Abstract
Background
Melioidosis, caused by Burkholderia pseudomallei, is an endemic disease in Bangladesh. No systematic study has yet been done to detect the environmental source of the organism and its true extent in Bangladesh. The present study attempted to isolate B. pseudomallei in soil samples and to determine its seroprevalence in several districts in Bangladesh.
Methodology and Results
Soil samples were collected from rural areas of four districts of Bangladesh from where culture confirmed melioidosis cases were detected earlier. Multiple soil samples, collected from 5–7 sampling points of 3–5 sites of each district, were cultured in Ashdown selective media. Suspected colonies of B. pseudomallei were identified by biochemical and serological test, and by polymerase chain reaction (PCR) using 16s rRNA specific primers. Blood samples were collected from 940 healthy individuals of four districts to determine anti- B. pseudomallei IgG antibody levels by indirect enzyme linked immunosorbent assay (ELISA) using sonicated crude antigen. Out of 179 soil samples, B. pseudomallei was isolated from two samples of Gazipur district which is located 58 km north of capital Dhaka city. Both the isolates were phenotypically identical, arabinose negative and showed specific 550bp band in PCR. Out of 940 blood samples, anti- B. pseudomallei IgG antibody, higher than the cut-off value (>0.8), was detected in 21.5% individuals. Seropositivity rate was 22.6%-30.8% in three districts from where melioidosis cases were detected earlier, compared to 9.8% in a district where no melioidosis case was either detected or reported (p<0.01). Seropositivity increased with the advancement of age from 5.3% to 30.4% among individuals aged 1–10 years and > 50 years respectively. The seropositivity rates were 26.0% and 20.6% in male and female respectively, while it was 20–27% among different occupational groups. No significant association was observed with gender (χ2 = 3.441, p = 0.064) or any occupational group (χ2 = 3.835, p = 0.280).
Conclusion
This is the first study demonstrating the presence of B. pseudomallei in the environmental (soil) samples of Bangladesh. It also suggested that a large proportion of people, residing in these districts, were exposed to the organism.
Author Summary
Melioidosis, caused by B. pseudomallei, can be a fatal disease if not treated with appropriate antibiotics. The organism is mainly present in soil and water in endemic areas, and people become infected through skin inoculation, inhalation or ingestion. The disease has been sporadically detected in Bangladesh over last several decades. However, its actual prevalence in Bangladesh is largely unknown due to the lack of systematic study and awareness of the medical community about the disease and the organism. In order to address the issue, we have undertaken this study to assess the presence of the organism in the soil as well as its magnitude of exposure among the people of selected areas of the country. The study revealed the presence of B. pseudomallei in the soil and its exposure among the people of different areas. The information would increase the awareness of the medical community for prevention and correct diagnosis of the disease.
Introduction
Melioidosis is an endemic disease of public health and clinical importance in tropical and subtropical regions of the world [1]. It is caused by a Gram negative saprophytic bacterium called Burkholderia pseudomallei. Infection occurs through skin and by inhalation when susceptible individuals are exposed to contaminated water and soil [2,3]. Melioidosis accounts for about 20% of all community-acquired septicemias in north-eastern Thailand and 2000 to 3000 new cases are diagnosed every year [4,5]. Multiple cases have also been reported from India and several countries of South-East Asia, the Middle East, Africa and South America [6].
In 1964, melioidosis was reported in a foreign sailor who was travelling through Bangladesh [7]. However, the first case of melioidosis in Bangladesh was diagnosed in a native Bangladeshi infant in 1988 [8]. Later on several cases of melioidosis were reported up to 2014 [9]. From 1991 to 1999, five cases were detected in United Kingdom (UK) among Bangladeshi people who immigrated to UK from the Sylhet region (a northeastern district) of Bangladesh [10–12]. In 2001, we reported the second culture confirmed suppurative melioidosis case in a 48 years old diabetic patient who came from Sherpur district of Bangladesh [13]. The district is located about 140 km north of capital Dhaka. Later on, at least 10 cases were detected among the diabetic patients at Bangladesh Institute of Research and Rehabilitation in Diabetes, Endocrine and Metabolic Disorders (BIRDEM) Hospital, Dhaka from 2009 to 2014 [9]. Analyses of the reported cases strongly indicate that the disease is potentially endemic in ten districts of Bangladesh particularly in northern and northeastern parts of the country.
Recently in 2013, melioidosis endemic countries of the world have been categorized into ‘definite’ and ‘probable’ country based on the presence of B. pseudomallei in humans and in the environment in the respective countries [6]. According to the above categorization, Bangladesh falls into ‘probable’ category of country as the presence of the organism in the environment has not yet been identified or reported even though several culture-confirmed melioidosis cases have been detected. Probability of the presence of B. pseudomallei in soil and water of Bangladesh is very high as the climatic condition of the country is favorable for its growth in the environment. Therefore, isolation and identification of B. pseudomallei from environmental samples (e.g. soil or water) is important to determine the source of the organism of melioidosis cases in the country.
The true extent of the disease in Bangladesh is not known, as this disease is not familiar to most of the physicians and microbiologists of the country. Seroepidemiological studies showed that 80% of children in north-eastern Thailand were positive for antibodies against B. pseudomallei by the age of 4 years [1]. In Malaysia, reported seroprevalence in healthy individuals was 17–22% among rice farmers and 26% in blood donors [14]. In north Australia 0.6 to 16% of children had evidence of infection by B. pseudomallei [15]. A hospital based serological survey in Bangladesh reported 28.9% seropositive rate for B. pseudomallei antibody among patients attending several tertiary care hospitals for unrelated ailments. The study, however, used a very low cut off titer (1:10) of indirect haemagglutination assay (IHA) for defining seropositive cases without considering the presence of cross reactive background antibody among the local population. The study did not investigate the possible source of the organism [16].
In view of the above, detection of B. pseudomallei in the soil samples and determination of anti-pseudomallei antibody in healthy population would help to establish the environmental source of the organism as well as the extent of its exposure in Bangladesh. So far, no systematic study has been done to find out the presence of organisms in environmental samples of Bangladesh. Therefore, the present study was designed for detection of B. pseudomallei by culture and molecular method from soil as well as to determine the extent of exposure by detecting antibodies to B. pseudomallei among the healthy population of four districts of Bangladesh.
Materials and Methods
The present study was carried out to isolate and identify B. pseudomallei in soil samples from four selected geographical areas of Bangladesh. The study also determined the presence of anti- B. pseudomallei IgG antibody to find out the seroprevalence of the infection in four districts of the country. B. pseudomallei was isolated in selective media, identified by biochemical tests, and finally confirmed by specific antisera and PCR. Serum IgG antibody to B. pseudomallei was determined by ELISA. Details of the methods are described below.
Ethics statement
The Ethical Review Committee (ERC) of the Diabetic Association of Bangladesh (BADAS) has approved the study. Ibrahim Medical College (IMC) is an institution under the BADAS and its ERC is the approval body for research protocols of IMC. Informed written consent was obtained from all adult participants (age 18 years and above) and from the parents/guardians of all children (age up to 17 years) prior to collection of blood samples and demographic data.
Selection of sites and collection of soil samples
Soil samples were collected from rural areas of four districts of Bangladesh with diagnosed melioidosis cases [9]. Three districts namely Mymensingh, Sylhet and Gazipur are situated in the north and northeast of capital Dhaka city while one district (Narayangange) is located south of Dhaka city. The locations of soil sampling district and their distance from capital Dhaka is shown in Fig 1. In each district, 3–5 sites were selected for collection of soil samples. Each sampling site was about 5 km apart from the next sampling sites. At each site 5-7sampling points were identified which were about 30 meters apart from each other. The preferred collection site was moist area within a rice field. Approximately 200 g soil was taken from each point from a depth of about 20–30 cm using a shell augur disinfected with 70% alcohol in between soil collection. Collected soil was placed into a sterile plastic bag and sealed with rubber band to prevent moisture loss and was transported to the laboratory as soon as possible. All the soil samples were collected and processed for culture from June to September 2011.
Fig 1. Map of Bangladesh showing collection sites (districts) of soil and blood samples.
Shaded areas indicate districts with known melioidosis cases. M = Mymensingh, G = Gazipur, K = Kishoregange, S = Sylhet, N = Narayangange. The distance of Mymensingh, Gazipur, Kishoregange, Sylhet and Narayangange from capital Dhaka is 115, 40, 89, 198 and 14 km respectively. ■ = Site of soil collection; ▲ = Site of blood collection; ● = Capital Dhaka. Source of the map: [9].
Isolation and identification of B. pseudomallei from soil samples by culture
Soil samples were processed for culture as described by Brook et al [17]. Twenty grams of soil were mixed with 40 ml sterile distilled water and the suspension was shaken vigorously for one minute and allowed to settle for 5–10 minutes. The supernatant fluid was collected. For enrichment, 1 ml of supernatant fluid was inoculated into 9 ml of modified Ashdown’s selective enrichment broth (ASB) and incubated at 37°C for 48 hours [18]. After enrichment, 10 μl of broth was streaked onto modified Ashdown`s selective agar (ASA) medium. The plates were incubated for 48–72 hours to allow typical colonies to grow. Purple colored dry, wrinkled and oxidase positive colonies were then sub cultured on MacConkey`s agar medium and incubated at 42°C. The organisms which grew on MacConkey`s agar medium at 42°C were identified as B. pseudomallei by typical colony morphology, Gram staining (bipolar staining), motility, biochemical tests (including API 20NE), arabinose assimilation and resistance to colistin and aminoglycoside [19]. Monoclonal antibody based latex agglutination test (Melioidosis Research Center, Khon Kaen, Thailand) was performed for the final identification and confirmation of the suspected colonies of B. pseudomallei.
Confirmation of B. pseudomallei by PCR
Serologically confirmed B. pseudomallei isolates from soil samples were further confirmed by PCR using specific primers constructed from 16s rRNA region of B. pseudomallei [16]. The primers were constructed from 16s rRNA region of B. pseudomallei to amplify a fragment of 550 bp in length. Primers were—PPM3 forward primer (5`AATCATTCTGGCTAATACCCG 3`) and PPM4 reverse primer (5`CGGTTCTCTTTCGAGCTCG 3`).
Total genomic DNA was prepared by RealLine DNA Extraction Sample Kit” (BIORON Diagnostics GmbH, Germany). Briefly, 500 μl preheated (at 56°C) lysis reagent with sorbent was added to 100 μl bacterial suspension. The tube was vortexed for 10 seconds followed by incubation in thermo shaker for 10 minutes at 1300 rpm. DNA/RNA solution was added to the tube and again vortexed and centrifuged at 13000 x g for 5 minutes. Supernatant was discarded and Wash Solution No 1 was added to the tube, vortexed and centrifuged at 13000 x g for 5 minutes. Supernatant was again removed and 300 μl Wash Solution No 2 was added to the tube, vortexed and centrifuged at 13000 x g for 5 minutes. Supernatant was discarded and the pellet was dried by opening the cap for 2–3 minutes at room temperature. 200 μl specimen diluents was added to the air dried tube and vortexed vigorously for 10 seconds followed by incubation at thermo shaker for 10 minutes at 56°C at 1300 rpm and then centrifuged at 13000 x g for 1 minute. Supernatant containing DNA was finally collected and stored at -20°C until used.
PCR amplification was carried out in a 25 μl final volume containing 2.0 μl DNA, 2.5 μl 1 x PCR buffer, 1.5 mM MgCl2, 25 μM of each dNTP, 10 pM of each primer, and 1.25 unit of Taq DNA polymerase enzyme. Samples were subjected to initial denaturation at 94°C for 2 minutes followed by denaturation at 94°C for 60 sec, primer annealing at 55°C for 60 sec and extension at 72°C for 90 sec. Final extension was for 10 minutes at 72°C. Amplification was performed in Master Cycler (Eppendorf) programmed for 35 cycles.
Amplified PCR product was analyzed by electrophoresis in 1.5% agarose gel containing ethidium bromide (0.5 μg/ml) in TBE buffer (0.04 M Tris acetate, 0.001 M EDTA, (pH 8.6) and photographed under UV illumination. The bands were compared to the band obtained with a positive B. pseudomallei DNA control. In all assays, DNA from known B. pseudomallei was included as positive control. A tube without DNA served as no template DNA control. All suspected soil and clinical B. pseudomallei isolates from the present and previous study [9] were sent to the Emerging Pathogens Institute (EPI), University of Florida, USA for species identification using type III secretion system (TTS1) assay [20]
Study population and collection of blood samples
Relatives or attendants of patients attending the rural healthcare facilities of the four districts namely Mymensingh, Sylhet, Narayangange and Kishoregange were recruited for determining the anti- B. pseudomallei antibodies (Fig 1). Blood samples were collected from 940 healthy individuals with no history of fever, persistent cough, wasting or suppurative lesion. Age, sex and socio-economic conditions were recorded.
In order to determine the cut off optical density (OD) value of ELISA test, 51 healthy newborn babies of Dhaka city were enrolled in the study. About 1–2 ml of venous blood was collected from each individual with proper aseptic technique. Serum samples from 10 culture confirmed melioidosis cases admitted at BIRDEM Hospital were included in this study as positive controls.
Determination of anti- B. pseudomallei IgG antibody by ELISA
Serum anti- B. pseudomallei IgG antibody was determined by an indirect ELISA as described by Voller et al [21]. To prepare sonicated antigen, 50 ml of Trypticase Soya Broth (TSB) was inoculated with pure colonies of B. pseudomallei USM strain and incubated overnight at 37°C. Organisms were harvested by centrifugation for 30 minutes at 4000 x g at 10°C. Pellets were suspended with 3 ml of 25 mM Tris-HCL (pH 7.4) and washed three times with Tris-HCL for 30 minutes at 4000 x g at 10°C. Deposited pellet, suspended in 5 ml of ice-cold Tris-HCL, was sonicated at 40W for 8 minutes in each pulse inside the assigned biosafety cabinet. Sonicated bacterial suspension was then centrifuged at 5000 x g at 10°C for 30 minutes. After centrifugation, the supernatant containing the bacterial proteins was collected and its protein concentration was determined. The 96 well EIA plate (Linbro, USA) was coated with sonicated antigen 10 μg/ml in 0.5 M carbonate/bicarbonate buffer (pH 9.6). To each well 100 μl volume of coating buffer was added and incubated overnight at 4°C. The plate was washed three times with PBS-0.05% Tween 20 (PBS-T, pH 7.4)) and blocked by incubating for 2 hrs with PBS-T containing 2% BSA at 37°C. The plate was then washed three times with PBS-T. A volume of 100 μl serum (1:1600 dilutions) sample was added into each well and incubated for 4 hours at 37°C. After washing with PBST three times, 100 μl of horseradish peroxidase conjugated anti-human IgG antibodies (1:4000) was added and incubated at 37°C for 2 hours. After washing three times with PBST, 50 μl of TMB substrate was added to each well and incubated at room temperature for 30 minutes in dark. Then 50 μl of 1 M sulfuric acid was added in each well. The colour developed was measured by EIA plate reader (Human ELISA Reader) at 450 nm. Optimum concentration of the antigen (10 μg/ml) and serum dilution (1:1600) was predetermined by checkerboard titrations.
A cut off OD values for anti- B. pseudomallei IgG antibody was determined to find out the exposure rate to B. pseudomallei in the study population. ELISA was performed with sera from 51 healthy newborn babies of Dhaka city who were presumed not to be exposed to B. pseudomallei. The mean OD + 3xSD of these sera were taken as cut-off OD value to determine the exposure rate. The mean OD±SD of the 51 healthy newborn babies were 0.2±0.2. Therefore, the calculated cut-off OD value was 0.8 (0.2+3x0.2). Any sample showing OD above this cut-off value of 0.8 was considered positive and referred to as exposed to B. pseudomallei infection. The mean OD value of ten culture positive cases (positive control) was 2.26±0.2.
To determine the specificity of anti- B. pseudomallei IgG by ELISA, a sub-set of 24 known positive serum samples were adsorbed with whole cell killed Pseudomonas aeruginosa and B. pseudomallei USM strain (1x 108 organisms/ml) by incubating overnight at 4°C. The adsorbed serum samples were centrifuged at 10,000 x g for 5 minutes to remove the bacteria. Anti- B. pseudomallei IgG antibody was then determined in adsorbed serum by ELISA as described above. Decline of antibody concentration in terms of OD values after adsorption with B. pseudomallei indicated presence of specific antibody to B. pseudomallei in serum samples while decline with P. aeruginosa indicated antibodies cross reacting to pseudomonas antigens. The adsorption assay showed that mean antibody level of the positive sera reduced significantly, after adsorption with B. pseudomallei compared to pre-adsorbed value from OD 1.1 to 0.6 (Fig 2). The mean OD value decreased below the cut off OD of 0.8 following absorption. But, the OD value decreased insignificantly from 1.1 to 0.9 following adsorption with P. aeruginosa
Fig 2. The effect of adsorption with whole cell P. aeruginosa and B. pseudomallei on the anti- B. pseudomallei IgG antibodies.

Before Ad = Before adsorption; Ps Ad = Adsorption with P. aeruginosa; Bps Ad = Adsorption with B. pseudomallei. n = 24 seropositive sera in each group.
Results
B. pseudomallei in soil sample
Total 179 samples from four districts with diagnosed melioidosis cases were tested for the presence of B. pseudomallei. Out of 179 soil samples, 87 yielded growth of oxidase positive non-fermenting, aminoglycoside and colistin resistant suspected colonies on the Ashdown selective media after enrichment at 42°C. Out of these suspected isolates, only two isolates (K23 and K35) were identified as B. pseudomallei (Table 1). These two isolates were finally confirmed as B. pseudomallei by specific monoclonal anti-sera to B. pseudomallei and polymerase chain reaction (Fig 3 PCR gel). These two soil and all the clinical isolates (ten) were positive for TTS1 (S1 Table and S1 Fig). Both the isolates were arabinose negative suggesting that they were not B. thailandensis. The two soil samples that yielded growth of B. pseudomallei were collected from paddy field of Gazipur district. No other soil samples from any location yielded growth of B. pseudomallei. The remaining suspected isolates were identified as 12 different organisms (S2 Table).
Table 1. Results of culture of soil samples for the detection of B. pseudomallei from four different melioidosis endemic districts of Bangladesh.
| Place (Districts) | No. of Sites | No. of Sampling points | Location of sampling points | No of Samples | No. of suspected isolates | No. positive for Bps |
|---|---|---|---|---|---|---|
| Gazipur | 4 | 7 | 4 paddy field, 1 cattle shed, 1 poultry shed, 1 riverbank | 41 | 15 | 2 |
| Mymensingh | 3 | 5 | All paddy field | 26 | 4 | 0 |
| Narayangange | 5 | 5 | All paddy field | 31 | 8 | 0 |
| Sylhet | 5 | 5 | All paddy field | 81 | 60 | 0 |
| Total | 19 | 22 | - | 179 | 87 | 2 |
Bps = B. pseudomallei
Fig 3. PCR analysis of B. pseudomallei isolates from Gazipur district.
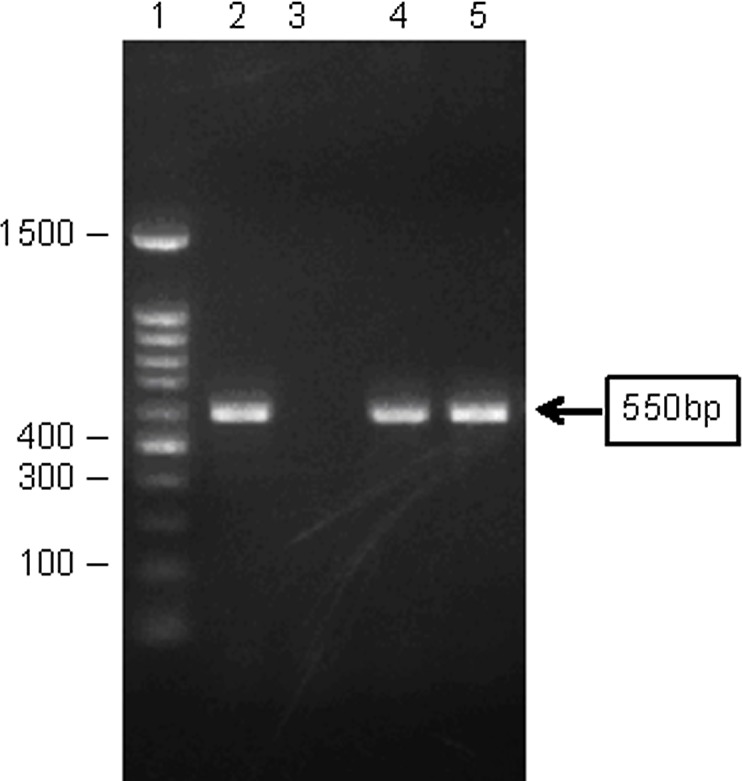
Lane 1- 100bp Marker, Lane 2- Positive control (USM strain of B. pseudomallei, Lane 3- Negative control (distilled water), Lane 4- K23 (Gazipur soil isolate) and Lane 5- K35 (Gazipur soil isolate).
Anti- B. pseudomallei IgG antibody in study population
Total 940 blood samples were collected from apparently healthy individuals residing in four districts of Bangladesh. Out of total 940 healthy subjects, anti- B. pseudomallei IgG antibody higher than the cut-off value (>0.8) were detected in 203 individuals (21.5%). Highest positive result was obtained from Mymensingh district (30.8%) while the rate was only 9.8% in Kishoregange district. The detail district wise rate of sero-positivity is shown in Table 2.
Table 2. Seroprevalence of anti- B. pseudomallei IgG antibodies among study population of four districts and mean OD values of positive and negative cases.
| Place (Districts) | Total No. of Samples | Positive for anti- B. pseudomallei IgG | Mean (±SE) OD of | ||
|---|---|---|---|---|---|
| Number | % (95% CI) | Positive cases | Negative cases | ||
| Kishoregange | 294 | 27 | 9.2 (5.8–12.5) | 1.1 (0.04) | 0.3 (0.01) |
| Mymensingh | 221 | 68 | 30.8 (24.7–36.8) | 1.2 (0.05) | 0.3 (0.02) |
| Narayangange | 214 | 58 | 22.6 (17.0–28.2) | 1.4 (0.05) | 0.4 (0.02) |
| Sylhet | 211 | 50 | 27.5 (21.5–33.5) | 1.1 (0.03) | 0.4 (0.02) |
| Total | 940 | 203 | 21.5 (18.9–24.9) | 1.2 (0.03) | 0.3 (0.01) |
Cut off OD value for positive cases = 0.8
CI = Confidence interval
OD = Optical density
The age distribution of the seropositive cases showed that the maximum number (30.4%) of positive cases belonged to > 50 years age group while the lowest rate was among the 1–10 years age group (5.3%; Table 3). The seropositivity rate of anti- B. pseudomallei IgG antibody in male and female population and among different occupational groups ranged from 20% to 27% (Table 4). No significant association was observed.
Table 3. Age specific seropositivity rate of anti- B. pseudomallei IgG antibodies of study population.
| Age group (years) | Total No of samples | Positive for anti- B. pseudomallei IgG | 95% CI | |
|---|---|---|---|---|
| No | % | |||
| 1–10 | 57 | 3 | 5.3 | 0–11.1 |
| 11–20 | 89 | 20 | 22.7 | 13.9–31.3 |
| 21–30 | 153 | 22 | 14.4 | 8.9–19.8 |
| 31–40 | 174 | 25 | 14.4 | 9.2–19.6 |
| 41–50 | 214 | 56 | 26.2 | 20.3–32.0 |
| >50 | 253 | 77 | 30.4 | 24.8–36.1 |
| Total | 940 | 203 | 21.5 | 18.8–24.1 |
Cut off OD value for positive cases = 0.8
CI = Confidence interval
Table 4. Distribution of occupation and sex of B. pseudomallei seropositive cases.
| Characteristics | Total No of samples | Positive for anti- B. pseudomallei IgG | 95% CI | |
|---|---|---|---|---|
| No | % | |||
| Male | 366 | 95 | 26.0 | 20.1–28.6 |
| Female | 523 | 108 | 20.7 | 16.3–23.0 |
| House wife | 473 | 97 | 20.5 | 16.8–24.2 |
| Farmer | 102 | 27 | 26.5 | 17.9–35.0 |
| Service holder | 137 | 33 | 24.1 | 16.9–31.2 |
| Business | 112 | 31 | 27.7 | 19.4–35.9 |
| Others | 115 | 15 | 13.0 | 6.9–19.1 |
No association with gender (χ2 = 3.441, p = 0.064) and with any occupational group (χ2 = 3.835, p = 0.280)
CI = Confidence interval
Discussion
In Bangladesh, melioidosis has been infrequently detected for last the 25 years but no systematic epidemiologic information regarding its true magnitude and source is available. Isolation of the organism, B. pseudomallei, from clinical specimens indicates that the organism is present in our environment. However, its actual source has never been identified. The present study has been designed to isolate B. pseudomallei from the soil as well as to determine the seroprevalence among an apparently healthy population residing in four northern and northeastern districts of Bangladesh from where melioidosis cases were diagnosed previously.
In the first phase of the study, we aimed to find out the presence of B. pseudomallei in the soil samples from the melioidosis endemic districts of Bangladesh. This strategy of selecting four endemic districts to detect B. pseudomallei from environmental sources has been previously used throughout South-East Asia and northern Australia [22]. Out of 179 soil samples, only two soil samples (K23 and K35) from paddy fields of Gazipur district yielded growth of B. pseudomallei. Our failure to isolate B. pseudomallei from soil samples from other sites could be due to soil condition at the time of sample collection and limited number of samples. The presence of B. pseudomallei is influenced by low bacterial density, rain fall, load of organic materials and oxygen contents of the soil [23]. It is to be noted that the collection and culture of soil in our study was carried out from June to September 2011 which encompasses summer and rainy seasons. Moreover, if we could use the method of soil culture as outlined in international consensus guidelines of 2013 [6] then the rate of isolation of the organism might be higher than the observed result.
The isolation of B. pseudomallei from the soil samples in one district of Bangladesh indicates for the first time that the organism might be present in the local environment and is the source of human infections. However, we could not ascertain whether our soil and previous clinical isolates were similar and whether the organisms from the soil reservoir were the source of clinical infections. If we could perform multi locus sequence typing (MLST) or sequencing of the genome of the isolates then we could confirm that the soil organisms might be the source of the clinical infections in Bangladesh. But, the presence of the organism in the soil strongly indicates that it could be a potential reservoir. So far 18 countries of the world had been designated as definitive country for melioidosis based on the presence of culture confirmed B. pseudomallei in clinical case as well as in the environmental samples namely soil, water, etc of the locality [6,24]. Our finding of B. pseudomallei from the soil of one of the districts finally confirms that Bangladesh too is a definite endemic country for melioidosis.
In the second phase of the study, blood samples were collected from healthy population of four districts to determine the seroprevalence of B. pseudomallei infection. We have used ELISA assay using crude sonicated antigen to determine the presence of anti- B. pseudomallei IgG antibody. The rate of serological positive cases for anti- B. pseudomallei IgG antibody ranged from 22%-31% among study population of three districts where melioidosis cases were detected earlier. The seropositive rate was highest in Mymensingh (30.8%) district while it was lowest (9.2%) in Kishoregange district where no case of melioidosis has yet been diagnosed or reported. The serological findings suggest that there is a wide exposure of people to B. pseudomallei present in the soil or other environmental sources. Seroprevalence rates may vary widely according to the region within the endemic country [25]. Our overall seroprevalence rate was almost similar to rates reported from other countries of the region [26]. In 2012, a hospital based serological study in Bangladesh has reported sero-positive rate of 28.9% for B. pseudomallei antibody among various patients attending selective tertiary care hospitals of the Bangladesh [16]. The study used a very low cut off titer (1:10) of indirect haemagglutination assay (IHA) for defining seropositive cases. The use of the IHA in sero-epidemiological study is problematic in endemic areas, particularly where rates of background seropositivity may be high [26], presumably due to subclinical exposure to organisms related to B. pseudomallei [27,28].
It is important to note from our adsorption study that some degree of non-specific cross reactive antibody was present in the serum which reacted with crude whole cell antigen used in the ELISA as was seen by reduction of OD value following adsorption with P. aeruginosa antigen. This non-specific reactivity might contribute to higher rate of seropositivity. Therefore, we assume that the seropositivity rate could be a little lower if more defined and specific antigen from B. pseudomallei was used. Such specific antigen would eliminate the cross reaction with background antibody to related species of B. pseudomallei like B. thailandensis or B. cepacia.
In our study, seropositivity rate for B. pseudomallei antibody significantly increased with advancement of age. Seropositivity was highest (30.4%) among individuals more than 50 years of age. Lowest rate (5.3%) was observed in 1–10 years age group. The result suggests that the chance of exposure to B. pseudomallei increases with age. Most of the people residing in the rural settings are involved in agricultural work and frequently come in contact with soil, mud and contaminated water, as they grow older.
The rate of seroprevalence among male and female population varies in different studies [29,30]. However, we found no significant association of seropositivity with gender or any particular occupation. Probably our adult inhabitants in rural areas are equally exposed to the environment.
The present study has for the first time identified the presence of B. pseudomallei in the soil samples of Bangladesh. The study has also demonstrated that a large proportion of people residing in four districts are exposed to the organism and have a potential for developing overt diseases during their lifetime.
Supporting Information
(DOCX)
(DOCX)
(PDF)
Acknowledgments
B. pseudomallei species confirmation using TTS1 assay was done in Dr. A. Tuanyok’s laboratory, Emerging Pathogens Institute, University of Florida.
Data Availability
All relevant data are within the paper.
Funding Statement
The study was partially funded by Bangladesh Medical Research Council (30% of total cost) to MSAJ, JAH, CRA. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Limmathurotsakul D, Peacock SJ. Melioidosis: a clinical overview. Br Med Bull. 2011; 99: 125–139. 10.1093/bmb/ldr007 [DOI] [PubMed] [Google Scholar]
- 2.Dance DA. Melioidosis as an emerging global problem. Acta Trop. 2000;74: 115–119. [DOI] [PubMed] [Google Scholar]
- 3.White NJ.Melioidosis. Lancet. 2003; 361: 1715–1722. [DOI] [PubMed] [Google Scholar]
- 4.Limmathurotsakul D, Wongratanacheewin S, Teerawattanasook N, Wongsuvan G, Chaisuksant S, Chetchotisakd P, et al. Increasing incidence of human melioidosis in Northeast Thailand. Am J Trop Med Hyg. 2010; 82: 1113–1117. 10.4269/ajtmh.2010.10-0038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaowagul W, White NJ, Dance DA, Wattanagoon Y, Naigowit P, Davis TM, et al. Melioidosis: a major cause of community-acquired septicemia in northeastern Thailand. J Infect Dis. 1989; 159: 890–899. [DOI] [PubMed] [Google Scholar]
- 6.Limmathurotsakul D, Dance DA, Wuthiekanun V, Kaestli M, Mayo M, Warner J, et al. Systematic review and consensus guidelines for environmental sampling of Burkholderia pseudomallei. PLoS Negl Trop Dis. 2013; 7: e2105 10.1371/journal.pntd.0002105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maegraith BG, Leithead CS. Melioidosis: a case-report. Lancet. 1964; 7338: 862–863 [DOI] [PubMed] [Google Scholar]
- 8.Struelens MJ, Mondol G, Bennish M, Dance DA. Melioidosis in Bangladesh: a case report. Trans R Soc Trop Med Hyg. 1988; 82: 777–778. [DOI] [PubMed] [Google Scholar]
- 9.Barai L, Jilani MSA, Haq JA. Melioidosis–case reports and review of cases recorded among Bangladeshi population from 1988–2014. Ibrahim Med Coll J. 2014; 8: 25–31. [Google Scholar]
- 10.Dance DA, Smith MD, Aucken HM, Pitt TL. Imported melioidosis in England and Wales. Lancet. 1999; 353: 208. [DOI] [PubMed] [Google Scholar]
- 11.Kibbler CC, Roberts CM, Ridgway GL, Spiro SG. Melioidosis in a patient from Bangladesh. Postgrad Med J. 1991; 67: 764–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoque SN, Minassian M, Clipstone S, Lloyd-Owen SJ, Sheridan E, Lessing MP. Melioidosis presenting as septic arthritis in Bengali men in east London. Rheumatology (Oxford). 1999; 38: 1029–1031. [DOI] [PubMed] [Google Scholar]
- 13.Nazimuddin K, Hossain M, Mansur A, Haq J A, Khan A. Melioidosis- a case report. Journal of Bangladesh College of Physicians & Surgeons. 2001; 19: 71–74. [Google Scholar]
- 14.Hassan MR, Pani SP, Peng NP, Voralu K, Vijayalakshmi N, Mehanderkar R, et al. Incidence, risk factors and clinical epidemiology of melioidosis: a complex socio-ecological emerging infectious disease in the Alor Setar region of Kedah, Malaysia. BMC Infect Dis. 2010; 10: 302 10.1186/1471-2334-10-302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng AC, Currie BJ. Melioidosis: epidemiology, pathophysiology, and management. Clin Microbiol Rev. 2005; 18: 383–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maude R, Maude R, Ghose A, Amin M, Islam M, Ali M, et al. Seroepidemiological surveillance of B. pseudomallei in Bangladesh. Trans R Soc Trop Med Hyg. 2012; 106: 576–578. 10.1016/j.trstmh.2012.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brook MD, Currie B, Desmarchelier PM. Isolation and identification of B. pseudomallei from soil using selective culture techniques and the polymerase chain reaction. J Appl Microbiol. 1997; 82: 589–596. [PubMed] [Google Scholar]
- 18.Wuthiekanun V, Dance DA, Wattanagoon Y, Supputtamongkol Y, Chaowagul W, White NJ. The use of selective media for the isolation of Pseudomonas pseudomallei in clinical practice. J Med Microbiol. 1990; 33: 121–126. [DOI] [PubMed] [Google Scholar]
- 19.Lipuma JJ, Currie BJ, Lum GD, Vandamme PAR. Burkholderia, Stenotrophomonas, Ralstonia, Cupriavidus, Pandrea, Brevundimonus, Comamonas, Delftia, and Acidovorax In: Murray PR, Baron EJ, Jorgensen JH, Landry ML, Pfaller MA, editors. Manual of Clinical Microbiology. 9th ed. Washington, DC 20036–2904, USA: ASM Press; pp. 2007;749–769. [Google Scholar]
- 20.Novak RT, Glass MB, Gee JE, Gal D, Mayo MJ, Currie BJ, et al. Development and evaluation of a real-time PCR assay targeting the type III secretion system of B. pseudomallei. J Clin Microbiol. 2006; 44: 85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Voller A, Bartlett A, Bidwell DE. Enzyme immunoassays with special reference to ELISA techniques. J Clin Pathol. 1978; 31: 507–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vesaratchavest M, Tumapa S, Day NP, Wuthiekanun V, Chierakul W, Holden MT, et al. Nonrandom distribution of Burkholderia pseudomallei clones in relation to geographical location and virulence. J Clin Microbiol. 2006; 44: 2553–2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trung TT, Hetzer A, Gohler A, Topfstedt E, Wuthiekanun V, Limmathurotsakul D, et al. Highly sensitive direct detection and quantification of B. pseudomallei bacteria in environmental soil samples by using real-time PCR. Appl Environ Microbiol. 2011; 77: 6486–6494. 10.1128/AEM.00735-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prakash A, Thavaselvam D, Kumar A, Kumar A, Arora S, Tiwari S, et al. Isolation, identification and characterization of B. pseudomallei from soil of coastal region of India. Springerplus. 2014; 3: 438 10.1186/2193-1801-3-438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pitt T, Dance D. Burkholderia spp. and related genera In: Borriello S, Murray P, Funke G, editors. Topley and Wilson`s Microbiology and Microbial Infections, Bacteriology. 10 ed. Euston Road, London: Hodder Arnold; pp. 2005; 1607–1638. [Google Scholar]
- 26.Khupulsup K, Petchclai B. Application of indirect hemagglutination test and indirect fluorescent antibody test for IgM antibody for diagnosis of melioidosis in Thailand. Am J Trop Med Hyg. 1986; 35: 366–369. [DOI] [PubMed] [Google Scholar]
- 27.Kanaphun P, Thirawattanasuk N, Suputtamongkol Y, Naigowit P, Dance DA, Smith MD, et al. Serology and carriage of Pseudomonas pseudomallei: a prospective study in 1000 hospitalized children in northeast Thailand. J Infect Dis. 1993; 167: 230–233. [DOI] [PubMed] [Google Scholar]
- 28.Anuntagool N, Naigowit P, Petkanchanapong V, Aramsri P, Panichakul T, Sirisinha S. Monoclonal antibody-based rapid identification of B. pseudomallei in blood culture fluid from patients with community-acquired septicaemia. J Med Microbiol. 2000; 49: 1075–1078. [DOI] [PubMed] [Google Scholar]
- 29.Currie BJ, Fisher DA, Howard DM, Burrow JN, Selvanayagam S, Snelling PL, et al. The epidemiology of melioidosis in Australia and Papua New Guinea. Acta Trop. 2000; 74: 121–127. [DOI] [PubMed] [Google Scholar]
- 30.Armstrong PK, Anstey NM, Kelly PM, Currie BJ, Martins N, Dasari P, et al. Seroprevalence of B. pseudomallei in East Timorese refugees: implications for healthcare in East Timor. Southeast Asian J Trop Med Public Health. 2005; 36: 1496–1502. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(PDF)
Data Availability Statement
All relevant data are within the paper.



