Despite a significant improvement in the survival of patients with multiple myeloma (MM), the disease remains incurable and innovative strategies are needed. Interactions among BCL-2 family proteins mainly determines cellular fate decision in response to drug therapy. Thus, anti-apoptotic members such as BCL-2, BCL-XL or MCL-1, represent an attractive target for therapy1. BH3 profiling is a functional assay that identifies the tumor cell’s addiction to these anti-apoptotic members2. The oral BCL-2-specific BH3 mimetic ABT-199 demonstrated very promising results in BCL-2 dependent malignancies such as chronic lymphoid leukemia (CLL)3 and mantle cell lymphoma4,5. To date, there has been no determination of what proportion of MM cases are likely to be BCL-2 dependent. The present study demonstrated that MM is a heterogeneous disease with respect to BCL-2, BCL-XL or MCL-1 dependence. Moreover, identification by BH3 profiling of BCL-2 dependence predicted in vitro sensitivity to BH3 mimetics.
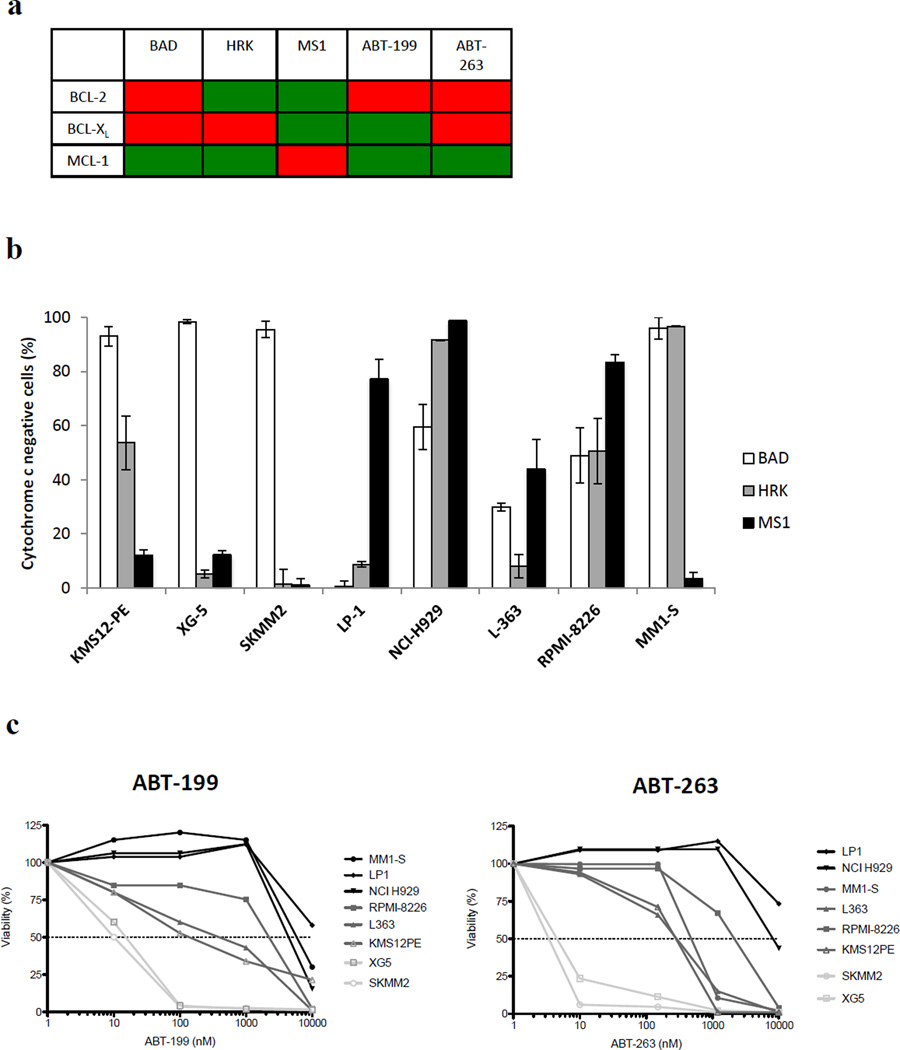
Dependence on BCL-2, BCL-XL or MCL-1 was measured in 8 human myeloma cell lines. After cell permeabilization, mitochondria were exposed to standardized amounts of BAD, HRK or MS1 peptides. The affinity of these peptides for the anti-apoptotic proteins are summarized in Figure 1a2,5,6. The release of cytochrome c induced by each peptide was quantified by FACS analysis. Dependence on individual anti-apoptotic proteins was found to be very heterogeneous from one cell line to another (Figure 1b). One cell line (LP-1) was found to be relatively purely MCL-1 dependent. XG-5 and SKMM2 were found to be relatively BCL-2 dependent and MM1-S was found to be relatively purely BCL-XL dependent. The remaining cell lines were characterized by co-dependency on anti-apoptotic proteins: BCL-2 and BCL-XL (KMS12-PE) or BCL-XL and MCL-1 (NCI-H929 and RPMI-8226) and BCL-2 and MCL-1 (L-363). We then determined the in vitro sensitivity of MM cell lines to the BH3 mimetics ABT-199 and ABT-263 (Figure 1c). The mitochondrial response to BAD peptide predicted the in vitro sensitivity to ABT-263 (Figure 1d). The BAD minus HRK mitochondrial response was used to reflect the specific BCL-2 dependency and significantly predicted sensitivity to ABT-199. (Figure 1d) Finally, the cytochrome c release in response to ABT-199 and ABT-263 strongly correlated with the in vitro sensitivity to the drug.
Figure 1. BH3 profiling of Myeloma cell lines demonstrates heterogeneous Bcl-2 dependancy and correlates with in vitro sensitivity to BH3 mimetics.
(a) Binding affinity of BH3 peptides, ABT-199 and ABT-263 for the anti-apoptotic proteins. Green and red colors indicate high and low affinity, respectively2,5,6. (b) KMS12-PE, XG-5, SKMM2, LP-1, L363, NCI-H929, RPMI-8226 and MM1-S identity was confirmed by DNA fingerprinting or HLA typing. Intracellular BH3 (iBH3) profiling was performed using BAD (100µM), HRK (100µM) and MS-1 (10µM) peptides. As previously described13 , tumor cells were pelleted and resuspended in DTEB buffer with addition of each BH3 peptide treatment with 0.002% w/v digitonin. BAD, HRK and MS1 peptides sequences have been previously described7,8. Mitochondria in the permeabilized cells were exposed to peptides for 45 min at 26°C before fixation with 2% formaldehyde at room temperature for 15 min. After addition of neutralizing buffer, cells were stained with anti-cytochrome c–Alexa488 (#560263, BD Biosciences) 1:100 in 0.1% Saponin/1%BSA/PBS overnight at 4 C. The quantification of cytochrome c loss induced by each peptide was analyzed by FACS (LSR Fortessa flow cytometer, BD Bioscience). Values indicate the percentage of cytochrome c negative cells. Indicated values are the mean of 3 independent experiments. (c) Sensitivity of Myeloma cell lines to the BH3 mimetics ABT-199 and ABT-263 was assessed using Cell Titer Glow® assay. Cell lines were cultured with increasing doses of ABT-199 or ABT-263 during 24 hours. Indicated values are the mean of 3 independent experiments. Light grey line indicates IC50 <100 nM, Dark grey line indicates IC50 between 100 and 5000 nM, black lines indicate IC50 > 5000 nM (d) IC50 of myeloma cell lines were correlated with the mitochondrial response to BAD (100µM), BAD (100µM) – HRK (100µM) peptides and with ABT-199 or 263 (1µM). All correlations were tested using a one-tailed Spearman r correlation using GraphPad Prism software.
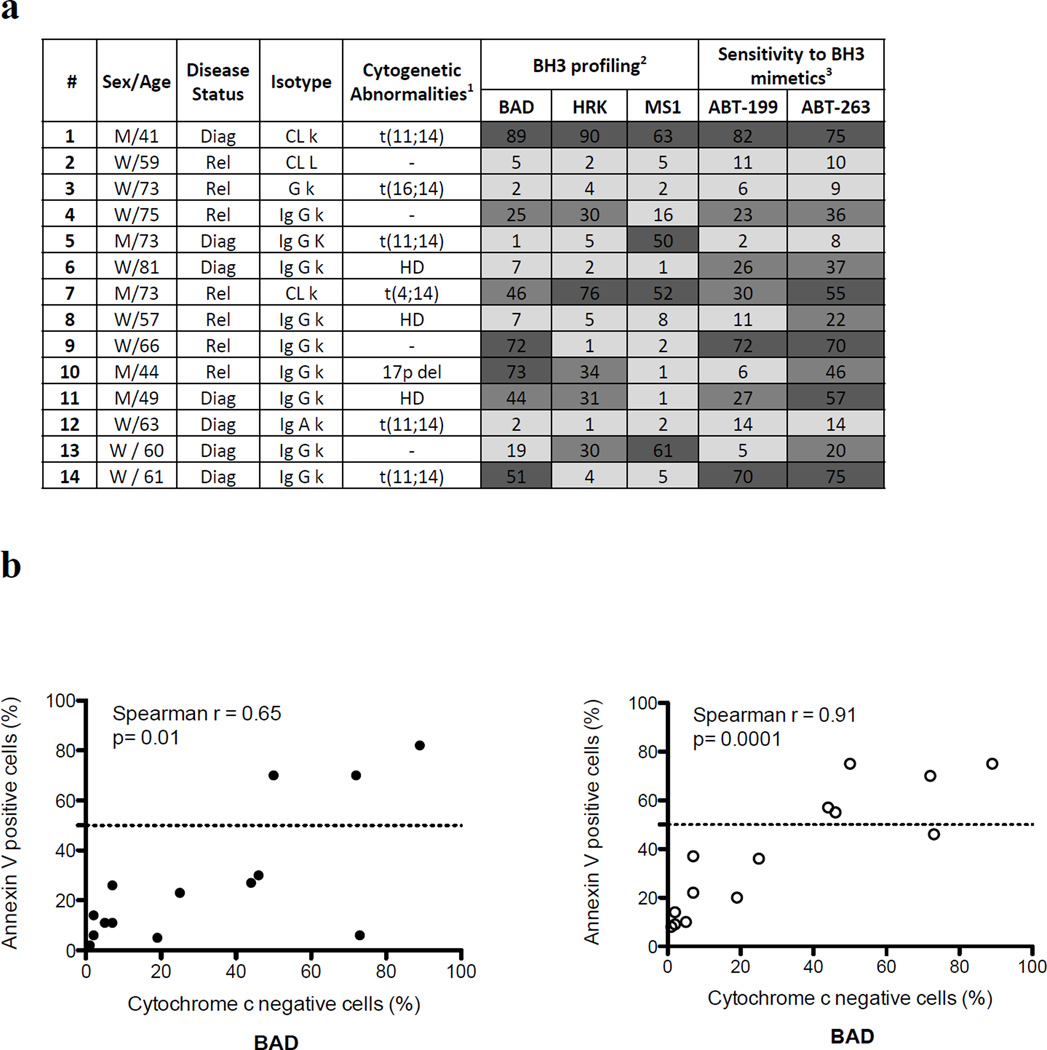
We then determined the mitochondrial priming of primary plasma cells obtained from 14 different patients with a diagnosis of de novo or relapsed MM. Patient characteristics are summarized in Figure 2a. As observed with cell lines, patient samples showed a heterogeneous dependence on anti-apoptotic proteins (Figure 2a). Of note, mitochondria of 5 patient samples were found to be relatively independent of BCL-2, BCL-XL or MCL-1. We also determined the in vitro sensitivity to ABT-199 and ABT-263. Three and 5 samples were found sensitive (LD50≤ 100 nM) to ABT-199 and ABT-263, respectively. As shown in Figure 2b, BH3 profiling using the BAD peptide correlated with in vitro sensitivity to ABT-199 and 263.
Figure 2. BH3 profiling of primary myeloma cells predicts lines in vitro sensitivity to BH3 mimetics.
(a) Mitochondrial priming of multiple myeloma primary cells. Primary cells were obtained after informed consent from MM patients treated at the Dana Farber Cancer Institute, Boston, USA. Intracellular BH3 (iBH3) profiling was performed using BAD (100µM), HRK (100µM) and MS-1 (10µM) peptides. Abreviations:Iso, immunoglobulin isotype; Diag, Newly diagnosed myeloma; Rel, relapsed myeloma. HD: hyperdiploidy. 1All samples were screened by FISH for the t(11;14), t(4;14), 17p deletion and caryotype was performed for hyperdiploidy status. 2Values indicate the percentage of cytochrome c negative cell. 3Values indicate the percentage of apoptotic cells. Cells were cultured with/without the drugs during 16 hours. Cell death was assessed by flow cytometry after annexin V staining Dark grey: ≥50%, Intermediate grey: 20–49, light grey: <20% (b) Primary samples were cultured for 16 hours with/without ABT-199 or ABT-263 (100 nM). Cell death was measured by FACS using Annexin V staining. Percentages of apoptotic cells were correlated with the BAD (100µM) peptide. All correlations were tested using a one-tailed Spearman r correlation using GraphPad Prism software.
BH3 profiling is a unique, functional method to measure the dependence to the anti-apoptotic proteins in live cancer cells. The present study demonstrates that Multiple Myeloma is a heterogeneous disease regarding its dependence on anti-apoptotic proteins and cannot be considered as monolithically BCL-2, BCL-XL or MCL-1 dependent. MCL-1 is expressed in MM cells at levels higher than normal plasma cells and its expression level has been shown to affect clinical outcome7,8. Here, mitochondria from half of MM cell lines (4/8) and from almost one third of primary samples (4/14) were found to be MCL-1 dependent. These results are consistent with the requirement for MCL-1 for survival of many myeloma cells. BH3 profiling also identified a subset of BCL-2 and/or BCL-XL dependent MM cells with relatively less dependence on MCL-1 and correctly predicted sensitivity to the BH3 mimetics ABT-199 and 263. In our series, one MM cell line (MM1-S) and 2 primary samples (#7 and #11) were found to be sensitive to ABT-263 but insensitive to ABT-199, that linked to their BCL-XL dependence determined using BH3 profiling. Even if these findings indicate that BCL-XL could be an attractive target for MM, the BCL-XL-related platelet toxicity has impaired the clinical development of ABT-2639. Previous studies identified MM cells sensitive to BH3 mimetics based on their BCL-2/MCL-1 mRNA ratio10,11 or interactions of BCL-XL and BCL-2 with BIM12. From a practical point of view, BH3 profiling can be performed in just a three hours with fewer cells (5.104 plasma cells to determine response to the BAD BH3 peptide). This consideration is of importance because bone marrow samples from MM patients usually contain a low percentage of plasma cells (the median percentage of plasma cell in BM aspirate was 9% in our series). It has been previously reported that sensitivity to ABT-199 was restricted in MM patients with t(11;14) translocation11. Here, among the three ABT-199 sensitive samples, two were found to be t(11;14). By including previous data from Touzeau et al.11 , sensitivity to ABT-199 from 29 different primary MM samples was analyzed. Overall, 7 samples (24%) were found to be sensitive to the drug (LD50<100 nM). Interestingly, 6 of these ABT-199 sensitive samples carried the t(11;14) translocation. Of note, the remaining sensitive sample was found to be BCL-2 dependent according to BH3 profiling. Moreover, one sensitive patient sample was found to be negative for the t(11;14) translocation suggesting that BCL-2 dependence may exist beyond this cytogenetic subgroup. The positive (PPV) and negative (NPV) predictive value of BH3 profiling to predict ABT-199 sensitivity were 75 and 100%, respectively (supplemental Table).
Overall, and as previously demonstrated in other hematologic malignancies2,13,14 , BH3 profiling identifies BCL-2 dependence in myeloma cells and predicts sensitivity to BH3 mimetics. Phase 1 clinical trials evaluating ABT-199 as a single agent (NCT01794520) or in combination with bortezomib plus dexamethasone (NCT01794507) in relapsed MM patients are ongoing. Preliminary results of these trials demonstrated significant anti myeloma response with a favorable safety profile.16,17 The promising predictive value of BH3 profiling needs to be confirmed in the context of prospective clinical studies. Because BCL-2 dependency was found only in a subset of myeloma patients, future clinical trials with an efficacy objective should integrate biomarkers such BH3 profiling to stratify potential candidates for ABT-199 therapy.
Supplementary Material
Legend: Se, sensitivity; Sp, specificity; PPV, positive predictive value; NPV, negative predictive value.
For the t(11;14) translocation test, a positive test was determined by the presence of the translocation. For the BH3 profiling test, a positive test was defined by a BAD (100 µM) response (% cytochrome c negative cells) > 50%
ACKNOWLEDGMENTS
The authors gratefully acknowledge support from NIH grants R01CA129974.
CT was supported by the Fondation Française pour la Recherche contre le Myélome et les Gammapathies monoclonales (F.F.R.M.G.).
Footnotes
CONFLICT-OF-INTEREST DISCLOSURE
AL has been a paid advisor to AbbVie. AL’s laboratory has received sponsorship for research with AbbVie.
AUTHORSHIP CONTRIBUTIONS
AL and CT designed research and wrote the manuscript. CT performed experiments. CT, AL, JR, TN analyzed the data. PR, KA, MA, SLG, PM provided myeloma cells. All the authors critically reviewed the manuscript.
REFERENCES
- 1.Letai AG. Diagnosing and exploiting cancer’s addiction to blocks in apoptosis. Nat Rev Cancer. 2008;8:121–132. doi: 10.1038/nrc2297. [DOI] [PubMed] [Google Scholar]
- 2.Certo M, Del Gaizo Moore V, Nishino M, Wei G, Korsmeyer S, Armstrong SA, et al. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 2006;9:351–365. doi: 10.1016/j.ccr.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 3.BC-2 inhibitor yields high response in CLL and SLL. Cancer Discov. 2014;4:OF5. doi: 10.1158/2159-8290.CD-NB2013-178. [DOI] [PubMed] [Google Scholar]
- 4.Davids MS, Roberts AW, Anderson MA, Pagel JM, Kahl BS, Gerecitano JF, et al. The BCL-2-Specific BH3-Mimetic ABT-199 (GDC-0199) Is Active and Well-Tolerated in Patients with Relapsed Non-Hodgkin Lymphoma: Interim Results of a Phase I Study. ASH Annu Meet Abstr. 2012;120:304. [Google Scholar]
- 5.Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013 doi: 10.1038/nm.3048. [DOI] [PubMed] [Google Scholar]
- 6.Foight GW, Ryan JA, Gullá SV, Letai A, Keating AE. Designed BH3 Peptides with High Affinity and Specificity for Targeting Mcl-1 in Cells. ACS Chem Biol. 2014 doi: 10.1021/cb500340w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Munshi NC, Hideshima T, Carrasco D, Shammas M, Auclair D, Davies F, et al. Identification of genes modulated in multiple myeloma using genetically identical twin samples. Blood. 2004;103:1799–1806. doi: 10.1182/blood-2003-02-0402. [DOI] [PubMed] [Google Scholar]
- 8.Wuillème-Toumi S, Robillard N, Gomez P, Moreau P, Le Gouill S, Avet-Loiseau H, et al. Mcl-1 is overexpressed in multiple myeloma and associated with relapse and shorter survival. Leukemia. 2005;19:1248–1252. doi: 10.1038/sj.leu.2403784. [DOI] [PubMed] [Google Scholar]
- 9.Roberts AW, Seymour JF, Brown JR, Wierda WG, Kipps TJ, Khaw SL, et al. Substantial susceptibility of chronic lymphocytic leukemia to BCL2 inhibition: results of a phase I study of navitoclax in patients with relapsed or refractory disease. J Clin Oncol Off J Am Soc Clin Oncol. 2012;30:488–496. doi: 10.1200/JCO.2011.34.7898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bodet L, Gomez-Bougie P, Touzeau C, Dousset C, Descamps G, Maïga S, et al. ABT-737 is highly effective against molecular subgroups of multiple myeloma. Blood. 2011;118:3901–3910. doi: 10.1182/blood-2010-11-317438. [DOI] [PubMed] [Google Scholar]
- 11.Touzeau C, Dousset C, Le Gouill S, Sampath D, Leverson JD, Souers AJ, et al. The Bcl-2 specific BH3 mimetic ABT-199: a promising targeted therapy for t(11;14) multiple myeloma. Leukemia. 2013 doi: 10.1038/leu.2013.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morales AA, Kurtoglu M, Matulis SM, Liu J, Siefker D, Gutman DM, et al. Distribution of Bim determines Mcl-1 dependence or codependence with Bcl-xL/Bcl-2 in Mcl-1-expressing myeloma cells. Blood. 2011;118:1329–1339. doi: 10.1182/blood-2011-01-327197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pan R, Hogdal LJ, Benito JM, Bucci D, Han L, Borthakur G, et al. Selective BCL-2 inhibition by ABT-199 causes on-target cell death in acute myeloid leukemia. Cancer Discov. 2014;4:362–375. doi: 10.1158/2159-8290.CD-13-0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chonghaile TN, Roderick JE, Glenfield C, Ryan J, Sallan SE, Silverman LB, et al. Maturation stage of T-cell acute lymphoblastic leukemia determines BCL-2 versus BCL-XL dependence and sensitivity to ABT-199. Cancer Discov. 2014;4:1074–1087. doi: 10.1158/2159-8290.CD-14-0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ryan J, Letai A. BH3 profiling in whole cells by fluorimeter or FACS. Methods San Diego Calif. 2013;61:156–164. doi: 10.1016/j.ymeth.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar S, Vij R, Kaufman J, Mikhael J, Facon T, Moreau P, et al. Phase I Interim Safety and Efficacy of Venetoclax (ABT-199/GDC-0199) Monotherapy for Relapsed/Refractory Multiple Myeloma; Abstract #8576, 2015 ASCO meeting. [Google Scholar]
- 17.Touzeau C, Chanan-Khan A, Roberts AW, Agarwal A, Facon T, Lebovic D, et al. Phase 1b Interim Results: Venetoclax (ABT-199/GDC-0199) in Combination with Bortezomib and Dexamethasone in Relapsed/Refractory Multiple Myeloma; Abstract #8580, 2015 ASCO meeting. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Legend: Se, sensitivity; Sp, specificity; PPV, positive predictive value; NPV, negative predictive value.
For the t(11;14) translocation test, a positive test was determined by the presence of the translocation. For the BH3 profiling test, a positive test was defined by a BAD (100 µM) response (% cytochrome c negative cells) > 50%