Abstract
Background
Some drugs that are actively taken up into the liver exhibit greater than dose proportional increases in plasma exposure, although human liver-to-plasma concentration ratios have rarely been evaluated. Understanding these relationships has implications for drug concentrations at the target site for certain classes of compounds, such as direct-acting antivirals, targeted towards HCV.
Methods
Treatment-experienced, chronic HCV non-cirrhotic patients (n=3) received vaniprevir (600 mg or 300 mg twice daily) on days 1–3 and (600 mg or 300 mg single dose) on day 4. Core needle biopsy was performed at 6 or 12 h post-dose on day 4. Blood samples were collected pre-dose on days 1 and 4, and for 24 h post-dose on day 4. The primary study objective was the hepatic concentration of vaniprevir at 6 and 12 h post-dose.
Results
Vaniprevir plasma pharmacokinetic parameters increased in a greater than dose-proportional manner between the 300 mg and 600 mg doses, with approximately fivefold increases in AUC0–12 and Cmax associated with a twofold increase in dose (AUC0–12, 10.6 µM/h to 59.5 µM/h; Cmax, 2.60 µM to 13.5 µM). In the 300 mg and 600 mg dose groups, mean liver concentrations of vaniprevir were 84.6 µM and 169 µM at 6 h post-dose, and 29.4 µM and 53.7 µM at 12 h post-dose. Liver concentrations were higher than plasma with liver-to-plasma concentration ratios of approximately 20–280.
Conclusions
These data confirm higher vaniprevir concentrations in human liver compared with plasma and demonstrate that measurement of human liver drug concentration using needle biopsy is feasible.
Introduction
Although the liver is the major organ for drug metabolism and excretion, little is known about these processes as they relate to intrahepatic drug concentrations in humans [1]. In drug development, dose selection is often based upon the estimation of liver concentrations extrapolated from plasma pharmacokinetics (PK) or on data derived from preclinical species. The relevance of these measurements to human liver drug partitioning, however, has not been widely investigated. The consequences of potentially inaccurate predictions of liver drug concentrations may be considerable for agents such as vaniprevir (HCV-NS3/4A protease inhibitor) that exhibit non-linear PK characterized by a greater-than-dose-proportional increase in plasma exposure and by single-dose plasma exposures that are not predictive of steady-state exposures [2]. Saturable hepatic uptake mechanisms or transporter specificity may partially underlie the substantially larger liver-to-plasma concentration ratios than would be predicted.
Liver drug concentrations for an HCV targeted agent were assessed in one study: liver telaprevir concentrations were lower, relative to plasma and substantially different than preclinical studies [3–5]. We sought to determine vaniprevir steady-state hepatic concentration in comparison to plasma.
Methods
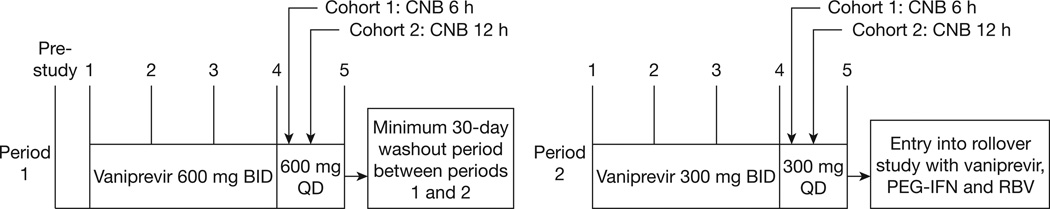
We conducted an open-label, 2-period, fixed-sequence, multiple-dose study (Figure 1) according to an institutional review board approved protocol. All patients provided written informed consent.
Figure 1. Study design.
Each patient received two separate doses of vaniprevir (600 mg followed by 300 mg) for 4 days followed by a liver biopsy performed at either 6 or 12 h after the last dose was administered on day 4. A minimum of 30 days ‘wash-out period’ separated each dosing period. BID, twice-daily dosing; CNB, core needle biopsy; PEG-IFN, pegylated interferon; QD, once-daily dosing; RBV, ribavirin.
Study subjects
Patients aged 40–65 years, with body mass index ≥18.5 to ≤36.0 kg/m2, and with genotype 1 chronic HCV infection, defined by HCV seropositivity and HCV RNA positivity, were enrolled. Patients were required to be treatment-experienced but not null responders (<2 log10 decline in HCV RNA) following 12 weeks continuous therapy with interferon or pegylated interferon plus ribavirin. Exclusion criteria included cirrhosis, prior treatment with an NS3/4A HCV protease inhibitor, chronic hepatitis due to a non-HCV-related aetiology, other liver diseases including liver cancer and coinfection with HIV or HBV.
Study design
In period 1, patients received vaniprevir (600 mg twice daily) on days 1–3 and vaniprevir (600 mg single dose) on day 4. Following a minimum 30-day period without vaniprevir, patients received vaniprevir (300 mg twice daily) on days 1–3 and vaniprevir (300 mg single dose) on day 4 (period 2). Nursing staff confirmed administration of each dose. Subsequently, all patients immediately commenced long-term treatment with vaniprevir, pegylated interferon/ribavirin.
Ultrasound-guided core needle biopsies (CNB) of the right hepatic lobe were performed either 6 or 12 h after drug administration on day 4. Liver histology was assessed by staff pathologists on samples of ≥2.5 cm in length using the Ishak classification [6]. The remaining sample for hepatic vaniprevir concentration was immediately weighed and stored at −70°C.
Vaniprevir assays
Plasma vaniprevir concentration was measured using liquid chromatography and tandem mass spectrometry (LC-MS/MS) validated over a linear calibration range of 1–1,000 ng/ml [7]. Assay reliability was demonstrated by adequate precision and accuracy when analysing six replicates of standard curve samples prepared using six lots of control plasma. Assay precision was better than 10% coefficient of variation (CV) and accuracy was within ±10.0%.
Human liver samples, obtained by CNB, were homogenized in human EDTA plasma using a Misonix liquid processor (Newtown, CT, USA), considered the most efficient device for handling small biopsy samples [8,9]. The homogenate was cleaned by liquid–liquid extraction at acidic pH prior to LC-MS/MS analysis under conditions similar to those described for the plasma assay. The assay performance was assessed over the range 0.1–200 µg/g with satisfactory accuracy and precision. No endogenous interferences were found at the retention windows of vaniprevir and the internal standard. Because of difficulties in obtaining human liver samples from multiple sources, validation was performed using standards prepared from a single lot of human control liver homogenate.
Statistical analysis
The primary study objective was the determination of the hepatic vaniprevir concentration 6 and 12 h post-dose. Secondary end points included liver/plasma vaniprevir concentration ratios. Plasma PK end points included the day 4 area under the plasma concentration versus time curve over the 12-h dosing interval area under the curve (AUC0–12), maximum plasma vaniprevir concentration (Cmax), trough plasma concentration (C12h), time to reach Cmax (Tmax), and apparent terminal half-life (t½).
Results
Patients
Three Caucasian males, aged 43–60 years, were enrolled (Table 1). In all patients, vaniprevir administration resulted in rapid decline of HCV RNA by day 2 (approximately 4 log in 600 mg and approximately 3 log in 300 mg treated patients).
Table 1.
Individual vaniprevir liver and plasma concentrations following administration of seven oral doses of vaniprevir administered twice daily to genotype 1 chronic HCV patients
| Subject | HCV RNA, IU/ml | Stagea | Twice daily dose, mg |
Liver concentration | Plasma concentration | Liver:plasma ratio | |||
|---|---|---|---|---|---|---|---|---|---|
| C6h, µM | C12h, µM | C6h, µM | C12h, µM | C6h | C12h | ||||
| 1 | 13.4×106 | F5 | 600 | – | 53.7 | – | 0.19 | – | 277.3 |
| 2 | 3.56×106 | F4-5 | 79.1 | – | 0.70 | – | 113.2 | – | |
| 3 | 5.54×106 | F1 | 258 | – | 12.1 | – | 21.4 | – | |
| Mean | 169 | 53.7 | 6.4 | 0.19 | 67.3 | 277.3 | |||
| 1 | – | – | 300 | – | 29.4 | – | 0.12 | – | 245.2 |
| 2 | – | – | 67.5 | – | 0.30 | – | 223.1 | – | |
| 3 | – | – | 102 | – | 0.93 | – | 108.9 | – | |
| Mean | – | – | 84.6 | 29.4 | 0.62 | 0.12 | 166.0 | 246.2 | |
Fibrosis stage assessed according to Ishak classification [5].
Vaniprevir plasma and liver concentrations
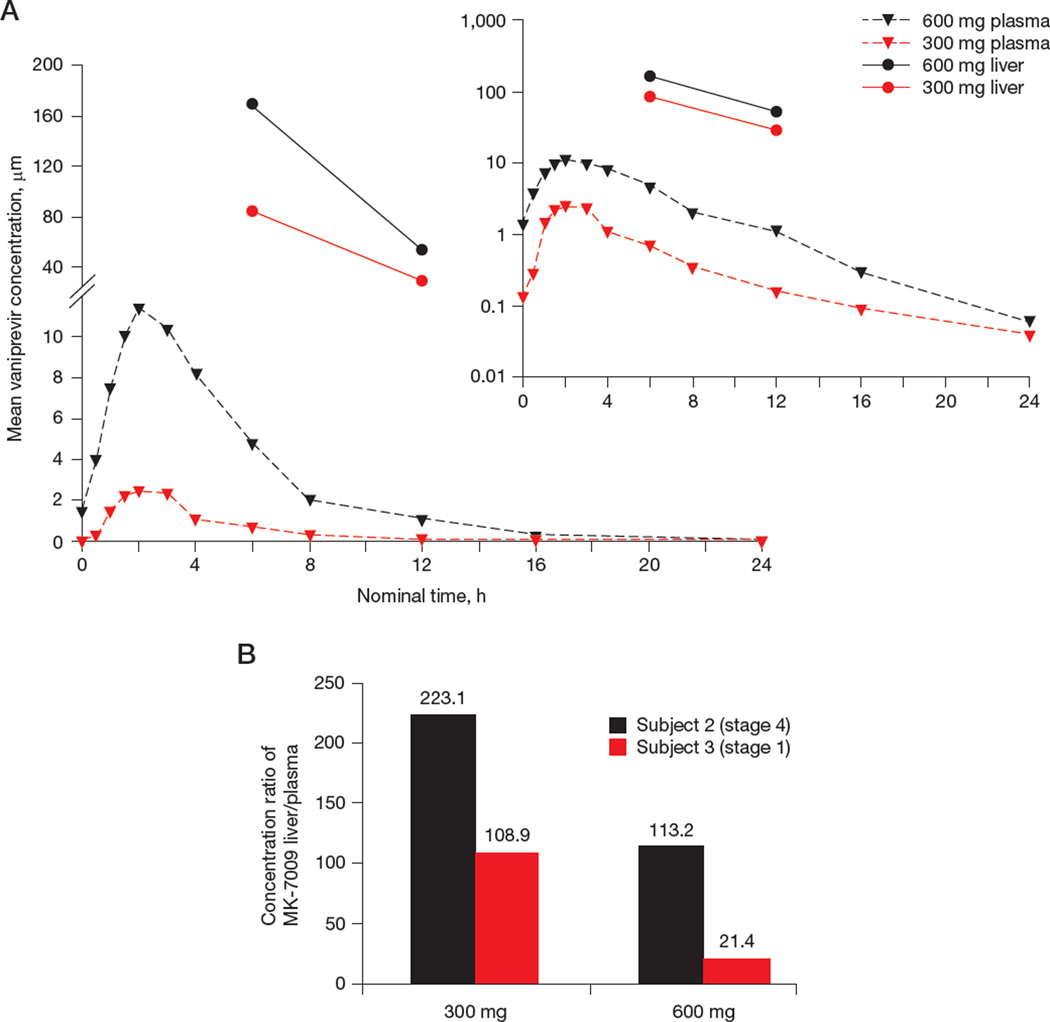
Mean liver vaniprevir concentrations at 6 and 12 h post-dose were dose-proportional, with twofold higher hepatic concentrations following the 600 mg twice daily than the 300 mg twice daily regimen (Table 1; twice daily). 6 h post-dose mean liver vaniprevir concentrations were 169 µM (period 1, 600 mg) and 84.6 µM (period 2, 300 mg). Similarly, liver concentrations were 53.7 µM and 29.4 µM at 12 h post-dose in periods 1 and 2, respectively. Intersubject variability was large for the individual liver concentrations at the 6 h time point, possibly attributable to non-uniform liver drug distribution.
Mean plasma steady-state AUC0–12 and Cmax values appeared to increase in a greater than dose-proportional manner between the two doses (Table 2). Both AUC0–12 and Cmax values increased approximately fivefold (AUC0–12, 10.6 µM/h to 59.5 µM/h; Cmax, 2.60 µM to 13.5 µM) and C12h was approximately sevenfold higher with a twofold increase in dose. Intersubject variability for day 4 plasma AUC0–12 and Cmax for the 300 mg and 600 mg doses was moderate (%CV of approximately 24–69%), but the variability of C12h was large (%CV 85–145%) because 12-h vaniprevir concentration was substantially higher in one patient (Table 2).
Table 2.
Summary of mean vaniprevir plasma pharmacokinetic parameters following administration of seven oral doses of vaniprevir administered twice daily to genotype 1 chronic hepatitis C virus patients
| Dose, mg | n | AUC0–12a, µM/h | C12 ha, µM | Cmaxa, µM | Tmaxb, h | Apparent t1/2c, h |
|---|---|---|---|---|---|---|
| 300 | 3 | 10.6 ±3.86 [36.4%] | 0.16 ±0.14 [85.2%] | 2.60 ±0.96 [37.0%] | 2.0 (1.5–3.0) | 6.3 ±1.4 |
| 600 | 3 | 59.5 ±41.0 [69.0%] | 1.11 ±1.61 [145.2%] | 13.5 ±3.22 [23.8%] | 3.0 (1.5–4.0) | 3.6 ±1.0 |
Arithmetic mean ±sd [CV%].
Median (min–max).
Harmonic mean ±pseudo sd. Nominal times used.
Liver concentrations were approximately 20–280 higher than plasma at both time points for both doses (Table 1; Figure 2A). Although mean liver-to-plasma ratios were higher for the 300 mg than the 600 mg dose (166.0 versus 67.3; Table 1) at 6 h post-dose, they were similar for both doses at 12 h (246.2 versus 277.3, respectively). The liver-to-plasma ratio was highest at the 12-h time point for both doses, suggesting that the distribution and/or elimination rate of vaniprevir may differ between liver and plasma.
Figure 2. Vaniprevir levels in plasma and liver and effect of hepatic fibrosis on intrahepatic levels.
(A) Mean plasma and liver concentration versus time (log-scale y-axis for inset plot). Plasma vaniprevir pharmacokinetics were obtained pre-dose on days 1 and 4, and post-dose on day 4 at the following time points: 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12, 16 and 24 h. (B) Relationship between fibrosis stage and liver concentration of vaniprevir for two patients, one with mild and one with advanced fibrosis. Subject 1 not included because liver concentration was measured only at 12 h post-dose.
Of note, the subject with mild fibrosis had markedly lower liver-to-plasma ratios at both doses than the subject with advanced fibrosis (Figure 2B).
Discussion
Increases in vaniprevir liver concentration were dose-proportional, which was distinctly different in plasma where a twofold increase in dose resulted in approximately fivefold increase in AUC0–12 and Cmax and approximately sevenfold increase in C12h at the clinically relevant dose in HCV patients. Liver-to-plasma drug concentration ratios were approximately 20–280. Greater than dose-proportional plasma PK have been observed previously with both the 300 mg and 600 mg twice daily doses (AUC0–12 5.15 and 23.36 µM/h, respectively) [10]. These results are consistent with the observation that liver organic anion-transporting polypeptides transport of vaniprevir in vitro is saturated at high concentrations [11]. While liver-to-plasma concentration ratios differed markedly between doses 6 h post-dose, they were strikingly similar by 12 h post-dose. In humans, the plasma vaniprevir Tmax was shown to be 1.5–3 h [10]. In chimpanzees dosed with vaniprevir (10 mg/kg oral), liver concentrations reached their maximum (31 µM) at 12 h after dosing, with a plasma AUC0–24 of 5.2 µM/h. Furthermore, substantial liver concentrations of vaniprevir (ranging between 200–600 nM) have also been reported in rat, dog and rhesus monkey at 24 h post-dose [12,13]. Together, the data suggest that the in vivo difference in liver-to-plasma ratios at various times may be attributed to high liver uptake of vaniprevir upon administration followed by a more rapid decline in plasma than liver (as observed in preclinical trials in other animal species).
Vaniprevir rapidly suppressed HCV RNA, and the plasma and intrahepatic concentrations of vaniprevir are substantially higher than the previously reported EC50 values of between 2.9 and 27 nM [12]. Collectively, these data imply sustained suppression of viral replication at the level of the hepatocyte. In another recent study with telaprevir, an agent without high liver partitioning, liver concentrations were lower than plasma [3].
Liver-to-plasma concentration ratios for both vaniprevir doses were substantially higher in a patient with advanced fibrosis while liver and plasma concentrations individually were higher in the patient with mild fibrosis. In rats with acute hepatic failure, a decrease in hepatic blood flow has been shown to be an important determinant of decreased clearance of the liver excreted drug 5-fluorouracil [14]. Liver damage in rats by bile duct ligation has been shown to differentially affect cytochrome P450 isoenzymes [15]. Diminished liver blood flow from increased portal pressure secondary to portal hypertension or effects on liver metabolizing enzymes resulting from advanced fibrosis may explain increased liver drug concentration. Fibrosis-related loss of efflux transporters, either through hepatocyte loss by replacement with fibrotic tissue or through the loss of transport proteins from the cell surface, might also explain the lower plasma drug concentration observed in the patient with advanced fibrosis. Alternatively, other mechanisms may also contribute.
Study limitations mostly relate to the small sample size resulting in low statistical power. An additional limitation is the large intersubject variability for the individual liver concentrations. Furthermore, while intersubject variability for plasma AUC and Cmax on day 4 was moderate (%CV= approximately 24–69%); the variability of C12h at the 600 mg dose was large (%CV=145%) due to one subject achieving a much higher trough concentration. In addition, liver samples were not collected up to 24 h post-dose, precluding calculation of the apparent terminal liver vaniprevir half-life and the steady-state hepatic AUC0–12.
Taken together, these data indicate that liver-to-plasma concentration ratios differ depending upon the agent and suggest that plasma PK may not adequately or consistently reflect liver PK especially for drugs with high liver partitioning. Further investigation should assess the relative importance of hepatic compared with plasma PK and the effect of fibrosis on intrahepatic drug concentration.
Acknowledgments
We thank the patients for their participation in this study and the nursing staff who assisted with the clinical conduct of the study. This study was funded by ULI RR024996 the Clinical and Translational Science Center (CTSC) at Weill Cornell and Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Whitehouse Station, NJ, USA. AHT acknowledges support of Kaleida Health Foundation’s Troup Fund in the preparation of the manuscript.
Disclosure statement
AHT received grant/research support from Merck/Schering Plough, Genentech/Roche, Vertex, Boerhinger-Ingelheim, Gilead, Tibotec, Abbott Diagnostics, AbbVie, and BMS, and has served as a consultant/advisor for Merck, Boerhinger-Ingelheim, Pfizer, Vertex, Genentech/Roche and Abbott Diagnostics. He was previously on the Speaker’s bureau for Genentech/Roche and Vertex and has received support from Medscape for development of educational presentations. MPM has received research support, consulting fees and honoraria for participation in review panels, and support for travel to meetings from Merck and Company. He has received grant support from Roche, Gilead, Novartis, Boehringer Ingelheim, BMS and Janssen; has served as a consultant to Roche, BMS, Gilead, Boehringer Ingelheim, Novartis, Janssen and GlaxoSmithKline; and has been on the Speaker’s bureau for Roche, Gilead, Novartis, Boehringer Ingelheim, BMS and Janssen. AP has been on the speaker’s bureaus for Falk Pharmaceuticals, Toshiba Medical Systems and BMS. Co-authors DHW, LC, MC, PP, LD, MA, RBN and JW are current employees of Merck and Company or were employees at the time when this study was performed. LC, JW, DHW, RBN, LD, PP, MA and MC may own stock or stock options with Merck.
Footnotes
This work was present at the 63rd Annual AASLD (9–13 November 2012, Boston, MA, USA; Abstract 1868) and was awarded Presidential Poster of Distinction as among top 10% presented.
DHW, LC, MC, PP, LD, MA, RBN, JW, MPM and AHT contributed significantly to study conception, design and planning. LC, MC, PP, LD, MA, AP, RBN, MPM and AHT collected/assembled the data. DHW, LC, AHT supervised the study. DHW, LC, JW, MPM and AHT interpreted the results. DHW, LC, MPM and AHT drafted the manuscript. All authors have approved of the final version of the article including the authorship list.
References
- 1.Chu X, Korzekwa K, Elsby R, et al. Intracellular drug concentrations and transporters: measurement, modeling, and implications for the liver. Clin Pharmacol Ther. 2013;94:126–141. doi: 10.1038/clpt.2013.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wright DH, Miller JL, Verlinden I, et al. Safety, tolerability, and pharmacokinetic data following single- and multiple-dose administration of MK-7009, a hepatitis C virus non-structural 3/4a protease inhibitor, to healthy male subjects. Hepatology. 2008;48:1165A. [Google Scholar]
- 3.Talal AH, Dimova RB, Zhang EZ, et al. Telaprevir-based treatment effects on hepatitis C virus in liver and blood. Hepatology. 2014;60:1826–1837. doi: 10.1002/hep.27202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perni RB, Almquist SJ, Byrn RA, et al. Preclinical profile of VX-950, a potent, selective, and orally bioavailable inhibitor of hepatitis C virus NS3-4A serine protease. Antimicrob Agents Chemother. 2006;50:899–909. doi: 10.1128/AAC.50.3.899-909.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reddy MB, Morcos PN, Le Pogam S, et al. Pharmacokinetic/pharmacodynamic predictors of clinical potency for hepatitis C virus nonnucleoside polymerase and protease inhibitors. Antimicrob Agents Chemother. 2012;56:3144–3156. doi: 10.1128/AAC.06283-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ishak K, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 7.Anderson MD, Breidinger SA, Woolf EJ. Effect of disease state on ionization during bioanalysis of MK-7009, a selective HCV NS3/NS4 protease inhibitor, in human plasma and human Tween-treated urine by high-performance liquid chromatography with tandem mass spectrometric detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:1047–1056. doi: 10.1016/j.jchromb.2009.02.069. [DOI] [PubMed] [Google Scholar]
- 8.Sun T, Xu Z, Godber JS. Ultrasound assisted extraction in quantifying lutein from chicken liver using high-performance liquid chromatography. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;830:158–160. doi: 10.1016/j.jchromb.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 9.Lee M-H, Lin C-C. Comparison of techniques for extraction of isoflavones from the root of Radix Puerariae: ultrasonic and pressurized solvent extractions. Food Chem. 2007;105:223–228. [Google Scholar]
- 10.Manns MP, Gane E, Rodriguez-Torres M, et al. Vaniprevir with pegylated interferon alpha-2a and ribavirin in treatment-naive patients with chronic hepatitis C: a randomized Phase II study. Hepatology. 2012;56:884–893. doi: 10.1002/hep.25743. [DOI] [PubMed] [Google Scholar]
- 11.Monteagudo E, Fonsi M, Chu X, et al. The metabolism and disposition of a potent inhibitor of hepatitis C virus NS3/4A protease. Xenobiotica. 2010;40:826–839. doi: 10.3109/00498254.2010.519061. [DOI] [PubMed] [Google Scholar]
- 12.Liverton NJ, Carroll SS, Dimuzio J, et al. MK-7009, a potent and selective inhibitor of hepatitis C virus NS3/4A protease. Antimicrob Agents Chemother. 2010;54:305–311. doi: 10.1128/AAC.00677-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCauley JA, McIntyre CJ, Rudd MT, et al. Discovery of vaniprevir (MK-7009), a macrocyclic hepatitis C virus NS3/4a protease inhibitor. J Med Chem. 2010;53:2443–2463. doi: 10.1021/jm9015526. [DOI] [PubMed] [Google Scholar]
- 14.Nagata M, Hidaka Y, Hidaka M, et al. Effect of acute hepatic failure on the hepatic first-pass effect of 5-fluorouracil in rats. J Pharm Pharmacol. 2010;62:598–603. doi: 10.1211/jpp.62.05.0006. [DOI] [PubMed] [Google Scholar]
- 15.Tateishi T, Watanabe M, Nakura H, Tanaka M, Kumai T, Kobayashi S. Liver damage induced by bile duct ligation affects CYP isoenzymes differently in rats. Pharmacol Toxicol. 1998;82:89–92. doi: 10.1111/j.1600-0773.1998.tb01403.x. [DOI] [PubMed] [Google Scholar]