMed] [Google Scholar]
- 15.Poh MSW, Yong PVC, Viseswaran N, Chia YY. Estrogenicity of glabridin in Ishikawa cells. PLoS One. 2015;10:e0121382. doi: 10.1371/journal.pone.0121382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi EM. The licorice root derived isoflavan glabridin increases the function of osteoblastic MC3T3-E1 cells. Biochem Pharmacol. 2005;70:363–8. doi: 10.1016/j.bcp.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 17.Ahn J, Lee H, Jang J, Kim S, Ha T. Anti-obesity effects of glabridin-rich supercritical carbon dioxide extract of licorice in high-fat-fed obese mice. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 2013;51:439–45. doi: 10.1016/j.fct.2012.08.048. [DOI] [PubMed] [Google Scholar]
- 18.Lee JW, Choe SS, Jang H, Kim J, Jeong HW, Jo H, et al. AMPK activation with glabridin ameliorates adiposity and lipid dysregulation in obesity. Journal of lipid research. 2012;53:1277–86. doi: 10.1194/jlr.M022897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aoki F, Honda S, Kishida H, Kitano M, Arai N, Tanaka H, et al. Suppression by licorice flavonoids of abdominal fat accumulation and body weight gain in high-fat diet-induced obese C57BL/6J mice. Bioscience, biotechnology, and biochemistry. 2007;71:206–14. doi: 10.1271/bbb.60463. [DOI] [PubMed] [Google Scholar]
- 20.Martinkovich S, Shah D, Planey SL, Arnott JA. Selective estrogen receptor modulators: tissue specificity and clinical utility. Clinical interventions in aging. 2014;9:1437–52. doi: 10.2147/CIA.S66690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oseni T, Patel R, Pyle J, Jordan VC. Selective estrogen receptor modulators and phytoestrogens. Planta Med. 2008;74:1656–65. doi: 10.1055/s-0028-1088304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hall JM, McDonnell DP. Coregulators in nuclear estrogen receptor action: from concept to therapeutic targeting. Molecular interventions. 2005;5:343–57. doi: 10.1124/mi.5.6.7. [DOI] [PubMed] [Google Scholar]
- 23.Madak-Erdogan Z, Kieser KJ, Kim SH, Komm B, Katzenellenbogen JA, Katzenellenbogen BS. Nuclear and extranuclear pathway inputs in the regulation of global gene expression by estrogen receptors. Mol Endocrinol. 2008;22:2116–27. doi: 10.1210/me.2008-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simmler C, Hajirahimkhan A, Lankin DC, Bolton JL, Jones T, Soejarto DD, et al. Dynamic residual complexity of the isoliquiritigenin-liquiritigenin interconversion during bioassay. J Agric Food Chem. 2013;61:2146–57. doi: 10.1021/jf304445p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Menati L, Khaleghinezhad K, Tadayon M, Siahpoosh A. Evaluation of contextual and demographic factors on licorice effects on reducing hot flashes in postmenopause women. Health care for women international. 2014;35:87–99. doi: 10.1080/07399332.2013.770001. [DOI] [PubMed] [Google Scholar]
- 26.Nahidi F, Zare E, Mojab F, Alavi-Majd H. Effects of licorice on relief and recurrence of menopausal hot flashes. Iranian journal of pharmaceutical research : IJPR. 2012;11:541–8. [PMC free article] [PubMed] [Google Scholar]
- 27.Choi YJ, Lim SS, Jung JY, Choi JS, Kim JK, Han SJ, et al. Blockade of nitroxidative stress by roasted licorice extracts in high glucose-exposed endothelial cells. J Cardiovasc Pharmacol. 2008;52:344–54. doi: 10.1097/FJC.0b013e3181888898. [DOI] [PubMed] [Google Scholar]
- 28.Gaur R, Yadav KS, Verma RK, Yadav NP, Bhakuni RS. In vivo anti-diabetic activity of derivatives of isoliquiritigenin and liquiritigenin. Phytomedicine. 2013 doi: 10.1016/j.phymed.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 29.Saunier EF, Vivar OI, Rubenstein A, Zhao X, Olshansky M, Baggett S, et al. Estrogenic plant extracts reverse weight gain and fat accumulation without causing mammary gland or uterine proliferation. PLoS One. 2011;6:e28333. doi: 10.1371/journal.pone.0028333. [DOI] [PMC free article] [PubMed] [Google Scholar]