Abstract
Diabetes can lead to vision loss because of progressive degeneration of the neurovascular unit in the retina, a condition known as diabetic retinopathy. In its early stages, the pathology is characterized by microangiopathies, including microaneurysms, microhemorrhages, and nerve layer infarcts known as cotton-wool spots. Analyses of postmortem human retinal tissue and retinas from animal models indicate that degeneration of the pericytes, which constitute the outer layer of capillaries, is an early event in diabetic retinopathy; however, the relative contribution of specific cellular components to the pathobiology of diabetic retinopathy remains to be defined. We investigated the phenotypic consequences of pericyte death on retinal microvascular integrity by using nondiabetic mice conditionally expressing a diphtheria toxin receptor in mural cells. Five days after administering diphtheria toxin in these adult mice, changes were observed in the retinal vasculature that were similar to those observed in diabetes, including microaneurysms and increased vascular permeability, suggesting that pericyte cell loss is sufficient to trigger retinal microvascular degeneration. Therapies aimed at preventing or delaying pericyte dropout may avoid or attenuate the retinal microangiopathy associated with diabetes.
Diabetic retinopathy (DR) is among the most common complications of both type 1 and type 2 diabetes and is a leading cause of permanent vision loss worldwide.1, 2 The early stage of DR is characterized by morphological and functional abnormalities in glial and neuronal cells and degenerative changes in retinal vessels and the choriocapillaris.3, 4 As the disease progresses, vascular degeneration leads to ischemia, which induces the expression of vascular endothelial growth factor (VEGF) and retinal angiogenesis, a process called proliferative diabetic retinopathy (PDR).4, 5, 6
Both clinical and experimental data identify hyperglycemia as a large cause of the microvascular complications of DR.7 Mounting evidence also suggests that inflammation plays a key role in the pathophysiology of DR, and it has been found that VEGF is crucial for the transition from DR to PDR.8, 9, 10, 11, 12, 13, 14, 15 Although some treatments for PDR have been developed, namely pan-retinal photocoagulation, surgical removal of the vascular outgrowths, and, more recently, anti-VEGF therapy,16, 17 these treatments are not without complication. Pan-retinal photocoagulation is associated with loss of peripheral vision,18 whereas anti-VEGF therapy is approved by the Food and Drug Administration only for treatment of macular edema, which may appear at any stage of the disease. Anti-VEGF is only rarely used off-label in PDR and then mainly as a surgical adjuvant because it was found to trigger retinal detachment.19, 20 Despite some progress in the treatment of DR, the understanding of the molecular and cellular mechanisms underlying the development of DR remains incomplete. To identify more effective therapies and, perhaps, preventative interventions, it is essential to identify molecular and cellular targets that initiate the cascade of events leading to DR. It is therefore essential to develop models that allow for experimental dissection of key players.
Pericyte loss, commonly referred to as pericyte drop-out, was first described in human DR by Cogan21 in 1961 and is understood to be an early event in developing DR. The reduced number of pericytes associated with the retinal vasculature in eyes from persons with DR was found by quantifying the ratio of endothelial cells (ECs) to pericytes in normal versus diabetic retinal vasculature (1:1 in normal and 4:1 in diabetes).22 The loss of pericytes precedes the development of other microangiopathies, including microaneurysms, acellular capillaries,23, 24 vessel tortuosity, hyperpermeability, and capillary nonperfusion.21, 25, 26, 27, 28 The temporal association between pericyte loss and microangiopathy is also supported by analyses of several animal models of diabetes,29 although the analysis of causality in these models is confounded by the effects of comorbidities and obesity. Similarly, in the platelet-derived growth factor (PDGF)-B/PDGF receptor (PDGFR)β knockout mice, the developmental absence of pericytes is associated with microaneurysms and embryonic lethality.30, 31 Conditional ablation of PDGF-B in ECs,32 PDGF-B heterozygosity, and transgenic mice expressing PDGF-B hypomorphic mutants33, 34 all result in animals that are postnatally viable and indicate various degrees of pericyte deficiency (28% to 50% pericyte loss) and other microangiopathies; however, in each case the postnatal phenotypes cannot be distinguished from the developmental abnormalities. Thus, experimental demonstration of a link between pericyte loss and microvascular degeneration in the retina is lacking.
Our aim was to develop a model that would allow us to investigate the role of the pericyte in retinal microangiopathies in nondiabetic mice. We accomplished this by directly inducing death of mural cells in adult mice. With the use of this approach we have examined the acute effects of pericyte loss on the integrity and function of the adult retinal microvasculature.
Materials and Methods
Mice
Experimental (iDTR;M-Cre) and control (M-Cre) mice were generated by breeding Cre-inducible diphtheria toxin (DT) receptor transgenic mice (C57BL/6-Gt(ROSA)26Sortm1(HBEGF)Awai/J; annotated as iDTR)35 with mice expressing tamoxifen-inducible Cre under the control of the smooth muscle myosin heavy chain (SMMHC) promoter [B6.FVB-Tg(Myh11-cre/ERT2)1Soff/J; annotated as M-Cre36, 37; stock numbers 007900 and 019079; The Jackson Laboratory, Bar Harbor, ME]. Because the SMMHC promoter is Y-chromosome linked, all mice used in this study were male. The presence of the RD1 mutation was ruled out by genotyping for all experimental animal lines. Both experimental and control mice (8 weeks of age) were injected i.p. daily for 3 days with 500 ng of tamoxifen (Sigma-Aldrich, St. Louis, MO) in 225 μL of corn oil. Eight to 10 weeks after tamoxifen administration, mice were injected i.p.with 500 ng of DT (Sigma-Aldrich) in 100 μL of saline daily for 2 days. The corn oil used to dissolve tamoxifen was completely absorbed to prevent interference with the bioavailability of DT. As a negative control, some iDTR;M-Cre mice did not receive DT. Mice were euthanized for analysis 5 days after the first DT injection. Littermates were used for comparative analysis throughout. Power analysis calculations were conducted to determine the number of animals to be used on the basis of previous data from induced diabetes in mice.38 All animal protocols were approved by the Schepens Eye Research Institutional Animal Care and Use Committee.
RNA Isolation and Reverse Transcription PCR
Total RNA was isolated from mouse tissues by using phenol-chloroform extraction (TRIzol; Life Technologies, Grand Island, NY). Reverse transcription was performed with iSCRIPT (Bio-Rad, Hercules, CA). The primers used included murine Hprt1 forward, 5′-TCAGTCAACGGGGGACATAAA-3′, and reverse, 5′-GGGGCTGTACTGCTTAACCAG-3′, and simian heparin-binding epidermal growth factor-like growth factor receptor forward, 5′-GCAGATCTGGACCTTTTGAGA-3′, and reverse, 5′-CCCGGAGCTCCTTCACATATT-3′.
Real-time quantitative PCR was performed on a LightCycler 480II (Roche Diagnostics, Indianapolis, IN) by using SYBR Green PCR Master Mix (Life Technologies). To calculate the fold-change by using the standard 2-ΔΔCt formula, a Ct value of 35, corresponding to the lowest limit for detection, was used when a signal was not detected for simian heparin-binding epidermal growth factor-like growth factor.
Biochemical Measurements
Blood was drawn by submandibular bleeding, and blood glucose was measured with an ACCU-CHEK Compact Plus Glucometer (Roche Diagnostics). Blood plasma was collected postmortem, and plasma insulin was measured by ELISA (ALPCO Diagnostics, Salem, NH).
Immunostaining
Flat-mounted retinas were incubated overnight at 4°C with Griffonia simplicifolia isolectin-B4 conjugated to Alexa Fluor 488 (dilution 1:100; I21411; Life Technologies) to detect ECs or with monoclonal antibody anti–smooth muscle actin conjugated to fluorescein isothiocyanate (dilution 1:100; F3777; Sigma-Aldrich) to detect pericytes and smooth muscle cells and were visualized with an Axioskop 2 Mot Plus microscope (Carl Zeiss Inc., Thornwood, NY; used for imaging throughout). These images were used to assess the presence of microvascular abnormalities, including microaneurysms. Cell identity was established by positive staining in conjunction with morphological cues, including vascular localization to capillaries. Microaneurysms were identified as saccular out-pouchings from the vascular wall.
TUNEL Assay
After overnight immunostaining as described above, retinas were washed with phosphate-buffered saline, fixed for 10 minutes at room temperature with 4% paraformaldehyde, and washed again with phosphate-buffered saline. Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) was performed with the In Situ Cell Death Detection TMR red kit (1215672910; Roche Diagnostics) according to the manufacturer's guidelines. As positive and negative controls for each experiment, retinas from C57BL/6 mice were treated with DNase before TUNEL staining or were stained with only the TUNEL label solution without the terminal transferase, respectively. The retinas were then flat-mounted, and TUNEL-positive cells were visualized with fluorescence microscopy. The number of TUNEL-positive cells per mouse retina was quantified in images that included every visible vessel in the retina.
Elastase Digests
Eyes were fixed in 10% formalin for 3 days at 4°C. Retinas were dissected from eyes and incubated in 0.15 mol/L glycine in phosphate-buffered saline overnight at 4°C to remove excess formalin. The retinal vasculature was then isolated with elastase digestion as previously described.39, 40 Briefly, retinas were then incubated in elastase (324682; EMD Millipore, Billerica, MA) (40 U/mL elastase in 100 mmol/L sodium phosphate buffer, pH 6.5, containing 150 mmol/L sodium chloride and 5 mmol/L EDTA) for 1.5 to 2 hours at 37°C. Retinas were placed in a Petri dish filled with sterile filtered water, and the vessels were cleaned by gently removing the neural retina with the use of rat whisker brushes. Isolated vessels were then mounted to a Superfrost Plus microscope slide (12-550-15; Fisher Scientific, Pittsburg, PA), dried overnight at room temperature, then stained with periodic acid-Schiff (Sigma-Aldrich) and counterstained with hematoxylin (Sigma-Aldrich). Elastase digests were imaged by light microscopy.
Morphometry
Isolated retinal networks were analyzed to determine the number of pericytes, ECs, and acellular capillaries. The total number of pericytes and ECs in 15 images (magnification, ×700) and acellular capillaries in 10 images were quantified with an automated quantitative image analysis method that considered size and morphological cues for ECs and pericytes (Wim_EC_Perycite_Count_Beta_1.02; Wimasis Imaging Analysis, Munich, Germany). Fields near the optic nerve and near the external border were excluded from analysis. ECs and pericytes were classified on the basis of size and morphology by using previously described morphological characteristics.22, 41, 42 Acellular capillaries were defined as being a minimum of one-fourth the width of a normal capillary43, 44 and displaying a nucleus-free length that was greater than the average internuclei distance calculated as 4 (average + 2 × SD) (Wim_Acellular_Capillaries_Beta_1.03; Wimasis Imaging Analysis).
Fluorescein Dextran Perfusion
Mice were perfused with fluorescein dextran as previously described.45 Briefly, mice were anesthetized i.p with a mixture of 120 mg/kg ketamine and 20 mg/kg xylazine and perfused with 50 mg/mL fluorescein dextran (2 × 106 mw; FD2000S; Sigma-Aldrich) in 4% paraformaldehyde in phosphate-buffered saline. Eyes were then collected and fixed overnight in 4% paraformaldehyde at 4°C before whole-mount preparation.
Results
Induction of Mural Cell Death in Adult Tissues
Mice carrying a floxed allele of the iDTR, which was found by others to render mouse tissues susceptible to DT,35, 46 were bred with mice expressing tamoxifen-inducible Cre recombinase under the control of the SMMHC promoter (M-Cre).37 This promoter was chosen because of its specificity to mural cells, which include pericytes and smooth muscle cells.47 DT (1 μg in saline over 2 days) was administered 8 to 10 weeks after tamoxifen administration. Treatments for induction (tamoxifen or DT) did not influence major physiological end points; there were no significant changes to blood glucose or plasma insulin levels (Figure 1, A and B). This design allowed us to examine the effects of DT-induced cell death in retinas from adult (16- to 18-week-old), nondiabetic mice with spatial and temporal resolution.
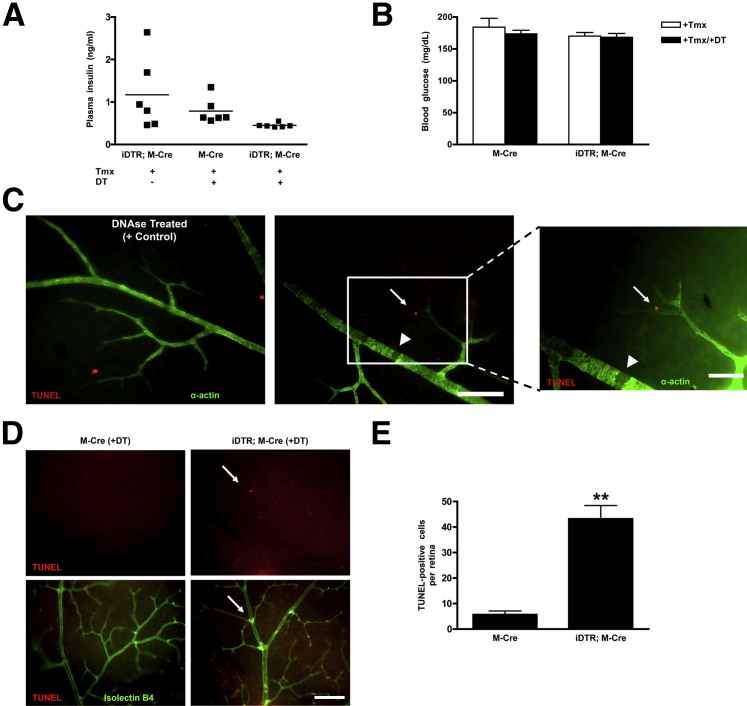
Figure 1.
Characterization of inducible mouse model of mural cell dropout. Levels of plasma insulin (A) and blood glucose (B) in M-Cre and iDTR;M-Cre mice are similar to those observed in C57BL/6J mice (0.637 ng/mL and 159 mg/dL, respectively). C: Retinal flat mounts in M-Cre (left panel) and iDTR;M-Cre (middle and right panels) mice stained for α-actin (green) to detect mural vascular cells and with TUNEL (using an in situ cell death detection kit, red). As a positive control for TUNEL staining, retinal flat mounts from M-Cre mice were treated with DNase. TUNEL-positive pericytes (arrow) as well as missing mural vascular cells (arrowhead) are observed in iDTR;M-Cre mice (middle and right panels) 5 days after DT injection. D: A TUNEL-positive cell, localized to capillary bifurcation, is observed in iDTR;M-Cre mice (arrow), demonstrating specificity of cell death in this model. E: Quantification of TUNEL-positive nuclei accomplished by counting positive cells in M-Cre and iDTR;M-Cre mice. Data are expressed as means ± SEM (A, B, and E). n = 3 M-Cre mice (E); n = 5 iDTR;M-Cre mice (E). ∗∗P < 0.01. Scale bars: 100 μm (C and D).
Five days after DT administration, TUNEL-positive cells were detected in retinal flat mounts from iDTR;M-Cre animals but were largely absent from mice lacking the iDTR transgene (Figure 1). TUNEL-positive cells were identified as mural cells on the basis of their location and costaining with α-smooth muscle actin (α-actin) antibodies, indicating the specificity of our genetic approach (Figure 1C). TUNEL-positive pericytes were identified by α-actin staining in conjunction with localization to capillary bifurcations and along the abluminal surface of the microvasculature (Figure 1D). Consistent with the expression pattern expected from the SMMHC promoter-Cre driver, TUNEL-positive staining was also observed in vascular smooth muscle cells on retinal arteries (data not shown). More than 40 TUNEL-positive mural vascular cells per retina were observed 5 days after the first DT injection (Figure 1E); these were often seen near vessels that lacked mural cell investment (Figure 1C), indicating that cell death was a dynamic process (M-Cre + DT, 5.67 ± 1.53 TUNEL-positive cells/retina, versus iDTR;M-Cre + DT, 43.20 ± 5.23 TUNEL-positive cells/retina; P = 0.0018) (Figure 1E).
Microangiopathy in Mouse Model of Inducible Mural Cell Death
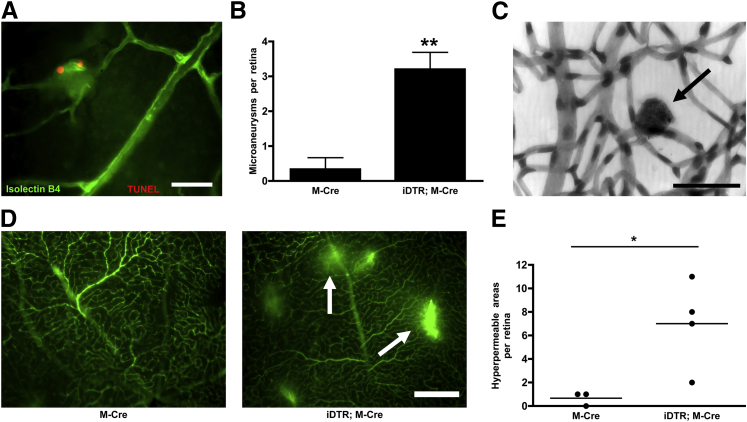
To determine whether retinal microangiopathy is directly linked to mural cell death, we examined retinas from the iDTR;M-Cre mice for the presence of microaneurysms and vascular leakage (Figure 2), both hallmarks of diabetic retinopathy.21, 25, 26, 27 An average of three microaneurysms were observed per retina in iDTR;M-Cre mice, and these were often seen in capillaries and in association with TUNEL-positive cells (M-Cre + DT, 0.33 ± 0.33 microaneurysms/retina, versus iDTR;M-Cre + DT, 3.20 ± 0.49 microaneurysms/retina; P = 0.0063) (Figure 2B). This observation indicated that microaneurysms may appear acutely after pericyte death, considering that DNA fragmentation is a late-stage event during apoptosis.48 To examine the effect of pericyte loss on barrier function of the retinal vasculature, mice were perfused with fluorescein-dextran before analysis of retina flat mounts (Figure 2D). An average of six hyperpermeable areas identified as regions of fluorescein-dextran leakage were observed per retina in iDTR;M-Cre mice, indicating breakage of the retinal-blood barrier (M-Cre + DT, 0.66 ± 0.33 hyperpermeable areas/retina, versus iDTR;M-Cre + DT, 7.00 ± 1.87 hyperpermeable areas/retina; P = 0.0363) (Figure 2E). The number of leakage events was highly variable between mice but generally affected only the capillaries (data not shown) (Figure 2).
Figure 2.
Pericyte loss in the retinal microvasculature is associated with microaneurysms and microvascular permeability. Microaneurysms (A) and their quantification (B) in retinal flat mounts labeled with isolectin-B4 to detect ECs (green) and TUNEL (red). Microaneurysms were quantified by assessing fluorescence microscopy images of M-Cre mice given tamoxifen and DT and iDTR;M-Cre mice given tamoxifen and DT. C: Microaneurysm (arrow) in retinal elastase digests stained for PAS and counterstained with hematoxylin from an iDTR;M-Cre mouse given tamoxifen and DT. D: DT-treated M-Cre and iDTR;M-Cre mice were perfused with fluorescein dextran before retina imaging by using fluorescence microscopy. E: Areas of vascular leakage (arrows) are observed in DT-treated iDTR;M-Cre mice. E: Hyperpermeable areas per retina quantified in M-Cre mice given tamoxifen and DT and iDTR;M-Cre mice given tamoxifen and DT. Data are expressed as means ± SEM (B and E). n = 3 M-Cre mice (B and E); n = 5 iDTR;M-Cre mice (B); n = 4 iDTR;M-Cre mice (E). ∗P < 0.05, ∗∗P < 0.01. Scale bars: 50 μm (A and C); 250 μm (D). PAS, periodic acid-Schiff.
Quantification of Pericyte Loss
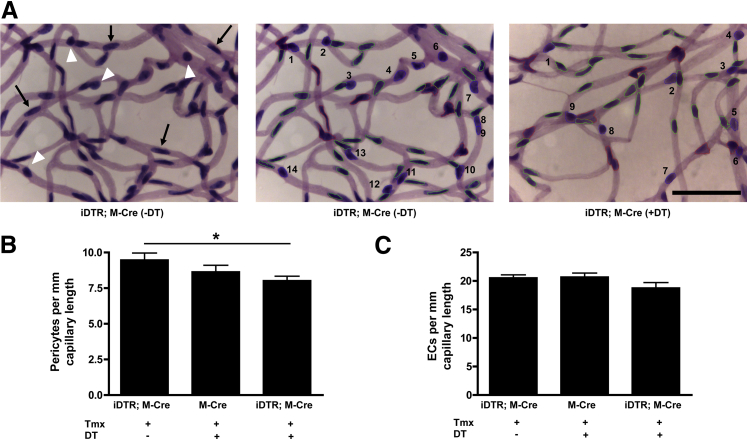
TUNEL staining of retinal flat mounts in the mouse model of inducible mural cell loss detected only the cell death occurring within that snapshot of time (Figure 1C). Some mural cell loss has already occurred in the retinas of these mice, as evidenced by a lack α-actin staining (Figure 1C). Taken together, these data indicated that the cell death process in this model of inducible mural cell loss is on-going. To quantify the total loss of retinal pericytes in this model, retinal vasculatures were isolated with elastase digestion before cell counting by using automated image analysis (Figure 3A). This analysis found that 5 days after DT administration about 7% to 15% of pericytes were lost from the retinal microvasculatures of DT-treated iDTR;M-Cre mice compared with the M-Cre and iDTR;M-Cre controls, respectively (M-Cre + DT, 8.647 ± 0.459 pericytes per mm; iDTR;M-Cre with no DT, 9.475 ± 0.490 pericytes per mm; iDTR;M-Cre + DT, 8.031 ± 0.302 pericytes per mm) (Figure 3B). This finding indicated that a relatively small loss of pericytes was sufficient to trigger microangiopathy (Figures 2 and 3). Interestingly, the number of ECs appeared to be reduced in DT-treated iDTR;M-Cre mice (8% to 9%) (Figure 3C), although this difference was not statistically significant, and we did not observe TUNEL-positive ECs in the analysis. No difference was found in capillary density among the groups (M-Cre + DT, 23.558 ± 0.583 mm capillary length per field; iDTR;M-Cre with no DT, 23.191 ± 0.397 mm capillary length per field; iDTR;M-Cre + DT, 21.913 ± 0.560 mm capillary length per field).
Figure 3.
Loss of pericytes in retinal microvasculature of an inducible model of mural cell loss. Vessels were digested from retinas by using elastase and stained with PAS and hematoxylin. A: Elastase-digested vasculature from iDTR;M-Cre retina showed pericytes (white arrowheads) and ECs (black arrows). Automated image analysis was used to quantify ECs (green) and pericytes (blue, numbered) in isolated vasculatures from iDTR;M-Cre and DT-treated iDTR;M-Cre mice. Red represents cells with overlapping nuclei that were not included in analysis. Only cells from capillaries were counted. The number of pericytes per millimeter capillary length (B) and the number of ECs per millimeter capillary length (C) were analyzed as in A by using 15 selected images per retina in iDTR;M-Cre mice given tamoxifen and no DT, M-Cre mice given tamoxifen and DT, and iDTR;M-Cre mice given tamoxifen and DT. Data are expressed as means ± SEM (B and C). n = 8 iDTR;M-Cre (−DT) mice (B and C); n = 7 M-Cre mice (B and C); n = 8 iDTR;M-Cre (+DT) mice (B and C). ∗P = 0.0251. Scale bar = 50 μm. PAS, periodic acid-Schiff.
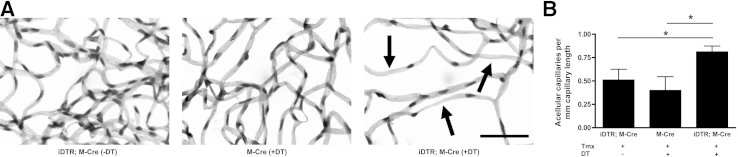
We therefore sought to determine whether this model could cause acellular capillaries. Image analysis automation was used to define acellular capillaries as those lacking nuclei in a length greater than the average internuclei distance and being a minimum of one-fourth the width of a normal capillary.43, 44 Representative images of acellular capillaries in iDTR;M-Cre mice given no DT, M-Cre mice given DT, and iDTR;M-Cre mice given DT are shown in Figure 4A. This analysis found that DT-treated iDTR;M-Cre mice had about twice as many acellular capillaries per millimeter of capillary length than the control groups (M-Cre + DT, 0.400 ± 0.147 acellular capillaries/mm; iDTR;M-Cre without DT, 0.511 ± 0.114 acellular capillaries/mm; iDTR;M-Cre + DT, 0.812 ± 0.062 acellular capillaries/mm; P < 0.05) (Figure 4B). Taken together, these findings indicated that this model of inducible mural cell death leads to vascular abnormalities, including microaneurysms, increased vascular permeability, and acellular capillaries temporally and spatially associated with the loss of pericytes.
Figure 4.
Microvascular abnormalities of retinal vessels in the retinal vasculature of DT-inducible mural cell loss. A: The vasculatures were digested from iDTR;M-Cre, DT-treated M-Cre, and DT-induced iDTR;M-Cre retinas and stained with PAS and hematoxylin. Arrows indicate acellular capillaries in elastase digests of retinal vessels of DT-treated iDTR;M Cre+ mice. B: The number of acellular capillaries per millimeter capillary length evaluated in elastase digests by using quantitative image analysis automation in 10 selected images per retina in iDTR;M-Cre mice given tamoxifen and no DT, M-Cre mice given tamoxifen and DT, and iDTR;M-Cre mice given tamoxifen and DT. Data are expressed as means ± SEM (B). n = 8 iDTR;M-Cre (−DT) mice (B); n = 7 M-Cre mice (B); n = 8 iDTR;M-Cre (+DT) mice (B). ∗P < 0.05. Scale bar = 50 μm. PAS, periodic acid-Schiff.
Discussion
Pericyte loss is an early pathological finding in DR and is temporally correlated with several other features of DR microangiopathy.21 However, whether the absence of pericytes is causally related to these changes has yet to be determined and presents a question with important clinical implications. To date, no models allow the investigation of pericyte loss in the adult retinal vasculature without the added complexity of diabetes, hyperglycemia, and/or obesity. Although mouse models that target the PDGF-B/PDGFRβ signaling pathway are available and indicate pericyte deficiency and microangiopathy,30, 49 these are models of developmental impairment, and the observations cannot be extrapolated to the adult.
To address this gap, we characterized mice that would allow us to investigate whether pericyte loss in adult mice, independent of diabetes, was sufficient to cause structural and functional deficits seen in DR. This model used a genetic approach that rendered mural cells susceptible to DT-induced cell death. Our analyses of this model found rapid and specific induction of mural cell death 5 days after DT administration. Pericyte loss was spatially and temporally associated with developing microaneurysms, acellular capillaries, and increased vascular permeability. However, a role for losing smooth muscle cells from retinal arteries cannot be formally excluded from our analysis, because these cells were also targeted in this approach.47, 50, 51 Alternative Cre drivers under the control of the neural/glial antigen 2 may further develop this model, although their use may be complicated by expression in other cell types, including neurons and glial cells.52
The analysis of the pathobiology of microaneurysms is relevant because their presence is diagnostic of early-stage DR in an ophthalmological examination.53 Microaneurysms were found in the retinas of diabetic mice fed high galactose for 21 months,43 and Akimba (Ins2Akita VEGF+/−) mice display retinal microaneurysms as early as 8 weeks of age.54 With the use of the model of induced mural cell death, saccular microaneurysms were clearly identified in the retina 5 days after the administration of DT, indicating that pericyte loss can lead to the acute formation of microaneurysms. Our data indicated that microaneursyms form at the site of pericyte loss, and we speculate that these are also the sites of leakage.
Mechanistically, the association of the pericytes with the ECs leads to deposition of the basement membrane and inhibition of both EC and pericyte migration and proliferation,55, 56, 57, 58 all hallmarks of vessel maturation. How pericytes contribute to vascular stability in adult vessels is a topic of much investigation. Relevant mechanisms may include pericyte regulation of EC–gene expression, of which an example was reported in PDGF-B mutant mice, and regulation of matrix metalloproteinases, including matrix metalloproteinases 1 and 10, via pericyte-derived expression of tissue inhibitor of metalloproteinases-3.34, 59, 60 Our model of inducible mural cell loss will allow us to look at global changes in gene expression on acute pericyte loss.
Loss of pericytes also precedes the appearance of acellular capillaries in human DR23, 24 and in many mouse models of diabetes (STZ, galactosemia, db/db, Ins2Akita).43, 44, 54, 61, 62, 63 In our model, long stretches of capillaries lacking pericytes and ECs were readily observed in iDTR;M-Cre mice treated with DT, whereas they were rare in controls. This finding supports the concept that mural cell loss alone contributes to the formation of acellular capillaries.
Acellular capillaries, regarded as one of the most significant changes to occur in DR, result in capillary nonperfusion and the development of retinal ischemia.23, 24 This retinal ischemia induces the expression of VEGF, which, in turn, leads to neovascularization in the retina.24 We speculate that the lack of pericytes results in the loss of the inhibitory effect that pericytes have on EC migration56 and proliferation,64, 65 thus making the retinal vasculature more vulnerable to VEGF stimulation.66, 67
Although macular edema is a common and vision-threatening complication of diabetes, its pathobiology remains unclear.4 Ins2Akita mice display increased vascular permeability after 12 weeks of hyperglycemia44 and the Akimba (Ins2AkitaVEGF+/−) mice exhibit vascular leakage between 8 and 16 weeks of age.54 We detected increased vascular permeability, as evidenced by the leakage of fluorescein-dextran 5 days after DT treatment, indicating that pericyte loss leads to breakdown of the blood–retinal barrier. Increased levels of VEGF appear to play a role in permeability changes associated with DR, because anti-VEGF therapy was found to reduce macular edema and is currently approved by the U.S. Food and Drug Administration for its treatment.19 However, anti-VEGF is effective in only approximately 40% of the patients,68 suggesting other mechanisms underlying the development of macular edema such as non-VEGF pathways and/or breakdown of the outer retinal barrier formed by the retinal pigment epithelium.4
A limitation of our approach is that this model does not allow for analyses of the chronic effects of pericyte loss in vascular stability. Approximately 1 week after DT injection, animals show signs of distress most likely caused by systemic effects of DT on nonvascular smooth muscle cells. Consistent with this interpretation, our mice did not show significant levels of mural cell loss from large vessels, such as the aorta (data not shown), which was found to occur in other mural cell death models.69
The inducible model of mural cell loss described here develops several features that are characteristics of early-stage or non-PDR. Despite the severity of the phenotype, the extent of pericyte loss underlying microangiopathy in this model was relatively low (7% to 15%) compared with that observed in the PDGF-B+/− mice (28%). An important difference in these two models is that in our system the mice are adults at the time of the induction of pericyte loss, and, as such, there is no opportunity for compensation, which may occur in developmental models. Thus, this inducible model of pericyte loss directly indicates the importance of pericytes in the adult retinal vasculature. Moreover, this model allows us to dissociate pericyte loss from the complex physiological aspects of diabetes to directly examine the role of pericyte loss in the development of diabetic microangiopathy. Our results indicate that pericyte protection, rescue, or replacement may be a viable therapeutic target.
Acknowledgments
We thank Dr. Magali Saint-Geniez (Schepens Eye Research Institute, Massachusetts Eye and Ear) for valuable discussions and advice, Dr. Tim Kern (Case Western Reserve University) for providing valuable advice and elastase digest methods, Dr. Christina Kaiser Marko for editorial assistance, and Vincent Primo for technical assistance to make this work possible.
C.N.V., J.F.A.-V., and P.A.D. designed experiments and wrote the manuscript. C.N.V., J.F.A.-V., L.A.K., and D.S.A. conducted research.
Footnotes
Supported by NIH grants EY005318 (P.A.D.), EY021624 (J.F.A.-V.), and K12-EY16335 (L.A.K.); American Diabetes Association Innovation Award 7-12-IN-11 (P.A.D.); American Heart Association Scientist Development grant 12SDG8960025 (J.F.A.-V.); and Massachusetts Lions Eye Research Fund (L.A.K.).
C.N.V. and J.F.A.-V. contributed equally to this work.
Disclosures: None declared.
References
- 1.Roy M.S., Klein R., O'Colmain B.J., Klein B.E., Moss S.E., Kempen J.H. The prevalence of diabetic retinopathy among adult type 1 diabetic persons in the United States. Arch Ophthalmol. 2004;122:546–551. doi: 10.1001/archopht.122.4.546. [DOI] [PubMed] [Google Scholar]
- 2.Cheung N., Mitchell P., Wong T.Y. Diabetic retinopathy. Lancet. 2010;376:124–136. doi: 10.1016/S0140-6736(09)62124-3. [DOI] [PubMed] [Google Scholar]
- 3.Cogan D.G. Diabetic retinopathy. N Engl J Med. 1964;270:787–788. doi: 10.1056/NEJM196404092701508. [DOI] [PubMed] [Google Scholar]
- 4.Antonetti D.A., Klein R., Gardner T.W. Diabetic retinopathy. N Engl J Med. 2012;366:1227–1239. doi: 10.1056/NEJMra1005073. [DOI] [PubMed] [Google Scholar]
- 5.Taylor E., Dobree J.H. Proliferative diabetic retinopathy. Site and size of initial lesions. Br J Ophthalmol. 1970;54:11–18. doi: 10.1136/bjo.54.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sapieha P., Hamel D., Shao Z., Rivera J.C., Zaniolo K., Joyal J.S., Chemtob S. Proliferative retinopathies: angiogenesis that blinds. Int J Biochem Cell Biol. 2010;42:5–12. doi: 10.1016/j.biocel.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. N Engl J Med. 2000;342:381–389. doi: 10.1056/NEJM200002103420603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adamis A.P., Miller J.W., Bernal M.T., D'Amico D.J., Folkman J., Yeo T.K., Yeo K.T. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol. 1994;118:445–450. doi: 10.1016/s0002-9394(14)75794-0. [DOI] [PubMed] [Google Scholar]
- 9.Aiello L.P., Avery R.L., Arrigg P.G., Keyt B.A., Jampel H.D., Shah S.T., Pasquale L.R., Thieme H., Iwamoto M.A., Park J.E. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331:1480–1487. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- 10.Miller J.W., Adamis A.P., Aiello L.P. Vascular endothelial growth factor in ocular neovascularization and proliferative diabetic retinopathy. Diabetes Metab Rev. 1997;13:37–50. doi: 10.1002/(sici)1099-0895(199703)13:1<37::aid-dmr174>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 11.Joussen A.M., Poulaki V., Le M.L., Koizumi K., Esser C., Janicki H., Schraermeyer U., Kociok N., Fauser S., Kirchhof B., Kern T.S., Adamis A.P. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J. 2004;18:1450–1452. doi: 10.1096/fj.03-1476fje. [DOI] [PubMed] [Google Scholar]
- 12.Antonetti D.A., Barber A.J., Bronson S.K., Freeman W.M., Gardner T.W., Jefferson L.S., Kester M., Kimball S.R., Krady J.K., LaNoue K.F., Norbury C.C., Quinn P.G., Sandirasegarane L., Simpson I.A., JDRF Diabetic Retinopathy Center Group Diabetic retinopathy: seeing beyond glucose-induced microvascular disease. Diabetes. 2006;55:2401–2411. doi: 10.2337/db05-1635. [DOI] [PubMed] [Google Scholar]
- 13.Avery R.L. Regression of retinal and iris neovascularization after intravitreal bevacizumab (Avastin) treatment. Retina. 2006;26:352–354. doi: 10.1097/00006982-200603000-00016. [DOI] [PubMed] [Google Scholar]
- 14.Mason J.O., 3rd, Nixon P.A., White M.F. Intravitreal injection of bevacizumab (Avastin) as adjunctive treatment of proliferative diabetic retinopathy. Am J Ophthalmol. 2006;142:685–688. doi: 10.1016/j.ajo.2006.04.058. [DOI] [PubMed] [Google Scholar]
- 15.Gologorsky D., Thanos A., Vavvas D. Therapeutic interventions against inflammatory and angiogenic mediators in proliferative diabetic retinopathy. Mediators Inflamm. 2012;2012:629452. doi: 10.1155/2012/629452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jardeleza M.S., Miller J.W. Review of anti-VEGF therapy in proliferative diabetic retinopathy. Semin Ophthalmol. 2009;24:87–92. doi: 10.1080/08820530902800330. [DOI] [PubMed] [Google Scholar]
- 17.Newman D.K. Surgical management of the late complications of proliferative diabetic retinopathy. Eye (Lond) 2010;24:441–449. doi: 10.1038/eye.2009.325. [DOI] [PubMed] [Google Scholar]
- 18.Tonello M., Costa R.A., Almeida F.P., Barbosa J.C., Scott I.U., Jorge R. Panretinal photocoagulation versus PRP plus intravitreal bevacizumab for high-risk proliferative diabetic retinopathy (IBeHi study) Acta Ophthalmol. 2008;86:385–389. doi: 10.1111/j.1600-0420.2007.01056.x. [DOI] [PubMed] [Google Scholar]
- 19.Massin P., Bandello F., Garweg J.G., Hansen L.L., Harding S.P., Larsen M., Mitchell P., Sharp D., Wolf-Schnurrbusch U.E., Gekkieva M., Weichselberger A., Wolf S. Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE Study): a 12-month, randomized, controlled, double-masked, multicenter phase II study. Diabetes Care. 2010;33:2399–2405. doi: 10.2337/dc10-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sohn E.H., He S., Kim L.A., Salehi-Had H., Javaheri M., Spee C., Dustin L., Hinton D.R., Eliott D. Angiofibrotic response to vascular endothelial growth factor inhibition in diabetic retinal detachment: report no. 1. Arch Ophthalmol. 2012;130:1127–1134. doi: 10.1001/archophthalmol.2012.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cogan D.G., Kuwabara T. The mural cell in perspective. Arch Ophthalmol. 1967;78:133–139. doi: 10.1001/archopht.1967.00980030135005. [DOI] [PubMed] [Google Scholar]
- 22.Speiser P., Gittelsohn A.M., Patz A. Studies on diabetic retinopathy. 3. Influence of diabetes on intramural pericytes. Arch Ophthalmol. 1968;80:332–337. doi: 10.1001/archopht.1968.00980050334007. [DOI] [PubMed] [Google Scholar]
- 23.Cogan D.G., Toussaint D., Kuwabara T. Retinal vascular patterns. IV. Diabetic retinopathy. Arch Ophthalmol. 1961;66:366–378. doi: 10.1001/archopht.1961.00960010368014. [DOI] [PubMed] [Google Scholar]
- 24.Engerman R.L. Pathogenesis of diabetic retinopathy. Diabetes. 1989;38:1203–1206. doi: 10.2337/diab.38.10.1203. [DOI] [PubMed] [Google Scholar]
- 25.Klein R., Klein B.E., Moss S.E., Davis M.D., DeMets D.L. The Wisconsin epidemiologic study of diabetic retinopathy. III. Prevalence and risk of diabetic retinopathy when age at diagnosis is 30 or more years. Arch Ophthalmol. 1984;102:527–532. doi: 10.1001/archopht.1984.01040030405011. [DOI] [PubMed] [Google Scholar]
- 26.Klein R., Klein B.E., Moss S.E., Davis M.D., DeMets D.L. The Wisconsin epidemiologic study of diabetic retinopathy. II. Prevalence and risk of diabetic retinopathy when age at diagnosis is less than 30 years. Arch Ophthalmol. 1984;102:520–526. doi: 10.1001/archopht.1984.01040030398010. [DOI] [PubMed] [Google Scholar]
- 27.Hammes H.P., Lin J., Renner O., Shani M., Lundqvist A., Betsholtz C., Brownlee M., Deutsch U. Pericytes and the pathogenesis of diabetic retinopathy. Diabetes. 2002;51:3107–3112. doi: 10.2337/diabetes.51.10.3107. [DOI] [PubMed] [Google Scholar]
- 28.Sasongko M.B., Wong T.Y., Nguyen T.T., Cheung C.Y., Shaw J.E., Wang J.J. Retinal vascular tortuosity in persons with diabetes and diabetic retinopathy. Diabetologia. 2011;54:2409–2416. doi: 10.1007/s00125-011-2200-y. [DOI] [PubMed] [Google Scholar]
- 29.Robinson R., Barathi V.A., Chaurasia S.S., Wong T.Y., Kern T.S. Update on animal models of diabetic retinopathy: from molecular approaches to mice and higher mammals. Dis Model Mech. 2012;5:444–456. doi: 10.1242/dmm.009597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Armulik A., Genove G., Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21:193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 31.Lindahl P., Johansson B.R., Leveen P., Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277:242–245. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- 32.Enge M., Bjarnegard M., Gerhardt H., Gustafsson E., Kalen M., Asker N., Hammes H.P., Shani M., Fassler R., Betsholtz C. Endothelium-specific platelet-derived growth factor-B ablation mimics diabetic retinopathy. EMBO J. 2002;21:4307–4316. doi: 10.1093/emboj/cdf418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bell R.D., Winkler E.A., Sagare A.P., Singh I., LaRue B., Deane R., Zlokovic B.V. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68:409–427. doi: 10.1016/j.neuron.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Armulik A., Genove G., Mae M., Nisancioglu M.H., Wallgard E., Niaudet C., He L., Norlin J., Lindblom P., Strittmatter K., Johansson B.R., Betsholtz C. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- 35.Buch T., Heppner F.L., Tertilt C., Heinen T.J., Kremer M., Wunderlich F.T., Jung S., Waisman A. A Cre-inducible diphtheria toxin receptor mediates cell lineage ablation after toxin administration. Nat Methods. 2005;2:419–426. doi: 10.1038/nmeth762. [DOI] [PubMed] [Google Scholar]
- 36.Indra A.K., Warot X., Brocard J., Bornert J.M., Xiao J.H., Chambon P., Metzger D. Temporally-controlled site-specific mutagenesis in the basal layer of the epidermis: comparison of the recombinase activity of the tamoxifen-inducible Cre-ER(T) and Cre-ER(T2) recombinases. Nucleic Acids Res. 1999;27:4324–4327. doi: 10.1093/nar/27.22.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wirth A., Benyo Z., Lukasova M., Leutgeb B., Wettschureck N., Gorbey S., Orsy P., Horvath B., Maser-Gluth C., Greiner E., Lemmer B., Schutz G., Gutkind J.S., Offermanns S. G12-G13-LARG-mediated signaling in vascular smooth muscle is required for salt-induced hypertension. Nat Med. 2008;14:64–68. doi: 10.1038/nm1666. [DOI] [PubMed] [Google Scholar]
- 38.Gerhardinger C., Dagher Z., Sebastiani P., Park Y.S., Lorenzi M. The transforming growth factor-beta pathway is a common target of drugs that prevent experimental diabetic retinopathy. Diabetes. 2009;58:1659–1667. doi: 10.2337/db08-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laver N.M., Robison W.G., Jr., Pfeffer B.A. Novel procedures for isolating intact retinal vascular beds from diabetic humans and animal models. Invest Ophthalmol Vis Sci. 1993;34:2097–2104. [PubMed] [Google Scholar]
- 40.Zhang Q., Guy K., Pagadala J., Jiang Y., Walker R.J., Liu L., Soderland C., Kern T.S., Ferry R., Jr., He H., Yates C.R., Miller D.D., Steinle J.J. Compound 49b prevents diabetes-induced apoptosis through increased IGFBP-3 levels. Invest Ophthalmol Vis Sci. 2012;53:3004–3013. doi: 10.1167/iovs.11-8779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuwabara T., Cogan D.G. Studies of retinal vascular patterns. I. Normal architecture. Arch Ophthalmol. 1960;64:904–911. doi: 10.1001/archopht.1960.01840010906012. [DOI] [PubMed] [Google Scholar]
- 42.Kuwabara T., Cogan D.G. Retinal vascular patterns. VI. Mural cells of the retinal capillaries. Arch Ophthalmol. 1963;69:492–502. doi: 10.1001/archopht.1963.00960040498013. [DOI] [PubMed] [Google Scholar]
- 43.Kern T.S., Engerman R.L. A mouse model of diabetic retinopathy. Arch Ophthalmol. 1996;114:986–990. doi: 10.1001/archopht.1996.01100140194013. [DOI] [PubMed] [Google Scholar]
- 44.Barber A.J., Antonetti D.A., Kern T.S., Reiter C.E., Soans R.S., Krady J.K., Levison S.W., Gardner T.W., Bronson S.K. The Ins2Akita mouse as a model of early retinal complications in diabetes. Invest Ophthalmol Vis Sci. 2005;46:2210–2218. doi: 10.1167/iovs.04-1340. [DOI] [PubMed] [Google Scholar]
- 45.Saint-Geniez M., Maharaj A.S., Walshe T.E., Tucker B.A., Sekiyama E., Kurihara T., Darland D.C., Young M.J., D'Amore P.A. Endogenous VEGF is required for visual function: evidence for a survival role on muller cells and photoreceptors. PLoS One. 2008;3:e3554. doi: 10.1371/journal.pone.0003554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brockschnieder D., Lappe-Siefke C., Goebbels S., Boesl M.R., Nave K.A., Riethmacher D. Cell depletion due to diphtheria toxin fragment A after Cre-mediated recombination. Mol Cell Biol. 2004;24:7636–7642. doi: 10.1128/MCB.24.17.7636-7642.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Joyce N.C., Haire M.F., Palade G.E. Contractile proteins in pericytes. II. Immunocytochemical evidence for the presence of two isomyosins in graded concentrations. J Cell Biol. 1985;100:1387–1395. doi: 10.1083/jcb.100.5.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Collins J.A., Schandi C.A., Young K.K., Vesely J., Willingham M.C. Major DNA fragmentation is a late event in apoptosis. J Histochem Cytochem. 1997;45:923–934. doi: 10.1177/002215549704500702. [DOI] [PubMed] [Google Scholar]
- 49.Wilkinson-Berka J.L., Babic S., De Gooyer T., Stitt A.W., Jaworski K., Ong L.G., Kelly D.J., Gilbert R.E. Inhibition of platelet-derived growth factor promotes pericyte loss and angiogenesis in ischemic retinopathy. Am J Pathol. 2004;164:1263–1273. doi: 10.1016/s0002-9440(10)63214-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.vom Hagen F., Feng Y., Hillenbrand A., Hoffmann S., Shani M., Deutsch U., Hammes H.P. Early loss of arteriolar smooth muscle cells: more than just a pericyte loss in diabetic retinopathy. Exp Clin Endocrinol Diabetes. 2005;113:573–576. doi: 10.1055/s-2005-872894. [DOI] [PubMed] [Google Scholar]
- 51.Gardiner T.A., Archer D.B., Curtis T.M., Stitt A.W. Arteriolar involvement in the microvascular lesions of diabetic retinopathy: implications for pathogenesis. Microcirculation. 2007;14:25–38. doi: 10.1080/10739680601072123. [DOI] [PubMed] [Google Scholar]
- 52.Karram K., Chatterjee N., Trotter J. NG2-expressing cells in the nervous system: role of the proteoglycan in migration and glial-neuron interaction. J Anat. 2005;207:735–744. doi: 10.1111/j.1469-7580.2005.00461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kinyoun J.L., Martin D.C., Fujimoto W.Y., Leonetti D.L. Ophthalmoscopy versus fundus photographs for detecting and grading diabetic retinopathy. Invest Ophthalmol Vis Sci. 1992;33:1888–1893. [PubMed] [Google Scholar]
- 54.Rakoczy E.P., Ali Rahman I.S., Binz N., Li C.R., Vagaja N.N., de Pinho M., Lai C.M. Characterization of a mouse model of hyperglycemia and retinal neovascularization. Am J Pathol. 2010;177:2659–2670. doi: 10.2353/ajpath.2010.090883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Orlidge A., D'Amore P.A. Inhibition of capillary endothelial cell growth by pericytes and smooth muscle cells. J Cell Biol. 1987;105:1455–1462. doi: 10.1083/jcb.105.3.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sato Y., Rifkin D.B. Inhibition of endothelial cell movement by pericytes and smooth muscle cells: activation of a latent transforming growth factor-beta 1-like molecule by plasmin during co-culture. J Cell Biol. 1989;109:309–315. doi: 10.1083/jcb.109.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stratman A.N., Malotte K.M., Mahan R.D., Davis M.J., Davis G.E. Pericyte recruitment during vasculogenic tube assembly stimulates endothelial basement membrane matrix formation. Blood. 2009;114:5091–5101. doi: 10.1182/blood-2009-05-222364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stratman A.N., Schwindt A.E., Malotte K.M., Davis G.E. Endothelial-derived PDGF-BB and HB-EGF coordinately regulate pericyte recruitment during vasculogenic tube assembly and stabilization. Blood. 2010;116:4720–4730. doi: 10.1182/blood-2010-05-286872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saunders W.B., Bohnsack B.L., Faske J.B., Anthis N.J., Bayless K.J., Hirschi K.K., Davis G.E. Coregulation of vascular tube stabilization by endothelial cell TIMP-2 and pericyte TIMP-3. J Cell Biol. 2006;175:179–191. doi: 10.1083/jcb.200603176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Davis G.E., Senger D.R. Extracellular matrix mediates a molecular balance between vascular morphogenesis and regression. Curr Opin Hematol. 2008;15:197–203. doi: 10.1097/MOH.0b013e3282fcc321. [DOI] [PubMed] [Google Scholar]
- 61.Midena E., Segato T., Radin S., di Giorgio G., Meneghini F., Piermarocchi S., Belloni A.S. Studies on the retina of the diabetic db/db mouse. I. Endothelial cell-pericyte ratio. Ophthalmic Res. 1989;21:106–111. doi: 10.1159/000266787. [DOI] [PubMed] [Google Scholar]
- 62.Martin P.M., Roon P., Van Ells T.K., Ganapathy V., Smith S.B. Death of retinal neurons in streptozotocin-induced diabetic mice. Invest Ophthalmol Vis Sci. 2004;45:3330–3336. doi: 10.1167/iovs.04-0247. [DOI] [PubMed] [Google Scholar]
- 63.Feit-Leichman R.A., Kinouchi R., Takeda M., Fan Z., Mohr S., Kern T.S., Chen D.F. Vascular damage in a mouse model of diabetic retinopathy: relation to neuronal and glial changes. Invest Ophthalmol Vis Sci. 2005;46:4281–4287. doi: 10.1167/iovs.04-1361. [DOI] [PubMed] [Google Scholar]
- 64.Antonelli-Orlidge A., Saunders K.B., Smith S.R., D'Amore P.A. An activated form of transforming growth factor beta is produced by cocultures of endothelial cells and pericytes. Proc Natl Acad Sci U S A. 1989;86:4544–4548. doi: 10.1073/pnas.86.12.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hirschi K.K., Rohovsky S.A., Beck L.H., Smith S.R., D'Amore P.A. Endothelial cells modulate the proliferation of mural cell precursors via platelet-derived growth factor-BB and heterotypic cell contact. Circ Res. 1999;84:298–305. doi: 10.1161/01.res.84.3.298. [DOI] [PubMed] [Google Scholar]
- 66.Darland D.C., Massingham L.J., Smith S.R., Piek E., Saint-Geniez M., D'Amore P.A. Pericyte production of cell-associated VEGF is differentiation-dependent and is associated with endothelial survival. Dev Biol. 2003;264:275–288. doi: 10.1016/j.ydbio.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 67.Ejaz S., Chekarova I., Ejaz A., Sohail A., Lim C.W. Importance of pericytes and mechanisms of pericyte loss during diabetes retinopathy. Diabetes Obes Metab. 2008;10:53–63. doi: 10.1111/j.1463-1326.2007.00795.x. [DOI] [PubMed] [Google Scholar]
- 68.Ho A.C., Scott I.U., Kim S.J., Brown G.C., Brown M.M., Ip M.S., Recchia F.M. Anti-vascular endothelial growth factor pharmacotherapy for diabetic macular edema: a report by the American Academy of Ophthalmology. Ophthalmology. 2012;119:2179–2188. doi: 10.1016/j.ophtha.2012.07.058. [DOI] [PubMed] [Google Scholar]
- 69.Clarke M.C., Figg N., Maguire J.J., Davenport A.P., Goddard M., Littlewood T.D., Bennett M.R. Apoptosis of vascular smooth muscle cells induces features of plaque vulnerability in atherosclerosis. Nat Med. 2006;12:1075–1080. doi: 10.1038/nm1459. [DOI] [PubMed] [Google Scholar]