Abstract
The fusiform gyrus (FG) is commonly included in anatomical atlases and is considered a key structure for functionally-specialized computations of high-level vision such as face perception, object recognition, and reading. However, it is not widely known that the FG has a contentious history. In this review, we first provide a historical analysis of the discovery of the FG and why certain features, such as the mid-fusiform sulcus, were discovered and then forgotten. We then discuss how observer-independent methods for identifying cytoarchitectonical boundaries of the cortex revolutionized our understanding of cytoarchitecture and the correspondence between those boundaries and cortical folding patterns of the FG. We further explain that the co-occurrence between cortical folding patterns and cytoarchitectonical boundaries are more common than classically thought and also, are functionally meaningful especially on the FG and probably in high-level visual cortex more generally. We conclude by proposing a series of alternatives for how the anatomical organization of the FG can accommodate seemingly different theoretical aspects of functional processing, such as domain specificity and perceptual expertise.
Keywords: Fusiform gyrus, Mid-fusiform sulcus, Cytoarchitecture, Domain specificity, Perceptual expertise
1. Introduction
Human ventral temporal cortex (VTC) is a key-structure in high-level visual processing, such as face perception (Kanwisher et al., 1997), object recognition (Grill-Spector et al., 2001), and reading (Cohen et al., 2000; Wandell et al., 2012). VTC is also involved in different aspects of memory (Henson et al., 2000; Wagner et al., 1999), multi-sensory integration (Amedi et al., 2002; James et al., 2002; Kitada, Johnsrude et al., 2009), and perceptual expertise (McGugin et al., 2012; McGugin et al., 2014). Recent research shows that many of these functions associated with VTC result in multiple large-scale and fine-scale maps with a predictable spatial relationship to cortical folding patterns (Grill-Spector and Weiner, 2014). Interestingly, recent cytoarchitectonic mapping (Caspers et al., 2013), diffusion weighted imaging (Saygin et al., 2012), receptor architectonic studies (Caspers et al., 2015), and myelination analyses (Glasser and Van Essen, 2011) result in maps that also align with cortical folding patterns in and around VTC. This alignment of both anatomical and functional features of VTC suggests that anatomical specialization may provide an underlying substrate for functional specialization. As Shlomo Bentin pioneered how temporal specializations in the brain enable rapid face perception (Bentin et al., 1996), for this Special Issue, we focus on the underlying microscopic organization of VTC that likely contribute to different aspects of functional specialization of VTC.
Since our present purpose is to link anatomical aspects of specialization to functional specialization within VTC, we focus on the fusiform gyrus (FG) because it is (a) the largest macro-anatomical structure within high-level visual cortex and (b) a highly contentious structure with many seemingly different proposed functions (Cohen et al., 2000; Cukur et al., 2013; Haxby et al., 2001, 2011; Huth et al., 2012; Kanwisher, 2000; Mahon and Caramazza, 2011; Tarr and Gauthier, 2000). In order to link microstructural features of anatomical organization such as cytoarchitecture to large-scale functional features across spatial scales (from microns to centimeters), it is imperative to precisely understand the topology of sulci within and around the FG. In order to do so, we first provide a historical analysis of both the FG and a sagittal sulcus known as the mid-fusiform sulcus (MFS), which divides the FG into lateral and medial partitions. Important components of this history are cross-species comparisons and the development of observer-independent methods for determining cytoarchitectonic boundaries of the cortex, which are included in our historical analysis. As the history of the macroanatomical and cytoarchitectonic organization of the FG has never been consolidated into one place before, we hope this paper serves as a reference for any researcher interested in measuring anatomical or functional features of the FG. It should also be stressed that the points we raise in the present work are not specific to the FG and MFS or high-level visual cortex more generally. On the contrary, the historical and methodological concerns have implications for understanding structural–functional organization and theories for any portion of cortex.
Altogether, this paper can be divided into four main sections. First, we provide a historical analysis of the discovery of both the FG, as well as the often neglected MFS. Second, we describe how observer-independent and statistically testable cytoarchitectonic analyses have uncovered cytoarchitectonic parcellations of the FG that were unidentifiable with prior observer-dependent methods such as those used by Brodmann (1909), von Economo and Koskinas (1925), and Bailey and von Bonin (1951). Third, we describe a series of recent findings that reveal the functionality of cytoarchitectonic regions, as well as the potential correspondence between cytoarchitectonic and functional FG regions. Fourth, based off of these recent new insights from anatomical features of the FG, we propose a series of alternatives for how the anatomical organization of the FG can accommodate seemingly different theoretical aspects of functional specialization, such as domain specificity and perceptual expertise. While we use domain specificity and perceptual expertise as examples for how the anatomical construction of the FG can accommodate multiple functions, there are many other functions of the FG, which are beyond the scope of the present manuscript.
2. The fusiform gyrus: a historical analysis
‘…just as it is desirable before studying the geology of a district to have the surface carefully surveyed and accurately mapped out, so it is advisable that the topography of the convolutions of the human cerebrum should be satisfactorily ascertained before an analysis of the intimate structure and deep connexions of the gray matter can be put forth with the necessary exactness’. Sir William Turner, 1866 P. 28
As eloquently described by Turner (1866) nearly 150 years ago, it is imperative to accurately identify the macroanatomical landmarks within a cortical extent of interest before one can fully understand the microstructure and connections within it – and especially, how such a structure may contribute to a corresponding function. In the last two years, many methodological advancements have been made, which have improved our understanding of structural–functional correspondences in human ventral temporal cortex (VTC) with a particular emphasis on the FG. In our previous work (Weiner et al., 2014), we noted that the longitudinal sulcus dividing the fusiform into lateral and medial partitions first appeared in modern writing by Puce and colleagues (Puce et al., 1996) and was later named the MFS (Allison et al., 1998). Since we were able to easily identify the MFS in 158 hemispheres (Weiner et al., 2014), it seemed surprising that the MFS had not been documented until the end of the 20th century.
Indeed, the 19th century saw a real paradigm shift in the localization and documentation of cortical convolutions and fissures across species. As reflected by Edinger in his twelve lectures (Edinger, 1891), 19th century anatomists began to make sense of the ‘chaos of convolutions’ (p. 45, introduction to Lecture IV) that in prior centuries were considered to be as irregular as the coils of the small intestine – a comparison which began with Erasistratos in the second century (Gross, 1998). In the sections below, we provide a historical analysis of the FG and the identification of the MFS. We then discuss two main reasons as to why the MFS was forgotten: (1) the end of the 19th century was a contentious time for anatomists, including a movement to omit minor anatomical structures from anatomical terminology altogether and (2) the evolution of the brain maps in the late 19th and early 20th centuries generally negated a correspondence between sulci and architectural transitions (Brodmann, 1909; Vogt and Vogt, 1919), which tended to devaluate attention to macroanatomical features of the cortex.
2.1. The fusiform gyrus is first labeled in 1854 and the mid-fusiform sulcus in 1896
The fusiform gyrus was first labeled in 1854 by Emil Huschke of Jena (Huschke, 1854; Fig. 1). Huschke specifically referred to the fusiform as ‘Spindelwulst’ (fusiform gyrus) due to the fact that it was wider in the middle than at its ends, similar to the shape of a spindle. Huschke writes:
‘Noch weiter nach aussen vom zungenförmigen Wulst folgt der von mir spindelförmiges Läppchen (Lobulus fusiformis) genannte Gyrus, ein hinten und vorn zugespitztes Läppchen (Wulst), das von verschiedener Länge (2-3” lang) und an seiner Oberfläche mit verschieden gestalteten Querfurchen oder inselartigen Vertiefungen versehen ist. Sein hinteres Ende hängt mit dem unteren Ende der beiden concentrisch in einander liegenden Schlingen von dem hinteren Aste des Zwickels zusammen. Hierauf läuft er, sich ausbreitend, vorwärts und endet früher oder später in den Windungen des Schläfenlappens bald dem Ende des Hakens gegenüber, bald auch noch 1” weiter vorwärts, spitz’. P. 144
Fig. 1.
The first lithograph of the fusiform (γ) and lingual (β) gyri in humans from Huschke (1854). Image courtesy of Stanford Medical History Center (http://lane.stanford.edu/med-history/index.html).
“More external from the lingual gyrus follows the fusiform lobule (Lobulus fusiformis) – as I call it – which is a gyrus with a sharp beginning and end. This gyrus has a variable length (2–3″ long) and various transverse grooves or insular-like indentations. Its posterior end reaches the inferior end of the two concentrically interposed loops of the posterior branch of the cuneus. From there, the fusiform gyrus runs anteriorly, becomes wider, and sharply ends sooner or later in the convolutions of the temporal lobe, sometime at the end of the opposite hook-like convolution, sometimes 1″ more rostrally”.
Immediately relevant is that even in this first labeling of the FG, Huschke highlighted the fact that there were sulci along the length of the FG. It is equally as important to highlight that Huschke did not label such sulci, but did label the sulcus in the neighboring lingual gyrus (LG), which he found more prominent. He writes:
‘Auf den Zwickel, weiter nach aussen, folgt hierauf der von mir zungenförmiges Läppchen (Gyrus s. lobulus lingualis) genannte Theil. Er ist hinten und vorn spitz, in der Mitte seiner Länge ½″ breit und hier mit einer verschieden gestalteten flachen Längenfurche (Sulcus lobuli lingualis) versehen. Sein hinterer Anfang ist die Fortsetzung der im hinteren Aste des Zwickels eingeschachtelten zweiten Schlinge, die sich nun als Spindel-läppchen gerade vorwärts fortsetzt. Seine vordere Spitze hin-gegen endet 1″ unter dem Anschlusse des Vorzwickels, dem Corpus geniculatum internum gegenüber im bogenförmigen Rande des Bogenwulstes, nachdem er nach aussen hin einige kurze, oft strahlenförmige, den Gyri operti ähnliche Aestchen abgegeben hat, die in der tiefen und langen Längenfurche an seiner äusseren Seite sich verlieren’. P. 143–144
“I call this part, which follows the cuneus more laterally, lingual-like lobule (Gyrus s. lobules lingualis). It is narrow at its anterior and posterior ends, ½″ wide in the middle, and shows a variably shaped longitudinal groove (Sulcus lobuli lingualis). Its posterior beginning is the continuation of the second loop nested in the posterior branch of the cuneus, and continues rostrally as the fusiform lobule. Its rostral end reaches 1” below the precuneus and opposite to the medial geniculate body in the arcuate-like rim of the fornicate gyrus, which has given some short, ray-like twigs in exterior direction. The twigs are similar to the Gyri operti, and disappear in the deep and long longitudinal sulcus at its external side”.
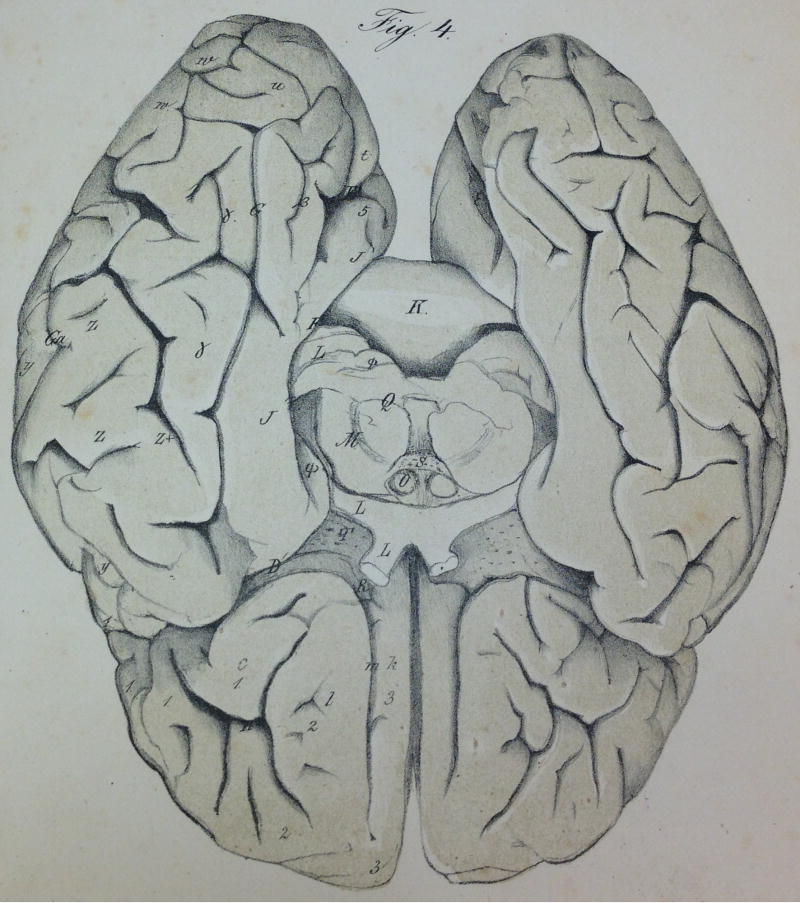
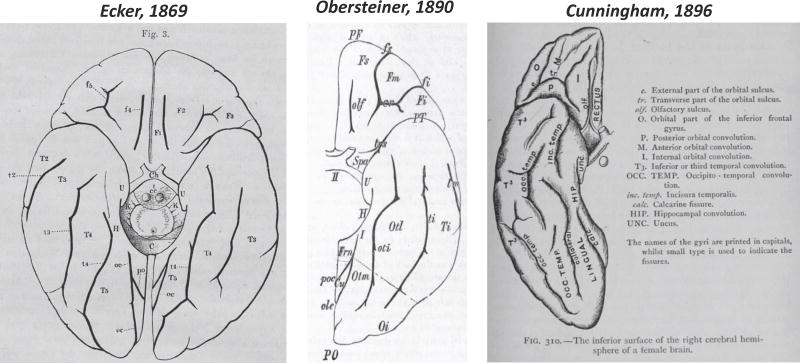
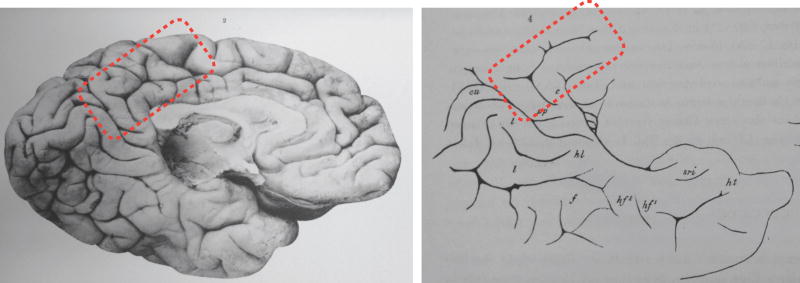
Huschke's atlas is important not only for labeling the FG, LG, and the lingual sulcus, but also for the fact that it was the first to have lithographs (Fig. 1) instead of the commonly used hand-drawn or woodcut schematics. Such vivid visualizations allowed the detailed labeling of the gyri and sulci, which was not common for the time period. The sulcal branches on the FG and the lingual sulcus identified by Huschke often went unlabeled by anatomists. This was largely due to a general confusion regarding the extent of the FG and how many sulci actually existed in the temporal lobe. This confusion can be attributed to the fact that the simplicity of the most widely used schematics depicting gyri and sulci in the temporal lobe did not match the complexity of cortical folding patterns (Fig. 2; Supplementary Table 1). Specifically, the most common approach of the time period was to label the gyri of the temporal lobe with a capital ‘T’ and to label the sulci of the temporal lobe with a lowercase ‘t’. Numbers were then used to refer to each gyrus and sulcus in a superior to inferior dimension (Fig. 2; Supplementary Table 1). Thus, the superior temporal gyrus (STG) was often referred to as T1 and the sulcus immediately to its inferior as t1. Consequently, the MTG was T2 and the ITS was t2, the ITG became T3 and the OTS, t3, the FG, T4 and the collateral sulcus, t4. And finally, the LG was T5. Thus, based on such schematics, there was only room for five gyri and four sulci in the temporal lobe, which left no room for additional macroanatomical structures. To add to the confusion, not all atlases had the same nomenclature or schematics (Fig. 2 for three such examples from Cunningham (1896), Ecker (1869), Obersteiner (1890).
Fig. 2.
Widely used schematics in the late 19th century depicting gyri and sulci in ventral temporal cortex. Left to right: Ecker (1869), Obersteiner (1890), and Cunningham (1896). Note the simplicity of the schematics compared to the complexity of the cortical folding patterns in Fig. 1.
Interestingly, this confusion of gyral and sulcal definitions among anatomists resulted in the first appearance of a sulcus in the FG. To our knowledge, the Fissura fusiformis first appeared in 1876 in a journal article by Carl Wernicke (Fig. 3; Supplementary Table 1). Specifically, Wernicke labeled the fusiform fissure in humans and several primate species (Wernicke, 1876). After further inspection, it is clear that Wernicke's Fissura fusiformis is actually the occipito-temporal sulcus. This correction was already made in 1881 by Benedikt in his examination of the brains of criminals (Benedikt, 1881).
Fig. 3.
The first images of Fissura fusiformis (f) in humans from Wernicke (1876). Left: fetal brain. Right: adult brain.
Just fifteen years later, the modern definition of the MFS surfaced in an atlas by Gustaf Retzius (Retzius, 1896). For the first time, Retzius describes the Sulcus sagittalis gyri fusiformis with nearly the exact definition used by modern authors (Nasr et al., 2011; Nobre et al., 1998; Puce et al., 1996; Puce et al., 1999; Weiner et al., 2014; Weiner and Grill-Spector, 2010, 2013; Weiner et al., 2010). Retzius writes:
‘Die untere Fläche des Temporallappens ist bekanntlich von vorn nach hinten ausgehöhlt; dies gilt ganz besonders von dem Gyrus fusiformis. An dieser ihrer Fläche lässt sich sehr oft längs der Mittellinie eine sagittale Furche, die Sulcus sagittalis gyri fusiformis heissen mag, nachweisen. Diese Furche kann zuweilen einheitlich und eine weite Strecke verfolgbar sein, doch ist sie öfter in zwei oder mehrere Furchenstücke zerklüftet, welche auch verästelt und mit den Nachbarfurchen vereinigt sein können, wodurch das Furchenbild der Windung compliciert wird; wenn aber der Sulcus sagittalis rein und stark ausgeprägt ist, zerfällt ihre Oberfläche in zwei parallele, sagittale Windungen, die Gyrus medius und lateralis, welche, bald ohne Verbindung, bald durch Brücken vereinigt, weit nach hinten verfolgbar sind, bis über die Mantelkante hinaus treten und in der einen oder anderen Weise mit den Windungen des Occipitallappens, dem Gyrus temporalis inferior und dem Gyrus lingualis, Verbindungen eingehen’. P. 142
“It is known, that the inferior surface of the temporal lobe is concave in anterior–posterior direction; this is particularly true for the fusiform gyrus. A sagittal sulcus along the midline can be very frequently found on its surface, which might be called Sulcus sagittalis gyri fusiformis. This sulcus can be uninterrupted and visible for a long distance, but is often subdivided into two or more parts, which may have branches and join neighboring sulci, which leads to a complicated pattern of the gyrus; if, however the Sulcus sagittalis is clearly visible and well developed, the surface of the gyrus can be subdivided into two parallel, sagittal convolutions, the Gyrus medius and lateralis, which are found separated in some cases or connected by bridges in other cases. These gyri can be followed far posterior, where they extend over the edge of the hemisphere and merge with the convolution of the occipital lobe, inferior temporal gyrus, and lingual gyrus in one or the other way”.
Thus, in 1896, Retzius eloquently defined a longitudinal sulcus bisecting the FG into lateral and medial partitions. This definition was buried within a several-hundred page atlas. And surprisingly, Retzius only described the MFS in the text, but not in the figures, which may have contributed to it being overlooked. Nevertheless, Retzius' collection of human brains was exemplary. Based off of the large sample size of his collection and clear photographs (Fig. 4), it is not surprising that he was able to clearly define this sulcus with a modern definition.
Fig. 4.
Photographs of the medial surface of the human brain from Retzius (1896). The collection of photographs from Retzius' atlas is exemplary. Given the large extent of brains, of which four are included here, it is unsurprising that he described the sulcus sagittalis gyri fusiformis for the first time. The outline of the fusiform (in white) has been added. Images courtesy of Stanford Medical History Center (http://lane.stanford.edu/med-history/index.html).
Just one year later, Mickle also referenced this sulcus by yet a different name in the Journal of Mental Science (Mickle, 1897). Specifically, Mickle describes the chaos of the temporal sulci in relation to the FG, this time referring to the MFS as the ‘intra-gyral sulcus of the fusiform lobule’. Mickle writes:
‘The t3 is sometimes a very well marked sulcus, and more so than the t2 (and therefore its furrow-connections more important). But in some cases the t3 is ill-marked. This is particularly apt to occur when there is a seeming compensation by an unusually developed deep and bold intra-gyral sulcus of the fusiform lobule. Yet in some examples it is exceedingly difficult to satisfy oneself as to the validity of this explanation of the appearances observed; and the alternative possible explanation must be at least kept in mind, and is to the effect that, in such case, the fusiform lobule is abnormally small, and that, after all, the sulcus in question is an irregular and unusually disposed t3’. P. 11–12
Less than a decade later, the expert anatomist Oskar Vogt (Vogt, 1904) offered a different approach to the labeling of sulci in the occipito-temporal lobe. In particular, he used ‘ot’ to indicate occipito-temporal and a number to indicate the number of sulci in this cortical expanse. Vogt's ot corresponds to the collateral sulcus and his ot1 to the modern day occipito-temporal sulcus. Then, ot2, ot3, and so forth were used to indicate additional sulci in this expanse – ot3 is likely the MFS and ot2 likely the lateral branch of the CoS (Supplementary Fig. 1; Supplementary Table 1).
To our knowledge, following 1904, the MFS did not reappear until the early 1950s in separate work by Connolly (1950), as well as Bailey and von Bonin (1951), who both accurately credit Retzius with the discovery. Specifically, Connolly (1950) writes:
‘When the caudal end of the occipito-temporal turns laterally or a larger fusiform gyrus forms by the extent and direction of the collateral (Fig. 47 left hemisphere), a sagittal sulcus is formed, often proceeding from the transverse occipital. This is Retzius' sulcus of the fusiform gyrus. A further variation is seen when this sulcus along with the collateral and the occipito-temporal runs approximately parallel, but this is more frequently seen in the large anthropoids’. P. 61
The latter sentence refers to the fact that Connolly's description is in gibbons, not in humans. Even though Connolly also extensively discusses fissural patterns in humans, he does not again address the MFS in the context of the human brain. Instead, it would be Bailey and von Bonin (1951) the following year who identify the intra-fusiform sulcus in humans:
‘In the fusiform gyrus, i.e., between the collateral and the third temporal sulcus, there may be the sulcus sagittalis gyri fusiformis of Retzius. There may be one straight sulcus, or there may be several smaller sulci, often fusing by oblique or transverse branches with the collateral or the inferior temporal sulcus’. P. 55
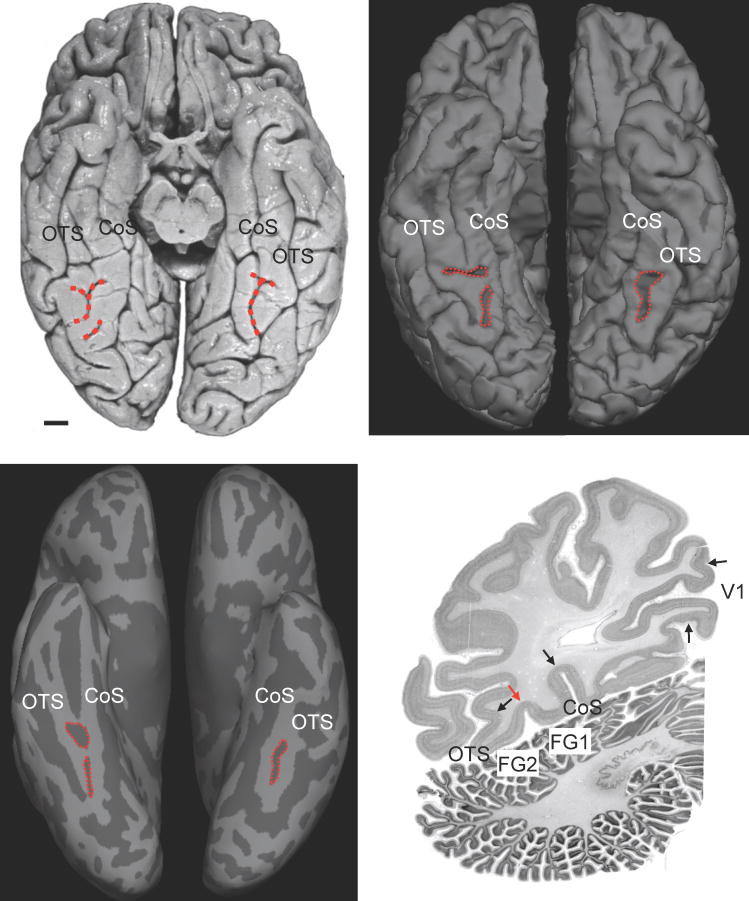
To our knowledge, after 1951, the MFS again disappeared from the literature for almost another half century until 1996 (Supplementary Table 1) and a full morphological analysis of the MFS was not conducted until our work nearly twenty years after (Weiner et al., 2014; Supplementary Table 1). The benefit of modern methods is the ability to examine large sample sizes in both in vivo and post mortem brains. Implementing such an approach, we were able to determine two main morphological types of the MFS based on its fractionation, as well as two subtypes based on intersections with neighboring sulci. Three of these four types are depicted in Fig. 5. The MFS is most often (68.1% of the time) either a single (Fig. 5b-c, left hemisphere) or fractionated (Fig. 5a-c, right hemisphere) longitudinal sulcus distinct from the OTS and CoS. In a minority of cases (31.9%), the MFS shares a sulcal bed with either the OTS (Fig. 5a, left hemisphere) or the CoS. Importantly, despite the different ways which the MFS may appear on the cortical surface – for example, in a post mortem brain freshly removed from the skull compared to a computational reconstruction of the cortical surface – the identifying feature of the MFS is its shallowness relative to the surrounding OTS and CoS. Specifically, the OTS and CoS are more than twice as deep as the MFS. This difference in depth results in an omega pattern on single coronal slices, which is easily identifiable within in vivo magnetic resonance images or post mortem histological sections (Fig. 5d). Importantly, this difference in depth is stable from childhood to adulthood (Weiner et al., 2014) and across research groups (Nasr et al., 2011), indicating that the shallowness of the MFS relative to the OTS and CoS is a reliable macroanatomical landmark identifiable in every brain.
Fig. 5.
A modern view of the MFS. (a) Ventral view of a post mortem brain. Red: MFS. Scale bar: 10 mm. (b) Ventral view of an in vivo brain. (c) Inflated version of the same brain in (b). The MFS has four morphological types, three of which are depicted here (see main text). (d) Cell body stained coronal histological section through the fusiform gyrus. Black arrows indicate outer cytoarchitectonic borders of fusiform areas FG1 and FG2 as well as of primary visual area V1. The red arrow labels the border between FG1 and FG2 in the depth of the MFS. The CoS and OTS are more than twice as deep as the MFS. This difference in depth generates a distinctive omega pattern in single slices despite morphological differences on the cortical surface. CoS: collateral sulcus; MFS: mid-fusiform sulcus; OTS: occipitotemporal sulcus.
Taken together, in a handful of instances (Supplementary-Table 1) prior to 1996, the MFS has been identified throughout history with a variety of different names. From our historical analysis, we agree that Huschke (1854) should be credited with the labeling of the FG, but that Retzius (1896) should be credited with the definitive labeling of the MFS. We suggest ‘MFS’ as a label as it is discriminatory from the surrounding OTS and CoS.
2.2. The fusiform – and perhaps the MFS – is hominoid-specific and not present in all mammals
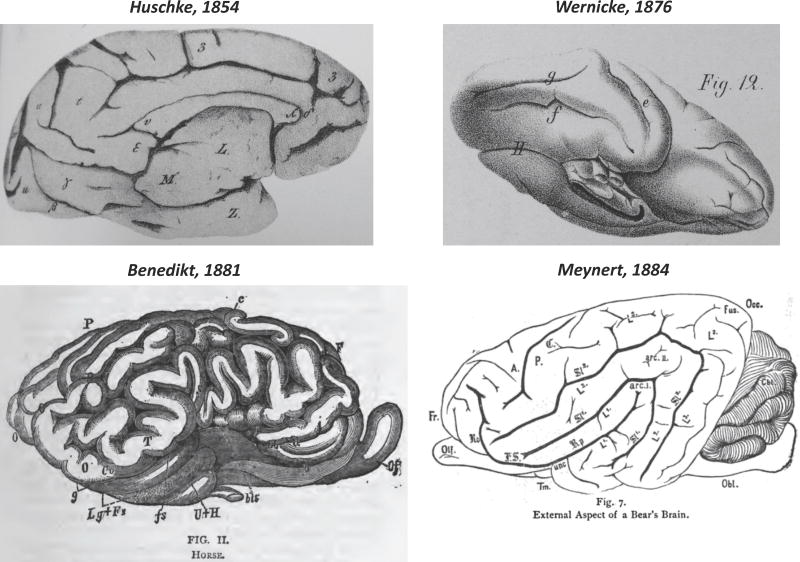
Differences in labeling of the FG and MFS in combination with disappearances and reappearances of these labels in the literature were not the only source of confusion for accurately identifying these macroanatomical structures. As evolution was a fashionable topic during this historical time period, cross-species comparisons exacerbated the confusion of identifying the FG and MFS. Specifically, scientists often attempted to instill the human anatomical organization onto other species despite the fact that (a) the gross organization of the brain was vastly different and (b) the FG is now understood to be a hominoid-specific structure. We highlight this confusion in the section below using images and descriptions of the FG in different mammals generated by well-known neuroanatomists.
The confusion actually began in Huschke's seminal work. In addition to identifying the FG in humans, Huschke also identified the FG in baboons. Nevertheless, he mixed up the location of the FG and LG across species (a common occurring issue which fueled Burt Wilder to re-label the fusiform and lingual gyri as we discuss in the next section). In humans, Huschke identified the FG consistent with its modern definition, which is inferior to the LG. However, in baboons, this topological relationship is inverted where the LG is inferior to the FG (Fig. 6). We believe this to be a mistake and not an intentional difference between baboons and humans. However, as evolution was the major concept of the time period, we believe that further confusions perpetuated as subsequent scientists often attempted to instill the human anatomical organization onto other species. This is a complicated endeavor when the overall organization of the brain looks entirely different – such as in bears and in horses (Fig. 6). And yet, Meynert (1885) and Benedikt (1881) identify the fusiform in bears and horses, respectively. Likewise, Benedikt even went so far as to identify the fusiform sulcus in horses based on Wernicke's definition. To remind the reader, Wernicke believed the fusiform sulcus to be a sulcus immediately inferior to the ITG (e.g. the OTS). Based on this definition, Wernicke identified the fusiform and its associated fissure in macaques, chimpanzees, cebus, and gorillas (Fig. 6).
Fig. 6.
The fusiform gyrus and sulcus in baboons, macaques, horses, and bears. Top: the fusiform (γ) and lingual (β) gyri in a baboon from Huschke (1854). Image courtesy of Stanford Medical History Center (http://lane.stanford.edu/med-history/index.html). The Fissura fusiformis (f) in macaques from Wernicke (1876). Bottom: the fusiform gyrus (Fs and Fus, respectively) in a horse (left) and bear (right). Note that Benedikt also identified the fusiform sulcus (fs) in the horse. Contrary to these identifications, we now know that the FG is a hominoid-specific structure.
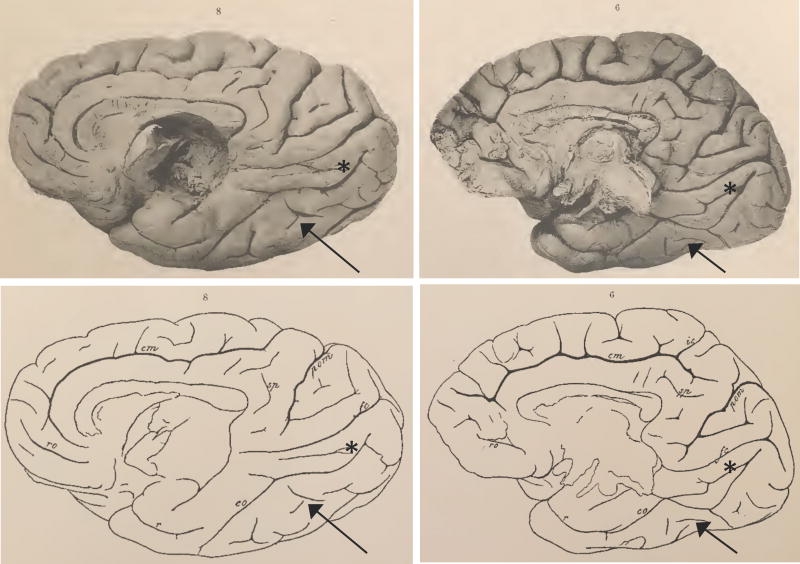
In modern times, however, it is now accepted that the FG is indeed a fourth temporal gyrus specific to hominoids. For example, Nasr and colleagues clearly state that the macaque only has three temporal gyri and ‘do not have a fusiform gyrus’ (Nasr et al., 2011). While the FG is accepted to be hominoid-specific, it is unknown if the MFS is common to all hominoids. Examining images of the inferior surface of the chimpanzee from Retzius (1906), the MFS looks to be present even in non-human hominoids (Fig. 7). Even though Retzius first labeled the MFS in humans (Retzius, 1896), he does not mention this sulcus in the atlas from which this image was taken (Retzius, 1906). In sum, while it was once believed that the FG was common across all mammals, it is now understood that the FG is specific to hominoids. This is important for determining if types of structural–functional coupling associated with functional specialization of the FG such as face-selectivity (Weiner et al., 2014) are specific to humans or general of all hominoids (Parr et al., 2009).
Fig. 7.
The fusiform gyrus in chimpanzee. The medial surface of two chimpanzees (Troglodytes niger) from Retzius (1906). While Retzius only labeled the collateral sulcus (co, bottom), we identify the mid-fusiform sulcus (black arrow). The lingual sulcus is also clearly identifiable (asterisk).
2.3. Wilhelm His vs. Burt Wilder: where did all of the anatomical landmarks go?
Even though there was a lot of variability in nomenclature for the fusiform and surrounding sulci as discussed in the prior sections, we speculate that a large movement to reduce anatomical labels contributed to the MFS being written out of history until the 1950s. Specifically, between 1880 and 1900, there was a large push by Burt Wilder from Cornell to generate rules for naming anatomical structures in order to make it easier for all scientists to remember these names. Wilder was not quiet. He has several Science papers on this subject matter (Wilder, 1881a, 1881b 1896b), the most relevant of which were in 1881 and 1896 (his 1881 paper was titled ‘A partial revision of Anatomical Nomenclature with especial reference to that of the brain’). It is important to stress that naming was an important topic between the mid to late 1800s. Because this time period was revolutionary for the exploration of the brain, researchers would identify the same structure with (a) different names in the same language, (b) similar names in different languages, and (c) by eponyms. As anatomical parcellations for the brain were often described in practical atlases for surgeons and medical students, it was an important goal to produce an internationally understood nomenclature not only for brain structures, but also for the human body in general. Such a universal nomenclature would produce the least amount of terms for researchers and surgeons to memorize. Though there was general agreement and enthusiasm behind Wilder's intention to generate a truncated list of neutral terms in order to make the life of scientists and surgeons easier by minimizing the amount of terms they needed to memorize, perhaps Benjamin Wheeler, one of Wilder's colleagues, said it best in response to Wilder's proposed list of changes to neural terms:
‘The last thing an older teacher wants is a new set of terms for familiar objects’. (Wilder, 1896, p. 237).
Consistent with this sentiment, the different ideas and opinions of neuroanatomists generated resistance rather than full acceptance of a universal nomenclature. Due to this contention and the general importance of nomenclature, Wilder's 1881 article received space in two different issues of Science with an introduction by the editor.
In addition to Wilder, Wilhelm His from Germany also got involved and started the Basel Anatomical Nomenclature (BNA; Barker, 1907; His, 1895). Between 1888 and 1895, the BNA cut the list of 30,000 names of anatomical structures (including eponyms and synonyms) to 4500 (Barker, 1907; His, 1895). When atlases were published after 1895, they started to include these terms in the beginning of the atlas (e.g., Wilder's terms in one column with BNA terms in another for comparison). Huschke's fusiform and lingual labels were points of contention. The BNA sided with Huschke, while Wilder desired Latin terms that were less descriptive, or ‘simile’ in nature as he describes. Specifically, buried within a 136 page paper on the subject matter (Wilder, 1896a, 1896b), Wilder writes:
‘Gyrus subcollateralis and G. subcalcarinus. – So slight is the resemblance of these cortical strips to the forms indicated in the commonly accepted simile names, fusiformis and lingualis, that I have never been able to remember their relative locations. It seems probable that the fissural names calcarine and collateralis are to persist. If so, is it not both logical and convenient to designate the gyres just ventrad of them by locatives indicating their positions, viz., G subcalcarinus and G. subcollateralis?’ P. 322
This attempt to rid the macroscopic nomenclature of the fusiform and lingual labels was again tried three years later in the same journal. The article was written by the secretary of the Association of American Anatomists, D.S. Lamb (Lamb, 1899). Nearly the same description is written except now the naming convention of fusiform and lingual is likened to a ‘puerile mnemonic device’.
‘Gyrus subcalcarinus (60) and Gyrus subcollateralis (61). These terms are recommended in place of “Gyrus lingualis” and “Gyrus fusiformis” respectively. The difficulty of applying the latter is well-known; indeed, so vague are the resemblances implied in them that certainty can hardly be insured without resort to the rather puerile mnemonic device of associating the letter n in calcarine and lingualis. But since Fissura calcarine and Fissura collateralis are now almost universally employed, and no new words have to be introduced, there seem to us to be several advantages and no disadvantages in designating the gyri just ventrad of the two fissures respectively by terms indicating their relative positions’. P. 49-50
Based on Wilder's own accounts (Wilder, 1896a, 1896b), he started using these terms in an 1885 conference proceedings (Wilder, 1885) with the first images the following year (Wilder, 1886; Fig. 8a). This subcollateral and subcalcarine nomenclature for the fusiform and lingual gyri, respectively, were used by other neuroanatomists mostly in the United States, appearing in both textbooks (Mills, 1898) and journal articles (Spitzka, 1907; Fig. 8b).
Fig. 8.
Wilder's subcollateral gyrus. (a) Schematic illustration from Wilder (1886). (b) Example brain from Spitzka (1907) also using the subcollateral (SBCLT G.) and subcalcarine (SBCLC G.) nomenclature.
Nevertheless, the BNA labels of fusiform and lingual were more widely accepted than Wilder's suggestions. Due to this global movement to improve the clarity of anatomical nomenclature, subsequent volumes of anatomical atlases would only have the edits to the BNA terms as they continued to meet and edit these terms. We speculate that cutting the list of 30,000 anatomical terms to 4,500 required focusing on the major structures agreed upon by most anatomists. Thus, the MFS, as well as important white matter pathways such as the vertical occipital fasciculus (Yeatman et al., 2014), which also has quite a convoluted history, were not on this list because they were considered more ‘minor’ in nature, and consequently, more easily dispensible.
2.4. Are there other forgotten landmarks throughout the brain?
As our present purpose is to link anatomical aspects of specialization (e.g. cortical folding and cytoarchitecture) to examples of functional specialization (e.g. face-selectivity and expertise) within high-level visual cortex, we focus on the historical contentions of the FG and MFS. Nevertheless, it is important to ask: Is the historical confusion described in the present paper specific to the MFS or applicable to other parts of the brain?
We speculate that the MFS is not an outlier, but that this confusion is likely applicable to other portions of the brain for two main reasons (aside from the historical movement to reduce the number of anatomical labels by Wilder and His, which was a movement that obviously influenced all portions of the brain). First, historically, minor anatomical features that were considered less replicable from one hemisphere to the next were excluded from summary schematics and discussions in atlases. Three such features are: (1) rami, or branches, of known sulci, (2) sulculi which was a term used to refer to furrows that were ‘inconstant’ and thus, seemingly irreplicable from one hemisphere to the next, and (3) gyruli, which like sulculi, was a term used to refer to small, inconsistent gyri. Sulculi is a label that Parker (1896) credits to Pansch (1866), while, to our knowledge, gyruli may have appeared first in Retzius' (1896) atlas. Only rami is still commonly used today. We bring up this point because the anatomists of the time were limited by their methods and rather small sample sizes. As such, it was complicated to accurately identify tertiary sulci just from the outer surface of the cerebrum in post mortem brains. Since the anatomists in the 19th century had a hard enough time agreeing on the definition of primary fissures as discussed in the prior section, labels for smaller tertiary sulci or gyri were simply not attempted and were neither included in summary schematics nor commonly discussed at length in atlases.
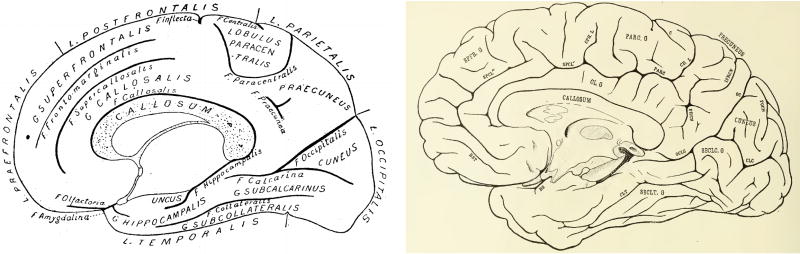
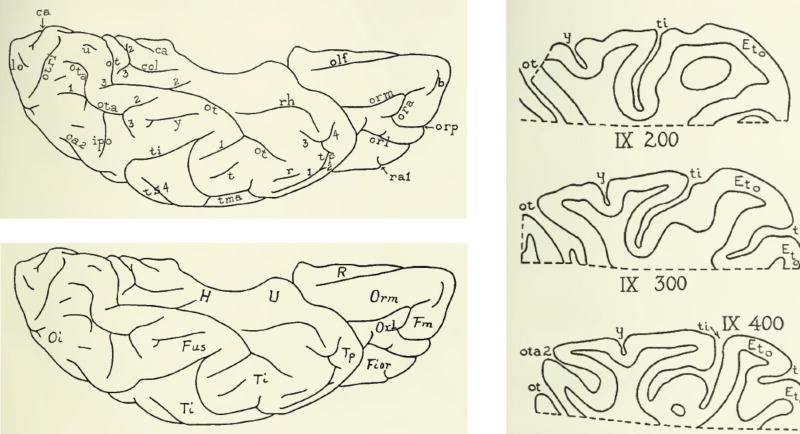
Second, while these schematics (such as those in Fig. 2) are supposed to encapsulate the consistencies of sulcal and gyral patterns across brains, the simplicity of schematic drawings minimizes the complexity of the true cortical folding patterns. Consequently, there are inconsistencies between the ‘raw’ data (e.g. photographs) and the summary (e.g. handdrawings). Take for example Fig. 9. Both images show the medial view of a left hemisphere – one is the photograph and the other is a handdrawn schematic from Retzius (1896). What should be obvious to the reader is the fact that there are clear discrepancies between the two. We highlight the main difference with the red rectangle. But, there are other issues – for example, shallow sulci (likely considered sulculi) are excluded from the schematic, the length of certain sulci are underestimated, and while the global sulcal pattern is coarsely preserved, the specifics are vastly under-represented. Thus, due to inaccuracies occurring in translation from photograph to drawing, we speculate that other tertiary sulci in addition to the MFS may be missing from present atlases.
Fig. 9.
The eye of the beholder in observer-dependent neuroanatomy. Left: a photograph of the medial surface of a left hemisphere from Retzius (1896). Right: a drawing of the photograph at left. Red rectangle indicates the clearest discrepancy between the observer-dependent schematic (right) and the true photograph (left). We encourage the reader to compare and contrast the other differences between the two images. Due to inaccuracies occurring from photographs to drawings, we speculate that many other tertiary sulci in addition to the MFS may be missing from present atlases. Images courtesy of Stanford Medical History Center (http://lane.stanford.edu/med-history/index.html). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
It should be noted that morphological features of primary sulci may also be under-represented in these atlases. Two immediate examples have been clarified recently within the high-level visual cortex of the occipito-temporal lobe. On the ventral surface of the hemisphere, the morphology of the collateral sulcus was recently clarified (Huntgeburth and Petrides, 2012). Specifically, the CoS was divided into three different partitions. Likewise, laterally, three clear morphological parcellations of the superior temporal sulcus have been proposed (Segal and Petrides, 2012). Whether or not these morphological parcellations have been proposed already throughout history and if these parcellations are cytoarchiteconically and functionally relevant like the MFS are open questions. As with our examination of the MFS, the clarification of the CoS and STS were uncovered by meticulously examining hundreds of hemispheres. To ensure that history is not repeated, the only way for these new morphological features to be generally accepted and remembered is through replication and standardized methods for identification. The chance to scan hundreds and even thousands of brains in the Human Connectome Project (humanconnectome-project.org) and additional data sharing and scientific transparency projects (scitran.stanford.edu) collectively offer opportunities to do just that and potentially clarify or uncover the macroanatomical structure of additional sulci and gyri that may be presently excluded from classical and modern atlases.
2.5. Classical cytoarchitectonic studies challenge the value of macroanatomical structures as reliable landmarks for localization of borders of cortical areas
The advent of cytoarchitectonical exploration may have also contributed to the omission of gyri and sulci because it diverted interest from the outer surface of the cerebrum to the cellular organization of the cortical ribbon (Meynert, 1885; Flechsig, 1896; Campbell, 1905; Brodmann, 1909; Vogt and Vogt, 1919; von Economo and Koskinas, 1925). For example, during the same time period as the controversy between His and Wilder, the concept of architectonic brain mapping began to gain momentum. Though different methods for brain mapping were used (cytoarchitecture: Meynert (1885), Campbell (1905), Brodmann (1909), von Economo and Koskinas (1925); myeloarchitecture: Vogt and Vogt (1919); myelogenesis: Flechsig (1896)), the goal was the same: to travel along the cortical ribbon and to identify boundaries between areas that differ in cellular or fiber structure, which were considered to be indicative of potential differences in function. The motivation of their endeavors was admired by the best anatomists of the time and hints of the His/Wilder debate still remained. His pushed hard and generated the first Commission of Brain Research, which consisted of 35 members representing 14 nationalities to normalize methodologies and to induce potential uniformity in the presentation of results. He died three weeks prior to the inaugural meeting of this commission. A prominent neuroanatomist, Elliot Smith, reflected on these details in a 1905 issue of The Journal of Comparative Neurology and Psychology (Smith, 1905). It is in this very article where Elliot Smith reflects on the fact that they still held the inaugural meeting despite His' death in order to discuss prevalent issues of the time. One of those issues was the idea of brain maps and correspondence of areal boundaries with sulci. Smith writes:
‘…In time it will probably be possible to describe all the important furrows of the hemisphere in terms of their relationship to certain definite cortical areas and so to correlate the data of morphology and physiology. The excellent researches of Dr. A. W. Campbell of Liverpool and the well-known work of Professor Flechsig are rapidly preparing the way for such an advance.
In the discussion of this matter, in which Professors Henschen, Retzius, von Monakow, Edinger and Langley took part, it was agreed that it was too early to adopt the proposed method of describing sulci.’ P. 64
This means that Retzius, who was the first to label the MFS, was in that very room agreeing that the topographic organization of sulci was complicated and far from being understood, and thus, was not fighting for his approach of labeling tertiary sulci such as the MFS. In addition to the complicated understanding of the gyral and sulcal definitions of the human brain, enthusiasm for identifying gyri and sulci was further depleted by the fact that there was often not a 1:1 mapping between cytoarchitectonic parcellations and sulci throughout the brain. Bailey and colleagues (Bailey et al., 1950) specifically point fingers at Brodmann and other neuroanatomists between 1905 and 1915 for this very reason. Bailey et al. write:
“About the turn of the century the advent of cortical cytoarchitecture tended to devaluate the gross configuration of the cortex, particularly when Brodmann (1906) and Ariens-Kappers (1913) practically denied a correlation between sulci and cortical areas. Hence, we possess comparatively few modern studies of cerebral sulci”. P. 15.
Thus, the combination of the His/Wilder movement to deemphasize minor anatomical structures in combination with the fact that sulci were not very good predictors of observer-dependent microarchitectural features of the cortex diverted attention from the outer surface of the brain to the inner architecture. In the process, discoveries of more minor anatomical structures, such as the MFS, were lost.
3. From poetics to statistics: evolving from visual inspection and verbal descriptions to observer-independent metrics of cytoarchitectonics
By pure visual inspection of histologically stained sections, the cytoarchitecture of the FG was considered rather uniform. Specifically, Brodmann (1909), von Economo and Koskinas (1925), and Sarkisov (1949) were only able to identify one or two cytoarchitectonically different areas on the FG. Though they did not have more luck seeing additional cytoarchitectonic differences of the FG, Bailey and von Bonin (1951) were the first to explicitly examine the cytoarchitectonic organization of the FG with respect to the MFS (Fig. 10). While they did not include any stained histological sections of the FG and instead, just hand-drawn schematics, such drawings of their intra-fusiform sulcus tightly match the omega pattern we identified over sixty years later (Weiner et al., 2014; Fig. 5), which discriminates the MFS from the surrounding CoS and OTS. Nevertheless, though they incorporate the MFS into their cytoarchitectonical analysis of the FG, they were still limited by the methods of the time, which largely depended on descriptive accounts of what they saw within the structure of the cortex. For example, Bailey and von Bonin could not see a difference in lateral and medial portions of the MFS. Here is a direct relay of that account:
Fig. 10.
Observer-dependent methods could not cytoarchitectonically dissociate the lateral from medial fusiform. Left: schematic illustration of gyri and sulci from Bailey and von Bonin (1951). They write: ‘Short, isolated dimples and sulci are given letters a–z.’ The MFS is dimple y, or the intra-fusiform sulcus (Supplementary Table 1). Right: schematic of histological sections (inferior is at the top of the image). Note that their depiction of the intra-fusiform sulcus displays a similar shallowness relative to surrounding sulci that we have previously described (Weiner et al., 2014). For cytoarchitectonic description, see main text.
‘Section 400. Fig. 56. The cortex over the fusiform gyrus measures 1.54mm; it is the same on both sides of the intrafusiform sulcus (y). It has a patchy appearance. Layer iv is built like a rail fence with iiic and v projecting alternately into it from either side. The border between i and ii is fairly smooth’. P.132
Other descriptions of the cortex are included as ‘moth-eaten’ to describe patchy appearances of clumps of cells. Consequently, there was a large need to evolve from poetic descriptions of what the anatomist saw in each histological section to observer-independent approaches that determined cytoarchitectonic boundaries in an unbiased and statistically-testable fashion.
The introduction of quantitative analyses of Nissl-stained histological sections was an absolute requirement to realize such approaches. However, this was not feasible during the first half of the 20th century due to a lack of efficient hardware and methods to automatically segregate the cell bodies from the “background” (i.e., neuropil) in the histological sections and measure their volume proportion per unit nervous tissue at sufficient speed and spatial resolution. The definition of the stereologically defined “gray cell coefficient” was an important and excellent first step to quantification of nervous tissue components (Haug, 1956), but it required a laborious time consuming procedure which is based on microscopic inspections by an observer and does not enable the definition of cytoarchitectonic borders between cortical areas.
A solution to this time constraint was achieved through the automatic segmentation of cell bodies by Schleicher and Zilles in a series of publications (Schleicher and Zilles, 1990; Schleicher et al., 1986; Zilles, 1978; Zilles et al., 1978; Zilles et al., 1980). This progress became possible only with the introduction of automated image analysis of nervous tissue (Ahrens et al., 1990; Istomin and Shkliarov, 1984; Rauch et al., 1989). The availability of hardware, e.g. image analyzers and the first affordable lab computers, and the development software, as well as the application of mathematical morphology (Istomin and Amunts, 1992; Serra, 1986), enabled the rapid measurement of tissue properties at relatively high spatial resolution (Schleicher and Zilles, 1990; Schleicher et al., 1986). The establishment of the “gray level index (GLI)” as a reliable estimate of cell body packing density (Wree et al., 1982) was a major step forward, since it provided a very fast and automated approach to the quantification of the major feature of cytoarchitecture, i.e. the regional-, laminar-, and cell column-dependent variation of cell packing density throughout the cortical ribbon (Amunts et al., 1995; Schlaug et al., 1995; Schleicher et al., 1999; Schleicher et al., 2005; Zilles et al., 1986).
In the following years, the development of a computer-controlled scanning procedure and of adequate statistics to determine localized transitions in cytoarchitecture independent from the pattern recognition abilities of the observer was the next and final crucial step for the development of the required observer-independent and statistically testable parcellation of the cerebral cortex based on Nissl-stained histological sections. Specifically, in stark contrast to the qualitative representation of observer-dependent cytoarchitectonics, such a methodological development enabled a quantitative representation of cytoarchitectonics for the first time. In this methodology, cytoarchitecture is represented as GLI profiles running vertical to the cortical surface from the layer I/II border to the border between layer VI and the white matter. Thus, each profile represents increasing and decreasing cell packing density of the different cortical layers, their widths, and the sharpness of the transition in cell density between the layers (Fig. 11). All these cytoarchitectonic features are represented in each profile, and can be described as a 10-parametric feature vector. By automatically moving the profile registration along the cortical ribbon, it becomes evident that blocks of profiles seem to be more similar for some distance, and then change to a different level of similarity. The distances between each adjacent block of profiles are quantified as Mahalanobis distances (Mahalanobis et al., 1949), and can be registered as a Mahalanobis curve along the cortical ribbon. The maxima of the Mahalanobis curve can then be tested for significant changes, which indicate a completely observer-independent detection of cytoarchitectonic borders between cortical areas (Schleicher et al., 1998; Schleicher et al., 1999, 2005). This parcellation of a cortical area is then performed in adjacent sections of the same brain as well as in sections through other brains to study reliability, reproducibility, and intersubject variability of size and extent of cortical areas. The results can be registered as volume data sets and visualized as cortical maps on 3D reconstructed brains. The first applications to the cytoarchitectonic parcellation of the Broca region and the primary and secondary visual cortex were published by Amunts and colleagues (Amunts et al., 2000; Amunts et al., 1999). For a review of this paradigm shift in cytoarchitectonic analysis see Zilles and Amunts (2010).
Fig. 11.
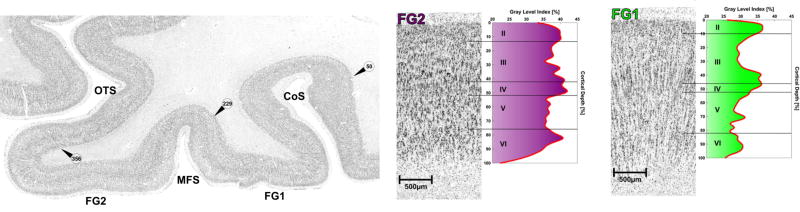
Observer-independent methods cytoarchitectonically dissociate the lateral from medial fusiform. Left: cell body stained histological coronal section through the fusiform gyrus of an adult brain. Arrowheads with numbers indicate the observer-independent positions of the cytoarchitectonically defined borders between FG1 and FG2. This observer-independent approach identifies the boundary within the MFS. From Weiner et al., 2014. Right: cytoarchitecture of areas FG1 and FG2 with the corresponding GLI profiles. Roman numerals indicate cortical layers. FG2 illustrates a greater neuronal density than FG1. FG1 displays a columnar structure that FG2 does not. From Caspers et al. (2013).
By comparison to this new approach, the classical cytoarchi-tectonic maps were most commonly a schematic parcellation of the cerebral cortex, which neglected the intersubject variability of the extent of cortical areas (e.g., Brodmann, 1909; von Economo and Koskinas, 1925). The observer-independent methodology generates maps based on measurements of cytoarchitectonic features and statistically identified definitions of borders separating cortical areas that are always based on the analysis of ten or more brains to achieve an impression of the intersubject variability. To achieve this goal, the maps of the single brains are registered to a common reference brain, e.g. the single subject MNI brain (Evans et al., 1992), and visualized as probability maps (e.g., Amunts et al., 1999, 2000; Amunts et al., 2007; Caspers et al., 2006, 2013; Bludau et al., 2014; Grefkes et al., 2001; Malikovic et al., 2007; Palomero-Gallagher et al., 2008, 2015; Scheperjans et al., 2008; Zaborszky et al., 2008) in order to accurately delineate the macroanatomical location of the cytoarchitectonic region shared across subjects. Thus, altogether, the evolution of cytoarchitectonic methods simultaneously pushed toward both observer-independent approaches, as well as assessments of intersubject variability – each of which were not possible with the classic approaches.
3.1. Cytoarchitectonically defined borders match macroscopical landmarks
Despite the above mentioned statements in previous sections regarding the mismatch between architectonically defined borders of cortical areas and macroscopical landmarks (Brodmann, 1909; Vogt and Vogt, 1919), the attempts to challenge this general verdict never ceased. Fischl and colleagues (Fischl et al., 2008) provided an analysis of the relationships between cytoarchitectonic maps of primary visual cortex (V1) and sulcal patterns. Additionally, several other studies analyzed the correspondence between motor areas, somatosensory area 2, as well as areas 44 and 45 of the Broca region and sulcal patterns (Amunts et al., 1999, 2000; Geyer et al., 1996; Grefkes et al., 2001; Rademacher et al., 2001; Zilles et al., 1996, 1995). Together, these studies found that sulci are better predictors of cytoarchitectonic borders than had been previously assumed, and multimodal areas (44, 45) vary more with regard to the sulcal pattern than unimodal sensory areas (e.g., V1 or V2).
Directly applicable to the FG, there is a correspondence between macroanatomical landmarks and cytoarchitectonic transitions. For example, our most recent clarification of the morphological patterns of the MFS revealed a tight correspondence between cytoarchitectonic transitions and sulcal patterns in high-level visual cortex (Weiner et al., 2014; Figs. 11–12). Specifically, Caspers and colleagues (Caspers et al., 2013) identified two regions in the posterior FG: FG1 and FG2. Blind to these cytoarchitectonic parcellations, the MFS was then defined in each of these brains. Contrary to the observer-dependent approach by Bailey and von Bonin (1951) as described in a previous section, this observer-independent approach revealed that this cytoarchitectonic transition occurred within the MFS (Fig. 11).
Fig. 12.
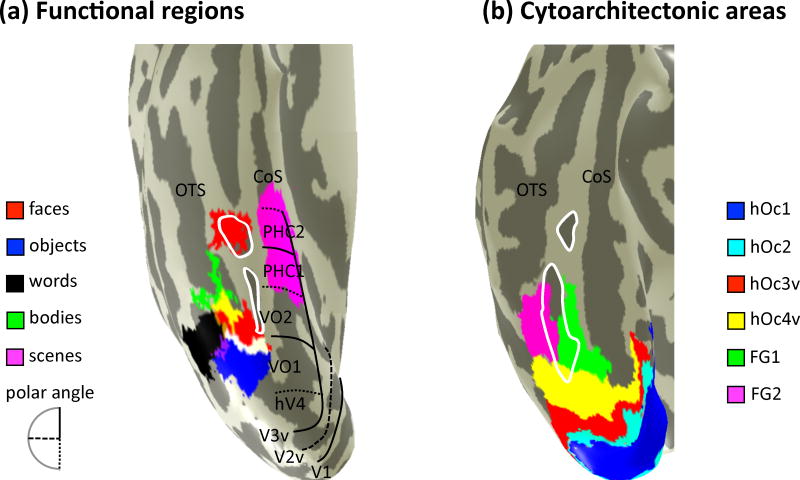
The relationship between functional and cytoarchitectonic organization of ventral occipito-temporal cortex. (a) Cortical surface reconstruction of an example living subject. Colors indicate category-selective regions (see legend). Lines indicate visual field maps. Yellow: overlap between face- and body-selective regions. White: overlap between face- and object-selective regions. Purple: overlap between word- and object-selective regions. (b) Cortical surface reconstruction of an example post mortem subject. Colors indicate cytoarchitectonic areas. White: MFS. See main text for hypotheses of how functional regions relate to cytoarchitectonic areas.
While it is anatomically interesting if a cytoarchitectonic transition coincides with a particular sulcus (Rakic, 2009; Van Essen, 1997; Zilles et al., 2013), it is even more interesting if this co-occurrence is functionally meaningful. This is also theoretically meaningful given the classic hypothesis that a cytoarchitectonic area is representative of a functional unit of the brain that performs specific neural computations (Brodmann, 1909; Vogt and Vogt, 1919). Recent findings show that this hypothesis is more complicated than once thought. Specifically, present findings show that cytoarchitectonic areas may not only perform particular functions, but may also be involved in many different computations and contain more than one functional area (Grill-Spector and Weiner, 2014; Van Essen et al., 2012). In Appendix A, we summarize the functional heterogeneity of cytoarchitectonically defined FG regions using a multitude of approaches. In the section below, we use the cytoarchitectonic organization of the human FG to make sense of the functional heterogeneity of high-level visual cortex. Specifically, we propose four alternatives for how the neuronal organization across the six layers of the FG can accommodate seemingly different functional processes – such as face processing and perceptual expertise.
4. Four alternatives for how the anatomical construction of the FG may contribute to different functional processes
A hypothesis implicitly considered by many is that there is a 1:1 mapping between a cyoarchitectonic area and a particular function. However, with an increasing amount of identified functional multimodal and anatomical areas, as well as variants therein such as zones, clusters, globs, blobs, and modules as eloquently described by Van Essen and colleagues (Van Essen, 2003; Van Essen et al., 2012), there is not such a simple 1:1 mapping between a cytoarchitectonic area and a functionally-defined region. To capture the complex relationship between anatomical and functional specialization, we propose a series of alternatives for the relationship between anatomical construction and functional processing of the FG using cytoarchitectonic areas as an example. Three structural–functional alternatives are explained with either a known example or a testable hypothesis for future studies.
Different area, different computation across domains. This alternative is consistent with the classical hypothesis of a 1:1 relationship between cytoarchitecture and function as well as newer functional hypotheses such as domain specificity. For example, FG2 and FG1 are located on lateral and medial sides of the MFS, as are face- and place-selective areas, respectively (Fig. 12). Thus, it is likely that face- and place-selective, as well as other large-scale functional domains displaying a similar lateral-medial cortical layout in VTC (Grill-Spector and Weiner, 2014), are cytoarchitectonically dissociable – a dissociation which likely reflects different computations underlying domain-specific processing.
Different area, different computation within domain. This alternative is based on the fact that several functional areas are composed of anatomically separate components. For example, the fusiform face area contains at least two separate components: pFus-faces/FFA-1 and mFus-faces/FFA-2 (Fig. 12). As FG2 is located in the lateral aspect of the posterior FG, it is likely that only one of these functional regions, pFus-faces/FFA-1, is located within FG2 and the more anterior component, mFus-faces/FFA-2, is located in a different cytoarchitectonic territory, which is not identified up to now. Thus, it is probable that these two fusiform face-selective regions are cytoarchitectonically different and also potentially display separate receptor fingerprints reflective of different underlying functions despite the fact that they are both regions involved in the domain of face processing located on the same macroanatomical structure.
Same area, different computations across domains. This alternative is based on the evidence that domain-specific regions are adjacent to one another. For example, face- and body-selective regions neighbor one another on the lateral aspect of VTC (Fig. 12). Thus, it is possible that these adjacent regions are located within the same cytoarchitectonic area. This would indicate that similar computations common to the cytoarchitectonic territory are performed in these regions irrespective of the functional domain. Other anatomical differences beyond cytoarchitecture may explain why these regions are adjacent in the same cytoarchitectonic area. For example, connectivity or receptor architecture.
A fourth alternative considers a special case in which a functional area is flexible and can perform different computations based on different combinations of neurons across cortical layers. This alternative may explain how domain-specific regions (e.g. the fusiform face area(s)) can also be involved in perceptual expertise. Specifically, the cortex contains six layers. Thus, each functional area has a vast number of combinations (e.g. factorial) of neurons to access when performing specific computations and not all neurons in every cortical layer within a functional cluster may be involved in every computation. For example, in medial aspects of entorhinal cortex in mice, tiled grid cells are superimposed across cortical layers in which layer II contains grid cells that encode only position and deeper layers contain cells with conjunctive properties coding both position and head orientation (McNaughton et al., 2006). Though in a different species and a different anatomical structure, this structural–functional correspondence across cortical layers illustrates the feasibility of our hypothesis.
For the FG, this scenario is appealing because it can resolve seemingly different theoretical findings in humans. Specifically, it is still presently debated if the fusiform face-selective regions are domain-specific or if they are also involved in perceptual expertise more generally (McGugin et al., 2012, 2014; Tarr and Gauthier, 2000). However, both functions are possible in this scenario because either face perception or expertise would rely on different combinations of neurons across cortical layers within face-selective regions – potentially in different patterns of activated cells across layers like the tiling of grid cells in entorhinal cortex as just described.
5. Conclusion
In this paper, we have reviewed the anatomical construction of the fusiform gyrus (FG) beginning with the contentious history of the FG and the mid-fusiform sulcus (MFS). We have further discussed how observer-independent cytoarchitectonic methods revolutionized our understanding of the microarchitecture of the FG and how clarifying the morphological patterns of the MFS set the stage to relate the microarchitectural features of the FG to the functional landscape of the FG as Turner advised nearly 150 years before. We ended with proposing a number of novel alternatives explaining how the anatomical construction of the FG may contribute to different types of functional specialization. These alternatives do not only have implications for testing and resolving theoretical debates for high-level visual processes and their underlying neural correlates, but also have implications for testing the relationship between anatomical and functional specialization in other parts of the cortex.
Supplementary Material
Acknowledgments
This work was supported by 1R01EY02391501A1, the European Union Seventh Framework Programme (FP7/2007-2013) under grant agreement no. 604102 (Human Brain Project), and the Portfolio Theme “Supercomputing and Modeling for the Human Brain” of the Helmholtz Association, Germany.
Appendix A
Examining the functional heterogeneity of cytoarchitectonic areas in the human brain
Three general approaches have been implemented to examine the function of cytoarchitectonic areas in the human brain. Contrary to classic hypotheses (Brodmann, 1909), each cytoarchitectonic area is functionally heterogeneous. We summarize present understanding of this heterogeneity for two areas recently identified in the posterior FG, FG1 and FG2.
The first approach relies on the analysis of regional and laminar neurotransmitter receptor expression patterns using quantitative in vitro receptor autoradiography (Zilles et al., 2002). Since receptors are key molecules of signal transmission, the balance between the densities of the different receptors in each cytoarchitectonically defined area (“receptor fingerprint”) provides insight into the neurochemical mechanisms underlying their functions at the cellular and systemic level (Caspers et al., 2015; Geyer et al., 1996; Morosan et al., 2005; Palomero-Gallagher et al., 2008; Scheperjans et al., 2005; Vogt et al., 2013; Zilles and Amunts, 2009; Zilles and Clarke, 1997; Zilles et al., 2002; Zilles et al., 2004). Caspers et al. (2015) demonstrated that areas FG1 and FG2 significantly differ between each other by the densities of excitatory NMDA, inhibitory GABAA and GABAB, and modulatory M3, nicotinic α4/β2 and 5-HT1A receptors. Therefore, both areas differ not only by their cellular architecture, but also by their receptor fingerprints, which indicates a difference in signal processing between both areas.
The second approach relies on a coordinate-based meta-analysis using meta-analytic connectivity modeling (MACM; Robinson et al., 2010), which is a revised activation likelihood estimation (ALE) technique (Eickhoff et al., 2012; Eickhoff et al., 2009; Laird et al., 2005) also involving the BrainMap database (www.brainmap.org; Fox et al., 2005; Fox and Lancaster, 2002; Laird et al., 2005). Applying this approach to FG1 and FG2 (Caspers et al., 2014, 2013) revealed that both areas are part of a network subserving object recognition, visual language perception, or visual attention. These approaches further suggest that FG1 is a transitional area between early and higher visual cortex, while FG2 is a higher-order cortical area and functionally lateralized. Left FG2 is related to the visual language network in the left hemisphere, while right FG2 is more strongly associated with the face-selective regions on the right FG. Since left–right differences in cytoarchitecture of FG2 have not been found (Caspers et al., 2013), the functional lateralization of FG2 relies on a different connectivity pattern (Caspers et al., 2014).
The third approach uses macroanatomical landmarks to link and map cytoarchitectonic and functional areas to one another in individual subjects (Weiner et al., 2014). Just as the MFS identifies the cytoarchitectonic transition from the lateral to the medial FG (Fig. 11), this sulcus also indicates transitions in several different large-scale functional maps in VTC. For instance, examples of functional representations in VTC are eccentricity bias, retinotopy, real-world object size, conceptual knowledge, and semantics. Each of these functional representations displays a common relationship to cortical folding patterns and the MFS often identifies lateral-medial transitions in these maps (Weiner et al., 2014; Grill-Spector and Weiner, 2014). The common lateral-medial functional division in VTC is likely constrained by cytoarchitectonic differences. For example, the columnar organization of FG1 likely contributes to the many functional representations in medial VTC, while the increased neuronal density of FG2 compared to FG1 likely contributes to the many functional representations in lateral VTC. The MFS also divides face- from place-selective regions, indicating that face- and place-selective regions are likely cytoarchitectonically dissociable. Additionally, the MFS more tightly couples with mFus-faces/FFA-2 than with pFus-faces/FFA-1 (Weiner et al., 2014; Grill-Spector and Weiner, 2014). As pFus-faces/FFA-1 and mFus-faces/FFA-2 are located in different locations relative to the MFS and so are the cytoarchitectonic regions FG2 and FG4 in the lateral FG (Lorenz et al., 2015), it is likely that these face-selective regions are also cytoarchitectonically dissociable.
Footnotes
Appendix B. Supplementary material: Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.neuropsychologia.2015.06.033.
References
- Ahrens P, Schleicher A, Zilles K, Werner L. Image analysis of Nissl-stained neuronal perikarya in the primary visual cortex of the rat: automatic detection and segmentation of neuronal profiles with nuclei and nucleoli. J Microsc. 1990;157:349–365. doi: 10.1111/j.1365-2818.1990.tb02970.x. [DOI] [PubMed] [Google Scholar]
- Allison T, Puce A, Spencer DD, McCarthy G. Electrophysiological studies of human face perception. I: Potentials generated in occipitotemporal cortex by face and non-face stimuli. Cereb Cortex. 1999;9:415–430. doi: 10.1093/cercor/9.5.415. [DOI] [PubMed] [Google Scholar]
- Amedi A, Jacobson G, Hendler T, Malach R, Zohary E. Convergence of visual and tactile shape processing in the human lateral occipital complex. Cereb Cortex. 2002;12:1202–1212. doi: 10.1093/cercor/12.11.1202. [DOI] [PubMed] [Google Scholar]
- Amunts K, Istomin V, Schleicher A, Zilles K. Postnatal development of the human primary motor cortex: a quantitative cytoarchitectonic analysis. Anat Embryol (Berl) 1995;192:557–571. doi: 10.1007/BF00187186. [DOI] [PubMed] [Google Scholar]
- Amunts K, Malikovic A, Mohlberg H, Schormann T, Zilles K. Brodmann's areas 17 and 18 brought into stereotaxic space-where and how variable? NeuroImage. 2000;11:66–84. doi: 10.1006/nimg.1999.0516. [DOI] [PubMed] [Google Scholar]
- Amunts K, Schleicher A, Burgel U, Mohlberg H, Uylings HB, Zilles K. Broca's region revisited: cytoarchitecture and intersubject variability. J Comp Neurol. 1999;412:319–341. doi: 10.1002/(sici)1096-9861(19990920)412:2<319::aid-cne10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Amunts K, Schleicher A, Zilles K. Cytoarchitecture of the cerebral cortex—more than localization. NeuroImage. 2007;37:1061–1065. doi: 10.1016/j.neuroimage.2007.02.037. discussion 1066–1068. [DOI] [PubMed] [Google Scholar]
- Bailey P, von Bonin G. The Isocortex of Man. University of Illinois Press; Urbana: 1951. [Google Scholar]
- Bailey P, von Bonin G, McCulloch WS. The Isocortex of the Chimpanzee. University of Illinois Press; Urbana: 1950. [Google Scholar]
- Barker LF. Anatomical Terminology with Specical Reference to the BNA. P. Blakiston's Son & Co.; Philadelphia: 1907. [Google Scholar]
- Benedikt M. A Contribution to Anthropology, Medicine, Jurisprudence, and Psychology. Wood & Company; New York: 1881. [Google Scholar]
- Bentin S, Allison T, Puce A, Perez E, McCarthy G. Electrophysiological studies of face perception in humans. J Cogn Neurosci. 1996;8:551–565. doi: 10.1162/jocn.1996.8.6.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bludau S, Eickhoff SB, Mohlberg H, Caspers S, Laird AR, Fox PT, Schleicher A, Zilles K, Amunts K. Cytoarchitecture, probability maps and functions of the human frontal pole. NeuroImage. 2014;93:260–275. doi: 10.1016/j.neuroimage.2013.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodmann K. Uber den allgemeinen Bauplan des Cortex pallii bei den Mammaliern und zwei homologe Rindenfelder im besonderen. Zugleich ein Beitrag zur Furchenlehre. J f Psychol, u Neurol. 1906;6:275–400. [Google Scholar]
- Brodmann K. Vergleichende Lokalisationslehre der Großhirnrinde in ihren Prinzipien dargestellt auf Grund des Zellbaues. Johann Ambrosius Barth Verlag; Leipzig: 1909. [Google Scholar]
- Campbell AW. Histological Studies on the Localisation of Cerebral Function. Cambridge University Press; Cambridge: 1905. [Google Scholar]
- Caspers J, Palomero-Gallagher N, Caspers S, Schleicher A, Amunts K, Zilles K. Receptor architecture of visual areas in the face and word-form recognition region of the posterior fusiform gyrus. Brain Struct Funct. 2015;220:205–219. doi: 10.1007/s00429-013-0646-z. [DOI] [PubMed] [Google Scholar]
- Caspers J, Zilles K, Amunts K, Laird AR, Fox PT, Eickhoff SB. Functional characterization and differential coactivation patterns of two cytoarchitectonic visual areas on the human posterior fusiform gyrus. Hum Brain Mapp. 2014;35:2754–2767. doi: 10.1002/hbm.22364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspers J, Zilles K, Eickhoff SB, Schleicher A, Mohlberg H, Amunts K. Cytoarchitectonical analysis and probabilistic mapping of two extrastriate areas of the human posterior fusiform gyrus. Brain Struct Funct. 2013;218:511–526. doi: 10.1007/s00429-012-0411-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspers S, Geyer S, Schleicher A, Mohlberg H, Amunts K, Zilles K. The human inferior parietal cortex: cytoarchitectonic parcellation and inter-individual variability. NeuroImage. 2006;33:430–448. doi: 10.1016/j.neuroimage.2006.06.054. [DOI] [PubMed] [Google Scholar]
- Cohen L, Dehaene S, Naccache L, Lehericy S, Dehaene-Lambertz G, Henaff MA, Michel F. The visual word form area: spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split-brain patients. Brain. 2000;123:291–307. doi: 10.1093/brain/123.2.291. [DOI] [PubMed] [Google Scholar]
- Connolly JC. External Morphology of the Primate Brain. C. C. Thomas; Springfield: 1950. [Google Scholar]
- Cukur T, Huth AG, Nishimoto S, Gallant JL. Functional subdomains within human FFA. J Neurosci. 2013;33(42):16748–16766. doi: 10.1523/JNEUROSCI.1259-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham DJ. In: Manual of Practical Anatomy. second. Pentland YJ, editor. Edingburgh and London; 1896. [Google Scholar]
- Ecker A. Die Hirnwindungen des Menschen. F. Vieweg u. Sohn; Braunschweig: 1869. [Google Scholar]
- Fleschsig P. Gehirn und Seele. Veit u. Co; Leipzig: 1896. [Google Scholar]
- Edinger L. Twelve Lectures on the Structure of the Central Nervous System for Physicians and Students. 2nd. F.A. Davis; Philadelphia: 1891. [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT. Activation likelihood estimation meta-analysis revisited. NeuroImage. 2012;59:2349–2361. doi: 10.1016/j.neuroimage.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp. 2009;30:2907–2926. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans AC, Marrett S, Neelin P, Collins L, Worsley K, Dai W, Milot S, Meyer E, Bub D. Anatomical mapping of functional activation in stereotactic coordinate space. NeuroImage. 1992;1:43–53. doi: 10.1016/1053-8119(92)90006-9. [DOI] [PubMed] [Google Scholar]
- Fischl B, Rajendran N, Busa E, Augustinack J, Hinds O, Yeo BT, Mohlberg H, Amunts K, Zilles K. Cortical folding patterns and predicting cytoarchitecture. Cereb Cortex. 2008;18:1973–1980. doi: 10.1093/cercor/bhm225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleschsig P. Gehirn und Seele. Leipzig: Veit u. Co; 1896. [Google Scholar]
- Fox PT, Laird AR, Fox SP, Fox PM, Uecker AM, Crank M, Koenig SF, Lancaster JL. BrainMap taxonomy of experimental design: description and evaluation. Hum Brain Mapp. 2005;25:185–198. doi: 10.1002/hbm.20141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox PT, Lancaster JL. Opinion: mapping context and content: the BrainMap model. Nat Rev Neurosci. 2002;3:319–321. doi: 10.1038/nrn789. [DOI] [PubMed] [Google Scholar]
- Geyer S, Ledberg A, Schleicher A, Kinomura S, Schormann T, Burgel U, Klingberg T, Larsson J, Zilles K, Roland PE. Two different areas within the primary motor cortex of man. Nature. 1996;382:805–807. doi: 10.1038/382805a0. [DOI] [PubMed] [Google Scholar]
- Glasser MF, Van Essen DC. Mapping human cortical areas in vivo based on myelin content as revealed by T1- and T2-weighted MRI. J Neurosci. 2011;31:11597–11616. doi: 10.1523/JNEUROSCI.2180-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grefkes C, Geyer S, Schormann T, Roland P, Zilles K. Human somatosensory area 2: observer-independent cytoarchitectonic mapping, inter-individual variability, and population map. NeuroImage. 2001;14:617–631. doi: 10.1006/nimg.2001.0858. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Kourtzi Z, Kanwisher N. The lateral occipital complex and its role in object recognition. Vis Res. 2001;41:1409–1422. doi: 10.1016/s0042-6989(01)00073-6. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Weiner KS. The functional architecture of the ventral temporal cortex and its role in categorization. Nat Rev Neurosci. 2014;15:536–548. doi: 10.1038/nrn3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross CG. Brain, Vision, Memory: Tales in the History of Neuroscience. MIT Press; Cambridge: 1998. [Google Scholar]
- Haug H. Remarks on the determination and significance of the gray cell coefficient. J Comp Neurol. 1956;104:473–492. doi: 10.1002/cne.901040306. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Gobbini MI, Furey ML, Ishai A, Schouten JL, Pietrini P. Distributed and overlapping representations of faces and objects in ventral temporal cortex. Science. 2001;293:2425–2430. doi: 10.1126/science.1063736. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Guntupalli JS, Connolly AC, Halchenko YO, Conroy BR, Gobbini MI, Hanke M, Ramadge PJ. A common, high-dimensional model of the representational space in human ventral temporal cortex. Neuron. 2011;72(2):404–416. doi: 10.1016/j.neuron.2011.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson R, Shallice T, Dolan R. Neuroimaging evidence for dissociable forms of repetition priming. Science. 2000;287:1269–1272. doi: 10.1126/science.287.5456.1269. [DOI] [PubMed] [Google Scholar]
- His W. Versammlung in Basel angenommenen Namen. Leipzig: Veit; 1895. Die anatomische Nomenclatur. [Google Scholar]
- Huntgeburth SC, Petrides M. Morphological patterns of the collateral sulcus in the human brain. Eur J Neurosci. 2012;35:1295–1311. doi: 10.1111/j.1460-9568.2012.08031.x. [DOI] [PubMed] [Google Scholar]
- Huschke E. Schaedel, Hirn und Seele des Menschen und der Thiere nach Alter, Geschlecht und Race, dargestellt nach neuen Methoden und Untersuchungen. Mauke, Jena: 1854. [Google Scholar]
- Huth AG, Nishimoto S, Vu AT, Gallant JL. A continous semantic space describes the representation of thousands of object and action categories across the human brain. Neuron. 2012;76(6):1210–1224. doi: 10.1016/j.neuron.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Istomin V, Amunts K. Application of mathematical morphology algorithms for automatic quantification of the cytoarchitecture of human neocortex. Vis Voice Mag. 1992;6:142–153. [Google Scholar]
- Istomin VV, Shkliarov MI. Automated investigation of the human cerebral cortex with a TV-based image analyse system (russ) Zh Nevropatol Psikhiatr im S S Korsakova. 1984;7:969–974. [PubMed] [Google Scholar]
- James TW, Humphrey GK, Gati JS, Servos P, Menon RS, Goodale MA. Haptic study of three-dimensional objects activates extrastriate visual areas. Neuropsychologia. 2002;40:1706–1714. doi: 10.1016/s0028-3932(02)00017-9. [DOI] [PubMed] [Google Scholar]
- Kanwisher N. Domain specificity in face perception. Nat Neurosci. 2000;3:759–763. doi: 10.1038/77664. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada R, Johnsrude IS, Kochiyama T, Lederman SJ. Functional specialization and convergence in the occipito-temporal cortex supporting haptic and visual identification of human faces and body parts: an fMRI study. J Cogn Neurosci. 2009;21:2027–2045. doi: 10.1162/jocn.2009.21115. [DOI] [PubMed] [Google Scholar]
- Laird AR, Fox PM, Price CJ, Glahn DC, Uecker AM, Lancaster JL, Turkeltaub PE, Kochunov P, Fox PT. ALE meta-analysis: controlling the false discovery rate and performing statistical contrasts. Hum Brain Mapp. 2005;25:155–164. doi: 10.1002/hbm.20136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb DS. Report of the association of american anatomists. J Comp Neurol. 1899;9:46–52. [Google Scholar]
- Lorenz S, Weiner KS, Caspers J, Mohlberg H, Schleicher A, Bludau S, Eickhoff S, Grill-Spector K, Zilles K, Amunts K. Two new cytoarchitectonic areas on the human mid-fusiform gyrus. 2015 doi: 10.1093/cercor/bhv225. in revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahalanobis PC, Majumda DN, Rao CR. Anthropometric survey of the united provinces: a statistical study. Sankhya. 1949;9:89–324. [Google Scholar]
- Mahon BZ, Caramazza A. What drives the organization of object knowledge in the brain? Trends Cogn Sci. 2011;15(3):97–103. doi: 10.1016/j.tics.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malikovic A, Amunts K, Schleicher A, Mohlberg H, Eickhoff SB, Wilms M, Palomero-Gallagher N, Armstrong E, Zilles K. Cytoarchitectonic analysis of the human extrastriate cortex in the region of V5/MT+: a probabilistic, stereotaxic map of area hOc5. Cereb Cortex. 2007;17:562–574. doi: 10.1093/cercor/bhj181. [DOI] [PubMed] [Google Scholar]
- McGugin RW, Gatenby JC, Gore JC, Gauthier I. High-resolution imaging of expertise reveals reliable object selectivity in the fusiform face area related to perceptual performance. Proc Natl Acad Sci USA. 2012;109:17063–17068. doi: 10.1073/pnas.1116333109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGugin RW, Newton AT, Gore JC, Gauthier I. Robust expertise effects in right FFA. Neuropsychologia. 2014;63:135–144. doi: 10.1016/j.neuropsychologia.2014.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton BL, Battaglia FP, Jensen O, Moser EI, Moser MB. Path integration and the neural basis of the ‘cognitive map’. Nat Rev Neurosci. 2006;7:663–678. doi: 10.1038/nrn1932. [DOI] [PubMed] [Google Scholar]
- Meynert T. A clinical treatise on diseases of the fore-brain based upon a study of its structure, functions, and nutrition. G.P. Putnam's Sons; New York and London: 1885. Psychiatry. [Google Scholar]
- Mickle WJ. Atypical and unusual brain-forms, especially in relation to mental status: a study on brain-surface morphology. J Ment Sci. 1897;43(180):1–32. [Google Scholar]
- Mills CK. The Nervous System and its Diseases: A Practical Treatise on Neurology for the Use of Physians and Students. J.B. Lippincott Company; Philadelphia: 1898. [Google Scholar]
- Morosan P, Schleicher A, Amunts K, Zilles K. Multimodal architectonic mapping of human superior temporal gyrus. Anat Embryol (Berl) 2005;210:401–406. doi: 10.1007/s00429-005-0029-1. [DOI] [PubMed] [Google Scholar]
- Nasr S, Liu N, Devaney KJ, Yue X, Rajimehr R, Ungerleider LG, Tootell RB. Scene-selective cortical regions in human and nonhuman primates. J Neurosci. 2011;31:13771–13785. doi: 10.1523/JNEUROSCI.2792-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobre AC, Allison T, McCarthy G. Modulation of human extrastriate visual processing by selective attention to colours and words. Brain. 1998;121(Pt 7):1357–1368. doi: 10.1093/brain/121.7.1357. [DOI] [PubMed] [Google Scholar]
- Obersteiner H. The anatomy of the central nervous organs in health and disease. Charles Griffin & Co.; London: 1890. [Google Scholar]
- Palomero-Gallagher N, Mohlberg H, Zilles K, Vogt B. Cytology and receptor architecture of human anterior cingulate cortex. J Comp Neurol. 2008;508:906–926. doi: 10.1002/cne.21684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomero-Gallagher N, Eickhoff SB, Hoffstaedter F, Schleicher A, Mohlberg H, Vogt BA, Amunts K, Zilles K. Functional organization of human subgenual cortical areas: relationship between architectonical segregation and connectional heterogeneity. NeuroImage. 2015;115:177–190. doi: 10.1016/j.neuroimage.2015.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pansch A. De Sulcis et Gyris in Cerebris: Simiarum et Hominum. Mohr, Kiliae; 1866. [Google Scholar]
- Parker AJ. Morphology of the cerebral convolutions with special reference to the order of primates. Journal of the Academy of the National Sciences of Philadelphia. 1896:247–366. [Google Scholar]
- Sarkisov S, Filimonoff IN, Preobrashenskaya NS. Cytoarchitecture of the human cortex cerebri. Medgiz; Moscow: 1949. [Google Scholar]
- Parr LA, Hecht E, Barks SK, Preuss TM, Votaw JR. Face processing in the chimpanzee brain. Curr Biol. 2009;19:50–53. doi: 10.1016/j.cub.2008.11.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puce A, Allison T, Asgari M, Gore JC, McCarthy G. Differential sensitivity of human visual cortex to faces, letterstrings, and textures: a functional magnetic resonance imaging study. J Neurosci. 1996;16:5205–5215. doi: 10.1523/JNEUROSCI.16-16-05205.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puce A, Allison T, McCarthy G. Electrophysiological studies of human face perception. III: Effects of top-down processing on face-specific potentials. Cereb Cortex. 1999;9:445–458. doi: 10.1093/cercor/9.5.445. [DOI] [PubMed] [Google Scholar]
- Rademacher J, Burgel U, Geyer S, Schormann T, Schleicher A, Freund HJ, Zilles K. Variability and asymmetry in the human precentral motor system. A cytoarchitectonic and myeloarchitectonic brain mapping study. Brain. 2001;124:2232–2258. doi: 10.1093/brain/124.11.2232. [DOI] [PubMed] [Google Scholar]
- Rakic P. Evolution of the neocortex: a perspective from developmental biology. Nat Rev Neurosci. 2009;10:724–735. doi: 10.1038/nrn2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch F, Schleicher A, Zilles K. Recognition of the retrograde reaction in motoneurons using an image analysing system. J Neurosci Methods. 1989;30:255–262. doi: 10.1016/0165-0270(89)90136-2. [DOI] [PubMed] [Google Scholar]
- Retzius G. Kgl Buchdr. P. A. Norstedt and Söner; Stockholm: 1896. Das Menschenhirn: Studien in der makroskopischen Morphologie. [Google Scholar]
- Retzius G. Cerebra Simiarum Illustrata: Das Affenhirn in Bildlicher Aarstellung. G. Fischer; Stockholm: 1906. [Google Scholar]
- Robinson JL, Laird AR, Glahn DC, Lovallo WR, Fox PT. Metaanalytic connectivity modeling: delineating the functional connectivity of the human amygdala. Hum Brain Mapp. 2010;31:173–184. doi: 10.1002/hbm.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saygin ZM, Osher DE, Koldewyn K, Reynolds G, Gabrieli JD, Saxe RR. Anatomical connectivity patterns predict face selectivity in the fusiform gyrus. Nat Neurosci. 2012;15:321–327. doi: 10.1038/nn.3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheperjans F, Grefkes C, Palomero-Gallagher N, Schleicher A, Zilles K. Subdivisions of human parietal area 5 revealed by quantitative receptor autoradiography: a parietal region between motor, somatosensory, and cingulate cortical areas. NeuroImage. 2005;25:975–992. doi: 10.1016/j.neuroimage.2004.12.017. [DOI] [PubMed] [Google Scholar]
- Scheperjans F, Hermann K, Eickhoff SB, Amunts K, Schleicher A, Zilles K. Observer-independent cytoarchitectonic mapping of the human superior parietal cortex. Cereb Cortex. 2008;18:846–867. doi: 10.1093/cercor/bhm116. [DOI] [PubMed] [Google Scholar]
- Schlaug G, Schleicher A, Zilles K. Quantitative analysis of the columnar arrangement of neurons in the human cingulate cortex. J Comp Neurol. 1995;351:441–452. doi: 10.1002/cne.903510310. [DOI] [PubMed] [Google Scholar]
- Schleicher A, Amunts K, Geyer S, Kowalski T, Zilles K. An observer-independent cytoarchitectonic mapping of the human cortex using a stereo-logical approach. Acta Stereol. 1998;17:75–82. [Google Scholar]
- Schleicher A, Amunts K, Geyer S, Morosan P, Zilles K. Observer-independent method for microstructural parcellation of cerebral cortex: a quantitative approach to cytoarchitectonics. NeuroImage. 1999;9:165–177. doi: 10.1006/nimg.1998.0385. [DOI] [PubMed] [Google Scholar]
- Schleicher A, Palomero-Gallagher N, Morosan P, Eickhoff SB, Kowalski T, de Vos K, Amunts K, Zilles K. Quantitative architectural analysis: a new approach to cortical mapping. Anat Embryol (Berl) 2005;210:373–386. doi: 10.1007/s00429-005-0028-2. [DOI] [PubMed] [Google Scholar]
- Schleicher A, Zilles K. A quantitative approach to cytoarchitectonics: analysis of structural inhomogeneities in nervous tissue using an image analyser. J Microsc. 1990;157:367–381. doi: 10.1111/j.1365-2818.1990.tb02971.x. [DOI] [PubMed] [Google Scholar]
- Schleicher A, Zilles K, Wree A. A quantitative approach to cytoarchitectonics: software and hardware aspects of a system for the evaluation and analysis of structural inhomogeneities in nervous tissue. J Neurosci Methods. 1986;18:221–235. doi: 10.1016/0165-0270(86)90121-4. [DOI] [PubMed] [Google Scholar]
- Segal E, Petrides M. The morphology and variability of the caudal rami of the superior temporal sulcus. Eur J Neurosci. 2012;36:2035–2053. doi: 10.1111/j.1460-9568.2012.08109.x. [DOI] [PubMed] [Google Scholar]
- Serra J. Mathematical morphology. Comput Vis Graph Image Process. 1986;35:283–305. [Google Scholar]
- Smith GE. The international commision of brain research. J Comp Neurol Psychol. 1905;15:62–64. [Google Scholar]
- Spitzka EA. A study of the brains of six eminent scientists and scholars belonging to the american anthropometric society together with a description of the skull of Professor E. D Cope. Trans Am Philos Soc. 1907;XXI:175–308. [Google Scholar]
- Tarr MJ, Gauthier I. FFA: a flexible fusiform area for subordinate-level visual processing automatized by expertise. Nat Neurosci. 2000;3:764–769. doi: 10.1038/77666. [DOI] [PubMed] [Google Scholar]
- Turner W. The Convolutions of the Human Cerebrum Topographically Considered. Maclachlan and Stewart; Edinburgh: 1866. [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC. A tension-based theory of morphogenesis and compact wiring in the central nervous system. Nature. 1997;385:313–318. doi: 10.1038/385313a0. [DOI] [PubMed] [Google Scholar]
- Van Essen DC. Organization of visual areas in macaque and human cerebral cortex. In: Chalupa L, Werner J, editors. Visual Neurosciences. 2003. [Google Scholar]
- Van Essen DC, Glasser MF, Dierker DL, Harwell J, Coalson T. Parcellations and hemispheric asymmetries of human cerebral cortex analyzed on surface-based atlases. Cereb Cortex. 2012;22:2241–2262. doi: 10.1093/cercor/bhr291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Hof PR, Zilles K, Vogt LJ, Herold C, Palomero-Gallagher N. Cingulate area 32 homologies in mouse, rat, macaque and human: cytoarchitecture and receptor architecture. J Comp Neurol. 2013;521:4189–4204. doi: 10.1002/cne.23409. [DOI] [PubMed] [Google Scholar]
- Vogt C, Vogt O. Allgemeinere Ergebnisse unserer Hirnforschung. J Psychol Neurol. 1919;25:279–462. [Google Scholar]
- Vogt O. Neurobiologische Arbeiten Verlag von Gustav. Fischer; Jena: 1904. [Google Scholar]
- von Economo C, Koskinas G. Die Cytoarchitektonik der Hirnrinde des Erwachsenen Menschen. Springer; Berlin: 1925. [Google Scholar]
- Wagner AD, Koutstaal W, Schacter DL. When encoding yields remembering: insights from event-related neuroimaging. Philos Trans R Soc Lond B: Biol Sci. 1999;354:1307–1324. doi: 10.1098/rstb.1999.0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandell BA, Rauschecker AM, Yeatman JD. Learning to see words. Annu Rev Psychol. 2012;63:31–53. doi: 10.1146/annurev-psych-120710-100434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner KS, Golarai G, Caspers J, Chuapoco MR, Mohlberg H, Zilles K, Amunts K, Grill-Spector K. The mid-fusiform sulcus: a landmark identifying both cytoarchitectonic and functional divisions of human ventral temporal cortex. NeuroImage. 2014;84:453–465. doi: 10.1016/j.neuroimage.2013.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner KS, Grill-Spector K. Sparsely-distributed organization of face and limb activations in human ventral temporal cortex. NeuroImage. 2010;52:1559–1573. doi: 10.1016/j.neuroimage.2010.04.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner KS, Grill-Spector K. Neural representations of faces and limbs neighbor in human high-level visual cortex: evidence for a new organization principle. Psychol Res. 2013;77:74–97. doi: 10.1007/s00426-011-0392-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner KS, Sayres R, Vinberg J, Grill-Spector K. fMRI-adaptation and category selectivity in human ventral temporal cortex: regional differences across time scales. J Neurophysiol. 2010;103:3349–3365. doi: 10.1152/jn.01108.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernicke C. Das urwindungssystem des menschlichen gehirns. Arch Psychiatr Nervenkr. 1876;6:298–326. [Google Scholar]
- Wilder BG. A partial revision of anatomical nomenclature with especial reference to that of the brain. Science. 1881a;2:133–138. doi: 10.1126/science.os-2.40.133-a. [DOI] [PubMed] [Google Scholar]
- Wilder BG. A partial revision of anatomical nomenclature, with especial reference to that of the brain. Science. 1881b;2:122–126. doi: 10.1126/science.os-2.39.122. [DOI] [PubMed] [Google Scholar]
- Wilder BG. On two little-known cerebral fissures, with suggestions as to fissural and gyral names. J Nerv Ment Dis. 1885;12:350–352. [Google Scholar]
- Wilder BG. Human cerebral fissures, their relations and names and the methods of studying them. Am Nat. 1886;XX:901–902. [Google Scholar]
- Wilder BG. Neural terms, international and national. J Comp Neurol. 1896a;6:216–352. [Google Scholar]
- Wilder BG. Some neural and descriptive terms. Science. 1896b;4:947. doi: 10.1126/science.4.104.947. [DOI] [PubMed] [Google Scholar]
- Wree A, Schleicher A, Zilles K. Estimation of volume fractions in nervous tissue with an image analyzer. J Neurosci Methods. 1982;6:29–43. doi: 10.1016/0165-0270(82)90014-0. [DOI] [PubMed] [Google Scholar]
- Yeatman JD, Weiner KS, Pestilli F, Rokem A, Mezer A, Wandell BA. The vertical occipital fasciculus: A century of controversy resolved by in vivo measurements. Proc Natl Acad Sci USA. 2014;111(48):E5214–E5223. doi: 10.1073/pnas.1418503111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaborszky L, Hoemke L, Mohlberg H, Schleicher A, Amunts K, Zilles K. Stereotaxic probabilistic maps of the magnocellular cell groups in human basal forebrain. Neuroimage. 2008;42:1127–1141. doi: 10.1016/j.neuroimage.2008.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilles K. A quantitative approach to cytoarchiterctonics. II: the allocortex of Tupaia belangeri. Anat Embryol (Berl) 1978;154:335–352. doi: 10.1007/BF00345660. [DOI] [PubMed] [Google Scholar]
- Zilles K, Amunts K. Receptor mapping: architecture of the human cerebral cortex. Curr Opin Neurol. 2009;22:331–339. doi: 10.1097/WCO.0b013e32832d95db. [DOI] [PubMed] [Google Scholar]
- Zilles K, Amunts K. Centenary of Brodmann's map: conception and fate. Nat Rev Neurosci. 2010;11:139–145. doi: 10.1038/nrn2776. [DOI] [PubMed] [Google Scholar]
- Zilles K, Clarke S. Architecture, connectivity and transmitter receptors of human extrastriate visual cortex. Comparison with non-human primates. In: Rockland KS, editor. Cerebral Cortex. Plenum Press; New York: 1997. pp. 673–742. [Google Scholar]
- Zilles K, Palomero-Gallagher N, Amunts K. Development of cortical folding during evolution and ontogeny. Trends Neurosci. 2013;36:275–284. doi: 10.1016/j.tins.2013.01.006. [DOI] [PubMed] [Google Scholar]
- Zilles K, Palomero-Gallagher N, Grefkes C, Scheperjans F, Boy C, Amunts K, Schleicher A. Architectonics of the human cerebral cortex and transmitter receptor fingerprints: reconciling functional neuroanatomy and neurochemistry. Eur Neuropsychopharmacol. 2002;12:587–599. doi: 10.1016/s0924-977x(02)00108-6. [DOI] [PubMed] [Google Scholar]
- Zilles K, Palomero-Gallagher N, Schleicher A. Transmitter receptors and functional anatomy of the cerebral cortex. J Anat. 2004;205:417–432. doi: 10.1111/j.0021-8782.2004.00357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilles K, Schlaug G, Geyer S, Luppino G, Matelli M, Qü M, Schleicher A, Schormann T. Anatomy and transmitter receptors of the supplementary motor areas in the human and nonhuman primate brain. In: Lüders HO, editor. Advances in Neurology, Volume 70: Supplementary Sensorimotor Area. Lippincott-Raven; Philadelphia: 1996. pp. 29–43. [PubMed] [Google Scholar]
- Zilles K, Schlaug G, Matelli M, Luppino G, Schleicher A, Qu M, Dabringhaus A, Seitz R, Roland PE. Mapping of human and macaque sensorimotor areas by integrating architectonic, transmitter receptor, MRI and PET data. J Anat. 1995;187(Pt 3):515–537. [PMC free article] [PubMed] [Google Scholar]
- Zilles K, Schleicher A, Kretschmann HJ. Automatic morphometric analysis of retrograde changes in the nucleus n. facialis at different ontogenetic stages in the rat. Cell Tissue Res. 1978;190:285–299. doi: 10.1007/BF00218176. [DOI] [PubMed] [Google Scholar]
- Zilles K, Schleicher A, Palomero-Gallagher N, Amunts K. Quantitative analysis of cyto- and receptorarchitecture of the human brain. In: Toga AW, Mazziotta JC, editors. Brain Mapping: The Methods. 2nd. Academic Press; San Diego, CA: 2002. [Google Scholar]
- Zilles K, Werners R, Büsching U, Schleicher A. Ontogenesis of the laminar structure in areas 17 and 18 of the human visual cortex: a quantitative study. Anat Embryol (Berl) 1986;174:339–353. doi: 10.1007/BF00698784. [DOI] [PubMed] [Google Scholar]
- Zilles K, Zilles B, Schleicher A. A quantitative approach to cytoarchitectonics. VI: the areal pattern of the cortex of the albino rat. Anat Embryol. 1980;159:335–360. doi: 10.1007/BF00317655. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.