Abstract
TORC1 activity in all eukaryotes is dependent on amino acids availability. However, the mechanism through which TORC1 senses amino acids is still a mystery unfolding like a detective story. Two new studies, implicate leucyl-tRNA synthetase in this evolving story.
The evolutionarily conserved target of rapamycin complex 1 (TORC1) plays a central role in promoting eukaryotic cell growth and proliferation, while negatively regulating autophagy (Bhaskar and Hay, 2007). TORC1 promotes growth and proliferation largely through protein synthesis and other anabolic activities. In all eukaryotic organisms, TORC1 is regulated by multiple inputs mostly emanating from the extracellular environment. However, the most evolutionarily conserved input is governed by amino acids (AA). Regardless of the stimulus that activates TORC1, AA availability, in particular branched-chain AA, is required for TORC1 activity in organisms from yeast to man. Currently, the exact mechanism by which AA exert their effect on TORC1 is not fully understood. In the past few years research focused on the effect of AA on TORC1 unraveled several regulators, including the Gtr/Rag small GTPases (Kim and Guan, 2011). The two new studies offer an additional step forward; they show that Leucyl-tRNA synthetase (LeuRS), the enzyme responsible for charging leucine to its cognate tRNA, functions as a sensor of leucine signaling to TORC1 through the Gtr/Rag GTPases in both yeast and mammalian cells (Bonfils et al., 2012; Han et al., 2012).
The Rag GTPase family in mammalian cells and in Drosophila, RagA, RagB, RagC, and RagD, and their homologues Gtr1 and Gtr2 in budding yeast have recently emerged as key regulators in the pathway by which AA regulate TORC1 (Kim and Guan, 2011). Gtr1 and its homologues RagA and RagB share amino sequence similarity significantly distinct from that shared by Gtr2 and its homologues RagC and RagD. These small GTPases function as heterodimers, whereby GTP-bound Gtr1 (Gtr1-GTP) forms a functional heterodimer with GDP-bound Gtr2 (Gtr2-GDP). By analogy, RagA-GTP and RagB-GTP heterodimerize with either RagC-GDP or RagD-GDP. However, exactly how this functional configuration, which maintains the GTP/GDP loading status of Gtr1/2 heterodimer or RagA,B/C,D heterodimers is achieved, is not fully understood. This configuration is probably mediated by specific positive regulators, guanine nucleotide exchange factors (GEFs), and negative regulators, GTPase activating proteins (GAPs). Importantly, how the GTP/GDP loading and activity of the Gtr/Rag heterodimers is mediated by AA remains to be determined.
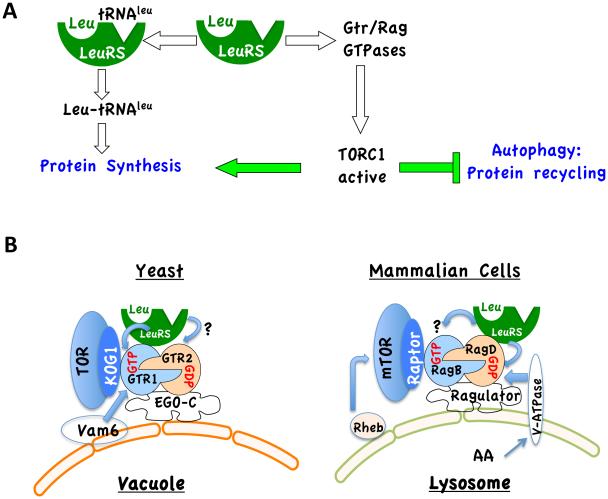
The new work by Bonfils et al (Bonfils et al., 2012) and Han et al (Han et al., 2012) may provide a clue to how AA signal to Gtr/Rag heterodimers. Although the two studies differ in mechanistic details, they both show that LeuRS, which is absolutely required for protein synthesis, is also essential for determining the activities of Gtr/Rag GTPases and TORC1 in response to AA. Therefore, LeuRS couples AA availability to protein synthesis and autophagy (Fig. 1A)
Figure 1. LeuRS, as a sensor of AA, transmits a signal to TORC1.
A. In both yeast and mammalian cells, leucine loaded LeuRS, which is required for protein synthesis, binds and activates Gtr/Rag GTPases, which in turn activate TORC1. Activated TORC1 then facilitates protein synthesis, while turning off the protein recycling process, autophagy. B. A comparison between TORC1 activation in yeast and in mammalian cells. In yeast LeuRS was shown to bind and preserve GTP-bound Gtr1, while in mammalian cells LeuRS was shown to bind RagD and to act as a GAP that preserves the GDP-bound RagD. It is not known whether, through close proximity, the yeast LueRS acts as GAP for Gtr2 or the mammalian LeuRS protects the GTP-bound RagB (see text for details).
One intriguing aspect of TORC1 regulation is its intracellular co-localization with the Rag or Gtr1 GTPases, which appears to play a role in TORC1 activation. Both the yeast TORC1 and mammalian TORC1 (mTORC1) physically interact with their respective GTP/GDP-bound Gtr/Rag heterodimers through their critical component Kog1 and Raptor, respectively. The yeast Gtr1/Gtr2 heterodimer is part of a complex that also includes Ego1, Ego3, and Ltv1 and is localized to the vacuole or late endosomes (Binda et al., 2009) (Fig. 1B). The mammalian Rag heterodimers interact with a tetrameric complex MP1/p14/p18 termed Ragulator, which is localized to the lysosmomal membrane and tethers GTP-bound RagB to the lysosomal membrane (Sancak et al., 2010) (Fig. 1B). One aspect that differs between the yeast and mammalian TORC1 is that, while the yeast complex does not change its intracellular localization in response to AA, the mammalian complex translocates to the lysosome in AA-dependent manner (Sancak et al., 2010). Two findings could explain the translocation of the mammalian TORC1 and its regulators to the lysosomal membrane. First, the mammalian TORC1 is also dependent on another small GTPase, Rheb, which is localized to the lysosome (Fig. 1B), whereas in yeast TORC1 is not dependent on Rheb. Second, it was recently shown in both Drosophila and mammalian cells that the vacuolar-type H+-ATPase (v-ATPase), which pumps protons into the lysosome, also interacts with the Ragulator on the lysosomal membrane. It was proposed that AA sensing in the lysosmal lumen by v-ATPase promotes its conformational change, which in turn transmits a signal to the Rag GTPases (Zoncu et al., 2011).
In yeast, Vam6 was identified as a GEF for Gtr1, which promotes GTP binding by Gtr1 (Binda et al., 2009). However, this finding does not address how AA facilitate the GTP/GDP configuration of the Gtr1/Gtr2 heterodimer. The new work by Bonfils et al (Bonfils et al., 2012) shows that in the presence of leucine, LeuRS binds and preserves the GTP-bound Gtr1, thereby delivering AA signaling to TORC1. LueRS and Gtr1 interaction occurs through its editing domain and is possibly dependent on a conformational change induced by leucine (Bonfils et al., 2012). Bonfills et al propose that LeuRS binding blocks the access to an elusive GAP, specific for GTP-bound Gtr1, thus preventing GTP hydrolysis. These results, however, do not explain how Gtr2 is kept in a GDP-bound form. A role for a Vam6 homologue as a Rag GEF has not been shown yet in Drosophila or in mammalian cells. Therefore, it is possible that another Rag-specific GEF dependent on AA couples AA signaling to TORC1 activation.
Meanwhile, Han et al found that LeuRS also funnels AA signaling to the mammalian TORC1 (Han et al., 2012). They found that upon addition of leucine to leucine-starved cells, LeuRS translocates to the lysosomal membrane together with TORC1. Furthermore, knockdown of LeuRS impairs the translocation of TORC1 to the lysosome. Surprisingly, Han et al found that LeuRS binds exclusively to RagD and not to any other member of the Rag family, even though RagC shares a high degree of amino acid sequence similarity with RagD. Perhaps RagC interacts with a different branched-chain aminoacyl tRNA synthetase. Regardless, Han et al showed that LueRS not only interacts with RagD but also possesses a GAP activity towards RagD-GTP, facilitating GTP hydrolysis and maintaining GDP-bound RagD in the active RagB/RagD heterodimer. These results cannot be reconciled easily with the results of Bonfils et al, who showed that LeuRS could not physically interact with Gtr2, the yeast homologue of RagD. One possible explanation for this discrepancy is that in yeast although LeuRS binds only to Gtr1 and protects its GTP-bound form, through its close proximity to Gtr2, it may also facilitate Gtr2-GTP hydrolysis (Fig. 1B). Similarly, in mammalian cells, although LeuRS binds only to RagD to stimulate its GTP hydrolysis, it may also protect GTP-bound RagB from GTP hydrolysis (Fig. 1B). If this scenarios were correct, it would be important to determine why the yeast LueRS exclusively binds to Gtr1 while the mammalian LeuRS exclusively binds to RagD, the Gtr2 homologue.
Both studies showed that leucine binding is required for the effect of LeuRS on TORC1. However, it is not clear what other activities of this enzyme are required. LeuRS normally binds leucine, ATP, and tRNA; like other amioacyl-tRNA synthetases (AARS), it possesses two activities, an ATP-dependent catalytic activity, which charges AA to its cognate tRNA (Fig.1 A), and an editing activity to ensure that the correct amino acid was loaded and charged. The charging occurs in two steps. Upon binding of leucine and ATP, leucyl-adenylate is formed and then transferred to its cognate tRNA to generate leucyl-tRNA. Han et al. showed that the charging activity of Leu-RS is not required. Because it appears that ATP binding is required to elicit a major LeuRS conformational change in addition to leucine, Han et al. speculate that only Leu and ATP binding, but not tRNA binding, is required for leucine-induced TORC1 activation.
One major question raised by these studies is why LeuRS was singled out to transmit the signal to TORC1. There are 20 amino acids and 20 AARS enzymes. The TORC1 pathway samples one of these 20 enzymes, LeuRS, to act as an “abundance” sensor; LeuRS needs to be in excess so that the limiting factor would be leucine. Indeed, LeuRS is one of the most abundant proteins in yeast (Ghaemmaghami et al., 2003). If the cell has a sufficient supply of leucine, chances are high that the other 19 amino acids are present as well. In addition, Bonfils et al. mention that leucine is the most frequently used amino acid in proteins, and thus represents the best link between AA availability and protein synthesis. Moreover, Han et al. suggest that LeuRS binds other branched-chain AA such as isoleucine, valine, and possibly methionine, albeit with lower affinity, thus sensing and transmitting their availability to TORC1. Therefore, cells need to have only one of the AARS enzymes in such excess. Under AA starvation when TORC1 is inactive, mRNA translation is blocked at the initiation step, before the absence of Leu-tRNAleustalls protein elongation and accumulation of partial proteins. Thus, this explanation makes economical sense.
In summary, regardless of the mechanistic details, both studies placed LeuRS as a critical mediator of AA sensing by TORC1. Thus, LeuRS is the newest member of a growing number of enzymes, such as Vam6 and v-ATPase, which in addition to their normal function possess a moonlighting activity required for TORC1 activation by AA. Finally, if indeed LueRS plays such a critical role in TORC1 activation, a new dimension for cancer therapy is opened. Since TORC1 inhibitors are used for cancer therapy, competitive inhibitors of LueRS could be used as new therapeutic regimens for cancer.
Acknowledgment
Work in the Segev laboratory is supported by NIH grant R01GM045444, and work in the Hay laboratory is supported by NIH grants R01AG016927 and R01CA090764.
References
- Bhaskar PT, Hay N. The Two TORCs and Akt. Dev Cell. 2007;12:487–502. doi: 10.1016/j.devcel.2007.03.020. [DOI] [PubMed] [Google Scholar]
- Binda M, Peli-Gulli MP, Bonfils G, Panchaud N, Urban J, Sturgill TW, Loewith R, De Virgilio C. The Vam6 GEF controls TORC1 by activating the EGO complex. Mol Cell. 2009;35:563–573. doi: 10.1016/j.molcel.2009.06.033. [DOI] [PubMed] [Google Scholar]
- Bonfils G, Jaquenoud M, Bontron S, Ostrowicz C, Ungermann C, De Virgilio C. Leucyl-tRNA Synthetase Controls TORC1 via the EGO Complex. Mol Cell. 2012 doi: 10.1016/j.molcel.2012.02.009. [DOI] [PubMed] [Google Scholar]
- Ghaemmaghami S, Huh WK, Bower K, Howson RW, Belle A, Dephoure N, O'Shea EK, Weissman JS. Global analysis of protein expression in yeast. Nature. 2003;425:737–741. doi: 10.1038/nature02046. [DOI] [PubMed] [Google Scholar]
- Han JM, Jeong SJ, Park MC, Kim G, Kwon NH, Kim HK, Ha SH, Ryu SH, Kim S. Leucyl-tRNA Synthetase Is an Intracellular Leucine Sensor for the mTORC1-Signaling Pathway. Cell. 2012 doi: 10.1016/j.cell.2012.02.044. [DOI] [PubMed] [Google Scholar]
- Kim J, Guan KL. Amino acid signaling in TOR activation. Annu Rev Biochem. 2011;80:1001–1032. doi: 10.1146/annurev-biochem-062209-094414. [DOI] [PubMed] [Google Scholar]
- Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, Sabatini DM. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell. 2010;141:290–303. doi: 10.1016/j.cell.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoncu R, Bar-Peled L, Efeyan A, Wang S, Sancak Y, Sabatini DM. mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H(+)-ATPase. Science. 2011;334:678–683. doi: 10.1126/science.1207056. [DOI] [PMC free article] [PubMed] [Google Scholar]