Abstract
Background
Vascular access failure, a major cause of morbidity in hemodialysis (HD) patients, occurs mainly at stenotic endothelium following an acute thrombotic event. Microparticles (MPs) are fragments derived from injured cell membrane and are closely associated with coagulation and vascular inflammatory responses.
Methods
We investigated the relationship between levels of circulating MPs and vascular access patency in HD patients. A total of 82 HD patients and 28 healthy patients were enrolled. We used flow cytometry to measure endothelial MPs (EMPs) identified by CD31+CD42− or CD51+ and platelet-derived MPs (PMPs) identified by CD31+CD42+ in plasma samples of participants. Vascular access patency was defined as an interval from the time of access formation to the time of first access stenosis in each patient. MP counts were compared according to access patent duration.
Results
The levels of EMP (both CD31+CD42− and CD51+) and CD31+CD42+PMP were significantly higher in patients than in healthy participants. Levels of CD31+CD42−EMP and CD31+CD42+PMP showed a positive correlation. In non-diabetic HD patients, CD31+CD42−EMPs and CD31+CD42+PMPs were more elevated in the shorter access survival group (access survival <1 year) than in the longer survival group (access survival ≥ 4 years).
Conclusion
Elevated circulating EMP or PMP counts are influenced by end-stage renal disease and increased levels of EMP and PMP may be associated with vascular access failure in HD patients.
Keywords: Cell derived, Endothelial cells, Hemodialysis, Microparticles, Platelets, Vascular access failure
Introduction
Vascular access failure is the single-most important cause of morbidity and hospitalization in patients receiving hemodialysis (HD) [1]. Vascular stenosis and subsequent thrombosis precede access failure, and venous neointimal hyperplasia (VNH) is a main pathology of vascular stenosis in both native arteriovenous fistulas (AVF) and polytetrafluoroethylene grafts [2], [3], [4]. In VNH, injured endothelial cells accelerate the expression of adhesion molecules and tissue factors and increase activated platelet aggregation, finally leading to regional stenosis and thrombosis [1]. Dialysis patients tend to show thrombotic tendencies such as enhanced platelet aggregation and hypercoagulability [5], [6]. However, despite many efforts to clarify the relationship between end-stage renal disease (ESRD) patients undergoing hemodialysis and thrombotic tendencies, there are insufficient data about what determines vulnerability to vascular access failure in HD patients.
Elevated circulating microparticles (MPs) are associated with many thrombotic diseases [7]. MPs are fragments ranging in the size of 0.2–1.0 μm shed from the plasma membrane in response to various stimuli such as activation or apoptosis [8], [9], [10]. MPs have no nucleus, contain a membrane skeleton, and express surface antigens specific for their parental cells of platelets, endothelial cells, leukocytes, lymphocytes, and erythrocytes [11], [12], [13]. MPs were first described in 1967 by Wolf, and were called “platelet dusts” from activated platelets [14]. However, MPs are not just byproducts of activated or apoptotic cellular processes, but are actively involved in the pathogenesis of many procoagulant diseases, especially vascular diseases [7]. Platelet-derived MPs (PMPs) were increased in myocardial infarction [15], hypertension (HTN) [16], and diabetes mellitus (DM) [17], and elevated endothelial MPs (EMPs) were closely associated with endothelial dysfunction [10], [18], [19] and occurred in acute coronary syndrome (ACS) [15], [20], DM [21], chronic renal failure (CRF) [22], and ESRD [10]. Thus, elevated EMPs or PMPs could play an important role in cardiovascular diseases. However, there are insufficient studies about roles of elevated MPs in ESRD patients, particularly the association between MPs and vascular access failure.
In this study, we hypothesized that elevated levels of EMP or PMP derived from activated injured endothelial cells or platelets in patients receiving HD could be associated with vascular access failure. We measured MP counts (EMP and PMP) in HD patients and compared them according to the duration of vascular access patency.
Methods
Study population and study design
For our retrospective cross-sectional study, 82 clinically stable patients receiving maintenance HD for more than 3 months in 00 Hospital were enrolled. The duration of HD per session was 4–6 h and its frequency was individually tailored to achieve a Kt/V>1.2. The patients were treated with synthetic membranes (polysulfone or polyamide) and without dialyzer reuse. Heparinization during dialysis session was done by continuous infusion using unfractionated heparin at a dose of 500–1000 U/h, according to patient weight. All patients did not have acute diseases such as recent myocardial infarction, unstable angina, acute pulmonary embolism, acute neurologic disorder, malignancies, or overt systemic infections during the last 6 months. Vascular access patency was defined as an interval from the time of access formation to the time of first vascular stenosis in each patient. To analyze the relationship between levels of MP and vascular access patency, we divided the patients into two subgroups with a reference point of 1-year access survival. In detail, patients with access patency of more than 1 year at enrollment were placed into Group A, and the others were included in Group B (access survival<1 year). In Group A, vascular access patency of more than 4 years was defined as Group C. We reviewed medical records of each patient and examined the medical histories of DM, HTN, and drug histories of renin-angiotensin system (RAS) blockers such as angiotensin converting enzyme inhibitors (ACEi) or angiotensin receptor blockers (ARB), statins, and antiplatelet agents. We assessed the nutritional status of each patient by measuring normalized protein catabolic rate (nPCR). A total of 28 healthy people without a medical history of DM or HTN were enrolled as controls. The study was performed according to the principles of the Declaration of Helsinki after our Institutional Review Board approval. Written informed consent was obtained from all participants.
Blood sampling
Venous blood samples were taken from each patient before starting the dialysis session and they were immediately analyzed. Samples were obtained from the HD-needle puncture site 72 h after the last dialysis. All patients were required to have midnight fasting for 6 h or more. Standard laboratory tests included complete blood cell and platelet counts and blood chemistry including serum albumin, protein, blood urea nitrogen (BUN), creatinine, cholesterol, and low-density lipoprotein (LDL). High sensitive C-reactive protein (hsCRP) was measured by a nephelometric immunoassay (Handok Pharm, Seoul, Korea). Interleukin-6 (IL-6) levels were checked using a commercial ELISA kit (Pierce Biotechnology, Rockford, IL, USA).
Preparation of microparticles (EMPs and PMPs)
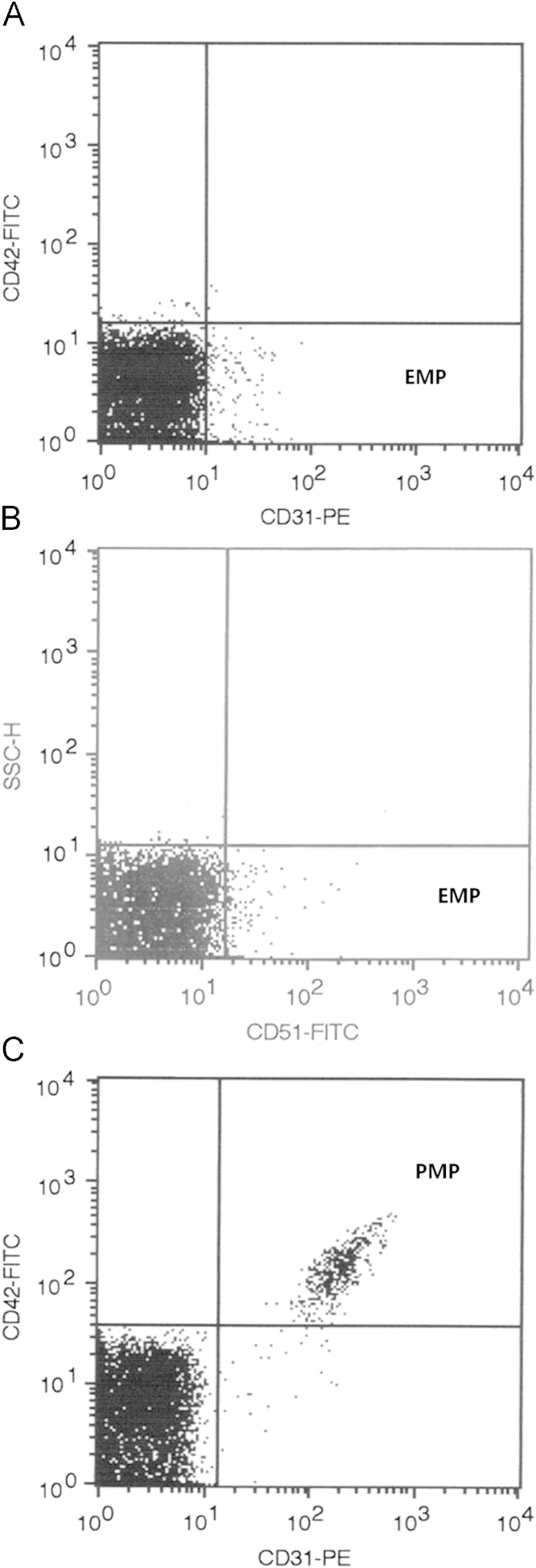
Blood samples (5 mL) were drawn into citrated (blue-top) Vacutainer tubes centrifuged for 10 min at 160g at 4 °C to prepare platelet-rich plasma (PRP). The PRP was then centrifuged for 6 min at 1200g at 4 °C to prepare platelet-poor plasma (PPP). Supernatant was collected and assays of EMPs and PMPs were performed within 1–2 h after obtaining samples [20]. We detected EMPs using two different markers, i.e., CD31 and CD51. PMPs were identified by the marker CD42 [23]. CD31 is expressed on both EMPs and PMPs, whereas CD42 is not expressed on endothelial cells, so double labeling was required. CD51 expression is extremely weak on platelets and is not detected with flow cytometry on PMPs; therefore, double-labeling was not required [20]. In brief, EMPs were defined as particles with CD31+CD42− or CD51+ and PMPs were defined as particles with CD31+CD42+ [24] (Fig. 1).
Figure 1.
Representative graph of flow cytometry analysis of microparticles. (A) The points in the right lower quadrant are CD31+CD42−EMPs. (B) The points in the right lower quadrant are CD51+EMPs. (C) The right upper quadrant region contains CD31+CD42+PMPs. EMPs, endothelial microparticles; PMPs, platelet-derived microparticles.
Materials
Fluorescein isothiocyanate (FITC)-conjugated human monoclonal antibody against αvβ3 [anti-CD51-FITC (clone 23C6, IgG1κ)], phycoerythrin (PE)-conjugated human monoclonal antibody against PECAM-1 [anti-CD31-PE (clone WM59, IgG1κ)], and FITC-conjugate human monoclonal antibody against leukocyte common antigen [anti-CD45-FITC (clone HI30, IgG1κ)] were purchased from BD Bioscience (San Jose, CA, USA). FITC-conjugated human monoclonal antibody against GPIbα [anti-CD42b-FITC (clone SZ2, IgG1)] from Beckman & Coulter (Marseillue, France), FITC- and PE-conjugated, isotype-matched monoclonal antibodies (clone MOPI-21, IgG1κ) of irrelevant specificity were purchased from BD Bioscience. Annexin V (An-V)-FITC apoptosis detection kit was purchased from Calbiochem (Darmstadt, Germany).
Flow cytometry analysis of MPs
We performed an analysis of MPs using flow cytometry with FACScalibur (BD Biosciences). Each 40 μL of prepared PPP in a 12 mm×75 mm polypropylene tube was incubated with either 4 μL of anti-CD42-FITC plus 4 μL of anti-CD31-PE or 4 μL of anti-CD51-FITC for 20 min with gentle regular shaking at room temperature. Then, 500 μL of phosphate buffered saline (PBS) was added, and MPs of each sample were analyzed by flow cytometry at the medium flow setting. Light scatter and fluorescence channels were set at logarithmic gain. Particles ≤1 μm defined by 1-μm calibrator beads (Polysciences, Warrington, PA, USA) were identified in forward scatter and side scatter intensity dot representation and gated as MPs. The fluorescence-positive particles were further separated on another histogram based on the size of this range. Sample analysis was stopped after 10,000 events. Data were analyzed using CELLQuest software (version 5.2, BD Bioscience). FITC- and PE-conjugated isotype-matched mouse monoclonal IgG was used for negative controls in each sample. Because CD31 is also expressed on leukocytes, flow cytometric analysis with anti-CD45-FITC was performed for exclusion of MPs from leukocytes.
Statistical analysis
Data are expressed as means ±SEM. The Student t test or the χ2 test was used for comparison between the two groups. The Mann–Whitney U test was used for comparison between the two groups for the nonparametric analysis. The multiple linear regression analysis was performed to evaluate correlated factors for increased microparticle counts. The logistic regression analysis was used to evaluate risk factors affecting vascular access failure. Pearson's correlation method was used for an analysis of association between MP counts and continuous variables. A P value of <0.05 was considered to be statistically significant. Statistical analysis was performed with SPSS, version 13.0 (Chicago, IL, USA).
Results
Comparison of levels of EMP and PMP between HD patients and healthy participants
The baseline characteristics of enrolled participants are shown in Table 1. The mean age of patients was higher than controls and sex distribution was different. Mean levels of serum albumin, cholesterol, LDL cholesterol, hemoglobin, and platelets were higher in healthy participants compared with HD patients.
Table 1.
Baseline Characteristics of HD Patients and Controlsa
| Variables | HD patients (n=82) | Healthy controls (n=28) | P |
|---|---|---|---|
| Sex (men, %) | 30 (36.6) | 18 (64.3) | 0.008 |
| Age (years) | 61.2±1.5 | 45.1±1.4 | <0.001 |
| Hypertension (%) | 67 (81.7) | 0 | |
| Diabetes (%) | 41 (50.0) | 0 | |
| Causes of ESRD (%) | 0 | ||
| Diabetes mellitus | 42 (51.2) | ||
| Hypertension | 26 (31.7) | ||
| Glomerulonephritis | 5 (6.1) | ||
| Other causes | 9 (11.0) | ||
| HD duration (years) | 4.0 (1.1–21.6) | 0 | |
| Usage of RAS blocker (%) | 34 (41.5) | 0 | |
| Usage of statin (%) | 10 (12.2) | 0 | |
| Usage of antiplatelet (%) | 38 (46.3) | ||
| HD access type (%) | 0 | ||
| AVF | 79 (96.3) | ||
| Kt/V | 1.46 (1.05–2.20) | ND | |
| nPCR (g/kg/day) | 1.06 (0.56–1.91) | ND | |
| BUN (mg/dL) | 69.5±2.1 | 13.3±0.6 | <0.001 |
| Creatinine (mg/dL) | 10.02±0.34 | 0.96±0.04 | <0.001 |
| Albumin (g/dL) | 3.59±0.07 | 4.51±0.04 | <0.001 |
| Total cholesterol (mg/dL) | 175.6±4.5 | 197.0±6.1 | 0.002 |
| LDL cholesterol (mg/dL) | 111.0±3.9 | 121.6±5.0 | <0.001 |
| Hemoglobin (g/dL) | 10.43±1.02 | 14.77±1.68 | <0.001 |
| Platelet (×103/μL) | 188.6±9.8 | 221.9±9.0 | 0.003 |
AVF, arteriovenous fistula; AVG, arteriovenous graft; BUN, blood urea nitrogen; ESRD, end-stage renal disease; HD, hemodialysis; LDL, low density lipoprotein; ND, not determined; nPCR, normalized protein catabolic rate; RAS, renin–angiotensin system; SEM, standard error of mean.
Data are presented as means±SEM or number (%).
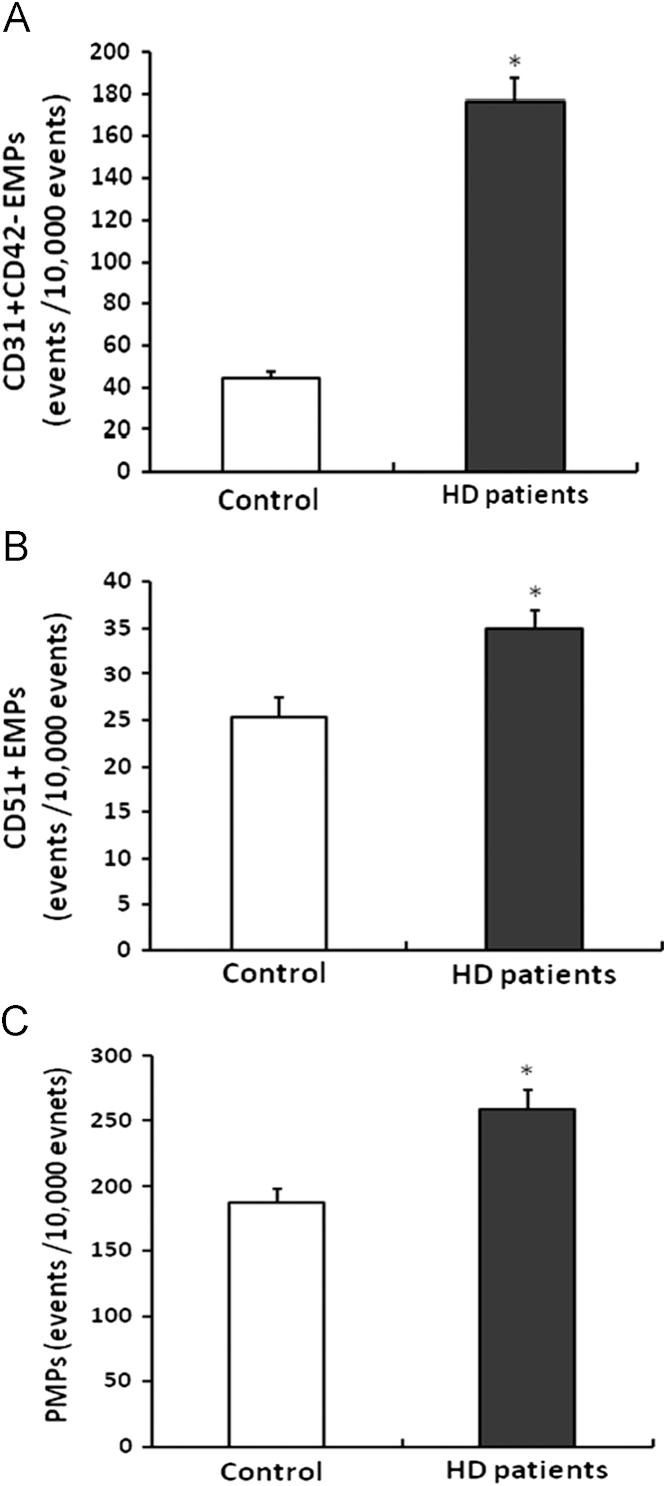
Both CD31+CD42−EMP and CD51+EMP levels were significantly higher in HD patients than controls (CD31+CD42−EMPs: 176.4±11.0 vs. 44.8±3.1 events/10,000 events; P<0.001, CD51+EMPs: 34.9±2.0 vs. 25.4±2.1 events/10,000 events; P=0.006). These results are shown in Fig. 2A and B. Levels of CD31+CD42−EMP showed higher values than that of CD51+EMP. CD31+CD42+PMP levels were also higher in HD patients than in controls (258.6±15.4 vs. 187.1±10.7 events/10,000 events; P<0.001). These results are shown in Fig. 2C. In measured MPs, CD45+MPs (common leukocyte marker) were rare (0–0.05%), suggesting that detected CD31+CD42-MP originated from endothelial cells.
Figure 2.
Comparison of EMP and PMP counts between patients on hemodialysis and healthy controls. CD31+CD42−EMPs (a); CD51+EMPs (b); and CD31+CD42+PMPs (c) levels were significantly higher in patients on hemodialysis compared with controls. Data are expressed as means ±SEM. ⁎P<0.01 vs. control. EMPs, endothelial microparticles; PMPs, platelet-derived microparticles; SEM, standard error of mean.
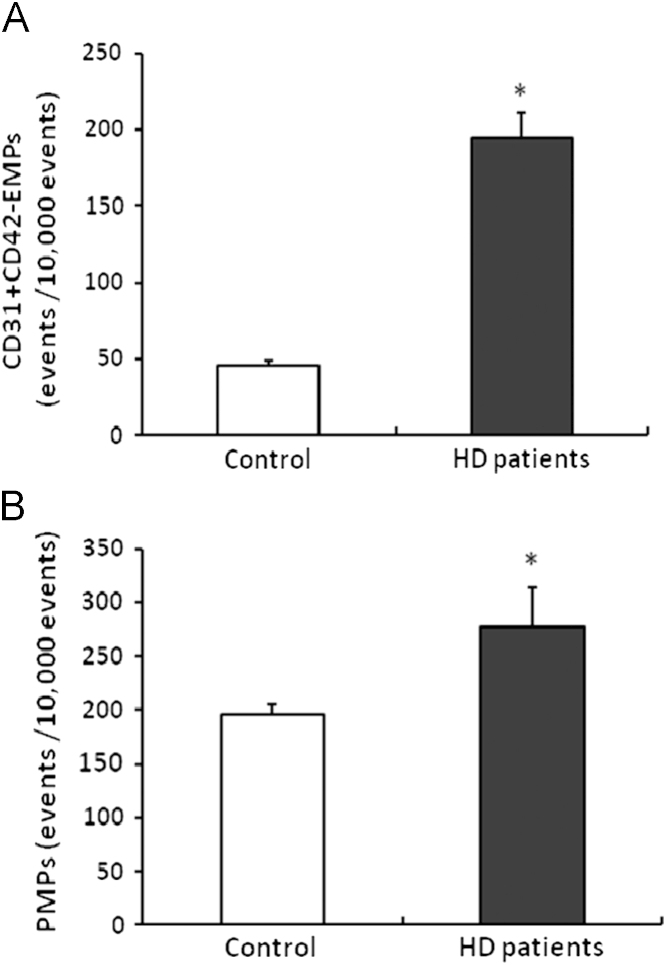
An age-matched subgroup analysis was also performed after excluding participants above 50 years old (n=20 in HD patients group; n=23 in control group), which provided similar mean ages for both groups (44.2±1.0 years in patients vs. 42.3±0.7 years in controls; P=0.118). Levels of CD31+CD42−EMP and CD31+CD42+PMP were significantly higher in HD patients than controls (CD31+CD42−EMPs: 194.1±16.7 vs. 45.8±3.3 events/10,000 events; P<0.001, CD31+CD42+PMPs: 277.4±37.5 vs. 195.6±9.5 events/10,000 events; P<0.001), but levels of CD51+EMP were not significantly different between the two groups (30.0±2.3 vs. 25.7±2.4 events/10,000 events; P=0.196). These results are shown in Fig. 3.
Figure 3.
Comparison of EMP and PMP levels between patients on hemodialysis and healthy participants < 50 years old. CD31+CD42−EMPs (a) and CD31+CD42+PMPs (b) levels were significantly higher in patients on hemodialysis compared with controls. Data are expressed as means ±SEM. ⁎P<0.01 vs. control. EMPs, endothelial microparticles; PMPs, platelet-derived microparticles; SEM, standard error of mean.
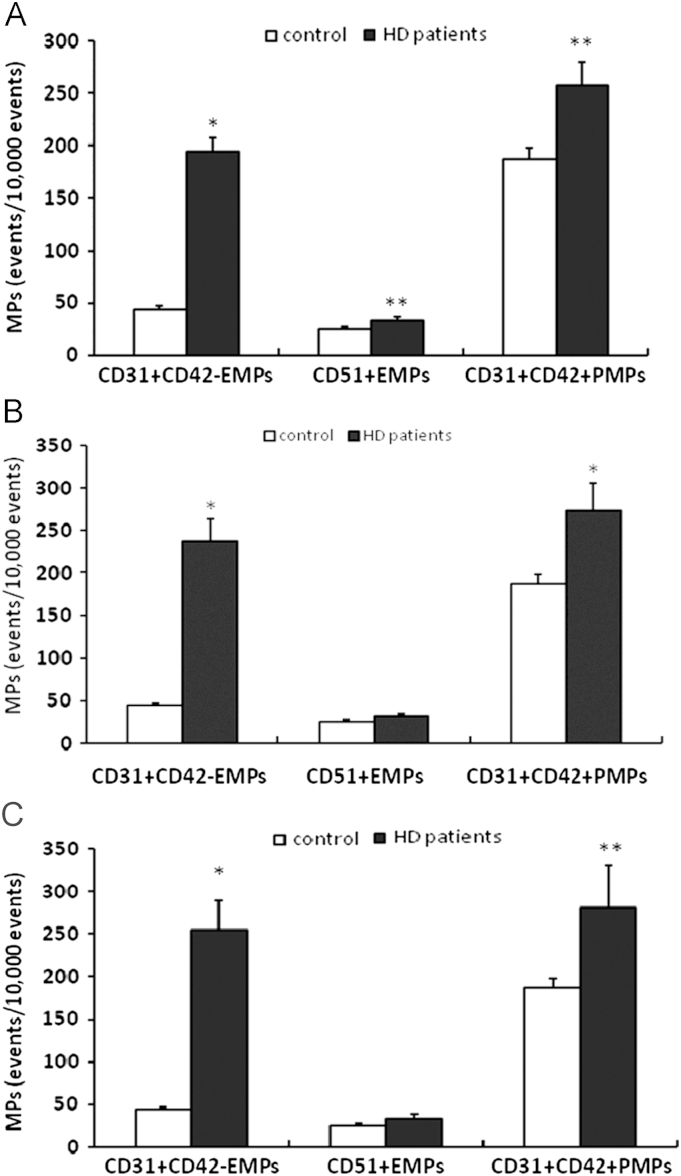
Because both DM and HTN can elevate circulating EMP or PMP counts [16], [17], [25], [26] a subgroup analysis was performed after excluding patients with DM or HTN. The levels of CD31+CD42−EMP, CD51+EMP, and CD31+CD42+PMP in non-DM HD patients were also higher than controls (CD31+CD42−EMPs: 194.0±13.9 vs. 44.0±3.1 events/10,000 events; P=0.006, CD51+EMPs: 34.3±2.7 vs. 25.4±2.1 events/10,000 events; P=0.014, CD31+CD42+PMPs: 257.5±22.1 vs. 187.1±10.7 events/10,000 events; P=0.006). These results are shown in Fig. 4A. When HTN patients were excluded, the levels of CD31+CD42−EMP and CD31+CD42+PMP were higher in patients than controls (CD31+CD42−EMPs: 238.6±26.4 vs. 44.0±3.1 events/10,000 events; P<0.001, CD31+CD42+PMPs: 274.3±31.9 vs. 187.1±10.7 events/10,000 events; P=0.019). These results are shown in Fig. 4B. CD51+EMPs were also slightly higher in patients (32.4±2.8 vs. 25.4±2.1 events/10,000 events; P=0.057). Excluding DM and HTN patients, CD31+CD42−EMP and CD31+CD42+PMP counts were still higher in HD patients (CD31+CD42−EMPs: 255.1±3.9 vs. 44.0±3.1 events/10,000 events; P<0.001, CD31+CD42+PMPs: 281.1±50.1 vs. 187.1±10.7 events/10,000 events, P=0.007). These results are shown in Fig. 4C. In an age-matched subgroup reanalysis that excluded DM patients, only CD31+CD42−EMP counts were significantly higher in non-DM HD patients group than in healthy participants (201.2±26.9 vs. 45.8±3.3 events/10,000 events, P<0.001). In multivariate analysis regarding age, sex, DM, HTN, ESRD, use of antiplatelet agent, ESRD was significantly associated with both CD31+CD41−EMP and CD31+CD41+PMP, but not CD51+EMP counts (Table 2).
Figure 4.
Comparison of EMP and PMP levels between patients on hemodialysis and healthy participants after excluding patients with diabetes mellitus or hypertension. (A) MPs of 3 markers in patients without diabetes mellitus (n=41) were significantly higher than healthy participants (n=28). (B) The levels of CD31+CD42−EMP and CD31+CD42+PMP were significantly higher in normotensive patients on hemodialysis (n=15) compared with the controls (n=28). (C) When patients with diabetes mellitus and hypertension were excluded, levels of CD31+CD42−EMP and CD31+CD42+PMP were significantly higher in patients on hemodialsis (patients, n=8; controls, n=28). Data are expressed as means ±SEM. ⁎P<0.01 vs. control. ⁎⁎P<0.05 vs. control. EMP, endothelial microparticles; MP, microparticle; PMP, platelet-derived microparticles; SEM, standard error of mean.
Table 2.
Multivariate Analysis of Risk Factors for Increased microparticles
| Risk factors | β | 95% CI | P |
|---|---|---|---|
| (A) Risk factors for increased CD31+CD42−EMP | |||
| Age | 0.055 | –0.90–1.71 | 0.539 |
| Male sex | 0.006 | –31.30–33.83 | 0.937 |
| ESRD | 0.766 | 122.69–241.12 | <0.001 |
| DM | –0.111 | –60.55–13.23 | 0.206 |
| HTN | 0.298 | 16.33–110.11 | 0.009 |
| Use of antiplatelet agent | 0.179 | 0.82–77.05 | 0.054 |
| (B) Risk factors for increased CD51+EMP | |||
| Age | 0.094 | –0.16–0.37 | 0.422 |
| Male sex | –0.172 | –12.12–1.05 | 0.098 |
| ESRD | 0.157 | –6.14–17.31 | 0.347 |
| DM | 0.032 | –6.92–9.02 | 0.794 |
| HTN | 0.053 | –7.89–11.33 | 0.723 |
| Use of antiplatelet agent | 0.172 | –2.39–14.09 | 0.164 |
| (C) Risk factors for increased CD31+CD42+PMP | |||
| Age | 0.052 | –1.46–2.37 | 0.640 |
| Male sex | 0.080 | –28.02–67.50 | 0.414 |
| ESRD | 0.225 | 2.13–129.50 | 0.041 |
| DM | 0.007 | –52.34–55.97 | 0.947 |
| HTN | –0.024 | –74.93–62.75 | 0.861 |
| Use of antiplatelet agent | 0.230 | 3.21–115.13 | 0.038 |
CI, confidence interval; EMP, endothelial microparticle; ESRD, end-stage renal disease; DM, diabetes mellitus; HTN, hypertension; PMP, platelet-derived microparticle.
Correlation between MP counts and values in HD patients
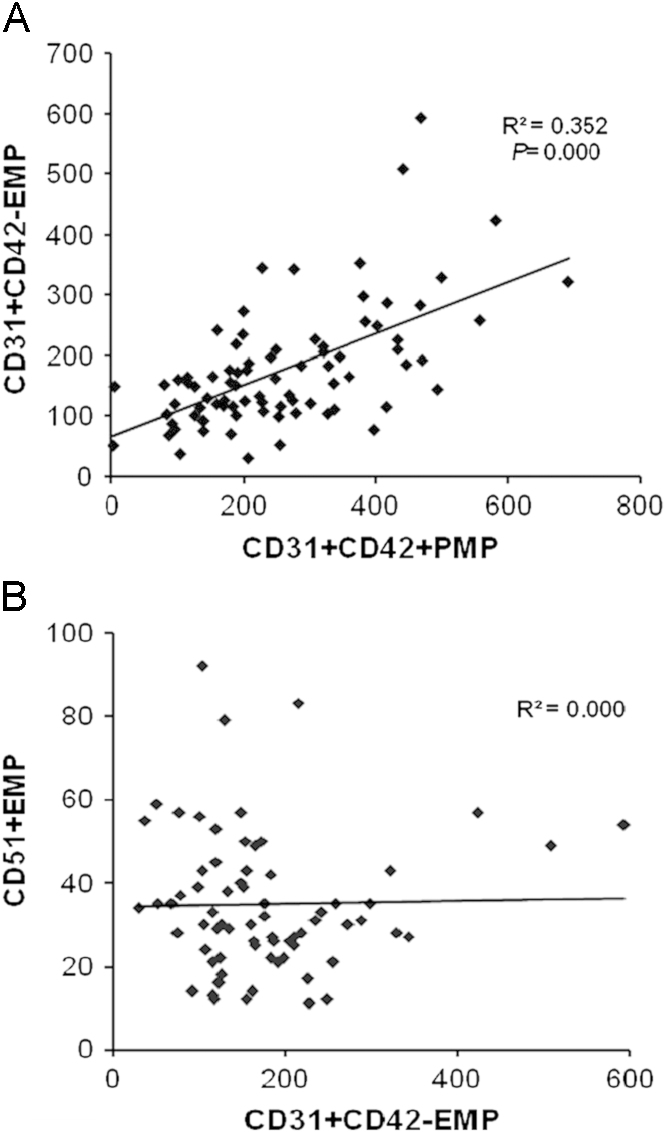
In HD patients, there were no significant correlations between MP (both EMP and PMP) counts and blood pressure, serum glucose, albumin, cholesterol, LDL, hemoglobin, platelet, hsCRP, and IL-6 levels (data not shown). Neither EMP counts nor PMP counts were different between the DM and non-DM patients groups. Interestingly, CD31+CD42−EMP and CD31+D42+PMP levels showed a positive correlation (β=0.672, P<0.001). These results are shown in Fig. 5.
Figure 5.
Correlations between markers of microparticles. (A) CD31+CD42−EMP and CD31+CD42+PMP showed positive correlation. (B) CD31+CD42−EMP and CD51+EMP showed no significant correlation. EMP, endothelial microparticle; PMP, platelet-derived microparticle.
Association between MP levels and vascular access patency in HD patients
A total of 77 patients were included for analysis of the relationship between MP levels and vascular access patency. The clinical characteristics and laboratory data of these participants are shown in Table 2. Neither EMP nor PMP levels showed significant differences between Group A (vascular access patency longer than 1 year) and Group B (vascular access patency shorter than 1 year). The percentage of patients with DM was higher in Group B, but Kt/V, BUN, creatinine, total cholesterol, LDL cholesterol, hemoglobin, platelets, hsCRP, and IL-6 levels were similar.
Next, we compared patients with Group B (shorter access survival group, access survival<1 year with one vascular access creation) with Group C (longer access survival group, access survival≥4 years with one vascular access creation). Clinical characteristics and laboratory data of the participants are summarized in Table 3. CD31+CD42−EMP counts were slightly higher in Group B than group C (P=0.068). Age and the percentage of patients with DM were higher in Group B. IL-6 levels were significantly higher in Group B, and HD duration was longer in Group C. Excluding DM patients, the levels of both CD31+CD42−EMP and CD31+CD4+PMP as well as IL-6 levels were significantly elevated in Group B (Table 4).
Table 3.
Comparison of Variables Between Shorter Vascular Access Survival Group (< 1 year) and the longer survival group (≥ 4 years)a
| Variables | Vascular access patency |
P | ||
|---|---|---|---|---|
| Group B | Group C | |||
| (<1 year, n=18) | (> 4 years, n=18) | |||
| Male sex (%) | 8 (44.4) | 3 (16.7) | 0.070 | |
| Age (years) | 65.3±3.1 | 54.9±3.0 | 0.022 | |
| Hypertension (%) | 14 (77.8) | 14 (77.8) | 1.000 | |
| Diabetes (%) | 13 (72.2) | 7 (38.9) | 0.044 | |
| HD duration (years) | 4.54±0.76 | 7.91±1.02 | 0.012 | |
| Access patent interval (M) | 4.7±0.8 | 95.9±7.0 | <0.001 | |
| Usage of RAS blocker (%) | 7 (38.9) | 8 (44.4) | 0.735 | |
| Usage of statin (%) | 0 (0) | 2 (11.1) | 0.486 | |
| Usage of antiplatelet (%) | 11 (61.1) | 6 (33.3) | 0.095 | |
| AVF (%) | 17 (94.4) | 18 (100) | 1.000 | |
| Kt/V | 1.49±0.07 | 1.62±0.06 | 0.191 | |
| nPCR (g/kg/day) | 1.18±0.08 | 1.14±0.06 | 0.624 | |
| BUN (mg/dL) | 69.7±5.2 | 72.1±2.9 | 0.689 | |
| Creatinine (mg/dL) | 9.8±0.6 | 10.4±0.6 | 0.462 | |
| Albumin (g/dL) | 3.62±0.10 | 3.86±0.06 | 0.043 | |
| Total cholesterol (mg/dL) | 172.7±9.7 | 162.1±16.4 | 0.582 | |
| LDL cholesterol (mg/dL) | 98.6±15.7 | 100.2±11.2 | 0.934 | |
| Hemoglobin (g/dL) | 10.6±0.3 | 10.5±0.2 | 0.873 | |
| Platelet (×103/μL) | 191.5±9.7 | 174.4±13.7 | 0.314 | |
| EMP | CD31+CD42- | 206.1±23.1 | 154.6±14.6 | 0.068 |
| CD51+ | 37.5±2.9 | 31.8±3.5 | 0.226 | |
| PMP CD31+CD42+ | 296.6±33.7 | 229.7±32.1 | 0.159 | |
| hsCRP (mg/dL) | 0.32±0.07 | 0.15±0.06 | 0.091 | |
| IL-6 ELISA (pg/mL) | 13.24±4.85 | 2.81±1.01 | 0.044 | |
AVF, arteriovenous fistula; BUN, blood urea nitrogen; ELISA, enzyme-linked immunosorbent assay; EMP, endothelial microparticle; HD, hemodialysis; hsCRP, high sensitive C-reactive protein; IL-6, interleukin-6; LDL, low-density lipoprotein; nPCR, normalized protein catabolic rate; PMP, platelet-derived microparticle; RAS, renin–angiotensin system; SEM, standard error of mean.
Data are presented as means±SEM or number (%).
Table 4.
Comparison of MPs and IL-6 Levels Between Shorter Vascular Access Survival group (<1 year) and Longer Survival Group (≥4 years) in HD Patients without Diabetes Mellitusa
| Variables | Vascular access patency |
P | ||
|---|---|---|---|---|
| Group B without DM | Group C without DM | |||
| (<1 year, n=5) | (> 4 years, n=11) | |||
| EMP | CD31+CD42− | 265.4±33.8 | 151.9±19.4 | 0.007 |
| CD51+ | 39.0±2.4 | 29.5±4.7 | 0.188 | |
| PMP CD31+CD42+ | 393.6±54.9 | 187.5±34.5 | 0.005 | |
| IL-6 ELISA (pg/mL) | 5.18±1.36 | 1.52±0.40 | 0.036 | |
| hsCRP (mg/dL) | 0.20±0.09 | 0.07±0.02 | 0.115 | |
DM, diabetes mellitus; ELISA, enzyme-linked immunosorbent assay; EMP, endothelial microparticle; hsCRP, high sensitive C-reactive protein; IL-6, interleukin-6; PMP, platelet-derived microparticle; SEM, standard error of mean.
Data are presented as means±SEM.
MP as a risk factor for 2-year vascular access failure
We performed univariate and multivariate logistic analysis about risk factors such as DM, HTN, old age (>50 years old), and levels of each MP (EMP or PMP) for vascular access failure. This analysis was based on the vascular access failure within 2 years from the first access creation because the median value of access patent interval in HD patients group was 2 years. We divided the MP levels of three markers into the quartile group. Among the risk factors, old age and the fourth quartile (>75%) group of CD31+CD42−EMP levels were significant risk factors for 2-year vascular access failure (Table 5).
Table 5.
Logistic Analysis of Risk Factors for 2-year Vascular Access Failure in the HD Patients Group
| Risk factors | Simple model |
Multiple model |
||||
|---|---|---|---|---|---|---|
| OR |
95% CI |
P |
OR |
95% CI |
P |
|
| Logistic analysis with EMP | ||||||
| DM | 1.79 | 0.69–4.65 | 0.233 | 1.92 | 0.65–5.69 | 0.238 |
| HTN | 1.18 | 0.36–3.88 | 0.791 | 1.32 | 0.29–5.98 | 0.719 |
| Old age (>50 years) | 5.31 | 1.13–24.98 | 0.035 | 6.86 | 1.21–38.84 | 0.029 |
| CD31+CD42−EMP | ||||||
| <25% | 1.00 | 1.00 | ||||
| 25–50% | 0.94 | 0.23–3.90 | 0.929 | 1.14 | 0.23–5.59 | 0.877 |
| 50–75% | 1.00 | 0.24–4.18 | 1.000 | 1.02 | 0.21–4.95 | 0.981 |
| >75% | 2.73 | 0.72–10.27 | 0.138 | 5.81 | 1.11–23.48 | 0.037 |
| CD51+EMP | ||||||
| <25% | 1.00 | 1.00 | ||||
| 25–50% | 1.41 | 0.33–5.98 | 0.644 | 0.57 | 0.09–3.65 | 0.551 |
| 50–75% | 2.50 | 0.60–10.34 | 0.206 | 1.25 | 0.25–6.33 | 0.785 |
| >75% | 1.88 | 0.45–7.82 | 0.388 | 0.74 | 0.14–4.09 | 0.731 |
| Logistic analysis with PMP* | ||||||
| DM | 1.79 | 0.69–4.65 | 0.233 | 1.55 | 0.56–4.32 | 0.472 |
| HTN | 1.18 | 0.36–3.88 | 0.791 | 1.71 | 0.44–6.65 | 0.437 |
| Old age (>50 years) | 5.31 | 1.13–24.98 | 0.035 | 6.10 | 1.19–31.39 | 0.030 |
| CD31+CD42+PMP | ||||||
| <25% | 1.00 | 1.00 | ||||
| 25–50% | 0.94 | 0.20–4.41 | 0.939 | 0.72 | 0.14–3.63 | 0.692 |
| 50–75% | 2.67 | 0.65–10.97 | 0.174 | 2.06 | 0.47–9.12 | 0.341 |
| >75% | 3.00 | 0.74–12.11 | 0.123 | 3.00 | 0.68–13.16 | 0.145 |
DM, diabetes mellitus; EMP, endothelial microparticle; HTN, hypertension; PMP, platelet-derived microparticle.
Discussion
The present study showed that HD patients had higher levels of circulating EMP and PMP than healthy participants. When diabetic or hypertensive patients were excluded, this association was also preserved. In an age-matched subgroup analysis, CD31+CD42−EMP and PMP counts were also elevated in HD patients whereas CD51+EMP counts were not. This study suggests the possibility that ESRD induces EMP and PMP generation. These findings correspond with the results from several previous studies. Levels of circulating EMP (CD144+ and CD146+) in CRF or HD patients were more elevated than in healthy controls [22]. In another study, levels of circulating MP derived from platelets (CD31+CD41+), red blood cells, and endothelial cells (CD31+CD41− and CD144+) derived from patients with ESRD were higher than healthy participants [10]. Elevated PMP counts also related to uremia [27]. It is not clear why PMP or EMP counts are higher in uremic patients. Reduced shear stress on vessel walls in ESRD patients was suggested as a determining factor for circulating EMP counts in vivo [28]. Alternatively, high shear stress in atherosclerotic arteries of most ESRD patients activated platelets, generating PMPs [29]. Moreover, PMP levels were not different according to renal replacement therapy (HD or peritoneal dialysis) or before and after HD [30]. Further work is necessary to determine the factors that directly influence MP generation in ESRD patients.
Increased levels of EMP were associated with vascular diseases such as ACS [15], [20] DM [21], lupus [31], and preeclampsia [32]. Increased PMP counts were also associated with thrombotic diseases such as venous thromboembolism [33], peripheral artery disease [34], coronary artery disease [35], and cerebrovascular infarction [36]. High levels of EMP or PMP reflect impaired endothelial dysfunction or abnormally increased thrombotic propensity. Vascular diseases showed elevated circulating EMP and PMP counts and cardiovascular diseases are known as a leading cause of death in uremic patients [37]; thus, increased EMPs or PMPs might be important markers of thrombogenic propensities in HD patients.
We found elevated CD31+CD42−EMP levels in HD patients, but CD51+EMP counts were not in age-matched subgroup analysis, even when excluding DM and HTN patients. CD31+CD42−EMP values were higher than CD51+EMP in each sample; CD51+EMP counts were only approximately 20% of CD31+CD42−EMP counts, consistent with a previous result of Bernal–Mizrachi's study [20]. Moreover, there was no significant relationship between CD31+CD42−EMPs and CD51+EMPs. There are two possible explanations for these findings. Firstly, CD31+CD42− could be better marker of EMP than CD51+ to discriminate the pathologic condition. Second, different species of EMP exist, and there are discrepancies in phenotypes of surface antigens expressed on MPs derived from endothelial cells [20], [38]. Different EMP species can reflect different kinds of endothelial injury, e.g., elevated CD31+EMP levels reflect acute endothelial injury, whereas CD51+EMP levels are associated chronic inflammation [20]. Because we did not measure acute thrombosis, it is not certain whether CD31+CD42−EMP may also reflect acute vascular events in ESRD patients. However, CD31+CD42−EMP counts were increased in the shorter vascular access survival group, suggesting that it may be a better marker for reflecting vulnerability to vascular access failure in HD patients.
However, there was a significant correlation between CD31+CD42−EMP counts and CD31+CD42+PMP counts. This finding can imply that both CD31+CD42−EMP and CD31+CD42+PMP are proportionally induced via coincidental endothelial and platelet injury in uremic condition. So, uremia can induce EMP and PMP simultaneously. However, more investigations are required to prove a definite correlation between EMP and PMP because it has been reported that EMPs can be induced by hemodialysis itself [28].
In this study, we aimed to determine the relationship between levels of MP and vascular access survival. In non-DM HD patients, CD31+CD42−EMP and CD31+CD42+PMP were significantly higher in the shorter access survival group (<1 year) than the longer access survival group (≥4 years). Despite the relatively small sample size of the shorter survival group, to our knowledge this study is the first report to show the positive relationship between high circulating MP (both EMP and PMP) levels and shorter vascular access survival. There were several previous studies to define the association between increased levels of circulating MP in uremic patients and vascular diseases. Increased EMP counts in ESRD patients are closely associated with vascular dysfunction [10] and elevated endothelial adhesion molecules [39], [40]. Higher circulating EMPs can inhibit endothelial NO pathway and surrogate markers of endothelial dysfunction in cardiovascular diseases in ESRD [10]. Elevated levels of PMP also relate to thrombotic diseases. For example, elevated PMP counts were higher in uremic patients with thrombotic events [30], whereas another recent study reported that PMP counts were not associated with shorter vascular access survival in HD patients [41]. It was not clear whether PMP counts relate to vascular access patency in HD patients. However, we found a relationship between high PMP counts and shorter vascular access survival, as well as a positive correlation between CD31+CD42−EMP and CD31+CD42+PMP. The uremic environment of coexistent uremic toxins, increased proinflammatory cytokines, and systemic atherosclerosis increases EMP counts due to endothelial injury and increased PMP counts from activated platelets may trigger thrombotic accidents in uremic patients [30].
There are some limitations to this study. First, this study was a retrospective cross-sectional study. Therefore, the effect of increased MP levels as a risk factor for vascular access failure was not definitively investigated in our study. Second, MP levels at the point of obtaining blood samples from each patient may not represent exactly the point of vascular access failure. Circulating MPs are cleared by phagocytes to prevent tissue inflammation [42], and this process may be quick. Measuring MPs at the time that stenotic or occlusive problems of vascular access developed may show a better correlation to vascular access failure. Third, MP levels at the time of access formation were not measured; thus, the baseline degrees of MP release of patients could not be provided. Finally, we did not measure objective indexes for vascular function other than taking histories of vascular access failure or physical examination.
Despite the fact that flow cytometry is an easy and fast methodology for MP measurement, there is no standardized absolute value to define pathologic MPs. However, measurement of EMP and PMP counts could allow noninvasive study of endothelial injuries and act as a good diagnostic tool for detection of thrombotic propensities in cardiovascular diseases, including renal disease.
In conclusion, our study showed that ESRD increased EMP and PMP counts in HD patients. Elevated levels of circulating EMPs and PMPs were associated with early vascular access failure in HD patients.
Conflict of interest
No conflict of interest has been declared.
Acknowledgments
This study was supported by a grant from the Korean Society of Nephrology (Baxter, 2009).
References
- 1.Roy-Chaudhury P., Kelly B.S., Zhang J., Narayana A., Desai P., Melham M., Duncan H., Heffelfinger S.C. Hemodialysis vascular access dysfunction: from pathophysiology to novel therapies. Blood Purif. 2003;21:99–110. doi: 10.1159/000067863. [DOI] [PubMed] [Google Scholar]
- 2.Roy-Chaudhury P., Sukhatme V.P., Cheung A.K. Hemodialysis vascular access dysfunction: a cellular and molecular viewpoint. J Am Soc Nephrol. 2006;17:1112–1127. doi: 10.1681/ASN.2005050615. [DOI] [PubMed] [Google Scholar]
- 3.Rekhter M., Nicholls S., Ferguson M., Gordon D. Cell proliferation in human arteriovenous fistulas used for hemodialysis. Arterioscler Thromb. 1993;13:609–617. doi: 10.1161/01.atv.13.4.609. [DOI] [PubMed] [Google Scholar]
- 4.Roy-Chaudhury P., Kelly B.S., Miller M.A., Reaves A., Armstrong J., Nanayakkara N., Heffelfinger S.C. Venous neointimal hyperplasia in polytetrafluoroethylene dialysis grafts. Kidney Int. 2001;59:2325–2334. doi: 10.1046/j.1523-1755.2001.00750.x. [DOI] [PubMed] [Google Scholar]
- 5.Viener A., Aviram M., Better O.S., Brook J.G. Enhanced in vitro platelet aggregation in hemodialysis patients. Nephron. 1986;43:139–143. doi: 10.1159/000183813. [DOI] [PubMed] [Google Scholar]
- 6.Nakamura Y., Chida Y., Tomura S. Enhanced coagulation-fibrinolysis in patients on regular hemodialysis treatment. Nephron. 1991;58:201–204. doi: 10.1159/000186415. [DOI] [PubMed] [Google Scholar]
- 7.VanWijk M.J., VanBavel E., Sturk A., Nieuwland R. Microparticles in cardiovascular diseases. Cardiovasc Res. 2003;59:277–287. doi: 10.1016/s0008-6363(03)00367-5. [DOI] [PubMed] [Google Scholar]
- 8.Diamant M., Tushuizen M.E., Sturk A., Nieuwland R. Cellular microparticles: new players in the field of vascular disease? Eur J Clin Invest. 2004;34:392–401. doi: 10.1111/j.1365-2362.2004.01355.x. [DOI] [PubMed] [Google Scholar]
- 9.Freyssinet J.M. Cellular microparticles: what are they bad or good for? J Thromb Haemost. 2003;1:1655–1662. doi: 10.1046/j.1538-7836.2003.00309.x. [DOI] [PubMed] [Google Scholar]
- 10.Amabile N., Guerin A.P., Leroyer A., Mallat Z., Nguyen C., Boddaert J., London G.M., Tedgui A., Boulanger C.M. Circulating endothelial microparticles are associated with vascular dysfunction in patients with end-stage renal failure. J Am Soc Nephrol. 2005;16:3381–3388. doi: 10.1681/ASN.2005050535. [DOI] [PubMed] [Google Scholar]
- 11.Jy W., Horstman L.L., Jimenez J.J., Ahn Y.S., Biro E., Nieuwland R., Sturk A., Dignat-George F., Sabatier F., Camoin-Jau L., Sampol J., Hugel B., Zobairi F., Freyssinet J.M., Nomura S., Shet A.S., Key N.S., Hebbel R.P. Measuring circulating cell-derived microparticles. J Thromb Haemost. 2004;2:1842–1851. doi: 10.1111/j.1538-7836.2004.00936.x. [DOI] [PubMed] [Google Scholar]
- 12.Morel O., Morel N., Hugel B., Jesel L., Vinzio S., Goichot B., Bakouboula B., Grunebaum L., Freyssinet J.M., Toti F. The significance of circulating microparticles in physiology, inflammatory and thrombotic diseases. Rev Med Int. 2005;26:791–801. doi: 10.1016/j.revmed.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 13.Zwaal R.F., Comfurius P., Bevers E.M. Platelet procoagulant activity and microvesicle formation. Its putative role in hemostasis and thrombosis. Biochim Biophys Acta. 1992;1180:1–8. doi: 10.1016/0925-4439(92)90019-j. [DOI] [PubMed] [Google Scholar]
- 14.Wolf P. The nature and significance of platelet products in human plasma. Br J Haematol. 1967;13:269–288. doi: 10.1111/j.1365-2141.1967.tb08741.x. [DOI] [PubMed] [Google Scholar]
- 15.Mallat Z., Benamer H., Hugel B., Benessiano J., Steg P.G., Freyssinet J.M., Tedgui A. Elevated levels of shed membrane microparticles with procoagulant potential in the peripheral circulating blood of patients with acute coronary syndromes. Circulation. 2000;101:841–843. doi: 10.1161/01.cir.101.8.841. [DOI] [PubMed] [Google Scholar]
- 16.Preston R.A., Jy W., Jimenez J.J., Mauro L.M., Horstman L.L., Valle M., Aime G., Ahn Y.S. Effects of severe hypertension on endothelial and platelet microparticles. Hypertension. 2003;41:211–217. doi: 10.1161/01.hyp.0000049760.15764.2d. [DOI] [PubMed] [Google Scholar]
- 17.Nomura S., Suzuki M., Katsura K., Xie G.L., Miyazaki Y., Miyake T., Kido H., Kagawa H., Fukuhara S. Platelet-derived microparticles may influence the development of atherosclerosis in diabetes mellitus. Atherosclerosis. 1995;116:235–240. doi: 10.1016/0021-9150(95)05551-7. [DOI] [PubMed] [Google Scholar]
- 18.Boulanger C.M., Scoazec A., Ebrahimian T., Henry P., Mathieu E., Tedgui A., Mallat Z. Circulating microparticles from patients with myocardial infarction cause endothelial dysfunction. Circulation. 2001;104:2649–2652. doi: 10.1161/hc4701.100516. [DOI] [PubMed] [Google Scholar]
- 19.Brodsky S.V., Zhang F., Nasjletti A., Goligorsky M.S. Endothelium-derived microparticles impair endothelial function in vitro. Am J Physiol Heart Circ Physiol. 2004;286:H1910–H1915. doi: 10.1152/ajpheart.01172.2003. [DOI] [PubMed] [Google Scholar]
- 20.Bernal-Mizrachi L., Jy W., Jimenez J.J., Pastor J., Mauro L.M., Horstman L.L., de Marchena E., Ahn Y.S. High levels of circulating endothelial microparticles in patients with acute coronary syndromes. Am Heart J. 2003;145:962–970. doi: 10.1016/S0002-8703(03)00103-0. [DOI] [PubMed] [Google Scholar]
- 21.Koga H., Sugiyama S., Kugiyama K., Watanabe K., Fukushima H., Tanaka T., Sakamoto T., Yoshimura M., Jinnouchi H., Ogawa H. Elevated levels of ve-cadherin-positive endothelial microparticles in patients with type 2 diabetes mellitus and coronary artery disease. J Am Coll Cardiol. 2005;45:1622–1630. doi: 10.1016/j.jacc.2005.02.047. [DOI] [PubMed] [Google Scholar]
- 22.Faure V., Dou L., Sabatier F., Cerini C., Sampol J., Berland Y., Brunet P., Dignat-George F. Elevation of circulating endothelial microparticles in patients with chronic renal failure. J Thromb Haemost. 2006;4:566–573. doi: 10.1111/j.1538-7836.2005.01780.x. [DOI] [PubMed] [Google Scholar]
- 23.Jy W., Horstman L.L., Arce M., Ahn Y.S. Clinical significance of platelet microparticles in autoimmune thrombocytopenias. J Lab Clin Med. 1992;119:334–345. [PubMed] [Google Scholar]
- 24.Jimenez J.J., Jy W., Mauro L.M., Horstman L.L., Ahn Y.S. Elevated endothelial microparticles in thrombotic thrombocytopenic purpura: findings from brain and renal microvascular cell culture and patients with active disease. Br J Haematol. 2001;112:81–90. doi: 10.1046/j.1365-2141.2001.02516.x. [DOI] [PubMed] [Google Scholar]
- 25.Nomura S., Shouzu A., Omoto S., Nishikawa M., Iwasaka T. Effects of losartan and simvastatin on monocyte-derived microparticles in hypertensive patients with and without type 2 diabetes mellitus. Clin Appl Thromb Hemost. 2004;10:133–141. doi: 10.1177/107602960401000203. [DOI] [PubMed] [Google Scholar]
- 26.Sabatier F., Darmon P., Hugel B., Combes V., Sanmarco M., Velut J.G., Arnoux D., Charpiot P., Freyssinet J.M., Oliver C., Sampol J., Dignat-George F. Type 1 and type 2 diabetic patients display different patterns of cellular microparticles. Diabetes. 2002;51:2840–2845. doi: 10.2337/diabetes.51.9.2840. [DOI] [PubMed] [Google Scholar]
- 27.Nomura S., Shouzu A., Nishikawa M., Kokawa T., Yasunaga K. Significance of platelet-derived microparticles in uremia. Nephron. 1993;63:485. doi: 10.1159/000187269. [DOI] [PubMed] [Google Scholar]
- 28.Boulanger C.M., Amabile N., Guerin A.P., Pannier B., Leroyer A.S., Mallat C.N., Tedgui A., London G.M. In vivo shear stress determines circulating levels of endothelial microparticles in end-stage renal disease. Hypertension. 2007;49:902–908. doi: 10.1161/01.HYP.0000259667.22309.df. [DOI] [PubMed] [Google Scholar]
- 29.Holme P.A., Orvim U., Hamers M.J., Solum N.O., Brosstad F.R., Barstad R.M., Sakariassen K.S. Shear-induced platelet activation and platelet microparticle formation at blood flow conditions as in arteries with a severe stenosis. Arterioscler Thromb Vasc Biol. 1997;17:646–653. doi: 10.1161/01.atv.17.4.646. [DOI] [PubMed] [Google Scholar]
- 30.Ando M., Iwata A., Ozeki Y., Tsuchiya K., Akiba T., Nihei H. Circulating platelet-derived microparticles with procoagulant activity may be a potential cause of thrombosis in uremic patients. Kidney Int. 2002;62:1757–1763. doi: 10.1046/j.1523-1755.2002.00627.x. [DOI] [PubMed] [Google Scholar]
- 31.Combes V., Simon A.C., Grau G.E., Arnoux D., Camoin L., Sabatier F., Mutin M., Sanmarco M., Sampol J., Dignat-George F. In vitro generation of endothelial microparticles and possible prothrombotic activity in patients with lupus anticoagulant. J Clin Invest. 1999;104:93–102. doi: 10.1172/JCI4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.VanWijk M.J., Nieuwland R., Boer K., van der Post J.A., VanBavel E., Sturk A. Microparticle subpopulations are increased in preeclampsia: possible involvement in vascular dysfunction? Am J Obstet Gynecol. 2002;187:450–456. doi: 10.1067/mob.2002.124279. [DOI] [PubMed] [Google Scholar]
- 33.Chirinos J.A., Heresi G.A., Velasquez H., Jy W., Jimenez J.J., Ahn E., Horstman L.L., Soriano A.O., Zambrano J.P., Ahn Y.S. Elevation of endothelial microparticles, platelets, and leukocyte activation in patients with venous thromboembolism. J Am Coll Cardiol. 2005;45:1467–1471. doi: 10.1016/j.jacc.2004.12.075. [DOI] [PubMed] [Google Scholar]
- 34.van der Zee P.M., Biro E., Ko Y., de Winter R.J., Hack C.E., Sturk A., Nieuwland R. P-selectin- and cd63-exposing platelet microparticles reflect platelet activation in peripheral arterial disease and myocardial infarction. Clin Chem. 2006;52:657–664. doi: 10.1373/clinchem.2005.057414. [DOI] [PubMed] [Google Scholar]
- 35.Tan K.T., Tayebjee M.H., Macfadyen R.J., Lip G.Y., Blann A.D. Elevated platelet microparticles in stable coronary artery disease are unrelated to disease severity or to indices of inflammation. Platelets. 2005;16:368–371. doi: 10.1080/00207230500120401. [DOI] [PubMed] [Google Scholar]
- 36.Lee Y.J., Jy W., Horstman L.L., Janania J., Reyes Y., Kelley R.E., Ahn Y.S. Elevated platelet microparticles in transient ischemic attacks, lacunar infarcts, and multiinfarct dementias. Thromb Res. 1993;72:295–304. doi: 10.1016/0049-3848(93)90138-e. [DOI] [PubMed] [Google Scholar]
- 37.Furie B., Furie B.C. Role of platelet p-selectin and microparticle psgl-1 in thrombus formation. Trends Mol Med. 2004;10:171–178. doi: 10.1016/j.molmed.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 38.Abid Hussein M.N., Meesters E.W., Osmanovic N., Romijn F.P., Nieuwland R., Sturk A. Antigenic characterization of endothelial cell-derived microparticles and their detection ex vivo. J Thromb Haemost. 2003;1:2434–2443. doi: 10.1046/j.1538-7836.2003.00455.x. [DOI] [PubMed] [Google Scholar]
- 39.Bonomini M., Reale M., Santarelli P., Stuard S., Settefrati N., Albertazzi A. Serum levels of soluble adhesion molecules in chronic renal failure and dialysis patients. Nephron. 1998;79:399–407. doi: 10.1159/000045084. [DOI] [PubMed] [Google Scholar]
- 40.Mezzano D., Tagle R., Pais E., Panes O., Perez M., Downey P., Munoz B., Aranda E., Barja P., Thambo S., Gonzalez F., Mezzano S., Pereira J. Endothelial cell markers in chronic uremia: relationship with hemostatic defects and severity of renal failure. Thromb Res. 1997;88:465–472. doi: 10.1016/s0049-3848(97)00280-6. [DOI] [PubMed] [Google Scholar]
- 41.Chuang Y.C., Chen J.B., Yang L.C., Kuo C.Y. Significance of platelet activation in vascular access survival of haemodialysis patients. Nephrol Dial Transplant. 2003;18:947–954. doi: 10.1093/ndt/gfg056. [DOI] [PubMed] [Google Scholar]
- 42.Morel O., Toti F., Hugel B., Bakouboula B., Camoin-Jau L., Dignat-George F., Freyssinet J.M. Procoagulant microparticles: disrupting the vascular homeostasis equation? Arterioscler Thromb Vasc Biol. 2006;26:2594–2604. doi: 10.1161/01.ATV.0000246775.14471.26. [DOI] [PubMed] [Google Scholar]