Abstract
Several cell types secrete small membranous vesicles that contain cell-specific collections of proteins, lipids, and genetic material. The function of these vesicles is to allow cell-to-cell signaling and the horizontal transfer of their cargo molecules. Here, we demonstrate that muscle cells secrete nano-sized vesicles and that their release increases during muscle differentiation. Analysis of these nanovesicles allowed us to characterize them as exosome-like particles and to define the potential role of the multifunctional protein Alix in their biogenesis.
Keywords: exosome, nanovesicle, Alix, Ozz-E3 ubiquitin ligase, muscle cells
1. Introduction
Animal cells release nano-sized single membrane vesicles that function in signaling and the horizontal transfer of their cargo molecules (proteins, mRNAs, and microRNAs) to other cells [1–3]. Research on extracellular nanovesicles has focused primarily on the immune system and tumor cells. Recently, it has also been reported that a skeletal muscle cell line (C2C12) can release vesicles [4]; however, how muscle cells generate these vesicles and what their effectors are remain largely unknown.
Alix is an evolutionarily conserved adaptor protein that has been implicated in cytoskeleton and membrane remodeling [5–7]. In line with these reports, we have recently shown that Alix also plays a part in actin cytoskeleton remodeling in muscle cells, and that the Ozz-E3 ubiquitin ligase is crucial for the regulation of this function of Alix [8]. In addition, it has been suggested that, because Alix can interact with both a lipid and proteins and retains a characteristic “banana-shaped” structure, it can generate or scaffold a negative curvature within the membrane as part of the inward budding process within the endocytic pathway [9–10]. An interplay between F-actin and membrane-bending proteins like Alix is thought to occur, in order to promote a negative curvature of the membrane during processes such as filopodia formation, vesicle budding and midbody abscission. In fact, we and others have demonstrated that Alix downregulation leads to a decrease in the number of late endosomes and muscle podia, and to alterations in their composition [7,8]; thus, Alix loss of function may interfere with mechanisms that regulate membrane dynamics. Here, we demonstrate that differentiated muscle cells release nanovesicles extracellularly and that the loss of Alix alters the budding and composition of these vesicles.
2. Materials and methods
2.1. Antibodies and reagents
Commercial antibodies included mouse anti-AIP1/Alix for immunoblotting (BD Transduction Labs) and anti-Alix (Santa Cruz Biotechnology) for immunogold electron microscopy, alpha-enolase (Santa Cruz Biotechnology), anti-CD63 (Santa Cruz Biotechnology), anti-Hsp70/Hsc70 (Novus Biologicals), anti-Elongin C (BD Biosciences), anti-MyHc (MF20, Developmental Studies Hybridoma Bank), anti-Myogenin (Santa Cruz Biotechnology), anti-MyoD (Santa Cruz Biotechnology), anti-Bcl-2 (Calbiochem), anti-Bax (Calbiochem), anti-PARP (Cell Signaling Technology). Rabbit anti-Ozz antibody was prepared as described [11].
siRNAs targeting Alix and standard negative controls, and the transfection reagent were purchased from Dharmacon, as reported [8].
2.2. Cell culture methods
For three-dimensional cultures, C2C12 were cultured as reported [8]. Primary myoblast cultures were established as described previously [11].
2.3. Purification of nanovesicles by differential ultracentrifugation
Nanovesicles were isolated and quantified according to a previously published method [12]. This isolation method included a penultimate centrifugation step (10,000×g for 30 min) that allowed the removal of small cell debris and larger microvesicles for the subsequent pelleting of nanovesicles, comprised mainly of exosomes [13]. After washing, the pellet is resuspended either in RIPA buffer or 4% PFA, for further immunoblot or electron microscope analyses respectively. To have an estimation of the amount of secreted nanovesicles, we quantified and compared the total protein content of the vesicle lysates using the Bradford assay [12].
2.4. Electron microscope analysis and immunogold labeling of muscle-derived nanovesicles
Nanovesicle pellets fixed in 4% PFA were mounted on Formvar-carbon coated EM gold grids by layering 10-µl drops of vesicle preparations, and letting grids air dry. Grid-mounted preparations were stained with uranyl acetate and lead citrate, and subsequently observed under the JEM-1220 (Jeol) electron microscope at 120 kV. Muscle cells were 3D cultured [8] and inserts were fixed in PFA 4%. Inserts were then dehydrated in alcohol and embedded in liquid LR White Medium Grade Resin before inclusion in gelatine capsules (EMS). Samples were then cut into 70nm-thick ultrathin sections and layered onto Formavar coated gold grids (EMS). For immunogold staining, the grids were rinsed in water, incubated in citrate buffer, and blocked in 3% BSA-c in T-PBS. Grids were then incubated with anti-Alix, washed in T-PBS, incubated with AuroProbe EM secondary antibody. The grids were post-fixed with 2% glutaraldhyde in PBS, and contrasted using conventional techniques.
3. Results and Discussion
3.1. Budding of nanovesicles from the plasma membrane of differentiated muscle cells
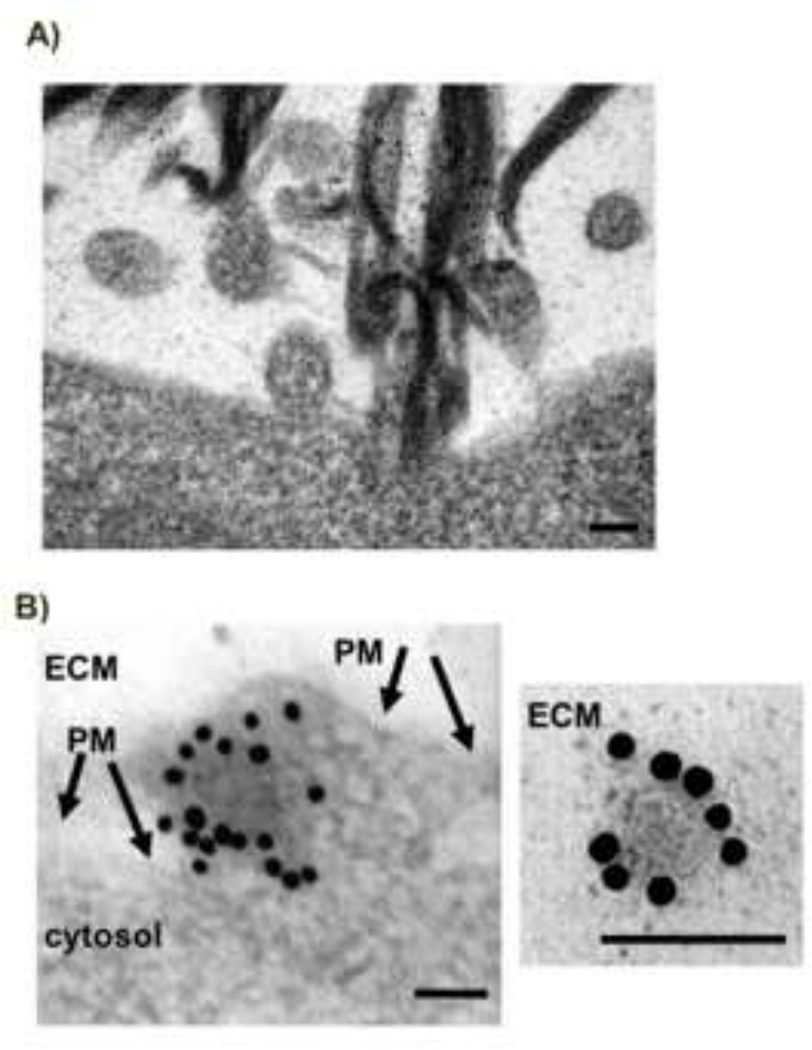
Exosomes are nano-sized (50–100 nm in diameter) membrane-bound vesicles released by a variety of cell types and specified by a plethora of protein markers, including Alix [1–3]. In contrast, microvesicles tend to constitute a larger and more heterogeneous population of extracellular vesicles, ranging from 50 to 1000 nm in diameter [14]. Transmission electron microscopy analyses of 3D cultures of C2C12 myotubes revealed that nanovesicles, ranging from 50–100 nm and with an average diameter of 80 nm, were released by direct budding from the plasma membrane (Fig. 1A). 3D cell cultures exhibit more in vivo-like responses allowing us to visualize, by immunogold labelling, the distribution of Alix in close proximity to the surface of budding nanovesicles and decorating released nanovesicles (Fig. 1B). The size of these muscle-derived vesicles and the presence of Alix are compatible with what has been described for exosomes from other cells [12–15].
Figure 1. Muscle cells release nanovesicles.
A) Electron microscope analyses of 3D cultures of differentiating C2C12 cells show the presence of outward budding intermediates (nascent vesicles) at the plasma membrane with an average diameter of 80 nm (scale bar=100 nm).
B) Transmission electron microscopy shows immunogold labeling of Alix in the nanovesicles from 3D cultures of differentiated C2C12 myotubes (10 nm gold particles). Left panel shows a nascent nanovesicle, right panel shows a released vesicle (PM=plasma membrane, ECM=extra-cellular matrix) (scale bar=100 nm).
Together, these observations indicate that skeletal muscle cells can produce and release nanovesicles directly from the plasma membrane. This modality of vesicle secretion is consistent with that previously reported for both microvesicle [16] and nanovesicle biogenesis; in the latter, human CD4+ T cells and certain T cell lines release nanovesicles—defined as exosomes—directly from subdomains of their plasma membrane [15,17,18]. However, it contrasts with the canonical view of exosome release as a delayed process in which vesicles bud first into an intermediate structure, the multivesicular bodies (MVB), and are subsequently released upon endosome–plasma membrane fusion [1,3,13]. Nevertheless, both modes of vesicle secretion involve outward nanovesicle budding and a negative curvature of specialized endosome-like membranes, either within the MVB or at the plasma membrane [14]. Whether the nanovesicles originating from these two mechanisms carry the same cargo molecules from the cell and are associated with the same protein sorting machinery is currently unknown. In this respect, it is conceivable that Alix, in addition to its canonical involvement in the biogenesis of MVBs and in viral budding, processes that involve a negative curvature of the membrane, may also participate in the direct budding of nanovesicles from the plasma membrane of muscle cells. Supporting this idea, Alix, which is mainly cytoplasmic, was found associated not only with actin and the membrane of internal organelles but also with sites of exosome biogenesis at the plasma membrane of T-cells, together with other exosomal proteins and the lipid lysobisphosphatidic acid (LBPA) [6–8,15].
3.2. Nanovesicle purification from muscle cell-conditioned media
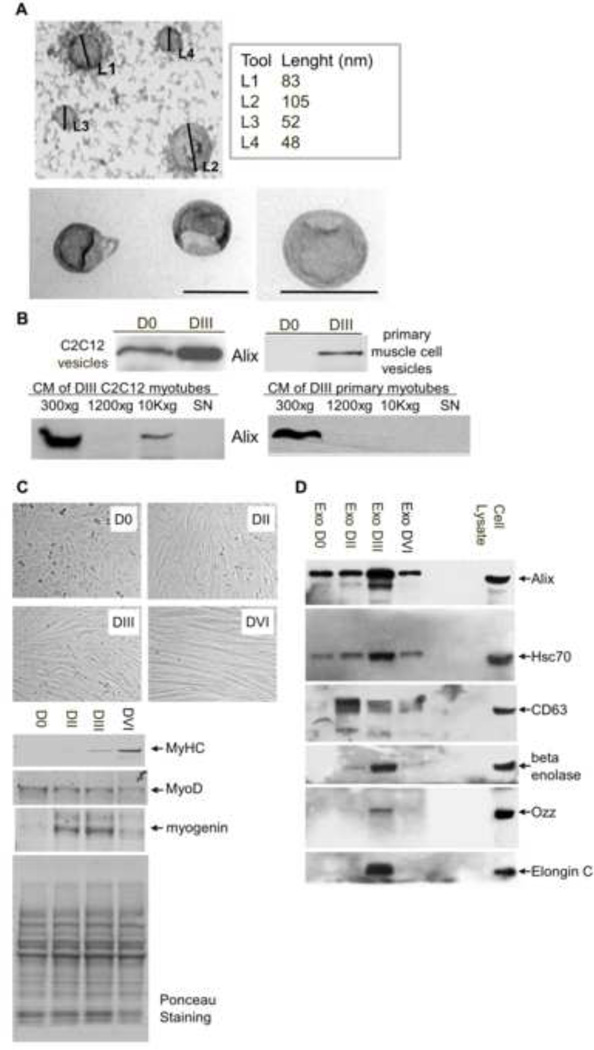
To further characterize these muscle-released vesicles, we employed a standard protocol [12] to isolate extracellular nanovesicles from larger vesicles (e.g., shedding microvesicles, apoptotic bodies) using a 24-hour culture media (CM) of C2C12 and primary muscle cells. In line with the ultrastructural analyses of the nanovesicles released by the 3D cultures, electron microscopy of negatively stained purified preparations revealed “cup-shaped” nanovesicles ranging in diameter from 50–100 nm (Fig. 2A), a size which is consistent with the previously characterized ultrastructural morphology of exosomes [12,15]. It is noteworthy that these vesicles were mainly observed in medium collected from myotubes at DIII of differentiation, compared to proliferating myoblasts. In addition, both the total protein content (data not shown) and the amount of Alix in these vesicle lysates were significantly higher in nanovesicles released from differentiated myotubes than those from proliferating myoblasts (Fig 2B, top panel). Noteworthy, Alix is present in small amount (CM of C2C12 myotubes) or almost undetectable (CM of primary myotubes) in the fraction containing small plasma debris and the largest shedding microvesicles, removed by the 10,000 × g centrifugation (10K×g, in Fig. 2B bottom panel).
Figure 2. Characterization of nanovesicles released by differentiated muscle cells.
A) Electron-microscope analysis of whole-mounted vesicles purified from conditioned-media (CM) of C2C12 myotubes (DIII) (scale bar=100 nm).
B) Equal amounts of total proteins (5 µg) extracted from the nanovesicle preparations were immunoblotted for Alix. Alix is mainly present in the nanovesicle preparation from differentiated muscle cells (DIII). Controls of the procedure show the purity of the nanovesicle preparation. Note, the 10,000 × g pellets (10k×g), containing cell debris and large microvesicles, were almost negative for Alix.
C) Assessment of myogenic differentiation of C2C12 cells. (Top) Myoblasts (D0) and myotubes after two (DII), three (DIII) and six days (DVI) of differentiation. (Bottom) Western blots showing the expression of MyHC, myogenin, and MyoD in lysates of C2C12 cells after the indicated days of differentiation. Ponceau staining is shown as a control of the total protein loaded per lane.
D) Characterization of the protein content of muscle-released nanovesicles showed the presence of proteins, indicated by gene symbol, that are identified more often in exosomes of different cell types.
3.3. Characterization of muscle-derived nanovesicles
Dramatic changes occur during myogenesis, and the C2C12 cell system mimics these events in vitro. Fig. 2C shows the morphological and molecular modifications that distinguish D0–DVI C2C12 muscle cells; we have used MyoD and myogenin as early markers of muscle differentiation and Myosin Heavy Chain (MyHC) as a marker of terminal differentiation. Next, we analyzed the protein contents of these vesicles released by C2C12 muscle cells at these different days of differentiation (D0, DII, DIII and DVI) by immunoblotting, using antibodies specific for three proteins that, in addition to Alix, are more often identified in exosomes of different cell origin: CD63, enolase, and the Heat shock 70 kDa protein 8 (Hsc70) [3].
Immunoblot analysis of equal quantity of D0–DVI vesicle proteins showed that the amounts of Alix, Hsc70, and enolase peaked in vesicles released from differentiated myotubes at DIII (Fig. 2D). In contrast, the CD63 expression profile was different, as its highest expression was at DII of differentiation, when many myocytes are still fusing and the contractile apparatus is being formed (Fig. 2C–D). This result suggests that the nanovesicles produced by myoblasts (D0), myocytes (DI–DII), and myotubes (DIII) could differ in their protein content as a result of a different biogenesis/function of the extracellular vesicles during myogenesis. This finding is in line with recent data showing that distinct populations of exosomes (apical and basolateral exosomes) from the same cell population do exist, and that CD63 is the marker for only one of them [19].
We have shown previously that, in muscle cells, Alix is one of the substrates of the RING-type ubiquitin ligase, Ozz-E3 [8]. This ligase complex includes the substrate-recognition module Ozz, as well as Elongin B and C, Rbx1 and Cullin 5 [11]. We have also shown that Ozz-E3 plays an active role in muscle differentiation and homeostasis, by targeting and ubiquitinating selective substrates associated with the actin cytoskeleton [8,11,20]. We therefore tested whether Ozz and Elongin C were present in the muscle-derived nanovesicles. We detected Ozz and Elongin C exclusively in nanovesicles released by differentiated muscle cells at DIII (Fig. 2C). This result is particularly relevant in view of the fact that the ubiquitin pathway plays a crucial role in the membrane trafficking and actin remodeling processes. Overall our analyses showed that the muscle-derived nanovesicles, depleted of larger microvesicles, were positive for components of the ubiquitination pathway and for all the exosomal markers tested. These data, together with the findings that the muscle-derived extracellular vesicles are homogenous in size and shape, allowed us to conclude that our preparation of nanovesicles is mainly composed of exosome-like particles.
3.4 Effect of Alix loss of function on the muscle cell release of nanovesicles
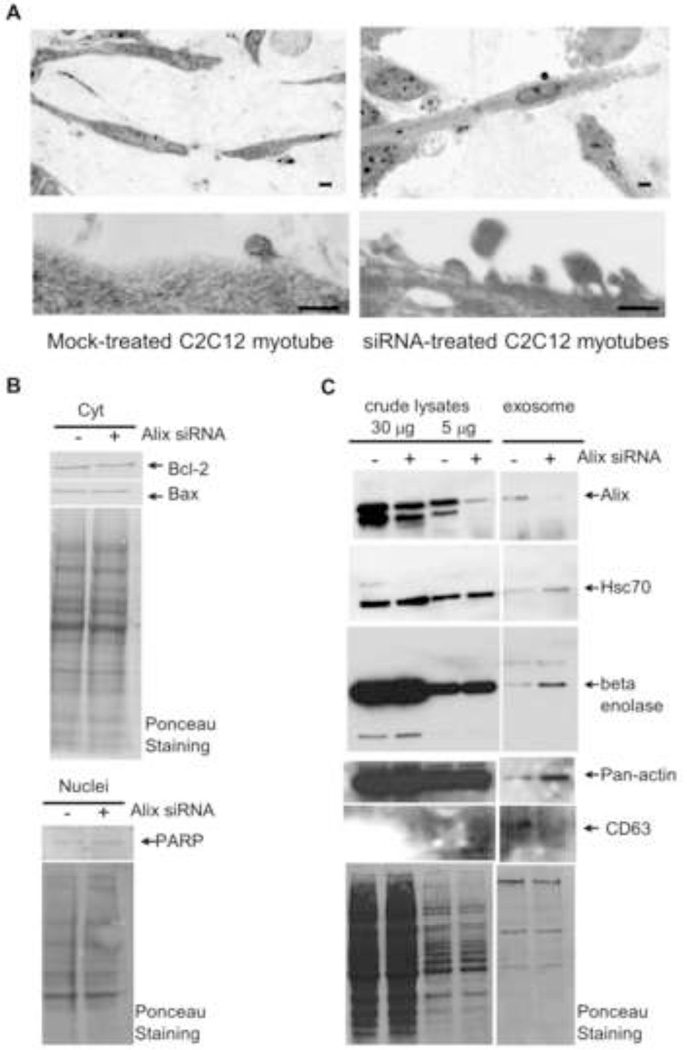
Finally, we investigated the role of Alix in the biogenesis of the muscle-released vesicles by analyzing 3D-cultured C2C12 cells in which Alix expression was silenced by siRNAs. Downregulation of Alix, obtained using two independent siRNA pools with different sequences, was specific and effective, as we routinely obtained 70–90% knockdown of Alix expression compared to mock-transfected cells (Fig. 3B, left panel). The viability and morphology of the cells treated with two different non-targeting siRNA pools were comparable to those of the mock-transfected cells that served as negative control (data not shown). Light microscopy analysis of semithin sections of 3D muscle cell inserts showed that Alix-silenced cells had an altered morphology, displaying extensive membrane budding of enlarged vesicles compared to controls (Fig. 3A). The extracellular vesicles released by Alix-silenced myotubes were heterogeneous in size, ranging from 70–200 nm, and were irregularly shaped (Fig. 3A). We can exclude that these aberrant vesicles were merely the result of Alix-induced apoptotic events for several reasons: they were smaller than apoptotic bodies (usually 500–2000 nm); we have never observed any apoptotic nuclear changes in Alix-silenced cells [8]; and the level of apoptosis markers such as nuclear PARP, Bcl-2, and Bax did not change between Alix- and mock-silenced myotubes (Fig. 3B). The plasma membrane alteration of the Alix-silenced extracellular vesicles was in line with previous reports [8,20–21], suggesting that Alix has a primary role in regulating membrane dynamics in muscle cells not only in the formation of filopodia [8] but also in the biogenesis of muscle-released vesicles. This was further explored by analyzing the protein composition of the nanovesicles purified from the 2D culture of C2C12 myotubes (DIII) after knockdown of Alix. We recovered the same amount of total proteins from the nanovesicles released by mock- and Alix-silenced cells. However, immunoblot analysis of equal protein amounts of the nanovesicle proteins showed that the downregulation of Alix led to a significant accumulation of the cytoplasmic proteins Hsc70, enolase and actin, and a reduction of the endosome/exosome associated protein CD63 in the nanovesicles released from Alix-silenced myotubes, compared to mock-transfected myotubes (Fig. 3C). We hypothesize that loss of Alix leads to deregulated budding and/or scission of vesicles from the plasma membrane. This effect can culminate in vesicles abnormally loaded with cargos of cytoplasmic origin (increased level of Hsc70, enolase and actin). CD63, like Alix, has been detected in the endosome-like domains of the plasma membrane [14], and its decrease in the Alix-depleted nanovesicles suggests that these membrane domains may not be well structured and are not involved in the biogenesis of the abnormal vesicles from the Alix-silenced myotubes. Altogether, our findings highlight the role of Alix in modulating the proper biogenesis of a major class of muscle-released nanovesicles, the loading of these vesicles with specific cargo, or both.
Figure 3. Alix-depletion affects release and protein content of muscle-derived nanovesicles.
A) Light microscopy analysis (top panel) and transmission electron microscopy (bottom panel) of 3D cultures of differentiated C2C12 cells (DIII) silenced for Alix showed an altered phenotype.
B) Cytoplasmic (top) and nuclear (bottom) fractions from Mock- and Alix-silenced myotubes were immunoblotted for the indicated apoptotic markers. Ponceau staining is shown as a control of the total protein loaded per lane.
C) Treatment of C2C12 myoblast with Alix-specific double stranded siRNA pools led to a significant reduction (≈80%) in Alix expression compared to mock-transfected cells. Nanovesicles released by Alix-silenced C2C12 cells exhibited a significant accumulation of Hsc70, enolase and actin, and a reduction of CD63.
Highlights.
Muscle cells secrete nano-sized vesicles
The release of nanovesicles increases during muscle differentiation
Exosome-like particles bud from the plasma membrane of differentiated muscle cells
Loss of Alix alters the budding and composition of these nanovesicles
Acknowledgments
We thank Giovanna Barbieri, Agata Giallongo, John Harris, and Gerard Grosveld for comments and suggestions; Vito Marcenò and Xiaohui Qiu for help with the electron microscopy analyses and the purification of the antibody; Richard Burket for editing the manuscript. The research leading to these results has been partly funded by the Italian Ministry for Education, University, and Research in the framework of the Flagship Project NanoMAX. This work was also supported in part by NIH grant AR049867, the Cancer Center Support Grant CA021765, and the American Lebanese Syrian Associated Charities (ALSAC) of SJCRH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Simons M, Raposo G. Exosomes--vesicular carriers for intercellular communication. Curr Opin Cell Biol. 2009;21:575–581. doi: 10.1016/j.ceb.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 2.Gould SJ, Booth AM, Hildreth JE. The Trojan exosome hypothesis. Proc Natl Acad Sci U S A. 2003;100:10592–10597. doi: 10.1073/pnas.1831413100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalra H, Simpson RJ, Ji H, Aikawa E, Altevogt P, et al. Vesiclepedia: A Compendium for Extracellular Vesicles with Continuous Community Annotation. PLoS Biol. 2012;10(12):e1001450. doi: 10.1371/journal.pbio.1001450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guescini M, Guidolin D, Vallorani L, Casadei L, Gioacchini AM, Tibollo P, Battistelli M, Falcieri E, Battistin L, Agnati LF, Stocchi V. C2C12 myoblasts release micro-vesicles containing mtDNA and proteins involved in signal transduction. Exp Cell Res. 2010;316:1977–1984. doi: 10.1016/j.yexcr.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Odorizzi G. The multiple personalities of Alix. J Cell Sci. 2006;119:3025–3032. doi: 10.1242/jcs.03072. [DOI] [PubMed] [Google Scholar]
- 6.Cabezas A, Bache KG, Brech A, Stenmark H. Alix regulates cortical actin and the spatial distribution of endosomes. J Cell Sci. 2005;118:2625–2635. doi: 10.1242/jcs.02382. [DOI] [PubMed] [Google Scholar]
- 7.Matsuo H, Chevallier J, Mayran N, Le Blanc I, Ferguson C, Fauré J, Blanc NS, Matile S, Dubochet J, Sadoul R, Parton RG, Vilbois F, Gruenberg J. Role of LBPA and Alix in multivesicular liposome formation and endosome organization. Science. 2004;303:531–534. doi: 10.1126/science.1092425. [DOI] [PubMed] [Google Scholar]
- 8.Bongiovanni A, Romancino DP, Campos Y, Paterniti G, Qiu X, Moshiach S, Di Felice V, Vergani N, Ustek D, d'Azzo A. Alix protein is substrate of Ozz-E3 ligase and modulates actin remodeling in skeletal muscle. J Biol Chem. 2012;287:12159–12171. doi: 10.1074/jbc.M111.297036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cory GO, Cullen PJ. Membrane curvature: the power of bananas, zeppelins and boomerangs. Curr Biol. 2007;17:R455–R457. doi: 10.1016/j.cub.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 10.Kim J, Sitaraman S, Hierro A, Beach BM, Odorizzi G, Hurley JH. Structural basis for endosomal targeting by the Bro1 domain. Dev Cell. 2005;8:937–947. doi: 10.1016/j.devcel.2005.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nastasi T, Bongiovanni A, Campos Y, Mann L, Toy JN, Bostrom J, Rottier R, Hahn C, Conaway JW, Harris AJ, D'Azzo A. Ozz-E3, a muscle-specific ubiquitin ligase, regulates beta-catenin degradation during myogenesis. Dev Cell. 2004;6:269–282. doi: 10.1016/s1534-5807(04)00020-6. [DOI] [PubMed] [Google Scholar]
- 12.Thery C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006;Chapter 3(Unit 3):22. doi: 10.1002/0471143030.cb0322s30. [DOI] [PubMed] [Google Scholar]
- 13.Bobrie A, Colombo M, Raposo G, Théry C. Exosome secretion: molecular mechanisms and roles in immune responses. Traffic. 2011;12(12):1659–1668. doi: 10.1111/j.1600-0854.2011.01225.x. [DOI] [PubMed] [Google Scholar]
- 14.Shen B, Wu N, Yang JM, Gould SJ. Protein targeting to exosomes/microvesicles by plasma membrane anchors. J Biol Chem. 2011;286(16):14383–14395. doi: 10.1074/jbc.M110.208660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Booth AM, Fang Y, Fallon JK, Yang JM, Hildreth JE, Gould SJ. Exosomes and HIV Gag bud from endosome-like domains of the T cell plasma membrane. J Cell Biol. 2006;172:923–935. doi: 10.1083/jcb.200508014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cocucci E, Racchetti G, Meldolesi J. Shedding microvesicles: artefacts no more. Trends Cell Biol. 2009;19:43–51. doi: 10.1016/j.tcb.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Lenassi M, Cagney G, Liao M, Vaupotic T, Bartholomeeusen K, Cheng Y, Krogan NJ, Plemenitas A, Peterlin BM. HIV Nef is secreted in exosomes and triggers apoptosis in bystander CD4 T cells. Traffic. 2010;11:110–122. doi: 10.1111/j.1600-0854.2009.01006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fang Y, Ning W, Xin G, Wanhua Y, James CM, Stephen JG. Higher-order oligomerization targets plasma membrane proteins and HIV gag to exosomes. PLoS Biol. 2007;5(6):e158. doi: 10.1371/journal.pbio.0050158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tauro BJ, Greening DW, Mathias RA, Mathivanan S, Ji H, Simpson RJ. Two distinct populations of exosomes are released from LIM1863 colon carcinoma cell-derived organoids. Mol Cell Proteomics. 2012 Dec 10; doi: 10.1074/mcp.M112.021303. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campos Y, Qiu X, Zanoteli E, Moshiach S, Vergani N, Bongiovanni A, Harris AJ, d'Azzo A. Ozz-E3 ubiquitin ligase targets sarcomeric embryonic myosin heavy chain during muscle development. PLoS One. 2010;5:e9866. doi: 10.1371/journal.pone.0009866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raiborg C, Stenmark H. A helix for the final cut. Science. 2011;331:1533–1534. doi: 10.1126/science.1204208. [DOI] [PubMed] [Google Scholar]
- 22.Baietti MF, Zhang Z, Mortier E, Melchior A, Degeest G, Geeraerts A, Ivarsson Y, Depoortere F, Coomans C, Vermeiren E, Zimmermann P, David G. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat Cell Biol. 2012;14(7):677–685. doi: 10.1038/ncb2502. [DOI] [PubMed] [Google Scholar]