Abstract
Background
This study was performed to evaluate their 5-year survival rates and identify the factors affecting the prognosis of oral cancer patients who had undergone surgical treatment only.
Methods
Among 130 patients who were diagnosed with malignant tumor of oral, maxillofacial, and surgical treated in the Department of Oral and Maxillofacial Surgery at Chonnam National University Hospital within a period from January 2000 to December 2010, for 11 years, 84 patients were investigated who were followed up for more than 5 years after radical surgery; oral cancer is primary and received only surgical treatment. The survival rate according to gender, age, type and site of cancer, TNM stage, cervical lymph node metastasis and its stage, recurrence or metastasis, time of recurrence and metastasis, and differentiation were investigated and analyzed.
Results
Overall, 5-year survival rate in patients who received only surgical treatment was 81.2 %, and disease-specific 5-year survival rate was 83.1 %. The disease-specific 5-year survival rate based on TNM stage, metastasis of cervical lymph node, N stage, and presence of recurrence/metastasis was a significant difference (p < 0.05). The disease-specific 5-year survival rate based on sex, age, type of tumor, primary site, and differentiation was not a significant difference (p > 0.05).
Conclusions
These results suggest that good survival rate can be obtained with surgical treatment only, and stage of oral cancer, cervical lymph node metastasis and stage, recurrence or metastasis, time of recurrence, and metastasis have a significant effect on survival rate in oral cancer patients.
Keywords: Neoplasm metastasis, Oral cancer, Recurrence, Survival rate, TNM classification
Background
For the treatment of oral cancer, it is still controversial, but usually, surgical treatment is preferred in the initial oral cancer and the cases of progressed oral cancer like cervical lymph node metastasis or extracapsular spread have been performed with surgical treatment along with combination therapy and radiation therapy [1]. Recently, it has emerged as an important factor of treatment decisions, quality of life in addition to the possibility of oral cancer recovery [2].
Radiation therapy and chemotherapy in patients with oral cancer performed separately and also performed before surgery or after surgery. Radiation therapy may be used for tongue cancer effectively but is performed limitedly because of the influence on the adjacent normal tissues [3]. It induces side effects such as induction of malignant neoplasm, osteoradionecrosis (ORN), pronunciation disorders, dysphagia, dry mouth, and dental caries [4]. In addition, it has limited radiation therapy to perform radiation therapy again in the same site and it makes more complicated that there is a salvage treatment through surgery after radiotherapy [3]. Chemotherapy is applied to advanced stage, extracapsular spread, recurrence or metastasis, and the case of palliative therapy.
It is reported that the prognosis of oral cancer patients undergoing radiation therapy or combination therapy after surgical treatment is not significantly better than those who received only surgical treatment [4]. Performing only surgical treatment is preferred since it prevents the side effects of chemotherapy and radiation therapy and obtains a good result. Wolfensberger et al. reported the disease-specific 4-year survival rate of 94 % and recurrence or metastasis rate of 18 % in the 93 cases only through surgical procedures in oral cancer patients, and Lim et al. reported the disease-specific 5-year survival rate of 83 % and recurrence or metastasis rate of 21 % in the 76 cases [4, 5]. Liu et al. reported a 5-year survival rate of 72 % and recurrence or metastasis rate of 25 % in 72 cases [6].
There are a few reports on the factors that affect the prognosis of oral cancer patients. Massano et al. reported that TNM stage, extracapsular spread, resection margins of lesions, and the thickness of the tumor have high relevance to the prognosis of oral squamous carcinoma patients; Rajapakshe et al. reported that factors which affect the prognosis and survival of oral squamous carcinoma patients are TNM staging, lymph node metastasis, and the status of the resection margin of lesions [7–9].
This study was performed to evaluate their 5-year survival rates and identify the factors affecting the prognosis of oral cancer patients who had undergone surgical treatment only.
Methods
- Patients
- Among oral cancer patients who have received the surgical treatment in the Department of Oral and Maxillofacial Surgery at Chonnam National University Hospital within a period from January 2000 to December 2010, for 11 years, 84 patients were investigated who were followed up for more than 5 years, had primary oral cancer, and received only surgical treatment.
- Methods
-
Examination of patients’ medical recordsThe patient’s medical records were examined. The clinical, pathological, and medical care information were collected retrospectively. Biopsy for diagnosis, computed tomography (CT), whole body bone scan (WBBS), and positron emission computed tomography-computed tomography (PET-CT) findings of such were examined. Overall survival rates, etc. were investigated after categorization referring to the classification table (AJCC cancer staging manual 7th edition) recommended by AJCC for the distribution of the survival status and location of the oral cavity of the patient [10].
-
Surgical treatmentPatients with tumor-free dissection boundaries and who cannot get radiation treatment for cancer underwent surgical treatment. Radical resection was performed including a safety margin of 10 mm. Neck dissection was performed in 82 patients among 84 patients, bilateral supraomohyoid neck dissection (SOHND) in 75 patients, ipsilateral SOHND in 5 patients, ipsilateral SOHND and opposite SND (Selective neck dissection: level I only) in a patient, and bilateral modified radical neck dissection (MRND) in a patient. Direct closure was performed in 46 cases (55 %) of 84 cases and reconstruction in 38 cases (45 %). Local flap (16 %) for reconstruction was in 6 cases, and microvascular free flap was performed in 32 cases (84 %).
-
Prognosis assessment of patientsThe 5-year survival rate and the disease-specific 5-year survival rate were calculated. Factors affecting the prognosis of oral cancer patients, including gender and age of patients, type, stage and location of cancer, lymph node metastasis, stage of lymph node, recurrence and metastasis, time of recurrence and metastasis, and differentiation, were investigated.Standard of classification table (AJCC cancer staging manual 7th edition) recommended by the AJCC is applied to TNM classification for staging of cancer [10].
-
Statistical assessmentThe survival rate was calculated using the Kaplan Meier method, and the log rank test was performed for significance test of the predicted factors that affect the prognosis. Each analysis was performed using the SPSS 20 (IBM, Chicago, Illinois, USA).
-
Results
-
Prognosis according to gender and age
Oral cancer patients receiving surgery treatment were 58 male and 26 female, and the rate was higher 2.3 times in men, and overall, 5-year survival rate was 81.2 %. The disease-specific 5-year survival rate according to gender (women 84.6 %, men 82.5 %) showed no significant difference, and results of log rank test showed that sex does not affect prognosis (Table 1).
According to the age distribution of oral cancer patients, affected age was 60 or more (55 patients, 65 %). Disease specific 5-year survival rate by age decreased slightly with age, but there was not statistically significant difference, and the result of log rank test showed that age does not affect the prognosis (Fig. 1).
-
Prognosis according to type, site, and stage of cancer
Squamous cell carcinoma among oral cancer was the most common with 62 cases (71.2 %); the disease-specific 5-year survival rate of these patients was 82.0 %. The disease-specific 5-year survival rate according to the type of oral cancer ranged from 100 to 0 %. Results of Kaplan Meier method and log rank test showed that the type of oral cancer does not affect prognosis (Table 2).
Areas most affected by oral cancer were anterior two-thirds of the tongue followed by floor of the mouth, inferior alveolar ridge. The disease-specific 5-year survival rate with the site of oral cancer ranged from 100.0 to 69.2 %, and results of log rank test showed that site of oral cancer does not affect prognosis (Table 3).
In the stage of patients, 25 people belong to stage I, 23 people to stage II, 16 people to stage III, and 20 people to stage IV, as stage progresses, the disease-specific 5-year survival rate decreases as follows: 96.0 % of patients belong to stage I, 90.9 % of patients to stage II, 86.7 % of patients to stage III, 57.1 % of patients to stage IV, and the p value is 0.003 by log rank test results, which showed the stage was significant factor in the survival rate (Fig 2).
-
Prognosis according to metastasis of cervical lymph node and stage of cervical lymph node
The disease-specific 5-year survival rate without cervical lymph node metastasis was significantly higher than that with cervical lymph node metastasis (93.2 vs 58.3 % (Fig 3)). As N stage progressed, the disease-specific 5-year survival rate significantly decreased (p < 0.05) (Fig 4).
-
Prognosis according to recurrence/metastasis or timing of recurrence/metastasis
The disease-specific 5-year survival rate according to recurrence or metastasis recurrence or metastasis was 89.1 % in the case without recurrence or metastasis and 63.2 % with recurrence or metastasis, and whether or not, recurrence and metastasis were significant factors, since significant probability was 0.011 by log rank test results, which showed that recurrence and metastasis were significant factors in the survival rate (Fig 5). The survival rate varied according to the time of recurrence or metastasis of 19 patients who experienced postoperative recurrence or metastasis. The disease-specific 5-year survival rate of patients who experienced recurrence or metastasis within 1 year after surgery was 45.5 %, within 1–2 years was 85.7 %, and within 2–3 years was 100 %, and significant probability was 0.002 by log rank test results, which showed that the time of recurrence or metastasis after surgery was a significant factor in the survival rate (Fig 6).
Recurrence or metastasis occurs to all 18 patients (21.4 % of 84 patients), with local recurrence only occurring to 7 patients, regional recurrence only to 8 patients, local recurrence and regional recurrence to 1 patient, and with distant metastasis to 2 patients. One of 7 patients has local recurrence only, 3 of 8 patients with regional recurrence only, one patient with locoregional recurrence, and 1 of 2 patients with distant metastasis died (Table 4). It was most common that recurrence or metastasis occurred within 1 year to 10 (58 % of 18 patients) of 18 cases, within 1–2 years to seven cases (37 % of the 18 patients), within 2–3 years to one case (5 % of 18 patients), and most recurrence or metastasis occurred within 2 years (95 %) after surgery.
-
Prognosis according to differentiation
Depending on the histopathological differentiation, the disease specific 5-year survival rate was 81.7 % (58 out of 73 patients survival) for the well differentiated type, 100.0 % (10 out of 10 patients survival) for the moderately differentiated type, and 100.0 % (1 out of 1 patients survival) for the poorly differentiated type, and p value was 0.189 by log rank test results, which showed that the histopathological differentiation was not a significant factor in the survival rate (Fig 7).
Out of the 7 patients with local recurrence, 5 patients were T1-2 stage and 2 patients were T3-4 stage. Categorizing according to differentiation, well differentiated type was 5 % (4 of 73 patients) and moderately differentiated type was 30 % (3 out of 10 patients).
Table 1.
Vital status and disease specific 5-year survival rate according to gender
| Gender | No. of cases | Alive | Dead | DSS | ||||
|---|---|---|---|---|---|---|---|---|
| TFNR | TFAR | AWPRM | DTF | DWC | DPO | |||
| Male | 58 | 38 | 5 | 4 | 4 | 5 | 2 | 82.5 |
| Female | 26 | 17 | 2 | 2 | 2 | 2 | 1 | 84.6 |
| Total | 84 | 55 | 7 | 6 | 6 | 7 | 3 | 83.1 |
ATFNR alive; tumor-free; no recurrence, ATFAR alive; tumor-free; after recurrence, AWPRM alive; with persistent/recurrent/metastatic disease, DTF dead; tumor-free, DWC dead; with cancer-primary/recurrent/metastatic, DPO dead; postoperative, DSS disease specific 5-year survival rate
Fig. 1.
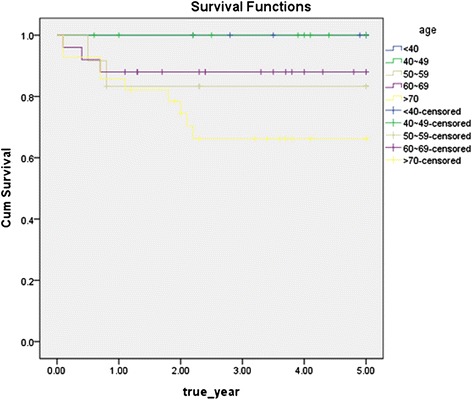
Disease specific 5-year survival rate by age group (p = 0.093)
Table 2.
Disease specific 5-year survival rate according to type of neoplasm
| Type | No. of cases (%) | Recur or meta | DSS |
|---|---|---|---|
| SCC | 62 (71.2) | 15 | 82 |
| ACC | 7 (8.2) | 1 | 100 |
| MM | 4 (4.7) | 1 | 75 |
| VC | 4 (4.7) | 0 | 100 |
| Sar | 3 (3.5) | 1 | 66.6 |
| MC | 2 (2.3) | 1 | 100 |
| BCC | 1 (1.8) | 0 | 100 |
| GCC | 1 (1.8) | 0 | 100 |
| Total | 84 (100.0) | 18 | 83.1 |
SCC squamous cell carcinoma, ACC adenoid cystic carcinoma, MM malignant melanoma, VC verrucous carcinoma, Sar sarcoma, MC mucoepidernoid carcinoma, BCC basal cell carcinoma, GCC ghost cell carcinoma, Recur recurrence, meta metastasis, DSS disease specific 5-year survival rate)
Table 3.
Disease specific 5-year survival rate according to primary site of neoplasm
| Primary site | No. of cases (%) | Recur or meta | DSS |
|---|---|---|---|
| Tongue | 18 (21.4) | 4 | 88.9 |
| FOM | 14 (16.7) | 4 | 76.9 |
| LAR | 13 (15.5) | 3 | 69.2 |
| HP | 9 (10.7) | 1 | 87.5 |
| ML | 9 (10.7) | 1 | 100 |
| RT | 7 (8.3) | 3 | 71.4 |
| SP | 6 (7.1) | 0 | 83.3 |
| BM | 5 (6.0) | 1 | 100 |
| UAR | 3 (3.6) | 1 | 100 |
| Total | 84 (100.0) | 18 | 83.1 |
Tongue anterior 2/3 of the tongue, FOM floor of mouth, LAR lower alveolar ridge, HP hard palate, ML mucosal lip, RT retromolar trigone, SP soft palate, BM buccal mucosa, UAR upper alveolar ridge
Fig. 2.
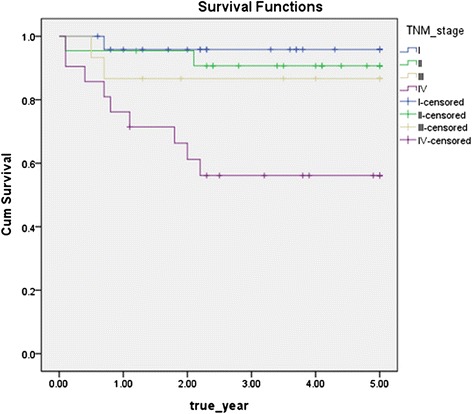
Disease specific 5-year survival rate by TNM stage (p = 0.003)
Fig. 3.
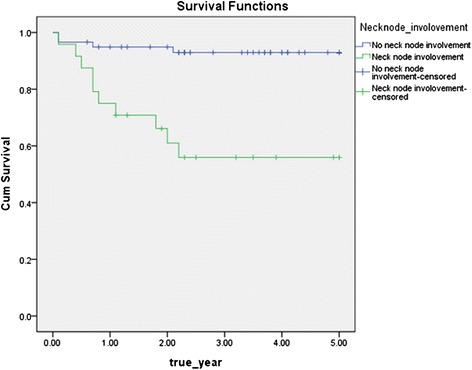
Disease specific 5-year survival rate by positive neck node (p = 0.000)
Fig. 4.
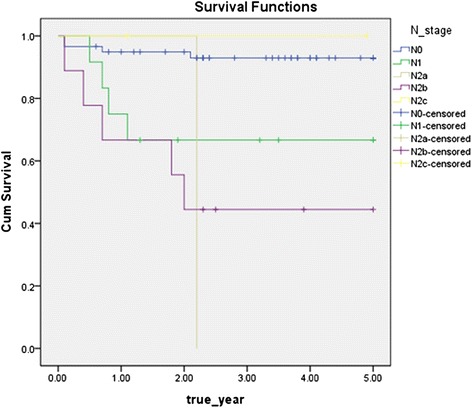
Disease specific 5-year survival rate by N stage (p = 0.000)
Fig. 5.
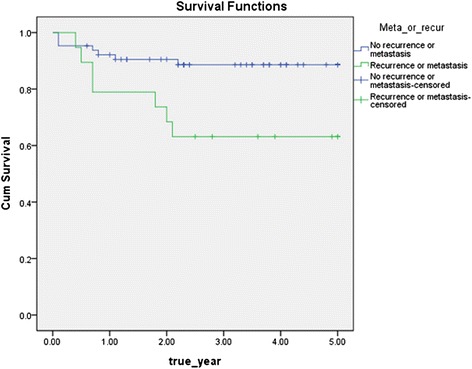
Disease specific 5-year survival rate by recurrence / metastasis (p = 0.011)
Fig. 6.
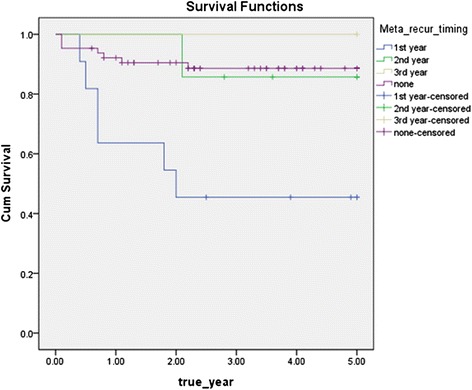
Disease specific 5-year survival rate by timing of recurrence/metastasis (p = 0.002)
Table 4.
The number of cases and death of the patients who had recurrence or meta
| Recur or meta | No. of cases (%) | No. of death |
|---|---|---|
| Local recur only | 7 (38.9) | 1 |
| Regional recur only | 8 (44.4) | 3 |
| Locoregional recur | 1 (55.6) | 1 |
| Distant meta | 2 (11.1) | 1 |
| Total | 18 (100.0) | 6 |
Recur recurrence, meta metastasis, DSS disease specific 5-year survival rate
Fig. 7.
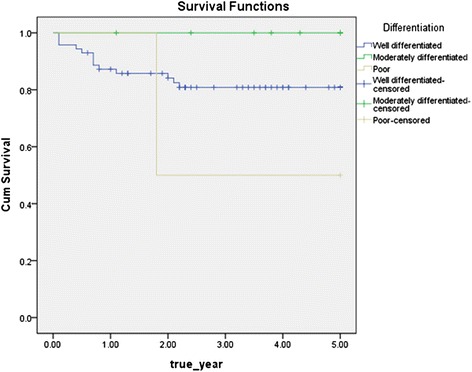
Disease specific 5-year survival rate by histopathological differentiation (p = 0.189)
Discussion
Radiotherapy or chemotherapy after the surgical procedure is largely determined by the histopathological findings of the lesion in the treatment decision of oral cancer [11, 12]. According to Brown et al., who reported that the overall 5-year survival rate of surgical treatment and surgical treatment accompanied by postoperative radiotherapy was 71 and 54 %, respectively, for 193 patients with oral squamous cell carcinoma of TNM stage I-II, good result can be obtained only through surgical treatment in the clean resection boundaries and the lesion of low stage (Stage I-II) with low recurrence probability [13]. In this study, the effect that sex, age, type of oral cancer, primary site, stage, cervical metastasis, stage of lymph node, metastasis depending on neck level, recurrence or metastasis, time of recurrence or metastasis, etc. has on survival rate was evaluated.
There are reports that showed excellent prognosis only through surgical treatment in oral cancer patients. Lim et al. reported a disease-specific 5-year survival rate of 83 % with only performing surgical procedure in 76 oral cancer patients [4]. Magge et al. reported that the prognosis of surgical treatment accompanied by postoperative radiotherapy compared to only surgical treatment did not improve [9]. In this study, disease-specific 5-year survival rate was 83.1 % only through surgical treatment for the oral cancer patients. This is similar when compared to the results reported in the other literature so far [14, 15].
In this study, with the gender distribution of oral cancer patients about 2.3:1 ratio (58 male, 26 female), the proportion of men was higher. The result was similar to gender distribution of the literature researched in Korea [4, 16]. There was no significant difference in the disease-specific 5-year survival rate by gender (male 82.5 %, female 84.6 %) as other reports (Liu et al., Roger et al.) [6, 17].
The effect that the age of oral cancer patients with surgical treatment has on prognosis has been controversial. Rogers et al. reported that as the age of the patient increase, disease-specific 5-year survival rate decreases, but Liu et al. reported that there was no significant differences statistically [6, 17]. In this study, year survival rate was slightly lower in the elderly after 50, but there was no significant difference.
With the result that squamous cell carcinoma patients only got surgical treatment, Lim et al. reported that 5-year survival rate was 83 % out of 76 patients, and Liu et al. reported that 5-year survival rate was 77 % out of 72 patients [4, 6]. In this study, the disease-specific 5-year survival rate was 82.0 % in the squamous cell carcinoma, melanoma, and sarcoma compared to the other tumor that showed a slightly lower survival rate, so the result was similar to the other literature [18–20], which was not statistically significant. The disease-specific 5-year survival rate based on type of tumor was not a significant difference.
Shah et al. reported that oral cancer showed another biological aspect according to primary site [21]. On the other hand, carcinomas on mucosal lip showed a good prognosis; carcinomas on anterior 2/3 of the tongue, floor of the mouth, and the lower alveolar ridge have high risk of metastasis to adjacent lymph nodes and showed a relatively poor prognosis. Rogers et al. reported that the disease-specific 5-year survival rate depending on primary site was 64–44 %, which was not statistically significant in the 489 oral cancer patients [17]. In this study, the disease-specific 5-year survival rate depending on primary site varied from 100.0 to 69.2 %, and there was no significant difference by the log rank test results.
Rajapakshe et al. and Geum et al. reported that TNM stage is the factor that has significant influence on the prognosis of oral cancer patients [8, 22]. In this study, as the stage increases, the disease-specific 5-year survival rate decreases (p = 0.003).
With the result only treated surgically for the 489 oral cancer patients, Rogers et al. reported that the disease specific 5-year survival rate (87 %) of the case without cervical lymph node metastasis was significantly higher than that of the case (54 %) with cervical lymph node metastasis [17]. In this study, the disease specific 5-year survival rate of the case (93.2 %) without cervical lymph node metastasis was significantly higher than that of the case (58.3 %) with cervical lymph node metastasis, which accorded with previous researches [4, 6, 17].
In study of Rogers et al., the disease-specific 5-year survival rate of N0, N1, and N2-3 stage was 87, 68, and 40 %, respectively [17]. In this study, the disease-specific 5-year survival rate according to cervical lymph node stage was 93.2 % for the N0 (60 patients), 66.7 % for the N1 (13 patients), 0 % for the N2a (a patient), 50.0 % for the N2b (8 patients), 100.0 % for the N2c (2 patients), and by the log rank test results, cervical lymph node stage had significant effects on oral cancer prognosis (p = 0.000).
Geum et al. reported that the disease-specific 5-year survival rate was 90.5 % for the patients without recurrence or metastasis and 30.0 % for the patients with recurrence or metastasis out of 37 oral cancer patients [22]. In this study, the disease-specific 5-year survival rate depending on recurrence or metastasis was 89.1 % for the case without recurrence or metastasis, was 63.2 % for the case with recurrence or metastasis, and by the log rank test results, recurrence or metastasis had an significant impact on oral cancer prognosis (p = 0.011).
Liu et al. reported that 72.2 % experienced a recurrence or metastasis after surgery within 2 years and 100 % did within 3 years out of patients with recurrence or metastasis [6]. In this study, 95 % experienced a recurrence or metastasis after surgery within 2 years and 100 % did within 3 years out of 18 cases with recurrence and metastasis, which was similar to report by Liu et al. [6].
Schwartz et al. reported that survival rate and prognosis of patients who experienced recurrence or metastasis after 6 months of primary operation were satisfactory than those of within 6 months in the study for 350 oral squamous cell carcinoma patients [23]. The disease-specific 5-year survival rate of those who experienced recurrence or metastasis within 1 year after surgery was 45.5 %, within 1~2 years was 85.7 %, and within 2~3 years was 100 %. As the recurrence or metastasis occurred early, prognosis was significantly poor, in results of the log rank test (p = 0.002).
Geum et al. reported that the disease-specific 5-year survival rate was 94.7 % for the well-differentiated type, 57.1 % for the moderately differentiated type, and 25.0 % for the poorly differentiated type related to survival rate of oral squamous cell carcinoma according to histopathological differentiation [22]. But Liu et al. reported that overall 5-year survival rate was 77.3 % for the well-differentiated type and 76.7 % for the moderately differentiated type, which was not statistically significant [6]. In this study, histologic differentiation did not have a significant impact on survival rate by the log rank test results.
Conclusions
These results suggest that good survival rate can be obtained with surgical treatment only, and stage of oral cancer, cervical lymph node metastasis and stage, recurrence or metastasis, time of recurrence, and metastasis have a significant effect on survival rate in oral cancer patients.
Consent
The authors declare that they have no competing interests.
Abbreviations
- ACC
adenoid cystic carcinoma
- ATFAR
alive; tumor-free; after recurrence
- ATFNR
alive; tumor-free; no recurrence
- AWPRM
alive with persistent/recurrent/metastatic disease
- BCC
basal cell carcinoma
- DPO
dead; postoperative
- DSS
disease specific 5-year survival rate
- DTF
dead; tumor-free
- DWC
dead; with cancer (primary/recurrent/metastatic)
- GCC
ghost cell carcinoma
- MC
mucoepidernoid carcinoma
- Meta
metastasis
- MM
malignant melanoma
- Recur
recurrence
- Sar
sarcoma
- SCC
squamous cell carcinoma
- VC
verrucous carcinoma
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
BGK obtained the measurements and data and wrote the manuscript. JHK helped in obtaining the data. MIK helped in drafting the manuscript. JJH made substantial contributions to the analysis and interpretation of the data. SGJ made substantial contributions to the analysis and interpretation of the data. HJP was involved in revising the manuscript. MSK participated in its design and coordination. SYR gave final approval of the manuscript to be published. HKO participated in its design and coordination and carefully reviewed and revised the manuscript. All authors read and approved the final manuscript.
References
- 1.Chen AY, Myers JN. Cancer of oral cavity. Curr Probl Surg. 2000;37(10):633–731. doi: 10.1016/S0011-3840(00)80018-0. [DOI] [PubMed] [Google Scholar]
- 2.Miloro M, Ghali GE, Larsen PE, Waite PD, editors. Peterson’s principles of oral and maxillofacial surgery. 3. Shelton: McGraw-Hill; 2012. p. 696. [Google Scholar]
- 3.Omura K. Current status of oral cancer treatment strategies: surgical treatments for oral squamous cell carcinoma. Int J Clin Oncol. 2014;19(3):423–30. doi: 10.1007/s10147-014-0689-z. [DOI] [PubMed] [Google Scholar]
- 4.Lim YC, Choi EC. Surgery alone for squamous cell carcinoma of the oral cavity: survival rates, recurrence patterns, and salvage treatment. Acta Otolaryngol. 2008;128:1132–1137. doi: 10.1080/00016480801901691. [DOI] [PubMed] [Google Scholar]
- 5.Wolfensberger M, Zbaeren P, Dulguerov P, Mu¨ller W, Arnoux A, Schmid S. Surgical treatment of early oral carcinoma—results of a prospective controlled multicenter study. Head Neck. 2001;23:525e–30. doi: 10.1002/hed.1073. [DOI] [PubMed] [Google Scholar]
- 6.Liu CH, Chen HJ, Wang PC, Chen HS, Chang YL. Patterns of recurrence and second primary tumors in oral squamous cell carcinoma treated with surgery alone. Kaohsiung J Med Sci. 2013;29:554–559. doi: 10.1016/j.kjms.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Massano J, Regateiro FS, Janua’rio G, Ferreira A. Oral squamous cell carcinoma: review of prognostic and predictive factors. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:67–76. doi: 10.1016/j.tripleo.2005.07.038. [DOI] [PubMed] [Google Scholar]
- 8.Rajapakshe RM, Pallegama RW, Jayasooriya PR, Siriwardena BS, Attygalla AM, Hewapathirana S, et al. A retrospective analysis to determine factors contributing to the survival of patients with oral squamous cell carcinoma. Cancer Epidemiol. 2015;39(3):360–366. doi: 10.1016/j.canep.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 9.Magge KT, Myers EN, Johnson JT. Radiation following surgery for oral cancer: impact on local control. Laryngoscope. 2003;113(6):933–935. doi: 10.1097/00005537-200306000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC cancer staging manual. 7. Chicago: Springer; 2010. pp. 29–40. [Google Scholar]
- 11.Amdur RJ, Parsons JT, Mendenhall WM, Million RR, Stringer SP, Cassisi NJ. Postoperative irradiation for squamous cell carcinoma of the head and neck: an analysis of treatment results and complications. Int J Radiat Oncol Biol Phys. 1989;16:25–36. doi: 10.1016/0360-3016(89)90006-0. [DOI] [PubMed] [Google Scholar]
- 12.Dinshaw KA, Agarwal JP, Laskar SG, Gupta T, Shrivastava SK, Cruz AD. Head and neck squamous cell carcinoma: the role of postoperative adjuvant radiotherapy. J Surg Oncol. 2005;91:48–55. doi: 10.1002/jso.20274. [DOI] [PubMed] [Google Scholar]
- 13.Brown JS, Shaw JR, Bekiroglu F, Rogers SN. Systematic review of the current evidence in the use of postoperative radiotherapy for oral squamous cell carcinoma. Br J Oral Maxillofac Surg. 2012;6:481–489. doi: 10.1016/j.bjoms.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 14.Hall J, Angele S. Radiation, DNA damage and cancer. Mol Med Today. 1999;5(4):157–164. doi: 10.1016/S1357-4310(99)01435-5. [DOI] [PubMed] [Google Scholar]
- 15.Finlay PM, Dawson F, Robertson AG, Soutar DS. An evaluation of functional outcome after surgery and radiotherapy for intraoral cancer. Br J Oral Maxillofac Surg. 1992;30:14–17. doi: 10.1016/0266-4356(92)90130-B. [DOI] [PubMed] [Google Scholar]
- 16.Lee JW, Kim JW, Kim CS. A clinic-statistical study on cervical lymph node metastasis of oral squamous cell carcinoma. J Kor Oral Maxillofac Surg. 2008;34:594–601. [Google Scholar]
- 17.Rogers SN, Brown JS, Woolgar JA, Lowe D, Magennis P, Shaw RJ, et al. Survival following primary surgery for oral cancer. Oral Oncol. 2009;45:201–211. doi: 10.1016/j.oraloncology.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 18.Padhye A, D’souza J. Oral malignant melanoma: a silent killer? J of Ind Soci of Perio. 2011;4:425–428. doi: 10.4103/0972-124X.92587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Auluck A, Zhang L, Desai R, Rosin MP. Primary malignant melanoma of maxillary gingiva—a case report and review of the literature. J Can Dent Assoc. 2008;74(4):367–371. [PubMed] [Google Scholar]
- 20.Yamaguchi S, Nagasawa H, Suzuki T, Fujii E, Iwaki H, Takagi M, et al. Sarcomas of the oral and maxillofacial region: a review of 32 cases in 25 years. Clin Oral Investig. 2004;8:52–55. doi: 10.1007/s00784-003-0233-4. [DOI] [PubMed] [Google Scholar]
- 21.Shah J, Gil Z. Current concepts in management of oral cancer—surgery. Oral Oncol. 2009;45:394–401. doi: 10.1016/j.oraloncology.2008.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geum DH, Roh YC, Yoon SY, Kim HG, Lee JH, Song JM, et al. The impact factors on 5-year survival rate in patients operated with oral cancer. J Korean Assoc Oral Maxillofac Surg. 2013;5:207–216. doi: 10.5125/jkaoms.2013.39.5.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwartz GJ, Mehta RH, Wenig BL, Shaligram C, Louis G. Salvage treatment for recurrent squamous cell carcinoma of the oral cavity. Head Neck. 2000;22(1):34–41. doi: 10.1002/(SICI)1097-0347(200001)22:1<34::AID-HED6>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]


